Abstract
Background
PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors represent a promising class of lipid‐lowering therapy, although their use has been limited by cost concerns.
Methods and Results
A retrospective cohort study was conducted using a nationwide commercial claims database comprising patients with atherosclerotic cardiovascular disease (ASCVD), aged 18 to 64 years. We identified the number of patients with ASCVD started on a PCSK9 inhibitor from the dates of US Food and Drug Administration approval in quarter 3 2015 through quarter 2 2019. Secondary objectives identified the proportions of patients started on a PCSK9 inhibitor in various ASCVD risk groups based on statin use and baseline low‐density lipoprotein cholesterol. We identified 126 419 patients with ASCVD on either PCSK9 inhibitor or statin therapy. Among these patients, 1168 (0.9%) filled a prescription for a PCSK9 inhibitor. The number of patients initiating a PCSK9 inhibitor increased from 2 patients in quarter 3 2015 to 119 patients in quarter 2 2019, corresponding to an increase from 0.05% to 2.5% of patients with ASCVD already on statins who started PCSK9 inhibitor therapy. Of patients with ASCVD with high adherence to a high‐intensity statin, 13 643 had low‐density lipoprotein cholesterol ≥70 mg/dL, and in this subgroup, 119 (0.9%) patients initiated a PCSK9 inhibitor.
Conclusions
Few patients started PCSK9 inhibitors from 2015 through mid‐2019, despite increasing trial evidence of efficacy, guidelines recommending PCSK9 inhibitors in high‐risk patients with ASCVD, and price reductions during this period. The magnitude of price reductions may not yet be sufficient to influence use management strategies aimed to limit PCSK9 inhibitor use.
Keywords: access to care, drug adoption, PCSK9 inhibitors, secondary prevention
Subject Categories: Quality and Outcomes, Health Services
Nonstandard Abbreviations and Acronyms
- MACE
major adverse cardiovascular event
- MPR
medication possession ratio
- PCSK9
proprotein convertase subtilisin/kexin type 9
Clinical Perspective
What Is New?
Despite increasing evidence demonstrating PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors provide improved cardiovascular outcomes, clinical guidelines recommending their use, and drug price reductions, few eligible patients with atherosclerotic disease are initiated on PCSK9 inhibitors.
The magnitude of price reductions may not yet be sufficient to influence use management strategies aimed to limit PCSK9 inhibitor use.
What Are the Clinical Implications?
The clinical benefits of novel therapies, such as PCSK9 inhibitors, may not be realized if barriers to access persist and adoption remains low.
PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors represent a novel class of lipid‐lowering therapy that has been demonstrated to reduce low‐density lipoprotein cholesterol (LDL‐C) as well as reduce the risk of major adverse cardiovascular events (MACEs). 1 , 2 The PCSK9 inhibitors currently available are alirocumab and evolocumab, both approved by the US Food and Drug Administration in 2015 for use in patients with familial hypercholesterolemia or preexisting atherosclerotic cardiovascular disease (ASCVD) who require additional lowering of LDL‐C despite maximally tolerated doses of statins. 3
The enthusiasm over the clinical promise of PCSK9 inhibitors was tempered by average treatment prices of $14 000 per year, combined with studies concluding that these drugs do not meet generally acceptable cost‐effectiveness thresholds. 4 , 5 Moreover, the high costs of PCSK9 inhibitors caused health insurers and pharmacy benefit managers to implement use management processes, such as prior authorizations and increased patient cost sharing, which have been associated with slower uptake of novel pharmaceuticals in select populations. 6 , 7
In 2017, the American College of Cardiology published guidelines on the role of nonstatin therapies for management of ASCVD, and in 2018, the American College of Cardiology/American Heart Association Multisociety Guideline on the Management of Blood Cholesterol was published. Both guidelines recommended PCSK9 inhibitors in patients with high‐risk ASCVD with LDL‐C ≥70 mg/dL after adoption of lifestyle modifications and treatment with standard background therapy. 8 , 9 In 2018, the manufacturers for both alirocumab and evolocumab announced price reductions to <$6000 per year in an attempt to meet cost‐effectiveness benchmarks and improve patient access to these therapies. 10 , 11
In this analysis, we identified trends in the number of patients with ASCVD initiated on PCSK9 inhibitor therapy as well as the proportion of patients with ASCVD on statin therapy who were started on a PCSK9 inhibitor from approval in 2015 through mid‐2019. We also identified different patient groups with ASCVD and calculated the proportion who were initiated on a PCSK9 inhibitor.
Methods
Study Data
Data were obtained from Optum’s deidentified Clinformatics Data Mart Database. The database consists of comprehensive inpatient, outpatient, laboratory, and pharmacy claims for >17 million patients annually throughout the United States and includes each patient’s dates of insurance coverage. Demographic data were also available through ZIP code linked enrollment data from the US Census Bureau. The study protocol was deemed exempt by the University of Pennsylvania Institutional Review Board. The proprietary data that support the findings of this study are available from the corresponding author on reasonable request, although they will be subject to data privacy rules and licensing requirements of Optum.
Study Cohort
Using administrative claims from January 1, 2015, through June 30, 2019, we included patients with ASCVD, aged 18 to 64 years, who filled a prescription for either a PCSK9 inhibitor or a statin. Pharmacy claims, including National Drug Codes, fill dates, and days supplied, were used to identify PCSK9 inhibitor and/or statin use. Drug classes were identified using National Drug Codes. 12 To identify ASCVD, we examined all inpatient and outpatient claims occurring 12 months before the index prescription, and included patients with a history of coronary artery disease, stroke or transient ischemic attack, and peripheral arterial disease. ASCVD diagnoses were identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM), codes and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM), codes from summaries of the Medicare Chronic Conditions Warehouse. 13 We excluded patients who had <12 months of continuous insurance enrollment before the index prescription for either a PCSK9 inhibitor or a statin during the study period to accurately capture patient comorbidities as well as assess for medication adherence. We also excluded patients with no serum lipid measurement in the 12 months before the index prescription for a PCSK9 inhibitor or a statin so we could describe baseline LDL‐C levels for patients initiated or not initiated on a PCSK9 inhibitor.
Outcomes
The primary outcome was the number of patients with ASCVD who filled a first prescription for a PCSK9 inhibitor during the study period of January 1, 2015, through June 30, 2019. We also compared the proportion of patients with ASCVD who filled a first prescription for a PCSK9 inhibitor with patients with ASCVD who were filling statin prescriptions over the same time period.
Among patients with ASCVD, we also identified 3 subgroups of patients based on statin use and LDL‐C levels: (1) patients with concurrent high‐intensity statin use, (2) patients highly adherent to a high‐intensity statin, and (3) patients highly adherent to a high‐intensity statin with measured serum LDL‐C ≥70 mg/dL. We identified these subgroups because those with high cardiovascular risk who were adherent to maximal statin therapy were potentially the patients who would benefit the most from PCSK9 inhibitor initiation, and thus we expected the proportion of these patients who were treated with a PCSK9 inhibitor to be higher than the general group of patients with ASCVD.
High‐intensity statin use was defined as filling prescriptions for either atorvastatin, 40 to 80 mg, or rosuvastatin, 20 to 40 mg. 9 High adherence to a statin was indicated by a medication possession ratio (MPR) ≥0.80 in the past 12 months. MPR is defined by the sum of the days supplied of all prescriptions filled for a drug in a given time period, divided by the number of days in the time period, and MPR ≥0.80 is commonly used as a marker for high adherence. 14 , 15 Last, we identified serum LDL‐C. If serum LDL‐C was measured more than once in the prior year, we included the most recent LDL‐C measurement (ie, the laboratory result closest in date to the index PCSK9 inhibitor or high‐intensity statin prescription).
Among patients with ASCVD, demographic characteristics, including age, sex, race, and US geographic region, were compared by the following groups: patients adherent to a high‐intensity statin with LDL‐C ≥70 mg/dL who were not started on a PCSK9 inhibitor, patients adherent to a high‐intensity statin with LDL‐C ≥70 mg/dL who were started on a PCSK9 inhibitor, and patients without consistent use of a high‐intensity statin (ie, MPR <0.8) and/or with an LDL‐C <70 mg/dL who were started on a PCSK9 inhibitor. Using ICD‐9‐CM and ICD‐10‐CM diagnosis codes from data summaries of the Medicare Chronic Conditions Warehouse, history of hypertension, diabetes mellitus, and premature ASCVD (aged <55 years in men and aged <60 years in women) was also compared. MACEs in the past year, defined as an inpatient hospitalization with a diagnosis code for acute myocardial infarction or transient ischemic attack/stroke, were compared by group.
Using National Drug Codes from pharmacy claims, we identified other lipid‐lowering therapies (ie, ezetimibe, bile acid sequestrants, and fibrates) that patients filled in the prior 12 months, given guidelines also recommend using ezetimibe in patients with ASCVD with high cardiovascular risk and LDL‐C ≥70 mg/dL. 8 , 9 We also determined the proportion of patients who had an outpatient cardiologist visit as well as the number of outpatient cardiologist visits in the past year to assess for an association between intensity of specialist care and PCSK9 inhibitor initiation. Specialty of provider was identified on the outpatient claim by Medicare taxonomy codes. 16 Last, we compared serum LDL‐C.
Last, we assessed patient factors associated with being initiated on a PCSK9 inhibitor. Using a multivariable logistic regression model with PCSK9 inhibitor initiation as the dependent variable, the independent variables measured were age in years, sex, race, US region, annual household income, history of hypertension, history of diabetes mellitus, MACE in the past year, baseline LDL‐C in mg/dL, and having a cardiology outpatient encounter in the past year. To assess the patients likely to have the most benefit from PCSK9 inhibitor initiation, we included patients with ASCVD who were highly adherent to a high‐intensity statin and with LDL‐C ≥70 mg/dL and compared them with the patients with ASCVD initiated on a PCSK9 inhibitor with LDL‐C ≥70 mg/dL in the logistic regression model.
Statistical Analysis
Differences in characteristics and outcomes between groups were compared using χ2 tests for categorical variables and ANOVA for continuous variables. All statistical tests were 2 sided, with P<0.05 indicating statistical significance. In the multivariable logistic regression model, estimated adjusted odds ratios (ORs) are reported with 95% CIs, and each OR reported is adjusted for all other covariables in the model. Analyses were performed using Stata, version 15.1 (StataCorp).
Results
Among patients with at least 1 year of continuous insurance eligibility, we identified 1 696 007 patients on statin therapy and 3463 patients initiated on a PCSK9 inhibitor. After excluding patients without serum lipid measurement data in the 12 months before the index prescription for a PCSK9 inhibitor or statin, we identified 569 572 patients on a statin and 1520 patients on PCSK9 inhibitor therapy.
After restricting the cohort to patients with a history of ASCVD, we identified 126 419 patients with ASCVD on either PCSK9 inhibitor or statin therapy from January 1, 2015, to June 30, 2019. Among these patients, we identified a total of 1168 (0.9%) who filled a prescription for a PCSK9 inhibitor. The number of patients filling a first prescription for a PCSK9 inhibitor increased from 2 patients in the third quarter of 2015 to 119 patients in the second quarter of 2019, corresponding to an increase from 0.05% to 2.5% of patients with ASCVD already on statins who started PCSK9 inhibitor therapy (Figure 1).
Figure 1. Trends in patients with atherosclerotic cardiovascular disease (ASCVD) initiated on PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor therapy, 2015 to 2019.
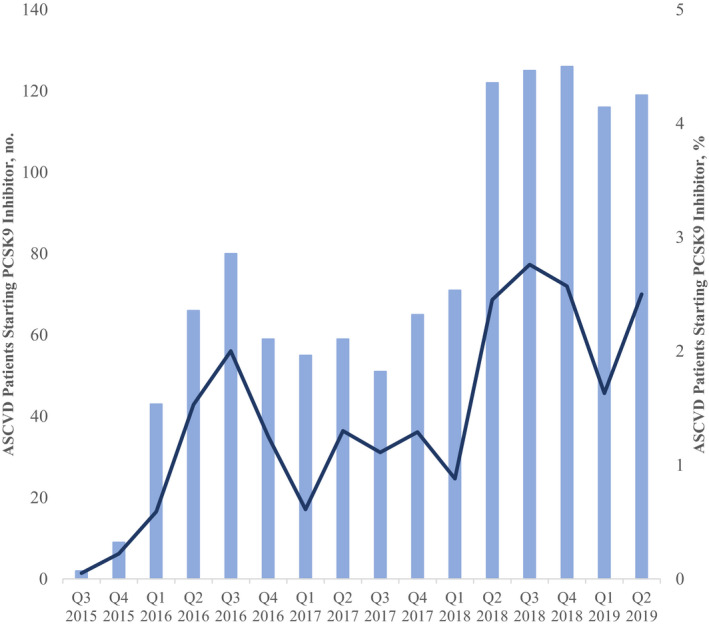
The bars represent the number of patients with ASCVD initiated on a PCSK9 inhibitor from US drug approval in second quarter (Q) 2015 to the second quarter of 2019. The line represents the proportion of patients with ASCVD started on a PCSK9 inhibitor among patients with incident ASCVD on statin therapy.
In our subgroup analysis, we identified 54 815 patients with ASCVD on a high‐intensity statin; of these patients, 478 (0.9%) were initiated on a PCSK9 inhibitor (Figure 2). Among the 27 122 patients who were highly adherent to a high‐intensity statin, 143 (0.5%) were started on a PCSK9 inhibitor. Of these patients with ASCVD with high adherence to a high‐intensity statin, 13 643 had LDL‐C ≥70 mg/dL; and in this subgroup, 119 (0.9%) patients were started on a PCSK9 inhibitor.
Figure 2. Number of patients initiated on a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor among different patient groups with atherosclerotic cardiovascular disease (ASCVD).
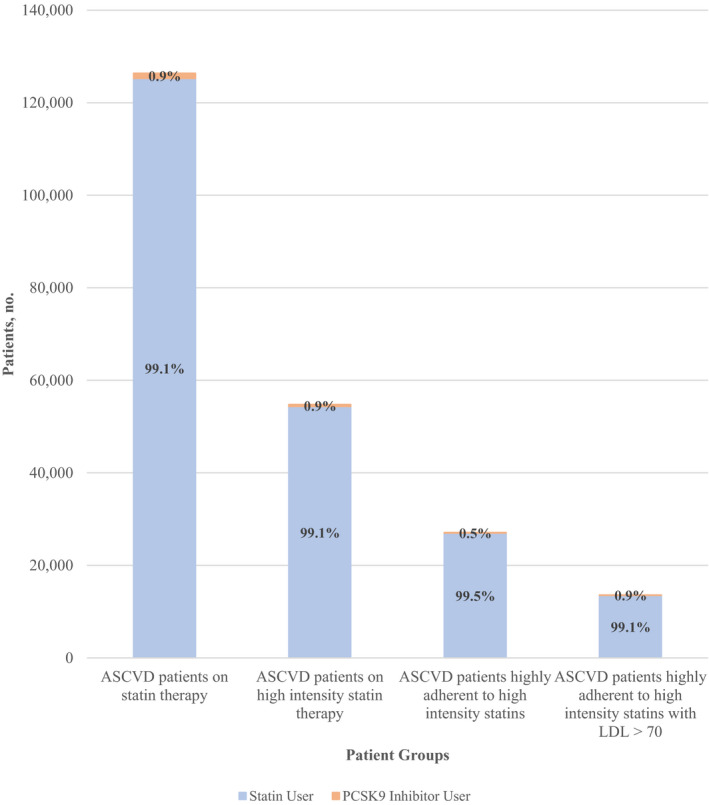
The orange bars represent the number of patients started on a PCSK9 inhibitor, and the blue bars represent the number of patients not started on PCSK9 inhibitor therapy, among different patient groups with ASCVD. LDL indicates low‐density lipoprotein.
Clinical Characteristics of PCSK9 Inhibitor Initiation
Patients with ASCVD who were adherent to high‐intensity statins, with LDL‐C ≥70 mg/dL and not started on a PCSK9 inhibitor (ie, high‐intensity statin only), were more likely to be women compared with patients with ASCVD who were adherent to a high‐intensity statin, with LDL ≥70 mg/dL and started on a PCSK9 inhibitor (ie, PCSK9 inhibitor and high‐intensity statin). Both of these groups were less likely to be women when compared with patients with ASCVD started on a PCSK9 inhibitor without consistent prior high‐intensity statin use and/or LDL‐C <70 mg/dL (ie, PCSK9 inhibitor only) (29.7% versus 26.9% versus 38.2%; P<0.001) (Table 1). There were no significant differences in age or US geographical region among the 3 groups. Patients in the high‐intensity statin‐only group were less likely to be White patients compared with the PCSK9 inhibitor and high‐intensity statin group and the PCSK9 inhibitor only group (63.3% versus 77.3% versus 66.7%; P<0.001). Compared with the 2 groups started on a PCSK9 inhibitor, the high‐intensity statin only group was more likely to have experienced MACEs in the past year (28.2% versus 19.3% versus 18.7%; P<0.001), more likely to have a history of stroke (17.7% versus 5.9% versus 9.0%; P<0.001), more likely to have diabetes mellitus (43.8% versus 32.8% versus 39.2%; P=0.001), and more likely to have hypertension (86.2% versus 81.5% versus 83.9%; P=0.04). There were no significant differences in prevalence of premature ASCVD or peripheral arterial disease among the 3 groups.
Table 1.
Demographic and Clinical Characteristics of Patients With ASCVD by Adherence to a High‐Intensity Statin, Baseline LDL‐C, and PCSK9 Inhibitor Initiation
| Characteristic | Patient Group With ASCVD | |||
|---|---|---|---|---|
|
Adherent to High‐Intensity Statin With LDL‐C ≥70 mg/dL and Not Started on PCSK9 Inhibitor (n=13 524) |
Adherent to High‐Intensity Statin With LDL‐C ≥70 mg/dL and Started on PCSK9 Inhibitor (n=119) |
Started on PCSK9 Inhibitor Without Adherence to High‐Intensity Statin and/or With LDL‐C <70 mg/dL (n=1049) |
P Value | |
| Age, mean (SD), y | 57.0 (5.9) | 56.0 (6.5) | 57.0 (6.3) | 0.053 |
| Women | 4015 (29.7) | 32 (26.9) | 401 (38.2) | <0.001 |
| Region | 0.63 | |||
| Northeast | 1368 (10.1) | 13 (10.9) | 108 (10.3) | |
| Midwest | 1767 (13.1) | 13 (10.9) | 118 (11.3) | |
| South | 7858 (58.1) | 68 (57.1) | 615 (58.6) | |
| West | 2503 (18.5) | 24 (20.2) | 205 (19.5) | |
| Unknown | 28 (0.2) | 1 (0.8) | 3 (0.3) | |
| Race/ethnicity | <0.001 | |||
| White | 8566 (63.3) | 92 (77.3) | 700 (66.7) | |
| Black | 1384 (10.2) | 10 (8.4) | 91 (8.7) | |
| Hispanic | 1258 (9.3) | 9 (7.6) | 116 (11.1) | |
| Asian | 321 (2.4) | 1 (0.8) | 30 (2.9) | |
| Unknown | 1995 (14.8) | 7 (5.9) | 112 (10.7) | |
| Cardiovascular risk factors | ||||
| Premature ASCVD | 5053 (37.4) | 51 (42.9) | 405 (38.6) | 0.35 |
| MACE in past year | 3811 (28.2) | 22 (19.3) | 196 (18.7) | <0.001 |
| Coronary artery disease | 11 251 (83.2) | 114 (95.8) | 975 (93.0) | <0.001 |
| Stroke/TIA | 2395 (17.7) | 7 (5.9) | 94 (9.0) | <0.001 |
| Peripheral arterial disease | 3006 (22.2) | 24 (20.2) | 219 (20.9) | 0.52 |
| Diabetes mellitus | 5923 (43.8) | 39 (32.8) | 411 (39.2) | 0.001 |
| Hypertension | 11 661 (86.2) | 97 (81.5) | 880 (83.9) | 0.04 |
| Baseline medications | ||||
| Ezetimibe | 911 (6.7) | 66 (55.5) | 331 (31.6) | <0.001 |
| Bile acid sequestrants | 148 (1.1) | 10 (8.4) | 53 (5.1) | <.001 |
| Fibrates | 1478 (10.9) | 17 (14.3) | 123 (11.7) | 0.38 |
| Use | ||||
| Outpatient cardiology visit | 10 137 (75.0) | 95 (79.8) | 930 (88.7) | <0.001 |
| No. of cardiology visits, median (IQR) | 2 (0–4) | 3 (1–5) | 3 (2–6) | <0.001 |
| Baseline LDL‐C, mg/dL | <0.001 | |||
| <70 | N/A | N/A | 154 (14.7) | |
| 70–99 | 9920 (73.4) | 33 (27.7) | 146 (13.9) | |
| 100–129 | 2610 (19.3) | 38 (31.9) | 220 (21.0) | |
| ≥130 | 994 (7.4) | 48 (40.3) | 529 (50.4) | |
Data are given as number (percentage) of each group, unless otherwise indicated. ASCVD indicates atherosclerotic cardiovascular disease; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; MACE, major adverse cardiovascular event; N/A, not applicable; PCSK9, proprotein convertase subtilisin/kexin type 9; and TIA, transient ischemic attack.
For use of nonstatin lipid‐lowering therapies in the preceding 12 months, the high‐intensity statin only group was less likely to have filled prescriptions for ezetimibe (6.7% versus 55.5% versus 31.6%; P<0.001) and bile acid sequestrants (1.1% versus 8.4% versus 5.1%; P<0.001) compared with the 2 groups started on a PCSK9 inhibitor.
The high‐intensity statin only group had a lower median number of outpatient cardiology visits in the preceding 12 months (2; interquartile range, 0–4), compared with the PCSK9 inhibitor and high‐intensity statin group (3; interquartile range, 1–5) and the PCSK9 inhibitor only group (3; interquartile range, 2–6) (P<0.001).
Factors Associated With PCSK9 Inhibitor Initiation
In multivariable analysis (Table 2), female patients were more likely to be started on a PCSK9 inhibitor than male patients (OR, 1.44; 95% CI, 1.22–1.68; P<0.001). Patients with annual household income between $50 000 and $99 999 were more likely to be started on PCSK9 inhibitors than patients with annual household income <$50 000 (OR, 1.31; 95% CI, 1.05–1.65; P=0.02); patients with household income >$100 000 were also more likely to be started on PCSK9 inhibitors than patients with annual household income <$50 000 (OR, 2.06; 95% CI, 1.66–2.55; P<0.001). Patients with MACEs in the past year were less likely to be initiated on a PCSK9 inhibitor (OR, 0.59; 95% CI, 0.49–0.71; P<0.001), and patients with a cardiology outpatient visit in the past year were more likely to be started on a PCSK9 inhibitor (OR, 2.89; 95% CI, 2.32–3.60; P<0.001). Relative to patients with a baseline LDL‐C of 70 to 99 mg/dL, patients with baseline LDL‐C of 100 to 129 mg/dL (OR, 5.79; 95% CI, 4.75–7.06; P<0.001) and baseline LDL‐C ≥130 mg/dL (OR, 36.29; 95% CI, 30.09–43.77; P<0.001) were more likely to be initiated on a PCSK9 inhibitor. There was no significant association between PCSK9 inhibitor initiation and age in years, race, and history of hypertension.
Table 2.
Multivariable Logistic Regression on Factors Associated With PCSK9 Inhibitor Initiation
| Patient Characteristic | PCSK9 Inhibitor Initiation | |
|---|---|---|
| OR (95% CI) | P Value | |
| Age, y | 1.00 (0.99–1.02) | 0.58 |
| Women | 1.44 (1.22–1.68) | <0.001 |
| Race/ethnicity | ||
| White | 1 (Reference) | |
| Black | 0.79 (0.61–1.03) | 0.08 |
| Hispanic | 1.03 (0.80–1.31) | 0.83 |
| Asian | 0.64 (0.39–1.07) | 0.09 |
| Unknown | 0.76 (0.58–1.01) | 0.06 |
| US region | ||
| Northeast | 1 (Reference) | |
| South | 0.92 (0.72–1.17) | 0.50 |
| Midwest | 0.73 (0.53–0.99) | 0.04 |
| West | 1.09 (0.83–1.44) | 0.53 |
| Unknown | 2.12 (0.58–7.71) | 0.25 |
| Annual household income, $ | ||
| <50 000 | 1 (Reference) | |
| 50 000–99 999 | 1.31 (1.05–1.65) | 0.02 |
| >$100 000 | 2.06 (1.66–2.55) | <0.001 |
| Unknown | 1.00 (0.78–1.28) | 0.98 |
| Hypertension | 0.85 (0.69–1.04) | 0.11 |
| Diabetes mellitus | 0.85 (0.73–1.00) | 0.05 |
| MACE in past year | 0.60 (0.50–0.72) | <0.001 |
| Baseline LDL‐C, mg/dL | ||
| 70–99 | 1 (Reference) | |
| 100–129 | 5.79 (4.75–7.06) | <0.001 |
| ≥ 130 | 36.29 (30.09–43.77) | <0.001 |
| Cardiology outpatient visit in past year | 2.80 (2.27–3.45) | <0.001 |
LDL‐C indicates low‐density lipoprotein cholesterol; MACE, major adverse cardiovascular event; OR, odds ratio; and PCSK9, proprotein convertase subtilisin/kexin type 9.
Patients included in the model were patients with ASCVD with high adherence to a high‐intensity statin and LDL‐C ≥70 mg/dL who were not started on a PCSK9 inhibitor as well as patients with ASCVD with LDL‐C ≥70 mg/dL who were initiated on a PCSK9 inhibitor.
Discussion
In a sample of 126 419 privately insured patients with a history of ASCVD and on statin therapy, <1% of patients were started on a PCSK9 inhibitor in the 4 years after the introduction of the first US Food and Drug Administration–approved agents in this therapeutic class. Over this time period, the proportion of potentially eligible patients with ASCVD on statin therapy who were initiated on a PCSK9 inhibitor modestly increased. Before 2018, the proportion of these patients started on a PCSK9 inhibitor was approximately 1%, and in 2018 through mid‐2019, the proportion of patients started on PCSK9 inhibitor therapy was approximately 2%. This was despite clinical trials showing improvement in cardiovascular outcomes, guidelines recommending PCSK9 inhibitors in patients with ASCVD with high cardiovascular risk, and price reductions in these drugs over this time period. The low use of PCSK9 inhibitors among patients with ASCVD was not substantially different among the different patient groups with ASCVD identified over the study period; the most inclusive group (ie, patients with ASCVD on a statin) and the most stringent group (ie, patients with ASCVD who were highly adherent to a high‐intensity statin for a year with suboptimal LDL‐C levels) both had <1% PCSK9 inhibitor initiation. In addition, we found that after factoring for demographic, socioeconomic, and clinical characteristics, patients with MACEs in the past year were less likely to be initiated on a PCSK9 inhibitor, suggesting the highest‐risk patients may not be receiving PCSK9 inhibitor therapy.
Our findings highlight the fact that patients who could potentially benefit from PCSK9 inhibitors far outnumber the patients currently receiving PCSK9 inhibitor therapy, even after drug price reductions and society guidelines recommended their use. A national estimate using American College of Cardiology registry data found 700 000 to 10 million patients would be eligible for PCSK9 inhibitor therapy, depending on LDL‐C threshold. 17 Another study using American College of Cardiology registry data found <2% of patients with an LDL‐C ≥190 mg/dL were receiving PCSK9 inhibitors. 18 Even among the highest‐risk patients with ASCVD who were highly adherent to high‐intensity statins and had suboptimal LDL‐C levels, the number of patients aged 18 to 64 years who were eligible for PCSK9 inhibitors far outnumbered the number of patients receiving a PCSK9 inhibitor.
Investigation into the barriers to PCSK9 inhibitor use has largely been focused on prior authorization requirements and patient cost sharing implemented by payers, which are themselves a result of high drug prices. 6 , 7 Less than half of patients prescribed a PCSK9 inhibitor receive prior authorization approval from their insurer; of the patients who receive approval, more than a third never fill a prescription, with high copayments appearing to play a substantial role in primary nonadherence. 6 It had been anticipated that insurer requirements and high out‐of‐pocket costs would become lower barriers after the manufacturers of alirocumab and evolocumab announced plans to reduce the price of both drugs in 2018. 16 However, our analysis found only a modest increase in patients initiated on a PCSK9 inhibitor in the year after these announcements were made. Moreover, our multivariable analysis found that annual household income was one of the strongest factors associated with PCSK9 inhibitor initiation, with a graded association for the low‐, middle‐, and high‐income groups. Thus, relative to higher‐income patients, the costs of PCSK9 inhibitors for the lower‐ and middle‐income insured patients in our study may be a barrier to their use.
PCSK9 inhibitors are representative of the growing number of biologic drug classes that comprise a greater share of new drug approvals by the US Food and Drug Administration each year. 19 Biologics are used by only 2% of Americans but have nonetheless been a large factor in increased drug spending in recent years. 20 In 2015, biologics accounted for nearly 40% of US prescription drug spending and for 70% of prescription drug spending growth from 2010 to 2015. 21 Inherently, complex manufacturing processes compared with small‐molecule drug production as well as patent protections and patent disputes have limited generic drug manufacturers from producing and marketing biosimilars, which would increase market competition and potentially reduce prices for biologics. 20 , 22 Thus, the likelihood of increased competition reducing PCSK9 inhibitor prices in the near future appears low.
However, the phenomenon of drug prices limiting the adoption of novel therapies is not limited to biologics. Within cardiovascular medicine, sacubitril/valsartan and sodium‐glucose cotransporter‐2 inhibitors are examples of small‐molecule drugs that, despite having substantial clinical benefit and being guideline‐recommended therapies, have had slow adoption because of insurance coverage restrictions linked to high‐priced novel therapies. 23 , 24 Payers are likely to impose coverage restrictions and strict use management strategies for high‐priced novel therapies for common conditions, such as ASCVD, diabetes mellitus, or heart failure, given the potential large budgetary impact inherent in providing these new therapies to millions of eligible patients. Ultimately, the potential clinical benefits these novel therapies might provide to patients may not be realized if significant underuse remains.
Aside from drug pricing, patient access to specialists and clinician variation in prescribing practices may be important barriers to PCSK9 inhibitor prescribing. Two thirds of commercial insurers restrict PCSK9 inhibitor approval to specialty prescribers, 7 and in our sample, patients prescribed PCSK9 inhibitors had a higher intensity of cardiology outpatient visits. Specialist access may be a significant barrier for some patients, particularly rural, low‐income, and minority patients. 25 , 26 Furthermore, PCSK9 inhibitor prescribing varies among lipid specialists, with 17% reporting never having prescribed a PCSK9 inhibitor when ASCVD is the indication, and more than half reporting they rarely prescribe PCSK9 inhibitors for ASCVD (and more commonly prescribe to patients with familial hypercholesterolemia). 27 In our sample, we found 1 in 8 patients started on a PCSK9 inhibitor had an LDL‐C <70 mg/dL before initiation, whereas more than half of the cohort highly adherent to a high‐intensity statin had suboptimal LDL‐C control, which may partly be reflective of differences in clinician practice, such as treating to a specific LDL‐C target versus treating to a specific statin dose.
We also found that, after factoring for multiple demographic, socioeconomic, and clinical characteristics, patients with MACEs in the past year were less likely to be initiated on PCSK9 inhibitors, suggesting that many of the highest‐risk patients are not being initiated on PCSK9 inhibitor therapy. In addition, even though patients with an LDL‐C ≥70 mg/dL are potential candidates for PCSK9 inhibitors, patients with LDL‐C ≥100 mg/dL were much more likely to be initiated on PCSK9 inhibitors compared with patients with baseline LDL‐C between 70 and 99 mg/dL. This difference may be explained by clinician prescribing practices and/or by insurer coverage.
The rate of suboptimal statin use (ie, low‐ or moderate‐intensity statin use in patients with ASCVD) in our overall patient sample is consistent with rates found in various populations. 28 , 29 , 30 Although most patients using PCSK9 inhibitors had filled a statin prescription in the previous year, notably only 41% had filled a high‐intensity statin prescription and <15% demonstrated high adherence rates to a high‐intensity statin in the prior year. The nature of administrative claims precludes determination of the causes for relatively low statin use, whether it be adverse effects/intolerance, patient preferences for nonstatin medications, other reasons for patient‐level nonadherence, or variation in clinician prescribing practices. Moreover, there was low use of ezetimibe in our sample of patients with ASCVD with suboptimal LDL‐C, despite guidelines recommending its use. Given ezetimibe was available as a generic medication for most of the study period, its underuse may be attributable to nonfinancial factors, such as low physician prescribing.
Limitations
This study has limitations inherent to retrospective studies using administrative claims. Our study data lacked detailed clinical information, which would have more accurately confirmed ASCVD and other baseline comorbidities. Although we identified high‐risk patients given they had ASCVD with prevalent comorbidities and suboptimal LDL‐C despite adherence to high‐intensity statins, we did not implement the guideline definition of “very‐high‐risk ASCVD” given the administrative claims database used did not allow us to observe all the criteria included in the guideline definition. However, we used well‐validated administrative codes to optimally identify patients with the data available. Notably, specific ICD‐10‐CM codes for familial hypercholesterolemia became effective in late 2016 and were not widely used in our data set during the period studied, which precluded us from identifying the prevalence of these patients within our sample. However, familial hypercholesterolemia is estimated to only represent 5% of the target population for PCSK9 inhibitors, and studies suggest barriers to access are similar to patients with ASCVD. 31 , 32 In addition, although we used National Drug Codes to identify prescription claims, past analyses have noted that statin use is underestimated in administrative claims, often attributed to patients filling their prescription through generic drug discount programs offered at major retail pharmacies rather than through their insurer 33 , 34 ; thus, this analysis may have underestimated statin use and adherence in our study population.
Our demographic measures were derived from ZIP code–linked US Census data rather than direct patient measurement, and therefore these measures may not accurately reflect individual patients’ demographic information; nevertheless, similar measures have been used extensively in the literature as a reasonable proxy for group‐level analysis. Our measure of adherence (MPR) is a commonly used, although indirect, measure of adherence. It does not indicate how many days a patient actually took a medication; hence, MPR tends to overestimate medication adherence. 35 , 36 Finally, although the study sample came from a large, nationally representative commercially insured population, our results may not be representative for patients with public health insurance.
Conclusions
We observed small numbers of potentially eligible patients starting PCSK9 inhibitors from US Food and Drug Administration approval in 2015 through mid‐2019, a period during which increasing clinical evidence demonstrated PCSK9 inhibitors improved cardiovascular outcomes, guidelines recommended PCSK9 inhibitors in patients with ASCVD with high cardiovascular risk, and manufacturers reduced prices of the drugs. Among various patient subgroups identified as potentially greatly benefiting from PCSK9 inhibitors, we still found <1% were started on PCSK9 inhibitor therapy. Thus, the magnitude of price reductions may not yet be sufficient to influence use management strategies aimed to limit PCSK9 inhibitor use, despite increased guideline recommendations for their use. The potential clinical benefits of novel therapies, such as PCSK9 inhibitors, may not be realized if barriers to access remain.
Sources of Funding
None.
Disclosures
None.
(J Am Heart Assoc. 2021;10:e019331. DOI: 10.1161/JAHA.120.019331.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. DOI: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 2. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. DOI: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 3. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol. 2015;65:2638–2651. DOI: 10.1016/j.jacc.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 4. Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, Tice JA, Guzman D, Bibbins‐Domingo K. Cost‐effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743–753. DOI: 10.1001/jama.2016.11004. [DOI] [PubMed] [Google Scholar]
- 5. Arrieta A, Hong JC, Khera R, Virani SS, Krumholz HM, Nassir K. Updated cost‐effectiveness assessments of PCSK9 inhibitors from the perspectives of the health system and private payers: insights derived from the FOURIER trial. JAMA Cardiol. 2017;2:1369–1374. DOI: 10.1001/jamacardio.2017.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Navar AM, Taylor B, Mulder H, Fievitz E, Monda KL, Fievitz A, Maya JF, Lopez JAG, Peterson ED. Association of prior authorization and out‐of‐pocket costs with patient access to PCSK9 inhibitor therapy. JAMA Cardiol. 2017;2:1217–1225. DOI: 10.1001/jamacardio.2017.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doshi JA, Puckett JT, Parmacek MS, Rader DJ. Prior authorization requirements for proprotein convertase subtilisin/kexin type 9 inhibitors across US private and public payers. Circ Cardiovasc Qual Outcomes. 2018;11:e003939. DOI: 10.1161/CIRCOUTCOMES.117.003939. [DOI] [PubMed] [Google Scholar]
- 8. Lloyd‐Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr, DePalma SM, Minissian MB, Orringer CE, Smith SC Jr. 2017 Focused update of the 2016 ACC expert consensus decision pathway on the role of non‐statin therapies for LDL‐cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol. 2017;70:1785–1822. DOI: 10.1016/j.jacc.2017.07.745. [DOI] [PubMed] [Google Scholar]
- 9. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. DOI: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dhruva SS, Ross JS, Desai NR. Alirocumab's price reduction. Circulation. 2018;138:1502–1504. DOI: 10.1161/CIRCULATIONAHA.118.036069. [DOI] [PubMed] [Google Scholar]
- 11. Fonarow GC, van Hout B, Villa G, Arellano J, Lindgren P. Updated cost‐effectiveness analysis of evolocumab in patients with very high‐risk atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4:691–695. DOI: 10.1001/jamacardio.2019.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. U.S. Food and Drug Administration . National Drug Code Directory. https://www.accessdata.fda.gov/scripts/cder/ndc/. Accessed January 10, 2020.
- 13. Centers for Medicare and Medicaid Services . Chronic Conditions Data Warehouse. https://www.ccwdata.org/web/guest/condition‐categories. Accessed January 10, 2020.
- 14. Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11:449–457. [PubMed] [Google Scholar]
- 15. Zhang Y, Wu SH, Fendrick AM, Baicker K. Variation in medication adherence in heart failure. JAMA Intern Med. 2013;173:468–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Centers for Medicare and Medicaid Services . Healthcare Provider Taxonomy Code Set. https://www.cms.gov/Medicare/Provider‐Enrollment‐and‐Certification/MedicareProviderSupEnroll/Taxonomy.html. Accessed January 10, 2020.
- 17. Hess PL, Kennedy K, Cowherd M, Virani SS, Masoudi FA, Navar AM, Yeh RW, Ho PM, Maddox TM. Implications of the FDA approval of PCSK9 inhibitors and FOURIER results for contemporary cardiovascular practice: an NCDR Research to Practice (R2P) project. Am Heart J. 2018;195:151–152. DOI: 10.1016/j.ahj.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Virani SS, Kennedy KF, Akeroyd JM. Variation in lipid‐lowering therapy use in patients with low‐density lipoprotein cholesterol ≥190 mg/dL: insights from the national cardiovascular data registry‐practice innovation and clinical excellence registry. Circ Cardiovasc Qual Outcomes. 2018;11:e004652. DOI: 10.1161/CIRCOUTCOMES.118.004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mullard A. 2018 FDA drug approvals. Nat Rev Drug Discov. 2019;18:85–89. [DOI] [PubMed] [Google Scholar]
- 20. Zhai MZ, Sarpatwari A, Kesselheim AS. Why are biosimilars not living up to their promise in the US? AMA J Ethics. 2019;21:E668–E678. DOI: 10.1001/amajethics.2019.668. [DOI] [PubMed] [Google Scholar]
- 21. Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q. 2018;7:3. [PMC free article] [PubMed] [Google Scholar]
- 22. Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469–478. [PMC free article] [PubMed] [Google Scholar]
- 23. DeJong C, Kazi DS, Dudley RA, Chen R, Tseng CW. Assessment of national coverage and out‐of‐pocket costs for sacubitril/valsartan under medicare Part D. JAMA Cardiol. 2019;4:828–830. DOI: 10.1001/jamacardio.2019.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McCoy RG, Dykhoff HJ, Sangaralingham L, Ross JS, Karaca‐Mandic P, Montori VM, Shah ND. Adoption of new glucose‐lowering medications in the U.S.—the case of SGLT2 inhibitors: nationwide cohort study. Diabetes Technol Ther. 2019;21:702–712. DOI: 10.1089/dia.2019.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crowley R. American College of Physicians. Racial and ethnic disparities in health care, updated 2010. https://www.acponline.org/system/files/documents/advocacy/current_policy_papers/assets/racial_disparities.pdf. Accessed March 2, 2020.
- 26. Reschovsky JD, Staiti AB. Access and quality: does rural America lag behind? Health Aff (Millwood). 2005;24:1128–1139. DOI: 10.1377/hlthaff.24.4.1128. [DOI] [PubMed] [Google Scholar]
- 27. Cohen JD, Cziraky MJ, Jacobson TA, Maki KC, Karalis DG. Barriers to PCSK9 inhibitor prescriptions for patients with high cardiovascular risk: results of a healthcare provider survey conducted by the national lipid association. J Clin Lipidol. 2017;11:891–900. DOI: 10.1016/j.jacl.2017.04.120. [DOI] [PubMed] [Google Scholar]
- 28. Salami JA, Warraich H, Valero‐Elizondo J, Spatz ES, Desai NR, Rana JS, Virani SS, Blankstein R, Khera A, Blaha MJ, et al. National trends in statin use and expenditures in the US adult population from 2002 to 2013: insights from the medical expenditure panel survey. JAMA Cardiol. 2017;2:56–65. DOI: 10.1001/jamacardio.2016.4700. [DOI] [PubMed] [Google Scholar]
- 29. Colantonio LD, Huang L, Monda KL, Bittner V, Serban MC, Taylor B, Brown TM, Glasser SP, Muntner P, Rosenson RS. Adherence to high‐intensity statins following a myocardial infarction hospitalization among Medicare beneficiaries. JAMA Cardiol. 2017;2:890–895. DOI: 10.1001/jamacardio.2017.0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodriguez F, Lin S, Maron DJ, Knowles JW, Virani SS, Heidenreich PA. Use of high‐intensity statins for patients with atherosclerotic cardiovascular disease in the veterans affairs health system: practice impact of the new cholesterol guidelines. Am Heart J. 2016;182:97–102. DOI: 10.1016/j.ahj.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 31. Doshi JA, Puckett JT, Parmacek MS, Rader DJ. Balancing affordability and access: lessons from new cholesterol‐lowering drugs. Health Affairs Blog. https://www.healthaffairs.org/do/10.1377/hblog20180604.549026/full. Accessed March 2, 2020.
- 32. Knowles JW, Howard WB, Karayan L, Baum SJ, Wilemon KA, Ballantyne CM, Myers KD. Access to nonstatin lipid‐lowering therapies in patients at high risk of atherosclerotic cardiovascular disease. Circulation. 2017;135:2204–2206. DOI: 10.1161/CIRCULATIONAHA.117.027705. [DOI] [PubMed] [Google Scholar]
- 33. Wade RL, Patel JG, Hill JW, De AP, Harrison DJ. Estimation of missed statin prescription use in an administrative claims dataset. J Manag Care Spec Pharm. 2017;23:936–942. DOI: 10.18553/jmcp.2017.23.9.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prada‐Ramallal G, Takkouche B, Figueiras A. Bias in pharmacoepidemiologic studies using secondary health care databases: a scoping review. BMC Med Res Methodol. 2019;19:53. DOI: 10.1186/s12874-019-0695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. DOI: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 36. Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:217047. DOI: 10.1155/2015/217047. [DOI] [PMC free article] [PubMed] [Google Scholar]


