Abstract
Complications of delirium and dementia increase mortality; however, it is difficult to diagnose delirium accurately, especially among dementia patients. The bispectral electroencephalography score can detect delirium and predict mortality in elderly patients. We aimed to develop an efficient and reliable bispectral electroencephalography device for high-throughput screening. We also hypothesized that bispectral electroencephalography score can predict mortality among dementia patients. A prospective cohort study was conducted between January 2016 and December 2018 to measure bispectral electroencephalography from elderly patients and correlate with outcomes. A total of 502 elderly (55 years old or older) patients with and without dementia were enrolled. For a replication of the utility of bispectral electroencephalography, mortalities between bispectral electroencephalography-positive and bispectral electroencephalography-negative group were compared. In addition, patients with and without dementia status were added to examine the utility of bispectral electroencephalography among dementia patients. The mortality within 180 days in the bispectral electroencephalography-positive group was higher than that of the bispectral electroencephalography-negative group in both the replication and the total cohorts. Mortality of those in the bispectral electroencephalography-positive group showed a dose-dependent increase in both cohorts. When the dementia patients showed bispectral electroencephalography positive, their mortality was significantly higher than those with dementia but who were bispectral electroencephalography-negative. Mortality within 30 days in the bispectral electroencephalography-positive group was significantly higher than that of the bispectral electroencephalography-negative group. The utility of the bispectral electroencephalography to predict mortality among large sample of 502 elderly patients was shown. The bispectral electroencephalography score can predict mortality among elderly patients in general, and even among dementia patients, as soon as 30 days.
Keywords: delirium, dementia, bispectral electroencephalogram, BSEEG, mortality
Saito et al. aimed to show that their bispectral electroencephalography (BSEEG) method can capture brain wave abnormalities indicative of delirium and predict mortality among elderly patients with and without dementia. They previously showed the usefulness of BSEEG with 274 inpatients, and this time they studied an additional 228 subjects to replicate their findings.
Graphical Abstract
Graphical Abstract.
Introduction
The relationship between delirium and dementia is complicated because dementia is one of the risk factors of delirium.1,2 In addition, delirium is known to accelerate the progression of dementia.2 Furthermore, delirium and dementia are associated with patients’ outcomes including mortality.3 Especially if patients have both delirium and dementia, their mortality would increase.4,5
Although it is important to diagnose delirium accurately for prompt intervention in the elderly, it is often difficult.6,7 We previously developed a method to detect delirium by using the bispectral electroencephalography (BSEEG) score.7,8 Higher BSEEG scores were associated with higher mortality independent of delirium status.9 In fact, we showed that BSEEG score can predict mortality of elderly inpatients better than clinical categorization of delirium.9 Consistent with our previous findings of higher BSEEG score indicative of slow brain wave and its association with increased mortality, a recent prospective cohort study10 and a retrospective cohort study11 showed that clinical electroencephalography slowing is associated with higher mortality. However, there is neither a replication study for the utility of BSEEG nor an investigation of the utility of BSEEG for subjects with dementia. Without detecting delirium, patients remain at high risk for poor outcomes including mortality.4,12
Therefore, first we conducted a replication study to confirm the utility of BSEEG. Next, we hypothesized that BSEEG score can predict mortality among dementia patients. To examine this hypothesis, we compared mortality in dementia patients by dividing subjects into two groups: Those with high and low BSEEG scores.
Materials and methods
Participants
We expanded the original prospective cohort study as reported previously.9 Briefly, patients were recruited from January 2016 to December 2018 in general medicine and orthopaedic services in the University of Iowa Hospitals and Clinics. Written informed consent was acquired from all participants or from their legally authorized representatives in cases in which patients were judged to be delirious or demented and lacked capacity to consent. In the present study, 228 patients (replication cohort) were selected to be consistent with the 274 subjects (discovery cohort) from the original study.9 These were a convenience sample. The participants of the discovery cohort were recruited from January 2016 to October 2017, and the replication cohort was from the additional cohort recruited up to December 2018. Both cohorts were enrolled in a similar way following the same protocol from January 2016 to December 2018 who aged 55 years or older. Taken together, 502 patients (discovery and replication cohorts) were subjected to analysis in the present study. All subjects provided written informed consent after receiving a complete description of the study. This research was approved by the University of Iowa Human Subjects Research Institutional Review Board and carried out in accordance with the Declaration of Helsinki.
Clinical outcomes
The details about delirium status definition were described previously.9 Briefly, we screened for delirium by using the following: The Confusion Assessment Method for the Intensive Care Unit,13,14 the Delirium Rating Scale-Revised-9815 and the Delirium Observation Screening Scale.16 Delirium status was defined according to the results of the following screening tests: Confusion Assessment Method for the Intensive Care Unit positive, Delirium Rating Scale-Revised-98 score ≥19 or Delirium Observation Screening Scale score ≥3. Baseline cognitive function was measured by using the Montreal Cognitive Assessment17 when it was possible based on patients’ capacity and willingness to administer. Dementia was recorded based on chart review. Delirium and dementia statuses were finally determined by a board-certified consultation-liaison psychiatrist (G.S.) with the results of the measures and detailed chart review. Mortality status information was identified by electronic medical record review and/or obituary record. We have assessed mortality status up to 180 days for 100% of the 502 enrolled participants.
BSEEG data collection and score definition
Details about BSEEG data collection and score definition have been described previously.7,9 Briefly, BSEEG data were collected by using a portable electroencephalography device (CMS2100, CONTEC, Qinhuangdao, Hebei, China) by trained research assistants twice daily at the same time of the clinical symptom assessment. One electrode was placed on the centre of patients’ forehead as a ground, and two electrodes were placed on the left and right sides of the forehead in case of two-channel recording, and one electrode was placed on the one side of the forehead in case of one-channel recording to obtain BSEEG signals for up to 10 min. While recording data, patients were instructed to keep their eyes closed and jaw relaxed. The obtained data were converted into spectral density plots, and a BSEEG score was produced by using the signal-processing algorithm. The cut-off score used was BSEEG score = 0 as reported in the previous study.9
Statistical analysis
All statistical analyses were conducted using R.18 A t-test was conducted to compare continuous data, and a chi-square test or a Fisher's exact test was conducted to compare categorical data between cases and controls for delirium and dementia, and positive and negative for BSEEG. A log-rank test was conducted to compare two survival functions in 180 days. Moreover, mortality of both BSEEG-positive and -negative groups at the time of 30 days was compared to test how soon BSEEG can differentiate mortality risk. In addition, relative risk of the mortality in 30 days was calculated between the BSEEG-positive and -negative groups. Cox proportional hazards regression analysis was conducted to calculate the hazard ratio adjusting age, sex and the Charlson Comorbidity Index (CCI).19 A P-value of <0.05 was determined to be statistically significant.
Data availability
The data will be shared by the corresponding author upon request with a reasonable institutional approval.
Results
Replication of the utility of BSEEG in prediction of mortality
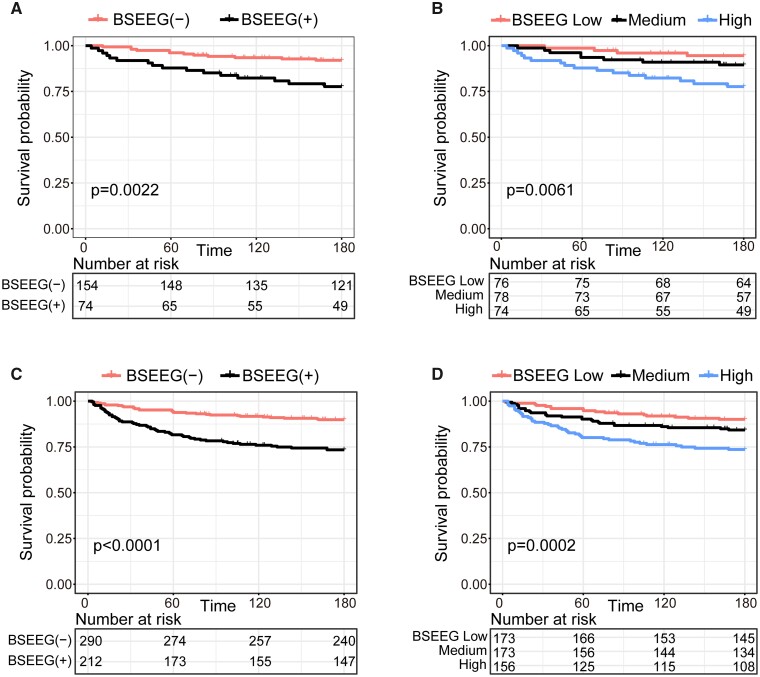
We first analysed 228 subjects (replication cohort) for a replication study to confirm the utility of BSEEG in prediction of mortality. Their demographic characteristics are shown in Supplementary Table 1. Age and CCI were significantly higher in patients with delirium, dementia and BSEEG-positive groups, compared to each of the control groups (Supplementary Table 1). The proportion of female subjects was significantly higher in patients with dementia compared to the control group (Supplementary Table 1). The unadjusted mortality in 180 days in the BSEEG-positive group was higher than that of the BSEEG-negative group (Fig. 1A). When the patients were divided into three categories to become approximately equal sample sizes with BSEEG low, medium and high based on BSEEG scores, their mortality showed a dose-dependent increase based on the BSEEG categories (Fig. 1B). According to the result of the Cox proportional hazard model adjusted for age, sex, CCI and delirium, BSEEG score was shown to be a significant predictive factor for mortality in 180 days (95% confidence interval, 1.33–6.00; P = 0.007) (Supplementary Table 2). In addition, age and CCI were significant predictors for mortality (Supplementary Table 2).
Figure 1.
Survival curve in 180 days based on the BSEEG category. (A) Two BSEEG categories in 228 subjects (replication cohort). (B) Three BSEEG categories in 228 subjects (replication cohort). (C) Two BSEEG categories in 502 subjects (discovery and replication cohorts). (D) Three BSEEG categories in 502 subjects (discovery and replication cohorts). BSEEG, bispectral electroencephalography; B (-), BSEEG-negative; B (+), BSEEG-positive.
Next, we analysed 502 subjects (discovery and replication cohorts). Their demographic characteristics are shown in Table 1. The subjects were recruited from the following units: 273 (54.4%) were general medicine, 141 (28.1%) were orthopaedics, 66 (13.2%) were emergency department and 22 (4.4%) were intensive care unit. The average number of the BSEEG recordings was 4.1 ± 3.3. Age and CCI were significantly higher in patients with delirium, dementia and in BSEEG-positive groups, compared to respective control groups (Table 1). The unadjusted mortality in 180 days in the BSEEG-positive group was higher than that of the BSEEG-negative group (Fig. 1C). Moreover, when the patients were divided into three categories to become approximately equal sample sizes with BSEEG low, medium and high based on the BSEEG scores, their mortality showed a score-dependent increase based on the BSEEG categories (Fig. 1D). According to results of the Cox proportional hazard model adjusted for age, sex, CCI and delirium, BSEEG showed significant predictive factor for mortality in 180 days (95% confidence interval, 1.55–3.82; P < 0.001) (Supplementary Table 3). Age, delirium status and CCI were significant predictors for mortality (Supplementary Table 3).
Table 1.
Demographic characteristic of the discovery and replication cohorts (N = 502)
| Delirium |
Dementia |
Day1 BSEEG |
||||
|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Positive | Negative | |
| n | 168 | 334 | 114 | 388 | 212 | 290 |
| Female, n (%) | 81 (48.2) | 177 (53.0) | 68 (59.6) | 190 (49.0) | 106 (50.0) | 152 (52.4) |
| Age, mean (SD), (year) | 73.5 (9.4)* | 71.6 (9.8) | 76.3 (9.0)* | 71.0 (9.6) | 72.8 (9.0)* | 71.8 (10.2) |
| CCI, mean (SD) | 4.3 (2.8)* | 3.0 (2.9) | 4.3 (3.0)* | 3.1 (2.9) | 3.9 (2.9)* | 3.0 (2.9) |
P < 0.01 vs. Control or Negative.
BSEEG, bispectral electroencephalography; CCI, Charlson Comorbidity Index.
Utility of BSEEG in predicting mortality among patients with and without dementia
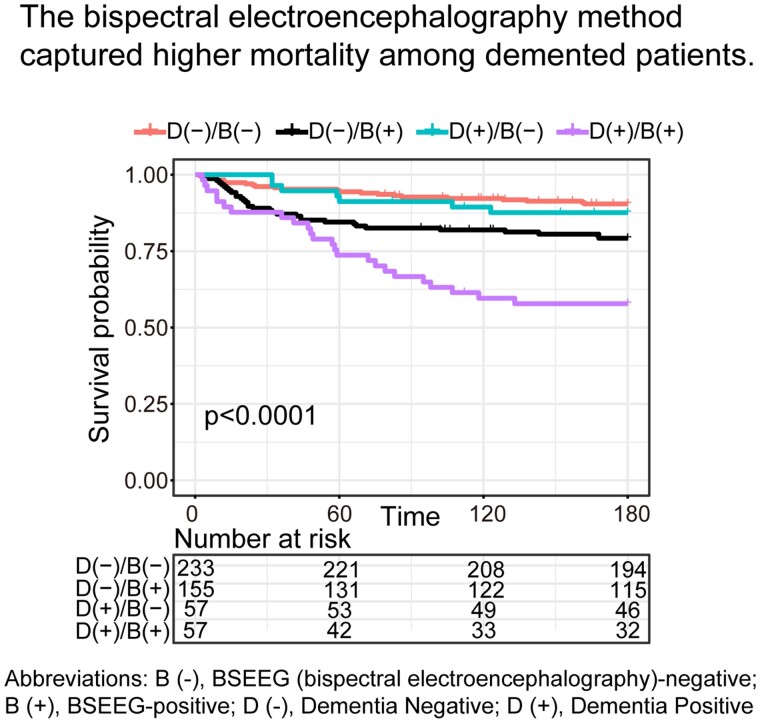
Next, we analysed the total 502 subjects (discovery and replication cohorts) to test the utility of BSEEG for predicting mortality in patients with dementia. When patients with dementia were shown to be BSEEG-positive, their mortality was higher than those with dementia but who were BSEEG-negative (Fig. 2). When dementia was added as a covariate in the Cox proportional hazard model, BSEEG was still shown to be a significant predictive factor for mortality in 180 days (95% confidence interval, 1.55 to 3.82; P < 0.001) (Supplementary Table 4). Similarly, age, delirium and CCI were significant predictors for mortality (Supplementary Table 4).
Figure 2.
Survival curve in 180 days based on the dementia and BSEEG categories. BSEEG, bispectral electroencephalography; B (-), BSEEG-negative; B (+), BSEEG-positive; D (-), Dementia Negative; D (+), Dementia Positive.
Utility of BSEEG in predicting short-term mortality
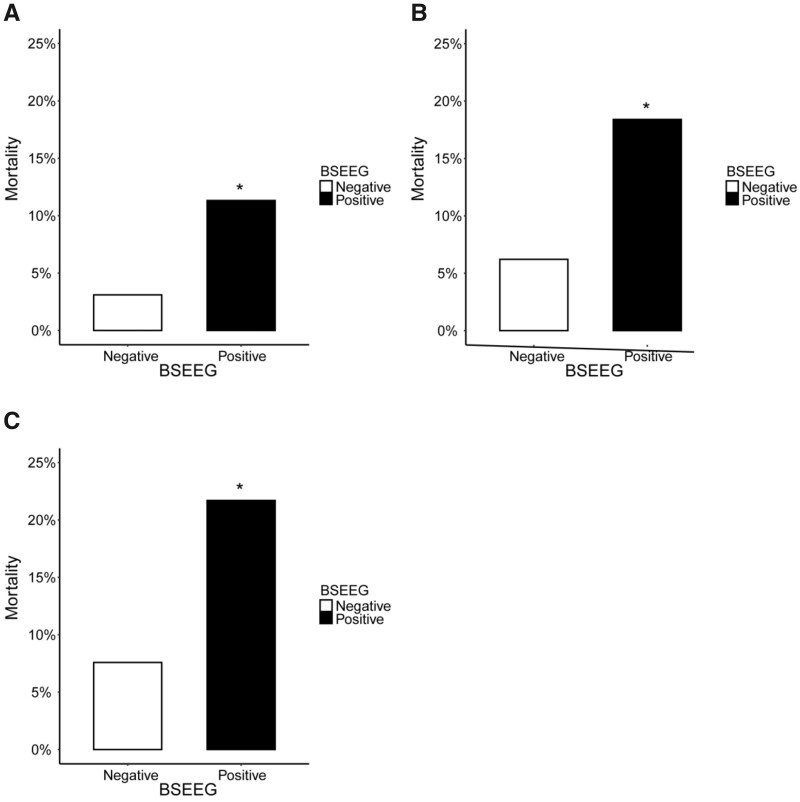
The mortality in 30, 60 and 90 days was compared to test how soon BSEEG can differentiate mortality risk among the total 502 subjects (discovery and replication cohorts). The 30-day mortality in the BSEEG-positive group was significantly higher than that of the BSEEG-negative group (relative risk = 3.65; 95% confidence interval, 1.73–7.69; P < 0.001) (Fig. 3A). Similarly, 60-day mortality in the BSEEG-positive group was significantly higher than that of the BSEEG-negative group (relative risk = 2.96; 95% confidence interval, 1.74–5.03; P < 0.001) (Fig. 3B), as well as 90-day mortality in the BSEEG-positive group compared to the negative group (relative risk = 2.86; 95% confidence interval, 1.78–4.60; P < 0.001) (Fig. 3C).
Figure 3.
Short-term mortality based on the BSEEG category in 502 subjects (discovery and replication cohorts). (A) Thirty days; (B) 60 days; and (C) 90 days. Notes: *Relative risk was significantly higher than those in the BSEEG-negative group. BSEEG, bispectral electroencephalography.
Furthermore, short-term mortalities were analysed to show the difference between patients with and without dementia among the total 502 subjects (discovery and replication cohorts). The 60-day mortality with dementia in the BSEEG-positive group was significantly higher than that of the BSEEG-negative group (relative risk = 3.00; 95% confidence interval, 1.17–7.70; P = 0.025) as well as those without dementia (relative risk = 2.78; 95% confidence interval, 1.46–5.28; P = 0.001) (Supplementary Fig. 1). Similarly, the 90-day mortality in those with dementia in the BSEEG-positive group was significantly higher than those with dementia in the BSEEG-negative group (relative risk = 3.80; 95% confidence interval, 1.52–9.48; P = 0.002) as well as in those without dementia (relative risk = 2.39; 95% confidence interval, 1.35–4.22; P = 0.003) (Supplementary Fig. 1).
Discussion
The present study shows the utility of BSEEG in predicting mortality in an independent cohort by conducting a replication study. Furthermore, the mortality in patients with dementia who showed a high BSEEG score was higher than those with dementia who showed a negative BSEEG score. The result was consistent with our hypothesis that BSEEG score can predict mortality among dementia patients. This is the first study to show the utility of BSEEG score in predicting mortality in dementia patients.
Previously, findings about the usefulness of the BSEEG had been limited to a study of delirium and mortality in 274 elderly inpatients.9 In the present study, we showed the utility of the BSEEG in predicting mortality with an independent cohort and a cohort in an increased sample size. Moreover, a score-dependent increase in mortality as identified by BSEEG score was replicated as shown in a previous cohort.9 As it is important to assess risks of outcomes including mortality in elderly inpatients to optimize intervention and care planning, numerous measures to evaluate the risk of mortality have been developed as shown below. For example, the CCI is used for predicting mortality by evaluating comorbidity.19 Similarly, various measures such as the Multidimensional Prognostic Index,20 the Elixhauser Comorbidity System21 and the single general self-rated health22 are used for predicting mortality. However, these measures mentioned above have the shared limitation of lacking biological basis. In addition to the above measures, the BSEEG score has the potential to be used for predicting mortality as an electrophysiological biomarker. We believe that the BSEEG is sensitive to detect brain signal abnormality with slow-wave characteristic to delirium or inflammatory process in brain, which is sometimes hard to capture in clinical settings. In fact, our group recently developed a pre-clinical model to assess neuroinflammation measured by BSEEG method applied to a mouse in response to systemic inflammation induced by lipopolysaccharides.23 The model showed clear dose-dependent increase of BSEEG proportionate to dose of lipopolysaccharides and the effect was more drastic in aged mouse compared to young mouse.23 We speculate that is why the BSEEG is capable to predict patients’ mortality.
In the present study, the utility of BSEEG for predicting mortality was shown for dementia patients as well. This result suggests that we may be able to predict mortality among dementia patients by using BSEEG score rather than just relying on clinical diagnoses for delirium. Although an appropriate intervention can improve outcomes of patients with delirium,24,25 it is well known that detection of delirium in patients with dementia is challenging.26–29 Therefore, detection of patients with a BSEEG-positive score followed by prompt intervention may improve patient outcomes whether or not they have dementia.
Importantly, there was a significant difference of mortality even in 30 days between BSEEG-positive and BSEEG-negative groups. According to the result of the present study, approximately one in eight who were BSEEG-positive died in 30 days, whereas one in 32 who were BSEEG-negative died in 30 days. It is important to predict short-term outcomes in elderly inpatients because their outcome may be directly related to death. In previous studies, there are significant relationships not only between a long-term mortality longer than a year and delirium,30,31 but also a short-term mortality within several months and delirium.32,33 The present finding suggests that BSEEG may be useful for predicting both a short-term and a long-term mortality in elderly inpatients. This result is consistent with the recent prospective cohort study10 and retrospective cohort study11 showing that clinical electroencephalography slowing is associated with an increased mortality. Furthermore, short-term mortalities and relative risks in BSEEG-positive patients were higher in patients with dementia compared to those without dementia. According to the results of the present study, approximately one in three with a BSEEG-positive score and dementia died in 90 days, whereas one in six with a BSEEG-positive score but without dementia. These results indicate that BSEEG may be useful for patients with dementia to predict short-term mortality.
There are several limitations of this study. First, determination of dementia status relied on chart review. Thus, there might be many cases of over- or under-diagnoses. In addition, we did not evaluate severity of dementia in the present study. A previous study showed that severity of dementia predicts mortality of patients with dementia.34 To overcome this limitation, we need to diagnose dementia with a more detailed approach in future study. Second, the average ages in cases with delirium and dementia, and in the BSEEG-positive group, were higher than those in controls and in the BSEEG-negative group. However, delirium and BSEEG were significant predictors for mortality according to results of the Cox proportional hazards model even after adjusting for age. Lastly, because this study was conducted only in a general hospital located in the Midwest of the USA, the results may not be generalizable. To overcome this limitation, multi-centre research is needed in the future.
Even with these limitations, this study suggests that BSEEG score can predict mortality among elderly patients in general, and even among dementia patients, as soon as 30 days after their hospital admission.
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
This study was funded by research grants from National Science Foundation 1664364.
Competing interests
G.S. is a co-founder of Predelix Medical LLC, and reports U.S. Provisional Patent Application No. 62/829411, titled ‘Prediction of patient outcomes with a novel electroencephalography device’. The other authors report no biomedical financial interests or potential conflicts of interest.
Supplementary Material
Glossary
- BSEEG =
bispectral electroencephalography;
- CCI =
Charlson Comorbidity Index
References
- 1. Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK.. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015;14(8):823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inouye SK, Westendorp RG, Saczynski JS.. Delirium in elderly people. Lancet. 2014;383(9920):911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA.. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: A meta-analysis. JAMA. 2010;304(4):443–451. [DOI] [PubMed] [Google Scholar]
- 4. Jackson TA, Wilson D, Richardson S, Lord JM.. Predicting outcome in older hospital patients with delirium: A systematic literature review. Int J Geriatr Psychiatry. 2016;31(4):392–399. [DOI] [PubMed] [Google Scholar]
- 5. Lee HB, Oldham MA, Sieber FE, Oh ES.. Impact of delirium after hip fracture surgery on one-year mortality in patients with or without dementia: A case of effect modification. Am J Geriatr Psychiatry. 2017;25(3):308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuczmarska A, Ngo LH, Guess J, et al. Detection of delirium in hospitalized older general medicine patients: A comparison of the 3D-CAM and CAM-ICU. J Gen Intern Med. 2016;31(3):297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shinozaki G, Chan AC, Sparr NA, et al. Delirium detection by a novel bispectral electroencephalography device in general hospital. Psychiatry Clin Neurosci. 2018;72(12):856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamanashi T, Kajitani M, Iwata M, et al. Topological data analysis (TDA) enhances bispectral EEG (BSEEG) algorithm for detection of delirium. Sci Rep. 2021;11(1):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shinozaki G, Bormann NL, Chan AC, et al. Identification of patients with high mortality risk and prediction of outcomes in delirium by bispectral EEG. J Clin Psychiatry. 2019;80(5):19m12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kimchi EY, Neelagiri A, Whitt W, et al. Clinical EEG slowing correlates with delirium severity and predicts poor clinical outcomes. Neurology. 2019;93(13):e1260–e1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wanzek R, Bormann N, Shabbir Y, Saito T, Yamada T, Shinozaki G.. Increased mortality in patients with standard EEG findings of ‘diffuse slowing’. Ann Clin Psychiatry. 2021;33(1):e14–e21. [DOI] [PubMed] [Google Scholar]
- 12. Young J, Inouye SK.. Delirium in older people. BMJ. 2007;334(7598):842–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703–2710. [DOI] [PubMed] [Google Scholar]
- 14. Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: Validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370–1379. [DOI] [PubMed] [Google Scholar]
- 15. Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N.. Validation of the Delirium Rating Scale-revised-98: Comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–242. [DOI] [PubMed] [Google Scholar]
- 16. Schuurmans MJ, Shortridge-Baggett LM, Duursma SA.. The Delirium Observation Screening Scale: A screening instrument for delirium. Res Theory Nurs Pract. 2003;17(1):31–50. [DOI] [PubMed] [Google Scholar]
- 17. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 18. R Core Team. R: A language and environment for statistical computing. 2019. https://www.R-project.org/. Accessed 13 February 2019.
- 19. Charlson M, Szatrowski TP, Peterson J, Gold J.. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. [DOI] [PubMed] [Google Scholar]
- 20. Pilotto A, Ferrucci L, Franceschi M, et al. Development and validation of a Multidimensional Prognostic Index for one-year mortality from comprehensive geriatric assessment in hospitalized older patients. Rejuvenation Res. 2008;11(1):151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ.. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633. [DOI] [PubMed] [Google Scholar]
- 22. Idler EL, Russell LB, Davis D.. Survival, functional limitations, and self-rated health in the NHANES I Epidemiologic Follow-up Study, 1992. First National Health and Nutrition Examination Survey. Am J Epidemiol. 2000;152(9):874–883. [DOI] [PubMed] [Google Scholar]
- 23. Yamanashi T, Malicoat JR, Steffen KT, et al. Bispectral EEG (BSEEG) quantifying neuro-inflammation in mice induced by systemic inflammation: A potential mouse model of delirium. J Psychiatr Res. 2021;133:205–211. [DOI] [PubMed] [Google Scholar]
- 24. Lundström M, Edlund A, Karlsson S, Brännström B, Bucht G, Gustafson Y.. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622–628. [DOI] [PubMed] [Google Scholar]
- 25. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: A randomized intervention study. Aging Clin Exp Res. 2007;19(3):178–186. [DOI] [PubMed] [Google Scholar]
- 26. Fick DM, Steis MR, Waller JL, Inouye SK.. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J Hosp Med. 2013;8(9):500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leonard M, McInerney S, McFarland J, et al. Comparison of cognitive and neuropsychiatric profiles in hospitalised elderly medical patients with delirium, dementia and comorbid delirium-dementia. BMJ Open. 2016;6(3):e009212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meagher DJ, Leonard M, Donnelly S, Conroy M, Saunders J, Trzepacz PT.. A comparison of neuropsychiatric and cognitive profiles in delirium, dementia, comorbid delirium-dementia and cognitively intact controls. J Neurol Neurosurg Psychiatry. 2010;81(8):876–881. [DOI] [PubMed] [Google Scholar]
- 29. Morandi A, Davis D, Bellelli G, Arora RC, et al. The diagnosis of delirium superimposed on dementia: An emerging challenge. J Am Med Dir Assoc. 2017;18(1):12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kiely DK, Marcantonio ER, Inouye SK, et al. Persistent delirium predicts greater mortality. J Am Geriatr Soc. 2009;57(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E.. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457–463. [DOI] [PubMed] [Google Scholar]
- 32. Gonzalez M, Martinez G, Calderon J, et al. Impact of delirium on short-term mortality in elderly inpatients: A prospective cohort study. Psychosomatics. 2009;50(3):234–238. [DOI] [PubMed] [Google Scholar]
- 33. van den Boogaard M, Schoonhoven L, van der Hoeven JG, van Achterberg T, Pickkers P.. Incidence and short-term consequences of delirium in critically ill patients: A prospective observational cohort study. Int J Nurs Stud. 2012;49(7):775–783. [DOI] [PubMed] [Google Scholar]
- 34. Connors MH, Ames D, Boundy K, et al. Predictors of mortality in dementia: The PRIME study. J Alzheimers Dis. 2016;52(3):967–974. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be shared by the corresponding author upon request with a reasonable institutional approval.