Abstract
Simple Summary
This narrative review first describes from several points of view the complex interrelationship between cancer and neurodegeneration, with special attention to the mechanisms that might underlie an inverse relationship between them. In particular, the mechanisms that might induce an imbalance between cell apoptotic and proliferative stimuli are discussed. Second, the review summarizes findings on orexins and their involvement in narcolepsy, neurodegenerative diseases, and cancer, starting from epidemiological data then addressing laboratory findings, animal models, and human clinical observational and interventional investigations. Important research efforts are warranted on these topics, as they might lead to novel therapeutic approaches to both neurodegenerative diseases and cancer.
Abstract
Conditions such as Alzheimer’s (AD) and Parkinson’s diseases (PD) are less prevalent in cancer survivors and, overall, cancer is less prevalent in subjects with these neurodegenerative disorders. This seems to suggest that a propensity towards one type of disease may decrease the risk of the other. In addition to epidemiologic data, there is also evidence of a complex biological interconnection, with genes, proteins, and pathways often showing opposite dysregulation in cancer and neurodegenerative diseases. In this narrative review, we focus on the possible role played by orexin signaling, which is altered in patients with narcolepsy type 1 and in those with AD and PD, and which has been linked to β-amyloid brain levels and inflammation in mouse models and to cancer in cell lines. Taken together, these lines of evidence depict a possible case of inverse comorbidity between cancer and neurodegenerative disorders, with a role played by orexins. These considerations suggest a therapeutic potential of orexin modulation in diverse pathologies such as narcolepsy, neurodegenerative disorders, and cancer.
Keywords: cancer, neurodegeneration, orexin, narcolepsy, inverse comorbidity
1. Cancer and Neurodegenerative Diseases
The World Health Organization (WHO) highlights cancer as one of the most common causes of death, which accounted for almost 10 million deaths worldwide in 2020 [1]. Overall, estimates indicate that one in five persons will get cancer in their lifetime before 75 years of age and one in ten will die from the disease.
The incidence and prevalence of neurodegenerative diseases are also high. Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by brain β-amyloid plaques and neurofibrillary tangles formed by phosphorylated tau (P-Tau) protein deposits. AD is the most frequent neurodegenerative disease and affects 24 million people worldwide. The second most frequent neurodegenerative disease is Parkinson’s disease (PD), characterized by Lewy bodies and neurites formed by alpha-synuclein deposits. PD has a prevalence of 1% in people older than age 60, and of 3% in people aged 80 years or older [2]. There is also increasing interest, in the literature, on multiple sclerosis (MS). Despite being an inflammatory demyelinating disease, MS can also be viewed as a neurodegenerative disease because of the cascade of events triggered by neuroinflammation and leading to neurodegeneration [3,4,5]. The key elements that induce neurodegeneration include activation of microglia, chronic oxidative damage, and altered mitochondrial function in axons, leading to chronic cellular stress and imbalance of ion homeostasis, resulting in axonal and neuronal death [6]. The total prevalence of MS in Europe is 83 per 100,000, which is lower than the prevalence of AD and PD, but still associated with substantial social and economic costs [7]. Amyotrophic lateral sclerosis (ALS), which is characterized by the progressive loss of motor neurons in the brain and spinal cord, is also rare, with a prevalence of 2–3 per 100,000 [8].
1.1. The Connection between Cancer and Neurodegenerative Diseases
The incidence of many common cancers and neurodegenerative diseases including AD and PD increases with age [9,10]. MS also typically occurs in adults, although its prevalence peaks for subjects between 35 and 64 years of age and does not further increase thereafter [7]. Similarly, the risk for ALS peaks at 50–75 years of age [8]. It is essential to focus attention on the development of alternative treatments that address age-related diseases, also in consideration of the increase in age in the population.
Despite the common age-related trends in the incidence of cancer and neurodegenerative disorders, a meta-analysis of observational studies including 577,013 participants concluded that there was a significantly lower co-occurrence of cancer in patients with neurodegenerative disorders. In particular, patients with AD had a markedly reduced co-occurrence of cancer in general, but no data were available for specific cancers [11]. The Framingham heart study, a longitudinal community based cohort study, in which 221 cases were evaluated for a 10-year follow-up, also indicated a lower risk of AD for cancer survivors: the risk of AD was lower in survivors of smoking-related cancers; this model for cancer is similar to that seen in PD and suggests an inverse association between cancer and neurodegeneration; the effect was stronger for lung cancer and preserved for participants who survived at least to 80 years of age [12]. Furthermore, in evaluating the interconnection between AD and lung cancer, it is important to consider that cigarette smoking appears to play a neuroprotective influence for both AD and PD [13], while it represents a known risk factor for cancer of the lung.
A recent systematic review and meta-analysis confirmed a weak but significant decrease in AD risk comparing older adults with vs. without a previous cancer diagnosis, but it could not rule out a role of survival bias [14]. Epidemiological studies indicate that AD patients have a lower risk of developing lung cancer and a higher risk of developing glioblastoma: transcriptomic meta-analyses reveal a significant number of genes with reverse expression patterns in AD and lung cancer, compared to AD and glioblastoma [15]. Meta-analytical data indicate that patients with PD and MS have a reduced co-occurrence of lung and prostate cancers, and patients with PD also have reduced co-occurrence of colorectal cancer. These associations are not consistent across all cancer types, with an increased cooccurrence of melanoma in patients with PD and of brain cancers in patients with MS [11]. On the other hand, a recent population-based case-control study reported negative point estimates of the odds of developing PD in survivors of most cancers, with the notable exception of skin and female breast cancer but with wide confidence intervals overlapping with zero [16]. A recent meta-analysis has shown that patients with MS have a lower risk of contracting tumors than the general population [17], with an inverse comorbidity between MS and cancer. The authors also stressed that the identification of inverse comorbidity and its underlying mechanisms could provide important new insights into the understanding of MS [18]. No significant association has been found between ALS and overall cancer occurrence [11].
These epidemiologic associations are complex and challenged by confounders and exceptions [19]. Cancer treatment may also modify the relationship; some studies suggest that patients who received chemotherapy may have lower white matter organization and connectivity when compared with healthy controls [20]. Other studies suggest that chemotherapy correlates with a lower risk of AD [21]. Inverse comorbidity does not involve all types of neurodegenerative disorders and all types of cancers. Thus, the concept of inverse comorbidity, as it is discussed in this review, should always be considered in its imperfect nature rather than as a fixed rule. Nevertheless, the epidemiological data suggest a pattern of lower-than-expected combined probability of cancer and neurodegenerative diseases, which has been defined as “inverse comorbidity” [22]. The study of the mechanisms behind this pattern of inverse comorbidity could influence therapeutic interventions and provide strategies that prevent or delay both cancer and neurodegenerative diseases [19,23,24,25].
There are multiple factors that play a central role both in cancers and neurodegeneration through the same metabolic pathways that are inversely regulated and altered, as outlined in a comprehensive review published in recent years [19]. One key feature highlighted by that review was the occurrence of two patterns of association between cancer and neurodegeneration, which were summarized as “proliferation” and “apoptosis”, respectively. This implied that neurodegenerative diseases and cancers may be viewed as two sets of diseases with “too little” and “too much” apoptosis, respectively. The review also highlighted multiple factors that may underlie this difference: oxidative stress, DNA damage, inflammation, genomic instability and epigenetic alterations, mitochondrial and telomere dysfunction, metabolic dysregulation, depletion of stem cells, aberrant activation of the cell cycle, and cellular interconnections [19]. Many of these factors overlap with those that have been proposed as the pillars of aging: macromolecular damage, proteostasis, inflammation, epigenetics, metabolism, stem cells and regeneration, and adaptation to stress [26].
Another potential example of a mechanism relevant to both cancer and neurodegeneration is the non-classical, non-enzymatic binding of acetylcholinesterase (AChE) acting at an allosteric site on the nicotinic alpha-7 receptor. AChE is expressed not only in the brain but also in epithelial, endothelial, immune, and cancer cells. This form of inter-cellular communication may represent a system for triggering the entry of calcium into a wide range of cells to promote their growth. Furthermore, the AChE peptide might play a fundamental role in cell migration; therefore, through this pathway (common to neurodegeneration and carcinogenesis), there might be an interconnection between the nervous, endocrine, and immune control systems [27].
Although neurodegeneration is typical of old age, neurodevelopmental disorders might share at least some mechanisms with neurodegenerative diseases, with cognitive delay representing the counterpart of dementia. It has been estimated that 40–100% of brain tumor survivors have neurocognitive problems [28], not necessarily connected with the direct brain damage associated with the tumor and/or its treatment, and a recent review has highlighted the bidirectional correlation between cancer and neurodevelopmental disorders in pediatric age [29]. Although pediatric studies conducted on this topic are still very few, a role in the neurodevelopment deficit (especially in a fundamental period for cerebral maturation and neuronal plasticity) is played by anticancer therapies, due to their neurotoxicity [30]. Over the years, various chemotherapeutic agents used in clinical practice for the treatment of brain tumors have shown severe effects on cognitive functions, and recent studies on nanotechnology have shown that nanomaterials that could be exploited for the treatment of brain cancer are able to induce neurotoxic effects and neurodegeneration [31]. However, another important role is definitely played by inflammatory processes related to the tumor and to the considerable increase of reactive oxygen species (ROS); thus, cytokine-mediated inflammatory mechanisms might act as a trigger that initiates a cascade of events responsible for neurotoxicity [32]. In support of this, a study on children with acute lymphatic leukemia showed damage to brain structures both before and after chemotherapy, with an increase in Tau protein (suggestive of axonal damage) and in glial fibrillary acidic protein (GFAP) in patients with alterations of the apolipoprotein E gene (associated with attention deficit) and an increase in leukoencephalopathy, with impairment of the white matter [32,33].
1.2. The Biological Bases of the Inverse Comorbidity between Cancer and Neurodegeneration
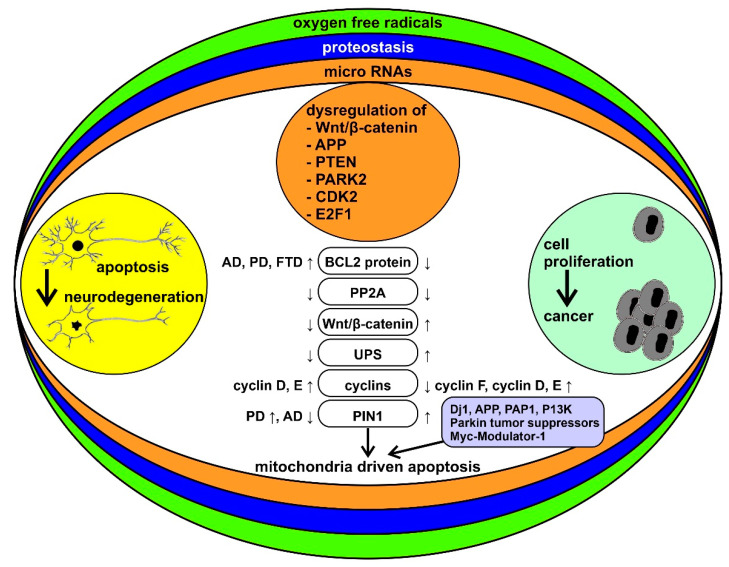
Inverse comorbidity between cancer and neurodegeneration may be influenced by environmental, pharmacological, and dietary factors. Genetic factors can additionally contribute to the inverse comorbidity between complex diseases [22,34,35,36]. At the base of the bidirectional interactions between cancer and neurodegenerative diseases there may be complex mechanisms that include genes, proteins, and mitochondrial function, the study of which could provide important therapeutic novelties for both diseases (Figure 1).
Figure 1.
Schematic representation of the bidirectional interactions between cancer and neurodegenerative diseases and their modulatory processes. Neurodegenerative diseases and cancer are framed as two sets of disorders with “too much” or “too little” apoptosis, respectively. Reactive oxygen species (ROS), alterations in proteostasis, and microRNA (miRNAs) are among the key factors that may underlie the differences between the two sets of disorders. Some of the specific molecular mechanisms thought to be involved in this pattern of inverse comorbidity are highlighted in the middle column (see the text of the paper for abbreviations). ↓ = downregulated, ↑ = upregulated.
Mutations in PRKN (PARK2, Parkin), PARK7 (DJ-1), and PINK1 genes, which are among the genes contributing to familial cases of PD, lead to the mutation of both tumor suppressor genes, PTEN and TP53 [25]. On the other hand, PRKN and PARK5 have antiproliferative properties and are often inactivated in tumors [37]; PINK1 can also have antiproliferative functions [38]. Another mechanism potentially contributing to the inverse comorbidity is related to the brain expression of PARP1. The PARP1 protein is underexpressed in the brains of subjects with PD [39], while it is overexpressed in glioblastoma multiforme [39]. Interestingly, the PARP1 protein is also overexpressed in prostate cancer [40].
The genetic variants shared between AD and cancer are less known. Recently, a genome-wide association study (GWAS) has highlighted genes potentially involved in AD and in five different cancers (colon, breast, prostate, ovary, lung), with some shared variants modulating disease risk in a concordant way and others exerting effects in opposite directions [41]. On the other hand, the transcription factor P53 (a tumor suppressor) has multiple functions common to cancer and neurodegenerative disorders such as HD, PD, and AD [25,42], and it is crucial for cell growth control and apoptosis. Its expression is upregulated in AD, PD, and HD but downregulated in the vast majority of tumors [19,23,25].
Aggregation of superoxide dismutase (SOD1) causes cell death in ALS; however, SOD1 also has a role in breast cancer and the ability to increase estrogen reactive gene expression [19]. The reduced activity of SOD1 and glutathione reductase (GR) induces an increase in the production of reactive oxygen species (ROS), which leads to a conformational change of P53. The modification of P53, in turn, induces the production of ROS, thus activating oncogenic functions, such as tumor cell invasion and metastases. P53 also induces the upregulation of apoptotic proteins, such as X associated with BCL2 (BAX) and caspase 3 (observed in PD) [43]. This cascade of events, depicted also in Figure 2, seems to be an example of unclear or absent inverse comorbidity between neurodegeneration and cancer, perhaps based on the physiological and ubiquitous ROS signaling.
Figure 2.
Schematic representation of the possible role of superoxide dismutase (SOD1) in amyothrophic lateral sclerosis (ALS), Parkinson’s disease (PD), and cancer metastasis. See the text of the paper for abbreviations. ↓ = decrease, ↑ = increase, ⊕ = stimulation.
Interestingly, drugs used in the treatment of symptoms of neurodegenerative diseases, such as thioridazine, have been shown to have anticancer effects, while anticancer drugs, such as cyclin-dependent kinase inhibitors and mithramycin, are neuroprotective; these data reinforce the existence of a link between cancer and central nervous system (CNS) diseases and indicate that future studies will need to focus on specific molecular pathways [23].
1.3. The Role of Mitochondria
The BCL2 protein, involved in the mitochondrial outer membrane permeabilization, is overexpressed in CNS disorders, such as AD, PD, and frontotemporal dementia (FTD), whereas it undergoes downregulation in tumors [23]. On the other hand, cyclin D and cyclin E are overexpressed in both cancer and neurodegenerative diseases, while PP2A is downregulated in both diseases. Cyclin F is downregulated in cancer, and a mutation has been found in neurodegenerative diseases [43]. PIN1, a multifunctional gene that is hypothesized to function as a molecular timer, is overexpressed in a number of tumors and in PD but is underexpressed in AD [19,43]. Inhibition of PIN1 suppresses the growth of various tumor cells and is therefore considered as a promising therapeutic target in the oncological field [43]. PIN1, through its interactions with P53 and BCL2, can have a pro- or anti-apoptotic role depending on the cellular context. Its role in mitochondria-driven apoptosis could therefore provide a link between cancer and AD [43]. Other potentially common factors in cancer and neurodegenerative diseases include the PARK7 (DJ-1) and APP oncogenes (the first is involved in mitochondrial regulation, the second is a precursor of the β-amyloid protein), the PFDN5 (MM-1) and PRKN (Parkin) tumor suppressors (the first inhibits transcription and protein aggregation, involved in the onset of PD, cerebellar atrophy and HD, the latter plays a role in mitophagy and ubiquitination), and the PDAP1 (PAP1) and PINK1 modulators (the former is involved in splicing and the latter in mitophagy) [44].
Growing evidence suggests that age-related changes in bioenergetics at the mitochondrial level and the resulting metabolic compensation may be an important driver of both cancer and neurodegeneration and a potential target for prevention and therapy; dysfunction of mitochondria leads to disruption of DNA repair and the malfunction of metabolic pathways (such as the PI3K pathway), increasing the risk of cancer [45]. Alterations in mitochondrial DNA (mtDNA) decrease the efficiency of the respiratory chain with aging, and the prevalence of mtDNA deletions seems particularly high in neurodegenerative disorders such as PD and AD [46,47]. It has also been shown that mutations in mitochondrial DNA and alterations in mitochondrial energy metabolism can be correlated with the onset or progression of glioblastoma, following alteration of the pathways involved in apoptosis [48].
Recent data highlight an important role of the intestinal microbiome, intestinal permeability, and alterations in the functioning of mitochondria in the pathophysiology of MS: orexins, melatonin, and butyrate increase oxidative phosphorylation in mitochondria through the disinhibition of the pyruvate dehydrogenase complex, leading to an increase in acetyl-coenzyme A, a co-substrate necessary for the activation of the melatoninergic pathway of the mitochondria. Loss of mitochondrial melatonin coupled with an increase in N-acetylserotonin has implications for impaired mitochondrial function and appears to play a role in the pathophysiology of MS [49].
1.4. Other Factors
Telomere alterations have been recognized as a risk factor for both age-related carcinogenesis and neurodegeneration. In AD, telomere shortening has been implicated in oxidative stress and inflammation, with cognitive impairment, amyloid pathology, and hyperphosphorylation of the Tau protein. A decrease in telomere length was found in peripheral blood leukocytes in patients with AD, compared with age-matched controls, and was proposed as a potential biomarker for AD [50,51]. As introduced above, this should be considered in the framework of the imperfect inverse comorbidity between AD and cancer. Several studies have shown that telomeres shorten in the early stages of carcinogenesis and that tumor cells need to activate telomerases (which synthesize telomeres) to become immortal [52,53].
The Wnt family of secreted glycolipoprotein signaling pathway molecules takes part in the regulation of cell proliferation, polarity, and fate during the embryonic phase and in tissue homeostasis. Changes in the Wnt pathway are involved in congenital defects, cancer, and other diseases [54]. The Wnt/β-catenin signaling pathway appears to play a critical role in neural stem cell proliferation [55]. However, the dysregulation of this pathway has also been associated with cancer and neurodegenerative disorders, such as AD and PD, through an inverse correlation. In particular, the activation of Wnt signaling could be protective in neurodegenerative diseases but could promote the onset and progression of cancer [56,57]. Several molecular components of the signaling pathway have been proposed as innovative targets for cancer therapy, and very recently, some have also been evaluated as potential therapeutic targets for PD [57].
Protein homeostasis (or proteostasis) indicates the maintenance of the correct concentration of proteins of regular conformation and subcellular compartmentalization. The loss of protein homeostasis is another very important process in neurodegeneration. In particular, the family of heat shock proteins, chaperones, and the ubiquitin–proteasome system (UPS), which decrease with aging, result in aggregation of synuclein in Lewy bodies, of β-amyloid, and of Tau [58,59]. Additionally, neoplastic cells show a loss of protein homeostasis but often in the opposite direction: in cancer cells there is an overexpression of UPS and heat shock proteins [60,61]. Global hypomethylation and hypohydroxymethylation, alterations of histone proteins, and high expression of some non-coding RNAs were found in AD [62,63]. The same mechanisms are also implicated in carcinogenesis, and epigenetic therapy has already been suggested as a potential method to correct the expression levels of dysregulated genes in neurodegenerative disorders and tumors [19].
The miR-34 and miR-122 miRNAs have been used in the treatment of certain types of cancer and hepatitis, with promising results [64]. On the other hand, some miRNAs (miRNA-9, miRNA-34a, miRNA-125b, miRNA-146a, and miRNA-155) may be involved in the physiopathology of AD, and some act on the Wnt/β-catenin pathway [65], which is involved in both neurodegenerative diseases and tumors. A recent paper suggested a potential anti-β-amyloid protective effect of miRNA-15b and a biological link between miRNA-125b and neurotoxic pathways, hypothesizing that these miRNAs may play a role as biomarkers of AD physiopathology with therapeutic potential [66]. The amyloid precursor protein (APP) is connected to both AD and malignant growth. APP is concentrated in neuronal synapses and is the major component of amyloid plaques associated with AD. APP increases the proliferation and migration of epithelial cells (although the mechanism has not been fully defined) and is overexpressed in various tumors (oral cavity, esophagus, pancreatic, neuroendocrine, thyroid, and colorectal cancers) [67]. These results suggest the potential role of APP in cancer pathogenesis and reinforce our concept of imperfect inverse comorbidity between AD and cancer. Aberrant expression of miRNAs could also be involved in both neurodegeneration and tumor pathologies through the downregulation of PTEN, involved in PD. Many genes involved in both types of pathologies, including PARK2, CDK2, and E2F1, are potential targets of multiple miRNAs; however, further studies are needed to better understand their roles [67]. Taken together, these data suggest that miRNAs may be the basis for common therapeutic approaches to both cancer and neurodegenerative diseases [67,68].
2. Cancer, Narcolepsy, and Other Neurodegenerative Diseases
2.1. Narcolepsy and Cancer
Narcolepsy type 1 (NT1) is a rare neurological disease that reflects a selective loss or dysfunction of the orexin (also known as hypocretin) neurons of the lateral hypothalamus. NT1 is typically characterized by excessive daytime sleepiness and cataplexy, accompanied by sleep-wake symptoms, such as hallucinations, sleep paralysis, and sleep disturbances. Its etiopathogenesis is still under study, and a likely autoimmune genesis has recently been convincingly proposed [69]. Furthermore, a meta-analysis has highlighted an increase in serum/plasma levels of some cytokines (IL-6 and TNF-α) in patients with narcolepsy, further supporting the involvement of inflammatory mechanisms in its pathophysiology [70]. However, it should be considered that while inflammation may be involved during the disease onset period as part of an autoimmune challenge, its significance in later disease stages is under debate.
In recent years, an increased cancer risk has been reported, albeit not invariably [71], in both adults [72] and children and adolescents [73] with narcolepsy. In particular, in a study conducted on 2833 patients with narcolepsy with an observation period of 10 years, a connection with cancer risk was reported, especially for head-neck and gastrointestinal cancers and, to a lesser extent, for hematological and genitourinary cancers. The authors hypothesized genetic and immunological factors underlying the interconnection between narcolepsy and cancer [74]. These data are of interest because they suggest a link between narcolepsy and cancers in organs and tissues in which there is evidence of functional responses to orexins (brain, gastrointestinal, and genitourinary tract) [75,76,77]. Orexin might, therefore, play a role in some types of tumors as well as in NT1. However, studies on this topic are few, also due to the rarity of narcolepsy, even though they were conducted on a large registry series. On the other hand, registry data may not allow the discrimination between NT1 with orexin deficiency and narcolepsy type 2 (NT2) without orexin deficiency [73]. One study based on retrospective medical claims data found an increased comorbidity with neoplasms in both subjects with NT1 and subjects with NT2, compared with controls [72]. Thus, the epidemiological link between orexin deficiency in NT1 and cancers is still unclear and warrants further research, which may pave the way to a better understanding of these pathological processes and to new therapeutic perspectives.
2.2. Narcolepsy and Neurodegenerative Diseases
NT1 may coexist with AD or PD [78] or with MS [79]. The prevalence of AD in patients with NT1 was similar to that in control subjects based on postmortem brain pathology in 12 patients [80]. While these data indicate that NT1 does not confer absolute protection from neurodegenerative diseases, they are too limited to conclude for or against an association. In other studies, attention was focused on cerebrospinal fluid (CSF) neurodegeneration markers, revealing a decrease in CSF β-amyloid1-42 (the amyloid beta peptide with greatest propensity to aggregation) [81] in patients with narcolepsy (both NT1 and NT2) close to disease onset, which progressively recovered with time. On the other hand, patients with long disease duration of narcolepsy had higher P-Tau CSF levels than patients with short disease duration or control subjects [82]. Lower CSF levels of β-amyloid1-42 [83,84] and P-Tau [84] in patients with NT1 than in control subjects were reported by other studies, although not invariably [85]. Reduced brain β-amyloid burden was also detected with positron emission tomography in elderly patients with NT1, suggesting a reduced risk of progression to AD; furthermore, the authors hypothesized a protective role of orexin antagonists against neurodegeneration [86]. This suggestion is supported by preclinical evidence that in mice, the amount of β-amyloid in the brain interstitial fluid increased during orexin infusion and decreased with infusion of an orexin receptor antagonist, which also decreased β-amyloid plaque formation in amyloid precursor protein transgenic mice [87].
3. Orexin, Cancer, and Neurodegenerative Diseases
Although many critical pieces of evidence are still missing, the results summarized in the previous paragraphs raise the hypothesis that orexins mediate, to some extent, the links between cancer and narcolepsy and other neurodegenerative diseases. In particular, orexin deficiency is a key pathophysiological feature of patients with NT1 and might explain a greater occurrence of some types of tumors and reduced markers of neurodegenerative diseases in these patients.
To better understand this aspect, it is necessary to focus attention on the potential mechanisms of inverse comorbidity that were discussed previously, which include genes, proteins, and mitochondrial function.
3.1. The Orexin System
Orexin A and B (OXA and OXB) [88], also named hypocretin 1 and 2 [89], are peptides expressed by hypothalamic neurons that are most active during active wakefulness and have sparse activity during rapid-eye-movement (REM) sleep [90].
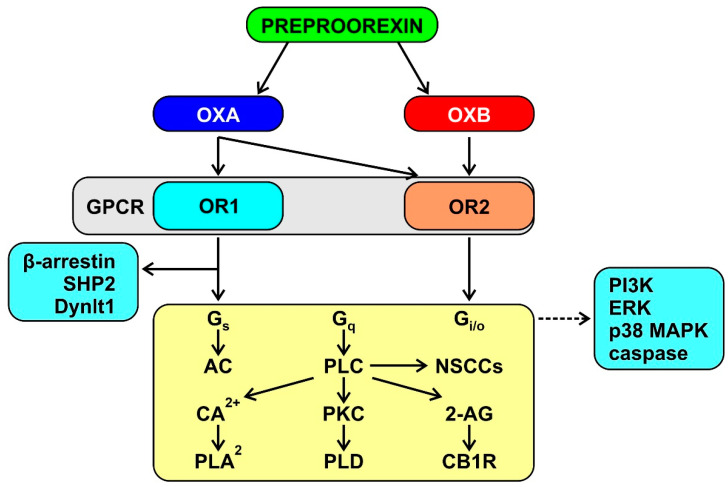
The orexins bind two G-protein-coupled receptors named OR1, which is selective for OXA, and OR2, which is non-selective for OXA and OXB [88]. OR1 and OR2 are widely expressed in the CNS [76], in line with the widespread projections of the orexin neurons [91]. The available evidence indicates that the orexin signaling is multifaceted and complex, with similar mechanisms elicited by OR1 and OR2 (Figure 3) [92,93]. Interpretation of this evidence is complicated by the fact that data were mostly obtained in recombinant cell lines that overexpress orexin receptors, with limited direct relevance to orexin physiology and pathophysiology. Nevertheless, the relevance of these expression systems is very considerable by implication because they reflect the intrinsic signaling capabilities of orexin receptors [94]. These capabilities underlie the in vivo modulation by the CNS orexin system of multiple physiological functions, including wake-sleep behavior, energy homeostasis, and autonomic cardiovascular control [95,96,97,98].
Figure 3.
Schematic representation of the signaling potential of orexin receptors, as demonstrated by experiments on recombinant cell lines. Continuous arrows indicate demonstrated or hypothesized causal links between messengers and/or enzymes. The broken arrow indicates an undetermined causal pathway. GPCR: G-protein coupled receptors. AC: adenylate cyclase. PLC, PLA2, PLD: phospholipase C, A2, and D, respectively. NSCCs: non-selective cation channels. 2-AC: 2-acyl-glycerol. CB1R: cannabinoid type 1 receptor. See the text of the paper for the other abbreviations.
OR1 and OR2 are also expressed outside the CNS, including in the gastrointestinal tract, adipose tissue, male reproductive tissue [76], as well as in the heart [99] and bone marrow [77], where they may be relevant to the pathophysiology of heart failure [99] and atherosclerosis [77]. As peripheral orexin synthesis is debated [75], these receptors may bind orexins that spill over to the systemic circulation after their release in the CNS by hypothalamic neurons [77]. In this respect, it should be noted that while OXA is relatively protected from inactivating peptidases by its chemical structure, OXB is not [88] and is rapidly metabolized in blood [100] and even in CSF [101]. Any peripheral effect of circulating orexins may thus be mediated largely if not solely by OXA. However, even the blood–brain barrier permeability to OXA is still debated. OXA was reported to rapidly enter the mouse brain by simple diffusion [100], but another study reported negligible (< 1%) brain penetration of OXA in mice and rats [102].
3.2. Orexin and Mitochondrial Function
There seems to be no studies in the literature on the functioning of mitochondria in narcolepsy. However, a recent in vivo study reported that OXA might reduce mitochondrial biogenesis, increase mitophagy, and damage mitochondrial structure in AD. These authors also envisaged inhibition of OXA as a potential approach to the treatment of AD [103]. Similar data were reported by a study on mouse models, which showed that OXA aggravated mitochondrial deterioration, the accumulation of β-amyloid, and cognitive deficits [104]. This might imply that OXA deficit, as in NT1, would preserve the integrity and functionality of the mitochondria, reducing the risk of progression to AD. These data might also explain why NT1 and MS seem to be only rarely associated, contrary to what one would expect, since both conditions may represent autoimmune diseases [69,70,79,105]. Lack of OXA in NT1 could exert a protective effect against mitochondrial damage, preserving them from the neurodegeneration mechanisms that occur in MS [6].
Emerging studies highlight that a mitochondrial dysfunction can enhance inflammatory and immune processes, also depending on environmental factors [106].
3.3. Microbiota
There is promising but initial evidence that the local immune response and systemic inflammation might play a role both in tumor progression and in the survival of cancer patients [107], in neurodegeneration associated with diseases such as AD, PD, ALS, and FTD [108], as well as in NT1, a pathology with a likely autoimmune pathogenesis [69,70]. In this respect, it is also worth mentioning alterations in the structure of the intestinal microbial community that may be linked to inflammation in narcolepsy, although the data on this topic are still limited. Further broader and longitudinal studies are necessary to replicate and clarify the relationship between the gut microbiota, immunity dysregulation, and narcolepsy [109].
It seems that the intestinal microbiota plays a key role in the interaction between the brain and the gastrointestinal tract [110], due to the ability of probiotic agents to alter cytokine levels and influence brain function. The intestinal microbiota has recently been linked to the pathogenesis of neurodegenerative diseases such as PD [111] and AD [112,113], in support of a close interconnection between inflammatory, immunological, neurodegenerative, and carcinogenic processes. Recent studies suggest that the modulation of the intestinal microbiome is able to influence the immune response and numerous forms of cancer therapy by reducing their toxicity [114].
3.4. Genetic Factors
In a study on 426 patients with NT1, a significant association was found with increased copy number variation in the PARK2 region [115]. However, the study was conducted only on the Japanese population, and there are no data on other ethnicities. It was previously mentioned that PARK2 has antiproliferative properties and is often dysregulated due to somatic mutations in tumors [37]. A variable expression of this gene in different narcoleptic patients could therefore contribute to explanations of the increased prevalence of neoplasms in narcolepsy [74]. These findings suggest that further studies are needed on the possible genetic variations common to cancers and narcolepsy.
Mitochondria are one of the targets of miRNAs [116]; thus, alterations in miRNAs might modify mitochondrial function. Alterations in the expression of some miRNAs may be involved in the pathophysiology of narcolepsy [117]. A study revealed significant differences in the plasma levels of four miRNAs in patients with NT1 compared with healthy controls: levels of miR-30c, let-7f, and miR-26a were higher, whereas the level of miR-130a was lower in NT1. However, these differences were not specific for NT1 but also occurred in patients with NT2 or idiopathic hypersomnia. The levels of miR-26a, miR-30c, and let-7f are relatively high in the brain. miR-30c is also highly expressed in the heart, thyroid gland, blood cells, skeletal muscle, kidney, and lung. miR-26a is also expressed in the heart, thyroid, skeletal muscle, colon, and reproductive system. let-7f is also expressed in the heart, thyroid, blood cells, skeletal muscle, and reproductive system. miR-130a is expressed in the heart, lung, kidney, jejunum, colon, thyroid gland, and the reproductive system. The authors raised the hypothesis that these miRNA play a pathophysiological role and speculated that let-7f might influence T cell proliferation [118]. An alteration of miRNAs in patients with narcolepsy might be correlated with metabolic dysfunctions and carcinogenesis, especially in the gastrointestinal and genitourinary tracts, where increased tumor occurrence has been reported in these patients [74]. Very recent and interesting data indicate that miRNA specifically located in brain orexin neurons are also involved in the regulation of their viability [119].
3.5. Orexin in Cancer
In recent years, a series of studies have been conducted on the role of orexins not only in the field of sleep disorders but also in oncology, discovering that modulation of the orexin signaling may also unexpectedly play a therapeutic role in the treatment of some types of cancer [120,121]. One study demonstrated that OXA stimulates neovascularization [122]. Angiogenesis is the formation of new capillaries from preexisting blood vessels, a critical step in physiologic and pathologic events such as embryonic development, wound healing, chronic inflammation, and tumor growth [123]. Recent studies on the relationship between the brain and the gastrointestinal tract have suggested that OXA may play an immunomodulatory role, reducing the production of pro-inflammatory cytokines (tumor necrosis factor α, interleukin-6, and chemotactic protein of monocytes-1). Therefore, it has been proposed that the modulation of the orexinergic system may be useful in the treatment of hyperalgesia and fatigue due to chemotherapy, as well as in inflammatory bowel diseases [124].
Early work revealed a pro-apoptotic effect of OR1 signaling in colon cancer and neuroblastoma cell lines [125]. These results were later confirmed on cell lines from human colon cancer and liver metastases, both in vitro and in vivo, after xenograft in nude mice [126]. Most interestingly, OXA also promotes robust apoptosis in cells that are resistant to 5-fluorouracil, the most widely used chemotherapy in colon cancer, and reverses the development of established tumors when administered seven days after cell inoculation [125]. OXA might promote tumor apoptosis in vivo by directly activating caspase-3. These findings seem to suggest OR1 agonists for future research on colon cancer therapy [125]. More recent data on human colon cancer cell lines indicate that OXA induces autophagy [127] and that dual-agonist occupancy of OR1 and cholecystokinin A receptor heterodimers decreases migration [128]. Orexin-dependent apoptosis might be mediated by two immunoreceptor tyrosine-based inhibitory motifs in both OR1 and OR2, involvement of the phosphotyrosine phosphatase SHP2, and induction of mitochondrial apoptosis [120].
The evidence in favor of a therapeutic role of orexins on cancers other than colon cancer is more limited and partly contrasting [129]. OXA was found to suppress the growth of rat glioma cells [130]. Conversely, OXA was found to inhibit gastric cancer cell apoptosis via OR1 [131] and to enhance proliferation by upregulating the protein expression of OR1 [132], which is opposite to what was reported for colon cancer cells. Data are also contrasting for pancreatic ductal cancer and prostate carcinoma. One study reported that OR1 signaling promotes cell proliferation in pancreatic ductal cancer cells [133], whereas another study indicated that both agonism (OXA) and antagonism (almorexant) of OR1 exert an antitumoral proapoptotic effect on pancreatic ductal cancer in vitro and in vivo [134]. On the other hand, OR1 was found to be overexpressed and to mediate apoptosis in advanced prostate cancer with a neuroendocrine differentiation [135]. Accordingly, OXA administration to a human androgen-dependent prostate carcinoma cell line was later found to upregulate OR1 expression, resulting in a decrease of cell survival [136]. The same year, however, lack of expression of orexin receptors genes was reported in human normal and prostate cancer cell lines [137].
3.6. Orexin in Neurodegenerative Diseases
Neurodegenerative disorders may be associated with decreases in orexin neuron number and orexin system activity [97]. The orexin neuron number was found decreased by 40–72% [138,139] in the brains of patients with AD, whereas CSF OXA levels were found reduced only by 14% [138], not significantly changed [140] or even increased [141] in these patients. This discrepancy suggests either that orexin neuron loss is inconstant or that the residual orexin neurons are overactive.
PD and dementia with Lewy bodies (DLB) are neurodegenerative disorders characterized by Lewy bodies and neurites formed by alpha-synuclein deposits, whereas multiple system atrophy (MSA) is characterized by neuroglial alpha-synuclein cytoplasmic inclusions [142]. The number of orexin neurons was found dramatically reduced by 62–75% in the brain of subjects with PD, DLB, and MSA [143,144,145]). However, similar to what has been reported for subjects with AD, reductions in orexin neuron number in subjects with PD, DLB, or MSA were found generally insufficient to entail significant decreases in CSF levels of orexin [146,147,148].
OXA levels in the CSF are not significantly decreased in subjects with MS [149], unless MS entails hypothalamic lesions [150]. Nervous system inflammation is central to MS, albeit possibly secondary to neurodegeneration [5], and is also involved in the pathophysiology of the tau- and synucleinopathy neurodegenerative disorders [151,152]. Interestingly, there is evidence that OXA may decrease inflammation at neural and systemic levels. In mouse models, OXA acts on the CNS to modulate inflammation and increase survival in septic shock [153] and to alleviate inflammation after intracerebral hemorrhage [154] and in experimental immune encephalomyelitis [155]. OXA may also act on intestinal OR1 to prevent lipopolysaccharide-induced neuroinflammation at the level of the intestinal barrier [156] and to decrease inflammation in ulcerative colitis [129]. Moreover, OXA acts on OR1 bone marrow pre-neutrophils to tune down myelopoiesis, restraining the nighttime increase in circulating inflammatory monocytes and neutrophils and limiting atherosclerosis burden [77]. Thus, OXA has an immunoregulatory and neuroprotective action, inhibiting apoptosis and reducing inflammation. Moreover, OXA seems to have an action on microglia, with promising implications not only for tumors and inflammatory diseases but also for neurodegenerative diseases, although data on these are still scarce [157]. The orexin receptor antagonist suvorexant might be useful for the prevention and treatment of AD because of its neuroprotective effect, with reduction of β-amyloid plaques and improvement of synaptic plasticity [158]. At least in part, this effect may result from enhanced sleep-related brain glymphatic clearance of metabolic by-products, such as amyloid-β [159,160]. On the other hand, studies on mouse models show that orexins can ameliorate parkinsonian motor deficits by increasing the spontaneous activation of pallidal neurons [161].
4. Conclusions
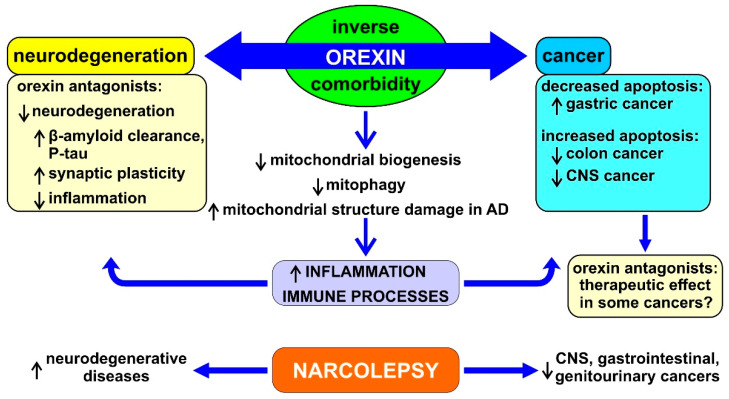
The studies on the potential modulation of the orexin system in cancer and in neurodegenerative diseases are still pioneering and further human data are needed, although they have already shown promising results. In consideration of the concept of inverse comorbidity and in order to understand more effective therapeutic options in the future for both neurodegenerative diseases and cancer, it is important to focus on the common metabolic pathways involved in these processes. Figure 4 summarizes the possible mechanistic role of orexin in neurodegeneration and cancer.
Figure 4.
Schematic representation of the possible mechanisms underlying the role of orexin and its influences on neurodegeneration and cancer. See the text of the paper for abbreviations. ↓ = decrease, ↑ = increase.
The findings reported in this paper point to a theoretical framework in which the cell fate is determined by the (un)balance between factors favoring apoptotic or proliferative processes in reciprocal opposite directions. This ideal, although not perfect, framework has been called inverse comorbidity and indicates a lower-than-expected probability that a disease will occur in people who have another disease. A convincing amount of data have been published in recent years supporting the existence of a general inverse comorbidity between neurodegenerative diseases—such as AD, PD, MS, and others—and several types of cancer, with some exceptions. On the other hand, there are few published studies about narcolepsy in this context. Studies aimed at evaluating the role of proteins, miRNAs, and mitochondria in this area could shed more light on the etiopathogenesis of this disease, provide more answers on the biological basis of the interconnection with related pathologies, suggest new possible therapeutic perspectives, and provide new data on risk factors for the disorder.
Inverse comorbidity has its biological bases in a probably very complex mechanism in which more general and ubiquitous processes, such as ROS, miRNAs, mitochondrial function, etc., as well as more specific factors, such as orexins, play a combined role with different weights in order to favor neurodegeneration or cancer, alternatively and in mutual (quasi)exclusion.
The findings summarized in this narrative review on orexin start from epidemiological data, then include support by laboratory findings, animal models, and human clinical observational and interventional investigations. Taken together, these different lines of evidence have a relevant possible translational value, which might lead to the arrangement of novel therapeutic approaches to both neurodegenerative disease and cancer by modulating orexin pathways. This perspective warrants an important research effort on this topic in the near future.
Author Contributions
Conceptualization, methodology, formal analysis, writing—original draft preparation, and writing—review and editing: M.P.M., A.S., L.M.D., M.S. and R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by a grant of the Italian Ministry of Health to R.F. and M.S. (“Ricerca Corrente” RC n. 2764026).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Colombet M., Soerjomataram I., Parkin D.M., Piñeros M., Znaor A., Bray F. Cancer statistics for the year 2020: An overview. Int. J. Cancer. 2021 doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 2.Erkkinen M.G., Kim M.-O., Geschwind M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018;10 doi: 10.1101/cshperspect.a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trapp B.D., Nave K.-A. Multiple Sclerosis: An Immune or Neurodegenerative Disorder? Annu. Rev. Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 4.Giovannoni G. The neurodegenerative prodrome in multiple sclerosis. Lancet Neurol. 2017;16:413–414. doi: 10.1016/S1474-4422(17)30127-8. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri A. Multiple sclerosis is primarily a neurodegenerative disease. J. Neural Transm. 2013;120:1463–1466. doi: 10.1007/s00702-013-1080-3. [DOI] [PubMed] [Google Scholar]
- 6.Mahad D.H., Trapp B.D., Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14:183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 7.Pugliatti M., Rosati G., Carton H., Riise T., Drulovic J., Vecsei L., Milanov I. The epidemiology of multiple sclerosis in Europe. Eur. J. Neurol. 2006;13:700–722. doi: 10.1111/j.1468-1331.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 8.van Es M.A., Hardiman O., Chio A., Al-Chalabi A., Pasterkamp R.J., Veldink J.H., Van den Berg L.H. Amyotrophic Lateral Sclerosis. Lancet. 2017;390:2084–2098. doi: 10.1016/S0140-6736(17)31287-4. [DOI] [PubMed] [Google Scholar]
- 9.Palmer S., Albergante L., Blackburn C.C., Newman T.J. Thymic involution and rising disease incidence with age. Proc. Natl. Acad. Sci. USA. 2018;115:1883–1888. doi: 10.1073/pnas.1714478115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Pedro-Cuesta J., Rabano A., Martinez-Martin P., Ruiz-Tovar M., Alcalde-Cabero E., Almazan-Isla J., Avellanal F., Calero M. Comparative Incidence of Conformational, Neurodegenerative Disorders. PLoS ONE. 2015;10:e0137342. doi: 10.1371/journal.pone.0137342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catalá-López F., Suárez-Pinilla M., Suárez-Pinilla P., Valderas J.M., Gómez-Beneyto M., Martinez S., Balanzá-Martínez V., Climent J., Valencia A., McGrath J., et al. Inverse and Direct Cancer Comorbidity in People with Central Nervous System Disorders: A Meta-Analysis of Cancer Incidence in 577,013 Participants of 50 Observational Studies. Psychother. Psychosom. 2014;83:89–105. doi: 10.1159/000356498. [DOI] [PubMed] [Google Scholar]
- 12.Driver J.A., Beiser A., Au R., Kreger B.E., Splansky G.L., Kurth T., Kiel D.P., Lu K.P., Seshadri S., Wolf P.A. Inverse Association between Cancer and Alzheimer’s Disease: Results from the Framingham Heart Study. BMJ. 2012;344:e1442. doi: 10.1136/bmj.e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fratiglioni L., Wang H.-X. Smoking and Parkinson’s and Alzheimer’s disease: Review of the epidemiological studies. Behav. Brain Res. 2000;113:117–120. doi: 10.1016/S0166-4328(00)00206-0. [DOI] [PubMed] [Google Scholar]
- 14.Ospina-Romero M., Glymour M.M., Hayes-Larson E., Mayeda E.R., Graff R.E., Brenowitz W.D., Ackley S.F., Witte J.S., Kobayashi L.C. Association between Alzheimer Disease and Cancer with Evaluation of Study Biases: A Systematic Review and Meta-Analysis. JAMA Netw. Open. 2020;3:e2025515. doi: 10.1001/jamanetworkopen.2020.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sánchez-Valle J., Tejero H., Ibáñez K., Portero J.L., Krallinger M., Al-Shahrour F., Tabares-Seisdedos R., Baudot A., Valencia A. A molecular hypothesis to explain direct and inverse co-morbidities between Alzheimer’s Disease, Glioblastoma and Lung cancer. Sci. Rep. 2017;7:4474. doi: 10.1038/s41598-017-04400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui X., Liew Z., Hansen J., Lee P.-C., Arah O.A., Ritz B. Cancers Preceding Parkinson’s Disease after Adjustment for Bias in a Danish Population-Based Case-Control Study. Neuroepidemiology. 2019;52:136–143. doi: 10.1159/000494292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghajarzadeh M., Mohammadi A., Sahraian M.A. Risk of cancer in multiple sclerosis (MS): A systematic review and meta-analysis. Autoimmun. Rev. 2020;19:102650. doi: 10.1016/j.autrev.2020.102650. [DOI] [PubMed] [Google Scholar]
- 18.Thormann A., Koch-Henriksen N., Laursen B., Sørensen P.S., Magyari M. Inverse comorbidity in multiple sclerosis: Findings in a complete nationwide cohort. Mult. Scler. Relat. Disord. 2016;10:181–186. doi: 10.1016/j.msard.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Houck A.L., Seddighi S., Driver J.A. At the Crossroads between Neurodegeneration and Cancer: A Review of Overlapping Biology and Its Implications. Curr. Aging Sci. 2018;11:77–89. doi: 10.2174/1874609811666180223154436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kesler S.R., Watson C.L., Blayney D.W. Brain network alterations and vulnerability to simulated neurodegeneration in breast cancer. Neurobiol. Aging. 2015;36:2429–2442. doi: 10.1016/j.neurobiolaging.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frain L., Swanson D., Cho K., Gagnon D., Lu K.P., Betensky R.A., Driver J. Association of Cancer and Alzheimer’s Disease Risk in a National Cohort of Veterans. Alzheimer’s Dement. 2017;13:1364–1370. doi: 10.1016/j.jalz.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabarés-Seisdedos R., Rubenstein J.L. Inverse cancer comorbidity: A serendipitous opportunity to gain insight into CNS disorders. Nat. Rev. Neurosci. 2013;14:293–304. doi: 10.1038/nrn3464. [DOI] [PubMed] [Google Scholar]
- 23.Klus P., Cirillo D., Orfila T.B., Tartaglia G.G. Neurodegeneration and Cancer: Where the Disorder Prevails. Sci. Rep. 2015;5:15390. doi: 10.1038/srep15390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tallaksen C.M., Muller U. Cancer and Neurodegeneration: Time to Move Beyond Janus? Neurology. 2017;88:1106–1107. doi: 10.1212/WNL.0000000000003727. [DOI] [PubMed] [Google Scholar]
- 25.Rojas N.G., Cesarini M., Etcheverry J.L., Da Prat G.A., Arciuch V.A., Gatto E.M. Neurodegenerative diseases and cancer: Sharing common mechanisms in complex interactions. J. Integr. Neurosci. 2020;19:187–199. doi: 10.31083/j.jin.2020.01.3. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy B.K., Berger S.L., Brunet A., Campisi J., Cuervo A.M., Epel E.S., Franceschi C., Lithgow G.J., Morimoto R.I., Pessin J.E., et al. Geroscience: Linking Aging to Chronic Disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Ratés S., Greenfield S. Cancer and neurodegeneration: Two sides, same coin? Oncotarget. 2017;8:22307–22308. doi: 10.18632/oncotarget.16190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duffner P.K. Risk factors for cognitive decline in children treated for brain tumors. Eur. J. Paediatr. Neurol. 2010;14:106–115. doi: 10.1016/j.ejpn.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Mogavero M.P., Bruni O., DelRosso L.M., Ferri R. Neurodevelopmental Consequences of Pediatric Cancer and Its Treatment: The Role of Sleep. Brain Sci. 2020;10:411. doi: 10.3390/brainsci10070411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marusak H.A., Iadipaolo A.S., Harper F.W., Elrahal F., Taub J.W., Goldberg E., Rabinak C.A. Neurodevelopmental consequences of pediatric cancer and its treatment: Applying an early adversity framework to understanding cognitive, behavioral, and emotional outcomes. Neuropsychol. Rev. 2018;28:123–175. doi: 10.1007/s11065-017-9365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catalan-Figueroa J., Palma-Florez S., Álvarez G., Fritz H.F., O Jara M., O Morales J. Nanomedicine and nanotoxicology: The pros and cons for neurodegeneration and brain cancer. Nanomedicine. 2016;11:171–187. doi: 10.2217/nnm.15.189. [DOI] [PubMed] [Google Scholar]
- 32.Cheung Y.T., Brinkman T.M., Mulrooney D.A., Mzayek Y., Liu W., Banerjee P., Panoskaltsis-Mortari A., Srivastava D., Pui C.-H., Robison L.L., et al. Impact of sleep, fatigue, and systemic inflammation on neurocognitive and behavioral outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Cancer. 2017;123:3410–3419. doi: 10.1002/cncr.30742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung Y.T., Khan R.B., Liu W., Brinkman T.M., Edelmann M.N., Reddick W.E., Pei D., Panoskaltsis-Mortari A., Srivastava D., Cheng C., et al. Association of Cerebrospinal Fluid Biomarkers of Central Nervous System Injury with Neurocognitive and Brain Imaging Outcomes in Children Receiving Chemotherapy for Acute Lymphoblastic Leukemia. JAMA Oncol. 2018;4:e180089. doi: 10.1001/jamaoncol.2018.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabarés-Seisdedos R., Dumont N., Baudot A., Valderas J.M., Climent J., Valencia A., Crespo-Facorro B., Vieta E., Gómez-Beneyto M., Martinez S., et al. No paradox, no progress: Inverse cancer comorbidity in people with other complex diseases. Lancet Oncol. 2011;12:604–608. doi: 10.1016/S1470-2045(11)70041-9. [DOI] [PubMed] [Google Scholar]
- 35.Devine M.J., Plun-Favreau H., Wood N.W. Parkinson’s Disease and Cancer: Two Wars, One Front. Nat. Rev. Cancer. 2011;11:812–823. doi: 10.1038/nrc3150. [DOI] [PubMed] [Google Scholar]
- 36.West A.B., Dawson V.L., Dawson T.M. To Die or Grow: Parkinson’s Disease and Cancer. Trends Neurosci. 2005;28:348–352. doi: 10.1016/j.tins.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Inzelberg R., Samuels Y., Azizi E., Qutob N., Inzelberg L., Domany E., Schechtman E., Friedman E. Parkinson disease (PARK) genes are somatically mutated in cutaneous melanoma. Neurol. Genet. 2016;2:e70. doi: 10.1212/NXG.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsushima-Nishiu M., Unoki M., Ono K., Tsunoda T., Minaguchi T., Kuramoto H., Nishida M., Satoh T., Tanaka T., Nakamura Y. Growth and gene expression profile analyses of endometrial cancer cells expressing exogenous PTEN. Cancer Res. 2001;61:3741–3749. [PubMed] [Google Scholar]
- 39.Salemi M., Mazzetti S., de Leonardis M., Giampietro F., Medici V., Poloni T.E., Cannarella R., Giaccone G., Pezzoli G., Cappelletti G., et al. Poly (Adp-Ribose) Polymerase 1 and Parkinson’s Disease: A Study in Post-Mortem Human Brain. Neurochem. Int. 2021;144:104978. doi: 10.1016/j.neuint.2021.104978. [DOI] [PubMed] [Google Scholar]
- 40.Salemi M., Galia A., Fraggetta F., La Corte C., Pepe P., La Vignera S., Improta G., Bosco P., Calogero A. Poly (ADP-ribose) polymerase 1 protein expression in normal and neoplastic prostatic tissue. Eur. J. Histochem. 2013;57:e13. doi: 10.4081/ejh.2013.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng Y.-C.A., Cho K., Lindstrom S., Kraft P., Cormack J., Liang L., Driver J.A. Investigating the genetic relationship between Alzheimer’s disease and cancer using GWAS summary statistics. Hum. Genet. 2017;136:1341–1351. doi: 10.1007/s00439-017-1831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Checler F., da Costa C.A. P53 in Neurodegenerative Diseases and Brain Cancers. Pharmacol. Ther. 2014;142:99–113. doi: 10.1016/j.pharmthera.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Seo J., Park M. Molecular crosstalk between cancer and neurodegenerative diseases. Cell. Mol. Life Sci. 2020;77:2659–2680. doi: 10.1007/s00018-019-03428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ariga H. Common Mechanisms of Onset of Cancer and Neurodegenerative Diseases. Biol. Pharm. Bull. 2015;38:795–808. doi: 10.1248/bpb.b15-00125. [DOI] [PubMed] [Google Scholar]
- 45.Fulda S. Modulation of mitochondrial apoptosis by PI3K inhibitors. Mitochondrion. 2013;13:195–198. doi: 10.1016/j.mito.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Yin F., Boveris A., Cadenas E. Mitochondrial Energy Metabolism and Redox Signaling in Brain Aging and Neurodegeneration. Antioxid. Redox Signal. 2014;20:353–371. doi: 10.1089/ars.2012.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bender A., Krishnan K.J., Morris C.M., Taylor G.A., Reeve A.K., Perry R.H., Jaros E., Hersheson J.S., Betts J., Klopstock T., et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 48.Nagy A., Eder K., Selak M.A., Kalman B. Mitochondrial energy metabolism and apoptosis regulation in glioblastoma. Brain Res. 2015;1595:127–142. doi: 10.1016/j.brainres.2014.10.062. [DOI] [PubMed] [Google Scholar]
- 49.Anderson G., Maes M. Gut Dysbiosis Dysregulates Central and Systemic Homeostasis Via Suboptimal Mitochondrial Function: Assessment, Treatment and Classification Implications. Curr. Top Med. Chem. 2020;20:524–539. doi: 10.2174/1568026620666200131094445. [DOI] [PubMed] [Google Scholar]
- 50.Cai Z., Yan L.-J., Ratka A. Telomere Shortening and Alzheimer’s Disease. Neuromol. Med. 2013;15:25–48. doi: 10.1007/s12017-012-8207-9. [DOI] [PubMed] [Google Scholar]
- 51.Panossian L.A., Porter V.R., Valenzuela H.F., Zhu X., Reback E., Masterman D., Cummings J.L., Effros R.B. Telomere Shortening in T Cells Correlates with Alzheimer’s Disease Status. Neurobiol. Aging. 2003;24:77–84. doi: 10.1016/S0197-4580(02)00043-X. [DOI] [PubMed] [Google Scholar]
- 52.Depinho R.A. The age of cancer. Nat. Cell Biol. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 53.Kim N., Piatyszek M., Prowse K., Harley C., West M., Ho P., Coviello G., Wright W., Weinrich S., Shay J. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 54.Clevers H. Wnt/Beta-Catenin Signaling in Development and Disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 55.Yao H., Sun L., Lian J., Zhang M., Liu D. Correlation of Alzheimer’s Disease with Wnt Signaling Pathway and Neural Stem Cells; Proceedings of the 4th International Conference on Mechatronics, Materials, Chemistry and Computer Engineering; Xi’an, China. 12–13 December 2015; pp. 119–122. [Google Scholar]
- 56.Reya T., Clevers H. Wnt signalling in stem cells and cancer. Nat. Cell Biol. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 57.Serafino A., Sferrazza G., Baldeschi A.C., Nicotera G., Andreola F., Pittaluga E., Pierimarchi P. Developing drugs that target the Wnt pathway: Recent approaches in cancer and neurodegenerative diseases. Expert Opin. Drug Discov. 2017;12:169–186. doi: 10.1080/17460441.2017.1271321. [DOI] [PubMed] [Google Scholar]
- 58.Spillantini M.G., Schmidt M.L., Lee V.M., Trojanowski J.Q., Jakes R., Goedert M. Alpha-Synuclein in Lewy Bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 59.Houck A.L., Hernández F., Ávila J. A Simple Model to Study Tau Pathology. J. Exp. Neurosci. 2016;10:31–38. doi: 10.4137/JEN.S25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neckers L. Heat shock protein 90: The cancer chaperone. J. Biosci. 2007;32:517–530. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 61.Frezza M., Schmitt S., Dou Q.P. Targeting the ubiquitin-proteasome pathway: An emerging concept in cancer therapy. Curr. Top. Med. Chem. 2011;11:2888–2905. doi: 10.2174/156802611798281311. [DOI] [PubMed] [Google Scholar]
- 62.Chouliaras L., Mastroeni D., Delvaux E., Grover A., Kenis G., Hof P.R., Steinbusch H.W., Coleman P.D., Rutten B.P., van den Hove D.L. Consistent Decrease in Global DNA Methylation and Hydroxymethylation in the Hippocampus of Alzheimer’s Disease Patients. Neurobiol. Aging. 2013;34:2091–2099. doi: 10.1016/j.neurobiolaging.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Faghihi M.A., Modarresi F., Khalil A.M., Wood D.E., Sahagan B.G., Morgan T.E., Finch C.E., Laurent G.S., 3rd, Kenny P.J., Wahlestedt C. Expression of a Noncoding Rna Is Elevated in Alzheimer’s Disease and Drives Rapid Feed-Forward Regulation of Beta-Secretase. Nat. Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rupaimoole R., Slack F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y., Jaber V., Alexandrov P.N., Vergallo A., Lista S., Hampel H., Lukiw W.J. Microrna-Based Biomarkers in Alzheimer’s Disease (Ad) Front. Neurosci. 2020;14:585432. doi: 10.3389/fnins.2020.585432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vergallo A., Lista S., Zhao Y., Lemercier P., Teipel S.J., Potier M.C., Habert M.O., Dubois B., Lukiw W.J., Hampel H., et al. Mirna-15b and Mirna-125b Are Associated with Regional Abeta-Pet and Fdg-Pet Uptake in Cognitively Normal Individuals with Subjective Memory Complaints. Transl. Psychiatry. 2021;11:78. doi: 10.1038/s41398-020-01184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du L., Pertsemlidis A. Cancer and neurodegenerative disorders: Pathogenic convergence through microRNA regulation. J. Mol. Cell Biol. 2011;3:176–180. doi: 10.1093/jmcb/mjq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vishnoi A., Rani S. MiRNA Biogenesis and Regulation of Diseases: An Overview. Methods Mol. Biol. 2017;1509:1–10. doi: 10.1007/978-1-4939-6524-3_1. [DOI] [PubMed] [Google Scholar]
- 69.Bassetti C.L.A., Adamantidis A., Burdakov D., Han F., Gay S., Kallweit U., Khatami R., Koning F., Kornum B.R., Lammers G.J., et al. Narcolepsy—Clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2019;15:519–539. doi: 10.1038/s41582-019-0226-9. [DOI] [PubMed] [Google Scholar]
- 70.Mohammadi S., Mayeli M., Saghazadeh A., Rezaei N. Cytokines in narcolepsy: A systematic review and meta-analysis. Cytokine. 2020;131:155103. doi: 10.1016/j.cyto.2020.155103. [DOI] [PubMed] [Google Scholar]
- 71.Jennum P., Ibsen R., Knudsen S., Kjellberg J. Comorbidity and Mortality of Narcolepsy: A Controlled Retro- and Prospective National Study. Sleep. 2013;36:835–840. doi: 10.5665/sleep.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Black J., Reaven N., Funk S., McGaughey K., Ohayon M., Guilleminault C., Ruoff C. Medical comorbidity in narcolepsy: Findings from the Burden of Narcolepsy Disease (BOND) study. Sleep Med. 2017;33:13–18. doi: 10.1016/j.sleep.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 73.Jennum P., Pickering L., Thorstensen E.W., Ibsen R., Kjellberg J. Morbidity of childhood onset narcolepsy: A controlled national study. Sleep Med. 2017;29:13–17. doi: 10.1016/j.sleep.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 74.Tseng C.-M., Chen Y.-T., Tao C.-W., Ou S.-M., Hsiao Y.-H., Li S.-Y., Chen T.-J., Perng D.-W., Chou K.-T. Adult narcoleptic patients have increased risk of cancer: A nationwide population-based study. Cancer Epidemiol. 2015;39:793–797. doi: 10.1016/j.canep.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 75.Kukkonen J.P. Physiology of the orexinergic/hypocretinergic system: A revisit in 2012. Am. J. Physiol. Physiol. 2013;304:C2–C32. doi: 10.1152/ajpcell.00227.2012. [DOI] [PubMed] [Google Scholar]
- 76.Leonard C.S., Kukkonen J.P. Orexin/hypocretin receptor signalling: A functional perspective. Br. J. Pharmacol. 2014;171:294–313. doi: 10.1111/bph.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McAlpine C.S., Kiss M.G., Rattik S., He S., Vassalli A., Valet C., Anzai A., Chan C.T., Mindur J.E., Kahles F., et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nat. Cell Biol. 2019;566:383–387. doi: 10.1038/s41586-019-0948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Economou N.-T., Manconi M., Ghika J., Raimondi M., Bassetti C.L. Development of Parkinson and Alzheimer Diseases in Two Cases of Narcolepsy-Cataplexy. Eur. Neurol. 2012;67:48–50. doi: 10.1159/000334733. [DOI] [PubMed] [Google Scholar]
- 79.Kallweit U., Bassetti C.L.A., Oberholzer M., Fronczek R., Beguin M., Strub M., Lammers G.J. Coexisting Narcolepsy (with and without Cataplexy) and Multiple Sclerosis: Six New Cases and a Literature Review. J. Neurol. 2018;265:2071–2078. doi: 10.1007/s00415-018-8949-x. [DOI] [PubMed] [Google Scholar]
- 80.Scammell T.E., Matheson J.K., Honda M., Thannickal T.C., Siegel J.M. Coexistence of Narcolepsy and Alzheimer’s Disease. Neurobiol. Aging. 2012;33:1318–1319. doi: 10.1016/j.neurobiolaging.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Long J., Holtzman D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell. 2019;179:312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liguori C., Placidi F., Izzi F., Nuccetelli M., Bernardini S., Sarpa M.G., Cum F., Marciani M.G., Mercuri N.B., Romigi A. Beta-amyloid and phosphorylated tau metabolism changes in narcolepsy over time. Sleep Breath. 2016;20:277–283. doi: 10.1007/s11325-015-1305-9. [DOI] [PubMed] [Google Scholar]
- 83.Liguori C., Placidi F., Albanese M., Nuccetelli M., Izzi F., Marciani M.G., Mercuri N.B., Bernardini S., Romigi A. CSF beta-amyloid levels are altered in narcolepsy: A link with the inflammatory hypothesis? J. Sleep Res. 2014;23:420–424. doi: 10.1111/jsr.12130. [DOI] [PubMed] [Google Scholar]
- 84.Jennum P.J., Østergaard Pedersen L., Bahl J.M.C., Modvig S., Fog K., Holm A., Kornum B.R., Gammeltoft S. Cerebrospinal Fluid Biomarkers of Neurodegeneration Are Decreased or Normal in Narcolepsy. Sleep. 2017;40 doi: 10.1093/sleep/zsw006. [DOI] [PubMed] [Google Scholar]
- 85.Baiardi S., Pizza F., Polischi B., Moresco M., Abu-Rumeileh S., Plazzi G., Parchi P. Cerebrospinal Fluid Biomarkers of Neurodegeneration in Narcolepsy Type 1. Sleep. 2020;43 doi: 10.1093/sleep/zsz215. [DOI] [PubMed] [Google Scholar]
- 86.Gabelle A., Jaussent I., Ben Bouallègue F., Lehmann S., Lopez R., Barateau L., Grasselli C., Pesenti C., De Verbizier D., Béziat S., et al. Reduced brain amyloid burden in elderly patients with narcolepsy type. Ann. Neurol. 2019;85:74–83. doi: 10.1002/ana.25373. [DOI] [PubMed] [Google Scholar]
- 87.Kang J.E., Lim M.M., Bateman R.J., Lee J.J., Smyth L.P., Cirrito J.R., Fujiki N., Nishino S., Holtzman D.M. Amyloid-Beta Dynamics Are Regulated by Orexin and the Sleep-Wake Cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R.M., Tanaka H., Williams S., A Richardson J., Kozlowski G.P., Wilson S., et al. Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors that Regulate Feeding Behavior. Cell. 1998;92:573–585. doi: 10.1016/S0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 89.de Lecea L., Kilduff T.S., Peyron C., Gao X., Foye P.E., Danielson P.E., Fukuhara C., Battenberg E.L., Gautvik V.T., Bartlett F.S., 2nd, et al. The Hypocretins: Hypothalamus-Specific Peptides with Neuroexcitatory Activity. Proc. Natl. Acad. Sci. USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mileykovskiy B.Y., Kiyashchenko L.I., Siegel J.M. Behavioral Correlates of Activity in Identified Hypocretin/Orexin Neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peyron C., Tighe D.K., Pol A.N.V.D., De Lecea L., Heller H.C., Sutcliffe J.G., Kilduff T. Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kukkonen J.P., Leonard C.S. Orexin/hypocretin receptor signalling cascades. Br. J. Pharmacol. 2014;171:314–331. doi: 10.1111/bph.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kukkonen J.P. OX2 orexin/hypocretin receptor signal transduction in recombinant Chinese hamster ovary cells. Cell. Signal. 2016;28:51–60. doi: 10.1016/j.cellsig.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 94.Kukkonen J.P. G-Protein-Dependency of Orexin/Hypocretin Receptor Signalling in Recombinant Chinese Hamster Ovary Cells. Biochem. Biophys. Res. Commun. 2016;476:379–385. doi: 10.1016/j.bbrc.2016.05.130. [DOI] [PubMed] [Google Scholar]
- 95.Sakurai T. The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nat. Rev. Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 96.Grimaldi D., Silvani A., Benarroch E.E., Cortelli P. Orexin/hypocretin system and autonomic control: New insights and clinical correlations. Neurology. 2014;82:271–278. doi: 10.1212/WNL.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 97.Bastianini S., Silvani A. Clinical Implications of Basic Research: The Role of Hypocretin/Orexin Neurons in the Central Autonomic Network. Clin. Transl. Neurosci. 2018;2 doi: 10.1177/2514183X18789327. [DOI] [Google Scholar]
- 98.Jennum P.J., Plazzi G., Silvani A., Surkin L.A., Dauvilliers Y. Cardiovascular disorders in narcolepsy: Review of associations and determinants. Sleep Med. Rev. 2021;58:101440. doi: 10.1016/j.smrv.2021.101440. [DOI] [PubMed] [Google Scholar]
- 99.Perez M.V., Pavlovic A., Shang C., Wheeler M.T., Miller C.L., Liu J., Dewey F.E., Pan S., Thanaporn P.K., Absher D., et al. Systems Genomics Identifies a Key Role for Hypocretin/Orexin Receptor-2 in Human Heart Failure. J. Am. Coll. Cardiol. 2015;66:2522–2533. doi: 10.1016/j.jacc.2015.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kastin A.J., Akerstrom V. Orexin A but not orexin B rapidly enters brain from blood by simple diffusion. J. Pharmacol. Exp. Ther. 1999;289:219–223. [PubMed] [Google Scholar]
- 101.Yoshida Y., Fujiki N., Maki R.A., Schwarz D., Nishino S. Differential kinetics of hypocretins in the cerebrospinal fluid after intracerebroventricular administration in rats. Neurosci. Lett. 2003;346:182–186. doi: 10.1016/S0304-3940(03)00571-8. [DOI] [PubMed] [Google Scholar]
- 102.Bingham S., Davey P.T., Babbs A.J., Irving E.A., Sammons M.J., Wyles M., Jeffrey P., Cutler L., Riba I., Johns A., et al. Orexin-A, an hypothalamic peptide with analgesic properties. Pain. 2001;92:81–90. doi: 10.1016/S0304-3959(00)00470-X. [DOI] [PubMed] [Google Scholar]
- 103.Zhu Z., Xu L., Cao D., Song C., Wang Y., Li M., Yan J., Xie Z. Effect of Orexin-a on Mitochondrial Biogenesis, Mitophagy and Structure in Hek293-Appswe Cell Model of Alzheimer’s Disease. Clin. Exp. Pharmacol. Physiol. 2021;48:355–360. doi: 10.1111/1440-1681.13424. [DOI] [PubMed] [Google Scholar]
- 104.Li M., Meng Y., Chu B., Shen Y., Xue X., Song C., Liu X., Ding M., Cao X., Wang P., et al. Orexin-a Exacerbates Alzheimer’s Disease by Inducing Mitochondrial Impairment. Neurosci. Lett. 2020;718:134741. doi: 10.1016/j.neulet.2020.134741. [DOI] [PubMed] [Google Scholar]
- 105.Lorenzoni P.J., Werneck L.C., Crippa A.C.D.S., Zanatta A., Kay C.S.K., Silvado C.E.S., Scola R.H. Is there a relationship between narcolepsy, multiple sclerosis and HLA-DQB1*06:02? Arq. Neuro-Psiquiatr. 2017;75:345–348. doi: 10.1590/0004-282x20170063. [DOI] [PubMed] [Google Scholar]
- 106.West A.P. Mitochondrial dysfunction as a trigger of innate immune responses and inflammation. Toxicology. 2017;391:54–63. doi: 10.1016/j.tox.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 107.Diakos C., A Charles K., McMillan D.C., Clarke S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 108.Ransohoff R.M. How neuroinflammation contributes to neurodegeneration. Science. 2016;353:777–783. doi: 10.1126/science.aag2590. [DOI] [PubMed] [Google Scholar]
- 109.LeComte A., Barateau L., Pereira P., Paulin L., Auvinen P., Scheperjans F., Dauvilliers Y. Gut microbiota composition is associated with narcolepsy type. Neurol. Neuroimmunol. Neuroinflamm. 2020;7:e896. doi: 10.1212/NXI.0000000000000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Foster J.A., Lyte M., Meyer E., Cryan J.F. Gut Microbiota and Brain Function: An Evolving Field in Neuroscience. Int. J. Neuropsychopharmacol. 2016;19:pyv114. doi: 10.1093/ijnp/pyv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., Challis C., Schretter C.E., Rocha S., Gradinaru V., et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167:1469–1480. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vogt N.M., Kerby R.L., Dill-McFarland K.A., Harding S.J., Merluzzi A.P., Johnson S.C., Carlsson C.M., Asthana S., Zetterberg H., Blennow K., et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spielman L.J., Gibson D.L., Klegeris A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018;120:149–163. doi: 10.1016/j.neuint.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 114.Gopalakrishnan V., Helmink B.A., Spencer C.N., Reuben A., Wargo J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell. 2018;33:570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamasaki M., Miyagawa T., Toyoda H., Khor S.-S., Koike A., Nitta A., Akiyama K., Sasaki T., Honda Y., Honda M., et al. Genome-wide analysis of CNV (copy number variation) and their associations with narcolepsy in a Japanese population. J. Hum. Genet. 2014;59:235–240. doi: 10.1038/jhg.2014.13. [DOI] [PubMed] [Google Scholar]
- 116.Sripada L., Tomar D., Singh R. Mitochondria: One of the destinations of miRNAs. Mitochondrion. 2012;12:593–599. doi: 10.1016/j.mito.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 117.Liu Z., Yang L., Zhao Y., Tang M., Wang F., Wang X., Li G., Du Y. Reproducibility of quantitative real-time PCR assay in microRNA expression profiling and comparison with microarray analysis in narcolepsy. SpringerPlus. 2015;4:812. doi: 10.1186/s40064-015-1613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holm A., Bang-Berthelsen C.H., Knudsen S., Kornum B.R., Modvig S., Jennum P., Gammeltoft S. miRNA Profiles in Plasma from Patients with Sleep Disorders Reveal Dysregulation of miRNAs in Narcolepsy and Other Central Hypersomnias. Sleep. 2014;37:1525–1533. doi: 10.5665/sleep.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Possovre M.-L., Tafti M. Conditional Deletion of Mirna in Hypocretin Neurons: A New Mouse Model of Narcolepsy. J. Sleep Res. 2020;29:e13181. [Google Scholar]
- 120.Mogavero M.P., DelRosso L.M., Fanfulla F., Bruni O., Ferri R. Sleep disorders and cancer: State of the art and future perspectives. Sleep Med. Rev. 2020;56:101409. doi: 10.1016/j.smrv.2020.101409. [DOI] [PubMed] [Google Scholar]
- 121.Graybill N.L., Weissig V. A review of orexin’s unprecedented potential as a novel, highly-specific treatment for various localized and metastatic cancers. SAGE Open Med. 2017;5 doi: 10.1177/2050312117735774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim M.-K., Park H.-J., Kim S.-R., Choi Y.K., Bae S.-K., Bae M.-K. Involvement of Heme Oxygenase-1 in Orexin-A-induced Angiogenesis in Vascular Endothelial Cells. Korean J. Physiol. Pharmacol. 2015;19:327–334. doi: 10.4196/kjpp.2015.19.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maines M.D. The Heme Oxygenase System: A Regulator of Second Messenger Gases. Annu. Rev. Pharmacol. Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 124.Mediavilla C. Bidirectional gut-brain communication: A role for orexin-A. Neurochem. Int. 2020;141:104882. doi: 10.1016/j.neuint.2020.104882. [DOI] [PubMed] [Google Scholar]
- 125.Rouet-Benzineb P., Rouyer-Fessard C., Jarry A., Avondo V., Pouzet C., Yanagisawa M., Laboisse C., Laburthe M., Voisin T. Orexins Acting at Native OX1 Receptor in Colon Cancer and Neuroblastoma Cells or at Recombinant OX1 Receptor Suppress Cell Growth by Inducing Apoptosis. J. Biol. Chem. 2004;279:45875–45886. doi: 10.1074/jbc.M404136200. [DOI] [PubMed] [Google Scholar]
- 126.Voisin T., El Firar A., Fasseu M., Rouyer-Fessard C., Descatoire V., Walker F., Paradis V., Bedossa P., Henin D., Lehy T., et al. Aberrant Expression of OX1 Receptors for Orexins in Colon Cancers and Liver Metastases: An Openable Gate to Apoptosis. Cancer Res. 2011;71:3341–3351. doi: 10.1158/0008-5472.CAN-10-3473. [DOI] [PubMed] [Google Scholar]
- 127.Wen J., Zhao Y., Guo L. Orexin A induces autophagy in HCT-116 human colon cancer cells through the ERK signaling pathway. Int. J. Mol. Med. 2016;37:126–132. doi: 10.3892/ijmm.2015.2409. [DOI] [PubMed] [Google Scholar]
- 128.Bai B., Chen X., Zhang R., Wang X., Jiang Y., Li D., Wang Z., Chen J. Dual-agonist occupancy of orexin receptor 1 and cholecystokinin A receptor heterodimers decreases G-protein–dependent signaling and migration in the human colon cancer cell line HT-29. Biochim. Biophys. Acta (BBA)-Mol. Bioenerg. 2017;1864:1153–1164. doi: 10.1016/j.bbamcr.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 129.Messal N., Fernandez N., Dayot S., Gratio V., Nicole P., Prochasson C., Chantret I., Leguilloux G., Jarry A., Couvelard A., et al. Ectopic expression of OX1R in ulcerative colitis mediates anti-inflammatory effect of orexin-A. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018;1864:3618–3628. doi: 10.1016/j.bbadis.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 130.Biegańska K., Sokołowska P., Joehren O., Zawilska J.B. Orexin A Suppresses the Growth of Rat C6 Glioma Cells via a Caspase-Dependent Mechanism. J. Mol. Neurosci. 2012;48:706–712. doi: 10.1007/s12031-012-9799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wen J., Zhao Y., Shen Y., Guo L. Effect of orexin A on apoptosis in BGC-823 gastric cancer cells via OX1R through the AKT signaling pathway. Mol. Med. Rep. 2015;11:3439–3444. doi: 10.3892/mmr.2015.3190. [DOI] [PubMed] [Google Scholar]
- 132.Liu Y., Zhao Y., Ju S., Guo L. Orexin A upregulates the protein expression of OX1R and enhances the proliferation of SGC-7901 gastric cancer cells through the ERK signaling pathway. Int. J. Mol. Med. 2015;35:539–545. doi: 10.3892/ijmm.2014.2038. [DOI] [PubMed] [Google Scholar]
- 133.Suo L., Chang X., Zhao Y. The Orexin-A-Regulated Akt/mTOR Pathway Promotes Cell Proliferation through Inhibiting Apoptosis in Pancreatic Cancer Cells. Front. Endocrinol. 2018;9:647. doi: 10.3389/fendo.2018.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dayot S., Speisky D., Couvelard A., Bourgoin P., Gratio V., Cros J., Rebours V., Sauvanet A., Bedossa P., Paradis V., et al. In vitro, in vivo and ex vivo demonstration of the antitumoral role of hypocretin-1/orexin-A and almorexant in pancreatic ductal adenocarcinoma. Oncotarget. 2018;9:6952–6967. doi: 10.18632/oncotarget.24084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alexandre D., Hautot C., Mehio M., Jeandel L., Courel M., Voisin T., Couvineau A., Gobet F., Leprince J., Pfister C., et al. The orexin type 1 receptor is overexpressed in advanced prostate cancer with a neuroendocrine differentiation, and mediates apoptosis. Eur. J. Cancer. 2014;50:2126–2133. doi: 10.1016/j.ejca.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 136.Valiante S., Liguori G., Tafuri S., Pavone L.M., Campese R., Monaco R., Iachetta G., Assisi L., Mirabella N., Forte M., et al. Expression and potential role of the peptide orexin-A in prostate cancer. Biochem. Biophys. Res. Commun. 2015;464:1290–1296. doi: 10.1016/j.bbrc.2015.07.124. [DOI] [PubMed] [Google Scholar]
- 137.Szyszka M., Paschke L., Tyczewska M., Rucinski M., Grabowska P., Malendowicz L.K. Lack of expression of preproorexin and orexin receptors genes in human normal and prostate cancer cell lines. Folia Histochem. Cytobiol. 2015;53:333–341. doi: 10.5603/fhc.a2015.0035. [DOI] [PubMed] [Google Scholar]
- 138.Fronczek R., van Geest S., Frolich M., Overeem S., Roelandse F.W., Lammers G.J., Swaab D.F. Hypocretin (Orexin) Loss in Alzheimer’s Disease. Neurobiol. Aging. 2012;33:1642–1650. doi: 10.1016/j.neurobiolaging.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 139.Oh J., Eser R.A., Ehrenberg A.J., Morales D., Petersen C., Kudlacek J., Dunlop S.R., Theofilas P., Resende E., Cosme C., et al. Profound Degeneration of Wake-Promoting Neurons in Alzheimer’s Disease. Alzheimer’s Dement. 2019;15:1253–1263. doi: 10.1016/j.jalz.2019.06.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shimizu S., Takenoshita N., Inagawa Y., Tsugawa A., Hirose D., Kaneko Y., Ogawa Y., Serisawa S., Sakurai S., Hirao K., et al. Positive Association Between Cognitive Function and Cerebrospinal Fluid Orexin A Levels in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020;73:117–123. doi: 10.3233/JAD-190958. [DOI] [PubMed] [Google Scholar]
- 141.Gabelle A., Jaussent I., Hirtz C., Vialaret J., Navucet S., Grasselli C., Robert P., Lehmann S., Dauvilliers Y. Cerebrospinal fluid levels of orexin-A and histamine, and sleep profile within the Alzheimer process. Neurobiol. Aging. 2017;53:59–66. doi: 10.1016/j.neurobiolaging.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 142.Goedert M., Jakes R., Spillantini M.G. The Synucleinopathies: Twenty Years On. J. Park. Dis. 2017;7:S51–S69. doi: 10.3233/JPD-179005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thannickal T.C., Lai Y.Y., Siegel J.M. Hypocretin (Orexin) Cell Loss in Parkinson’s Disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kasanuki K., Iseki E., Kondo D., Fujishiro H., Minegishi M., Sato K., Katsuse O., Hino H., Kosaka K., Arai H. Neuropathological investigation of hypocretin expression in brains of dementia with Lewy bodies. Neurosci. Lett. 2014;569:68–73. doi: 10.1016/j.neulet.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 145.Coon E.A., Cutsforth-Gregory J.K., Benarroch E.E. Neuropathology of autonomic dysfunction in synucleinopathies. Mov. Disord. 2018;33:349–358. doi: 10.1002/mds.27186. [DOI] [PubMed] [Google Scholar]
- 146.Wienecke M., Werth E., Poryazova R., Baumann-Vogel H., Bassetti C.L., Weller M., Waldvogel D., Storch A., Baumann C.R. Progressive dopamine and hypocretin deficiencies in Parkinson’s disease: Is there an impact on sleep and wakefulness? J. Sleep Res. 2012;21:710–717. doi: 10.1111/j.1365-2869.2012.01027.x. [DOI] [PubMed] [Google Scholar]
- 147.Baumann C.R., Dauvilliers Y., Mignot E., Bassetti C.L. Normal CSF Hypocretin-1 (Orexin A) Levels in Dementia with Lewy Bodies Associated with Excessive Daytime Sleepiness. Eur. Neurol. 2004;52:73–76. doi: 10.1159/000079749. [DOI] [PubMed] [Google Scholar]
- 148.Abdo W., Bloem B., Kremer H., Lammers G., Verbeek M., Overeem S. CSF hypocretin-1 levels are normal in multiple-system atrophy. Park. Relat. Disord. 2008;14:342–344. doi: 10.1016/j.parkreldis.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 149.Constantinescu C.S., Niepel G., Patterson M., Judd A., Braitch M., Fahey A.J., Harikrishnan S., Edwards L.J., Tench C.R., Bennett G.W., et al. Orexin A (hypocretin-1) levels are not reduced while cocaine/amphetamine regulated transcript levels are increased in the cerebrospinal fluid of patients with multiple sclerosis: No correlation with fatigue and sleepiness. J. Neurol. Sci. 2011;307:127–131. doi: 10.1016/j.jns.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 150.Oka Y., Kanbayashi T., Mezaki T., Iseki K., Matsubayashi J., Murakami G., Matsui M., Shimizu T., Shibasaki H. Low CSF hypocretin-1/orexin-A associated with hypersomnia secondary to hypothalamic lesion in a case of multiple sclerosis. J. Neurol. 2004;251:885–886. doi: 10.1007/s00415-004-0442-z. [DOI] [PubMed] [Google Scholar]
- 151.Mehrotra P., Sturek M., Neyra J.A., Basile D.P. Calcium channel Orai1 promotes lymphocyte IL-17 expression and progressive kidney injury. J. Clin. Investig. 2019;129:4951–4961. doi: 10.1172/JCI126108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Leng F., Edison P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021;17:157–172. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- 153.Ogawa Y., Irukayama-Tomobe Y., Murakoshi N., Kiyama M., Ishikawa Y., Hosokawa N., Tominaga H., Uchida S., Kimura S., Kanuka M., et al. Peripherally administered orexin improves survival of mice with endotoxin shock. Elife. 2016;5:e21055. doi: 10.7554/eLife.21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li T., Xu W., Ouyang J., Lu X., Sherchan P., Lenahan C., Irio G., Zhang J.H., Zhao J., Zhang Y., et al. Orexin a Alleviates Neuroinflammation Via Oxr2/Camkkbeta/Ampk Signaling Pathway after Ich in Mice. J. Neuroinflamm. 2020;17:187. doi: 10.1186/s12974-020-01841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Becquet L., Abad C., Leclercq M., Miel C., Jean L., Riou G., Couvineau A., Boyer O., Tan Y.-V. Systemic administration of orexin A ameliorates established experimental autoimmune encephalomyelitis by diminishing neuroinflammation. J. Neuroinflamm. 2019;16:64. doi: 10.1186/s12974-019-1447-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tunisi L., Forte N., Fernández-Rilo A.C., Mavaro I., Capasso R., D’Angelo L., Milić N., Cristino L., Di Marzo V., Palomba L. Orexin-A Prevents Lipopolysaccharide-Induced Neuroinflammation at the Level of the Intestinal Barrier. Front. Endocrinol. 2019;10:219. doi: 10.3389/fendo.2019.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Couvineau A., Voisin T., Nicole P., Gratio V., Abad C., Tan Y.-V. Orexins as Novel Therapeutic Targets in Inflammatory and Neurodegenerative Diseases. Front. Endocrinol. 2019;10:709. doi: 10.3389/fendo.2019.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou F., Yan X.-D., Wang C., He Y.-X., Li Y.-Y., Zhang J., Wang Z.-J., Cai H.-Y., Qi J.-S., Wu M.-N. Suvorexant ameliorates cognitive impairments and pathology in APP/PS1 transgenic mice. Neurobiol. Aging. 2020;91:66–75. doi: 10.1016/j.neurobiolaging.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 159.Kylkilahti T.M., Berends E., Ramos M., Shanbhag N.C., Töger J., Bloch K.M., Lundgaard I. Achieving brain clearance and preventing neurodegenerative diseases—A glymphatic perspective. Br. J. Pharmacol. 2021 doi: 10.1177/0271678x20982388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bishir M., Bhat A., Essa M.M., Ekpo O., Ihunwo A.O., Veeraraghavan V.P., Mohan S.K., Mahalakshmi A.M., Ray B., Tuladhar S., et al. Sleep Deprivation and Neurological Disorders. BioMed Res. Int. 2020;2020:5764017. doi: 10.1155/2020/5764017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang Y., Chen A.Q., Xue Y., Liu M.F., Liu C., Liu Y.H., Pan Y.P., Diao H.L., Chen L. Orexins Alleviate Motor Deficits via Increasing Firing Activity of Pallidal Neurons in a Mouse Model of Parkinson’s Disease. Am. J. Physiol. Cell. Physiol. 2019;317:C800–C812. doi: 10.1152/ajpcell.00125.2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.