Abstract
Background
Recurrent Clostridioides difficile infection (rCDI) is associated with loss of microbial diversity and microbe-derived secondary bile acids, which inhibit C. difficile germination and growth. SER-109, an investigational microbiome drug of donor-derived, purified spores, reduced recurrence in a dose-ranging, phase (P) 1 study in subjects with multiple rCDIs.
Methods
In a P2 double-blind trial, subjects with clinical resolution on standard-of-care antibiotics were stratified by age (< or ≥65 years) and randomized 2:1 to single-dose SER-109 or placebo. Subjects were diagnosed at study entry by PCR or toxin testing. Safety, C. difficile–positive diarrhea through week 8, SER-109 engraftment, and bile acid changes were assessed.
Results
89 subjects enrolled (67% female; 80.9% diagnosed by PCR). rCDI rates were lower in the SER-109 arm than placebo (44.1% vs 53.3%) but did not meet statistical significance. In a preplanned analysis, rates were reduced among subjects ≥65 years (45.2% vs 80%, respectively; RR, 1.77; 95% CI, 1.11–2.81), while the <65 group showed no benefit. Early engraftment of SER-109 was associated with nonrecurrence (P < .05) and increased secondary bile acid concentrations (P < .0001). Whole-metagenomic sequencing from this study and the P1 study revealed previously unappreciated dose-dependent engraftment kinetics and confirmed an association between early engraftment and nonrecurrence. Engraftment kinetics suggest that P2 dosing was suboptimal. Adverse events were generally mild to moderate in severity.
Conclusions
Early SER-109 engraftment was associated with reduced CDI recurrence and favorable safety was observed. A higher dose of SER-109 and requirements for toxin testing were implemented in the current P3 trial.
Clinical Trials Registration
NCT02437487, https://clinicaltrials.gov/ct2/show/NCT02437487?term=SER-109&draw= 2&rank=4.
Keywords: Clostridioides difficile infection, microbiome, dysbiosis, fecal microbiota transplantation, Clostridium difficile diagnostics
In a phase 2 trial, SER-109, an investigational microbiome drug, did not reduce rates of recurrent CDI, despite a previously successful open-label study. Key contributing factors, which led to a redesign of the currently enrolling phase 3 trial, are highlighted.
(See the Editorial Commentary by Bensan Young on pages 2141–3.)
Recurrence of Clostridioides difficile infection (CDI) occurs in 40–60% of patients with prior infection, with most recurrences following within 3 weeks of antibiotic discontinuation [1, 2]. Antibiotic exposure leads to low microbial diversity (ie, dysbiosis), impairing colonization resistance, a major function of the healthy microbiome [3, 4]. Although antibiotics kill toxin-producing C. difficile vegetative bacteria, they have no impact on dormant spores, which germinate when dysbiosis persists [4].
Firmicutes and Bacteroidetes are 2 dominant phyla in the gastrointestinal microbiome, while proinflammatory Proteobacteria, comprise a limited fraction of healthy microbiota [5]. Depletion of Firmicutes species, and their metabolites, facilitates CDI recurrence. Primary and secondary bile acids (BAs) are microbe-associated metabolites that play a key role in the 2-phase life cycle of C. difficile (Supplementary Figure 1). Primary BAs are synthesized in the liver and secreted into the intestine where they are converted into secondary BAs by commensal microbes, including spore-forming Firmicutes. In vitro, primary BAs promote C. difficile spore germination, while certain secondary BAs inhibit vegetative growth [6, 7]. Depletion of Firmicutes leads to an increase in the relative concentration of primary versus secondary BAs, supporting favorable conditions for spore germination, bacterial replication, and toxin production [8]. Although antibiotics kill vegetative C. difficile, microbiome therapeutics may promote a durable response by restoring microbial diversity and functions that prevent spore germination and vegetative growth.
Studies of fecal microbiota transplantation (FMT) in patients with recurrent CDI (rCDI) demonstrate a “proof-of-concept” that replenishment of key microbes, including the spore-forming Firmicutes bacteria (eg, Ruminococcaceae, Lachnospiraceae, and beneficial Clostridiaceae), is associated with clinical resolution [9] and a relative increase in secondary BA concentrations [6]. However, the paucity of placebo-controlled studies leads to uncertain estimates of FMT efficacy, and optimal dosing and administration routes are unknown [10–13]. Safety concerns have been amplified by recent reports of transmission of bacterial infections and emerging viral infections (eg, coronavirus disease 2019 [COVID-19]), highlighting the need for mandatory donor screening guidelines, Food and Drug Administration (FDA)–regulated FMT products, and development of microbiome therapeutics with manufacturing steps that inactivate pathogens to mitigate risk [14–17].
While Bacteroidetes and Proteobacteria are not spore-formers, a large number of Firmicutes are spore-formers, a characteristic that we exploited in our drug development process.
Based on the observation that Firmicutes play an important role in colonization resistance, we developed SER-109, an investigational microbiome drug consisting of a consortium of bacterial spores from healthy donors. Our nonclinical studies comparing the efficacy of fecal suspensions with matched spore fractions supported the use of Firmicutes to reduce CDI recurrence (Lombardo M-J, Litcofsky K, Cook D, Henn M, 2012, unpublished data). We developed a manufacturing process to reduce potential pathogens and fecal matter and debris, while enriching for spore-forming Firmicutes. Purified spores are resistant to gastric acid, enabling oral formulation. Metabolically active bacteria that germinate can establish residence in the gastrointestinal tract, a process termed “engraftment.” We hypothesized that engraftment of SER-109 dose-species leads to a succession of compositional and functional changes in the microbiome that reduce the risk of CDI recurrence.
In a 2-cohort open-label phase 1 dose-ranging study, SER-109 led to clinical resolution in 26 of 30 (86.7%) subjects with rCDI. The same response rates were observed in the dose-ranging (1 × 107–1010 spores for 2 days) and fixed-dose cohorts (single dose of 1.1 × 108 spores) and the magnitude of SER-109 engraftment (using 16S ribosomal RNA [rRNA] gene sequencing) was similar between the 2 cohorts at the 8-week endpoint [18]. Based on these outcomes, we conducted a phase 2 trial evaluating the same fixed dose (108) of SER-109. Herein, we report on the efficacy, safety, and engraftment analyses and change in BAs in the phase 2 study, which support the biologic activity of SER-109. We complement these data with an analysis of engraftment from the prior phase 1 study to enhance insights into the clinical outcomes, which guided our phase 3 trial design.
METHODS
Study Subjects
This multicenter, randomized, double-blind, placebo-controlled phase 2 study evaluated the safety and efficacy of SER-109 versus placebo to reduce rCDI. The protocol was approved by institutional review boards and conducted at 40 US sites.
Eligible adults aged 18 years or older with 3 or more CDI episodes within 9 months provided written informed consent. The qualifying episode was defined as follows: (1) more than 3 stools/day for 2 or more consecutive days, (2) a positive C. difficile stool test by either polymerase chain reaction (PCR) or toxin testing (by enzyme immunoassay), and (3) clinical response to standard-of-care antibiotics (ie, 10–21 days of vancomycin or fidaxomicin). See the Supplementary Materials for inclusion/exclusion criteria.
Donor Screening and Preparation of SER-109
Our donor screening and manufacturing protocols have been reviewed by the FDA and are consistent with their published considerations [19]. Before donating stool, donors underwent a medical history, physical examination, laboratory testing (ie, chemistry, hematology, urinalysis), and viral, parasite, and bacterial pathogen testing from blood and fecal samples. Donors were re-screened before material was released for manufacturing.
Stool donations underwent Good Manufacturing Process–compliant manufacturing steps including clearance of vegetative bacteria, fungi, parasites, and viruses via solvent treatments and purification steps (Supplementary Table 1). Nonproduct stool matter was reduced by over 200-fold to less than 5 mg/dose, with final product purity of approximately 10–20% spores by weight. Three drug lots were evaluated in this study; each lot was derived from donations from a single donor. Genera identified within the lots are found in Supplementary Table 2. A total of 111 species (mean: 86 species per lot) were observed by direct whole-metagenomics shotgun sequencing (WMS). Seventy-four percent of species were shared across 2 or more lots and 59% were observed in all lots.
SER-109 potency was assessed by 2 complementary quantification methods by enumerating the number of (1) spores (SporQ) by calculating the concentration of dipicolinic acid in the spore capsid and (2) the viable colony-forming units generated after applying germinant. Hereafter, potency of SER-109 will be measured via SporQ and noted as “spores.”
Study Protocol
Subjects completed antibiotics 2–4 days prior to dosing. One day prior to dosing, subjects underwent a bowel preparation (10 ounces magnesium citrate) to minimize residual antibiotic and fasted overnight. Subjects were stratified by age (<65 years, ≥65 years) and randomly assigned 2:1 to an observed dose of 4 capsules of SER-109 (1 × 108 spores) or placebo.
Study Outcomes
Diarrhea was evaluated day 1 postdosing through week 8 or an early termination visit. Recurrence was defined as 3 or more unformed stools per day for 2 consecutive days, with a positive C. difficile stool test and an investigator decision to treat. Diagnostic testing included either (1) glutamate dehydrogenase antigen followed by either a PCR or toxin enzyme immunoassay (EIA) or (2) PCR alone.
Subjects with rCDI were eligible for screening for an open-label extension study; those who elected not to enroll received antibiotics and were followed for safety assessments through 24 weeks posttreatment.
Adverse events (AEs) were monitored from randomization through 12 weeks and serious AEs through 24 weeks. Safety was assessed by history, physical examination, and laboratory testing.
Clinical Statistical Analysis
The primary outcome measure was the relative risk (RR) of CDI recurrence among subjects randomized to placebo versus SER-109 up to 8 weeks after treatment, analyzed using the Mantel-Haenszel method and stratified by age [20]. The corresponding 2-sided 95% confidence interval (CI) for the common RR was based on the Greenland and Robins variance estimate [21]. To determine if SER-109 was statistically superior to placebo, the lower bound of the CI was compared to the null value of 1. Efficacy analyses were conducted in the intention-to-treat (ITT) population; safety analyses were conducted in the safety population. The ITT population included all subjects randomized, and the safety population included all who received the study drug. Time to CDI recurrence was analyzed per the Kaplan-Meier method [22].
Microbiome and Metabolomic Post Hoc Analyses
Whole-metagenomics shotgun sequencing and liquid chromatography/mass spectrometry analyses were conducted post hoc on phase 1 and 2 datasets to evaluate the impact of SER-109 on clinical outcome.
Whole-Metagenomics Sequencing of Subject Stool and Drug Lots
Bacterial genomic DNA was extracted from stool and drug lot samples using the MagAttract PowerSoil DNA Kit (Qiagen), and WMS was performed (Illumina HiSeq; Diversign) to a target depth of 5 gigabases (Gb) for subject samples and SER-109 drug product lots (see Supplementary Materials for further details).
Microbe taxonomic profiling was performed using a custom update of MetaPhlAn2 software [23, 24] that included additional taxonomic markers from genomes of spore-forming species from public databases and Seres strain isolates. Data were subsampled to 470 000 mapped reads to ensure a similar limit of detection across samples.
Microbiome and Metabolomic Analyses
The output from the MetaPhlAn2 taxonomic profiling pipeline is referred to as the “microbiome profile” of a sample. SER-109 dose-species were defined as the set of spore-forming Firmicutes species identified by WMS analysis of the drug lots.
We quantified the number of SER-109 dose-species in the fecal samples of SER-109 subjects as compared with placebo subjects as a measure of SER-109 engraftment. We quantified the abundance of Bacteroidetes as a measure of the impact of SER-109 treatment on non–dose-species abundance. Statistical tests for comparisons across treatment and outcome groups included 2-sided Mann-Whitney U test and Spearman’s correlation and were conducted as noted in the Results section [25, 26].
Bile acid metabolomics were performed at Metabolon, Inc (Durham, NC). Details on sample handling and quantification are found in the Supplementary Materials.
RESULTS
Patient Demographics
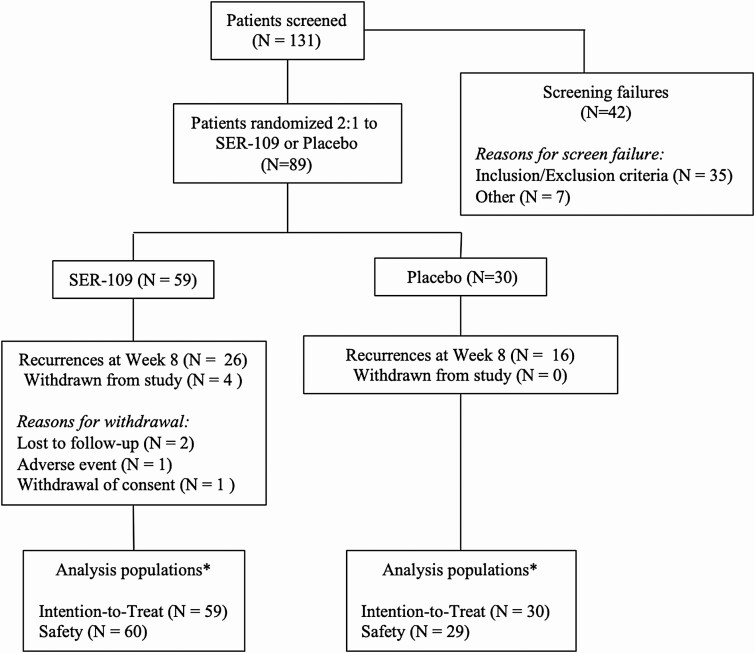
Recruitment occurred between May 2015 and October 2016; 89 of 131 (67.9%) subjects screened were randomized (59: SER-109; 30: placebo). The most common reasons for screen failures were an inadequate antibiotic response and fewer than 3 documented CDI episodes within 9 months (Figure 1). The baseline characteristics in the SER-109 and placebo arms were well balanced as all comparisons were nonsignificant (Table 1). Of the study candidates who were enrolled based on a positive C. difficile test, 19.1% were tested with an EIA toxin assay (Table 1).
Figure 1.
CONSORT diagram. One subject randomized to placebo was dosed with SER-109; this subject was analyzed with the placebo intention-to-treat population in all efficacy analyses* and with the SER-109 safety population in all safety analyses* (see Supplementary Materials for further details). Abbreviation: CONSORT, Consolidated Standards of Reporting Trials.
Table 1.
Demographics and Baseline Disease Characteristics of Subjects in the Intent-to-Treat Population
| Characteristics | SER-109 (n = 59) | Placebo (n = 30) | Total (n = 89) |
|---|---|---|---|
| Age class | |||
| <65 years | 28 (47.5) | 15 (50.0) | 43 (48.3) |
| ≥65 years | 31 (52.5) | 15 (50.0) | 46 (51.7) |
| Female sex | 40 (67.8) | 20 (66.7) | 60 (67.4) |
| White race | 54 (91.5) | 29 (96.7) | 83 (93.3) |
| Non-Hispanic/Latino ethnicity | 57 (96.6) | 28 (93.3) | 85 (95.5) |
| Number of previous CDI episodes (inclusive of the qualifying episode) | |||
| 3 | 28 (47.5) | 20 (66.7) | 48 (53.9) |
| 4 | 21 (35.6) | 5 (16.7) | 26 (29.2) |
| ≥5 | 10 (16.9) | 5 (16.7) | 15 (16.9) |
| Antibiotic used for qualifying episode | |||
| Vancomycin | 47 (79.7) | 23 (76.7) | 70 (78.7) |
| Fidaxomicin | 12 (20.3) | 7 (23.3) | 19 (21.3) |
| Diagnostic test used at study entry | |||
| PCR+ | 47 (79.7) | 25 (83.3) | 72 (80.9) |
| GDH+/toxin+ | 12 (20.3) | 5 (16.7) | 17 (19.1) |
| PPI use at baseline | 19 (32.2) | 8 (26.7) | 27 (30.3) |
Data are presented as n (%). The placebo ITT population includes 1 subject randomized to placebo who was administered SER-109.
Abbreviations: CDI, Clostridioides difficile infection; GDH, glutamate dehydrogenase; ITT, intent-to-treat; PCR, polymerase chain reaction; PPI, proton pump inhibitor.
Efficacy of SER-109
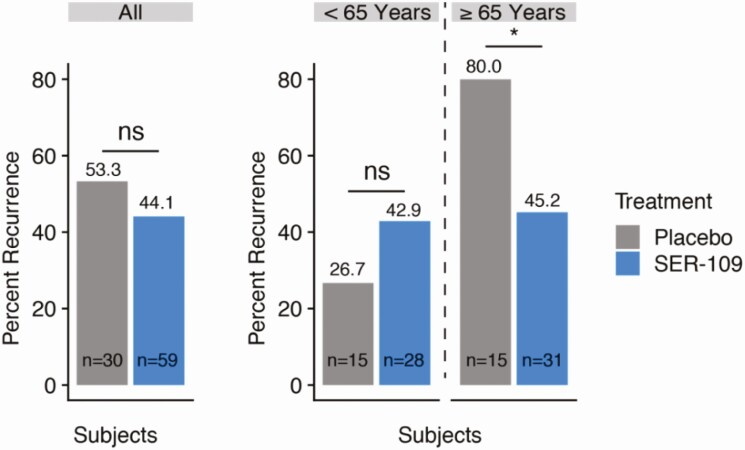
In the overall population, there was no significant difference in CDI recurrence rates between SER-109 or placebo subjects (44.1% vs 53.3%; RR, 1.2; 95% CI, .8–1.9) (Figure 2). However, the primary endpoint by age stratum showed that SER-109 significantly reduced recurrence, compared with placebo, among those aged 65 years or older (45.2% vs 80%, respectively; RR, 1.8; 95% CI, 1.1–2.8). In contrast, among subjects younger than 65 years, no significant difference in CDI recurrence rates were observed in SER-109 subjects compared with placebo (42.9% vs 26.7%, respectively; RR, .62; 95% CI, .24–1.6).
Figure 2.
Rates of recurrence of CDI within 8 weeks of study drug treatment in the ITT population of the phase 2 population. Among all study subjects, SER-109 was not associated with a statistically significant reduction in risk of recurrence. In an age-based subgroup analysis, SER-109 was associated with a significant reduction in recurrence among subjects aged ≥65 years (Mantel-Haenszel test: RR, 1.77; *95% CI, 1.1–2.8) but not in subjects <age 65. Abbreviations: CDI, Clostridioides difficile infection; CI, confidence interval; ns, nonsignificant; RR, relative risk.
Efficacy was similar across drug lots with overlap of 95% CIs; however, the trial was not powered for this analysis. An analysis of outcomes by diagnostic test was uninformative due to the low numbers of subjects tested with EIA toxin (Supplementary Table 3). Time to recurrence was not different, with 25% of subjects recurring in 11 or 12 days in the SER-109 versus placebo groups, respectively. Notably, 50% of recurrences occurred within 11 days.
Safety
SER-109 was generally well tolerated. Adverse events occurred in 46 of 60 (76.7%) subjects on SER-109 and 20 of 29 (69.0%) subjects on placebo; AEs were generally mild to moderate in severity (Table 2). Six subjects (10.0%) on SER-109 experienced a severe AE; none were considered related to study drug. Gastrointestinal disorders were the most commonly reported but did not differ significantly by treatment arm (55.0% SER-109 vs 44.8% placebo; P = .44).
Table 2.
Summary of Adverse Events
| AE Categories | SER-109 (n = 60) | Placebo (n = 29) |
|---|---|---|
| Subjects with AEsa | 46 (76.7) | 20 (69.0) |
| Subjects with mild AEs | 24 (40.0) | 11 (37.9) |
| Subjects with moderate AEs | 16 (26.7) | 9 (31.0) |
| Subjects with severe AEsb | 6 (10.0) | 0 |
| Subjects with study drug–related or possibly related AEs | 11 (18.3) | 4 (13.8) |
| Subjects with serious AEs | 9 (15.0) | 3 (10.3) |
| Subjects with serious AEs related or possibly related to study drug | 0 | 0 |
| Number of subjects with AEs leading to death | 1 (1.7) | 0 |
Data are presented as n (%). SER-109 safety group includes 1 subject randomized to placebo and administered SER-109; this subject was included in the placebo ITT population for efficacy analysis. Abbreviations: AE, adverse event; ITT, intent-to-treat.
aReported AEs occurred following initiation of study treatment with SER-109 or placebo.
bSevere AEs included a single subject each with severe diarrhea, deep vein thrombosis, and a drug overdose; 2 subjects experienced abdominal pain; 1 subject had multiple severe AEs, including abdominal and flank pain, constipation, pelvic mass and deep vein thrombosis, chest pain, and agitation. This subject subsequently died with a diagnosis of metastatic non–small cell lung cancer, which was deemed by the investigator to be a serious AE unrelated to the drug.
Overall, 16.9% of subjects experienced an AE considered by the investigator to be related or possibly related to the study drug, including 18.3% on SER-109 and 13.8% on placebo.
Twelve of 89 subjects (13.5%) experienced a serious AE: 9 of 60 (15.0%) subjects who received SER-109 and 3 of 29 (10.3%) who received placebo. None were considered treatment related.
SER-109 Engraftment and Clinical Outcome
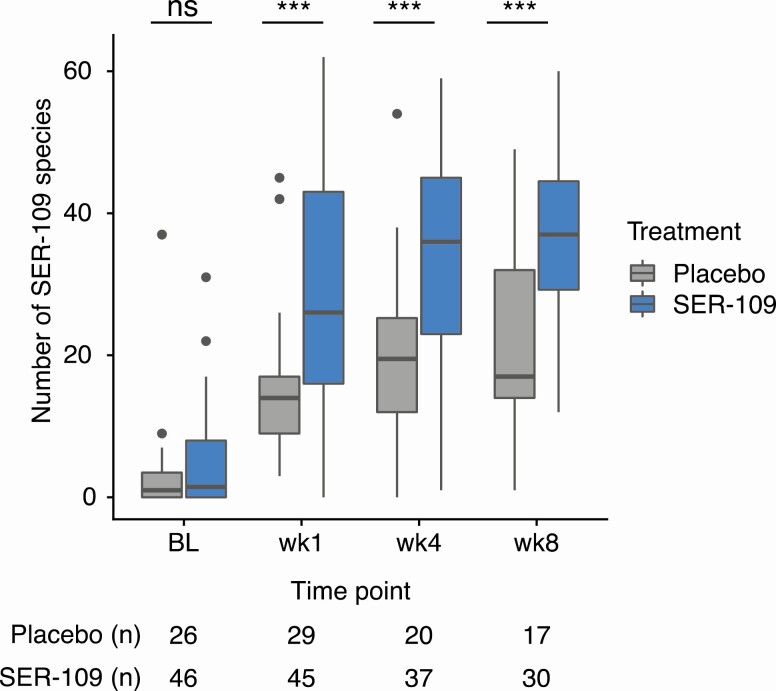
We assessed engraftment by comparing the number of dose-species in stool samples at 3 time points. As expected, minimal SER-109 dose-species were detected at baseline (ie, following cessation of antibiotic) in either treatment arm. As early as week 1 following dosing, subjects receiving SER-109 had significantly more dose-species than those on placebo; this response was durable through 8 weeks (P < .001 for all comparisons; Mann-Whitney U test) (Figure 3). Engraftment at week 1 did not vary significantly by drug lot received (P = .34, Kruskal-Wallis test). We evaluated the relative abundance of Bacteroidetes as a measure of the impact of SER-109 on recovery of non–dose-species and observed significantly greater relative abundance of these species at week 8 in subjects treated with SER-109 compared with placebo (P = .04) (Supplementary Figure 2).
Figure 3.
Number of SER-109 species stratified by time point and treatment in the phase 2 study. In the phase 2 study, subjects receiving treatment had significantly more SER-109 species than those receiving placebo at weeks 1, 4, and 8 posttreatment (P < .001, all comparisons; Mann-Whitney U test). At pretreatment baseline, SER-109 diversity was not significantly different between subjects receiving placebo or SER-109 (Mann-Whitney U test). Boxplots display the median (horizontal line), 25th and 75th percentiles of distribution (box edges), range of nonoutlier observations (whiskers), and outlier observations (dots; >1.5 times the interquartile range). Sample sizes are shown below the x axis. ***P < .001. Abbreviations: BL, baseline; ns, nonsignificant; wk, week.
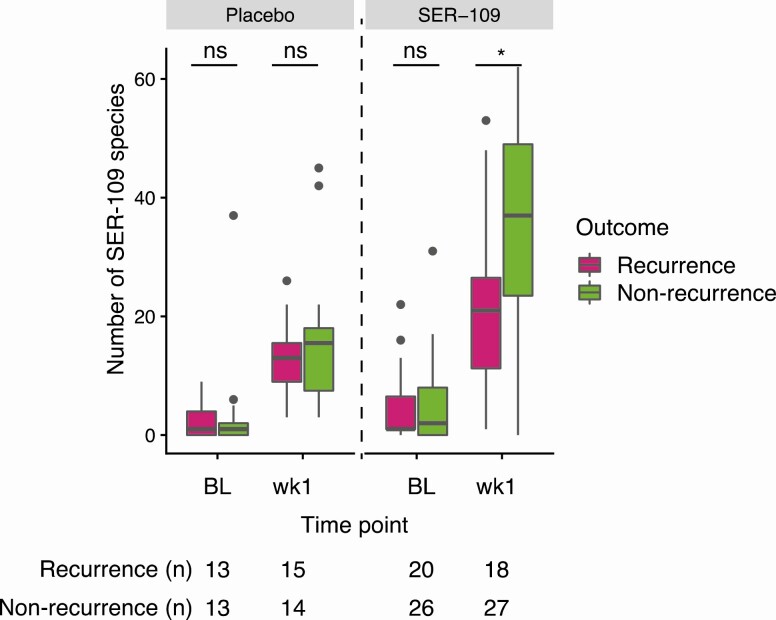
We next evaluated whether the degree of engraftment differed by clinical outcome. Since 50% of recurrences were observed by day 11, we compared dose-species diversity at baseline and week 1 by clinical outcome. Before SER-109 treatment, dose-species diversity was not associated with outcome in either treatment group (Mann-Whitney U test). At week 1, SER-109–treated subjects with nonrecurrence had significantly more dose-species than those who did experience a recurrence (P < .05, Mann-Whitney U test) (Figure 4). This association was not observed in placebo recipients at week 1. Although SER-109 was associated with a significant reduction in recurrence among subjects aged 65 years or older, age had no impact on the magnitude of engraftment (Supplementary Figure 3).
Figure 4.
Number of SER-109 species stratified by outcome in the phase 2 study. Nonrecurrent subjects had significantly more SER-109 species than recurrent subjects within the treatment arm 1 week postdosing (wk1; P < .05, Mann-Whitney U test) but not at baseline (Mann-Whitney U test). SER-109 species diversity was not significantly different for subjects receiving placebo at either baseline or 1 week posttreatment (Mann-Whitney U test). Boxplots display the median (horizontal line), 25th and 75th percentiles of distribution (box edges), range of nonoutlier observations (whiskers), and outlier observations (dots; >1.5 times the interquartile range). Sample sizes are shown below the x axis, *P < .05. Abbreviations: BL, baseline; ns, nonsignificant; wk1, week 1.
SER-109 Engraftment and Secondary Bile Acids
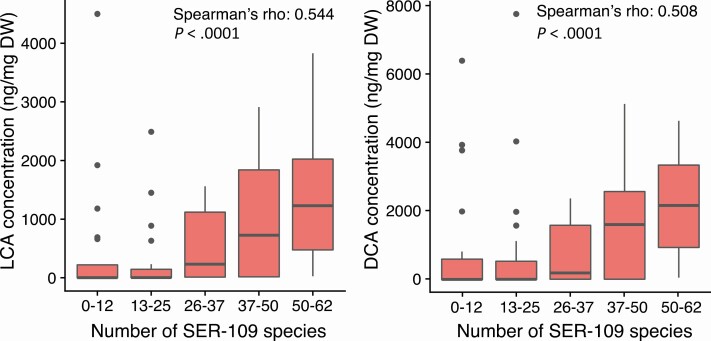
To understand the association of SER-109 engraftment with nonrecurrence, we evaluated the relationship between engraftment and the abundance of secondary BAs, previously shown to inhibit C. difficile germination [27]. At week 1, there was a significant positive correlation between the number of SER-109 species and the abundance of secondary BAs lithocholic acid (LCA) and deoxycholic acid (DCA), as shown in Figure 5 (Spearman correlation, P < .0001 for both comparisons). In subjects receiving SER-109, DCA and LCA levels were higher in subjects with nonrecurrent CDI compared with subjects who experienced recurrence before week 8; however, these observations were not significant (P = .08 and .10, respectively; Mann-Whitney U test).
Figure 5.
Relationship of the engraftment of SER-109 species and concentration of secondary bile acids in the phase 2 study. In the phase 2 study, the number of SER-109 species, assessed in both placebo and SER-109 subjects at week 1, was significantly correlated with the concentration (ng/mg DW of lyophilized samples) of secondary bile acids LCA and DCA. Spearman correlation significance and ρ are shown for each comparison. In the boxplots shown, samples were binned by number of dose-species detected. The concentration of LCA and DCA is shown on the y axis. Boxplots display the median (horizontal line), 25th and 75th percentiles of distribution (box edges), range of nonoutlier observations (whiskers), and outlier observations (dots; >1.5 times the interquartile range). Abbreviations: DCA, deoxycholic acid; DW, dry weight; LCA, lithocholic acid.
Post Hoc Comparison of Phase 1b and Phase 2 Trials
Since the CDI recurrence rate for SER-109 subjects was higher in this phase 2 trial (44%) than in the phase 1 study (13.4%), we evaluated both studies to identify potential factors that may have led to this discordance in clinical outcomes, including the following: (1) demographics (eg, duration of antibiotics prior to SER-109 dosing, number of prior CDI episodes, time from antibiotic cessation to study drug dosing), (2) study design (eg, number of study sites, diagnostics, dose regimen), and (3) drug manufacturing (eg, process changes, potency assays, drug diversity and composition, and drug activity in an animal model). The primary differences we identified in this analysis were related to number of sites (40 vs 4), study design (open-label vs randomized-controlled) and dose regimen (dose-ranging vs fixed-dose). Based on these findings, we focused our subsequent analyses on the impact of the dose regimen on SER-109 engraftment.
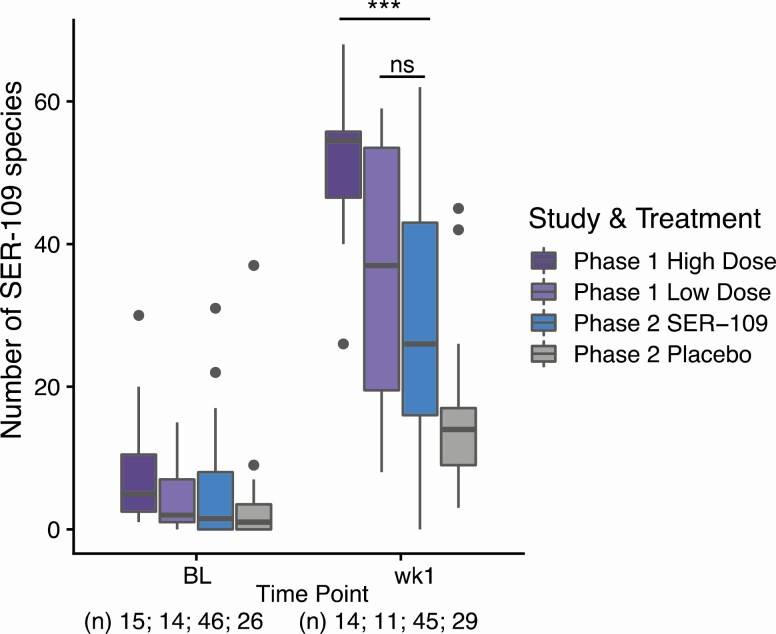
We assessed the magnitude of engraftment at week 1 as a function of dose across 3 groups: phase 1 “low-dose” (3.4 × 107–1.3 × 108 spores), phase 1 “high-dose” (1.5 × 108–2.3 × 1010 spores), and phase 2 “fixed-dose” (1 × 108 spores) (Figure 6). There was no significant difference in the number of SER-109 species between those who received the low-dose or fixed-dose regimens. However, phase 1 subjects who received the high dose had a greater number of SER-109 species by week 1 than the other 2 dosing cohorts, consistent with a dose-dependent response. This result is consistent, regardless of whether the SporQ or the spore colony-forming units methodology is used for quantifying SER-109 potency (Supplementary Figure 4).
Figure 6.
Relationship of engraftment of SER-109 species to dose administered in the phase 1 and phase 2 studies. Subjects receiving the high dose, as defined by SporQ (see Methods), in the open-label phase 1 dose-ranging study (SERES- 001) had significantly more SER-109 species than subjects in the treatment arm of the phase 2 study (SERES-004), who received a fixed dose (wk1; P < .0001, Mann-Whitney U test). The number of SER-109 species was not significantly different between subjects receiving the low dose in the phase 1 trial and subjects receiving the fixed dose in the phase 2 trial (Mann-Whitney U test). The number of SER-109 species stratified by treatment and time point is shown; dots represent outliers. Sample sizes are shown below the x axis. ***P < .0001. Abbreviations: BL, baseline; ns, nonsignificant; wk1, week 1.
DISCUSSION
We propose that sustained clinical resolution of CDI requires a new 2-pronged treatment approach: antibiotics to kill vegetative C. difficile bacteria followed by a microbiome therapeutic to prevent spore germination and rapidly restore colonization resistance. SER-109 was developed to provide a scaffolding of Firmicutes bacteria that lead to an ecologic succession of compositional and functional changes in the microbiome, which reduce the risk of CDI recurrence after antibiotic-mediated disruption.
The safety profile of SER-109 was similar to placebo with mainly mild or moderate AEs. Commonly reported AEs were gastrointestinal disorders, as reported with FMT [13]. None of the serious AEs were considered drug related by the investigators. This favorable safety profile might be expected given that SER-109 is composed of Firmicutes bacteria, which normally reside within the healthy microbiome. Furthermore, compared to FMT, SER-109 manufacturing and quality-control processes mitigate the risk of transmitting undetected pathogens, beyond donor screening alone (Supplementary Table 1). Viral inactivation is particularly important in light of the emerging epidemic of COVID-19 and the current absence of commercially available diagnostics for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in stool [16].
Potential explanations for failure of SER-109 to achieve a significant therapeutic effect in the overall phase 2 study population were 2-fold: underdosing of SER-109 and use of PCR testing in most subjects.
From our reanalysis of the phase 1 and 2 trials, we learned that there is a dose-dependent response governing early SER-109 pharmacokinetics, with increased engraftment associated with successful CDI resolution through 8 weeks. In this phase 2 trial, SER-109 was dosed at 1 × 108 spores based on equivalent clinical outcomes and week 8 engraftment measures observed between the phase 1 dosing cohorts. However, our integrated analysis of both trials revealed that (1) engraftment kinetics at week 1 were of greater importance for reducing rCDI than later time points, (2) week 1 engraftment was highly variable in phase 2 subjects, and (3) rapid engraftment was dependent on dose, which was clearly suboptimal in the phase 2 trial. Notably, in our phase 1 study, we observed a shift in the microbiome as early as 4 days postdosing with SER-109 [18]. We hypothesize that rapid engraftment of a microbiome therapeutic may be critical to efficacy since CDI recurrence usually occurs within 1–3 weeks of antibiotic discontinuation, the “window of vulnerability” [28, 29]. Consistent with this hypothesis, in the phase 2 trial, greater engraftment of SER-109 species at week 1 was correlated with reduced CDI rates. This correlation was not previously appreciated due to the use of lower resolution 16S rRNA gene amplicon–based methods in the phase 1 study [18]. These observations highlight the potential importance of early engraftment, when speed is essential in preventing C. difficile spore germination and vegetative outgrowth.
We further sought to understand the impact of SER-109 engraftment on overall microbiome composition. As observed in the phase 1 study, dosing of this purified Firmicutes product also led to changes in non–dose-species, including increased abundance of Bacteroidetes, at the 8-week time point, suggesting that SER-109 can promote broad compositional changes in the microbiome [18]. Reconstitution of Bacteroidetes was not associated with clinical outcome, suggesting it is not the primary driver of clinical resolution.
We hypothesize that SER-109 engraftment leads to important functional changes via key microbe-associated metabolites, which break the vicious cycle of rCDI. In our phase 2 study, we have shown that SER-109 engraftment enhanced microbe-associated conversion of primary to secondary BAs, a process limited to spore-forming Firmicutes. These BA metabolites inhibit spore germination and vegetative growth. Taken together, these data highlight the importance of evaluating microbiome pharmacokinetics (ie, engraftment of SER-109 species) and pharmacodynamics (ie, changes in microbe-associated metabolites and overall microbiome changes) in parallel with assessment of clinical efficacy.
In our phase 2 study, use of PCR testing to determine eligibility and to diagnose recurrence may have attenuated the ability to define a therapeutic effect of SER-109. Infectious Diseases Society of America/Society for Healthcare Epidemiology of America guidelines specifically recommend toxin testing for suspected recurrence since it has superior specificity and positive predictive value for true infection compared to PCR, which cannot differentiate infection from colonization [30, 31]. In addition, prolonged excretion of C. difficile occurs for months in a large proportion of patients with CDI after clinical resolution [32]. Thus, alternative etiologies for diarrheal symptoms can be missed if PCR testing is used without other clinical investigation [33, 34]. The problem of CDI overdiagnosis by PCR has been highlighted in epidemiologic studies, observational clinical studies, and in the clinical trial setting [35–37]. Unfortunately, retesting of stool samples that led to qualification of study candidates was not feasible since those tests were performed at local laboratories, while specimens used for diagnosis of recurrence were limited by sample availability and stability, limiting firm conclusions.
We postulate that PCR had a greater positive predictive value among older subjects since the diagnostic performance of any laboratory test is influenced by the population being sampled [38]. CDI is highly prevalent in the elderly, who are also at increased risk of recurrence [39, 40]. In contrast, other noninfectious etiologies for diarrhea are more prevalent in younger subjects, such as irritable bowel syndrome [41]. The marked difference in CDI risk between the 2 subgroups is well illustrated by the placebo arms, where a high recurrence rate of 80% was observed among those aged 65 years and older, compared with the unexpectedly low recurrence rate of 27% among those younger than 65 years. Interestingly, in a preplanned analysis, subjects 65 years and older had significantly lower rates of CDI recurrence on SER-109 compared with placebo, while those younger than 65 years derived no treatment benefit. We strongly suspect that universal toxin testing would have led to more accurate selection of study candidates, regardless of age, due to its high positive predictive value for true disease.
Toxin testing is required for the SER-109 phase 3 trial (A Phase 3 Multicenter, RandomizEd, Double Blind, Placebo-COntrolled, Parallel-Group Study to Evaluate the Safety, Tolerability, and Efficacy of SER-109 vs. Placebo to Reduce Recurrence of ClOstRidium difficile Infection [CDI] in Adults Who Have Received Antibacterial Drug Treatment for Recurrent CDI [RCDI] [ECOSPOR III]) at study entry and at the time of suspected recurrence for optimal diagnostic accuracy. Notably, no published placebo-controlled FMT trial in rCDI has required toxin testing, which raises concerns about subject selection and estimates of efficacy [13, 42]. At the time of this manuscript submission, more than one-third of subjects entering ECOSPOR III with a history of presumptive rCDI have failed screening due to a negative toxin test, highlighting the importance of appropriate diagnostic methodologies.
Since we do not have certainty about the clinical diagnosis of all subjects, our analysis of compositional and functional changes by outcome may be prone to classification errors. However, the biological activity of SER-109 is directly supported by the relationship between SER-109 engraftment and change in secondary BA concentrations. Additionally, a post hoc analysis of the phase 2 study showed a decreased abundance of antibiotic-resistance genes in association with SER-109 engraftment [43]. These objective measures would not be impacted by CDI misclassification.
In conclusion, we propose that SER-109 expedites microbiome repair during the window of vulnerability, thereby limiting C. difficile spore germination and clinical recurrence. SER-109 engraftment and associated changes in BA concentrations provide insight into the mechanism of action of SER-109, although broader metabolite analyses will be performed when the phase 3 trial is completed. Based on these results, a higher dose was selected for our phase 3 trial to optimize efficacy. Finally, the incorporation of toxin testing will allow accurate assessment of efficacy and safety of this investigational microbiome agent [30, 37]. The lessons learned from these early studies of SER-109 have implications not only for microbiome therapeutics but for all CDI clinical trials [37].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the patients and clinical investigators who participated, including Drs Herbert DuPont John Pullman, Richard Nathan, Ian Baird, Leonard Weinstock, and Matthew Sims.
Financial support. This work was supported by Seres Therapeutics.
Potential conflicts of interest: B. H. M, C. B. F., M. R. H., E. J. O., C. A. D., J. R. W., M.-J. L., K. D. L., J. A. W., C. W. J. M., S. S. L., and J. G. A. are employees and stockholders of Seres Therapeutics. R. J. P. was past Chief Executive Officer and M. T. was past Chief Medical Officer and is currently a consultant for Seres Therapeutics. D. S. P., S. K., and E. L. H. were clinical trial investigators; P. B., A. D. T., M. N., D. N. C., and R. J. P. were past employees and stock holders of Seres Therapeutics. D. N. C. was past Chief Scientific Officer and R. J. P. was past Chief Executive Officer. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gerding DN, Meyer T, Lee C, et al. Administration of spores of nontoxigenic Clostridium difficile strain M3 for prevention of recurrent C. difficile infection: a randomized clinical trial. JAMA 2015; 313:1719–27. [DOI] [PubMed] [Google Scholar]
- 2. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010; 362:197–205. [DOI] [PubMed] [Google Scholar]
- 3. Seekatz AM, Theriot CM, Molloy CT, Wozniak KL, Bergin IL, Young VB. Fecal microbiota transplantation eliminates clostridium difficile in a murine model of relapsing disease. Infect Immun 2015; 83:3838–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis 2008; 197:435–8. [DOI] [PubMed] [Google Scholar]
- 5. Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science 2005; 308:1635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weingarden AR, Dosa PI, DeWinter E, et al. Changes in colonic bile acid composition following fecal microbiota transplantation are sufficient to control clostridium difficile germination and growth. PLoS One 2016; 11:e0147210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorg JA, Sonenshein AL. Chenodeoxycholate is an inhibitor of Clostridium difficile spore germination. J Bacteriol 2009; 191:1115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Theriot CM, Young VB. Interactions between the gastrointestinal microbiome and Clostridium difficile. Annu Rev Microbiol 2015; 69:445–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang ZD, Ajami NJ, Petrosino JF, et al. Randomised clinical trial: faecal microbiota transplantation for recurrent Clostridum difficile infection—fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment Pharmacol Ther 2017; 45:899–908. [DOI] [PubMed] [Google Scholar]
- 10. Tariq R, Pardi DS, Bartlett MG, Khanna S. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Clin Infect Dis 2019; 68:1351–8. [DOI] [PubMed] [Google Scholar]
- 11. Bafeta A, Yavchitz A, Riveros C, Batista R, Ravaud P. Methods and reporting studies assessing fecal microbiota transplantation: a systematic review. Ann Intern Med 2017; 167:34–9. [DOI] [PubMed] [Google Scholar]
- 12. Dubberke ER, Lee CH, Orenstein R, Khanna S, Hecht G, Gerding DN. Results from a randomized, placebo-controlled clinical trial of a RBX2660-A microbiota-based drug for the prevention of recurrent clostridium difficile infection. Clin Infect Dis 2018; 67:1198–204. [DOI] [PubMed] [Google Scholar]
- 13. Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent clostridium difficile infection: a randomized trial. Ann Intern Med 2016; 165:609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med 2019; 381:2043–50. [DOI] [PubMed] [Google Scholar]
- 15. Glover SC, Burstiner S, Jones D, Teymoorian A, et al. E. coli sepsis following FMT in an IgA deficient IBD subject. Available at: https://www.eventscribe.com/2019/ACG/fsPopup.asp?efp=RUxTQkhWU1g2NDI1&PresentationID=594098&mode=presinfo. Accessed 9 March 2020.
- 16. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 Novel coronavirus in the United States. N Engl J Med 2020; 382:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Almomani SA, Button J, Schuster BM, Auniņš JA, Lombardo MJ, McKenzie GJ. Inactivation of vegetative bacteria during production of SER-109, a microbiome-based therapeutic for recurrent Clostridium difficile Infection. Poster 450. Presented at: American Society for Microbiology;Boston, MA; 16–20 June 2016. [Google Scholar]
- 18. Khanna S, Pardi DS, Kelly CR, et al. A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent Clostridium difficile infection. J Infect Dis 2016; 214:173–81. [DOI] [PubMed] [Google Scholar]
- 19. Carlson PE Jr. Regulatory considerations for fecal microbiota transplantation products. Cell Host Microbe 2020; 27:173–5. [DOI] [PubMed] [Google Scholar]
- 20. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22:719–48. [PubMed] [Google Scholar]
- 21. Robins J, Breslow N, Greenland S. Estimators of the Mantel-Haenszel variance consistent in both sparse data and large-strata limiting models. Biometrics 1986; 42:311–23. [PubMed] [Google Scholar]
- 22. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–81. [Google Scholar]
- 23. Segata N, Waldron L, Ballarini A, Narasimhan V, Jousson O, Huttenhower C. Metagenomic microbial community profiling using unique clade-specific marker genes. Nat Methods 2012; 9:811–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Truong DT, Franzosa EA, Tickle TL, et al. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Methods 2015; 12:902–3. [DOI] [PubMed] [Google Scholar]
- 25. Mann HB, Whitney DR.. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Statist 1947; 18:50–60. [Google Scholar]
- 26. Spearman C. The proof and measurement of association between two things. Am J Psychology 1904; 15:72–101. [PubMed] [Google Scholar]
- 27. Theriot CM, Bowman AA, Young VB. Antibiotic-induced alterations of the gut microbiota alter secondary bile acid production and allow for clostridium difficile spore germination and outgrowth in the large intestine. mSphere 2016; 1:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abujamel T, Cadnum JL, Jury LA, et al. Defining the vulnerable period for re-establishment of Clostridium difficile colonization after treatment of C. difficile infection with oral vancomycin or metronidazole. PLoS One 2013; 8:e76269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect 2012; 18(Suppl 6):21–7. [DOI] [PubMed] [Google Scholar]
- 30. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Planche TD, Davies KA, Coen PG, et al. Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C difficile infection. Lancet Infect Dis 2013; 13:936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gonzalez-Orta M, Saldana C, Cadnum J, Donskey CJ. Are patients with prior Clostridium difficile infection (CDI) a potential source of transmission during hospitalization ? Presented at IDWeek in San Diego, CA, 4–8 October 2017. Poster 494.
- 33. Jackson M, Olefson S, Machan JT, Kelly CR. A high rate of alternative diagnoses in patients referred for presumed clostridium difficile infection. J Clin Gastroenterol 2016; 50:742–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wadhwa A, Al Nahhas MF, Dierkhising RA, et al. High risk of post-infectious irritable bowel syndrome in patients with Clostridium difficile infection. Aliment Pharmacol Ther 2016; 44:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beaulieu C, Dionne LL, Julien AS, Longtin Y. Clinical characteristics and outcome of patients with Clostridium difficile infection diagnosed by PCR versus a three-step algorithm. Clin Microbiol Infect 2014; 20:1067–73. [DOI] [PubMed] [Google Scholar]
- 36. Polage CR, Gyorke CE, Kennedy MA, et al. Overdiagnosis of Clostridium difficile infection in the molecular test era. JAMA Intern Med 2015; 175:1792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kong LY, Davies K, Wilcox MH. The perils of PCR-based diagnosis of Clostridioides difficile infections: painful lessons from clinical trials. Anaerobe 2019; 60:102048. [DOI] [PubMed] [Google Scholar]
- 38. Eastwood K, Else P, Charlett A, Wilcox M. Comparison of nine commercially available Clostridium difficile toxin detection assays, a real-time PCR assay for C. difficile tcdB, and a glutamate dehydrogenase detection assay to cytotoxin testing and cytotoxigenic culture methods. J Clin Microbiol 2009; 47:3211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma GK, Brensinger CM, Wu Q, Lewis JD. Increasing incidence of multiply recurrent Clostridium difficile infection in the United States: a cohort study. Ann Intern Med 2017; 167:152–8. [DOI] [PubMed] [Google Scholar]
- 41. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014; 6:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hohmann EL. Are microbial politics local? Ann Intern Med 2016; 165:667–8. [DOI] [PubMed] [Google Scholar]
- 43. Ford C, Henn M, Bryant J, et al. Treatment of recurrent Clostridium difficile infection with SER-109 reduces gastrointestinal carriage of antimicrobial resistance genes. Available at: https://idsa.confex.com/idsa/2018/webprogram/Paper73452.html. Accessed 9 March 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.