Abstract
Aim
People with rheumatic diseases (PRD) remain vulnerable in the era of the COVID‐19 pandemic. We formulated recommendations to meet the urgent need for a consensus for vaccination against SARS‐CoV‐2 in PRD.
Methods
Systematic literature reviews were performed to evaluate: (a) outcomes in PRD with COVID‐19; (b) efficacy, immunogenicity and safety of COVID‐19 vaccination; and (c) published guidelines/recommendations for non‐live, non‐COVID‐19 vaccinations in PRD. Recommendations were formulated based on the evidence and expert opinion according to the Grading of Recommendations Assessment, Development and Evaluation methodology.
Results
The consensus comprises 2 overarching principles and 7 recommendations. Vaccination against SARS‐CoV‐2 in PRD should be aligned with prevailing national policy and should be individualized through shared decision between the healthcare provider and patient. We strongly recommend that eligible PRD and household contacts be vaccinated against SARS‐CoV‐2. We conditionally recommended that the COVID‐19 vaccine be administered during quiescent disease if possible. Immunomodulatory drugs, other than rituximab, can be continued alongside vaccination. We conditionally recommend that the COVID‐19 vaccine be administered prior to commencing rituximab if possible. For patients on rituximab, the vaccine should be administered a minimum of 6 months after the last dose and/or 4 weeks prior to the next dose of rituximab. Post‐vaccination antibody titers against SARS‐CoV‐2 need not be measured. Any of the approved COVID‐19 vaccines may be used, with no particular preference.
Conclusion
These recommendations provide guidance for COVID‐19 vaccination in PRD. Most recommendations in this consensus are conditional, reflecting a lack of evidence or low‐level evidence.
Keywords: COVID‐19, immunosuppression, people with rheumatic diseases, SARS‐CoV‐2, vaccination
1. INTRODUCTION
The global novel coronavirus disease 2019 (COVID‐19) pandemic has posed many uncertainties among physicians treating people with rheumatic diseases (PRD). Such patients are considered high risk due to their diseases and the immunosuppressive nature of their medications. A recent meta‐analysis demonstrated that PRD had a 2‐fold risk of COVID‐19 compared to control patients. 1 In addition, PRD with COVID‐19 had a higher fatality rate and were at significant risk of suffering poor outcomes such as the need for hospitalization, care in the intensive care unit (ICU) and mechanical ventilation. 2 , 3
Various candidate vaccines against severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) are in development. The first 3 COVID‐19 vaccines to receive Emergency Use Authorization (EUA) from the United States Food and Drug Administration (US FDA), the Pfizer‐BioNTech® COVID‐19 vaccine (BNT162b2), the Moderna® COVID‐19 vaccine (messenger RNA [mRNA]‐1273) and the Johnson & Johnson® vaccine (JNJ‐78436735), reported good vaccine efficacy at 95%, 94.1% and 66.9%, respectively. 4 , 5 , 6 However, patients on immunosuppressive therapy were excluded from all 3 trials. Additionally, patients with autoimmune diseases were excluded from 2 of the trials, and only 62 PRD (0.3% of the total study population, but without detailed information) were included in the treatment arm of the Pfizer‐BioNTech® trial. Thus, there is a paucity of evidence for PRD and their managing physicians to guide COVID‐19 vaccination in this population.
The Singapore Health Sciences Authority (HSA) has approved the Pfizer‐BioNTech® and Moderna® COVID‐19 mRNA vaccines via the Pandemic Special Access Route, and the Ministry of Health, Singapore (MOH) Expert Committee on COVID‐19 Vaccination (EC19V) has published recommendations for their use 7 , 8 with other vaccines to be evaluated at a later date. Worldwide, 4 additional vaccines, namely from Gamaleya Research Institute of Epidemiology and Microbiology (Gam‐COVID‐Vac or Sputnik V®), 9 Oxford‐Astra‐Zeneca® (AZD1222), 10 Novartis (Novavax® or NVX‐CoV2373) 11 and Bharat Biotech (BB‐152 or Covaxin®), 12 have so far published or announced interim Phase 3 efficacy data and are either already authorized or expected to apply for EUA in several countries. In this consensus recommendation, the Chapter of Rheumatologists, College of Physicians, Academy of Medicine, Singapore seeks to address questions regarding the suitability of COVID‐19 vaccination in PRD and provide consensus recommendations on COVID‐19 vaccination among PRD.
2. METHODS
A Core Working Group (CWG) was established (AS, CX, WF, ML). Members of the CWG reviewed published primary clinical trials and performed a systematic literature review to answer 4 research questions. Where appropriate, in lieu of a systematic review of the primary literature, international best practice guidelines and recommendations from rheumatology societies on vaccinations in PRD were reviewed. Other academic bodies’ recommendations for COVID‐19 vaccination and other non‐live, non‐COVID‐19 vaccinations in PRD and / or immunocompromizing conditions were also considered. The CWG developed draft recommendations for rating by an invited task force panel (TFP). A modified Delphi approach, similar to what has been applied by other organizations, was used. 13 , 14 The TFP (TA, KOK, AL, THL, KHL, AHLL, MKS, TCT, GGT, BYT) comprised 8 locally recognized adult rheumatologists from public and private healthcare institutions in Singapore, 1 pediatric rheumatologist and 1 infectious diseases specialist. A conflict‐of‐interest declaration was required from all members of the CWG and TFP prior to the consensus process. All members declared no conflicts of interest.
2.1. Review of the literature
The CWG sent out preselected topics to the TFP and sought their input on additional clinically important topics. Considering the TFP's input, the CWG selected the following core topics relevant to clinical decision‐making for COVID‐19 vaccination:
-
1.
Are PRD at increased risk of adverse outcomes from COVID‐19?
A recent systematic review and meta‐analysis of global data showed that PRD remain vulnerable, with substantial rates of severe outcomes. 3 The overall rates of hospitalization, oxygen support, ICU admission and fatality among COVID‐19 infected patients with rheumatic diseases were 58% (95% CI 48%‐67%), 33% (95% CI 21%‐47%), 9% (95% CI 5%‐15%) and 7% (95% CI 3%‐11%), respectively, which are comparable with data from the COVID‐19 Global Rheumatology Alliance (GRA) physician registry. The fatality rate was higher both in this meta‐analysis and the COVID‐19 GRA (7.0% and 6.7%, respectively) than that (3.4%) of general population infected with COVID‐19 in the WHO database, although age, gender and comorbidities were not matched. 3 D'Silva et al reported a higher risk of hospitalization, ICU admission, mechanical ventilation, acute kidney injury, renal replacement therapy and death based on TriNetX, a multi‐center research network with real‐time electronic health record data across 35 healthcare organizations in the US. 15 The authors concluded that these outcomes were likely mediated by a higher comorbidities burden in PRD, such as hypertension, diabetes mellitus, chronic kidney disease and asthma.
-
2.
Are existing approved vaccines against SARS CoV2 safe, immunogenic and efficacious in PRD?
Two mRNA vaccines are currently approved by the US FDA and Singapore HSA. It is known that selected DNA and RNA molecules have the unique property to activate the immune system, through activation of Toll‐like receptors. 16 It has been shown that the innate immune response would be suppressed by nucleoside modification of RNA, as the innate immune system detects RNA lacking nucleoside modification as a means of selectively responding to bacteria or viruses. 17 , 18 Both mRNA COVID‐19 vaccines from Pfizer/BioNTech® and Moderna® are nucleoside‐modified RNA. Thus, the risk of autoimmune disease flare after receiving mRNA COVID‐19 vaccine may more likely result from the adaptive immune response to spike protein synthesized by mRNA, rather than the innate immune response to nucleoside‐modified RNA. Theoretically, this is no different from the risk from other protein / conjugate vaccines, which have been in use for many years and have been confirmed to be safe in PRD.
There were 62 (0.3%) participants who had rheumatic disease and received BNT162b2 mRNA COVID‐19 vaccine in the Pfizer/BioNTech® trial. 4 No flare of autoimmune disease was reported. Certainly, larger sample size and long‐term follow‐up studies are needed to further ascertain the risk of flares in autoimmune diseases.
Other vaccine strategies, including inactivated virus vaccines (such as the CoronaVac developed by Sinovac® 19 and Covaxin® developed by Bharat Biotech 12 ), virus vector vaccines (such as the COVID‐19 vaccines by AstraZeneca®, 10 the Johnson & Johnson® vaccine 6 and the Sputnik V® Russian vaccine by Gamaleya 9 ) and protein subunit vaccines (such as the Novavax® vaccine 11 ) similarly provide little data in PRD. Pertinent information from primary COVID‐19 vaccine trials to date are summarized in Table 1. 4 , 5 , 6 , 9 , 10 , 12 , 19 , 20
TABLE 1.
Primary COVID‐19 vaccine trials
| Pfizer‐BioNTech® 4 | Moderna® 5 | Sinovac® 19 | Oxford‐Astra Zeneca® 10 | Gamaleya® 9 | Johnson & Johnson® 6 | Novartis 20 | Bharat Biotech12 | |
|---|---|---|---|---|---|---|---|---|
| Trial sites | US, Brazil, Argentina, South Africa, Germany, Turkey | US | China | UK, Brazil, South Africa | Russia | US, Central and South America, South Africa | UK, South Africa | India |
| MOA | Lipid nanoparticle–formulated, nucleoside‐modified mRNA | Adsorbed SARS‐CoV‐2 (inactivated) vaccine | Replication deficient viral vector with SARS COV2 spike protein | Adjuvant recombinant protein particle | Whole‐virion inactivated SARS‐CoV‐2 vaccine with a Toll‐like receptor 7/8 agonist molecule adsorbed to alum | |||
| Storage | Freezer −70°C | Freezer −15 to −25°C | Refrigerator 2 to 8°C | Refrigerator 2 to 8°C | Refrigerator 2 to 8°C | Refrigerator 2 to 8°C | Refrigerator 2 to 8°C | Refrigerator 2 to 8°C |
| Dosing | Two 30 µg (0.3 mL) IM doses 21 d apart | Two 100 µg (0.5 mL) IM doses 28 d apart | Two doses 2 wk apart | Two doses 4‐12 wk apart | Two (0.5 mL) IM doses 21 d apart | Single dose | Two (0.5 mL) IM doses 21 d apart | Two 6 µg IM doses 28 d apart |
| Inclusion | Adults (>16 y) | Adults (≥18 y) | Adults (≥18 y) | Adults (≥18 y) | Adults (≥18 y) | Adults (≥18 y) | Adults (18‐84 y) | Adults (18‐98 y) |
| Relevant Exclusions | Immunodeficient state and use of immunosuppressant medication. |
Autoimmune disease Immunodeficient state and use of immunosuppressant medication within the past 6 mo |
Autoimmune disease Immunodeficient state and use of immunosuppressant medication within the preceding 3 mo |
Autoimmune disease Immunodeficient state and use of immunosuppressant medication within the past 6 mo |
Immunodeficient state and use of immunosuppressant medication within the past 3 mo | Immunodeficient state and use of immunosuppressant medication. |
Autoimmune disease Immunodeficient state and use of immunosuppressant medication within the preceding 3 mo |
NA |
| N a | 37 706 | 28 207 | 13 060 | 11 636 | 19 866 | 43 783 | 15 000 | 25 800 |
| Follow‐up (median) after last dose | 2 mo | 64 d | NA | 2 mo | 27 d | 8 wk | NA | NA |
| Asian participants | 1608 (4.3%) | 1382 (4.6%) | NA | 517 (4.4%) | 286 (1.4%) | 3.5% | NA | 100% |
| Comorbidities | 20.5% | 22.3% | NA | 24.7% | 24.8% | 41% (including obesity) | NA | 17.4% |
| PRD | 62 (0.3%) | Excluded | NA | Excluded | Likely excluded | Allowed, likely none included | NA | NA |
| Elderly | >55 y (42.2%) | >65 y (25.3%) | NA | >55 y (12.2%) | >60 y (10.8%) | >65 y (20.4%) | >65 y (27%) | >60 y (9.4%) |
| Outcomes | 8 vs 162 cases of symptomatic and PCR confirmed COVID‐19, vaccine efficacy 95% | 11 vs 185 cases of symptomatic and PCR confirmed COVID‐19, vaccine efficacy 94.1% | NA | 30 vs 101 cases of symptomatic and PCR confirmed COVID‐19, vaccine efficacy 70.4% | 16/14 964 vs 62/4902 cases of symptomatic and PCR confirmed COVID‐19, vaccine efficacy 91.6% | 116/19 514 vs 348/19 544 moderate / severe PCR confirmed COVID‐19, vaccine efficacy 66.9% | 6 vs 56 cases of symptomatic and PCR confirmed COVID‐19, vaccine efficacy 89.3% | 7 vs 36 cases of symptomatic and PCR confirmed COVID‐19, vaccine efficacy 80.6% |
| Common adverse events | Expected reactogenicity: fatigue, headache. Rare anaphylaxis, lymphadenopathy | Expected reactogenicity: injection site pain, fatigue, headache, muscle pain, joint pain and chills. Rare lymphadenopathy and hypersensitivity. | NA | Not different from placebo arm. No anaphylaxis | Injection site reactions, flu‐like illness, headache, asthenia. No anaphylaxis | Expected reactogenicity: injection site pain, fatigue, headache, myalgia and fever. No anaphylaxis | NA | Not different from placebo arm. No anaphylaxis |
Abbreviations: MOA, mechanism of action; NA, not available; PRD, patients with rheumatic disease.
Patients included in published interim analysis
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
There are currently no available data on the immunogenicity and efficacy of COVID‐19 vaccination in PRD.
-
3.
Are other (non‐COVID‐19) recommended non‐live vaccines safe, immunogenic and efficacious in PRD?
-
4.
What is the effect of various drugs used in PRD on immunogenicity of (non‐COVID‐19) vaccines in PRD?
To review the evidence in non‐live, non‐COVID‐19 vaccinations in PRD, a systematic review of international best practice guidelines and recommendations from rheumatology societies on vaccinations in PRD was performed, in lieu of a systematic review of the primary literature. We searched PubMed for publications using the Medical Subject Headings (MeSH) terms ("Consensus" [MeSH] OR "Consensus Development Conference, NIH" [Publication Type] OR "Consensus Development Conference" [Publication Type] OR "Consensus Development Conferences, NIH" [MeSH] OR "Consensus Development Conferences" [MeSH]) OR ("Guidelines as Topic" [MeSH] OR "Practice Guidelines as Topic" [MeSH] OR "Guideline" [Publication Type] OR "Health Planning Guidelines" [MeSH] OR "Standard of Care" [MeSH] OR "Practice Guideline" [Publication Type] OR "Clinical Protocols" [MeSH] AND ((vaccine [MeSH Terms]) OR (vaccination [MeSH Terms])) OR (active immunization [MeSH Terms]) AND (autoimmune disease [MeSH Terms]) OR (rheumatology [MeSH Terms]) OR (host, immunocompromised [MeSH Terms]) OR (immunocompromised host [MeSH Terms]) OR (immunocompromised patient[MeSH Terms]). The filters English (language) and Humans were applied. This search yielded 191 citations. One member of the CWG (ML) screened through the titles and/or abstracts and excluded those that were not a practice guideline, not targeted to PRD, only addressed live vaccines, were only targeted to childhood vaccines, did not undertake a systematic literature review, were duplicates, or were outdated recommendations from the same body. Four additional citations were added from manual search. We then reviewed the remaining 21 full text articles and excluded best practice guidelines that did not undertake a consensus methodology and evidence grading or strength of recommendations. Eleven full text articles were finally included (Figure 1, Table 2). 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 The definitions of PRD and immunomodulatory drugs considered in this recommendation are summarized in Table 3.
FIGURE 1.
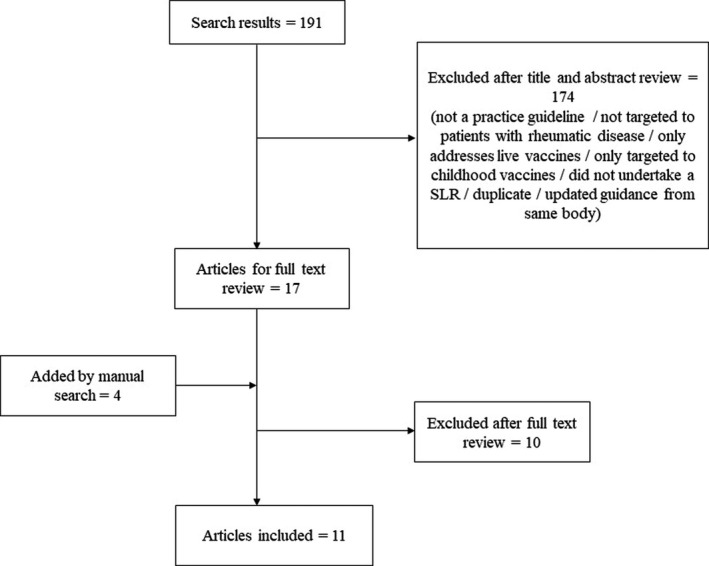
Flowchart for study selection
TABLE 2.
Reviewed practice guidelines citations, with focus on non‐live vaccinations
| Article | Vaccine type | Patient population | Safety | Immunogenicity | Efficacy | Timing /DMARD cessation | Post‐vaccination antibody testing | Vaccination of household contacts |
|---|---|---|---|---|---|---|---|---|
| Furer V, et al. 29 | Non‐live | PRD on IS/ DMARD/ GC | Influenza (LOE a 2b‐4) and PCV (LOE 4) deemed safe |
Good for influenza (LOE 1b‐4) and PPSV23 (LOE 1b‐4) Influenza: reduced by RTX, ABA PPSV23: reduced by RTX, ABA, TOF, GOL PCV13: reduced by MTX |
Influenza (LOE 2a‐5), PPSV23 (LOE 1b‐4) No data for MTX, TNFi, B cell depletion, belimumab, tocilizumab, abatacept, tofacitinib, glucocorticoids |
Quiescent dx Prior to IS, in particular B cell depleting therapy (6 mo post‐RTX, 4 wk before next dose of RTX) No DMARD cessation |
‐ | Yes, except for oral polio (LOE NA) |
| Seo YB, et al. 22 | Non‐live | PRD on IS/ DMARD/ GC | Similar risk as general population (Influenza LOE: b mod; pneumococcal LOE : low) |
Similar or slightly lower than that of healthy individuals. Pneumococcal: reduced by MTX, RTX, ABA |
Influenza and pneumococcal |
Stable dx (LOE: very low) Prior to IS (LOE : very low) Before ABA and ≥4 wk before RTX No DMARD cessation |
‐ | Yes |
| Guerrini G, et a. 28 | Influenza and pneumococcal | PRD on IS/ DMARD/ GC | Influenza and pneumococcal deemed safe (LOE c 2) |
Pneumococcal: reduced by MTX, RTX, ABA, TOF, MMF, AZA, CyC, high dose GC (LOE 2) Influenza: reduced by RTX, ABA, high dose GC (LOE 2) |
‐ |
Stable dx (LOE 2) Pneumococcal: before IS and ≥4 wk before RTX (LOE 2) |
‐ | ‐ |
| Papp KA, et al. 24 | Non‐live | PRD on IS/ DMARD/ GC | ‐ | ‐ | ‐ |
2 wk before IS (LOE 4 mod) RTX: 5 mo post‐RTX and ≥4 wk prior to RTX (LOE low) |
‐ | ‐ |
| Holroyd CR, et al. 26 | Non‐live | RA, PsA, axSpA | No flare of RA with Influenza |
Influenza: reduced by ETN and INF, RTX, ABA Pneumococcal: reduced by MTX, RTX, ABA (LOE d 1C) |
‐ | ‐ | ‐ | ‐ |
| Keeling SO, et al. 25 | Influenza | SLE | Trivial number of SLE flares with influenza (LOE e mod) | ‐ | Influenza (LOE mod) | ‐ | ‐ | ‐ |
| Singh JA, et al. 21 | Non‐live | RA on DMARD/ GC | ‐ | Reduced by RTX and possibly MTX (LOE d very low) | Killed vaccine (LOE very low) | No DMARD cessation needed (LOE very low) | ||
| Bühler S, et al. 30 | Non‐live | PRD on IS/ DMARD/ GC | No flare nor trigger of rheumatic disease, (LOE d low) | Reduced by DMARD/ GC especially MTX, RTX, ABA (LOE mod) | ‐ |
When the IS lowest (LOE low) Before ABA RTX: 6 mo post‐RTX for revaccination, 12 mo post‐RTX for primary vaccination |
4‐6 wk post vaccine (LOE NA) | Yes (LOE NA) |
| Rubin LQ, et al. 23 | Non‐live | IC | ‐ | Influenza: reduced within 6 mo of RTX (LOE 5 mod) | ‐ | ≥2 wk before IS (LOE mod) | ‐ | Yes (LOE high) |
| Centers for Disease Control & Prevention 31 | Pneumococcal | IC | ‐ | ‐ | ‐ | ‐ | ||
| Heijstek MW, et al. 27 | Non‐live | PRD on DMARD /GC | No flare of rheumatic disease or serious adverse events in comparison to healthy subjects |
Influenza: reduced by GC > 10 mg/d (LOE c 3), AZA, HCQ, CYC (LOE 2), RTX (LOE 2) Pneumococcal: reduced by MTX (LOE 2), RTX (LOE 1b) |
‐ | Before RTX (LOE 1b‐2) |
Influenza and pneumococcal: on RTX (LOE 1b‐2), GC ≥ 2 mg/kg or 20 mg/d for ≥2 wk (LOE 3), ±TNFi (LOE 2) PPSV23: On MTX (LOE 2) |
‐ |
Abbreviations: ABA, abatacept; axSpA, axial spondyloarthritis; Aza, azathioprine; CYC, cyclophosphamide; DMARD, disease modifying anti‐rheumatic drugs; dx, disease; GC, glucocorticoid; IC, immunocompromised; IS, immunosuppression; JAKi, inhibitors of Janus kinase; LOE, level of evidence; MMF, mycophenolate mofetil; mod, moderate; mo, months; MTX, methotrexate; NA, non‐available; PCV, pneumococcal vaccination; PRD, people with rheumatic diseases; PsA, psoriatic arthritis; RA, rheumatoid arthritis; RTX, rituximab; SLE, systemic lupus erythematosus; TNFi, tumor necrosis factor inhibitors; TOC, tocilizumab; wk, weeks.
Oxford Centre for Evidence‐Based Medicine – levels of evidence. 45
Level of evidence as defined: High – very unlikely to change confidence in the estimate of effect by an additional study; Moderate – likely to change confidence in the estimate of effect by an additional study; Low – highly likely to change confidence in the estimate of effect by an additional study; Very low – not sure about confidence in the estimate of effect
Level of evidence as defined: 1a – meta‐analysis of randomized controlled trials (RCT); 1b – RCT; 2 – prospective controlled intervention study without randomization; 3 – descriptive/analytic study (including case‐control, cross‐sectional, case series); 4 – expert committee reports or opinion or clinical experience of respected authorities or both
GRADE level of evidence. 32
Level of evidence as defined: High – consistent evidence from well performed RCTs or exceptionally strong evidence from unbiased observational studies; Moderate – evidence from RCTs with important limitations (inconsistent results, methodological flaws, indirect, or imprecise) or exceptionally strong evidence from unbiased observational studies; Low – evidence for at least 1 critical outcome from observational studies, RCTs with serious flaws or indirect evidence; Very low – evidence for at least 1 critical outcome from unsystematic clinical observations or very indirect evidence.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
TABLE 3.
Definition of PRD (people with rheumatic diseases) and immunomodulatory treatment
| PRD include, but are not limited to, those diagnosed with: |
| 1. Chronic inflammatory arthritides (eg rheumatoid arthritis, psoriatic arthritis, spondyloarthritides, juvenile idiopathic arthritis, adult onset Still’s disease) |
| 2. Connective tissue diseases (eg systemic lupus erythematosus, immune‐mediated inflammatory myositis, Sjӧgren’s syndrome, systemic sclerosis) |
| 3. Primary systemic vasculitides |
| 4. Autoinflammatory diseases |
| Immunomodulatory drugs considered for this guidance include: |
| 1. Conventional synthetic disease modifying anti‐rheumatic drugs (DMARDs) (methotrexate, sulphasalazine, leflunomide, hydroxychloroquine) |
| 2. Biologic DMARDs (anti‐tumor necrosis factor, tocilizumab, rituximab, abatacept, secukinumab, ixekizumab, anakinra, belimumab) |
| 3. Targeted synthetic DMARDs (tofacitinib, baricitinib, upadacitinib a ) |
| 4. Immunosuppressive drugs (cyclophosphamide, mycophenolate mofetil, azathioprine, cyclosporin A, tacrolimus) |
| 5. Glucocorticoids |
Not included in any of the searched literature on vaccines, hence recommendation is by extrapolation.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2.2. Creation of preliminary statements and rating
The CWG met to formulate and finalize preliminary statements for rating by the TFP, which was conducted on an online survey platform. The TFP were provided with summarized evidence from the reviewed trials and guidelines, a link to an online rating form and rating instructions. Based on their expertise and the provided literature, each TFP member independently rated each statement on a 5‐point Likert scale (1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, 5 = strongly agree); an agreement was defined as a score of 4 or 5. A consensus was obtained if there was ≥70% agreement. The CWG and the TFP convened via a teleconferencing platform, where the aggregated findings were presented and discussed. Definitions were clarified and statements were reworded, if needed. As there was consensus on all statements following the online voting round, no further round of voting was conducted. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology 32 was used to determine the strength of recommendations. In determining the strength of recommendations, the TFP considered the level of evidence available, as well as the balance between the potentially expected benefits and risks from COVID‐19 vaccination/ omission of vaccination in PRD. Recommendations were categorized as “strong” when benefits/risks clearly outweighed the other, and “conditional” when benefits/risks were closely balanced or uncertain.
2.3. Finalizing consensus statements
The final consensus statement was circulated to the TFP after the consensus meeting and was approved by all members.
3. RESULTS
The final consensus statements consist of 2 overarching principles and 7 recommendations. They are summarized in Table 4.
TABLE 4.
Final consensus statements
| Median Likert score | % agreement | Strength of recommendation | |
|---|---|---|---|
| Overarching principles | |||
| Vaccination in people with rheumatic diseases should be aligned with prevailing national policy. | 4.5 | 100 | ‐ |
| The decision for vaccination should be individualized, and should be explained to the patient, to provide a basis for shared decision‐making between the healthcare provider and the patient. | 5 | 100 | ‐ |
| Recommendations | |||
| 1. We strongly recommend that eligible patients be vaccinated against SARS‐CoV2. | 5 | 100 | Strong |
| 2. We conditionally recommend that the COVID‐19 vaccine be administered during quiescent disease, if possible. | 4.5 | 100 | Conditional |
| 3. We conditionally recommend that immunomodulatory drugs, other than rituximab, can be continued alongside COVID‐19 vaccination. | 5 | 80 | Conditional |
| 4. We conditionally recommend that the COVID‐19 vaccine be administered prior to commencing rituximab, if possible. For patients on rituximab, the vaccine should be administered a minimum of 6 mo after the last dose, and/or 4 wk prior to the next dose of rituximab. | 4 | 90 | Conditional |
| 5. We conditionally recommend that post‐COVID‐19 vaccination antibody titers need not be measured. | 4 | 90 | Conditional |
| 6. We strongly recommend that household contacts be vaccinated against SARS‐CoV2. | 4.5 | 100 | Strong |
| 7. We conditionally recommend that any of the approved COVID‐19 vaccines may be used, with no particular preference. | 4 | 70 | Conditional |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.1. Overarching principles
-
1.
Vaccination in PRD should be aligned with prevailing national policy.
The knowledge on COVID‐19 vaccination is rapidly evolving with various candidate vaccines still undergoing clinical trials. As new evidence becomes available, the landscape of vaccine availability in each country will likely differ. It is important that healthcare professionals align their recommendations to prevailing national policy, to ensure consistency of messages to patients and maintain streamlined safety workflows. Vaccine safety monitoring systems, such as the vaccine adverse event reporting system are in place to detect possible safety signals in the vaccinated population. Locally, the HSA reviews all reports of post‐vaccination reactions, to inform national policy of vaccine eligibility, monitoring and precautions.
-
2.
The decision for vaccination should be individualized, and should be explained to the patient, to provide a basis for shared decision‐making between the healthcare provider and the patient.
Rheumatologists’ decision for offering vaccinations to their patients should take into account the individual patient's disease state, medications, as well as their risk profile and preferences. Patients should be provided with evidenced‐based information to enable them to participate in a shared decision‐making process. Information should include the potential risks and benefits from vaccination (or its omission), the vaccination schedule and a discussion of the various available vaccines.
3.2. Recommendations
-
1.
We strongly recommend that eligible patients be vaccinated against SARS‐CoV2.
PRD are a vulnerable patient population at increased risk of acquiring COVID‐19 1 and suffering severe outcomes. 3 , 15 While there are little data on mRNA vaccination in PRD, there are no reports of autoimmune disease flares in the small group of PRD included in the Pfizer/BioNTech® trial. 4 There is an isolated report of a healthy individual who was diagnosed with fatal immune thrombocytopenia 6 days after COVID‐19 vaccination with no clear evidence of the development of a new autoimmune disease. While there was temporal association, it could not be fully concluded that the vaccine was definitely the cause for the patient's presentation. 33 To our knowledge, there are no other published reports of autoimmune disease induction or flare after COVID‐19 vaccination in the more than 300 million people vaccinated worldwide to date. COVID‐19 vaccination should therefore be strongly recommended for PRD given the vulnerability of PRD along with good efficacy, immunogenicity and favorable safety profile of COVID‐19 vaccination in healthy patients. This is in line with recommendations endorsed by the British Society of Rheumatology for clinically extremely vulnerable patients, 34 which includes PRD and the recent press release from the American College of Rheumatology (ACR). 35 The United States Centers for Disease Control and Prevention (US CDC) similarly places immunocompromised persons at an increased risk for severe COVID‐19 and recommends that these patients receive vaccination as long as there are no contraindications. 36
-
2.
We conditionally recommend that the COVID‐19 vaccine be administered during quiescent disease, if possible.
This recommendation is extrapolated from other vaccine recommendations in PRD, and is largely based on expert opinion, hence the conditional strength of recommendation. Vaccination studies in PRD have been largely conducted during quiescent disease state 28 , 29 and thus have limited generalizability to the PRD population with active disease, although isolated studies have shown similar vaccine immunogenicity regardless of disease state. 37 The decision for vaccination in patients whose disease is not quiescent should be considered on an individual patient level.
-
3.
We conditionally recommend that immunomodulatory drugs, other than rituximab, can be continued alongside COVID‐19 vaccination.
Vaccination studies in PRD on immunomodulatory drugs (other than B cell depleting therapy) have shown sufficient protective efficacy with common non‐live vaccines including influenza and pneumococcal vaccines, despite somewhat reduced immunogenicity, particularly with methotrexate and abatacept. 22 , 26 , 30
-
4.
We conditionally recommend that the COVID‐19 vaccine be administered prior to commencing rituximab, if possible. For patients on rituximab, the vaccine should be administered a minimum of 6 months after the last dose, and/or 4 weeks prior to the next dose of rituximab.
B cell depleting therapy with rituximab is associated with significant reduction in immunogenicity. Despite reduced humoral immune response, cellular immune response is still preserved after influenza vaccination in patients who were treated with rituximab. 38 Satisfactory immunogenicity has been shown in rituximab treated patients when influenza and pneumococcal vaccines were administered 6 months after a previous dose 21 , 23 , 29 and at least 4 weeks prior to a subsequent dose, 24 , 28 forming the basis of this conditional recommendation. Of note, the British Arthritis and Musculoskeletal Alliance recommends that vaccination should not be delayed in patients on or planned for rituximab, with an ideal interval of vaccination 4‐8 weeks after the last dose of rituximab or 2 weeks prior to a planned dose of rituximab, if possible. 34
-
5.
We conditionally recommend that post‐COVID‐19 vaccination antibody titers need not be measured.
Outside of pediatric care, 27 post‐vaccination antibody titer measurement is not part of routine clinical practice and is not part of other vaccination guidelines in adult PRD. As the correlation between antibody titers post‐COVID‐19 vaccination and clinical protection is not well established at present, we conditionally recommend that titers not be measured.
-
6.
We strongly recommend that household contacts be vaccinated against SARS‐CoV‐2.
Vaccination of household contacts has been advocated by societies such as European Alliance of Associations for Rheumatology (EULAR) 29 and Infectious Diseases Society of America (IDSA) 23 for a variety of inactivated and live vaccines (except for the oral polio vaccination 22 , 23 , 29 ). Increasingly, epidemiologic studies have demonstrated SARS‐CoV‐2 transmission in close contacts due to asymptomatic and presymptomatic infections, 39 , 40 , 41 highlighting the importance of extending vaccinations to household contacts in order to protect vulnerable patients.
-
7.
We conditionally recommend that any of the approved COVID‐19 vaccines may be used, with no particular preference.
The various SARS‐CoV‐2 vaccines in development are non‐live vaccines. The anticipated risk‐benefit ratio should therefore be similar for vaccinations to be recommended without preference for any particular vaccine. However, long‐term follow‐up in PRD will be needed to ascertain longer term efficacy and safety of the various vaccines.
4. DISCUSSION
The consensus recommendations for COVID‐19 vaccination in PRD presented in this article were based on review of the limited currently available literature with these vaccines, supplemented by the more extensive knowledge that is available for other non‐live vaccines in PRD. It is noteworthy that the absence of evidence is not evidence of absence, and practical recommendations for PRD need to be made despite the scarcity of literature in these vulnerable patients. Experts in the specialty were consulted, in order to synthesize the available literature into clinically meaningful recommendations. Available evidence on the risk of COVID‐19 in PRD was weighed against the potential risks / benefits of vaccination with a new vaccine technology, borrowing from the principles of vaccination with non‐live viruses in PRD and the available knowledge on mRNA drug delivery systems.
In formulating these recommendations, the TFP were cognizant of the heightened risk of COVID‐19 in our patients. Therefore, recommendations were formulated to aid practicing rheumatologists in their decision‐making without being overly restrictive, while allowing individualized decision‐making for each patient. These should take into account patient's disease status, ongoing treatment, risk profiles, preferences and local community transmission risk.
Our consensus recommendations for COVID‐19 vaccinations in PRD were developed employing a systematic literature review and Delphi method. The process of recommendation development incorporated all components of the Appraisal of Guidelines for Research & Evaluation (AGREE) instrument, 42 other than patient/ allied health involvement, for practicality. The AGREE framework was developed to ensure the rigor of guideline formulations which are feasible for clinical practice. The only other consensus recommendations developed using a standardized Delphi method for COVID‐19 vaccination in PRD were recently announced in a press release by the ACR. 35 Importantly, the broad principles for COVID‐19 vaccination in PRD in our recommendations are similar to what the ACR has outlined, in spite of the vastly different pandemic situations (and therefore the balance of risk / benefit of the vaccine) in Asia vs North America. Vaccination is strongly encouraged, may be given while on immunomodulatory therapy, preferably during quiescent disease, and without the need for testing for post‐vaccination antibody titers. The ACR recommended that COVID‐19 vaccination should be timed according to the dosing of certain immunomodulatory treatments (rituximab, intravenous abatacept and intravenous cyclophosphamide) and that treatment with methotrexate, Janus kinase inhibitors and abatacept should be temporarily interrupted prior to or after COVID‐19 vaccination. However, as discussed, while there may be reduced vaccine immunogenicity in patients on these medications, sufficient protective efficacy has been demonstrated, 22 , 26 , 30 thus forming the basis of our recommendation to vaccinate without treatment interruption or consideration for timing of doses.
As of the latest WHO update on March 5 2021, 79 candidate vaccines are in clinical development, with a further 182 in pre‐clinical development. 43 Since the rollout of vaccination campaigns in various regions in mid‐December 2020 up to March 9 2021, more than 312 million vaccine doses have been administered worldwide 44 and our collective experience with the new vaccines continues to evolve. It is important that governing institutions and healthcare providers continue to keep abreast of the latest evidence, so that recommendations can be reviewed and/or revised as new knowledge emerges. Particularly, data on safety and efficacy of vaccination in PRD are urgently needed to update recommendations in this vulnerable population.
Santosa A, Xu C, Arkachaisri T, et al. Recommendations for COVID‐19 vaccination in people with rheumatic disease: Developed by the Singapore Chapter of Rheumatologists. Int J Rheum Dis. 2021;24:746–757. 10.1111/1756-185X.14107
REFERENCES
- 1. Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID‐19 in patients with autoimmune diseases: a systematic review and meta‐analysis. Ann Rheum Dis. 2020;80:384‐391. [DOI] [PubMed] [Google Scholar]
- 2. D'Silva KM, Jorge A, Cohen A, et al. COVID‐19 outcomes in patients with Systemic Autoimmune Rheumatic Diseases (SARDs) compared to the general population: A US multi‐center comparative cohort study. Arthritis Rheumatol. 2020. 10.1002/art.41619. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu C, Yi Z, Cai R, Chen R, Thong BY, Mu R. Clinical outcomes of COVID‐19 in patients with rheumatic diseases: a systematic review and meta‐analysis of global data. Autoimmun Rev. 2021. 10.1016/j.autrev.2021.102778. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383(27):2603‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. FDA Briefing Document Janssen Ad26 . COV2.S vaccine for the prevention of COVID‐19. 2021. https://www.fda.gov/media/146217/download. Accessed on March 9, 2021.
- 7. Reccommendations on Singapore's COVID‐19 vaccination strategy by the expert committee on COVID‐19 vaccination. 2021. https://www.moh.gov.sg/docs/librariesprovider5/pressroom/press‐releases/annex‐b‐ec19v‐27‐dec.pdf. Accessed on March 9, 2021.
- 8. HSA Grants Interim Authorisation for Moderna COVID‐19 vaccine in Singapore. 2021. https://www.hsa.gov.sg/announcements/press‐release/hsa‐grants‐interim‐authorisation‐for‐moderna‐covid‐19‐vaccine‐in‐singapore. Accessed on March 9, 2021.
- 9. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Novavax COVID‐19 vaccine demonstrates 89.3% efficacy in UK phase 3 trial. 2021. https://ir.novavax.com/node/15506/pdf. Accessed on March 9, 2021.
- 12. Bharat biotech announces phase 3 results of COVAXIN®: India’s first COVID‐19 vaccine demonstrates interim clinical efficacy of 81%. 2021. https://www.bharatbiotech.com/images/press/covaxin‐phase3‐efficacy‐results.pdf. Accessed on March 9, 2021.
- 13. van der Heijde D, Aletaha D, Carmona L, et al. 2014 Update of the EULAR standardised operating procedures for EULAR‐endorsed recommendations. Ann Rheum Dis. 2015;74(1):8‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American College of Rheumatology . Policy and Procedure Manual for Clinical Practice Guidelines. Atlanta, Georgia: American College of Rheumatology; 2015. [Google Scholar]
- 15. D'Silva K, Jorge A, Lu N, Zhang Y, Wallace Z, Choi H. Outcomes of coronavirus disease 2019 infection among patients living with rheumatic diseases: a matched cohort study from a US multi‐center research network [abstract]. Arthritis Rheumatol. 2020;72(Suppl 10). https://acrabstracts.org/abstract/outcomes‐of‐coronavirus‐disease‐2019‐infection‐among‐patients‐living‐with‐rheumatic‐diseases‐a‐matched‐cohort‐study‐from‐a‐us‐multi‐center‐research‐network/. Accessed March 5, 2021. [Google Scholar]
- 16. Jiménez‐Dalmaroni MJ, Gerswhin ME, Adamopoulos IE. The critical role of toll‐like receptors — from microbial recognition to autoimmunity: a comprehensive review. Autoimmun Rev. 2016;15(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll‐like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23(2):165‐175. [DOI] [PubMed] [Google Scholar]
- 18. Koski GK, Kariko K, Xu S, Weissman D, Cohen PA, Czerniecki BJ. Cutting edge: innate immune system discriminates between RNA containing bacterial versus eukaryotic structural features that prime for high‐level IL‐12 secretion by dendritic cells. J Immunol. 2004;172(7):3989‐3993. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS‐CoV‐2 vaccine in healthy adults aged 18–59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clinical study protocol of a phase 3, randomised, observer‐blinded, placebo‐controlled trial to evaluate the efficacy and safety of a SARS‐CoV‐2 recombinant spike protein nanoparticle vaccine (SARS‐CoV‐2 rS) with matrix‐M1 TM adjuvant in adult participants 18‐84 years of age in the United Kingdom. 2021. https://www.novavax.com/sites/default/files/2020‐11/2019nCoV302Phase3UKVersion2FinalCleanRedacted.pdf. Accessed on March 9, 2021.
- 21. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2016;68(1):1‐25. [DOI] [PubMed] [Google Scholar]
- 22. Seo YB, Moon SJ, Jeon CH, et al. The practice guideline for vaccinating Korean patients with autoimmune inflammatory rheumatic disease. Infect Chemother. 2020;52(2):252‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):e44‐100. [DOI] [PubMed] [Google Scholar]
- 24. Papp KA, Haraoui B, Kumar D, et al. Vaccination guidelines for patients with immune‐mediated disorders taking immunosuppressive therapies: executive summary. J Rheumatol. 2019;46(7):751‐754. [DOI] [PubMed] [Google Scholar]
- 25. Keeling SO, Alabdurubalnabi Z, Avina‐Zubieta A, et al. Canadian rheumatology association recommendations for the assessment and monitoring of systemic lupus erythematosus. J Rheumatol. 2018;45(10):1426‐1439. [DOI] [PubMed] [Google Scholar]
- 26. Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology. 2019;58(2):372. [DOI] [PubMed] [Google Scholar]
- 27. Heijstek MW, Ott de Bruin LM, Bijl M, et al. EULAR recommendations for vaccination in paediatric patients with rheumatic diseases. Ann Rheum Dis. 2011;70(10):1704‐1712. [DOI] [PubMed] [Google Scholar]
- 28. Guerrini G, Franzetti F, Giacomelli R, et al. Italian recommendations for influenza and pneumococcal vaccination in adult patients with autoimmune rheumatic diseases. Clin Exp Rheumatol. 2020;38(2):245‐256. [PubMed] [Google Scholar]
- 29. Furer V, Rondaan C, Heijstek MW, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79(1):39‐52. [DOI] [PubMed] [Google Scholar]
- 30. Buhler S, Eperon G, Ribi C, et al. Vaccination recommendations for adult patients with autoimmune inflammatory rheumatic diseases. Swiss Med Wkly. 2015;145:w14159. [DOI] [PubMed] [Google Scholar]
- 31. Centers for Disease Control and Prevention . Use of 13‐Valent Pneumococcal Conjugate Vaccine and 23‐Valent Pneumococcal Conjugate Vaccine for Adults with Immunocompromising Conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2012;61:816‐819. [PubMed] [Google Scholar]
- 32. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tarawneh O, Tarawneh H. Immune thrombocytopenia in a 22‐year‐old post Covid‐19 vaccine. Am J Hematol. 2021. 10.1002/ajh.26106. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Principles for COVID‐19 vaccination in musculoskeletal and rheumatology for clinicians. 2021. http://arma.uk.net/covid‐19‐vaccination‐and‐msk/. Accessed on March 9, 2021.
- 35. American College of Rheumatology. COVID‐19 vaccine clinical guidance summary for patients with rheumatic and musculoskeletal diseases. 2021. https://www.rheumatology.org/Portals/0/Files/COVID‐19‐Vaccine‐Clinical‐Guidance‐Rheumatic‐Diseases‐Summary.pdf. Accessed on March 9, 2021.
- 36. Centres for Disease Control and Prevention . Interim clinical considerations for use of COVID‐19 vaccines currently authorized in the United States. 2021. https://www.cdc.gov/vaccines/covid‐19/info‐by‐product/clinical‐considerations.html. Accessed on March 9, 2021.
- 37. Campos LM, Silva CA, Aikawa NE, et al. High disease activity: an independent factor for reduced immunogenicity of the pandemic influenza a vaccine in patients with juvenile systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2013;65(7):1121‐1127. [DOI] [PubMed] [Google Scholar]
- 38. Arad U, Tzadok S, Amir S, et al. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine. 2011;29(8):1643‐1648. [DOI] [PubMed] [Google Scholar]
- 39. Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS‐CoV‐2 infection. Lancet Infect Dis. 2020;20(4):410‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qian G, Yang N, Ma AHY, et al. COVID‐19 transmission within a family cluster by presymptomatic carriers in China. Clin Infect Dis. 2020;71(15):861‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mizumoto K, Chowell G. Transmission potential of the novel coronavirus (COVID‐19) onboard the diamond Princess Cruises Ship, 2020. Infect Dis Model. 2020;5:264‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Collaboration A. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. 2003;12(1):18‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. World Health Organization: draft landscape and tracker of COVID‐19 candidate vaccines. 2021. https://www.who.int/publications/m/item/draft‐landscape‐of‐covid‐19‐candidate‐vaccines. Accessed on March 9, 2021.
- 44. Our world in data. 2021. https://ourworldindata.org/grapher/cumulative‐covid‐vaccinations?tab=chart&stackMode=absolute&time=earliest..latest®ion=World. Accessed on March 9, 2021.
- 45. Oxford centre for evidence‐based medicine: levels of evidence (March 2009). https://www.cebm.ox.ac.uk/resources/levels‐of‐evidence/oxford‐centre‐for‐evidence‐based‐medicine‐levels‐of‐evidence‐march‐2009. Accessed on March 9, 2021.


