Abstract
Objective
To determine whether (1) immunofluorescence is a reproducible technique in detecting misfolded α-synuclein in skin nerves and subsequently whether (2) immunofluorescence and real-time quaking-induced conversion (RT-QuIC) (both in skin and CSF) show a comparable in vivo diagnostic accuracy in distinguishing synucleinopathies from non-synucleinopathies in a large cohort of patients.
Methods
We prospectively recruited 90 patients fulfilling clinical and instrumental diagnostic criteria for all synucleinopathies variants and non-synucleinopathies (mainly including Alzheimer disease, tauopathies, and vascular parkinsonism or dementia). Twenty-four patients with mainly peripheral neuropathies were used as controls. Patients underwent skin biopsy for immunofluorescence and RT-QuIC; CSF was examined in patients who underwent lumbar puncture for diagnostic purposes. Immunofluorescence and RT-QuIC analysis were made blinded to the clinical diagnosis.
Results
Immunofluorescence showed reproducible results between 2 pairs of neighboring skin samples. Both immunofluorescence and RT-QuIC showed high sensitivity and specificity in discriminating synucleinopathies from non-synucleinopathies and controls but immunofluorescence presented higher diagnostic accuracy. Immunofluorescence presented a good level of agreement with RT-QuIC in both skin and CSF in synucleinopathies.
Conclusions
Both immunofluorescence and RT-QuIC showed high diagnostic accuracy, although immunofluorescence displayed the better value as well as optimal reproducibility; they presented a good level of agreement in synucleinopathies, supporting the use of less invasive tests such as skin immunofluorescence or RT-QuIC instead of CSF RT-QuIC as a diagnostic tool for synucleinopathies.
Classification of Evidence
This study provides Class III evidence that immunofluorescence or RT-QuIC accurately distinguish synucleinopathies from non-synucleinopathies.
The diagnosis of synucleinopathies is mainly based on clinical criteria, leading to frequent misdiagnosis, as underlined by autopsy studies.1-3
Recent advances have been made by the development of tests able to detect in vivo pathologic α-synuclein such as immunofluorescence analysis of skin nerves4-8 and real-time quaking-induced conversion (RT-QuIC) of CSF.9,10 An important advantage of RT-QuIC is the possibility to perform the simultaneous analysis of different samples from the same patient such as the easily available skin together with CSF obtainable by an invasive procedure although a direct comparative study has not been reported.
Immunofluorescence and RT-QuIC explore different aspects of pathologic α-synuclein. Immunofluorescence may detect the morphology of pathologic aggregates in skin nerves whereas RT-QuIC can determine their seeding activity, a prion-like biological trait of misfolded proteins. Accordingly, the diagnostic accuracy of each test in disclosing the pathologic form of α-synuclein may differ but a direct comparison is not yet available.
This study aimed to establish the reproducibility of immunofluorescence and subsequently to compare the diagnostic accuracy of immunofluorescence and RT-QuIC as biomarkers for synucleinopathies. Specifically, this study aims at comparing (1) the sensitivity and specificity of immunofluorescence and RT-QuIC to disclose pathologic α-synuclein in different variants of synucleinopathies and (2) the diagnostic accuracy of RT-QuIC in disclosing the seeding activity of pathologic α-synuclein in skin samples vs CSF.
Methods
We prospectively screened at the IRCCS Institute of Neurologic Sciences of Bologna (Italy), from 2018 to 2020, 100 consecutive patients with possible neurodegenerative disorders; only 90 reaching a high level of diagnostic accuracy fulfilling current clinical and instrumental diagnostic criteria for a specific α-synucleinopathies or non-synucleinopathies were recruited for this study. The reproducibility of immunofluorescence findings was ascertained in 21 patients: 9 synucleinopathies (4 fulfilling diagnostic criteria for Parkinson disease [PD],11 4 for probable multiple system atrophy [MSA],12 and 1 for dementia with Lewy bodies [DLB]2) and 12 non-synucleinopathies (1 fulfilling diagnostic criteria for Alzheimer disease [AD],13 8 for progressive supranuclear palsy [PSP],14 and 3 for corticobasal syndrome [CBD]15). Immunofluorescence and RT-QuIC were then compared in an additional 69 patients. They included 31 patients with synucleinopathies: 17 with PD, 5 with DLB, 8 with probable MSA, and 3 with pure autonomic failure (PAF)12; and 38 patients with non-synucleinopathies, comprising 15 with AD with typical CSF findings,13 6 with vascular parkinsonism,16 1 with iatrogenic parkinsonism secondary to high dosage of neuroleptic drugs in treatment of psychotic disorders, 2 with vascular dementia,17 7 with tauopathies or TDP proteinopathy (1 patient with frontotemporal dementia,18 3 with PSP, 2 with CBD, and 1 with anti-IgLON5 antibody), and 6 fulfilling diagnostic criteria for amyotrophic lateral sclerosis19 (table 1). Recruited patients had no familial history for neurologic disorders.
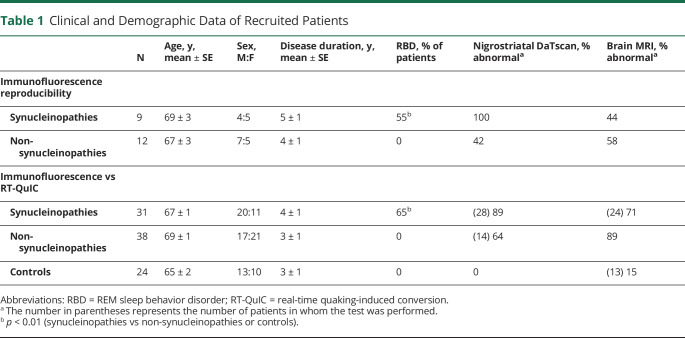
Table 1.
Clinical and Demographic Data of Recruited Patients
Neurogenic orthostatic hypotension was established by a decrease of at least 20/10 mm Hg without significant change of heart rate on the head-up tilt test (3 minutes at 65°)12 in PD with orthostatic hypotension. Cognitive deterioration was ascertained by corrected Mini-Mental State Examination (≤24) and by Brief Mental Deterioration Battery, a neuropsychological test standardized in the Italian population expressing global cognitive impairment when the final score is negative (<0).20
The clinical diagnosis of recruited patients was supported by appropriate diagnostic instrumental tests (table 1). Ioflupane [123I] SPECT of nigrostriatal dopaminergic activity showed abnormal findings in all synucleinopathies except in patients with PAF. The low rate of nigrostriatal SPECT scan in non-synucleinopathies was due to the lack of motor involvement in almost half of these patients showing an isolated dementia. Brain MRI showed typical abnormalities supporting the clinical diagnosis in DLB and AD (cortical atrophy), MSA (putaminal atrophy or hyperintense putaminal rim, hot cross bun sign, and brainstem atrophy), and tauopathies or TDP proteinopathy (cortical or brainstem atrophy). In addition, cardiac uptake of [123I]-MIBG SPECT was done in 11 patients because sufficient diagnostic accuracy had already been achieved by means of other diagnostic tests or because patients were unable to perform the test. Cardiac MIBG SPECT showed a sympathetic denervation in all synucleinopathies (7 patients), except patients with MSA (4), displaying normal findings.
The presence of REM sleep behavior disorder (RBD) has been verified in all recruited patients by the clinical interview of patients and their bed partners. RBD was determined in 17 patients with synucleinopathies by the bed partner describing a history of frequent clear dream enactment (table 1). Polysomnographic study was performed in 5 patients reporting RBD (3 PD and 2 MSA) and confirmed REM sleep without atonia. Recruited patients were treated with levodopa with or without dopamine agonists for patients with parkinsonism and a variable combination of cholinesterase inhibitors, memantine, and selective serotonin reuptake inhibitor in patients with dementia.
For the comparative study, 24 patients with neurologic disorders without evidence of neurodegenerative diseases were also recruited. They included 7 patients with small fiber neuropathy due to diabetes or autoimmune diseases, 5 with idiopathic peripheral neuropathy, 1 with chronic inflammatory demyelinating polyneuropathy, 1 with Wernicke encephalopathy, 2 with cerebral vasculitis, 1 with stiff-person syndrome, 2 with subjective cognitive disorder, and 5 with severe depression showing normal cerebral MRI, cognitive tests, and CSF markers of neurodegeneration (table 1). All recruited patients underwent skin biopsy; lumbar puncture to collect CSF was performed only in patients when required for diagnostic purposes. CSF was thus available in 9 patients with synucleinopathies, 24 patients with non-synucleinopathies, and 16 controls. Immunofluorescence and RT-QuIC analysis were made blinded to the clinical diagnosis.
Primary research questions to be addressed in this study were the immunofluorescence reproducibility and the diagnostic accuracy of immunofluorescence and RT-QuIC to differentiate synucleinopathies from non-synucleinopathies and control patients. This study provides Class III evidence that immunofluorescence is reproducible and that immunofluorescence or RT-QuIC accurately distinguish synucleinopathies from non-synucleinopathies.
Standard Protocol Approvals, Registrations, and Patient Consents
This study received approval from an ethical standards committee on human experimentation (institutional) for any experiments using human subjects and the procedures followed the Helsinki Declaration related to clinical research of human beings. Written informed consent was obtained from all patients (or guardians) participating in the study (consent for research).
Skin Biopsy
Three-millimeter punch biopsies were taken from proximal and distal hairy skin sites. Patients recruited for this study underwent both routine diagnostic skin biopsy protocol and the study protocol used for this research (figure 1). The routine diagnostic protocol included a pair of skin samples, spaced about 3 cm on a horizontal line, taken from proximal (C7 paravertebral: 5 cm from the midline) and distal sites (thigh: 15 cm above the patella; and leg: 10 cm above the lateral malleolus). Two skin samples were taken in every single site to increase the rate of phosphorylated α-synuclein (p-syn) positivity as previously described.8,21 The study protocol provided 2 additional pairs of skin samples taken from a single skin site 2–3 cm apart for a total of 8 samples/patient (figure 1). The skin site from the study protocol was chosen for the study considering the probability of finding p-syn in skin nerves, which is influenced by the specific clinical phenotype.8 Accordingly, the proximal skin site was mainly chosen in patients with parkinsonism and dementia (C7, which corresponds to protocol III of figure 1), while in patients with autonomic or neuropathic disorders, the distal sites (L = distal leg and T = thigh, which correspond to protocols I and II, respectively, of figure 1) were mainly selected. However, when the patient was particularly compliant, 2 different skin sites were analyzed for the study protocol (10 samples/patient) in 1 patient with PD and 4 patients with PSP for the immunofluorescence reproducibility study and 2 patients with PD and 1 patient with AD for the comparative study. The immunofluorescence reproducibility was ascertained by analyzing the extra pair of skin samples taken close to the routine pair samples by the same immunofluorescence method explained in detail below. By contrast, for the comparative study the routine pair of skin samples analyzed by immunofluorescence were compared with the extra pair of skin samples analyzed by RT-QuIC. Each of the pairs of skin samples selected for the immunofluorescence or comparative studies has been anonymized through the use of a numerical code to allow a blinded analysis.
Figure 1. Skin Biopsy Protocols.
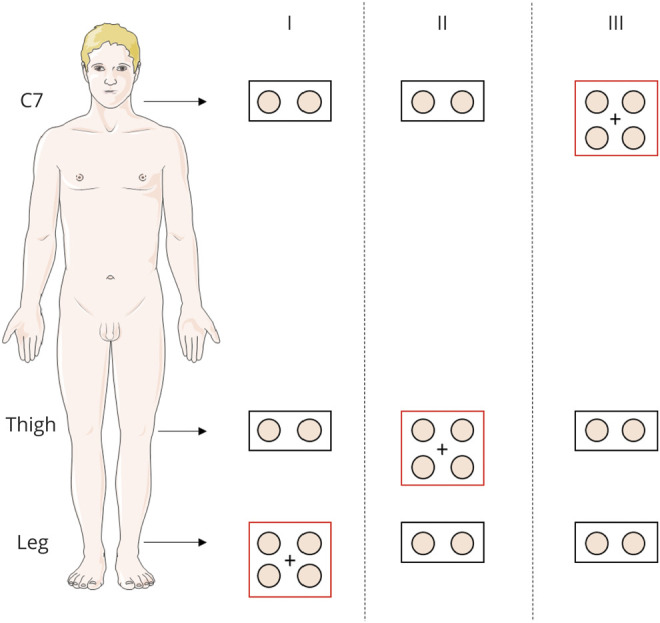
Skin samples were taken from C7, thigh, and distal leg. We took in each skin site a second biopsy approximately 3 centimeters away from the first sample to increase the rate of phosphorylated α-synuclein (p-syn) positivity. The study protocol includes 2 pairs of skin samples taken from a single skin site (i.e., C7, thigh, or leg) 2–3 centimeters apart (red rectangle). In addition, in the remaining 2 skin sites, a pair of skin samples were taken for routine purpose (black rectangle). The skin site was chosen for the study considering the probability of finding p-syn in skin nerves, which is influenced by the specific clinical phenotype. Accordingly, the distal skin sites were mainly chosen in patients with autonomic or neuropathic disorders (protocols I and II), while in patients with parkinsonism and dementia, the proximal site was mainly used (protocol III). In 1 patient with Parkinson disease (PD) and 4 patients with progressive supranuclear palsy for the immunofluorescence reproducibility study and 2 patients with PD and 1 patient with Alzheimer disease for the comparative study 2 different skin sites were analyzed for the study protocol (10 samples/patient).
IF Analysis to Search for P-Syn Deposits in Skin Nerves
According to previously published procedures,4,6,21 skin samples were immediately fixed in cold Zamboni fixative and kept at 4 °C overnight.
Ten-micrometer sections were obtained using a cryostat (CM 1950; Leica, Deerfield, IL). Three sections 500 μm apart from each skin sample were analyzed. They were double-immunostained overnight with a panel of primary antibodies including rabbit monoclonal phosphorylated α-synuclein at Ser 129 (p-syn; 1:500, Abcam, Cambridge, UK; cat. no. ab-51253) and mouse pan-neuronal marker protein gene product 9.5 (1:750; Abcam; cat. no. ab72911). Sections were then washed and secondary antibodies were added for an incubation of 1 hour. As for secondary antibodies, an anti-mouse Alexa Fluor 488 (1:400; Jackson ImmunoResearch, West Grove, PA; cat. num. 715-545-150) and rabbit cyanine dye fluorophores 3.18 (1:200, Jackson ImmunoResearch; cat. num. 711-165-152) were used. The microscope analysis and criteria followed to define a p-syn positivity were described previously and mainly based on the correspondence between rabbit p-syn and mouse PGP staining.4,6,8,21 Two authors with expertise in immunofluorescent analysis (V.D. and A.I.) analyzed immunofluorescence images blinded to the clinical diagnosis. The intralaboratory analysis revealed an excellent reproducibility, with 100% concordance of classification in all patients (K = 1), in agreement with recently reported data.22 P-syn staining was rated as positive when a single skin nerve fiber showed a positive staining at high magnification (×40) in the 2 close skin samples. Because of clear-cut differences among different groups with no p-syn positivity found in non-synucleinopathies and control patients, quantitative analysis of p-syn distribution was not performed for this study. However, for the co-localization of p-syn with PGP, we performed analysis by acquiring digital images using a laser-scanning confocal microscope (Nikon confocal microscopy, Eclipse Ti A1, Japan) as previously described.21
RT-QuIC Analysis of Seeding Activity of Pathologic α-Synuclein
Preparation of Skin and CSF Samples
The skin punch biopsy samples (∼30 mg, ∼3×3 mm) included epidermis and dermis layers. The 2 frozen skin samples were prepared for RT-QuIC analysis as described previously10,23,24 with minor modifications. Each single skin sample was analyzed individually. The skin tissues washed for 3 times in 1× Tris-buffered saline (TBS) were separated into small pieces in dishes. The skin homogenates at 10% (w/vol) were prepared in skin lysis buffer containing 2 mM CaCl2 and 0.25% (w/vol) collagenase A (Roche) in TBS and incubated at 37°C for 4 hours with shaking, followed by homogenization in a Mini-Beadbeater (BioSpec; Laboratory Supply Network, Inc., Atkinson, NH) for 1 minute. After sonication, the samples were centrifuged at 500g for 3 minutes to collect the supernatant fraction (S1). A total of ∼60–100 µL aliquot of CSF for each patient was kept at −80°C in a freezer for RT-QuIC assay.
RT-QuIC Analysis
The RT-QuIC analysis of skin or CSF samples was made as described previously,10,23,24 with minor modifications. In brief, RT-QuIC reaction mix was composed of 40 mM phosphate buffer (pH 8.0), 170 mM NaCl, 0.1 mg/mL recombinant human wild-type α-synuclein purchased commercially (rPeptide, Watkinsville, GA), 10 µM Thioflavin T (ThT), and 0.00125% sodium dodecyl sulfate. Aliquots of the 98 or 85 µL reaction mix/each were loaded into each well of a black 96-well plate with a clear bottom (Nunc) preloaded with ∼5 glass beads (diameter 1 mm) and seeded with 2 µL of skin homogenate S1 or 15 µL of CSF. The skin homogenates or CSF samples were spun at 2000g for 2 minutes at 4°C prior to making serial dilutions. The plate sealed with a plate sealer film (Nalgene Nunc International) was incubated at 42°C in a BMG FLUOstar Omega plate reader with cycles of 1 minute shaking (400 rpm double orbital) and 1 minute rest for the indicated incubation time. The measurements of ThT fluorescence (450 ± 10 nm excitation and 480 ± 10 nm emission; bottom read) were conducted every 45 minutes. Four replicate reactions were seeded with the same dilution for each individual sample. The average fluorescence value of each sample was calculated using fluorescence values from all 4 replicate wells regardless of whether these values crossed the threshold described below. The maximal fluorescence response (260,000 rfu) of the plate readers was plotted vs different time points of measurements. Seeding activity was classified as RT-QuIC positive based on criteria similar to those previously described for RT-QuIC analysis of brain specimens.10 Skin RT-QuIC was considered positive in case of reaction of at least 1 of 2 samples analyzed for each skin site. The fluorescence threshold for a positive reaction was based on the mean ThT value of all negative control samples at 60 hours, plus 3 SDs. At least 2 of 4 replicate wells must cross this threshold and lag phase in less than 50 hours in order for a sample to be considered positive. The RT-QuIC fluorescence signal reported for the analysis from each skin site was the largest value of the 2 samples analyzed.
Statistical Analysis
This analysis was performed using SPSS 25.0. Kolmogorov-Smirnov test was used to verify the normal distribution of continuous variables. Student t test or 1-way analysis of variance followed by a post hoc Bonferroni test were used for comparison of normally distributed data. Mann-Whitney U test or Kruskal-Wallis test were utilized to compare variables without a normal distribution. Categorical variables were compared using χ2 test and the results were presented as absolute and relative frequency (%). To adjust the results for age, sex, and disease duration, we further used logistic or multivariate linear regression depending on the data. Agreement was tested using Cohen k statistics. For all analyses, significance was assumed for p < 0.05, Bonferroni-corrected for multiple comparisons. Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the different diagnostic techniques were calculated using the clinical diagnosis as the gold standard.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Results
Recruited patients and controls were matched for age and disease duration. Males were more prevalent in each group except for patients with non-synucleinopathies used for comparison between immunofluorescence vs RT-QuIC, in which females were more represented (table 1). As expected, RBD incidence was highly prevalent in synucleinopathies, and none of the non-synucleinopathies or control patients presented with this sleep disorder. Also as expected, nigrostriatal DaTscan abnormalities were more prevalent in patients with synucleinopathies than in those with non-synucleinopathies and controls, but cerebral MRI abnormalities were similar among recruited patients (table 1), although the specific type of abnormality differed in each group, as expected.
Immunofluorescence Staining of Pathologic P-Syn in Skin Nerves
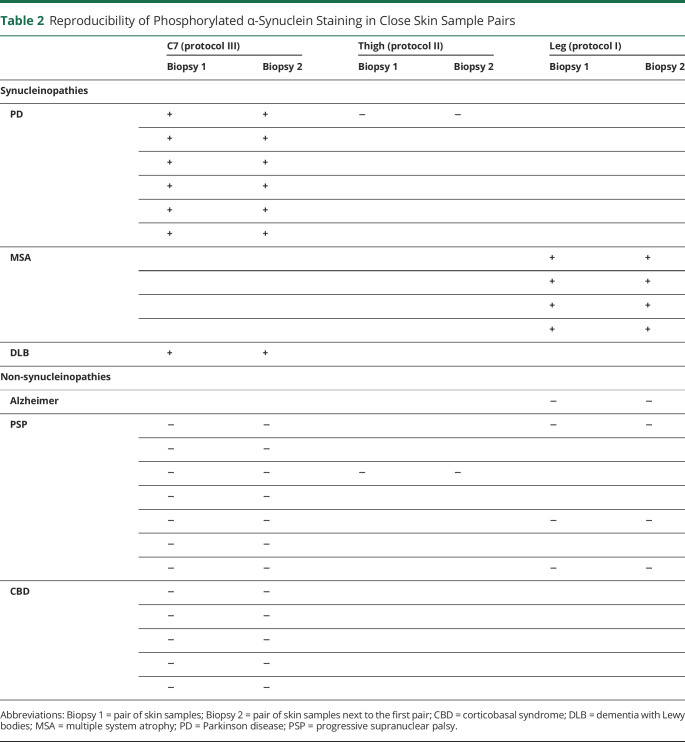
P-syn staining in skin nerves was reproducible between 2 pairs of neighboring skin samples in all sites (table 2). In all 29 skin sites analyzed (19 C7, 8 distal leg, and 2 thigh), p-syn showed the same results, supporting the comparison between a pair of skin samples analyzed with immunofluorescence with a neighboring pair analyzed with a different technique.
Table 2.
Reproducibility of Phosphorylated α-Synuclein Staining in Close Skin Sample Pairs
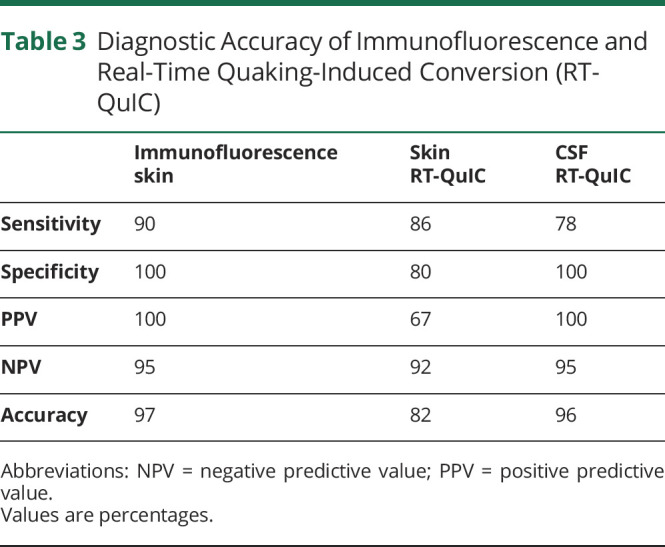
Considering samples selected for the comparison study, p-syn positivity was disclosed in all synucleinopathies except 3 patients (2 with MSA and 1 with PAF), but importantly, it was not found in any non-synucleinopathies or control patients. The patient with PAF negative for p-syn in the selected skin sample was instead positive in both additional routinely analyzed skin sites, that is, the C7 and leg, whereas patients with MSA showing no p-syn in selected samples were negative even in routinely analyzed C7 and thigh sites. Routine immunofluorescence analysis (including additional 2 or 4 skin samples and 6 or 12 skin sections) did not reveal any p-syn positivity in non-synucleinopathies and control patients in agreement with the results of skin samples selected for the study. The p-syn positivity differed among patients with synucleinopathies, being mainly detected in autonomic fibers of PD, DLB, and PAF, but detected in somatic fibers of the upper dermis in MSA, confirming previous data.7,25,26 The analysis of sensitivity and specificity showed that p-syn presented a high diagnostic accuracy in discriminating synucleinopathies from non-synucleinopathies and controls with 90% sensitivity and 100% specificity (table 3). Logistic regression model showed no influence of age, sex, or disease duration on the results.
Table 3.
Diagnostic Accuracy of Immunofluorescence and Real-Time Quaking-Induced Conversion (RT-QuIC)
RT-QuIC Evaluation of Pathologic α-Synuclein Seeding Activity
RT-QuIC Assay of Skin Samples
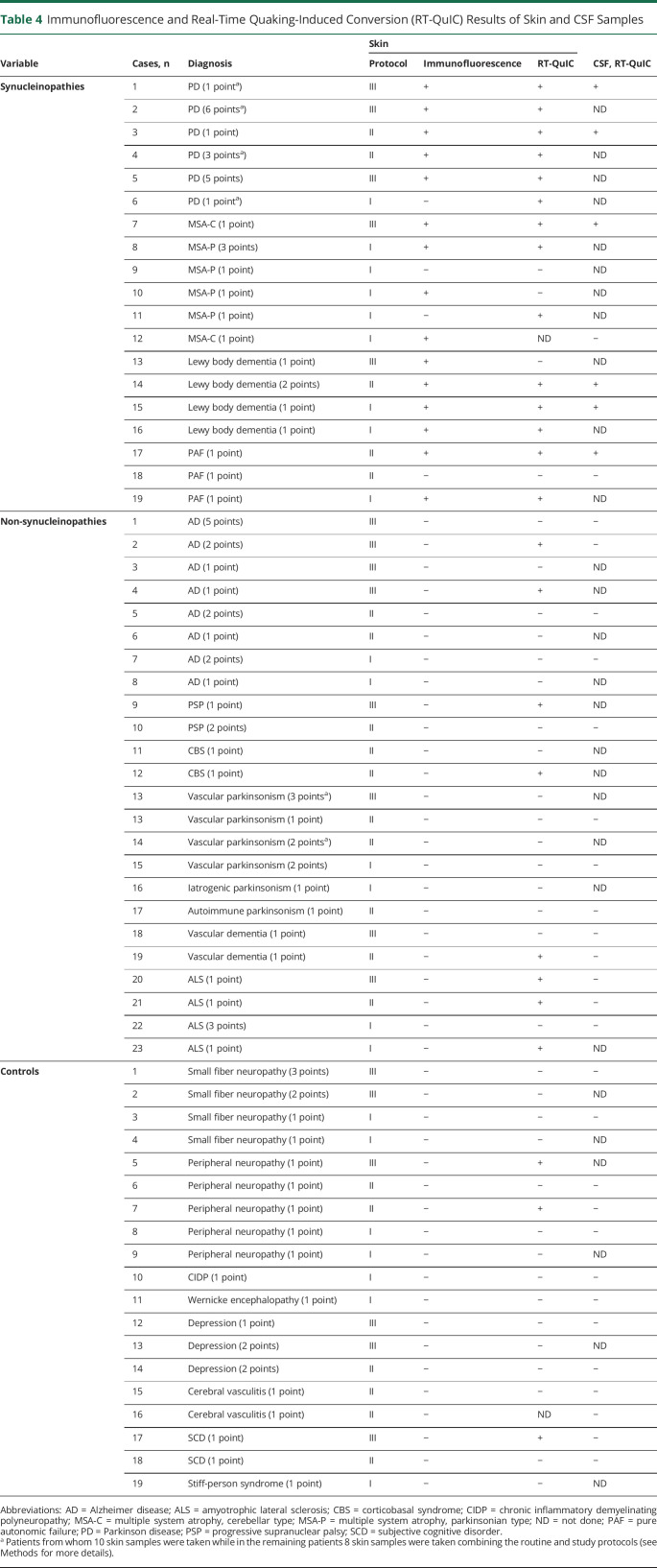
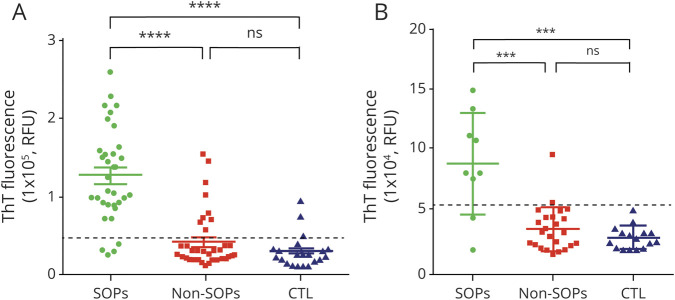
The pathologic α-synuclein seeding activity of the skin punch biopsy samples from the 3 groups of patients was detected by RT-QuIC assay. The cutoff value for defining the positive seeding activity was 45,414 (attribute unit [au]) based on the mean α-synuclein seeding activity of control plus 3 SDs. The skin RT-QuIC assay yielded 86% sensitivity and 80% specificity (table 3) in disclosing synucleinopathies, lower than immunofluorescence due to 9 out of 38 patients with non-synucleinopathies showing positive α-synuclein ThT fluorescence reaction. In addition, 3 out of 24 control patients showed a positive α-synuclein ThT fluorescence reaction (table 4). Positive patients with non-synucleinopathies and controls did not show any clinical sign of parkinsonism, dysautonomia, or cerebellar dysfunction at the time of recruitment or careful clinical reassessment carried out after 14 ± 3 months (mean ± SE). The mean skin α-synuclein ThT fluorescence reaction from patients with synucleinopathies (n = 31) was significantly higher than that from patients with non-synucleinopathies (n = 38) (125.575 ± 10.525 [SE] vs 41.295 ± 5.674, au, p < 0.001, figure 2) and that from controls (n = 24) (125.575 ± 10.525 vs 29.073 ± 4.064, au, p < 0.001) (figure 3A), respectively. RT-QuIC reaction was higher than in control patients even considering only the 3 positive controls (125.575 ± 10.525 vs 73.744 ± 13.047; p = 0.10). In contrast, there was no significant difference in skin α-synuclein ThT fluorescence intensity between non-synucleinopathies and controls (41.295 ± 5.674 vs 29.073 ± 4.064, au, p = 0.359) (figure 3A). Age, sex, and disease duration did not influence the results by using a logistic regression model.
Table 4.
Immunofluorescence and Real-Time Quaking-Induced Conversion (RT-QuIC) Results of Skin and CSF Samples
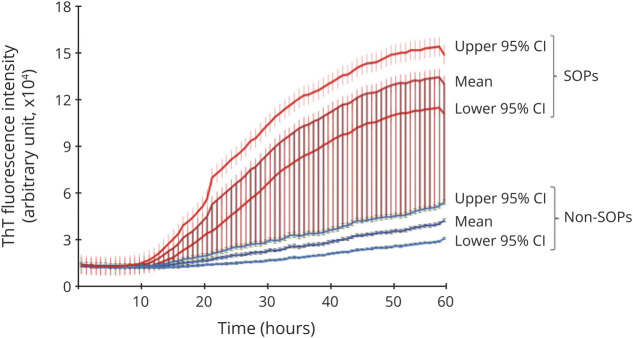
Figure 2. Real-Time Quaking-Induced Conversion (RT-QuIC) Spectra of Average Thioflavin T (ThT) Fluorescence Intensity of Skin α-Synuclein of Patients With Synucleinopathies or Non-Synucleinopathies.
α-Synuclein-seeding activity of biopsied skin samples from patients with synucleinopathy (synucleinopathies, n = 31) and non-synucleinopathy (non-synucleinopathies, n = 38) was blindly examined by RT-QuIC assay. The mean ThT fluorescence intensity in arbitrary units of all the synucleinopathies and non-synucleinopathies along with the associated 95% confidence interval are shown at different time points. SOPs = synucelinopathies.
Figure 3. Real-Time Quaking-Induced Conversion (RT-QuIC) Assay of Skin and CSF Samples From Living Patients.
(A) RT-QuIC assay of skin punch biopsy samples. The pathologic α-synuclein ThT fluorescence response of skin biopsy samples from α-synucleinopathies (synucleinopathies, n = 31), non-synucleinopathies (non-synucleinopathies, n = 38), and controls (n = 24) were detected by RT-QuIC assay. The dotted line represents the threshold that defines the positive ThT fluorescence response, which was Thioflavin T (ThT) fluorescence intensity of 45,414 attribute units (au). ****p < 0.0001. (B) RT-QuIC assay of CSF samples. The pathologic α-synuclein ThT fluorescence response of CSF samples from living patients with α-synucleinopathies (synucleinopathies, n = 9), non-synucleinopathies (non-synucleinopathies, n = 24), and controls (n = 16) were detected by RT-QuIC assay. The dotted line represents the threshold that defines the positive ThT fluorescence response, which was ThT fluorescence intensity of 54,473 au. ***p < 0.005. CTRL = controls; ns = no statistically significant difference; SOPs = synucelinopathies.
RT-QuIC Assay of CSF Samples
Next, the α-synuclein seeding activity of CSF was also detected by RT-QuIC assay. The cutoff point for defining the positive seeding activity was ThT fluorescence of 54.473 (au) based on the mean CSF α-synuclein ThT fluorescence intensity of control plus 3 SDs. The CSF RT-QuIC result yielded a sensitivity of 78% and specificity of 100% (table 2) in disclosing synucleinopathies without any non-synucleinopathies and control patients showing positive α-synuclein seeding activity (table 3). The mean CSF α-synuclein ThT fluorescence intensity from patients with synucleinopathies (n = 9) was significantly higher than that from patients with non-synucleinopathies (n = 24) (88.137 ± 14.177 [SE] vs 32.695 ± 3.819, au, p < 0.01) and controls (n = 16) (88.137 ± 14.177 vs 25.723 ± 2.442, au, p < 0.01). In contrast, there was no significant difference in CSF α-synuclein ThT fluorescence intensity between non-synucleinopathies and controls (32.695 ± 3.819 vs 25.723 ± 2.442, au, p = 0.45) (figure 3B). Regression models showed no influence of age, sex, or disease duration on the results.
Immunofluorescence vs RT-QuIC to Detect Pathologic α-Synuclein
The p-syn staining in skin nerves disclosed by immunofluorescence showed good agreement with the CSF RT-QuIC assay (κ = 0.9; p < 0.001) but the agreement was lower with skin RT-QuIC (κ = 0.6; p < 0.001) due to positive detection in skin samples by RT-QuIC in non-synucleinopathies (κ = 0.2; p = 0.08) and control patients (κ = 0.3; p < 0.05). For the same reason, skin RT-QuIC showed low agreement with CSF RT-QuIC considering all recruited patients (κ = 0.6; p < 0.001) but the agreement in synucleinopathies was optimal (κ = 1; p < 0.001).
χ2 test showed that immunofluorescence better discriminates than RT-QuIC between positive vs negative patients among synucleinopathies, non-synucleinopathies, and control patients (χ2 = 80.1 for immunofluorescence, χ2 = 37.8 for skin RT-QuIC, and χ2 = 36.3 for CSF RT-QuIC; p < 0.0001).
Discussion
The main findings of our study are that (1) both immunofluorescence and RT-QuIC showed a high diagnostic accuracy in differentiating synucleinopathies from different neurodegenerative and other neurologic disorders, although immunofluorescence displayed the better value as well as optimal reproducibility; and (2) these techniques presented a good level of agreement, supporting their equivalency for diagnostic purpose, although the agreement with skin RT-QuIC was lower in patients with non-synucleinopathies.
The correct diagnosis of synucleinopathies in living patients is a prominent challenge with several implications. The search for several biomarkers is in progress but a single diagnostic test with high sensitivity and specificity is not currently available for synucleinopathies, although urgently needed. Because autonomic symptoms may precede the classical symptoms of synucleinopathy, such as motor dysfunction in PD or dementia in DLB,4 reflecting the early damage of postganglionic sympathetic branches, peripheral tissues with extensive autonomic innervation, such as the skin, could be a useful target site in the search of pathologic forms of α-synuclein aggregates. The use of skin samples to identify biomarkers for synucleinopathies is also promising considering that skin biopsy is a minimally invasive procedure. The morphologic identification of misfolded α-synuclein aggregates as p-syn in skin nerves by means of immunofluorescence has been reported in all variants of synucleinopathies, allowing the distinction of these disorders from diseases of different origins or from healthy controls.4-8,25 Additional data showed that p-syn in skin nerves may also be used to differentiate MSA from PD7,26 or uncomplicated PD from PD associated with orthostatic hypotension.21 Furthermore, it has recently been demonstrated by means of a meta-analysis that skin biopsy presented the best diagnostic accuracy in disclosing abnormal α-synuclein deposits in patients with PD.27 In addition, the reliability of immunofluorescence as a diagnostic tool has been supported by excellent interlaboratory and intralaboratory reproducibility.22
RT-QuIC was formerly developed as a diagnostic tool for prion diseases using CSF9 or skin tissue.23,24 This test was recently adapted to detect the seeding activity of pathologic α-synuclein with brain, CSF, or skin samples10,28,29 from PD and LBD with high diagnostic accuracy. Although results are promising and support the clinical use of this technique, reproducibility studies with more cases are lacking.
Immunofluorescence and RT-QuIC explore different forms of pathologic α-synuclein. Immunofluorescence discloses the morphology of the pathologic form of α-synuclein in skin nerve fibers as it identifies the phosphorylation at ser129 of α-synuclein correlated to the change of conformation of the native state with specific antibodies.25 In contrast, RT-QuIC reveals the aggregation behavior of pathologic α-synuclein,23,24 a prion-like feature of misfolded proteins. For this reason, it is important to compare the diagnostic accuracy of the 2 approaches in disclosing a synucleinopathy. The clarification of this aspect may improve their adoption as a diagnostic tool for synucleinopathies in clinical practice.
Our study demonstrates that immunofluorescence staining of p-syn in skin nerves showed side-to-side optimal reproducibility in a large number of samples of both synucleinopathies and non-synucleinopathies. We found that immunofluorescence exhibited a good level of agreement with RT-QuIC (mainly CSF), supporting their equivalency as diagnostic tools for synucleinopathies. The exact reasons for the discrepancy between positive skin RT-QuIC and negative immunofluorescence, even in routine analysis, in some non-synucleinopathies and control patients remain to be clarified. Because the 2 approaches determine 2 different aspects of the α-synuclein aggregates as mentioned above, a possibility needs to be excluded in the future that there are unphosphorylated α-synuclein aggregates in the skin of patients with certain conditions and those aggregates may be detectable by only RT-QuIC for their prion-like seeding activity but not by immunofluorescence with antibodies for their phosphorylation. Accordingly, this finding could be the expression of a copathology (mixed pathology) in non-synucleinopathies.29 Similarly, α-synuclein aggregates in the skin sample of patients with neuropathies could be explained by an incidental synucleinopathy occasionally found on autopsy of decedents without showing clinical phenotypes. In addition, the intraneural p-syn deposits detected by immunofluorescence may not have been found in patients with peripheral neuropathy because of the decrease of the peripheral nerve fibers. However, this hypothesis is denied by the absence of p-syn in these patients even in skin sites with likely preserved skin innervation as cervical and thigh sites in routine analysis. We carefully re-evaluated patients with non-synucleinopathies and control patients found to be positive at the skin by RT-QuIC assay, but no clinical signs attributable to a synucleinopathy could be found, although to exclude this possibility patients should undergo a more extensive clinical follow-up.
The analysis of the diagnostic accuracy of single tests underlined that RT-QuIC presented high sensitivity and specificity in identifying synucleinopathies, as recently reported.30 However, in our study, we found that the greater diagnostic accuracy was given by immunofluorescence. In fact, this test provided higher sensitivity, with 90% of patients with synucleinopathies positive for p-syn, and the highest specificity, as p-syn staining was negative in all non-synucleinopathies and control patients. However, it should be underlined that the low number of patients undergoing CSF RT-QuIC may be responsible for the lower sensitivity of this test compared to immunofluorescence.
The current study presents the following limitations: (1) the lack of autopsy-confirmed diagnosis of recruited patients. This is a significant limitation, although we recruited for this study only patients fulfilling accepted clinical criteria in agreement with the results of appropriated diagnostic tools. However, immunofluorescence or CSF RT-QuIC assay found a clear-cut difference among the 3 groups of investigated patients, supporting a correct selection of recruited patients; (2) the diagnostic accuracy in detecting a synucleinopathy in skin samples may be underestimated since the analysis has almost always been done in a single skin site addressed by the specific clinical phenotype, e.g., PD and DLB in proximal skin sites25 and MSA in distal sites,25,26,31,32 to simplify the study protocol and decrease the number of biopsies taken from each patient. However, abnormal aggregates of α-synuclein may not be evenly distributed in skin nerves and a single site may not be sufficient to detect it precisely. A similar situation has been observed in RT-QuIC assay of prion-seeding activity in skin tissues from different body areas of cadavers with Creutzfeldt-Jakob disease diagnosed neuropathologically; in some cases, positive prion-seeding activity was not always detected in all 3 different skin samples and 1 or 2 skin areas could exhibit no detectable prion-seeding activity23; (3) the lack of quantitative MRI analysis to better differentiate tauopathies from synucleinopathies or TDP proteinopathies. However, a high diagnostic accuracy for synucleinopathies was reached by means of characteristic abnormalities on different diagnostic tools such as MRI, nigrostriatal SPECT, cardiac MIBG SPECT, or head-up tilt test.
This study deals with the important topic of the in vivo diagnosis of synucleinopathies by comparing immunofluorescence and RT-QuIC, 2 recently described advanced techniques to disclose pathologic aggregates of α-synuclein. Our data demonstrate that both techniques displayed high diagnostic accuracy in differentiating synucleinopathies from other neurodegenerative and neurologic disorders, although immunofluorescence displayed the better value as well as an optimal reproducibility. In addition, these 2 diagnostic tools showed good agreement in disclosing a synucleinopathy, supporting the use in clinical practice of less invasive tests such as skin immunofluorescence or RT-QuIC instead of CSF as diagnostic tool for synucleinopathies.
Acknowledgment
The authors thank Johnny Dang for proofreading the manuscript.
Glossary
- AD
Alzheimer disease
- ALS
amyotrophic lateral sclerosis
- au
attribute unit
- CBD
corticobasal syndrome
- DLB
dementia with Lewy bodies
- MSA
multiple system atrophy
- p-syn
phosphorylated α-synuclein
- PAF
pure autonomic failure
- PD
Parkinson disease
- PSP
progressive supranuclear palsy
- RBD
REM sleep behavior disorder
- RT-QuIC
real-time quaking-induced conversion
- TBS
Tris-buffered saline
- ThT
Thioflavin T
Appendix. Authors
Footnotes
Editorial, page 925
Class of Evidence: NPub.org/coe
Study Funding
Ricerca Finalizzata Ministero della Salute Grant RF-2016-02362047 to V.D. and A.I. and NIH (NS112010, NIH NS09532, NS096626) and ALZ/ARUK/MJFF/Weston to W.Z.
Disclosure
The authors report no conflicts of interest. Go to Neurology.org/N for full disclosures.
References
- 1.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007;68:384–386. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koga S, Aoki N, Uitti RJ, et al. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology 2015;85:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donadio V, Incensi A, Cortelli P, et al. Skin sympathetic fiber α-synuclein deposits: a potential biomarker for pure autonomic failure. Neurology 2013;80:725–732. [DOI] [PubMed] [Google Scholar]
- 5.Doppler K, Ebert S, Uçeyler N, et al. Cutaneous neuropathy in Parkinson's disease: a window into brain pathology. Acta Neuropathol 2014;128:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donadio V, Incensi A, Rizzo G, et al. A new potential biomarker for dementia with Lewy bodies: skin nerve α-synuclein deposits. Neurology 2017;89:318–326. [DOI] [PubMed] [Google Scholar]
- 7.Doppler K, Weis J, Karl K, et al. Distinctive distribution of phospho-alpha-synuclein in dermal nerves in multiple system atrophy. Mov Disord 2015;30:1688–1692. [DOI] [PubMed] [Google Scholar]
- 8.Donadio V. Skin nerve α-synuclein deposits in Parkinson's disease and other synucleinopathies: a review. Clin Auton Res 2019;29:577–585. [DOI] [PubMed] [Google Scholar]
- 9.Atarashi R, Satoh K, Sano K, et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat Med 2011;17:175–178. [DOI] [PubMed] [Google Scholar]
- 10.Groveman BR, Orrù CD, Hughson AG, et al. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun 2018;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 1988;51:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008;71:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duits FH, Teunissen CE, Bouwman FH, et al. The cerebrospinal fluid "Alzheimer profile": easily said, but what does it mean? Alzheimers Dement 2014;10:713–723. [DOI] [PubMed] [Google Scholar]
- 14.Litvan I, Agid Y, Calne D, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996;47:1–9. [DOI] [PubMed] [Google Scholar]
- 15.Bak TH, Hodges JR. Corticobasal degeneration. In: Vinken PJ, Bruyn GW, eds. Handbook of Clinical Neurology, vol 89. Elsevier Science; 2008:509–511. [DOI] [PubMed] [Google Scholar]
- 16.Kalra S, Grosset DG, Benamer HTS. Differentiating vascular parkinsonism from idiopathic Parkinson's disease: a systematic review. Mov Disord 2010;25:149–156. [DOI] [PubMed] [Google Scholar]
- 17.Román GC, Sachdev P, Royall DR, et al. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci 2004;226:81–87. [DOI] [PubMed] [Google Scholar]
- 18.The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. J Neurol Neurosurg Psychiatry 1994;57:416–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De CarvalhoDengler MR, Eisen A, England JD, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol 2008;119:497–503. [DOI] [PubMed] [Google Scholar]
- 20.Gallassi R, Lenzi P, Stracciari A, et al. Neuropsychological assessment of mental deterioration: purpose of a brief battery and a probabilistic definition of “normality” and “non-normality”. Acta Psychiatr Scan 1986;75:62–67. [DOI] [PubMed] [Google Scholar]
- 21.Donadio V, Incensi A, Del Sorbo F, et al. Skin nerve phosphorylated α-synuclein deposits in Parkinson's disease with orthostatic hypotension. J Neuropathol Exp Neurol 2018;77:942–949. [DOI] [PubMed] [Google Scholar]
- 22.Donadio V, Doppler K, Incensi A, et al. Abnormal α-synuclein deposits in skin nerves: intra- and inter-laboratory reproducibility. Eur J Neurol 2019;26:1245–1251. [DOI] [PubMed] [Google Scholar]
- 23.Orrú CD, Yuan J, Appleby BS, et al. Prion seeding activity and infectivity in skin samples from patients with sporadic Creutzfeldt-Jakob disease. Sci Transl Med. 2017;9:eaam7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Manca M, Foutz A, et al. Early preclinical detection of prions in the skin of prion-infected animals. Nat Commun 2019;10:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donadio V, Incensi A, El-Agnaf O, et al. Skin α-synuclein deposits differ in clinical variants of synucleinopathy: an in vivo study. Sci Rep 2018;8:14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donadio V, Incensi A, Rizzo G, et al. Skin biopsy may help to distinguishing MSA-P from Parkinson's disease with orthostatic hypotension. Mov Disord 2020;35:1649–1657. [DOI] [PubMed] [Google Scholar]
- 27.Tsukita K, Sakamaki-Tsukita H, Tanaka K, Suenaga T, Takahashi R. Value of in vivo α-synuclein deposits in Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2019;34:1452–1463. [DOI] [PubMed] [Google Scholar]
- 28.Fairfoul G, McGuire LI, Pal S, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 2016;3:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Becker K, Donadio V, et al. Skin α-synuclein aggregation seeding activity as a novel biomarker for Parkinson Disease. JAMA Neurol 2020;78:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi M, Candelise N, Baiardi S, et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol 2020;40:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isonaka R, Rosenberg AZ, Sullivan P, et al. Alpha-synuclein deposition within sympathetic noradrenergic neurons is associated with myocardial noradrenergic deficiency in neurogenic orthostatic hypotension. Hypertension 2019;73:910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isonaka R, Gibbons CH, Wang N, Freeman R, Goldstein DS. Association of innervation-adjusted alpha-synuclein in arrector pili muscles with cardiac noradrenergic deficiency in autonomic synucleinopathies. Clin Auton Res 2019;29:587–593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.