Short abstract
With every emergent pathogen, an immediate concern to blood collection organizations (BCOs) is to determine if the new agent represents a risk to the safety of the blood supply. If suspected, prompt efforts are introduced to prevent the spread of infection to blood transfusion recipients. This is done initially by setting donor eligibility criteria, modifying donor educational materials, introducing new questions to the medical history questionnaire, encouraging donors to report post‐donation illness, and finally by implementing blood donor screening tests if warranted along with lookback procedures. When severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) first emerged in late 2019/early 2020, it was believed based on our experiences with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) that there was no risk to the blood supply and that SARS‐CoV‐2 would only minimally impact BCOs and transfusion services. We soon realized such wishful thinking was far from the truth. Although coronavirus disease 2019 (COVID‐19) presents primarily as a lower respiratory tract infection transmitted via air droplets, increasing evidence points to multiorgan involvement in infected patients. This systemic involvement is postulated to be mainly related to SARS‐CoV‐2 binding to angiotensin‐converting enzyme 2 (ACE2) receptors located on several different human cells. 1 Consequently, hematology services 2 , 3 had to manage patients with COVID‐19‐associated immune thrombocytopenia, 4 hyperviscosity, 5 and coagulopathy. 6 , 7 , 8 With the arrival of the first COVID‐19 case into Washington State on January 21, 2020, Pagano, et al. were first to give a glimpse into what became the norm at hospitals, transfusion services, and blood centers – that is, preparing to adapt to changes in the blood supply and transfusion support during the pandemic. 9 One aim of this piece is to demonstrate the resilience and readiness of BCOs facing the COVID‐19 pandemic.
The history of serum therapy or convalescent plasma therapy dates to the 1890s, when the German physician Emil von Behring intentionally exposed horses to the toxic bacteria that causes diphtheria. 10 After the animals had recovered, Behring used their antibody‐rich blood to successfully immunize humans against the deadly disease. For this work, Behring won the first Nobel Prize in Physiology or Medicine in 1901. 11 Afterwards, in the late 19th and early 20th century, convalescent serum or plasma was used to treat measles, Spanish influenza, and many other diseases. Interest in this therapy waned in the antibiotic era but remained on stand‐by for resurrection when an infectious agent emerged for which there was no match in our therapeutic armamentarium, e.g., influenza, SARS, MERS, etc. 12 COVID‐19 convalescent plasma (CCP) took center stage worldwide in the current pandemic due to our lack of directly effective antiviral medications or other therapies.
The SARS‐CoV‐2 pandemic forced significant changes to BCOs. Every mitigation measure to curb the spread of infection directly impacted BCO operations. Stay‐at‐home orders and school and business closures immediately constrained the size of the donor pool. Blood collection, processing, and distribution activities were modified to accommodate enhanced personal protective equipment, social distancing, sanitizing collection areas, and screening individuals for elevated temperature prior to entry to facilities. While aided by the substantial elimination of elective surgeries, the fewer donations collected were barely adequate to meet the decreasing demand.
Then came the need to collect convalescent plasma from patients who had recently recovered from COVID‐19 infections. Faced with a rapidly increasing demand, BCOs had limited time to establish a complex process: from recruiting patients/donors, to screening donors for eligibility, to collecting and processing plasma, to distributing CCP to transfusion services. All activities had to follow strict clinical trial criteria and regulatory requirements. This required developing new processes with all that entails, from creating computer controls, writing procedures, and training staff to establishing screening tests for SARS‐CoV‐2 antibodies to donor notifications. In addition, BCOs had to keep up with the frequent regulatory updates made before and after the emergency use authorization issued on August 23, 2020; some required almost immediate implementation (Figure 1).
FIGURE 1.
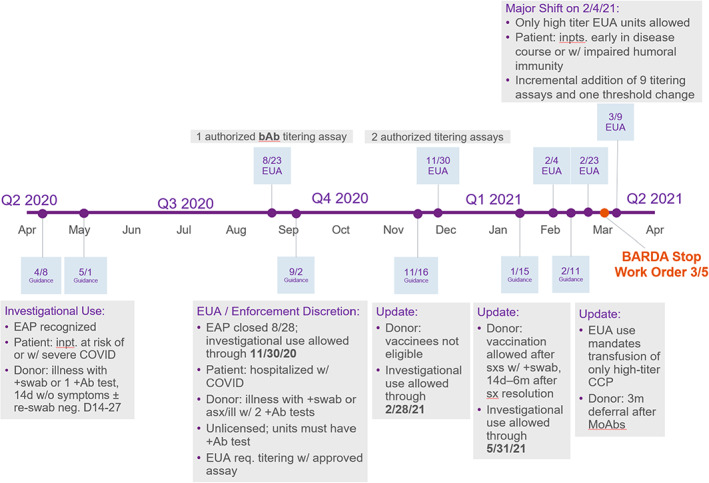
CCP regulatory timeline. Abbreviations: BARDA, The Biomedical Advanced Research and Development Authority; EAP, expanded access protocol; EUA, emergency use authorization; MoAbs, monoclonal antibodies; sx/asx, symptomatic/asymptomatic [Color figure can be viewed at wileyonlinelibrary.com]
In this issue of TRANSFUSION, Lasky et al., describe the American Red Cross's experience with establishing a CCP donor/donation program in response to information favoring the efficacy of this therapeutic modality in managing hospitalized patients with COVID‐19 infection. 13 The analysis covers an approximately 14‐week period. The study describes CCP donor characteristics; the majority (56.2%) were first‐time donors in contrast to standard plasma/platelet apheresis (SA) or whole blood (WB) donor populations. The proportion of female donors was 12 percentage points higher than in the SA donor group. Older (>65 years) and younger (16–19 years) individuals were comparatively underrepresented among CCP donors. Presenting CCP donations had deferral rates and Quantity Not Sufficient rates of 10.2 and 6.4%, respectively, compared to 8.2 and 1.1% for SA donations. HLA antibody reactivity comprised the most frequent cause of product loss for CCP donations versus SA donations (9.6 vs. 1.3%). Acute adverse events also occurred at a higher rate among both first‐time and repeat CCP donations compared to SA. The overall pattern of reaction rates between both first‐time and repeat CCP and SA donors were similar, with small hematomas and prefaint reactions being the most common reactions. Two points of interest may warrant additional exploration. First, the CCP donor racial profile was similar to that of SA and WB donors but differed from that of COVID‐19 patient populations. The second is the higher rate of reactions observed in both first‐time and repeat CCP donors compared to first‐time and repeat SA donors. A small previous study showed a higher frequency of mild adverse reactions in CCP donors with a per‐donation volume of >8 ml/kg body weight or ≥600 ml, <100 mm Hg in pre‐donation systolic blood pressure, or less than 28 days from the onset of COVID‐19 symptoms. There was no correlation with donation history, weight, sex, ABO blood type, pre‐donation diastolic blood pressure, pulse, or hemoglobin level. 14
This manuscript is a welcome addition to the many multifaceted manuscripts related to COVID‐19 already published in TRANSFUSION over the past year: from how to establish a program 15 , 16 to donor studies, 17 , 18 , 19 , 20 to transfusion optimization, 21 , 22 , 23 to CCP. 24 , 25
The collection and distribution of CCP ramped up steadily, peaking in December 2020 and January 2021, before starting to decline in February 2021 (Figure 2). Multiple studies and meta‐analyses of CCP have been performed that overall have failed to show significant benefits in patient outcomes. 26 , 27 If anything, the observation made by von Behring more than 130 years ago (see quote) remains valid. The earlier the administration of CCP, the better – in one randomized trial, early administration of high‐titer CCP (within 72 h after the onset of mild Covid‐19 symptoms) to mildly ill infected older adults reduced the progression of Covid‐19. 28 , 29 At present, the Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID‐19 recommendations state, “Among patients who have been admitted to the hospital with COVID‐19, the IDSA guideline panel recommends COVID‐19 convalescent plasma only in the context of a clinical trial“. 30 Are there lessons learned? 31 Recent convalescent plasma trials to treat Ebola, SARS, and MERS were criticized as being small, non‐randomized trials. A search for Covid‐19 convalescent plasma on clinicalTrials.gov returned 146 (49 in the United States) registered trials at various stages of completion. Will these trials provide the answer or will they create new questions?
FIGURE 2.
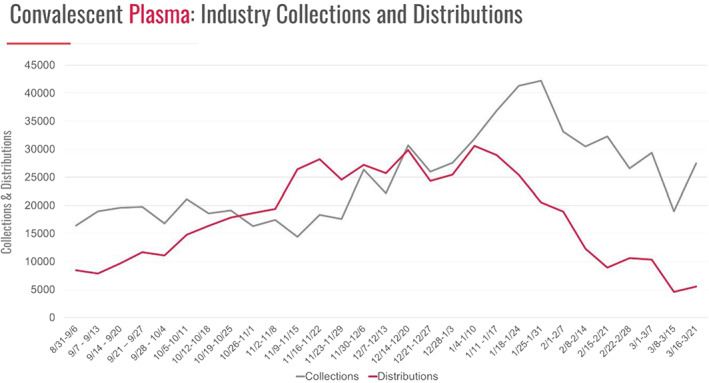
COVID convalescent plasma*. * Collection and distribution data reported from AABB, America's Blood Centers, American Red Cross, and Blood Centers of America. With permission, ABC Newsletter – March 26, 2021 [Color figure can be viewed at wileyonlinelibrary.com]
Before closing, it would be remiss not to mention the significant role BCOs across the globe are playing to further our understanding of the epidemiology and the immunologic response to SARS‐CoV‐2 by introducing various serological testing for SARS‐CoV‐2 antibodies. 32 , 33 , 34
What is next? Will the pandemic regress with the development of vaccines and the population reaching herd immunity? Will a new wave of the pandemic emerge with the spread of the ever‐increasing variants along with the relaxation of restrictions by various states and localities? Will there be a resurgence of CCP with neutralizing antibodies against variants resistant to current CCP inventory? 35 Will BCOs successfully retain most of the first‐time CCP donors as blood donors supporting the community blood supply post‐pandemic? Regardless, there are reasons to assure the public that BCOs will be standing ready to meet the challenges of the next crisis.
CONFLICT OF INTEREST
The author declares no conflict of interest.
ACKNOWLEDGMENT
The author thanks Dr. Ralph Vassallo for reviewing the editorial and contributing Figure 1 (The CCP Regulatory Timeline).
REFERENCES
- 1. Gavriatopoulou M, Korompoki E, Fotiou D, Ntanasis‐Stathopoulos I, Psaltopoulou T, Kastritis E, et al. Organ‐specific manifestations of COVID‐19 infection. Clin Exp Med. 2020;20:493–506. 10.1007/s10238-020-00648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sahu KK, Cerny J. A review on how to do hematology consults during COVID‐19 pandemic. Blood Rev. 2020;100777. Available online 8 November 2020. 10.1016/j.blre.2020.100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bell R, Zini G, d'Onofrio G, Rogers HJ, Lee YS, Frater JL. The hematology laboratory's response to the COVID‐19 pandemic: a scoping review. Int J Lab Hematol. 2021;43:148–59. 10.1111/ijlh.13381. [DOI] [PubMed] [Google Scholar]
- 4. Tariq Kewan T, Gunaratne TN, Mushtaq K, Alayan D, Daw H, Haddad A. Outcomes and management of immune thrombocytopenia secondary to COVID‐19: Cleveland clinic experience. Transfusion. 2021;1–5. 10.1111/trf.16368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Truong AD, Auld SC, Barker NA, Friend S, Wynn AT, Cobb J, et al. Therapeutic plasma exchange for COVID‐19‐associated hyperviscosity. Transfusion. 2020;1–6. 10.1111/trf.16218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iba T, Levy JH, Levi M, Thachil J. Coagulopathy in COVID‐19. J Thromb Haemost. 2020;18:2103–9. 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thachil J, Cushman M, Srivastava A. A proposal for staging COVID‐19 coagulopathy. Res Pract Thromb Haemost. 2020;4:731–6. 10.1002/rth2.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18:1023–6. 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pagano MB, Hess JR, Tsang HC, Staley E, Gernsheimer T, Sen N, et al. Prepare to adapt: blood supply and transfusion support during the first 2 weeks of the 2019 novel coronavirus (COVID‐19) pandemic affecting Washington State. Transfusion. 2020;60:908–11. 10.1111/trf.15789. [DOI] [PubMed] [Google Scholar]
- 10. Mock J. The peculiar 100‐plus‐year history of convalescent plasma. smithsonianmag.com. 2020. https://www.nobelprize.org/prizes/medicine/1901/behring/biographical/. Accessed 12 April 2021.
- 11. Emil von Behring Biographical . https://www.nobelprize.org/prizes/medicine/1901/behring/biographical/. Accessed 22 Mar 2021.
- 12. Sullivan HC, Roback JD. Convalescent plasma: therapeutic hope or hopeless strategy in the SARS‐CoV‐2 pandemic. Transfus Med Rev. 2020;34:145–50. 10.1016/j.tmrv.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lasky B, Meyer EG, Steele WR, Crowder LA, Young PP. COVID‐19 convalescent plasma donor characteristics, product disposition, and comparison with standard apheresis donors. Tranfusion. 2021; 1–8. 10.1111/trf.16286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He R, Lin H, Xie S, Lv Q, Kong Y, Li L, et al. Donor tolerability of convalescent plasma donation. J Clin Apher. 2021;1–8. 10.1002/jca.21882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blackall D, Wulff S, Roettger T, Jacobs L, Lacasse A, Patri M, et al. Rapid establishment of a COVID‐19 convalescent plasma program in a regional health care delivery network. Transfusion. 2020;60:2203–9. 10.1111/trf.16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Budhai A, Wu AA, Hall L, Strauss D, Paradiso S, Alberigo J, et al. How did we rapidly implement a convalescent plasma program? Transfusion. 2020;60:1348–55. 10.1111/trf.15910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vassallo RR, Bravo MD, Kamel H. Pandemic blood donor demographics – do changes impact blood safety? Transfusion. 2021;1–5. 10.1111/trf.16320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gammon RR, Prichard AB, Gannett MS, Yordanov B. The effect of COVID‐19 on blood donation habits. Transfusion. 2021;1–7. 10.1111/trf.16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Del Fante C, Franchini M, Baldanti F, Percivalle E, Glingani C, Marano G, et al. A retrospective study assessing the characteristics of COVID‐19 convalescent plasma donors and donations. Transfusion. 2021;61:830–8. 10.1111/trf.16208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang HE, Ostrosky‐Zeichner L, Katz J, Wanger A, Bai Y, Sridhar S, et al. Screening donors for COVID‐19 convalescent plasma. Transfusion. 2021;1–6. 10.1111/trf.16253. [DOI] [PubMed] [Google Scholar]
- 21. DeSimone RA, Costa VA, Kane K, Sepulveda JL, Ellsworth GB, Gulick RM, et al. Blood component utilization in COVID‐19 patients in New York City: transfusions do not follow the curve. Transfusion. 2021;61:692–8. 10.1111/trf.16202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Velázquez‐Kennedy K, Luna A, Sánchez‐Tornero A, Jiménez‐Chillón C, Jiménez‐Martín A, Vallés Carboneras A, et al. Transfusion support in COVID‐19 patients: impact on hospital blood component supply during the outbreak. Transfusion. 2021;61:361–7. 10.1111/trf.16171. [DOI] [PubMed] [Google Scholar]
- 23. Barriteau CM, Bochey P, Lindholm PF, Hartman K, Sumugod R, Ramsey G. Blood transfusion utilization in hospitalized COVID‐19 patients. Transfusion. 2020;60:1919–23. 10.1111/trf.15947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barone P, DeSimone RA. Convalescent plasma to treat coronavirus disease 2019 (COVID‐19): considerations for clinical trial design. Transfusion. 2020;60:1123–7. 10.1111/trf.15843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jungbauer C, Weseslindtner L, Weidner L, Gänsdorfer S, Farcet MR, Gschaider‐Reichhart E, et al. Characterization of 100 sequential SARS‐CoV‐2 convalescent plasma donations. Transfusion. 2021;61:12–6. 10.1111/trf.16119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Horby PW, Landray MJ, The RECOVERY Collaborative Group . Convalescent plasma in 2 patients admitted to hospital with COVID‐19 (RECOVERY): a randomised, controlled, open‐label, platform trial. medRxiv. 2021. 10.1101/2021.03.09.21252736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Janiaud P, Axfors C, Schmitt AM, Gloy V, Ebrahimi F, Hepprich M, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID‐19 a systematic review and meta‐analysis. JAMA. 2021;325(12):1185–95. 10.1001/jama.2021.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tobian AA, Shaz BH. Earlier the better: convalescent plasma. Blood. 2020;136(6):652–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Libster R, Marc GP, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high‐titer plasma therapy to prevent severe Covid‐19 in older adults. N Engl J Med. 2021;384:610–8. 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID‐19. Please check website for most updated version of these guidelines. www.idsociety.org/COVID19guidelines. Accessed 18 Mar 2021
- 31. Angus DC, Gordon AC, Bauchner H. Emerging lessons from COVID‐19 for the US clinical research enterprise. JAMA. 2021;325(12):1159–61. 10.1001/jama.2021.3284. [DOI] [PubMed] [Google Scholar]
- 32. Erikstrup C, Hother CE, Pedersen OBV, Mølbak K, Skov RL, Holm DK, et al. Estimation of SARS‐CoV‐2 infection fatality rate by realtime antibody screening of blood donors. Clin Infect Dis. 2021;72(2):249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Busch MP, Stone M. Serosurveillance for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) incidence using global blood donor populations. Clin Infect Dis. 2021;72(2):254–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vassallo RR, Bravo MD, Dumont LJ, Hazegh K, Kamel H. Seroprevalence of antibodies to SARS‐CoV‐2 in US blood donors. Medrxiv. 2020. 10.1101/2020.09.17.20195131. [DOI] [Google Scholar]
- 35. Garcia‐Beltran WF, Lam EC, Denis KS, Nitido AD, Garcia ZH, Hauser BM, et al. Multiple SARS‐CoV‐2 variants escape neutralization by vaccine‐induced humoral immunity. Cell. 2021. 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


