Abstract
Abdominal aortic aneurysm (AAA) is a chronic dilated disease of the aorta that is characterized by chronic inflammation. Curcumin (Cur) is previously showed to attenuate AAA by inhibiting inflammatory response in ApoE −/− mice. Since Cur has the limitations of aqueous solubility and instability. Here, we focus on the role of curcumin nicotinate (CurTn), a Cur derivative is derived from Cur and nicotinate. An in vitro model of AAA was established by treating vascular smooth muscle cells (VSMCs) with II (Ang-II). Gene and protein expressions were examined by quantitative real-time PCR (qPCR) or western blotting. Cell migration and pyroptosis were determined by transwell assay and flow cytometry. The interaction between plasmacytoma variant translocation 1 (PVT1), miR-26a and krüppel-like factor 4 (KLF4) was predicted by online prediction tool and confirmed by luciferase reporter assay. CurTn reduced Ang-II-induced AAA-associated proteins, inflammatory cytokine expressions, and attenuated pyroptosis in VSMCs. PVT1 overexpression suppressed the inhibitory effect of CurTn on AngII-induced pyroptosis and inflammatory in VSMCs by sponging miR-26a. miR-26a directly targeted KLF4 and suppressed its expression, which eventually led to the deactivation of the PI3K/AKT signaling pathway. Besides, the regulatory effect of CurTn on pyroptosis of VSMCs induced by Ang-II was reversed through the PVT1/miR-26a/KLF4 pathway. In short, CurTn suppressed VSMCs pyroptosis and inflammation though mediation PVT1/miR-26a/KLF4 axis by regulating the PI3K/AKT signaling pathway, CurTn might consider as a potential therapeutic target in the treatment of AAA.
Keywords: Abdominal aortic aneurysms, curcumin nicotinate, pyroptosis, PVT1, miR-26a
Introduction
Abdominal aortic aneurysms (AAA) is a chronic vascular degenerative disease characterized by localized enlargement of the abdominal aorta. The total mortality rate of patients with a ruptured AAA is between 65 and 80% and approximately 200 000 patients die of AAA rupture per year in the worldwide [1–3]. Currently, surgical treatment is the only effective therapeutic method for treating large or symptomatic AAAs [4]. Nevertheless, surgical repair does not reduce mortality in AAA patients. Therefore, it is urgent to explore medical therapies to prevent AAA.
Curcumin (Cur), a major bioactive component of turmeric, has been previously shown to have many pharmacological effects, including anti-inflammatory, anti-oxidant and anti-angiogenesis [5]. However, the application of Cur is limited because of its aqueous solubility and instability [6]. Curcumin nicotinate (CurTn) is a Cur derivative derived from Cur and nicotinate, which has superior bioavailability and aqueous solubility and exhibits anti-atherosclerosis and anti-inflammation properties [7–9]. The key role of Cur in inhibiting AAA had been reported in several studies [10, 11]. However, whether CurTn can be used to prevent AAA remains unclear.
Long non-coding RNAs (lncRNAs) are non-coding RNA that transcripts more than 200 nucleotides. It has been proved to regulate the genome at the transcriptional and post-transcriptional level [12, 13]. Plasmacytoma variant translocation 1 (PVT1), one of the lncRNAs, was upregulated in AAA patients tissue samples [14]. And overexpression of PVT1 promoted cell apoptosis and apoptosis-associated proteins expression [14]. A previous study showed that PVT1 was involved in Cur regulated LPS-induced septic acute kidney injury [15]. Furthermore, it was previously documented that CurTn mediated cancer cell apoptosis and cell cycle arrest [5]. Therefore, the interaction between CurTn and PVT1 as well as their functional role in mediating AAA still remains to be explored.
microRNAs (miRNAs) are small non-coding RNA with a length of about 20–24 nucleotides, which play key roles in gene expression regulation and involve in the regulation of a variety of physiological processes [16]. Previous studies showed that miRNAs regulated the development of various human diseases, such as cardiovascular diseases [17], myocardial infarction [18] and many others. It was reported that miR-26a was showed to prevent cell apoptosis, including endothelial cells, cardiac fibroblasts and podocytes [19–21]. It was proved that miR-26a was significantly downregulated in the AAA formation process [22]. Additionally, it was also demonstrated that miR-26a reduced smooth muscle cells (SMCs) apoptosis while enhanced its regeneration [22]. Krüppel-like factor 4 (KLF4) was demonstrated to play a crucial role in the maintenance of embryonic stem cell pluripotency [23]. KLF4 was also showed to be a key factor required for the switching of the SMCs phenotypic [2]. Salmon et al. indicated that KLF4 and KLF2 activated autophagy-associated genes in VSMCs during AAA formation [24]. Furthermore, knockdown of KLF4 suppressed AAA formation [2]. In addition, according to bioinformatics analysis, we predicted that there were potentially bind sites between PVT1 and miR-26a, KLF4 was a target of miR-26a. Based on these data, we hypothesized that CurTn prevented AAA pathological development though regulating the PVT1/miR-26a/KLF4 axis.
In the present study, we first established an in vitro cell model of AAA by treating VSMCs with angiotensin II (Ang-II) as reported in previous studies [25, 26]. We investigated the regulatory effect of CurTn on cell pyroptosis and inflammatory injury in AAA in vitro. We found that CurTn suppressed cell pyroptosis and promoted migration in AAA by inhibiting PVT1-mediated miR-26a/KLF4/PI3K/AKT signaling pathway.
Materials and Methods
Ethical approval
All described experiments in this study strictly adhered to the local ethics committees of our hospital.
Cell culture and treatment
Human primary VSMCs were purchased from American type culture collection (ATCC, Manassas, VA, USA) and cultured in a VSMC growth kit according to the manufacturer’s instructions, which was also obtained from ATCC. Only VSMCs of passage 3–6 were used in this study. VSMCs were divided into three groups, and Cur and CurTn are dissolved in dimethyl sulfoxide (DMSO). The first group was cultured in VSMC growth medium with or without Ang-II (Sigma-Aldrich, St. Louis, MO, 15 μM) for 24 h. Ang-II has been widely used to stimulate AAA formation [27, 28]. The second group was cultured in VSMC growth medium with Ang-II (15 μM) and Cur (20 μM) for 24 h. The third group was cultured in VSMC growth medium with Ang-II (15 μM) and CurTn (20 μM). All cells were culture in T175 flasks in 37°C and 5% CO2 incubator for 24 h.
The transwell migration assay
VSMCs were digested, washed and 4 × 106 cells were resuspended in 500 μl serum-free VSMCs basal medium. The cells were seeded into the upper chamber of transwell assays containing inserts (Thermo Scientific, Waltham, MA) that pre-coated with collagen (Sigma-Aldrich). The inserts were with .4 μm pores. After 18 h incubation, the culture medium was removed and cells were gently washed three times with phosphate-buffered saline (PBS, Gibco, Shanghai, China) and fixed with 4% formalin for 15 min. Afterward, the cells were stained with 1 μl/ml 4,6-diamidino-2-phenyl indole (DAPI, Sigma-Aldrich). After washing of PBS for three times, the membrane was cut from the inserts and mounted onto the glass coverslips. Images were taken using an LSM730 confocal microscope (Carl Zeiss, Oberkochen, Germany).
Cell transfection
The miR-26a mimics, miR-26a inhibitor, mimics/inhibitor negative control (NC), KLF4 short hairpin RNA (shRNA) and the NC vector were purchased from Genepharma (shanghai, China). For PVT1 overexpression, the full sequence of PVT1 was amplified by PCR and inserted into the pcDNA3.1 (Invitrogen, CA, USA) vector. The construct was named pcDNA 3.1-PVT1. VSMCs were seeded into 96-well plates and transfected with miR-26a mimics or miR-26a inhibitor, KLF4 shRNA, pcDNA 3.1-PVT1 and NC vectors were carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction.
Real-time polymerase chain reaction
Total RNA was extracted using Trizol according to the manufacture’s instruction (Qiagen; Valencia, California). The quantity and the quality of RNA were measured using a Nanodrop spectrophotometer (ND-100; Thermo Scientific). About, 1 μg of total RNA of each sample was reverse-transcribed into cDNA using the TaqMan miRNA reverse transcription kit (Invitrogen) according to the manufacturer protocol. Real-time polymerase chain reaction (qPCR) was performed on transcription DNA using an SYBR Green PCR kit based on the manufacturer manuals. The relative messenger RNA (mRNA) levels were quantified using the comparative 2-ΔΔCt method and normalized to the housekeeping gene D-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and U6. The primers used were as follows: GAPDH, forward primer: 5’-GCACCGTCAAGGCTGAGAAC-3′, reverse primer: 5’-TGGTGAAGACGCCAGTGGA-3′; U6, forward primer: 5’-GCTTCGGCAGCACATATACTAAAAT-3′, reverse Primer: 5’-CGCTTCACGAATTTGCGTGTCAT-3′; caspase-1, forward primer: 5’-GCCTGTTCCTGTGATGTGGAG-3′, reverse primer: 5’-TGCCCACAGACATTCATACAGTTTC-3′; interleukin-1β (IL-1β), forward primer: 5’-CCAGGGACAGGATATGGAGCA-3′, reverse primer: 5′- TTCAACACGCAGGACAGGTACAG-3′; IL-18, forward primer: 5’-GCTTGAATCTAAATTATCAGTC-3′, reverse primer: 5’-GAAGATTCAAATTGCATCTTAT-3′.
Western blotting
Total proteins were isolated from VSMCs using RIPA buffer (Sigma-Aldrich) supplemented with protease and phosphatase inhibitor cocktail. The total protein was determined with a BCA protein assay kit (Solarbio, Beijing, China) and separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE, Bio-Rad Laboratories, Inc., Hercules, CA, USA). Subsequently, the proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane (Schleicher & Schuell, Germany). The membranes were blocked with PBS containing 5% skimmed milk at room temperature. Afterward, the membranes were incubated with primary antibodies against caspase-1, IL-1β, IL-18, PI3K, p-PI3K, AKT, p-AKT and GAPDH for 4°C overnight. All antibodies were purchased from Abcam. The membranes were next incubated with secondary antibodies for 1 h at room temperature. The protein bands were measured using an ECL detection system (Thermo Fisher Scientific, Waltham, MA).
Flow cytometry
VSMCs were digested and washed twice with PBS. VSMCs were strained using a caspase-1 antibody according to the manufacturer’s instructions. After the staining, the detection of cell pyroptosis was performed using flow cytometry and calculated based on the percentage of caspase-1 positive cells.
Dual-luciferase reporter assay
A luciferase reporter assay was used to determine the interaction between miR-26a and PVT1 or KLF4. The sequence of nucleotides of the PVT1 or KLF4 was amplified by PCR and inserted into downstream of the luciferase gene in the pLuc luciferase vector (Ambion, Austen, Texas, USA). Site-directed mutagenesis of the miR-26a target site of PVT1 or KLF4 was performed utilizing the QuickChange mutagenesis kit (Stratagene, Heidelberg, Germany). The resulting constructs were sequenced and named pLuc-PVT1-wt/pLuc-PVT1-mut or pLuc-KLF4-wt/pLuc-KLF4-mut. VSMCs were cultured in 24-well plates and co-transfected with 100 ng of pLuc-PVT1-wt/pLuc- PVT1-mut or pLuc-KLF4-wt/pLuc-KLF4-mut fand 50 nM of miR-26a mimics or mimics NC using Lipofectamine™ 2000 (Invitrogen). About, 2 days after transfection, the cells were harvested. Luciferase activity was measured using the dual-luciferase reporter assay kit (Promega, Madison, Wisconsin, USA) according to the manufacturer’s protocol.
Statistical analysis
All statistical analysis was carried out using GraphPad Prism 5.0 (GraphPad Software, Ind., San Diego, C.A., USA). Data were presented as the mean ± standard deviation (SD). Data were compared using Student’s t-test and one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Each experiment was performed at least in triplications. P < .05 was considered as a statistically significant difference.
Results
CurTn attenuated Ang-II-induced AAA formation
We analyzed the effect of Cur and CurTn on VSMCs behavior following Ang-II treatment to investigate the role of Cur and CurTn in AAA formation. Western blotting results showed that Ang-II treatment significantly increased MMP2, MMP9 and OPN expressions, but decreased the expression of α-SMA (Fig. 1A). However, the Cur and CurTn treatments reversed the stimulation effects on MMP2, MMP9 and OPN levels and the inhibitory effect on the α-SMA expression by Ang-II (Fig. 1A). The transwell assay results showed that treatment with Ang-II-inhibited VSMCs migration, but Cur and CurTn reversed the effect of Ang-II treatment on VSMCs migration (Fig. 1B). Furthermore, it was found that Cur and CurTn treatments attenuated the promotion effect of Ang-II on IL-1β, IL-18 and caspase-1 gene and protein expressions in the VSMCs, as determined by the qPCR and western blotting data (Fig. 1C and D). Cur and CurTn also suppressed the promotion effect on VSMC pyroptosis induced by Ang-II (Fig. 1E). Taken together, these results indicate that Cur and CurTn inhibits MMP protein expression and cell pyroptosis in VSMCs following Ang-II treatment.
Figure 1.
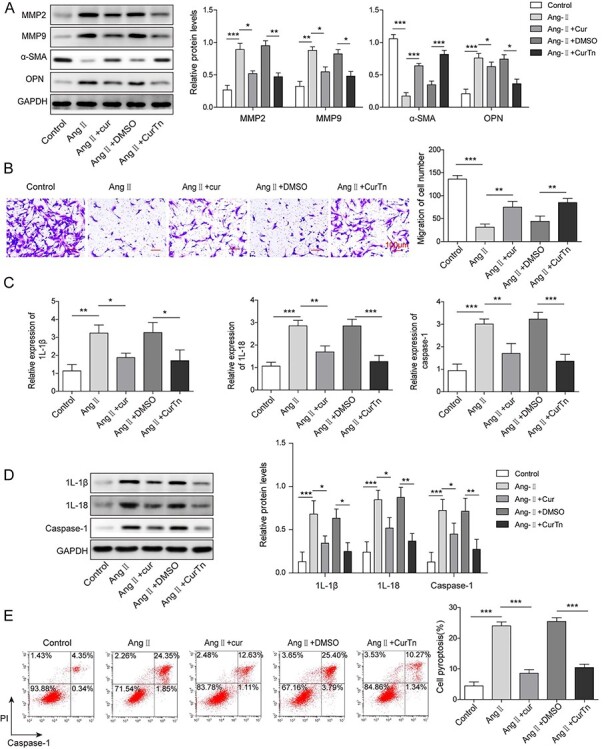
CurTn attenuated Ang-II-induced AAA formation. (A) Western blotting detected MMP2, MMP9, α-SMA, OPN expressions in VSMCs. (B) VSMCs migration was assessed using the transwell assay. (C) qPCR determined the expressions of caspase-1, IL-1β, IL-18. (D) Protein levels of caspase-1, IL-1β, IL-18 were tested by western blotting. (E) Pyroptosis of VSMCs was measured using flow cytometry and the quantitive data were calculated accordingly. *P < .05, **P < .01, ***P < .001.
CurTn inhibited PVT1, KLF4 and PI3K/AKT signaling pathway but promoted miR-26a expression in Ang-II-treated VSMCs
To further explore the underlying mechanism of CurTn suppressed AAA formation, we examined expressions of PVT1, KLF4, phosphorylated-PI3K (p-PI3K) and p-AKT following CurTn and Ang-II treatment of VSMCs. As shown in Fig. 2A and C, the expression levels of PVT1 and KLF4 were significantly upregulated in the Ang-II-treated VSMCs compared to the control group, whereas Ang-II-induced upregulation was decreased by Cur or CurTn treatment. Similarly, Ang-II treatment significantly downregulated miR-26a in VSMCs compared to the control (Fig. 2B). However, Cur or CurTn reversed Ang-II treatment-induced miR-26a downregulation in VSMCs. In addition, Cur or CurTn reduced the promotion effect of Ang-II on KLF4, p-PI3K and p-AKT expressions in the VSMCs (Fig. 2C and D). Altogether, Cur and CurTn inhibits PVT1, KLF4 and PI3K/AKT signaling pathway but promotes miR-26a expression in the Ang-II-treated VSMCs.
Figure 2.
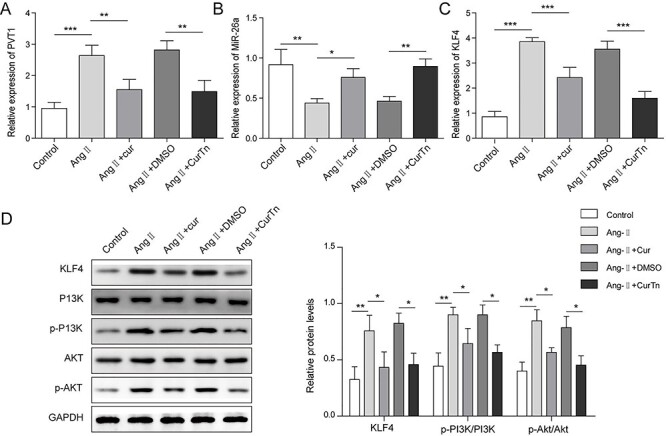
CurTn inhibited PVT1 and KLF4/PI3K/AKT signaling pathways but promoted miR-26a expression in Ang-II-stimulated VSMCs. QPCR was used to detect levels of PVT1 (A), miR-26a (B) and KLF4 (C) in VSMCs following the treatment of Ang-II, or Ang-II + Cur or Ang-II + CurTn. (D) Protein levels of KLF4, PI3K, p-PI3K, AKT and p-AKT in VSMCs were assessed using western blotting. *P < .05, **P < .01, ***P < .001.
PVT1 overexpression reversed CurTn-induced inhibitory effect on AAA formation
To prove that PVT1 was involved in CurTn-preventing AAA development, PVT1 was overexpressed in VSMCs by transfecting with pcDNA3.1-PVT1. Figure 3A showed that overexpression of PVT1 significantly increased the expression of PVT1, indicating the success of the transfection. Overexpression of PVT1 reversed CurTn-induced VSMCs migration after AngII treatment (Fig. 3B). In addition, it was found that the upregulation of PVT1 attenuated the inhibitory effect of CurTn on the expression of caspase-1, IL-1β and IL-18, in the Ang-II-treated VSMCs, as determined through qPCR and western blot data (Fig. 3C and D). Moreover, the PVT1 overexpression eliminated the protective effect of CurTn on VSMCs pyroptosis that induced by Ang-II treatment (Fig. 3E). In summary, these data prove that PVT1 overexpression reverses CurTn-induced inhibitory effect on AAA formation.
Figure 3.
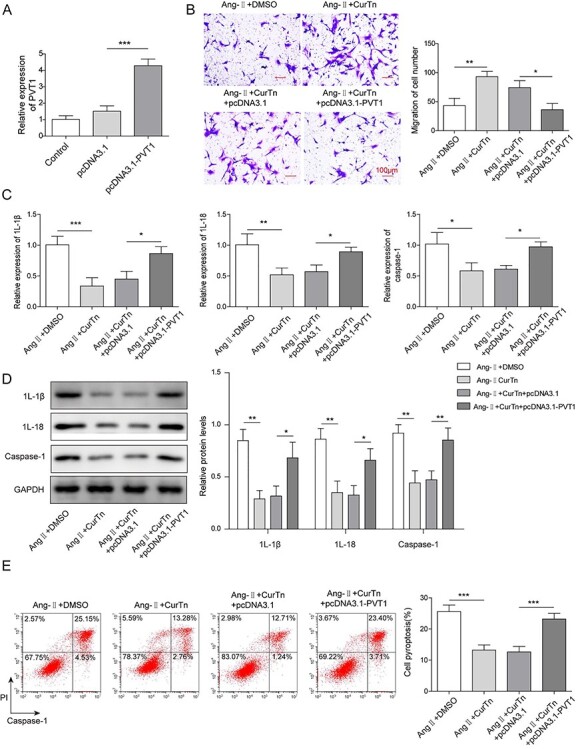
PVT1 overexpression reversed CurTn-induced inhibitory effect on AAA formation. (A) The expression of PVT1 was detected by qPCR. (B) The migration of PVT1 overexpressed VSMCs was assessed using the transwell assay. (C) Gene expressions of caspase-1,IL-1β,IL-18 in PVT1 overexpressed VSMCs were detected by qPCR. (D) Protein levels of caspase-1,IL-1β,IL-18 in PVT1 overexpressed VSMCs were determined by western blot assay. (E) PVT1 overexpressed VSMCs pyroptosis was measured using flow cytometry, and the quantitive data were calculated accordingly. *P < .05, **P < .01, ***P < .001.
PVT1 regulated KLF4 expression and PI3K/AKT signaling pathway by targeting miR-26a
We next used the online prediction tool to predict the potential targets of PTV1. Bioinformatics analyses revealed that there were potentially bind sites between miR-26a and PVT1 or KLF4 (Fig. 4A). Measurement of luciferase activity demonstrated that miR-26a mimics significantly decreased luciferase activity of cells transfected with PVT1-wt but not PVT1-mut (Fig. 4B). Similarly, miR-26a mimics significantly reduced the luciferase activity of cells transfected with KLF4-wt but not KLF4-mut (Fig. 4B). In addition, overexpression of PVT1 significantly reduced miR-26a expression (Fig. 4C).VSMCs were transfected with miR-26a mimics or miR-26a inhibitor to overexpress miR-26a or suppress miR-26a, respectively. qPCR results confirmed that overexpression of miR-26a and depletion of miR-26a were successfully performed (Fig. 4D). miR-26a overexpression significantly inhibited KLF4 expression. In contrast, depletion of miR-26a promoted KLF4 expression, (Fig. 4D and E). Furthermore, miR-26a overexpression reduced protein levels of p-PI3K and p-AKT, whereas PVT1 overexpression or silencing of miR-26a promoted p-PI3K and p-AKT protein levels (Fig. 4E and F). Altogether, PVT1 targetes miR-26a to promote KLF4 expression and activate PI3K/AKT signaling pathway.
Figure 4.
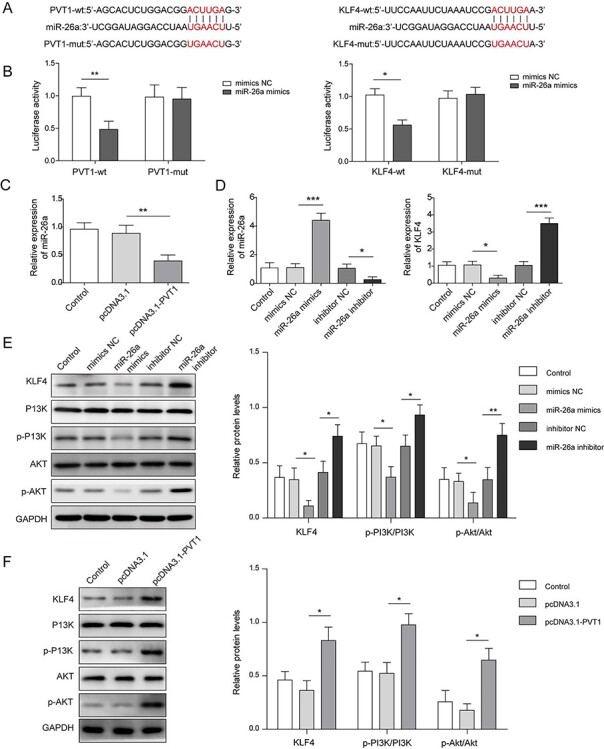
PVT1 regulated KLF4 and PI3K/AKT signaling pathways by targeting miR-26a. (A) Schematic diagram of the miR-26a binding site on PVT1 and the miR-26a binding site on KLF4. (B) Luciferase activity was performed by a dual luciferase reporter. (C) The relative gene expressions of miR-26a in PVT1 overexpressed VSMCs were detected by qPCR. (D) The relative gene expression of miR-26a and KLF4 was measured by qPCR. (E and F) Western blot assay detected protein expressions of KLF4, PI3K, p-PI3K, AKT and p-AKT in miR-26a overexpressed VSMCs, miR-26a depleted VSMCs and PVT1 overexpressed VSMCs. *P < .05, **P < .01, ***P < .001.
Knockdown of miR-26a attenuated protective effect of CurTn on Ang-II-induced AAA formation
To examine the effect of miR-26a on CurTn regulating AAA, we knocked down miR-26a in VSMCs following treatment with CurTn and Ang-II. VSMCs were transfected with KLF4 shRNA and the transfection was successfully performed as evidenced by qPCR results shown in Fig. 5A. As indicated in Fig. 5B, silencing of miR-26a reversed CurTn-induced stimulation effect on VSMCs migration upon the treatment of Ang-II, but knockdown of KLF4 led to the opposite effect. qPCR and western blotting results proved that depletion of miR-26a also attenuated the inhibitory effect of CurTn on caspase-1, IL-1β and IL-18 expressions in the Ang-II treated VSMCs, whereas KLF4 downregulation promoted the expression of caspase-1, IL-1β and IL-18 in VSMCs induced by CurTn (Fig. 5C and D). In consistent with it, suppression of miR-26a attenuated the protective effect of CurTn on VSMC pyroptosis following Ang-II treatment and the co-transfection of sh-KLF4 displayed the opposite trend (Fig. 5E). Taken together, knockdown of miR-26a regulates the protective effect of CurTn on VSMCs pyroptosis.
Figure 5.
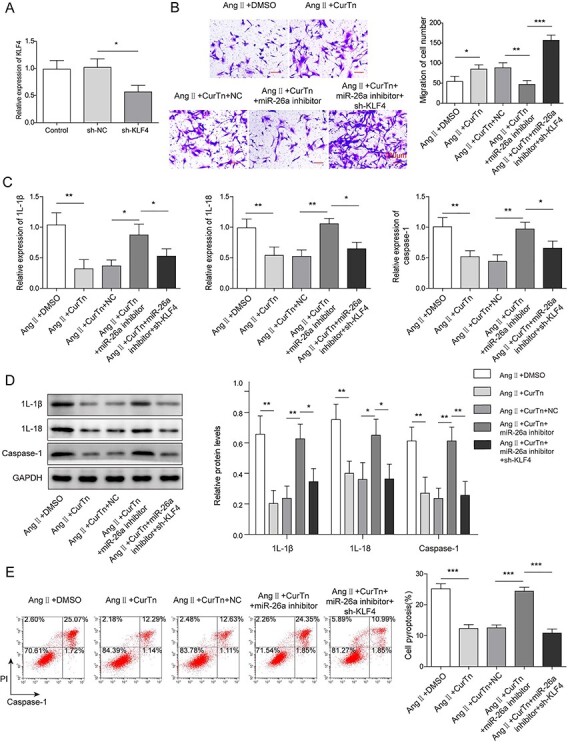
Knockdown of miR-26a attenuated protective effect of CurTn on Ang-II-induced AAA formation. (A) The relative gene expression of KLF4 in VSMCs after knockdown of KLF4 was tested by qPCR. (B) Migration of miR-26a depleted VSMCs was assessed using transwell assay. (C) qPCR determined gene expressions of inflammatory cytokines in miR-26a depleted VSMCs. (D) Western blotting detected protein levels of inflammatory cytokines in miR-26a-depleted VSMCs. (E) The impact of miR-26a depletion on VSMCs pyroptosis was measured using flow cytometry, and the quantitive data were calculated accordingly. *P < .05, **P < .01, ***P < .001.
PVT1 overexpression diminished the inhibitory effect of CurTn on VSMCs pyroptosis through miR-26a/KLF4/PI3K/AKT axis
The relationship between PVT1 and miR-26a/KLF4 and PI3K/AKT signaling pathway was further explored in Ang-II-stimulated VSMCs. As proved in the present study, PVT1 overexpression attenuated CurTn-induced regulatory effect on VSMC migration and pyroptosis upon Ang-II treatment. However, miR-26a overexpression or knockdown of KLF4 dampened the effect of PVT1 overexpression on regulating CurTn-induced deactivation of PI3K/AKT signaling pathway in Ang-II treated VSMCs, as indicated by downregulated p-PI3K and p-AKT (Fig. 6A). And overexpression of miR-26a or depletion of KLF4 diminished the regulatory effect of PVT1 overexpression on CurTn induced-VSMCs migration and anti-inflammation upon Ang-II treatment, as evidenced by decreased caspase-1, IL-1β and IL-18 (Fig. 6B-D). In addition, miR-26a overexpression or knockdown of KLF4 attenuated the effect of PVT1 overexpression in regulating CurTn-inhibited VSMCs pyroptosis upon Ang-II treatment (Fig. 6E). Collectively, these data further indicate that miR-26a and KLF4 locates downstream of PVT1 and PVT1 overexpression diminishes the inhibitory effect of CurTn on Ang-II-induced AAA formation through miR-26a/KLF4 axis.
Figure 6.
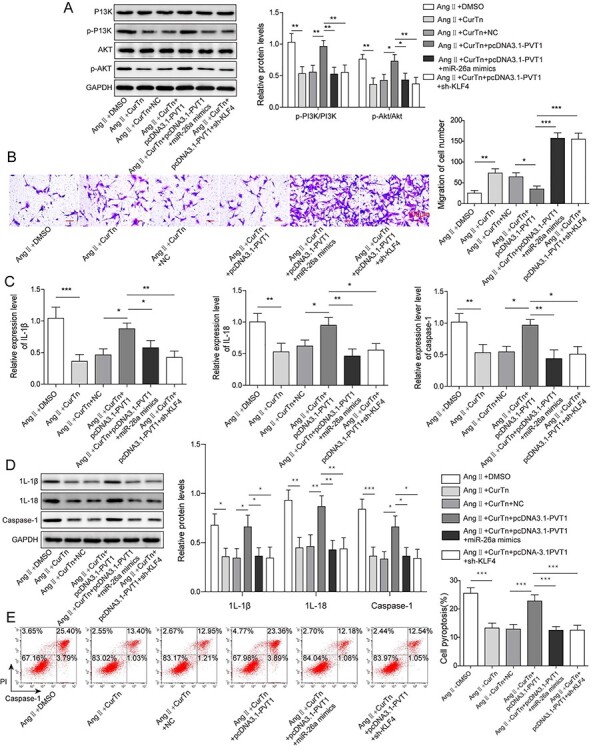
PVT1 overexpression diminished the inhibitory effect of CurTn on AAA formation by regulating miR-26a/KLF4/PI3K/AKT axis. (A) Western blotting measured the expressions of PI3K, p-PI3K, AKT and p-AKT. (B) VSMCs migration was assessed using the transwell assay. Gene expressions (C) and protein levels (D) of inflammatory cytokines were detected by qPCR or western blotting. (E) VSMCs pyroptosis was measured using flow cytometry, and the quantitive data were calculated accordingly. *P < .05, **P < .01, ***P < .001.
Discussion
As one of the serious, life-threatening vascular diseases, AAA has become one of the top ten causes of mortality in the elderly all over the world [1, 2, 28]. Hence, it is necessary to explore effective drug candidates for treating AAA. This study first revealed the critical role of CurTn in regulating AAA cell pyroptosis and demonstrated the regulatory mechanism of the PVT1/miR-26a/KLF4 axis and PI3K/AKT signaling pathway.
Cur has long been used to treat age-related diseases in China and India, including atherosclerosis and coronary heart disease [29] and its pharmacological effects, including anti-inflammatory, anti-oxidant and anti-angiogenesis effects, have been well reported [7–9]. A recent study published by Hao et al. showed that Cur attenuated AAA by inhibiting the inflammatory response and ERK signaling pathways in ApoE −/− mice [11]. However, considering the application of Cur was limited due to its aqueous solubility and instability, we examined the role of CurTn, a Cur derivative derived from Cur and nicotinate and had superior bioavailability and aqueous solubility on AAA formation [6, 11]. Here, we proved that CurTn attenuated Ang-II-induced AAA-associated proteins, including MMP2, MMP9 and OPN in VSMCs. CurTn dampened Ang-II-induced promotion effects on the expressions of caspase-1, IL-1β, IL-18 and pyroptosis in VSMCs. CurTn also diminished the inhibitory effect of Ang-II on VSMCs migration. It is well known that AAA is characterized by chronic inflammation and VSMCs pyroptosis is a pro-inflammatory form of regulated VSMCs death [28, 30, 31]. Hence, we concluded that CurTn had a protective effect against Ang-II-induced VSMC pytoptosis and AAA formation.
Evidence suggested that lncRNAs acted as key modulators in VSMCs de-differentiation, proliferation, migration and apoptosis [12]. The study found that knockdown of PVT1 inhibited the apoptosis and inflammatory response of IL-1β-induced chondrocytes though downregulating TRAF3 expression via miR-27b-3p [32]. Cardamom inhibited the proliferation and induced apoptosis in gastric cancer by inhibiting the activity of STAT3 mediated by PVT1 [33]. In addition, it was reported PVT1 was increased in AAA patients tissue samples and promoted VSMCs apoptosis and phenotypic switching [14]. Notably, Yoshida et al. found that Cur increased the response of pancreatic cancer cells to gemcitabine through regulating PVT1 [34]. Consistently, in this present study, we found that CurTn significantly reduced the expression of IL-18, IL-1β and caspase-1 and pyrocytosis in Ang-II-induced VSMCs. Furthermore, CurTn decreased PVT1 expression in Ang-II-induced VSMCs, but the inhibitory effect of CurTn on Ang-II-induced VSMCs pyroptosis was abrogated by upregulation of PVT1, indicating PVT1 might act as an important modulator in CurTn-mediated AAA formation.
It is well accepted that lncRNAs act as a competitive endogenous RNA to segregate miRNAs away from their target mRNA [35, 36]. Numerous studies demonstrated that miRNAs played crucial roles in the pathogenesis of AAA, and it was suggested miRNAs as promising therapeutic targets [1, 3]. Among these miRNAs, miR-26a was found to be downregulated in the H2O2-induced VSMCs injury model, and overexpression of miR-26a attenuated H2O2-induced cell injury [3]. It was also documented by Leeper et al. that miR-26a was abnormally downregulated in the mouse AAA model and suppressed SMCs differentiation and apoptosis [22]. Recently, miR-26a was delivered to rat models by ultrasound-targeted microbubble destruction and it was found that miR-26a decreased apoptosis and promoted regeneration of SMCs in the aortic wall [37]. In agreement with it, our results showed that miR-26a was downregulated in Ang-II-treated VSMCs. Furthermore, we proved for the first time that PVT1 was directly bound to miR-26a, as evidenced by the dual-luciferase assay result. Knockdown of miR-26a attenuated the inhibitory effect of CurTn on Ang-II-induced VSMCs pyroptosis, suggesting the important functional roles of miR-26a in CurTn-regulated AAA formation.
miRNA usually displays its post-transcription through binding to mRNA [1, 3]. Salmon et al. also documented that KLF4 was significantly upregulated in human AAA tissues and KLF4 played a critical role in AAA formation by affecting SMCs [2]. In this study, the results revealed that KLF4 was a downstream target of miR-26a. The treatment of CurTn significantly decreased KLF4 expression in VSMCs. Furthermore, silencing of KLF4 attenuated the effect of PVT1 overexpression in regulating CurTn-inhibited VSMC pyroptosis upon Ang-II treatment, suggesting KLF4 was located downstream of PVT1 and was functionally involved in PVT1 regulating AAA formation. PI3K/AKT signaling pathway is vital for the survival, proliferation, differentiation and apoptosis of cells [1, 3]. A recent study also documented that miR-195 suppressed AAA inflammation through the PI3K/AKT pathway [30]. We found that p-PI3K and p-AKT levels could be repressed by CurTn but increased by PVT1 overexpression. In addition, overexpression of miR-26a or depletion of KLF4 reversed the regulatory effect of PVT1 overexpression on CurTn-induced deactivation of PI3K/AKT signaling. Therefore, we believed that CurTn regulated AAA formation, at least partly through the PVT1/miR-26a/KLF4/PI3K-AKT signaling pathways.
Conclusion
In conclusion, in the present study, we demonstrated that CurTn has been protective effect against AAA formation via regulating PVT1/miR-26a/KLF4 to deactivate PI3K/AKT signaling pathway. Thus, CurTn may be a promising therapeutic target for the treatment of AAA.
Ethical Approval
All described experiments in this study strictly adhered to the local ethics committees of our hospital.
Authors’ Contribution
Conception and study design: J.M.X.; data acquisition: H.L.; data analysis: J.C., Q.Q.Z.; manuscript drafting: Y.Y.J.; manuscript revising: G.S.B. All authors have read and approved the final version of this manuscript to be published.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Funding
This work was supported by Hunan Province Clinical Medical Technology Innovation Guidance Project (2018SK52202), General guidance project of Hunan Provincial Health Commission (20201949), Project of Hunan Provincial Health Commission (B2019111), Hunan Provincial Natural Science Foundation Project (2020JJ5504) and Hengyang Science and Technology Bureau Guidance Project (2020161).
Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Contributor Information
Jian-Ming Xiong, Department of Vascular Surgery, Yiyang Central Hospital, Yiyang 413000, Hunan Province, P.R. China.
Hui Liu, Department of Vascular Surgery, Yiyang Central Hospital, Yiyang 413000, Hunan Province, P.R. China.
Jie Chen, Department of Vascular Surgery, The Second Affiliated Hospital, University of South China, Hengyang 421000, Hunan Province, P.R. China.
Qing-Qing Zou, Department of Vascular Surgery, The Second Affiliated Hospital, University of South China, Hengyang 421000, Hunan Province, P.R. China.
Yang-Yi-Jing Wang, Department of Vascular Surgery, The Second Affiliated Hospital, University of South China, Hengyang 421000, Hunan Province, P.R. China.
Guo-Shan Bi, Department of Vascular Surgery, The Second Affiliated Hospital, University of South China, Hengyang 421000, Hunan Province, P.R. China.
Consent for Publication
The informed consent was obtained from study participants.
Availability of Data and Material
All data generated or analyzed during this study are included in this article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Ma X, Yao H, Yang Y et al. miR-195 suppresses abdominal aortic aneurysm through the TNF-α/NF-κB and VEGF/PI3K/Akt pathway. Int J Mol Med 2018;41:2350–8. [DOI] [PubMed] [Google Scholar]
- 2. Salmon M, Johnston WF, Woo A et al. KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation 2013;128:S163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peng J, He X, Zhang L, Liu P. MicroRNA-26a protects vascular smooth muscle cells against H2O2-induced injury through activation of the PTEN/AKT/mTOR pathway. Int J Mol Med 2018;42:1367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Golledge J, Moxon JV, Singh TP et al. Lack of an effective drug therapy for abdominal aortic aneurysm. J Intern Med 2020;288:6–22. [DOI] [PubMed] [Google Scholar]
- 5. He Y-C, He L, Khoshaba R et al. Curcumin nicotinate selectively induces cancer cell apoptosis and cycle arrest through a P53-mediated mechanism. Molecules 2019;24:4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wei X-Q, Zhu J-F, Wang X-B, Ba K. Improving the stability of liposomal curcumin by adjusting the inner aqueous chamber pH of liposomes. ACS Omega 2020;5:1120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alok A, Singh ID, Singh S et al. Curcumin-pharmacological actions and its role in oral submucous fibrosis: a review. J Clin Diagn Res 2015;9:ZE01–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El-Azab M, Hishe H, Moustafa Y, El-Awady E-S. Anti-angiogenic effect of resveratrol or curcumin in Ehrlich ascites carcinoma-bearing mice. Eur J Pharmacol 2011;652:7–14. [DOI] [PubMed] [Google Scholar]
- 9. Chen C-Y, Kao C-L, Liu C-M. The cancer prevention, anti-inflammatory and anti-oxidation of bioactive phytochemicals targeting the TLR4 signaling pathway. Int J Mol Sci 2018;19:2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garg AX, Devereaux PJ, Hill A et al. Oral curcumin in elective abdominal aortic aneurysm repair: a multicentre randomized controlled trial. CMAJ 2018;190:E1273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hao Q, Chen X, Wang X et al. Curcumin attenuates angiotensin II-induced abdominal aortic aneurysm by inhibition of inflammatory response and ERK signaling pathways. Evid Based Complement Alternat Med 2014;2014:270930–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu Z-Y, Trenner M, Boon RA et al. Long noncoding RNAs in key cellular processes involved in aortic aneurysms. Atherosclerosis 2020;292:112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cao J. The functional role of long non-coding RNAs and epigenetics. Biol Proced Online 2014;16:11–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Z, Zou G, Chen X et al. Knockdown of lncRNA PVT1 inhibits vascular smooth muscle cell apoptosis and extracellular matrix disruption in a murine abdominal aortic aneurysm model. Mol Cells 2019;42:218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang W, Li X, Wang D et al. Curcumin reduces LPS-induced septic acute kidney injury through suppression of lncRNA PVT1 in mice. Life Sci 2020;254:117340. [DOI] [PubMed] [Google Scholar]
- 16. Joviliano EE, Ribeiro MS, Tenorio EJR. MicroRNAs and current concepts on the pathogenesis of abdominal aortic aneurysm. Braz J Cardiovasc Surg 2017;32:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou SS, Jin JP, Wang JQ et al. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin 2018;39:1073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gabisonia K, Prosdocimo G, Aquaro GD et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 2019;569:418–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Icli B, Dorbala P, Feinberg MW. An emerging role for the miR-26 family in cardiovascular disease. Trends Cardiovasc Med 2014;24:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li X, Pan X, Fu X et al. MicroRNA-26a: an emerging regulator of renal biology and disease. Kidney Blood Press Res 2019;44:287–97. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Y, Qin W, Zhang L et al. MicroRNA-26a prevents endothelial cell apoptosis by directly targeting TRPC6 in the setting of atherosclerosis. Sci Rep 2015;5:9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leeper NJ, Raiesdana A, Kojima Y et al. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol 2011;226:1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grande G, Milardi D, Martini M et al. Protein expression of PTTG-1, OCT-4, and KLF-4 in seminoma: a pilot study. Front Endocrinol (Lausanne) 2019;10:619–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salmon M, Spinosa M, Zehner ZE et al. Klf 4, Klf 2, and Zfp 148 activate autophagy-related genes in smooth muscle cells during aortic aneurysm formation. Physiol Rep 2019;7:e14058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cai Z, Huang J, Yang J et al. Lnc RNA SENCR suppresses abdominal aortic aneurysm formation by inhibiting smooth muscle cells apoptosis and extracellular matrix degradation. Bosn J Basic Med Sci 2020. 2020;21:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li FF, Shang XK, Du XL, Chen S. Rapamycin treatment attenuates angiotensin II -induced abdominal aortic aneurysm formation via VSMC phenotypic modulation and down-regulation of ERK1/2 activity. Curr Med Sci 2018;38:93–100. [DOI] [PubMed] [Google Scholar]
- 27. Zhou Y, Wu W, Lindholt JS et al. Regulatory T cells in human and angiotensin II-induced mouse abdominal aortic aneurysms. Cardiovasc Res 2015;107:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hou X, Yang S, Zheng Y. Licochalcone a attenuates abdominal aortic aneurysm induced by angiotensin II via regulating the miR-181b/SIRT1/HO-1 signaling. J Cell Physiol 2019;234:7560–8. [DOI] [PubMed] [Google Scholar]
- 29. Gu H-F, Li N, Tang Y-L et al. Nicotinate-curcumin ameliorates cognitive impairment in diabetic rats by rescuing autophagic flux in CA1 hippocampus. CNS Neurosci Ther 2019;25:430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liang B, Che J, Zhao H et al. MiR-195 promotes abdominal aortic aneurysm media remodeling by targeting Smad 3. Cardiovasc Ther 2017;35:e12286. [DOI] [PubMed] [Google Scholar]
- 31. Zhang X, Li F, Wang W et al. Macrophage pyroptosis is mediated by immunoproteasome subunit β5i (LMP7) in abdominal aortic aneurysm. Biochem Biophys Res Commun 2020;533:1012–1020. [DOI] [PubMed] [Google Scholar]
- 32. Lu X, Yu Y, Yin F et al. Knockdown of PVT1 inhibits IL-1beta-induced injury in chondrocytes by regulating miR-27b-3p/TRAF3 axis. Int Immunopharmacol 2020;79:106052. [DOI] [PubMed] [Google Scholar]
- 33. Wang Z, Tang X, Wu X et al. Cardamonin exerts anti-gastric cancer activity via inhibiting Lnc RNA-PVT1-STAT3 axis. Biosci Rep 2019;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoshida K, Toden S, Ravindranathan P et al. Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis 2017;38:1036–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kong Y, Huang T, Zhang H et al. The lncRNA NEAT1/miR-29b/Atg 9a axis regulates IGFBPrP1-induced autophagy and activation of mouse hepatic stellate cells. Life Sci 2019;39:BSR20190357. [DOI] [PubMed] [Google Scholar]
- 36. Xie ZY, Wang FF, Xiao ZH et al. Long noncoding RNA XIST enhances ethanol-induced hepatic stellate cells autophagy and activation via miR-29b/HMGB1 axis. IUBMB Life 2019;71:1962–72. [DOI] [PubMed] [Google Scholar]
- 37. Elfaki LA, Pirani RA, Afrasiabi Z et al. Abstract 16459: MicroRNA-26a targeted therapy for abdominal aortic aneurysms. Circulation 2019;140:A16459–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


