Dispersion of repolarization has been associated with life‐threatening arrhythmias. Already in 1940, Wiggers related regional disparities in ventricular repolarization times to susceptibility for ventricular arrhythmias. 1 Nonuniform repolarization induces nonuniform recovery of excitability and consequently asymmetrical conduction (“unidirectional block”) of a premature beat that can initiate circus‐movement reentry. 2 A second mechanism, termed phase‐2 reentry, 3 has also been proposed as an arrhythmic mechanism in ischemia and Brugada syndrome. In phase‐2 reentry, electrotonic current flows intracellularly from still depolarized tissue to tissue that has already repolarized, providing depolarizing charge for spontaneous generation of a premature action potential (AP). For both mechanisms, greater disparity in repolarization over a shorter distance (steep dispersion) is likely to be more arrhythmogenic. The spatial steepness of repolarization gradients is also affected by temporal repolarization properties, including restitution properties of the tissue 4 and presence of discordant repolarization alternans. 5
At the cellular level, the depolarization phase of a normal AP (its upstroke) relies on a single ionic current, the fast sodium current. It is a large current that generates the AP with a large margin of safety. This mechanism is consistent with the requirement that AP generation should be a robust “all or none” process. In contrast, AP repolarization relies on complex multicurrent interactions. A delicate balance of depolarizing and repolarizing currents during the plateau and repolarization phases of the AP provides for sensitive and tight control of the AP duration (APD) and its dependence on rate. Although this property is essential for normal function, the delicate balance of currents can be easily perturbed by pathological features (channelopathies) or drugs, causing a nonlinear large change in repolarization and the APD. Typically, the distribution of pathological features or drug in the myocardium is nonuniform, inducing regional differences in repolarization. Even if the distribution were uniform, it occurs on the background of a heterogeneous substrate (ion‐channel expression levels vary with location), perturbing the delicate balance of currents in a location‐dependent manner. Given the nonlinear, large response of repolarization to perturbations in the delicate balance of ionic currents, it is not surprising that steep dispersion of repolarization is the arrhythmic substrate in many cardiac pathological conditions and a consequence of the effects of drugs that block cardiac repolarizing ion channels, in particular the rapid delayed rectifier.
The first mechanism of reentry described above is the more common, universal mechanism. It requires a substrate of steep repolarization gradients and a trigger in the form of a premature beat. Abnormal repolarization can cause both. The substrate was described above. In the case of abnormally prolonged repolarization (eg, the long‐QT syndrome [LQTS]), calcium channels can recover and reactivate during the plateau phase, generating a secondary depolarization called early afterdepolarization. In case of abnormally abbreviated repolarization, the early return to baseline favors generation of delayed afterdepolarizations following the AP under conditions of abnormal calcium cycling (eg, in heart failure). If the early afterdepolarization or delayed afterdepolarization reaches the threshold for synchronized AP generation in sufficient number of cells, a premature beat is generated, providing the arrhythmogenic trigger. It should be recognized that the occurrence of a reentrant arrhythmia is a probabilistic event; the premature wave front must encounter the steep‐dispersion substrate from the right direction, with the right orientation and at the right time during a “vulnerable window” for reentry to occur. 6 , 7
The arrhythmic properties of steep repolarization dispersion identify detection of this substrate as a diagnostic strategy and as a potential prognostic strategy for identifying patients at risk of sudden cardiac death. This approach requires a noninvasive method for detection and quantification of disparity of repolarization in the myocardium. Indeed, QT‐interval dispersion in the body‐surface ECG was proposed and tried as an index of dispersion of repolarization in the heart. 8 , 9 Unfortunately (but predictably), this approach has met with limited success because the signal in each body‐surface electrode measures the integrated activity over the entire heart. Therefore, geometrical relationships between cardiac wave fronts and electrical sources in the heart are not preserved in the body surface potential distribution. Clearly, a noninvasive method for mapping potentials and electrograms in the heart is needed for evaluating the spatiotemporal properties of myocardial repolarization and quantifying its dispersion. Electrocardiographic imaging (ECGI) is a novel method for noninvasive mapping of activation and repolarization on the epicardial surface of the heart; it is suitable for this purpose.
ECGI, when formulated in terms of potentials, constructs electrograms over the entire epicardial surface of the heart with high resolution. From the electrograms, local activation and recovery times can be determined and activation‐recovery intervals (a measure of local APD) can be computed, providing activation‐isochrone maps and repolarization‐pattern maps on the heart surface noninvasively. 10 , 11 Early demonstration of ECGI's ability to detect and map myocardial dispersion of repolarization was obtained using a hybrid experimental‐modeling approach. 12 Dispersion of repolarization was introduced by regional myocardial warming (shortening APD) and cooling (prolonging APD). The repolarization patterns and dispersion were faithfully reconstructed by ECGI. In this issue of the Journal of the American Heart Association (JAHA), Bear et al 13 report on a fully experimental and well‐controlled approach, designed to evaluate ECGI reconstruction of repolarization abnormalities and in particular myocardial dispersion of repolarization. Isolated pig hearts (n=8) and a human donor heart were suspended in a human torso‐shaped electrolytic tank. Another, in vivo set of experiments was conducted in closed‐chest pigs (n=5). Torso and epicardial potentials were recorded simultaneously, with the directly measured epicardial data providing a gold standard for quantitative evaluation of ECGI. Repolarization gradients were introduced through perfusion of dofetilide (prolongation) and pinacidil (abbreviation) into separate perfusion beds. Results show strong correlation between ECGI‐reconstructed and directly measured T‐wave electrogram morphological features and repolarization time distributions, with a slight shift (8.3 mm) in the position of the repolarization gradient. The authors conclude that “ECGI reliably and accurately maps potentially critical repolarization abnormalities.”
This is the first fully experimental evaluation of ECGI's ability to map noninvasively repolarization abnormalities, including repolarization dispersion. The elegant torso‐tank experiments provide excellent control over the experimental conditions, and allow for detailed comparison between ECGI reconstructions and direct mapping with high resolution. The isolated human heart experiment adds clinical relevance to the tank‐torso experiments, as do the in vivo experiments in the closed‐chest pigs. Taken together, this detailed and well‐controlled study provides strong support for ECGI as a method for noninvasive mapping of cardiac abnormalities, with potential as a diagnostic tool and a method for arrhythmic risk stratification.
ECGI was applied to map the arrhythmic substrate in several hereditary syndromes. Steep dispersion of repolarization was detected and mapped in LQTS, 14 Brugada syndrome, 15 early repolarization syndrome, 16 and arrhythmogenic cardiomyopathy. 17 An example of ECGI repolarization maps from patients with LQTS is provided in the Figure. Epicardial activation‐recovery interval maps are shown for normal control (left column) and for the most common types of LQTS: long QT [LQT] 1, LQT2, and LQT3 (left to right, respectively). All subjects with LQTS have regions with abnormally long activation‐recovery intervals (magenta and white regions). The maximum activation‐recovery interval value in LQTS is 450 ms compared with only 340 ms in control. In contrast to the uniform repolarization map in control, the LQTS maps are heterogeneous and contain steep repolarization gradients (shown by black arrows in the Figure), with 2 orders of magnitude greater steepness than control (control, 7 ms/cm; LQT1, 104 ms/cm; LQT2, 146 ms/cm; and LQT3, 140 ms/cm). More important, for the entire study population of 25 patients, 12 were symptomatic with documented arrhythmic events and 13 were asymptomatic. There was a statistically significant difference in the mean steepness of repolarization gradients between these 2 groups; it was 130 ms/cm in the symptomatic group, compared with only 98 ms/cm in the asymptomatic group (P=0.002). If this finding were found to be consistent in a larger study, it could be the basis for noninvasive arrhythmia risk stratification with ECGI. With the examples of other studies 14 , 15 , 16 , 17 in mind, one can envision other future clinical applications for ECGI in the context of repolarization abnormalities. Being noninvasive, it could be used for follow‐up mapping of the arrhythmic substrate to evaluate disease progression. It could assess the effects of drug therapy on the substrate (ie, does it reduce or increase repolarization heterogeneities), and with future developments in targeted drug delivery and targeted gene therapy, it could be used to direct an intervention to myocardial regions with steep dispersion of repolarization.
Figure 1. Electrocardiographic imaging maps (superior and inferior views) of repolarization activation‐recovery intervals (ARIs) in the most common types of long‐QT syndrome (LQTS).
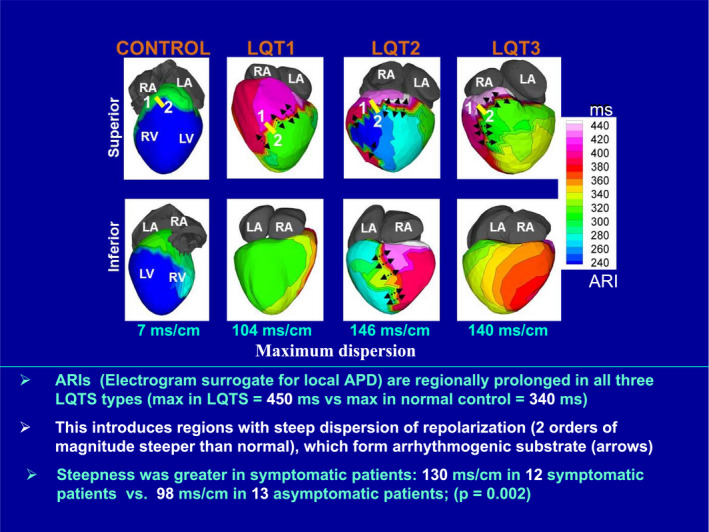
Normal control is shown in the left column. Steep repolarization gradients are indicated by arrows. Adapted with permission from Vijayakumar et al, 14 ©2014, Wolters Kluwer Health, Inc. APD indicates action potential duration; LA, left atrium; LQT, long QT; LV, left ventricle; max, maximum; RA, right atrium; and RV, right ventricle.
Disclosures
Dr Rudy receives royalties from CardioInsight Technologies (CIT). CIT does not support any research conducted in Dr Rudy's laboratory.
(J Am Heart Assoc. 2021;10:e021396. DOI: 10.1161/JAHA.121.021396.)
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Disclosures, see page 3.
See Article by Bear et al.
References
- 1. Wiggers CJ. The mechanism and nature of ventricular fibrillation. Am Heart J. 1940;399–412. DOI: 10.1016/S0002-8703(40)90874-2. [DOI] [Google Scholar]
- 2. Allessie MA, Bonke FIM, Schopman FJG. Circus movement in rabbit atrial muscle as a mechanism of tachycardia, 2: role of nonuniform recovery of excitability in occurrence of unidirectional block, as studied with multiple microelectrodes. Circ Res. 1976;168–177. DOI: 10.1161/01.res.39.2.168. [DOI] [PubMed] [Google Scholar]
- 3. Lukas A, Antzelevitch C. Phase 2 reentry as a mechanism of initiation of circus movement reentry in canine epicardium exposed to simulated ischemia. Cardiovasc Res. 1996;593–603. DOI: 10.1016/S0008-6363(96)00115-0. [DOI] [PubMed] [Google Scholar]
- 4. Coronel R, Wilms‐Schopman FJG, Opthof T, Janse MJ. Dispersion of repolarization and arrhythmogenesis. Heart Rhythm. 2009;537–543. DOI: 10.1016/j.hrthm.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 5. Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T‐wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;1385–1394. DOI: 10.1161/01.CIR.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 6. Quan W, Rudy Y. Unidirectional block and reentry of cardiac excitation–a model study. Circ Res. 1990;367–382. DOI: 10.1161/01.RES.66.2.367. [DOI] [PubMed] [Google Scholar]
- 7. Shaw RM, Rudy Y. The vulnerable window for unidirectional block in cardiac tissue: characterization and dependence on membrane excitability and cellular coupling. J Cardiovasc Electrophysiol. 1995;115–131. DOI: 10.1111/j.1540-8167.1995.tb00763.x. [DOI] [PubMed] [Google Scholar]
- 8. Mirvis DM. Spatial variation of QT intervals in normal persons and patients with acute myocardial infarction. Am Coll Cardiol. 1985;625–631. DOI: 10.1016/S0735-1097(85)80387-9. [DOI] [PubMed] [Google Scholar]
- 9. Day CP, McComb JM, Campbell RW. QT dispersion: an indication of arrhythmia risk in patients with long QT intervals. Br Heart J. 1990;342–344. DOI: 10.1136/hrt.63.6.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramanathan C, Ghanem RN, Jia P, Ryu K, Rudy Y. Electrocardiographic imaging (ECGI): a noninvasive imaging modality for cardiac electrophysiology and arrhythmia. Nat Med. 2004;422–428. DOI: 10.1038/nm1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramanathan C, Jia P, Ghanem RN, Ryu K, Rudy Y. Activation and repolarization of the normal human heart under complete physiological conditions. Proc Natl Acad Sci USA. 2006;6309–6314. DOI: 10.1073/pnas.0601533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghanem RN, Burnes JE, Waldo AL, Rudy Y. Imaging dispersion of myocardial repolarization II: noninvasive reconstruction of epicardial measures. Circulation. 2001;1306–1312. DOI: 10.1161/hc3601.094277. [DOI] [PubMed] [Google Scholar]
- 13. Bear LR, Cluitmans M, Abell E, Rogier J, Labrousse L, Cheng LK, LeGrice I, Lever N, Sands GB, Smaill B, et al. Electrocardiographic imaging of repolarization abnormalities. J Am Heart Assoc. 2021;e020153. DOI: 10.1161/JAHA.120.020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vijayakumar R, Silva JNA, Desouza KA, Abraham RL, Strom M, Sacher F, Van Hare GF, Haïssaguerre M, Roden DM, Rudy Y. Electrophysiologic substrate in congenital long QT syndrome: noninvasive mapping with electrocardiographic imaging (ECGI). Circulation. 2014;1936–1943. DOI: 10.1161/CIRCULATIONAHA.114.011359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J, Sacher F, Hoffmayer K, O’Hara T, Strom M, Cuculich P, Silva J, Cooper D, Faddis M, Hocini M, et al. The cardiac electrophysiologic substrate underlying the ECG phenotype and electrogram abnormalities in Brugada syndrome patients. Circulation. 2015;1950–1959. DOI: 10.1161/CIRCULATIONAHA.114.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang J, Hocini M, Strom M, Cuculich PS, Cooper DH, Sacher F, Haïssaguerre M, Rudy Y. The electrophysiological substrate of early repolarization syndrome: noninvasive mapping in patients. JACC Clin Electrophysiol. 2017;894–904. DOI: 10.1016/j.jacep.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andrews CM, Srinivasan NT, Rosmini S, Bulluck H, Orini M, Jenkins S, Pantazis A, McKenna WJ, Moon JC, Lambiase PD, et al. The electrical and structural substrate of arrhythmogenic right ventricular cardiomyopathy determined using noninvasive electrocardiographic imaging and late gadolinium magnetic resonance imaging. Circ Arrhythm Electrophysiol. 2017;e005105. DOI: 10.1161/CIRCEP.116.005105. [DOI] [PMC free article] [PubMed] [Google Scholar]


