Abstract
The efficacy of standard antidepressants is limited for many patients with mood disorders such as major depressive disorder (MDD) and bipolar depression, underscoring the urgent need to develop novel therapeutics. Both clinical and preclinical studies have implicated glutamatergic system dysfunction in the pathophysiology of mood disorders. In particular, rapid reductions in depressive symptoms have been observed in response to subanesthetic doses of the glutamatergic modulator racemic (R,S)-ketamine in individuals with mood disorders. These results have prompted investigation into other glutamatergic modulators for depression, both as monotherapy and adjunctively. Several glutamate receptor-modulating agents have been tested in proof-of-concept studies for mood disorders. This manuscript gives a brief overview of the glutamate system and its relevance to rapid antidepressant response and discusses the existing clinical evidence for glutamate receptor-modulating agents, including (1) broad glutamatergic modulators ((R,S)-ketamine, esketamine, (R)-ketamine, (2R,6R)-hydroxynorketamine [HNK], dextromethorphan, Nuedexta [a combination of dextromethorphan and quinidine], deudextromethorphan [AVP-786], axsome [AXS-05], dextromethadone [REL-1017], nitrous oxide, AZD6765, CLE100, AGN-241751); (2) glycine site modulators (d-cycloserine [DCS], NRX-101, rapastinel [GLYX-13], apimostinel [NRX-1074], sarcosine, 4-chlorokynurenine [4-Cl-KYN/AV-101]); (3) subunit (NR2B)-specific N-methyl-d-aspartate (NMDA) receptor antagonists (eliprodil [EVT-101], traxoprodil [CP-101,606], rislenemdaz [MK-0657/CERC-301]); (4) metabotropic glutamate receptor (mGluR) modulators (basimglurant, AZD2066, RG1578, TS-161); and (5) mammalian target of rapamycin complex 1 (mTORC1) activators (NV-5138). Many of these agents are still in the preliminary stages of development. Furthermore, to date, most have demonstrated relatively modest effects compared with (R,S)-ketamine and esketamine, though some have shown more favorable characteristics. Of these novel agents, the most promising, and the ones for which the most evidence exists, appear to be those targeting ionotropic glutamate receptors.
1. Overview: The Glutamate Receptor and its Role in Mood Disorders
Mood disorders, including major depressive disorder (MDD) and bipolar disorder (BD), are among the most common mental illnesses and, worldwide, cause enormous disability [1]. While the discovery and dissemination of first-generation (monoamine oxidase inhibitors [MAOIs] and tricyclic antidepressants [TCAs]) and second-generation antidepressants (selective serotonin reuptake inhibitors [SSRIs] and serotonin-norepinephrine reuptake inhibitors [SNRIs], among others) initially revolutionized the treatment of depression, these treatments are not effective for all individuals with MDD [2]. In addition, these compounds often take weeks or months to reach full effectiveness, underscoring the critical need to develop more effective and rapid-acting interventions for MDD [3].
Over the last two decades, clinical and preclinical evidence has demonstrated that the glutamatergic system contributes to the pathophysiology of MDD as well as a number of other psychiatric disorders, such as schizophrenia, Alzheimer’s disease, and bipolar disorder, though these are not believed to share a common underlying mechanism [4]. The glutamatergic system is also believed to be key to the mechanism of action underlying the rapid antidepressant effects associated with a number of novel agents [5], most notably the glutamatergic modulator racemic (R,S)-ketamine. Numerous controlled studies have consistently described rapid, robust, and relatively sustained antidepressant effects in response to a single, subanesthetic-dose (0.5 mg/kg) infusion of (R,S)-ketamine [6–9] as well as repeated administration [10–12] (reviewed in greater detail below). These findings fundamentally altered our underlying perceptions of the pathophysiology of depression and refocused research interest on the glutamatergic system as a source for possible treatments [13, 14] as well as new biomarkers for depression [15, 16]. The rapid antidepressant effects associated with (R,S)-ketamine (within one day of administration) also altered our expectation regarding the speed of antidepressant response and bolstered efforts to identify more rapid-acting treatments. In contrast to standard antidepressant treatments whose delayed effects may take weeks to manifest, rapid-acting antidepressants (RAADs) such as (R,S)-ketamine may demonstrate almost immediate effects with maximal improvement observed within one day of initiating treatment [17].
Glutamate is the major excitatory neurotransmitter in the central nervous system. The two main classes of glutamatergic receptors—ionotropic and metabotropic (mGluR)—consist of various subtype families based on structure, ion selectivity, and mechanism of action of downstream effectors [18]. Ionotropic receptors are fast-acting, ligand-gated ion channels that open when an agonist binds to them. The ionotropic receptor subtypes are N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy- 5-methyl-4-isoxazolepropionic acid (AMPA), and kainate. mGluRs are G protein-coupled receptors located on presynaptic and postsynaptic neurons as well as on glial cells and mediate slower glutamatergic activity via intracellular messenger systems. Glutamate appears to be reduced in the dorsolateral prefrontal cortex (PFC) of individuals with MDD [19] as well as in other areas of the PFC, such as the dorsomedial and dorsoanterolateral PFC [20] and the anterior cingulate cortex [21]; increased glutamate levels have also been observed in the occipital cortex [22]. One meta-analysis and systematic review of 1H-proton magnetic resonance spectroscopy (MRS) studies concluded that decreased levels of glutamatergic metabolites in the medial frontal cortex were associated with the pathophysiology of depression [23].
Ketamine’s antidepressant effects are thought to be related to its glutamatergic activity. For instance, one randomized, blinded, parallel design study of MDD participants treated with intravenous (R,S)-ketamine found that Glx response (as assessed by 1H-MRS) in the ventromedial prefrontal cortex was inversely correlated with (R,S)-ketamine dose and antidepressant effect; that is, greater antidepressant response was associated with lower Glx response [24]. In particular, (R,S)-ketamine is known to be a potent NMDA receptor antagonist, and this quality is believed to underlie its rapid antidepressant effects [25]. Theories posited to explain (R,S)-ketamine’s mechanism of action include (i) inhibition of NMDA receptor-mediated transmission at the synapse; (ii) direct inhibition of extrasynaptic NMDA receptors; (iii) inhibition of NMDA receptor-dependent bursting activity in the lateral habenula; and (iv) inhibition of NMDA receptors on gamma aminobutyric acid (GABA)-ergic interneurons [26]. The greatest attention has been paid to the last of these hypotheses—the inhibition of NMDA receptors on GABAergic interneurons; indeed, a recent preclinical study found that inhibition of cortical GABAergic interneurons was necessary as well as sufficient to engender a rapid antidepressant-like response in mice [27]. In this process, referred to as the disinhibition hypothesis of antidepressant response, (R,S)-ketamine’s effect on GABAergic interneurons would be to decrease inhibitory tone on pyramidal neurons, leading to increased synaptic glutamate release. Disinhibition of the pyramidal neuron would thus result in a transient, but significant, increase of extracellular glutamate—sometimes referred to as a ‘glutamate burst’—which is thought to be critical for antidepressant response [28], though it remains unclear how the glutamate burst leads to antidepressant effects. One theory proposes that (R,S)-ketamine blocks synaptic NMDA receptors involved in spontaneous synaptic transmission. This, in turn, deactivates calcium/calmodulin-dependent kinase and eukaryotic elongation factor 2 (eEF2). The end result is dephosphorylation of eEF2, which leads to hippocampal de-suppression of brain-derived neurotrophic factor (BDNF) synthesis [29]. Other research suggests that AMPA receptor modulation may play a role in antidepressant response, independent of NMDA receptor inhibition. In animal models, AMPA agonists exerted antidepressant-like properties that were extinguished with the administration of AMPA antagonists [30]. AMPA activation is also known to modulate BDNF, tropomyosin receptor kinase B (TrkB), glycogen synthase kinase 3 (GSK3), eEF2 [31], and mammalian target of rapamycin complex 1 (mTORC1) to increase neuroplastic processes [32]. In addition, two recent studies suggest other theories that may underlie the mechanism of action of RAADs. One study found that 4E-BP1 and 4E-BP2 are key effectors of the antidepressant activity of both (R,S)-ketamine and its metabolite (2R,6R)-hydroxynorketamine (HNK) [33]. The other study reported that direct binding of both conventional antidepressants and RAADs to the BDNF receptor TrkB accounted for the behavioral and cell-related actions of antidepressants [34].
Thus, the prevailing hypotheses suggest direct and indirect antagonism at the NMDA receptor as well as AMPA throughput modulation [5, 35, 36] as the neurochemical substrate of antidepressant effects. These converging mechanisms appear to induce rapid and sustained changes in synaptic plasticity that result in synaptic spine remodeling that, in turn, propagate (R,S)-ketamine’s antidepressant-like effects over time [37]. However, it should also be acknowledged that other targets implicated in (R,S)-ketamine’s mechanism of action are also currently being studied. For example, the opioid system has been suggested as a potential target [38], though not all studies are in agreement. We refer the interested reader to a recent detailed review of relevant mechanisms [39].
This review discusses novel glutamatergic compounds with clinical antidepressant efficacy in mood disorders. Rather than systematically review the evidence, this review seeks to summarize and update published clinical studies and ongoing clinical trials investigating the antidepressant efficacy of multiple glutamatergic agents. It should also be noted that this review emphasizes clinical results rather than preclinical work. Notably, a fundamental assumption of the recent clinical evidence reviewed below is that rapid antidepressant effects are indeed achievable in humans, a paradigm shift that lends additional urgency to the development of novel treatments for mood disorders, particularly for individuals with treatment-resistant depression (TRD) who have not responded to currently available therapies.
Any grouping of glutamate-modulating agents is by necessity fairly broad as, in some cases, the full extent of the mechanism of action of these compounds is not known and subject to reclassification with further discoveries, and the involvement of additional neurotransmitter systems and downstream mechanisms is likely. For instance, as described above, one plausible theory for (R,S)-ketamine’s mechanism of action is that NMDA receptor blockade by (R,S)-ketamine leads to a glutamate burst after which AMPA activation is required, underscoring the complex and nuanced interplay of glutamatergic mechanisms involved. The glutamate receptor-modulating agents reviewed here include: (1) broad glutamatergic modulators ((R,S)-ketamine, esketamine, (R)-ketamine, (2R,6R)-HNK, dextromethorphan, Nuedexta [a combination of dextromethorphan and quinidine], deudextromethorphan [AVP-786], axsome [AXS-05], dextromethadone [REL-1017], nitrous oxide, AZD6765, CLE100, AGN-241751); (2) glycine site modulators (d-cycloserine [DCS], NRX-101, rapastinel [GLYX-13], apimostinel [NRX-1074], sarcosine, 4-Chlorokynurenine [4-Cl-KYN/AV-101]); (3) subunit (NR2B)-specific NMDA receptor antagonists (eliprodil [EVT-101], traxoprodil [CP-101,606], rislenemdaz [MK-0657/CERC-301]); (4) mGluR modulators (basimglurant, AZD2066, RG1578, TS-161); and (5) mTORC1 activators (NV-5138). These agents are listed in Table 1 and presented in Fig. 1.
Table 1.
Novel glutamatergic modulators in human studies for depression
| Compound | Reference or NCT | Completed studies | Current studies | Notes |
|---|---|---|---|---|
| Broad NMDA receptor modulators | ||||
| (R,S)-Ketamine | Reviewed in [43–47]; see ClinicalTrials.gov* | Used in the clinic | Ongoing* (Phase II, III, and IV) | |
| (S)-Ketamine (esketamine) | [59–61]; NCT03965871; NCT03185819; NCT04425473; NCT02782104; NCT03434041; NCT04338321; NCT04476446 | FDA-approved | Ongoing (Phase II, III, and IV) | FDA approved for adults with TRD or major depression with acute suicidal ideation/behavior (March 2019); also approved by the European Union for the same indications |
| (R)-Ketamine (arketamine) | [76]; NCT04108234 | Phase I and II | Phase I | |
| Hydroxynorketamine (HNK) | NCT03977675 | No completed studies | Phase I | |
| Dextromethorphan | [82]; NCT04226352 | Phase I and II | Phase II | |
| Nuedexta | [84–86] | Phase II | No active trials | FDA approved for treatment of pseudobulbar affect (October 2010); EU approval for treatment of pseudobulbar affect (June 2013) |
| Deudextromethorphan (AVP-786) | NCT02153502 | Phase I and II (results not posted) | Phase III for Alzheimer’s; no active trials for depression | FDA Fast-Track designation for agitation in Alzheimer’s disease (November 2015) |
| Axsome (AXS-05) | NCT04019704; NCT04039022; NCT02741791; NCT03495579 | No completed studies | Phase III | FDA Fast-Track designation for TRD and for agitation in Alzheimer’s disease (February 2017) |
| Dextromethadone (REL-1017) | NCT03051256 | Phase II | Phase III | FDA Fast-Track designation as adjunctive treatment for MDD (April 2017) |
| Nitrous oxide | [94]; NCT03283670; NCT03869736; NCT03932825; NCT02757521; NCT03736538 | Phase II | Phase I and II | |
| AZD6765 | [95–97] | Phase II | No active trials | |
| CLE100 | NCT04103892 | No completed studies | Phase II | |
| AGN-241751 | NCT03726658; NCT03486427 | No completed studies | Phase II and III | FDA Fast-Track designation for TRD (July 2018) |
| Glycine site modulators | ||||
| D-cycloserine (DCS) | [101–103] NCT03937596 with rTMS | Phase II and IV | Phase II | |
| NRX-101 | NCT02974010; NCT03396068; NCT03396601; NCT03395392 | No published studies | Phase II and III | FDA Breakthrough Therapy (November 2018) and Fast-Track Therapy (September 2017) designations for suicide in bipolar depression |
| Rapastinel (GLYX-13) | [105, 106, 134] | Phase II and III | No active trials | FDA Fast-Track (March 2014) and Breakthrough Therapy (January 2016) designations for adjunctive treatment of MDD |
| Apimostinel (NRX-1074) | NCT02067793; NCT02366364 | Phase I and II (results not posted) | No active trials | |
| Sarcosine | [108] | Phase II | No active trials | |
| 4-Chlorokynurenine (4-Cl-KYN/AV-101) | [109]; NCT03078322 | Phase II | Phase II | FDA Fast-Track designation as adjunctive treatment for MDD (January 2018) |
| Subunit (NR2B)-specific NMDA receptor antagonists | ||||
| Eliprodil (EVT-101) | NCT01128452 | No completed studies | No active trials | Trial terminated early due to an FDA-issued clinical hold |
| Traxoprodil (CP-101,606) | [111] | Phase II | No active trials | Development halted due to potential cardiovascular effects |
| Rislenemdaz (MK-0657/CERC-301) | [112, 113] | Phase II | No active trials | |
| mGIuR modulators | ||||
| Basimglurant | [114] | Phase II | No active trials | |
| AZD2066 | NCT01145755 | Phase II | No active trials | |
| RG1578 (decoglurant) | [116] | Phase II | No active trials | |
| TS-161 | NCT03919409 | Phase I (results not posted) | Phase II for TRD | |
Ongoing NCTs for (R,S)-ketamine are too numerous to list here; see ClinicalTrials.gov for a full listing
FDA US Food and Drug Administration, MDD major depressive disorder, NCT National Clinical Trial, NMDA N-methyl-D-aspartate, rTMS repetitive transcranial magnetic stimulation, TRD treatment-resistant depression
Fig. 1.
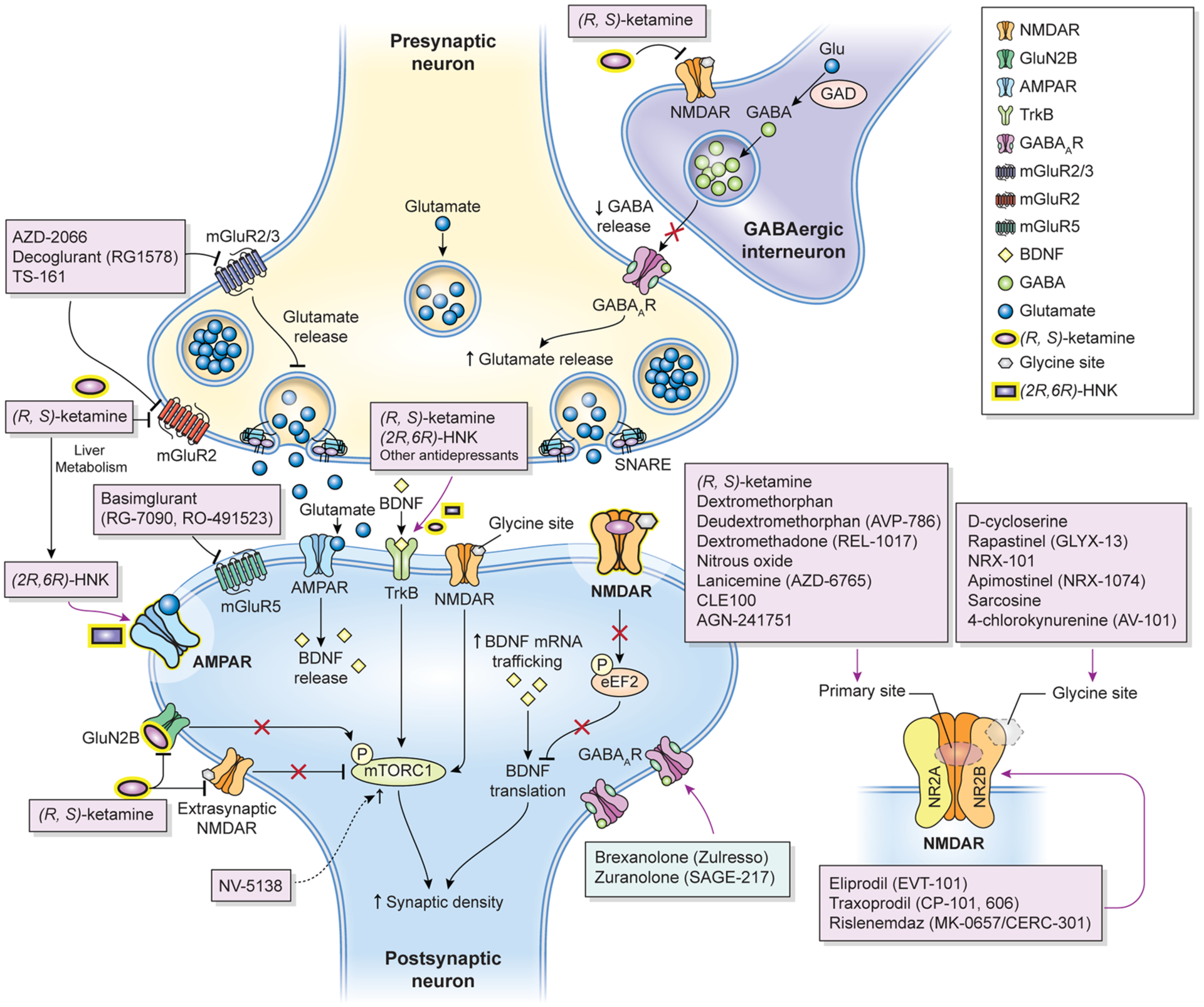
Proposed mechanisms of action of glutamatergic modulators and other putative rapid-acting antidepressants. Disinhibition hypothesis: Racemic (R,S)-ketamine and esketamine selectively block N-methyl-D-aspartate receptors (NMDARs). These are expressed on γ-aminobutyric acid (GABA)-ergic inhibitory interneurons, and their blockade decreases interneuron activity that, in turn, leads to disinhibition of pyramidal neurons and enhanced glutamatergic firing. Glutamate then binds to and activates post-synaptic α-amino-3- hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs). Inhibition of spontaneous NMDAR-mediated transmission: Alternatively, (R,S)-ketamine may induce rapid brain-derived neurotrophic factor (BDNF) translation in the hippocampus, reduce phosphorylation, and activate eukaryotic elongation factor 2 (eEF2). Ketamine may also preferentially bind to NMDARs and affect neuronal NMDAR-mediating spontaneous excitatory transmission, which at rest keeps eEF2 phosphorylated and inhibits BDNF synaptic translation. De-suppression of BDNF translation then contributes to changes in synaptic plasticity that mediate (R,S)-ketamine’s antidepressant effects. AMPAR activation is also necessary for these effects. Inhibition of extra-synaptic NMDARs: Ketamine selectively blocks extra-synaptic GluN2B-containing NMDARs, which are tonically activated by low levels of ambient glutamate regulated by the excitatory amino acid transporter 2 (EAAT2) located on astrocytes. Inhibition of the extra-synaptic GluN2B-NMDARs de-suppresses mammalian target of rapamycin complex 1 (mTORC1) function, which in turn induces protein synthesis. Blockade of spontaneous NMDAR activation inhibits eEF2 kinase (eEF2K) activity, thus preventing phosphorylation of its eEF2 substrate. This effect subsequently enhances BDNF translation and, ultimately, protein synthesis. The role of ketamine metabolites: (2R,6R)-hydroxynorketamine (HNK) exerts NMDAR inhibition-independent antidepressant actions by activating AMPAR-mediated synaptic potentiation. Other glutamatergic modulators: Metabotropic glutamate receptor (mGluR) 2/3 antagonists are thought to enhance synaptic glutamate levels, thereby boosting AMPAR transmission and firing rates and extracellular monoamine levels. GLYX-13, which has partial agonist properties at NMDARs, is hypothesized to activate mTORC and subsequently induce protein synthesis. GLYX-13 requires AMPA and activity-dependent BDNF release but, unlike (R,S)-ketamine, it does not produce glutamate bursts. AMPAR activation enhances BDNF release, activates the tropomyosin receptor kinase B (TrkB) receptor, and subsequently promotes protein synthesis by activating the mTORC complex. GABAAR GABA type A receptor, GAD glutamate decarboxylase, Glu glutamate, SNARE soluble N-ethylmaleimide-sensitive factor attachment protein receptor Adapted with permission from Kadriu et al. [5]
2. (R,S)-Ketamine, the Prototypical Glutamatergic Rapid-Acting Antidepressant, and Its Derivates: Esketamine, R-Ketamine, and Hydroxynorketamine (HNK)
Building on preclinical work [25], Berman and colleagues conducted a small, controlled trial in humans demonstrating that a single, subanesthetic-dose (0.5 mg/kg) infusion of the NMDA receptor antagonist (R,S)-ketamine given as monotherapy exerted antidepressant effects that began within 4 h of infusion and peaked at 72 h in a group of seven participants with major depression (seven with MDD and one with bipolar depression) [7]. A subsequent series of randomized controlled studies corroborated and extended these findings and identified a 24-hour response rate of > 70% to a single, monotherapeutic (R,S)-ketamine infusion in 18 participants with TRD; this response lasted one week or longer in some patients [8]. Since then, most of the placebo-controlled, double-blind, randomized trials examining this agent have demonstrated the antidepressant efficacy of subanesthetic-dose (R,S)-ketamine in individuals with TRD [8, 40] as well as bipolar depression [41, 42]. One meta-analysis of nine randomized, placebo-controlled studies found that (R,S)-ketamine’s antidepressant effects after a single administration typically begin 40 min post-infusion, peak approximately 24 h later, and lose superiority to placebo 10–12 days post-infusion [43]. Other meta-analyses have corroborated these findings [44–47]. In addition to these robust antidepressant effects, a single subanesthetic-dose (R,S)-ketamine infusion has also been found to have significant and rapid (1–4 h) anti-suicidal ideation effects [48–53], anti-anhedonic properties [54, 55], and therapeutic potential in other disorders such as post-traumatic stress disorder (PTSD) [56, 57] and obsessive compulsive disorder (OCD) [58]. Researchers have also been able to successfully prolong (R,S)-ketamine’s otherwise transient antidepressant effects via repeated infusions [10–12]. These groundbreaking findings culminated in the successful development of [59–61] and 2019 FDA approval of [62] intranasal esketamine (Spravato™)—the (S +) enantiomer of ketamine—for adults with TRD or major depression with acute suicidal ideation or behavior; this agent has also been approved for the same indications by the European Union. Additional clinical trials with esketamine are ongoing, including phase II trials for treatment-resistant bipolar depression (ClinicalTrials.gov identifier NCT03965871), pediatric depression with imminent risk for suicide (NCT03185819), and perioperative depression (ClinicalTrials.gov identifier NCT04425473) as well as phase III (NCT02782104; NCT03434041; NCT04338321) and phase IV (NCT04476446) monotherapy and adjunctive-use trials in TRD.
It should be noted that (R,S)-ketamine is associated with psychotomimetic effects that peak at about 40 min post-infusion, and it requires medical supervision during administration [63]. In addition to these psychotomimetic/dissociative effects, (R,S)-ketamine also carries abuse liability and is associated with clinical side effects that include blood pressure changes, cognitive effects, risk of cystitis and other chronic bladder issues, and hepatotoxicity (though the latter two are less common at the lower sub-anesthetic doses used to treat depression) [64]. As a result, Spravato™ can only be dispensed and administered to individuals in medically supervised healthcare settings that provide monitoring (Risk Evaluation and Mitigation Strategies [REMS]). These issues remain a concern, particularly in the context of long-term (R,S)-ketamine/esketamine use, and suggest that the side effect, safety, and addiction profile of these agents should be explored in larger studies designed to better identify the limitations associated with long-term use [65, 66]. Current research is examining these concerns in an attempt to separate them from (R,S)-ketamine and esketamine’s broader efficacy profile.
To date, only one study has examined the differences between esketamine (0.25 mg/kg) and (R,S)-ketamine (0.5 mg/kg); though underpowered, it found no differences in efficacy, tolerability, or psychotomimetic profile between the two agents [67]. A recent meta-analysis suggests the need to compare these two agents head-to-head [68]. In addition, though these agents are comparatively new, consensus-building guideline documents are beginning to emerge that describe where to employ (R,S)-ketamine and esketamine in the treatment algorithm (for instance, for TRD, or for individuals who cannot tolerate standard treatments) [65, 69–71].
Another related agent under preliminary investigation is (R)-ketamine (arketamine), the R enantiomer of ketamine. While (R)-ketamine is a less potent NMDA receptor inhibitor [30, 72], initial animal studies found that it had greater and longer-lasting antidepressant-like properties than esketamine [30, 73–75]. Building on this work, an open-label trial in seven human participants with TRD (several of whom were taking concomitant antidepressant and antipsychotic medications) found that a single (R)-ketamine infusion (0.5 mg/kg) had rapid and significant antidepressant effects, leading to a 20-point reduction in Montgomery-Åsberg Depression Rating Scale (MADRS) score at 24 h; the investigators noted only mild dissociative side effects after (R)-ketamine infusion [76]. Nevertheless, larger, randomized controlled studies are needed to definitively establish (R)-ketamine’s effects, and a phase I, randomized, placebo-controlled study (NCT04108234) is currently ongoing.
Finally, although this manuscript focuses almost entirely on clinical findings, the paradigm-shifting nature of one recent line of inquiry leads us to present more preliminary evidence. In particular, a recent series of studies called into question whether NMDA receptor inhibition is indeed the primary mechanism of (R,S)-ketamine’s antidepressant action. In preclinical studies, researchers found that the antidepressant effects of (R,S)-ketamine appear to be produced not by (R,S)-ketamine itself but by one of its metabolites: (2R,6R)-HNK. Specifically, (2R,6R)-HNK exerts antidepressant-like properties via indirect early and sustained activation of AMPA receptors, likely resulting from a mechanism converging with mGluR2 signaling [30, 77–79]. These findings are particularly intriguing given that many of (R,S)-ketamine’s side effects appear to be related to NMDA receptor-dependent inhibition. Phase I safety and tolerability studies investigating the potential antidepressant efficacy and side effect profile of (2R,6R)-HNK in humans are underway (NCT03977675), and a phase II clinical trial is planned. The work underscores the current rapid pace of research in glutamatergic drug development and further highlights the critical role of (R,S)-ketamine as a proof-of-concept agent for exploring new RAADs.
As noted above, despite the established body of evidence for (R,S)-ketamine’s antidepressant efficacy, its underlying mechanisms of action have yet to be conclusively established. However, as our theoretical understanding of (R,S)-ketamine’s mechanisms of action grow, researchers have sought to establish whether other glutamatergic modulators might exert antidepressant effects similar to those of (R,S)-ketamine. Several candidate drugs whose mechanistic processes overlap with those of (R,S)-ketamine have been explored to see whether they could mimic its rapid and robust antidepressant properties while avoiding its dissociative and psychotomimetic side effects. As reviewed in the following section and in Table 1, these compounds are in varying stages of development and testing.
3. The Antidepressant Efficacy of Novel Glutamatergic Modulators
3.1. Broad Glutamatergic Modulators
3.1.1. Dextromethorphan, Nuedexta, Deudextromethorphan (AVP 786), and AXS-05 (Axsome)
The non-selective, non-competitive NMDA receptor antagonist dextromethorphan is a cough suppressant with sedative and dissociative properties; this agent also acts on opioid receptors and, at higher doses, is an antagonist at the sigma-1 receptor. Dextromethorphan and its enantiomers have been considered as potential RAADs [80, 81], and various formulations are currently under investigation for MDD. To date, there have been no positive phase III results.
With regard to bipolar depression, dextromethorphan was studied adjunctively with valproic acid in a 12-week, randomized, placebo-controlled trial of 250 individuals. That study found that dextromethorphan had no significant antidepressant effects, as assessed by mean Hamilton Depression Rating Scale (HAM-D) and Young Mania Rating Scale (YMRS) scores [82]. A phase II, open-label pilot study is currently exploring three dosing regimens of dextromethorphan in MDD and TRD (NCT04226352).
Nuedexta is a related drug comprising dextromethorphan 20 mg and quinidine 10 mg and is FDA approved for the treatment of pseudobulbar affect. The rationale for combining dextromethorphan with quinidine is that quinidine is a potent inhibitor of the cytochrome P450 2D6 isoform, which is the predominant metabolic pathway for dextromethorphan. Co-administration yields significantly greater dextromethorphan plasma concentrations and CNS bioavailability [83]. Nuedexta was also found to have antidepressant effects in a case report describing a depressed patient with emotional lability [84]. In a retrospective chart review of 77 participants with BD-II or BD not otherwise specified (BD-NOS), adding Nuedexta once or twice daily to a current medication regimen over 90 days significantly improved Clinical Global Impression (CGI) scale scores [85]. Finally, a prospective, open-label, 10-week, phase IIA clinical trial examined the tolerability and efficacy of Nuedexta in 20 participants with TRD, half of whom were receiving concomitant medications; the investigators found that response and remission rates were 45% and 35%, respectively, as measured by pre- and post-administration MADRS and Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR) scores [86]. No active clinical trials are underway.
Another related agent is deudextromethorphan (AVP-786), an orally administered compound formed by combining deuterated (d6)-dextromethorphan hydrobromide (to reduce first-pass metabolism) and ultra-low-dose quinidine sulfate; it is a weak NMDA receptor antagonist. A phase II, 10-week, multi-center, randomized, double-blind, placebo-controlled, clinical trial (NCT02153502) evaluated the efficacy, safety, and tolerability of AVP-786 as adjunctive therapy in patients with TRD. The study was completed in 2016, but no results have been posted. No additional studies for mood disorders are currently underway, and it appears that AVP-786 is largely being pursued as a therapeutic option for the negative symptoms of schizophrenia and for agitation in Alzheimer’s disease; it has received FDA Fast-Track designation for the latter, and four phase III trials are underway (NCT02446132; NCT03393520; NCT02442765; NCT02442778).
Finally, another related compound is AXS-05 (Axsome), a combination of dextromethorphan and bupropion. The antidepressant efficacy of AXS-05, which has received FDA Fast-Track designation for both TRD and agitation in Alzheimer’s disease, is currently being explored in ongoing phase III clinical trials in TRD (NCT04019704; NCT04039022; NCT02741791; NCT03495579). Initial results of a 6-week, double-blind, bupropion-controlled, multi-center study in 80 participants with depression designed to assess a primary safety endpoint found that oral AXS-05 as monotherapy was associated with statistically significant mean reductions in MADRS total score [87]. At study endpoint (Week 6), 47% of MDD participants taking AXS-05 had achieved remission compared with 16% taking bupropion. Statistically significant antidepressant effects were observed within 2 weeks of treatment. In addition, AXS-05 was not associated with serious adverse events, including psychotomimetic symptoms. A subsequent phase III randomized, double-blind, bupropion-controlled study of 312 adult TRD patients found no significant improvement at the primary 6-week endpoint, though it did demonstrate significant improvement at earlier time points (Week 1) [88]. Based on these results, a second phase III trial is being planned, though no active efficacy trials are currently listed in ClinicalTrials.gov.
3.1.2. Dextromethadone (REL-1017)
REL-1017, an isomer of racemic methadone (d-methadone), is a non-competitive NMDA receptor antagonist [89, 90]. The d-isomer of methadone demonstrates primary activity at the NMDA receptor. It should be noted that although it demonstrates much lower affinity to the mu and delta opioid receptors than the l-isomer, abuse and dependence concerns persist. REL-1017 has demonstrated antidepressant activity in animal models [90]. In human trials, after a small, phase I, open-label study found that adjunctive use of REL-1017 effectively reduced pain in eight chronic pain sufferers [91], REL-1017 was subsequently developed as a rapid-acting oral agent for the treatment of depression as well as neuropathic pain; it subsequently received FDA Fast-Track designation as an adjunctive treatment for MDD. A phase IIA, multicenter, randomized, double-blind, placebo-controlled clinical trial assessed the safety and efficacy of adjunctive-dose (either 25 mg or 50 mg daily for 2 weeks) REL-1017 in 62 participants with TRD. Within the first 4 days, participants in both the 25-mg and 50-mg treatment groups experienced statistically significant and clinically meaningful improvements in depressive symptoms, as assessed via the MADRS, CGI-Severity (CGI-S), and CGI-Improvement (CGI-I) scales. These improvements continued for the remainder of the 7-day treatment period and, interestingly, improvements were sustained for an additional 7 days after participants were no longer taking REL-1017 [92, 93]. While REL-1017 was well tolerated, and participants experienced no psychotomimetic symptoms, as an enantiomer of the opioid methadone, concerns persist about its abuse and dependence potential. A phase III trial is now underway (NCT04688164).
3.1.3. Nitrous Oxide
Nitrous oxide is a non-competitive NMDA receptor antagonist and an inhaled general anesthetic most often used in obstetrics or dentistry. In a placebo-controlled, double-blind, crossover pilot study, 20 individuals with TRD received an hour of adjunctive-use inhaled nitrous oxide (50% nitrous oxide and 50% oxygen) or placebo (50% nitrogen and 50% oxygen) during two treatment sessions separated by 1 week [94]. As assessed via the HAM-D, depressive symptoms improved within 2 h in response to nitrous oxide versus placebo, an effect that remained for 24 h; a median reduction of 5.5 points was observed on the HAM-D. Transient side effects included anxiety, headache, and nausea/vomiting. Several additional phase I and II double-blind clinical trials are actively assessing the efficacy and safety of nitrous oxide in MDD (NCT03283670; NCT03869736; NCT03932825) as well as bipolar depression (NCT02757521) and suicide ideation (NCT03736538), though none have yet posted results. Such studies should provide more information regarding the rapidity of nitrous oxide’s antidepressant effects and its overall feasibility as an RAAD, especially given that no studies have yet determined the safety and efficacy of repeated doses of this agent.
3.1.4. AZD6765
The non-selective NMDA receptor channel blocker AZD6765 (lanicemine) is thought to have a mechanism of action similar to that of (R,S)-ketamine. It was found to have promising, though modest, antidepressant effects in early clinical studies [95]. A 3-week, placebo-controlled, phase IIb trial of repeated-dose adjunctive lanicemine (at daily doses of either 100 mg or 150 mg) in 34 participants with TRD then found that this agent’s antidepressant effects were not rapid [96]. A larger (n = 302), 6-week, phase IIb study of adjunctive repeated-dose lanicemine (50 mg and 100 mg) then found no separation from placebo on primary endpoint measures [97], though this may have been due to a large placebo effect (39%). As a result, clinical development was halted due to lack of efficacy [97], and no active trials are underway. However, Bio-Haven Pharmaceuticals recently licensed an orally available compound (BHV-5000) whose active metabolite is lanicemine. The planned study intends to explore the efficacy of BHV-5000 for TRD, but these trials are not yet underway [98].
3.2. Glycine Site Modulators
3.2.1. D-Cycloserine (DCS) and NRX-101
D-cycloserine (DCS) is a broad-spectrum antibiotic that, at doses > 100 mg/day, also acts as a functional NMDA glycine site modulator [99]. Like (R,S)-ketamine, DCS administration (1000 mg) in healthy volunteers has been associated with acute (35 and 79–90 min) increases in Glx as measured by 1H-MRS [100]. A small (n = 22), 6-week, placebo-controlled, crossover study of adjunctive DCS (250 mg/day) in individuals with TRD found that DCS reduced depressive symptoms, but the high placebo response rate precluded separation from placebo [101]. A subsequent double-blind, placebo-controlled, 6-week parallel group study enrolled 26 participants with TRD and assessed the efficacy of escalating dose-adjunctive DCS, up to 1000 mg/day [102]. The investigators found that higher-dose DCS had significant antidepressant effects. Specifically, DCS treatment significantly improved depressive symptoms as assessed by the HAM-D (p = 0.005) and the Beck Depression Inventory (BDI) (p = 0.046), which were the primary outcome measures. Fifty-four percent of the participants randomized to receive high-dose DCS had > 50% reductions in their HAM-D scores by the end of the trial, though this antidepressant response was not rapid.
An 8-week, open-label study then investigated adjunctive DCS (1000 mg/day) as a relapse prevention strategy after six intravenous (R,S)-ketamine infusions in 12 individuals with bipolar depression, only seven of whom completed the study [103]. With the exception of the 2-week time point, depressive symptoms improved from baseline through Week 8, and a large effect size was seen at Day 1, comparable to the magnitude of improvement observed 24 h after administration of (R,S)-ketamine. Furthermore, four of the seven study completers remained in remission after 8 weeks. Finally, a recent meta-analysis that included data drawn from the studies described above found that high-dose (1000 mg/day), but not low-dose (250 mg/day), DCS had acute antidepressant effects [46]. One ongoing placebo-controlled study is also investigating the use of adjunctive DCS as an augmentation strategy for repetitive transcranial magnetic stimulation (rTMS) (NCT03937596).
NRX-101 (DCS and the antipsychotic lurasidone, which has D2/5-HT2A receptor antagonist activity) is a related compound used in a sequential treatment regimen comprising a single dose of intravenous (R,S)-ketamine followed by an oral formulation of NRX-101. A recent phase II/III trial in 22 individuals with BD and recent suicidal ideation randomized participants to receive a single dose of (R,S)-ketamine; those who achieved response were randomized to receive NRX-101 or lurasidone alone (NCT02974010). Participants in the NRX-101 group had statistically significant reductions in both depression and suicidal ideation scores and, furthermore, maintained stable remission from depressive symptoms without recurrence of suicidality [104]. These promising preliminary results led the FDA to award both Breakthrough Therapy and Fast-Track Therapy designation to NRX-101 for suicide in bipolar depression. One ongoing investigation in participants with bipolar depression is examining whether NRX-101 increases Glx in the anterior cingulate cortex and whether changes in Glx correlate with clinical treatment effects (NCT03402152). The antidepressant efficacy of NRX-101 is also being investigated in phase III trials of patients with bipolar depression and suicidal ideation (NCT03396068; NCT03396601; NCT03395392).
3.2.2. GLYX-13 (Rapastinel) and NRX-1074 (Apimostinel)
GLYX-13 (rapastinel) is an NMDA glycine site partial agonist that is administered intravenously. A phase IIb study randomized 116 unmedicated inpatients with TRD to receive a single intravenous administration of saline placebo or GLYX-13 as monotherapy (1 mg/kg, 5 mg/kg, 10 mg/kg, or 30 mg/kg) over 3–15 min. One week post-administration, participants who received the 5 and 10 mg/kg doses of GLYX-13 had a significant antidepressant response, as assessed by the HAM-D, compared with placebo [105]; antidepressant effects manifested within 2 h. GLYX-13 infusion was not associated with psychotomimetic properties at any dose, nor were any serious adverse events reported. GLYX-13 subsequently received both Fast-Track and Breakthrough Therapy designations from the FDA for adjunctive treatment of MDD but, disappointingly, did not meet primary or secondary endpoints in three large phase III trials [106]. No active studies with this agent are presently underway.
NRX-1074 (apimostinel) is an orally available derivative of GLYX-13; although the mechanism of action of these two agents is similar, NRX-1074 is orally active and appears to be several thousand-fold more potent than GLYX-13 at the glycine site. It was developed as an adjunctive therapy for TRD, appeared to be well tolerated, and did not cause psychotomimetic symptoms [107]. An intravenous formulation of apimostinel has been investigated in phase II trials for MDD (NCT02067793), and an oral formulation has been investigated in phase I trials of healthy volunteers (NCT02366364); however, while the studies have been completed, results have not yet been posted. No active studies with this agent are presently listed on ClinicalTrials.gov.
3.2.3. Sarcosine
Sarcosine is a glycine site modulator whose mechanism of action is thought to depend on NMDA receptor modulation. A 6-week, double-blind, randomized, citalopram-controlled phase II clinical trial examined the efficacy of sarcosine as monotherapy in 40 individuals with MDD; the HAM-D, CGI, and global assessment of functioning (GAF) rating scales were the primary outcome measures. Compared with those treated with the SSRI citalopram, participants who received sarcosine had greater improvements in depressive symptoms, as measured by all of the rating scales, and better remission (65% vs 5% for sarcosine vs citalopram, respectively) and response rates (70% vs 20% for sarcosine vs citalopram, respectively) [108]. Sarcosine-treated patients were also less likely to drop out of the study (30% vs 45% for sarcosine vs citalopram, respectively) [108]. No significant side effects were reported. Presently, no active studies are investigating sarcosine for the treatment of mood disorders.
3.2.4. 4-Chlorokynurenine (AV-101)
4-Chlorokynurenine (4-Cl-KYN), also known as AV-101, is a novel prodrug that crosses the blood–brain barrier, where it is converted to 7-chlorokynurenic acid (7-Cl-KYNA), a potent and highly selective glycine site modulator. This agent received FDA Fast-Track designation in 2018 for the adjunctive treatment of MDD. However, a recent phase II, double-blind, 2-week, placebo-controlled, crossover study of 19 participants with TRD found that AV-101 monotherapy (either 1080 mg/day or 1440 mg/day) did not improve participants’ overall depressive symptoms (as assessed by HAM-D total score) [109]. This study, which obtained CSF drug levels, found very low levels of 7-Cl-KYNA, suggesting that 4-Cl-KYN did not convert to 7-Cl-KYNA. Another recent study explored potential mechanisms for this lack of conversion and found that co-administration of probenecid with 4-Cl-KYN led to dose-dependent increases in 7-Cl-KYNA concentrations by as much as ~ 800-fold in the prefrontal cortex of rats, suggesting a path forward for humans with depression [110]. In the aforementioned phase II trial, no differences in any of the peripheral or central biological indices or in frequency of adverse effects were observed at any time between groups [109]. A larger, multicenter, randomized, placebo-controlled phase II study recently examined the efficacy and safety of adjunctive oral AV-101 in 180 participants with TRD concomitantly treated with either an SSRI or an SNRI, with change in MADRS score as the primary outcome measure (NCT03078322); no results have yet been posted.
3.3. Subunit (NR2B)-Specific NMDA Receptor Antagonists
3.3.1. CP-101,606 (Traxoprodil)
Traxoprodil (CP-101,606) acts as an NR2B subunit-selective NMDA receptor antagonist. One placebo-controlled, double-blind, randomized study investigated 30 individuals with TRD [111] who received 6 weeks of open-label paroxetine followed by a single CP-101,606 infusion plus paroxetine 40 mg/day for 30 days. Despite initially promising results, significant dissociative side effects were noted and development of the compound was ultimately halted. No replication studies were conducted.
3.3.2. Rislenemdaz (CERC-301/MK-0657)
Rislenemdaz (also known as CERC-301 and MK-0657) is an NR2B antagonist. The efficacy of orally administered rislenemdaz (4–8 mg/day for 12 days) monotherapy for TRD was assessed in a small, placebo-controlled, double-blind, randomized, crossover pilot study [112]. Only five of the planned 21 participants completed the study, and no antidepressant efficacy over placebo was observed on the primary outcome measure (MADRS score); however, improvement was observed using other depression rating scales. A subsequent phase II, 5-week trial explored the antidepressant efficacy of adjunctive rislenemdaz 8 mg/day in 137 participants with TRD and recent suicidal ideation. Although well tolerated, this agent had no significant antidepressant effects compared with placebo, as assessed by the HAM-D [113]. No active clinical trials are currently exploring the antidepressant efficacy of this agent.
3.4. mGluR Modulators
3.4.1. Basimglurant
Basimglurant is an mGluR5 negative allosteric modulator; while they are not NMDA receptor modulators, mGluRs can modulate glutamatergic function outside of the NMDA receptor and AMPA receptor pathways. A phase II, placebo-controlled, randomized, multi-center trial investigated the antidepressant efficacy of adjunctive, modified-release basimglurant (0.5 or 1.5 mg) versus adjunctive placebo in 333 individuals with TRD. The 9-week study included 6 weeks of double-blind treatment and 3 weeks of follow-up [114]. No significant difference was observed between the three groups (adjunctive basimglurant 0.5 mg, adjunctive basimglurant 1.5 mg, or placebo) on change in MADRS score (the primary endpoint), although potentially promising results were observed on several of the exploratory secondary endpoints at 1.5 mg. No active studies are presently investigating use of this agent for depression.
3.4.2. AZD2066
AZD2066 is an mGluR5 negative allosteric modulator that has most recently been investigated for pain-related conditions. A 6-week, randomized, phase II study of 131 participants with MDD compared the efficacy of 12–18 mg/day of AZD2066 monotherapy with both placebo and the SNRI duloxetine (NCT01145755; results available on ClinicalTrials.gov). No statistically significant differences were observed between the three treatment arms with regard to the primary outcome measure (MADRS score), antidepressant response, or remission. No active studies with AZD2066 for the treatment of mood disorders are currently underway.
3.4.3. RG1578 (decoglurant) and TS-161
In preclinical studies, mGlu2/3 receptor inhibition elicits rapid antidepressant effects that resemble (R,S)-ketamine’s effects in rodents [115]. A recent phase II, 6-week clinical trial of adjunctive-use RG1578 (decoglurant), a negative allosteric modulator of the mGlu2/3 receptor, in 357 MDD individuals with partial TRD found no antidepressant response compared with placebo; however, no measure of target engagement was included in this trial, so it cannot be definitively concluded that the target was engaged [116]. Although both orthosteric and allosteric mGlu2/3 receptor modulators have been shown to exert antidepressant effects, the potency of allosteric modulators was found to be weaker than that of orthosteric antagonists [117]. Currently, the potent and selective mGlu2/3 receptor orthosteric antagonist TS-161 has completed phase I (NCT03919409) trials in 70 healthy volunteers to evaluate its safety profile, tolerability, and pharmacokinetics. This agent is presently in phase II studies for TRD at the NIMH.
3.5. Drugs in Early Development: CLE100, AGN-241751, EVT-101, and NV-5138
Other agents currently being explored for rapid-acting antidepressant efficacy in MDD are in various stages of exploration. They include two NMDA receptor modulators—CLE100 and AGN-241751—as well as the selective NMDA receptor NR2B antagonist EVT-101 and the mTORC1 activator NV-5138.
In 2011, a phase II clinical trial for MDD of EVT-101 was terminated early due to a clinical hold issued by the FDA (NCT01128452), and no results have been posted; no further clinical trials for this agent are underway.
A randomized, double-blind, placebo-controlled phase IIA trial is currently exploring an oral formulation of the NMDA receptor modulator CLE100 for adjunctive use in individuals with TRD (NCT04103892), but no results have yet been posted.
The rapid-acting antidepressant efficacy of AGN-241751, believed to function as an orally bioavailable small molecule NMDA receptor modulator and positive allosteric modulator [118], is currently being studied in a double-blind, placebo-controlled, phase II trial (NCT03726658) examining single-dose versus multiple-dose AGN-241751 as well as twice-daily dosing, but results from the trial have not been posted. Another double-blind, placebo-controlled, fixed-dose study in TRD (NCT03486427) was also recently completed, but results are not yet available. Based on promising preliminary data, AGN-241751 received FDA Fast-Track designation for TRD in July 2018.
Finally, although they do not technically encompass glutamate modulation, strategies are also being developed to directly activate the intercellular mTORC1 signaling cascade. As noted above, mTORC1 has been implicated as a downstream pathway connecting glutamate modulation with the induction of neuroplastic processes [32]. NV-5138, an analog of the amino acid leucine, was developed as an mTORC1 activator and potential antidepressant [119]. Animal models subsequently found that NV-5138 had rapid antidepressant effects [120, 121]. In light of these promising preliminary results, human studies are currently under consideration.
4. Challenges and Future Directions
As the evidence reviewed here demonstrates, the clinical pipeline for novel glutamatergic treatments for mood disorders, though varied, is encouraging [98]. Nevertheless, it should also be cautioned that despite the number of agents currently in the clinical pipeline, no other glutamatergic modulators tested to date have shown the same rapid, robust, and sustained antidepressant effects as (R,S)-ketamine and esketamine. Furthermore, none appear to possess the breadth of (R,S)-ketamine’s therapeutic effects including its antisuicidal properties, anti-anhedonic effects, and therapeutic potential in other disorders, though certainly not all novel compounds have yet been investigated for these indications. The failure to expand glutamatergic treatment strategies past (R,S)-ketamine underscores the complexity of the biological substrate of depression. It also bears noting that while this manuscript has focused on glutamatergic mechanisms of antidepressant response, advances in depression treatment are occurring with other molecular targets.
For instance, GABA modulation strategies, specifically GABA type A (GABAA) receptor-positive allosteric modulators, have also yielded positive results. Brexanolone (Zulresso), a neurosteroid derivative of allopregnanolone, acts as a positive allosteric modulator of GABAA receptor function [122]; in contrast to benzodiazepines, positive allosteric modulators bind and trigger the GABAA receptor to open more frequently or for longer periods, but they do not work independently, requiring the presence of either GABA or a GABA agonist [123]. Brexanolone’s mechanism of rapid antidepressant action is presently unclear, but is thought to bind to both synaptic and extrasynaptic GABAA receptors, thus increasing receptor functionality [124]. This agent, which is administered intravenously over a period of 60 h, received FDA approval as the first drug specifically indicated for postpartum depression (PPD) and was found to exert rapid (within 2.5 days) antidepressant properties in a number of placebo-controlled, double-blind, randomized trials [125, 126]. Zuranolone (SAGE-217) is a next-generation GABAA receptor enhancer with a pharmacokinetic profile similar to that of brexanolone but intended for once-daily oral dosing. Zuranolone has shown positive results in phase II [127] and phase III [128] trials. Additional phase III trials are underway for both MDD and PPD (NCT03864614; NCT04442490; NCT04442503). Though contemporaneous findings of effective glutamate and GABA modulating agents for depression may be coincidental, it is notable that the interaction and balance between excitatory glutamate and inhibitory GABA in corticolimbic systems has been implicated in the pathophysiology and treatment of depression [129, 130].
Overall, challenges remain for developing novel potential therapies. The recent failure of several novel agents for MDD reviewed here (GLYX-13 is a particularly striking example) deserves careful scrutiny by the scientific community as a whole, particularly with regard to the overall importance of bench-to-bedside translational paradigms that lead from basic science research to clinical trials. The fact that many of the agents reviewed above focused on eliminating (R,S)-ketamine’s side effects and observed decreased antidepressant efficacy as a result may help the field reconceptualize the challenging and complex processes involved with drug discovery in psychiatry.
Furthermore, questions remain about the ideal outcome measures used in clinical trials to evaluate RAADs [98], particularly as regards the best way to assess the rapid changes observed in response to a variety of these compounds. Many outcome measures currently in use—including the HAM-D and MADRS—were designed to measure change in symptoms that occur over weeks to months rather than hours to days. For instance, changes in insomnia or appetite cannot reasonably be assessed over a period of hours. Yet, most trials of RAADs use these scales, suggesting that more accurate ways to capture the clinical effects of these agents are needed. It is possible that identifying unidimensional constructs that can help parse the heterogeneity of depressive symptoms will ultimately create more refined rating scale scores to more successfully illuminate connections between specific symptoms and underlying pathophysiology [131].
Additional concerns include the broad heterogeneity of mood disorders and the fact that our understanding of their pathophysiology remains incomplete. In this context, recruiting a relatively homogeneous sample of participants for clinical trials may prove challenging. In addition, placebo response rates are highly variable [132], difficult to predict, and can hinder drug development. Wilkinson and Sanacora (2019) note that studies have attempted to constrain the heterogeneity of participant samples in order to limit placebo response, often by limiting participants to those with TRD [98]. As they point out, however, it is not clear whether this strategy consistently limits placebo response, and little consensus exists on the type of trial design that might limit placebo response rate. Finally, given that there may be some sex-related differences in antidepressant efficacy and treatment response [133], including with (R,S)-ketamine and other NMDA modulators, clinical trials exploring sex differences are critical to establishing the potential therapeutic implications of this variable and to developing novel therapeutics.
5. Conclusions
Significant challenges and low rates of success have historically been associated with drug development in this area. In this context, we remain encouraged by this area of research despite the sometimes mixed results described herein. Most fundamentally, the discovery of (R,S)-ketamine’s rapid and robust antidepressant effects ushered in a new era of paradigm-shifting research focused on developing or repurposing older agents as new antidepressant therapies that may be capable of working within hours or days versus weeks or months. Notably, the FDA’s 2019 approval of esketamine for TRD and of brexanolone for PPD marks the first time in 50 years that two antidepressants with distinct, novel mechanisms of action have reached the market. Furthermore, as reviewed here, the current pipeline of potential glutamatergic compounds for use in mood disorders is strong. Exploration of these agents has shed valuable light on novel treatment avenues and advanced the ultimate goal of developing much-needed, novel, rapid-acting, safe, and effective treatment options for the millions of individuals worldwide suffering from mood disorders.
Key Points.
Many individuals with mood disorders do not respond to currently available antidepressants.
Rapid reductions in depressive symptoms have been observed in response to subanesthetic doses of the glutamatergic modulator (R,S)-ketamine in individuals with mood disorders, which has prompted the investigation of other glutamatergic modulators.
This article reviews these novel glutamatergic agents, which are in various stages of development.
Acknowledgements
The authors thank the 7SE research unit and staff for their support.
Funding
Funding for this work was provided by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIAMH002857). The work was completed as part of the authors’ official duties as Government employees. The views expressed do not necessarily reflect the views of the NIH, the Department of Health and Human Services, or the United States Government.
Footnotes
Conflicts of interest Dr Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the US government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
Availability of data and material Not applicable.
Code availability Not applicable.
Ethics approval Not applicable.
References
- 1.World Health Organization. Depression Fact Sheet. [Website] 2018. [cited 2019 January 8]; https://www.who.int/news-room/fact-sheets/detail/depression. Accessed 31 July 2020.
- 2.Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439–45. [DOI] [PubMed] [Google Scholar]
- 3.Machado-Vieira R, Salvadore G, Luckenbaugh D, Manji HK, Zarate CAJ. Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry. 2008;69:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9:984–7. [DOI] [PubMed] [Google Scholar]
- 5.Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA Jr. Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int J Neuropsychopharmacol. 2019;22(2):119–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–93. [DOI] [PubMed] [Google Scholar]
- 7.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–4. [DOI] [PubMed] [Google Scholar]
- 8.Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64. [DOI] [PubMed] [Google Scholar]
- 9.Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, et al. Ketamine and other N-methyl-d-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6:97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139–45. [DOI] [PubMed] [Google Scholar]
- 11.Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry. 2019;176:401–9. [DOI] [PubMed] [Google Scholar]
- 12.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013;74(4):250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park M, Niciu MJ, Zarate CAJ. Novel glutamatergic treatments for severe mood disorders. Curr Behav Neurosci Rep. 2015;2:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niciu MJ, Henter ID, Luckenbaugh DA, Zarate CAJ, Charney DS. Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu Rev Pharmacol Toxicol. 2014;54:119–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lener M, Iosifescu DV. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Ann N Y Acad Sci. 2015;1344:50–65. [DOI] [PubMed] [Google Scholar]
- 16.Kadriu B, Ballard ED, Henter ID, Murata S, Gerlus N, Zarate CAJ. Neurobiological biomarkers of response to ketamine. Adv Pharmacol. 2020;89:195–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malhi GS, Morris G, Bell E, Hamilton A. A new paradigm for achieving a rapid antidepressant response. Drugs. 2020;80:755–64. [DOI] [PubMed] [Google Scholar]
- 18.Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav. 2012;100(4):656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. 2006;147(1):1–25. [DOI] [PubMed] [Google Scholar]
- 20.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64(2):193–200. [DOI] [PubMed] [Google Scholar]
- 21.Auer DP, Putz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305–13. [DOI] [PubMed] [Google Scholar]
- 22.Sanacora G, Gueorguieva R, Epperson CN, Wu YT, Appel M, Rothman DL, et al. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–13. [DOI] [PubMed] [Google Scholar]
- 23.Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, et al. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic responance spectroscopy studies. Mol Psychiatry. 2019;24:952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milak MS, Rashid R, Dong Z, Kegeles LS, Grunebaum MF, Ogden RT, et al. Assessment of relationship of ketamine dose with magnetic resonance spectroscopy of Glx and GABA responses in adults with major depression: a andomized clinical trial. JAMA Netw Open. 2020;3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185(1):1–10. [DOI] [PubMed] [Google Scholar]
- 26.Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fogaça MV, Wu M, Li C, Li X-Y, Picciotto MR, Duman RS. Inhibition of GABA interneurons in the mPFC is sufficient and necessary for rapid antidepressant responses. Mol Psychiatry. 2020;October 17 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moghadam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2912–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki K, Monteggia LM. The role of eEF2 kinase in the rapid antidepressant actions of ketamine. Adv Pharmacol. 2020;89:79–99. [DOI] [PubMed] [Google Scholar]
- 30.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taha E, Gildish I, Gal-Ben-Ari S, Rosenblum K. The role of eEF2 pathway in learning and synaptic plasticity. Neurobiol Learn Mem. 2013;105:100–6. [DOI] [PubMed] [Google Scholar]
- 32.Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aguilar-Valles A, De Gregorio D, Matta-Camacho E, Eslamizade MJ, Khlaifia A, Skaleka A, et al. Antidepressant actions of ketamine engage cell-specific translation via eIF4E. Nature. 2020;December 16 [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34.Casarotto PC, Girych M, Fred SM, Kovaleva V, Moliner R, Enkavi G, et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell. 2021;184:1299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarate CAJ, Machado-Vieira R. Ketamine: translating mechanistic discoveries into the next generation of glutamate modulators for mood disorders. Mol Psychiatry. 2017;22:324–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–52. [DOI] [PubMed] [Google Scholar]
- 37.Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364(6436). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018;175:1205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riggs LM, Gould TD. Ketamine and the future of rapid-acting antidepressants. Annu Rev Clin Psychol. 2021;February 9 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67(8):793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71(11):939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med. 2016;46(7):1459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caddy C, Giaroli G, White TP, Shergill SS, Tracy DK. Ketamine as the prototype glutamatergic antidepressant: pharma-codynamic actions, and a systematic review and meta-analysis of efficacy. Ther Adv Psychopharmacol. 2014;4(2):75–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGirr A, Berlim MT, Bond DJ, Fleck MP, Yatham LN, Lam RW. A systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials of ketamine in the rapid treatment of major depressive episodes. Psychol Med. 2015;45(4):693–704. [DOI] [PubMed] [Google Scholar]
- 46.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950–66. [DOI] [PubMed] [Google Scholar]
- 47.Romeo B, Choucha W, Fossati P, Rotge JY. Meta-analysis of short- and mid-term efficacy of ketamine in unipolar and bipolar depression. Psychiatry Res. 2015;230(2):682–8. [DOI] [PubMed] [Google Scholar]
- 48.Diazgranados N, Ibrahim L, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71(12):1605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murrough JW, Soleimani L, DeWilde KE, Collins KA, Lapidus KA, Iacoviello BM, et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med. 2015;45(16):3571–80. [DOI] [PubMed] [Google Scholar]
- 50.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66(5):522–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry. 2018;3(175):150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grunebaum MF, Galalvy HC, Choo TH, Keilp JG, Moitra VK, Parris MS, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175:327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grunebaum MF, Ellis SP, Keilp JG, Moitra VK, Cooper TB, Marver JE, et al. Ketamine versus midazolam in bipolar depression with suicidal thoughts: a pilot midazolam-controlled randomized clinical trial. Bipolar Disord. 2017;19:176–83. [DOI] [PubMed] [Google Scholar]
- 54.Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;14(4): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mkrtchian A, Evans JW, Kraus C, Yuan P, Kadriu B, Nugent AC, et al. Ketamine modulates fronto-striatal circuitry in depressed and healthy individuals. Mol Psychiatry. 2020;September 14 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2014;71(6):681–8. [DOI] [PubMed] [Google Scholar]
- 57.Feder A, Costi S, Rutter SB, Collins AB, Govindarajulu U, Jha MK, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178:193–202. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38:2475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am J Psychiatry. 2018;16(175):620–30. [DOI] [PubMed] [Google Scholar]
- 60.Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018;75(2):139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.U.S. Food & Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression. 2019. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm632761.htm. Accessed 12 June 2020.
- 63.Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, et al. Administration of ketamine for unipolar and bipolar depression. Int J Psychiatry Clin Pract. 2017;18:1–12. [DOI] [PubMed] [Google Scholar]
- 64.Acevedo-Diaz EE, Cavanaugh GW, Greenstein D, Kraus C, Kadriu B, Zarate CAJ, et al. Comprehensive assessment of side effects associated with a single dose of ketamine in treatment-resistant depression. J Affect Disord. 2020;263:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swainson J, McGirr A, Blier P, Brietzke E, Richard-Devantoy S, Ravindran N, et al. The Canadian Network for Mood and Anxiety Treatments (CANMAT) Task Force Recommendations for the Use of Racemic Ketamine in Adults with Major Depressive Disorder: Recommandations Du Groupe De Travail Du Réseau Canadien Pour Les Traitements De L’humeur Et De L’anxiété (Canmat) Concernant L’utilisation De La Kétamine Racémique Chez Les Adultes Souffrant De Trouble Dépressif Majeur. Can J Psychiatry. 2020;November 11 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wajs E, Aluisio L, Holder R, Daly EJ, Lane R, Lim P, et al. Esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression: assessment of long-term safety in a Phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry. 2020;81:19m12891. [DOI] [PubMed] [Google Scholar]
- 67.Correia-Melo FS, Leal GC, Vieira F, Jesus-Nunes AP, Mello RP, Magnavita G, et al. Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: a randomized, double-blind, non-inferiority study. J Affect Disord. 2020;264:527–34. [DOI] [PubMed] [Google Scholar]
- 68.Bahji A, Vazquez GH. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2020;278:542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanacora G, Frye MA, McDonald WM, Mathew SJ, Turner MS, Schatzberg AF, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74:399–405. [DOI] [PubMed] [Google Scholar]
- 70.McIntyre RS, Rosenblat JD, Nemeroff CB, Sanacora G, Murrough JW, Berk M, et al. Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: an international expert opinion on the available evidence and implementation. Am J Psychiatry. 2021;March 17 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zarate CAJ. Commentary on the Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the use of racemic ketamine in adults with major depressive disorder. Can J Psychiatry. 2021;March 19 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morris PJ, Moaddel R, Zanos P, Moore CE, Gould TD, Zarate CAJ, et al. Synthesis and N-methyl-d-aspartate (NMDA) receptor activity of ketamine metabolites. Org Lett. 2017;19:4572–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang JC, Li SX, Hashimoto K. R(−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–41. [DOI] [PubMed] [Google Scholar]
- 74.Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi J-I, Hashimoto K, et al. Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther. 2017;361:9–16. [DOI] [PubMed] [Google Scholar]
- 76.Leal GC, Bandeira I, Correia-Melo FS, Telles M, Mello RP, Vieira F, et al. Intravenous arketamine for treatment resistant depression: open-label pilot study. Eur Arch Psychiatry Clin Neurosci. 2021;271:577–82. [DOI] [PubMed] [Google Scholar]
- 77.Zanos P, Thompson SM, Duman RS, Zarate CA Jr, Gould TD. Convergent mechanisms underlying rapid antidepressant action. CNS Drugs. 2018;32(3):197–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zanos P, Highland JN, Stewart BW, Georgiou P, Jenne CE, Lovett J, et al. (2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions. Proc Natl Acad Sci USA. 2019;116:6441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lumsden EW, Troppoli TA, Myers SJ, Zanos P, Aracava Y, Kehr J, et al. Antidepressant-relevant concentrations of the ketamine metabolite (2R,6R)-hydroxynorketamine do not block NMDA receptor function. Proc Natl Acad Sci USA. 2019;116:5160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lauterbach EC. Dextromethorphan as a potential rapid-acting antidepressant. Med Hypotheses. 2011;76(5):717–9. [DOI] [PubMed] [Google Scholar]
- 81.Lauterbach EC. An extension of hypotheses regarding rapid-acting, treatment-refractory, and conventional antidepressant activity of dextromethorphan and dextrorphan. Med Hypotheses. 2012;78(6):693–702. [DOI] [PubMed] [Google Scholar]
- 82.Lee SY, Chen SL, Chang YH, Chen SH, Chu CH, Huang SY, et al. The DRD2/ANKK1 gene is associated with response to add-on dextromethorphan treatment in bipolar disorder. J Affect Disord. 2012;138:295–300. [DOI] [PubMed] [Google Scholar]
- 83.Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR. Pharmacology of dextromethorphan: relevance to dextromethorphan/quinidine (Nuedexta) clinical use. Pharmacol Ther. 2016;164:170–82. [DOI] [PubMed] [Google Scholar]
- 84.Messias E, Everett B. Dextromethorphan and quinidine combination in emotional lability associated with depression: a case report. Prim Care Companion CNS Disord. 2012;14:PCC.12I01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kelly TF, Lieberman DZ. The utility of the combination of dextromethorphan and quinidine in the treatment of bipolar II and bipolar NOS. J Affect Disord. 2014;167:333–5. [DOI] [PubMed] [Google Scholar]
- 86.Murrough JW, Wade E, Sayed S, Ahle G, Kiraly DD, Welch A, et al. Dextromethorphan/quinidine pharmacotherapy in patients with treatment-resistant depression: a proof of concept trial. J Affect Disord. 2017;218:277–83. [DOI] [PubMed] [Google Scholar]
- 87.Axsome Therapeutics. Axsome Therapeutics announces AXS-05 achieves primary endpoint in Phase 2 trial in major depressive disorder. January 7, 2019. 2019 [cited; https://www.globenewswire.com/news-release/2019/01/07/1681055/0/en/Axsome-Therapeutics-Announces-AXS-05-Achieves-Primary-Endpoint-in-Phase-2-Trial-in-Major-Depressive-Disorder.html. Accessed 31 July 2020.
- 88.The Pharmaletter. Axsome sees mixed top-line Ph III results with AXS-05 in TR depression. March 31, 2020. https://www.thepharmaletter.com/article/axsome-sees-mixed-top-line-ph-iii-results-with-axs-05-in-tr-depression. Accessed 31 July 2020.
- 89.Bernstein G, Davis K, Mills C, Wang L, McDonnell M, Oldenhof J, et al. Characterization of the safety and pharmacokinetic profile of D-methadone, a novel N-methyl-d-aspartate receptor antagonist in healthy, opioid-naive subjects: results of two Phase 1 studies. J Clin Psychopharmacol. 2019;39:226–37. [DOI] [PubMed] [Google Scholar]
- 90.Fogaca MV, Fukumoto K, Franklin T, Liu R-J, Duman CH, Vitolo OV, et al. N-methyl-d-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology. 2019;44:2230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moryl N, Tamasdan C, Tarcatu D, Thaler HT, Correa D, Steingart R, et al. A phase I study of d-methadone in patients with chronic pain. J Opioid Manag. 2016;12:47–55. [DOI] [PubMed] [Google Scholar]
- 92.Hagler G BioSpace: Relmada Therapeutics developing REL-1017 to treat major depression. April 21, 2020. https://www.biospace.com/article/relmada-developing-rel-1017-to-treat-major-depression/. Accessed 31 July 2020.
- 93.Relmada Therapeutics I Relmada Therapeutics announces top-line results from REL-1017 Phase 2 study in individual with treatment resistant deprssion. October 15, 2019. https://www.prnewswire.com/news-releases/relmada-therapeutics-announces-top-line-results-from-rel-1017-phase-2-study-in-individuals-with-treatment-resistant-depression-300938577.html. Accessed 31 July 2020.
- 94.Nagele P, Duma A, Kopec M, Gebara MA, Parsoei A, Walker M, et al. Nitrous oxide for treatment-resistant major depression: a proof-of-concept trial. Biol Psychiatry. 2015;78:10–8. [DOI] [PubMed] [Google Scholar]
- 95.Zarate CA, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, et al. A randomized trial of a low-trapping nonselective N-methyl-d-aspartate channel blocker in major depression. Biol Psychiatry. 2013;74:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, et al. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol Psychiatry. 2014;19(9):978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP, et al. Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: a randomized, placebo-controlled study. Neuropsychopharmacology. 2017;42(4):844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilkinson ST, Sanacora G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov Today. 2019;24:606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Millan MJ. N-methyl-d-aspartate receptor-coupled glycineB receptors in the pathogenesis and treatment of schizophrenia: a critical review. Curr Drug Targets CNS Neurol Disord. 2002;1(2):191–213. [DOI] [PubMed] [Google Scholar]
- 100.Kantrowitz JT, Milak MS, Mao X, Shungu DC, Mann JJ. d-Cycloserine, an NMDA glutamate receptor glycine site partial agonist, induces acute increases in brain glutamate plus glutamine and GABA comparable to ketamine. Am J Psychiatry. 2016;173:1241–2. [DOI] [PubMed] [Google Scholar]
- 101.Heresco-Levy U, Javitt DC, Gelfin Y, Gorelik E, Bar M, Blanaru M, et al. Controlled trial of d-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. J Affect Disord. 2006;93(1–3):239–43. [DOI] [PubMed] [Google Scholar]
- 102.Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, et al. A randomized add-on trial of high-dose d-cycloserine for treatment-resistant depression. Int J Neuropsychopharmacol. 2013;16(3):501–6. [DOI] [PubMed] [Google Scholar]
- 103.Kantrowitz JT, Halberstam B, Gangwisch J. Single-dose ketamine followed by daily d-Cycloserine in treatment-resistant bipolar depression. J Clin Psychiatry. 2015;76(6):737–8. [DOI] [PubMed] [Google Scholar]
- 104.NeuroRx. NeuroRx: Phase 3 drug for suicidal bipolar depression to present at BIO CEO conference. February 10, 2020. 2020. https://www.prnewswire.com/news-releases/neurorx-phase-3-drug-for-suicidal-bipolar-depression-to-present-at-bio-ceo-conference-301001882.html. Accessed 12 Aug 2020.
- 105.Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM. Randomized proof of concept trial of GLYX-13, an N-methyl-d-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015;21(2):140–9. [DOI] [PubMed] [Google Scholar]
- 106.Allergan. Allergan announces Phase 3 results for rapastinel as an adjunctive treatment of major depressive disorder (MDD). 2019.
- 107.Fasipe OJ. The emergence of new antidepressants for clinical use: agomelatine paradox versus other novel agents. IBRO Reports. 2019;6:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang C-C, Hua Wei I, Huang C-L, Chen K-T, Tsai M-H, Tsai P, et al. Inhibition of glycine transporter-I as a novel mechanism for the treatment of depression. Biol Psychiatry. 2013;74:734–41. [DOI] [PubMed] [Google Scholar]
- 109.Park LT, Kadriu B, Gould TD, Zanos P, Greenstein D, Evans JW, et al. A randomized trial of the N-methyl-d-aspartate receptor glycine site antagonist prodrug 4-chlorokynurenine in treatment-resistant depression. Int J Neuropsychopharmacol. 2020;March 31 [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patel W, Rimmer L, Smith M, Moss L, Smith MA, Snodgrass HR, et al. Probenecid increases the concentration of 7-chlo-rokynurenic acid derived from the prodrug 4-chlorokynurenine within the prefrontal cortex. Mol Pharm. 2021;18:113–23. [DOI] [PubMed] [Google Scholar]
- 111.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-d-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–7. [DOI] [PubMed] [Google Scholar]
- 112.Ibrahim L, Diazgranados N, Jolkovsky L, Brutsche N, Luckenbaugh D, Herring WJ, et al. A randomized, placebo-controlled, crossover pilot trial of the oral selective NR2B antagonist MK-0657 in patients with treatment-resistant major depressive disorder. J Clin Psychopharmacol. 2012;32:551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paterson B, Fraser H, Wang C, Marcus R. A randomized, double-blind, placebo-controlled, sequential parallel study of CERC-301 in the adjunctive treatment of subjects with severe depression and recent active suicidal ideation despite antidepressant treatment. National Network of Depression Centers Annual Conference; 2015 November 5–6; Ann Arbor, Michigan; 2015. http://c.eqcdn.com/_2a1b537df6a6e1398b6806c9b30635ad/cerecor/db/322/603/file/Poster+-+Study+Clin301-201_FINAL.pdf. Accessed 12 June 2020. [Google Scholar]
- 114.Quiroz JA, Tamburri P, Deptula D, Banken L, Beyer U, Rabbia M, et al. Efficacy and safety of basimglurant as adjunctive therapy for major depression: a randomized clinical trial. JAMA Psychiatry. 2016;73:675–84. [DOI] [PubMed] [Google Scholar]
- 115.Chaki S mGlu2/3 receptor as a novel target for rapid acting antidepressants. Adv Pharmacol 2020;89:289–309. [DOI] [PubMed] [Google Scholar]
- 116.Umbricht D, Niggli M, Sanwald-Ducray P, Deptula D, Moore R, Grünbauer W, et al. Randomized, double-blind, placebo-controlled trial of the mGlu2/3 negative allosteric modulator decoglurant in partically refractory major depressive disorder. J Clin Psychiatry. 2020;81:18m12470. [DOI] [PubMed] [Google Scholar]
- 117.Campo B, Kalinichev M, Lambeng N, El Yacoubi M, Royer-Urios I, Schneider M, et al. Characterization of an mGluR2/3 negative allosteric modulator in rodent models of depression. J Neurogenet. 2011;25:152–66. [DOI] [PubMed] [Google Scholar]
- 118.Pothula S, Liu R-J, Wu M, Sliby AN, Picciotto MR, Banerjee P, et al. Positive modulation of NMDA receptors by AGN-241751 exerts rapid antidepressant-like effects via excitatory neurons. Neuropsychopharmacology. 2020;October 15 [online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sengupta S, Giaime E, Narayan S, Hahm S, Howell J, O’Neill D, et al. Discovery of NV-5138, the first selective Brain mTORC1 activator. Sci Rep. 2019;9:4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hasegawa Y, Zhu X, Kamiya A. NV-5138 as a fast-acting antidepressant via direct activation of mTORC1 signaling. J Clin Invest. 2019;129:2207–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kato T, Pothula S, Liu R-J, Duman CH, Terwilliger R, Vlasuk GP, et al. Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation. J Clin Invest. 2019;129:2542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(4753):1004–7. [DOI] [PubMed] [Google Scholar]
- 123.Kleinman RA, Schatzberg AF. Understanding the clinical effects and mechanisms of action of neurosteroids. Am J Psychiatry. 2021;178:221–3. [DOI] [PubMed] [Google Scholar]
- 124.Frieder A, Fersh M, Hainline R, Deligiannidis KM. Pharmacotherapy of postpartum depression: current approaches and novel drug development. CNS Drugs. 2019;33:265–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, et al. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390(10093):480–9. [DOI] [PubMed] [Google Scholar]
- 126.Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058–70. [DOI] [PubMed] [Google Scholar]
- 127.Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, et al. Trial of SAGE-217 in patients with major depressive disorder. N Engl J Med. 2019;381:903–11. [DOI] [PubMed] [Google Scholar]
- 128.Sage Therapeutics. Sage announces pivotal Phase 3 trial status for SAGE-217 in major depressive disorder and postpartum depression based on FDA breakthrough therapy meeting. 2018. https://investor.sagerx.com/news-releases/news-release-details/sage-announces-pivotal-phase-3-trial-status-sage-217-major. Accessed 12 June 2020.
- 129.Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, et al. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry. 2017;81:886–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fogaca MV, Duman RS. Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interverntions. Front Cell Neurosci. 2019;13:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ballard ED, Yarrington JS, Farmer CA, Lener MS, Kadriu B, Lally N, et al. Parsing the heterogeneity of depression: an exploratory factor analysis across commonly used depression rating scales. J Affect Disord. 2018;231:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. JAMA. 2002;287:1840–7. [DOI] [PubMed] [Google Scholar]
- 133.Williams AV, Trainor BC. The impact of sex as a biological variable in the search for novel antidepressants. Front Neuroen-docrinol. 2018;50:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, et al. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs. 2014;23:243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]


