Abstract
Background
As obesity rates continue to rise, it is increasingly important to understand factors that can influence body weight and growth, especially from an early age. The infant gut microbiota has broad effects on a variety of bodily processes, but its relation to infant growth is not yet fully characterized. Since the infant gut microbiota is closely related to breastfeeding practices and maternal health, understanding the relationship between these factors and infant growth may provide insight into the origins of childhood obesity.
Objectives
Identify the relationship between human milk exposure, maternal pre-pregnancy body mass index (BMI), the infant gut microbiota, and 12-month-old BMI-for-age z-scores (12M BAZ) to identify key factors that shape infant growth.
Methods
Two Michigan cohorts (ARCHGUT and BABYGUT) comprised of a total of 33 mother-infant dyads provided infant fecal samples at 12M. After DNA extraction, amplification, and sequencing of the V4 16S rRNA region using Illumina MiSeq v2 Chemistry, gut bacterial diversity metrics were analyzed in relation to human milk exposure, maternal pre-pregnancy BMI, and infant growth parameters.
Results
Recent human milk exposure was inversely related to maternal pre-pregnancy BMI and most strongly associated with infant gut bacterial community membership and individual gut microbiota richness differences. Maternal pre-pregnancy BMI was not associated with the infant gut microbiota after adjusting for human milk exposure. However, maternal pre-pregnancy BMI was the only factor significantly associated with 12M BAZ.
Conclusions
Human milk exposure is one of the central influences on the infant gut microbiota at 12M of age. However, the lack of association between the infant gut microbiota and 12M-old infant BAZ suggests that genetic, physiological, dietary, and other environmental factors may play a more direct role than the gut microbiota in determining infant BAZ at 12M.
Keywords: Pregnancy, Infant growth, Infant development, Infant health, Breastfeeding, Human milk, Gut microbiota, Maternal health, Alpha diversity, Beta diversity
Abbreviations: 6M, 6 months; 12M, 12 months; BAZ, BMI-for-age z-score; HM, Human Milk; LAZ, Length-for-age z-score; LWZ, Length-for-weight z-score; PCoA, Principal Coordinates Analysis; WAZ, Weight-for-age z-score
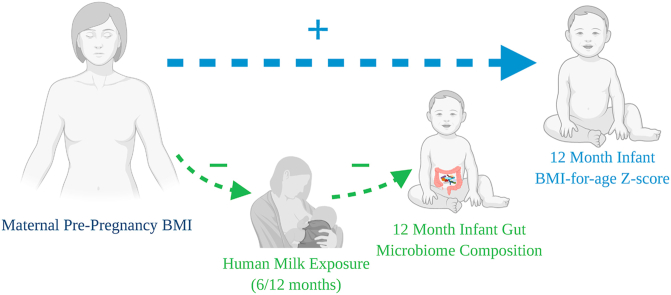
Graphical abstract
Highlights
-
•
Infant 12M BMI-for-age z-score positively correlated with maternal pre-pregnancy BMI.
-
•
Infant 12M BMI-for-age z-score was not associated with the infant gut microbiota.
-
•
Pre-pregnancy BMI was associated with the infant gut microbiota but not after adjusting for recent exposure to human milk.
-
•
Human milk exposure immediately prior to stool collection is an important covariate.
The 12-month infant gut microbiota strongly associates with human milk exposure but not with maternal BMI. 12-month infant BAZ positively correlates with maternal BMI, but not with human milk exposure.
1. Introduction
Between 1980 and 2013, worldwide obesity rates for children rose nearly 50% (Ng et al., 2014). Obesity is strongly associated with a plethora of medical complications including type II diabetes, cancer, and cardiovascular disease (Guh et al., 2009) and produces significant global economic costs of around $2 trillion (Dobbs et al., 2014). It is therefore of utmost importance to understand the potential causes of obesity and to continue to develop novel, efficient solutions to reduce the prevalence of obesity. This is especially relevant for childhood obesity, which correlates with adult obesity/overweight status (Simmonds et al., 2016). Beginning at age five, the body mass index (BMI) category of a child is likely to continue on the same or similar trajectory throughout their development into adulthood (Geserick et al., 2018). Furthermore, there is a significant positive correlation between an individual’s BMI at 4 months of age and their BMI at 5 years of age (Gittner et al., 2014). Therefore, understanding the causes and variables associated with the development of childhood obesity can provide important information to tackle the obesity epidemic from its roots.
From birth, an infant is inoculated with their mother’s bacterial communities (Bäckhed et al., 2015). Several factors may play a role in altering the infant gut microbiota, with some of the most important being mode of delivery, human milk exposure/cessation, maternal health, and antibiotic exposure (Bäckhed et al., 2015; Koleva et al., 2015). Examining the relationship between maternal pre-pregnancy BMI and the gut microbiota can reveal important insights into the development of the infant gut microbiota, especially in light of the well-established energy-harvesting effects of the gut microbiota demonstrated in mice and humans (Backhed et al., 2004; Schwiertz et al., 2010).
Breastfeeding is associated with positive health outcomes in infants (Bashiardes et al., 2016; van den Elsen et al., 2019; Miliku and Azad, 2018), and has been shown to distinctly alter the infant gut microbiota (Bode et al., 2014; Wang et al., 2015). As such, understanding the ways in which human milk shapes the 12-month (12M) infant gut microbiota can have important public health implications. Furthermore, maternal BMI has been reported to influence bacterial communities in human milk (Cabrera-Rubio et al., 2012). Hence, by examining the gut microbiota of infants and important covariates such as human milk exposure and maternal pre-pregnancy BMI, valuable information may be gained concerning the origins of childhood obesity. The objective of this study is to define the ways that the 12M infant gut microbiota is associated with the interrelated factors of maternal BMI, infant human milk exposure, and infant BMI-for-age z-scores, a measure of infant growth.
2. Methods
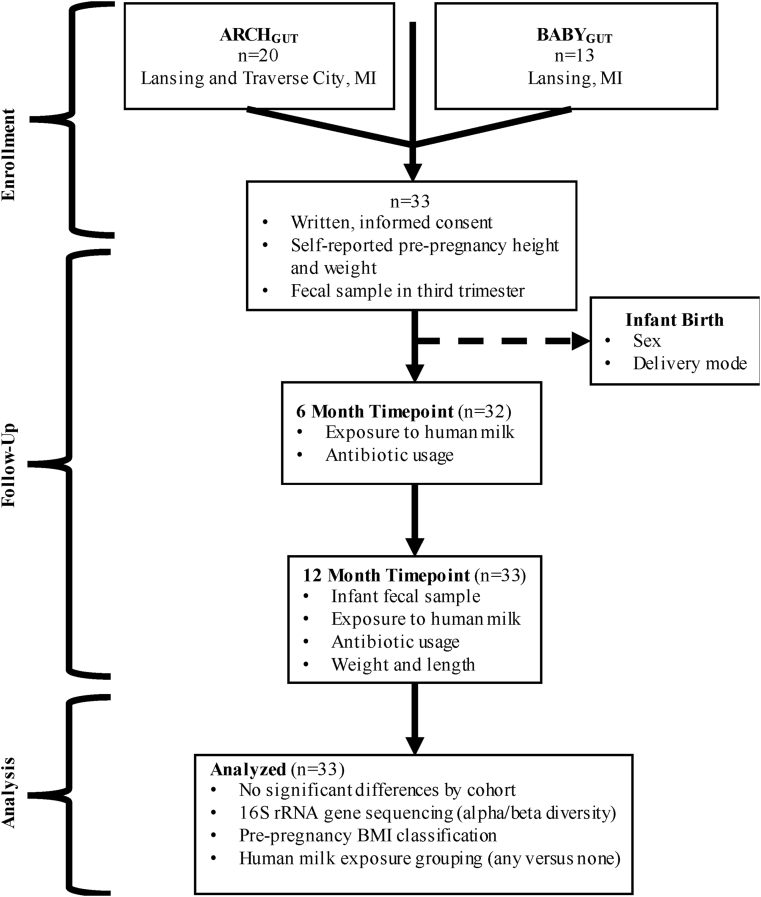
2.1. Subjects (Fig. 1)
Fig. 1.
Participant flowchart showing enrollment, follow-up, and analysis of data from all participants who provided a 12-month infant fecal sample in addition to 6 month and pre-pregnancy data.
Two cohorts (ARCHGUT and BABYGUT) of Michigan women were enrolled for this study. ARCHGUT, a subset of the Archive for Research in Child Health, recruited pregnant women at a prenatal clinic in Traverse City and another in Lansing, Michigan. Meanwhile, BABYGUT recruitment occurred in several prenatal clinics in the greater Lansing area. All participants provided written, informed consent and study activities were approved by the Michigan State University Human Research Protection Program (IRB 15–1240 and 14–170M) (Sugino et al., 2019).
2.2. Sample collection
The pregnant women provided fecal samples in their third pregnancy trimester, and later from infants at 1 week, 6 months, 12 months and 24 months of age. Samples were sent to the lab by way of mail or collected from the participant’s home. Stool was collected from the diaper and put into a Para-Pak Clean Vial collection tube (Meridian Biosciences, Cincinnati, OH). Fecal aliquots were stored at −80 °C upon arrival to the lab. The focus of this paper is the relationship between pre-pregnancy BMI, human milk exposure, and the 12M infant stool samples (n = 33). Results from the pregnancy, 1-week and 6-month timepoints have been reported elsewhere (Sugino et al., 2019, 2020; Sosa-Moreno et al., 2020). For the 12M infant stool samples, the mean time from sample collection to receipt by the laboratory was 3.7 ± 1.9 days (median of 3 days).
2.3. DNA extraction and rRNA gene amplification
DNA extraction, 16S rRNA gene amplification, and sequencing were as presented in (Sugino et al., 2019).
2.4. Processing and analysis of sequence data
Processing and analysis of sequence data also followed the same, previously-described protocol (Sugino et al., 2019). In this study, samples were each rarefied to 10,000 reads 999 times, followed by averaging and rounding of each OTU to the nearest integer prior to subsequent analysis.
2.5. Data analysis
The women who participated in this study were classified by maternal pre-pregnancy BMI (calculated from self-reported height and weight) as normal (18.5≤BMI<25; n = 10), overweight (25≤BMI<30; n = 11) or obese (BMI≥30; n = 12). Participants estimated the fraction of their infant’s diet, in the past week, that was human breast milk as 100%, 80%, 50–80%, 50%, 20–50%, 20% or 0% (Bonuck et al., 2005). To increase power, we collapsed all breast milk categories from 20% or more (n = 13) and contrasted findings to the no human milk group (n = 20). Infant length and weight at 12M were reported by the mother. BMI-for-age (BAZ), length-for-age (LAZ), weight-for-age (WAZ), and length-for-weight (LWZ) z-scores were calculated using infant length, weight, sex and age using the WHO’s Anthro Software (WHO, 2011). Comparison of population characteristics was done using a chi-square test for categorical variables or ANOVA for continuous variables. Post hoc analysis of chi-square values for HM exposure versus maternal pre-pregnancy BMI was done in R (R Core Team, 2011) using a pairwise comparison through the rcompanion package (Mangiafico, 2020).
Alpha (within-sample) diversity, measured with Chao1 and Shannon indices, was calculated using R via the vegan package (Oksanen et al., 2015). Normality of the alpha diversity was confirmed using the Shapiro-Wilk test and ANOVA was used to test for significant differences across groups. Post-hoc comparison between HM exposure categories was performed using a Tukey’s Honest Significant Difference test. Sorensen, which pertains to community composition, and Bray-Curtis, which pertains to community structure, dissimilarities were calculated from the abundance data via the vegan package in R and plotted using principal coordinate analysis (PCoA). Using the adonis function, PERMANOVA was performed to identify significant beta-diversity differences, specifically with taxa driving Sorensen distance separation. The p-values for the separation driven by the taxa were adjusted for false positives by the Benjamini-Hochberg method. To identify differences in sample dispersion, PERMDISP (betadisper function in the vegan package) was utilized. For both alpha and beta diversity, multivariable linear regression models were used to test the associations between maternal BMI category and human milk exposure. Alpha diversity models were tested using a type II ANOVA. Analyses of beta diversity used PERMANOVA (adonis2 function in the vegan package) to fit the models. Individual taxa were compared by human milk exposure groups using a negative binomial model in the MASS package (Venables and Ripley, 2002). Post-hoc power analysis was done through the G∗power version 3.1.9.2 (Faul et al., 2007) for alpha diversity and through the micropower package (Kelly et al., 2015) in R for beta diversity. Significance levels were set at p < 0.05.
3. Results
Participant (n = 33) characteristics did not differ by cohort (Table 1). Ten (30.3%) mothers were normal weight, 11 (33.3%) were overweight and 12 (36.4%) were obese prior to becoming pregnant. The majority of infants were male (n = 24; 72.7%), and 12 (36.4%) infants were born via C-section. Mode of delivery did not significantly associate with maternal BMI category (chi-square, p = 0.09). At 6M, 20 (62.5%) infants consumed at least some human milk. Whereas at 12M, 13 (39.4%) infants were still consuming at least some human milk. No infants were exposed to antibiotics within 30 days of providing the stool sample. Infants averaged 379.8±16.4 days of age, 76.0±3.3 cm in length, and 10.2±1.1 kg of weight. Infant anthropometrics were as follows: BAZ (0.7±1.4), LAZ (−0.04±1.33), LWZ (0.7±1.3), and WAZ (0.4±1.2).
Table 1.
Population characteristics. Abbreviations: 6M: 6 months, 12M: 12 months, BAZ: BMI-for-age z-score, HM: Human Milk, LAZ: Length-for-age z-score, LWZ: Length-for-weight z-score, WAZ: Weight-for-age z-score.
| ARCHGUT (n = 20) | BABYGUT (n = 13) | p-value | |
|---|---|---|---|
| Pre-pregnancy BMI category | 0.35 | ||
| Normal | 6 (30.0) | 4 (30.8) | |
| Overweight | 5 (25.0) | 6 (46.2) | |
| Obese | 9 (45.0) | 3 (23.1) | |
| Girls | 7 (35.0) | 2 (15.4) | 0.4 |
| Vaginal delivery | 14 (70.0) | 7 (53.8) | 0.57 |
| Any HM – 6M | 10 (52.6)a | 10 (76.9) | 0.31 |
| Any HM – 12M | 9 (45.0) | 4 (30.8) | 0.65 |
| Antibiotic exposure in the past monthb | 0 (0.0) | 0 (0.0) | – |
| Infant age, days | 379.4 ± 15.7 | 380.3 ± 18.0 | 0.51 |
| Infant length, cm | 74.4 ± 3.5 | 75.4 ± 3.1 | 0.54 |
| Infant weight, kg | 10.5 ± 1.0 | 9.8 ± 1.2 | 0.43 |
| BAZ | 0.9 ± 1.3 | 0.4 ± 1.6 | 0.46 |
| LAZ | 0.1 ± 1.3 | −0.3 ± 1.4 | 0.46 |
| LWZ | 1.0 ± 12 | 0.4 ± 1.4 | 0.52 |
| WAZ | 0.7 ± 0.9 | 0.04 ± 1.04 | 0.47 |
One value missing.
Reported antibiotic exposure is for infants; maternal antibiotic use was not collected at 6M or 12M.
3.1. Human milk exposure
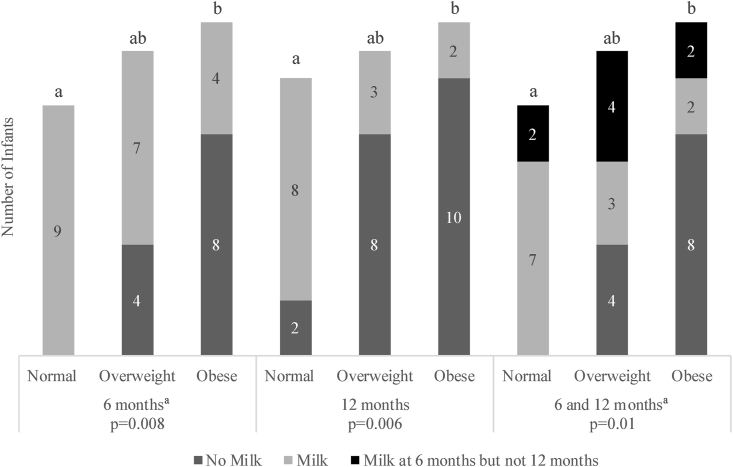
Infant human milk exposure was inversely associated with maternal pre-pregnancy BMI (Fig. 2). At each set of time periods, a higher ratio of women with pre-pregnancy obesity did not give their infants any human milk compared to women of normal weight. In fact, infants with mothers of normal BMI all received some exposure to human milk, whereas four of the infants with overweight mothers and eight of the infants with obese mothers were never exposed to human milk at 6M or 12M.
Fig. 2.
Stacked bar chart demonstrating the number of infants (n = 33) that were exposed to human milk at 6 months, 12 months, and both timepoints together versus maternal pre-pregnancy BMI category. Bars within each age grouping that share a superscript letter do not differ from each other.
a 6 months and 6 + 12 months data exclude one value in the normal pre-pregnancy BMI category due to incomplete data collection.
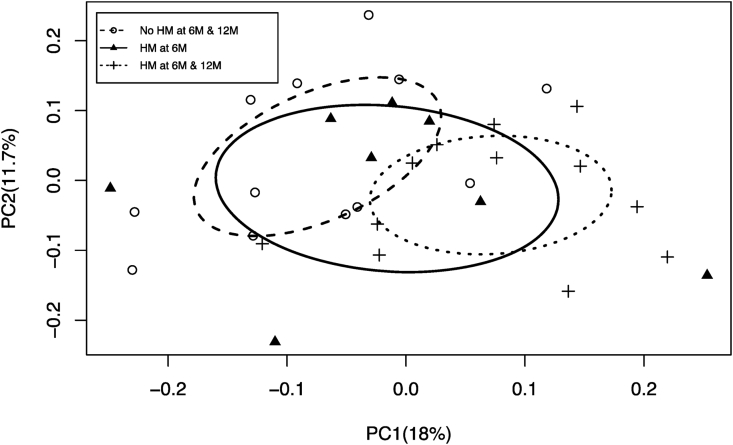
Human milk exposure at 6M and 12M was associated with 12M infants’ gut microbiota beta diversity as determined by Sorensen distances (Fig. 3) but not with Bray-Curtis distances (Supplementary Fig. 1). Gut microbiota membership in infants who were not exposed to human milk at 6M or 12M differed significantly from that of infants who were exposed to some human milk at 6M and 12M. Infants exposed to human milk at only 6M had specific members of the gut microbiota that resembled those found in infants who were never exposed to human milk and others similar to those found in infants exposed to human milk at both 6M and 12M. Not all genera found in the infant gut microbiota differed by human milk exposure. The taxa whose presence/absence drove Sorensen distance separation are included (Supplementary Table 1).
Fig. 3.
PCoA of the Sorensen distances for the 12M-old microbiota (p = 0.003). The comparison is across infants without human milk exposure, with human milk exposure at 6M timepoint only, and human milk exposure at both 6M and 12M. A principal coordinates analysis plot represents each microbial community from a given sample as a single point on a plot with axes that account for the major difference-driving factors. Sorensen distance accounts for the presence or absence of bacterial types between samples. The farther apart the points on the plot, the more dissimilar the samples. Abbreviations: 6M: 6 months, 12M: 12 months, HM: Human Milk, PCoA: Principal Coordinates Analysis.
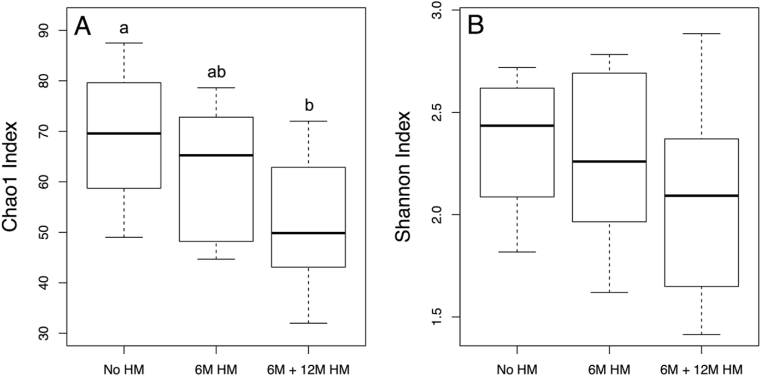
Infants exposed to human milk at both 6M and 12M of age had significantly lower alpha diversity, as measured by the Chao1 index, than infants who were not exposed at 6M or 12M (Fig. 4). However, overall bacterial alpha diversity represented by Shannon index values did not differ by human milk exposure.
Fig. 4.
Boxplots of A) Chao1 indices (p = 0.01) and B) Shannon indices (p = 0.22) for 12M infant gut microbial samples stratified based on timing of human milk exposure. Chao1 is an estimator of the species richness in a community, and the Shannon index considers both evenness and abundance to produce a measure of alpha diversity. Boxplots with shared superscript letters are not significantly different from one another. Abbreviations: 6M: 6 months, 12M: 12 months, HM: Human Milk.
3.2. Maternal pre-pregnancy BMI
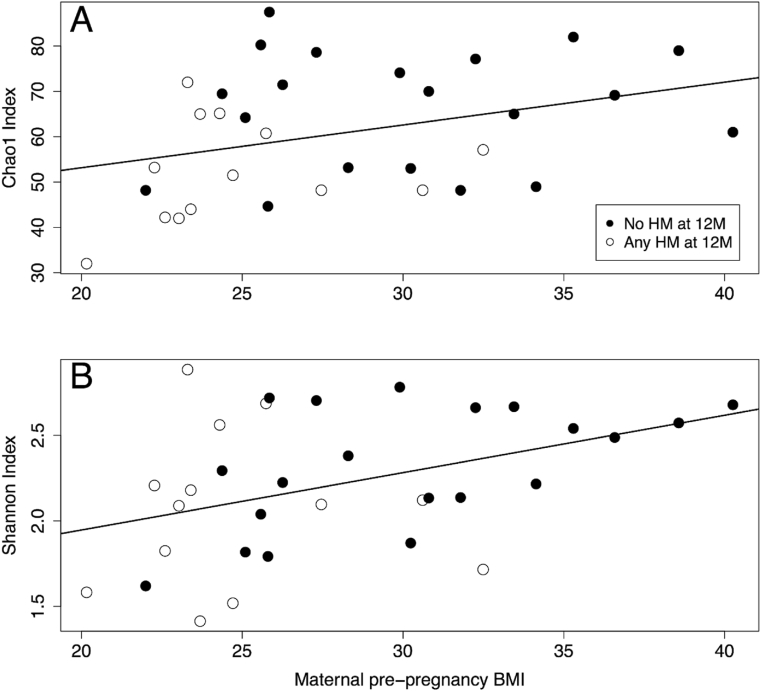
Infants with mothers who had higher pre-pregnancy BMIs also had a greater richness of gut microbes at 12M (r = 0.50, p = 0.01) (Fig. 5a), but this significant association between maternal pre-pregnancy BMI and alpha diversity richness (Chao1) disappeared (p = 0.49) when adjusting for human milk exposure through a bivariate analysis. Indeed, human milk exposure (p = 0.03) drove the association with the Chao1 index. Similarly, maternal pre-pregnancy BMI was positively associated with 12M infant alpha diversity represented through the Shannon index (r = 0.43, p = 0.04) (Fig. 5b), which factors richness and evenness, but this significant association between pre-pregnancy BMI and Shannon scores became a trend (p = 0.07) when adjusting for human milk exposure. In this case, human milk exposure was not associated with infant Shannon index scores (p = 0.54). Maternal pre-pregnancy BMI was not associated with infant 12M beta diversity measures (Supplementary Fig. 2).
Fig. 5.
A) Chao1 index values for each infant versus maternal pre-pregnancy BMI (r = 0.50, p = 0.01). B) Shannon index versus maternal pre-pregnancy BMI (r = 0.43, p = 0.04). Abbreviations: 6M: 6 months, 12M: 12 months, HM: Human Milk.
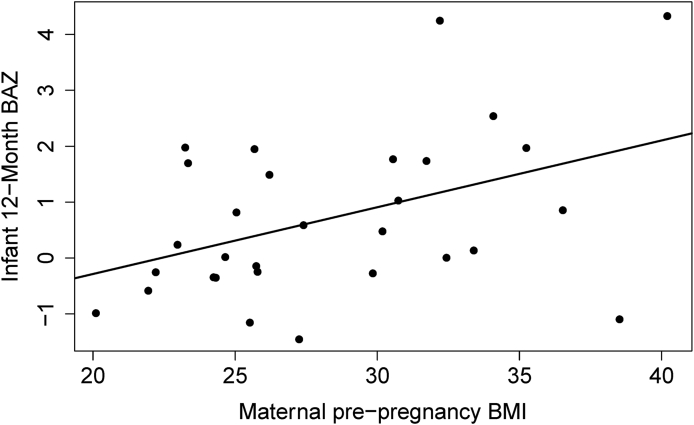
Infant BAZ was positively associated with maternal pre-pregnancy BMI (Fig. 6) but not associated with gut microbial alpha/beta diversity measures (Supplementary Figs. 3 and 4) or human milk exposure (Supplementary Fig. 5). Additionally, neither 1-week nor 6M infant gut microbial diversities were associated with 12M infant BAZ (Supplementary Figs. 6 and 7). Effect sizes and power for all analyses are included (Supplementary Table 2).
Fig. 6.
12-month infant BAZ is positively associated with maternal pre-pregnancy BMI (r = 0.43, p = 0.01). BAZ is a statistical measure of the number of standard deviations an infant’s BMI is away from the mean for that age. Abbreviations: BAZ: BMI-for-age z-score.
4. Discussion
This study examined the associations between the infant gut microbiota, maternal pre-pregnancy BMI, infant human milk exposure, and infant BAZ at 12M of age. Mothers with higher pre-pregnancy BMI did not provide as much human milk at both 6M and 12M as did women with BMIs ≤25. Human milk exposure was associated with decreased alpha diversity richness within infant fecal samples at 12M. We observed a positive relationship between maternal pre-pregnancy BMI and infant gut microbial alpha diversity. However, this association was driven by differences in human milk exposure. When assessing 12M infant growth, as characterized by BAZ scores, both the infant gut microbiota and human milk exposure were unassociated with BAZ. Mothers with higher BMIs were more likely to have infants with a greater BAZ at 12M of age. These results underscore the importance of variables apart from the gut microbiota in shaping infant growth at 12M of age. These factors are postulated to be environmental, dietary, physiological, and genetic in origin.
Infant human milk exposure was inversely associated with maternal pre-pregnancy BMI (Fig. 2). Mothers with a pre-pregnancy BMI falling in the normal range provided their infants with more breastmilk at 6M and at 12M than mothers with pre-pregnancy obesity. This is consistent with previous research, which has repeatedly found that mothers with a BMI over 25 are less likely to breastfeed than mothers with lower BMI (Lucas et al., 2015; Donath and Amir, 2008; Guelinckx et al., 2012). This is likely due to a combination of physical, physiological and psychological obstacles that decrease the probability that an overweight or obese mother will attempt to initiate or continue breastfeeding their infant (Garner et al., 2014; Jevitt et al., 2007). Some of these obstacles include delayed lactogenesis, edema in breasts, hormonal imbalances, and body-image insecurities (Bever Babendure et al., 2015).
Of all factors analyzed in this study, human milk exposure was most consistently associated with the gut microbiota composition (Bäckhed et al., 2015; Stewart et al., 2018). Infants not exposed to human milk at or beyond 6M had very distinct microbiotas—characterized by Sorensen distance which accounts for presence/absence—from infants who continued breastfeeding (Fig. 3). This result is consistent with a previous meta-analysis that concluded that breastfed infants do indeed have a significantly distinct gut microbiota from non-breastfed infants that is duration/exposure-dependent, with significant differences persisting from 6M to two years of age (Ho et al., 2018). The presence of a wide variety of taxa drove separation of gut microbial communities of infants with little to no recent human milk exposure (Supplementary Table 1), which is in line with the well-established notion that infants with lesser degrees of exposure to human milk exhibit more diverse gut microbial compositions (Bäckhed et al., 2015; O’Sullivan et al., 2015). However, community differences were not significant when analyzed on the basis of Bray-Curtis distance (Supplementary Fig. 1), which takes bacterial abundance, as well as presence/absence, into account.
Increased breastmilk exposure was associated with a reduction in microbial richness in this sample of infants (Fig. 4a). Although it is not yet clear which bacteria are necessary and/or sufficient to assemble a healthy gut microbiota (McBurney et al., 2019), high microbial diversity has classically been thought to indicate a healthier gut in adults, whose gut microbiota is relatively stable (Valdes et al., 2018; Yassour et al., 2016; Lloyd-Price et al., 2016; Lozupone et al., 2012; Turnbaugh et al., 2009; Caporaso et al., 2011). The infant gut microbiota, in contrast, is highly variable, especially in the first three years of life (Yatsunenko et al., 2012). Nonetheless, it is commonly reported that HM-fed infants have less diverse gut bacterial communities (Ho et al., 2018; Azad et al., 2013), and this aligns with the results herein. Maturation of the infant gut through an increase in microbial diversity to result in an adult-like gut microbiota is brought about by the cessation of breastfeeding (Bäckhed et al., 2015; De Filippo et al., 2010). Lower gut microbial richness at 12M in association with HM exposure is likely due to the selective nature of human milk oligosaccharides which promote the growth of a limited number of beneficial microbes such as Bifidobacterium longum subsp. infantis and some Bacteroides species (Marcobal et al., 2010). Although the bacterial gut microbiota of 12M breastfed infants in this study had decreased richness, their overall community alpha diversity was not significantly different from infants not exposed to HM or to those only exposed at 6M, as shown by the Shannon scores (Fig. 4b). This is potentially due to the greatly increased counts of Bifidobacteria, and to a lesser degree Bacteroides, that are present in breastfed infant guts (Bezirtzoglou et al., 2011). The elevated abundances of these genera may serve to counterbalance overall community diversity changes in spite of a richness reduction.
Maternal pre-pregnancy BMI was significantly correlated with alpha diversity in the microbiota of 12M infants, but once human milk exposure was included in the bivariate model, the significant positive association disappeared (Fig. 5). Instead, human milk exposure became the main factor accounting for the differences in richness (Fig. 5a), but not Shannon diversity scores (Fig. 5b), which suggests that the influence of maternal pre-pregnancy BMI on the infant gut microbiota at 12M was likely indirect and driven by other complex factors, especially breastfeeding. This possibility is supported by the lack of association between maternal pre-pregnancy BMI and infant gut microbial beta diversity (Supplementary Fig. 2). Considering the environmental and genetic factors that influence development within an infant’s first year, it is plausible that maternal pre-pregnancy BMI no longer plays as important of a role in modulating the infant gut microbiota at 12M. In early infancy, however, maternal pre-pregnancy BMI is significantly associated with several parameters of the gut microbiota (Sugino et al., 2019).
Infant 12M BAZ scores were significantly associated with maternal pre-pregnancy BMI (Fig. 6), but not infant gut microbial alpha and beta diversity (Supplementary Figs. 3 and 4) or human milk exposure (Supplementary Fig. 5). Previous studies have demonstrated a positive association between maternal BMI and infant BMI at birth and throughout later development (Williams et al., 2014; Voerman et al., 2019; Taveras et al., 2009; Kjaer et al., 2019; Bonakdar et al., 2019; Heslehurst et al., 2019). In adults, the gut microbiota has been shown to be significantly associated with obesity (Davis, 2016; Muscogiuri et al., 2019; Turnbaugh et al., 2006; Huttenhower et al., 2012), but associations with 12M infant risk for obesity are less clear (Stanislawski et al., 2018; Moossavi and Azad, 2019; Gohir et al., 2015; Mohammadkhah et al., 2018). Although one previous study identified a negative correlation between 12M Staphylococcus counts and BAZ at 1–3 years of age (Vael et al., 2011), our results suggest that, overall, 12M-old infant BAZ is most correlated with maternal BMI, rather than the gut microbiota. We postulate that genetic, physiological, dietary, and environmental factors mediate this observed association between maternal BMI and 12M infant BAZ, but future investigations accounting for an extensive range of confounders including socioeconomic status, physical activity, and health history are required to truly ascertain this. Additionally, 12M of age is a time of significant dietary changes and developmental strides (Dwyer, 2018), which may limit the ability to detect a signal in the noise. When testing for possible associations of the gut microbiota at 1 week or 6M with 12M BAZ, no associations were found (Supplementary Figs. 6 and 7). Previous research has indicated that the gut microbiota at 3–4 months but not 12M is significantly associated with 12M growth parameters (Forbes et al., 2018), and our study confirms the lack of association at timepoints before or after this 3–4 month “key” timeframe.
This study has some limitations to consider. BMI is a flawed indicator of fat mass and even health, but it is an acceptable metric due to its practicality and cost-efficiency (Gurunathan and Myles, 2016; Stefan et al., 2013; Daniels, 2009). Furthermore, the weight and height measurements for both mothers and infants, as well as breastfeeding status, were self-reported, but this has been shown to be accurate in similar populations (Shin et al., 2014; Li et al., 2005). Since gut microbial changes are most affected by only a few days’ dietary history (Johnson et al., 2019), recent reports of intake at 6M and 12M were assumed to be sufficient for the analyses described in this paper. Nonetheless, historical breastfeeding and other dietary patterns for both the infant and mother may be valuable in providing a more granular understanding of the associations of human milk exposure and diet with the gut microbiota in future studies. For example, it may be useful for such considerations as whether the weaning diet of infants differs by maternal BMI status and whether this also potentiates infant growth at 12M. Infant size was only assessed at 12M of age, which may not be fully representative of growth over time. The infant gut microbiota can be assessed at a greater range of time points to more accurately determine whether a potential relationship exists and whether the relationship is time dependent. Additionally, the power for some statistical analyses was low (Supplementary Table 2), which may be due to small effect sizes or the number of participants. However, this does not diminish the value of the overarching results since associations between human milk with the infant gut microbiota and BAZ with pre-pregnancy BMI were sufficiently powered. Finally, samples were collected by the women in their homes and mailed to the laboratory, an approach which may be liable to error since the microbial composition of the samples can change during shipment. Nonetheless, the analyses and results can still be considered valid since differences in microbial communities between samples are preserved when all samples are collected and processed under the same conditions (Song et al., 2016; Lauber et al., 2010; Tedjo et al., 2015).
In conclusion, human milk exposure is associated with the infant gut microbiota whereas infant 12M BAZ is correlated with maternal pre-pregnancy BMI but not the infant gut microbiota, suggesting that genetics, physiology, and the environment play a more pronounced role in shaping 12M BAZ. These results underscore the importance of maternal wellbeing and healthy child-rearing practices on the establishment of the gut microbial community and the influence on infant BAZ, which have implications on health outcomes in later life and public health in general.
Financial support
KYS was supported by a graduate research assistantship from the Michigan State University Department of Food Science and Human Nutrition. ENH was supported by the Professorial Assistantship program run by the Honors College at Michigan State University. This research was financially supported by funds provided to SSC by Michigan State University as part of her start-up package and by funds from the Environmental influences on Child Health Outcomes (ECHO) program, which is a nationwide research program supported by the National Institutes of Health (NIH), Office of the Director, to enhance child health (UG3 OD023285 and UH3OD023285) as well as an investigator grant to SSC from the Offices of Vice Presidents for Research from three universities (Michigan State University, the University of Michigan and Wayne State University) within the Child Health Advances from Research with Mothers Coalition (CHARM).
CRediT authorship contribution statement
Eliot N. Haddad: interpreted results and, Writing – original draft, wrote the paper, Writing – review & editing, All authors have edited, read and approved the final manuscript. Kameron Y. Sugino: conducted the research, Formal analysis, analyzed the, Data curation, data and interpreted the results, Writing – review & editing, All authors have edited, read and approved the final manuscript. Jean M. Kerver: designed the research, Writing – review & editing, All authors have edited, read and approved the final manuscript. Nigel Paneth: designed the research, Writing – review & editing, All authors have edited, read and approved the final manuscript. Sarah S. Comstock: designed the research, conducted the research, Formal analysis, analyzed the, Data curation, data, and interpreted the results, had primary responsibility for final content, Writing – review & editing, All authors have edited, read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
JMK, NP, and SSC designed the research; KYS and SSC conducted the research, analyzed the data and interpreted the results; and ENH interpreted results and wrote the paper. SSC had primary responsibility for final content. All authors have edited, read and approved the final manuscript. Graphical abstract created with BioRender.com.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crphys.2021.03.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Azad M.B., Konya T., Maughan H., Guttman D.S., Field C.J., Chari R.S., Sears M.R., Becker A.B., Scott J.A., Kozyrskyj A.L. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. Canadian Medical Association. 2013;185:385–394. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A., Semenkovich C.F., Gordon J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. India. 2004 doi: 10.1073/pnas.0407076101. http://www.pnas.org/cgi/doi/10.1073/pnas.0407076101 [cited 2019 Nov 20];101:15718–23. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F., Roswall J., Peng Y., Feng Q., Jia H., Kovatcheva-Datchary P., Li Y., Xia Y., Xie H., Zhong H. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015 doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Bashiardes S., Thaiss C.A., Elinav E. It’s in the milk: feeding the microbiome to promote infant growth. Cell Metabol. 2016:393–394. doi: 10.1016/j.cmet.2016.02.015. Cell Press. [DOI] [PubMed] [Google Scholar]
- Bever Babendure J., Reifsnider E., Mendias E., Moramarco M.W., Davila Y.R. Reduced breastfeeding rates among obese mothers: a review of contributing factors, clinical considerations and future directions. Int. Breastfeed. J. 2015 doi: 10.1186/s13006-015-0046-5. BioMed Central Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezirtzoglou E., Tsiotsias A., Welling G.W. Microbiota profile in feces of breast- and formula-fed newborns by using fluorescence in situ hybridization (FISH) Anaerobe. 2011;17:478–482. doi: 10.1016/j.anaerobe.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Bode L., McGuire M., Rodriguez J.M., Geddes D.T., Hassiotou F., Hartmann P.E., McGuire M.K. It’s alive: microbes and cells in human milk and their potential benefits to mother and infant. Adv Nutr. 2014 doi: 10.3945/an.114.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdar S.A., Dorosty Motlagh A.R., Bagherniya M., Ranjbar G., Daryabeygi-Khotbehsara R., Mohajeri S.A.R., Safarian M. Pre-pregnancy body mass index and maternal nutrition in relation to infant birth size. Clin Nutr Res. Korean Society of Clinical Nutrition (KAMJE) 2019;8:129. doi: 10.7762/cnr.2019.8.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonuck K.A., Trombley M., Freeman K., McKee D. Randomized, controlled trial of a prenatal and postnatal lactation consultant intervention on duration and intensity of breastfeeding up to 12 months. Pediatrics. 2005 doi: 10.1542/peds.2005-0435. [DOI] [PubMed] [Google Scholar]
- Cabrera-Rubio R., Collado M.C., Laitinen K., Salminen S., Isolauri E., Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012 doi: 10.3945/ajcn.112.037382. [DOI] [PubMed] [Google Scholar]
- Caporaso J.G., Lauber C.L., Costello E.K., Berg-Lyons D., Gonzalez A., Stombaugh J., Knights D., Gajer P., Ravel J., Fierer N. Moving pictures of the human microbiome. Genome Biol. 2011;12 doi: 10.1186/gb-2011-12-5-r50. BioMed Central Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels S.R. The use of BMI in the clinical setting. Pediatrics. 2009 doi: 10.1542/peds.2008-3586F. [DOI] [PubMed] [Google Scholar]
- Davis C.D. The gut microbiome and its role in obesity. Nutr. Today. 2016;51:167–174. doi: 10.1097/NT.0000000000000167. Lippincott Williams and Wilkins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S., Collini S., Pieraccini G., Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U. S. A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs R., Sawers C., Thompson F., Manyika J., Woetzel J., Child P., McKenna S., Spatharou A. McKinsey Global Institute; 2014. Overcoming Obesity: an Initial Economic Analysis. [Google Scholar]
- Donath S.M., Amir L.H. Maternal obesity and initiation and duration of breastfeeding: data from the longitudinal study of Australian children. Matern Child Nutr [Internet] 2008 doi: 10.1111/j.1740-8709.2008.00134.x. http://www.ncbi.nlm.nih.gov/pubmed/18582350 [cited 2020 Jan 13];4:163–70. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J.T. The feeding infants and toddlers study (FITS) 2016: moving forward. J. Nutr. 2018:1575S–1580S. doi: 10.1093/jn/nxy159. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.-G., Buchner A. GPOWER: a general power analysis program. Behav. Res. Methods. 2007 doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Forbes J.D., Azad M.B., Vehling L., Tun H.M., Konya T.B., Guttman D.S., Field C.J., Lefebvre D., Sears M.R., Becker A.B. Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. American Medical Association. 2018;172 doi: 10.1001/jamapediatrics.2018.1161. e181161–e181161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner C.D., Ratcliff S.L., Devine C.M., Thornburg L.L., Rasmussen K.M. Health professionals’ experiences providing breastfeeding-related care for obese women. Breastfeed. Med. 2014;9:503–509. doi: 10.1089/bfm.2014.0104. Mary Ann Liebert Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geserick M., Vogel M., Gausche R., Lipek T., Spielau U., Keller E., Pfäffle R., Kiess W., Körner A. Acceleration of BMI in early childhood and risk of sustained obesity. N. Engl. J. Med. 2018 doi: 10.1056/NEJMoa1803527. [DOI] [PubMed] [Google Scholar]
- Gittner L.S., Ludington-Hoe S.M., Haller H.S. Kluwer Academic/Plenum Press; New York: 2014. Infant Obesity and Severe Obesity Growth Patterns in the First Two Years of Life. Maternal and Child Health Journal; pp. 613–624. [DOI] [PubMed] [Google Scholar]
- Gohir W., Ratcliffe E.M., Sloboda D.M. Of the bugs that shape us: maternal obesity, the gut microbiome, and long-term disease risk. Pediatr. Res. 2015 doi: 10.1038/pr.2014.169. [DOI] [PubMed] [Google Scholar]
- Guelinckx I., Devlieger R., Bogaerts A., Pauwels S., Vansant G. The effect of pre-pregnancy BMI on intention, initiation and duration of breast-feeding. Publ. Health Nutr. 2012;15:840–848. doi: 10.1017/S1368980011002667. [DOI] [PubMed] [Google Scholar]
- Guh D.P., Zhang W., Bansback N., Amarsi Z., Birmingham C.L., Anis A.H. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health [Internet] 2009 doi: 10.1186/1471-2458-9-88. http://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-9-88 [cited 2019 Nov 20];9:88. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurunathan U., Myles P.S. Limitations of body mass index as an obesity measure of perioperative risk. Br J Anaesth [Internet] 2016 doi: 10.1093/bja/aev541. https://linkinghub.elsevier.com/retrieve/pii/S0007091217304385 [cited 2020 Feb 3];116:319–21. Available from: [DOI] [PubMed] [Google Scholar]
- Heslehurst N., Vieira R., Akhter Z., Bailey H., Slack E., Ngongalah L., Pemu A., Rankin J. The association between maternal body mass index and child obesity: a systematic review and meta-analysis. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002817. Public Library of Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho N.T., Li F., Lee-Sarwar K.A., Tun H.M., Brown B.P., Pannaraj P.S., Bender J.M., Azad M.B., Thompson A.L., Weiss S.T. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-06473-x. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenhower C., Gevers D., Knight R., Abubucker S., Badger J.H., Chinwalla A.T., Creasy H.H., Earl A.M., Fitzgerald M.G., Fulton R.S. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevitt C., Hernandez I., Groër M. Lactation complicated by overweight and obesity: supporting the mother and newborn. J Midwifery Women’s Heal [Internet] 2007 doi: 10.1016/j.jmwh.2007.04.006. https://pubmed.ncbi.nlm.nih.gov/17983998/ J Midwifery Womens Health. [cited 2021 Jan 26];52:606–13. Available from: [DOI] [PubMed] [Google Scholar]
- Johnson A.J., Vangay P., Al-Ghalith G.A., Hillmann B.M., Ward T.L., Shields-Cutler R.R., Kim A.D., Shmagel A.K., Syed A.N., Walter J. Daily sampling reveals personalized diet-microbiome associations in humans. Cell Host Microbe. 2019 doi: 10.1016/j.chom.2019.05.005. [DOI] [PubMed] [Google Scholar]
- Kelly B.J., Gross R., Bittinger K., Sherrill-Mix S., Lewis J.D., Collman R.G., Bushman F.D., Li H. Power and sample-size estimation for microbiome studies using pairwise distances and PERMANOVA. Bioinformatics. 2015;31:2461–2468. doi: 10.1093/bioinformatics/btv183. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer T.W., Faurholt-Jepsen D., Medrano R., Elwan D., Mehta K., Christensen V.B., Wojcicki J.M. Higher birthweight and maternal pre-pregnancy BMI persist with obesity association at age 9 in high risk latino children. J. Immigr. Minority Health. 2019;21:89–97. doi: 10.1007/s10903-018-0702-0. Springer New York LLC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleva P.T., Bridgman S.L., Kozyrskyj A.L. The infant gut microbiome: evidence for obesity risk and dietary intervention. Nutrients. MDPI AG. 2015:2237–2260. doi: 10.3390/nu7042237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber C.L., Zhou N., Gordon J.I., Knight R., Fierer N. Effect of storage conditions on the assessment of bacterial community structure in soil and human-associated samples. FEMS Microbiol Lett [Internet] 2010 doi: 10.1111/j.1574-6968.2010.01965.x. https://academic.oup.com/femsle/article-lookup/doi/10.1111/j.1574-6968.2010.01965.x [cited 2020 Feb 3];307:80–6. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Scanlon K.S., Serdula M.K. The validity and reliability of maternal recall of breastfeeding practice. Nutr Rev [Internet] 2005 doi: 10.1111/j.1753-4887.2005.tb00128.x. https://academic.oup.com/nutritionreviews/article-lookup/doi/10.1111/j.1753-4887.2005.tb00128.x Oxford University Press (OUP) [cited 2020 Jun 3];63:103–10. Available from: [DOI] [PubMed] [Google Scholar]
- Lloyd-Price J., Abu-Ali G., Huttenhower C. The healthy human microbiome. Genome Medicine. BioMed Central Ltd. 2016 doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas R., Judge M., Sajdlowska J., Cong X., Mcgrath J.M., Brandon D. Effect of maternal body mass index on infant breastfeeding behaviors and exclusive direct breastfeeding. JOGNN - J Obstet Gynecol Neonatal Nurs. 2015;44:772–783. doi: 10.1111/1552-6909.12755. Blackwell Publishing Ltd. [DOI] [PubMed] [Google Scholar]
- Mangiafico S. Rcompanion: functions to support extension education program evaluation [internet]. R package version 2.3.25. 2020. https://cran.r-project.org/package=rcompanion Available from:
- Marcobal A., Barboza M., Froehlich J.W., Block D.E., German J.B., Lebrilla C.B., Mills D.A. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem. 2010;58:5334–5340. doi: 10.1021/jf9044205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney M.I., Davis C., Fraser C.M., Schneeman B.O., Huttenhower C., Verbeke K., Walter J., Latulippe M.E. Establishing what constitutes a healthy human gut microbiome: state of the science, regulatory considerations, and future directions. J. Nutr. 2019:1882–1895. doi: 10.1093/jn/nxz154. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliku K., Azad M.B. Breastfeeding and the developmental origins of asthma: current evidence, possible mechanisms, and future research priorities. Nutrients. MDPI AG. 2018 doi: 10.3390/nu10080995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadkhah A.I., Simpson E.B., Patterson S.G., Ferguson J.F. Development of the gut microbiome in children, and lifetime implications for obesity and cardiometabolic disease. Children. MDPI AG. 2018;5:160. doi: 10.3390/children5120160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moossavi S., Azad M.B. Quantifying and interpreting the association between early- life gut microbiota composition and childhood obesity. mBio. American Society for Microbiology. 2019 doi: 10.1128/mBio.02787-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscogiuri G., Cantone E., Cassarano S., Tuccinardi D., Barrea L., Savastano S., Colao A. Gut microbiota: a new path to treat obesity. Int. J. Obes. Suppl. 2019;9:10–19. doi: 10.1038/s41367-019-0011-7. Springer Science and Business Media LLC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E.C., Biryukov S., Abbafati C., Abera S.F. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. Lancet Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen J., Blanchet F., Kindt R., Legendre P., Minchin P., O’Hara R., Simpson G., Solymos P., Stevens M., Wagner H. 2015. Vegan: Community Ecology. R Package Version 2.2-1. [Google Scholar]
- O’Sullivan A., Farver M., Smilowitz J.T. The Influence of early infant-feeding practices on the intestinal microbiome and body composition in infants. Nutr. Metab. Insights. 2015 doi: 10.4137/NMI.S29530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Found Stat Comput; 2011. Team R. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Schwiertz A., Taras D., Schäfer K., Beijer S., Bos N.A., Donus C., Hardt P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity [Internet] 2010 doi: 10.1038/oby.2009.167. [cited 2020 Feb 3];18:190–5. Available from: [DOI] [PubMed] [Google Scholar]
- Shin D., Chung H., Weatherspoon L., Song W.O. Validity of prepregnancy weight status estimated from self-reported height and weight. Matern Child Health J [Internet] 2014 doi: 10.1007/s10995-013-1407-6. http://link.springer.com/10.1007/s10995-013-1407-6 Springer. [cited 2020 Jun 2];18:1667–74. Available from: [DOI] [PubMed] [Google Scholar]
- Simmonds M., Llewellyn A., Owen C.G., Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes Rev [Internet] 2016 doi: 10.1111/obr.12334. Blackwell Publishing Ltd. cited 2020 Feb 18];17:95–107. Available from: [DOI] [PubMed] [Google Scholar]
- Song S.J., Amir A., Metcalf J.L., Amato K.R., Xu Z.Z., Humphrey G., Knight R. Preservation methods differ in fecal microbiome stability, affecting suitability for field studies. mSystems. American Society for Microbiology. 2016;1 doi: 10.1128/mSystems.00021-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa-Moreno A., Comstock S.S., Sugino K.Y., Ma T.F., Paneth N., Davis Y., Olivero R., Schein R., Maurer J., Zhang L. In: PLoS One [Internet] Luo Y., editor. Public Library of Science; 2020. Perinatal risk factors for fecal antibiotic resistance gene patterns in pregnant women and their infants.https://dx.plos.org/10.1371/journal.pone.0234751 [cited 2020 Sep 3];15:e0234751. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislawski M.A., Dabelea D., Wagner B.D., Iszatt N., Dahl C., Sontag M.K., Knight R., Lozupone C.A., Eggesbø M. Gut microbiota in the first 2 Years of life and the association with body mass index at age 12 in a Norwegian birth cohort. MBio. NLM (Medline) 2018;9 doi: 10.1128/mBio.01751-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N., Häring H.U., Hu F.B., Schulze M.B. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. The Lancet Diabetes and Endocrinology. 2013:152–162. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- Stewart C.J., Ajami N.J., O’Brien J.L., Hutchinson D.S., Smith D.P., Wong M.C., Ross M.C., Lloyd R.E., Doddapaneni H.V., Metcalf G.A. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018:583–588. doi: 10.1038/s41586-018-0617-x. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K.Y., Paneth N., Comstock S.S. In: PLoS One [Internet] Rosenfeld C.S., editor. Public Library of Science; 2019. Michigan cohorts to determine associations of maternal pre-pregnancy body mass index with pregnancy and infant gastrointestinal microbial communities: late pregnancy and early infancy.http://dx.plos.org/10.1371/journal.pone.0213733 cited 2019 Sep 4];14:e0213733. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K.Y., Ma T., Kerver J.M., Paneth N., Comstock S.S. Human milk feeding patterns at 6 Months of age are a major determinant of fecal bacterial diversity in infants. J Hum Lact [Internet] 2020 doi: 10.1177/0890334420957571. http://journals.sagepub.com/doi/10.1177/0890334420957571 SAGE Publications Inc. [cited 2020 Oct 7];089033442095757. Available from: [DOI] [PubMed] [Google Scholar]
- Taveras E.M., Rifas-Shiman S.L., Belfort M.B., Kleinman K.P., Oken E., Gillman M.W. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123:1177–1183. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedjo D.I., Jonkers D.M.A.E., Savelkoul P.H., Masclee A.A., van Best N., Pierik M.J., Penders J. In: PLoS One [Internet] Favia G., editor. 2015. The effect of sampling and storage on the fecal microbiota composition in healthy and diseased subjects.https://dx.plos.org/10.1371/journal.pone.0126685 [cited 2020 Feb 3];10:e0126685. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P.J., Hamady M., Yatsunenko T., Cantarel B.L., Duncan A., Ley R.E., Sogin M.L., Jones W.J., Roe B.A., Affourtit J.P. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vael C., Verhulst S.L., Nelen V., Goossens H., Desager K.N. Intestinal microflora and body mass index during the first three years of life: an observational study. Gut Pathog. 2011;3 doi: 10.1186/1757-4749-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes A.M., Walter J., Segal E., Spector T.D. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:36–44. doi: 10.1136/bmj.k2179. BMJ Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elsen L.W.J., Garssen J., Burcelin R., Verhasselt V. Shaping the gut microbiota by breastfeeding: the gateway to allergy prevention? Frontiers in Pediatrics. 2019 doi: 10.3389/fped.2019.00047. Frontiers Media S.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables W.N., Ripley B.D. 2002. Modern Applied Statistics with S-Plus. World. [Google Scholar]
- Voerman E., Santos S., Golab B.P., Amiano P., Ballester F., Barros H., Bergström A., Charles M.A., Chatzi L., Chevrier C. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: an individual participant data meta-analysis. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002744. Public Library of Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Li M., Wu S., Lebrilla C.B., Chapkin R.S., Ivanov I., Donovan S.M. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J. Pediatr. Gastroenterol. Nutr. 2015;60:825–833. doi: 10.1097/MPG.0000000000000752. Lippincott Williams and Wilkins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO WHO Anthro for personal computers, version 3.2.2: Software for assessing growth and development of the world’s children. World Health. 2011 [Google Scholar]
- Williams C.B., MacKenzie K.C., Gahagan S. The effect of maternal obesity on the offspring. Clin. Obstet. Gynecol. 2014:508–515. doi: 10.1097/GRF.0000000000000043. Lippincott Williams and Wilkins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassour M., Vatanen T., Siljander H., Hämäläinen A.M., Härkönen T., Ryhänen S.J., Franzosa E.A., Vlamakis H., Huttenhower C., Gevers D. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. American Association for the Advancement of Science. 2016;8 doi: 10.1126/scitranslmed.aad0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P. Human gut microbiome viewed across age and geography. Nature. 2012:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.