Abstract
Advancements in measurement and modeling capabilities are providing unprecedented access to estimates of chemical exposure and bioactivity. With this influx of new data, there is a need for frameworks that help organize and disseminate information on chemical hazard and exposure in a manner that is accessible and transparent. A case study approach was used to demonstrate integration of the Adverse Outcome Pathway (AOP) and Aggregate Exposure Pathway (AEP) frameworks to support cumulative risk assessment of co-exposure to two phthalate esters that are ubiquitous in the environment and that are associated with disruption of male sexual development in the rat: di(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DnBP). A putative AOP was developed to guide selection of an in vitro assay for derivation of bioactivity values for DEHP and DnBP and their metabolites. AEPs for DEHP and DnBP were used to extract key exposure data as inputs for a physiologically based pharmacokinetic (PBPK) model to predict internal metabolite concentrations. These metabolite concentrations were then combined using in vitro-based relative potency factors for comparison with an internal dose metric, resulting in an estimated margin of safety of ~13,000. This case study provides an adaptable workflow for integrating exposure and toxicity data by coupling AEP and AOP frameworks and using in vitro and in silico methodologies for cumulative risk assessment.
Keywords: DEHP, DnBP, high throughput, environmental chemicals
2. INTRODUCTION
Adverse outcome pathways (AOPs) represent compound-independent motifs of biological responses initiated by the interaction of a chemical with a biological target (i.e., molecular initiating event; MIE) within an organism that, when sufficiently perturbed, may lead to adverse outcomes (AOs) at the organism or population level (Edwards et al., 2016). Globally, AOPs are being developed to provide a toxicological knowledge framework to support chemical safety assessment, such as grouping chemicals for read-across and cumulative risk assessments and mapping/organizing mechanistic data for the development of alternative testing strategies. For example, the AOP for skin sensitization is the foundation of the development of an integrated approach to testing and assessment (NICEATM, 2016) for chemical sensitizers (Patlewicz et al., 2016; Patlewicz et al., 2007).
Just as the AOP framework that describes the underlying biological processes between a molecular initiating event and an adverse outcome can facilitate more efficient organization and evaluation of mechanistic information in the toxicological sciences, a similar framework has been proposed to organize exposure information. The field of exposure science faces the challenge of accessing and integrating exposure data that exists across a wide variety of resources and repositories, in addition to new data that are rapidly being generated through advances in computational approaches, biomonitoring, and analytical methods (Egeghy et al., 2011; Egeghy et al., 2012; Egeghy and Lorber, 2011). As a result, the Aggregate Exposure Pathway (AEP) framework was introduced to advance the application of systems-based approaches for more efficient and meaningful integration of exposure measurements and predictions (ICF International, 2016; Tan et al., 2018a; Tan et al., 2018b; Teeguarden et al., 2016).
The AEP framework mirrors the general scheme of the AOP framework in that it seeks to organize exposure information generated across a wide and fragmented landscape of sub-disciplines in exposure science, in order to follow a chemical from its source (i.e., its state at the site of origination) to a target site(s) exposure (TSE; its state at a site of action). The AEP framework consists of AOP-like nodes (key exposure states) connected by key transitional relationships. The key exposure state is defined as the state of a stressor in time and space (e.g., the presence or concentration of a chemical in a particular medium). A key transitional relationship represents a transition in the stressor’s transport to a different medium within the same AEP (e.g., from sediment to overlying surface water in a stream) or its transformation (e.g., degradation in soil or metabolism). AEP networks can also be established for different chemicals that are known to act on the same target site, which is critical knowledge that can aid in better cumulative risk assessments based on realistic exposures.
The AEP framework purposely includes a target site exposure (TSE), which is an estimate of the concentration of a chemical at a site of action, to ensure that exposure-relevant concentrations can be integrated with the AOP framework when assessing health outcomes from real-world exposures to multiple chemicals. An integrated AEP-AOP framework has recently been demonstrated for cumulative risk assessment of the perchlorate ion, by focusing on aggregate exposure and resulting adverse outcomes across different species (Hines et al., 2018). In the current study, a similar approach has been conducted to investigate cumulative risk assessments for multiple chemical entities (phthalates) within a single species (humans). Here, a case study with two phthalates, di(2-ethylhexyl) phthalate (DEHP) and di-n-butyl phthalate (DnBP), is used to demonstrate the utility of the AEP and AOP frameworks in developing a comprehensive cumulative risk assessment strategy (Figure 1). DEHP and DnBP, which are both ubiquitous in the environment and also two of the more potent phthalates associated with disruption of testosterone-mediated fetal development in the male rat, provide a convenient case study for several reasons. First, the phthalate family is a focus of many ongoing risk assessment efforts. Several global regulatory agencies are already working to develop cumulative risk assessment methods that account for combined exposure to multiple members of the phthalate family (Health Canada, 2015b; National Research Council Committee on the Health Risks of Phthalates, 2008; U.S. CPSC, 2014). Second, as the phthalate esters are a well-studied class of chemicals, there are substantial published data, including exposure, dosimetry, and toxicological effects that can be used to populate AEP and AOP frameworks (Centers for Disease Control and Prevention, 2014; Chang et al., 2017; Christen et al., 2012; Clewell and Clewell, 2008; Ferrara et al., 2006; Gentry et al., 2011; Health Canada, 2015a; Howdeshell et al., 2017; Howdeshell et al., 2008a; Howdeshell et al., 2008b; Pan et al., 2006; Rider et al., 2010; Rider et al., 2009b; U.S. CPSC, 2014).
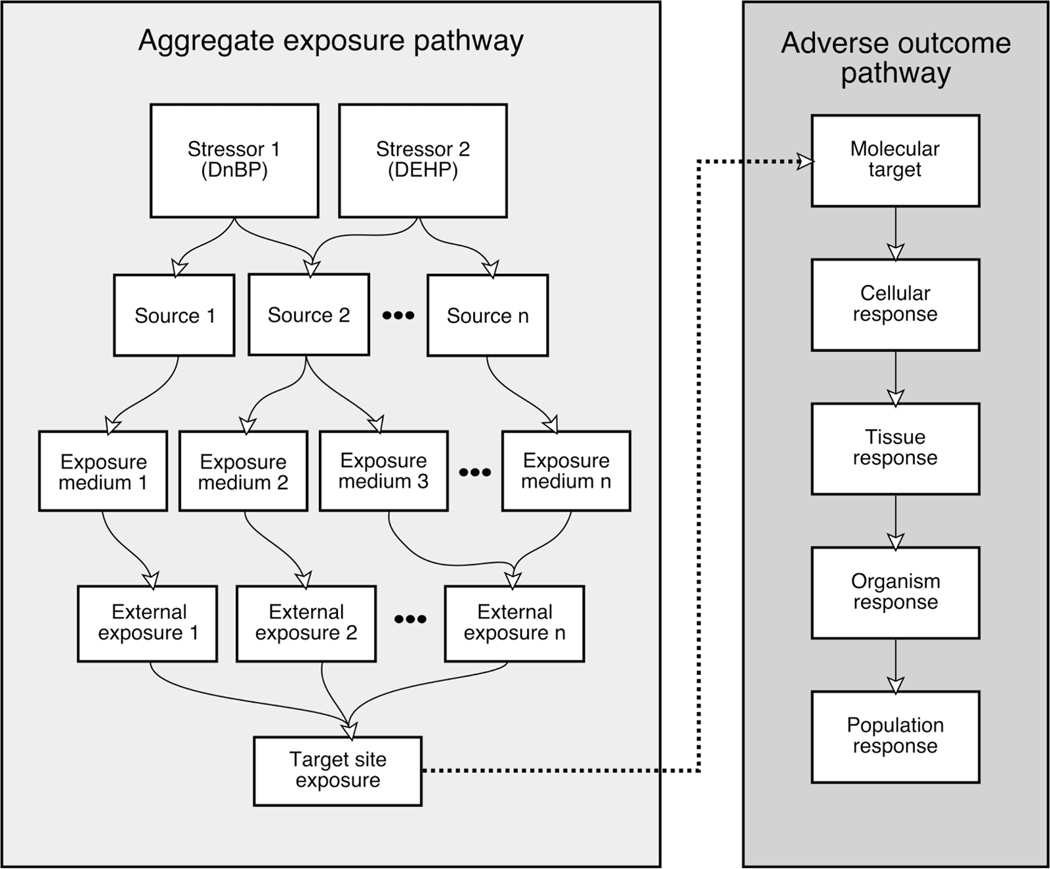
Figure 1.
Adverse Outcome Pathway (AOP) and Aggregate Exposure Pathway (AEP) frameworks. The dotted line represents the target tissue dose calculated from AEP that interacts with the MIE of the AOP leading to cellular effects. (Figure is adapted from Teeguarden, Tan, et al., 2016 (Teeguarden et al., 2016)).
3. MATERIALS AND METHODS
3.1. Developing the AEP
In order to develop examples of AEPs for DEHP, DnBP, and their metabolites, we searched the following databases: PubMed, ProQuest Environmental Science Collection, and Science Direct. The search terms used were ([“substance”] OR [“synonym”] OR [“synonym] …), where substance referred to the phthalate of interest, “diethylhexyl phthalate” and “dibutyl phthalate”. The synonyms used were “bis(2-ethylhexl) phthalate”, “Di(2-ethylhexyl) phthalate”, and “DEHP” for the former substance and “Di-n-butyl phthalate” and “DnBP” for the latter substance. In addition, because dibutyl phthalate can have multiple isomers (e.g., diisobutyl phthalate or DiBP), care was taken during literature review to ensure that only elements related to DnBP were taken into account. Finally, each of the strings stated above for substance or synonym was added to the following terms to search for exposure information:
“…AND (air OR water OR soil OR dust OR dirt OR sediment OR atmosphere OR plants OR vegetation)” for environmental media
“…AND (consumer OR products OR toy OR “building materials” OR “personal care” OR cosmetics OR “medical devices” OR PVC OR food OR beverages OR water OR vegetables)” for consumer and personal care products
“…AND (blood OR urine OR feces OR tissue OR bone OR hair OR “breast milk” OR semen OR biomarker OR bioaccumulation OR bioconcentration OR bioavailability)” for biological media.
The DEHP and DnBP diesters are readily metabolized to toxicologically active constitutive monoesters in vivo and collection of these metabolites is possible in the urine. As such, the same database keyword search was conducted for the metabolite monoesters of DEHP and DnBP and included the abbreviation for the respective metabolite monoester as well. The metabolites of DEHP included mono(2-ethylhexyl) phthalate (MEHP) and its three oxidative metabolites: mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), and mono-(2-ethyl-5-carboxy-pentyl) phthalate (MECPP). The metabolite of DnBP was mono-n-butyl phthalate (MBP). See supplemental text for additional detail on the queries used.
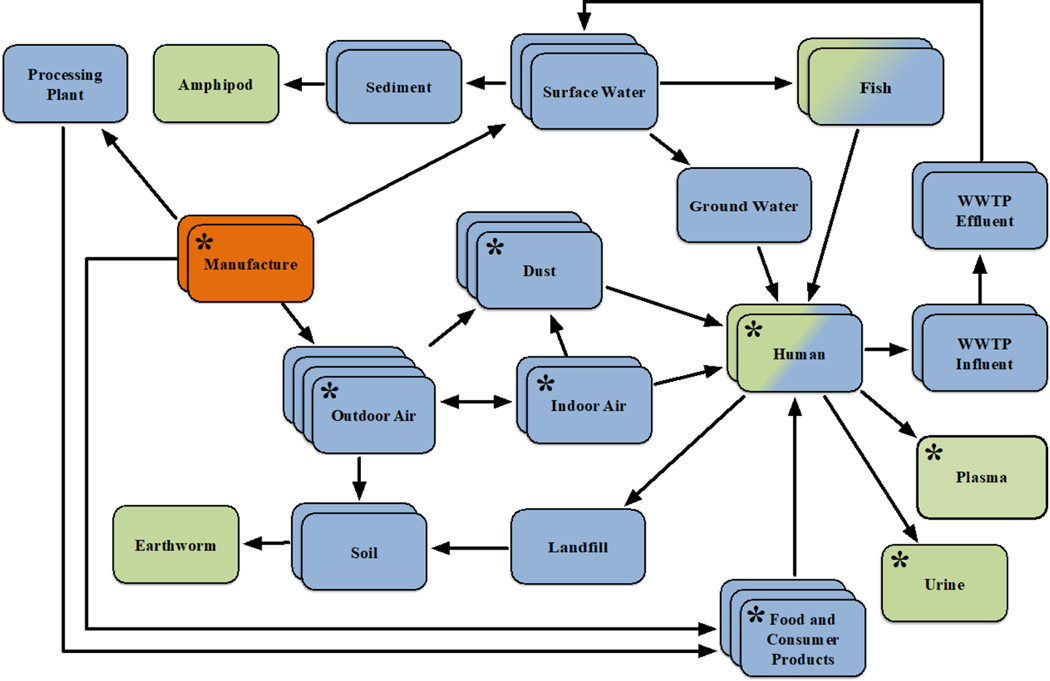
Key exposure information extracted from each reference included the medium in which the chemical was found (e.g., dust, surface water, body fluid), any specific information related to the medium (e.g., daycare centers, rivers, breast milk), the method of instrumental analysis conducted and limits of detection or quantification for these instruments, the concentrations with their respective statistical metric (e.g., mean, median, range), and any additional statistical metrics or information describing the exposure information provided in a given reference (see Supplemental Tables S1–S4 for details). Upon completion, each dataset was checked for accuracy by a separate investigator in the laboratory. Exposure information comprising a visual example of a phthalate AEP is shown in Figure 2. All environmental and biological media found during the literature search are represented in the figure, as are likely, but hypothetical, organisms associated with those media. Information from different resources (e.g., different papers in the primary literature, database resources, epidemiological surveys) is encoded in Figure 2 as depth (e.g., stacked boxes). For simplicity, the individual concentrations found in a given medium are not presented, as these differed across those reviewed resources. For the purposes of this case study, only the media most relevant to human exposure, as determined by results of the literature search, are focused upon (Fig. 2, boxes labeled with asterisks).
Figure 2.
A visual Aggregate Exposure Pathway for phthalates, as determined from information extracted from literature. Key exposure states (KESs) are represented as stacks of boxes, with each stack meant to describe temporal and spatial information (i.e., date and location recorded for the samples, as extracted from different literature resources) for that particular KES. Key transitional relationships (KTRs) are represented as arrows, and for the purpose of this figure, are only transport processes from one KES to another for a parent phthalate. The source is represented by orange stacks, while the target site exposure (TSE) is represented by green stacks. A blue-green box is meant to demonstrate that a TSE from one species can act as an intermediate KES for another species, such as consumption of contaminated fish tissue. Note that most TSEs unrelated to humans are inferred from laboratory studies artificially exposing the given species to the medium in question, under the assumption that that particular species is susceptible to exposure due to knowledge of its and the chemicals’ presence in that medium. While multiple empirically-derived KESs are represented in this figure, for the purposes of our case study, only eight are of focus here (i.e., those boxes that include asterisks) due to their relative importance in regards to what was found during the literature review.
3.2. Identifying a mode of action for phthalate-mediated toxicity
The International Programme on Chemical Safety mode of action (MOA) evaluation framework (Sonich-Mullin et al., 2001), which modified the Bradford Hill criteria (Hill, 1965), recommends a formal process that should be applied to evaluate the evidence for toxicological endpoints of interest. The process of building the weight of evidence for a chemical’s MOA and associated key biological events helps to ensure that the regulatory decisions are based on appropriate toxicological endpoints. Further, by establishing that the chemical’s MOA is relevant to humans, we ensure that the endpoint used as the point of departure is appropriate for regulatory decisions concerning human health. We evaluated the literature to determine the toxicological endpoints for the phthalates, and present here the best-described toxicological responses that would be suitable for a cumulative risk assessment (i.e., those responses that are shared amongst multiple phthalates). The two most extensively documented effects of phthalates in mammals are liver tumors and disruption of male sexual development observed during fetal and neonatal exposures in rats. Here, we consider the relevance of these endpoints to human health to inform a suitable MOA.
One of the primary toxicological effects observed in response to phthalate exposure in rodent studies is liver hypertrophy, hyperplasia and tumor induction in the adult rat as a direct result of peroxisome proliferator activated receptor α (PPARα) agonism. The association of rodent liver toxicity with binding and activation of PPARα has been clearly established in rodents for both DEHP and DBP, as well as a number of other PPARα agonists (Cattley, 2004; Cattley et al., 1998; Klaunig et al., 2003). However, acute and chronic exposures to PPARα agonists are not associated with tumors in humans or non-human primates (Cattley et al., 1998). The weight of evidence indicates that the PPARα MOA for rodent tumors is not relevant to humans (Felter et al., 2018). Because the human relevance of phthalate-mediated liver effects is not established, we would not recommend the use of liver carcinogenicity as the critical effect in risk assessment efforts. However, it should be noted that a similar approach to that described here could easily be undertaken to consider the impact of liver effects, should they be determined to be relevant to humans.
The other toxicological endpoint that has been extensively documented in rats for several phthalates is disruption of male rat sexual development in utero (Gray et al., 2009; Gray et al., 2000; Mylchreest et al., 1999). High doses in late gestation cause a number of effects related to reduced testosterone production in the Leydig cell in the testis of the male fetal rat. These effects include reduced anogenital distance (AGD), nipple retention, hypospadias (malformed penis), and other malformations of the genital tract. Additional effects have also been observed that are either partially related to testosterone disruption or entirely independent of testosterone. Cryptorchidism (undescended testes), for example, results from simultaneous disruption of testosterone and insulin-like 3 (INSL3) production (Bay and Anand-Ivell, 2014). Multinucleated germ cells, increased seminiferous tubule diameter, and Sertoli-germ cell detachment are also observed in rodents following perinatal phthalate exposure, and while the mode of action is unknown, the majority of evidence indicates that these effects are not causally related to testosterone disruption (Chang et al., 2005; Fisher, 2004; Fisher et al., 2003; Gaido et al., 2007). Human relevance of the steroidogenic endpoints, however, is still a matter of debate in the scientific community: studies with xenograft transplant models using human fetal testes have shown little testosterone response to phthalates (Heger et al., 2012), while epidemiological data and some ex vivo studies focusing on testosterone effects indicate similarity in human and rat responses (French, 2013; Swan et al., 2005). Currently, there is substantial public and regulatory concern that the steroidogenic effects may be relevant to human fetal development based on similarity between the steroidogenic pathway in rats (where toxicity has been observed) and humans (Foster, 2006)- (Foster, 2005), and observation of similar pathologies in the human (e.g., testicular dysgenesis syndrome) (Skakkebaek et al., 2001).
Based on our review of the literature, we determined that the testosterone-mediated male reproductive tract effects are the most appropriate endpoints of concern for our cumulative risk assessment case study because they are: 1) potentially relevant to humans, 2) causally associated with adverse outcomes, 3) well described in terms of possible mode of action, with sufficient data to support development of an AOP, and 4) observed with both DEHP and DnBP administration. The mode of action of the two phthalates in this case involves their transformation to their respective metabolite monoesters, which are then responsible for disruption of testosterone homeostasis. The choice of this endpoint as the basis for a risk assessment is supported by its use in ongoing regulatory efforts by the United States Environmental Protection Agency, Health Canada, and the Consumer Product Safety Commission, among other agencies (Health Canada, 2015b; Schoeny, 2014; U.S. CPSC, 2014).
3.3. Developing an AOP for testosterone reduction leading to disruption of male sexual development
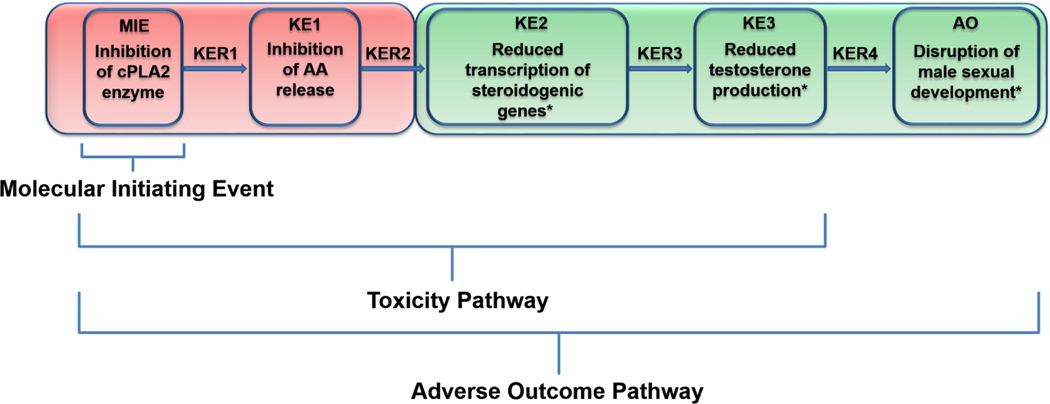
An AOP based on PPARα inhibition has been proposed for the antisteroidogenic effects in rodents (AOPWiki), based primarily on the coincidence of testicular Leydig cells (the testosterone producing cell) and liver cancer in PPARα inducers (Corton and Lapinskas, 2005; Gazouli et al., 2002). However, over the last few decades, evaluation of the relationship between fetal testis/Leydig cell response and PPARα agonism do not support the hypothesized link between PPARα interaction and testosterone inhibition in the fetal Leydig cell (Clewell et al., 2009; Gazouli et al., 2002). Studies in PPARα knockout mice indicate that PPARα alone cannot account for the full range of steroidogenic effects (Gazouli et al., 2002). Further, the trend in potency is not consistent between PPARα agonism and testosterone inhibition; while MBP is slightly more potent inhibitor of testosterone than MEHP (Balbuena et al., 2013; Clewell et al., 2010), MEHP is a much more potent PPARα agonist than MBP (Hurst and Waxman, 2003; Seo et al., 2004). Here, we propose a novel AOP (Figure 3) based on a comprehensive review of literature for the testosterone-mediated sexual development. While AOPs are chemical independent, their development necessarily depends on studies using specific compounds and case examples. We therefore used some phthalate-related literature to support the key events (KEs) and key event relationships (KERs) (Table 1). The weight of evidence for the proposed key events varies widely across the pathway. For example, while disruption of testosterone production independent of the androgen receptor (KER3) and a resulting feminization of male rats (KER4), are well established, the inhibition of transporter and metabolism genes along the arachidonic acid pathway (KER2) have less literature support. The AOP diagram (Figure 3) is color coded to portray the different level of confidence in the proposed key events.
Figure 3.
Proposed AOP for testosterone-mediated male sexual development in the rat fetus: the target site chemical dose (stressor) is provided as input from the AEP. Strength of evidence for each key event is illustrated by color: red = low confidence and green = high confidence. cPLA2: calcium-dependent phospholipase A2; AA: arachidonic acid.
Table 1.
Summary Table for key event relationships in the proposed AOP for disruption of testosterone-mediated male sexual development.
| Key Event Relationships (KERs) | Evidence |
|---|---|
| KER1 | Active monoesters (MEHP, MBP) interact with phospholipase A2 (PLA2) proteins (e.g., calcium-dependent phospholipase A2 (cPLA2) (Six and Dennis, 2000). |
| cPLA2 is responsible for intracellular arachidonic acid (AA) release from cell membranes (Kudo and Murakami, 2002). | |
| The C2 domain of the CPLA2 C-terminus binds to calcium ions (Ca++), targeting the head group of the phosphatidylcholine (PC) phospholipid membranes in the endoplasmic reticulum. This causes the activated enzyme to cleave the phospholipid and release arachidonic acid (AA) (Clewell et al., 2009). | |
| In platelets, MEHP inhibits the PLA2 release of AA, which can be metabolized into eicosanoids (Labow et al., 1988). | |
| DEHP inhibits cPLA2 in vivo (Kim et al., 2004). | |
| Active monoesters inhibit the release of AA that is incorporated through the plasma in Leydig cells (Clewell et al., 2009). | |
| KER2 | Protein kinase A (PKA) and cyclic adenosine monophosphate (c-AMP) are intermediate signaling molecules between luteinizing hormone (LH) and testosterone production. However, the signaling cascade that leads to the release of AA is independent of the c-AMP/PKA cascade (Clewell et al., 2009; Cooke et al., 1991; Kudo and Murakami, 2002; Moraga et al., 1997; Six and Dennis, 2000; Wang and Stocco, 1999). |
| MBP disrupts the LH pathway prior to up-regulation of steroidogenic acute regulatory protein (StAR) gene transcription, and independent of PKA/c-AMP pathway, suggesting phthalates work through AA pathway rather than PKA/c-AMP (Didolkar and Sundaram, 1989; Lin, 1985; Wang and Stocco, 1999; Wang et al., 2000). | |
| Phthalates inhibit AA release (see evidence for KER1). | |
| Phthalates inhibit transcription of key transport and steroid metabolism genes (e.g., scavenger receptor class B member 1 (SRB1), StAR, cholesterol side-chain cleavage enzyme (P450scc), cytochrome P450 17A1 (CYP17)) in fetal rat testes (Gaido et al., 2007; Gray et al., 1982; Lehmann et al., 2004) and in rodent Leydig cells (Balbuena et al., 2013; Clewell et al., 2013; Wang and Stocco, 1999). | |
| KER3 | Phthalates disrupt testosterone signaling independent of androgen receptor (AR) (Parks et al., 2000). |
| Active phthalates reduce testosterone production in rat testes in vivo and in rodent Leydig cells in vitro (Balbuena et al., 2013; Clewell and Clewell, 2008; Clewell et al., 2009; Clewell et al., 2013; Gaido et al., 2007; Gray et al., 1982; Lehmann et al., 2004; Wang and Stocco, 1999). | |
| KER4 | Inhibition of fetal testosterone synthesis leads to hypospadias and feminization of male rat demonstrated by delayed testes descent (cryptorchidism), vaginal pouch development (Foster, 2005, 2006; Gray et al., 2009; Rider et al., 2009b). |
3.4. Using the AOP framework to define an appropriate in vitro assay
We next asked whether the putative AOP framework could inform an in vitro testing strategy for defining chemical dose-response. An individual AOP describes a hypothesized series of key events that are causally related to the apical outcome of interest. A quantitative description of the key events and associated transitions would ideally allow mathematical modeling of the progression between initiating event and adverse outcome; however, in general this quantitative description is not sufficiently well-understood for all KERs and for many cases even the qualitative aspects of the AOP are contentious. However, where the in vitro concentration-response for a key event has been appropriately demonstrated to be consistent with the in vivo dose-response for the apical outcome, the key event assay can provide a means of performing quantitative assessment in vitro. Indeed, one of the great benefits of building AOPs is that they inherently provide a map for a successful in vitro testing strategy. Key elements to a successful, predictive assay are (1) consistency with key biological processes (i.e., the AOP), (2) a design based on current understanding of biological signaling networks, and (3) the capability of returning robust information on the dose-response for chemical perturbation. Ideally, an appropriate in vitro fit-for-purpose assay coupled with an appropriately quantitative AOP would suffice to determine the effect of a candidate chemical, thus eliminating the need for further animal testing.
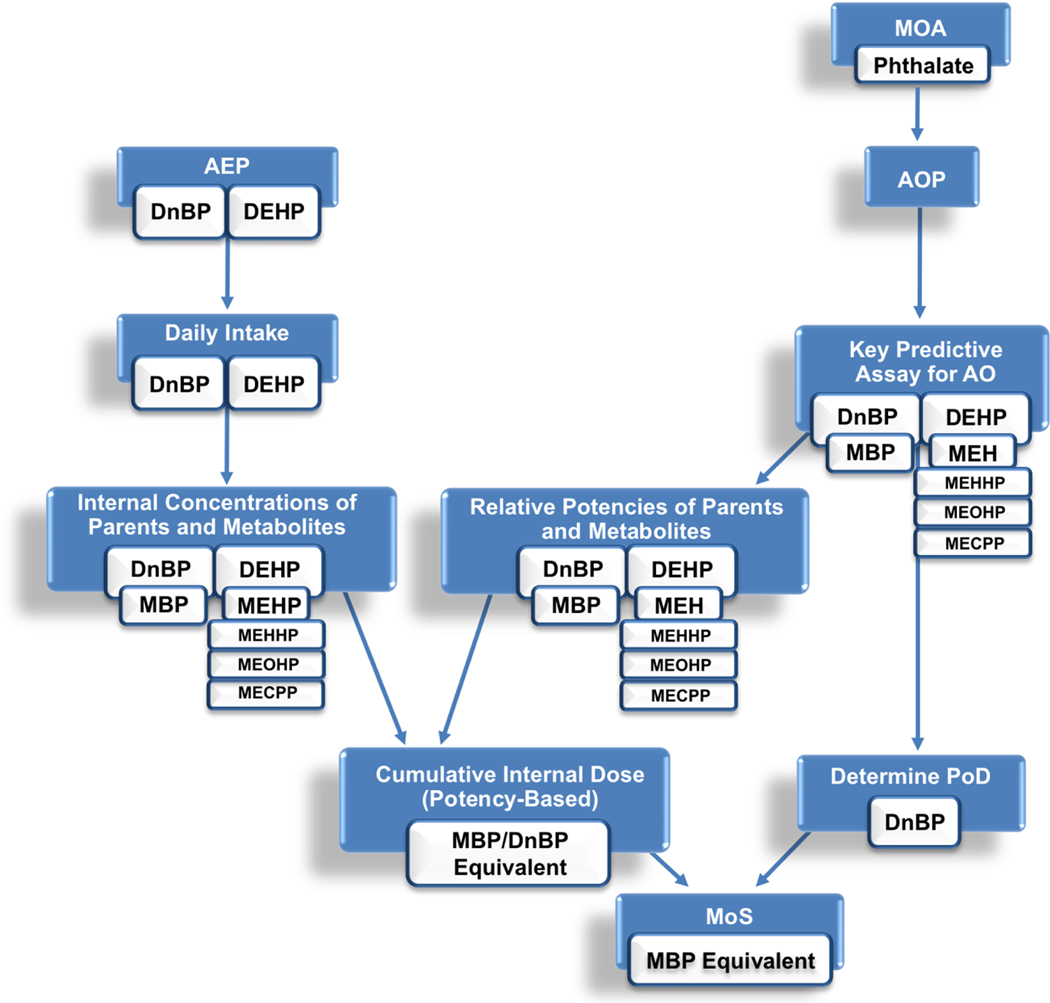
We interrogated the key events of the AOP for testosterone disruption and feminization to determine whether a suitable in vitro assay existed, and found that an in vitro assay was available for recapitulating the effect of phthalate compounds on testosterone production in the rat Leydig cell (KE3) (Balbuena et al., 2013). The R2C rat Leydig tumor cell line demonstrates robust dose-response behavior, with inhibition of testosterone at biologically relevant doses and relative potency estimates consistent with in vivo data. (Balbuena et al., 2013) Monoester concentrations causing 50% inhibition of testosterone production in the R2C cells were 3 and 6 μM for MBP and MEHP vs. 3 and 7 μM in vivo. (Balbuena et al., 2013) The inactive phthalates, such as mono-ethyl phthalate or mono-methyl phthalate, did not affect testosterone synthesis in vitro at non-cytotoxic concentrations (>> 100 μM) (Balbuena et al., 2013). Because this assay recapitulates in vivo dose-response, we concluded that this testosterone inhibition assay could be used to predict relative potencies for the different phthalates and to derive points of departure for DEHP, DnBP, and their metabolites (Figure 4).
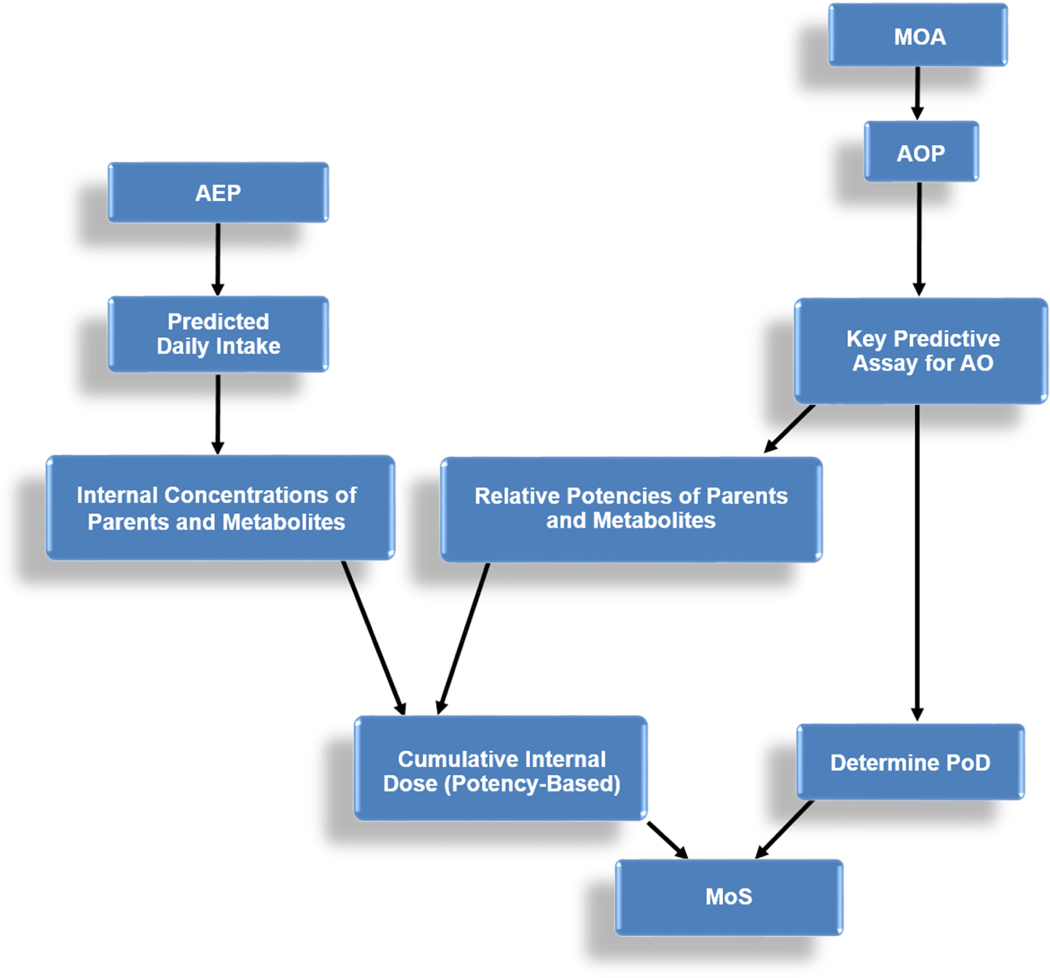
Figure 4.
Proposed general scheme for the determination of a cumulative margin of safety (MoS) for combined exposures.
3.5. Cumulative Risk Assessment
For this case study, we first reviewed exposure data taken from literature in order to determine reasonable estimates of exposures for DEHP and DnBP under real world conditions. DEHP and DnBP have been measured in indoor and outdoor air, indoor sources such as dust and vinyl flooring, food, and personal care products. From this literature review, we identified the article(s) best incorporating these major exposure routes that are relevant to humans and focus on plasma metabolites as shown in Figure 2, in order to derive predicted daily intake estimates. These predicted values, derived from a combination of sources that were considered to be most relevant to human health (see below results), were then used as input for a previously published human PBPK model for DEHP, DnBP, and their metabolites (Gentry et al., 2011; Moreau et al., 2017) to estimate the plasma concentrations of phthalate parents and their metabolite(s) at steady-state, assuming an average body weight of 70 kg. For DEHP, the concentrations of the 3 oxidative metabolites of MEHP were explicitly simulated along with DEHP and MEHP, whereas for DnBP, only DnBP and MBP concentrations were simulated. Due to the lack of distribution data in the human fetus, maternal plasma concentrations were used as a surrogate for target tissue concentrations in the fetus based on the fact that the monoesters are water soluble small molecules, and the observation of rapid equilibration of MBP between the dam and fetus in rat studies (Clewell et al., 2009). The adequacy of the in vitro assay to predict in vivo activity was evaluated previously (Balbuena et al., 2013). In that study, published dose–response data for the reduction of fetal testis testosterone for the MBP and MEHP parent chemicals DBP and DEHP (Clewell et al., 2009; Kurata et al., 2012) were analyzed to estimate the administered dose associated with 50% reduction in testosterone. Existing PBPK models (Clewell et al., 2009; Gentry et al., 2011) were used to calculate the average fetal testes concentrations associated with the ED50s. As shown in Table 2, the in vitro IC50 values were remarkably similar to the in vivo IC50s for the two active phthalates, supporting the use of nominal concentrations in the in vitro assay as a surrogate for in vivo target tissue concentrations.
Table 2.
Comparison of IC50 values in vivo (fetal rat testes) to IC50 values in vitro (R2Cs) from Balbuena et al., 2013 (Balbuena et al., 2013).
| In vivo IC50 (μM) | In vitro IC50 (μM) | RPF | |
|---|---|---|---|
| monobutyl phthalate (MBP) | 3 | 3 | 1 |
| mono-2-ethylhexyl phthalate (MEHP) | 7 | 6 | 0.53 |
| mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) | - | 9 | 0.32 |
| mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) | - | 37 | 0.08 |
| mono-(2-ethyl-5-carboxy-pentyl) phthalate (MECPP) | - | 122 | 0.024 |
We used the toxic equivalency (TEQ) approach popularized for estimating the potency of mixtures of dioxins, furans, and polychlorobiphenyls to characterize mixtures of the phthalates and their monoesters (Van den Berg et al., 1998; Van den Berg et al., 2006). Published in vitro data were available for the metabolites of DEHP and DnBP (Balbuena et al., 2013), and the in vitro assay has also been run with the parent compounds (unpublished data). We used IC50 (concentrations resulting in 50% inhibition of testosterone synthesis) to develop relative potency factors for DEHP and DnBP and their respective monoesters. Because MBP is the most potent of the metabolites measured, it was used as the reference compound. Thus, the Relative Potency Factor for MBP was 1, and all other Relative Potency Factors were a value between 0 and 1 depending on their relative potency. MBP, MEHP, and the oxidative metabolites of MEHP demonstrated in vitro activity, while DEHP and DnBP did not demonstrate any activity in the in vitro assay. As such, the Relative Potency Factors for DEHP and DnBP were given values of zero. A combined internal exposure estimate was obtained by converting the PBPK-predicted plasma concentrations of parent and metabolite compounds to “MBP equivalents”. A MBP equivalent concentration was calculated by multiplying steady-state plasma concentration of each chemical species by its Relative Potency Factor. The sum of these MBP equivalent concentrations provided an estimate of total target site exposure normalized by the potency of each species for testosterone inhibition. Finally, the margin of safety (MoS) for cumulative DEHP and DnBP exposure was calculated by dividing the estimated point of departure from the in vitro testosterone assay by the estimated total phthalate concentration in plasma (in MBP equivalents) at the total phthalate intake estimated for real-world conditions. In this case, the MoS essentially represents a margin of exposure based on internal dosimetry, where the exposure is represented by the steady-state plasma concentration in MBP equivalents, and the dose associated with a particular effect is represented by the media concentration of the in vitro assay. The point of departure was estimated based on the in vitro IC50 for MBP (3 μM). The detailed formula and spreadsheet is provided in the Supplemental Materials.
4. RESULTS
4.1. Using the AEP framework to estimate exposure to DEHP and DnBP
Supplemental tables S1 and S2 provide AEPs for DEHP and DnBP, respectively, which were obtained from over 100 references describing studies conducted in over 25 countries from 2004–2016. Nearly half of the references described studies conducted in Indo-Asian countries, followed by Europe with 20–25% and the United States with 10%, with the remaining coming from other locales throughout the world. At least 10 unique types of environmental or biological media were found to contain DEHP and DnBP. Most media types pertained to food and beverage items (22% for DEHP and 18% for DnBP), followed by dust (17% for DEHP and 18% for DnBP), soil and sediment (15% for DEHP and 17% for DnBP), and air (12% for DEHP and 16% for DnBP). Municipal waste and water concentrations comprised 8 to 10% of the total number of media types for both phthalates, and only 4 to 6% were related to concentrations in articles or products for both. The media most relevant to human health are outdoor and indoor air, indoor dust, numerous food items (e.g., dairy, fish, vegetables), articles and consumer products. Further details of key exposure states for DEHP and DnBP can be found in the supplemental materials (Aragon et al., 2012; Bu, 2016; Jeon et al., 2016; Ji et al., 2014; Kim et al., 2013; Pei et al., 2013; Rakkestad et al., 2007; Wang et al., 2015b; Xie, 2006; Xie et al., 2007; Zhu et al., 2012).
Supplemental tables S3 and S4 provide AEPs for DEHP and DnBP metabolites, respectively, taken from studies conducted in nearly 20 countries from 2000–2016, with almost half conducted in the United States. All but one of the 124 DEHP metabolite-related references and all but one of the 70 MBP-related related references described concentrations in body fluids or tissues, such as breast milk, amniotic fluid, and urine (Choi et al., 2012; Choi et al., 2014; Hait et al., 2014; Hines et al., 2009; Huang et al., 2009; Kim et al., 2015; La Rocca et al., 2014; Liou et al., 2014; Main et al., 2006; McConnell, 2007; Minatoya et al., 2016; Wang et al., 2015a; Wittassek et al., 2009; Yan et al., 2009; Zhang et al., 2015; Zhao et al., 2014; Ziv-Gal et al., 2016).
For our purpose, the ideal TSE would be the toxic metabolites (MEHP and MBP) in human plasma, as a surrogate to the target tissue dose. Since such data do not currently exist in our AEPs, we extracted an upstream key exposure state that is closest to the target site exposure from one existing manuscript listed within the AEPs for both phthalate diesters (See Supplemental Materials), the 2014 article by Shin and colleagues (Shin et al., 2014), which provides a quantitative daily intake estimate. A PBPK model can take such a daily intake rate as an input to predict TSE. One major reason for selecting that particular research article was that it provided a convenient key exposure state, which integrated exposures from several major sources most relevant to humans. Within that article, daily intake amounts of DEHP and DnBP were predicted based on the following parameters: 1) total production volume (i.e., manufacture) and outdoor emissions, as determined by the United States Environmental Protection Agency’s Inventory Update Reporting system and the National-Scale Air Toxics Assessments database; 2) outdoor air releases, as determined by the multimedia fate and transport model created by the California Department of Toxic Substances Control (CalTOX) (McKone and Enoch, 1993); 3) indoor air releases, dust ingestion, dermal uptake, and exposure to building materials like vinyl flooring, as determined using a fugacity-based indoor exposure model; 4) food ingestion, also determined by application of the CalTOX model; and 5) direct dermal exposure to cosmetic or consumer products, as determined by extrapolation from absorption measurements in rat skin to those in humans. The predicted daily intake rates were 16.1 μg/d and 64.5 μg/d for DnBP and DEHP, respectively (Supplemental Table A12 of Shin et al. (Shin et al., 2014)).
4.2. Evaluating the proposed use of the PBPK models to predict human biomarker concentrations
To ground-truth the prediction of metabolite concentrations in humans using the PBPK model, we used the estimated daily intake values described above for DEHP and DnBP to predict urinary concentrations of the four DEHP metabolites (MEHP, MEHHP, MEOHP, MECPP) assuming an average adult body weight of 70 kg. These values were then compared to several key exposure states, specifically, the measured urinary concentrations of those metabolites from the reported NHANES 2013–2014 (Centers for Disease Control and Prevention, 2014). While there are other urinary data in the AEPs, the NHANES 2013–2014 data were used because this study period corresponded to the same time period as the exposure studies used to generate the daily intakes of DEHP and DnBP in the Shin et al. study. In addition, as women of reproductive age represent the exposure population of interest for the adverse outcome used in the current case study, the reported NHANES 2013–2014 (Centers for Disease Control and Prevention, 2014) had data specific for in this subpopulation. In general, predicted urinary concentrations were within the 25th and 75th percentile of the NHANES data and were within a factor of 2 of median urinary concentrations for MEHP (2.3 vs. 1.3 μg/L), MEHHP (3.7 vs. 7.2 μg/L), and MEOHP (3.7 vs. 5 μg/L). The model prediction of MECPP was equivalent to the 17th percentile of the NHANES data and was slightly more than 2-fold higher than the median of measured values in the NHANES study group (4.93 vs. 11.25 μg/L). In general, the PBPK model predicted urinary metabolite concentrations based on the AEP estimated daily intake values for DEHP and DnBP were consistent with measured concentrations in human biomonitoring studies. Thus, the model appears to be a useful tool for predicting metabolite distribution for use in the cumulative risk assessment.
4.3. Using the AOP-defined in vitro test system
The available in vitro assay for testosterone production (Balbuena et al., 2013) was used to predict relative potency of the phthalates for inhibition of testosterone synthesis as described in the Methods. The predicted in vitro IC50’s for MBP, MEHP, MEHHP, MEOHP, and MECPP are 3, 6, 9, 37, and 122 μM, respectively. The parent compounds, DEHP and DnBP, were assumed to have Relative Potency Factor values of zero based on a lack of activity in vitro. Corresponding in vivo IC50’s and Relative Potency Factor are summarized in Table 2.
4.4. Calculating a combined exposure for phthalates
Defining the combined exposure for compounds with different potencies is the first step in estimating cumulative risk from exposures to multiple chemicals that cause the same adverse outcome. In this case, we simplified the task of comparing potencies by choosing compounds with a shared AOP. Summing MBP equivalents allowed for estimating an aggregate phthalate plasma concentration, while accounting for the different potencies of the various metabolites. The combined phthalate exposure, accounting for relative potency of the different chemical species, was equivalent to 2.17×10−4 μM MBP in plasma, or a 0.66 μg/kg/day DnBP external exposure (Table 3). The detailed calculation is provided in Supplemental Table S5.
Table 3.
Calculated parameters for estimated cumulative exposure with relative potency factors (RPFs) taken from Balbuena et al., 2013 (Balbuena et al., 2013).
| Plasma Concentration at 1 μg/kg/day (μM) | Daily Intake of Parent Diester Phthalate (μg/kg/day) | Plasma Concentration at Daily Intake (μM) | RPF (−) | MBP Equivalent Plasma Concentration (μM) | |
|---|---|---|---|---|---|
| DEHP | 8.22E-05 | 0.92 | 7.58E-05 | 0.000 | 0.00E+00 |
| MEHP | 1.82E-04 | 0.92 | 1.68E-04 | 0.530 | 8.90E-05 |
| MEHHP | 1.45E-04 | 0.92 | 1.33E-04 | 0.320 | 4.27E-05 |
| MEOHP | 9.93E-05 | 0.92 | 9.15E-05 | 0.080 | 7.32E-06 |
| MECPP | 1.24E-04 | 0.92 | 1.14E-04 | 0.024 | 2.75E-06 |
| DnBP | 9.16E-08 | 0.23 | 2.11E-08 | 0.000 | 0.00E+00 |
| MBP | 3.26E-04 | 0.23 | 7.50E-05 | 1.000 | 7.50E-05 |
| Total | 2.17E-04 | ||||
4.5. Estimating the margin of safety for combined DEHP/DnBP exposure
We calculated a point of departure (PoD) by estimating the IC50 for MBP (3 μM) with the in vitro assay for testosterone production. Assuming the media concentration is equivalent to the blood concentration in vivo, the IC50 can be compared to the cumulative MBP internal dose (2.17×10−4 μM). Thus, the margin of safety (MoS) for the combined DEHP and DnBP exposure using this in vitro toxic equivalency approach was calculated to be 13,000.
A scheme for the determination of a MoS (Figure 4) was adapted for combined DEHP and DnBP exposure and is depicted in Figure 5. It should be noted that this MoS is a point estimate and does not take into account uncertainty in the estimates of exposure or PoD. One approach to incorporating uncertainty would be to apply a probabilistic approach to exposure estimation, though this would not account for uncertainty in the PoD. Ultimately, primary cell-based assays may allow for in vitro based determination of pharmacodynamic susceptibility and interindividual variability, though such models do not yet exist for this particular endpoint. A more traditional approach could also be adopted to deal with uncertainty, i.e., applying traditional uncertainty factors for interspecies and intraspecies variability in pharmacokinetics and pharmacodynamics to ensure health protection. An argument can be made for eliminating interspecies uncertainty factors because (1) ex vivo studies indicate the rat is more sensitive than the human to testosterone inhibition and rodent cells were used for PoD determination, and (2) the cross-species dosimetry was compared based on internal dose. If a standard 10-fold uncertainty factor for human interindividual variability (3 for intraspecies pharmacokinetics, 3 for intraspecies pharmacokinetics) was applied, the MoS would become 1,300.
Figure 5.
Proposed method for determining cumulative margin of safety for phthalates, using DEHP and DnBP as a case study example.
5. DISCUSSION
The case study in this current work focused on conducting a safety assessment for combined exposures to the phthalate esters DEHP and DnBP. A putative AOP was developed for disruption of male sexual development using the framework established by OECD and the EPA (AOPWiki, 2016), based on knowledge of the MOA for the two phthalates. In turn, knowledge of the key events (i.e., decreased testosterone production) facilitates development of in vitro assays relevant to an AOP. By establishing a dose-response relationship and invoking appropriate in vitro-to-in vivo extrapolation techniques, these in vitro assays could inform PoD estimates for phthalates, as an alternative to animal testing. These PoD estimates then can be compared to internal concentrations related to real-world exposures, which are informed by AEPs or other exposure frameworks, as appropriate. In this case study, the closest key exposure state to the TSE, which were estimated daily intake rates (Shin et al., 2014), were selected as inputs to a published PBPK model (Gentry et al., 2011) to estimate the TSEs of both parent chemicals and their metabolites. These steady state plasma concentrations then act as the dose metric for the adverse outcome (fetal testosterone inhibition). In this manner, our study involved coverage of two AEP networks: 1) DEHP, its metabolite MEHP, and the three oxidative metabolites of MEHP, and 2) DnBP and its metabolite MBP. The testosterone inhibition AOP framework allowed us to identify a relevant in vitro assay for dose-response assessments. Combining the dose-response data from the AOP with expected blood concentrations in representative populations, as determined from a quantitative estimate of intake extracted from two AEP networks and inputted into a PBPK model, allowed us to estimate the MoS for combined DEHP and DnBP exposure.
This study follows on a related work by Hines et al. that paired AEP and AOP networks to assess human health and ecotoxicological effects from perchlorate exposure (Hines et al., 2018). While conceptually similar, our work builds on the foundation of this work with perchlorate while differing in several respects. First, our study was designed in part to show the utility of AOP framework to drive selection of in vitro endpoints relevant to the adverse outcome. We demonstrated how pharmacokinetic modeling can be used to translate a PoD derived from an appropriate in vitro assay to a context relevant to human exposure. Phthalates, in addition to arising from a number of sources that can be captured by the AEP framework, are metabolized into a series of active metabolites that all act via the same TSE. As such, the phthalate case study provided the opportunity to model multiple stressors. Lastly, the quantitative characterization of both exposure and toxicity allowed an estimation of risk (via a MoS) in our work. Hines et al. considered an extensive network of AOPs covering a variety of endpoints in several species relevant to the importance of perchlorate contamination to ecotoxicity. This integration across species demonstrates another advantage of the AEP-AOP framework that our case study has not directly considered, and may provide an opportunity for additional exploration for phthalates.
Throughout this case study, decisions were made that would affect the estimated MoS. For example, the assumption that the media concentration of the metabolites could be directly related to the in vivo blood levels and external exposure via a PBPK model (Figure 5), assumes that no significant binding, degradation or metabolism occurs within the in vitro system. If this assumption did not hold true, the prediction of risk would likely be underestimated due to a comparatively lower concentration of the phthalate causing the observed response. This and other assumptions that could affect the MoS (choice of exposure scenarios, choice of adverse effect, use of toxic equivalence approach and assumption of dose-additivity) are discussed further below.
5.1. Determining confidence in the AOP.
There is much debate regarding the human relevance of testosterone-mediated effects caused by phthalates based on studies with human fetal testis xenograft and in vitro models (Heger et al., 2012; Swan et al., 2005). We used disruption of testosterone-mediated development as the toxicity endpoint of interest, assuming that this particular endpoint would be conservative (i.e., rat tissues are more sensitive than human). The choice of this endpoint is also consistent with its use in current ongoing regulatory assessment efforts by EPA, Consumer Product Safety Commission, and Health Canada, among others (National Research Council, 2009). An AOP describing the phthalate toxicological outcome of interest was developed by defining the key biological events first arising from the chemical-molecular interaction at the target site and ending at the adverse outcome. It should be noted that a proposed AOP for phthalate-induced disruption of male sexual development, mediated through PPARα, currently exists and is available on AOPWiki (AOPWiki), though it has not yet undergone peer review. The publicly available AOP (AOPWiki) proposes a MIE that involves binding of the phthalate monoester to PPARα, that then leads to disruption of testosterone. However, the current literature has largely disproved that hypothesis (Clewell et al., 2009; Gazouli et al., 2002), as the weight of evidence demonstrates that PPARα is not associated with testosterone reduction in the fetal testes (Clewell et al., 2009; Gazouli et al., 2002). Based on available literature and in-house studies, we have proposed an alternative AOP, wherein the phthalate ester inhibits phospholipase A2 release of arachidonic acid, leading to a reduction in steroidogenic gene expression. This hypothesis is consistent with the current literature, though other mechanisms have also been proposed (Main et al., 2006; van den Driesche et al., 2015; van den Driesche et al., 2012). The efforts by EPA and OECD to use crowd-sourcing and peer review via AOPWiki (AOPWiki), may help to identify data gaps and reduce uncertainty (AOPWiki). However, this peer review mechanism does not avoid the issues of 1) human relevance, which is poorly addressed by the wealth of AOPs developed for animal-based endpoints, 2) differing scientific opinions on the relevant key events, and 3) the lack of mechanistic data for many reported chemical toxicities.
To deal with the variability in the data available to support key events within the AOP in this case study, rather than omitting key events with small bodies of evidence, we took the approach of providing the evidence for the proposed AOP and ranking confidence of each key event. While the early key events, particularly the molecular initiating event, are yet unproven, the cellular and tissue-level key events have substantial support and are widely accepted among the scientific and regulatory community. Fortunately, the in vitro assay that was used to derive the point of departure for the cumulative risk assessment coincides with a key event that is particularly well established based on in vivo, ex vivo, and in vitro studies and the in vitro testosterone reduction has been shown to quantitatively predict in vivo dose response when compared based on internal dose (Balbuena et al., 2013). Certainly, if the points of departure were derived using an in vitro model for a less established key event, the uncertainty around the points of departure would be much greater. Moving towards AOP-based risk assessments, we face significant challenges in identifying the appropriate non-animal models for use in deriving estimates of safety given the lack of mechanistic data for most regulated compounds. An alternative approach may be the use of broad coverage, high throughput in vitro based screens, such as the Toxicity Forecaster (ToxCast) (Judson et al., 2014), or high throughput transcriptomics. However, the utility of these screens for defining MOA, let alone developing AOPs, has yet to be demonstrated.
5.2. Determining confidence in the AEP
We note than in this case study daily intake rates predicted for DEHP and DnBP were obtained from a published study (Shin et al., 2014), rather than building new predictive models to assemble KESs and KTRs for our purpose. Only a fraction of the media types (i.e., KESs) was included in the Shin et al. study for predicting daily intake rates, compared to the number of KESs determined through our literature search and development of the phthalate AEP visual construct (Figure 2). Additionally, the examples of the phthalate AEPs we assembled (Supplemental Tables S1 and S2) indicate that consumer products comprise only a small number of total references related to DEHP and DnBP exposure, especially compared to those references for soil, sediment, and water to a lesser degree. Using exposure estimates that are aggregated from a variety of sources, as opposed to just a given external exposure concentration, allows consideration of values that might not otherwise be included in a single external exposure estimate. Thus, greater confidence is provided through an aggregate exposure estimate, based on the weight of evidence that all exposure sources considered relevant to a given target are considered for that estimate. While it is acknowledged that the number of references for a given medium by no means indicates its importance relative to others, and that the selected media used within the Shin et al. models are likely the most relevant to human exposures, it is worth considering the potential of these other media to represent substantial sources of exposure. This is especially the case for individuals continuously exposed to aquatic environments or soil, in which case, exclusion of predicted intake values derived from other immediate sources may significantly underestimate exposure.
A primary reason for which an AEP is developed is to provide a means for disseminating exposure information for a given chemical so that users of that AEP can conduct their studies on a fit-for-purpose basis, by selecting the KESs and KTRs most relevant to them at their discretion. While under the recognition that we could include KESs and KTRs other than those found in the models developed by Shin et al. (2014) into our own exposure model to predict daily intakes of the two phthalate compounds, the estimates we obtained from Shin et al. (2014) are sufficient for our purpose for the following reasons. Total production volume was provided in the article by Shin et al. (2014), with values of 100,000 metric tons for DEHP and 10,000 metric tons for DBP. While environmental media concentrations were not provided in the selected article, the authors referenced models such as the fugacity-based indoor exposure model (Bennett and Furtaw, 2004; Shin et al., 2012) and the multimedia fate, transport, and exposure model CalTOX (McKone and Enoch, 1993), with which they used to estimate intake fractions based on measured concentrations found in the literature. It is worth noting that predicted rate of food intake using the CalTOX model was similar to measured concentrations reported by (Schecter et al., 2013), at 0.138 μg/kg/d compared to 0.184 μg/kg/d for DBP and 0.712 μg/kg/d compared to 0.673 μg/kg/d for DEHP, indicating the accuracy of the CalTOX model. Daily intake rates calculated by Shin et al. (2014) from NHANES biomarkers resulted in a nearly one-to-one ratio with exposure model-based predictions, with the former being 14.2 μg/d for DBP and 57.5 μg/d for DEHP. Thus, we contend that even though our selected intake values of 16.1 and 64.5 μg/d are point estimates, they are appropriate for use as input into the PBPK model. That three of the four metabolites of DEHP and MBP exhibited similar concentrations as measured NHANES biomarker values, indicates the PBPK model performed in a reasonable manner. It is unclear why MCPP would be predicted at a value 2–3-fold lower than measured concentrations when this is not the case for the other three DEHP metabolites, and so rates involved in its metabolism or in its transfer from blood to urine could be looked at further to improve the model’s performance in regards to this particular metabolite.
5.3. Considerations for a cumulative risk assessment.
DEHP and DnBP demonstrate substantial differences in their kinetic profiles and in their potency for inducing in vivo toxicological outcomes. Both diesters are rapidly hydrolyzed to their monoester metabolites in vivo. However, while MEHP undergoes extensive secondary metabolism, MBP does not. The metabolites of DEHP and DnBP also differ in potency for in vivo effects by as much as 50-fold. In order to develop an estimate of cumulative risk, the external doses of the two phthalates require normalization through a toxic equivalency approach to account for both differences in pharmacokinetics and pharmacodynamics. In this case study, published PBPK models were available (i.e., the AEP) to translate external dose to internal blood concentrations. We used a fit-for-purpose in vitro assay for testosterone inhibition to directly measure potency for a widely-accepted key event. This assay was shown previously to quantitatively predict in vivo activity. However, as we move forward into assessments of less well-studied compounds, we will likely use methods that are not as extensively validated to obtain such estimates. This case study with phthalates demonstrates the urgent need to develop better fit for purpose in vitro assays for specific responses and computational approaches (Yoon et al., 2016).
The toxic equivalency approach used here to account for pharmacokinetic and pharmacodynamic differences in the phthalate diesters depends on a number of assumptions that combine to fulfill the principles of dose additivity (Safe, 1998). First, the relevant chemical species operate via the same adverse outcome pathway. Transcriptomics experiments have shown consistency in the mode of action between for the phthalates that result in sexual dysregulation in rodents (Liu et al., 2005). We have demonstrated here and in previous studies (Balbuena et al., 2013; Clewell et al., 2010) that the relevant monoester metabolic products of DEHP (MEHP, MEHHP, MEOHP, and MECPP) and DnBP (MBP) are active in the testosterone inhibition assay, suggesting that they have a consistent mode of action. Second, dose additivity assumes a quantitative concordance between the testosterone inhibition assay and the apical effect. We have demonstrated this previously for the two most potent metabolite products, MBP and MEHP (Table 2), as well as for two other monoesters (monoethyl phthalate and monomethyl phthalate) for which in vivo data existed (Balbuena et al., 2013). Additionally, the dose additivity assumption requires that the concentration-response curves for the species considered are similarly shaped, allowing for variation of the potency. This has been demonstrated in vivo (Rider et al., 2009a) for the developmental effects of several phthalates including DEHP and DBP, and has been demonstrated in vitro for the testosterone inhibition key event for the active metabolites of DEHP and DBP, as well as several other phthalates. Several studies (Balbuena et al., 2013; Clewell et al., 2010) showed that concentration-response curves were parallel. Lastly, dose additivity assumes that concentrations of components in the mixture are well below saturation of the concentration response curve. For each monoester predicted in plasma via the AEP, the predicted plasma concentration (Table 4) was at least 1000-fold lower than the IC50 in the testosterone inhibition assay (Table 3). Together, these data indicate that the assumption of dose additivity, and therefore the toxic equivalence approach, is appropriately used in this case study.
As mentioned previously, an assumption was made that in vitro nominal concentrations could be used as a surrogate for in vivo blood levels. This assumption assumes negligible binding, degradation, or metabolism of the chemical in vitro. Ideally, measured free concentrations in the media would be available. However, this data rarely exists for in vitro studies and was not available here. Nonetheless, in this case data is available that directly compares in vitro nominal concentration-response to in vivo concentration response (Balbuena et al., 2013). The in vitro IC50 values were remarkably similar to the in vivo IC50s for the two active phthalates, supporting the use of nominal concentrations in the in vitro assay as a surrogate for in vivo target tissue concentrations.
5.4. Advantages to an AEP-AOP cumulative risk assessment approach
The main advantage using frameworks such as AEPs and AOPs is the built-in transparency and explicit evaluation of the uncertainties in the decision-making process involved in cumulative risk assessment. The structure of AEPs, regarding the organization of exposure information for chemicals from their source to the target site exposures, supports cumulative risk assessments for multiple chemicals with a common mechanism. In the current case study, the two phthalates are often found in the same media due to their similar physicochemical properties and functional use, but at different concentrations. Co-occurrence data, such as concentrations for multiple chemicals that are measured within the same medium, are readily captured as AEP networks to enable predictions of target site exposures. Here we have shown that in some cases these networks may suitably simplify into exposure estimates that can be related to in vitro measures of activity. To estimate the cumulative risk resulting from such combined exposures, chemical-specific target site exposures were normalized based on their relative potencies determined from dose-response analysis conducted in an in vitro system. As a result, it can be predicted whether the combined concentrations of additional chemicals are likely to lead to an adverse health outcome even if the concentration of any one chemical is found to be lower than that required for triggering a response.
Much like has been implemented for AOPs, we envision the creation of a public repository for AEPs and their composite key exposure states and key transitional relationships. This would provide an extensible database of elements that could be easily reused or adapted to different target substances or exposure routes. Furthermore, organization of AEP elements in this manner allows sharing of information across multiple disciplines and for a variety of investigator needs, in addition to allowing generation of computational models that can be customized when appropriate and sufficient exposure data are available. For example, if bioaccumulation is observed in a target species, a database of AEPs could identify what other human and ecological health effects are possible concerns. Lastly, this resource would provide a forum for aggregating and sharing relevant exposure data as it becomes available.
Explicitly defining an AOP provides a framework that can be systematically evaluated for error and implicit or explicit bias. This systematic evaluation of the framework, supplemented by comprehensive literature review and peer-review of mechanisms such as the AOPWiki approach (AOPWiki), increases confidence in the basis of the choice of toxicological endpoints. Further, as the AOP is comprised of events that are causal, not merely associated with an effect, it explicitly defines biological endpoints that may be tested for chemical effect, and ultimately used for point of departure estimate. Moving forward in a public health landscape that is looking to incorporate in vitro based assays into chemical safety assessment, AOPs will be invaluable in defining the characteristics of in vitro models necessary for predicting in vivo dose-response and for deriving points of departure to support public health decision making. Finally, the ability to combine these concepts and the available 21st century technologies, including in silico and in vitro approaches, allows for more comprehensive evaluation of human risk than the traditional animal-based, single chemical assessments. In this case, we estimated a margin of safety for simultaneous exposure to DEHP and DnBP and found the current exposure to be lower than an in vitro-estimated point of departure. Given that both phthalates are ubiquitous in the environment, a cumulative risk estimate might provide a more comprehensive evaluation of their potential to impact human health, rather than investigating the risk of each phthalate individually.
In general, a chemical or metabolite could be associated with different AOPs at different levels of exposure. For example, there is evidence that some of the developmental effects of DEHP reverse direction at low concentrations (Gentry et al., 2011). For this reason, a quantitative understanding of the relationship between key events and the adverse outcome is essential for matching the internal target site exposure with the response of the relevant AOP. In this case study, we have used an in vitro assay that coincides with a well-supported key event in the AOP to define the relationship of the concentration of a particular phthalate or metabolite with the expected adverse outcome. Significantly, the concentration response of the in vitro assay and the adverse outcome are concordant. Based on the internal PoD and the in vivo cumulative MBP dose derived through integration of predicted daily intake values with the PBPK model, the MoS was at least 10-fold above that generally considered to be of high concern. Therefore, it appears that the cumulative MBP-equivalent dose does not elicit a strong reduction in testosterone production. While the assay used here has some limitations (e.g., it assumes rodent responses are a reasonable proxy for the human), as knowledge of the AOP for human effects increases, new in vitro assays may be developed and used to improve the estimates of human risk. Thus, by explicitly defining the level of confidence in each underlying assumption for the AOP and AEP, we can identify key areas of uncertainty and focus ongoing research on the areas that would provide the most benefit to risk assessors.
Supplementary Material
HIGHLIGHTS.
Major exposure routes extracted from an AEP were used to estimate exposures to DEHP and DnBP.
A new AOP was developed for disruption of male development through reduced testosterone production via inhibition of c-PLA2.
A margin of safety was determined for aggregate exposures to DEHP and DnBP.
These AEP-AOP frameworks will inform decisions in cumulative risk assessment.
6. ACKNOWLEDGEMENTS AND DISCLAIMER
This work was performed under a Memorandum of Understanding between ScitoVation and the EPA. The participation of the ScitoVation authors in this research was funded by the American Chemistry Council Long-range Research Initiative. Dr. Jeremy Leonard was supported by the Research Participation Program at the Office of Research and Development, US Environmental Protection Agency, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between US Department of Energy and US Environmental Protection Agency.
Disclaimer: The U.S. Environmental Protection Agency has provided administrative review and has approved this paper for publication. The views expressed in this paper are those of the authors and do not necessarily reflect the views of the U.S. Environmental Protection Agency.
Parts of this work were supported by American Chemistry Council’s Long Range Research Initiative.
Footnotes
Declaration of interests
☐ The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
7 REFERENCES
- AOPWiki, PPARα activation leading to impaired fertility in adult male rodents. [Google Scholar]
- Aragon M, Marce RM, Borrull F, 2012. Determination of phthalates and organophosphate esters in particulated material from harbour air samples by pressurised liquid extraction and gas chromatography-mass spectrometry. Talanta 101, 473–478. [DOI] [PubMed] [Google Scholar]
- Balbuena P, Campbell J Jr., Clewell HJ 3rd, Clewell RA, 2013. Evaluation of a predictive in vitro Leydig cell assay for anti-androgenicity of phthalate esters in the rat. Toxicol In Vitro 27, 1711–1718. [DOI] [PubMed] [Google Scholar]
- Bay K, Anand-Ivell R, 2014. Human testicular insulin-like factor 3 and endocrine disrupters. Vitam Horm 94, 327–348. [DOI] [PubMed] [Google Scholar]
- Bennett DH, Furtaw EJ Jr., 2004. Fugacity-based indoor residential pesticide fate model. Environ Sci Technol 38, 2142–2152. [DOI] [PubMed] [Google Scholar]
- Bu Z, et al. , 2016. Indoor phthalate concentration in residential apartments in Chongqing, China: Implications for preschool children’s exposure and risk assessment. Atmospheric Environment 127, 34–45. [Google Scholar]
- Cattley RC, 2004. Peroxisome proliferators and receptor-mediated hepatic carcinogenesis. Toxicol Pathol 32 Suppl 2, 6–11. [DOI] [PubMed] [Google Scholar]
- Cattley RC, DeLuca J, Elcombe C, Fenner-Crisp P, Lake BG, Marsman DS, Pastoor TA, Popp JA, Robinson DE, Schwetz B, Tugwood J, Wahli W, 1998. Do peroxisome proliferating compounds pose a hepatocarcinogenic hazard to humans? Regul Toxicol Pharmacol 27, 47–60. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2014. National Health and Nutrition Examination Survey Data. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- Chang BV, Liao CS, Yuan SY, 2005. Anaerobic degradation of diethyl phthalate, di-n-butyl phthalate, and di-(2-ethylhexyl) phthalate from river sediment in Taiwan. Chemosphere 58, 1601–1607. [DOI] [PubMed] [Google Scholar]
- Chang WH, Wu MH, Pan HA, Guo PL, Lee CC, 2017. Semen quality and insulin-like factor 3: Associations with urinary and seminal levels of phthalate metabolites in adult males. Chemosphere 173, 594–602. [DOI] [PubMed] [Google Scholar]
- Choi H, Kim J, Im Y, Lee S, Kim Y, 2012. The association between some endocrine disruptors and hypospadias in biological samples. J Environ Sci Health A Tox Hazard Subst Environ Eng 47, 2173–2179. [DOI] [PubMed] [Google Scholar]
- Choi J, Eom J, Kim J, Lee S, Kim Y, 2014. Association between some endocrine-disrupting chemicals and childhood obesity in biological samples of young girls: a cross-sectional study. Environ Toxicol Pharmacol 38, 51–57. [DOI] [PubMed] [Google Scholar]
- Christen V, Crettaz P, Oberli-Schrammli A, Fent K, 2012. Antiandrogenic activity of phthalate mixtures: validity of concentration addition. Toxicol Appl Pharmacol 259, 169–176. [DOI] [PubMed] [Google Scholar]
- Clewell RA, Campbell JL, Ross SM, Gaido KW, Clewell HJ 3rd, Andersen ME, 2010. Assessing the relevance of in vitro measures of phthalate inhibition of steroidogenesis for in vivo response. Toxicol In Vitro 24, 327–334. [DOI] [PubMed] [Google Scholar]
- Clewell RA, Clewell HJ 3rd, 2008. Development and specification of physiologically based pharmacokinetic models for use in risk assessment. Regul Toxicol Pharmacol 50, 129–143. [DOI] [PubMed] [Google Scholar]
- Clewell RA, Kremer JJ, Williams CC, Campbell JL, Sochaski MA, Andersen ME, Borghoff SJ, 2009. Kinetics of selected di-n-butyl phthalate metabolites and fetal testosterone following repeated and single administration in pregnant rats. Toxicology 255, 80–90. [DOI] [PubMed] [Google Scholar]
- Clewell RA, Sochaski M, Edwards K, Creasy DM, Willson G, Andersen ME, 2013. Disposition of diiosononyl phthalate and its effects on sexual development of the male fetus following repeated dosing in pregnant rats. Reprod Toxicol 35, 56–69. [DOI] [PubMed] [Google Scholar]
- Cooke BA, Dirami G, Chaudry L, Choi MS, Abayasekara DR, Phipp L, 1991. Release of arachidonic acid and the effects of corticosteroids on steroidogenesis in rat testis Leydig cells. J Steroid Biochem Mol Biol 40, 465–471. [DOI] [PubMed] [Google Scholar]
- Corton JC, Cunningham ML, Hummer BT, Lau C, Meek B, Peters JM, Popp JA, Rhomberg L, Seed J, Klaunig JE, 2014. Mode of action framework analysis for receptor-mediated toxicity: The peroxisome proliferator-activated receptor alpha (PPARalpha) as a case study. Crit Rev Toxicol 44, 1–49. [DOI] [PubMed] [Google Scholar]
- Corton JC, Lapinskas PJ, 2005. Peroxisome proliferator-activated receptors: mediators of phthalate ester-induced effects in the male reproductive tract? Toxicol Sci 83, 4–17. [DOI] [PubMed] [Google Scholar]
- Didolkar AK, Sundaram K, 1989. Mechanism of LHRH-stimulated steroidogenesis in rat Leydig cells: lipoxygenase products of arachidonic acid may not be involved. J Androl 10, 449–455. [DOI] [PubMed] [Google Scholar]
- Edwards SW, Tan YM, Villeneuve DL, Meek ME, McQueen CA, 2016. Adverse Outcome Pathways-Organizing Toxicological Information to Improve Decision Making. J Pharmacol Exp Ther 356, 170–181. [DOI] [PubMed] [Google Scholar]
- Egeghy PP, Cohen Hubal EA, Tulve NS, Melnyk LJ, Morgan MK, Fortmann RC, Sheldon LS, 2011. Review of pesticide urinary biomarker measurements from selected US EPA children’s observational exposure studies. Int J Environ Res Public Health 8, 1727–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeghy PP, Judson R, Gangwal S, Mosher S, Smith D, Vail J, Cohen Hubal EA, 2012. The exposure data landscape for manufactured chemicals. Sci Total Environ 414, 159–166. [DOI] [PubMed] [Google Scholar]
- Egeghy PP, Lorber M, 2011. An assessment of the exposure of Americans to perfluorooctane sulfonate: a comparison of estimated intake with values inferred from NHANES data. J Expo Sci Environ Epidemiol 21, 150–168. [DOI] [PubMed] [Google Scholar]
- Felter SP, Foreman JE, Boobis A, Corton JC, Doi AM, Flowers L, Goodman J, Haber LT, Jacobs A, Klaunig JE, Lynch AM, Moggs J, Pandiri A, 2018. Human relevance of rodent liver tumors: Key insights from a Toxicology Forum workshop on nongenotoxic modes of action. Regul Toxicol Pharmacol 92, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara D, Hallmark N, Scott H, Brown R, McKinnell C, Mahood IK, Sharpe RM, 2006. Acute and long-term effects of in utero exposure of rats to di(n-butyl) phthalate on testicular germ cell development and proliferation. Endocrinology 147, 5352–5362. [DOI] [PubMed] [Google Scholar]
- Fisher JS, 2004. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction 127, 305–315. [DOI] [PubMed] [Google Scholar]
- Fisher JS, Macpherson S, Marchetti N, Sharpe RM, 2003. Human ‘testicular dysgenesis syndrome’: a possible model using in-utero exposure of the rat to dibutyl phthalate. Hum Reprod 18, 1383–1394. [DOI] [PubMed] [Google Scholar]
- Foster PM, 2005. Mode of action: impaired fetal leydig cell function--effects on male reproductive development produced by certain phthalate esters. Crit Rev Toxicol 35, 713–719. [DOI] [PubMed] [Google Scholar]
- Foster PM, 2006. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl 29, 140–147; discussion 181–145. [DOI] [PubMed] [Google Scholar]
- French D, 2013. Development and validation of a serum total testosterone liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay calibrated to NIST SRM 971. Clin Chim Acta 415, 109–117. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Hensley JB, Liu D, Wallace DG, Borghoff S, Johnson KJ, Hall SJ, Boekelheide K, 2007. Fetal mouse phthalate exposure shows that Gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol Sci 97, 491–503. [DOI] [PubMed] [Google Scholar]
- Gazouli M, Yao ZX, Boujrad N, Corton JC, Culty M, Papadopoulos V, 2002. Effect of peroxisome proliferators on Leydig cell peripheral-type benzodiazepine receptor gene expression, hormone-stimulated cholesterol transport, and steroidogenesis: role of the peroxisome proliferator-activator receptor alpha. Endocrinology 143, 2571–2583. [DOI] [PubMed] [Google Scholar]
- Gentry PR, Clewell HJ 3rd, Clewell R, Campbell J, Van Landingham C, Shipp AM, 2011. Challenges in the application of quantitative approaches in risk assessment: a case study with di-(2-ethylhexyl)phthalate. Crit Rev Toxicol 41 Suppl 2, 1–72. [DOI] [PubMed] [Google Scholar]
- Gray LE Jr., Barlow NJ, Howdeshell KL, Ostby JS, Furr JR, Gray CL, 2009. Transgenerational effects of Di (2-ethylhexyl) phthalate in the male CRL:CD(SD) rat: added value of assessing multiple offspring per litter. Toxicol Sci 110, 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE Jr., Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L, 2000. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci 58, 350–365. [DOI] [PubMed] [Google Scholar]
- Gray TJ, Rowland IR, Foster PM, Gangolli SD, 1982. Species differences in the testicular toxicity of phthalate esters. Toxicol Lett 11, 141–147. [DOI] [PubMed] [Google Scholar]
- Hait EJ, Calafat AM, Hauser R, 2014. Urinary phthalate metabolite concentrations among men with inflammatory bowel disease on mesalamine therapy. Endocr Disruptors (Austin) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada, 2015a. Proposed approach for cumulative risk assessment of certain phthalates under the chemicals management plan. [Google Scholar]
- Health Canada, 2015b. Proposed approach for cumulative risk assessment of certain phthalates under the chemicals management plan. [Google Scholar]
- Heger NE, Hall SJ, Sandrof MA, McDonnell EV, Hensley JB, McDowell EN, Martin KA, Gaido KW, Johnson KJ, Boekelheide K, 2012. Human fetal testis xenografts are resistant to phthalate-induced endocrine disruption. Environ Health Perspect 120, 1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AB, 1965. The Environment and Disease: Association or Causation? Proc R Soc Med 58, 295–300. [PMC free article] [PubMed] [Google Scholar]
- Hines CJ, Nilsen Hopf NB, Deddens JA, Calafat AM, Silva MJ, Grote AA, Sammons DL, 2009. Urinary phthalate metabolite concentrations among workers in selected industries: a pilot biomonitoring study. Ann Occup Hyg 53, 1–17. [DOI] [PubMed] [Google Scholar]
- Hines DE, Edwards SW, Conolly RB, Jarabek AM, 2018. A Case Study Application of the Aggregate Exposure Pathway (AEP) and Adverse Outcome Pathway (AOP) Frameworks to Facilitate the Integration of Human Health and Ecological End Points for Cumulative Risk Assessment (CRA). Environ Sci Technol 52, 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK, Gray LE Jr., 2017. Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. Int J Hyg Environ Health 220, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Rider CV, Wilson VS, Gray LE Jr., 2008a. Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in male laboratory rats. Environ Research 108, 168–176. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK, Gray LE Jr., 2008b. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol Sci 105, 153–165. [DOI] [PubMed] [Google Scholar]
- Huang PC, Kuo PL, Chou YY, Lin SJ, Lee CC, 2009. Association between prenatal exposure to phthalates and the health of newborns. Environ Int 35, 14–20. [DOI] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ, 2003. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci 74, 297–308. [DOI] [PubMed] [Google Scholar]
- ICF International, 2016. Aggregate Exposure Pathway Workshop, AEP workshop, Durham, NC. [Google Scholar]
- Jeon S, Kim KT, Choi K, 2016. Migration of DEHP and DINP into dust from PVC flooring products at different surface temperature. Sci Total Environ 547, 441–446. [DOI] [PubMed] [Google Scholar]
- Ji Y, Wang F, Zhang L, Shan C, Bai Z, Sun Z, Liu L, Shen B, 2014. A comprehensive assessment of human exposure to phthalates from environmental media and food in Tianjin, China. J Hazard Mater 279, 133–140. [DOI] [PubMed] [Google Scholar]
- Judson R, Houck K, Martin M, Knudsen T, Thomas RS, Sipes N, Shah I, Wambaugh J, Crofton K, 2014. In vitro and modelling approaches to risk assessment from the U.S. Environmental Protection Agency ToxCast programme. Basic Clin Pharmacol Toxicol 115, 69–76. [DOI] [PubMed] [Google Scholar]
- Kim HH, Lim YW, Yang JY, Shin DC, Ham HS, Choi BS, Lee JY, 2013. Health risk assessment of exposure to chlorpyrifos and dichlorvos in children at childcare facilities. Sci Total Environ 444, 441–450. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kim TS, Shin JH, Moon HJ, Kang IH, Kim IY, Oh JY, Han SY, 2004. Neonatal exposure to di(n-butyl) phthalate (DBP) alters male reproductive-tract development. J Toxicol Environ Health A 67, 2045–2060. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee J, Park J, Kim HJ, Cho G, Kim GH, Eun SH, Lee JJ, Choi G, Suh E, Choi S, Kim S, Kim YD, Kim SK, Kim SY, Kim S, Eom S, Moon HB, Kim S, Choi K, 2015. Concentrations of phthalate metabolites in breast milk in Korea: estimating exposure to phthalates and potential risks among breast-fed infants. Sci Total Environ 508, 13–19. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Babich MA, Baetcke KP, Cook JC, Corton JC, David RM, DeLuca JG, Lai DY, McKee RH, Peters JM, Roberts RA, Fenner-Crisp PA, 2003. PPARalpha agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol 33, 655–780. [DOI] [PubMed] [Google Scholar]
- Kudo I, Murakami M, 2002. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat 68–69, 3–58. [DOI] [PubMed] [Google Scholar]
- Kurata Y, Makinodan F, Shimamura N, Katoh M, 2012. Metabolism of di (2-ethylhexyl) phthalate (DEHP): comparative study in juvenile and fetal marmosets and rats. J Toxicol Sci 37, 33–49. [DOI] [PubMed] [Google Scholar]
- La Rocca C, Tait S, Guerranti C, Busani L, Ciardo F, Bergamasco B, Stecca L, Perra G, Mancini FR, Marci R, Bordi G, Caserta D, Focardi S, Moscarini M, Mantovani A, 2014. Exposure to endocrine disrupters and nuclear receptor gene expression in infertile and fertile women from different Italian areas. Int J Environ Res Public Health 11, 10146–10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labow RS, Meek E, Adams GA, Rock G, 1988. Inhibition of human platelet phospholipase A2 by mono(2-ethylhexyl)phthalate. Environ Health Perspect 78, 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann KP, Phillips S, Sar M, Foster PM, Gaido KW, 2004. Dose-dependent alterations in gene expression and testosterone synthesis in the fetal testes of male rats exposed to di (n-butyl) phthalate. Toxicol Sci 81, 60–68. [DOI] [PubMed] [Google Scholar]
- Lin T, 1985. Mechanism of action of gonadotropin-releasing hormone stimulated Leydig cell steroidogenesis. III. The role of arachidonic acid and calcium/phospholipid dependent protein kinase. Life Sci 36, 1255–1264. [DOI] [PubMed] [Google Scholar]
- Liou SH, Yang GC, Wang CL, Chiu YH, 2014. Monitoring of PAEMs and beta-agonists in urine for a small group of experimental subjects and PAEs and beta-agonists in drinking water consumed by the same subjects. J Hazard Mater 277, 169–179. [DOI] [PubMed] [Google Scholar]
- Liu K, Lehmann KP, Sar M, Young SS, Gaido KW, 2005. Gene expression profiling following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis. Biol Reprod 73, 180–192. [DOI] [PubMed] [Google Scholar]
- Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, Schmidt IM, Suomi AM, Virtanen HE, Petersen DV, Andersson AM, Toppari J, Skakkebaek NE, 2006. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect 114, 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell ML, 2007. Distribution of phthalate monoesters in an aquatic food web, School of Resource and Environmental Management. Simon Fraser University. [Google Scholar]
- McKone TE, Enoch KG, 1993. CalTOX: A Multimedia Total Exposure Model for Hazardous-waste Sites. California Environmental Protection Agency. Dept. of Toxic Substances Control. [Google Scholar]
- McMullen PD, Bhattacharya S, Woods CG, Pendse SN, McBride MT, Soldatow VY, Deisenroth C, LeCluyse EL, Clewell RA, Andersen ME, 2019. Identifying qualitative differences in PPARα signaling networks in human and rat hepatocytes and their significance for next generation chemical risk assessment methods. Toxicol. In Vitro. In press. [DOI] [PubMed] [Google Scholar]
- Minatoya M, Naka Jima S, Sasaki S, Araki A, Miyashita C, Ikeno T, Nakajima T, Goto Y, Kishi R, 2016. Effects of prenatal phthalate exposure on thyroid hormone levels, mental and psychomotor development of infants: The Hokkaido Study on Environment and Children’s Health. Sci Total Environ 565, 1037–1043. [DOI] [PubMed] [Google Scholar]
- Moraga PF, Llanos MN, Ronco AM, 1997. Arachidonic acid release from rat Leydig cells depends on the presence of luteinizing hormone/human chorionic gonadotrophin receptors. J Endocrinol 154, 201–209. [DOI] [PubMed] [Google Scholar]
- Moreau M, Leonard J, Phillips KA, Campbell J, Pendse SN, Nicolas CI, Phillips M, Yoon M, Tan YM, Smith S, Pudukodu H, Isaacs K, Clewell H, 2017. Using exposure prediction tools to link exposure and dosimetry for risk-based decisions: a case study with phthalates. Chemosphere 184, 1194–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Cattley RC, Foster PM, 1999. Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol Appl Pharmacol 156, 81–95. [DOI] [PubMed] [Google Scholar]
- National Research Council, 2009. Science and Decisions: Advancing Risk Assessment. National Academies Press (US), Copyright 2009 by the National Academy of Sciences. All rights reserved., Washington (DC). [Google Scholar]
- National Research Council Committee on the Health Risks of Phthalates, 2008. Phthalates and Cumulative Risk Assessment: The Tasks Ahead. National Academies Press; (US) Copyright 2008 by the National Academy of Sciences. All rights reserved., Washington (DC). [Google Scholar]
- NICEATM, 2016. Integrated Approaches to Testing and Assessment. [Google Scholar]
- Palmer CN, Hsu MH, Griffin KJ, Raucy JL, Johnson EF, 1998. Peroxisome proliferator activated receptor-alpha expression in human liver. Mol Pharmacol 53, 14–22. [PubMed] [Google Scholar]
- Pan G, Hanaoka T, Yoshimura M, Zhang S, Wang P, Tsukino H, Inoue K, Nakazawa H, Tsugane S, Takahashi K, 2006. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environmental Health Perspectives 114, 1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE Jr., 2000. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci 58, 339–349. [DOI] [PubMed] [Google Scholar]
- Patlewicz G, Casati S, Basketter DA, Asturiol D, Roberts DW, Lepoittevin JP, Worth AP, Aschberger K, 2016. Can currently available non-animal methods detect pre and pro-haptens relevant for skin sensitization? Regul Toxicol Pharmacol 82, 147–155. [DOI] [PubMed] [Google Scholar]
- Patlewicz G, Dimitrov SD, Low LK, Kern PS, Dimitrova GD, Comber MI, Aptula AO, Phillips RD, Niemela J, Madsen C, Wedebye EB, Roberts DW, Bailey PT, Mekenyan OG, 2007. TIMES-SS--a promising tool for the assessment of skin sensitization hazard. A characterization with respect to the OECD validation principles for (Q)SARs and an external evaluation for predictivity. Regul Toxicol Pharmacol 48, 225–239. [DOI] [PubMed] [Google Scholar]
- Pei XQ, Song M, Guo M, Mo FF, Shen XY, 2013. Concentration and risk assessment of phthalates present in indoor air from newly decorated apartments. Atmospheric Environment 68, 17–23. [Google Scholar]
- Rakkestad KE, Dye CJ, Yttri KE, Holme JA, Hongslo JK, Schwarze PE, Becher R, 2007. Phthalate levels in Norwegian indoor air related to particle size fraction. J Environ Monit 9, 1419–1425. [DOI] [PubMed] [Google Scholar]
- Rider CV, Furr JR, Wilson VS, Gray LE Jr., 2010. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. International Journal of Andrology 33, 443–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CV, Wilson VS, Howdeshell KL, Hotchkiss AK, Furr JR, Lambright CR, Gray LE Jr., 2009a. Cumulative effects of in utero administration of mixtures of “antiandrogens” on male rat reproductive development. Toxicol Pathol 37, 100–113. [DOI] [PubMed] [Google Scholar]
- Rider CV, Wilson VS, Howdeshell KL, Hotchkiss AK, Furr JR, Lambright CR, Gray LE Jr., 2009b. Cumulative effects of in utero administration of mixtures of “antiandrogens” on male rat reproductive development. Toxicologic Pathology 37, 100–113. [DOI] [PubMed] [Google Scholar]
- Safe SH, 1998. Hazard and Risk Assessment of Chemical Mixtures Using the Toxic Equivalency Factor Approach. Environ Health Perspect 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Lorber M, Guo Y, Wu Q, Yun SH, Kannan K, Hommel M, Imran N, Hynan LS, Cheng D, Colacino JA, Birnbaum LS, 2013. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ Health Perspect 121, 473–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeny R, 2014. AOP Workshop: AOPs and Regulatory Decisions: Problem Formulation, Development and Acceptance. [Google Scholar]
- Seo KW, Kim KB, Kim YJ, Choi JY, Lee KT, Choi KS, 2004. Comparison of oxidative stress and changes of xenobiotic metabolizing enzymes induced by phthalates in rats. Food Chem Toxicol 42, 107–114. [DOI] [PubMed] [Google Scholar]
- Shin HM, McKone TE, Bennett DH, 2012. Intake fraction for the indoor environment: a tool for prioritizing indoor chemical sources. Environ Sci Technol 46, 10063–10072. [DOI] [PubMed] [Google Scholar]
- Shin HM, McKone TE, Bennett DH, 2014. Attributing population-scale human exposure to various source categories: merging exposure models and biomonitoring data. Environment International 70, 183–191. [DOI] [PubMed] [Google Scholar]
- Six DA, Dennis EA, 2000. The expanding superfamily of phospholipase A(2) enzymes: classification and characterization. Biochim Biophys Acta 1488, 1–19. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM, 2001. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Human Reproduction 16, 972–978. [DOI] [PubMed] [Google Scholar]
- Sonich-Mullin C, Fielder R, Wiltse J, Baetcke K, Dempsey J, Fenner-Crisp P, Grant D, Hartley M, Knaap A, Kroese D, Mangelsdorf I, Meek E, Rice JM, Younes M, 2001. IPCS conceptual framework for evaluating a mode of action for chemical carcinogenesis. Regul Toxicol Pharmacol 34, 146–152. [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL, 2005. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 113, 1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YM, Leonard JA, Edwards S, Teeguarden J, Egeghy P, 2018a. Refining the aggregate exposure pathway. Environ Sci Process Impacts 20, 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YM, Leonard JA, Edwards S, Teeguarden J, Paini A, Egeghy P, 2018b. Aggregate Exposure Pathways in Support of Risk Assessment. Curr Opin Toxicol 9, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeguarden JG, Tan YM, Edwards SW, Leonard JA, Anderson KA, Corley RA, Kile ML, Simonich SM, Stone D, Tanguay RL, Waters KM, Harper SL, Williams DE, 2016. Completing the Link between Exposure Science and Toxicology for Improved Environmental Health Decision Making: The Aggregate Exposure Pathway Framework. Environ Sci Technol 50, 4579–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. CPSC, 2014. Chronic hazard advisory panel on phthalates and phthalate alternatives. [Google Scholar]
- Van den Berg M, Birnbaum L, Bosveld AT, Brunström B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, van Leeuwen FX, Liem AK, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Waern F, Zacharewski T, 1998. Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect 106, 775–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE, 2006. The 2005 World Health Organization Reevaluation of Human and Mammalian Toxic Equivalency Factors for Dioxins and Dioxin-Like Compounds. Toxicological Sciences 93, 223–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Driesche S, McKinnell C, Calarrao A, Kennedy L, Hutchison GR, Hrabalkova L, Jobling MS, Macpherson S, Anderson RA, Sharpe RM, Mitchell RT, 2015. Comparative effects of di(n-butyl) phthalate exposure on fetal germ cell development in the rat and in human fetal testis xenografts. Environ Health Perspect 123, 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Driesche S, Walker M, McKinnell C, Scott HM, Eddie SL, Mitchell RT, Seckl JR, Drake AJ, Smith LB, Anderson RA, Sharpe RM, 2012. Proposed role for COUP-TFII in regulating fetal Leydig cell steroidogenesis, perturbation of which leads to masculinization disorders in rodents. PLoS One 7, e37064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Wang H, Zhou W, Chen Y, Zhou Y, Jiang Q, 2015a. Urinary excretion of phthalate metabolites in school children of China: implication for cumulative risk assessment of phthalate exposure. Environ Sci Technol 49, 1120–1129. [DOI] [PubMed] [Google Scholar]
- Wang X, Song M, Guo M, Chi C, Mo F, Shen X, 2015b. Pollution levels and characteristics of phthalate esters in indoor air in hospitals. J Environ Sci (China) 37, 67–74. [DOI] [PubMed] [Google Scholar]
- Wang X, Stocco DM, 1999. Cyclic AMP and arachidonic acid: a tale of two pathways. Mol Cell Endocrinol 158, 7–12. [DOI] [PubMed] [Google Scholar]
- Wang X, Walsh LP, Reinhart AJ, Stocco DM, 2000. The role of arachidonic acid in steroidogenesis and steroidogenic acute regulatory (StAR) gene and protein expression. J Biol Chem 275, 20204–20209. [DOI] [PubMed] [Google Scholar]
- Wittassek M, Angerer J, Kolossa-Gehring M, Schafer SD, Klockenbusch W, Dobler L, Gunsel AK, Muller A, Wiesmuller GA, 2009. Fetal exposure to phthalates--a pilot study. Int J Hyg Environ Health 212, 492–498. [DOI] [PubMed] [Google Scholar]
- Xie Z, Ebinghaus R, Temme C, Lohmann R, Caba A, Ruck W, 2007. Occurrence and air-sea exchange of phthalates in the Arctic. Environ Sci Technol 41, 4555–4560. [DOI] [PubMed] [Google Scholar]
- Xie Z, et al. , 2006. Atmospheric concentrations and air-sea exchanges of phthalates in the North Sea (German Bight). Atmospheric Environment 39, 3209–3219. [Google Scholar]
- Yan X, Calafat A, Lashley S, Smulian J, Ananth C, Barr D, Silva M, Ledoux T, Hore P, Robson MG, 2009. Phthalates Biomarker Identification and Exposure Estimates in a Population of Pregnant Women. Hum Ecol Risk Assess 15, 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon M, Adeleye Y, Clewell R, Jennings P, Whelan M, 2016. Moving Beyond Prioritization Toward True In Vitro Safety Assessment. Applied In Vitro Toxicology 2, 67–73. [Google Scholar]
- Zhang Y, Cao Y, Shi H, Jiang X, Zhao Y, Fang X, Xie C, 2015. Could exposure to phthalates speed up or delay pubertal onset and development? A 1.5-year follow-up of a school-based population. Environ Int 83, 41–49. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chen L, Li LX, Xie CM, Li D, Shi HJ, Zhang YH, 2014. Gender-specific relationship between prenatal exposure to phthalates and intrauterine growth restriction. Pediatr Res 76, 401–408. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tian J, Wu G, Wei F, 2012. Estimation of the air-soil exchange of phthalates. Environmental Chemistry - Huanjing Huaxue 31, 1535–1541. [Google Scholar]
- Ziv-Gal A, Gallicchio L, Chiang C, Ther SN, Miller SR, Zacur HA, Dills RL, Flaws JA, 2016. Phthalate metabolite levels and menopausal hot flashes in midlife women. Reprod Toxicol 60, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.