Abstract
Staphylococcus aureus (S. aureus) is a major human pathogen that requires new antibiotics with unique mechanism. A new pleuromutilin derivative, 14-O-[(4,6-Diamino-pyrimidine-2-yl) thioacetyl] mutilin (DPTM), has been synthesized and proved as a potent antibacterial agent using in vitro and in vivo assays. In the present study, DPTM was further in vitro evaluated against methicillin-resistant Staphylococcus aureus (MRSA) isolated from dairy farms and outperformed tiamulin fumarate, a pleuromutilin drug used for veterinary. Moreover, a murine skin wound model caused by MRSA infection was established, and the healing effect of DPTM was investigated. The results showed that DPTM could promote the healing of MRSA skin infection, reduce the bacterial burden of infected skin MRSA and decrease the secretion of IL-6 and TNF-α inflammatory cytokines in plasma. These results provided the basis for further in-depth drug targeted studies of DPTM as a novel antibacterial agent.
Keywords: DPTM, methicillin-resistant Staphylococcus aureus (MRSA), antibacterial activity, murine skin wound model, MIC
1. Introduction
Staphylococcus aureus (S. aureus) is a major human pathogen associated with increased morbidity, mortality, and excess hospital costs [1,2]. It also causes skin and soft tissue infections (SSTI), including impetigo, folliculitis, furuncles, and subcutaneous abscesses [3,4]. Methicillin-resistant Staphylococcus aureus (MRSA) has aroused a growing concern and became a significant public health threat. Every year there are approximately 80,000 invasive infections individuals in the United States, resulting in 11,000 deaths annually [3,5,6]. Furthermore, decreased susceptibility and even resistance to vancomycin, daptomycin, linezolid, and other antibiotics, have been reported in many parts of the world [7,8,9]. As such, there is pressing need to develop novel antibiotics with unique mechanism of action against this dreadful pathogen.
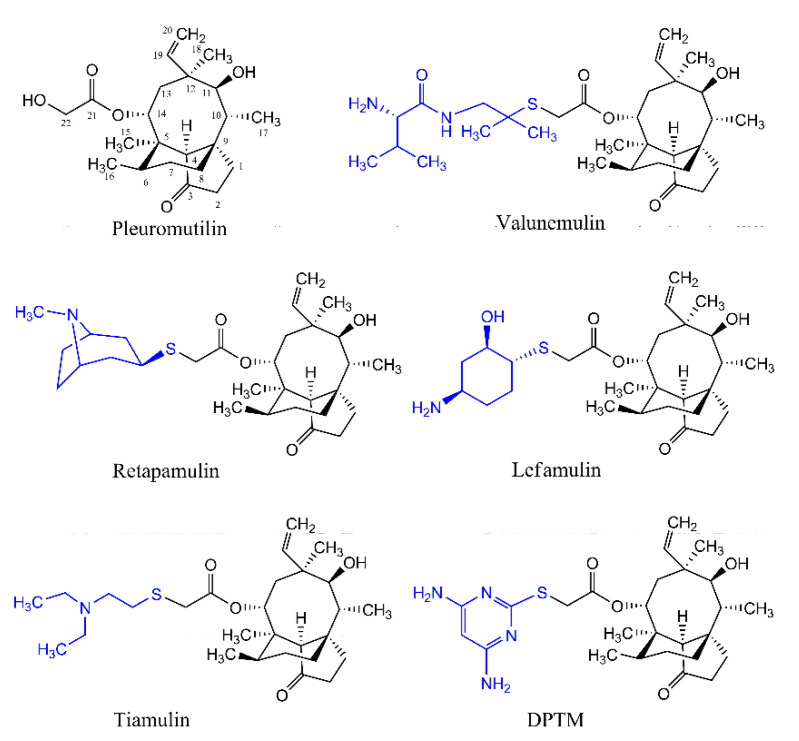
Pleuromutilin (Figure 1) is a natural compound that was first discovered and isolated from cultures of two species of basidiomycetes, Pleurotus mutilus and P. passeckerianus in 1951 [10]. Pleuromutilin derivatives selectively inhibited bacterial protein synthesis through interaction with prokaryotic ribosomes at the acceptor and donor site [11,12,13]. Modification of the glycolic ester side chain in pleuromutilin has been shown to give derivatives with improved antibacterial activities, and has led to tiamulin, valnemulin, retapamulin, and lefamulin (Figure 1) [14,15,16,17].
Figure 1.
Chemical structures of pleuromutilin tiamulin, valnemulin, retapamulin, Lefamulin, and DPTM.
14-O-[(4,6-Diamino-pyrimidine-2-yl) thioacetyl] mutilin (DPTM, Figure 1) is a new pleuromutilin derivative with a pyrimidine moiety. It was first synthesized and has been shown excellent antibacterial activity, suggesting its potential as a promising antimicrobial drug [18,19]. In this study, we further investigated the activity of DPTM against MRSA using in vitro and in vivo assays.
2. Results
2.1. Effect of DPTM In Vitro
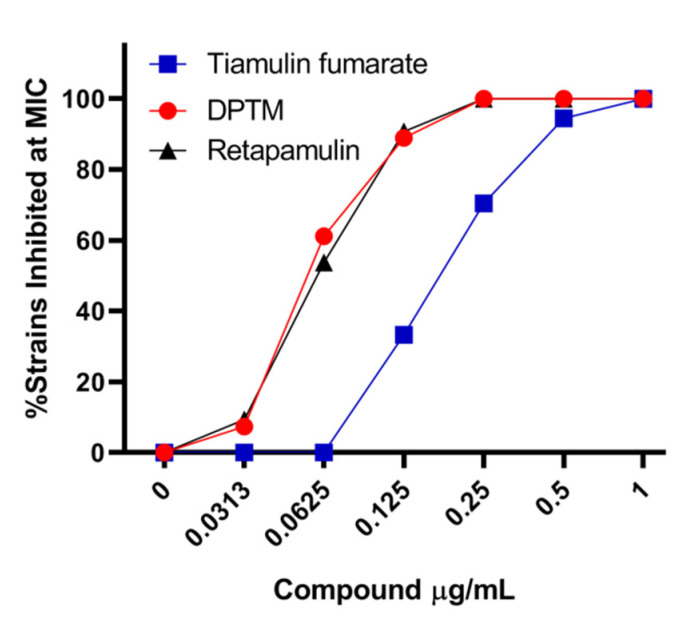
The minimum inhibitory concentrations (MICs) of DPTM against two standard quality control strains of MRSA (ATCC 29213 and ATCC 33591) and a S. aureus (CMCC 26003) have been reported in our previous study [18]. Extensive panels of clinical isolates of MRSA (n = 54) were assessed for their susceptibility to DPTM. We chose tiamulin fumarate as reference drug because it was used primarily in veterinary medicine [20]. MICs for DPTM ranged from 0.0313 to 0.25 μg/mL, while that of tiamulin fumarate were 0.125 to 1 μg/mL (a full listing of this MIC data is in Table S1). Within these collections of clinical isolates, MICs of DPTM were lower than that of tiamulin fumarate (Figure 2).
Figure 2.
Assessment of DPTM and tiamulin fumarate against clinical isolates of MRSA. Full listing of this MIC data is in Table S1.
2.2. Macroscopic Evaluation of Efficacy in a Murine Skin Wound Model
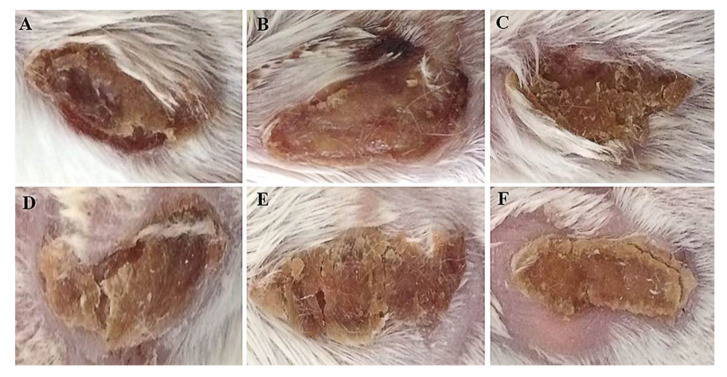
Bearing the excellent protection efficacy of mice infected with MRSA-29213 [18], DPTM was further assessed for its healing effect on murine skin wound caused by MRSA infections. After inoculation, symptoms of the skin wound and mouse viability were monitored daily for 6 days. Mice treated with three dosages of DPTM and retapamulin ointments had improved survival (100%) compared to positive control mice (80%). Mice that were not infected showed no death and normal wound with a small amount of thin exudate (Figure 3A). Most murine wounds in positive control group presented symptoms including the increased exudate, worsened with bloody skin, and heavy or purulent drainage (Figure 3B). Treatment with retapamulin and 1% and 2% DPTM caused improved symptoms with some exudate remaining, and the wounds begun healing at the margin with some crust (Figure 3C−E). However, wounds in 3% DPTM ointment treatment group exhibited partial thickening and the crust had peeled away from the margin leaving behind fresh skin (Figure 3F).
Figure 3.
Macroscopic evaluation of wound at the 6th day in the infection model. Mice (n = 10 per group) were infected in the excision wound with MRSA ATCC43300 strain. (A) uninfected group, (B) control group, (C) 1% DPTM ointment treatment group, (D) 2% DPTM ointment treatment group, (E) 2% Retapamulin ointment treatment group, and (F) 3% DPTM ointment treatment group.
2.3. Bacterial Count in Treated Skin Wound
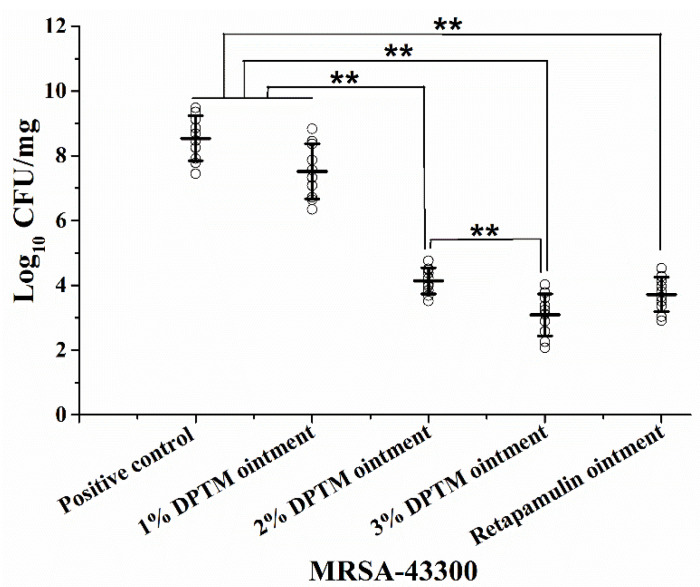
Next, we test the efficacy of a topical antibiotic treatment in the skin wound model in which mice infected with MRSA were treated with three dosages of DPTM ointment (Figure 4). The bacterial counts in 2% DPTM, 3% DPTM, and retapamulin ointments treated mice were 4.40, 5.42, and 4.82 logs lower, respectively, (p < 0.01) than that in positive control mice, whereas the 1% DPTM ointment treated group was 1.02 log lower. The 3% DPTM ointment treated group had the bacterial count lowered by 1.05 log compared to 2% DPTM ointment treated group (p < 0.01), but only lowered by 0.63 log compared to retapamulin ointment treated group.
Figure 4.
Efficacy of DPTM (1%, 2%, and 3% ointment) and retapamulin (2% ointment) against an experimental surgical wound infection in mice (n = 5) caused by MRSA. Values are means and standard errors of the means. ** p < 0.01.
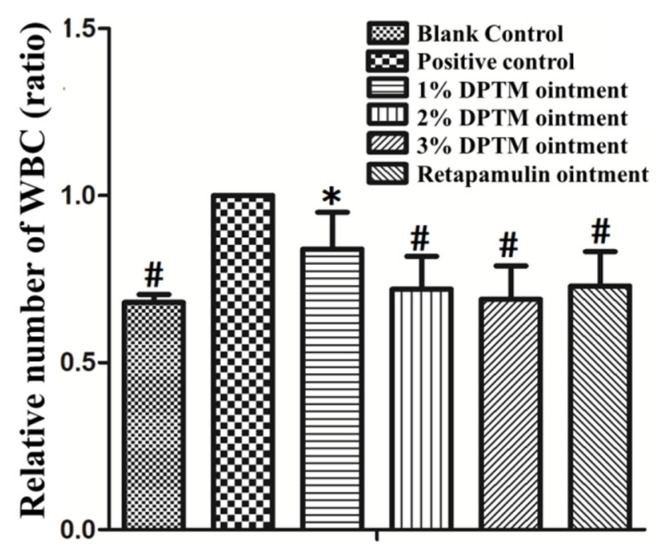
2.4. White Blood Cell (WBC) Level
To further investigate the efficacy of DPTM on the murine skin wound infected with MRSA, we analyzed the WBC which are involved in protecting the body against both infectious disease and foreign invaders [21] in blood, using a blood biochemistry analyzer (Hefei Jianneng Optical Instrument Co., Ltd., Hefei, China). The numbers of WBC in blank control group, three treatment groups and retapamulin ointment group decreased significantly compared with that in positive control group (p < 0.05). There was more WBC in the 1% DPTM ointment group compared with that in the retapamulin ointment group (p < 0.05). However, no significant difference was found among the 2% DPTM, 3% DPTM, and retapamulin ointment groups (Figure 5).
Figure 5.
Effect of DPTM (1%, 2%, and 3% ointment) and retapamulin (2% ointment) on mice WBC. All values are mean ± SD. # p < 0.05 indicates that the blank group, 2% DPTM, 3% DPTM, and retapamulin ointment treatment group vs. control group; * p < 0.05 indicates that 1% DPTM ointment treatment group vs. 2% retapamulin group.
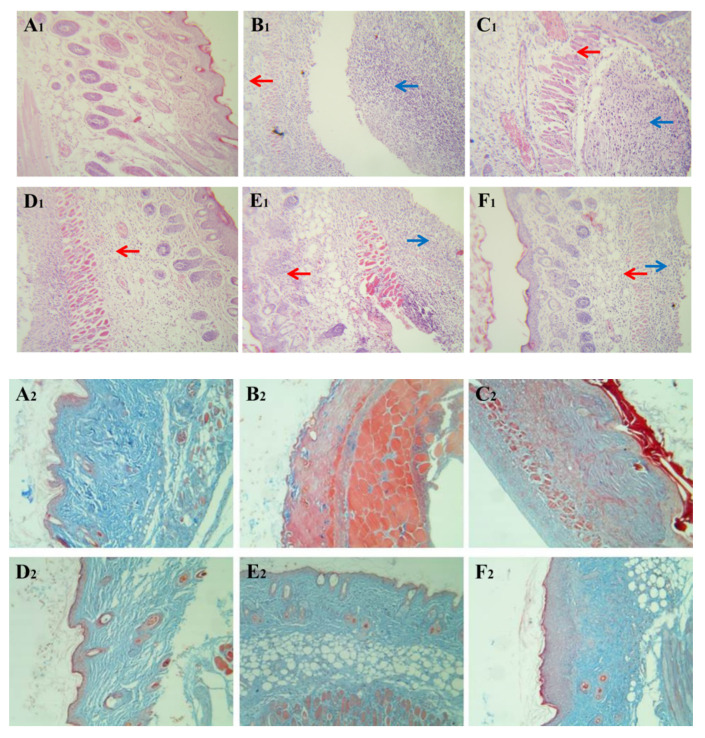
2.5. Histopathological Observation
The healing tissues obtained from all the six groups of animals in our excision wound model were processed for histopathological analysis by H&E and Masson staining (Figure 6). At 6 d post-infection, severe inflammatory cell infiltration, inflammatory cell accumulation, and tissue necrosis, as well as swelling and granular degeneration of muscle fiber were commonly observed in the wound skin of mice at the positive control group at the sixth day (Figure 6B1). It was worth noting that proliferation of myofibroblast, increase of new capillary, and attenuation of inflammatory reaction were noticeable after treatment with 2% and 3% DPTM and retapamulin ointments (Figure 6D1,E1,F1). Furthermore, obvious blue collagen deposition appeared in the wound after treatment with three dosages of DPTM and retapamulin ointments (Figure 6C2,D2,E2,F2). The connection of collagen fiber (dyed blue in Figure 6A2–F2) in treatments groups was closer than that in control group, especially the connection of collagen fiber in 3% DPTM ointment treatment group which showed significantly higher tightness than that of retapamulin ointment treatment group (Figure 6E2,F2).
Figure 6.
Effect of DPTM on pathological changes in MRSA infected skin (HE: 200×, Masson: 100×). These microscopic photographs of the wound healing skins were obtained from mice in the blank control group (A1 and A2), positive control group (B1 and B2), 1% DPTM ointment treatment group (C1 and C2), 2% DPTM ointment treatment group (D1 and D2), 2% retapamulin ointment treatment group (E1 and E2), and 3% DPTM ointment treatment group (F1 and F2). Red arrows point at inflammatory cell infiltration and the blue arrows point at inflammatory cell accumulation.
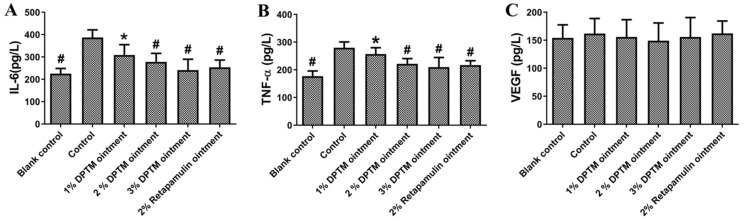
2.6. IL-6, TNF-α, and VEGF Levels
Primary skin infections stimulate inflammatory response which plays an essential role in the defense against pathogens [22]. This response involves a complex interplay of cytokines and chemokines, such as the pro-inflammatory cytokines interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF) [23]. Therefore, we used the ELISA method to detect the changes of IL-6, TNF-α, and VEGF after drug treatment. As illustrated in Figure 7, all treated groups, except 1% DPTM ointment, effectively decreased IL-6 and TNF-α induced by the inflammation in comparison with that in the control group (Figure 7A,B). To our surprise, no significant difference of VEGF secreted in serum between treatment groups and control group was observed (Figure 7C).
Figure 7.
Effect of DPTM and retapamulin ointments on secretions of IL-6, TNF-α, and VEGF. IL-6 (A), TNF-α (B), and VEGF (C). All values are mean ± SD. # p < 0.05 indicates that the blank group, 2% DPTM, 3% DPTM, and 2% retapamulin ointment treatment group vs. the control group; * p < 0.05 indicates 1% DPTM ointment treatment group vs. the 2% retapamulin ointment group.
3. Discussion
At present, MRSA displays resistance to most of β-lactam antibiotics, including oxacillin, methicillin, amoxicillin, and penicillin [24,25]. Drug resistance acquired by MRSA has increased clinical risk and caused serious problems worldwide [26]. Therefore, it is of great significance to develop new drugs for the diseases infected by MRSA.
This study evaluated the potent antibacterial activities of DPTM, a derivative of pleuromutilin, against MRSA isolated from dairy farms and against MRSA ATCC43300 in a murine skin wound model. While DPTM showed excellent in vitro inhibition against standard strains of S. aureus and MRSA at previous MIC testing [18], activity against MRSA isolated from clinic is lacking. We found that DPTM inhibited 61.1% strains with the concentration of 0.0625 μg/mL, while no strain was inhibited with the same concentration of tiamulin fumarate.
S. aureus is the top infectious pathogens responsible for SSTIs in children and adults [27]. Retapamulin, a semi-synthetic member of pleuromutilin, has been licensed in USA and Europe as 1% ointment (Altabax) for the topical treatment of SSTIs caused by MRSA and Streptococcus pyogenes [28]. Therefore, we established the mouse MRSA ATCC43300 skin infection model to compare the therapeutic effect of DPTM with that of retapamulin. After treatment, DPTM reduced the exudation of the infected skin and accelerate the healing. Furthermore, 2% and 3% DPTM ointment significantly reduced the number of white blood cells in the blood, indicating that DPTM displayed a certain therapeutic effect on MRSA skin infections. The ability of drugs to promote healing of infectious wounds is related to its antibacterial activities, reducing inflammatory reactions, and promoting epithelial formation [22]. Therefore, the number of bacteria in the infected skin was counted to evaluate the antibacterial effect of DPTM in live animals. The results showed that 2% and 3% DPTM ointment significantly reduced the number of MRSA.
Inflammation is one of key stages for the healing of skin wounds proceeds. S. aureus activate the STAT3, MAPK, and NF-κB signaling pathways, which promote the expression and secretion of pro-inflammatory cytokines, such as IL-6 and TNF-α, in keratinocytes [29,30]. It is helpful for the recovery of skin wounds to inhibit the secretion of excessive pro-inflammatory cytokines. After treatment, DPTM ointment significantly reduced serum IL-6 and TNF-αsecretion, which indicates that DPTM could improve the inflammatory response caused by MRSA. As a chemoattractant, VEGF recruits macrophages and granulocytes and participates in nitric oxide-mediated vasodilation, which induces endothelial cells to participate wound healing, thereby promoting blood vessel formation and vascular remodeling [31,32]. However, in this study there is no significant difference of secreted VEGF in the serum between the control group and the treatment groups. Because we did not detect the concentration of VEGF during the initial time of administration, we could not conclude whether VEGF had reached the peak of expression, or DPTM could not affect the secretion of VEGF. This needs to be further studied in detail.
4. Materials and Methods
4.1. Reagents
The synthesis method of DPTM was described previously [18] in our lab. The purity of this compound was checked by Waters 2695 HPLC (Massachusetts, USA) and quantitative NMR analyses at 98.72%, and its structure was confirmed by IR, NMR (Supplementary data), and HR-MS spectrometry. Tiamulin fumarate (purity: 98.5%) was purchased from Labor Dr. Ehrenstorfer-Schäfers (Augsburg, Germany) and retapamulin (98.0%) was purchased from (BOC Sciences, New York, NY, USA).
4.2. Bacterial Strain
All MRSA (n = 54) were isolated using brain-heart infusion (BHI) broth from fresh milk samples which were collected from different dairy farms in northwest China at 2017–2018. The isolates were identified by sequencing the 16S rRNA universal primer and Vitek 2 Compact (BioMerieux, Lyon, France), followed by typing the Staphylococcal chromosomal cassette mec (SCCmec) gene (Supplementary data). Susceptibility testing was performed as per Clinical Laboratory and Standards Institute (CLSI) recommendations. MRSA ATCC43300 was purchased from Beijing Beina Science & Technology Co., Ltd. (Beijing, China).
4.3. Animals
Sixty healthy BALB/c mice (weight of 23 to 25 g; Centre of Experimental Animals of Lanzhou University, Lanzhou, China) were housed in a comfortable room and were given free access to standard diet and water. Mice were maintained on a 12 h light/dark cycle at the temperature of 25 °C and relative humidity of 55–65%. All animals were handled in strict accordance with good animal practice according to the Animal Ethics Procedures and Guidelines of the People’s Republic of China, and the study was approved by The Animal Administration and Ethics Committee of Lanzhou Institute of Husbandry and Pharmaceutical Sciences of CAAS (No. SYXK-2018-002).
4.4. MIC Determination
The MICs of DPTM were determined by micro-dilution technique in Mueller-Hinton broth (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) according to the Clinical and Laboratory Standards Institute (CLSI) guidelines [33]. The experiments were performed in triplicate.
4.5. Skin Infection Model
MRSA ATCC43300 was grown in tryptone soy broth (TSB, Beijing Solarbio Science & Technology Co., ltd. Beijing, China) at 37 °C overnight and harvested by centrifugation at 3000 g for 10 min, followed by being washed twice in phosphate buffer saline (PBS). Bacteria were suspended in sterile PBS at a concentration of 1012 CFU/mL. Different amounts of DPTM (0.05, 0.10, and 0.15 g) and retapamulin (0.10 g) used in this study were made as ointments (1%, 2%, and 3% DPTM and 2% retapamulin, respectively) with matrix including albolene (3 g), liquid paraffin (1.5 mL), lanolin (0.1 g), and anhydrous alcohol (0.4 mL). Wound preparation and infection protocol were modified from published report [34,35]. In brief, mice were randomly divided into six groups and were anaesthetized intraperitoneally with 10% chloral hydrate. The fur on mice back was shaved and the shaved area was cleaned with gauze sponges and water. An incision deep to sarcolemma was made through the shaved area after the alcohol disinfection. The blood from the wound was cleaned with cotton ball and an inoculum of 0.1 mL of MRSA (at a final concentration of approximately 1012 CFU/mL which was obtained by pre-test) in PBS was smeared evenly on the whole wound of mice except for the blank control group which was only treated with blank ointment matrix. After inoculation with MRSA for 4 h, the positive control group was treated with blank ointment matrix. The treatment groups were treated with 2% retapamulin and 1%, 2%, and 3% DPTM ointment, respectively. For each treatment 15 mg per mice of ointment was applied. The dosage for each group was continued for 5 days with 24 h intervals. After inoculation, the mice were caged separately and observed twice daily for their clinical signs and mortality. For all groups, the experiments were terminated 24 h (on day 6 after infection) after the last topical treatment in order to avoid carryover effects in vitro.
4.6. Detection of WBC, IL-6, TNF-α and VEGF in Blood Serum
On day 6 after infection, the blood was taken, by excising their eyeballs, for counting white blood cells and detecting the IL-6, TNF-α, and VEGF in blood serum by enzyme-linked immunosorbent assay (ELISA) using the commercially available kit (IL-16: Mouse IL-16 ELISA Kit, Boster Biological Technology co.ltd., Wuhan, China; TNF-α: Mouse TNF-αELISA Kit, Elabscience Biotechnology Co.,Ltd., Wuhan, China; VEGF: Mouse VEGF-B ELISA Kit, Elabscience Biotechnology Co.,Ltd., Wuhan, China).
4.7. Counting the Colonies of MRSA and Histological Examination
To examine CFU burden within the wound skin, the survival of the mice at 6 d after infection was used as the end-point and anaesthetized. The infected skins of five mice in treatment groups and positive control (including one dead mouse after infection) were sterilizing collected, homogenized, diluted (10×) and plated onto Baird-Parker agar (Hope Bio-Technology Co., Ltd., Qingdao, China) to count the colonies of MRSA after incubation for 16–24 h at 37 °C. The infected skins of the remaining five mice (including the other one dead mouse after infection) used for hematoxylin and eosin (H&E) and Masson trichrome staining after soaking in 10% formalin, respectively [19], and their lesions were observed using a Nikon DS-Fi2 fluorescent microscope (Nikon, Tokyo, Japan).
4.8. Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows version 24.0 (SPSS Inc., Chicago, IL, USA). The data were analyzed by One-way analysis of variance (ANOVA), followed by Dunnett’s post-hoc tests as appropriate. Statistically significant difference was defined as a p < 0.05 and the extremely significant difference was defined as a p < 0.01.
5. Conclusions
DPTM demonstrated potent in vitro activity against clinical isolates of MRSA with lower MICs than that of tiamulin fumarate. In in vivo efficacy using a murine skin wound model, 2% and 3% DPTM ointment displayed similar effect to retapamulin to significantly promote the healing of wound caused by MRSA and reduce bacterial count. In addition, DPTM decrease relative number of WBC and the secretion of IL-6 and TNF-α inflammatory cytokines in plasma. Thus, DPTM represents a promising treatment option for MRSA infections.
Supplementary Materials
The following are available online, Figure S1: IR, 1H-NMR and 13C-NMR spectra of DPTM, Table S1: MIC values of DPTM and tiamulin fumarate against clinical isolates of MRSA.
Author Contributions
Conceptualization, Y.F. (Yunxing Fu) and R.S.; methodology, Y.F. (Yunxing Fu), C.L., Y.F. (Yuan Fan), X.L. and X.M.; software, R.S.; validation, Y.F. (Yunxing Fu), Z.G. and X.W. (Xiujun Wang); formal analysis, Y.F. (Yuan Fan), X.W. (Xuefei Wang), Z.G. and R.S.; investigation, X.L., X.W. (Xiujun Wang) and Y.F. (Yunxing Fu); resources, Y.F. (Yunxing Fu); data curation, Y.F. (Yuan Fan); writing—original draft preparation, Y.F. (Yunxing Fu), C.L. and R.S.; writing—review and editing, Y.F. (Yunxing Fu) and R.S.; visualization, Y.F. (Yunxing Fu); supervision, Y.F. (Yunxing Fu); project administration, Y.F. (Yunxing Fu) and R.S.; funding acquisition, Y.F. (Yunxing Fu). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ph.D. research start-up funds of Henan University of Animal Husbandry and Economy (No. 2020HNUAHEDF019).
Institutional Review Board Statement
The experimental procedures were performed in accordance with the Ethical Principles in Animal Research and were approved by The Animal Administration and Ethics Committee of Lanzhou Institute of Husbandry and Pharmaceutical Sciences of CAAS (No. SYXK-2018-002).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang C.J., Ye C., Liao L.L., Wang Z.H., Hu Y., Deng C., Liu L. Adjuvant β-lactam therapy combined with vancomycin or daptomycin for methicillin-resistant Staphylococcus aureus bacteraemia: A systematic review and meta-analysis. Antimicrob. Agents Chemoth. 2020;64:e01377-20. doi: 10.1128/AAC.01377-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong S.I., Lee Y.M., Park K.H., Ryu B.H., Hong K.W., Kim S., Bae I.G., Cho O.H. Clinical and molecular characteristics of qacA and qacB-positive methicillin-resistant Staphylococcus aureus causing bloodstream infections. Antimicrob. Agents Chemother. 2019;63:e02157-18. doi: 10.1128/AAC.02157-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raz A., Serrano A., Thaker M., Alston T., Fischettia V.A. Lysostaphin lysibody leads to effective opsonization and killing of Methicillin-resistant Staphylococcus aureus in a murine model. Antimicrob. Agents Chemother. 2018;62:e01056-18. doi: 10.1128/AAC.01056-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guardabassi L., Moodley A., Williams A., Stegger M., Damborg P., Halliday-Simmonds I., Butaye P. High prevalence of USA300 among clinical isolates of Methicillin-resistant Staphylococcus aureus on St. Kitts and Nevis, West Indies. Front. Microbiol. 2019;10:1123. doi: 10.3389/fmicb.2019.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher H.W., Corey G.R. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008;5:S344–S349. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 6.Malani P.N. National burden of invasive methicillin-resistant Staphylococcus aureus infection. JAMA. 2014;311:1438–1439. doi: 10.1001/jama.2014.1666. [DOI] [PubMed] [Google Scholar]
- 7.Gardete S., Tomasz A. Vancomycin resistant Staphylococcus aureus infections: A review of case updating and clinical features. J. Adv. Res. 2020;21:169–176. doi: 10.1016/j.jare.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu B., Kelesidis T., Tsiodras S., Hindler J., Humphries R.M. The emerging problem of linezolid-resistant Staphylococcus. J. Antimicrob. Chemother. 2013;68:4–11. doi: 10.1093/jac/dks354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shariati A., Dadashi M., Chegini Z., Belkum A., Mirzaii M., Khoramrooz S.S., Darban-Sarokhalil D. The global prevalence of Daptomycin, Tigecycline, Quinupristin/Dalfopristin, and Linezolid-resistant Staphylococcus aureus and coagulase-negative staphylococci strains: A systematic review and meta-analysis. Antimicrob. Resist. In. 2020;9:56. doi: 10.1186/s13756-020-00714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kavanagh F., Hervey A., Robbins W.J. Antibiotic substances from Basidiomycetes: VIII. Pleurotus multilus (Fr.) Sacc. and Pleurotus passeckerianus Pilat. Proc. Natl. Acad. Sci. USA. 1951;37:570–574. doi: 10.1073/pnas.37.9.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak R., Shlaes D.M. The pleuromutilin antibiotics: A new class for human use. Curr. Opin. Invest. Drugs. 2010;11:182–191. [PubMed] [Google Scholar]
- 12.Schlunzen F., Pyetan E., Fucini P., Yonath A., Harms J.M. Inhibition of peptide bond formation by pleuromutilins: The structure of the 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin. Mol. Microbiol. 2004;54:1287–1294. doi: 10.1111/j.1365-2958.2004.04346.x. [DOI] [PubMed] [Google Scholar]
- 13.Davidovich C., Bashan A., Auerbach-Nevo T., Yaggie R.D., Gontarek R.R., Yonath A. Induced-fit tightens pleuromutilins, binding to ribosomes and remote interactions enable their selectivity. Proc. Natl. Acad. Sci. USA. 2007;104:4291–4296. doi: 10.1073/pnas.0700041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burch D.G. Tiamulin activity against Brachyspira hyodysenteriae. Vet. Rec. 2008;163:760. [PubMed] [Google Scholar]
- 15.Stipkovits L., Ripley P.H., Tenk M., Glavits R., Molnar T., Fodor L. The efficacy of valnemulin (Econor) in the control of disease caused by experimental infection of calves with Mycoplasma bovis. Res. Vet. Sci. 2005;78:207–215. doi: 10.1016/j.rvsc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Scangarella-Oman N.E., Shawar R.M., Bouchillon S., Hoban D. Microbiological profile of a new topical antibacterial: Retapamulin ointment 1% Expert. Rev. Anti. Infect. Ther. 2009;7:269–279. doi: 10.1586/eri.09.7. [DOI] [PubMed] [Google Scholar]
- 17.Powell D., Donato A. In community-acquired bacterial pneumonia, lefamulin was noninferior to moxifloxacin at 96 h after the first dose. Ann. Intern. Med. 2020;4:Jc22. doi: 10.7326/ACPJ202002180-022. [DOI] [PubMed] [Google Scholar]
- 18.Yi Y., Xu X., Liu Y., Xu S., Huang X., Liang J., Shang R. Synthesis and antibacterial activities of novel pleuromutilin derivatives with a substituted pyrimidine moiety. Eur. J. Med. Chem. 2017;126:687–695. doi: 10.1016/j.ejmech.2016.11.054. [DOI] [PubMed] [Google Scholar]
- 19.Fu Y., Ma L., Yi Y., Fan Y., Liang J., Shang R. A new pleuromutilin candidate with potent antibacterial activity against Pasteurella multocida. Microb. Pathogenesis. 2019;127:202–207. doi: 10.1016/j.micpath.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Killeavy E.E., Jogl G., Gregory S.T. Tiamulin-resistant mutants of the thermophilic bacteriumt Thermus thermophiles. Antibiotics. 2020;9:313. doi: 10.3390/antibiotics9060313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng Z.H., Yin J.J., Luo W., Kotian R.N., Gao S.S., Yi Z.Q., Xiao W.F., Li W.P., Li Y.S. The effect of earthworm extract on promoting skin wound healing. Biosci. Rep. 2018;38:20171366. doi: 10.1042/BSR20171366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han H.M., Ko S., Cheong M.J., Bang J.K., Seo C.H., Luchian T., Park Y. Myxinidin 2 and myxinidin 3 suppress inflammatory responses through STAT3 and MAPKs to promote wound healing. Oncotarget. 2017;8:87582–87597. doi: 10.18632/oncotarget.20908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaczynska K., Kogut E., Zajac D., Jampolska M., Andrzejewski K., Sulejczak D., Lipkowski A.W., Kleczkowska P. Neurotensin-based hybrid peptide’s anti-inflammatory activity in murine model of a contact sensitivity response. Eur. J. Pharm. Sci. 2016;93:84–89. doi: 10.1016/j.ejps.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Gorwitz R.J., Kruszon-Moran D., Mcallister S.K., Mcquillan G., McDougal L.K., Fosheim G.E., Jensen B.J., Killgore G., Tenover F.C., Kuehnert M.J. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001. J. Infect. Dis. 2008;197:1226–1234. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 25.Vestergaard M., Frees D., Ingmer H. Antibiotic resistance and the MRSA problem. Microbiol. Spectrum. 2019;7:1–23. doi: 10.1128/microbiolspec.GPP3-0057-2018. [DOI] [PubMed] [Google Scholar]
- 26.Angebault C., Andremont A. Antimicrobial agent exposure and the emergence and spread of resistant microorganisms: Issues associated with study design. Eur. J. Clin. Microbiol. Infect. Dis. 2013;32:581–595. doi: 10.1007/s10096-012-1795-3. [DOI] [PubMed] [Google Scholar]
- 27.Papastefan S.T., Buonpane C., Ares G., Benyamen B., Helenowski I., Hunter C.J. Impact of decolonization protocols and recurrence in pediatric MRSA skin and soft-tissue infections. J. Surg. Res. 2019;242:70–77. doi: 10.1016/j.jss.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W.J., He C.Y., Yang H., Shu W., Cui Z.L., Tang R., Zhang C., Liu Q. Prevalence and molecular characterization of methicillin-resistant Staphylococcus aureus with mupirocin, fusidic acid and/or retapamulin resistance. BMC. Microbiol. 2020;20:183. doi: 10.1186/s12866-020-01862-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia X., Li Z., Liu K., Wu Y., Jiang D., Lai Y. Staphylococcal LTA-induced miR-143 inhibits Propionibacterium acnesmediated inflammatory response in skin. J. Invest. Dermatol. 2016;136:621–630. doi: 10.1016/j.jid.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Li R., Wang J., Wang X., Zhou J., Wang M., Ma H., Xiao S. Increased betaTrCP are associated with miquimod-induced psoriasis-like skin inflammation in mice via NF-kappaB signaling pathway. Gene. 2016;592:164–171. doi: 10.1016/j.gene.2016.07.066. [DOI] [PubMed] [Google Scholar]
- 31.Pan C.Y., Chen J.Y., Lin T.L., Lin C.H. In vitro activities of three synthetic peptides derived from epinecidin-1 and an anti-lipopolysaccharide factor against Propionibacterium acnes, Candida albicans, and Trichomonas vaginalis. Peptides. 2009;30:1058–1068. doi: 10.1016/j.peptides.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Lee S.Y., Seong I.W., Kim J.S., Cheon K.A., Gu S.H., Kim H.H., Park K.H. Enhancement of cutaneous immune response to bacterial infection after low-level light therapy with 1072 nm infrared light: A preliminary study. J. Photoch. Photobio. B. 2011;105:175–182. doi: 10.1016/j.jphotobiol.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Clinical Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. approved standard-ninth edition. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2012. [Google Scholar]
- 34.Boon R.J., Beale A.S. Response of Streptococcus pyogenes to therapy with amoxicillin or amoxicillin-clavulanic acid in a mouse model of mixed infection caused by Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 1987;31:1204–1209. doi: 10.1128/AAC.31.8.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kugelberg E., Norstrom T., Petersen T.K., Duvold T., Andersson D.I., Hughes D. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 2005;49:3435–3441. doi: 10.1128/AAC.49.8.3435-3441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.