Abstract
Background
Intensive systolic blood pressure treatment (<120 mm Hg) in SPRINT (Systolic Blood Pressure Intervention Trial) improved survival compared with standard treatment (<140 mm Hg) over a median follow‐up of 3.3 years. We projected life expectancy after observed follow‐up in SPRINT using SPRINT‐eligible participants in the NHLBI‐PCS (National Heart, Lung, and Blood Institute Pooled Cohorts Study).
Methods and Results
We used propensity scores to weight SPRINT‐eligible NHLBI‐PCS participants to resemble SPRINT participants. In SPRINT participants, we estimated in‐trial survival (<4 years) using a time‐based flexible parametric survival model. In SPRINT‐eligible NHLBI‐PCS participants, we estimated posttrial survival (≥4 years) using an age‐based flexible parametric survival model and applied the formula to SPRINT participants to predict posttrial survival. We projected overall life expectancy for each SPRINT participant and compared it to parametric regression (eg, Gompertz) projections based on SPRINT data alone. We included 8584 SPRINT and 10 593 SPRINT‐eligible NHLBI‐PCS participants. After propensity weighting, mean (SD) age was 67.9 (9.4) and 68.2 (8.8) years, and 35.5% and 37.6% were women in SPRINT and NHLBI‐PCS, respectively. Using the NHLBI‐PCS–based method, projected mean life expectancy from randomization was 21.0 (7.4) years with intensive and 19.1 (7.2) years with standard treatment. Using the Gompertz regression, life expectancy was 11.2 (2.3) years with intensive and 10.5 (2.2) years with standard treatment.
Conclusions
Combining SPRINT and NHLBI‐PCS observed data likely offers a more realistic estimate of life expectancy than parametrically extrapolating SPRINT data alone. These results offer insight into the potential long‐term effectiveness of intensive SBP goals.
Keywords: hypertension, life expectancy, survival
Nonstandard Abbreviations and Acronyms
- ARIC
Atherosclerosis Risk in Communities
- ASMD
absolute standardized mean difference
- CARDIA
Coronary Artery Risk Development in Young Adults
- CHS
Cardiovascular Health Study
- FHS‐O
Framingham Offspring Study
- Health ABC
Health, Aging, and Body Composition Study
- MESA
Multi‐Ethnic Study of Atherosclerosis
- NHLBI‐PCS
National Heart, Lung, and Blood Institute Pooled Cohorts Study
- SBP
systolic blood pressure
- SPRINT
Systolic Blood Pressure Intervention Trial
Clinical Perspective
What Is New?
Using individual participant data from SPRINT (Systolic Blood Pressure Intervention Trial) and from large cardiovascular epidemiologic US cohorts with long‐term follow‐up, we estimated that SPRINT participants in the intensive systolic blood pressure arm would survive 21.0 years compared with 19.1 years in the standard arm.
The mean estimated survival was sensitive to the intensity of the treatment effect with intensive systolic blood pressure, varying from 19.3 to 22.1 years.
The combined data approach resulted in substantially longer and more realistic life expectancy estimates than those generated using typical methods based on SPRINT participant data alone (ie, Gompertz regression).
What Are the Clinical Implications?
Estimating long‐term survival (eg, 40–50 years) based on only short‐term data from clinical trials (eg, 3–5 years) poses a critical challenge for clinicians.
Using participant‐level data observed in cohorts with similar characteristics provides an opportunity to extrapolate in‐trial event rates and examine treatment‐effect assumptions.
Accurately estimating the life expectancy of SPRINT participants may better inform treatment decisions by patients and healthcare providers as well as the economic consequences and cost‐effectiveness of intensive systolic blood pressure treatment.
Intensive systolic blood pressure (SBP) treatment (targeting <120 mm Hg) of patients with high cardiovascular disease (CVD) risk in SPRINT (Systolic Blood Pressure Intervention Trial) is safe, reduces CVD morbidity and mortality, and is cost‐effective compared with standard treatment (<140 mm Hg). 1 , 2 , 3 In the United States, over 16 million adults meet the SPRINT eligibility criteria, and it is estimated that over 107 000 deaths per year could be prevented if intensive SBP treatment was fully adopted. 4 However, blood pressure control rates among US adults with hypertension remain suboptimal. 5 , 6 Achieving more intensive SBP goals may be difficult and costly for local healthcare systems, but it may yield health and economic gains over time. Accurately estimating the long‐term costs, health consequences, and cost‐effectiveness of the intensive target strategy may aid in implementation decisions. However, in‐trial results with relatively short follow‐up (ie, <5 years) do not reflect the effect of intensive SBP treatment over the remaining life course 2 ; appropriate methods must be used to extrapolate effectiveness and facilitate long‐term cost–benefit analyses.
Alongside trial cost‐effectiveness analyses use participant‐level trial data to estimate economic and health consequences within and beyond the trial follow‐up. Estimates of survival, event rates, and costs beyond the duration of the observed trial are needed to project future costs and benefits. 7 , 8 Typical methods to estimate long‐term survival include Gompertz, Weibull, generalized gamma, and other parametric survival models, survival tables, and actuarial methods. 8 , 9 , 10 , 11 , 12 , 13 These methods are limited by requiring hypothetical assumptions about continuation of treatment effects and event rates that may not necessarily represent the trial‐eligible populations. In the United States, there are several high‐quality longitudinal CVD cohort studies that may be used to estimate longer‐term survival and CVD outcomes over the lifecourse. 14 , 15 , 16 By combining SPRINT participant data with long‐term epidemiologic cohort data, we can estimate survival for each SPRINT participant and, adjusting for identified covariates, estimate the effects of intensive versus standard SBP treatment on long‐term survival and cost‐effectiveness.
Therefore, we estimated life expectancy among SPRINT participants beyond the end of the trial by using individual participant data from SPRINT and the NHLBI‐PCS (National Heart, Lung, and Blood Institute Pooled Cohorts Study). 2 , 15 , 16 We then compared this approach to parametric survival extrapolation methods using the observed SPRINT trial data alone.
METHODS
All analyses were performed using Stata version 16 (StataCorp, College Station, TX). All data used in this study were obtained from each study coordinating center. Limited versions of the data sets may be available through the Biologic Specimen and Data Repository Information Coordinating Center from the National Heart, Lung, and Blood Institute. Our analytic code is available to interested researchers from the corresponding author upon reasonable request. This study was reviewed and approved by the Columbia University Medical Center Institutional Review Board. The study protocols for SPRINT and the included cohorts were approved by the institutional review boards at each participating institution, and all participants provided written informed consent. The funders had no role in study design, analysis, preparation of the article, or the decision to submit the article for publication.
Overview
We used a 4‐step process to estimate overall life expectancy for each SPRINT participant (Figure S1). First, we identified SPRINT‐eligible NHLBI‐PCS participants and used propensity‐score weighting to ensure the NHLBI‐PCS cohort resembled SPRINT participants. Second, we estimated in‐trial survival for SPRINT participants using a time‐based flexible parametric survival model. 17 Third, we similarly estimated posttrial survival in SPRINT‐eligible NHLBI‐PCS participants using an age‐based flexible parametric survival model, and then applied the model estimates back to SPRINT participants to estimate their posttrial survival with standard treatment. We used the published SPRINT hazard ratio (HR) for all‐cause mortality to estimate survival in the intensive arm and varied the HR in sensitivity analysis. Fourth, we combined in‐trial and posttrial survival estimates to generate life expectancy estimates for each SPRINT participant.
Data
We used individual‐level data from SPRINT and from 41 360 individuals in pooled, harmonized data from across 6 epidemiologic cohorts included in the NHLBI‐PCS : (1) ARIC (Atherosclerosis Risk in Communities), (2) CARDIA (Coronary Artery Risk Development in Young Adults), (3) MESA (Multi‐Ethnic Study of Atherosclerosis), (4) FHS‐O (Framingham Offspring Study), (5) Health ABC (Health, Aging, and Body Composition Study), and (6) CHS (Cardiovascular Health Study). 2 , 15 , 16
Inclusion Criteria
We included individuals from the NHLBI‐PCS who met all of the following SPRINT eligibility criteria at ≥1 study visit (Figure 1): (1) age ≥50 years, (2) SBP 130 to 180 mm Hg, and (3) ≥1 high CVD‐risk condition (ie, clinical coronary heart disease, estimated glomerular filtration rate [eGFR] 20 to 59 mL/min per 1.73 m2, Framingham 10‐year generalized CVD risk score ≥15%, or age ≥75 years). 18 As in SPRINT, we excluded individuals with diabetes mellitus, a history of stroke, a history of heart failure, or an eGFR <20 mL/min per 1.73 m2. In both the SPRINT and NHLBI‐PCS data sets, we excluded individuals with missing data on key covariates: age, sex, race/ethnicity (non‐Hispanic White, non‐Hispanic Black, Hispanic, and other ‐ Asian, American Indian/Alaska Native, and Native Hawaiian/Pacific Islander), body mass index, smoking status (current, former, never), SBP, diastolic blood pressure, antihypertensive medication use, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, eGFR, history of coronary heart disease, and Framingham 10‐year CVD risk score. To estimate posttrial survival, we further restricted to NHLBI‐PCS participants who survived at least 4 years after first meeting SPRINT eligibility criteria. In the secondary analysis, we compared the observed survival during the first 4 years of follow‐up between SPRINT and SPRINT‐eligible NHLBI‐PCS participants by removing the requirement to survive at least 4 years and included all SPRINT‐eligible NHLBI‐PCS participants.
Figure 1. Population selection flowchart.

We included 6 epidemiologic cohort studies from the National Heart, Lung, and Blood Institute Pooled Cohorts Study: Atherosclerosis Risk in Communities; Coronary Artery Risk Development in Young Adults; Multi‐Ethnic Study of Atherosclerosis; Framingham Offspring Study; Health, Aging, and Body Composition Study; and Cardiovascular Health Study. CHD indicates coronary heart disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; NHLBI, National Heart, Lung, and Blood Institute; SBP, systolic blood pressure; and SPRINT, Systolic Blood Pressure Intervention Trial.
Statistical Analysis
Propensity‐Score Weighting
We used propensity‐score weighting to balance covariates between SPRINT and SPRINT‐eligible NHLBI‐PCS participants. We used the characteristics of SPRINT participants at their randomization visit and NHLBI‐PCS participants at the first study visit in which they met the SPRINT‐eligibility criteria. The propensity‐score model was derived using the key covariates noted above. We used logistic regression with weights derived using average treatment effect among treated participants (ie, SPRINT participants weight=1; NHLBI‐PCS participants weight=propensity score/[1−propensity score]). We assessed covariate balance between groups using absolute standardized mean difference (ASMD), with an ASMD <0.1 indicating good balance. To avoid having participants with extreme propensity‐score weights unduly influence the results, we truncated weights below the first percentile and above the 99th percentile, resulting in weights between 0.01 and 8.22 for all participants. 19 In developing the propensity score, we examined interaction terms (up to 3‐way interactions), spline terms, and log transformation of SBP. We also tried generalized boosted models but did not find it improved the balance of the covariates.
In‐Trial Survival
We used a flexible parametric survival model to estimate the survival probability of SPRINT participants in each study arm over the first 4 years of SPRINT, which approximates the duration of the trial (median follow‐up, 3.3 years). 2 Flexible parametric survival models use restricted cubic spline functions to flexibly model the baseline cumulative hazard. 17 We used follow‐up time as the time scale. Participants were censored at time of death or end of 4 years follow‐up. The model was adjusted for the following baseline covariates: age, sex, race/ethnicity, body mass index, smoking status, SBP, diastolic blood pressure, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, eGFR, and history of coronary heart disease. We visually compared survival for each intervention arm predicted by the flexible parametric survival model to Kaplan‐Meier curves of the observed SPRINT data.
To ensure similar survival during the in‐trial period, and thus indicate that NHLBI‐PCS participants are an appropriate source from which to estimate posttrial survival of SPRINT participants, we visually compared Kaplan‐Meier curves over the first 4 years between SPRINT participants and all SPRINT‐eligible NHLBI‐PCS participants, including those who did not survive the first 4 years.
Posttrial Survival
We again used a propensity‐score weighted flexible parametric survival model to estimate survival in NHLBI‐PCS participants who survived for at least 4 years after their first SPRINT‐eligible visit. In contrast to the in‐trial survival, which used follow‐up time, the posttrial analysis used age as the time scale to make more efficient use of the available data for extrapolation of survival beyond the empirical follow‐up time period. 9 The model was adjusted for the same baseline covariates as the in‐trial survival analysis. We then applied the β coefficients from the model to each SPRINT participant, using the baseline covariate levels from the SPRINT participants, to estimate the survival probability beyond 4 years for each SPRINT participant with standard treatment. We assumed a constant treatment effect for the intensive arm of SPRINT (HR, 0.73) in the base case. 2 In sensitivity analysis, we varied this to the lower (HR, 0.60) and upper (HR, 0.90) limits of the reported 95% CI and to no continued treatment effect (HR, 1.00).
To obtain the overall survival from randomization for each SPRINT participant out to 40 years, we combined the predicted survival probability from the in‐trial and posttrial periods. The overall survival distribution for each intervention arm was estimated by averaging across the survival distributions for each SPRINT participant. Mean life expectancy was calculated as the area under the survival curve.
Comparison of Methods to Extrapolate Survival
We compared the survival estimates obtained by the flexible parametric survival model using both SPRINT and NHLBI‐PCS data with estimates derived on the basis of SPRINT data alone. Additionally, we compared survival estimates derived using other commonly used parametric survival models to extrapolate survival, including Gompertz, Weibull, and generalized gamma survival models, all fitted using only the observed SPRINT data and projecting lifetime overall survival. 10 , 20 , 21 , 22 , 23 All models were adjusted for the same covariates mentioned above in the main analysis.
RESULTS
Population Characteristics
Of the 9361 SPRINT participants, we included 8584 who had complete data on key covariates used in the propensity score. Of the 41 360 NHLBI‐PCS participants, 10 593 had ≥1 study visit in which they met all SPRINT eligibility criteria and had data on all key covariates (Figure 1). The median follow‐up of SPRINT‐eligible NHLBI‐PCS participants was 21 years in ARIC, 5 years in CARDIA, 13 years in MESA, 18 years in FHS‐O, 13 years in Health ABC, and 14 years in CHS. Before propensity‐score weighting, SPRINT and SPRINT‐eligible NHLBI‐PCS participants were not well‐balanced on age, sex, race/ethnicity, body mass index, SBP, antihypertensive medication use, low‐density lipoprotein cholesterol, eGFR, and history of coronary heart disease (Table 1). Covariate balance was substantially improved after propensity‐score weighting. In SPRINT, participant medication regimens were allowed to be adjusted to meet eligibility criteria, and nearly 26% of SPRINT participants had a baseline SBP outside the 130‐ to 180‐mm Hg inclusion criterion (Figure S2). 2 We applied the SBP 130‐ to 180‐mm Hg inclusion criterion strictly in NHLBI‐PCS participants. As such, SBP (mean, SPRINT 139.7 mm Hg versus NHLBI‐PCS 142.7 mm Hg; ASMD, 0.21) and related covariates diastolic blood pressure (mean, SPRINT 78.2 mm Hg versus NHLBI‐PCS 79.7 mm Hg; ASMD, 0.13), and 10‐year Framingham Risk Score ≥15% (SPRINT 76.3% versus NHLBI‐PCS 87.8%; ASMD, 0.28) remained not well‐balanced after propensity‐score weighting. All models were adjusted for these and other covariates noted above. The mean (SD) age in the propensity‐score–weighted NHLBI‐PCS was 68.2 (8.8) years, and 37.6% were women, compared with 67.9 (9.4) years (ASMD, 0.03) and 35.5% in SPRINT (ASMD, 0.04).
Table 1.
Comparison of Baseline Characteristics Between the SPRINT and NHLBI‐PCS Pooled Cohort
| Characteristics | Before Propensity‐Score Weighting | After Propensity‐Score Weighting | ||||
|---|---|---|---|---|---|---|
|
SPRINT (N=8584) |
SPRINT‐Eligible NHLBI‐PCS (N=10 593) |
ASMD |
SPRINT (N=8584) |
SPRINT‐Eligible NHLBI‐PCS (N=10 593) |
ASMD | |
| Demographics | ||||||
| Age, y | 67.9±9.4 | 66.2±9.0 | 0.18 | 67.9±9.4 | 68.2±8.8 | 0.03 |
| 50–59 | 21.1 | 27.3 | 21.1 | 19.9 | ||
| 60–69 | 36.6 | 32.8 | 36.6 | 31.1 | ||
| 70–79 | 30.0 | 33.6 | 30.0 | 40.9 | ||
| ≥80 | 12.4 | 6.3 | 12.4 | 8.1 | ||
| Women | 35.5 | 50.4 | 0.31 | 35.5 | 37.6 | 0.04 |
| Race/ethnicity | 0.32 | 0.07 | ||||
| Non‐Hispanic White | 57.6 | 73.6 | 57.6 | 53.2 | ||
| Non‐Hispanic Black | 29.6 | 20.5 | 29.6 | 33.5 | ||
| Hispanic | 10.9 | 3.6 | 10.9 | 11.5 | ||
| Other* | 1.9 | 2.3 | 1.9 | 1.8 | ||
| Clinical characteristics | ||||||
| Current smoker | 13.1 | 16.5 | 0.10 | 13.1 | 14.1 | 0.03 |
| Body mass index, kg/m2 | 29.9±5.8 | 27.8±5.0 | 0.38 | 29.9±5.8 | 29.7±5.8 | 0.04 |
| Systolic blood pressure, mm Hg | 139.7±15.6 | 144.7±12.0 | 0.36 | 139.7±15.6 | 142.7±10.8 | 0.21 |
| Diastolic blood pressure, mm Hg | 78.2±12.0 | 78.0±10.7 | 0.01 | 78.2±12.0 | 79.7±10.3 | 0.13 |
| Antihypertensive medication use | 90.9 | 44.8 | 1.14 | 90.9 | 90.3 | 0.02 |
| Low‐density lipoprotein cholesterol, mg/dL | 112.5±35.0 | 132.5±36.2 | 0.56 | 112.5±35.0 | 114.4±32.8 | 0.05 |
| High‐density lipoprotein cholesterol, mg/dL | 53.0±14.3 | 51.9±15.8 | 0.07 | 53.0±14.3 | 54.3±17.1 | 0.09 |
| Estimated glomerular filtration rate, mL/min per 1.73 m2 | 71.9±20.5 | 67.3±18.9 | 0.24 | 71.9±20.5 | 73.2±21.0 | 0.06 |
| 10‐y Framingham Risk Score, % | 24.7±12.3 | 23.6±12.0 | 0.09 | 24.7±12.3 | 25.6±11.3 | 0.08 |
| High cardiovascular disease risk criteria | ||||||
| Clinical coronary heart disease | 19.9 | 9.5 | 0.30 | 19.9 | 17.5 | 0.07 |
| Estimated glomerular filtration rate, 20–59 mL/min per 1.73 m2 | 27.9 | 41.2 | 0.28 | 27.9 | 28.0 | 0.00 |
| 10‐y Framingham Risk Score ≥15%, | 76.2 | 79.8 | 0.09 | 76.3 | 87.8 | 0.28 |
| Age ≥75 y | 28.2 | 20.7 | 0.18 | 28.2 | 26.5 | 0.04 |
Values are mean±SD or percent. To convert low‐ or high‐density lipoprotein cholesterol from mg/dL to mmol/L, multiply by 0.0259. ASMD indicates absolute standardized mean difference; NHLBI‐PCS, National Heart, Lung, and Blood Institute Pooled Cohorts Study; and SPRINT, Systolic Blood Pressure Intervention Trial.
Other includes Asian, American Indian/Alaskan Native, and Native Hawaiian/Pacific Islander.
In‐Trial Survival
The proportion of SPRINT participants surviving at least 4 years was 96% in the intensive arm and 95% in the standard arm. SPRINT participants in the standard arm and SPRINT‐eligible NHLBI‐PCS participants had similar survival during the first 4 years (Figure 2). The predicted proportion surviving 4 years based on the in‐trial flexible parametric survival model was 95% in the intensive arm and 94% in the standard arm (Figure S3 and Table S1).
Figure 2. SPRINT (Systolic Blood Pressure Intervention Trial) observed in‐trial vs NHLBI‐PCS (National Heart, Lung, and Blood Institute Pooled Cohorts Study) observed 4‐year survival.

The figure shows the Kaplan‐Meier (KM) curves for observed survival during the in‐trial period of pooled SPRINT participants (ie, both intensive and standard arms, N=8584) compared with the observed survival during the first 4 years after meeting SPRINT eligibility criteria in the propensity‐score weighted NHLBI‐PCS (N=12 485). For this analysis, we used the same propensity‐score development process as in the primary analysis, but we did not exclude individuals who survived <4 years; thus, the NHLBI‐PCS sample size is larger than in the primary analysis.
All of the parametric survival models, including Gompertz, Weibull, and generalized gamma, produced similar survival estimates for the in‐trial period and were similar to the SPRINT observed data (Figure S4). The Gompertz model fit the in‐trial data marginally better, with a closer approximation of the Kaplan‐Meier curve upon visual inspection and a slightly lower Akaike information criterion (Gompertz 3417.2, Weibull 3423.0, generalized gamma 3423.3). The multivariable Gompertz regression model produced results consistent with the observed in‐trial survival. The mean survival and percent surviving 4 years was 3.9 (0.1) years and 95% in the intensive arm and 3.9 (0.1) years and 94% in the standard arm, respectively.
Posttrial Survival
When extrapolating survival to 40 years using the flexible parametric survival model based on NHLBI‐PCS participants, and assuming a constant treatment effect for the intensive arm of SPRINT (HR, 0.73), we estimated that the intensive arm would survive a mean of 21.0 (7.4) years from randomization compared with 19.1 (7.2) years in the standard arm (mean difference, 1.9 years; P<0.001) (Table 2 and Table S2, Figure 3). 2 As expected, the survival predicted by the flexible parametric survival model using the NHLBI‐PCS data was similar to the observed overall survival in the NHLBI‐PCS weighted to resemble SPRINT participants (Figure S5). Based on the SPRINT observed data alone, the Gompertz model resulted in substantially shorter long‐term survival estimates than the Weibull and generalized gamma survival models (Figure S6; Weibull and generalized gamma estimated ≈20% still surviving at 50 years follow‐up). However, the Gompertz model predicted much shorter posttrial survival estimates when extrapolated over a lifetime than the flexible parametric survival model based on NHLBI‐PCS participants. We estimated a mean survival of 11.2 (2.3) years in the intervention arm and 10.5 (2.2) years in the standard arm. At 20 years of follow‐up, the Gompertz model predicted all SPRINT participants in both the intensive and standard arms would die, but the flexible parametric survival model estimated that only 48% in the intensive arm and 55% in the standard arm would die by 20 years. The flexible parametric survival model using only SPRINT participant data resulted in similar but slightly longer survival estimates (Figure S7).
Table 2.
Predicted Mean Life Expectancy by Subgroup in SPRINT Participants
| Characteristics | Standard Arm | Intensive Arm |
|---|---|---|
| Overall | 19.1±7.2 | 21.0±7.4 |
| Baseline age, y | ||
| 50–59 | 28.0±4.3 | 30.1±3.8 |
| 60–69 | 21.6±3.8 | 23.6±3.9 |
| 70–79 | 14.1±3.1 | 15.8±3.4 |
| ≥80 | 8.8±2.1 | 10.3±2.3 |
| Sex | ||
| Men | 18.6±7.1 | 20.4±7.4 |
| Women | 20.0±7.3 | 22.2±7.4 |
| Race/ethnicity* | ||
| White | 17.1±6.5 | 18.8±6.8 |
| Black | 20.7±6.8 | 22.8±6.9 |
| Hispanic | 25.3±7.3 | 26.9±7.4 |
| Other | 22.7±6.8 | 24.4±6.9 |
Values are mean±SD. Estimates were derived by combining (1) the in‐trial period (<4 years) estimates from a flexible parametric survival model of SPRINT participants and (2) the posttrial period (≥4 years) estimates by applying to SPRINT participants the baseline hazards and coefficients of a flexible parametric survival model in National Heart, Lung, and Blood Institute Pooled Cohorts Study participants propensity‐score weighted to resemble SPRINT participants. SPRINT indicates Systolic Blood Pressure Intervention Trial.
Mean baseline age was significantly different within race/ethnicity groups: White 70.3 (9.0) years, Black 64.1 (9.0) years, Hispanic 65.3 (9.1) years, and other 68.2 (8.7) years.
Figure 3. Predicted overall survival in SPRINT (Systolic Blood Pressure Intervention Trial) participants.
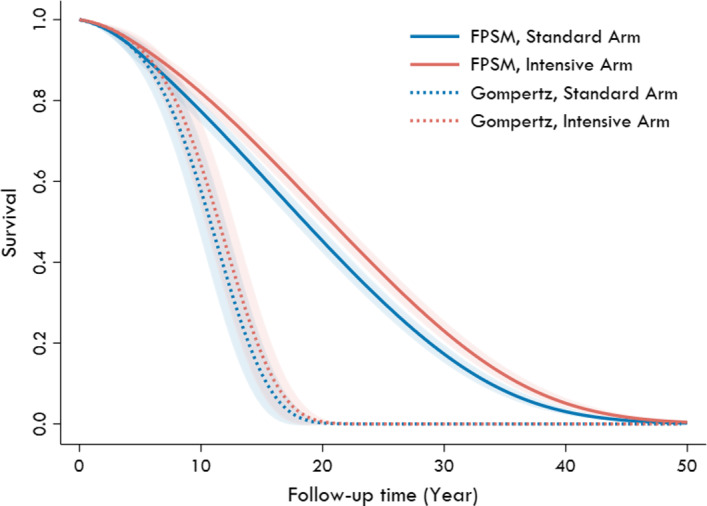
The figure shows the overall survival of SPRINT participants from randomization predicted by the multivariable flexible parametric survival model (FPSM) and Gompertz survival regression models. The shaded regions represent the 95% CIs.
We found clinically meaningful differences in predicted mean life expectancy within age subgroups (Table 2). Compared with the standard arm, the intensive arm gained 2.1 years when baseline age was between 50 and 59 years (intensive 30.1 [3.8] versus standard 28.0 [4.3] years), but only 1.5 years when baseline age was ≥80 years (intensive 10.3 [2.3] versus standard 8.8 [2.1] years). Other observed differences between the intensive and standard arms may be a result of differences in baseline age between groups. For example, the mean baseline age in non‐Hispanic White participants was 70.3 (9.0) and 64.1 (9.0) in non‐Hispanic Black participants. The difference in predicted mean survival was 1.7 in White participants (versus 1.7 with baseline age 70–79 years) and 2.1 in Black participants (versus 2.0 with baseline age 60–69 years).
Sensitivity Analysis
When varying the treatment effect beyond the 4‐year trial period, assuming an HR of 0.60 (the lower bound of the published 95% CI of the SPRINT treatment effect), mean survival in the intensive arm increased to 22.1 (7.5) years, and the upper bound (HR, 0.90) decreased to 19.9 (7.3) years (Figure 4). When assuming no difference beyond the duration of the trial (HR, 1), mean survival in the intensive arm was 19.3 (7.2) years.
Figure 4. Predicted survival and life expectancy in SPRINT (Systolic Blood Pressure Intervention Trial) participants when varying the posttrial survival benefit with intensive treatment.

A, Predicted survival. B, Predicted mean life expectancy. A, The overall survival from randomization for SPRINT participants predicted by the multivariable flexible parametric survival model with varying assumptions about the treatment effect (hazard ratio [HR]) after the end of the trial. B, The mean±SD life expectancy for each treatment‐effect scenario.
DISCUSSION
We used a combination of individual‐level data from SPRINT participants and long‐term follow‐up data from a pooled cohort of epidemiologic study participants who were propensity‐score weighted to resemble SPRINT participants to extrapolate survival and overall life expectancy beyond the observed SPRINT follow‐up (median of 3.3 years). Assuming the treatment effects observed in SPRINT persisted (HR, 0.73) beyond the trial period, we estimated that SPRINT participants in the intensive arm would survive a mean of 21.0 (7.4) years from randomization, compared with 19.1 (7.2) years in the standard arm. However, mean estimated survival was sensitive to the intensity of the treatment effect. These estimates were substantially longer than life expectancy estimates generated using Gompertz regression models, which estimated a mean survival of 11.2 (2.3) years in the intensive arm and 10.5 (2.2) years in the standard arm from randomization. Accurately estimating life expectancy of SPRINT participants may better inform treatment decisions by patients and healthcare providers, as well as the long‐term economic consequences and cost‐effectiveness of intensive SBP treatment.
Our life expectancy results for SPRINT participants are similar to estimates using actuarial methods, a simulation model, and average life tables from the Centers for Disease Control and Prevention and the Social Security Administration, but are longer than other simulation models. 1 , 3 , 11 , 24 For SPRINT participants, life tables from the Social Security Administration project a mean life of expectancy of 17.1 (6.7) years, compared with 19.1 (7.2) years for the standard arm from our flexible parametric survival model with NHLBI‐PCS data. Some of this difference may be explained by SPRINT excluding individuals with diabetes mellitus, a history of stroke, symptomatic heart failure, cancer, conditions that limit expected survival to <3 years, or individuals who may not be adherent to the intervention. 2 Using actuarial methods, the predicted mean survival was 24.5 years in the intensive and 23.3 years in the control arm for individuals aged 65 years, which compares with 23.6 and 21.6 years, respectively, for participants aged 60 to 69 years in our analysis. 11 Actuarial methods assume survival is independent of how long the treatment is given and independent of patient adherence. The approach used in the current analysis allows for exploration of these assumptions by modifying the HR after the end of the trial. Additionally, actuarial methods are designed for population‐level estimates and do not incorporate individual characteristics. Using data from external cohorts to extrapolate survival allows for adjustment for individual characteristics, but also assumes that cohort participants with similar characteristics have the same risk for outcomes. In our analysis, some of the included cohorts began following participants decades before SPRINT and could have differences in overall survival for period or birth cohort effect reasons not fully explained by the included covariates.
Alongside trial cost‐effectiveness analyses of chronic health conditions, such as hypertension, often require that event rates and treatment effects be extrapolated well beyond the observed trial data. 7 Estimating long‐term survival (eg, 40–50 years) based on only short‐term data from clinical trials (eg, 3–5 years) poses a critical challenge for clinicians, and the approach used by economic analyses may substantially impact projected outcomes and uncertainty. 21 , 25 , 26 , 27 Although parametric survival models are a convenient way to extrapolate long‐term outcomes, even models that fit short‐term survival curves well may result in inaccurate long‐term estimations of survival and treatment effects. 27 Careful assessment of the extrapolated outcomes is critical, and multiple approaches should be considered. Using participant‐level data observed in cohorts with similar characteristics provides an opportunity to extrapolate in‐trial event rates and examine treatment‐effect assumptions. Participant‐level data can generate risk functions that adjust for covariates and may be used to generate individualized cost‐effectiveness estimates (Data S1, Tables S3 and S4).
Limitations
The primary limitation to our approach is that it requires access to participant‐level data from both the clinical trial and a sufficiently large cohort with long‐term follow‐up data on the outcomes of interest, similar participant characteristics, and inclusion of key covariates. This may not be feasible for trials without public access to participant‐level data or where sufficiently similar long‐term data resembling the trial are not available. Additionally, individual cohorts may be too small and, as in our analysis, pooling of multiple data sources may be required. We pooled data from over 40 000 participants across 6 large epidemiologic studies to obtain a cohort of SPRINT‐eligible participants with a sample size similar to that of SPRINT. Without a comparable external cohort, the life‐expectancy estimates generated from this approach may not be applicable or reliable. Additionally, because our approach focused on establishing a cohort similar to SPRINT, the generalizability of our estimates to non–SPRINT‐eligible patients may be limited. We excluded participants with missing data on key covariates in both SPRINT and NHLBI‐PCS. However, our included SPRINT population had similar baseline characteristics as the overall SPRINT population (Table S5) and a similar proportion of participants in the NHLBI‐PCS met SPRINT eligibility criteria as estimated in US adults. 2 , 4 , 28 Estimates based on cohort studies assume that observed survival in these cohorts, developed over a period of the past several decades, remains applicable to projections decades in the future. However, any projection of survival into the future must in some way be informed by existing survival estimates. Finally, we assumed a constant treatment effect for intensive SBP treatment projections and explored the magnitude of the effect in sensitivity analysis, including no effect after the trial period. Although treatment effect may actually wane over time, our analyses provide the boundaries within which such a waning effect would lie.
CONCLUSIONS
Estimating long‐term survival based on short‐term data from clinical trials is a challenge for clinicians and long‐term economic projections. We combined individual participant‐level data from SPRINT and 6 large epidemiologic cohorts to estimate the life expectancy of SPRINT participants beyond the trial follow‐up. Assuming that the treatment effects from the trial continued afterward, we estimated that participants with intensive SBP treatment would survive about 2 years longer than with standard treatment. Accurate life expectancy estimates for SPRINT participants can better inform treatment decisions and the economic consequences of intensive SBP treatment.
Sources of Funding
Dr Bellows received funding for this analysis from the National Heart, Lung, and Blood Institute (NHLBI) (K01HL140170). Drs Moran, Bress, and Weintraub received funding for this analysis from the NHLBI (R01HL139837). Dr Bress also received funding from the NHLBI (K01HL133468). Dr Oelsner received funding for the NHLBI Pooled Cohorts Study from the NHLBI (R03HL132590). SPRINT (ClinicalTrials.gov number, NCT01206062) is funded by the NHLBI, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, and the National Institute of Neurological Disorders and Stroke (contract numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, and HHSN268200900049C, and Interagency Agreement Number A‐HL‐13‐002‐001), through the Department of Veterans Affairs, and through Clinical and Translational Science Awards Programs funded by the National Center for Advancing Translational Sciences. The ARIC (Atherosclerosis Risk in Communities) has been funded in whole or in part with federal funds from the NHLBI, National Institutes of Health, Department of Health and Human Services, under contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I. CARDIA (Coronary Artery Risk Development in Young Adults Study) is conducted and supported by the NHLBI in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This article has been reviewed by CARDIA for scientific content. CHS (Cardiovascular Health Study) was supported by NHLBI contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from the National Institute on Aging. A full list of principal CHS investigators and institutions can be found at CHS‐NHLBI.org. The FHS‐O (Framingham Offspring) study is conducted and supported by the NHLBI in collaboration with Boston University (Contract No. N01‐HC‐25195 and HSN268201500001I). The Health ABC (Health, Aging, and Body Composition Study) study was supported by National Institute on Aging contracts N01‐AG‐6‐2101, N01‐AG‐6‐2103, N01‐AG‐6‐2106, grant R01‐AG028050, and National Institute of Nursing Research grant R01‐NR012459. The MESA study (Multi‐Ethnic Study of Atherosclerosis) was supported by NHLBI contracts HHSN268201500003I, N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168 and N01‐HC‐95169, and by National Center for Advancing Translational Sciences grants UL1‐TR‐000040, UL1‐TR‐001079, and UL1‐TR‐001420.
Disclosures
Outside of the current work, Dr Cushman has received research support from Eli Lilly and personal fees from Sanofi and Dr Weintraub has received research support from Amarin and is a consultant for Amarin and Astra‐Zeneca. The remaining authors have no relevant disclosures to report.
Supporting information
Data S1
Tables S1–S5
Figures S1–S7
Acknowledgments
A full list of participating MESA (Multi‐Ethnic Study of Atherosclerosis) investigators and institutions can be found at http://www.mesa‐nhlbi.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
(J Am Heart Assoc. 2021;10:e020361. DOI: 10.1161/JAHA.120.020361.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.020361
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Bress AP, Bellows BK, King JB, Hess R, Beddhu S, Zhang Z, Berlowitz DR, Conroy MB, Fine L, Oparil S, et al. Cost‐effectiveness of intensive versus standard blood‐pressure control. N Engl J Med. 2017;377:745–755. DOI: 10.1056/NEJMsa1616035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. SPRINT Research Group , Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. DOI: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richman IB, Fairley M, Jorgensen ME, Schuler A, Owens DK, Goldhaber‐Fiebert JD. Cost‐effectiveness of intensive blood pressure management. JAMA Cardiol. 2016;1:872–879. DOI: 10.1001/jamacardio.2016.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bress AP, Kramer H, Khatib R, Beddhu S, Cheung AK, Hess R, Bansal VK, Cao G, Yee J, Moran AE, et al. Potential deaths averted and serious adverse events incurred from adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) intensive blood pressure regimen in the united states: projections from NHANES (National Health and Nutrition Examination Survey). Circulation. 2017;135:1617–1628. DOI: 10.1161/CIRCULATIONAHA.116.025322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. DOI: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 6. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. DOI: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 7. Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost‐Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 2016. https://global.oup.com/academic/product/cost‐effectiveness‐in‐health‐and‐medicine‐9780190492939?cc=us&lang=en& [Google Scholar]
- 8. Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, Briggs A, Sullivan SD. Cost‐effectiveness analysis alongside clinical trials ii‐an ispor good research practices task force report. Value Health. 2015;18:161–172. DOI: 10.1016/j.jval.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 9. Nelson CL, Sun JL, Tsiatis AA, Mark DB. Empirical estimation of life expectancy from large clinical trials: use of left‐truncated, right‐censored survival analysis methodology. Stat Med. 2008;27:5525–5555. DOI: 10.1002/sim.3355. [DOI] [PubMed] [Google Scholar]
- 10. Gray AM, Clarke PM, Wolstenholme JL, Wordsworth S. Applied Methods of Cost‐Effectiveness Analysis in Health Care. Oxford, United Kingdom: Oxford University Press; 2011. [Google Scholar]
- 11. Vaduganathan M, Claggett BL, Juraschek SP, Solomon SD. Assessment of long‐term benefit of intensive blood pressure control on residual life span: secondary analysis of the Systolic Blood Pressure Intervention Trial (SPRINT). JAMA Cardiol. 2020;5:576–581. DOI: 10.1001/jamacardio.2019.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang Z, Kolm P, Grau‐Sepulveda MV, Ponirakis A, O'Brien SM, Klein LW, Shaw RE, McKay C, Shahian DM, Grover FL, et al. Cost‐effectiveness of revascularization strategies: the ASCERT study. J Am Coll Cardiol. 2015;65:1–11. DOI: 10.1016/j.jacc.2014.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Claggett B, Lachin JM, Hantel S, Fitchett D, Inzucchi SE, Woerle HJ, George JT, Zinman B. Long‐term benefit of empagliflozin on life expectancy in patients with type 2 diabetes mellitus and established cardiovascular disease. Circulation. 2018;138:1599–1601. DOI: 10.1161/CIRCULATIONAHA.118.033810. [DOI] [PubMed] [Google Scholar]
- 14. Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, Greenland P, Van Horn L, Tracy RP, Lloyd‐Jones DM. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366:321–329. DOI: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oelsner EC, Balte PP, Cassano PA, Couper D, Enright PL, Folsom AR, Hankinson J, Jacobs DR Jr, Kalhan R, Kaplan R, et al. Harmonization of respiratory data from 9 US population‐based cohorts: the NHLBI pooled cohorts study. Am J Epidemiol. 2018;187:2265–2278. DOI: 10.1093/aje/kwy139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Vittinghoff E, Pletcher MJ, Allen NB, Zeki Al Hazzouri A, Yaffe K, Balte PP, Alonso A, Newman AB, Ives DG, et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 2019;74:330–341. DOI: 10.1016/j.jacc.2019.03.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lambert PC, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9:265–290. DOI: 10.1177/1536867X0900900206. [DOI] [Google Scholar]
- 18. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. DOI: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 19. Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. DOI: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bagust A, Beale S. Survival analysis and extrapolation modeling of time‐to‐event clinical trial data for economic evaluation: an alternative approach. Med Decis Making. 2014;34:343–351. DOI: 10.1177/0272989X13497998. [DOI] [PubMed] [Google Scholar]
- 21. Kearns B, Stevens J, Ren S, Brennan A. How uncertain is the survival extrapolation? A study of the impact of different parametric survival models on extrapolated uncertainty about hazard functions, lifetime mean survival and cost effectiveness. Pharmacoeconomics. 2020;38:193–204. DOI: 10.1007/s40273-019-00853-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Latimer N. National Institute for Health and Care Excellence decision support unit technical support document 14: undertaking survival analysis for economic evaluations alongside clinical trials—extrapolation with patient‐level data. National Institute for Health and Care Excellence Decision Support Unit. Available at: http://www.nicedsu.org.uk. Accessed April 30, 2020. [PubMed] [Google Scholar]
- 23. Latimer NR. Survival analysis for economic evaluations alongside clinical trials–extrapolation with patient‐level data: inconsistencies, limitations, and a practical guide. Med Decis Making. 2013;33:743–754. DOI: 10.1177/0272989X12472398. [DOI] [PubMed] [Google Scholar]
- 24. Arias E, Xu J, Kochanek KD. United States life tables, 2016. Natl Vital Stat Rep. 2019;68:1–66. [PubMed] [Google Scholar]
- 25. Tremblay G, Livings C, Crowe L, Kapetanakis V, Briggs A. Determination of the most appropriate method for extrapolating overall survival data from a placebo‐controlled clinical trial of lenvatinib for progressive, radioiodine‐refractory differentiated thyroid cancer. Clinicoecon Outcomes Res. 2016;8:323–333. DOI: 10.2147/CEOR.S107498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cope S, Ayers D, Zhang J, Batt K, Jansen JP. Integrating expert opinion with clinical trial data to extrapolate long‐term survival: a case study of CAR‐T therapy for children and young adults with relapsed or refractory acute lymphoblastic leukemia. BMC Med Res Methodol. 2019;19:182. DOI: 10.1186/s12874-019-0823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davies C, Briggs A, Lorgelly P, Garellick G, Malchau H. The, "hazards" of extrapolating survival curves. Med Decis Making. 2013;33:369–380. DOI: 10.1177/0272989X12475091. [DOI] [PubMed] [Google Scholar]
- 28. Bress AP, Tanner RM, Hess R, Colantonio LD, Shimbo D, Muntner P. Generalizability of sprint results to the U.S. adult population. J Am Coll Cardiol. 2016;67:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S5
Figures S1–S7


