Abstract
Background
Abnormal cardiac repolarization is observed in patients with epilepsy and can be associated with sudden death. We investigated whether structural brain abnormalities are correlated with abnormal cardiac repolarizations in patients with seizure or epilepsy.
Methods and Results
We retrospectively analyzed and compared 12‐lead ECG parameters following seizures between patients with and without structural brain abnormalities. A total of 96 patients were included: 33 women (17 with and 16 without brain abnormality) and 63 men (44 with and 19 without brain abnormality). Brain abnormalities included past stroke, chronic hematoma, remote bleeding, tumor, trauma, and postsurgical state. ECG parameters were comparable for heart rate, PR interval, and QRS duration between groups. In contrast, corrected QT intervals evaluated by Fridericia, Framingham, and Bazett formulas were prolonged in patients with brain abnormality compared with those without (women: Fridericia [normal versus abnormal], 397.4±32.7 versus 470.9±48.9; P=0.002; Framingham, 351.0±40.1 versus 406.2±46.1; P=0.002; Bazett, 423.8±38.3 versus 507.7±56.6; P<0.0001; men: Fridericia, 403.8±30.4 versus 471.0±47.1; P<0.0001; Framingham, 342.7±36.4 versus 409.4±45.8; P<0.0001; Bazett, 439.3±38.6 versus 506.2±56.8; P<0.0001). QT dispersion and Tpeak−Tend intervals were comparable between groups. We also observed abnormal ST‐segment elevation in 5 patients. Importantly, no patients showed fatal arrhythmias during or after seizures.
Conclusions
Our study demonstrated that brain abnormalities can be associated with abnormal cardiac repolarization after seizures, which might be a manifestation of electrophysiological remodeling in the brain.
Keywords: QT prolongation, seizure, ST‐segment elevation, sudden death
Clinical Perspective
What Is New?
Our retrospective study showed that postictal QT intervals were longer in patients with structural brain abnormality compared with patients without structural brain abnormality.
Our study also showed that patients with postictal precordial ST‐segment elevation and incomplete right bundle‐branch block did not show fatal arrhythmias during and after seizure episodes.
What Are the Clinical Implications?
Our study suggests that careful cardiovascular follow‐up might be necessary in patients with seizures associated with structural brain abnormality.
Our study suggests that sudden unexpected death in epilepsy might not be caused by arrhythmias in patients without structural brain abnormality.
Patients with status epilepticus must be carefully treated regardless of ECG findings.
It has been proposed that epilepsy can be associated with sudden unexpected death, termed sudden unexpected death in epilepsy. 1 Nonsyndromic epilepsy is diagnosed when 2 unprovoked seizures occur 24 hours apart or the recurrence risk after the first unprovoked seizure is expected to be >60%. 2 The 2017 International League Against Epilepsy classification breaks seizures into 6 etiologic categories: genetic, structural, metabolic, immune, infectious, and unknown. 3 Structural causes refer to preexisting brain lesions or progressive nervous system disorders. These can be acquired (eg, hypoxic ischemic encephalopathy, stroke, trauma, infection, or tumors) or genetic (eg, tuberous sclerosis). 3 Regardless of its etiology, a seizure can be fatal, and the mechanism of death might be respiratory, cardiac, or autonomic. 4 , 5
There exists substantial interest in the underlying mechanisms of seizure‐related sudden death and abnormal cardiac repolarization. Interestingly, abnormal ECGs are often observed in patients with epilepsy even without ongoing seizures. For example, abnormal J‐wave and ST‐segment morphology and prolonged QT interval are more common in epileptic patients in comparison with control populations. 6 , 7 , 8 , 9 In contrast, abnormal ECG changes such as ST‐segment elevation, inverted T wave, and QT prolongation can often be seen after a brain insult such as ischemia or hemorrhage. 10 , 11 , 12 It has been hypothesized that these acute ECG changes might be attributable to the effects of increased sympathetic tone on the cardiovascular system. 13 , 14
The etiology of postictal ECG changes is currently unclear. It could be attributable to alterations in the autonomic nervous system coupling the brain and the heart. To test this hypothesis, the 2 organs need to be uncoupled anatomically or pharmacologically. The latter could be achieved via autonomic nervous system block using β‐blocking agents and atropine. 15 However, this is not practical in emergency situations. In this study, we sought to investigate whether chronic structural abnormalities in the brain can cause electrical remodeling in the autonomic regulation of the heart. To this end, we measured and compared ECG parameters in patients with and without structural brain abnormalities after seizure episodes.
Methods
Patient Selection
All data pertinent to this article are available upon request. This study was conducted in accordance with the Declaration of Helsinki and ethical guidelines for research involving human subjects. The study protocol was approved by the Ethical Committee at Tokyo Medical and Dental University (M2019‐341). As part of the approval, the Ethics Committee explicitly waived the need for an informed consent from individual patients since analyzed samples were deidentified.
Between January 1, 2017, and December 31, 2019, a total of 164 patients were transferred to our emergency department as a result of seizure. We included patients >15 years of age who had a witnessed seizure. Exclusion criteria were those with acute events such as stroke and brain trauma, known or suspected intoxication, preexisting cardiac disease, acute respiratory failure, electrolyte disturbances, hepatic disorders, and cardiopulmonary arrest. Patients taking levetiracetam and phenobarbital were excluded for QT interval measurement because of possible effects of these medications on cardiac repolarization. 16
Cases in which the ECGs showed intraventricular conduction disturbances such as complete right bundle‐branch block (RBBB) or left bundle‐branch block were excluded. Patients with incomplete RBBB with right precordial ST‐segment elevation were included in the study. However, their QT intervals were not analyzed. Patients with atrial fibrillation were also excluded because of relatively unreliable QT measurement.
The diagnosis of epilepsy was made by the patients' neurologist on the basis of the following definition: (1) at least 2 unprovoked seizures occurring >24 hours apart; or (2) one unprovoked seizure and a probability of further seizures similar to the general recurrence risk after 2 unprovoked seizures occurring over the next 10 years. This included the cases with remote structural lesions such as stroke, central nervous system infection, or certain types of traumatic brain injury. 3
ECG Recordings
Within 30 minutes after seizure onset, ECGs were recorded with CardiMax6 (Fukuda Denshi Co., Ltd., Tokyo, Japan) with a sampling rate of 8 kHz. Filter settings were alternating current, −20 dB or less at 50 to 60 Hz; electromyography, −3dB (−6 dB/octave) at 25 or 35 Hz; and drift, −3 dB at 0.25 or 0.5 Hz. QT intervals were evaluated in all 12 leads, and the following parameters were measured by a certified laboratory technologist and a cardiologist who was unaware of patients' history, and average values were used: the QT intervals, Tpeak−Tend intervals, QT dispersions, Tpeak−Tend, and Tpeak−Tend dispersions. 17 QT intervals were corrected by Fridericia, Framingham, and Bazett formulas. 18 , 19 ST‐segment elevation was defined as an upward deflection of the ST segment by ≥0.1 mV at the end of the QRS. 20 Saddle‐back ST‐segment elevation was defined as an upward deflection of the ST segment by ≥0.05 mV in leads V1–2. 21
Laboratory Testing
Venous blood samples were collected immediately after patients were transferred through a peripheral intravenous line. Leukocyte counts, pH, base excess, electrolytes, glucose, and lactate were measured.
Head Computed Tomography and Magnetic Resonance Imaging
Head computed tomography scans were performed in all patients by Aquilion 16 (Toshiba, Tokyo, Japan). To rule out acute cerebral ischemia, magnetic resonance imaging was checked depending on attending physicians' neurological evaluations. All imaging studies were separately adjudicated by board‐certified radiologists who were blind to patients' history.
Statistical Analysis
Data between the 2 groups were compared by Mann‐Whitney U test using Prism 8 (GraphPad Software, San Diego, CA). Linear regression fit was used for scatter plots using SPSS v26 (IBM, Armonk, NY). A P<0.05 was considered statistically significant.
Results
Patient Characteristics
Since women have longer QT intervals than men, 22 we divided patients into 4 groups: female or male patients with or without structural brain disease as diagnosed by medical history, physical examination, and imaging studies.
Table 1 shows the patient characteristics. Structural brain abnormalities included previous stroke, intracerebral hemorrhage, subarachnoid hemorrhage, brain tumors, and trauma. Patients with structural abnormalities were older than those without. Five (31.3%) female and 7 (36.8%) male patients without structural abnormalities had been previously diagnosed with epilepsy. Five (29.4%) female and 4 (9.1%) male patients with structural abnormality had been previously diagnosed with epilepsy. One male patient without structural abnormality had seizures previously without a diagnosis of epilepsy. All other patients in the study had their first seizure episode at the time of study conduct. Most patients who had a previous history of seizure or epilepsy were on antiepileptic drugs, listed in Table 1. Table 2 shows the computed tomography findings in patients with structural brain changes. Seven (41.2%) female and 24 (54.5%) male patients with structural brain changes showed ischemic findings. Table 3 shows the laboratory data following seizure episodes. All patients showed elevated lactate and creatine kinase, and low pH and base excess levels. Electrolytes were within normal limits. Laboratory findings were comparable between patients with and without brain abnormality in each sex.
Table 1.
Clinical Background
| Female Patients | Male Patients | |||
|---|---|---|---|---|
| Normal | Structural | Normal | Structural | |
| No. | 16 | 17 | 19 | 44 |
| Age | 47.3±24.7 | 66.8±11.9* | 49.4±16.5 | 61.5±14.1 † |
| Nonneurological diseases | ||||
| Yes | 1 | 0 | 3 | 3 |
| History of neural disease | ||||
| Epilepsy | 5 | 5 | 7 | 4 |
| Seizure (undiagnosed) | … | … | 1 | … |
| Infarction | … | 2 | … | 4 |
| Cerebral bleeding | … | 1 | … | 5 |
| Subarachnoid hemorrhage | … | 1 | … | 2 |
| Injury | … | … | … | 2 |
| Tumor | … | 5 | … | 4 |
| Hippocampal sclerosis | … | 2 | … | … |
| Aneurysm/AVM | … | … | … | 3 |
| Medication | ||||
| Phenytoin | … | … | 1 | 2 |
| Clonazepam | … | … | … | … |
| Diazepam | … | … | … | 1 |
| Lacosamide | … | 1 | 1 | … |
| Suvorexant | … | … | … | 1 |
| Valproic acid | 3 | 2 | 2 | … |
| Zonisamide | … | … | … | 2 |
AVM indicates arteriovenous malformation.
P=0.04 vs normal.
P=0.008 vs normal.
Table 2.
Computed Tomography Findings
| Female Patients | Male Patients | |||
|---|---|---|---|---|
| Normal | Structural | Normal | Structural | |
| No. | 16 | 17 (%) | 19 | 44 (%) |
| Infarction/ischemic change | … | 7 (41.2) | … | 24 (54.5) |
| Chronic hematoma | … | 1 (5.9) | … | 1 (2.3) |
| Remote bleeding | … | 4 (9.1) | ||
| Tumor | … | 3 (20.0) | … | 4 (9.1) |
| Injury | … | … | … | … |
| Postsurgery | … | 2 (11.8) | … | 7 (15.9) |
| Hemangioma/AVM | … | 1 (5.9) | … | 1 (2.3) |
| Brain defects/Atrophy | … | 2 (11.8) | … | … |
AVM indicates arteriovenous malformation.
Table 3.
Laboratory Data
| Female Patients | P Value | Normal | P Value | |||
|---|---|---|---|---|---|---|
| Normal | Structural | Normal | Structural | |||
| No. | 16 | 17 | 19 | 44 | ||
| Lactate, mmol/L | 6.32±5.49 | 6.41±3.26 | 0.660 | 8.91±6.55 | 9.01±8.20 | 0.568 |
| BE, mEq/L | −4.30±6.56 | −4.61±4.56 | 0.435 | −9.16±8.46 | −5.66±9.54 | 0.144 |
| pH | 7.27±0.17 | 7.19±0.15 | 0.147 | 7.23±0.19 | 7.22±0.20 | 0.865 |
| WBC, 103/mm3 | 9.320±4.443 | 8.464±2.582 | 0.704 | 8.205±2.731 | 9.996±4.120 | 0.904 |
| CK, g/dL | 216.8±460.2 | 148.7±107.7 | 0.352 | 240.6±158.6 | 426.7±38.5 | 0.131 |
| Na, mEq/L | 139.8±2.2 | 138.8±5.14 | 0.764 | 139.9±4.7 | 141.3±4.7 | 0.215 |
| K, mEq/L | 4.33±1.02 | 4.04±0.87 | 0.418 | 3.90±0.50 | 3.96±0.64 | 0.703 |
| Cl, mEq/L | 103.3±5.2 | 101.7±5.6 | 0.368 | 102.1±6.5 | 101.8±6.0 | 0.748 |
| Ca, mg/dL | 9.13±0.49 | 9.22±0.34 | 0.904 | 9.08±0.61 | 9.14±0.68 | 0.810 |
Normal ranges: lactate, 0.5 to 2.2; BE (base excess), −2 to +2; pH, 7.31 to 7.41; WBC (white blood cell), 4.5 to 11.0; CK (creatine kinase), 22 to 198; sodium (Na), 135 to 145; potassium (K), 3.6 to 5.2; chloride (Cl), 96 to 106; calcium (Ca), 8.6 to 10.3.
Prolonged QT Intervals After Seizure Episodes in Patients With Structural Brain Abnormalities
Figure 1 shows representative ECG traces in patients without and with structural brain abnormality. Compared with the female patients without structural brain abnormality (Figure 1A), female patients with structural brain abnormality showed longer postictal QT intervals (Figure 1B). Similarly, compared with the normal male patients (Figure 1C), the QT intervals were prolonged in the patients with brain abnormality (Figure 1D). Table 4 summarizes the ECG parameters in each group. Compared with the patients without brain abnormality, QT intervals and corrected QT intervals by Fridericia, Framingham, and Bazett formulas were significantly longer in patients with structural brain abnormality in both sexes (Table 4, Table S1). Figure S1 shows scatter plots of QTc intervals as a function of RR intervals. The plots were fitted with linear regression, yielding a slope of −0.087 for the QTc intervals with the Fridericia formula in male patients without brain abnormality (Figure S1C) and slopes of 0.153 to 0.171 for the Framingham formula in both sexes without brain abnormality and male patients with brain abnormality (Figure S1A, S1C and S1D). 18 , 23 QTc intervals were also plotted against lactate values for each group. Figure S2 shows that there was no clear relationship between QTc intervals and lactate values.
Figure 1. Representative ECG traces following seizure episodes.
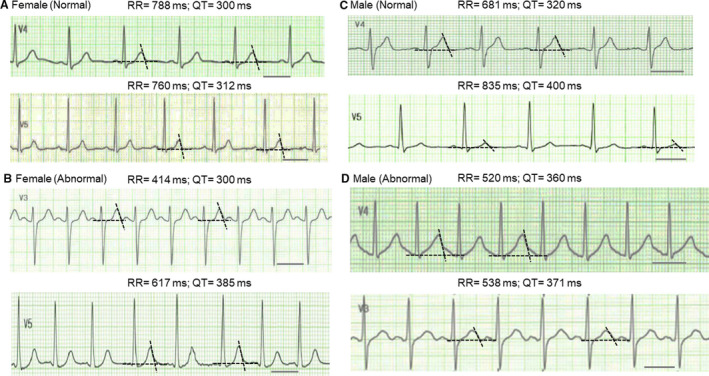
A, ECG traces from a 44‐year‐old female patient (upper panel) and a 37‐year‐old female patient (lower panel) without structural brain abnormality. The lead showing the longest QT interval was selected for each patient. The black bar indicates 400 ms. The average values of 2 measurements by 2 examiners were shown for RR and QT intervals. B, ECG traces from a 76‐year‐old female patient with brain abnormality (upper panel) and an 83‐year‐old female patient with cerebral infarction (lower panel). C, ECG traces from a 49‐year‐old male patient (upper panel) and a 48‐year‐old male patient (lower panel) without brain abnormality. D, ECG traces from a 73‐year‐old male patient with chronic subdural hematoma (upper panel) and a 48‐year‐old male patient who had surgery for brain tumor (lower panel).
Table 4.
ECG Parameters
| Female Patients | P Value | Male Patients | P Value | |||
|---|---|---|---|---|---|---|
| Normal | Structural | Normal | Structural | |||
| No. | 16 | 17 | 19 | 44 | ||
| RR, ms | 701.8±171.2 | 651.4±130.0 | 0.682 | 624.0±155.1 | 668.9±148.8 | 0.182 |
| PR, ms | 137.4±20.0 | 143.5±16.6 | 0.395 | 152.2±26.8 | 160.1±33.5 | 0.281 |
| QRS, ms | 101.9±16.5 | 95.8±10.6 | 0.483 | 101.7±9.2 | 99.6±13.2 | 0.264 |
| QT, ms | 351.0±40.0 | 406.2±46.1 | 0.003 | 342.7±36.4 | 409.3±45.8 | 0.000* |
| QT dispersion | 46.8±17.9 | 48.2±22.9 | 0.478 | 48.2±22.9 | 51.1±27.2 | 0.771 |
| Tpeak−Tend, ms | 128.6±48.0 | 123.4±50.3 | 0.697 | 125.6±40.1 | 128.0±32.0 | 0.373 |
| Tpeak−Tend dispersion | 30.4±25.3 | 32.8±30.2 | 0.689 | 37.9±22.2 | 40.1±22.3 | 0.435 |
| Tpeak−Tend/QT | 0.36±0.14 | 0.28±0.07 | 0.177 | 0.36±0.10 | 0.31±0.06 | 0.052 |
| Fridericia formula | ||||||
| Corrected QT | 397.4±32.7 | 470.9±48.9 | 0.002 | 403.8±30.4 | 471.0±47.1 | 0.000* |
| Corrected QT dispersion | 48.8±21.4 | 44.6±25.5 | 0.631 | 57.1±28.4 | 58.8±31.7 | 0.857 |
| Framingham formula | ||||||
| Corrected QT | 351.0±40.1 | 406.2±46.1 | 0.002 | 342.7±36.4 | 409.4±45.8 | 0.000* |
| Corrected QT dispersion | 43.6±19.4 | 39.7±22.6 | 0.818 | 48.3±22.9 | 51.2±27.2 | 0.865 |
| Bazzet formula | ||||||
| Corrected QT | 423.8±38.3 | 507.7±56.6 | 0.000* | 439.3±38.6 | 506.2±56.8 | 0.000* |
| Corrected QT dispersion | 48.8±27.4 | 48.0±27.4 | 0.841 | 55.7±35.6 | 57.4±37.6 | 0.818 |
<0.0001.
Other parameters including RR intervals, PR intervals, and QRS duration were comparable between patients with and without structural brain abnormality. In addition, QT dispersion and Tpeak−Tend intervals were comparable between the patients with and without brain abnormality.
Figure 2 shows initial and follow‐up ECGs in 6 patients. In 3 patients with no structural brain abnormality, QTc intervals in follow‐up ECGs did not show a consistent pattern (left panel). In contrast, in the 3 patients with structural brain abnormality, QTc intervals in follow‐up ECGs became shorter than the initial postictal ECGs (right panel).
Figure 2. Comparison of postictal ECG traces with follow‐up ECG traces.
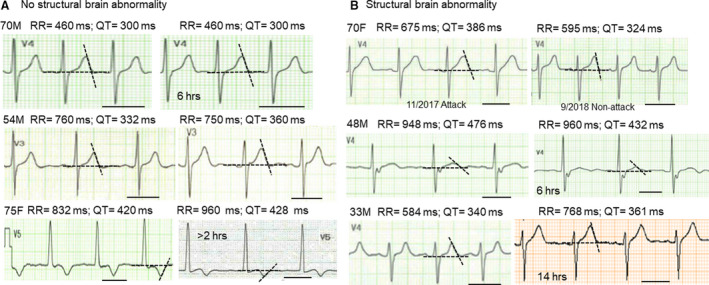
A, ECG traces obtained after seizure episodes and follow‐up ECG traces in 3 patients without brain abnormality. The lead showing the longest QT interval was selected for each patient. The black bar indicates 400 ms. The average values of 2 measurement (2 personnel) were shown for RR and QT intervals. B, ECG traces obtained after seizure episodes and follow‐up ECG traces in 3 patients with brain abnormality: brain tumor surgery (upper panel); brain trauma (mid panel); and metastatic brain tumor (lower panel). QTc intervals (Fridericia): initial, 443, 485, 407 ms vs follow‐up, 400, 438, 394 ms. Note that the follow‐up time varied depending on the patient. F indicates female; and M, male.
Abnormal ST‐T Changes After Seizure Episodes
We also observed ST‐segment elevation and abnormal T waves in 5 patients after seizure episodes. Since these patients showed an incomplete RBBB pattern, these patients were not included in the evaluation of QT intervals. Figure 3A shows ST‐segment elevation in leads V1–2 in a 53‐year‐old man without structural brain abnormality. Preexcitation cannot be ruled out. Figure 3B shows coved‐type (V1) and saddleback‐type (V2) ST‐segment elevation with incomplete RBBB in a 53‐year‐old man without apparent structural abnormality. Figure 3C shows nonspecific ST‐segment elevation in leads V2–4 and first‐degree atrioventricular block in an 87‐year‐old man with prior ischemic stroke. Figure 3D shows saddleback‐type ST‐segment elevation with incomplete RBBB in leads V2–3 in a 78‐year‐old man with prior ischemic stroke. Figure 3E shows nonspecific ST‐segment elevation in lead V2 observed in a 61‐year‐old man with epilepsy who was treated with levetiracetam and lacosamide. He was recommended admission for observation, but he refused. He was found dead 2 days later at his house. None of these patients showed arrhythmia in the ambulance or emergency department.
Figure 3. ECG traces and 12‐lead ECG after seizure attacks.
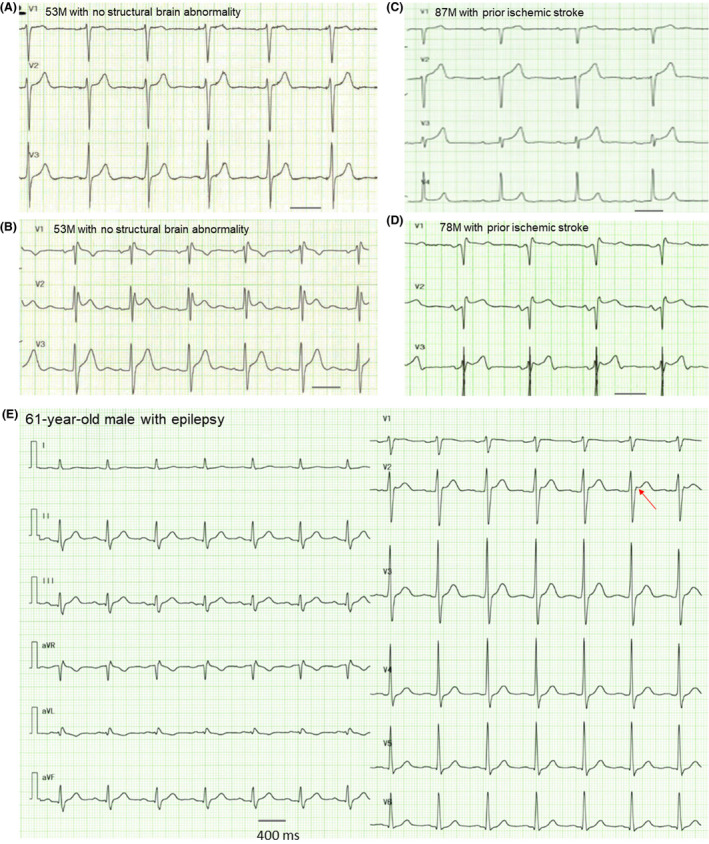
Precordial leads obtained from 4 patients showing ST‐segment elevation (A through D). A 12‐lead ECG obtained a patient who died suddenly 2 days after discharge. Black bars indicate 400 ms. (E). Clinical information is described in the text. F indicates female; and M, male.
Discussion
Sudden cardiac death can be caused by genetic arrhythmic syndromes such as long‐QT syndrome, Brugada syndrome, J‐wave syndrome, and short‐QT syndrome. In some cases, these syndromes can be diagnosed by a 12‐lead ECG. 24 However, prediction methods have not been established for sudden unexpected death in epilepsy. 25 In this study, we evaluated 12‐lead ECG parameters after seizure episodes in patients transferred to our emergency department who met our inclusion criteria. ECG parameters were compared between patients with or without structural brain abnormalities based on medical history and computed tomography and magnetic resonance imaging findings. Our data showed that QT intervals were longer in patients with structural brain abnormalities than in the patients without them. Additionally, patients with abnormal right precordial ST‐segment elevation did not show hemodynamically unstable arrhythmias during seizure episodes.
Abnormal ECG Parameters in Primary Seizure Episodes
In an attempt to predict arrhythmias in patients with epilepsy, various ECG parameters have been studied. Dagar et al 26 studied 103 patients with epilepsy and found that QTc, QTc dispersion, Tpeak−Tend intervals, and Tpeak−Tend/QTc ratio were significantly higher in patients with epilepsy than in control subjects. Although abnormal repolarization (eg, QT prolongation) can be a marker of sudden cardiac death, 27 the meaning of these parameter changes in seizures remains unknown. Hayashi et al 6 studied 354 patients with epilepsy and found that abnormal early repolarization such as J‐wave elevation (≥0.1 mV) was seen in ≈20% of patients with epilepsy. J‐wave changes such as notching and slurring, and ST‐segment elevation are known risk factors for sudden cardiac death, which are known as J‐wave syndromes. 28 However, it is not easy to differentiate primary epilepsy and seizures caused by arrhythmia‐induced brain ischemia. Indeed, Auerbach et al 9 reported that longer QT intervals were predictive of seizures in long‐QT syndrome cohorts. However, the authors stated that arrhythmia‐induced seizures could not be ruled out. Brotherstone et al 19 continuously monitored electroencephalographs and ECGs during seizure episodes. Analyses of single‐lead ECGs in 39 patients revealed that QTc intervals were prolonged in 21 of 156 seizure episodes. Interestingly, maximal prolongation was observed in cases of right temporal lobe seizures. In our study, patients without structural brain abnormality did not show significant QT prolongation in either sex following seizure episodes. We were able to observe follow‐up ECGs in 3 patients without structural abnormality. QTc intervals several hours after seizure episodes did not change in 1 patient, became longer in 1 patient, and became shorter in 1 patient (Figure 2). These data indicate that QT prolongation may not provoke seizures in patients with epilepsy. In addition, we observed abnormal right precordial ST‐segment changes in several male patients without structural abnormality (Figure 3A through 3D). Since none of them showed arrhythmias during seizures, arrhythmia can be ruled out as the cause of seizure in these patients. Importantly, a patient who showed nonspecific ST‐segment elevation in lead V2 died suddenly 2 days after discharge, indicating that a cardiac cause was less likely (Figure 3E).
Abnormal ECG Parameters in Seizures Associated With Structural Brain Abnormalities
It is well known that acute stroke can cause abnormal ECG patterns. For example, subarachnoid hemorrhage can cause ST‐segment elevation mimicking myocardial infarction, 29 and giant negative T waves. 30 Acute occlusion of the right middle cerebral artery can cause inverted T waves and QTc prolongation. 31 , 32 , 33 These changes are so‐called heart stress or excess activation of autonomic tone affecting cardiac electrophysiological characteristics. However, if sympathetic tone is increased, QT intervals are supposed to shorten because of activation of the slow component of the delayed rectifier K+ currents. 17 In contrast, if parasympathetic tone is increased, acetylcholine‐induced K+ channels can be activated, resulting in the shortening of QT intervals. 34 Nonetheless, stellate ganglionectomy has been performed for refractory torsades de pointes in long‐QT syndromes. 35 This strongly supports the hypothesis that brain and nervous system function contribute largely to the regulation of cardiac electrophysiological properties.
Although ECGs in the absence of seizures were not available in all patients, the prevalence of QT prolongation is far more than the expected prevalence of long‐QT syndrome, which is ≈1 of 2534. 36 These findings may indicate that functional remodeling might take place more often in the damaged brain than in structurally normal ones. Alternatively, if seizure‐related abnormal cardiac repolarization is attributable to the changes in autonomic tone, then the regulation of the autonomic nervous system might differ in injured brains and structurally normal ones. This concept has been well studied in heart disease and termed cardiac memory: “atrial fibrillation begets atrial fibrillation.” 37 It is reasonable to speculate that electrophysiological remodeling can occur in the brain regardless of any structural disease. Structural changes may form obstacle(s) to cause reentrant circuits or turbulences.
Can Abnormal Repolarization After Seizure Episodes Predict Sudden Death?
Another important question is “What does abnormal cardiac repolarization mean in patients with seizure?” Despite many previous studies, we still do not know the role of abnormal cardiac repolarization in sudden unexpected death in epilepsy. In our study, patients with structural brain abnormalities showed QT prolongation after episodes of seizure. Although prolonged QT intervals can be a risk factor for sudden cardiac death, 27 these patients did not show any signs of fatal arrhythmias. In addition, our patients did not show difference in QT dispersion or Tpeak–Tend intervals, which can predict sudden cardiac death, 38 in either group. Furthermore, all patients who showed abnormal right precordial ST‐segment elevation, which might be associated with arrhythmia syndromes, did not have any arrhythmias during seizure episodes. Importantly, a 61‐year‐old male patient without apparent structural brain abnormality died suddenly 2 days after his emergency department visit. His postictal ECG did not show QT prolongation, but it showed subtle ST‐segment elevation and notching of the J point in lead V2 (Figure 3E). Since an autopsy including molecular genetic studies could not be performed, we do not know the exact cause of his sudden cardiac death. These data indicate that abnormal repolarization after seizure episodes may be manifestations of short‐term brain remodeling. However, it is less likely that this neural remodeling causes sudden cardiac death.
Limitations
There are several limitations:
This is a single‐center study, and our sample size was small. In addition, since multiple ECGs (eg, at different heart rates) could not be obtained for most patients, QTc/RR plots (Figure S1) did not produce good fits except for the Framingham formula.
We could not analyze ECGs on the basis of the types of seizures since only patients with generalized seizures were transferred to our center.
The duration of seizure episodes varied; however, the laboratory parameters were not significantly different among the groups. Importantly, Figure S2 did not show any clear correlation between QTc intervals and lactate values.
Time to postictal ECGs were not exactly the same among patients since ECGs could not be obtained at the time after seizure in some cases.
Since QT intervals can prolong with age, 39 our comparison between normal and abnormal groups might have been overestimated. However, it has been reported that the difference of QTc intervals among different generations was less than a few milliseconds, 40 which was smaller than the difference in our data. 40 Also, it has been reported that QTc intervals shorten in older age groups in the Japanese population (>60 years). 39
We were able to obtain follow‐up ECGs for only a few patients, and we were unable to rule out congenital long‐QT syndrome in most of the patients.
Although seizure episodes may affect cardiac repolarization and conduction, we could not analyze the cases with RBBB or left bundle‐branch block since the number of patients was not adequate, and correction methods for QT intervals might not have been established despite the fact that some formulas exist. 34 , 41
Ischemia in microcirculation could not be completely ruled out.
It was difficult to prove that the seizures were associated with abnormal brain activity because electroencephalographs were not recorded in the ER.
Because this is a retrospective study, we do not have any genetic information including germline and somatic variants. Currently, we are analyzing genomic DNA information in a new study, and we are planning to obtain brain tissues from patients with seizure with structural abnormalities in the future.
In conclusion, our data indicate that structural brain abnormalities may play a role in abnormal cardiac repolarization after seizure episodes. Additionally, our findings show that abnormal repolarization is not obviously manifest in idiopathic seizures.
Sources of Funding
This study was partially supported by Japan Society for the Promotion of Science 18K08900 (Dr Ai) and by 5T32CA217834‐03 VIMORTP (Vanderbilt Integrated Molecular Oncology Research Training Program) (Dr Turker).
Disclosures
None.
Supporting information
Table S1
Figures S1–S2
Acknowledgments
The authors are grateful to the patients who participated in this study. The authors also thank Dr Seiko Ohno for her critical reading of manuscript.
(J Am Heart Assoc. 2021;10:e019778. DOI: 10.1161/JAHA.120.019778.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.019778
For Sources of Funding and Disclosures, see page 9.
References
- 1. Ryvlin P, Nashef L, Tomson T. Prevention of sudden unexpected death in epilepsy: a realistic goal? Epilepsia. 2013;54(suppl 2):23–28. DOI: 10.1111/epi.12180. [DOI] [PubMed] [Google Scholar]
- 2. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J Jr, Forsgren L, French JA, Glynn M, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. DOI: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 3. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, Hirsch E, Jain S, Mathern GW, Moshé SL, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. DOI: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aukland P, Lando M, Vilholm O, Christiansen EB, Beier CP. Predictive value of the Status Epilepticus Severity Score (STESS) and its components for long‐term survival. BMC Neurol. 2016;16:213. DOI: 10.1186/s12883-016-0730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nobis WP, Gonzalez Otarula KA, Templer JW, Gerard EE, VanHaerents S, Lane G, Zhou G, Rosenow JM, Zelano C, Schuele S. The effect of seizure spread to the amygdala on respiration and onset of ictal central apnea. J Neurosurg. 2019;132:1313–1323. DOI: 10.3171/2019.1.JNS183157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hayashi K, Kohno R, Akamatsu N, Benditt DG, Abe H. Abnormal repolarization: a common electrocardiographic finding in patients with epilepsy. J Cardiovasc Electrophysiol. 2019;30:109–115. DOI: 10.1111/jce.13746. [DOI] [PubMed] [Google Scholar]
- 7. Kishk NA, Sharaf Y, Ebraheim AM, Baghdady Y, Alieldin N, Afify A, Eldamaty A. Interictal cardiac repolarization abnormalities in people with epilepsy. Epilepsy Behav. 2018;79:106–111. DOI: 10.1016/j.yebeh.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 8. de Sousa JM, Fialho GL, Wolf P, Walz R, Lin K. Determining factors of electrocardiographic abnormalities in patients with epilepsy: a case‐control study. Epilepsy Res. 2017;129:106–116. DOI: 10.1016/j.eplepsyres.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 9. Auerbach DS, McNitt S, Gross RA, Zareba W, Dirksen RT, Moss AJ. Genetic biomarkers for the risk of seizures in long QT syndrome. Neurology. 2016;87:1660–1668. DOI: 10.1212/WNL.0000000000003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Komatsuzaki M, Takasusuki T, Kimura Y, Yamaguchi S. Assessment of the ECG T‐wave in patients with subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2021;33:58–64. DOI: 10.1097/ANA.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 11. Sugimoto K, Yamada A, Inamasu J, Hirose Y, Takada K, Sugimoto K, Tanaka R, Watanabe E, Ozaki Y. Electrocadiographic scoring helps predict left ventricular wall motion abnormality commonly observed after subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2018;27:3148–3154. DOI: 10.1016/j.jstrokecerebrovasdis.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 12. Laundon RK, Littmann L. Spiked helmet pattern ST elevation in subarachnoid hemorrhage. J Electrocardiol. 2019;52:96–98. DOI: 10.1016/j.jelectrocard.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 13. Lele A, Lakireddy V, Gorbachov S, Chaikittisilpa N, Krishnamoorthy V, Vavilala MS. A narrative review of cardiovascular abnormalities after spontaneous intracerebral hemorrhage. J Neurosurg Anesthesiol. 2019;31:199–211. DOI: 10.1097/ANA.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 14. Mulcahy J, Johnson P, James M. Electrocardiogram qt interval increases in acute stroke. Cerebrovasc Dis. 2010;29:178–180. DOI: 10.1159/000262315. [DOI] [PubMed] [Google Scholar]
- 15. Jose AD, Taylor RR. Autonomic blockade by propranolol and atropine to study intrinsic myocardial function in man. J Clin Invest. 1969;48:2019–2031. DOI: 10.1172/JCI106167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Siniscalchi A, Scaglione F, Sanzaro E, Iemolo F, Albertini G, Quirino G, Manes MT, Gratteri S, Mercuri NB, De Sarro G, et al. Effects of phenobarbital and levetiracetam on PR and QTc intervals in patients with post‐stroke seizure. Clin Drug Investig. 2014;34:879–886. DOI: 10.1007/s40261-014-0243-9. [DOI] [PubMed] [Google Scholar]
- 17. Takenaka K, Ai T, Shimizu W, Kobori A, Ninomiya T, Otani H, Kubota T, Takaki H, Kamakura S, Horie M. Exercise stress test amplifies genotype‐phenotype correlation in the LQT1 and LQT2 forms of the long‐QT syndrome. Circulation. 2003;107:838–844. DOI: 10.1161/01.CIR.0000048142.85076.A2. [DOI] [PubMed] [Google Scholar]
- 18. Vandenberk B, Vandael E, Robyns T, Vandenberghe J, Garweg C, Foulon V, Ector J, Willems R. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc. 2016;5:e004252. DOI: 10.1161/JAHA.117.004252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brotherstone R, Blackhall B, McLellan A. Lengthening of corrected QT during epileptic seizures. Epilepsia. 2010;51:221–232. DOI: 10.1111/j.1528-1167.2009.02281.x. [DOI] [PubMed] [Google Scholar]
- 20. Pargaonkar VS, Perez MV, Jindal A, Mathur MB, Myers J, Froelicher VF. Long‐term prognosis of early repolarization with J‐wave and QRS slur patterns on the resting electrocardiogram: a cohort study. Ann Intern Med. 2015;163:747–755. DOI: 10.7326/M15-0598. [DOI] [PubMed] [Google Scholar]
- 21. Curcio A, Mazzanti A, Bloise R, Monteforte N, Indolfi C, Priori SG, Napolitano C. Clinical presentation and outcome of Brugada syndrome diagnosed with the new 2013 criteria. J Cardiovasc Electrophysiol. 2016;27:937–943. DOI: 10.1111/jce.12997. [DOI] [PubMed] [Google Scholar]
- 22. Salama G, Bett GC. Sex differences in the mechanisms underlying long QT syndrome. Am J Physiol Heart Circ Physiol. 2014;307:H640–H648. DOI: 10.1152/ajpheart.00864.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Phan DQ, Silka MJ, Lan YT, Chang RK. Comparison of formulas for calculation of the corrected QT interval in infants and young children. J Pediatr. 2015;166:960–964.e961–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang C‐E, Huikuri H, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. DOI: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 25. Bagnall RD, Crompton DE, Semsarian C. Genetic basis of sudden unexpected death in epilepsy. Front Neurol. 2017;8:348. DOI: 10.3389/fneur.2017.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dagar S, Emektar E, Corbacioglu SK, Demirci OL, Tandogan M, Cevik Y. Evaluation of electrocardiographic parameters in patients with epileptic seizure. Acta Neurol Belg. 2020;120:321–327. DOI: 10.1007/s13760-019-01182-8. [DOI] [PubMed] [Google Scholar]
- 27. Schwartz PJ, Crotti L. QTc behavior during exercise and genetic testing for the long‐QT syndrome. Circulation. 2011;124:2181–2184. DOI: 10.1161/CIRCULATIONAHA.111.062182. [DOI] [PubMed] [Google Scholar]
- 28. Antzelevitch C, Yan GX, Ackerman MJ, Borggrefe M, Corrado D, Guo J, Gussak I, Hasdemir C, Horie M, Huikuri H, et al. J‐wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. Europace. 2017;19:665–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park I, Kim YJ, Ahn S, Sohn CH, Seo DW, Kim WY. Subarachnoid hemorrhage mimicking ST‐segment elevation myocardial infarction after return of spontaneous circulation. Clin Exp Emerg Med. 2015;2:260–263. DOI: 10.15441/ceem.15.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Said SA, Bloo R, de Nooijer R, Slootweg A. Cardiac and non‐cardiac causes of T‐wave inversion in the precordial leads in adult subjects: a Dutch case series and review of the literature. World J Cardiol. 2015;7:86–100. DOI: 10.4330/wjc.v7.i2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blech B, O'Carroll C. Acute right middle cerebral artery occlusion resulting in acute systolic heart failure, cerebral T‐waves, and QTc prolongation: a case report. Neurologist. 2018;23:135–137. DOI: 10.1097/NRL.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 32. Simula S, Muuronen AT, Taina M, Jakala P, Sipola P, Vanninen R, Hedman M. Effect of middle cerebral artery territory ischemic stroke on QT interval. J Stroke Cerebrovasc Dis. 2014;23:717–723. DOI: 10.1016/j.jstrokecerebrovasdis.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 33. Henninger N, Haussen DC, Kakouros N, Selim M, Searls DE, Kumar S, Schlaug G, Caplan LR. QTc‐prolongation in posterior circulation stroke. Neurocrit Care. 2013;19:167–175. DOI: 10.1007/s12028-013-9873-7. [DOI] [PubMed] [Google Scholar]
- 34. Yang Y, Yang Y, Liang BO, Liu J, Li J, Grunnet M, Olesen S‐P, Rasmussen HB, Ellinor PT, Gao L, et al. Identification of a Kir3.4 mutation in congenital long QT syndrome. Am J Hum Genet. 2010;86:872–880. DOI: 10.1016/j.ajhg.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reardon PR, Matthews BD, Scarborough TK, Preciado A, Marti JL, Conklin LD, Garson A Jr, Reardon MJ. Left thoracoscopic sympathectomy and stellate ganglionectomy for treatment of the long QT syndrome. Surg Endosc. 2000;14:86. DOI: 10.1007/s004649901209. [DOI] [PubMed] [Google Scholar]
- 36. Schwartz PJ, Stramba‐Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, Gabbarini F, Goulene K, Insolia R, Mannarino S, et al. Prevalence of the congenital long‐QT syndrome. Circulation. 2009;120:1761–1767. DOI: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–1968. DOI: 10.1161/01.CIR.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 38. Tse G, Yan BP. Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. Europace. 2017;19:712–721. DOI: 10.1093/europace/euw280. [DOI] [PubMed] [Google Scholar]
- 39. Vink AS, Clur SB, Wilde AAM, Blom NA. Effect of age and gender on the QTc‐interval in healthy individuals and patients with long‐QT syndrome. Trends Cardiovasc Med. 2018;28:64–75. DOI: 10.1016/j.tcm.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 40. Rabkin SW, Cheng XJ, Thompson DJ. Detailed analysis of the impact of age on the QT interval. J Geriatr Cardiol. 2016;13:740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Talbot S. QT interval in right and left bundle‐branch block. Br Heart J. 1973;35:288–291. DOI: 10.1136/hrt.35.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figures S1–S2


