Key Points
Question
What are the benefits and adverse events associated with acute treatments for episodic migraine in adults?
Findings
In this systematic review and meta-analysis that included 15 systematic reviews and 115 randomized clinical trials of 28 803 participants with migraine headache, multiple acute interventions, including nonsteroidal anti-inflammatory drugs, triptans, calcitonin gene-related peptide receptor antagonists, 5-HT1F receptor agonist, dihydroergotamine, acetaminophen, and remote electrical neuromodulation, were associated with improvements in short-term pain outcomes, with moderate to high strength of evidence. The evidence for these end points regarding opioids and other interventions was low or insufficient.
Meaning
For the acute treatment of migraine, several established and newer therapies were associated with improvements in short-term pain outcomes, with varying strengths of evidence.
Abstract
Importance
Migraine is common and can be associated with significant morbidity, and several treatment options exist for acute therapy.
Objective
To evaluate the benefits and harms associated with acute treatments for episodic migraine in adults.
Data Sources
Multiple databases from database inception to February 24, 2021.
Study Selection
Randomized clinical trials and systematic reviews that assessed effectiveness or harms of acute therapy for migraine attacks.
Data Extraction and Synthesis
Independent reviewers selected studies and extracted data. Meta-analysis was performed with the DerSimonian-Laird random-effects model with Hartung-Knapp-Sidik-Jonkman variance correction or by using a fixed-effect model based on the Mantel-Haenszel method if the number of studies was small.
Main Outcomes and Measures
The main outcomes included pain freedom, pain relief, sustained pain freedom, sustained pain relief, and adverse events. The strength of evidence (SOE) was graded with the Agency for Healthcare Research and Quality Methods Guide for Effectiveness and Comparative Effectiveness Reviews.
Findings
Evidence on triptans and nonsteroidal anti-inflammatory drugs was summarized from 15 systematic reviews. For other interventions, 115 randomized clinical trials with 28 803 patients were included. Compared with placebo, triptans and nonsteroidal anti-inflammatory drugs used individually were significantly associated with reduced pain at 2 hours and 1 day (moderate to high SOE) and increased risk of mild and transient adverse events. Compared with placebo, calcitonin gene-related peptide receptor antagonists (low to high SOE), lasmiditan (5-HT1F receptor agonist; high SOE), dihydroergotamine (moderate to high SOE), ergotamine plus caffeine (moderate SOE), acetaminophen (moderate SOE), antiemetics (low SOE), butorphanol (low SOE), and tramadol in combination with acetaminophen (low SOE) were significantly associated with pain reduction and increase in mild adverse events. The findings for opioids were based on low or insufficient SOE. Several nonpharmacologic treatments were significantly associated with improved pain, including remote electrical neuromodulation (moderate SOE), transcranial magnetic stimulation (low SOE), external trigeminal nerve stimulation (low SOE), and noninvasive vagus nerve stimulation (moderate SOE). No significant difference in adverse events was found between nonpharmacologic treatments and sham.
Conclusions and Relevance
There are several acute treatments for migraine, with varying strength of supporting evidence. Use of triptans, nonsteroidal anti-inflammatory drugs, acetaminophen, dihydroergotamine, calcitonin gene-related peptide antagonists, lasmiditan, and some nonpharmacologic treatments was associated with improved pain and function. The evidence for many other interventions, including opioids, was limited.
This systematic review and meta-analysis assesses the benefits and harms associated with acute treatments for episodic migraine, including pharmacologic and nonpharmacologic therapies, with a focus on pain-related outcomes, function, and adverse events.
Introduction
The 2016 Global Burden of Disease Study noted that migraine affected approximately 14.4% of the worldwide population1 and was ranked the second overall cause for years lived with disability and the leading cause of years lived with disability in young women.2 It is best conceptualized as a chronic neurologic disease punctuated by attacks of headache and accompanying symptoms such as photophobia, phonophobia, nausea/vomiting, and aura.3
In addition to modification of lifestyle and environmental factors, migraine management includes acute therapies (ie, interventions taken as needed for symptom relief during a migraine attack) and preventive therapies (ie, interventions taken to reduce the frequency and severity of migraine attacks). The need for preventive therapies depends on the frequency and severity of the migraine attacks and thus may not be required for every patient with migraine. However, all patients with migraine should be offered acute therapies with the goal of providing rapid, effective, and reliable pain and symptom relief with minimal adverse effects.
Patients with migraine encounter various barriers to receiving appropriate acute therapy. Prescribing data have demonstrated a mismatch between what patients receive for acute treatment of migraine and the evidence to support those treatments. In weighted results of National Ambulatory Medical Care Surveys conducted from 2006 to 2013 that included 2860 visits, use of any narcotic as an abortive medication was reported by 15.2% of responders compared with use of high-quality abortive medications reported by 18.9%.4
In this systematic review, the benefits and harms associated with acute treatments for episodic migraine were assessed, including pharmacologic and nonpharmacologic therapies. This review focused on pain-related outcomes, such as pain relief and pain freedom, as well as function and adverse events.
Methods
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statements and was part of a large systematic review of acute treatments for episodic migraine funded by the Agency for Healthcare Research and Quality. The study protocol was developed with input from clinical and research experts and registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42020163262). A 6-member technical expert panel was established at the beginning of the study to help protocol development. Given numerous systematic reviews that summarized evidence supporting the use of triptans and nonsteroidal anti-inflammatory drugs (NSAIDs) for acute treatment of migraine, an overview of previously published systematic reviews approach (also called umbrella systematic review) was used to synthesize the evidence for these 2 classes of drugs. For all other treatments, new systematic reviews were performed. This study was deemed exempt by the Mayo Clinic Institutional Review Board because only study-level published data were collected.
Data Sources and Searches
EMBASE, Epub Ahead of Print, In-Process & Other Non-Indexed Citations, MEDLINE Daily, MEDLINE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, PsycINFO, and Scopus from database inception to February 24, 2021, were searched. Clinical trial registries, government databases and websites, conference proceedings, patient advocate group websites, and medical society websites were also searched. Reference mining of existing systematic reviews/meta-analyses, clinical trial registries, and relevant primary studies was conducted to identify additional literature. The technical expert panel helped identify relevant literature. In addition, a Supplemental Evidence and Data for Systematic Reviews (SEADS) portal was posted on the Agency for Healthcare Research and Quality website to collect additional study-specific information. The literature search strategy was developed and executed by a medical reference librarian and peer reviewed by a second librarian. The detailed search strategy is provided in eTable 1 in the Supplement. A Federal Register notice was posted for this review (https://www.federalregister.gov/documents/2020/01/15/2020-00488/supplemental-evidence-and-data-request-on-treatments-for-acute-episodic-migraine).
Study Selection
Eligible studies (1) included adult patients (≥18 years) with episodic migraine; (2) evaluated abortive pharmacologic therapy or noninvasive nonpharmacologic abortive therapy; (3) involved comparisons of the intervention with placebo, usual care, another pharmacologic therapy, noninvasive nonpharmacologic therapy, wait list, no treatment, or attention control, (4) reported short-term outcomes of interest (≤4 weeks after the end of treatments); and (5) were published in English. Because the definition of migraine has changed, the definition used in the original studies was accepted as long as it also fit the current International Classification of Headache Disorders, Third Edition criteria for episodic migraine (defined as the presence of headache 14 or fewer days per month in someone who has migraine).3 Systematic reviews of triptans and NSAIDs, as well as randomized clinical trials (RCTs) of the other treatments, were included. Invasive treatments (defined as surgically implanted), preventive treatments, in vitro studies, studies without original data, and single-group studies were excluded. Therapies in development, with terminated development, or unavailable in the United States were also excluded (eTable 2 in the Supplement).
Pairs of independent reviewers screened the titles and abstracts of all citations. Studies included by either reviewer were retrieved for full-text screening. Independent pairs of reviewers screened the full-text version of eligible studies. Conflicts between the reviewers were resolved by a third senior investigator. For systematic reviews of triptans and NSAIDs, when more than 1 systematic review was available per drug, the most recent one with the largest number of included studies was chosen.
Data Extraction and Quality Assessment
A standardized data extraction form was developed to extract study characteristics. Reviewers worked independently to extract study details. An additional reviewer reviewed data extraction and resolved conflicts. Authors were contacted for missing or incomplete information.
The risk of bias of the included RCTs was evaluated with the Cochrane Collaboration’s Risk of Bias 2 tool.5 For systematic reviews of triptans and NSAIDs, credibility of the review process was assessed,6 which included the comprehensiveness of the search and rigor of study selection procedures, using items from the AMSTAR tool.7
Outcome Measures
The primary outcomes were pain freedom, pain relief, sustained pain freedom, sustained pain relief, and adverse events. Additional outcomes included improved function, restored function, pain as reported with a pain scale, function as reported with a function scale, opioid overdose, and medication overuse headache. In the Supplement, eTable 3 lists the definitions of the pain and function outcomes, eTable 4 summarizes pain scales and function scales included in the analyses, and eTable 5 describes categorization of adverse events. For serious adverse events, the definitions in the original studies were used.
Data Synthesis and Analysis
All statistical analyses for RCTs involved analyzing participants according to their original allocation group. For crossover RCTs, outcomes before crossover were used in meta-analysis.8 Studies that randomized migraine attacks instead of patients were not meta-analyzed because correlations between attacks could not be controlled for.
Relative risk (RR) and risk difference (RD) for binary outcomes were extracted or estimated. For ordinal outcomes (pain scale and function scale), standardized mean difference was calculated and the direction of all measures was converted so that a higher score represented better outcome. For adverse events, rate ratio (ie, ratio of the incidence rate of events within a given time between the intervention and the comparison) was calculated. Meta-analyses were conducted according to length of follow-up after treatment (2 hours, 1 day, and 1 week). The DerSimonian-Laird random-effects model with Hartung-Knapp-Sidik-Jonkman variance correction was used to combine direct comparisons between treatments if the number of studies included in the analysis was larger than 3.9 The fixed-effect method based on the Mantel-Haenszel method was adopted when the number of studies was 3 or fewer. Heterogeneity between studies was evaluated with the I2 indicator. To further explore heterogeneity, prespecified subgroup analyses were conducted according to age, sex, race, body mass index, route of administration, dose, study setting, and prior response to treatment. Race was defined according to the methods used by the original studies. Analyses were also stratified by risk of bias (low, moderate, and high). Publication bias could not be quantitatively evaluated due to the small number of studies included in meta-analyses (n < 10). Two-sided P < .05 was deemed to be statistically significant.
Grading the Strength of Evidence
The strength of evidence (SOE) was graded following the Agency for Healthcare Research and Quality Methods Guide for Effectiveness and Comparative Effectiveness Reviews and categorized as “high,” “moderate,” “low,” or “insufficient evidence.”10 eTable 6 in the Supplement provides a description of this approach.
Results
The literature search identified 16 319 citations. An additional 185 citations were identified through reference mining, through gray literature search, and by the technical experts panel. Evidence on triptans and NSAIDs was summarized from 15 systematic reviews. For other interventions, 115 RCTs from 121 articles with 28 803 patients were included in the analyses (Figure 1). Details of the interventions reported in each study can be found in eTables 7 and 8 in the Supplement.
Figure 1. Selection of Trials for Inclusion in the Review and Meta-analysis.
NSAIDs indicates nonsteroidal anti-inflammatory drugs.
aOther sources include reference mining of the included studies, gray literature search, and the technical experts panel.
bThe purpose of using these systematic reviews and clinical trial registries was to identify relevant studies that may have been missed in the database search and in other sources. The studies found matched those that were already included, and no additional studies were identified through existing systematic reviews and clinical trial registries.
Pharmacologic Therapy
Evidence on triptans and NSAIDs was summarized from 15 existing systematic reviews. For other pharmacologic interventions, 97 RCTs with 27 052 patients were included. Forty-nine studies were conducted in the emergency department, 46 in the outpatient setting, 1 in urgent care, and 1 in an inpatient setting. The results of the meta-analyses on effectiveness are summarized in the Table11,12,13,14,15,16,17,18,19,20,21; Figures 2, 3, and 4; and eFigures 1-8 in the Supplement. Adverse events and subgroup analyses are summarized in eTables 9 and 10 in the Supplement, respectively.
Table. Effectiveness of Triptans and Nonsteroidal Anti-inflammatory Drugs (NSAIDs) From Previous Systematic Reviewsa.
| Comparison | Outcome | Time | Findings, RR (95% CI)a | Study design and sample size |
|---|---|---|---|---|
| Triptans | ||||
| Naratriptan, 2.5 mg (oral) vs placebo11 | Pain free | 2 h | 2.52 (1.78-3.57) | 6 RCTs; 2358 patients |
| 1 d | 2.58 (1.99-3.35) | |||
| Pain relief | 2 h | 1.81 (1.55-2.11) | ||
| 1 d | 2.11 (1.75-2.54) | |||
| Sustained pain relief | 1 d | 2.43 (2.11-2.80) | ||
| Zolmitriptan, 2.5 mg (oral and nasal spray) vs placebo12 | Pain relief | 2 h | 2.06 (1.91-2.22) | 11 RCTs; 4904 patients |
| Sustained pain free | 1 d | 3.51 (2.12-5.79) | 2 RCTs; 984 patients | |
| Sustained pain relief | 1 d | 2.92 (2.37-3.61) | 4 RCTs; 2059 patients | |
| Rizatriptan, 10 mg (oral) vs placebo13 | Pain relief | 2 h | 71% vs 38%; P < .001 | 7 RCTs; 3328 patients |
| Pain free | 2 h | 41% vs 10%; P < .001 | ||
| Sustained pain relief | 1 d | 37% vs 18%; P < .001 | ||
| Sustained pain free | 1 d | 25% vs 7%; P < .001 | ||
| Frovatriptan, 2.5 mg (oral) vs placebo14 | Pain free | 2 h | 3.70 (2.59-5.29) | 5 RCTs; 2866 patients |
| RD, 0.09 (0.07-0.10) | ||||
| 1 d | 2.67 (2.21-3.22) | |||
| RD, 0.18 (0.15-0.21) | ||||
| Pain relief | 2 h | 1.66 (1.47-1.88) | ||
| 1 d | 1.83 (1.66-2.00) | |||
| Almotriptan, 12.5 mg (oral) vs placebo15 | Pain relief | 2 h | 1.68 (1.42-1.98); I2 = 41.90% | 5 RCTs; 1429 patients |
| RD, 0.25 (0.19-0.31) | ||||
| Pain free | 2 h | 2.15 (1.64-2.80); I2 = 39.60% | 5 RCTs; 1590 patients | |
| RD, 0.19 (0.14-0.25) | ||||
| Sustained pain free | 1 d | 2.12 (1.64-2.75) | 5 RCTs | |
| RD, 0.14 (0.11-0.18) | ||||
| Sumatriptan, 100 mg (oral) vs placebo16 | Pain free | 2 h | 3.20 (2.84-3.62); I2 = 37.00% | 15 RCTs and comparative observational studies; 6571 patients |
| Pain relief | 2 h | 1.93 (1.82-2.04); I2 = 67.00% | 20 RCTs and comparative observational studies; 7811 patients | |
| Sustained pain free | 1 d | 2.81 (2.30-3.44); I2 = 31.00% | 5 RCTs and comparative observational studies, 2891 patients | |
| Sustained pain relief | 1 d | 2.12 (1.87-2.39); I2 = 0.00% | 5 RCTs and comparative observational studies; 4116 patients | |
| Improved function | 2 h | 1.87 (1.65-2.11); I2 = 0.00% | 6 RCTs and comparative observational studies; 1827 patients | |
| Sumatriptan, 6 mg (subcutaneous) vs placebo17 | Pain free | 2 h | 3.85 (3.32-4.46); I2 = 62.00% | 11 RCTs and comparative observational studies; 2522 patients |
| Pain relief | 2 h | 2.50 (2.29-2.73); I2 = 75.00% | 12 RCTs and comparative observational studies; 2738 patients | |
| Sustained pain free | 1 d | 2.18 (1.61-2.95) | 2 RCTs and comparative observational studies; 752 patients | |
| Restored function | 2 h | 3.40 (2.66-4.35) | 2 RCTs and comparative observational studies; 750 patients | |
| Improved function | 2 h | 3.21 (2.68-3.84) | 3 RCTs and comparative observational studies; 1328 patients | |
| NSAIDs | ||||
| Diclofenac, 50 mg (oral) vs placebo18 | Pain free | 2 h | 2.02 (1.57-2.61); I2 = 63.00% | 2 RCTs; 1477 patients |
| Pain relief | 2 h | 1.47 (1.31-1.65); I2 = 0.00% | 2 RCTs; 1477 patients | |
| Sustained pain free | 1 d | 2.25 (1.68-3.01); I2 = 45.00% | 2 RCTs; 1578 patients | |
| Restored function | 2 h | 2.36 (1.80-3.08); I2 = 0.00% | 2 RCTs; 873 patients | |
| Ibuprofen, 400 mg (oral) vs placebo19 | Pain free | 2 h | 1.91 (1.60-2.28); I2 = 81.00% | 6 RCTs; 2575 patients |
| Pain relief | 2 h | 2.17 (1.92-2.45); I2 = 92.00% | 7 RCTs; 1815 patients | |
| Sustained pain relief | 1 d | 2.17 (1.76-2.69); I2 = 75.00% | 4 RCTs; 879 patients | |
| Improved function | 2 h | 1.61 (1.38-1.89); I2 = 78.00% | 3 RCTs; 1114 patients | |
| Aspirin (oral) vs placebo20 | Pain free | 2 h | 2.08 (1.70-2.55); I2 = 0.00% | 6 RCTs; 2027 patients |
| Pain relief | 2 h | 1.64 (1.48-1.83); I2 = 0.00% | 6 RCTs; 2027 patients | |
| Sustained pain relief | 1 d | 1.63 (1.37-1.95); I2 = 0.00% | 3 RCTs; 1142 patients | |
| Triptans plus NSAIDs | ||||
| Sumatriptan (oral) plus naproxen (oral) vs placebo21 | Pain free | 2 h | 3.65 (2.97-4.49); I2 = 38.00% | 4 RCTs; 2596 patients |
| Pain relief | 2 h | 2.16 (1.95-2.39); I2 = 0.00% | 4 RCTs; 2596 patients | |
| Sustained pain free | 1 d | 3.43 (2.69-4.36); I2 = 0.00% | 4 RCTs; 2596 patients | |
| Sustained pain relief | 1 d | 2.61 (2.27-2.99); I2 = 0.00% | 4 RCTs; 2596 patients | |
| Improved function | 2 h | 3.36 (2.63-4.29); I2 = 0.00% | 3 RCTs; 1984 patients | |
Abbreviations: NSAID, nonsteroidal anti-inflammatory drug; RCT, randomized clinical trial; RD, risk difference; RR, relative risk.
Results extracted from existing systematic reviews with various details. Data are RR (95% CI) unless otherwise noted.
Figure 2. Findings of Meta-analysis of Calcitonin Gene-Related Peptide Receptor Antagonists on Pain and Function Measured as Binary Outcomes for Episodic Migraine in Adults.
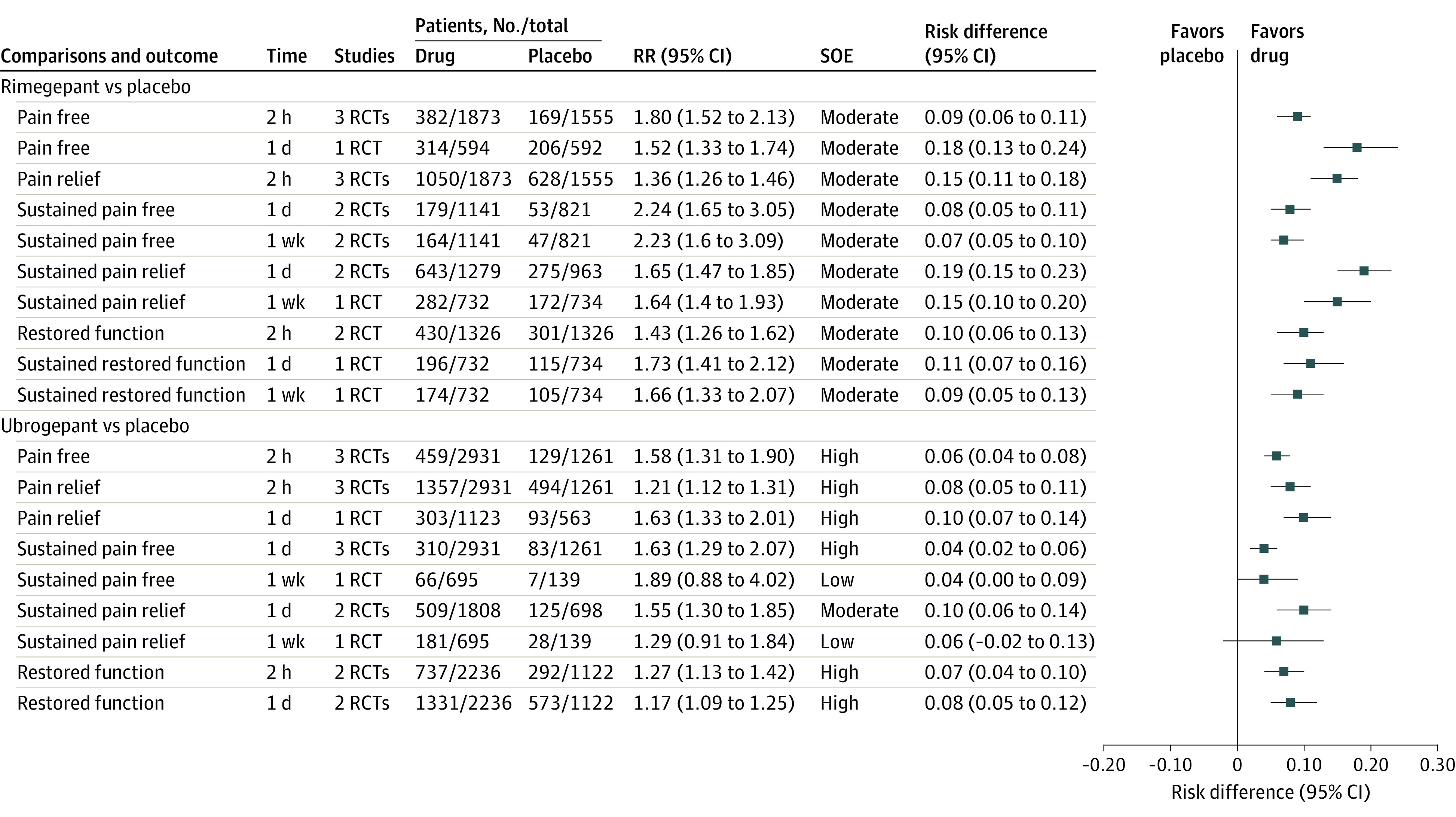
eTable 3 in the Supplement lists definitions of outcomes. eTable 6 in the Supplement lists definitions of strength of evidence (SOE) and approaches used to grade SOE. RCT indicates randomized clinical trial; RR, relative risk.
Figure 3. Findings of Meta-analysis of 5-HT1F Receptor Agonists on Pain and Function Measured as Binary Outcomes for Episodic Migraine in Adults.
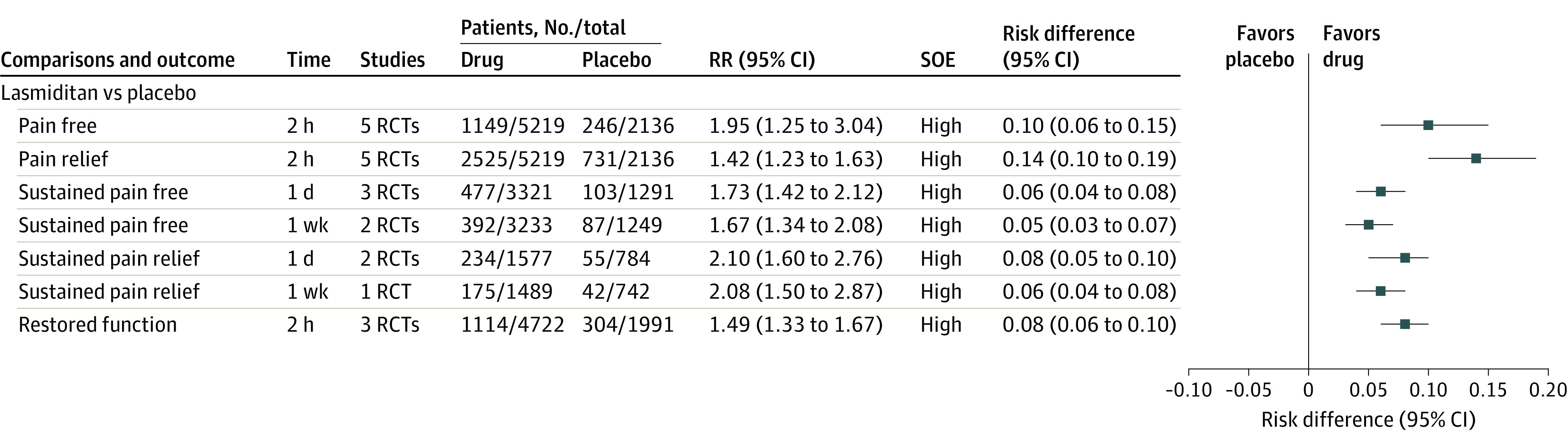
eTable 3 in the Supplement lists definitions of outcomes. eTable 6 in the Supplement lists definitions of strength of evidence (SOE) and approaches used to grade SOE. RCT indicates randomized clinical trial; RR, relative risk.
Figure 4. Findings of Meta-analysis of Antiemetics on Pain and Function Measured as Binary Outcomes for Episodic Migraine in Adults.
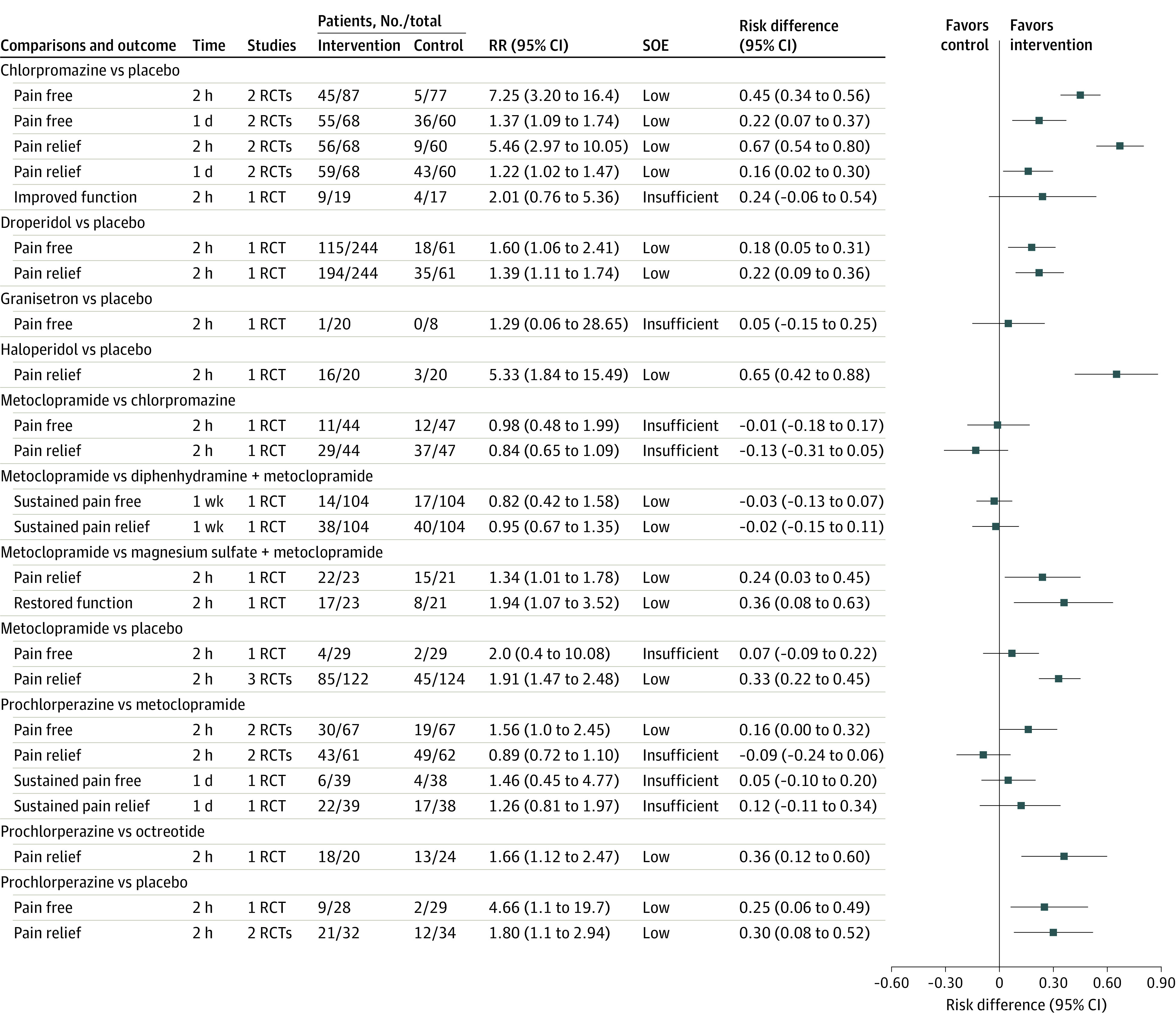
eTable 3 in the Supplement lists definitions of outcomes. eTable 6 in the Supplement lists definitions of strength of evidence (SOE) and approaches used to grade SOE. RCT indicates randomized clinical trial; RR, relative risk.
Triptans and NSAIDs
Numerous systematic reviews have been published evaluating triptans and NSAIDs (eTable 7 in the Supplement). Most of the systematic reviews were judged to have high credibility.6 The Table summarizes pain and function outcomes from 11 systematic reviews, when triptans and NSAIDs were compared with placebo.
According to 7 systematic reviews, triptans (various administration routes, including oral, subcutaneous, and intranasal) compared with placebo were significantly associated with increased pain freedom and pain relief at 2 hours and 1 day (high SOE), and increased risk of mild and transient adverse events (eg, malaise, nausea, chest pain, flushing sensation, palpitation, paresthesia).
According to 3 systematic reviews, NSAIDs (various administration routes, including oral, intravenous, and intramuscular) compared with placebo were significantly associated with increased pain freedom and pain relief at 2 hours and 1 day (moderate SOE), and increased risk of mild and transient adverse events (eg, dyspepsia, nausea, somnolence, dizziness).
According to 1 systematic review, the combination of sumatriptan (oral) and naproxen (oral) compared with placebo was significantly associated with improved pain freedom (high SOE) and pain relief (high SOE) at 2 hours, sustained pain freedom (high SOE) and sustained pain relief (high SOE) at 1 day, and improved function (high SOE) at 2 hours.
Calcitonin Gene-Related Peptide Receptor Antagonists (Gepants)
Six RCTs22,23,24,25,26,27 with 7620 patients evaluated calcitonin gene-related peptide receptor antagonists for the acute treatment of migraine. The overall risk of bias was low to moderate (2 RCTs with low risk; 2, moderate risk; and 2, high risk) (eTable 11 in the Supplement), without notable differences in findings based on risk of bias assessment (eTable 10.1 in the Supplement).
Compared with placebo, rimegepant (3 RCTs; oral and sublingual) and ubrogepant (3 RCTs; oral) were associated with significant improvement in pain freedom and pain relief at 2 hours (moderate to high SOE) and sustained pain freedom at 1 day and at 1 week (low to high SOE). Ubrogepant was associated with significantly more adverse events related to ear, nose, and throat symptoms. The results of the meta-analyses of the pain and function outcomes are summarized in Figure 2.
5-HT1F Receptor Agonists (Ditans)
Six RCTs from 7 articles with 8988 patients evaluated lasmiditan for the acute treatment of migraine.28,29,30,31,32,33,34 The overall risk of bias was low to moderate (3 RCTs with low risk; 1, moderate risk; and 2, high risk) (eTable 11 in the Supplement). Studies with different risk of bias demonstrated consistent findings (eTable 10.4 in the Supplement).
Compared with placebo, lasmiditan (oral and intravenous) was associated with significant improvement in pain freedom at 2 hours (5 RCTs; high SOE) and pain relief at 2 hours (5 RCTs; high SOE). It was also associated with significant improvement in sustained pain freedom at 1 day (3 RCTs; high SOE) and 1 week (2 RCTs; high SOE). Lasmiditan was associated with significantly increased risk of gastrointestinal adverse events, neurologic adverse events, serious adverse events, and total number of adverse events. The meta-analyses of pain and function outcomes, as well as adverse events, are summarized in Figure 3 and eFigure 1 and eTable 9.2 in the Supplement.
A subgroup analysis35 by prior response to triptans based on 2 RCTs31,32 demonstrated that, regardless of prior triptan response, lasmiditan was associated with significantly more pain freedom and pain relief at 2 hours compared with placebo (eTable 10.10 in the Supplement).
Antiemetics
Twenty-six RCTs36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61 with 2561 patients evaluated antiemetic medications. The overall risk of bias was moderate to high (4 RCTs with low risk; 8, moderate risk; and 14, high risk) (eTable 11 in the Supplement).
Compared with placebo, chlorpromazine (2 RCTs; intramuscular and intravenous) was associated with significantly more pain freedom and pain relief at 2 hours (low SOE) and 1 day (low SOE); prochlorperazine (2 RCTs; oral and rectal) and droperidol (1 RCT; intramuscular) individually were associated with significantly improved pain freedom (low SOE) and pain relief at 2 hours (low SOE); and metoclopramide (3 RCTs; intravenous) and haloperidol (1 RCT; intravenous) were each associated with improved pain relief at 2 hours (low SOE). Haloperidol, droperidol, and prochlorperazine significantly increased the risk of adverse events. Figure 4 and eFigure 2 and eTable 9.3 in the Supplement present the results of meta-analyses of pain and function outcomes and adverse events for the antiemetic drugs.
Ergot Alkaloids
Fifteen RCTs62,63,64,65,66,67,68,69,70,71,72,73,74,75,76 with 2535 patients were included in the analyses of ergot alkaloid medications. The overall risk of bias was high (2 RCTs with low risk; 2, moderate risk; and 11, high risk) (eTable 11 in the Supplement). No notable differences in findings between studies with low and moderate/high risk of bias (eTable 10.14 in the Supplement) were found.
Compared with placebo, dihydroergotamine (3 RCTs; intranasal) was associated with significantly more pain freedom and pain relief at 2 hours, 1 day, and 1 week (moderate to high SOE), sustained pain freedom and pain relief at 1 day and 1 week (high SOE), and gastrointestinal adverse events.
Compared with placebo, ergotamine plus caffeine (1 RCT; oral) was associated with significantly more pain relief at 2 hours (moderate SOE). The results of meta-analyses of pain and function outcomes and adverse events for the ergot alkaloid drugs are summarized in eFigures 3 and 4 and eTable 9.4, respectively, in the Supplement.
Opioids
Thirteen RCTs77,78,79,80,81,82,83,84,85,86,87,88,89 with 1847 patients were included in the analyses of opioid medications. The overall risk of bias was high (12 RCTs with high risk and 1 RCT with moderate risk) (eTable 11 in the Supplement).
Compared with placebo, tramadol in combination with acetaminophen (1 RCT; oral) was significantly associated with improved pain freedom at 2 hours (RR, 2.42 [95% CI, 1.34-4.35]; RD, 0.11 [95% CI, 0.04-0.17]; 1 RCT; 375 patients; low SOE) and 1 day (RR, 1.43 [95% CI, 1.09-1.88]; RD, 0.13 [95% CI, 0.03-0.23]; 1 RCT; 375 patients; low SOE), sustained pain freedom at 1 day (RR, 2.26 [95% CI, 1.15-4.46]; RD, 0.07 [95% CI, 0.02-0.13]; 1 RCT; 375 patients; low SOE), pain relief at 2 hours (RR, 1.68 [95% CI, 1.27-2.22]; RD, 0.18 [95% CI, 0.09-0.28]; 1 RCT; 375 patients; low SOE) and 1 day (RR, 1.75 [95% CI, 1.35-2.25]; RD, 0.23 [95% CI, 0.13-0.32]; 1 RCT; 375 patients; low SOE), and sustained pain relief at 1 day (RR, 1.56 [95% CI, 1.08-2.27]; RD, 0.11 [95% CI, 0.02-0.19]; 1 RCT; 375 patients; low SOE). Significantly more adverse events were reported in the group treated with tramadol and acetaminophen (rate ratio, 2.49; 95% CI, 1.48-4.18). Tramadol alone (1 RCT; intravenous) vs placebo failed to show a significant difference in pain freedom at 2 hours (RR, 2.50 [95% CI, 0.56-11.16]; RD, 0.18 [95% CI, −0.09 to 0.44]; 1 RCT; 34 patients; insufficient SOE) and pain relief at 2 hours (RR, 2.00 [95% CI, 0.98-4.08]; RD, 0.35 [95% CI, 0.04-0.67]; 1 RCT; 34 patients; insufficient SOE) or change in pain scale at 2 hours (standardized mean difference, 0.25; 95% CI, −0.43 to 0.92; 1 RCT; 34 patients; insufficient SOE).
Two RCTs compared butorphanol with placebo (intranasal). Butorphanol was associated with improvement over placebo regarding pain freedom at 2 hours (RR, 2.90 [95% CI, 1.20-7.01]; RD, 0.19 [95% CI, 0.07-0.31]; 1 RCT; 157 patients; low SOE) and 1 day (RR, 1.83 [95% CI, 1.10-3.05]; RD, 0.22 [95% CI, 0.06-0.37]; 1 RCT; 157 patients; low SOE), pain relief at 2 hours (RR, 3.37 [95% CI, 1.83-6.22]; RD, 0.43 [95% CI, 0.29-0.57]; 1 RCT; 157 patients; low SOE) and 1 day (RR, 2.07 [95% CI, 1.43-2.98]; RD, 0.41 [95% CI, 0.25-0.56]; 1 RCT; 157 patients; low SOE). Butorphanol was also associated with significantly increased total number of adverse events (rate ratio, 6.08; 95% CI, 4.19-8.82; I2 = 94.00%).
Meperidine (5 RCTs; intramuscular), morphine (1 RCT; intravenous), and hydromorphone (1 RCT; intravenous) failed to show superiority over various comparators regarding pain and function outcomes (eFigures 4 and 5 in the Supplement). There were increased numbers of adverse events associated with these medications (eTable 9.5 in the Supplement).
No study reported opioid overdose or opioid-specific adverse events, such as opioid misuse, opioid use disorder, or overdose.
Other Pharmacologic Interventions
eTable 9.6, eTables 10.16 to 10.19, and eFigures 7 and 8 in the Supplement list the findings for other pharmacologic therapies, including acetaminophen, dexamethasone, greater occipital nerve blocks, ketamine, lidocaine, magnesium sulfate, octreotide, propofol, secobarbital, and valproate.
Compared with placebo, acetaminophen was associated with significantly improved pain freedom at 2 hours (RR, 1.89 [95% CI, 1.24-2.86]; I2 = 0.00%; RD, 0.07 [95% CI, 0.03-0.12]; 2 RCTs; 729 patients; moderate SOE) and 1 day (RR, 1.78 [95% CI, 1.38-2.30]; I2 = 0.00%; RD, 0.15 [95% CI, 0.09-0.21]; 2 RCTs; 729 patients; moderate SOE) and pain relief at 2 hours (RR, 1.61 [95% CI, 1.33-1.95]; I2 = 0.00%; RD, 0.18 [95% CI, 0.11-0.25]; 2 RCTs; 729 patients; moderate SOE) and 1 day (RR, 1.71 [95% CI, 1.43-2.04]; I2 = 0.00%; RD, 0.22 [95% CI, 0.15-0.29]; 2 RCTs; 729 patients; moderate SOE). There was no significant difference on adverse events.
Nonpharmacologic Therapy
Eighteen RCTs in 19 articles with 1751 patients evaluated nonpharmacologic therapies.90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108 Five were conducted in the emergency department setting91,94,97,101,107 and 13 (in 14 articles) in the outpatient setting.90,92,93,95,96,98,100,102,103,104,105,106,108 The overall risk of bias was moderate (3 RCTs with low risk; 7, moderate risk; and 8, high risk) (eTable 11 in the Supplement). Nonpharmacologic studies with low risk of bias reported similar findings compared with studies with moderate or high risk of bias. The results of meta-analyses of pain and function outcomes, adverse events, and subgroup analyses are summarized in eTable 9.7 and eTable 10.20, and in eFigures 9 and 10 in the Supplement.
Compared with sham, remote electrical neuromodulation was significantly associated with improved pain freedom (RR, 1.95 [95% CI, 1.19-3.19]; RD, 0.14 [95% CI, 0.04-0.24]; 1 RCT; 252 patients; moderate SOE) and pain relief at 2 hours (RR, 1.65 [95% CI, 1.22-2.24]; RD, 0.21 [95% CI, 0.09-0.33]; 1 RCT; 252 patients; moderate SOE), and improved sustained pain freedom (RR, 2.57 [95% CI, 1.11-5.94]; RD, 0.09 [95% CI, 0.01-0.16]; 1 RCT; 252 patients; moderate SOE) and sustained pain relief at 1 week (RR, 2.27 [95% CI, 1.30-3.95]; RD, 0.15 [95% CI, 0.05-0.25]; 1 RCT; 252 patients; moderate SOE).
Compared with sham, external trigeminal nerve stimulation was significantly associated with improved ratings on the pain scale at 2 hours (standardized mean difference, 1.25; 95% CI, 0.90-1.60; I2 = 98.65%; 2 RCTs; 189 patients; low SOE) and 1 day (standardized mean difference, 0.53; 95% CI, 0.14-0.92; 1 RCT; 106 patients; moderate SOE). The RCT with low risk of bias reported notably smaller improvement on the pain scale at 2 hours than the RCT with high risk of bias.
Compared with sham, transcranial magnetic stimulation was significantly associated with more pain freedom at 2 hours (RR, 1.73 [95% CI, 1.04-2.86]; RD, 0.13 [95% CI, 0.01-0.25]; 1 RCT; 201 patients; low SOE). There was no significant difference in adverse events or other outcomes. No serious adverse events were reported in either group.
Noninvasive vagus nerve stimulation compared with sham was significantly associated with more pain relief at 2 hours (RR, 1.49 [95% CI, 1.04-2.13]; RD, 0.13 [95% CI, 0.02-0.25]; 1 RCT; 248 patients; moderate SOE). There was no significant difference in adverse events.
Discussion
The findings of this systematic review and meta-analysis demonstrated that several acute treatments for migraine were associated with improvements in pain and function and also with increased risk of adverse effects, with varying strengths of evidence to support their use. In particular, use of triptans, NSAIDs, acetaminophen, dihydroergotamine, calcitonin gene-related peptide antagonists, lasmiditan, and remote electrical neuromodulation was associated with improved pain and function with relatively robust SOE (eTable 12 in the Supplement). In contrast, the evidence for many other interventions, including opioids, was limited.
Choosing an acute treatment for migraine attacks requires an individualized approach for each patient; a number of factors must be considered such as patient characteristics (including age, comorbidities, and affordability/insurance coverage), migraine attack characteristics (such as severity, speed of onset, and presence of nausea/vomiting), and reported effectiveness and harms associated with available interventions. All these factors should be considered in a shared decision-making approach.
In 2015, the American Headache Society recommended either a triptan or an NSAID for migraine attacks, or acetaminophen for nonincapacitating attacks.109 This systematic review found high and moderate SOE in support of triptans and NSAIDs, respectively, and these drug classes should remain as the primary choice for the acute treatment of migraine in patients who do not have contraindications.109 The combination of triptans and NSAIDs was also effective and well tolerated, and can be used for patients with partial response to either agent. However, triptans and ergot alkaloids should be avoided in individuals who have a history of myocardial infarction, stroke, or multiple vascular risk factors because they are considered vasoactive.110,111,112 Additionally, NSAID use may be limited if patients have certain gastrointestinal, kidney, or cardiac comorbidities. A notable feature of the newest Food and Drug Administration–approved treatments, lasmiditan and calcitonin gene-related peptide receptor antagonists, is that, by their mechanisms of action, they are deemed to be nonvasoconstrictive.113 These drugs had moderate to high strength of supporting evidence, and can be considered for individuals who have not found triptans and NSAIDs to be effective or tolerated, or have contraindications to their use. Ubrogepant and rimegepant should not be used in patients who are receiving strong or moderate cytochrome P450 3A4 inducers or inhibitors.114 Given its central mechanism of action and risks of somnolence and dizziness, lasmiditan carries an 8-hour driving restriction postdose.115 Treatment guidelines will need to be updated to determine the place of lasmiditan and calcitonin gene-related peptide receptor antagonists among established therapies, given these unique features and adverse effect profiles, especially in people with vascular comorbidities.
The choice between pharmacologic and nonpharmacologic acute treatments of migraine depends on patient preference (ie, not wanting to receive a medication), tolerability, and contraindications in various patient populations. Compared with sham, several nonpharmacologic treatments may improve various measures of pain but had low to moderate SOE. Several noninvasive neuromodulation devices have gained Food and Drug Administration clearance for the acute treatment of migraine, such as remote electrical neuromodulation, transcranial magnetic stimulation, external trigeminal nerve stimulation, and noninvasive vagus nerve stimulation. When using remote electrical neuromodulation, individuals wear a wireless, battery-operated device on the lateral aspect of the upper arm that stimulates small skin nerves to induce conditioned pain modulation, an innate mechanism to inhibit perception of pain in other remote body areas.116 With transcranial magnetic stimulation, a handheld magnetic device is held against the occiput; when the tool is discharged, a brief magnetic pulse interrupts the pattern of neuronal firing that is believed to be associated with migraine. External trigeminal nerve stimulation is another noninvasive neuromodulation option that is applied to the forehead to stimulate the supraorbital nerves.91 Vagus nerve stimulation is a noninvasive device applied over the vagus nerve on either side of the neck. Although there is a role for these nonpharmacologic treatments, given their potential better tolerability, cost and lack of insurance coverage are barriers for many individuals. Patients who have access to these devices can use them at any stage of migraine treatment, including as initial therapy, adjunctive therapy, or options to consider when other acute medications have not been helpful or tolerated, or are contraindicated.
Current guidelines recommend against the use of opioids and butalbital-containing medications for acute treatment of migraine.116 Despite this, opioids are frequently prescribed for the acute treatment of migraine in varied clinical settings and patient populations.117,118,119,120,121,122,123 This review found the overall SOE to be low or insufficient for opioids, and opioids were associated with higher rates of adverse effects compared with other treatment options or placebo. As such, the current guideline recommendations against opioids stand.
The adverse events captured in this review are those experienced from the immediate exposure. Harms with frequent or long-term use of medications may relate to end-organ damage (eg, nephrotoxicity and cardiotoxicity with NSAIDs, hepatotoxicity with acetaminophen, ergotism or peritoneal fibrosis with ergot alkaloids), as well as secondary conditions that may develop in the setting of consuming medications (eg, medication overuse headache, opioid use disorder, overdose). Medication overuse headache is defined according to headache frequency (15 or more days per month for greater than 3 months) and days of use of specific medications per month.3 Opioids and butalbital-containing medications have a 2-fold higher risk of medication overuse headache development compared with simple analgesics and triptans.124 Concerns about risk of opioid gastrointestinal-related adverse events, as well as addiction and drug abuse secondary to treatment of migraine, have also been raised.121,125,126 No included studies in the systematic review evaluated risk mitigation strategies or instruments to predict the risk of abuse or dependency in patients treated with opioids for a migraine attack, which has implications for implementation of treatment algorithms that include opioids.
Limitations
This study has several limitations. First, many of the RCTs compared treatments against placebo, limiting comparative effectiveness inferences. Head-to-head trials of active therapies and trials of combinations of therapies are needed to support shared decision making among all of the available treatment options. Second, nonpain symptoms of migraine, such as nausea, photophobia, and phonophobia, can be as bothersome as or more bothersome than pain, and this can vary, depending on the patient. Recent migraine trials are therefore evaluating the most bothersome symptom as an end point. Third, many clinical trials excluded certain populations from enrollment, including older individuals; those with hemiplegic migraine, comorbid second headache conditions, or other primary headache disorders; and those with significant mental health or vascular diagnoses. Consequently, lack of efficacy and safety data in these populations was a limitation. Fourth, trials included various clinical settings (emergency department, outpatient, inpatient, and urgent care), which may indicate a differing degree of migraine attack severity or refractoriness, depending on presenting location. Fifth, although statistical heterogeneity was minor in most analyses (I2 < 30%), some heterogeneity that relates to patients’ characteristics and study settings did exist, and some important subgroup analyses (eg, based on migraine attack characteristics, comorbidities) to evaluate heterogeneity could not be conducted.
Conclusions
There are several acute treatments for migraine, with varying degrees of supporting evidence. Use of triptans, NSAIDs, acetaminophen, dihydroergotamine, calcitonin gene-related peptide antagonists, lasmiditan, and some nonpharmacologic treatments was associated with improved pain and function. The evidence for many other interventions, including opioids, was limited.
eTable 1. Search strategy
eTable 2. List of the excluded interventions
eTable 3. Definition of pain and function outcomes
eTable 4. Pain and function scales included in the anaysis
eTable 5. Categories of adverse events
eTable 6. Definition and approaches to grade strength of evidence
eTable 7. Results of systematic reviews evaluating triptans and nonsteroidal anti-inflammatory drugs (NSAIDs)
eTable 8. Characteristics of included studies evaluating CGRP, 5-HT1F, antiemetics, ergot alkaloids, opioids, other pharmacological interventions, and nonpharmacological interventions
eTable 9. Adverse events
eTable10. Subgroup analysis
eTable 11. Risk of bias (Cochrane Risk of Bias tool for randomized trials [RoB 2.0]) for randomized clinical trials
eTable 12. Effectiveness of treatments other than triptans and nonsteroidal anti-inflammatory drugs
eFigure 1. Findings of Meta-analysis of 5-HT1F Receptor Agonists on Pain and Function Outcomes measured as Continuous Outcomes for Episodic Migraine in Adults
eFigure 2. Findings of Meta-analysis of Antiemetics on Pain Outcomes measured as Continuous Outcomes for Episodic Migraine in Adults
eFigure 3. Findings of Meta-analysis of Ergot Alkaloids on Pain and Function Outcomes measured as Binary Outcomes for Episodic Migraine in Adults
eFigure 4. Findings of Meta-analysis of Ergot Alkaloids on Pain Outcomes measured as Continuous Outcomes for Episodic Migraine in Adults
eFigure 5. Findings of Meta-analysis of Opioids on Pain and Function Outcomes measured as Binary Outcomes for Episodic Migraine in Adults
eFigure 6. Findings of Meta-analysis of Opioids on Pain Outcomes measured as Continuous Outcomes for Episodic Migraine in Adults
eFigure 7. Findings of Meta-analysis of Other Pharmacological Interventions on Pain and Function Outcomes measured as Binary Outcomes for Episodic Migraine in Adults
eFigure 8. Findings of Meta-analysis of Other Pharmacological Interventions on Pain and Function Outcomes measured as Continuous Outcomes for Episodic Migraine in Adults
eFigure 9. Findings of Meta-analysis of Nonpharmacological Interventions on Pain Outcomes measured as Binary Outcomes for Episodic Migraine in Adults
eFigure 10. Findings of Meta-analysis of Nonpharmacological Interventions on Pain Outcomes measured as Continuous Outcomes for Episodic Migraine in Adults
References
References
- 1.GBD 2016 Headache Collaborators . Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954-976. doi: 10.1016/S1474-4422(18)30322-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Lim SS, Abbafati C, et al. . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204-1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Headache Classification Committee of the International Headache Society (IHS): the International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. doi: 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 4.Charleston IV L, Burke JF. Do racial/ethnic disparities exist in recommended migraine treatments in US ambulatory care? Cephalalgia. 2018;38(5):876-882. doi: 10.1177/0333102417716933 [DOI] [PubMed] [Google Scholar]
- 5.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 6.Viswanathan M, Patnode CD, Berkman ND, et al. Recommendations for assessing the risk of bias in systematic reviews of health-care interventions. J Clin Epidemiol. 2018;97:26-34. doi: 10.1016/j.jclinepi.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 7.Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi: 10.1186/1471-2288-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T, Yu T, Hawkins BS, Dickersin K. Design, analysis, and reporting of crossover trials for inclusion in a meta-analysis. PLoS One. 2015;10(8):e0133023. doi: 10.1371/journal.pone.0133023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Röver C, Knapp G, Friede T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol. 2015;15:99. doi: 10.1186/s12874-015-0091-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and Quality. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Agency for Healthcare Research and Quality; 2014. [PubMed]
- 11.Ashcroft DM, Millson D. Naratriptan for the treatment of acute migraine: meta-analysis of randomised controlled trials. Pharmacoepidemiol Drug Saf. 2004;13(2):73-82. doi: 10.1002/pds.890 [DOI] [PubMed] [Google Scholar]
- 12.Bird S, Derry S, Moore RA. Zolmitriptan for acute migraine attacks in adults. Cochrane Database Syst Rev. 2014;2014(5):CD008616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari MD, Loder E, McCarroll KA, Lines CR. Meta-analysis of rizatriptan efficacy in randomized controlled clinical trials. Cephalalgia. 2001;21(2):129-136. doi: 10.1046/j.1468-2982.2001.00169.x [DOI] [PubMed] [Google Scholar]
- 14.Poolsup N, Leelasangaluk V, Jittangtrong J, Rithlamlert C, Ratanapantamanee N, Khanthong M. Efficacy and tolerability of frovatriptan in acute migraine treatment: systematic review of randomized controlled trials. J Clin Pharm Ther. 2005;30(6):521-532. doi: 10.1111/j.1365-2710.2005.00677.x [DOI] [PubMed] [Google Scholar]
- 15.Chen L-C, Ashcroft DM. Meta-analysis examining the efficacy and safety of almotriptan in the acute treatment of migraine. Headache. 2007;47(8):1169-1177. doi: 10.1111/j.1526-4610.2007.00884.x [DOI] [PubMed] [Google Scholar]
- 16.Derry CJ, Derry S, Moore RA. Sumatriptan (oral route of administration) for acute migraine attacks in adults. Cochrane Database Syst Rev. 2012;(2):CD008615. doi: 10.1002/14651858.CD008615.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derry CJ, Derry S, Moore RA. Sumatriptan (subcutaneous route of administration) for acute migraine attacks in adults. Cochrane Database Syst Rev. 2012;(2):CD009665. doi: 10.1002/14651858.CD009665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Derry S, Rabbie R, Moore RA. Diclofenac with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2013;(4):CD008783. doi: 10.1002/14651858.CD008783.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabbie R, Derry S, Moore RA. Ibuprofen with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2013;2013(4):CD008039. doi: 10.1002/14651858.CD008039.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirthi V, Derry S, Moore RA. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev. 2013;(4):CD008041. doi: 10.1002/14651858.CD008041.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law S, Derry S, Moore RA. Sumatriptan plus naproxen for the treatment of acute migraine attacks in adults. Cochrane Database Syst Rev. 2016;4(4):CD008541. doi: 10.1002/14651858.CD008541.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croop R, Goadsby PJ, Stock DA, et al. Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised, phase 3, double-blind, placebo-controlled trial. Lancet. 2019;394(10200):737-745. doi: 10.1016/S0140-6736(19)31606-X [DOI] [PubMed] [Google Scholar]
- 23.Lipton RB, Croop R, Stock EG, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med. 2019;381(2):142-149. doi: 10.1056/NEJMoa1811090 [DOI] [PubMed] [Google Scholar]
- 24.Marcus R, Goadsby PJ, Dodick D, Stock D, Manos G, Fischer TZ. BMS-927711 for the acute treatment of migraine: a double-blind, randomized, placebo controlled, dose-ranging trial. Cephalalgia. 2014;34(2):114-125. doi: 10.1177/0333102413500727 [DOI] [PubMed] [Google Scholar]
- 25.Voss T, Lipton RB, Dodick DW, et al. A phase IIb randomized, double-blind, placebo-controlled trial of ubrogepant for the acute treatment of migraine. Cephalalgia. 2016;36(9):887-898. doi: 10.1177/0333102416653233 [DOI] [PubMed] [Google Scholar]
- 26.Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381(23):2230-2241. doi: 10.1056/NEJMoa1813049 [DOI] [PubMed] [Google Scholar]
- 27.Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant vs placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: the ACHIEVE II randomized clinical trial. JAMA. 2019;322(19):1887-1898. doi: 10.1001/jama.2019.16711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandes JL, Klise S, Krege JH, et al. Interim results of a prospective, randomized, open-label, phase 3 study of the long-term safety and efficacy of lasmiditan for acute treatment of migraine (the GLADIATOR study). Cephalalgia. 2019;39(11):1343-1357. doi: 10.1177/0333102419864132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Färkkilä M, Diener H-C, Géraud G, et al. ; COL MIG-202 Study Group . Efficacy and tolerability of lasmiditan, an oral 5-HT(1F) receptor agonist, for the acute treatment of migraine: a phase 2 randomised, placebo-controlled, parallel-group, dose-ranging study. Lancet Neurol. 2012;11(5):405-413. doi: 10.1016/S1474-4422(12)70047-9 [DOI] [PubMed] [Google Scholar]
- 30.Ferrari MD, Färkkilä M, Reuter U, et al. ; European COL-144 Investigators . Acute treatment of migraine with the selective 5-HT1F receptor agonist lasmiditan: a randomised proof-of-concept trial. Cephalalgia. 2010;30(10):1170-1178. doi: 10.1177/0333102410375512 [DOI] [PubMed] [Google Scholar]
- 31.Goadsby PJ, Wietecha LA, Dennehy EB, et al. Phase 3 randomized, placebo-controlled, double-blind study of lasmiditan for acute treatment of migraine. Brain. 2019;142(7):1894-1904. doi: 10.1093/brain/awz134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuca B, Silberstein SD, Wietecha L, Berg PH, Dozier G, Lipton RB; COL MIG-301 Study Group . Lasmiditan is an effective acute treatment for migraine: a phase 3 randomized study. Neurology. 2018;91(24):e2222-e2232. doi: 10.1212/WNL.0000000000006641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandes JL, Klise S, Krege JH, et al. . Long-term safety and efficacy of lasmiditan for acute treatment of migraine: final results of the GLADIATOR study. Cephalalgia Rep. 2020;3. doi: 10.1177/2515816320958176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashina M, Reuter U, Smith T, et al. Randomized, controlled trial of lasmiditan over four migraine attacks: findings from the CENTURION study. Cephalalgia. 2021;41(3):294-304. doi: 10.1177/0333102421989232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knievel K, Buchanan AS, Lombard L, et al. Lasmiditan for the acute treatment of migraine: subgroup analyses by prior response to triptans. Cephalalgia. 2020;40(1):19-27. doi: 10.1177/0333102419889350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amiri H, Ghodrati N, Nikuyeh M, Shams-Vahdati S, Jalilzadeh-Binazar M. Comparison of granisetron and metoclopramide in the treatment of pain and emesis in migraine patients: a randomized controlled trial study. Turk J Emerg Med. 2017;17(2):61-64. doi: 10.1016/j.tjem.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bigal ME, Bordini CA, Speciali JG. Intravenous chlorpromazine in the emergency department treatment of migraines: a randomized controlled trial. J Emerg Med. 2002;23(2):141-148. doi: 10.1016/S0736-4679(02)00502-4 [DOI] [PubMed] [Google Scholar]
- 38.Cameron JD, Lane PL, Speechley M. Intravenous chlorpromazine vs intravenous metoclopramide in acute migraine headache. Acad Emerg Med. 1995;2(7):597-602. doi: 10.1111/j.1553-2712.1995.tb03596.x [DOI] [PubMed] [Google Scholar]
- 39.Cete Y, Dora B, Ertan C, Ozdemir C, Oktay C. A randomized prospective placebo-controlled study of intravenous magnesium sulphate vs metoclopramide in the management of acute migraine attacks in the emergency department. Cephalalgia. 2005;25(3):199-204. doi: 10.1111/j.1468-2982.2004.00840.x [DOI] [PubMed] [Google Scholar]
- 40.Coppola M, Yealy DM, Leibold RA. Randomized, placebo-controlled evaluation of prochlorperazine versus metoclopramide for emergency department treatment of migraine headache. Ann Emerg Med. 1995;26(5):541-546. doi: 10.1016/S0196-0644(95)70001-3 [DOI] [PubMed] [Google Scholar]
- 41.Corbo J, Esses D, Bijur PE, Iannaccone R, Gallagher EJ. Randomized clinical trial of intravenous magnesium sulfate as an adjunctive medication for emergency department treatment of migraine headache. Ann Emerg Med. 2001;38(6):621-627. doi: 10.1067/mem.2001.119424 [DOI] [PubMed] [Google Scholar]
- 42.Dexter SL, Graham AN, Johnston ES, Ratcliffe DM, Wilkinson MI, Rose AJ. Double-blind controlled study of Paramax in the acute treatment of common and classical migraine. Br J Clin Pract. 1985;39(10):388-392. [PubMed] [Google Scholar]
- 43.Doğan NO, Pekdemir M, Yılmaz S, et al. Intravenous metoclopramide in the treatment of acute migraines: a randomized, placebo-controlled trial. Acta Neurol Scand. 2019;139(4):334-339. doi: 10.1111/ane.13063 [DOI] [PubMed] [Google Scholar]
- 44.Fernando T, Lumanauw DD, Youn S, et al. Buccally absorbed vs intravenous prochlorperazine for treatment of migraines headaches. Acta Neurol Scand. 2019;140(1):72-77. doi: 10.1111/ane.13104 [DOI] [PubMed] [Google Scholar]
- 45.Friedman BW, Esses D, Solorzano C, et al. A randomized controlled trial of prochlorperazine versus metoclopramide for treatment of acute migraine. Ann Emerg Med. 2008;52(4):399-406. doi: 10.1016/j.annemergmed.2007.09.027 [DOI] [PubMed] [Google Scholar]
- 46.Friedman BW, Mulvey L, Esses D, et al. Metoclopramide for acute migraine: a dose-finding randomized clinical trial. Ann Emerg Med. 2011;57(5):475-82.e1. doi: 10.1016/j.annemergmed.2010.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman BW, Cabral L, Adewunmi V, et al. Diphenhydramine as adjuvant therapy for acute migraine: an emergency department–based randomized clinical trial. Ann Emerg Med. 2016;67(1):32-39.e3. doi: 10.1016/j.annemergmed.2015.07.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaffigan ME, Bruner DI, Wason C, Pritchard A, Frumkin K; Intravenous Metoclopramide for Acute Migraine Therapy in the Emergency Department . A randomized controlled trial of intravenous haloperidol vs intravenous metoclopramide for acute migraine therapy in the emergency department. J Emerg Med. 2015;49(3):326-334. doi: 10.1016/j.jemermed.2015.03.023 [DOI] [PubMed] [Google Scholar]
- 49.Honkaniemi J, Liimatainen S, Rainesalo S, Sulavuori S. Haloperidol in the acute treatment of migraine: a randomized, double-blind, placebo-controlled study. Headache. 2006;46(5):781-787. doi: 10.1111/j.1526-4610.2006.00438.x [DOI] [PubMed] [Google Scholar]
- 50.Jones EB, Gonzalez ER, Boggs JG, Grillo JA, Elswick RK Jr. Safety and efficacy of rectal prochlorperazine for the treatment of migraine in the emergency department. Ann Emerg Med. 1994;24(2):237-241. doi: 10.1016/S0196-0644(94)70135-0 [DOI] [PubMed] [Google Scholar]
- 51.Jones J, Pack S, Chun E. Intramuscular prochlorperazine versus metoclopramide as single-agent therapy for the treatment of acute migraine headache. Am J Emerg Med. 1996;14(3):262-264. doi: 10.1016/S0735-6757(96)90171-0 [DOI] [PubMed] [Google Scholar]
- 52.McEwen JI, O’Connor HM, Dinsdale HB. Treatment of migraine with intramuscular chlorpromazine. Ann Emerg Med. 1987;16(7):758-763. doi: 10.1016/S0196-0644(87)80569-3 [DOI] [PubMed] [Google Scholar]
- 53.Miller MA, Levsky ME, Enslow W, Rosin A. Randomized evaluation of octreotide vs prochlorperazine for ED treatment of migraine headache. Am J Emerg Med. 2009;27(2):160-164. doi: 10.1016/j.ajem.2008.01.015 [DOI] [PubMed] [Google Scholar]
- 54.Rowat BM, Merrill CF, Davis A, South V. A double-blind comparison of granisetron and placebo for the treatment of acute migraine in the emergency department. Cephalalgia. 1991;11(5):207-213. doi: 10.1046/j.1468-2982.1991.1105207.x [DOI] [PubMed] [Google Scholar]
- 55.Salazar G, Fragoso M, Vergez L, Sergio P, Cuello D. Metoclopramide as an analgesic in severe migraine attacks: an open, single-blind, parallel control study. Recent Pat CNS Drug Discov. 2011;6(2):141-145. doi: 10.2174/157488911795933947 [DOI] [PubMed] [Google Scholar]
- 56.Shahrami A, Assarzadegan F, Hatamabadi HR, Asgarzadeh M, Sarehbandi B, Asgarzadeh S. Comparison of therapeutic effects of magnesium sulfate vs dexamethasone/metoclopramide on alleviating acute migraine headache. J Emerg Med. 2015;48(1):69-76. doi: 10.1016/j.jemermed.2014.06.055 [DOI] [PubMed] [Google Scholar]
- 57.Silberstein SD, Young WB, Mendizabal JE, Rothrock JF, Alam AS. Acute migraine treatment with droperidol: a randomized, double-blind, placebo-controlled trial. Neurology. 2003;60(2):315-321. doi: 10.1212/01.WNL.0000042477.63516.B2 [DOI] [PubMed] [Google Scholar]
- 58.Tek DS, McClellan DS, Olshaker JS, Allen CL, Arthur DC. A prospective, double-blind study of metoclopramide hydrochloride for the control of migraine in the emergency department. Ann Emerg Med. 1990;19(10):1083-1087. doi: 10.1016/S0196-0644(05)81508-2 [DOI] [PubMed] [Google Scholar]
- 59.Tanen DA, Miller S, French T, Riffenburgh RH. Intravenous sodium valproate versus prochlorperazine for the emergency department treatment of acute migraine headaches: a prospective, randomized, double-blind trial. Ann Emerg Med. 2003;41(6):847-853. doi: 10.1067/mem.2003.195 [DOI] [PubMed] [Google Scholar]
- 60.Kandil M, Jaber S, Desai D, et al. MAGraine: Magnesium compared to conventional therapy for treatment of migraines. Am J Emerg Med. 2021;39:28-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Motamed H, Mozafari J, Porozan S, Sasani M. Magnesium sulfate and acute migraine: a randomized clinical trial. Ann Clin Anal Med. 2020;11(5):369-373. [Google Scholar]
- 62.Aurora SK, Silberstein SD, Kori SH, et al. MAP0004, orally inhaled DHE: a randomized, controlled study in the acute treatment of migraine. Headache. 2011;51(4):507-517. doi: 10.1111/j.1526-4610.2011.01869.x [DOI] [PubMed] [Google Scholar]
- 63.Aurora SK, Rozen TD, Kori SH, Shrewsbury SB. A randomized, double blind, placebo-controlled study of MAP0004 in adult patients with migraine. Headache. 2009;49(6):826-837. doi: 10.1111/j.1526-4610.2009.01453.x [DOI] [PubMed] [Google Scholar]
- 64.Bell R, Montoya D, Shuaib A, Lee MA. A comparative trial of three agents in the treatment of acute migraine headache. Ann Emerg Med. 1990;19(10):1079-1082. doi: 10.1016/S0196-0644(05)81507-0 [DOI] [PubMed] [Google Scholar]
- 65.Callaham M, Raskin N. A controlled study of dihydroergotamine in the treatment of acute migraine headache. Headache. 1986;26(4):168-171. doi: 10.1111/j.1526-4610.1986.hed2604168.x [DOI] [PubMed] [Google Scholar]
- 66.Diener H-C, Jansen J-P, Reches A, Pascual J, Pitei D, Steiner TJ; Eletriptan and Cafergot Comparative Study Group . Efficacy, tolerability and safety of oral eletriptan and ergotamine plus caffeine (Cafergot) in the acute treatment of migraine: a multicentre, randomised, double-blind, placebo-controlled comparison. Eur Neurol. 2002;47(2):99-107. doi: 10.1159/000047960 [DOI] [PubMed] [Google Scholar]
- 67.Friedman AP, Di Serio FJ, Hwang DS. Symptomatic relief of migraine: multicenter comparison of Cafergot P-B, Cafergot, and placebo. Clin Ther. 1989;11(1):170-182. [PubMed] [Google Scholar]
- 68.Gallagher RM; Dihydroergotamine Working Group . Acute treatment of migraine with dihydroergotamine nasal spray. Arch Neurol. 1996;53(12):1285-1291. doi: 10.1001/archneur.1996.00550120097022 [DOI] [PubMed] [Google Scholar]
- 69.Hakkarainen H, Allonen H. Ergotamine vs metoclopramide vs their combination in acute migraine attacks. Headache. 1982;22(1):10-12. doi: 10.1111/j.1526-4610.1982.hed2201010.x [DOI] [PubMed] [Google Scholar]
- 70.Kangasniemi P, Kaaja R. Ketoprofen and ergotamine in acute migraine. J Intern Med. 1992;231(5):551-554. doi: 10.1111/j.1365-2796.1992.tb00973.x [DOI] [PubMed] [Google Scholar]
- 71.Rapoport A, Sheftell F, Couch J, et al. ; Dihydroergotamine Nasal Spray Multicenter Investigators . Efficacy, safety, and tolerability of dihydroergotamine nasal spray as monotherapy in the treatment of acute migraine. Headache. 1995;35(4):177-184. doi: 10.1111/j.1526-4610.1995.hed3504177.x [DOI] [PubMed] [Google Scholar]
- 72.Ryan RE. Double-blind clinical evaluation of the efficacy and safety of ergostine-caffeine, ergotamine-caffeine, and placebo in migraine headache. Headache. 1970;9(4):212-220. doi: 10.1111/j.1526-4610.1970.hed0904212.x [DOI] [PubMed] [Google Scholar]
- 73.Sharma S, Prasad A, Nehru R, et al. Efficacy and tolerability of prochlorperazine buccal tablets in treatment of acute migraine. Headache. 2002;42(9):896-902. doi: 10.1046/j.1526-4610.2002.02210.x [DOI] [PubMed] [Google Scholar]
- 74.Treves TA, Kuritzky A, Hering R, Korczyn AD. Dihydroergotamine nasal spray in the treatment of acute migraine. Headache. 1998;38(8):614-617. doi: 10.1046/j.1526-4610.1998.3808614.x [DOI] [PubMed] [Google Scholar]
- 75.Tulunay FC, Karan O, Aydin N, Culcuoglu A, Guvener A. Dihydroergotamine nasal spray during migraine attacks: a double-blind crossover study with placebo. Cephalalgia. 1987;7(2):131-133. doi: 10.1046/j.1468-2982.1987.0702131.x [DOI] [PubMed] [Google Scholar]
- 76.Ziegler D, Ford R, Kriegler J, et al. Dihydroergotamine nasal spray for the acute treatment of migraine. Neurology. 1994;44(3 pt 1):447-453. doi: 10.1212/WNL.44.3_Part_1.447 [DOI] [PubMed] [Google Scholar]
- 77.Alemdar M, Pekdemir M, Selekler HM. Single-dose intravenous tramadol for acute migraine pain in adults: a single-blind, prospective, randomized, placebo-controlled clinical trial. Clin Ther. 2007;29(7):1441-1447. doi: 10.1016/j.clinthera.2007.07.017 [DOI] [PubMed] [Google Scholar]
- 78.Boureau F, Joubert JM, Lasserre V, Prum B, Delecoeuillerie G. Double-blind comparison of an acetaminophen 400 mg–codeine 25 mg combination versus aspirin 1000 mg and placebo in acute migraine attack. Cephalalgia. 1994;14(2):156-161. doi: 10.1046/j.1468-2982.1994.1402156.x [DOI] [PubMed] [Google Scholar]
- 79.Carleton SC, Shesser RF, Pietrzak MP, et al. Double-blind, multicenter trial to compare the efficacy of intramuscular dihydroergotamine plus hydroxyzine versus intramuscular meperidine plus hydroxyzine for the emergency department treatment of acute migraine headache. Ann Emerg Med. 1998;32(2):129-138. doi: 10.1016/S0196-0644(98)70126-X [DOI] [PubMed] [Google Scholar]
- 80.Freitag FG. The acute treatment of migraine with transnasal butorphanol (TNB). Headache Q. 1993;4(suppl 3):22-28. [Google Scholar]
- 81.Friedman BW, Irizarry E, Solorzano C, et al. Randomized study of IV prochlorperazine plus diphenhydramine vs IV hydromorphone for migraine. Neurology. 2017;89(20):2075-2082. doi: 10.1212/WNL.0000000000004642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoffert MJ, Couch JR, Diamond S, et al. Transnasal butorphanol in the treatment of acute migraine. Headache. 1995;35(2):65-69. doi: 10.1111/j.1526-4610.1995.hed3502065.x [DOI] [PubMed] [Google Scholar]
- 83.Klapper JA, Stanton J. Current emergency treatment of severe migraine headaches. Headache. 1993;33(10):560-562. doi: 10.1111/j.1526-4610.1993.hed3310560.x [DOI] [PubMed] [Google Scholar]
- 84.Lane PL, McLellan BA, Baggoley CJ. Comparative efficacy of chlorpromazine and meperidine with dimenhydrinate in migraine headache. Ann Emerg Med. 1989;18(4):360-365. doi: 10.1016/S0196-0644(89)80570-0 [DOI] [PubMed] [Google Scholar]
- 85.Richman PB, Allegra J, Eskin B, et al. A randomized clinical trial to assess the efficacy of intramuscular droperidol for the treatment of acute migraine headache. Am J Emerg Med. 2002;20(1):39-42. doi: 10.1053/ajem.2002.30007 [DOI] [PubMed] [Google Scholar]
- 86.Scherl ER, Wilson JF. Comparison of dihydroergotamine with metoclopramide versus meperidine with promethazine in the treatment of acute migraine. Headache. 1995;35(5):256-259. doi: 10.1111/j.1526-4610.1995.hed3505256.x [DOI] [PubMed] [Google Scholar]
- 87.Silberstein SD, Freitag FG, Rozen TD, et al. ; CAPSS-223 Investigators . Tramadol/acetaminophen for the treatment of acute migraine pain: findings of a randomized, placebo-controlled trial. Headache. 2005;45(10):1317-1327. doi: 10.1111/j.1526-4610.2005.00264.x [DOI] [PubMed] [Google Scholar]
- 88.Stiell IG, Dufour DG, Moher D, Yen M, Beilby WJ, Smith NA. Methotrimeprazine versus meperidine and dimenhydrinate in the treatment of severe migraine: a randomized, controlled trial. Ann Emerg Med. 1991;20(11):1201-1205. doi: 10.1016/S0196-0644(05)81471-4 [DOI] [PubMed] [Google Scholar]
- 89.Taheraghdam AA, Amiri H, Shojaan H, Shamsvahdati S, Houshyar Y. Intravenous dexamethasone versus morphine in relieving of acute migraine headache. Pak J Biol Sci. 2011;14(12):682-687. doi: 10.3923/pjbs.2011.682.687 [DOI] [PubMed] [Google Scholar]
- 90.Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010;64(4):451-456. doi: 10.1111/j.1742-1241.2009.02215.x [DOI] [PubMed] [Google Scholar]
- 91.Chou DE, Shnayderman Yugrakh M, Winegarner D, Rowe V, Kuruvilla D, Schoenen J. Acute migraine therapy with external trigeminal neurostimulation (ACME): a randomized controlled trial. Cephalalgia. 2019;39(1):3-14. doi: 10.1177/0333102418811573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farahmand S, Shafazand S, Alinia E, Bagheri-Hariri S, Baratloo A. Pain management using acupuncture method in migraine headache patients: a single blinded randomized clinical trial. Anesth Pain Med. 2018;8(6):e81688. doi: 10.5812/aapm.81688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fuglsang CH, Johansen T, Kaila K, Kasch H, Bach FW. Treatment of acute migraine by a partial rebreathing device: a randomized controlled pilot study. Cephalalgia. 2018;38(10):1632-1643. doi: 10.1177/0333102418797285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Korucu O, Dagar S, Çorbacioglu SK, Emektar E, Cevik Y. The effectiveness of greater occipital nerve blockade in treating acute migraine-related headaches in emergency departments. Acta Neurol Scand. 2018;138(3):212-218. doi: 10.1111/ane.12952 [DOI] [PubMed] [Google Scholar]
- 95.Li Y, Liang F, Yang X, et al. Acupuncture for treating acute attacks of migraine: a randomized controlled trial. Headache. 2009;49(6):805-816. doi: 10.1111/j.1526-4610.2009.01424.x [DOI] [PubMed] [Google Scholar]
- 96.Lipton RB, Dodick DW, Silberstein SD, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. 2010;9(4):373-380. doi: 10.1016/S1474-4422(10)70054-5 [DOI] [PubMed] [Google Scholar]
- 97.Marcus SV. Phase 1 of integrated EMDR: an abortive treatment for migraine headaches. J EMDR Pract Res. 2008;2(1):15-25. doi: 10.1891/1933-3196.2.1.15 [DOI] [Google Scholar]
- 98.Niazi M, Hashempur MH, Taghizadeh M, Heydari M, Shariat A. Efficacy of topical rose (Rosa damascena Mill.) oil for migraine headache: a randomized double-blinded placebo-controlled cross-over trial. Complement Ther Med. 2017;34:35-41. doi: 10.1016/j.ctim.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 99.Sasannejad P, Saeedi M, Shoeibi A, Gorji A, Abbasi M, Foroughipour M. Lavender essential oil in the treatment of migraine headache: a placebo-controlled clinical trial. Eur Neurol. 2012;67(5):288-291. doi: 10.1159/000335249 [DOI] [PubMed] [Google Scholar]
- 100.Tassorelli C, Grazzi L, de Tommaso M, et al. ; PRESTO Study Group . Noninvasive vagus nerve stimulation as acute therapy for migraine: the randomized PRESTO study. Neurology. 2018;91(4):e364-e373. doi: 10.1212/WNL.0000000000005857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Triner WR, Bartfield JM, Birdwell M, Raccio-Robak N. Nitrous oxide for the treatment of acute migraine headache. Am J Emerg Med. 1999;17(3):252-254. doi: 10.1016/S0735-6757(99)90118-3 [DOI] [PubMed] [Google Scholar]
- 102.Wang L-P, Zhang X-Z, Guo J, et al. Efficacy of acupuncture for acute migraine attack: a multicenter single blinded, randomized controlled trial. Pain Med. 2012;13(5):623-630. doi: 10.1111/j.1526-4637.2012.01376.x [DOI] [PubMed] [Google Scholar]
- 103.Yang J, Zeng F, Feng Y, et al. A PET-CT study on the specificity of acupoints through acupuncture treatment in migraine patients. BMC Complement Altern Med. 2012;12:123. doi: 10.1186/1472-6882-12-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yarnitsky D, Dodick DW, Grosberg BM, et al. Remote electrical neuromodulation (REN) relieves acute migraine: a randomized, double-blind, placebo-controlled, multicenter trial. Headache. 2019;59(8):1240-1252. doi: 10.1111/head.13551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yarnitsky D, Volokh L, Ironi A, et al. Nonpainful remote electrical stimulation alleviates episodic migraine pain. Neurology. 2017;88(13):1250-1255. doi: 10.1212/WNL.0000000000003760 [DOI] [PubMed] [Google Scholar]
- 106.Zargaran A, Borhani-Haghighi A, Salehi-Marzijarani M, et al. Evaluation of the effect of topical chamomile (Matricaria chamomilla L.) oleogel as pain relief in migraine without aura: a randomized, double-blind, placebo-controlled, crossover study. Neurol Sci. 2018;39(8):1345-1353. doi: 10.1007/s10072-018-3415-1 [DOI] [PubMed] [Google Scholar]
- 107.Hokenek NM, Erdogan MO, Hokenek UD, Algin A, Tekyol D, Seyhan AU. Treatment of migraine attacks by transcutaneous electrical nerve stimulation in emergency department: a randomize controlled trial. Am J Emerg Med. 2021;39:80-85. doi: 10.1016/j.ajem.2020.01.024 [DOI] [PubMed] [Google Scholar]
- 108.Antal A, Bischoff R, Stephani C, et al. Low intensity, transcranial, alternating current stimulation reduces migraine attack burden in a home application set-up: a double-blinded, randomized feasibility study. Brain Sci. 2020;10(11):21. doi: 10.3390/brainsci10110888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3-20. doi: 10.1111/head.12499 [DOI] [PubMed] [Google Scholar]
- 110.Lipton RB, Reed ML, Kurth T, Fanning KM, Buse DC. Framingham-based cardiovascular risk estimates among people with episodic migraine in the US population: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2017;57(10):1507-1521. doi: 10.1111/head.13179 [DOI] [PubMed] [Google Scholar]
- 111.Buse DC, Reed ML, Fanning KM, Kurth T, Lipton RB. Cardiovascular events, conditions, and procedures among people with episodic migraine in the US population: results from the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2017;57(1):31-44. doi: 10.1111/head.12962 [DOI] [PubMed] [Google Scholar]
- 112.Pringsheim T, Davenport WJ, Marmura MJ, Schwedt TJ, Silberstein S. How to apply the AHS evidence assessment of the acute treatment of migraine in adults to your patient with migraine. Headache. 2016;56(7):1194-1200. doi: 10.1111/head.12870 [DOI] [PubMed] [Google Scholar]
- 113.Shapiro RE, Hochstetler HM, Dennehy EB, et al. Lasmiditan for acute treatment of migraine in patients with cardiovascular risk factors: post-hoc analysis of pooled results from 2 randomized, double-blind, placebo-controlled, phase 3 trials. J Headache Pain. 2019;20(1):90. doi: 10.1186/s10194-019-1044-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Szkutnik-Fiedler D. Pharmacokinetics, pharmacodynamics and drug-drug interactions of new anti-migraine drugs: lasmiditan, gepants, and calcitonin-gene-related peptide (CGRP) receptor monoclonal antibodies. Pharmaceutics. 2020;12(12):E1180. doi: 10.3390/pharmaceutics12121180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pearlman EM, Wilbraham D, Dennehy EB, et al. Effects of lasmiditan on simulated driving performance: results of two randomized, blinded, crossover studies with placebo and active controls. Hum Psychopharmacol. 2020;35(5):e2732. doi: 10.1002/hup.2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nir R-R, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care. 2015;9(2):131-137. doi: 10.1097/SPC.0000000000000126 [DOI] [PubMed] [Google Scholar]
- 117.Lipton RB, Schwedt TJ, Friedman BW, et al. . Demographics, headache characteristics, and other factors associated with opioid use in people with migraine: results from the CaMEO study. Neurology. 2019;92:S59.006. [Google Scholar]
- 118.Lipton RB, Araujo AB, Nicholson RA, et al. Patterns of diagnosis, consultation, and treatment of migraine in the US: results of the OVERCOME Study. Presented at: 61st Annual Scientific Meeting of the American Headache Society; July 11-14, 2019; Philadelphia, PA. [Google Scholar]
- 119.Ashina S, Foster SA, Nicholson RA, et al. Opioid use among people with migraine: results of the OVERCOME study. Presented at: 61st Annual Scientific Meeting of the American Headache Society; July 11-14, 2019; Philadelphia, PA. [Google Scholar]
- 120.Molina KC, Fairman KA, Sclar DA. Concomitant use of opioid medications with triptans or serotonergic antidepressants in US office-based physician visits. Drug Healthc Patient Saf. 2018;10:37-43. doi: 10.2147/DHPS.S151073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bonafede M, Wilson K, Xue F. Long-term treatment patterns of prophylactic and acute migraine medications and incidence of opioid-related adverse events in patients with migraine. Cephalalgia. 2019;39(9):1086-1098. doi: 10.1177/0333102419835465 [DOI] [PubMed] [Google Scholar]
- 122.Connelly M, Glynn EF, Hoffman MA, Bickel J. Rates and predictors of using opioids in the emergency department to treat migraine in adolescents and young adults. Pediatr Emerg Care. 2019. doi: 10.1097/PEC.0000000000001851 [DOI] [PubMed] [Google Scholar]
- 123.Vinson DR, Hurtado TR, Vandenberg JT, Banwart L. Variations among emergency departments in the treatment of benign headache. Ann Emerg Med. 2003;41(1):90-97. doi: 10.1067/mem.2003.24 [DOI] [PubMed] [Google Scholar]
- 124.Katsarava Z, Schneeweiss S, Kurth T, et al. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology. 2004;62(5):788-790. doi: 10.1212/01.WNL.0000113747.18760.D2 [DOI] [PubMed] [Google Scholar]
- 125.Langemark M, Olesen J. Drug abuse in migraine patients. Pain. 1984;19(1):81-86. doi: 10.1016/0304-3959(84)90067-8 [DOI] [PubMed] [Google Scholar]
- 126.Salomone JA III, Thomas RW, Althoff JR, Watson WA. An evaluation of the role of the ED in the management of migraine headaches. Am J Emerg Med. 1994;12(2):134-137. doi: 10.1016/0735-6757(94)90231-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Search strategy
eTable 2. List of the excluded interventions
eTable 3. Definition of pain and function outcomes
eTable 4. Pain and function scales included in the anaysis
eTable 5. Categories of adverse events
eTable 6. Definition and approaches to grade strength of evidence
eTable 7. Results of systematic reviews evaluating triptans and nonsteroidal anti-inflammatory drugs (NSAIDs)
eTable 8. Characteristics of included studies evaluating CGRP, 5-HT1F, antiemetics, ergot alkaloids, opioids, other pharmacological interventions, and nonpharmacological interventions
eTable 9. Adverse events
eTable10. Subgroup analysis
eTable 11. Risk of bias (Cochrane Risk of Bias tool for randomized trials [RoB 2.0]) for randomized clinical trials
eTable 12. Effectiveness of treatments other than triptans and nonsteroidal anti-inflammatory drugs
eFigure 1. Findings of Meta-analysis of 5-HT1F Receptor Agonists on Pain and Function Outcomes measured as Continuous Outcomes for Episodic Migraine in Adults
eFigure 2. Findings of Meta-analysis of Antiemetics on Pain Outcomes measured as Continuous Outcomes for Episodic Migraine in Adults
eFigure 3. Findings of Meta-analysis of Ergot Alkaloids on Pain and Function Outcomes measured as Binary Outcomes for Episodic Migraine in Adults
eFigure 4. Findings of Meta-analysis of Ergot Alkaloids on Pain Outcomes measured as Continuous Outcomes for Episodic Migraine in Adults
eFigure 5. Findings of Meta-analysis of Opioids on Pain and Function Outcomes measured as Binary Outcomes for Episodic Migraine in Adults
eFigure 6. Findings of Meta-analysis of Opioids on Pain Outcomes measured as Continuous Outcomes for Episodic Migraine in Adults
eFigure 7. Findings of Meta-analysis of Other Pharmacological Interventions on Pain and Function Outcomes measured as Binary Outcomes for Episodic Migraine in Adults
eFigure 8. Findings of Meta-analysis of Other Pharmacological Interventions on Pain and Function Outcomes measured as Continuous Outcomes for Episodic Migraine in Adults
eFigure 9. Findings of Meta-analysis of Nonpharmacological Interventions on Pain Outcomes measured as Binary Outcomes for Episodic Migraine in Adults
eFigure 10. Findings of Meta-analysis of Nonpharmacological Interventions on Pain Outcomes measured as Continuous Outcomes for Episodic Migraine in Adults
References



