Abstract
Obesity is a significant public health concern associated with high morbidity. Obese patients are at risk of severe COVID-19 infection, and obesity is a high-risk factor for admission to the intensive care unit. We aimed to write a narrative review of cardiac and pulmonary pathophysiological aspects of obese patients in the context of COVID-19 infection. Obesity affects lung volume, with a decrease in expiratory reserve volume, which is associated with a decrease in lung and chest wall compliance, an increase in airway resistance, and an increase in work of breathing. Obesity affects cardiac structure and hemodynamics. Obesity is a risk factor for hypertension and cardiovascular disorders. Moreover, obesity is associated with a low-grade inflammatory state, endothelial dysfunction, hyperinsulinemia, and metabolic disorders. Obesity is associated with severe COVID-19 and invasive mechanical ventilation. These previous cardiopulmonary pathological aspects may explain the clinical severity in obese patients with COVID-19. Obese patients are at risk of severe COVID-19 infection. Understanding cardiorespiratory pathophysiological aspects may help physicians manage patients in hospitals.
Keywords: COVID-19, Obesity, BMI, Heart, Lung, Severity
Introduction
Obesity, defined according to a body mass index (BMI) ≥30 kg/m2, is a significant public health problem with high morbidity and mortality [1]. Obesity is also associated with early mortality [2]. Obesity affects not only adults but also adolescents, with an estimated prevalence of 17% among children and adolescents in the USA [3]. Moreover, the prevalence of obesity is as high as 35% in adults [4]. Obesity is associated with endothelial dysfunction, hyperinsulinemia, a low-grade inflammatory state, cardiovascular risk factors, and cardiovascular events [5, 6]. In fact, the risk of hypertension is significantly higher among individuals with obesity [5], and obesity is associated with diabetes mellitus, dyslipidemia, cardiovascular disease, and stroke [5, 7]. In parallel, obesity affects the respiratory system with a reduction in lung capacity and compliance [8]. COVID-19 is relatively new, and we are studying it extensively. There have been concerns related to obese patients given what we know about the pathophysiology of this viral infection as well as obesity [9]. These concerns have led to a number of observation-based scientific investigations, and the evidence is growing that obese patients are at risk of severe COVID-19 [9–14]. In the USA, the prevalence of obesity in ICU patients may reach 46% [15], and in France, the prevalence of obesity reaches 25% in patients with severe COVID-19 [11]. Mortality is higher in obese intensive care unit (ICU) patients [16, 17] and remains higher in young obese patients [14].
We aimed to review the cardiorespiratory pathophysiological aspects involved in obesity in the context of COVID-19 and to propose clarifications in the clinical management of obese patients suffering from COVID-19.
Lung Function and Obesity
COVID-19 particularly affects the lungs with severe pneumonia and sometimes acute respiratory distress syndrome (ARDS) [18, 19]. The presence of heart failure, diabetes, a BMI >40 kg/m2, and older age are factors associated with critical illness severity [17]. SARS-CoV-2 has a high affinity for the lung via the ACE2 receptor present in respiratory epithelial cells [20]. One can assume that the underlying lung function status may worsen clinical status in the context of obesity because of a decrease in lung compliance, a decrease in chest wall compliance, and the recumbent position worsening respiratory compliance [21]. In fact, in the supine position, the reduction in the expiratory reserve volume (ERV) worsens due to the ascending of the diaphragm within the thorax and causes a length-tension disadvantage for the diaphragm due to fiber overstretching [22]. Patients with obesity disclose lower lung volumes, including reductions in forced vital capacity (VC), forced expiratory volume in one second (FEV1), and expiratory reserve volume (ERV) [8]. This is associated with an increase in airway resistance and an impairment of upper airway mechanics [22, 23]. Obese patients have to overcome this previous airway increase. Inspiratory time is also decreased with an increase in the respiratory rate [22, 24]. Abdominal obesity is also associated with lung volume reduction with a negative association between forced vital capacity (FVC) and abdominal adiposity [25].
In obese patients, the work of breathing is increased [23], and diaphragm motion is impeded by abdominal adipose tissue. Expiratory flow limitation leads to dynamic hyperinflation, creating an autoPEEP in relation to incomplete expiration [26]. Additionally, the strength of respiratory muscles is affected in obese individuals [27, 28]. Finally, in the context of obesity, lung bases are underventilated but overperfused, creating a ventilation-perfusion mismatch [29]. In addition, obesity may be associated with a mild widening of the A-aO2 gradient [30, 31]. Hypoxemia and the A-aO2 gradient are correlated with ERV [22].
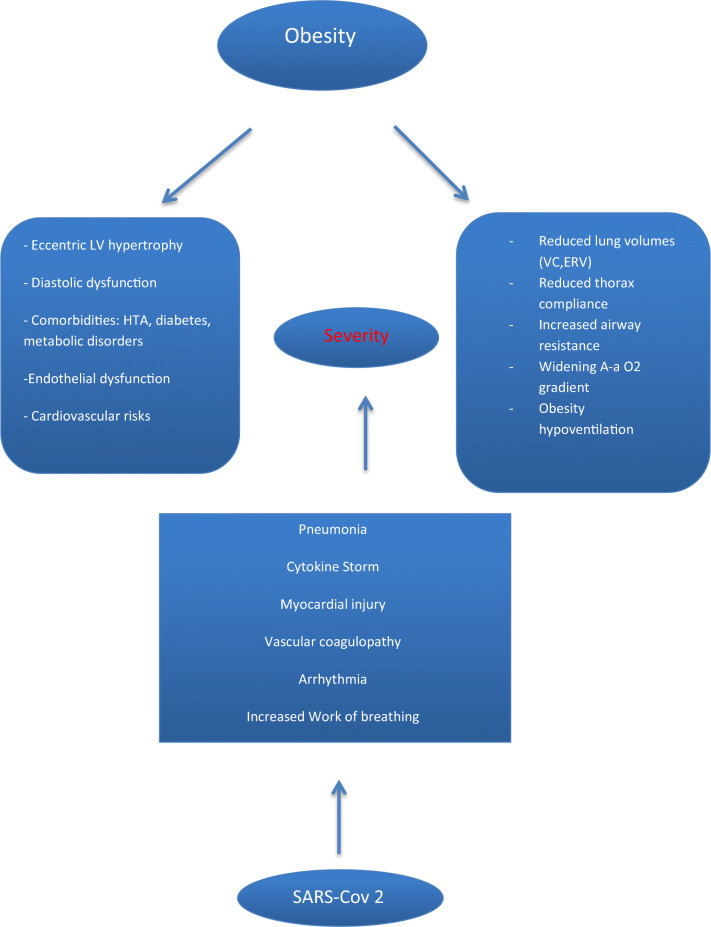
Obesity is also a risk factor for obstructive sleep apnea, and obesity hypoventilation syndrome is frequent. Obstructive sleep apnea can cause cardiovascular features that include hypertension, endothelial dysfunction, metabolic disorders, arrhythmia, and cardiac remodeling [32]. Obesity hypoventilation syndrome (OHS) is defined as a daytime PaCO2>45 mmHg associated with sleep-related breathing disorders in patients with BMI≥30 kg/m2 in the absence of known causes of hypoventilation [32]. The prevalence of OHS has been reported to range between 8 and 20% in obese patients, and the prevalence of hypertension reaches 55 to 88% [33]. Other comorbidities associated with OHS are metabolic derangement, coronary artery disease, and heart failure [33]. Figure 1 summarizes respiratory and cardiac pathophysiological aspects of obesity and COVID-19.
Fig. 1.
Pathophysiological aspects in obese patients. HTA: hypertension; VC: vital capacity; ERV: expiratory reserve volume
Mechanisms involved in respiratory injury in COVID-19 are multifactorial and include endothelial dysfunction, pulmonary vasoconstriction, ventilation/perfusion mismatch, microthrombosis, and macrothrombosis leading to an increase in dead space [34–36]. Collapsed alveoli and pulmonary injury edema may occur, and the persistence of respiratory effort can cause self-induced lung injury [36].
One can assume that the presence of these previous comorbidities in obese patients may worsen the clinical status of obese patients in the context of pulmonary COVID-19.
Heart Health in the Context of COVID-19 and Obesity
In obese individuals, diabetes mellitus, hypertension, insulin resistance, and dyslipidemia are common, leading to an increased risk of cardiovascular disease [7]. Metabolic disorders are associated with coronary microvascular impairment with a reduced coronary flow reserve, particularly in patients with diabetes and lipid profile abnormalities [37]. Patients with cardiovascular risk factors are at risk of severe COVID-19 infection resulting in myocardial injury, acute coronary syndrome, arrhythmia, heart failure, and microvascular thrombosis [18, 19, 38]. In addition, patients with COVID-19 may have alterations of the left ventricle and the right ventricle [39]. Pulmonary hypertension may also be present in the context of COVID-19 infection [40]. Myocardial abnormalities can be present, even after COVID-19 infection recovery, as previously shown [41–43]. There are multiple mechanisms involved in the onset of myocardial injury in the context of COVID-19 infection, including hypoxemia, cytokine storms, microvascular thrombi, coronary plaque instability, systemic and vessel inflammation, myocarditis, sepsis-related cardiomyopathy, stress-related cardiomyopathy, and arrhythmia [44, 45].
In the context of the COVID-19 outbreak, clinical severity may be partially attributable to the underlying myocardial abnormalities, metabolic disorders, and pre-existing cardiovascular disorders in obese patients. A subset of meta-analyses have recently been published, underscoring the association between obesity and critical illness in terms of the need for ICU admission or invasive mechanical ventilation and mortality from COVID-19 [46–49]. In the meta-analysis by Yang et al [47], obesity was associated with COVID-19 severity (OR 2.31; 95% CI, 1.3–4.12). In the meta-analysis by Chu et al [48], obesity was associated with severe COVID-19 (OR 4.17, 95% CI, 2.32–7.48) and invasive mechanical ventilation (OR 2.13, 95% CI 1.10–4.14). The association between obesity and severity is particularly relevant in younger obese COVID-19 patients (OR 3.3) [48]. Zhao et al [49] reported an OR of 2.07 (95% CI 1.53–2.81) between obesity and severe outcomes of COVID-19. Finally, obesity was found to be a risk factor for COVID-19 severity independent of cardiovascular disorders and diabetes [50].
From a mechanical point of view, obesity affects the left ventricular (LV) structure and function in the presence of subclinical myocardial impairments [51]. In fact, adaptive mechanisms occur in obese patients with an increase in several cardiac parameters: LV mass, stroke volume, cardiac output, and total and central blood volume [52, 53]. These hemodynamic changes may lead to LV dilation and to the onset of eccentric LV hypertrophy. In a multiethnic cohort study, obesity was found to be associated with LV concentric remodeling, and Turkbey et al [51] found a positive association between an index of LV geometry (LV mass-to-LV end-diastolic volume) and BMI. In fact, BMI is associated with LV tissue Doppler imaging velocities and global longitudinal strain features [54]. Diastolic function is also affected in patients with obesity with abnormal pulsed mitral inflow, abnormal tissue Doppler velocities, and decreased longitudinal strain [54, 55]. The prevalence of diastolic dysfunction may reach 19% in obese patients [56]. Furthermore, obesity affects left atrial (LA) size, LA reservoir, and conduit [57], with a significant correlation between LA size and LV mass [58]. In this context, obesity increases the risk of heart failure and the risk of atrial fibrillation [59]. In a long-term follow-up study (>20 years) that included 15,402 individuals, obesity was found to be significantly associated with cardiovascular hospital admissions and cardiovascular deaths [60]. Right-heart hemodynamics may also be impaired in a severely obese state, with increased right atrial and pulmonary pressure [61]. Obesity is also a risk factor for venous thromboembolism [62]. Conversely, weight loss is associated with positive cardiac effects that included improvement in cardiac systolic and diastolic function and reductions in LV mass, stroke volume, filling pressures, and resting oxygen consumption [63].
Finally, abdominal obesity, rather than general obesity, may have a high accuracy for predicting cardiac features [64]. In fact, an increase in abdominal obesity is associated with LV global longitudinal strain abnormalities and heart failure [55, 65]. Abdominal obesity is frequently associated with dyslipidemia, low high-density lipoprotein cholesterol, increased triglycerides, apolipoprotein B levels, insulin resistance, atherogenic abnormalities, and cardiovascular diseases [66]. Figure 1 summarizes cardiac and pulmonary pathophysiological aspects involved in obesity and COVID-19.
Obesity, Hypertension, Inflammation, Angiotensin 1-7, and COVID-19
Obese individuals frequently develop hypertension in relation to hyperactivation of the renin angiotensin aldosterone system (RAAS) [5]. The RAAS plays a key role in humans, controlling blood pressure, electrolyte balance, and cardiac remodeling. Angiotensin II is locally produced by abdominal adipose tissue [5]. Additionally, the sympathetic nervous system is activated in obese individuals, leading to hypertension, whereas parasympathetic tone is reduced [67]. Obesity is associated with an increase in renal sodium reabsorption [67]. This increased renal sodium reabsorption plays a key role in the onset of blood pressure increase in obese individuals [67]. Another mechanism involved in the onset of hypertension is the physical compression of kidneys in the context of obesity that impacts intrarenal pressure and natriuresis pressure [68]. Arterial stiffening occurs early in obese individuals and may precede systolic hypertension [68]. Blood flow reserve is reduced in obese individuals, which limits blood flow during exercise [67]. Finally, microcirculatory dysfunction may induce the onset of hypertension in obese individuals [5].
In parallel to neurohormonal modifications, low-grade chronic inflammation is present in obese patients and is related to adipose tissue dysfunction [69]. In obese individuals, proinflammatory cytokines, including interleukin (IL) 1, IL-6, IL-8, and TNF-alpha, are increased, whereas anti-inflammatory cytokines, including IL-10 and adiponectin, are decreased [69]. Leptin and adipokines are increased in obese individuals, whereas adiponectin is reduced [70].
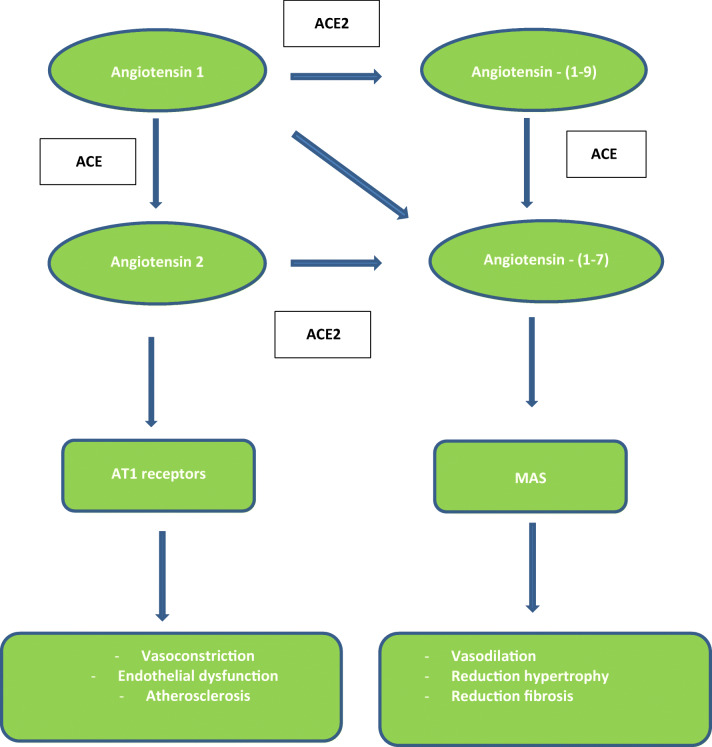
Locally, adipose tissue may play a role in COVID-19 infection severity. Adipocytes seem to be significant targets for SARS-CoV-2 entry via ACE2 receptors and might play a role as a viral reservoir [71]. Adipose tissue expresses ACE2, which catalyzes the conversion of angiotensin 2 to angiotensin (1-7) and displays an opposition to the proinflammatory, vasoconstrictive, and proatherosclerotic properties of angiotensin-2 [72]. These properties involve the ACE2/ang-(1-7)/MAS axis, with MAS being a G protein–coupled receptor for angiotensin (1-7) (Fig. 2). Dysfunction of the MAS can lead to endothelial dysfunction, hypertension, thrombosis, and metabolic syndrome–like states [72, 73].
Fig. 2.
Renin angiotensin system and angiotensin- [1–7]/Mas axis. ACE2: angiotensin converting enzyme type 2
In the context of COVID-19 infection, angiotensin (1-7) production can decrease in patients, contributing to clinical severity [74]. Insulin resistance, which can be assessed by the leptin/adiponectin ratio, may be a potential link between COVID-19 infection and clinical severity in obese patients [75]. Furthermore, the immunological system is overactivated in an inflammatory state and in the context of the cytokine release syndrome in COVID-19 patients [76]. In fact, patients with COVID-19 display a high level of IL-6, which is significantly associated with disease severity [76]. Deceased patients with COVID-19 disclosed higher concentrations of IL-6, IL-8, IL-10, and tumor necrosis factor (TNF)-alpha [77].
Clinical Implications for the Management of Obese COVID-19 Patients
Given the findings discussed so far, clinicians may consider not only classic tools used to stratify patients with pneumonia (for example, CURB-69 score) but also adipose tissue status. In patients with COVID-19 pneumonia, BMI may be assessed, and the need for cardiorespiratory monitoring may be recommended. Severe COVID-19 may affect not only lung status but also cardiovascular function, which can lead to acute myocardial injury, myocarditis, arrhythmia, heart failure, thromboembolic events, and cardiogenic shock.
Regarding the respiratory system, blood oxygen saturation, respiratory rate, and blood gas exchange need to be monitored. Identifying excessive respiratory effort is essential to adapt supportive oxygen therapy. High-flow nasal cannulas can be used as first-line therapy in patients with respiratory insufficiency since they may prevent invasive intubation [78]. Severe patients with obstructive sleep apnea and chronic obstructive pulmonary disease may benefit from noninvasive positive pressure ventilation [79]. The prone position may be used in selected nonintubated patients with severe respiratory forms [80]. Otherwise, it may be useful to avoid supine positions. In the context of COVID-19 infection, an imbalance between the demand on the respiratory muscles and their capacity to generate tension may worsen respiratory symptoms in obese patients [81]. In addition, since the rest gradient A-aO2 is mildly widening and because the gradient A-aO2 is associated with pneumonia severity [82], one may expect that obese COVID-19 patients would experience severe gradient A-aO2. Furthermore, thromboembolism events can be more common in obese patients with COVID-19 since obesity and COVID-19 are two risk factors for thromboembolic events [83, 84]. In this context, anticoagulation therapies should be used, particularly in obese patients, to avoid thromboembolic events.
In the ICU, for critically ill obese patients requiring invasive mechanical ventilation, difficulties in airway management during the induction-intubation phase need to be anticipated. Regarding the ventilation setting, tidal volume should be adapted using the predicted body weight [85]. Obese patients are at high risk of atelectasis during anesthesia in the supine position [86]. In fact, when under anesthesia and in the supine position, the large mass of abdominal adipose tissue leads to a cranial displacement of the diaphragm that can contribute to the onset of atelectasis [86]. Positive end-expiratory pressure (PEEP), which prevents atelectasis and is also used in patients with ARDS, should be adapted since PEEP can cause hemodynamic impairment and right ventricle dysfunction and can impede venous return [87, 88]. Monitoring plateau pressure and driving pressure is crucial, in addition to the search for the presence of intrinsic PEEP in patients on mechanical ventilation [85]. In fact, during mechanical ventilation, monitoring transpulmonary pressure, which is the difference between alveolar pressure and pleural pressure, can be useful to adapt ventilation settings and to avoid lung collapse [86]. Both obesity and ARDS result in elevated alveolar collapse and hypoxemia, although the mechanisms differ (pulmonary and extrapulmonary causes). The use of esophageal pressure measurements helps to separate the two mechanisms and therefore to optimize the PEEP level and insufflation to recruit alveoli and avoid overdistension of the lungs. Indeed, airway opening pressure and esophageal pressure measurements during an expiratory pause allow PEEP to be titrated to maintain the end-expiratory transpulmonary pressure (airway pressure–esophageal pressure) just above 0 cmH2O and avoid alveolar collapse (because in the supine position, the heart superior to the esophageal balloon falsely elevates the esophageal pressure) [89]. Similarly, because obesity participates in an increase in the airway opening plateau without inducing lung overdistension, in the obese population, it is more appropriate to adjust the driving pressure according to a transpulmonary plateau pressure threshold (usually less than 25 cmH2O) rather than an airway opening plateau pressure threshold (usually less than 30 cmH2O) [89].
Regarding the cardiovascular system, cardiovascular comorbidities, including hypertension and diabetes, are often present in obese patients. Cardiac function needs to be monitored. Diastolic function needs to be particularly evaluated in the context of COVID-19 pneumonia. Diastolic dysfunction is relatively frequent in critically ill patients in the context of sepsis and myocardial ischemia [90, 91]. Classic risk factors associated with left ventricular diastolic dysfunction are age, hypertension, obesity, coronary disease, arrhythmia, and kidney disease [92]. Cardiac biomarkers and electrocardiograms should be monitored to stratify patients and to search for eventual cardiovascular complications since these parameters affect prognosis [18, 72, 93]. Echocardiography that must be performed with health worker measures in place can help to stratify patients focusing on LV geometry, LV function, diastolic function, RV function, and arterial pulmonary pressures. In patients on invasive ventilation and particularly patients with ARDS, it is essential to monitor right ventricular function and right pulmonary pressure since acute core pulmonale is frequent in this population [94].
The weaning process in the ICU population requires specific considerations. Weaning success depends on cardiac function, respiratory function, and clinical status. Weaning failure is associated with an increase in morbidity, nosocomial pneumonia, severe outcomes, and mortality [95]. Weaning failure may be due to heart failure, respiratory muscle weakness, and sepsis. During the weaning process, the shift from mechanical ventilation to spontaneous breathing induces a negative intrathoracic pressure that can affect cardiac function [96]. This negative intrathoracic pressure caused by the transition from mechanical ventilation to spontaneous breathing increases venous return and left ventricular afterload and may cause ischemia, as reported initially by Lemaire et al [97]. In addition, adrenergic tone increases during spontaneous breathing trials and myocardial oxygen demand increases [96, 97].
ARDS is relatively frequent in severe COVID-19 patients and patients with severe ARDS experience a long ICU stay [79]. This parameter is known to affect diaphragm function. Since respiratory muscle inspiratory strength may be affected in obese patients, one may expect to have severe diaphragm function dysfunction in obese COVID-19 patients and a longer ICU length of stay.
Diaphragm ultrasound can be used to assess and monitor diaphragm function in this context. Diaphragm dysfunction is a classic cause of respiratory-related weaning failure. Diaphragm dysfunction can be evaluated using ultrasound by measuring diaphragm thickening and/or diaphragm motion during a spontaneous breathing trial (Fig. 3) [96].
Fig. 3.
Right diaphragmatic motion using ultrasound in a COVID-19 patient with obesity. Note the physiological displacement of the diaphragm during inspiration
Echocardiography should be performed to search for left ventricular impairment and left ventricular diastolic dysfunction combined with diaphragm ultrasound [98]. Diastolic dysfunction is a classic cause of weaning failure in ICU patients [99]. Liu et al [99] reported an incidence of weaning failure reaching 45% (among which 59% experienced weaning-induced pulmonary edema). Risk factors for weaning-induced pulmonary edema are obesity, previous cardiomyopathy, and COPD [99]. Physicians should be careful in the context of COVID-19 since myocardial ischemia and myocardial injury are frequent in critically ill COVID-19 patients [100] and myocardial ischemia can occur during the weaning process in ICU patients [101]. Plasma BNP level and plasma BNP variation during a spontaneous breathing trial can be used to predict weaning failure [102]. Finally, COPD can be associated with obesity [103]. COPD patients are at risk of weaning failure. In fact, in COPD patients, a high negative intrathoracic pressure is necessary to generate tidal volume since COPD is associated with airway obstruction. During the weaning trial, this phenomenon increases the work of breathing, venous return, and LV afterload and can induce RV dilation by increasing RV afterload [103]. All these previous pathophysiological aspects may explain the higher rate of critical illness in obese ICU patients.
Conclusion
Obesity is acknowledged to be a risk factor for severe COVID-19 presentation, which requires particular attention by physicians in cases of respiratory presentation. Understanding cardiorespiratory pathophysiological aspects may help physicians manage patients in hospitals.
Author Contribution
AF and BD conceived, wrote, and edited the manuscript. MCDC wrote and reviewed the manuscript and added substantial scientific modifications. FL reviewed the manuscript and added substantial scientific modifications. NM wrote and reviewed the manuscript and added substantial scientific modifications. All authors approved the final version of the manuscript.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors consent to publish the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on COVID-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg JA. Obesity and early mortality in the United States. Obesity (Silver Spring) 2013;21(2):405–412. doi: 10.1002/oby.20023. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, Flegal KM. Trends in obesity prevalence among children and adolescents in the United States, 1988-1994 Through 2013-2014. JAMA. 2016;315(21):2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schütten MT, Houben AJ, de Leeuw PW, Stehouwer CD. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology (Bethesda) 2017;32(3):197–209. doi: 10.1152/physiol.00037.2016. [DOI] [PubMed] [Google Scholar]
- 6.Levitan EB, Yang AZ, Wolk A, Mittleman MA. Adiposity and incidence of heart failure hospitalization and mortality: a population-based prospective study. Circ Heart Fail. 2009;2(3):202–208. doi: 10.1161/CIRCHEARTFAILURE.108.794099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–977. doi: 10.1161/01.CIR.67.5.968. [DOI] [PubMed] [Google Scholar]
- 8.Lazarus R, Sparrow D, Weiss ST. Effects of obesity and fat distribution on ventilatory function: the normative aging study. Chest. 1997;111(4):891–898. doi: 10.1378/chest.111.4.891. [DOI] [PubMed] [Google Scholar]
- 9.Gao F, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, Chen YP, Targher G, Byrne CD, George J, Zheng MH. Obesity Is a Risk Factor for Greater COVID-19 Severity. Diabetes Care. 2020;43(7):e72–e74. doi: 10.2337/dc20-0682. [DOI] [PubMed] [Google Scholar]
- 10.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, Labreuche J, Mathieu D, Pattou F, Jourdain M. LICORN and the Lille COVID-19 and Obesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020;28(7):1195–1199. doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caussy C, Pattou F, Wallet F, Simon C, Chalopin S, Telliam C, Mathieu D, Subtil F, Frobert E, Alligier M, Delaunay D, Vanhems P, Laville M, Jourdain M. Disse E; COVID Outcomes HCL Consortium and Lille COVID–Obesity Study Group. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8(7):562–564. doi: 10.1016/S2213-8587(20)30160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smati S, Tramunt B, Wargny M, Caussy C, Gaborit B, Vatier C, Vergès B, Ancelle D, Amadou C, Bachir LA, Bourron O, Coffin-Boutreux C, Barraud S, Dorange A, Fremy B, Gautier JF, Germain N, Larger E, Laugier-Robiolle S, Meyer L, Monier A, Moura I, Potier L, Sabbah N, Seret-Bégué D, Winiszewski P, Pichelin M, Saulnier PJ, Hadjadj S, Cariou B, Gourdy P. CORONADO investigators. Relationship between obesity and severe COVID-19 outcomes in patients with type 2 diabetes: Results from the CORONADO study. Diabetes Obes Metab. 2021;23(2):391–403. doi: 10.1111/dom.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh AK, Gillies CL, Singh R, Singh A, Chudasama Y, Coles B, Seidu S, Zaccardi F, Davies MJ, Khunti K. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: A systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1915–1924. doi: 10.1111/dom.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng M, Qi Y, Deng L, Wang H, Xu Y, Li Z, Meng Z, Tang J, Dai Z. Obesity as a potential predictor of disease severity in young COVID-19 patients: a retrospective study. Among authors: deng l. Obesity (Silver Spring) 2020;28(10):1815–1825. doi: 10.1002/oby.22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, Chang BP, Chau KH, Choi JJ, Gavin N, Goyal P, Mills AM, Patel AA, Romney MS, Safford MM, Schluger NW, Sengupta S, Sobieszczyk ME, Zucker JE, Asadourian PA, Bell FM, Boyd R, Cohen MF, Colquhoun MI, Colville LA, de Jonge JH, Dershowitz LB, Dey SA, Eiseman KA, Girvin ZP, Goni DT, Harb AA, Herzik N, Householder S, Karaaslan LE, Lee H, Lieberman E, Ling A, Lu R, Shou AY, Sisti AC, Snow ZE, Sperring CP, Xiong Y, Zhou HW, Natarajan K, Hripcsak G, Chen R. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H, Entz A, Demertzis Z, Hanna Z, Failla A, Dagher C, Chaudhry Z, Vahia A, Abreu Lanfranco O, Ramesh M, Zervos MJ, Alangaden G, Miller J, Brar I. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in Metropolitan Detroit. JAMA Netw Open. 2020;3(6):e2012270. doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, Tobin KA, Cerfolio RJ, Francois F, Horwitz LI. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingraham NE, Barakat AG, Reilkoff R, Bezdicek T, Schacker T, Chipman JG, Tignanelli CJ, Puskarich MA. Understanding the renin-angiotensin-aldosterone-SARS-CoV axis: a comprehensive review. Eur Respir J. 2020;56(1):2000912. doi: 10.1183/13993003.00912-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yap JC, Watson RA, Gilbey S, Pride NB. Effects of posture on respiratory mechanics in obesity. J Appl Physiol (1985) 1995;79(4):1199–1205. doi: 10.1152/jappl.1995.79.4.1199. [DOI] [PubMed] [Google Scholar]
- 22.Sharp JT, Druz WS, Kondragunta VR. Diaphragmatic responses to body position changes in obese patients with obstructive sleep apnea. Am Rev Respir Dis. 1986;133(1):32–37. doi: 10.1164/arrd.1986.133.1.32. [DOI] [PubMed] [Google Scholar]
- 23.Lin CK, Lin CC. Work of breathing and respiratory drive in obesity. Respirology. 2012;17(3):402–411. doi: 10.1111/j.1440-1843.2011.02124.x. [DOI] [PubMed] [Google Scholar]
- 24.Sampson MG, Grassino AE. Load compensation in obese patients during quiet tidal breathing. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(4):1269–1276. doi: 10.1152/jappl.1983.55.4.1269. [DOI] [PubMed] [Google Scholar]
- 25.Ochs-Balcom HM, Grant BJ, Muti P, Sempos CT, Freudenheim JL, Trevisan M, Cassano PA, Iacoviello L, Schünemann HJ. Pulmonary function and abdominal adiposity in the general population. Chest. 2006;129(4):853–862. doi: 10.1378/chest.129.4.853. [DOI] [PubMed] [Google Scholar]
- 26.Ferretti A, Giampiccolo P, Cavalli A, Milic-Emili J, Tantucci C. Expiratory flow limitation and orthopnea in massively obese subjects. Chest. 2001;119(5):1401–1408. doi: 10.1378/chest.119.5.1401. [DOI] [PubMed] [Google Scholar]
- 27.Kelly TM, Jensen RL, Elliott CG, Crapo RO. Maximum respiratory pressures in morbidly obese subjects. Respiration. 1988;54(2):73–77. doi: 10.1159/000195504. [DOI] [PubMed] [Google Scholar]
- 28.da Rosa GJ, Schivinski CI. Assessment of respiratory muscle strength in children according to the classification of body mass index. Rev Paul Pediatr. 2014;32(2):250–255. doi: 10.1590/0103-0582201432210313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurewitz AN, Susskind H, Harold WH. Obesity alters regional ventilation in lateral decubitus position. J Appl Physiol (1985) 1985;59(3):774–783. doi: 10.1152/jappl.1985.59.3.774. [DOI] [PubMed] [Google Scholar]
- 30.Vaughan RW, Cork RC, Hollander D. The effect of massive weight loss on arterial oxygenation and pulmonary function tests. Anesthesiology. 1981;54(4):325–328. doi: 10.1097/00000542-198104000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Littleton SW, Tulaimat A. The effects of obesity on lung volumes and oxygenation. Respir Med. 2017;124:15–20. doi: 10.1016/j.rmed.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Gopalakrishnan P, Tak T. Obstructive sleep apnea and cardiovascular disease. Cardiol Rev. 2011;19(6):279–290. doi: 10.1097/CRD.0b013e318223bd08. [DOI] [PubMed] [Google Scholar]
- 33.Masa JF, Pépin JL, Borel JC, Mokhlesi B, Murphy PB, Sánchez-Quiroga MÁ. Obesity hypoventilation syndrome. Eur Respir Rev. 2019;28(151):180097. doi: 10.1183/16000617.0097-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camporota L, Vasques F, Sanderson B, Barrett NA, Gattinoni L. Identifca- tion of pathophysiological patterns for triage and respiratory support in COVID-19. Lancet Respir Med. 2020;8(8):752–754. doi: 10.1016/S2213-2600(20)30279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciceri F, Beretta L, Scandroglio AM, Colombo S, Landoni G, Ruggeri A, Peccatori J, D’Angelo A, De Cobelli F, Rovere-Querini P, Tresoldi M, Dagna L, Zangrillo A. Microvascular COVID-19 lung vessels obstruc- tive thromboinfammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hajjar LA, Costa IBSDS, Rizk SI, Biselli B, Gomes BR, Bittar CS, de Oliveira GQ, de Almeida JP, de Oliveira Bello MV, Garzillo C, Leme AC, Elena M, Val F, de Almeida LM, Lacerda MVG, Ramires JAF, Kalil Filho R, Teboul JL, Landoni G. Intensive care management of patients with COVID-19: a practical approach. Ann Intensive Care. 2021;11(1):36. doi: 10.1186/s13613-021-00820-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosmala W, Sanders P, Marwick TH. Subclinical myocardial impairment in metabolic diseases. JACC Cardiovasc Imaging. 2017;10(6):692–703. doi: 10.1016/j.jcmg.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z, Guo T, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;27:e201017. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dweck MR, Bularga A, Hahn RT, Bing R, Lee KK, Chapman AR, White A, Salvo GD, Sade LE, Pearce K, Newby DE, Popescu BA, Donal E, Cosyns B, Edvardsen T, Mills NL, Haugaa K. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;18:jeaa178. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pagnesi M, Baldetti L, Beneduce A, Calvo F, Gramegna M, Pazzanese V, Ingallina G, Napolano A, Finazzi R, Ruggeri A, Ajello S, Melisurgo G, Camici PG, Scarpellini P, Tresoldi M, Landoni G, Ciceri F, Scandroglio AM, Agricola E, Cappelletti AM. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020;106(17):1324–1331. doi: 10.1136/heartjnl-2020-317355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, Liu W, Zeng H, Tao Q, Xia L. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020:S1936-878X(20)30403-4 [DOI] [PMC free article] [PubMed]
- 42.Knight DS, Kotecha T, Razvi Y, Chacko L, Brown JT, Jeetley PS, Goldring J, Jacobs M, Lamb LE, Negus R, Wolff A, Moon JC, Xue H, Kellman P, Patel N, Fontana M. COVID-19: Myocardial injury in survivors. Circulation. 2020;142(11):1120–2. [DOI] [PMC free article] [PubMed]
- 43.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rali AS, Ranka S, Shah Z, Sauer AJ. Mechanisms of myocardial injury in coronavirus disease 2019. Card Fail Rev. 2020;6:e15. doi: 10.15420/cfr.2020.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandoval Y, Januzzi JL Jr, Jaffe AS. Cardiac troponin for the diagnosis and risk-stratification of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020:S0735-1097(20)35888-5. [DOI] [PMC free article] [PubMed]
- 46.Sharma A, Garg A, Rout A, Lavie CJ. Association of obesity with more critical illness in COVID-19. Mayo Clin Proc. 2020;95(9):2040–2042. doi: 10.1016/j.mayocp.2020.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Hu J, Zhu CJ. Obesity aggravates COVID-19: a systematic review and meta-analysis. J Med Virol. 2021;93(1):257–261. doi: 10.1002/jmv.26237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chu Y, Yang J, Shi J, Zhang P, Wang X. Obesity is associated with increased severity of disease in COVID-19 pneumonia: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):64. doi: 10.1186/s40001-020-00464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao X, Gang X, He G, Li Z, Lv Y, Han Q, Wang G. Obesity increases the severity and mortality of influenza and COVID-19: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2020;11:595109. doi: 10.3389/fendo.2020.595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring) 2020;28(9):1595–1599. doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, Arai AE, Lima JA, Bluemke DA. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3(3):266–274. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dorbala S, Crugnale S, Yang D, Di Carli MF. Effect of body mass index on left ventricular cavity size and ejection fraction. Am J Cardiol. 2006;97(5):725–729. doi: 10.1016/j.amjcard.2005.09.122. [DOI] [PubMed] [Google Scholar]
- 53.Mangner N, Scheuermann K, Winzer E, Wagner I, Hoellriegel R, Sandri M, Zimmer M, Mende M, Linke A, Kiess W, Schuler G, Körner A, Erbs S. Childhood obesity: impact on cardiac geometry and function. JACC Cardiovasc Imaging. 2014;7(12):1198–1205. doi: 10.1016/j.jcmg.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 54.Lee HJ, Kim HL, Lim WH, Seo JB, Kim SH, Zo JH, Kim MA. Subclinical alterations in left ventricular structure and function according to obesity and metabolic health status. PLoS One. 2019;14(9):e0222118. doi: 10.1371/journal.pone.0222118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Russo C, Sera F, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Abdominal adiposity, general obesity, and subclinical systolic dysfunction in the elderly: a population-based cohort study. Eur J Heart Fail. 2016;18(5):537–544. doi: 10.1002/ejhf.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dini FL, Fabiani I, Miccoli M, Galeotti GG, Pugliese NR, D'Agostino A, Scartabelli A, Conte L, Salvetti G, Santini F, Pedrinelli R. Prevalence and determinants of left ventricular diastolic dysfunction in obese subjects and the role of left ventricular global longitudinal strain and mass normalized to height. Echocardiography. 2018;35(8):1124–1131. doi: 10.1111/echo.13890. [DOI] [PubMed] [Google Scholar]
- 57.Chirinos JA, Sardana M, Satija V, Gillebert TC, De Buyzere ML, Chahwala J, De Bacquer D, Segers P. Rietzschel ER; Asklepios investigators. Effect of obesity on left atrial strain in persons aged 35-55 years (The Asklepios Study) Am J Cardiol. 2019;123(5):854–861. doi: 10.1016/j.amjcard.2018.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sasson Z, Rasooly Y, Gupta R, Rasooly I. Left atrial enlargement in healthy obese: prevalence and relation to left ventricular mass and diastolic function. Can J Cardiol. 1996;12(3):257–263. [PubMed] [Google Scholar]
- 59.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 60.Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew-Paisley study) Eur Heart J. 2006;27(1):96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- 61.Alpert MA, Agrawal H, Aggarwal K, Kumar SA, Kumar A. Heart failure and obesity in adults: pathophysiology, clinical manifestations and management. Curr Heart Fail Rep. 2014;11(2):156–165. doi: 10.1007/s11897-014-0197-5. [DOI] [PubMed] [Google Scholar]
- 62.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 63.Shah RV, Murthy VL, Abbasi SA, Eng J, Wu C, Ouyang P, Kwong RY, Goldfine A, Bluemke DA, Lima J, Jerosch-Herold M. Weight loss and progressive left ventricular remodelling: The Multi-Ethnic Study of Atherosclerosis (MESA) Eur J Prev Cardiol. 2015;22(11):1408–1418. doi: 10.1177/2047487314541731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61(7):646–653. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Nicklas BJ, Cesari M, Penninx BW, Kritchevsky SB, Ding J, Newman A, Kitzman DW, Kanaya AM, Pahor M, Harris TB. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006;54(3):413–420. doi: 10.1111/j.1532-5415.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 66.Emerging Risk Factors Collaboration. Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377(9771):1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. doi: 10.1161/CIRCRESAHA.116.305697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. 2019;15(6):367–385. doi: 10.1038/s41581-019-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 71.Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21(6):e13034. doi: 10.1111/obr.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aidan V, Davido B, Mustafic H, Dinh A, Mansencal N, Fayssoil A. Cardiovascular disorders in patients infected with 2019 novel coronavirus. Ann Cardiol Angeiol (Paris) 2021;70(2):106–115. doi: 10.1016/j.ancard.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J Endocrinol. 2013;216(2):R1–R17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 74.Magalhaes GS, Rodrigues-Machado MDG, Motta-Santos D, Campagnole-Santos MJ, Santos RAS. Activation of Ang-(1-7)/Mas receptor is a possible strategy to treat coronavirus (SARS-CoV-2) infection. Front Physiol. 2020;11:730. doi: 10.3389/fphys.2020.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Finucane FM, Davenport C. Coronavirus and obesity: could insulin resistance mediate the severity of Covid-19 infection?Front. Public Health. 2020;8:184. doi: 10.3389/fpubh.2020.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q, Chen G, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;13:137244. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bonnet N, Martin O, Boubaya M, Levy V, Ebstein N, Karoubi P, Tandjaoui-Lambiotte Y, Van Der Meersch G, Oziel J, Soulie M, Ghalayini M, Winchenne A, Zahar JR, Ahmed P, Gaudry S, Cohen Y. High flow nasal oxygen therapy to avoid invasive mechanical ventilation in SARS-CoV-2 pneumonia: a retrospective study. Ann Intensive Care. 2021;11(1):37. doi: 10.1186/s13613-021-00825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 80.Elharrar X, Trigui Y, Dols AM, Touchon F, Martinez S, Prud’homme E, Papazian L. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323(22):2336–2338. doi: 10.1001/jama.2020.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharp JT, Henry JP, Sweany SK, Meadows WR, Pietras RJ. The total work of breathing in normal and obese men. J Clin Invest. 1964;43(4):728–739. doi: 10.1172/JCI104957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moammar MQ, Azam HM, Blamoun AI, Rashid AO, Ismail M, Khan MA, DeBari VA. Alveolar-arterial oxygen gradient, pneumonia severity index and outcomes in patients hospitalized with community acquired pneumonia. Clin Exp Pharmacol Physiol. 2008;35(9):1032–1037. doi: 10.1111/j.1440-1681.2008.04971.x. [DOI] [PubMed] [Google Scholar]
- 83.Goldhaber SZ, Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, Willett WC, Hennekens CH. A prospective study of risk factors for pulmonary embolism in women. JAMA. 1997;277(8):642–645. doi: 10.1001/jama.1997.03540320044033. [DOI] [PubMed] [Google Scholar]
- 84.Bompard F, Monnier H, Saab I, Tordjman M, Abdoul H, Fournier L, Sanchez O, Lorut C, Chassagnon G, Revel MP. Pulmonary embolism in patients with Covid-19 pneumonia. Eur Respir J. 2020;12:2001365. doi: 10.1183/13993003.01365-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ball L, Pelosi P. How I ventilate an obese patient. Crit Care. 2019;23(1):176. doi: 10.1186/s13054-019-2466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Imber DA, Pirrone M, Zhang C, Fisher DF, Kacmarek RM, Berra L. Respiratory management of perioperative obese patients. Respir Care. 2016;61(12):1681–1692. doi: 10.4187/respcare.04732. [DOI] [PubMed] [Google Scholar]
- 87.MacIntyre NR. Physiologic effects of noninvasive ventilation. Respir Care. 2019;64(6):617–624. doi: 10.4187/respcare.06635. [DOI] [PubMed] [Google Scholar]
- 88.Petrof BJ. Diaphragm weakness in the critically ill: basic mechanisms reveal therapeutic opportunities. Chest. 2018;154(6):1395–1403. doi: 10.1016/j.chest.2018.08.1028. [DOI] [PubMed] [Google Scholar]
- 89.Talmor D, Sarge T, Malhotra A, O'Donnell CR, Ritz R, Lisbon A, Novack V, Loring SH. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359(20):2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vignon P. Ventricular diastolic abnormalities in the critically ill. Curr Opin Crit Care. 2013;19(3):242–249. doi: 10.1097/MCC.0b013e32836091c3. [DOI] [PubMed] [Google Scholar]
- 91.Jha AK. Left ventricular diastolic dysfunction as a predictor of weaning failure from mechanical ventilation. Intensive Care Med. 2020;46(11):2121–2. [DOI] [PubMed]
- 92.Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124(11):1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Al-Wahaibi K, Al-Wahshi Y, Mohamed EO. Myocardial injury is associated with higher morbidity and mortality in patients with 2019 novel coronavirus disease (COVID-19)SN. Compr Clin Med. 2020;9:1–7. doi: 10.1007/s42399-020-00569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S, Cousin N, Durand A, el Kalioubie A, Favory R, Girardie P, Houard M, Jaillette E, Jourdain M, Ledoux G, Mathieu D, Moreau AS, Niles C, Nseir S, Onimus T, Préau S, Robriquet L, Rouzé A, Simonnet A, Six S, Toussaint A, Dupont A, Bauters A, Zawadzki C, Paris C, Trillot N, Wibaut B, Hochart A, Marichez C, Dalibard V, Vanderziepe S, Bourgeois L, Gaul A, Jospin A, Stepina N, Pradines B, Tournoys A, Brousseau T, Rémy M, Hutt A. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 95.Esteban A, Anzueto A, Frutos F, Alía I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguía C, Nightingale P, Arroliga AC, Tobin MJ. Mechanical Ventilation International Study Group. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 96.Mayo P, Volpicelli G, Lerolle N, Schreiber A, Doelken P, Vieillard-Baron A. Ultrasonography evaluation during the weaning process: the heart, the diaphragm, the pleura and the lung. Intensive Care Med. 2016;42(7):1107–1117. doi: 10.1007/s00134-016-4245-3. [DOI] [PubMed] [Google Scholar]
- 97.Lemaire F, Teboul JL, Cinotti L, Giotto G, Abrouk F, Steg G, Macquin-Mavier I, Zapol WM. Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology. 1988;69(2):171–179. doi: 10.1097/00000542-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 98.Tuinman PR, Jonkman AH, Dres M, Shi ZH, Goligher EC, Goffi A, de Korte C, Demoule A, Heunks L. Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients-a narrative review. Version 2. Intensive Care Med. 2020;46(4):594–605. doi: 10.1007/s00134-019-05892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu J, Shen F, Teboul JL, Anguel N, Beurton A, Bezaz N, Richard C, Monnet X. Cardiac dysfunction induced by weaning from mechanical ventilation: incidence, risk factors, and effects of fluid removal. Crit Care. 2016;20(1):369. doi: 10.1186/s13054-016-1533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stefanini GG, Montorfano M, Trabattoni D, Andreini D, Ferrante G, Ancona M, Metra M, Curello S, Maffeo D, Pero G, Cacucci M, Assanelli E, Bellini B, Russo F, Ielasi A, Tespili M, Danzi GB, Vandoni P, Bollati M, Barbieri L, Oreglia J, Lettieri C, Cremonesi A, Carugo S, Reimers B, Condorelli G, Chieffo A. ST-elevation myocardial infarction in patients with COVID-19: clinical and angiographic outcomes. Circulation. 2020;141(25):2113–2116. doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chatila W, Ani S, Guaglianone D, Jacob B, Amoateng-Adjepong Y, Manthous CA. Cardiac ischemia during weaning from mechanical ventilation. Chest. 1996;109(6):1577–1583. doi: 10.1378/chest.109.6.1577. [DOI] [PubMed] [Google Scholar]
- 102.Mekontso-Dessap A, de Prost N, Girou E, Braconnier F, Lemaire F, Brun-Buisson C, Brochard L. B-type natriuretic peptide and weaning from mechanical ventilation. Intensive Care Med. 2006;32(10):1529–1536. doi: 10.1007/s00134-006-0339-7. [DOI] [PubMed] [Google Scholar]
- 103.Teboul JL, Abrouk F, Lemaire F. Right ventricular function in COPD patients during weaning from mechanical ventilation. Intensive Care Med. 1988;14(Suppl 2):483–485. doi: 10.1007/BF00256965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.