This work uses controlled human cohorts to investigate urinary prostaglandin E2, the product of cyclooxygenase-2, as both a diagnostic and prognostic biomarker of recurrent UTI postmenopausal women.
Abstract
Urinary tract infection (UTI) is one of the most common adult bacterial infections and exhibits high recurrence rates, especially in postmenopausal women. Studies in mouse models suggest that cyclooxygenase-2 (COX-2)–mediated inflammation sensitizes the bladder to recurrent UTI (rUTI). However, COX-2–mediated inflammation has not been robustly studied in human rUTI. We used human cohorts to assess urothelial COX-2 production and evaluate its product, PGE2, as a biomarker for rUTI in postmenopausal women. We found that the percentage of COX-2–positive cells was elevated in inflamed versus uninflamed bladder regions. We analyzed the performance of urinary PGE2 as a biomarker for rUTI in a controlled cohort of 92 postmenopausal women and PGE2 consistently outperformed all other tested clinical variables as a predictor of rUTI status. Furthermore, time-to-relapse analysis indicated that the risk of rUTI relapse was 3.6 times higher in women with above median urinary PGE2 levels than with below median levels. Taken together, these data suggest that urinary PGE2 may be a clinically useful diagnostic and prognostic biomarker for rUTI in postmenopausal women.
Introduction
Urinary tract infection (UTI) affects more than 150 million people annually worldwide (Stamm & Norrby, 2001; Flores-Mireles et al, 2015). UTIs are a leading indication for prescription antibiotics and have a significant impact on women of all ages (Amna et al, 2013). Recurrent urinary tract infection (rUTI) is defined as ≥3 symptomatic UTIs in 12 mo or ≥2 symptomatic UTIs in 6 mo (Nicolle, 2001; Glover et al, 2014; Malik et al, 2018b). rUTI has a severe impact on quality of life causing pain, frequency, urgency, anxiety, and symptoms of clinical depression among affected women (Ellis & Verma, 2000; Renard et al, 2014). rUTI incidence increases with age with reported recurrence rates as high as 50% in postmenopausal women (Foxman, 2002, 2014; Raz, 2011). rUTI is caused by diverse bacterial species and less commonly by fungi (Flores-Mireles et al, 2015). The front-line therapy for rUTI is prescription of antibiotics such as trimethoprim sulfamethoxazole, nitrofurantoin, and fluoroquinolones (Jancel & Dudas, 2002). Resistance, allergy, or documented adverse reactions to antibiotics are frequent in the elderly and limit the safety and efficacy of antibiotics in this demographic (Jancel & Dudas, 2002; Malik et al, 2018a). Therefore, new therapies for rUTI must be developed.
rUTI is, in part, an inflammatory disease (Bjorling et al, 2011). Evidence of chronic inflammation has been reported in the bladders of rUTI patients during cystoscopy (Wu et al, 2016; Crivelli et al, 2019). Extensive work performed exclusively in mouse models has implicated host inflammation, specifically Cyclooxygenase-2 (COX-2)–mediated inflammation, as a key sensitizing factor for rUTI (Hannan et al, 2010; Hannan et al, 2014; O’Brien et al, 2016). Excessive urothelial neutrophil infiltration and COX-2–dependent inflammation were found to cause tissue damage and remodeling that sensitize the bladder to severe rUTI (Hannan et al, 2014; O’Brien et al, 2015; O’Brien et al, 2016). Importantly, Hannan et al (2014) demonstrated that treatment of mice with specific COX-2, but not COX-1, inhibitors protected the bladder against sensitization to severe rUTI (Hannan et al, 2014). Furthermore, treatment of mice with dexamethasone, another drug targeting the COX-2 pathway, suppressed development of chronic cystitis (Hannan et al, 2010). These data suggest that the COX-2 pathway may be a promising therapeutic target for rUTI; however, knowledge of the role of COX-2–mediated inflammation in human rUTI is limited.
Here, we used defined, well-curated human cohorts to evaluate activation of the COX-2–mediated inflammation during rUTI in postmenopausal women. We hypothesized that, as observed in mouse models of UTI, COX-2 would be expressed in urothelium of visibly inflamed bladder regions in human rUTI patients. To determine if COX-2 enzyme levels are elevated in regions of cystitis, we enumerated COX-2–expressing urothelial cells in bladder biopsies from inflamed and control regions. We assessed urinary PGE2 as a proxy for urothelial COX-2 expression in matched bladder biopsy and urine samples. We then evaluated PGE2 as a biomarker for rUTI in postmenopausal women by measuring urinary PGE2 levels across three groups with different UTI histories. Finally, we performed a time-to-relapse study to determine if urinary PGE2 levels were predictive of rUTI relapse.
Results
COX-2 expression is activated in the bladder urothelium during human rUTI
Previous work in mouse models has demonstrated that COX-2 expression triggers prolonged neutrophil recruitment resulting in tissue damage, changes to bladder wall morphology, and increased susceptibility to severe rUTI (Hannan et al, 2014; O’Brien et al, 2016; Yu et al, 2019). To determine if COX-2 expression was activated during human rUTI, we analyzed COX-2 expression and neutrophil recruitment in bladder urothelium in two cohorts of women undergoing cystoscopy with fulguration of trigonitis (CFT). For Cohort 1, antibiotic therapy was stopped after symptom resolution 7 d before CFT, and two biopsies, one from a visibly inflamed (I1) and one from a visibly normal control region (C1) of the bladder, were obtained (Fig 1A) (De Nisco et al, 2019). To determine if COX-2 expression was associated with neutrophil recruitment, we visualized COX-2 and elastase (ELA2), a neutrophil marker (Lammers et al, 1986), in the I1 and C1 regions using immunofluorescence (IF) confocal microscopy (Figs 1B and S1). COX-2–positive urothelial cells were not observed in every I1 biopsy. Representative images of I1 biopsies with high urothelial COX-2 (PNK006) versus undetectable urothelial COX-2 (PNK011) are presented in Fig 1B. Quantification of COX-2–expressing urothelial cells and neutrophil infiltration was performed and reported as percentage of total of urothelial cells (Fig 1C and D). Urothelial COX-2 expression was observed in the I1 regions of 85.7% (12/14) of patients (Fig 1B and C). Similarly, neutrophil infiltration was observed in the I1 regions of 71.4% (10/14) of patients (Fig 1B and D). The quantification data for COX-2 and neutrophils were summarized and classified into two groups: inflamed and control. The median percentage of urothelial COX-2–expressing cells and neutrophils was 6.1 and 24.1 times higher in inflamed versus control regions, respectively (Fig 1E). Correlation analysis between neutrophil infiltration and COX-2–expressing cells in the I1 area revealed a strong correlation between the two inflammatory markers (rS = 0.8877, P = 0.00006) (Fig 1F). In these biopsies we defined the suburothelium as the region underlying the urothelium that includes the lamina propria and in some cases the muscularis propria. Suburothelial neutrophil accumulation was found in patients PNK003, PNK006, PNK010, PNK011, and PNK014, indicating that neutrophil accumulation was not limited to the urothelium (Fig S2A–E). Suburothelial COX-2 expression was also observed but predominately in regions with severely damaged urothelium or co-localized with neutrophils (Fig S2A, C, E, and F).
Figure 1. COX-2 expression is activated in the bladder urothelium during human recurrent urinary tract infection.
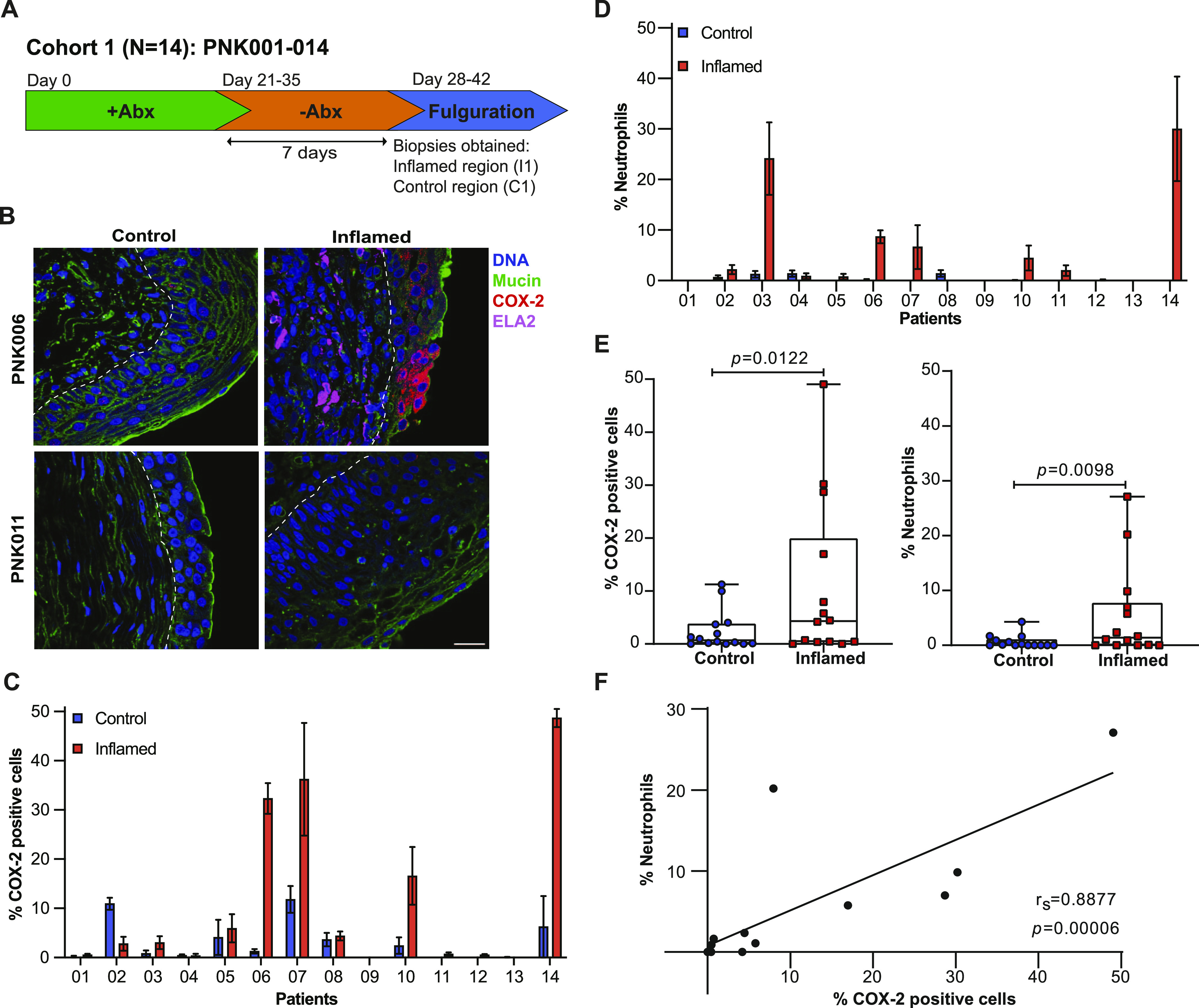
(A) Cohort 1 patient recruitment and procedure timeline. (B) Representative confocal micrographs (63×) of I1 and C1 regions of PNK006 and PNK011 with DNA (Hoechst) in blue, Mucin (WGA) in green, COX-2 in red and neutrophils (ELA-2) in magenta. Scale bar represents 10 μm. (C, D) Quantification of COX-2 expressing cells and (D) neutrophils within the urothelium of control (blue) and inflamed (red) region biopsies reported as percentage of total urothelial cells. 10 randomly sampled images were enumerated for each section. Bar graphs represent mean ± SEM. (E) Comparison of %COX-2–expressing cells and %neutrophils between control and inflamed regions. Whiskers drawn minimum to maximum, boxes represent interquartile range, and median denoted by horizontal line. P-values generated by Wilcoxon matched pairs signed-ranks test. (F) Linear regression with Spearman correlation between %neutrophils and %COX-2–positive cells.
Figure S1. COX-2 and ELA-2 detection in the bladder urothelium of patients PNK006 and PNK011.
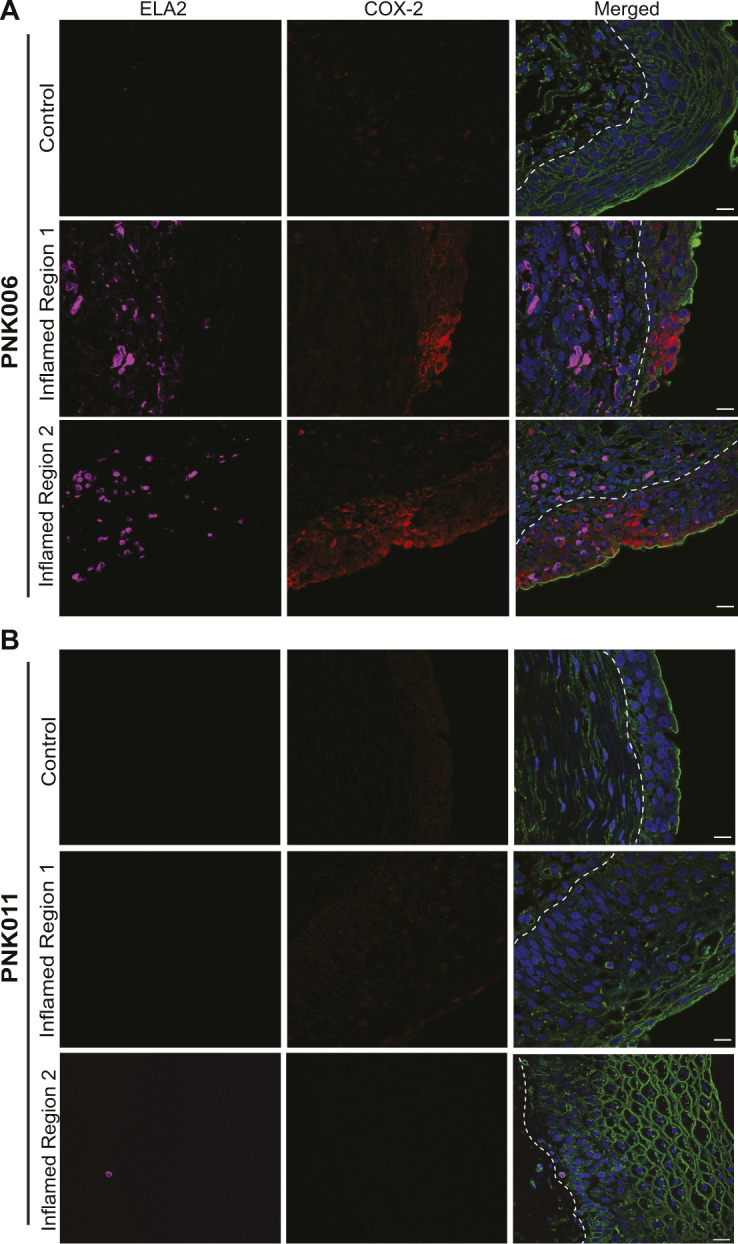
(A, B) Confocal micrographs (63×) from Fig 1B with split channels for ease of interpretation (control and Inflamed Region 1) as well as an additional representative inflamed region micrograph (Inflamed Region 2) from (A) PNK006 and (B) PNK011 biopsies. Independent channels for COX-2 (red) and ELA-2 (magenta) as well as the merged channel are shown. Scale bar represents 10 μm. White dashed line delineates urothelium and suburothelium. DNA (Hoechst) in blue, Mucin (WGA) in green, COX-2 in red, and neutrophils (ELA-2) in magenta.
Figure S2. Detection of COX-2–expressing cells and neutrophils in the bladder suburothelium.
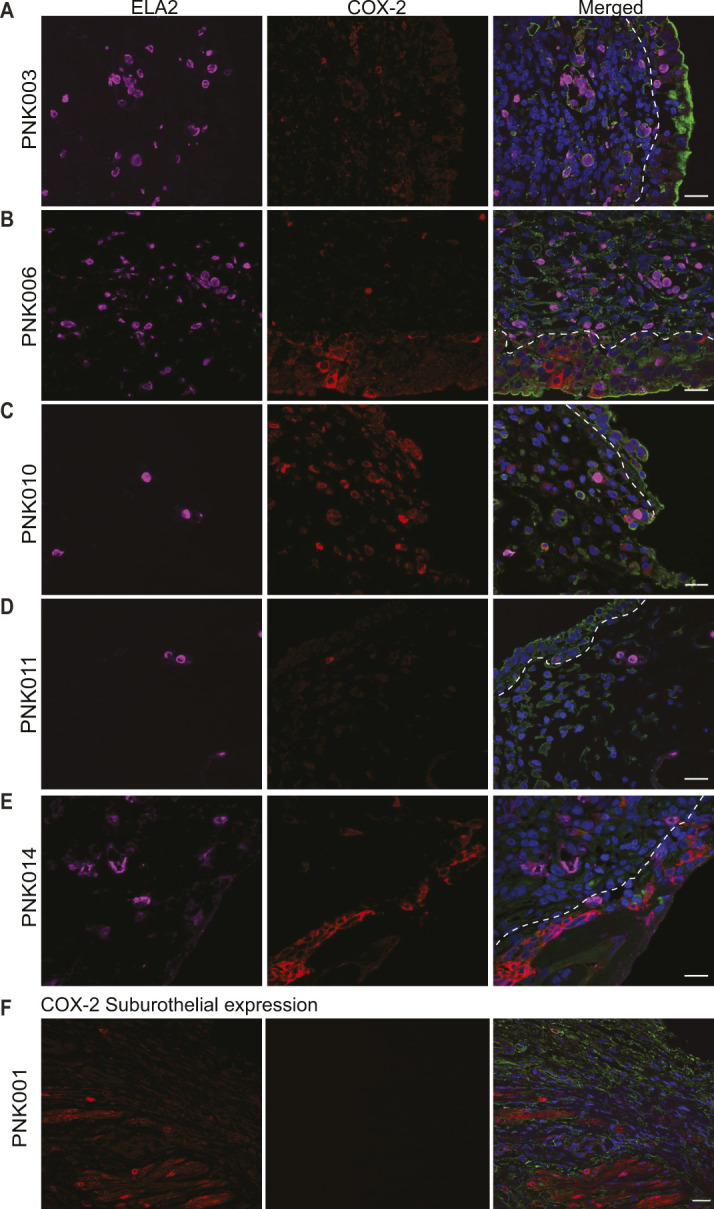
Cohort 1 bladder biopsy sections were analyzed for the presence of suburothelial COX-2 expression and neutrophil accumulation. (A, B, C, D, E) Representative confocal micrograph (63×) of I1 bladder biopsy sections of patients PNK003, 006, 010, 011, and 014 with suburothelial COX-2 expression and neutrophils. (F) Representative confocal micrograph (63×) of I1 bladder biopsy of PNK001 with suburothelial COX-2 expression in the absence of neutrophils. DNA (Hoechst) in blue, Mucin (WGA) in green, COX-2 in red, and neutrophils (ELA-2) in magenta. White dashed line delineates urothelium and suburothelium. Scale bar represents 10 μm.
Urinary PGE2 as a marker for COX-2–mediated bladder inflammation
We next sought to identify a marker of COX-2 activity that would be measurable in urine. Prostaglandin E2 (PGE2) is the product of arachidonic acid conversion by the COX-2 enzyme (Ricciotti & FitzGerald, 2011). Extracellular PGE2 elicits diverse cellular responses including cell proliferation, angiogenesis, pain sensation, and inflammation (Nakanishi & Rosenberg, 2013; Kawahara et al, 2015). Induced COX-2 expression results in higher secreted levels of PGE2 (Wheeler et al, 2002; Park et al, 2006). Therefore, we hypothesized that urinary PGE2 should be a reliable indicator of urothelial COX-2 expression (Wheeler et al, 2002). To test this hypothesis, we measured both urothelial COX-2 expression and urinary PGE2 in a second CFT cohort (Cohort 2, PNK016-27) (Fig 2A). For this cohort, due to changes in the IRB-approved protocol, antibiotic therapy was not ceased before CFT and one inflamed-region biopsy (I1) was obtained. Cohort 2 patient statistics and clinical urine culture (UC) results are reported in Table 1.
Figure 2. Urinary PGE2 as a marker for COX-2–mediated bladder inflammation.
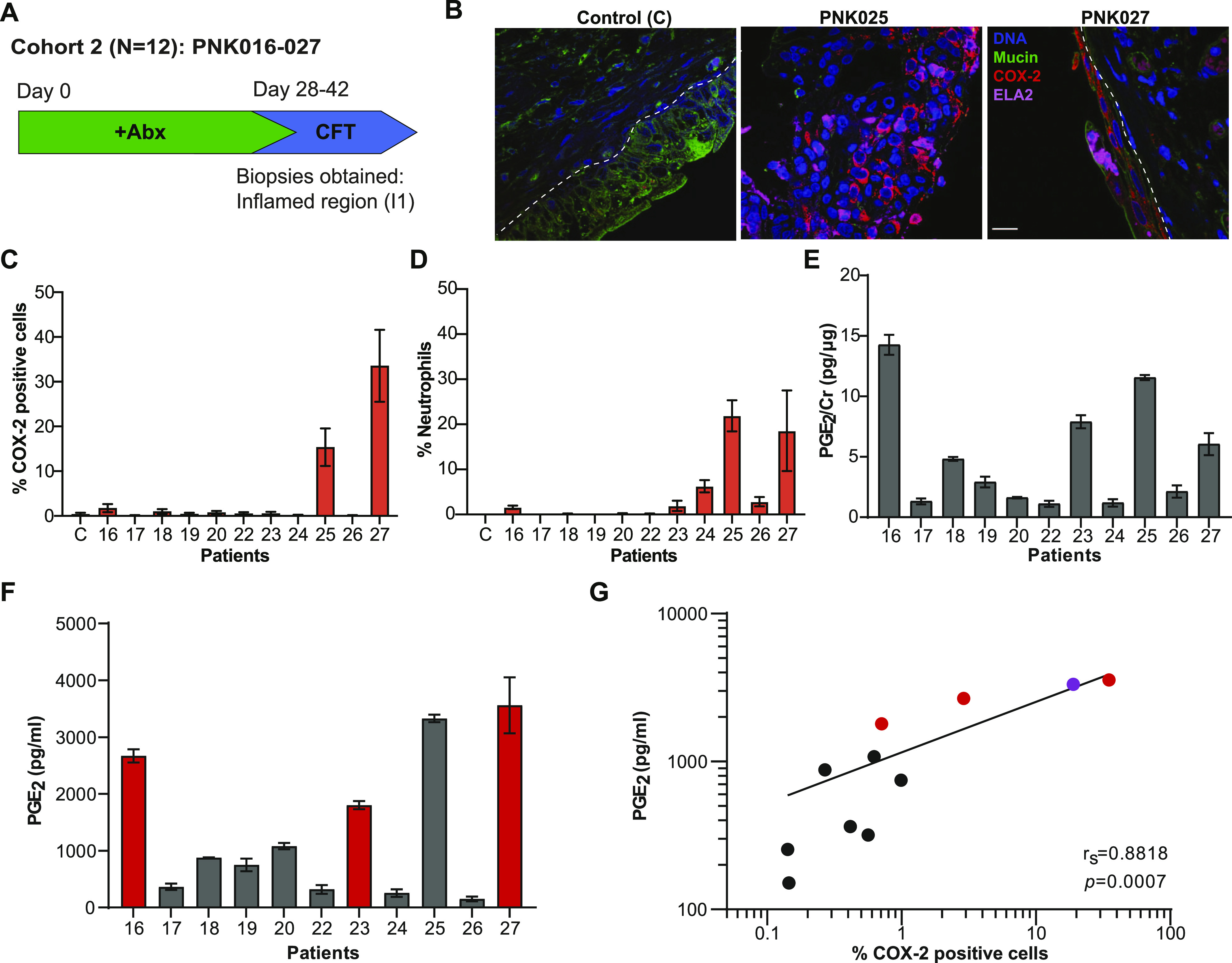
(A) Cohort 2 patient recruitment and procedure timeline. (B) Representative confocal micrographs for control and I1 region from patients PNK025 and PNK027 compared to commercially available human normal bladder section (control, US Biomax) with DNA in blue, Mucin in green, COX-2 in red and neutrophils in magenta. Scale bar represents 10 μm. (C, D) Quantification of COX-2 expressing cells and (D) neutrophils within the urothelium reported as percentage of total urothelial cells. 10 randomly sampled images taken with a 63× objective were enumerated for scoring. C denotes control. (E) Bar graphs represent mean ± SEM (E) Urinary PGE2 normalized to Cr. (F) Raw urinary PGE2 concentration. Bar graphs represent mean ± SD. (G) Linear regression with Spearman’s correlation between raw urinary PGE2 concentration and %COX-2–positive urothelial cells. Red circle denotes positive urine culture. Purple circle denotes PNK025.
Table 1.
Cohort 2 patient characteristics.
| Patients | Age (yr) | BMI (kg/m2) | Diabetes | Prior CFT | Urine culture history | Urine culture before CFT |
|---|---|---|---|---|---|---|
| PNK016 | 58 | 32.2 | IDDM | No | Pseudomonas aeruginosa and Proteus mirabilis | Enterococcus faecalis (105) |
| PNK017 | 65 | 25.3 | No | No | P. aeruginosa, Escherichia coli, and E. faecalis | No growth |
| PNK018 | 62 | 24.0 | No | No | Klebsiella pneumoniae, E. coli, and E. faecalis | No growth |
| PNK019 | 80 | 22.5 | No | No | E. faecalis and K. pneumoniae | No growth |
| PNK020 | 51 | 31.9 | No | No | E. coli | No growth |
| PNK022 | 88 | 27.5 | No | No | E. coli | No growth |
| PNK023 | 82 | 20.7 | No | No | K. pneumoniae | K. pneumoniae (105) |
| PNK024 | 58 | 30.9 | AODM | Yes | K. pneumoniae | No growth |
| PNK025 | 66 | 41.2 | No | No | E. coli | No growth |
| PNK026 | 65 | 35.0 | AODM | No | E. coli | No growth |
| PNK027 | 77 | 22.0 | No | Yes | E. coli and E. faecalis | Enterococcus faecium (105), Aerococcus urinae (55–99,000) |
Relevant patient data recorded for the 11 study participants. BMI, body mass index. Diabetes: no, nondiabetic; IDDM, diabetes mellitus type 1; AODM, adult-onset diabetes mellitus type 2; CFT, cystoscopy with fulguration of trigonitis. Urine cultures performed in clinical laboratories with a 104 CFU/ml detection limit.
We performed IF for COX-2 and neutrophils on I1 bladder biopsy tissues and commercially available normal bladder biopsy sections as a control (US Biomax). Representative images of control, PNK025 and PNK027 biopsy sections are shown in Figs 2B and S3. COX-2 expressing urothelial cells or neutrophils were counted and reported as percentage to total number of urothelial cells (Fig 2C and D). PNK025 and PNK027 biopsy sections showed the highest median percentages of COX-2 expressing cells (16.5% and 33.5%, respectively) (Fig 2C). Urothelial neutrophil infiltration was found in PNK016 and PNK023-27 but was highest in PNK025 (median = 21.9%) and PNK027 (median = 18.5%), mirroring the high percentage of COX-2–expressing cells detected in these biopsies (Fig 2D).
Figure S3. COX-2 and ELA-2 detection in the bladder urothelium of PNK025 and PNK027.
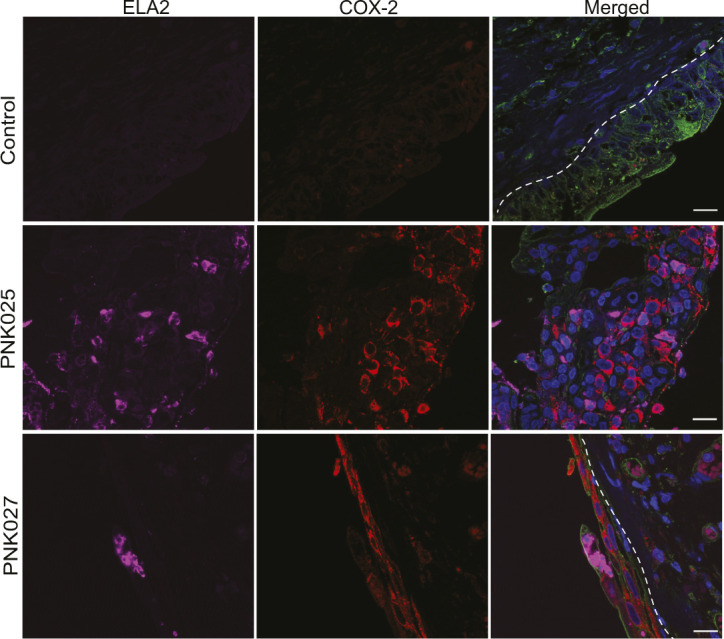
Confocal micrographs (63×) from Fig 2B with split channels for ease of interpretation. Independent channels for COX-2 (red) and ELA-2 (magenta) as well as the merged channel are shown for PNK025 and PNK027 versus Control (US Biomax) biopsy sections. Scale bar represents 10 μm. White dashed line delineates urothelium and suburothelium. DNA (Hoechst) in blue, Mucin (WGA) in green, COX-2 in red, and neutrophils (ELA-2) in magenta.
We next measured urinary PGE2 levels. To control for differences in urine concentration, urinary biomarkers are often normalized to creatinine (Cr). Urinary creatinine excretion rate is assumed to remain constant within and between individuals; however, recent reports indicate that urinary creatinine excretion rate may vary widely between individuals depending on different clinical factors (Tang et al, 2015). Accordingly, Tang et al (2015) suggest both normalized and non-normalized data should be reported. Urinary PGE2 concentration is presented normalized to creatinine (Fig 2E) and in raw values (Fig 2F). To determine if urinary PGE2 is associated with urothelial COX-2 expression, we performed linear regression analysis between PGE2 concentration and percentage of COX-2-positive urothelial cells and observed a robust positive association between urinary PGE2 concentration and percentage of urothelial COX-2–expressing cells (rS = 0.8818 P = 0.0007) (Fig 2G). Urinary PGE2 levels were highest in PNK016, 23, 25, and 27 (Fig 2F and G). Although the urinary PGE2 concentration of PNK016 was high (2,670.7 pg/ml), the percentage of COX-2–positive urothelial cells and neutrophils was relatively low in the biopsied tissue (Fig 2C and D). Because the selection of inflamed region biopsied was based on visible cues, one possible explanation is that the inflamed region biopsied for this patient was not representative of the bladder as a whole and regions with high numbers of COX-2–positive cells or neutrophils were not captured in the biopsy. For this reason, analysis of PGE2 concentrations in the urine, which is less prone to sampling bias, is important. Interestingly, three of the patients with high urinary PGE2 concentrations (PNK016, 23, and 27) were the only patients in this cohort who presented with positive UC on the day of CFT (Table 1).
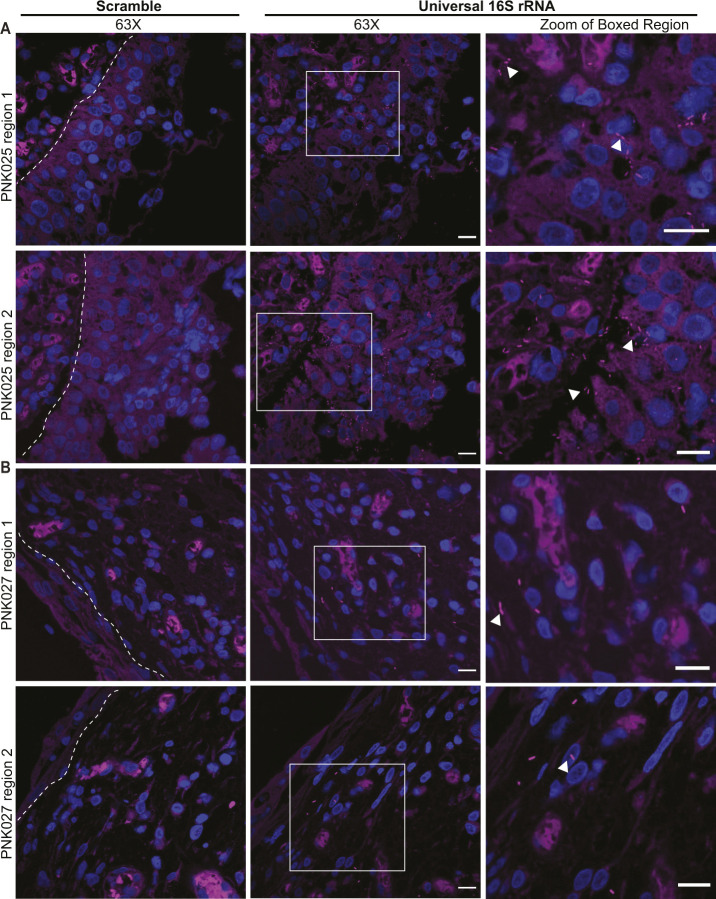
Although sample size is low, one possible interpretation is that urinary PGE2 levels are associated with bacteriuria. We observed a single outlier, PNK025, which was UC negative. We hypothesized that although antibiotics had eliminated urinary bacteria, bacteria may still be present within the bladder wall. To test this hypothesis, we performed FISH with a 16S rRNA universal bacterial probe on all Cohort 2 biopsies (Neugent et al, 2019). Representative images demonstrate the presence of tissue-associated bacteria in the bladder wall of PNK025 and PNK027 (Fig 3A). Quantification of bacterial community size revealed the largest load of tissue-resident bacteria in PNK025 (Fig 3B), suggesting that although the UC for PNK025 was negative, there was a high burden of tissue-resident bacteria (Fig 3A and B). In accordance with previously published observations, we detected suburothelial bacterial communities in the bladder wall of several patients including PNK016-19, 22, 25, and 26 (Figs 3B and S4). Taken together, higher urinary PGE2 levels were associated with urothelial COX-2 expression as well as with a higher bacterial load either in the urine or bladder wall.
Figure 3. FISH detects bladder-resident bacterial communities.
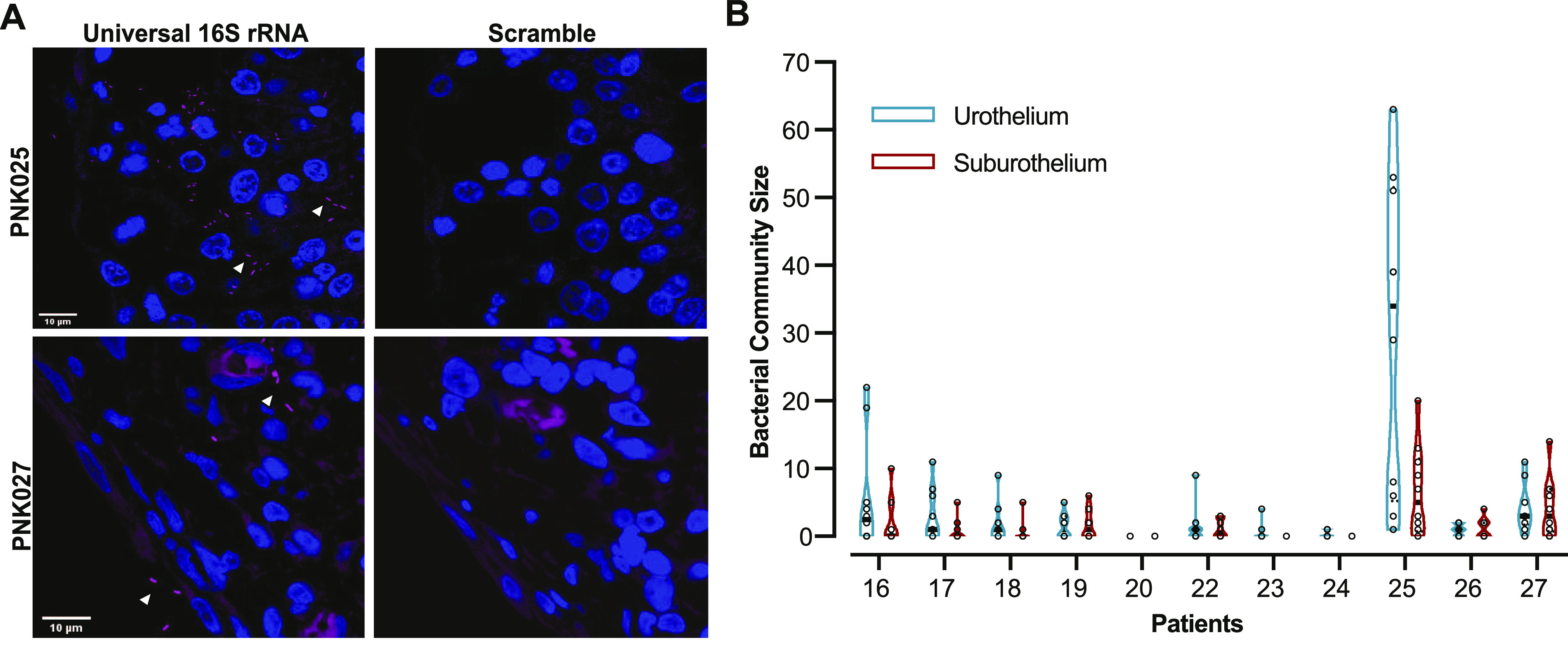
(A) Representative confocal micrograph of FISH performed on PNK025 and PNK027 I1 biopsies using universal 16S rRNA and scramble probes with bacteria in magenta and DNA in blue. White arrowheads point to bacteria. (B) Violin plot of urothelial and suburothelial bacterial community enumeration in each biopsy. 10 randomly sampled images were quantified per biopsy. Individual data points are open circles and black boxes depict the median.
Figure S4. 16S rRNA FISH detection of suburothelial bladder-resident bacteria.
(A, B) Representative confocal micrographs (63×) of FISH performed on (A) PNK025 and (B) PNK027 bladder biopsy sections using scramble (left panels) and universal 16S rRNA probes (center and right panels). Two regions per biopsy are shown for patients PNK025 and PNK027. Right-most column of panels are zoomed-in views of the boxed regions in the center panel. Bacteria are in magenta (Alexa-647) and DNA (Hoechst) in blue. White arrowheads point to bacteria. White dashed line delineates urothelium and suburothelium. Scale bar represents 10 μm.
PGE2 is elevated during rUTI relapse but not during remission
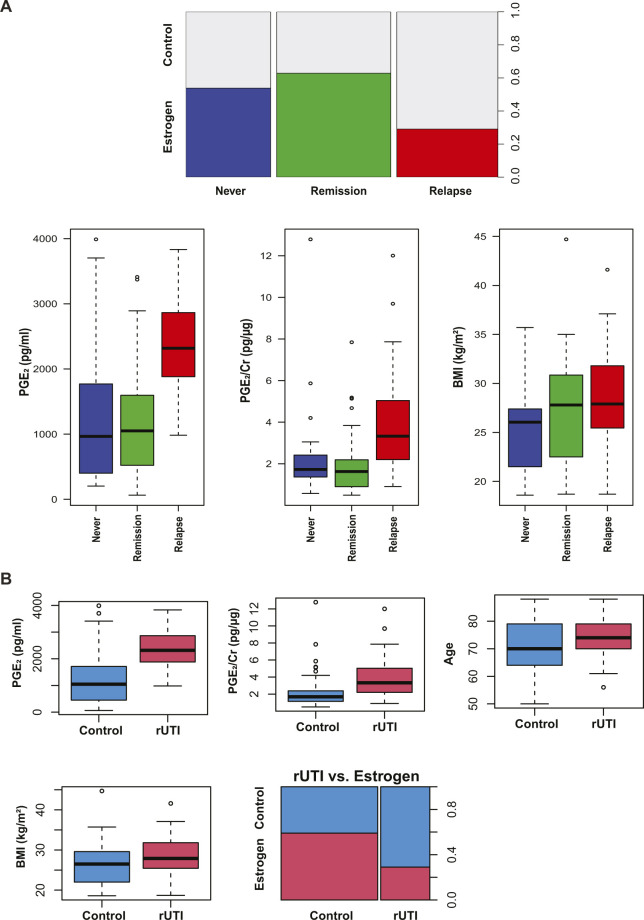
Previous studies have shown that expression of the gene encoding COX-2, Ptgs2, was elevated 24 h postinfection in rUTI-sensitized mice but did not remain elevated during convalescence (Hannan et al, 2014). These data along with our observed association between urinary PGE2 and bacterial load led us to hypothesize that urinary PGE2 may be a biomarker for active rUTI in postmenopausal women. To test this hypothesis, we measured urinary PGE2 in a third cohort (Cohort 3, n = 92) of postmenopausal women. rUTI patients typically oscillate between rUTI remission and relapse (Dason et al, 2011; Corriere et al, 2013). Accordingly, women were stratified into three groups based on their current infection status and rUTI history: Never (no UTI history), Remission (rUTI history, no current rUTI) and Relapse (rUTI history, current UTI) (Fig 4A). The Never group served as a control for normal variations in urinary PGE2 levels between individuals. The Remission group allowed determination of COX-2 activity in the absence of active infection in rUTI-sensitized individuals. None of the women in Cohort 3 were undergoing CFT and active rUTI in the Relapse group was managed by antibiotic therapy. Cohort summary statistics are reported in Table 2.
Figure 4. Urinary PGE2 is a biomarker for active recurrent urinary tract infection (rUTI) and is the best model to predict rUTI relapse in postmenopausal women.
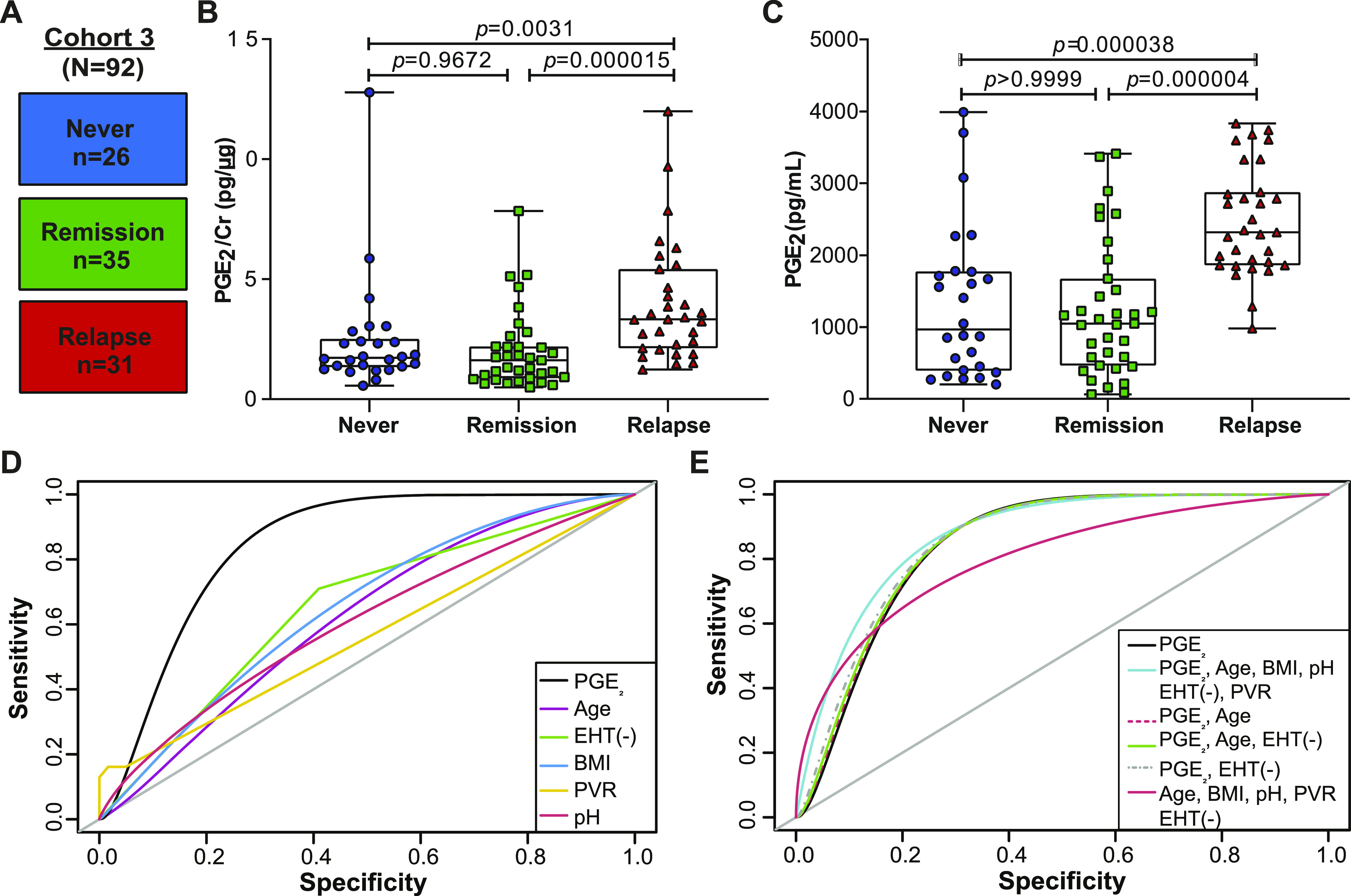
(A) Diagram depicting Cohort 3 design. Patients were stratified intro three cohorts: Never (no clinical history of urinary tract infection [UTI]), Remission (history of rUTI, no current UTI), and Relapse (history of rUTI, current UTI). (B, C) Urinary PGE2 normalized to creatinine and (C) raw urinary PGE2 concentration as measured by ELISA. P-values generated by Kruskal-Wallis test and Dunn’s multiple comparison test. Whiskers drawn to minimum and maximum, boxes represent interquartile range, and median is denoted by a horizontal line. (D) Receiver operating characteristic curves of single-variable logistic regression models illustrate the ability of each model to predict rUTI status. Area under the curve was calculated using leave-one-out cross validation. (E) Receiver operating characteristic curves comparing multivariable logistic regression models to a PGE2-only model for predicting rUTI. Area under the curve was calculated using leave-one-out cross validation.
Table 2.
Cohort 3 summary statistics.
| Never | Remission | Relapse | ||
|---|---|---|---|---|
| Number of women (n) | 26 | 35 | 31 | |
| Hx of fulguration | 0 | 20 | 19 | |
| Median Age (IQR) (yr) | 69.5 (63–77.75) | 70 (63–81) | 74 (70–80) | |
| Median BMI (IQR) (kg/m2) | 26.05 (21.4–27.5) | 27.8 (22–31) | 27.9 (25.4–32.1) | |
| Median urine pH (IQR) | 6 (5–7) | 5.82 (5–6.74) | 5.35 (5–6) | |
| AODM (%) | 1 (3%) | 7 (20%) | 6 (19.35%) | |
| EHT (%) | 14 (53%) | 22 (62%) | 9 (29%) | |
| NSAID | Selective (%) | 2 (7.69%) | 5 (14.28%) | 1 (3.22%) |
| Nonselective (%) | 6 (23.07%) | 7 (20%) | 2 (6.45%) | |
Median and interquartile range (IQR) for age; (BMI) body mass index, and urinary pH. AODM, adult-onset diabetes mellitus; EHT, estrogen hormone therapy; NSAID, nonsteroidal anti-inflammatory drugs.
We observed significantly elevated urinary PGE2 concentration, both raw and normalized to Cr, in Relapse patients as compared to the Remission and Never patients (Fig 4B and C). The median level of PGE2 in the Relapse group was 2,318 pg/ml (Interquartile range [IQR]: 1,859–2,879) compared to 1,050 pg/ml (IQR: 464.5–1,675) and 965.1 pg/ml (IQR: 391.9–1,773) in the Remission and Never groups, respectively (Fig 4C). We observed no significant difference in urinary PGE2 levels between the Remission and Never groups (Fig 4C). These data suggest that during rUTI remission urinary PGE2 returns to basal levels; however, longitudinal studies must be conducted to confirm this result.
PGE2 is a biomarker of active rUTI in postmenopausal women
We then analyzed cohort-associated clinical metadata to identify additional variables associated with group (Never, Remission, Relapse) membership or rUTI status. A total of 16 variables were tested including age, BMI, urine pH, estrogen hormone therapy (EHT), prolapse stage, post void residual (PVR) and diabetes (Tables S1 and S2). Besides urinary PGE2 concentration (P < 0.001) and PGE2/Cr ratio (P < 0.001), BMI was the only variable significantly associated with group membership (P = 0.0161, Fig S5A). PGE2 concentration, PGE2/Cr ratio, and BMI were positively associated with active rUTI, whereas EHT (P = 0.01246) was negatively associated with active rUTI (Table S1 and Fig S5B). We next used logistic regression to detect biomarkers of rUTI status within the clinical metadata (Fig S6). The PGE2 logistic regression model outperformed all other single-variable models in predicting rUTI status with an area under the curve (AUC) of 0.841 (Fig 4D and Table 3). Adding the covariates of age, BMI, EHT, PVR, and urine pH improved the model slightly (AUC = 0.880) but PGE2 was the primary driver of the model as dropping PGE2 from the model reduced the AUC to 0.805 (Fig 4E and Table 3). Taken together, these results indicate that urinary PGE2 is a strong predictor, or biomarker, of rUTI status in this cohort of postmenopausal women.
Figure S5. Significant Clinical Variables for Group and recurrent urinary tract infection comparisons in Cohort 3.
(A) Plots of statistically significant variables comparing Never, Remission, and Relapse groups within Cohort 3. (B) Plots of statistically significant variables comparing active recurrent urinary tract infection group versus Control (no active urinary tract infection). Significant numerical variables are displayed in boxplots and significant categorical variables in stacked bar plots.
Figure S6. Predicted probabilities of recurrent urinary tract infection for Cohort 3 patients.
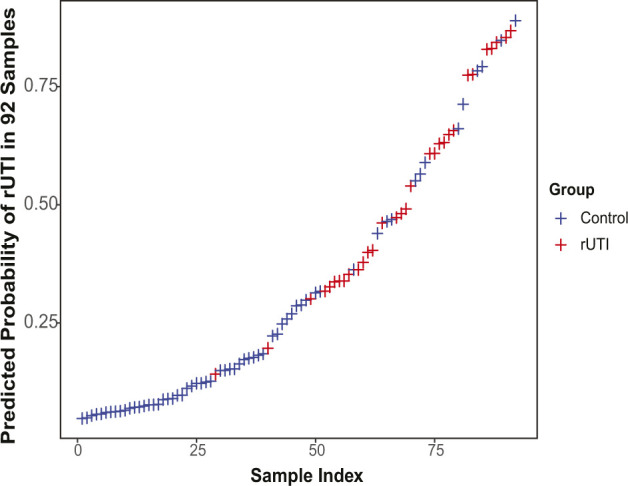
Prediction of recurrent urinary tract infection (red) and Control (blue) for each of the 92 samples of the logistic regression model containing PGE2 as the only explanatory variable.
Table 3.
Summary of cohort-associated clinical metadata analysis to assess the model prediction accuracy.
| Model variables | AUC | F score | Cutoff probability |
|---|---|---|---|
| PGE2 | 0.843 | 0.778 | 0.317 |
| Age | 0.623 | 0.574 | 0.289 |
| Estrogen | 0.65 | 0.564 | 0.468 |
| BMI | 0.627 | 0.594 | 0.266 |
| Post void residual (PVR) | 0.56 | 0.504 | 0.299 |
| pH | 0.606 | 0.538 | 0.304 |
| PGE2, age, BMI, estrogen, PVR, and pH | 0.882 | 0.761 | 0.308 |
| PGE2 and age | 0.844 | 0.75 | 0.224 |
| PGE2, age, and estrogen | 0.848 | 0.737 | 0.268 |
| PGE2, and estrogen | 0.855 | 0.776 | 0.43 |
| Age, BMI, estrogen, PVR, and pH | 0.805 | 0.675 | 0.287 |
Leave-one-out cross-validation (LOOCV) procedure used to calculate the area under the ROC curve (AUC), F-score (predictive accuracy of the model), and the cutoff probability.
PGE2 is associated with active rUTI in postmenopausal women with adult-onset diabetes mellitus (AODM)
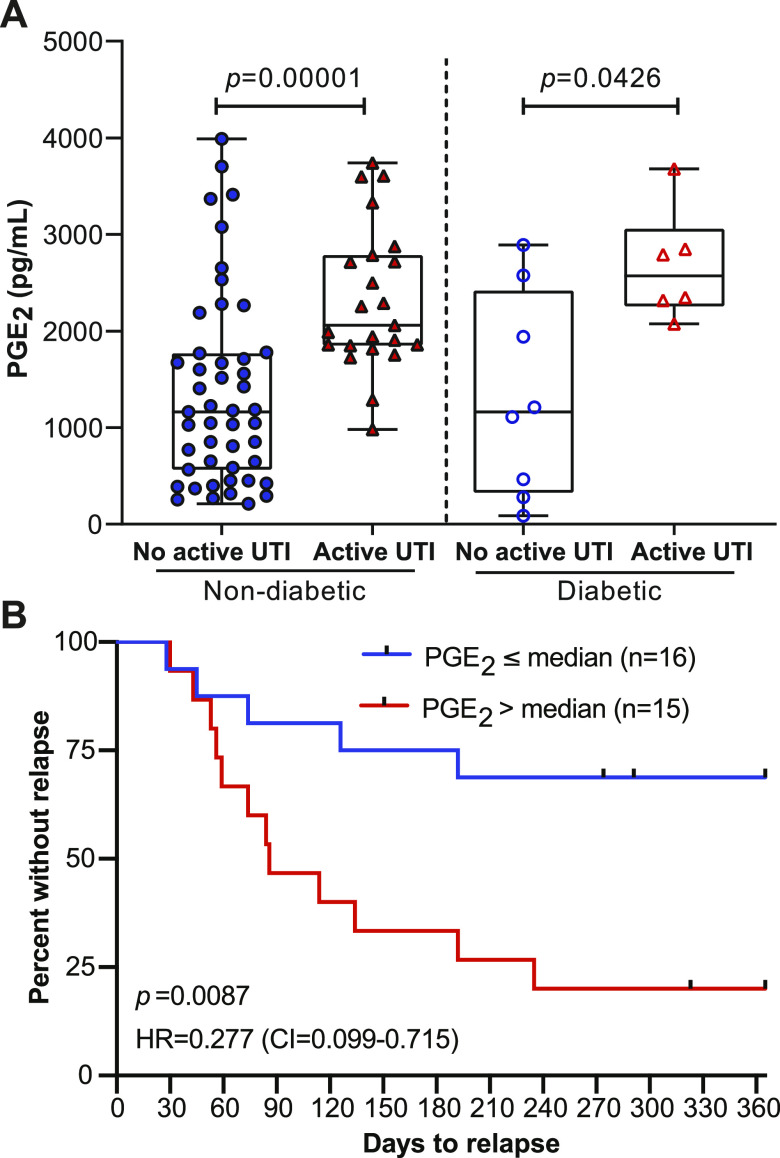
There is a high frequency (22–33%) of AODM in adults over the age of 65 years in the United States and a high incidence of rUTI in individuals with AODM (Kirkman et al, 2012; Corriere et al, 2013; Nitzan et al, 2015). Therefore, a clinically useful biomarker for rUTI would differentiate patients independent of AODM status. We identified Cohort 3 individuals with AODM and divided patients into groups defined by documented AODM diagnosis and UTI status at the time of urine donation: No active UTI and nondiabetic, Active UTI and nondiabetic, No active UTI and diabetic, and Active UTI and diabetic. Urinary PGE2 levels were significantly elevated in the Active UTI versus No active UTI in both diabetic and nondiabetic groups (Fig 5A). These results suggest that urinary PGE2 can be used as a biomarker of rUTI in individuals with and without AODM.
Figure 5. High urinary PGE2 is a prognostic marker for development of recurrent urinary tract infection relapse in a 12-mo follow-up study.
(A) Analysis of the urinary PGE2 concentrations between nondiabetic (filled) and diabetic (empty) No active urinary tract infection (blue circle) versus Active urinary tract infection (red triangle) patients. Mann–Whitney U test used to calculate P-values. (B) Kaplan–Meier analysis of time-to-relapse data for patients in the Relapse group dichotomized about the median PGE2 concentration. Red line depicts above median and blue line below median patients. Data were analyzed by log-rank (Mantel–Cox) test. HR, hazard ratio (below median/above median) and CI = 95% confidence interval.
Association between urinary PGE2 and relevant clinical variables
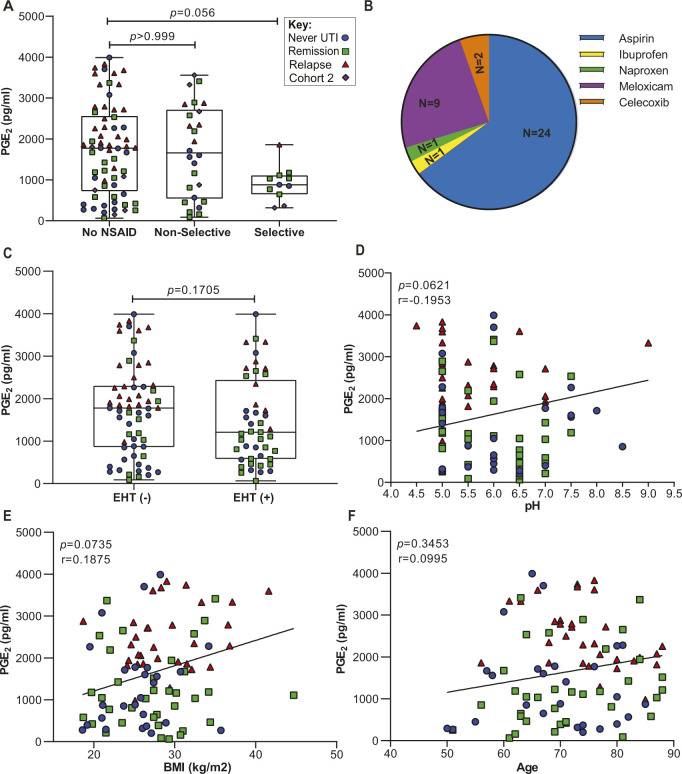
COX-2–mediated inflammation can be affected by many clinically relevant factors, such as, age, BMI, and the common use of nonsteroidal anti-inflammatory drugs (NSAIDs). In contrast to nonselective NSAIDs like ibuprofen, COX-2–selective inhibitors (e.g., Celecoxib) preferentially block COX-2 activity over COX-1 activity and are prescribed to adults for the management of osteoarthritis, rheumatoid arthritis, and acute pain (Schattenkirchner, 1997; Zarghi & Arfaei, 2011; Zweers et al, 2011). We found that 25% of women in Cohort 3 used nonselective or selective NSAIDs (Table 2). To determine if there was any association between NSAID use on urinary PGE2, we classified Cohort 2 and 3 patients into three groups based upon NSAID use: no NSAID, nonselective NSAID and selective NSAID. We observed no statistically significant difference in urinary PGE2 concentrations between these groups (Fig S7A). The distribution of specific NSAIDs used by patients in Cohorts 2 and 3 is reported in Fig S7B.
Figure S7. Correlation analysis between clinical variables and urinary PGE2 concentration.
Clinical variables were assessed to determine extent of correlation with urinary PGE2 concentration. (A) Urinary PGE2 in three groups of patients from Cohort 2 and Cohort 3 using different types of NSAIDs: No NSAID, nonselective, and selective. P-values calculated by Kruskal-Wallis and Dunn’s multiple comparison test. (B) Pie chart representing distribution of NSAIDs used by patients in Cohorts 2 and 3. (C) Association between PGE2 and estrogen hormone therapy in Cohort 3. Modality of estrogen hormone therapy was either vaginal, transdermal patch, or oral. Whiskers drawn to minimum and maximum, boxes represent interquartile range, and median is denoted by a horizontal line. Data analyzed by Mann–Whitney U test. (D, E, F) Spearman’s correlation between raw urinary PGE2 and pH, BMI, and age in Cohort 3. The line depicts the linear regression. The color and shape of the data points indicate original group assignment: Never (Blue circle), Remission (Green square), Relapse (Red triangle), and Cohort 2 (Purple diamond).
EHT has been shown to affect local immune responses (Porter et al, 2001). Since EHT is common among postmenopausal women (Stanton et al, 2020), we evaluated the association between urinary PGE2 and EHT. Although no association was found between EHT and urinary PGE2 concentration, only 29% of patients in the Relapse group used EHT compared to 62% and 53% of patients in the Remission and Never groups, respectively, (Fig S7C and Table 3). Similarly, no association was found between urinary PGE2 and urine pH (Fig S7D), BMI (Fig S7E), and age (Fig S7F).
Urinary PGE2 concentration is predictive of rUTI relapse
To further investigate the association between elevated urinary PGE2 and rUTI, we recorded time to relapse over a 12-mo period for the Relapse group. We dichotomized patients about the median PGE2 concentration into above median (n = 15, >2,318 pg/ml) and below median (n = 16, ≤2,318 pg/ml) groups. Proportional hazard analysis using the Mantel–Cox log-rank test indicated a much higher likelihood of rUTI relapse (HRhigh/low = 3.61, HRlow/high = 0.277 P = 0.0087) in the above median group compared to the below median group (Fig 5B). At the end of the follow-up period, 12/15 patients in the above median group experienced rUTI relapse versus 5/16 patients in the below median group (Fig 5B). These data suggest that urinary PGE2 concentration is predictive of rUTI relapse in this cohort of postmenopausal women.
Discussion
As the human population ages and antimicrobial resistance becomes more widespread, rUTI is becoming both more prevalent and more difficult to manage. Because development of new antibiotics has not kept pace with the rate of emergence of resistant organisms, alternate therapies that improve upon existing antibiotics must be developed (Gupta et al, 1999; Roca et al, 2015). One strategy is to encourage a more productive immune response by controlling excessive inflammation (Ingersoll & Albert, 2013). For rUTI, murine studies have identified COX-2–mediated inflammation as a key sensitizing factor and a possible target for alternate rUTI therapies.
In this study, we demonstrate that the product of COX-2, PGE2, is significantly elevated in the urine of postmenopausal women with active rUTI. Urinary PGE2 concentration outperformed all other clinical variables as a predictor of rUTI status and was not significantly associated with clinical variables other than active rUTI. These findings suggest that urinary PGE2 is a reliable biomarker for active rUTI in postmenopausal women. These observations are supported by previous reports of elevated urinary PGE2 in young patients with UTI before antibiotic therapy (Wheeler et al, 2002). Importantly, we found that elevated PGE2 concentration was strongly predictive of rUTI relapse in the studied cohort of postmenopausal women.
Previous clinical trials have evaluated the noninferiority of ibuprofen compared to antibiotic therapy, and, although over half of the ibuprofen treatment cohort recovered without antibiotic therapy, the incidence of complications like pyelonephritis was higher (Gagyor et al, 2015; Vik et al, 2018). Interestingly, neither nonselective COX inhibitors (i.e., ibuprofen) or selective COX-2 inhibitors (i.e., Celecoxib) have been evaluated in conjunction with antibiotics as adjunct therapies for UTI. Our finding that postmenopausal women with above median levels of urinary PGE2 have a significantly elevated risk of rUTI relapse alongside mechanistic evidence provided by studies in murine models support the hypothesis that high levels of COX-2–mediated inflammation sensitize the bladder to recurrent infection. These findings could be used to inform the design of future clinical trials evaluating the efficacy of adjunct antibiotic and selective COX-2 inhibitor therapies for rUTI.
Materials and Methods
All studies were performed between May 2018 and June 2020 following informed patient consent and institutional review board approval (STU 082010-016, STU 032016-006, MR 17-120).
Cohort 1 and 2 biopsy and urine sample collection
Cold cup biopsies and urine were collected from postmenopausal women under anesthesia undergoing outpatient cystoscopy with fulguration of trigonitis (CFT) for advanced management of antibiotic-refractory rUTI. Exclusion criteria were immunodeficiency, renal insufficiency, urogenital abnormality, and any surgery 1 mo prior. Qualification for antibiotic-refractory rUTI requires presentation of >3 antibiotic class allergies and >3 antibiotic class resistances (Chavez et al, 2020). For Cohort 1, one C1 (no visible cystitis, control) and one I1 (visible cystitis, inflamed) biopsy was obtained (n = 14, PNK001-14) (De Nisco et al, 2019). Cohort 1 patient statistics have been previously reported (De Nisco et al, 2019). For Cohort 2, one I1 biopsy was collected (n = 12, PNK016-27). PNK021 did not pass exclusion criteria. Biopsies were fixed immediately in 4% paraformaldehyde, paraffin-embedded, and longitudinally sectioned using sterile solutions and equipment (De Nisco et al, 2019). Paraffin-embedded normal bladder tissue sections (HUFPT108) were purchased from US Biomax.
Cohort 3 recruitment, urine collection, and classification
92 patients passing exclusion criteria of premenopausal, sporadic UTI, PVR > 150 ml, >stage 2 bladder prolapse, immune suppression, history of catheterization, and surgery less than a month prior were recruited from a tertiary care center into Cohort 3. Clean-catch midstream urine was collected, immediately chilled and processed within 2 h. Urine was handled aseptically and stored in liquid nitrogen. The 92 women were stratified into three groups based on their history of rUTI, UTI symptoms, and urinalysis (UA): Never (no clinical history of symptomatic UTI, n = 26), Remission (history of rUTI, no current UTI symptoms, and −UA, n = 35), and Relapse (history of rUTI, current UTI symptoms, and +UA, n = 31). The women in this cohort were distinct from cohorts 1 and 2 in that their rUTI was not yet refractory to antibiotics and they were therefore not undergoing CFT. For nonsteroidal anti-inflammatory drug (NSAID) classification, patients taking ibuprofen, aspirin or naproxen were placed in the nonselective NSAID group and patients taking Meloxicam or Celecoxib were placed in the selective NSAID group.
PGE2 and creatinine measurement
Urinary PGE2 levels were measured by highly sensitive PGE2 ELISA (Enzo). To inhibit the activity of prostaglandin synthase, 10 μg/ml indomethacin (Alfa Aesar) was added. PGE2 ELISA was performed on diluted urine (1:2) and standards. Optical density was measured at 405 nm with a Synergy H1 plate reader (BioTek). PGE2 concentration was calculated based on the standard curve. Creatinine level was measured in diluted urine (1:20) and standards with the Creatinine Urinary Detection kit (Thermo Fisher Scientific). Absorbance was read at 490 nm and creatinine concentration was calculated based upon the standard curve.
16S rRNA FISH
16S rRNA FISH was performed as described previously (Neugent et al, 2019). Biopsies were fixed immediately upon collection and were processed using sterile reagents and aseptic technique. Briefly, after deparaffinization and rehydration, tissues were incubated with 10 nM Alexa Fluor-647–conjugated probe overnight at 50°C, washed and stained with 1 μg/ml Hoechst (Thermo Fisher Scientific), and mounted.
Immunofluorescence (IF)
Tissues were deparaffinized and rehydrated as described previously (De Nisco et al, 2019). Following antigen retrieval in 10 mM citrate buffer, tissues were blocked in 5% goat serum (Krenacs et al, 2010). IF was performed with primary antibodies against COX-2 (D5H5) 1:500 (rabbit; Cell Signaling) and 2 μg/ml neutrophil elastase (ELA-2, 950334, mouse; Novus). Secondary antibodies Alexa Fluor-555 goat anti-rabbit IgG(H+L) and Alexa Fluor-647 goat anti-mouse IgG(H+L) (Thermo Fisher Scientific) were added to a final concentration of 4 and 2 μg/ml, respectively. Hoechst 33342 final concentration was 1 μg/ml. Alexa Fluor-488 phalloidin and Alexa Fluor-488 Wheat Germ Agglutinin were used at a 1:500 dilution.
Imaging and analysis
For both FISH and IF, confocal micrographs were taken with 63× objectives on a Zeiss LSM880. Images were of a single focal plane and were processed and analyzed with Zen Blue (Zeiss) and ImageJ. 10 randomly sampled images were enumerated from each biopsy section in a blinded manner to calculate the percentage of COX-2 expressing cells and neutrophils present in the urothelium. Cells with a mean fluorescence intensity >10 and integral fluorescence density >1,000 in the Alexa Fluor-555 channel were scored as COX-2 positive. Cells with nuclei surrounded by ELA-2 signal were counted as neutrophils and neutrophils were distinguished from neutrophil extracellular traps by morphology (Brinkmann et al, 2004; Kaplan & Radic, 2012). For 16S rRNA FISH, bacterial communities (Universal 16s rRNA probe) were enumerated in a blinded manner in 10 randomly sampled images taken from a single section of each biopsy and compared with control (Scramble) images taken from the same region of a serial section as previously described (De Nisco et al, 2019).
Time-to-relapse analysis
Time to relapse in the relapse group was assessed by Kaplan–Meier analysis. Patients were dichotomized about the median urinary PGE2 concentration (n = 16 ≤ median, n = 15 > median). Median PGE2 was chosen as the discriminator because it is an unbiased method for dichotomizing a group of individuals. Patients were censored at last follow-up time who did not complete a 12-mo follow-up interval (n = 3). The Mantel–Cox log-rank test was performed to test for a time-to-relapse difference between groups.
Statistical analysis
All statistical analyses were performed in GraphPad Prism 8.1.0 and R Studio version 4.0.2 with an α of 0.05. Hypothesis testing was performed using classical methods. Pairwise associations between continuous variables were performed using Spearman’s rho correlation with P-values generated by permutation. Differences between continuous variables by group were analyzed using ANOVA or t test if normally distributed, or the Mann–Whitney U test, Wilcoxon matched pairs signed rank test, or Kruskal–Wallis test with multiple comparison post hoc if not normally distributed. Differences between continuous variable by group were analyzed using ANOVA, t test, or Kruskal–Wallis test with multiple comparison post hoc. Differences between categorical variables were analyzed using chi-square, Fisher’s exact test, or ordinal logistic regression. Various logistic regression models were constructed to predict rUTI using combinations of all significant covariates. Model fit was assessed using McFadden’s pseudo-R2. The predictive power of each logistic regression model was assessed using F-scores and AUC attained through leave-one-out cross validation. Models were further compared using ANOVA.
Data Availability
All experimental source data are available upon request from the corresponding author.
Supplementary Material
Acknowledgements
This work was funded in part by the Felecia and John Cain Chair in Women’s Health in honor of PE Zimmern, the Welch Foundation grant AT-2030-20200401 (NJ De Nisco), the University of Texas System Rising STARs award (NJ De Nisco), the Welch Foundation grant I-1561 (K Orth), and Once Upon a Time Foundation (K Orth). K Orth is a Burroughs Welcome Investigator in Pathogenesis of Infectious Disease, a Beckman Young Investigator, and a W. W. Caruth, Jr., Biomedical Scholar and has an Earl A. Forsythe Chair in Biomedical Science.
Author Contributions
T Ebrahimzadeh: formal analysis, investigation, visualization, methodology, and writing—original draft.
A Kuprasertkul: data curation, investigation, and methodology.
ML Neugent: formal analysis, visualization, methodology, and writing—original draft.
KC Lutz: formal analysis, visualization, methodology, and writing—original draft.
JL Fuentes: data curation and investigation.
J Gadhvi: investigation and visualization.
F Khan: investigation.
C Zhang: formal analysis.
BM Sharon: validation and investigation.
K Orth: funding acquisition and writing—review and editing.
Q Li: conceptualization, supervision, and validation.
PE Zimmern: conceptualization, supervision, funding acquisition, and writing—review and editing.
NJ De Nisco: conceptualization, supervision, funding acquisition, validation, methodology, writing—original draft, and project administration.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Amna MA, Chazan B, Raz R, Edelstein H, Colodner R (2013) Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection 41: 473–477. 10.1007/s15010-012-0347-1 [DOI] [PubMed] [Google Scholar]
- Bjorling DE, Wang ZY, Bushman W (2011) Models of inflammation of the lower urinary tract. Neurourol Urodyn 30: 673–682. 10.1002/nau.21078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A (2004) Neutrophil extracellular traps kill bacteria. Science 303: 1532–1535. 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- Chavez JA, Christie AL, Zimmern PE (2020) Favorable outcomes of repeat electrofulguration procedures in women with antibiotic-refractory recurrent urinary tract infections. Urology 146: 83–89. 10.1016/j.urology.2020.08.030 [DOI] [PubMed] [Google Scholar]
- Corriere M, Rooparinesingh N, Kalyani RR (2013) Epidemiology of diabetes and diabetes complications in the elderly: An emerging public health burden. Curr Diab Rep 13: 805–813. 10.1007/s11892-013-0425-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivelli JJ, Alhalabi F, Zimmern PE (2019) Electrofulguration in the advanced management of antibiotic-refractory recurrent urinary tract infections in women. Int J Urol 26: 662–668. 10.1111/iju.13963 [DOI] [PubMed] [Google Scholar]
- Dason S, Dason JT, Kapoor A (2011) Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can Urol Assoc J 5: 316–322. 10.5489/cuaj.11214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nisco NJ, Neugent M, Mull J, Chen L, Kuprasertkul A, de Souza Santos M, Palmer KL, Zimmern P, Orth K (2019) Direct detection of tissue-resident bacteria and chronic inflammation in the bladder wall of postmenopausal women with recurrent urinary tract infection. J Mol Biol 431: 4368–4379. 10.1016/j.jmb.2019.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis AK, Verma S (2000) Quality of life in women with urinary tract infections: Is benign disease a misnomer? J Am Board Fam Pract 13: 392–397. 10.3122/15572625-13-6-392 [DOI] [PubMed] [Google Scholar]
- Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ (2015) Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13: 269–284. 10.1038/nrmicro3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxman B (2002) Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am J Med 113: 5S–13S. 10.1016/s0002-9343(02)01054-9 [DOI] [PubMed] [Google Scholar]
- Foxman B (2014) Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am 28: 1–13. 10.1016/j.idc.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Gágyor I, Bleidorn J, Kochen MM, Schmiemann G, Wegscheider K, Hummers-Pradier E (2015) Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: Randomised controlled trial. BMJ 351: h6544. 10.1136/bmj.h6544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover M, Moreira CG, Sperandio V, Zimmern P (2014) Recurrent urinary tract infections in healthy and nonpregnant women. Urol Sci 25: 1–8. 10.1016/j.urols.2013.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K, Scholes D, Stamm WE (1999) Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA 281: 736–738. 10.1001/jama.281.8.736 [DOI] [PubMed] [Google Scholar]
- Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ (2010) Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog 6: e1001042. 10.1371/journal.ppat.1001042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan TJ, Roberts PL, Riehl TE, van der Post S, Binkley JM, Schwartz DJ, Miyoshi H, Mack M, Schwendener RA, Hooton TM, et al. (2014) Inhibition of cyclooxygenase-2 prevents chronic and recurrent cystitis. EBioMedicine 1: 46–57. 10.1016/j.ebiom.2014.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll MA, Albert ML (2013) From infection to immunotherapy: Host immune responses to bacteria at the bladder mucosa. Mucosal Immunol 6: 1041–1053. 10.1038/mi.2013.72 [DOI] [PubMed] [Google Scholar]
- Jancel T, Dudas V (2002) Management of uncomplicated urinary tract infections. West J Med 176: 51–55. 10.1136/ewjm.176.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MJ, Radic M (2012) Neutrophil extracellular traps: Double-edged swords of innate immunity. J Immunol 189: 2689–2695. 10.4049/jimmunol.1201719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y (2015) Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim Biophys Acta 1851: 414–421. 10.1016/j.bbalip.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS, et al. (2012) Diabetes in older adults: A consensus report. J Am Geriatr Soc 60: 2342–2356. 10.1111/jgs.12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenacs L, Krenacs T, Stelkovics E, Raffeld M (2010) Heat-induced antigen retrieval for immunohistochemical reactions in routinely processed paraffin sections. Methods Mol Biol 588: 103–119. 10.1007/978-1-59745-324-0_14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers AM, van de Kerkhof PC, Schalwijk J, Mier PD (1986) Elastase, a marker for neutrophils in skin infiltrates. Br J Dermatol 115: 181–186. 10.1111/j.1365-2133.1986.tb05715.x [DOI] [PubMed] [Google Scholar]
- Malik RD, Wu YR, Christie AL, Alhalabi F, Zimmern PE (2018. a) Impact of allergy and resistance on antibiotic selection for recurrent urinary tract infections in older women. Urology 113: 26–33. 10.1016/j.urology.2017.08.070 [DOI] [PubMed] [Google Scholar]
- Malik RD, Wu YR, Zimmern PE (2018. b) Definition of recurrent urinary tract infections in women: Which one to adopt? Female Pelvic Med Reconstr Surg 24: 424–429. 10.1097/SPV.0000000000000509 [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Rosenberg DW (2013) Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol 35: 123–137. 10.1007/s00281-012-0342-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugent ML, Gadhvi J, Palmer KL, Zimmern PE, De Nisco NJ (2019) Detection of tissue-resident bacteria in bladder biopsies by 16S rRNA fluorescence in situ hybridization. J Vis Exp e60458. 10.3791/60458 [DOI] [PubMed] [Google Scholar]
- Nicolle LE (2001) A practical guide to antimicrobial management of complicated urinary tract infection. Drugs Aging 18: 243–254. 10.2165/00002512-200118040-00002 [DOI] [PubMed] [Google Scholar]
- Nitzan O, Elias M, Chazan B, Saliba W (2015) Urinary tract infections in patients with type 2 diabetes mellitus: Review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes 8: 129–136. 10.2147/DMSO.S51792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien VP, Hannan TJ, Schaeffer AJ, Hultgren SJ (2015) Are you experienced? Understanding bladder innate immunity in the context of recurrent urinary tract infection. Curr Opin Infect Dis 28: 97–105. 10.1097/QCO.0000000000000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien VP, Hannan TJ, Yu L, Livny J, Roberson ED, Schwartz DJ, Souza S, Mendelsohn CL, Colonna M, Lewis AL, et al. (2016) A mucosal imprint left by prior Escherichia coli bladder infection sensitizes to recurrent disease. Nat Microbiol 2: 16196. 10.1038/nmicrobiol.2016.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Pillinger MH, Abramson SB (2006) Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin Immunol 119: 229–240. 10.1016/j.clim.2006.01.016 [DOI] [PubMed] [Google Scholar]
- Porter VR, Greendale GA, Schocken M, Zhu X, Effros RB (2001) Immune effects of hormone replacement therapy in post-menopausal women. Exp Gerontol 36: 311–326. 10.1016/s0531-5565(00)00195-9 [DOI] [PubMed] [Google Scholar]
- Raz R (2011) Urinary tract infection in postmenopausal women. Korean J Urol 52: 801–808. 10.4111/kju.2011.52.12.801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J, Ballarini S, Mascarenhas T, Zahran M, Quimper E, Choucair J, Iselin CE (2014) Recurrent lower urinary tract infections have a detrimental effect on patient quality of life: A prospective, observational study. Infect Dis Ther 4: 125–135. 10.1007/s40121-014-0054-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA (2011) Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 31: 986–1000. 10.1161/ATVBAHA.110.207449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca I, Akova M, Baquero F, Carlet J, Cavaleri M, Coenen S, Cohen J, Findlay D, Gyssens I, Heure OE, et al. (2015) The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect 6: 22–29. 10.1016/j.nmni.2015.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattenkirchner M (1997) Meloxicam: A selective COX-2 inhibitor non-steroidal anti-inflammatory drug. Expert Opin Investig Drugs 6: 321–334. 10.1517/13543784.6.3.321 [DOI] [PubMed] [Google Scholar]
- Stamm WE, Norrby SR (2001) Urinary tract infections: Disease panorama and challenges. J Infect Dis 183: S1–S4. 10.1086/318850 [DOI] [PubMed] [Google Scholar]
- Stanton A, Mowbray C, Lanz M, Brown K, Hilton P, Tyson-Capper A, Pickard RS, Ali ASM, Hall J (2020) Topical estrogen treatment augments the vaginal response to Escherichia coli flagellin. Sci Rep 10: 8473. 10.1038/s41598-020-64291-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang KW, Toh QC, Teo BW (2015) Normalisation of urinary biomarkers to creatinine for clinical practice and research: When and why. Singapore Med J 56: 7–10. 10.11622/smedj.2015003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vik I, Bollestad M, Grude N, Bærheim A, Damsgaard E, Neumark T, Bjerrum L, Cordoba G, Olsen IC, Lindbæk M (2018) Ibuprofen versus pivmecillinam for uncomplicated urinary tract infection in women-A double-blind, randomized non-inferiority trial. PLoS Med 15: e1002569. 10.1371/journal.pmed.1002569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA, Hausladen DA, Yoon JH, Weiss RM (2002) Prostaglandin E2 production and cyclooxygenase-2 induction in human urinary tract infections and bladder cancer. J Urol 168: 1568–1573. 10.1097/01.ju.0000030583.31299.80 [DOI] [PubMed] [Google Scholar]
- Wu YR, Rego LL, Christie AL, Lavelle RS, Alhalabi F, Zimmern PE (2016) Recurrent urinary tract infections due to bacterial persistence or reinfection in women-does this factor impact upper tract imaging findings? J Urol 196: 422–428. 10.1016/j.juro.2016.01.111 [DOI] [PubMed] [Google Scholar]
- Yu L, O’Brien VP, Livny J, Dorsey D, Bandyopadhyay N, Colonna M, Caparon MG, Roberson ED, Hultgren SJ, Hannan TJ (2019) Mucosal infection rewires TNFɑ signaling dynamics to skew susceptibility to recurrence. Elife 8: e46677. 10.7554/eLife.46677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarghi A, Arfaei S (2011) Selective COX-2 inhibitors: A review of their structure-activity relationships. Iran J Pharm Res 10: 655–683. 10.22037/ijpr.2011.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweers MC, de Boer TN, van Roon J, Bijlsma JW, Lafeber FP, Mastbergen SC (2011) Celecoxib: Considerations regarding its potential disease-modifying properties in osteoarthritis. Arthritis Res Ther 13: 239. 10.1186/ar3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All experimental source data are available upon request from the corresponding author.