Abstract
Background:
CD47, a glycoprotein on red blood cell membranes, inhibits phagocytosis via interaction with signal regulatory protein α on phagocytes. Our previous research has demonstrated that blocking CD47 accelerates hematoma clearance and reduces brain injury after intracerebral hemorrhage. The current study investigated whether phagocytosis or erythrocyte CD47 impacts hematoma resolution and hydrocephalus development after intraventricular hemorrhage (IVH).
Methods:
Adult (3-month-old) male Fischer 344 rats were intraventricularly injected with 200 μl autologous blood, mixed with either CD47 blocking antibody or isotype IgG, or 200 μl saline as control. In subgroups of CD47 blocking antibody treated rats, clodronate liposomes (to deplete microglia/monocyte-derived macrophages) or control liposomes were co-injected. Magnetic resonance imaging (MRI) was used to evaluate ventricular volume and intraventricular T2* lesion volume (estimating hematoma volume). The brains were harvested after 4 or 72 hours for histology to evaluate phagocytosis.
Results:
In adult male rats, CD47 blocking antibody alleviated hydrocephalus development by day 3. In addition, the CD47 blocking antibody reduced intraventricular T2* lesion and T2* non-hypointense lesion size after IVH through day 1 to day 3. Erythrophagocytosis was observed as soon as 4 hours after IVH and was enhanced on day 3. Furthermore, intra-hematoma infiltration of CD68, heme oxygenase-1 and ferritin positive phagocytes were upregulated by CD47 blockade by day 3. Clodronate liposomes co-injection caused more severe hydrocephalus and weight loss.
Conclusion:
Blocking CD47 in the hematoma accelerated hematoma clearance and alleviated hemolysis and hydrocephalus development after IVH, suggesting CD47 might be valuable in the future treatment for IVH.
Keywords: CD47 Blocking antibody, Hydrocephalus, Intraventricular Hemorrhage, Macrophage
Background
Adult intraventricular hemorrhage (IVH) frequently occurs after intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH), and it is associated with worse functional outcomes. Thus, IVH is an independent indicator of higher mortality and morbidity in both ICH and SAH patients(Hanley, 2009, Rosen et al., 2007). Meanwhile, preterm neonates are at high risk of IVH due to germinal matrix hemorrhage(Strahle et al., 2012). Hydrocephalus is generally characterized as the excessive accumulation of cerebrospinal fluid (CSF) within the cranium, which may associate with dilation of cerebral ventricles. Post-hemorrhagic hydrocephalus (PHH) develops in up to 50–55% of adult IVH patients(Bhattathiri et al., 2006, Balami and Buchan, 2012) and 30% of infants with high-grade IVH(Christian et al., 2016). And it is a predictor of poor prognosis and worse neurodevelopmental outcomes, which require continuous care such as external ventricular drainage(Garton et al., 2017, Adams-Chapman et al., 2008).
Larger IVH volumes are associated with worse outcomes and an increased rate of PHH(Bisson et al., 2020, Balami and Buchan, 2012). As yet, IVH treatment approaches are limited to supportive care and complication management. Clinical trials have investigated whether intraventricular recombinant tissue plasminogen activator (rt-PA) administration could benefit IVH patients by removing blood clots from the ventricular system. However, the most recent multicenter randomized, placebo-controlled trial CLEAR III (NCT00784134) with 500 patients and 180-day follow-up data showed no significant neurofunctional outcome improvement between rt-PA treatment and saline control(Hanley et al., 2017). Further translational studies are needed to provide new therapeutic strategies for IVH. An alternative approach of surgically hematoma removal in ICH is accelerating phagocytosis via microglia/monocyte-derived macrophages (M/Mo-MΦ) system(Wilkinson et al., 2018, Tao et al., 2020, Jing et al., 2019, Chang et al., 2020). By enhancing erythrophagocytosis after ICH, the brain may be protected from neurotoxic components of the hematoma (e.g., hemoglobin, iron, peroxiredoxin-2). However, the role of the M/Mo-MΦ system in hematoma clearance after IVH is still incompletely understood.
CD47 is a glycoprotein expressed on red blood cell (RBC) membranes and other cell surfaces. It inhibits phagocytosis (i.e., it acts as a don’t-eat-me signal) by interacting with signal regulatory protein α (SIRP α) on phagocytes such as M/Mo-MΦ (Olsson et al., 2006). Our previous studies have demonstrated that genetic knock-out of CD47 or a CD47 blocking antibody can accelerate hematoma clearance, reduce brain edema, diminish neuronal death and improve neurofunctional outcome after ICH in both mice(Ni et al., 2016, Jing et al., 2019) and aged rats(Tao et al., 2020).
The current study investigated the role of M/Mo-MΦ in hematoma clearance after IVH. It particularly examined the effect of CD47 blocking antibody on hydrocephalus development and hematoma resolution after experimental IVH in rats.
Methods
Animals and IVH
Animal use protocol approval was obtained from the University of Michigan Committee on Use and Care of Animals. The study followed the ARRIVE guidelines for the reporting of in vivo animal experiments(Percie du Sert et al., 2020). Fifty-eight adult male Fischer 344 rats (3-month-old, bodyweight 230–330g, Envigo, Indianapolis, IN) were used in this research. Rats were housed on a 12 hours light-dark cycle, maintained in a temperature-controlled room, and provide with food and water. To establish an IVH model, rats were anesthetized with pentobarbital (50 mg/kg; intraperitoneally) and core body temperature was maintained by a feedback heated blanket. Autologous arterial blood was obtained by right femoral artery cannulation with a polyethylene catheter. After positioning the rat in a stereotactic frame (Kopf Instruments, Tujunga, CA), a 5 mm midline scalp incision was made, and a 1 mm cranial burr hole was drilled (0.6 mm posterior, 1.6 mm lateral to bregma). A 26-gauge needle was inserted through the burr hole into the right ventricle (4.5mm ventral to the cranium). A microinfusion pump was used to infuse 200 μl autologous arterial blood or saline at a rate of 14 μl/min. The needle was removed 5 min after injection, and the burr hole was sealed by bone wax and the incision was sutured.
Experimental Groups
Adult male F344 rats received an intraventricular injection of 200 μl autologous blood with either anti-CD47 blocking antibody (Clone OX101, 10 μg/ml, final concentration in blood, n=9) or IgG (10 μg/ml, final concentration in blood, n=9). Control rats received an intraventricular injection of 200 μl saline (n=7).
In subgroups of the anti-CD47 blocking antibody treatment group, rats received 200 μl autologous blood with anti-CD47 blocking antibody (10 μg/ml, final concentration in blood) co-injected with 5 μl clodronate liposomes (FormuMax, F70101C-A) or control liposomes (FormuMax, F70101-A).
MRI scans were performed at 4, 24 and 72 hours after injection and the rats were euthanized at 4 or 72 hours post-surgery. Simple randomization was used to assign animals into different groups. Brains were harvested after euthanasia for histology studies.
Eight rats died after intraventricular blood injection; the death rate of each group was: IVH + anti-CD47 group (2/11), IVH + IgG (1/10), saline group (0/7), IVH + clodronate liposomes group (3/10), IVH + control liposomes group (2/9). All dead animals were excluded, and one rat from the IgG group was excluded for unclear MRI images.
Magnetic Resonance Imaging and Measurements
MRI scans were performed by a 9.4 Tesla MR scanner (Varian Inc., Palo Alto, CA) at different time-point following IVH. Rats were anesthetized with 2% isoflurane and air mixture. T2 fast spin-echo and T2* gradient-echo sequences were performed with a view field of 35×35 mm, matrix 256×128 mm, thickness 0.5 mm (a total of 25 coronal slices were obtained).
Bilateral ventricle volume was measured and calculated with T2 sequence as described earlier(Chen et al., 2011). In brief, ventricle volume was calculated by summing the ventricular area in each slice and multiplying by slice thickness. The percent change in ventricle volume over time was calculated with the following formula: (72h ventricle volume – 24h ventricle volume)/ 24h ventricle volume × 100%. Intraventricular T2* lesion volume and T2* non-hypointense lesion volume were measured and calculated with T2* sequence. The intraventricular T2* lesion represents hematoma size in the acute phase (24h and 72h). T2* lesion volume was obtained by summing outlined intraventricular lesions in each slice and multiplying by slice thickness. The T2* non-hypointense lesion represents hemolysis as we previously reported(Dang et al., 2017). Iso- and hyper- intense signal inside the hematoma was outlined in each slice, summed and multiplied by slice thickness to obtain the T2* non-hypointense lesion volume. The ratio of T2* non-hypointense lesion to T2* lesion was calculated by T2* non-hypointense lesion volume/ T2* lesion volume × 100%. All MRI results were analyzed with NIH Image J by a blinded researcher.
Brain Histology and H&E Staining
Rats were euthanized with a fatal dose of pentobarbital (100 mg/kg, intraperitoneally) and underwent trans-cardiac perfusion with 4% paraformaldehyde in 0.1M phosphate-buffered saline (PBS, PH=7.4). The harvested brains were immersed in 4% paraformaldehyde for 24 hours before being transferred to 30% sucrose in 0.1M PBS at 4 °C until they fully sank to the bottom. Brains were embedded in a mixture of optimal cutting temperature compound (Thermo Fisher Scientific, Waltham, MA) and 30% sucrose before being coronally sectioned (18 μm) on a cryostat (Thermo Fisher Scientific). Hematoxylin and eosin (H&E) staining was performed following a standard protocol for observation of the intraventricular lesion.
Immunohistochemistry and Immunofluorescence Double Labeling
The protocols of immunohistochemistry and immunofluorescence staining have been described previously(Xi et al., 1999, Wan et al., 2020). The primary antibodies were rabbit anti-heme oxygenase-1 (HO-1, 1:400 dilution; Enzo, New York, NY), goat anti-Iba1 (1:400 dilution; Abcam, Cambridge, MI), mouse anti-CD68 (1:200 dilution; Abcam), rabbit anti-ferritin (1:200 dilution; Abcam). Negative controls were conducted by omitting the primary antibody. Secondary antibodies were horse anti-mouse (1:500 dilution, Vector Laboratories Inc, Burlingame, CA), goat anti-rabbit (1:500 dilution, Invitrogen, Eugene, OR), rabbit anti-goat (1:500 dilution, Invitrogen), Alexa Fluor 594 donkey anti-goat IgG (1:500 dilution; Invitrogen), Alexa Fluor 488 donkey anti-rabbit IgG (H+L) (1:500 dilution; Invitrogen). Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, St. Louis, MO) in immunofluorescence studies and hematoxylin for immunohistochemistry.
Cell Counting
Brain coronal sections were used for histology staining and observed under a microscope. Three high power images (40×) were obtained by a digital camera from the target area of each animal. Immuno-positive cells were counted 3 times by a blinded researcher using Image J, and the mean value in each animal was used for analysis.
Statistical analysis
All data in this article are presented as mean ± standard deviation (SD) and analyzed by Student t-test or one-way ANOVA using a Tukey post hoc test. Differences were considered significant at p < 0.05.
Results
CD47 Blocking Antibody Attenuates Hydrocephalus Development After Experimental IVH
Serial T2 weighted MRI scans were performed to estimate ventricle volume after intraventricular injection of 200 μl autologous arterial mixed with either CD47 blocking antibody or isotype IgG or 200 μl saline as control. Rats exhibited bilateral ventricular enlargement after IVH compared to intraventricular saline injection from day 1 to day 3 (p<0.001, Fig. 1A, B). Ventricular volumes were similar between IgG co-injection (IVH+IgG) group and anti-CD47 blocking antibody co-injection (IVH+anti-CD47) group at 4h post-surgery (72.7 ± 24.3 vs. 62.5 ± 16.8 mm3 in IVH+IgG, n=5–6 per group, p = 0.436, Supplemental Figure. 1A, B). 24 hours post-surgery, ventricular volumes were not significantly different between IVH+IgG group and IVH+anti-CD47 group (46.7 ± 22.7 vs. 65.9 ± 18.6 mm3 in IVH+IgG, n=9 per group, p = 0.068, Fig. 1B). However, at 72h post-hemorrhage, ventricle volumes in the IVH+anti-CD47 group (33.7 ± 16.8 mm3) were significantly smaller compared to the IVH+IgG group (56.9 ± 19.4 mm3, n=9 per group, p=0.015, Fig. 1B). To normalize the initial difference of ventricle volume between animals, percent changes in ventricle volumes were calculated as (72h ventricle volume – 24h ventricle volume)/ 24h ventricle volume × 100%. Anti-CD47 blockade significantly attenuated hydrocephalus development from day 1 to day 3 (−27.5 ± 10.5 % vs. −14.5 ± 10.2 % in IVH+IgG, n=9 per group, p=0.017, Fig. 1C).
Figure 1.
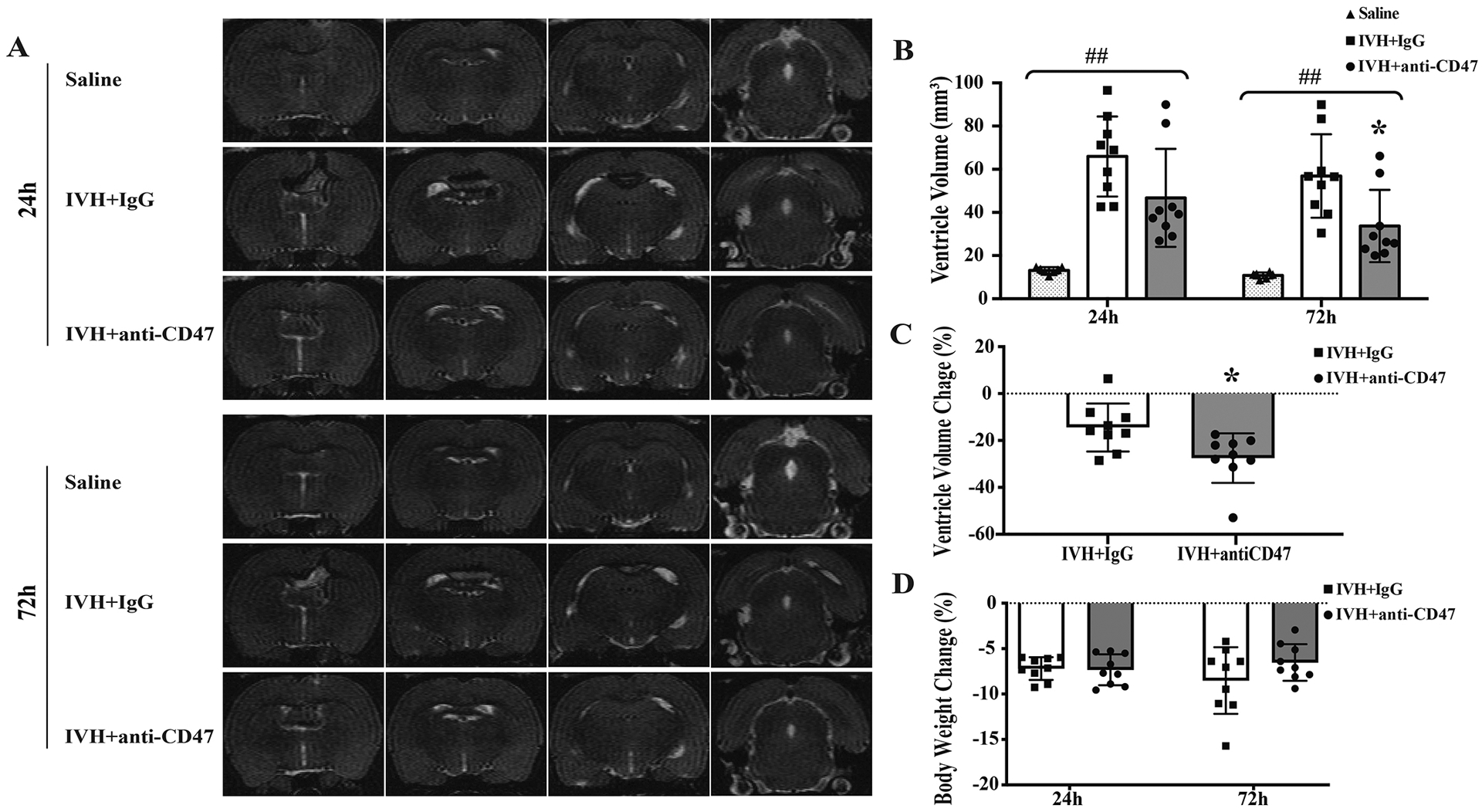
(A) Representative T2-weighted MRI scans at 24 and 72 hours after intraventricular injection of 200 μl saline (Saline) as control or 200 μl autologous blood with IgG (IVH+IgG) or CD47 blocking antibody (IVH+anti-CD47) in F344 rats. Note the significant ventricular dilation in rats with IVH compared to saline control. (B) Ventricular volumes were quantified using the T2-weighted images in the three groups. The CD47 blocking antibody significantly reduced the ventricular dilation after IVH. (C) The percent changes in ventricular dilation between 24 and 72 hours also significantly increased the CD47 antibody group. (D) Percentage changes from pre-IVH body weights 24 and 72 hours after IVH. For all graphs, data are shown as mean ± SD; n = 6–7 in saline group, n = 9 in IVH groups; ## p< 0.01 between groups by one-way ANOVA with Tukey post hoc test, * p < 0.05 IVH+anti-CD47 vs. IVH+IgG group.
Percent changes in body weight from pre-surgery were monitored 24h and 72h after IVH. All animals experienced weight loss after IVH. At 24 hours post-hemorrhage, percentage weight change was similar between IVH+anti-CD47 and IVH+IgG groups (−7.3 ± 1.7 vs. −7.2 ± 1.2 %, respectively, n=9 per group, p=0.842, Fig. 1D). At 72 hours post-hemorrhage, percent weight reductions tended to be smaller in the IVH+anti-CD47 group though not statistically significant (−6.5 ± 2.0 vs. −8.5 ± 3.7 % in IVH+IgG, n=9 per group, p=0.174, Fig. 1D).
CD47 Blocking Antibody Accelerates Hematoma Clearance After Experimental IVH
Serial T2* weighted MRI scans were used to assess intraventricular hematoma size after intraventricular injection of 200 μl autologous arterial blood (Fig. 2). Intraventricular lesion observed 4h post-surgery showed no difference IVH+anti-CD47 and IVH+IgG groups (51.1 ± 30.5 vs. 51.1 ± 12.4 mm3 respectively, n=5–6 per group, p=1.000, Fig. 2B). However, at 24h post-hemorrhage, hematoma volumes in the IVH+anti-CD47 group (22.5 ± 21.6 mm3) were significantly smaller than in the IVH+IgG group (49.6 ± 16.9 mm3, n=9 per group, p=0.009, Fig. 2B). This difference of intraventricular lesion persists for 72h post-hemorrhage, with the IVH+anti-CD47 group having significantly smaller intraventricular hematomas compared to the IVH+IgG group (17.4 ± 18.0 vs. 41.2 ± 13.6 mm3, respectively, n=9 per group, p=0.006, Fig. 2B).
Figure 2.
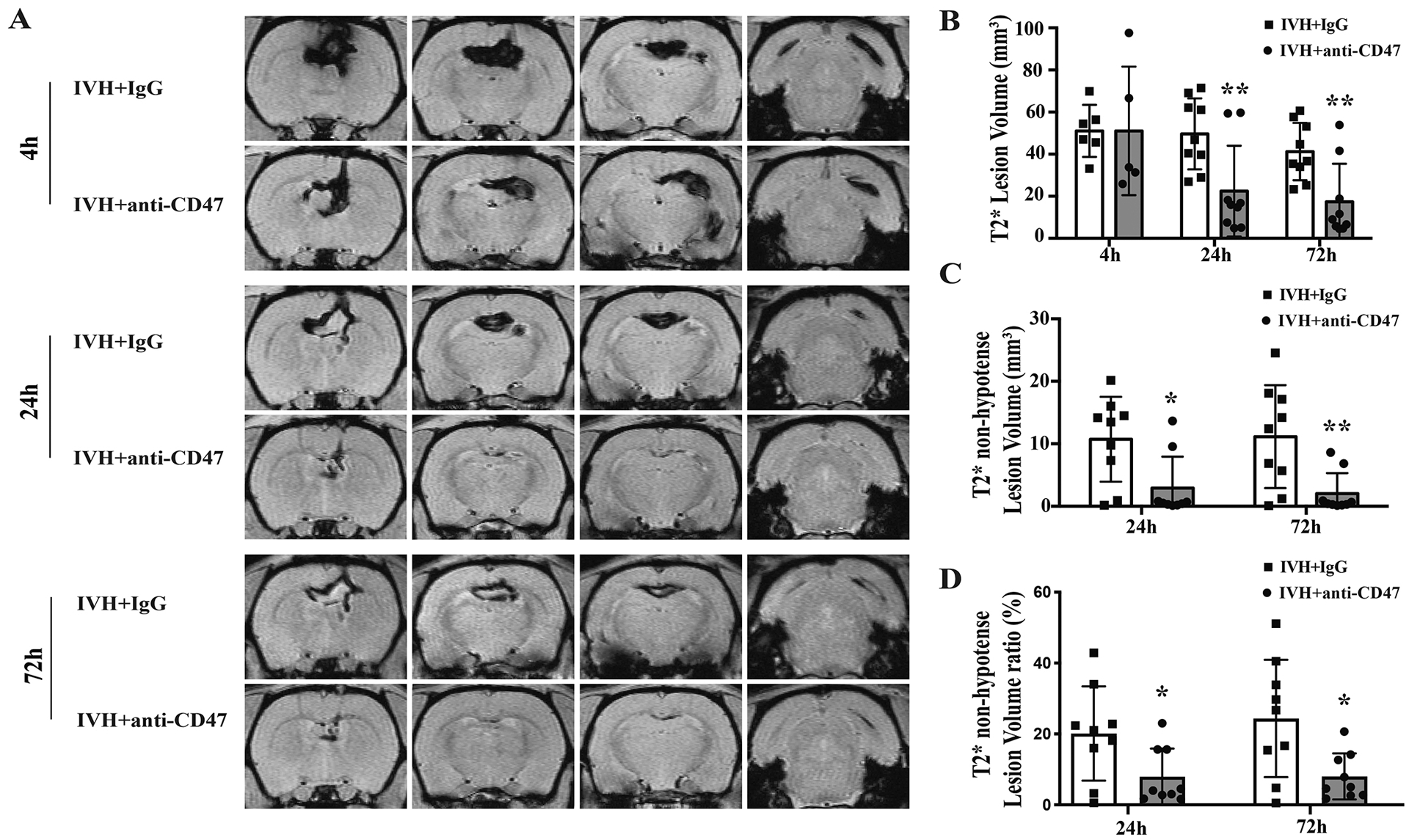
(A) Representative images of T2*-weighted MRI scans from 4, 24 and 72 hours after intraventricular injection of 200 μl autologous blood with IgG (IVH+IgG) or CD47 blocking antibody (IVH+anti-CD47) in F344 rats. Intraventricular lesion and intra-hematoma non-hypointense lesions can be noted. (B) Intraventricular lesion volumes were quantified using T2*-weighted images at 4, 24- and 72-hours post-surgery. Note the significant reduction at 24 and 72 hours in the anti-CD47 group. (C) Intraventricular non-hypointense lesion volumes were quantified using T2*-weighted images at 24- and 72-hours post-surgery. Note again the significant reduction at 24 and 72 hours in the anti-CD47 group. (D) The ratios of intraventricular non-hypointense lesion volume to T2* lesion volume were calculated at 24- and 72-hours post-surgery and showed the relative reduction in non-hypointense lesion volume in the anti-CD47 group. Values are shown as mean ± SD; n = 5–6 per group in 4h, n = 9 per group in 24h and 72h, * p < 0.05 IVH+anti-CD47 vs. IVH+IgG group; ** p < 0.01 IVH+anti-CD47 vs. IVH+IgG group.
CD47 Blocking Antibody Alleviated Hematoma Hemolysis After Experimental IVH
Our prior studies found early hemolysis in the hematoma core after ICH using histology and a T2* non-hypointense signal on MRI(Dang et al., 2017). In aged rats, CD47 blocking antibody could alleviate the early hemolysis after ICH(Tao et al., 2020). In the current study, a T2* non-hypointense signal was also found in the hematoma core (Figs. 2, 3) from 4h after IVH. T2* non-hypointense lesion volumes at 24 and 72 hours were calculated. The T2* non-hypointense lesion volume in the IVH+anti-CD47 group was significantly smaller than that in the IVH+IgG group at both 24 h (2.9 ± 5.0 vs. 10.7 ± 6.8 mm3 with IVH+IgG, n=9 per group, p=0.013, Fig. 2C) and 72h post-hemorrhage (2.0 ± 3.3 vs. 11.1 ± 8.2 mm3 in IVH+IgG, n=9 per group, p=0.007, Fig. 2C). Moreover, the ratios of T2* non-hypointense lesion volume to T2* lesion volume were significantly decreased in IVH+anti-CD47 group at both 24h (8.0 ± 8.0 vs. 20.1 ± 13.3% in IVH+IgG, n=9 per group, p=0.032, Fig. 2D) and 72 h (8.0 ± 6.5 vs. 24.5 ± 16.6% in IVH+IgG, n=9 per group, p=0.014, Fig. 2D) after IVH.
Figure 3.
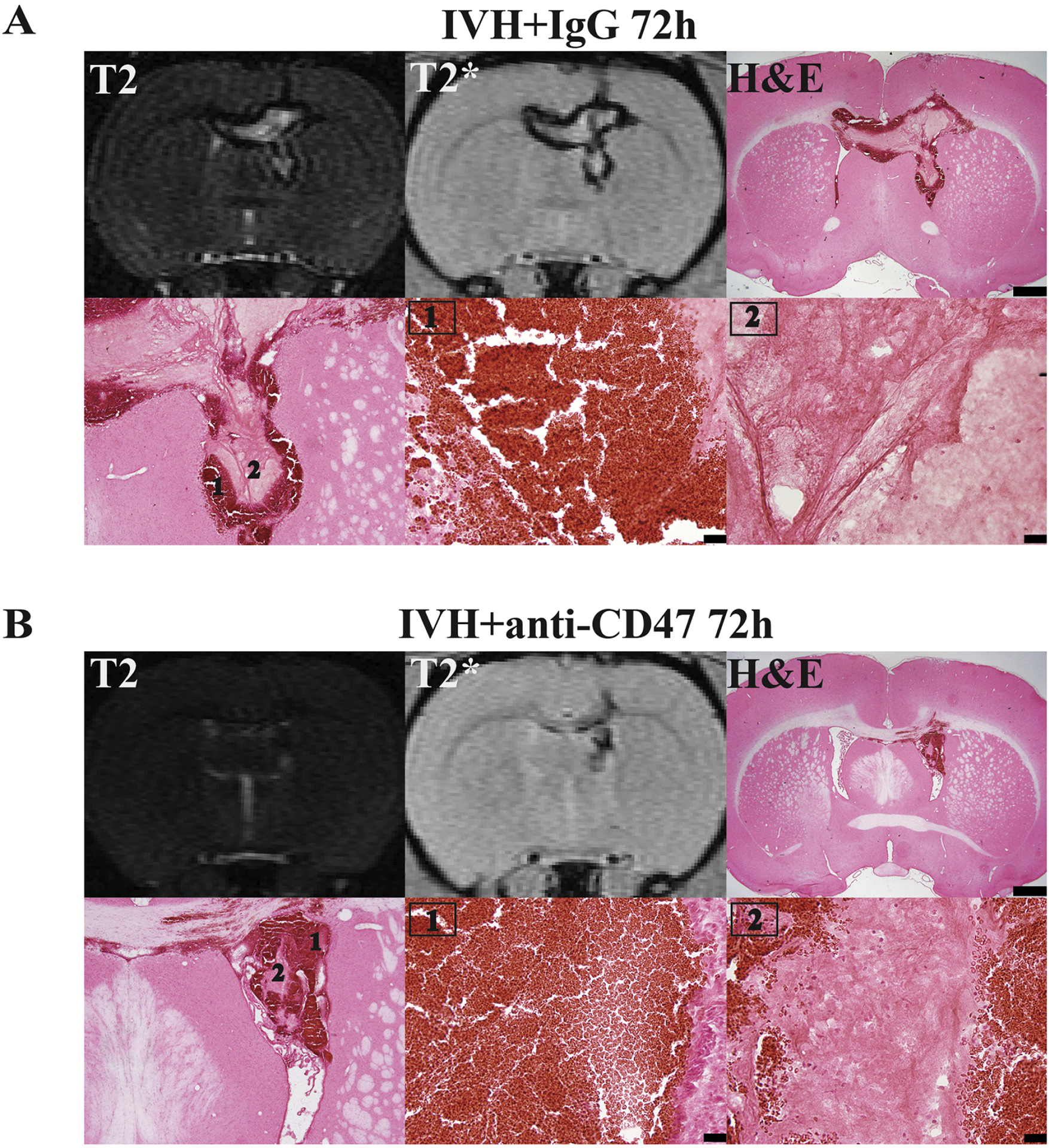
Representative images of T2 and T2* MRI scans and H&E staining at the same level of the hematoma at 72 hours after intraventricular injection of 200 μl autologous blood with (A) IgG (IVH+IgG) or (B) CD47 blocking antibody (IVH+anti-CD47). Higher magnification images of the hypo-T2* (1, periphery) and the non-hypo-T2* area (2, center) of the hematoma are also shown. Scale bars are 1 mm for top panels and 20 μm for lower middle and right images.
To examine the pathological changes in the T2* non-hypointense lesion, H&E staining was performed in corresponding brain slices. In hypointense areas (peripheral area), erythrocytes demonstrated typical double-disk morphologies (Fig. 3). However, in non-hypointense areas (commonly in the hematoma center), no erythrocyte morphology was appreciated. Instead, the hematoma appears to be homogeneous (Fig. 3) with occasional erythrocyte ghosts (small, round, pink, cell-like). This phenomenon was found in both IVH+IgG group (Fig. 3A) and IVH+anti-CD47 group (Fig. 3B) 72h post-hemorrhage. Figure 3 shows representative T2 and T2* weighted MRIs showing the intraventricular lesion and H&E staining of corresponding brain sections. We hypothesize that these changes represent hemolysis of intraventricular blood, which leads to a release of neurotoxic compounds (hemoglobin, iron, peroxiredoxin 2) from erythrocytes.
Phagocytosis Occurs 4 Hours After Experimental IVH and is Enhanced at 72 Hours
M/Mo-MΦ phagocytosis is an important mechanism of endogenous hematoma clearance and a potential therapeutic target after ICH(Wilkinson et al., 2018, Zhao et al., 2007). However, the role of phagocytosis for intraventricular hematoma clearance and the origin of the participating M/Mo-MΦ is uncertain. In the present study, intra- and peri-ventricular phagocytosis of RBC was observed as soon as 4 hours after IVH in both IVH+IgG and IVH+anti-CD47 groups (supplemental Figure 2). H&E staining showed cells containing a central round nucleus and abundant cytoplasm, RBCs and fragments of RBCs were observed inside those M/Mo-MΦ (sFig. 2A). At 72 hours post-IVH, M/Mo-MΦ cells were significantly increased in both IVH+IgG group and IVH+anti-CD47 group in all observed areas (Fig. 4A). Iba1 is an M/Mo-MΦ marker and HO-1 is an enzyme that catalyzes heme degradation, abundantly expressed in M/Mo-MΦ activation upon erythrocytosis(Xi et al., 2006). Immunohistochemistry showed Iba1 (Fig. 4B) and HO-1(Fig. 4C) positive cells along the ventricular face of the lateral ventricular wall and corpus callosum as well as the choroid plexus. This suggests that the M/Mo-MΦ involved in phagocytosis after IVH originated from the periventricular parenchyma, the corpus callosum white matter and choroid plexus. Interestingly, H&E staining showed some large multinuclear cells that engulfed with RBC appeared in the lateral ventricle wall and corpus callosum but not choroid plexus (Fig. 4A). Large multinuclear cells with RBC were also found in the lateral ventricle wall and corpus callosum but not choroid plexus with HO-1 immunostaining (Fig. 4C). The number of Iba1 positive cells was significantly higher in the IVH+anti-CD47 group in all three observed areas compare to IVH+IgG group (p=0.020 in lateral ventricle wall, p=0.043 in corpus callosum, p=0.043 in choroid plexus, n=9 per group, Fig. 4D). Similarly, the number of HO-1 positive cells in the IVH+anti-CD47 group was higher in lateral ventricle wall area (p=0.013) and choroid plexus area (p=0.043) but not statistically significant in corpus callosum (p=0.148; n=9 per group, Fig. 4E).
Figure 4.
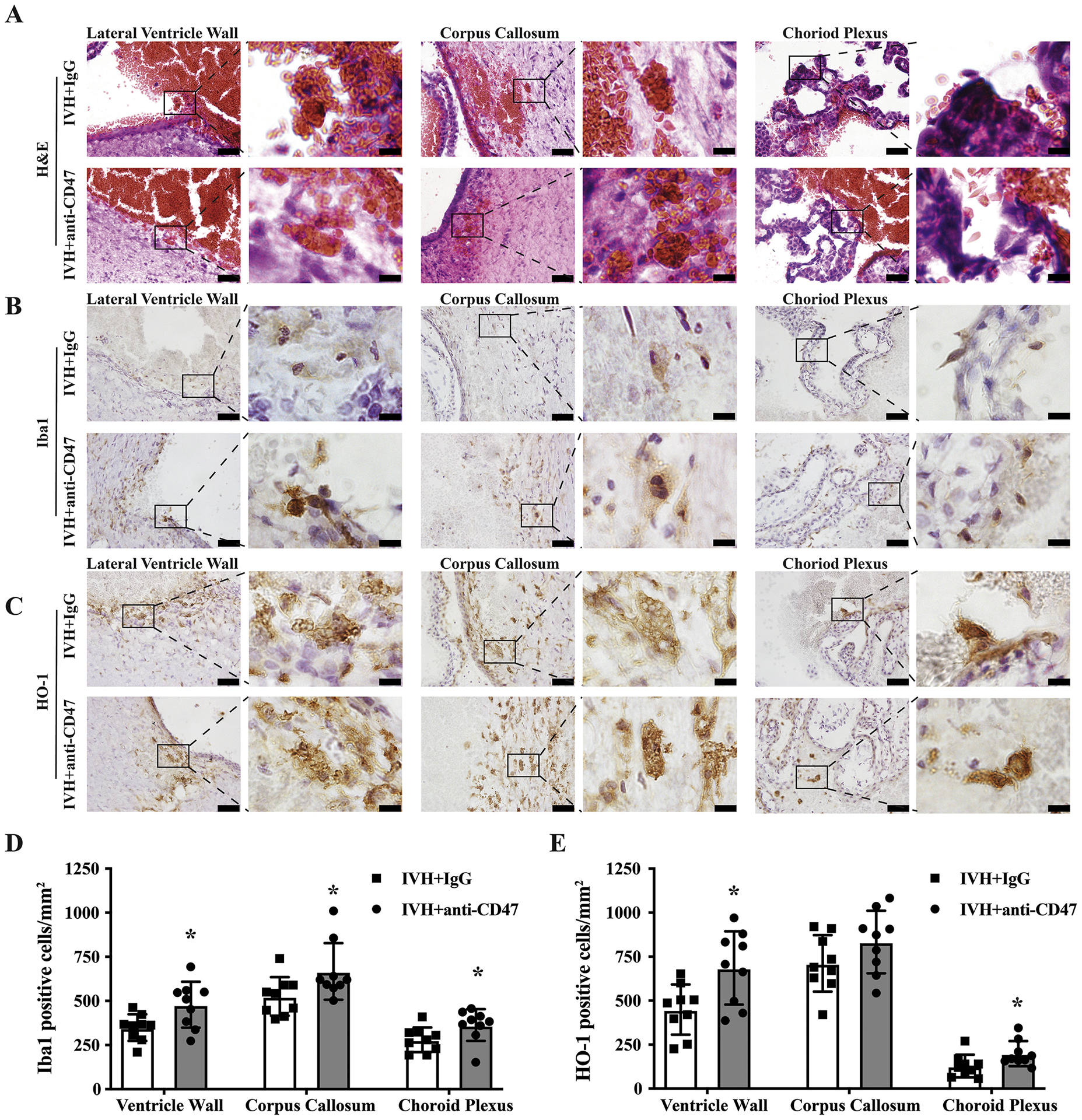
(A) Representative images of H&E staining 72 h after intraventricular injection of 200 μl autologous blood with IgG (IVH+IgG) or CD47 blocking antibody. Microglia/monocyte-derived macrophages were significantly increased in both IVH+IgG group and IVH+anti-CD47 group in all observed areas (A). Interestingly, some large multinuclear cells with engulfed RBCs were found at the lateral ventricle wall and corpus callosum. (B, C) Immunohistochemistry staining showing Iba1 positive cells (B) and HO-1 positive cells (C) along the CSF-facing side of the lateral ventricular wall, corpus callosum and choroid plexus 72 hours after IVH. Large multinuclear cells with RBC were also found at the lateral ventricle wall and corpus callosum with HO-1 immunostaining (C). Scale bars are 20 μm. (D) Iba1 immunopositive cells at lateral ventricle wall, corpus callosum and choroid plexus 72 hours after intraventricular injection of 200 μl autologous arterial blood with IgG (IVH+IgG) or CD47 blocking antibody (IVH+anti-CD47). (E) HO-1 immunopositive cells at lateral ventricle wall, corpus callosum and choroid plexus 72 hours after intraventricular injection of 200 μl autologous arterial blood with IgG (IVH+IgG) or CD47 blocking antibody (IVH+anti-CD47). Immuno-positive cells were counted and shown as mean ± SD; n= 9 per group; * p < 0.05 IVH+anti-CD47 vs. IVH+IgG group.
CD47 Blocking Antibody Enhance M/Mo-MΦ Phagocytosis After Experimental IVH
Our prior studies indicated that CD47 blocking antibody enhanced erythrophagocytosis after ICH which reduced brain injury in mice and rats(Jing et al., 2019, Tao et al., 2020). The current study found evidence of phagocytosis after IVH, which was enhanced by CD47 blocking antibody. CD68 is a maker of M/Mo-MΦ activation. At 72h post-hemorrhage, intra-hematoma CD68 immuno-positive cells were significantly upregulated in the IVH+anti-CD47 group (305 ± 131 vs. 138 ± 71 cells/mm2 in IVH+IgG, n=9 per group, p=0.004, Fig. 5A). HO-1, an enzyme that catalyzes heme degradation, is abundantly expressed after M/Mo-MΦ activation upon erythrocytosis(Xi et al., 2006). At 72h post-hemorrhage, intra-hematoma HO-1 immuno-positive cells were significantly upregulated in the IVH+anti-CD47 group (364 ± 132 vs. 216 ± 124 cells/mm2 in IVH+IgG, n=9 per group, p=0.026, Fig. 5B). Ferritin is an iron-storage protein in M/Mo-MΦ, and it plays an important role in protecting the brain after ICH(Wu et al., 2003). Immunofluorescent double staining of ferritin and microglia/macrophage marker Iba1 shows an increased number of intra-hematoma ferritin positive cells (618 ± 146 vs. 379 ± 105 cells/mm2 in IVH+IgG, n=9 per group, p=0.001, Fig. 6B) and ferritin/Iba1 double-positive cells (382 ± 136 vs. 209 ± 83 cells/mm2 in IVH+IgG, n=9 per group, p=0.005, Fig. 6C) in CD47 blocking antibody treated rats.
Figure 5.
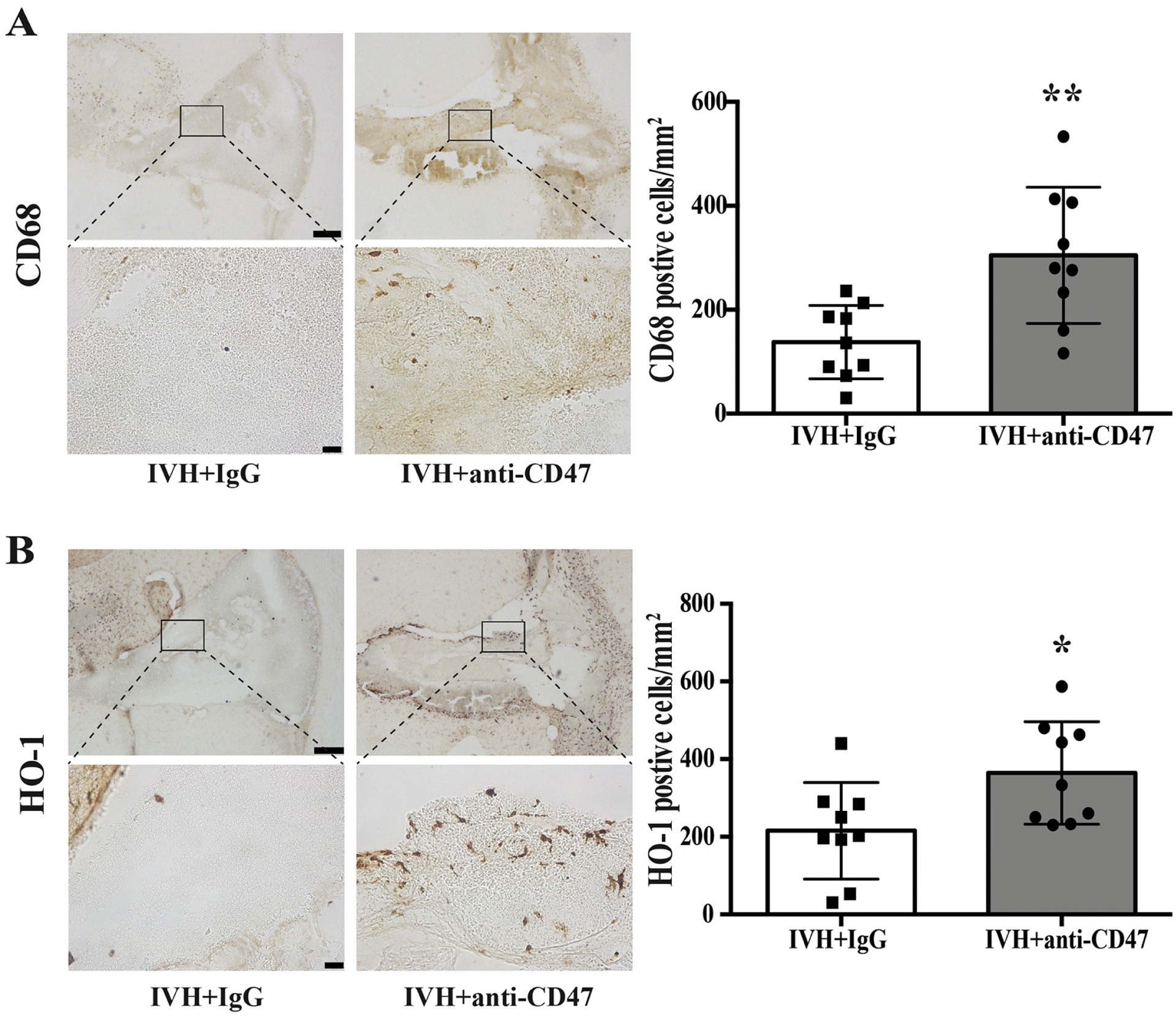
(A) CD68 immune positive cells 72 hours after intraventricular injection of 200 μl autologous blood with IgG (IVH+IgG) or CD47 blocking antibody (IVH+anti-CD47). The high magnification images correspond to the hematoma. (B) HO-1 immune positive cells 72 hours after intraventricular injection of 200 μl autologous arterial blood with IgG (IVH+IgG) or CD47 blocking antibody (IVH+anti-CD47). The high magnification images correspond to the hematoma. Upper panel scale bars are 200 μm, lower panel scale bars are 20 μm. Intra-hematoma positive cells were counted and shown as mean ± SD; n= 9 per group; * p < 0.05 IVH+anti-CD47 vs. IVH+IgG group, ** p < 0.01 IVH+anti-CD47 vs. IVH+IgG group.
Figure 6.
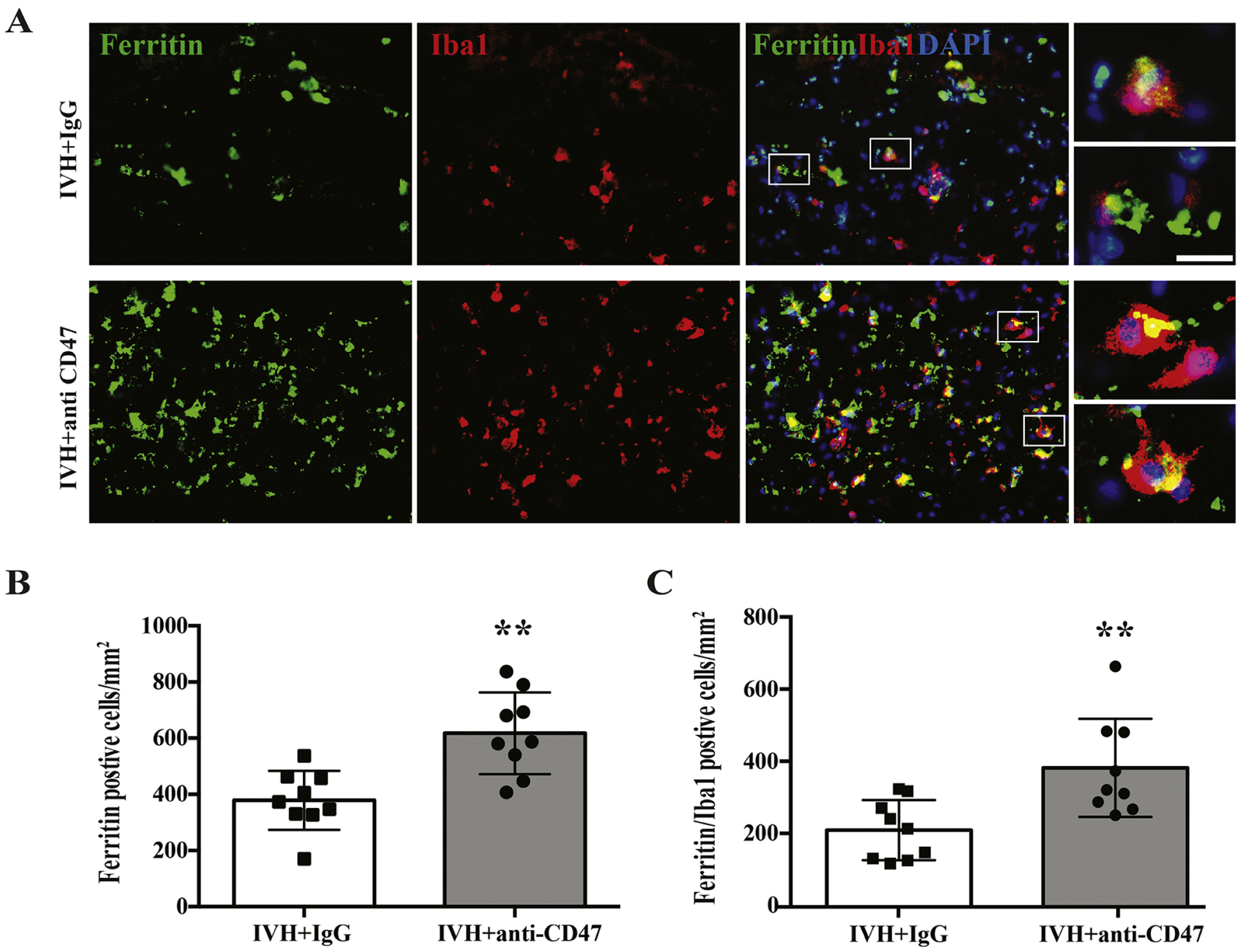
(A) Ferritin and Iba1 double labeling 72 hours after intraventricular injection of 200 μl autologous arterial blood with IgG (IVH+IgG). The high magnification images correspond to double labeling cells are shown on the right panels. Scale bar is 10 μm. (B) Intra-hematoma ferritin positive cells and ferritin/Iba1 double-positive cells were counted and shown as mean ± SD; n= 9 per group; ** p < 0.01 IVH+anti-CD47 vs. IVH+IgG group.
Clodronate Liposomes Worsened Hydrocephalus and Reduced the Number of Iba1 and HO-1 Positive Cells After Experimental IVH
Serial T2 and T2* weighted MRI scans were performed to estimate ventricle volume after intraventricular injection of 200 μl autologous arterial mixed with CD47 blocking antibody plus either clodronate liposomes or control liposomes. Clodronate liposomes were added to deplete phagocytic cells. All rats exhibited bilateral ventricular enlargement. At 24 hours post-surgery ventricular volumes in the clodronate liposomes co-injection group (IVH+anti-CD47+Clodronate) were significantly larger than control liposomes co-injection group (IVH+anti-CD47+Control) (61.1 ± 18.9 vs. 42.5 ± 11.4 mm3 in IVH+anti-CD47+Control, n=7 per group, p=0.046, Fig. 7A, C). This difference in ventricle volume was more distinctive at 72 hours after surgery (56.4 ± 22.3 vs. 29.3 ± 8.0 mm3 in IVH+anti-CD47+Control, n=7 per group, p=0.011, Fig. 7A, C). Notably, 3 of the 7 rats in the clodronate liposomes co-injection group exhibited progressive hydrocephalus from day 1 to day 3. In contrast, none of the animals in the control liposomes co-injection group exhibited this phenomenon. At 24h post-hemorrhage, intraventricular hematoma volumes in the IVH+anti-CD47+Control group (19.3 ± 9.0 mm3) were smaller than those in the IVH+anti-CD47+Clodronate group (29.7 ± 14.5 mm3), though the difference was not statistically different (n=7 per group, p=0.061, Fig. 7B, D). Similarly, in 72 hours post hemorrhage, the intraventricular lesions in the IVH+anti-CD47+Clodronate group tended to be larger but not statistically significant than the IVH+anti-CD47+Control group (18.5 ± 16.9 vs. 10.2 ± 6.0 mm3, n=7 per group, p=0.122, Fig. 7B, D).
Figure 7.
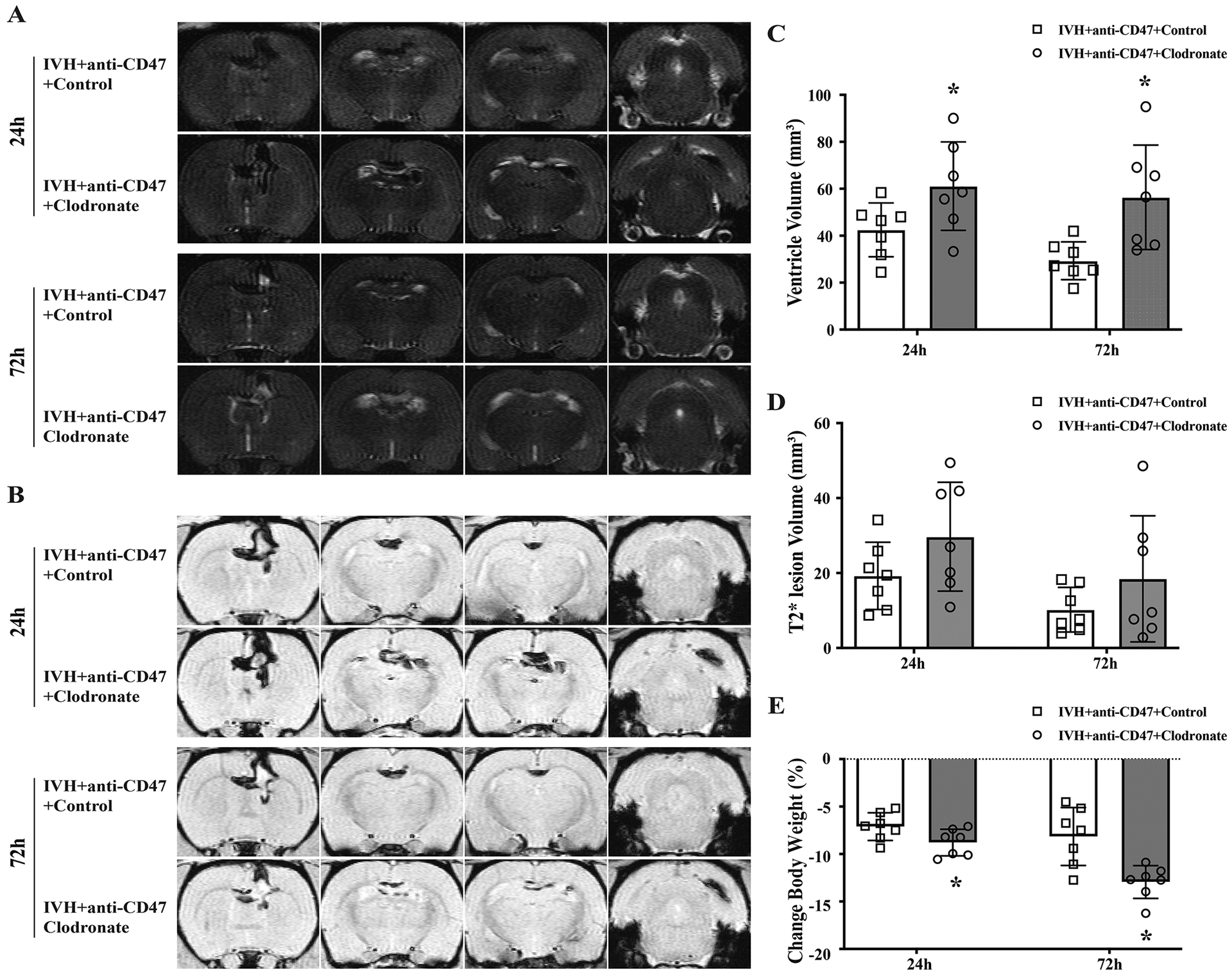
(A) Representative T2-weighted MRI scans at 24 and 72 hours after intraventricular injection of 200 μl autologous blood with CD47 blocking antibody plus clodronate liposomes (IVH+anti-CD47+Clodronate) or control liposomes (IVH+anti-CD47+Control) in F344 rats. (B) Representative images of T2*-weighted MRI scans at 24 and 72 hours after intraventricular injection of 200 μl autologous blood with CD47 blocking antibody plus clodronate liposomes (IVH+anti-CD47+Clodronate) or control liposomes (IVH+anti-CD47+Control) in F344 rats. (C) Ventricular volumes were quantified using the T2-weighted images. The clodronate liposomes treatment significantly increased ventricular dilation after IVH. (D) Intraventricular lesion volumes were quantified using T2*-weighted images at 24- and 72-hours post-surgery. (E) Weight changes from pre-IVH to 24 and 72 hours after IVH. For all graphs, data are shown as mean ± SD; n = 7 per group; * p < 0.05 IVH+anti-CD47+Clodronate vs. IVH+anti-CD47+Control group.
Changes in body weight from pre-surgery were monitored 24h and 72h after IVH. Weight loss was significantly more severe in the IVH+anti-CD47+Clodronate group than the IVH+anti-CD47+Control group at 24 hours (−8.7 ± 1.4 vs. −7.1 ± 1.5 %, respectively, n=7 per group, p=0.048, Fig. 7E). At 72 hours post-hemorrhage, continuous weight loss was observed in the IVH+anti-CD47+Control group (−12.19 ± 1.7 %) but less so in the IVH+anti-CD47+Control group (−8.1 ± 3.0 %, n=7 per group, p=0.004, Fig. 7E).
At 72h post-hemorrhage, intra-hematoma Iba1 immuno-positive cells were significantly downregulated in the IVH+anti-CD47+Clodronate group (173 ± 70 vs. 343 ± 190 cells/mm2 in IVH+anti-CD47+Control, n=7 per group, p=0.046, Fig. 8A). At 72h post-hemorrhage, intra-hematoma HO-1 immuno-positive cells were significantly downregulated in the IVH+anti-CD47 group (221 ± 91 vs. 423 ± 242 cells/mm2 in IVH+anti-CD47+Control, n=7 per group, p=0.026, Fig. 8B).
Figure 8.
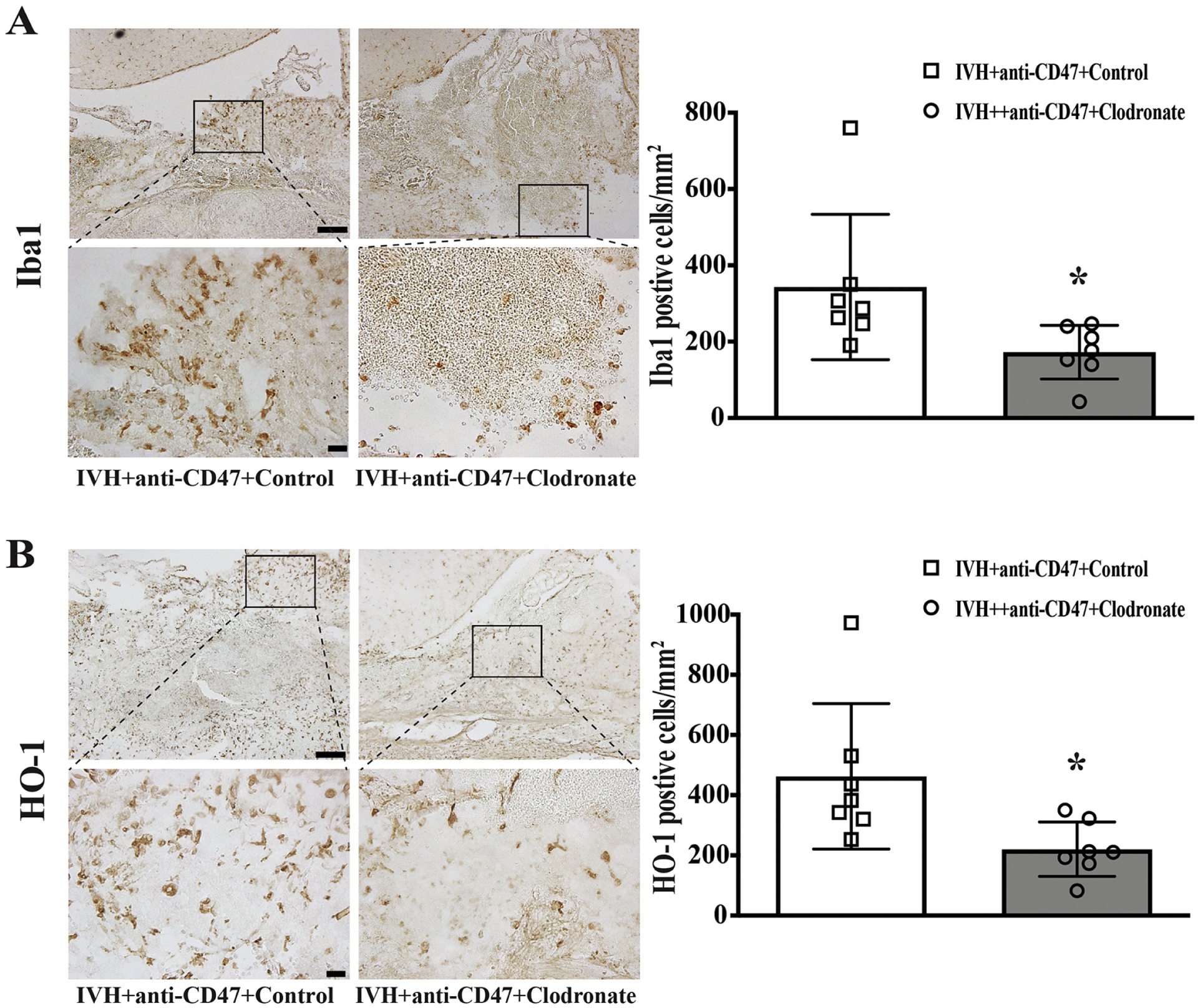
(A) Iba1 immune positive cells 72 hours after intraventricular injection of 200 μl autologous blood with CD47 blocking antibody plus clodronate liposomes (IVH+anti-CD47+Clodronate) or control liposomes (IVH+anti-CD47+Control) in F344 rats. The high magnification images correspond to the hematoma. (B) HO-1 immune positive cells 72 hours after intraventricular injection of 200 μl autologous arterial blood with CD47 blocking antibody plus clodronate liposomes (IVH+anti-CD47+Clodronate) or control liposomes (IVH+anti-CD47+Control). The high magnification images correspond to the hematoma. Upper panel scale bars are 100 μm, lower panel scale bars are 20 μm. Intra-hematoma positive cells were counted and shown as mean ± SD; n= 10 per group; * p < 0.05 IVH+anti-CD47+Clodronate vs. IVH+anti-CD47+Control group.
Discussion
The present study in adult male rats showed: (1) A CD47 blocking antibody alleviated hydrocephalus development by day 3 after IVH. (2) That antibody reduced hematoma size (intraventricular T2* lesion) after IVH from day 1. (3) It also reduced hemolysis following IVH. (4) Microglia/Monocyte-derived macrophage activation was enhanced by the CD47 blocking antibody, which may contribute to the acceleration of hematoma clearance.
Erythrocyte surface CD47 acts as a “don’t eat me” signal which interrupts M/Mo-MΦ phagocytosis under physiological and pathological conditions(Olsson et al., 2006). Our former studies found that monoclonal anti-CD47 blocking antibody co-injection with autologous arterial blood accelerated hematoma clearance, reduced brain injury, and improved neurofunctional outcomes in both mice (Jing et al., 2019) and aged rat ICH models(Tao et al., 2020) likely through enhanced M/Mo-MΦ phagocytosis. The current study used the same dose (blood concentration10 μg/mL) of blocking antibody as it was effective in ICH(Jing et al., 2019).
Hydrocephalus is one of the most common neurological disorders worldwide(Isaacs et al., 2018), with poor long-term outcomes including impaired overall intelligence, epilepsy, gait disturbance, urinary incontinence, and serious chronic headaches(Kahle et al., 2016, Kulkarni et al., 2013). It has brought in a huge global health expenditure burden(Kahle et al., 2016, Dewan et al., 2018).
Adult-onset hydrocephalus commonly results from IVH and SAH(Strahle et al., 2012, Chen et al., 2017), yet the pathophysiology of PHH remains largely unknown. Proposed mechanisms of PHH include damaged periventricular brain structures, initial mass effects resulting in obstructive hydrocephalus, disruption of cerebrospinal fluid flow dynamics, as well as IVH-induced neuroinflammation(Klebe et al., 2020, Karimy et al., 2020, Balami and Buchan, 2012). Here, we suggest that the hydrocephalus attenuation after IVH resulted from accelerated hematoma clearance by erythrocytes surface CD47 blockade.
Hematoma volume after IVH has been related to worse outcomes and increased PHH rate(Bisson et al., 2020, Balami and Buchan, 2012). Larger hematomas will have a larger mass effect and may release more RBC-derived neurotoxic compounds (including hemoglobin, iron and peroxiredoxin 2). Therefore, reducing hematoma volume has long been a goal in IVH treatment, including the recent CLEAR III trial. That trial found a decrease in mortality with rt-PA mediated intraventricular hematoma reduction, but no significant improvement in neurofunctional outcome(Hanley et al., 2017). Alternative or combination hematoma removal approaches may be beneficial. Though the M/Mo-M Φ system plays an important role in hematoma resolution(Wilkinson et al., 2018) and brain injury/recovery after ICH(Chang et al., 2018), the relationship between M/Mo-MΦ system activation and hematoma clearance after IVH was uncertain. In the current study, T2* MRI was used to evaluate intraventricular hematoma size. As soon as 24h after IVH, the intraventricular T2* lesion volumes in CD47 blocking antibody co-injection group were significantly smaller compared with isotype IgG co-injection controls, despite similar hematoma sizes 4 hours post-hemorrhage. On day 3, this difference in hematoma size was maintained. Thus, CD47 blockade markedly promotes hematoma clearance after IVH from day 1.
The current study also found non-hypointense areas inside intraventricular hematoma, which corresponded to hemolysis areas by H&E staining. We previously reported MRI findings of early hemolysis in both an animal ICH model(Dang et al., 2017) and spontaneous ICH patients(Liu et al., 2019) are correlated with neurological function outcomes. Here, early hemolysis was also observed on MRI of rats after IVH. Hemolysis and subsequent release of neurotoxic compounds have been shown to induce neuronal death and neurofunctional damage in ICH(Hatakeyama et al., 2013). In animal models of IVH, lysed RBC induced hydrocephalus development and intense neuroinflammation(Tan et al., 2020, Gao et al., 2014). We also noted hemolysis inhibition by CD47 blocking antibody, which may be associated with the relatively smaller hematoma size in the CD47 treated group. The ratio of hemolysis to hematoma volume was also decreased in the anti-CD47 treated group, which we suspect results from the enhancement of phagocytosis before hemolysis can happen. These findings make hematoma clearance through endogenous phagocytosis a potential therapeutic target for IVH, urging future translational studies.
Relatively little is known about when and where the M/Mo-MΦ system participates in hematoma resolution after IVH. Unexpectedly, we observed M/Mo-MΦ activation and phagocytosis just 4 hours after IVH, indicating phagocytosis is likely to play a part in the decreased hematoma size by 24 hours. At 72 hours, phagocytosis in all observed locations was increased, in terms of Iba1, HO-1 immune positive cells. Some large multinuclear M/Mo-MΦ were observed at the lateral ventricle wall and corpus callosum, but not in the choroid plexus. We suggest those large erythrophagocytosis large cells were activated resident microglia, similar to the multinucleated giant cells we observed in experimental ICH(Wei et al., 2020).
The origin of the M/Mo-MΦ involved in the hematoma clearance was not determined in this study. Microglia and macrophages are the primary resident phagocytes in the brain parenchyma, and monocytes can be recruit from the periphery and enter the ventricular system through its “gate” choroid plexus. Currently, no perfect marker has been found to distinguish these phagocytes by immunostaining. We suspect that resident phagocytes contribute to the intraventricular hematoma clearance via the damaged ventricle walls and disrupted periventricular white matter. At the same time, the epiplexus cells of the choroid plexus are activated by intraventricular blood and monocytes entering the ventricular system from peripheral through the choroid plexus may help intraventricular hematoma resolution. One of our study interests is to investigate the origin of the phagocytes involved in hematoma clearance and what happens to those cells after they phagocytose red blood cells.
To determine the effect of the anti-CD47 antibody for hematoma resolution, we used CD68, HO-1 and ferritin/Iba1 to mark the intra-hematoma cells. There was an increase in intra-hematoma infiltration of CD68, HO-1 and ferritin/Iba1 positive cells in the anti-CD47 treatment group 3 days after IVH. CD68 is frequently used to identify macrophages and activated microglia in brain(Walker and Lue, 2015) participating in phagocytosis. The upregulation of CD68-positive cells in the anti-CD47 group suggests increased M/Mo-MΦ activation. HO-1 and ferritin are important proteins taking part in heme degradation and iron storage and are abundantly expressed in M/Mo-MΦ activation upon erythrophagocytosis(Xi et al., 2006, Wu et al., 2003). The greater number of HO-1 and ferritin positive cells indicates increased involvement of M/Mo-MΦ in hemoglobin metabolism after IVH by CD47 blockade. Besides, clodronate liposomes eliminated the protective effect of CD47 blocking antibody and reduced the intra-hematoma infiltration of M/Mo-MΦ. These data further support our hypothesis that the M/Mo-MΦ could play a protective role after IVH by participating in hematoma clearance. Further studies are needed to explain the activation and polarization of M/Mo-MΦ following IVH.
This study has several limitations: (1) It was a short-term study with no evaluation of long term neurofunctional outcomes after administering CD47 blocking antibody. Further investigations of long term neurofunctional effects in aged rats are needed because progressive hydrocephalus only occurs in aged IVH rats. (2) The antibody was administered with the blood used to create the IVH model. The effects of delayed antibody administration are wanted for higher clinical significance. (3) Only young male rats were used.
Conclusions
An anti-CD47 blocking antibody was able to diminish hydrocephalus development, promote hematoma resolution, and inhibit hemolysis after IVH. Those effects were associated with the upregulated M/Mo-MΦ activation. Blocking erythrocyte surface CD47, a „don’t eat me‟ signal, could be a potential therapeutic strategy for IVH.
Supplementary Material
Supplemental Figure 1. (A) Representative T2-weighted MRI scans 4 hours after intraventricular injection of 200 μl autologous blood with IgG (IVH+IgG) or CD47 blocking antibody (IVH+anti-CD47) in F344 rats. (B) Ventricular volumes were quantified using the T2-weighted images.
Supplemental Figure 2. Representative images of H&E staining 4 hours after intraventricular injection of 200 μl autologous blood with IgG (IVH+IgG) or CD47 blocking antibody. Cells containing a central round nucleus and abundant cytoplasm were identified as microglia/monocyte-derived macrophages in areas adjacent to choroid. Black arrowheads indicating RBCs and RBC fragments inside microglia/monocyte-derived macrophages. Scale bars are 20 μm.
Highlights.
CD47 blocking antibody accelerated hematoma clearance following IVH
CD47 blocking antibody alleviated IVH-induced hydrocephalus
CD47 blocking antibody reduced hemolysis in the clot after IVH
Funding
YH, RFK and GX were supported by grants NS091545, NS090925, NS096917, NS106746 and NS116786 from the National Institutes of Health.
Abbreviations
- IVH
Intraventricular hemorrhage
- MRI
Magnetic resonance imaging
- ICH
Intracerebral hemorrhage
- SAH
Subarachnoid hemorrhage
- CSF
Cerebrospinal fluid
- PHH
Post-hemorrhagic hydrocephalus
- rt-PA
Recombinant tissue plasminogen activator
- M/Mo-MΦ
Microglia/Monocyte-derived macrophages
- RBC
Red blood cell
- SIRP α
Signal regulatory protein α
- H&E
Hematoxylin and eosin
- HO-1
Heme oxygenase-1
- DAPI
4′, 6-diamidino-2-phenylindole
- SD
Standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests: The authors declare no conflict of interests.
Ethical approval: All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- ADAMS-CHAPMAN I, HANSEN NI, STOLL BJ, HIGGINS R & NETWORK NR 2008. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics, 121, e1167–77, doi: 10.1542/peds.2007-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALAMI JS & BUCHAN AM 2012. Complications of intracerebral haemorrhage. Lancet Neurol, 11, 101–18, doi: 10.1016/S1474-4422(11)70264-2. [DOI] [PubMed] [Google Scholar]
- BHATTATHIRI PS, GREGSON B, PRASAD KS, MENDELOW AD & INVESTIGATORS, S. 2006. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir Suppl, 96, 65–8, doi: 10.1007/3-211-30714-1_16. [DOI] [PubMed] [Google Scholar]
- BISSON DA, FLAHERTY ML, SHATIL AS, GLADSTONE D, DOWLATSHAHI D, CARROZZELLA J, ZHANG L, HILL MD, DEMCHUCK A, AVIV RI, STOP IT & INVESTIGATORS, S. 2020. Original and Modified Graeb Score Correlation With Intraventricular Hemorrhage and Clinical Outcome Prediction in Hyperacute Intracranial Hemorrhage. Stroke, 51, 1696–1702, doi: 10.1161/STROKEAHA.120.029040. [DOI] [PubMed] [Google Scholar]
- CHANG CF, GOODS BA, ASKENASE MH, HAMMOND MD, RENFROE SC, STEINSCHNEIDER AF, LANDRENEAU MJ, AI Y, BEATTY HE, DA COSTA LHA, MACK M, SHETH KN, GREER DM, HUTTNER A, COMAN D, HYDER F, GHOSH S, ROTHLIN CV, LOVE JC & SANSING LH 2018. Erythrocyte efferocytosis modulates macrophages towards recovery after intracerebral hemorrhage. J Clin Invest, 128, 607–624, doi: 10.1172/JCI95612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG CF, MASSEY J, OSHEROV A, ANGENENDT DA COSTA LH & SANSING LH 2020. Bexarotene Enhances Macrophage Erythrophagocytosis and Hematoma Clearance in Experimental Intracerebral Hemorrhage. Stroke, 51, 612–618, doi: 10.1161/STROKEAHA.119.027037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN S, LUO J, REIS C, MANAENKO A & ZHANG J 2017. Hydrocephalus after Subarachnoid Hemorrhage: Pathophysiology, Diagnosis, and Treatment. Biomed Res Int, 2017, 8584753, doi: 10.1155/2017/8584753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Z, GAO C, HUA Y, KEEP RF, MURASZKO K & XI G 2011. Role of iron in brain injury after intraventricular hemorrhage. Stroke, 42, 465–70, doi: 10.1161/STROKEAHA.110.602755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIAN EA, JIN DL, ATTENELLO F, WEN T, CEN S, MACK WJ, KRIEGER MD & MCCOMB JG 2016. Trends in hospitalization of preterm infants with intraventricular hemorrhage and hydrocephalus in the United States, 2000–2010. J Neurosurg Pediatr, 17, 260–9, doi: 10.3171/2015.7.PEDS15140. [DOI] [PubMed] [Google Scholar]
- DANG G, YANG Y, WU G, HUA Y, KEEP RF & XI G 2017. Early Erythrolysis in the Hematoma After Experimental Intracerebral Hemorrhage. Transl Stroke Res, 8, 174–182, doi: 10.1007/s12975-016-0505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEWAN MC, RATTANI A, MEKARY R, GLANCZ LJ, YUNUSA I, BATICULON RE, FIEGGEN G, WELLONS JC, PARK KB & WARF BC 2018. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J Neurosurg, 1–15, doi: 10.3171/2017.10.JNS17439. [DOI] [PubMed] [Google Scholar]
- GAO C, DU H, HUA Y, KEEP RF, STRAHLE J & XI G 2014. Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab, 34, 1070–5, doi: 10.1038/jcbfm.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARTON T, HUA Y, XIANG J, XI G & KEEP RF 2017. Challenges for intraventricular hemorrhage research and emerging therapeutic targets. Expert Opin Ther Targets, 21, 1111–1122, doi: 10.1080/14728222.2017.1397628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANLEY DF 2009. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke, 40, 1533–8, doi: 10.1161/STROKEAHA.108.535419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANLEY DF, LANE K, MCBEE N, ZIAI W, TUHRIM S, LEES KR, DAWSON J, GANDHI D, ULLMAN N, MOULD WA, MAYO SW, MENDELOW AD, GREGSON B, BUTCHER K, VESPA P, WRIGHT DW, KASE CS, CARHUAPOMA JR, KEYL PM, DIENER-WEST M, MUSCHELLI J, BETZ JF, THOMPSON CB, SUGAR EA, YENOKYAN G, JANIS S, JOHN S, HARNOF S, LOPEZ GA, ALDRICH EF, HARRIGAN MR, ANSARI S, JALLO J, CARON JL, LEDOUX D, ADEOYE O, ZUCCARELLO M, ADAMS HP JR., ROSENBLUM M, THOMPSON RE, AWAD IA & INVESTIGATORS, C. I. 2017. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet, 389, 603–611, doi: 10.1016/S0140-6736(16)32410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HATAKEYAMA T, OKAUCHI M, HUA Y, KEEP RF & XI G 2013. Deferoxamine reduces neuronal death and hematoma lysis after intracerebral hemorrhage in aged rats. Transl Stroke Res, 4, 546–53, doi: 10.1007/s12975-013-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAACS AM, RIVA-CAMBRIN J, YAVIN D, HOCKLEY A, PRINGSHEIM TM, JETTE N, LETHEBE BC, LOWERISON M, DRONYK J & HAMILTON MG 2018. Age-specific global epidemiology of hydrocephalus: Systematic review, metanalysis and global birth surveillance. PLoS One, 13, e0204926, doi: 10.1371/journal.pone.0204926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JING C, BIAN L, WANG M, KEEP RF, XI G & HUA Y 2019. Enhancement of Hematoma Clearance With CD47 Blocking Antibody in Experimental Intracerebral Hemorrhage. Stroke, 50, 1539–1547, doi: 10.1161/STROKEAHA.118.024578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAHLE KT, KULKARNI AV, LIMBRICK DD JR. & WARF BC 2016. Hydrocephalus in children. Lancet, 387, 788–99, doi: 10.1016/S0140-6736(15)60694-8. [DOI] [PubMed] [Google Scholar]
- KARIMY JK, REEVES BC, DAMISAH E, DUY PQ, ANTWI P, DAVID W, WANG K, SCHIFF SJ, LIMBRICK DD JR., ALPER SL, WARF BC, NEDERGAARD M, SIMARD JM & KAHLE KT 2020. Inflammation in acquired hydrocephalus: pathogenic mechanisms and therapeutic targets. Nat Rev Neurol, 16, 285–296, doi: 10.1038/s41582-020-0321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEBE D, MCBRIDE D, KRAFFT PR, FLORES JJ, TANG J & ZHANG JH 2020. Posthemorrhagic hydrocephalus development after germinal matrix hemorrhage: Established mechanisms and proposed pathways. J Neurosci Res, 98, 105–120, doi: 10.1002/jnr.24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULKARNI AV, RIVA-CAMBRIN J, BUTLER J, BROWD SR, DRAKE JM, HOLUBKOV R, KESTLE JR, LIMBRICK DD, SIMON TD, TAMBER MS, WELLONS JC 3RD, WHITEHEAD WE & HYDROCEPHALUS CLINICAL RESEARCH, N. 2013. Outcomes of CSF shunting in children: comparison of Hydrocephalus Clinical Research Network cohort with historical controls: clinical article. J Neurosurg Pediatr, 12, 334–8, doi: 10.3171/2013.7.PEDS12637. [DOI] [PubMed] [Google Scholar]
- LIU R, LI H, HUA Y, KEEP RF, XIAO J, XI G & HUANG Y 2019. Early Hemolysis Within Human Intracerebral Hematomas: an MRI Study. Transl Stroke Res, 10, 52–56, doi: 10.1007/s12975-018-0630-2. [DOI] [PubMed] [Google Scholar]
- NI W, MAO S, XI G, KEEP RF & HUA Y 2016. Role of Erythrocyte CD47 in Intracerebral Hematoma Clearance. Stroke, 47, 505–11, doi: 10.1161/STROKEAHA.115.010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLSSON M, NILSSON A & OLDENBORG PA 2006. Target cell CD47 regulates macrophage activation and erythrophagocytosis. Transfus Clin Biol, 13, 39–43, doi: 10.1016/j.tracli.2006.02.013. [DOI] [PubMed] [Google Scholar]
- PERCIE DU SERT N, HURST V, AHLUWALIA A, ALAM S, AVEY MT, BAKER M, BROWNE WJ, CLARK A, CUTHILL IC, DIRNAGL U, EMERSON M, GARNER P, HOLGATE ST, HOWELLS DW, KARP NA, LAZIC SE, LIDSTER K, MACCALLUM CJ, MACLEOD M, PEARL EJ, PETERSEN OH, RAWLE F, REYNOLDS P, ROONEY K, SENA ES, SILBERBERG SD, STECKLER T & WURBEL H 2020. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J Cereb Blood Flow Metab, 40, 1769–1777, doi: 10.1177/0271678X20943823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSEN DS, MACDONALD RL, HUO D, GOLDENBERG FD, NOVAKOVIC RL, FRANK JI & ROSENGART AJ 2007. Intraventricular hemorrhage from ruptured aneurysm: clinical characteristics, complications, and outcomes in a large, prospective, multicenter study population. J Neurosurg, 107, 261–5, doi: 10.3171/JNS-07/08/0261. [DOI] [PubMed] [Google Scholar]
- STRAHLE J, GARTON HJ, MAHER CO, MURASZKO KM, KEEP RF & XI G 2012. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res, 3, 25–38, doi: 10.1007/s12975-012-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAN X, CHEN J, KEEP RF, XI G & HUA Y 2020. Prx2 (Peroxiredoxin 2) as a Cause of Hydrocephalus After Intraventricular Hemorrhage. Stroke, 51, 1578–1586, doi: 10.1161/STROKEAHA.119.028672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAO C, KEEP RF, XI G & HUA Y 2020. CD47 Blocking Antibody Accelerates Hematoma Clearance After Intracerebral Hemorrhage in Aged Rats. Transl Stroke Res, 11, 541–551, doi: 10.1007/s12975-019-00745-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER DG & LUE LF 2015. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther, 7, 56, doi: 10.1186/s13195-015-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAN Y, GAO F, YE F, YANG W, HUA Y, KEEP RF & XI G 2020. Effects of aging on hydrocephalus after intraventricular hemorrhage. Fluids Barriers CNS, 17, 8, doi: 10.1186/s12987-020-0169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEI J, WANG M, JING C, KEEP RF, HUA Y & XI G 2020. Multinucleated Giant Cells in Experimental Intracerebral Hemorrhage. Transl Stroke Res, 11, 1095–1102, doi: 10.1007/s12975-020-00790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON DA, KEEP RF, HUA Y & XI G 2018. Hematoma clearance as a therapeutic target in intracerebral hemorrhage: From macro to micro. J Cereb Blood Flow Metab, 38, 741–745, doi: 10.1177/0271678X17753590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU J, HUA Y, KEEP RF, NAKAMURA T, HOFF JT & XI G 2003. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke, 34, 2964–9, doi: 10.1161/01.STR.0000103140.52838.4501.STR.0000103140.52838.45 [pii]. [DOI] [PubMed] [Google Scholar]
- XI G, KEEP RF & HOFF JT 2006. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol, 5, 53–63, doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- XI G, KEEP RF, HUA Y, XIANG J & HOFF JT 1999. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke, 30, 1247–55, doi: 10.1161/01.str.30.6.1247. [DOI] [PubMed] [Google Scholar]
- ZHAO X, SUN G, ZHANG J, STRONG R, SONG W, GONZALES N, GROTTA JC & ARONOWSKI J 2007. Hematoma resolution as a target for intracerebral hemorrhage treatment: role for peroxisome proliferator-activated receptor gamma in microglia/macrophages. Ann Neurol, 61, 352–62, doi: 10.1002/ana.21097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. (A) Representative T2-weighted MRI scans 4 hours after intraventricular injection of 200 μl autologous blood with IgG (IVH+IgG) or CD47 blocking antibody (IVH+anti-CD47) in F344 rats. (B) Ventricular volumes were quantified using the T2-weighted images.
Supplemental Figure 2. Representative images of H&E staining 4 hours after intraventricular injection of 200 μl autologous blood with IgG (IVH+IgG) or CD47 blocking antibody. Cells containing a central round nucleus and abundant cytoplasm were identified as microglia/monocyte-derived macrophages in areas adjacent to choroid. Black arrowheads indicating RBCs and RBC fragments inside microglia/monocyte-derived macrophages. Scale bars are 20 μm.


