Abstract
The mechanisms controlling wiring of neuronal networks are not completely understood. The stereotypic architecture of the Drosophila mushroom body (MB) offers a unique system to study circuit assembly. The adult medial MB γ‐lobe is comprised of a long bundle of axons that wire with specific modulatory and output neurons in a tiled manner, defining five distinct zones. We found that the immunoglobulin superfamily protein Dpr12 is cell‐autonomously required in γ‐neurons for their developmental regrowth into the distal γ4/5 zones, where both Dpr12 and its interacting protein, DIP‐δ, are enriched. DIP‐δ functions in a subset of dopaminergic neurons that wire with γ‐neurons within the γ4/5 zone. During metamorphosis, these dopaminergic projections arrive to the γ4/5 zone prior to γ‐axons, suggesting that γ‐axons extend through a prepatterned region. Thus, Dpr12/DIP‐δ transneuronal interaction is required for γ4/5 zone formation. Our study sheds light onto molecular and cellular mechanisms underlying circuit formation within subcellular resolution.
Keywords: circuit formation, dopaminergic neurons, IgSF, mushroom body compartments, neuronal remodeling
Subject Categories: Neuroscience
Immunoglobulin‐superfamily adhesin Dpr12 and its interaction with DIP‐δ is required for developmental regrowth and targeting of neurons, providing subcellular‐resolution insights into molecular and cellular basis of circuit formation.
Introduction
The precise connectivity between neurons is crucial for the function of neural circuits in vertebrates and invertebrates. The formation of neural circuits is especially complex as it is a multi‐step process that involves guidance of axons and dendrites belonging to distinct neurons, as well as the identification of subcellular zones on the target cell onto which synapses are formed. Despite its fundamental nature, our knowledge of the molecular and cellular mechanisms underlying development of neural circuits remains incomplete.
Given its well‐studied development, connectivity, and function, the Drosophila mushroom body (MB) offers an attractive model to study the mechanisms of neuronal circuit formation and maturation. The MB complex, which functions as a center for associative learning and memory (Heisenberg, 2003; Gerber et al, 2004; Fiala, 2007; Owald & Waddell, 2015; Modi et al, 2020), is comprised of both intrinsic and extrinsic neurons (Tanaka et al, 2008; Aso et al, 2014a). The intrinsic MB neurons are derived from four identical neuroblasts which sequentially give rise to three major classes of unipolar neurons: γ, α’/β’, and α/β, which are collectively known as Kenyon cells (KCs). Axons from each KC type bundle together to form five MB lobes in the adult brain—the vertical α and α’ lobes, and the medial γ, β, and β’ lobes (Fig 1Q, Crittenden et al, 1998). KCs form well‐defined circuits with extrinsic MB neurons, including MB output neurons (MBONs) that relay sensory information to higher brain regions, as well as modulatory neurons, which are mostly dopaminergic (DANs). The adult MB KC lobes are innervated by typical MBONs of 22 types (and additionally newly discovered atypical MBON types; Li et al, 2020) and by over 150 DANs of 20 types which are divided into two major clusters: protocerebral posterior lateral 1 (PPL1) and protocerebral anterior medial (PAM; Aso et al, 2014a). The processes of MBONs and DANs innervate the intrinsic KCs at distinct and stereotypic locations along the MB lobes, thereby defining discrete zones, also known as compartments, within the lobes (due to a potential confusion between cell intrinsic compartments such as the axon initial segment, here we use the term zone to describe these lobe compartments; Tanaka et al, 2008; Aso et al, 2014a). In the case of the adult γ‐lobe, which is comprised of intrinsic γ‐KC axons, stereotypic innervations by DANs and MBONs define five distinct axonal zones, termed γ1‐γ5 (see scheme in Fig 1Q as well as models in Fig 1P and Movie EV1 which are both based on EM traces [Scheffer et al, 2020]; see methods for more details). Interestingly, recent analyses of EM data suggest further categorization of the PAM‐DANs innervating the γ5 zone into five anatomically distinct subtypes, suggested to perform different functions (Otto et al, 2020). Each γ‐KC axon extends throughout the entire lobe and forms synaptic boutons with the dendrites of distinct MBONs and processes of DANs within each zone. Remarkably, a recent study has shown that boutons within the same KC, but in different zones, often exhibit distinct calcium dynamics (Bilz et al, 2020). Finally, these zones have distinct functional roles; DANs innervating the γ1‐γ2 zones are associated with aversive memory, while DANs innervating the γ4–5 zones promote appetitive memory (Aso et al, 2014a; Cohn et al, 2015; Cognigni et al, 2018). Despite their functional importance, the cellular and molecular mechanisms that control MB circuit and zone formation are not known.
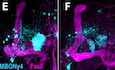
Figure 1. Dpr12 is required for full extension of γ‐KCs.
-
ASchematic representation of neuronal remodeling of γ‐KCs and its regulation by the nuclear receptors EcR and UNF. p: axon peduncle; m/v: medial and vertical lobes.
-
BDynamic expression of Dprs and DIPs during γ‐KC development. Left: Heatmap depicting the relative expression patterns of Dprs and DIPs in γ‐KCs during development. Middle: Magenta intensity depicts the peak expression of each gene during development relative to other Dprs and DIPs. Right: Expression change of Dprs and DIPs while knocking down the UNF transcription factor compared to WT γ‐KCs. Dprs highlighted in bold were tested in the RNAi mini‐screen (Fig EV1).
-
C–NConfocal z‐projections of the indicated genotypes and age, labeled with membrane‐bound GFP (mCD8‐GFP; CD8) driven by the γ‐specific Gal4 driver GMR71G10‐Gal4 (γ‐Gal4). While γ‐axons of control flies project through the entire lobe (C is the RNAi control; n = 12/12, E is the tsCRISPR control; n = 14/14), knockdown of dpr12 by RNAi (D; n = 12/12) or knockout by tsCRISPR (F; n = 14/14) resulted in short axons. At L3, γ‐axons in dpr12∆50‐81 homozygous mutant animals (K; n = 20/20) resemble WT γ‐axons (G; n = 20/20). At 48 h APF, WT γ‐axons normally re‐extend to form the adult lobe (H; n = 12/12). dpr12∆50‐81 γ‐axons (L; n = 14/14) fail to extend to the end of the lobe. This defect persists to adult (I; n = 11/11 vs. M; n = 18/18). Expressing a UAS‐Dpr12 transgene within γ‐KCs in dpr12∆50‐81 homozygote mutant animals rescued the axon regrowth defect (N; n = 23/24, J; n = 14/14). The adult γ‐lobe and α/β lobes are outlined in (C, D) in yellow and orange, respectively, for clarity. Asterisks demarcate the distal part of the lobe. Green and white indicate mCD8‐GFP. Magenta represents FasII staining. Scale bar is 20 µm.
-
OQuantification of the regrowth defects in (I, M, and N). The z‐projections were blindly classified into four classes of regrowth defect severity; see Fig EV1D for examples. Significance was calculated by Kruskal–Wallis test followed by a Mann–Whitney post hoc test; ***P < 0.001.
-
PModels based on hemibrain EM traces (Scheffer et al, 2020) of adult γ‐KCs (representative neurons shown in green), in relation to either selected PAM‐DANs (left; red and orange) or MBONs (right; cyan and blue) targeting the γ4 and γ5 zones. Note that the cell body of the γ4‐MBON is located in the contralateral hemisphere. The MB neuropil is shown in gray. See Materials and Methods for additional details.
-
QSchematic representation of the adult MB. The bundled γ‐KCs form the γ‐lobe (an example of a single γ‐KC is depicted in green). The γ1‐γ5 zones are defined by stereotyped and tiled innervations of the γ‐lobe by dopaminergic neurons (DANs; examples of DANs targeting the γ4 and γ5 zones are depicted in red and orange, respectively) and MB output neurons (MBONs; an example of the γ4 > γ1γ2 MBON innervation is shown in cyan to match the schematics in Fig 7E and F; note that its cell body and innervations are located in contralateral hemispheres). Black dashed line represents the midline. Magenta represents typical FasII staining (which stains the α/β lobes and the γ‐lobe but not the α’/β’ lobes).
The MB is attractive to study wiring of neural circuits not only due to its complex yet stereotypic nature, but also due to its multi‐step development. The larval MB is primarily comprised of γ‐KCs, which form two axonal lobes (vertical and medial γ‐lobes). While larval and adult MB lobes follow the same basic organizational principles in which DANs and MBONs define distinct zones, the actual zonation pattern differs between these two developmental stages (Rohwedder et al, 2016; Saumweber et al, 2018). The larval γ‐lobes undergo extensive remodeling during metamorphosis, including axon pruning followed by developmental regrowth (Lee et al, 1999; Fig 1A), to give rise to the adult medial γ‐lobe containing the γ1–5 zones (Fig 1Q). We have previously demonstrated that regrowth of the adult γ‐lobe is genetically controlled by the nuclear receptor Unfulfilled (UNF) functioning as a ligand‐dependent transcription factor, by mechanisms distinct from initial axon outgrowth (Yaniv et al, 2012). Importantly, while we found that UNF promotes axon regrowth partly via the TOR pathway, it is yet unclear through which mechanisms it promotes targeting, circuitry, and sub‐zone formation. Here, we exploit detailed expression profile analyses to focus on the Immunoglobulin superfamily (IgSF) proteins as potential mediators of zone formation and circuit wiring within the γ4/5 zones.
Results
Dpr12 is required for the full regrowth of γ‐KCs
To identify potential genes and pathways that mediate axon regrowth and circuit formation, we sequenced the RNA content of WT γ‐KCs during development (Alyagor et al, 2018) alongside γ‐KCs expressing RNAi targeting UNF, a known protein required for regrowth (Fig 1A). Dataset EV1 shows this comparison alongside the previously generated (Alyagor et al, 2018) expression profiles of γ‐KCs expressing a dominant‐negative form of the ecdysone receptor (EcRDN), which is required for pruning (Lee et al, 2000; data are freely available in: https://www.weizmann.ac.il/mcb/Schuldiner/resources). The immunoglobulin superfamily (IgSF) appeared as the protein family most significantly affected by UNF‐RNAi expression (P = 3 × 10−29; analyzed in http://www.flymine.org/). Within the IgSF, the defective proboscis response (Dpr) family stood out as 16 of 21 members were significantly expressed in dynamic patterns in developing γ‐KCs (Fig 1B, Dataset EV2). We found that the transcription of approximately half of the Dprs (7/16; Dataset EV2) was significantly reduced in UNF‐RNAi‐expressing flies. Interestingly, the interactions between the Dprs, containing two immunoglobulin (Ig) domains, and the Dpr‐interacting proteins (DIPs), containing three Ig domains, are important for proper development and synaptic connectivity of the Drosophila visual system and neuromuscular junction (NMJ; Xu et al, 2018; Ashley et al, 2019; Venkatasubramanian et al, 2019). Based on these data, we focused on the Dprs as potential candidates required for regrowth and circuit reformation.
We targeted eight different Dprs in γ‐KCs by RNAi (Fig EV1A) based on reagent availability (TRiP lines, https://fgr.hms.harvard.edu/fly‐in‐vivo‐rnai), using a γ‐specific driver (R71G10, which is predominantly and consistently expressed in γ‐KCs, but is also expressed in α/β‐KCs in a stochastic manner; see Appendix Table S2). Seven of these RNAis did not affect γ‐neuron development. While it is possible that these Dprs are indeed not required for γ‐KC development, the lack of phenotype may also result from inherent redundancies of Dpr‐DIP interactions (Cosmanescu et al, 2018), or, alternatively, from limited efficiency of the RNAi lines. In contrast, expressing dpr12 RNAi in γ‐KCs induced a pronounced regrowth defect, where axons did not occupy the distal portion of the γ‐lobe (Figs 1C and D, and EV1A). Interestingly, the expression of Dpr12 is markedly reduced in neurons expressing UNF‐RNAi at the relevant times for regrowth (Figs 1B, and EV1A, Dataset EV2), suggesting that UNF might positively regulate dpr12 transcription. These findings suggest that Dpr12 could promote developmental regrowth and circuit formation as a part of an UNF‐dependent transcriptional program.
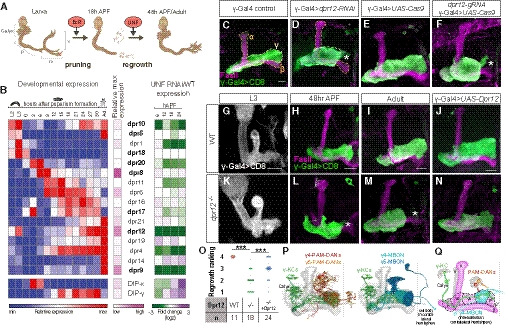
Figure EV1. Knockdown of dpr12 results in regrowth phenotype and description of Dpr12 alleles used in the study, related to Fig 1.
-
ALeft: Graphs depicting the normalized RNA expression levels of selected Dprs in WT γ‐KCs (black), and in γ‐KCs expressing EcRDN (green) or UNF‐RNAi (blue). *P < 0.05; Error bars indicate SEM; units on the y‐axis are arbitrary. Right: Confocal z‐projections of adult γ‐KCs expressing RNAi transgenes as indicated labeled with membrane‐bound GFP (mCD8‐GFP; CD8) driven by the γ‐specific Gal4 driver R71G10‐Gal4 (γ‐Gal4). Note that while R71G10 is consistently expressed in γ‐KCs, it is also expressed in α/β‐KCs in a stochastic manner.
-
BA schematic representation of the Dpr12 locus showing introns (black line) and coding and non‐coding exons (red and gray, respectively). The location of Dpr12 gRNA (arrow), dpr12∆50‐81 mutation (arrow), dpr12 RNAiJF03210 (black lines connected with dashed lines), and MiMICMI01695 (arrowhead) is indicated. SA and SD are splice acceptor and donor sites, respectively. Recombination‐mediated cassette exchange was used to transform Dpr12MI01695 into Dpr12GFSTF.
-
CA schematic representation of Dpr12 protein variants. Signal peptide (SP), Immunoglobulin (Ig), transmembrane (TM), and GPI anchor (GPI).
-
DRanking of regrowth: Confocal z‐projections of adult γ‐KCs labeled with membrane‐bound GFP (mCD8‐GFP; CD8) driven by the γ‐specific Gal4 driver GMR71G10‐Gal4 (γ‐Gal4). Representative images of the regrowth defect severity (1 = strong, 2 = intermediate, 3 = weak, 4 = WT) described in Fig 1O. The arrowhead demarcates an unusually short β‐lobe; morphologically abnormal β‐lobes (either short, thin or absent) appear in approximately 40% of either dpr12 or DIP‐δ homozygous mutant brains. Since β‐lobe morphology is rescued by overexpressing a UAS‐Dpr12 transgene within γKCs, it is most likely a non‐cell‐autonomous defect, which is beyond the scope of this study. Asterisk demarcates the distal tip of the γ‐lobe. Green is CD8‐GFP; magenta is FasII staining. Scale bar is 20 µm.
To validate the RNAi results, we next perturbed Dpr12 through tissue‐specific (ts)CRISPR using Gal4‐driven Cas9 expression (Port & Bullock, 2016; Meltzer et al, 2019; Port et al, 2020). tsCRISPR of dpr12 in γ‐KCs induced a defect closely resembling the RNAi phenotype (Fig 1E and F). Finally, we used CRISPR/Cas9 technology to generate a dpr12 loss‐of‐function mutant (dpr12∆50‐81, Fig EV1B and C). At 3rd instar larva (L3), γ‐KCs in dpr12∆50‐81 homozygotes exhibited WT morphology (Fig 1G and K) and subsequently pruned normally (as evident by the lack of unpruned axons in the adult, Fig 1L and M). These data indicate that Dpr12 is not required for initial axon extension or pruning. In contrast, γ‐KCs in dpr12∆50‐81 animals failed to extend to the distal part of the lobe during the mid‐pupal stage (at 48 h APF), a time when γ‐axons would have normally completed their regrowth (Lee et al, 1999; Rabinovich et al, 2016), and in the adult (Fig 1H,I,L,M,O). Importantly, γ‐specific expression of a Dpr12 transgene significantly rescued the regrowth defect within dpr12∆50‐81 homozygotes (Fig 1J,N,O, ranking examples shown in Fig EV1D). Together, these data demonstrate that Dpr12 is required for full axon regrowth during metamorphosis and that mutant γ‐axons stop prematurely and do not extend into the distal end of the lobe. Importantly, its requirement during metamorphosis does not rule out additional roles of Dpr12 in other developmental stages.
Dpr12 is cell‐autonomously required for γ‐axon regrowth into the γ4/5 zones
To determine whether Dpr12 functions in a cell‐autonomous manner, we used the mosaic analysis with a repressible cell marker (MARCM) technique to express dpr12‐RNAi within neuroblast (NB) or single‐cell (SC) clones. We found that both NB and SC γ‐KC clones expressing dpr12 RNAi exhibited normal growth at L3 but failed to fully extend axons at 48 h APF and in adult flies (Fig 2A–L). Based on these results, we conclude that Dpr12 is cell‐autonomously required in γ‐KCs for their full developmental regrowth.
Figure 2. Dpr12 is cell‐autonomously required for γ‐axon regrowth into the γ4/5 zones.
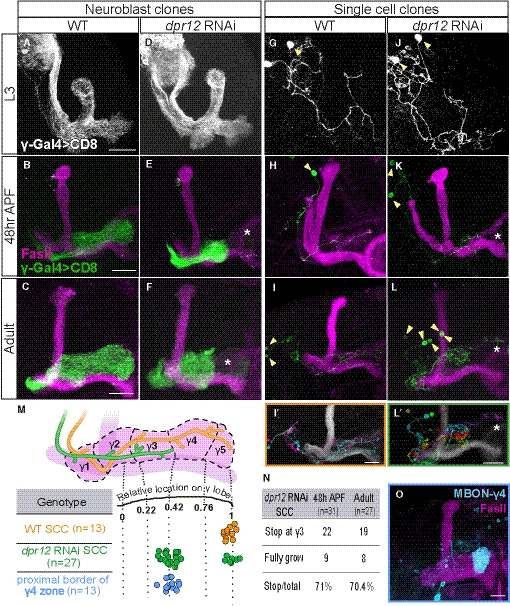
-
A–LConfocal z‐projections of MARCM neuroblast (NB, A‐F) and single‐cell (SC, G‐L) clones labeled with membrane‐bound GFP (mCD8‐GFP; CD8) driven by the γ‐specific Gal4 driver GMR71G10‐Gal4 (γ‐Gal4). At L3, NB and SC clones expressing dpr12 RNAi are similar to equivalent WT clones (A; n = 20/20, D; n = 15/15, G; n = 15/15 and J; n = 17/17). At 48 h APF and adult stage, WT NB (B; n = 15/15, C; n = 10/10) and SC (H; n = 16/16, I; n = 13/13) clones extend their axons to form the full adult lobe. In contrast, clones expressing dpr12 RNAi (E; n = 14/14, F; n = 22/2, K; n = 18/24, L; n = 19/27) fail to extend their axons to the distal part of the medial lobe (asterisks). (I’ and L’) are traces of multiple single‐cell clones depicting each cell in a different color.
-
MTop: Schematic representation of WT (orange) and dpr12 RNAi‐expressing (green) single γ‐KC axons. Bottom: Measurements of the relative location to which WT (I) and dpr12 RNAi (L) axons grow across the entire length of the adult γ lobe, alongside the relative position of the proximal border of the γ4 zone (see O, as well as Fig EV2).
-
NA table depicting the percentage of dpr12 RNAi‐expressing single‐cell clones (SCCs) which stop at the γ3‐γ4 border, at 48 h APF compared to the adult stage.
-
OConfocal z‐projection of MBONγ4 > γ1γ2 labeled by GMR18H09‐Gal4 driving the expression of mCD8‐GFP (CD8) shown in cyan.
Data information: Yellow arrowheads demarcate single cell bodies. Green, white, and cyan represent mCD8‐GFP. Magenta represents FasII. Scale bar is 20 µm.
Interestingly, unlike other mutants that affect developmental regrowth (Yaniv et al, 2012; Yaniv et al, 2020), dpr12 mutant axons seem to partially regrow but stop prematurely in a particular and stereotypic location along the lobe. Therefore, and given that the γ‐lobe is divided into distinct zones, we next mapped the location of the premature stopping in more detail. We measured the length of adult WT and mutant axons relative to the γ‐lobe span and superimposed these data onto the γ‐lobe zones (Fig 2M), as defined by distinct innervations of MBONs and DANs (Figs 2O and EV2; Aso et al, 2014a; Shuai et al, 2015). This analysis indicated that the premature stopping of clones expressing dpr12 RNAi correlates with the border between the γ3 and γ4 zones (Fig 2I’,L’,M, Movies EV2 and EV3). We found that the majority of SC clones stopped at the γ3/4 border, while only a minority extended to the end of the lobe (Fig 2M). We also observed brains containing multiple single‐cell clones, in which one axon extended to the edge of the lobe, while the remaining stopped prematurely, ruling out a brain‐specific effect (Fig 2L'). Importantly, the proportion of SCCs that stalled at the γ3/4 border was similar in both adults and at the earlier developmental stage of 48 h APF (70.4% and 71%, respectively; Fig 2N). Since 48 h APF is the time point in which axons normally complete their regrowth, this suggests that the phenotype observed in the adult stage is the result of arrested regrowth, rather than retraction of previously grown axons. Since axons always stall at a discrete location, our data suggest that the phenotype does not arise from reduced growth potential, per se, but rather through a failure to recognize a molecular signal at a designated and stereotypic location (see discussion). Overall, we conclude that Dpr12 is cell‐autonomously required for γ‐KC projection into the MB γ4/5 zones.
Figure EV2. Measurements of γ‐axon outgrowth, related to Fig 2.
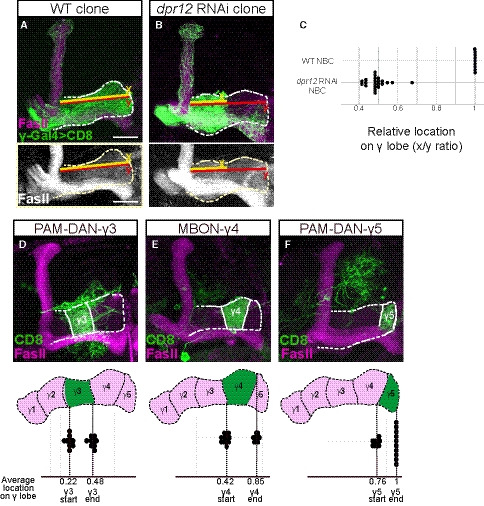
-
A, BConfocal z‐projections of WT (A) and dpr12 RNAi (B) MARCM neuroblast clones (NBC) labeled with membrane‐bound GFP (mCD8‐GFP; CD8) driven by the γ‐specific Gal4 driver GMR71G10‐Gal4 (γ‐Gal4). y (red) represents the length of the entire γ‐lobe as indicated; x (yellow) represents the extent of clonal γ‐axon outgrowth.
-
CMeasurements of the x/y ration as depicted in (A, B). While WT NBC always extends up to the end of the lobe, dpr12 RNAi NBC stops at about midway (x/y ratio of 0.48 ± 0.05).
-
D–FTop: Confocal z‐projections of PAM‐DAN‐γ3 (D, MB441B), MBON‐γ4 > γ1γ2 (E, R18H09), and PAM‐DAN‐γ5 (F, R48H11) Gal4s driving the expression of mCD8‐GFP (CD8). Bottom: start and end of the indicated zone is superimposed on a schematic representation of the adult γ lobe compartments. (D) γ3 zone begins at x/y ratio of 0.22 ± 0.03 and ends at 0.48 ± 0.03. (E) γ4 zone begins at x/y ratio of 0.42 ± 0.04 and ends at 0.85 ± 0.03. (F) γ5 zone begins at x/y ratio of 0.76 ± 0.03 and ends at a mean ratio of 1. Green is CD8‐GFP; magenta and white represent FasII. Scale bar is 20 µm.
Dpr12 and its putative interacting protein DIP‐δ localize at the γ4/5 zones
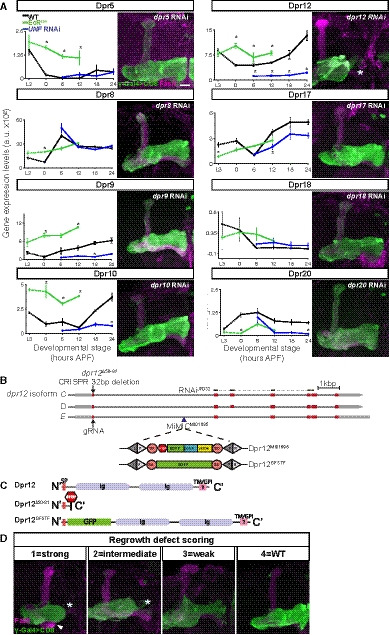
To assess Dpr12 protein localization, we used the Minos‐mediated integration cassette (MiMIC) transgene collection to obtain a GFP insertion within the endogenous Dpr12 locus, which should produce a Dpr12‐GFP fusion protein (Dpr12GFSTF; Fig EV1B and C; Nagarkar‐Jaiswal et al, 2015). We found that Dpr12‐GFP localized to the distal part of γ‐axons at late larva (L3; Fig 3A), then becoming diffuse at 24 h APF, when γ‐axons initiate their developmental regrowth (Fig 3B). Finally, at 48 h APF up until adulthood, Dpr12 relocalized to the distal part of the lobe (Fig 3C and D, Appendix Fig S1A) and was highly enriched within the adult γ4 and γ5 zones (Fig 3D; Appendix Fig S1E). Our data suggest that Dpr12 is expressed at the right time and place to mediate γ‐axon regrowth into the γ4/γ5 zones.
Figure 3. Both Dpr12 and its interacting protein DIP‐δ localize to the γ4/5 zones.
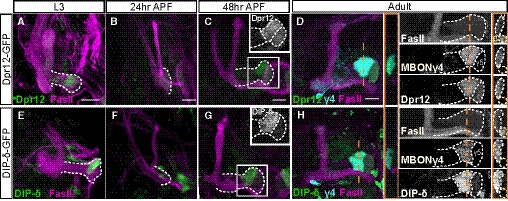
-
A–HConfocal z‐projections of brains expressing MiMIC mediated Dpr12GFSTF (Dpr12‐GFP; A‐D) and DIP‐δGFSTF (DIP‐δ‐GFP; E‐H) fusion proteins at the indicated time points. See Figs EV1 and EV3 for more details on the fusion protein structure. (A‐D) Dpr12‐GFP is localized to the distal part of the γ‐lobe at L3 (A; n = 10/10), 48 h APF (C; n = 12/12) and the adult stage (D; n = 20/20), where it colocalizes with the γ4 > γ1γ2 MBON (γ4; labeled by GMR18H09‐Gal4 driving the expression of CD4‐tdT). At 24hr APF (B; n = 10/10), Dpr12‐GFP appears diffuse. (E‐H) DIP‐δ‐GFP is localized to the distal part of the γ‐lobe throughout development: L3 (E; n = 16/16), 24 h APF (F; n = 10/10), 48 h APF (G; n = 12/12), and adult (H; n = 24/24). At the adult stage, DIP‐δ‐GFP is colocalized with the γ4 > γ1γ2 MBON (γ4).
Data information: White dashed lines depict the γ‐lobe. Green is GFP, cyan is CD4‐tdT, and magenta is FasII. Grayscale in the right panels of (D and H) represents single channels, as labeled. Insets in (C, G) represent grayscale magnifications of the GFP channel within the white boxes. In orange are transverse sections through the γ4 zone at the indicated locations; the γ‐lobe is outlined in white and the β‐lobe in yellow. Scale bar is 20 µm.
Dprs can form heterophilic interactions with DIPs in a rather promiscuous fashion, in which most Dprs can bind to multiple DIPs and most DIPs can bind to multiple Dprs (Ozkan et al, 2013; Carrillo et al, 2015; Cosmanescu et al, 2018). Interestingly, Dpr12 and DIP‐δ represent a unique case of “monogamous” binding. We found that DIP‐δ‐GFP (DIP‐δGFSTF; Fig EV3A and B) is also localized to both the γ4 and γ5 zones, although its expression is significantly stronger in γ4 compared to γ5 (Fig 3G and H; Appendix Fig S1F). In contrast to Dpr12, DIP‐δ was localized to the distal MB lobe at all developmental time points tested (Fig 3E–H, Appendix Fig S1B), including at 24 h APF, when γ‐axons are completely pruned and have not yet extended (Fig 3F). Transverse sections across the γ4 zone in the adult stage clearly demonstrate the colocalization of both the Dpr12 and DIP‐δ proteins with γ4 (as determined by the well‐characterized MBONγ4 > γ1γ2; Fig 3D and H). Taken together, these data indicate that DIP‐δ is localized to the γ4/γ5 zones throughout development and is expressed in cells that project to this region before γ‐KCs reach their terminal projections.
Figure EV3. Description of DIP‐δ alleles used in the study and additional DIP‐δ perturbation phenotypes, related to Fig 4.
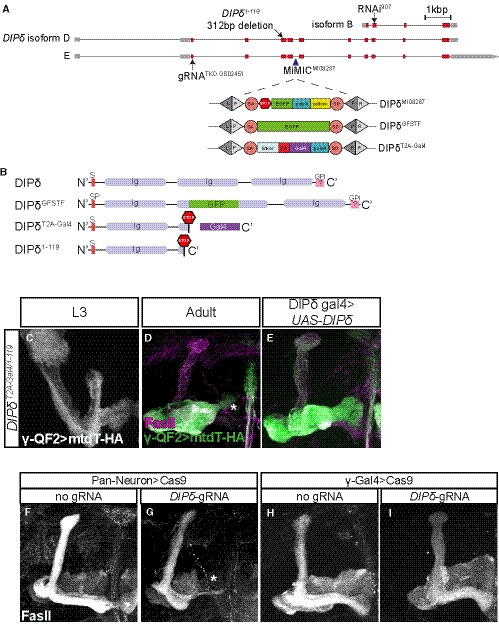
-
AA schematic representation of the DIP‐δ locus showing introns (black line) and coding and non‐coding exons (red and gray, respectively). The location of DIP‐δ gRNA, DIP‐δ1‐119 mutation, DIP‐δ RNAi, and MiMICMI08287 is indicated. SA and SD are splice acceptor and donor sites, respectively. Recombination‐mediated cassette exchange was used to transform DIP‐δMI08287 into DIP‐δGFSTF and DIP‐δT2A‐Gal4.
-
BA schematic description of DIP‐δ protein variants. Signal peptide (SP), Immunoglobulin (Ig), and GPI anchor (GPI).
-
C–EConfocal z‐projections of DIP‐δ transheterozygotes (DIP‐δT2A‐Gal4/1‐119) at L3 (C; n = 20/20) and adult (D; n = 12/12, E; n = 22/23), in which γ neurons were marked by expressing membrane‐bound tandem tomato (mtdT‐HA) driven by the γ‐specific QF2 driver GMR71G10‐QF2 (γ‐QF2). In DIP‐δ transheterozygotes, as in homozygous mutants (see Fig 4), γ‐KCs do not extend into the distal end of the lobe. Expression of DIP‐δ in DIP‐δ+ cells (E) rescues the growth defect present in DIP‐δT2A‐Gal4/1‐119 brains. Green and white represent mtdT‐HA; magenta represents FasII staining.
-
F–IConfocal z‐projections of brains expressing UAS‐Cas9 alone (F, H) or together with DIP‐δ‐gRNA (G, I). DIP‐δ knockout by tsCRISPR in all postmitotic neurons (G; n = 22/24) resulted in a defect in γ4/5 innervation by γ‐axons, while DIP‐δ knockout by tsCRISPR in γ‐KCs (I; n = 28/28) did not affect γ‐axon regrowth. Expression of Cas9 alone (F, n = 10/10; H, n = 14/14) did not affect γ‐axon regrowth. White represents FasII staining.
Data information: Scale bar is 20 µm.
DIP‐δ is non‐cell‐autonomously required for full γ‐axon regrowth
We next asked whether DIP‐δ is also required for the extension of γ‐axons into the γ4/5 zones. We therefore both generated and obtained DIP‐δ mutant alleles (DIP‐δT2A‐Gal4, DIP‐δ1–119, respectively; Fig EV3A and B). We marked the γ‐KCs by expressing a membrane‐bound tomato (QUAS‐mtdT‐3XHA) driven by the Gal4‐independent γ‐KC QF2 driver (71G10‐QF2; note that, as its Gal4 counterpart, in addition to consistent expression within γ‐KCs, 71G10‐QF2 is also stochastically expressed in α/β‐KCs; see Appendix Table S2). At L3, γ‐KCs within DIP‐δ mutant brains exhibited WT morphology (Figs 4A and E, and EV3C), indicating that DIP‐δ is not required for their initial axon extension. However, DIP‐δ mutant brains displayed a γ‐axon regrowth defect at 48 h APF and in adult (Figs 4B, C, F, G, and EV3D), which resembled the dpr12 mutant phenotype. To confirm that DIP‐δ loss of function induced this γ‐axon regrowth defect, we exploited the fact that the DIP‐δT2A‐Gal4 allele also expresses Gal4 in DIP‐δ+ neurons (Fig EV3A and B). Expressing a DIP‐δ transgene driven by DIP‐δT2A‐Gal4 rescued the γ‐axon extension defect (Fig 4D,H,I, and EV3E), confirming that DIP‐δ is required for γ‐axon innervation of the γ4/5 zones.
Figure 4. DIP‐δ is required for γ‐axon regrowth into the γ4/5 zones.
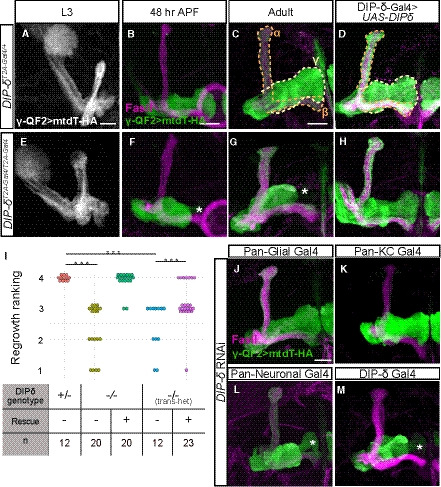
-
A–HConfocal z‐projections DIP‐δ hetero‐ and homozygous brains in which γ‐KCs were labeled by expressing membrane‐bound tandem tomato (mtdT‐HA) driven by the γ‐specific QF2 driver R71G10‐QF2 (γ‐QF2). Larval (L3) γ‐axons grow normally in DIP‐δT2A‐Gal4 heterozygotes (A; n = 16/16) and homozygotes (E; n = 24/24). In contrast, at 48 h APF and adult, γ‐axons within DIP‐δT2A‐Gal4 homozygotes do not enter the distal part of the lobe (asterisks; F; n = 8/8, G; n = 20/20), while they grow normally in heterozygotes (B; n = 12/12, C; n = 12/12). Overexpression of a DIP‐δ transgene driven by the Gal4 activity of DIP‐δT2A‐Gal4 (see also Fig EV3) does not affect normal growth (D; n = 10/10) and rescues mutant phenotypes (H; n = 20/20). Note that while the R71G10 driver is consistently expressed in γ‐KCs, it is also expressed in α/β‐KCs in a stochastic manner. The adult γ‐lobe and α/β lobes are outlined in (C, D) in yellow and orange, respectively, for clarity.
-
IRanking of regrowth for (C, G, H), and Fig EV3D and E. Regrowth defect severity and statistics were calculated as in Fig 1; Wilcoxon–Mann–Whitney test; ***P < 0.001.
-
J–MConfocal z‐projections of brains expressing DIP‐δ‐RNAi driven by the indicated Gal4. γ‐KCs are labeled by mtdT‐HA driven by R71G10‐QF2 (γ‐QF2). Expression of DIP‐δ‐RNAi in all glia (Repo‐Gal4, J; n = 28/28) or all KCs (OK107‐Gal4, K; n = 12/12) did not affect γ‐neuron regrowth. In contrast, expression of DIP‐δ‐RNAi in all postmitotic neurons (C155‐Gal4, L; n = 9/12) or DIP‐δ‐expressing neurons (DIP‐δT2A‐Gal4, M; n = 21/22) induced a defect in γ4/5 innervation by γ‐axons.
Data information: Asterisks demarcate distal part of the lobe. Green and white are mtdT‐HA, and magenta is FasII. Scale bar is 20 µm.
To identify these DIP‐δ‐expressing cells, we expressed DIP‐δ RNAi in different cell types, while simultaneously labeling γ‐KCs using the QF2 system described above. Driving the expression of DIP‐δ‐RNAi in all glia (using the Pan‐glial driver Repo‐Gal4) or all KCs (using OK107‐Gal4) did not affect γ‐axon extension (Fig 4J and K). In contrast, knocking down of DIP‐δ in all neurons (using the pan‐neuronal driver C155‐Gal4) or all DIP‐δ‐expressing neurons (using DIP‐δT2A‐Gal4) resulted in stalled γ‐axons that do not innervate the γ4/5 zones (Fig 4L and M; Appendix Table S2). Similarly, tsCRISPR of DIP‐δ in all neurons, but not when restricted to γ‐KCs, affected the extension of γ‐axons (Fig EV3, EV4, EV5, EV6). Together, these experiments indicate that DIP‐δ is not required in γ or other KCs. Rather, DIP‐δ likely functions in extrinsic MB neurons in a non‐cell‐autonomous manner to mediate γ‐axon extension into the γ4/5 zones.
Figure EV4. Characterization of PAM‐DAN and DIP‐δ Gal4s, related to Fig 5.
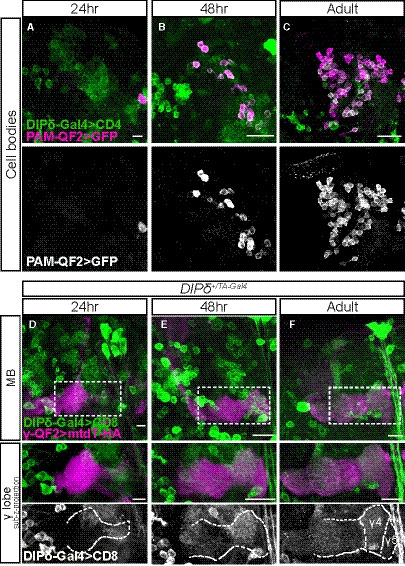
-
A–CConfocal z‐projections of the cell body region of the PAM‐DAN cluster demonstrating the expression of membrane‐bound tandem tomato (CD4‐tdT; CD4) driven by DIP‐δT2A‐Gal4 (DIP‐δ‐Gal4) in addition to GFP driven by the PAM‐DAN specific QF2 driver GMR58E02‐QF2 (PAM‐QF2). The PAM‐QF2 driver is not expressed at 24 h APF (A, n = 12), starts to be expressed at 48 h APF (B, n = 10) and fully expressed and localized with DIP‐δ‐Gal4 in adult (C, n = 14).
-
D–FConfocal z‐projections of heterozygous brains (DIP‐δ+/T2A‐Gal4) in which DIP‐δ positive neurons were labeled by membrane‐bound GFP (mCD8‐GFP; CD8) driven by DIP‐δ‐Gal4. γ‐KCs were marked by expressing membrane‐bound tandem tomato (mtdT‐HA) driven by the γ‐specific QF2 driver GMR71G10‐QF2 (γ‐QF2). Bottom: High magnification images as demarcated by dashed boxes in top panels, of sub‐z‐projections restricted to slices that contain the γ‐lobe.
Data information: Dashed outline demarcates γ‐lobe as depicted by FasII staining. γ4/5 zones are indicated in adult. (D), n = 20; (E), n = 16; (F), n = 26. Scale bar is 20 µm.
Figure EV5. Phenotypic analysis of PAM‐DAN and MBON innervation of the MB γ‐lobe in Dpr12 mutant brains, related to Fig 7.
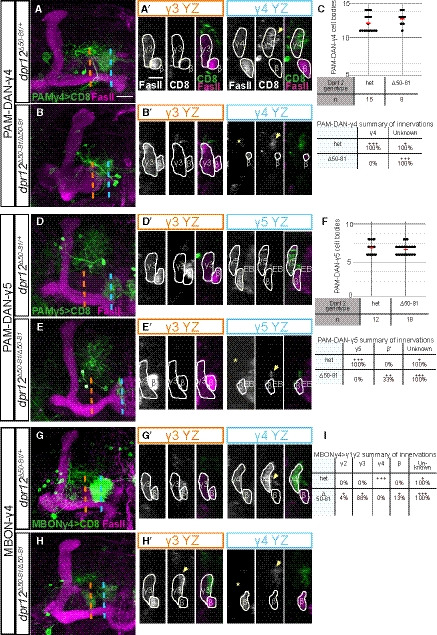
-
A–I(A, B, D, E, G, H) Left: Detailed analysis of the confocal z‐projections of dpr12 heterozygous or homozygous mutant brains that are presented in Fig 7A–F, which express membrane‐bound GFP (CD8) driven by: (A‐B) R10G03‐Gal4 is used to label PAM‐DANs innervating the γ4 compartment (PAM‐DAN‐γ4); (D‐E) R48H11‐Gal4 is used to label PAM‐DANs innervating the γ5 compartment (PAM‐DAN‐γ5); (G‐H) R18H09‐Gal4 is used to label the MBONγ4 > γ1γ2 which innervates the γ4 zone (MBON‐γ4). Right: YZ projections along the indicated lines in γ3 (orange) and γ4 or γ5 (blue) compartments. (C, F, I) Top: Cell body numbers of the indicated neurons in dpr12 heterozygous and homozygous brains. Bottom: Summary of innervation destinations. Unknown means stereotypic projections to unidentifiable domains.
Data information: Green is CD8‐GFP, magenta is FasII, and grayscale single channels are shown as indicated. Asterisks mark missing innervation and arrows mark innervations outside the γ lobe. Scale bar is 20 µm in (A‐B, D‐E, G‐H) and 10 µm in (A’‐B’,D’‐E’,G’‐H’).
Figure EV6. UAS‐DIP‐α function is Gal4‐dependent; FasII overexpression fails to suppress the DIP‐δ mutant phenotype, related to Fig 8.
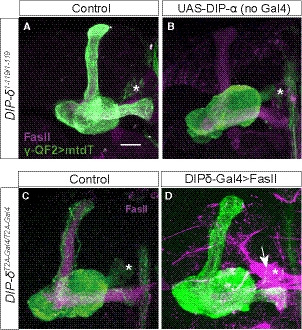
-
A, BConfocal z‐projections of DIP‐δ1‐119 homozygous mutant brains, in which γ‐KCs are labeled by membrane‐bound tandem tomato (mtdT‐HA; green) driven by R71G10‐QF2 (γ‐QF2), that either contain (B) or do not contain (A) a UAS‐DIP‐α transgene.
-
C, DConfocal z‐projections of DIP‐δT2A‐Gal4/T2A‐Gal4 homozygous mutant brains, in which γ‐KCs are labeled by mtdT‐HA (green) driven by γ‐QF2, that either express (D) or do not express (C) a UAS‐FasII transgene driven by DIP‐δ‐Gal4.
Data information: Magenta is FasII; arrow in (D) indicates FasII accumulation in DIP‐δ+ PAM‐DANs. Asterisks mark the distal edge of the lobe. Scale bar is 20 µm.
A sub‐population of DANs express DIP‐δ in the γ4/γ5 zones
Our data suggest that DIP‐δ is expressed in extrinsic MB neurons that innervate the γ4/γ5 zones (Figs 3 and 4). These zones are strongly innervated by a sub‐population of PAM‐DANs, as well as by three typical MBONs: MBONγ4 > γ1γ2 (also known as MBON‐05), MBONγ4γ5 (MBON‐21), and MBONγ5β’2a (MBON‐01; Aso et al, 2014a; Li et al, 2020; Tanaka et al, 2008). To investigate whether DIP‐δ is expressed in these cells, we selectively ablated PAM‐DANs or MBONs by cell type specific expression of Diphtheria toxin (UAS‐DTI) and assayed DIP‐δ‐GFP localization in adult flies. Ablating one of the two γ4‐MBONs, MBON‐γ4 > γ1γ2, did not affect DIP‐δ expression (Fig 5A and B). In contrast, ablating PAM‐DANs using R58E02‐Gal4 drastically reduced γ4/5 specific DIP‐δ expression (Fig 5C and D). While we cannot exclude the possibility that DIP‐δ is additionally expressed in the other γ4‐MBON (MBON‐γ4γ5), these results strongly suggest that at the adult stage, DIP‐δ protein that is localized to the γ4/5 zones is mainly, if not exclusively, expressed by PAM‐DANs. This finding is consistent with recent profiling experiments which showed that DIP‐δ is highly enriched in PAM‐DANs (and was in fact even suggested as a new marker for these neurons; Croset et al, 2018).
Figure 5. PAM‐DANs are the source of DIP‐δ in the γ4/5 zones.
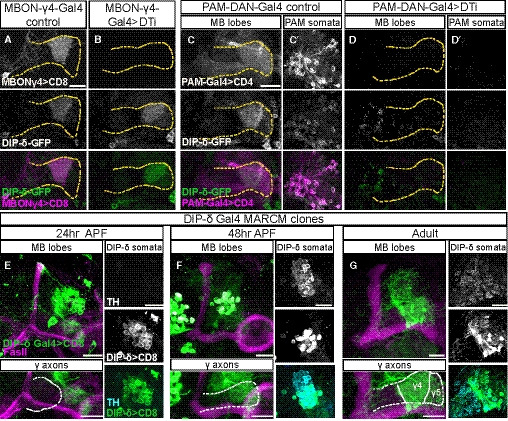
-
A–DConfocal z‐projections of brains expressing DIP‐δGFSTF (DIP‐δ‐GFP) together with the indicated Gal4s and transgenes. Expressing diphtheria toxin (DTi) and membrane‐bound RFP (mCD8‐RFP; CD8) driven by the γ4 > γ1γ2 MBON driver MB294B‐Gal4 (MBONγ4‐Gal4) did not affect DIP‐δ‐GFP expression (A, n = 16/16; B, n = 14/14). In contrast, similar expression of DTi and membrane‐bound tomato (CD4‐tdT; CD4) in PAM‐DANs (using the GMR58E02‐Gal4; PAM‐DAN‐Gal4) abolished the normal DIP‐δ‐GFP expression in the γ4/5 zone (compare D, n = 18/18, to C, n = 16/16) and within the PAM‐DAN cell bodies (compare D’ to C’). Magenta is CD8‐RFP (A, B) and CD4‐mtdT (C, D). Green is GFP, and grayscale depicts individual channels as labeled. Scale bar is 20 µm. Yellow dashed line demarcates the γ‐lobe based on FasII staining (not shown).
-
E–GConfocal z‐projections of MARCM clones labeled by DIP‐δT2A‐Gal4 (DIP‐δ Gal4) driving the expression of membrane‐bound GFP (mCD8‐GFP; CD8) and heat shocked at 24 h after egg laying. Clones innervate the γ4/5 zones at 24 h APF (E; n = 8), 48 h APF (F; n = 8), and adult (G; n = 16). Clones become tyrosine hydroxylase (TH) positive only at 48 h APF onwards (F, G). Magenta is FasII, green is mCD8‐GFP, cyan is TH antibody staining, and grayscale single channels are shown as indicated. White dashed line demarcates the γ‐lobe. Scale bar is 20 µm.
Next, we wanted to visualize PAM‐DANs during development to determine whether they may provide a template for γ‐axon growth, as suggested by DIP‐δ‐GFP expression (Fig 3). However, the “classical” PAM‐DAN driver, R58E02, is not expressed throughout development (Fig EV4, EV5, EV6, Appendix Table S2). We therefore analyzed the expression of DIP‐δT2A‐Gal4 and found that it is widespread throughout development. While it is expressed in cells innervating the γ4–5 zones (Fig EV4E and F), it is also, for example, expressed in cells that send processes to the MB vertical lobes. We thus decided to use the MARCM technique to label sparse DIP‐δT2A‐Gal4 clones (Fig 5E–G; of note, these clones remain heterozygous for DIP‐δ). We detected DIP‐δ‐expressing clones that innervate the γ4/γ5 zones as early as 24 h APF and up to adulthood. Interestingly, these clones do not seem to express tyrosine hydroxylase (TH), required for Dopamine biogenesis, at 24 h APF but become TH positive at 48 h APF onwards. Notably, while DIP‐δ‐expressing PAM‐DANs innervate both the γ4 and γ5 zones, their innervation of γ5 is sparser than that of γ4 (Fig 5G). This correlates with the reduced enrichment of the DIP‐δ protein in γ4 compared to γ5 (Fig 3G and H). In summary, our data suggest that DIP‐δ is expressed in PAM‐DANs, which innervate the future γ4/5 zones as early as 24 h APF, and support the speculation that DIP‐δ expressed in PAM‐DANs may provide a template for γ‐axon growth.
DIP‐δ is required and sufficient for Dpr12 localization
Both Dpr12 and DIP‐δ localize to the γ4/5 zones and are required for their formation likely by mediating interactions between the γ‐KCs and the PAM‐DANs. We therefore investigated how losing either dpr12 or DIP‐δ affects their binding partner localization and mature circuit architecture. First, we investigated whether the highly localized expression of Dpr12 and DIP‐δ is cell‐autonomous or requires interaction with their binding partner. We visualized Dpr12‐ and DIP‐δ‐GFP fusion proteins in brains homozygous mutant for their reciprocal Dpr/DIP partner. We found that Dpr12 expression appears diffuse in DIP‐δ mutant brains throughout development (Fig 6A–D, Appendix Fig S1C), indicating that Dpr12 protein localization requires interaction with DIP‐δ, likely on PAM‐DANs. In contrast, DIP‐δ localization seemed unperturbed during the early stages of development in dpr12 mutants (Fig 6E and F). At both 48 h and 72 h APF, while we still detected DIP‐δ at the distal part of the lobe, it occupied a smaller area than in WT brains and resembled the innervation pattern typical of 24 h APF (compare Figs 3, 6 and Appendix Fig S1D to S1B). Furthermore, we did not detect any DIP‐δ in the adult MB medial lobe (Fig 6H), suggesting that Dpr12 is required for the refinement and maintenance of DIP‐δ localization.
Figure 6. DIP‐δ is required and sufficient for Dpr12 localization.
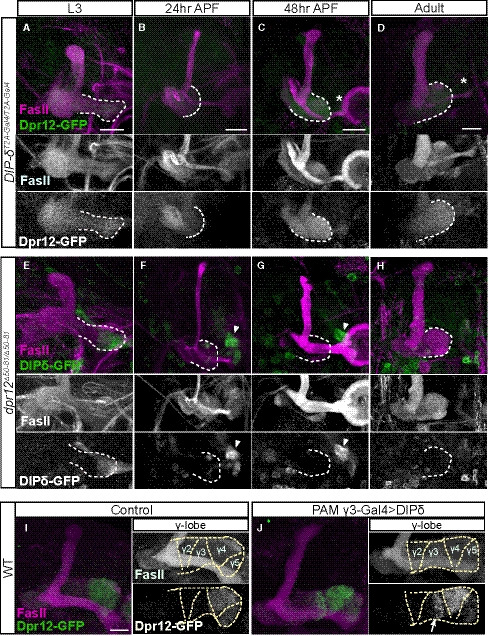
-
A–JConfocal z‐projections of brains expressing MiMIC mediated Dpr12GFSTF (Dpr12‐GFP) and DIP‐δGFSTF (DIP‐δ‐GFP) fusion proteins of the indicated genotypes and time points. (A‐D) Dpr12‐GFP expression is diffuse in DIP‐δT2A‐Gal4 homozygotes mutant brains at L3 (A; n = 20/20), 24 h APF (B; n = 14/14), 48 h APF (C; n = 28/28), and adult (D; n = 26/26). (E‐H) DIP‐δ‐GFP expression in dpr12∆50‐81 homozygotes mutant brains remains localized to the distal part of the γ‐lobe at L3 (E; n = 16/16), 24 h APF (F; n = 16/16), and 48 h APF (G; n = 10/10) but cannot be identified in adult brains (H; n = 16/16). (I, J) Dpr12‐GFP expression in WT animals (I, n = 8/8) or in those ectopically expressing DIP‐δ in PAM‐DANs that innervate the γ3 zone (J, n = 14/14) driven by MB441B‐Gal4 (PAM‐DAN‐γ3‐Gal4). DIP‐δ expression in PAM‐DAN‐γ3 resulted in Dpr12‐GFP localization within the γ3 zone (arrow), in addition to its normal γ4/γ5 localization.
Data information: Arrowheads demarcate DIP‐δ expression at the distal part of the lobe. Asterisks demarcate the distal part of the lobe. Dashed line depicts the medial γ‐lobe, as determined by FasII staining. See legend of Fig EV1D for an explanation regarding the β‐lobe morphological defects observed in (E, J). Green is GFP, and magenta is FasII. Grayscale panels represent single channels, as indicated. Scale bar is 20 µm.
To explore whether DIP‐δ expression is sufficient to regulate Dpr12 protein localization within the γ‐lobe, we misexpressed DIP‐δ in PAM‐DANs that innervate the γ3 zone, normally devoid of Dpr12 protein (Fig 6I). Remarkably, we found that DIP‐δ misexpression indeed caused Dpr12 protein to become localized to the adult γ3 zone, in addition to its endogenous γ4/γ5 localization (Fig 6J). Together, these results indicate that while DIP‐δ is both required and sufficient for Dpr12 localization throughout development, Dpr12 is required only for maintenance of the adult localization of DIP‐δ.
Dpr12‐DIP‐δ interaction mediates circuit re‐assembly
We next determined whether the loss of normal Dpr12‐DIP‐δ interaction induced axonal misrouting, cell loss, or other circuit reorganizations. In dpr12 mutants, we found that PAM‐DANs which normally target the γ4 or γ5 zones misrouted and failed to form substantial connections within the γ‐lobe (Figs 7A–D, and EV5A–E). Importantly, the number of PAM‐DAN cell bodies of the subtypes tested remained unaffected in dpr12 mutants, suggesting that the observed change is not associated with cell death (Fig EV5C and F). In contrast, the γ4 > γ1γ2‐MBON still innervated the γ‐lobe in dpr12 mutant brains, albeit in abnormal locations like the γ3 compartment (Figs 7E and F, and EV5G–I). As expected, the innervation pattern of an MBON targeting the γ3 zone (MBONγ3β’1 or MBON‐09) was unaffected by the dpr12 mutant phenotype (Appendix Fig S2). Finally, we examined the global neuropil structure in dpr12 mutant brains by following staining of the active zone protein bruchpilot (Brp), which demonstrated that the γ4/5 zones were largely missing (Fig 7G and H, Movies [Link], [Link]). Interestingly, the lack of these zones was accompanied by enlarged γ2/3 zones, as well as distortions in other, adjacent brain regions. This was demonstrated, for example, with Crepine (Cre), a neuropil that surrounds the medial MB lobes and functions as a convergence zone for DAN dendrites and MBON axons (Aso et al, 2014a; Fig 7G and H). Whether the change in Crepine anatomy is a result of a direct function of Dpr12‐DIP‐δ specifically within the Crepine, or a secondary effect due to the lack of the γ4–5 zones, remains to be determined.
Figure 7. Dpr12‐DIP‐δ interaction mediates circuit assembly.
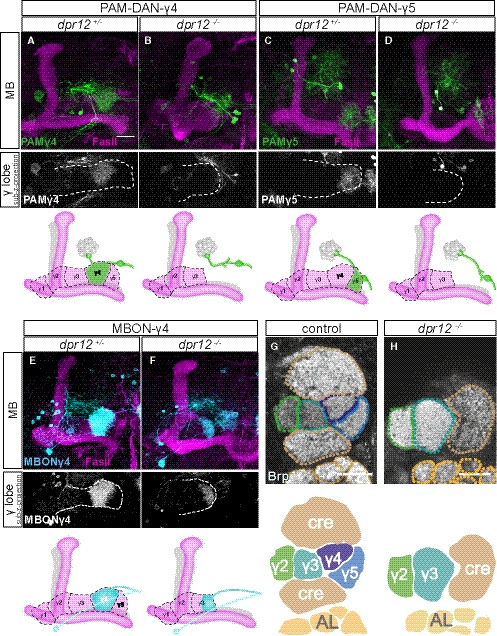
-
A–FTop: Confocal z‐projections of dpr12∆50‐81 heterozygous (A, n = 15; C, n = 10; E, n = 10) and homozygous brains (B, n = 8; D, n = 18; F, n = 24) expressing mCD8‐GFP (CD8) driven by: (A, B) R10G03‐Gal4 (PAM‐DAN‐γ4‐Gal4); (C, D) R48H11‐Gal4 (PAM‐DAN‐γ5‐Gal4), or (E, F) R18H09‐Gal4 (MBONγ4 > γ1γ2 ‐Gal4). The grayscale channels are sub‐z‐projections comprised of slices restricted to the γ‐lobe region. White dashed line demarcates the γ‐lobe. Bottom: Cartoons schematizing MB lobe structure and innervation by specific PAM‐DANs or MBON.
-
G, HSingle confocal slices of WT (G, n = 5/5) and dpr12∆50‐81 homozygous brains (H, n = 5/5) stained with anti‐Brp. Dashed lines demarcate neuropil boundaries, schematic shown below. Cre, crepine, a neuropil that surrounds the medial MB lobes; AL, antenna lobe.
Data information: Magenta is FasII, green and cyan are mCD8‐GFP, and grayscale depicts single channels as indicated. Scale bar is 20 µm.
Taken together, our data suggest that the Dpr12‐DIP‐δ interaction is required for circuit re‐assembly between γ‐KCs and PAM‐DANs and is not required for PAM‐DAN viability.
γ4/5 zone formation depends on matching Dpr‐DIP‐pairing between γ‐KCs and PAM‐DANs
To gain mechanistic insights into the Dpr12‐DIP‐δ interaction, we wanted to determine whether it is specifically required for the formation of the γ4/5 zones, or, alternatively, could other similar interactions between γ‐KCs and PAM‐DANs mediate this process. First, we tested whether loss of the Dpr12‐DIP‐δ interaction could be compensated by other matching Dpr‐DIPs. We focused on the interaction between Dpr6/Dpr10 and their interacting partner DIP‐α (Carrillo et al, 2015), since both Dpr6 and Dpr10 are endogenously expressed in γ‐KCs (Fig 1B; Alyagor et al, 2018), and DIP‐α is largely absent from PAM‐DANs (Croset et al, 2018; Aso et al, 2019). In a replacement experiment, we overexpressed DIP‐α within DIP‐δ‐expressing neurons (using DIP‐δ‐Gal4), on a DIP‐δ ‐/‐ background. Remarkably, this resulted in complete suppression of the DIP‐δ mutant phenotype, and γ‐axons now extended to the edge of the lobe (Fig 8A, B, and G, see additional controls in Fig EV6A and B). Thus, expression of DIP‐α instead of DIP‐δ is sufficient to rescue γ4/5 innervation. While DIP‐α likely interacts with Dpr6/Dpr10 in γ‐KCs, in theory DIP‐α could also function via Dpr12. To further explore this possibility, we performed two parallel experiments. First, we determined Dpr12 protein localization in this abnormal situation where DIP‐δ is missing but γ‐lobe morphology is seemingly normal. Indeed, we found that in this genetic background, Dpr12‐GFP is no longer enriched in the γ4/5 zones but instead diffused along the γ‐lobe, suggesting DIP‐α functions in a Dpr12‐independent manner (Fig 8C and D). Second, we wanted to determine whether expression of DIP‐α in addition to DIP‐δ can suppress the dpr12 mutant phenotype. Indeed, we found that overexpressing DIP‐α within DIP‐δ‐expressing neurons, this time on a dpr12 ‐/‐ background, also led to suppression of the wiring defect, as well as restored the normal innervation pattern of PAM‐DANs (see cyan in Fig 8E and F). Together, these findings suggest that formation of the γ4/5 zones can be driven by matching Dpr‐DIP‐mediated interactions between γ‐KCs and PAM‐DANs, regardless of their specific identity.
Figure 8. γ4/5 zone formation depends on matching Dpr‐DIP‐pairing between γ‐KCs and PAM‐DANs.
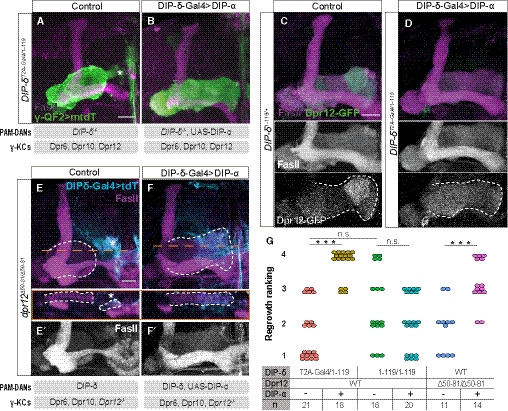
-
A, BConfocal z‐projections of adult DIP‐δT2A‐Gal4/ 1‐119 trans‐heterozygous mutant brains, in which γ‐KCs are labeled by membrane‐bound tandem tomato (mtdT‐HA; green) driven by R71G10‐QF2 (γ‐QF2), and DIP‐δ‐Gal4 (expressed in DIP‐δ+ neurons) either drives expression of UAS‐DIP‐α (B) or not (A). Gray boxes below the images describe the relevant components within PAM‐DANs and γ‐KCs.
-
C, DConfocal z‐projections of adult DIP‐δT2A‐Gal4/ 1‐119 trans‐heterozygous mutant (D) or DIP‐δ1‐119/ + heterozygous (C) brains, which express MiMIC mediated Dpr12GFSTF (Dpr12‐GFP; green). In (D), DIP‐δ‐Gal4 drives expression of UAS‐DIP‐α. Grayscale panels represent single channels, as indicated. The γ‐lobe is outlined in white.
-
E, FConfocal z‐projections of adult dpr12Δ50‐81 homozygous mutant brains, in which DIP‐δ‐Gal4 drives expression of either UAS‐mtdT (E) or UAS‐DIP‐α‐T2A‐tdT (F; expected to induce expression of DIP‐α as well as tdT encoded by a polycistronic message). In orange are longitudinal sections across the γ‐lobe at the indicated location. The γ‐lobe is outlined in white, as determined by FasII staining (gray in E’‐F’). Cyan is tdT within DIP‐δ+ cells. Gray boxes below the images describe the relevant components within PAM‐DANs and γ‐KCs.
-
GRanking of regrowth for (A, B, E, F) and Fig EV6A and B. Regrowth defect severity and statistics were calculated as in Fig 1; Wilcoxon–Mann–Whitney test; ***P < 0.001; ns, not significant.
Data information: In all images, magenta is FasII. Asterisks demarcate the distal part of the lobe. Scale bar is 20 µm.
Finally, since Dpr/DIPs are IgSF proteins, hence their interactions are expected to be, at least in principle, of adhesive nature, we examined whether another adhesive interaction between γ‐KCs and PAM‐DANs could replace the Dpr12‐DIP‐δ interaction. We focused on FasII, an IgSF adhesion molecule that forms homophilic interactions and is endogenously expressed in γ‐KCs (Kurusu et al, 2002; Bornstein et al, 2015). However, overexpressing FasII within DIP‐δ‐expressing cells was not sufficient to suppress the DIP‐δ ‐/‐ phenotype, and the γ4/5 zones failed to form (Fig EV6C and D), indicating the specific requirement of Dpr‐DIP‐mediated interactions.
Taken together, our data suggest that matching interactions between Dprs, expressed in γ‐KCs, and DIPs, expressed in PAM‐DANs, mediate the formation of the MB γ4/5 zones, via a mechanism that is not solely based on adhesion.
Discussion
Our understanding of the development of complex neural circuits remains largely unknown. Specifically, how long axons can make en passant synapses with different partners in a stereotypic manner is not well understood. The unique development and morphology of the Drosophila MB γ‐lobe, combined with the comprehensive genetic power of the fly, offer an excellent opportunity to dissect mechanisms required for wiring of complex neural networks, and specifically mechanisms that drive zonation within axonal bundles to allow for stereotypic localized innervation by distinct populations of neurons. Here, we identify a molecular mechanism that mediates neuron–neuron interactions which subsequently promote the formation of stereotypic circuits that define subcellular axonal zones.
The adult γ‐lobe is divided into zones (also known as compartments) due to specific and localized innervations by extrinsic MB neurons including MBONs and DANs. Here we show that the interaction between two IgSF proteins, Dpr12 on γ‐KCs and DIP‐δ on PAM‐DANs, underlies the formation of the MB γ4/5 zones. Within each zone, input from DANs can modify synaptic strength between the KC and MBON to provide specific valence to sensory information (Aso et al, 2014a, 2014b,2014a, 2014b; Cohn et al, 2015; Cognigni et al, 2018). Based on the results presented here, we speculate that various specific combinations of adhesion molecules may mediate target recognition events that occur between predefined synaptic pairs in other MB zones as well. γ‐neurons express a broad spectrum of IgSFs in tight temporal regulation (Dataset EV1; Alyagor et al, 2018), highlighting their potential role in circuit formation. However, many adhesion molecules, including Dpr/DIPs, can form promiscuous interactions, making their analyses challenging. Future studies could use CRISPR/Cas9 technology to generate multi‐gene mutations to further explore the adhesion code required for zone/compartment formation.
Here we used the interaction between Dpr12 and DIP‐δ to study the development of the γ4/5 zones. Our developmental analyses have concluded that DIP‐δ‐expressing PAM‐DANs arrive to the region of the γ4/5 zones before γ‐axons. Interestingly, our DIP‐δ localization experiments suggest that in dpr12 mutant animals, PAM‐DANs arrive to the right place (the future γ4/5 zones) during larval development, maintain their processes at least until 48 h APF, but eventually (at a yet unknown time point) eliminate or remodel their γ4/5 innervations, while maintaining and even strengthening/broadening other connections in this vicinity. Therefore, it is attractive to speculate that γ‐axons extend into a prepatterned lobe. More studies comparing the development of other compartment‐specific DANs as well as MBONs are however required.
Here we demonstrate that Dpr12 is cell‐autonomously required in γ‐KCs, while DIP‐δ is required in PAM‐DANs for the formation of the γ4/5 zones. To the best of our knowledge, this is the first case in which a Dpr molecule was shown to be cell‐autonomously required for correct wiring. However, the precise molecular mechanism by which the Dpr12‐DIP‐δ interaction mediates formation of the γ4/5 zones, or, in fact, how any wiring by Dpr‐DIPs is achieved, is yet to be determined. The robust phenotype associated with loss of the Dpr12‐DIP‐δ interaction offers an excellent opportunity to delve into the mechanistic basis, which could potentially shed light on similar mechanisms in the visual system and the NMJ. Further research should focus on several critical questions that remain unresolved: (i) Why do the γ‐axons stop prematurely? That γ‐axons stall at the γ3‐γ4 junction when we perturb the Dpr12‐DIP‐δ interaction—which at least in principle is expected to be of adhesive nature—is unintuitive. One possibility is that axon growth into the γ4/5 zones depends on Dpr12‐DIP‐δ interaction either because they overcome a yet undiscovered inhibitory signal, or because they are positively required for the progression of the growth cone. Alternatively, Dpr12‐DIP‐δ interaction could be important for the stabilization of the connections between γ‐axons and PAM‐DAN processes to result in the formation of the γ4/5 zones. At 48 h APF, the large majority of dpr12 mutant γ‐axons do not innervate the γ4/5 zones, arguing against the stability hypothesis; (ii) What are the signaling pathways that mediate Dpr/DIP targeting recognition? None of the Dprs or DIPs contain a large intracellular domain that is capable of signaling. Identifying the potential co‐receptor/s is a critical step in gaining a mechanistic understanding of axon targeting whether in the visual, motor or MB circuits. Our results that DIP‐α can replace DIP‐δ suggest that signaling may be conserved between different Dpr‐DIP pairs; (iii) What is the significance of the GPI anchor? Many of the Dprs and DIPs are predicted to be GPI‐anchored proteins (e.g., Cheng et al, 2019), suggesting that they can be cleaved to create a secreted soluble form. Whether this is an important step in targeting has not yet been investigated. Interestingly, the vertebrate homologs of the DIPs, the IgLON subfamily (Zinn & Ozkan, 2017), are GPI‐anchored proteins that were shown to be cut by metalloproteinases to promote axonal outgrowth (Sanz et al, 2015).
Expression patterns of Dpr and DIP molecules in the NMJ (Carrillo et al, 2015) and visual system (Carrillo et al, 2015; Tan et al, 2015) suggested a model where these molecules instruct target cell specificity. Recent loss‐of‐function experiments strengthened this target specificity hypothesis, as the DIP‐α‐Dpr10 interaction was shown to be important for motoneuron innervation of specific larval (Ashley et al, 2019) and adult (Venkatasubramanian et al, 2019) muscles, and DIP‐α‐Dpr10/Dpr6 interactions for specific layer targeting in the visual system (Xu et al, 2018). Our results suggest that mechanisms used to target axons and dendrites to specific cell types or layers may be further implicated to orchestrate the wiring of long axons to different pre‐ and postsynaptic partners along their route and thus the formation of axonal zones.
Here we describe that interaction between two IgSF proteins mediates transneuronal communication that is required for proper wiring within specific zones of the Drosophila MB. The anatomical organization of the MB suggests that these interactions may provide target specificity for the long KC axon, while it forms en passant synapses with different targets along its length. While the existence of such wiring architecture is known from invertebrates such as Drosophila and C. elegans, long axons making distinct yet stereotypic en passant connections are not widely described in vertebrates. Given the existence of long axons, that travel through dense neuropil structures, such as mossy fibers in the hippocampus, cholinergic axons in the basal forebrain, and parallel fibers in the cerebellar cortex, we posit that this type of connectivity exists in vertebrates but has not yet been described in detail due to technological limitations that are likely to be resolved soon. Pairwise IgSF‐mediated molecular interactions are conserved in vertebrates and invertebrates, implying similar mechanisms to dictate axon and dendrite targeting of subcellular neurite zones in other organisms.
Materials and Methods
Reagents and Tools Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Chicken anti GFP 1:500 | AVES |
GFP‐1020 RRID: AB_10000240 |
| Mouse monoclonal anti FasII 1:25 | Developmental Studies Hybridoma Bank (DSHB) |
1D4 RRID: AB_528235 |
| Mouse monoclonal anti Brp 1:5 | DSHB |
nc82 RRID: AB_2314866 |
| Rabbit anti TH 1:500 | Merck Millipore |
AB152 RRID: AB_390204 |
| Rat anti RFP 1:500 | ChromoTek |
5f8 RRID: AB_2336064 |
| Alexa fluor 568 Goat anti Rat 1:500 | Invitrogen |
A‐21247 RRID: AB_2534121 |
| Alexa fluor 647 Goat anti Mouse 1:500 | Invitrogen |
A‐32728 RRID: AB_2633277 |
| Alexa fluor 488 Goat anti Mouse 1:500 | Invitrogen |
A‐11001 RRID: AB_2534069 |
| FITC Goat anti Chicken 1:500 | Invitrogen |
A‐16055 RRID: AB_2534728 |
| Alexa fluor 568 Goat anti Rabbit 1:500 | Invitrogen |
A‐11036 RRID: AB_10563566 |
| Bacterial and Virus Strains | ||
| DH5ɑ | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Cell Dissociation Solution | Sigma Aldrich |
Cat# C1544 |
| Collagenase/Dispase mix | Roche | Cat# 10269638001 |
| poly‐L‐lysine | Sigma Aldrich | Cat# P1524‐25MG |
| Critical Commercial Assays | ||
| Pico pure RNA isolation kit | Thermo Fisher | Cat# KIT0204 |
| Gibson assembly | NEB | Cat# E5510S |
| Deposited Data | ||
| Raw data files for UNF Perturbation seq | Deposited in the NCBI Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE165896) | |
| Experimental Models: D. melanogaster | ||
|
w[1118]; P{y[+t7.7] w[+mC]=GMR71G10‐GAL4}attP2 (R71G10‐Gal4) |
Bloomington Drosophila Stock Center (BDSC) |
BDSC: 39604 FlyBase ID (FBID): FBsf0000166728 |
| y*,w*; P{y[+t7.7] w[+mC]=GMR71G10‐GAL4}attP40 | (Alyagor et al, 2018) | N/A |
| P{GawB}elavC155 (C155‐GAL4) | BDSC |
BDSC: 458 FBID: FBti0002575 |
| w1118; P{GAL4}repo/TM3, Sb1 (Repo‐Gal4) | BDSC |
BDSC: 7415 FBID: FBti0018692 |
| w[1118]; P{y[+t7.7] w[+mC]=GMR58E02‐GAL4}attP2 (R58E02‐Gal4) | BDSC |
BDSC: 41347 FBID: FBtp0061564 |
| w[1118]; P{y[+t7.7] w[+mC]=GMR18H09‐GAL4}attP2 (R18H09‐Gal4) | BDSC |
BDSC: 48830 FBID: FBti0133650 |
| w[1118]; P{y[+t7.7] w[+mC]=GMR48H11‐GAL4}attP2 (R48H11‐GAL4) | BDSC |
BDSC: 50396 FBID: FBti0136291 |
| w[1118]; P{y[+t7.7] w[+mC]=GMR10G03‐GAL4}attP2 (R10G03‐Gal4) | BDSC |
BDSC: 48271 FBID: FBti0132904 |
| P{y[+t7.7] w[+mC]= GMR71G10‐QF2HSP}attP40 (R71G10‐QF2) | This study | N/A |
| y[1] w[*]; PBac{y[+mDint2] w[+mC]=10XQUAS‐6XGFP}VK00018, P{w[+mC]=UAS‐mtdTomato‐3xHA}2; P{y[+t7.7] w[+mC]=GMR58E02‐QF2.L}attP2 (R58E02‐QF2) | BDSC |
BDSC: 66480 FBID: FBti0184753 |
| w[1118]; P{y[+t7.7] w[+mC]=R53C03‐p65.AD}attP40; P{y[+t7.7] w[+mC]=R24E12‐GAL4.DBD}attP2/TM6B, Tb[1] (MB298B‐Gal4) | BDSC |
BDSC: 68309 FBID: FBst0068309 |
| w[1118]; P{y[+t7.7] w[+mC]=R30G08‐p65.AD}attP40; P{y[+t7.7] w[+mC]=R48B03‐GAL4.DBD}attP2 (MB441B‐Gal4) | BDSC |
BDSC: 68251 FBID: FBst0068251 |
| w[*]; P{w[+mW.hs]=GawB}OK107 ey[OK107] (OK107‐Gal4) | BDSC |
BDSC: 854 FBID: FBti0004170 |
|
w[1118]; P{y[+t7.7] w[+mC]=R94B10‐GAL4.DBD}attP2 PBac{y[+mDint2] w[+mC]=R52G04‐p65.AD}VK00027 (MB083C‐Gal4) |
BDSC |
BDSC: 68287 FBID: FBst0068287 |
| y*,w*; DIP‐δ‐T2A‐Gal4 | This study | N/A |
|
w[*]; P{y[+t7.7] w[+mC]=10XUAS‐IVS‐mCD8::GFP}attP2 (UAS‐CD8‐GFP) |
BDSC |
BDSC: 32185 FBID: FBst0032185 |
| w[*]; P{y[+t7.7] w[+mC]=10XUAS‐IVS‐mCD8::GFP} attP40 (UAS‐CD8‐GFP) | BDSC |
BDSC: 32186 FBID: FBst0032185 |
| y[1] w[*] P{y[+t7.7] w[+mC]=10XUAS‐IVS‐mCD8::GFP}su(Hw)attP8 (UAS‐CD8‐GFP) | BDSC |
BDSC: 32189 FBID: FBst0032189: |
|
y[1] w[*]; P{w[+mC]=UAS‐CD4‐tdTom}7M1 (UAS‐CD4‐tdT) |
BDSC |
BDSC: 35841 FBID: FBst0035841 |
|
y[1] w[1118]; P{w[+mC]=QUAS‐mtdTomato‐3xHA}26 (QUAS‐mtdT) |
BDSC |
BDSC: 30005 FBID: FBti0129951 |
| w[1118]; P{w[+mC]=UAS‐Dcr‐2.D}10 | BDSC |
BDSC: 24651 FBID: FBst0024651 |
|
P{hsFLP}22, w*, P{w[+mC]=UAS‐mCD8::GFP.L}; P{w[+mC]=tubP‐GAL80}LL10 P{ry[+t7.2]=neoFRT}40A; GMR71G10‐Gal4, P{w[+mC]=UAS‐Dcr‐2.D}10 (recombination between R71G10‐Gal4 and UAS‐Dcr‐2) |
This study |
N/A FBIC: FBrf0227179 |
| P{hsFLP}22, w*, P{w[+mC]=UAS‐mCD8::GFP.L}; P{w[+mC]=tubP‐GAL80}LL10 P{ry[+t7.2]=neoFRT}40A; DIP‐δ‐T2A‐Gal4 | This study | N/A |
| y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.JF03306}attP2 (RNAi of Dpr5) | BDSC |
BDSC: 29627 FBID: FBst0029627 |
| y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.JF03172}attP2 (RNAi of Dpr8) | BDSC |
BDSC: 28744 FBID: FBst0028744 |
| y[1] sc[*] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.HMS00288}attP2 (RNAi of Dpr9) | BDSC |
BDSC: 33409 FBID: FBst0033409 |
| y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.JF02920}attP2 (RNAi of Dpr10) | BDSC |
BDSC: 27991 FBID: FBst0027991 |
| y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.JF03210}attP2 (RNAi of Dpr12) | BDSC |
BDSC: 28782 FBID: FBst0028782 |
| [1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.GL01238}attP2 (RNAi of Dpr17) | BDSC |
BDSC: 41656 FBID: FBst0041656 |
| y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.JF03283}attP2 (RNAi of Dpr18) | BDSC |
BDSC: 29604 FBID: FBst0029604 |
| [1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.JF02923}attP2 (RNAi of Dpr20) | BDSC |
BDSC: 28293 FBID: FBst0028293 |
| P{ry[+t7.2]=hsFLP}12, y[1] w[*]; P{y[+t7.7] w[+mC]=UAS‐Cas9.P2}attP40 | BDSC |
BDSC: 58985 FBID: FBst0058985 |
| w[1118]; P{y[+t7.7] w[+mC]=UAS‐Cas9.C}attP2 | BDSC |
BDSC: 54595 FBID:FBst0054595 |
| gRNA dpr12 | This study | N/A |
| y[1] sc[*] v[1]; P{y[+t7.7] v[+t1.8]=TKO.GS02451}attP40 (gRNA DIP‐δ) | BDSC |
BDSC: 78754 FBID: FBst0078754 |
| y*,w*;FRT40A, dpr12∆50‐81, FRTG13, cn,bw | This study | N/A |
| w*; DIPδ1‐119, FRT2A | A generous gift from Larry Zipursky, UCLA | N/A |
| y[1] w[67c23]; Mi{PT‐GFSTF.1}dpr12[MI01695‐GFSTF.1]/SM6a (dpr12GFSTF) | BDSC |
BDSC: 60171 FBID: FBst0060171 |
| y[1] w[*]; Mi{PT‐GFSTF.1}DIP‐delta[MI08287‐GFSTF.1] (DIP‐δGFSTF) | BDSC |
BDSC: 60558 FBID: FBst0060558 |
| y[1] w[*]; Mi{y[+mDint2]=MIC}DIP‐delta[MI08287] | BDSC |
BDSC: 51229 FBID: FBst0051229 |
| y[1] w[*]; P{w[+mC]=lox(Trojan‐GAL4)x3}10; Dr[1]/TM3, Sb[1] Ser[1] | BDSC |
BDSC: 60310 FBID: FBst0060310 |
| P{y[+mDint2]=Crey}1b, y[1] M{vas‐int. Dm}ZH‐2A w[*] | BDSC |
BDSC: 60299 FBID: FBst0060299 |
| w[*]; P{w[+mC]=UAS‐Cbbeta\DT‐A.I}18/CyO (UAS‐DTI) | BDSC |
BDSC: 25039 FBID: FBst0025039 |
| UAS‐Dpr12 (in86FB) | This study | N/A |
| UAS‐DIP‐δ (in attp40) | This study | N/A |
| UAS‐DIP‐δ‐T2A‐tdT | A generous gift from Larry Zipursky, UCLA | N/A |
| UAS‐DIP‐α | A generous gift from Larry Zipursky, UCLA | N/A |
| UAS‐DIP‐α‐T2A‐tdT | A generous gift from Larry Zipursky, UCLA | N/A |
| UAS‐FasII‐A | A generous gift form Brian McCabe (Beck et al, 2012) | N/A |
| y[1] M{w[+mC]=nos‐Cas9.P}ZH‐2A w[*] | BDSC |
BDSC: 54591 FBID: FBst0054591 |
| UAS‐DIP‐δ‐RNAi (in attp40) | This study | N/A |
| y[1] w[*] P{y[+t7.7] w[+mC]=10XUAS‐IVS‐mCD8::RFP}su(Hw)attP8 | BDSC |
BDSC: 32220 FBID: FBst0032220 |
| Oligonucleotides | ||
|
gRNAs for Dpr12 CRISPR deletion: CGCAGTTCCATCAGGTGCAGGGG CCATTAGACATATCTTCCTGACC |
This study | N/A |
|
Primers for dpr12∆50‐81 check PCR: F’:GTTGCCGTAGCTGAAAGGATT R’:TAAACCGGGTATCGGAGTGTC |
This study | N/A |
|
RNAi for DIPδ (SS) GACGAUAAGAAACCUACAAUA (AS) UUGUAGGUUUCUUAUCGUCAG |
This study | N/A |
|
Primers for QF2 cloning F’:TAAGCCAACTTTGAATCACAAGACGCATACCAAACGGTACATGCCACCCAAG R’:TGAATAATTTTCTATTTGGCTTTAGTCGACGGTATCGATAATCACTGTTCGT |
This study | N/A |
|
Primers for hsp70 cloning F’:AAGTGGTGATAAACGGCCGGCCGAGCGCCGGAGTATAAATAGAG R’:AAGTGGTGATAAACGGCCGGCCGAGCGCCGGAGTATAAATAGAG |
This study | N/A |
| Recombinant DNA | ||
| pCFD4 | (Port et al, 2014) | N/A |
| pVALIUM22 | Harvard Medical School | PlasmID: pVALIUM22 |
| pDEST‐UAS‐IVS‐Syn21‐p10aw | (Rabinovich et al, 2016) | N/A |
| pBPGUw | addgene | Plasmid #17575 |
| pBPGUw‐QF2 | This study | N/A |
| GMR71G10 entry vector | (Alyagor et al, 2018) | N/A |
| GMR71G10‐QF2hsp70 | This study | N/A |
| Plasmid: UAS‐IVS‐Syn21‐Dpr12‐p10 | This study | N/A |
| Plasmid: UAS‐IVS‐Syn21‐DIP‐δ‐p10 | This study | N/A |
| Plasmid: pCFD4‐dpr12 (Dpr12 gRNA) | This study | N/A |
| Plasmid: pVALIUM22‐DIP‐δ (DIP‐δ RNAi) | This study | N/A |
| Software and Algorithms | ||
| FIJI | Image J | https://imagej.net/Fiji/Downloads |
| VVD Viewer (a branch of FluoRender, Center for Integrative Biomedical Computing (CIBC), Utah) | Takashi Kawase | https://github.com/takashi310/VVD_Viewer/releases |
| MATLAB R2016a software | MathWorks | N/A |
| FlyMine | (Lyne et al, 2007) | http://www.flymine.org/ |
| HISAT v.0.1.5 | (Kim et al, 2015) | https://github.com/infphilo/hisat |
| DEseq2 | (Love et al, 2014) | N/A |
| Gene‐e v.3.0.215 | Broad Institute, Inc. | https://software.broadinstitute.org/GENE‐E/ |
| HOMER software | (Heinz et al, 2010) | |
| DSIR | (Vert et al, 2006) | http://biodev.extra.cea.fr/DSIR/DSIR.php |
| FlyCRISPR | (Gratz et al, 2014) | http://flycrispr.molbio.wisc.edu/ |
| neuPrint | (Clements et al, 2020) | https://neuprint.janelia.org/ |
| Other | ||
| Zeiss LSM 800 confocal microscope | Zeiss | |
| 40× 1.3 NA oil immersion lens | Zeiss | |
Methods and Protocols
Experimental model
Drosophila melanogaster flies were reared under standard laboratory conditions at 25°C on molasses containing food. Males and females were chosen at random. For developmental analysis, white pupae were collected and incubated for the indicated number of hours. For adult analysis, flies were dissected 3–5 days posteclosion.
See Appendix: List of genotypes for the detailed list of all fly genotypes used in this study.
RNA extraction
The RNA extraction of WT and UNF‐RNAi‐expressing MB γ‐KCs was performed as described in Alyagor et al (2018). In brief, brains were dissected in a cold Ringer’s solution and dissociated by incubation with collagenase/dispase mix at 29°C (Roche, 15 min for larval and pupal brains and 30 min for adult brains), washed in dissociation solution (Sigma‐Aldrich), and mechanically dissociated into single cells. Cells were transferred via 35 µm mesh (Falcon) to eliminate clusters and debris. 1,000 γ‐KCs (DsRed+) were sorted using a 100 mm nozzle and low pressure in BD FACSAria Fusion (BD Bioscience) directly into 100 µl Pico‐Pure RNA isolation kit extraction buffer (Life Technologies) followed by RNA extraction. mRNA was captured using 12 ml of Dynabeads oligo (Life Technologies), which were washed from unbound total RNA according to the protocol. mRNA was eluted from beads at 85°C with 10 ml of 10 mM Tris–HCl (pH 7.5). mRNA was barcoded, converted into cDNA, and linearly amplified by T7 in vitro transcription. The resulting RNA was fragmented and converted into an Illumina sequencing‐ready library through ligation, RT, and PCR. Prior to sequencing, libraries were evaluated by Qubit fluorometer and TapeStation (Agilent).
Analysis of RNA‐seq Data
Samples were sequenced using Illumina NextSeq 500, at a sequencing depth of an average of 5 million reads. We aligned the reads to D. melanogaster reference genome (DM6, UCSC) using Hisat v0.1.5 with “–sensitive ‐local” parameters (Kim et al, 2015). Gene annotation was taken from FlyBase.org (Dmel R6.01/Fb_2014_04). Duplicate reads were filtered if they aligned to the same base and had identical unique molecular identifiers (UMI). Expression levels were counted using HOMER software (http://homer.salk.edu) (Heinz et al, 2010). For general analyses, we considered genes with reads over the noise threshold (20 reads). Significant expression in γ‐KCs was considered for genes with reads over a second noise threshold (50 reads) in at least two γ‐KCs. For normalization and statistics, we performed DEseq2 algorithm (Love et al, 2014) on our samples on R platform, which took into account batch effects. All P‐values presented for RNA‐seq data are adjusted P‐values. Gene enrichment analysis was done using FlyMine (http://www.flymine.org/).
Generation of CRISPR‐mediated mutant
For Dpr12 mutation, two guide RNAs were designed using the FlyCRISPR algorithm (http://flycrispr.molbio.wisc.edu/) and cloned into pCFD4 using Transfer‐PCR (TPCR) (Unger et al, 2010; Meltzer et al, 2019). The pCFD4‐Dpr12 plasmid was injected into the 86FB landing site using ΦC31 integration (BestGene). Injected flies were crossed with nanos‐Cas9 flies (Bloomington stock #54591). After two generations, single males were crossed with balancers and checked for deletion using specific primers. The dpr12∆50‐81 allele is a 32bp deletion in the 5’ end of the transcript resulting in a premature stop after 37aa.
For tissue‐specific CRISPR (tsCRISPR), Dpr12‐ and DIP‐δ gRNA‐containing flies were crossed with UAS‐Cas9.C or UAS‐Cas9.P2, respectively, driven by the indicated Gal4s.
Generation of transgenes and transgenic flies
To generate UAS‐Dpr12 and UAS‐DIP‐δ transgenes, cDNA was cloned into the Gateway entry vector pDONR201. The Gateway entry vectors were then recombined into pDEST‐UAS‐IVS‐Syn21‐p10aw destination vector (Rabinovich et al, 2016) using LR recombinase (Invitrogen). UAS‐Dpr12 and UAS‐DIP‐δ plasmids were injected into the 86FB and attp40 landing sites, respectively, using ΦC31 integration (BestGene).
For the generation of UAS‐DIP‐δ RNAi, a 21 nucleotide sequence was selected using DSIR (http://biodev.extra.cea.fr/DSIR/DSIR.php). Off‐target results were eliminated by blast in NCBI. The RNAi hairpins were cloned into pVALIUM22 as described in https://fgr.hms.harvard.edu/cloning‐and‐sequencing. In brief, hairpin oligos, containing sense and anti‐sense nucleotide with overhang DNA fragment for NheI and EcoRI, were synthesized (Sigma). 10 µl of sense and anti‐sense strand oligos (10–20 µM each) were annealed into 80 µl annealing buffer (10 mM Tris–HCl, pH 7.5, 0.1 M NaCl, 1 mM EDTA) by incubation at 95°C for 5 min. 6 µl of the annealed oligos were directly cloned into the pVALIUM22 vector which has been linearized by NheI and EcoRI.
DIP‐δ hairpin oligos (CAPS represent gene‐specific sequences):
ctagcagtGACGATAAGAAACCTACAATAtagttatattcaagcataTTGTAGGTTTCTTATCGTCAGgcg.
aattcgcCTGACGATAAGAAACCTACAAtatgcttgaatataactaTATTGTAGGTTTCTTATCGTCactg.
UAS‐DIP‐δ RNAi plasmid was injected into the attp40 landing site, using ΦC31 integration (BestGene).
Generation of DIP‐δT2A‐Gal4
DIP‐δT2A‐Gal4 was generated as described in Diao et al (2015). In brief, flies carrying the MiMICMI08287 insertion were crossed with flies bearing the triplet donor cassettes (Trojan Gal4 cassettes of the three reading frames). Males from this progeny carrying both components were crossed to females carrying germline transgenic sources of Cre and ΦC31. Adult progeny with all relevant components were crossed to UAS‐GFP balanced on the 3rd chromosome. Single males from this final cross were screened by fluorescence microscopy for Gal4 expression.
Generation of QF2 driver
To generate the R71G10‐QF2 driver, the QF2 sequence was amplified from pattB‐DSCP_prom‐QF7‐hsp70_term (a gift from Chris potter) using the QF‐F and QF‐R primers, and cloned into pBPGUw plasmid, using the Gibson assembly kit (NEB) to create pBPGUw‐QF2. Then, the GMR71G10 entry vector (Alyagor et al, 2018) was recombined into pBPGUw‐QF2 using LR recombinase (Invitrogen). Finally, the DSCP promoter region within the GMR71G10‐QF2 was replaced with hsp70 promoter by RF cloning using the hspF and hspR primers. The GMR71G10‐QF2hsp70 plasmid was injected into the attp40 landing site, using ΦC31 integration (BestGene).
QF‐F TAAGCCAACTTTGAATCACAAGACGCATACCAAACGGTACATGCCACCCAAG
QF‐R
TGAATAATTTTCTATTTGGCTTTAGTCGACGGTATCGATAATCACTGTTCGT
HspF
AAGTGGTGATAAACGGCCGGCCGAGCGCCGGAGTATAAATAGAG
HspR
AAGTGGTGATAAACGGCCGGCCGAGCGCCGGAGTATAAATAGAG
Generation of MARCM clones
Due to centromeric chromosomal location of dpr12, we could not generate dpr12 mutant clones and instead expressed dpr12‐RNAi within γ‐KC clones. MB γ‐KC MARCM clones were generated as described in Lee and Luo (1999). In brief, flies were heat shocked (hs) for 40–60 min at 37°C at 24 h after egg laying and examined at the indicated developmental time points.
To discover the mitotic window which results in adult PAM‐DANs, we used the MARCM technique to generate DIP‐δT2A‐Gal4 clones by hs at different developmental times. Only hs at 0–24 h after egg laying resulted in clones containing PAM‐DANs, and therefore, this hs regime was used in this study. Importantly, here mitotic recombination was performed using FRT40A and used to eliminate Gal80 expression but DIP‐δ remained heterozygous as it is on another chromosome.
Immunostaining and imaging
Brains were dissected in ringer solution, fixed using 4% paraformaldehyde (PFA) for 20 min at room temperature (RT), and washed with PB with 0.3% Triton‐X (PBT, three immediate washes followed by 3 × 20 min washes). Non‐specific staining was blocked using 5% heat inactivated goat serum in PBT and then samples were subjected to primary antibodies (over‐night, 4°C) and secondary antibodies (2 h at RT) with PBT washes (three quick washed followed by 3 × 20 min washes). The brains were mounted on SlowFade (Invitrogen) and imaged using Zeiss LSM800 confocal microscope. Images were processed with ImageJ 1.51 (NIH). Individual neurons were traced manually through all focal planes using the Edge Detection Settings of the Analyze Paint Brush VVD selection tool (Takashi Kawase). Thresholding was individually adapted for each focal plane and neuronal structures and refined through the Analyze Erase tool whenever necessary.
For Brp staining, brains were blocked in 2% bovine serum albumin (BSA) in PBT for 2 h at RT, incubated with mouse anti‐Brp antibody for two days at 4°C, and then incubated with secondary antibody for an over‐night at 4°C. Next, brains were transferred to a poly‐L‐lysine (Sigma‐Aldrich. # P1524‐25MG) pre‐treated cover glasses (22 × 22 × 1; Fisher Scientific. # 12‐542B), fixed, dehydrated in ascending alcohol series (30%, 50%, 75%, 95%, and 3 × 100%, 10 min each), incubated in Xylene 2 × 10 min and embedded in DPX (Electron Microscopy Sciences; # 180627‐05) and incubated for at least 4 days. Confocal laser scanning microscopy was done using an Olympus microscope equipped with a Plan‐Apochromat 20x objective. Taken images were analyzed using VVD Viewer (Takashi Kawase).
Generation of EM‐based models
We screened the publicly available connectome neuPrint 1.1 EM dataset (Clements et al, 2020) at the Howard Hughes Medical Institute (HHMI) Janelia Research Campus (https://NeuPrint.janelia.org/) for intrinsic and extrinsic MB circuit entities projecting to the γ4 and γ5 zones of the MB (Scheffer et al, 2020). The resulting EM skeletons were projected with VVD Viewer 1.1 onto the JRC2018_UNISEX template brain (Bogovic et al, 2020). See Appendix Table S1 for the full list of EM skeletons used.
Quantification and statistical analysis
In all cases, statistical significance was calculated as follows: *** represents a P‐value lower than 0.001, ** represents a P‐value lower than 0.01, and * represents a P‐value lower than 0.05. Specific P‐values and sample sizes are indicated in the relevant figure legends.
For quantification of regrowth (Figs 1, 4 and 8), confocal Z‐stacks were given to an independent laboratory member who blindly ranked the severity of the regrowth defects. For statistical analysis, Kruskal–Wallis test was performed followed by a Wilcoxon–Mann–Whitney test.
Author contributions
BB designed, performed, and analyzed experiments, performed bioinformatic analyses, and wrote the manuscript. HM designed, performed, and analyzed experiments and wrote the revised manuscript. RA designed, performed, and analyzed experiments. IA designed and performed the RNA‐seq experiments and performed bioinformatic analyses. VB, GC, and FR performed specific experiments. HK‐S performed and analyzed the RNA‐seq experiments. ED performed bioinformatic analyses. TM performed image analyses and provided important conceptual insights. OS led the project, designed experiments, interpreted results, and wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Movie EV5
Review Process File
Acknowledgements
We thank Larry Zipursky and Brian McCabe for sharing reagents and the Bloomington Stock Centers for reagents; monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa. We thank R. Rothkopf for assistance with statistics; M. Schuldiner, A. Yaron, T. Misgeld, and the O.S laboratory for discussions and critical reading of this manuscript. We thank Life Science Editors for editing assistance. Funding: This work was supported by the European Research Council (erc), consolidator grant # 615906, “AxonGrowth”, the Volkswagen Stiftung (joint Lower Saxony—Israel) grant # ZN3459, the Sagol Institute for Longevity Research, and the Deutsche Forschungsgemeinschaft (DFG) grant RI 2419/4‐1. Fly food for this project was funded by the Women Health Research Center. O.S. is the Incumbent of the Prof. Erwin Netter Professorial Chair of Cell Biology, T.R. was supported by Axa as team member of the Axa Chair from genome to structure, and F.R is a GSfBS member and was supported by Evangelisches Studienwerk Villigst e.V.
The EMBO Journal (2021) 40: e105763.
Contributor Information
Hagar Meltzer, Email: hagar.meltzer@weizmann.ac.il.
Oren Schuldiner, Email: oren.schuldiner@weizmann.ac.il.
Data availability
The RNA‐seq data have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO series accession number GEO: GSE165896 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE165896).
References
- Alyagor I, Berkun V, Keren‐Shaul H, Marmor‐Kollet N, David E, Mayseless O, Issman‐Zecharya N, Amit I, Schuldiner O (2018) Combining developmental and perturbation‐seq uncovers transcriptional modules orchestrating neuronal remodeling. Dev Cell 47: 38–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley J, Sorrentino V, Lobb‐Rabe M, Nagarkar‐Jaiswal S, Tan L, Xu S, Xiao Q, Zinn K, Carrillo RA (2019) Transsynaptic interactions between IgSF proteins DIP‐alpha and Dpr10 are required for motor neuron targeting specificity. eLife 8: e42690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo TT, Dionne H, Abbott LF, Axel R, Tanimoto H et al (2014a) The neuronal architecture of the mushroom body provides a logic for associative learning. eLife 3: e04577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Sitaraman D, Ichinose T, Kaun KR, Vogt K, Belliart‐Guerin G, Placais PY, Robie AA, Yamagata N, Schnaitmann C et al (2014b) Mushroom body output neurons encode valence and guide memory‐based action selection in Drosophila . eLife 3: e04580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Ray RP, Long X, Bushey D, Cichewicz K, Ngo TT, Sharp B, Christoforou C, Hu A, Lemire AL et al (2019) Nitric oxide acts as a cotransmitter in a subset of dopaminergic neurons to diversify memory dynamics. eLife 8: e49257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck ES, Gasque G, Imlach WL, Jiao W, Jiwon Choi B, Wu PS, Kraushar ML, McCabe BD (2012) Regulation of Fasciclin II and synaptic terminal development by the splicing factor beag. J Neurosci 32: 7058–7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilz F, Geurten BRH, Hancock CE, Widmann A, Fiala A (2020) Visualization of a distributed synaptic memory code in the Drosophila brain. Neuron 106: 963–976 [DOI] [PubMed] [Google Scholar]
- Bogovic JA, Otsuna H, Heinrich L, Ito M, Jeter J, Meissner G, Nern A, Colonell J, Malkesman O, Ito K et al (2020) An unbiased template of the Drosophila brain and ventral nerve cord. PLoS One 15: e0236495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein B, Zahavi EE, Gelley S, Zoosman M, Yaniv SP, Fuchs O, Porat Z, Perlson E, Schuldiner O (2015) Developmental axon pruning requires destabilization of cell adhesion by JNK signaling. Neuron 88: 926–940 [DOI] [PubMed] [Google Scholar]
- Carrillo RA, Ozkan E, Menon KP, Nagarkar‐Jaiswal S, Lee PT, Jeon M, Birnbaum ME, Bellen HJ, Garcia KC, Zinn K (2015) Control of synaptic connectivity by a network of Drosophila IgSF cell surface proteins. Cell 163: 1770–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Ashley J, Kurleto JD, Lobb‐Rabe M, Park YJ, Carrillo RA, Ozkan E (2019) Molecular basis of synaptic specificity by immunoglobulin superfamily receptors in Drosophila . eLife 8: e41028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J, Dolafi T, Umayam L, Neubarth NL, Scheffer LK, Plaza SM (2020) neuPrint: analysis tools for EM connectomics. bioRxiv 10.1101/2020.01.16.909465 [PREPRINT] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognigni P, Felsenberg J, Waddell S (2018) Do the right thing: neural network mechanisms of memory formation, expression and update in Drosophila . Curr Opin Neurobiol 49: 51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn R, Morantte I, Ruta V (2015) Coordinated and compartmentalized neuromodulation shapes sensory processing in Drosophila . Cell 163: 1742–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmanescu F, Katsamba PS, Sergeeva AP, Ahlsen G, Patel SD, Brewer JJ, Tan L, Xu S, Xiao Q, Nagarkar‐Jaiswal S et al (2018) Neuron‐subtype‐specific expression, interaction affinities, and specificity determinants of DIP/Dpr cell recognition proteins. Neuron 100: 1385–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EM, Han KA, Kalderon D, Davis RL (1998) Tripartite mushroom body architecture revealed by antigenic markers. Learning & Memory 5: 38–51 [PMC free article] [PubMed] [Google Scholar]
- Croset V, Treiber CD, Waddell S (2018) Cellular diversity in the Drosophila midbrain revealed by single‐cell transcriptomics. eLife 7: e34550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao F, Ironfield H, Luan H, Diao F, Shropshire WC, Ewer J, Marr E, Potter CJ, Landgraf M, White BH (2015) Plug‐and‐play genetic access to Drosophila cell types using exchangeable exon cassettes. Cell Rep 10: 1410–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala A (2007) Olfaction and olfactory learning in Drosophila: recent progress. Curr Opin Neurobiol 17: 720–726 [DOI] [PubMed] [Google Scholar]
- Gerber B, Scherer S, Neuser K, Michels B, Hendel T, Stocker RF, Heisenberg M (2004) Visual learning in individually assayed Drosophila larvae. J Exp Biol 207: 179–188 [DOI] [PubMed] [Google Scholar]
- Gratz SJ, Ukken FP, Rubinstein CD, Thiede G, Donohue LK, Cummings AM, O'Connor‐Giles KM (2014) Highly specific and efficient CRISPR/Cas9‐catalyzed homology directed repair in Drosophila . Genetics 196: 961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK (2010) Simple combinations of lineage‐determining transcription factors prime cis‐regulatory elements required for macrophage and B cell identities. Mol Cell 38: 576–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M (2003) Mushroom body memoir: from maps to models. Nat Rev Neurosci 4: 266–275 [DOI] [PubMed] [Google Scholar]
- Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12: 357–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu M, Awasaki T, Masuda‐Nakagawa LM, Kawauchi H, Ito K, Furukubo‐Tokunaga K (2002) Embryonic and larval development of the Drosophila mushroom bodies: concentric layer subdivisions and the role of fasciclin II. Development 129: 409–419 [DOI] [PubMed] [Google Scholar]
- Lee T, Lee A, Luo L (1999) Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development 126: 4065–4076 [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L (1999) Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22: 451–461 [DOI] [PubMed] [Google Scholar]
- Lee T, Marticke S, Sung C, Robinow S, Luo L (2000) Cell‐autonomous requirement of the USP/EcR‐B ecdysone receptor for mushroom body neuronal remodeling in Drosophila . Neuron 28: 807–818 [DOI] [PubMed] [Google Scholar]
- Li F, Lindsey J, Marin EC, Otto N, Dreher M, Dempsey G, Stark I, Bates AS, Pleijzier MW, Schlegel P et al (2020) The connectome of the adult Drosophila mushroom body: implications for function. bioRxiv 10.1101/2020.08.29.273276 [PREPRINT] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biol 15: 550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyne R, Smith R, Rutherford K, Wakeling M, Varley A, Guillier F, Janssens H, Ji W, McLaren P, North P et al (2007) FlyMine: an integrated database for Drosophila and Anopheles genomics. Genome Biol 8: R129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer H, Marom E, Alyagor I, Mayseless O, Berkun V, Segal‐Gilboa N, Unger T, Luginbuhl D, Schuldiner O (2019) Tissue‐specific (ts)CRISPR as an efficient strategy for in vivo screening in Drosophila . Nat Commun 10: 2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi MN, Shuai Y, Turner GC (2020) The Drosophila mushroom body: from architecture to algorithm in a learning circuit. Annu Rev Neurosci 43: 465–484 [DOI] [PubMed] [Google Scholar]
- Nagarkar‐Jaiswal S, Lee PT, Campbell ME, Chen K, Anguiano‐Zarate S, Gutierrez MC, Busby T, Lin WW, He Y, Schulze KL et al (2015) A library of MiMICs allows tagging of genes and reversible, spatial and temporal knockdown of proteins in Drosophila . eLife 4: e05338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto N, Pleijzier MW, Morgan IC, Edmondson‐Stait AJ, Heinz KJ, Stark I, Dempsey G, Ito M, Kapoor I, Hsu J et al (2020) Input connectivity reveals additional heterogeneity of dopaminergic reinforcement in Drosophila . Curr Biol 30: 3200–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Waddell S (2015) Olfactory learning skews mushroom body output pathways to steer behavioral choice in Drosophila . Curr Opin Neurobiol 35: 178–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkan E, Carrillo RA, Eastman CL, Weiszmann R, Waghray D, Johnson KG, Zinn K, Celniker SE, Garcia KC (2013) An extracellular interactome of immunoglobulin and LRR proteins reveals receptor‐ligand networks. Cell 154: 228–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F, Bullock SL (2016) Augmenting CRISPR applications in Drosophila with tRNA‐flanked sgRNAs. Nat Methods 13: 852–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F, Chen HM, Lee T, Bullock SL (2014) Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila . Proc Natl Acad Sci USA 111: E2967–E2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port F, Strein C, Stricker M, Rauscher B, Heigwer F, Zhou J, Beyersdorffer C, Frei J, Hess A, Kern K et al (2020) A large‐scale resource for tissue‐specific CRISPR mutagenesis in Drosophila . eLife 9: e53865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich D, Yaniv SP, Alyagor I, Schuldiner O (2016) Nitric oxide as a switching mechanism between axon degeneration and regrowth during developmental remodeling. Cell 164: 170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwedder A, Wenz NL, Stehle B, Huser A, Yamagata N, Zlatic M, Truman JW, Tanimoto H, Saumweber T, Gerber B et al (2016) Four individually identified paired dopamine neurons signal reward in larval Drosophila . Curr Biol 26: 661–669 [DOI] [PubMed] [Google Scholar]
- Sanz R, Ferraro GB, Fournier AE (2015) IgLON cell adhesion molecules are shed from the cell surface of cortical neurons to promote neuronal growth. J Biol Chem 290: 4330–4342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saumweber T, Rohwedder A, Schleyer M, Eichler K, Chen YC, Aso Y, Cardona A, Eschbach C, Kobler O, Voigt A et al (2018) Functional architecture of reward learning in mushroom body extrinsic neurons of larval Drosophila . Nat Commun 9: 1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer LK, Xu CS, Januszewski M, Lu Z, Takemura SY, Hayworth KJ, Huang GB, Shinomiya K, Maitlin‐Shepard J, Berg S et al (2020) A connectome and analysis of the adult Drosophila central brain. eLife 9: e57443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai Y, Hirokawa A, Ai Y, Zhang M, Li W, Zhong Y (2015) Dissecting neural pathways for forgetting in Drosophila olfactory aversive memory. Proc Natl Acad Sci USA 112: E6663–E6672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Zhang KX, Pecot MY, Nagarkar‐Jaiswal S, Lee PT, Takemura SY, McEwen JM, Nern A, Xu S, Tadros W et al (2015) Ig superfamily ligand and receptor pairs expressed in synaptic partners in Drosophila . Cell 163: 1756–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka NK, Tanimoto H, Ito K (2008) Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol 508: 711–755 [DOI] [PubMed] [Google Scholar]
- Unger T, Jacobovitch Y, Dantes A, Bernheim R, Peleg Y (2010) Applications of the restriction free (RF) cloning procedure for molecular manipulations and protein expression. J Struct Biol 172: 34–44 [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian L, Guo Z, Xu S, Tan L, Xiao Q, Nagarkar‐Jaiswal S, Mann RS (2019) Stereotyped terminal axon branching of leg motor neurons mediated by IgSF proteins DIP‐alpha and Dpr10. eLife 8: e42692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert JP, Foveau N, Lajaunie C, Vandenbrouck Y (2006) An accurate and interpretable model for siRNA efficacy prediction. BMC Bioinformatics 7: 520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Xiao Q, Cosmanescu F, Sergeeva AP, Yoo J, Lin Y, Katsamba PS, Ahlsen G, Kaufman J, Linaval NT et al (2018) Interactions between the Ig‐superfamily proteins DIP‐alpha and Dpr6/10 regulate assembly of neural circuits. Neuron 100: 1369–1384.e1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv SP, Issman‐Zecharya N, Oren‐Suissa M, Podbilewicz B, Schuldiner O (2012) Axon regrowth during development and regeneration following injury share molecular mechanisms. Curr Biol 22: 1774–1782 [DOI] [PubMed] [Google Scholar]
- Yaniv SP, Meltzer H, Alyagor I, Schuldiner O (2020) Developmental axon regrowth and primary neuron sprouting utilize distinct actin elongation factors. J Cell Biol 219: e201903181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K, Ozkan E (2017) Neural immunoglobulin superfamily interaction networks. Curr Opin Neurobiol 45: 99–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Dataset EV1
Dataset EV2
Movie EV1
Movie EV2
Movie EV3
Movie EV4
Movie EV5
Review Process File
Data Availability Statement
The RNA‐seq data have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO series accession number GEO: GSE165896 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE165896).


