Abstract
Background
Serum levels of insulin-like growth factor 1 (IGF-1) and body mass index (BMI) are both associated with susceptibility to age-related diseases. Reports on the correlation between them have been conflicting, with both positive to negative correlations reported. However, the age ranges of the participants varied widely among these studies.
Methods
Using data on 4241 participants (aged 24–110) from the Long Life Family Study, we investigated the relationship between IGF-1 and BMI by age groups using regression analysis.
Results
When stratified by age quartile, the relationship between IGF-1 and BMI varied: in the first quartile (Q1, 20–58 years) the relationship was negative (β = −0.2, p = .002); in Q2 (58–66 years) and Q3 (67–86 years) the relationship was negative (β = −0.07, β = −0.01, respectively) but nonsignificant; and in Q4 (87–110 years) the relationship was positive (β = 0.31, p = .0002). This pattern did not differ by sex. We observed a similar age-related pattern between IGF-1 and BMI among participants in the third National Health and Nutritional Examination Survey.
Conclusions
Our results that the relationship between IGF-1 and BMI differs by age may explain some of the inconsistency in reports about their relationship and encourage additional studies to understand the mechanisms underlying it.
Keywords: Age-related diseases, Age-related pattern, Regression analysis
Insulin-like growth factor 1 (IGF-1) is a member of the IGF-1 pathway (1), which appears to play a key role in the processes underlying longevity (2). Many epidemiological studies report that serum IGF-1 levels are associated with an elevated risk of type 2 diabetes (3), cancer (4,5), cardiovascular disease (6,7), and mortality (8,9). IGF-1 has structural homology to insulin and characteristics of both a circulating hormone that mediates growth hormone (GH) actions in promoting growth, development, and metabolism (1) and a local tissue growth factor that promotes cellular growth, differentiation, and apoptosis (10). Serum IGF-1 levels are heritable, with estimated heritability ranging from 40% to 63% (11,12), and are influenced by obesity, age, sex, physical activity, GH level, and nutritional status (12). Across the life span, serum IGF-1 levels are low at birth, increase during childhood and puberty, and reach their highest concentration during early adulthood then start to decline in the third decade of life (13).
Body mass index (BMI) is also strongly associated with the risk for chronic disease development associated with aging (14). Because both IGF-1 and BMI are associated with disease risk and disease endpoints, several studies have assessed the relationship between them. Understanding the relationship between these 2 predictors could help categorizing those at risk of disease development or event. However, the relationship between BMI and IGF-1 across studies is neither consistent nor clear. Several studies have reported that IGF-1 levels are inversely correlated with BMI (15–20), whereas others report a positive correlation (21) or no correlation (22). Most of these studies estimated the relationship between IGF-1 and BMI by stratifying the samples based on BMI categories, and not age, although the study participants’ ages varied widely (17,19,20,22).
To date, no study has assessed the influence of age on the association between BMI and IGF-1. We hypothesize that the relationship between IGF-1 and BMI varies by age.
Here we present a cross-sectional study of the relationship between IGF-1 and BMI in a large sample of 4241 participants from the Long Life Family Study (LLFS) with validation of the relationships in a large sample of 2555 participants from the third National Health and Nutritional Examination Survey (NHANES III). In particular, we assessed whether the relationship between IGF-1 and BMI varies in an age- and sex-specific manner.
Method
Study Population
The primary sample for this study is a set of participants from LLFS. LLFS is a multicenter family-based cohort study of 539 families that was designed to determine the genetic and behavioral/environmental risk factors that promote exceptional longevity (23). The families were recruited between 2006 and 2009 from the United States and Denmark at 4 enrollment sites (New York, Boston, and Pittsburgh in the United States and nationwide in Denmark). The total number of enrolled participants is 4953, consisting of long-lived probands and their siblings (n = 1727), the offspring of this generation and their spouses (n = 3226). Participants without measurements of serum IGF-1 levels or BMI were excluded; therefore, the total sample size for this analysis is 4241 participants (aged 24–110 years) consisting of 1391 from the proband generation (49–110 years), 2119 from the offspring generation (32–87 years), and 731 offspring spouses (24–88 years). All participants self-identified as non-Hispanic White.
The findings in LLFS were replicated using participants from NHANES III (24). NHANES III is a sample of approximately 39 000 participants aged 2 months and older and was designed to be representative of the U.S. population. It was conducted from 1988 to 1994 in 2 phases. Of the total sample of adults (n = 20 024), we selected a subset of 2555 non-Hispanic White participants (20–90 years) with a complete record of the study variables. Parallel analyses were conducted with non-Hispanic Black participants (n = 1639, 20–90 years) and Mexican American participants (n = 1607, 20–90 years).
Participant Characteristics
In both LLFS and NHANES III, standing height, weight, and waist circumference (WC) were assessed by trained interviewers with a standardized protocol and skill level. BMI was calculated as weight in kilograms per square of the height in meters. Age, race, ethnicity, and sex were taken by self-report during the interview. For the analysis in LLFS, presence of diabetes was defined as the use of diabetes medications or fasting glucose ≥126 mg/dL. Presence of hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or self-report confirmed by use of antihypertensive medication. For NHANES III, presence of diabetes was defined as a history of diabetes diagnosis, or fasting glucose ≥126 mg/dL, or current use of oral hypoglycemics or insulin. Presence of hypertension was defined if a participant reported both ever being told that he or she had high blood pressure and current use of antihypertensive medication, or if the average measured BP was ≥140 mmHg systolic or ≥90 mmHg diastolic. We excluded smoking status as a covariate because of the large number of missingness within the NHANES III participants, in addition to the nonsignificant association between IGF-1 and smoking status in LLFS.
Laboratory Assays
In LLFS, fasting peripheral blood samples were obtained from participants and then shipped to the Advanced Diagnostics and Research Laboratory at the University of Minnesota (25). IGF-1 was measured in serum using a solid-phase enzyme-linked chemiluminescent immunoassay on an Immulite 2000 system (Siemens Healthcare Diagnostics, Inc.). The interassay coefficient of variability was 8.7%.
In NHANES III, fasting serum samples were collected from 1988 to 1994 and IGF-1 concentrations were quantified by IGF-I enzyme-linked immunosorbent assay (DSL 10-5600) including an extraction step that separates IGF-1 from its binding protein. The samples were reanalyzed if the coefficient of variation for replicate samples was greater than 15% (26). In the current study, given the differences in the assays used for serum IGF-1 measurements in LLFS and NHANES III, we only compared the relationships among traits between studies.
Statistical Approach
All data analyses were performed using R version 3.4.0 (27). To approximate normality, both IGF-1 and BMI were natural log–transformed for the analysis. We calculated the Pearson coefficient of correlation between IGF-1 levels and BMI, height, weight, WC, and age.
We used 2 sample t tests to assess the mean age difference and mean IGF-1 difference between the LLFS and NHANES III. Also, we used the lstrends function to estimate and compare the slopes of fitted lines between males and females in both studies.
We regressed log(IGF-1) on log(BMI) to get an overall assessment of their relationship in each cohort. We performed a linear mixed-effect model using coxme package (28) and adjusting for covariates. Covariates were chosen based on their known association with serum IGF-1 and included age, age (2), male sex, log(BMI), field center, diabetes, hypertension, log(BMI) × age, and log(BMI) × sex as fixed variables, and also adjusting for kinship as a random variable to account for the relatedness between LLFS participants. To assess whether the relationship differed by sex, for all analyses described below we also stratified by sex and regressed IGF-1 on BMI as above without the sex and log(BMI) × sex terms.
First, we regressed IGF-1 on BMI in all samples regardless of age, but including other covariates. Then, to assess the relationship between IGF-1 and BMI by age, we divided the LLFS sample into age quartiles and performed linear mixed-model regression of IGF-1 with BMI as a fixed variable and kinship as a random variable within each age quartile.
In addition, we also conducted all of the previous analyses with IGF-1 and WC, as another measure of adiposity.
To validate the results, the same approach was used with the NHANES III sample of 2555 non-Hispanic White participants, although we did not adjust for kinship, as the participants were assumed to be unrelated. The age quartile thresholds in LLFS were used as age group thresholds in NHANES III. We then assessed whether the relationship between IGF-1 and BMI also differed by age quartile in non-Hispanic Black (n = 1639) and Mexican American (n = 1607) participants.
Results
The means, standard deviations, and proportions of key characteristics of the study samples are presented in Table 1. The LLFS participants’ mean age was 70 years (range of 24–110 years) and the prevalence of diabetes and hypertension was 7% and 51%, respectively. The overall mean serum IGF-1 level was 128.3 ng/mL and ranged from 26 to 745 ng/mL. The mean BMI was 27 kg/m2. In NHANES III, the participants’ mean age was 53.2 years (range of 20–90 years) and the prevalence of diabetes and hypertension was 6% and 28%, respectively. The overall mean IGF-1 was 249.5 ng/mL and ranged from 25.3 to 863.8 ng/mL. The mean BMI was 26.48 kg/m2.
Table 1.
Descriptive Statistics of Age, IGF-1, and Anthropometric Measurements in LLFS and NHANES III
| Measurements | LLFS | NHANES III | ||||
|---|---|---|---|---|---|---|
| Male (45.3%) | Female (54.7%) | Total | Male (45.2%) | Female (54.8%) | Total | |
| Participants (n) | 1924 | 2317 | 4241 | 1155 | 1400 | 2555 |
| First age quartile/group (n) (20–58 years) | 421 | 640 | 1061 | 643 | 840 | 1483 |
| Second age quartile/group (n) (58–66 years) | 507 | 553 | 1060 | 140 | 144 | 284 |
| Third age quartile/group (n) (66–86 years) | 497 | 563 | 1060 | 346 | 381 | 727 |
| Fourth age quartile/group (n) (87–110 years) | 499 | 561 | 1060 | 26 | 35 | 61 |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age at enrollment (years) | 70.6 (15.3) | 69.5 (15.9) | 70.0 (15.6) | 54.0 (19.5) | 52.6 (19.7) | 53.2 (19.6) |
| IGF-1 (ng/mL) | 134.6 (54.0) | 123.2 (51.6) | 128.3 (52.9) | 264.7 (97.6) | 236.9 (103.6) | 249.5 (101.9) |
| BMI (kg/m2) | 27.5 (4.0) | 26.7 (5.3) | 27.8 (4.8) | 26.7 (4.8) | 26.3 (5.7) | 26.5 (5.3) |
| Height (cm) | 173.6 (7.7) | 159.6 (7.8) | 166.0 (10.5) | 175.5 (7.1) | 161.3 (7.0) | 167.7 (9.9) |
| Weight (kg) | 83.2 (14.9) | 68.2 (14.9) | 75.0 (16.5) | 82.5 (16.5) | 68.4 (15.4) | 74.8 (17.4) |
| WC (cm) | 99.5 (11.0) | 90.4 (13.9) | 94.5 (13.4) | 97.7 (12.7) | 89.5 (14.5) | 93.2 (14.3) |
| Hypertension | 24% | 27%. | 51% | 12% | 16% | 28% |
| Diabetes | 4% | 3% | 7% | 3% | 3% | 6% |
Notes: BMI = body mass index; IGF-1 = insulin-like growth factor 1; LLFS = Long Life Family Study; NHANES III = third National Health and Nutritional Examination Survey; WC = waist circumference. Enrollment for LLFS was 2006–2008, and for NHANES III was 1988–1994. IGF-1 and BMI are values on the natural scale to report the mean and standard deviation.
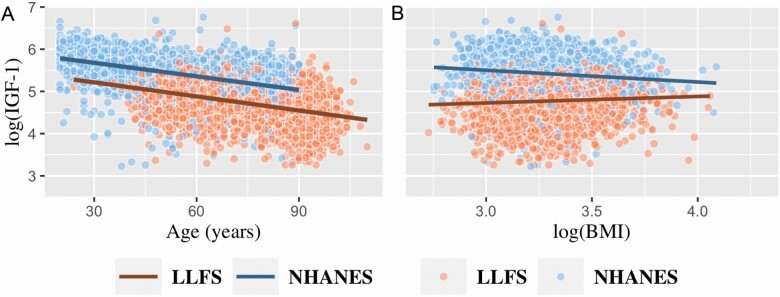
Both studies had a wide age range, but on average, NHANES III participants were 16.8 years younger than LLFS participants (p < .0001). The age distribution in LLFS is bimodal due to the family study design with some overlap between the LLFS generations; the age distribution in NHANES III is approximately uniform across its range (Supplementary Appendix Figure 1). As expected, log(IGF-1) levels were negatively correlated with age in both LLFS and NHANES III, r = −0.42 (p < .001) and r = −0.47 (p < .001), respectively (Figure 1A). In addition, mean serum IGF-1 levels were 121.2 ng/mL lower in LLFS compared to NHANES III (p < .0001).
Figure 1.
(A) Scatter plot of log(IGF-1) by age and (B) scatter plot of log(IGF-1) by log(BMI) in both LLFS and NHANES III.
In LLFS, across all participants, log(IGF-1) levels were positively correlated with both log(BMI) and WC, r = 0.06 (p < .001) and r = 0.03 (p = .06), respectively (Table 2). In contrast, in NHANES III, log(IGF-1) levels were negatively correlated with log(BMI) and WC, r = −0.12 (p < .001) and r = −0.18 (p < .001), respectively. However, log(IGF-1) was positively correlated with height among both LLFS and NHANES III participants (r = 0.27, p < .001 and r = 0.24, p < .001, respectively; Table 2).
Table 2.
Pearson Correlation Coefficients Between Log(IGF-1), Age, and Anthropometric Measures in LLFS and NHANES III
| LLFS | |||||||
|---|---|---|---|---|---|---|---|
| Log(IGF-1) | Age | Log(BMI) | Height | Weight | WC | ||
| NHANES III | Log(IGF-1) | 1 | −0.42*** | 0.06*** | 0.27*** | 0.19*** | 0.03 |
| Age | −0.47*** | 1 | −0.12*** | −0.38*** | −0.31*** | 0.03* | |
| Log(BMI) | −0.12*** | 0.10*** | 1 | 0.08*** | 0.82*** | 0.81*** | |
| Height | 0.24*** | −0.24*** | 0.01 | 1 | 0.62*** | 0.28*** | |
| Weight | 0.02 | −0.05*** | 0.85*** | 0.52*** | 1 | 0.80*** | |
| WC | −0.18*** | 0.28*** | 0.87*** | 0.23*** | 0.86*** | 1 |
Note: BMI = body mass index; IGF-1= insulin-like growth factor 1; LLFS = Long Life Family Study; NHANES III = third National Health and Nutritional Examination Survey; WC= waist circumference.
*p < .05, **p < .01, ***p < .001.
In the regression analysis—adjusting for sex, diabetes, and hypertension, and, in LLFS, field center and kinship, log(IGF-1) was associated positively with log(BMI) (β = 0.20, p = 4.4 × 10−12), whereas, in NHANES III, the relationship was significant and negative (β = −0.23, p = 1.4 × 10−6; Table 3, Figure 1B). In NHANES III, hypertension was negatively associated with log(IGF-1) in the overall sample (β = −0.12, p = 6.9 × 10−10) and in the overall sample stratified by sex (data not shown). However, in LLFS, hypertension was not associated with log(IGF-1) in the overall sample, but it was negatively associated with log(IGF-1) in the stratified overall sample by sex (both p = <.0001; data not shown).
Table 3.
Result of Linear Mixed-Model Regression of Association Between Log(IGF-1) as an Outcome and Log(BMI) in the Overall Sample and by Sex of LLFS and NHANES III and Then Stratified by Age
| LLFS | NHANES III | |||
|---|---|---|---|---|
| Log(IGF-1) | β (SE) | p Value | β (SE) | p Value |
| Overall | 0.20 (0.04) | 4.4 × 10−12 | −0.23 (0.05) | 1.4× 10−6 |
| Male | 0.45 (0.06) | 4.5 × 10−12 | −0.23 (0.07) | 1.4 × 10−3 |
| Female | 0.05 (0.05) | 2.8 × 10−1 | −0.22 (0.06) | 5.2 × 10−4 |
| First age quartile/group (20–58 years) | −0.20 (0.06) | 2.2 × 10−3 | −0.40 (0.05) | 2.6 × 10−12 |
| Second age quartile/group (58–66 years) | −0.07 (0.06) | 3.2 × 10−1 | 0.006 (0.13) | 9.6× 10−1 |
| Third age quartile/group (67–86 years) | −0.01 (0.07) | 8.4 × 10−1 | 0.03 (0.08) | 7.6× 10−1 |
| Fourth age quartile/group (87–110 years) | 0.31 (0.08) | 2.6 × 10−4 | 0.84 (0.39) | .031 |
Notes: BMI = body mass index; IGF-1= insulin-like growth factor 1; LLFS = Long Life Family Study; NHANES III = third National Health and Nutritional Examination Survey; SE = standard error; β = log(BMI) regression coefficient adjusting for diabetes, hypertension, sex (when applicable) in both studies and field center, and kinship in LLFS. The same age quartile thresholds for LLFS were applied onto NHANES III.
In LLFS, interaction for both log(BMI) and age and log(BMI) and sex had significant effects on log(IGF-1) (both P = <0.0001; data not shown). Whereas, in NHANES III, there was a significant interaction effect between log(BMI) and age (p = 7.3 × 10−5) on log(IGF-1), but no significant interaction between log(BMI) and sex (p = .4; data not shown).
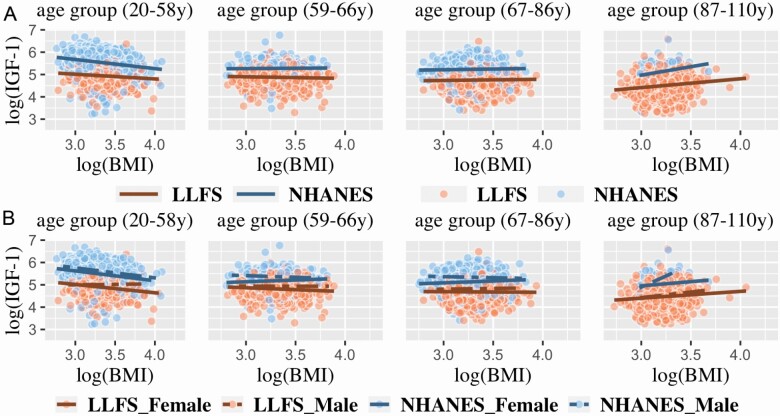
We further investigated the interaction between age and log(BMI) on log(IGF-1) in LLFS using age quartiles (Supplementary Appendix Table 1). As can be seen in Figure 2A, the relationship between log(IGF-1) and log(BMI) differed by age quartile. In the first (youngest) age quartile (20–58 years), the relationship was significant and negative (β = −0.2, p = .0022), in the second (59–66 years) and third (67–86 years) age quartiles the relationship was nonsignificant, but in the fourth (oldest) quartile (87–110 years), the relationship was significant and positive (β = 0.31, p = 2.6 × 10−4; Table 3). When the NHANES III data were stratified using the LLFS age quartile thresholds, a similar pattern was observed (Table 3, Figure 2A). We also stratified LLFS and NHANES III using age thresholds derived from NHANES III and applied them to LLFS; a similar pattern was observed (Supplementary Appendix Figure 2).
Figure 2.
(A) Scatter plot of log(IGF-1) by log(BMI) stratified by age groups and (B) scatter plot of log(IGF-1) by log(BMI) per age groups and stratified by sex in LLFS and NHANES III. The same age quartile thresholds for LLFS were applied onto NHANES III.
We next investigated the relationship between log(BMI) and sex on log(IGF-1) levels in LLFS by stratifying each age quartile by sex. Among females, log(IGF-1) was significantly and negatively associated with log(BMI) in the first (20–58 years) age quartiles (β = −0.28, p = 3 × 10−4), but there was no significant association in males (Figure 2B). In the second (59–66 years) and third age quartiles (67–86 years), log(IGF-1) was not associated with log(BMI) in either sex. However, in the oldest quartile (87–110 years), log(IGF-1) was significantly and positively associated with log(BMI) in both sexes (β = 0.2, p = .04 and β = 0.4, p = .0014, respectively; Figure 2B). The relationship between log(IGF-1) and log(BMI) did not significantly differ by sex in NHANES III, except in the fourth quartile (87–110 years) wherein the association was positive and significant in males (p = .002), but not in females (p = .4), though the sample sizes were small. In addition, we observed a significant slope difference by sex in the relationship between log(IGF-1) and log(BMI) in the first age quartile of LLFS only (p = .002).
Similar results were observed for the relationship between IGF-1 and WC in both studies (Supplementary Appendix Table 2). Also, similar patterns were seen among non-Hispanic Black and Mexican American participants in NHANES III (Supplementary Appendix Figure 3).
Discussion
In this cross-sectional study of LLFS, a unique family-based cohort of exceptional longevity, we examined the age- and sex-specific effects of the relationship between serum IGF-1 levels and BMI. Younger participants (24–58 years) had a negative relationship between IGF-1 and BMI, while older participants (87–110 years) had a positive relationship. There was no statistically significant relationship for the age groups in between. The same pattern was observed in an independent sample of non-Hispanic White adults of similar age range recruited from the general population in the NHANES III. In addition, we did not observe a consistent sex-specific difference in the relationship between IGF-1 and BMI across the age groups. The discrepancies in the relationship between serum IGF-1 and BMI among studies in the literature (15–22) may be explained by the ages of the cohorts used in the previous studies. Studies reporting the negative relationship between BMI and IGF-1 were primarily conducted in participants with ages ranging from 10 to 60 years (15–20). In contrast, studies that reported a positive relationship were often performed with older individuals with ages ranging from 45 to 90 years (21). These studies also showed a similar pattern between the relationship of IGF-1 and WC (17–19,21). In addition, several of these studies were comprised of highly selected groups, such as obese/overweight individuals, who might be experiencing a weight-related disruption in insulin and GH secretion (19).
The age-related difference in the relationship between IGF-1 and BMI might be due to height, which is a component of BMI. However, the pattern between IGF-1 and WC (a measure of central adiposity that is independent of height) was similar to that with BMI. This result indicates that the relationship is driven by adiposity rather than height (Supplementary Appendix Table 2).
In non-Hispanic Black and Mexican American participants, other investigators have reported an inverse association between IGF-1 and BMI (15,17), whereas others have reported no association (29). However, in our study, we saw similar patterns by age group in non-Hispanic Black and Mexican American participants within NHANES III as we saw in the non-Hispanic White participants, despite smaller sample sizes (Supplementary Appendix Figure 3). These results suggest that the relationship between IGF-1 and BMI by age group is similar among different racial/ethnic groups.
In this cohort, we observed that younger participants had a higher mean IGF-1 level compared to older participants, and this is consistent with known IGF-1 biology in adolescent and early adulthood (13). Although the interaction between log(BMI) and sex in predicting log(IGF-1) was statistically significant, the slope difference between male and female was not statistically significant except in the youngest LLFS age quartile. The latter results might reflect the sex differences in development during puberty and early adulthood.
LLFS and NHANES III data were collected 10 years apart; thus, period or cohort effects may exist, in addition to the age effect we demonstrate. However, despite this potential period effect, the patterns were consistent for both LLFS and NHANES III cohorts, for BMI and WC, and across different racial/ethnic groups. In addition, mean serum IGF-1 levels were 121.2 ng/mL lower in LLFS compared to NHANES III. The most likely reason for this difference is the use of different assay kits in the measurement of serum IGF-1 levels between the 2 studies. Previous studies have reported significant differences in serum IGF-1 levels when using different assay kits, even though the samples were from the same population (30–32). Different assay kits have different age- and sex-specific reference ranges, and this might affect the upper and lower limit of each study’s serum IGF-1 levels. Furthermore, NHANES III was conducted using a sample from the general population, whereas LLFS sampled healthy long-lived individuals (23). Thus, the study population and assay type are confounded, and it is impossible to determine whether the difference in mean IGF-1 levels between the cohorts is due to ascertainment differences or assay differences given these data. However, all statistical comparisons in this article were done within-study, so the mean differences in IGF-1 levels between studies should not affect our conclusions, especially given that the patterns across age groups were similar.
This current study was cross-sectional and not longitudinal; therefore, we could not measure the relationship between IGF-1 and BMI on the same participants throughout their life span in order to determine the patterns of change in this relationship. Instead, we stratified our samples by age group and are extrapolating these cross-sectional results to reflect individual changes related to aging. However, additional longitudinal data are needed to confirm these findings. Also, we relied on BMI and WC to measure adiposity, which capture body size but not body composition. These anthropometric measurements are not as precise as imaging-based measurements, such as fat mass as estimated from dual X-ray absorptiometry or peripheral quantitative computed tomography (33). Such measures were not available for these studies. Another limitation in our study is the lack of data on potential confounders such as physical activity, diet, and GH level. These factors can have major effects on BMI and IGF-1 level and may be important to consider as potential confounders of this association.
In summary, we identified age-related differences in the relationship between serum IGF-1 levels and BMI, as well as WC, in non-Hispanic White, non-Hispanic Black, and Mexican American participants. This finding clarifies that the apparent contradiction in the previous literature on the relationship between IGF-1 and adiposity is likely due to differences in cohort age ranges. As such, the clinical implication of this is that age should be considered when evaluating the relationship between adiposity and IGF-1. However, longitudinal studies and further investigation into the underlying biology affecting the relationship between serum IGF-1 and measures of adiposity across the life span are needed to understand these observations. Such an understanding might help categorize individuals at risk of disease or inform interventions to delay disease depending on their age-dependent BMI and IGF-1 level.
Supplementary Material
Acknowledgments
We would like to thank the LLFS and NHANES III participants as well as the research staff who recruited and survey them.
Funding
The work with the Long Life Family Study was supported by the National Institute on Aging at National Institutes of Health, USA cooperative agreements U01AG023712, U01AG23744, U01AG023746, U01AG023749, U01AG023755, and U19AG063893. Support and sponsorship for R.A.S. provided by the University of Zawia, Libya and the Libyan Ministry of Higher Education through the Libyan–North American Scholarship Program (LNASP).
Conflict of Interest
None declared.
References
- 1. Le Roith D. The insulin-like growth factor system. Exp Diabesity Res. 2003;4:205–212. doi: 10.1155/EDR.2003.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vitale G, Pellegrino G, Vollery M, Hofland LJ. ROLE of IGF-1 system in the modulation of longevity: controversies and new insights from a centenarians’ perspective. Front Endocrinol (Lausanne). 2019;10(FEB):1–11. doi: 10.3389/fendo.2019.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rajpathak SN, He M, Sun Q, et al. . Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes. 2012;61:2248–2254. doi: 10.2337/db11-1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3 [DOI] [PubMed] [Google Scholar]
- 5. Murphy N, Carreras-Torres R, Song M, et al. . Circulating levels of insulin-like growth factor 1 and insulin-like growth factor binding protein 3 associate with risk of colorectal cancer based on serologic and Mendelian randomization analyses. Gastroenterology. 2020;158:1300–1312.e20. doi: 10.1053/j.gastro.2019.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andreassen M, Raymond I, Kistorp C, Hildebrandt P, Faber J, Kristensen LØ. IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population. Eur J Endocrinol. 2009;160:25–31. doi: 10.1530/EJE-08-0452 [DOI] [PubMed] [Google Scholar]
- 7. Carlzon D, Svensson J, Petzold M, et al. . Both low and high serum IGF-1 levels associate with increased risk of cardiovascular events in elderly men. J Clin Endocrinol Metab. 2014;99:E2308–E2316. doi: 10.1210/jc.2014-1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schutte AE, Conti E, Mels CM, et al. . Attenuated IGF-1 predicts all-cause and cardiovascular mortality in a Black population: a five-year prospective study. Eur J Prev Cardiol. 2016;23:1690–1699. doi: 10.1177/2047487316661436 [DOI] [PubMed] [Google Scholar]
- 9. Zhang WB, Aleksic S, Gao T, et al. . Insulin-like growth factor-1 and IGF binding proteins predict all-cause mortality and morbidity in older adults. Cells. 2020;9(6):1–18. doi: 10.3390/cells9061368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stewart CE, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol Rev. 1996;76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005 [DOI] [PubMed] [Google Scholar]
- 11. Harrela M, Koistinen H, Kaprio J, et al. . Genetic and environmental components of interindividual variation in circulating levels of IGF-I, IGF-II, IGFBP-1, and IGFBP-3. J Clin Invest. 1996;98:2612–2615. doi: 10.1172/JCI119081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hong Y, Pedersen NL, Brismar K, Hall K, de Faire U. Quantitative genetic analyses of insulin-like growth factor I (IGF-I), IGF-binding protein-1, and insulin levels in middle-aged and elderly twins. J Clin Endocrinol Metab. 1996;81:1791–1797. doi: 10.1210/jcem.81.5.8626837 [DOI] [PubMed] [Google Scholar]
- 13. Le Roith D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. N Engl J Med. 1997;336:633–640. doi: 10.1056/NEJM199702273360907 [DOI] [PubMed] [Google Scholar]
- 14. Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95:727–748. doi: 10.1152/physrev.00030.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alderete TL, Byrd-Williams CE, Toledo-Corral CM, Conti DV, Weigensberg MJ, Goran MI. Relationships between IGF-1 and IGFBP-1 and adiposity in obese African-American and Latino adolescents. Obesity (Silver Spring). 2011;19:933–938. doi: 10.1038/oby.2010.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lam CS, Chen MH, Lacey SM, et al. . Circulating insulin-like growth factor-1 and its binding protein-3: metabolic and genetic correlates in the community. Arterioscler Thromb Vasc Biol. 2010;30:1479–1484. doi: 10.1161/ATVBAHA.110.203943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Faupel-Badger JM, Berrigan D, Ballard-Barbash R, Potischman N. Anthropometric correlates of insulin-like growth factor 1 (IGF-1) and IGF binding protein-3 (IGFBP-3) levels by race/ethnicity and gender. Ann Epidemiol. 2009;19:841–849. doi: 10.1016/j.annepidem.2009.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schernhammer ES, Tworoger SS, Eliassen AH, et al. . Body shape throughout life and correlations with IGFs and GH. Endocr Relat Cancer. 2007;14:721–732. doi: 10.1677/ERC-06-0080 [DOI] [PubMed] [Google Scholar]
- 19. Gram IT, Norat T, Rinaldi S, et al. . Body mass index, waist circumference and waist-hip ratio and serum levels of IGF-I and IGFBP-3 in European women. Int J Obes (Lond). 2006;30:1623–1631. doi: 10.1038/sj.ijo.0803324 [DOI] [PubMed] [Google Scholar]
- 20. Fowke JH, Matthews CE, Yu H, et al. . Racial differences in the association between body mass index and serum IGF1, IGF2, and IGFBP3. Endocr Relat Cancer. 2010;17:51–60. doi: 10.1677/ERC-09-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laughlin GA, Barrett-Connor E, Criqui MH, Kritz-Silverstein D. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89:114–120. doi: 10.1210/jc.2003-030967 [DOI] [PubMed] [Google Scholar]
- 22. Jernström H, Deal C, Wilkin F, et al. . Genetic and nongenetic factors associated with variation of plasma levels of insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in healthy premenopausal women. Cancer Epidemiol Biomarkers Prev. 2001;10:377–384. [PubMed] [Google Scholar]
- 23. Newman AB, Glynn NW, Taylor CA, et al. . Health and function of participants in the Long Life Family Study: a comparison with other cohorts. Aging (Albany NY). 2011;3:63–76. doi: 10.18632/aging.100242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. NHANES III (1988–1994). https://wwwn.cdc.gov/nchs/nhanes/nhanes3/default.aspx. Accessed April 6, 2019.
- 25. Sebastiani P, Thyagarajan B, Sun F, et al. . Age and sex distributions of age-related biomarker values in healthy older adults from the long life family study. J Am Geriatr Soc. 2016;64:e189–e194. doi: 10.1111/jgs.14522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berrigan D, Potischman N, Dodd KW, et al. . Race/ethnic variation in serum levels of IGF-I and IGFBP-3 in US adults. Growth Horm IGF Res. 2009;19:146–155. doi: 10.1016/j.ghir.2008.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. R Core Team. (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.https://www.R-project.org/ [Google Scholar]
- 28. Therneau TM. coxme: mixed effects Cox models.https://cran.r-project.org/web/packages/coxme/coxme.pdf
- 29. Henderson KD, Goran MI, Kolonel LN, Henderson BE, Le Marchand L. Ethnic disparity in the relationship between obesity and plasma insulin-like growth factors: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev. 2006;15:2298–2302. doi: 10.1158/1055-9965.EPI-06-0344 [DOI] [PubMed] [Google Scholar]
- 30. Zhu H, Xu Y, Gong F, et al. . Reference ranges for serum insulin-like growth factor I (IGF-I) in healthy Chinese adults. PLoS One. 2017;12(10):1–15. doi: 10.1371/journal.pone.0185561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pokrajac A, Wark G, Ellis AR, Wear J, Wieringa GE, Trainer PJ. Variation in GH and IGF-I assays limits the applicability of international consensus criteria to local practice. Clin Endocrinol (Oxf). 2007;67:65–70. doi: 10.1111/j.1365-2265.2007.02836.x [DOI] [PubMed] [Google Scholar]
- 32. Krebs A, Wallaschofski H, Spilcke-Liss E, et al. . Five commercially available insulin-like growth factor I (IGF-I) assays in comparison to the former Nichols Advantage IGF-I in a growth hormone treated population. Clin Chem Lab Med. 2008;46:1776–1783. doi: 10.1515/CCLM.2008.349 [DOI] [PubMed] [Google Scholar]
- 33. Kuriyan R. Body composition techniques. Indian J Med Res. 2018;148:648–658. doi: 10.4103/ijmr.IJMR_1777_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.