Abstract
Introduction
Management of chronic kidney disease (CKD) entails high medical complexity and often results in high hospitalization burden. There are limited data on the associations of longitudinal hospital utilization patterns with adverse clinical outcomes in individuals with CKD.
Methods
We derived cumulative all-cause hospitalization trajectory groups using latent class trajectory analysis in 3012 participants of the Chronic Renal Insufficiency Cohort (CRIC) Study who were alive and did not reach end-stage kidney disease (ESKD) within 4 years of study entry. Cox proportional hazards models tested the associations between hospitalization trajectory groups and risks of ESKD and death prior to the onset of ESKD (ESKD-censored death).
Results
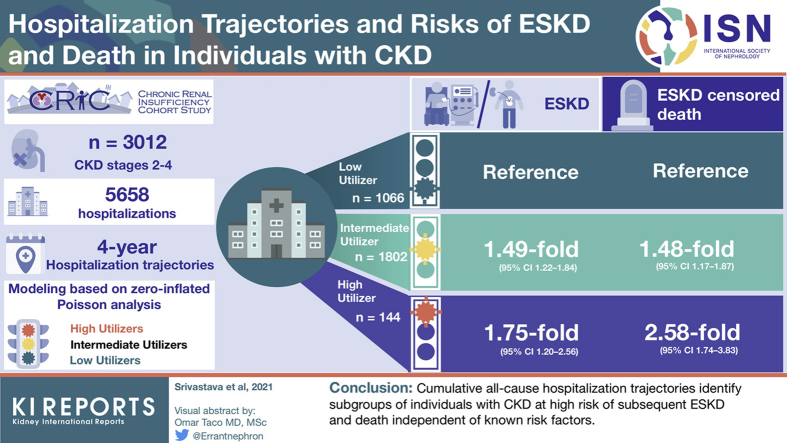
Within 4 years of study entry, there were 5658 hospitalizations among 3012 participants. We identified 3 distinct subgroups of individuals with CKD based on cumulative all-cause hospitalization trajectories over 4 years: low-utilizer (n = 1066), intermediate-utilizer (n = 1802), and high-utilizer (n = 144). High-utilizers represented a patient population of lower socioeconomic status who had a greater prevalence of comorbid conditions and lower kidney function compared with intermediate- and low-utilizers. After the 4-year ascertainment period to form the trajectory subgroups, there were 544 ESKD events and 437 ESKD-censored deaths during a median follow-up time of 5.1 years. Compared with low-utilizers, intermediate-utilizers and high-utilizers were at 1.49-fold (95% confidence interval [CI] 1.22–1.84) and 1.75-fold (95% CI 1.20–2.56) higher risk of ESKD in adjusted analyses, respectively. Compared with low-utilizers, intermediate-utilizers and high-utilizers were at 1.48-fold (95% CI 1.17–1.87) and 2.58-fold (95% CI 1.74–3.83) higher risk of ESKD-censored death in adjusted analyses, respectively.
Conclusions
Trajectories of cumulative all-cause hospitalization identify subgroups of individuals with CKD who are at high risk of ESKD and death.
Keywords: chronic kidney disease, end-stage kidney disease, hospital utilization, hospitalization, trajectory
Graphical abstract
See Commentary on Page 1492
More than 35 million American adults have CKD,1 which places them at high risks of adverse clinical outcomes.2, 3, 4, 5 Medicare spending to manage the high medical complexity of CKD amounts to nearly $80 billion annually, and the costs are increasing each year.6,7 Hospitalizations greatly contribute to rising health care costs8, 9, 10, 11 and result in significant distress for patients and their families.
Individuals with CKD are hospitalized at least twice as often as individuals without CKD,6,12 and hospital utilization increases with worsening kidney function.3,13 Existing literature reports on population-level hospitalization rates over time6 and risk factors for time to first hospitalization in individuals with CKD.11,14 These data do not provide information for patients and stakeholders regarding the global hospitalization burden over time. Analysis of hospitalization trajectories may detect heterogeneity in a CKD population and identify subgroups of individuals whose evolution of hospital utilization is much greater than the population mean and who may be at the highest risk of adverse outcomes. Enhanced understanding of distinct subgroups of individuals with CKD who have increasing hospitalization trajectories is critical to develop and test interventions15, 16, 17 that may reduce hospitalizations. If longitudinal hospitalization utilization patterns are associated with risks of mortality and ESKD, then investigators may consider using hospitalization rates as outcomes in trials or as means to enrich study populations. We performed a prospective cohort study among participants with CKD stages 2 to 4 in the CRIC Study to test the hypotheses that trajectories of cumulative all-cause hospitalization would identify discrete subgroups of individuals with CKD, and that the subgroups would be associated with varying risks of subsequent ESKD and death.
Methods
Source Population
The CRIC Study is a prospective, observational cohort study of individuals with mild to severe CKD that was designed to investigate risk factors for progression of CKD, development of cardiovascular disease, and mortality.18 During phase 1, the CRIC Study enrolled 3939 men and women aged 21 to 74 years between June 2003 and September 2008 across 7 clinical centers in the United States. More detailed information about the CRIC Study is provided in the Supplementary Methods.18, 19, 20 The study protocol was approved by the Institutional Review Boards of the participating centers and is in accordance with the principles of the Declaration of Helsinki. All CRIC Study participants provided informed consent.
Study Population
To describe the longitudinal evolution of hospitalization utilization in individuals with CKD stages 2 to 4, we first examined hospitalization incidence densities among all CRIC Study participants (N = 3939). Because many CRIC Study participants have stable or slowly progressive CKD,21 we next described longitudinal hospitalization trends among subgroups with extreme phenotypes. These included individuals who progressed to ESKD (n = 1084) or who died during follow-up (n = 710). Among these subgroups, we examined hospitalization incidence densities before development of ESKD or death (Supplementary Figure S1).
To identify subgroups of individuals with different patterns of hospital utilization over the 4-year exposure ascertainment period and to relate the subgroups to risks of adverse outcomes, we studied 3012 CRIC Study participants who survived beyond their fifth annual study visit (baseline visit through the year 4 visit) without progressing to ESKD (Supplementary Figure S2).
Exposure
The primary exposures were grouped trajectories of hospitalization, which we formed from the number of cumulative all-cause hospitalizations. Information regarding hospitalizations were ascertained every 6 months by self-report and confirmed by hospital queries. We included all hospitalizations, independent of duration or station (emergency department, observation, and inpatient), to determine if any type of hospitalization provided information about an individual’s health.22 More detailed information about ascertainment of hospitalization and covariate data is provided in the Supplementary Methods.
Outcomes
The primary outcomes were ESKD, defined as initiation of dialysis or kidney transplantation, and death before the onset of ESKD (ESKD-censored death). The latter outcome was chosen because the frequency of hospitalization may change after the onset of ESKD.6,13 Ascertainment of ESKD status was confirmed by cross-linkage of participants with the United States Renal Data System.18 All deaths were confirmed by death certificate review. Participants were followed up until the occurrence of event of interest, voluntary study withdrawal, loss to follow-up, or end of the follow-up period on September 30, 2015.
Statistical Analysis
To describe the longitudinal evolution of hospitalization utilization in all participants of the CRIC Study, we calculated incidence densities of all-cause hospitalizations over time. In high-risk individuals who experienced ESKD and ESKD-censored death, we calculated incidence densities across time in relation to the number of years prior to the onset of the outcome.
Next, we used group-based trajectory modeling to categorize participants who were alive and did not reach ESKD within 4 years of study entry into subgroups of 4-year hospitalization utilization.23 We assumed the distribution of cumulative number of hospitalizations conditional on time to be zero-inflated Poisson as there was no severe overdispersion observed. We used SAS PROC TRAJ to fit the longitudinal hospitalization data as a discrete mixture of 2 or more trajectories via maximum likelihood.23, 24, 25 This method relies on a semiparametric group-based modeling strategy, which incorporates hierarchical and latent growth curve modeling, and it assumes that there are multiple trajectory groups within a population. We evaluated models with different numbers of trajectory groups for model fit, which we assessed with the Bayesian information criterion. Based on the model fit criteria and the visual appearance of the trajectories, we identified 3 hospitalization trajectory groups. We assigned participants to the trajectory group for which they had the highest posterior predicted probability.23 The mean posterior probabilities and 95% CIs were 1.0 (1.0–1.0), 0.98 (0.97–0.98), and 0.89 (0.87–0.92) for the low-, intermediate-, and high-utilizer groups, respectively (Supplementary Figure S3).
After derivation of the hospitalization trajectory groups within 4 years of study entry, we summarized descriptive statistics according to trajectory group membership as mean ± standard deviation (SD) or median and interquartile range (IQR) for continuous variables and as percentages for frequency distribution for categorical variables. For skewed data distributions, we performed natural logarithmic transformation. We used chi-square tests to compare frequency distributions of categorical variables by hospitalization trajectory groups. For evaluations between continuous variables and hospitalization trajectory groups, we used analysis of variance (for normally distributed variables) and Kruskal-Wallis tests (for non-normally distributed variables).
We used Cox proportional hazards regression to test the associations between the hospitalization trajectory groups and risks of ESKD and ESKD-censored death following the 4-year ascertainment period. We set the survival time (time 0) to begin with the participant’s fifth annual visit (year 4 visit). All covariates were ascertained at the time survival follow-up began. For each outcome, we fit a series of hierarchically adjusted models based on the biological and clinical plausibility of covariates as potential confounders: model 1 was unadjusted; model 2 was stratified by site and adjusted for age, sex, race, ethnicity, income level, education level, and insurance type; model 3 included covariates from model 2 and further adjusted for systolic blood pressure, body mass index, smoking status, diabetes mellitus, prior cardiovascular disease, and medications (angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, β-blocker, statin, and anti-platelet agent); model 4 included covariates from model 3 and further adjusted for hemoglobin, serum albumin, natural log transformed proteinuria, and estimated glomerular filtration rate (eGFR). To test whether the associations of hospitalization trajectory groups with each respective outcome were modified by eGFR, we tested for statistical interaction between hospitalization trajectory groups and eGFR for each outcome through multiplicative interaction terms. To account for missing covariate data, we used multiple imputation (Supplementary Methods). We confirmed no violations of the proportional hazards assumption using the Kolmogorov-type supremum test and visual inspection by checking martingale residuals.
Sensitivity Analyses
Because the primary trajectory analysis included all hospitalizations and many hospitalizations may be short stay visits, we reformed the trajectory groups after excluding hospitalizations with a length of stay ≤1 day. Because the onset of ESKD may require a hospitalization to initiate dialysis, reverse causality is possible. To address this potential confounding, we repeated the primary trajectory analysis after incorporating a 1-year lag, such that only individuals that were free of ESKD and survived beyond the year 5 visit were included and follow-up began at the time of the year 5 visit. Because 4 years of hospitalization data may not be available to all health care providers, we repeated the primary trajectory analysis in individuals that were free of ESKD and survived beyond the year 2 visit with start of survival time at the year 2 visit. To determine whether hospitalization trajectories remained significant predictors of future adverse outcomes following adjustment for hospitalizations during the first year of the CRIC Study, we adjusted the primary trajectory analysis for whether a participant was hospitalized between years 0 and 1. To determine whether a single year of hospitalization data provided information about the future risk of adverse outcomes, we categorized the number of hospitalizations (0, 1, or >1 hospitalization) in the first year of the CRIC Study to determine the association between number of hospitalizations and future risk of ESKD and ESKD-censored death.
Statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc). All statistical tests were 2-sided, and P <0.05 was considered significant.
Results
Hospitalization Incidence Densities
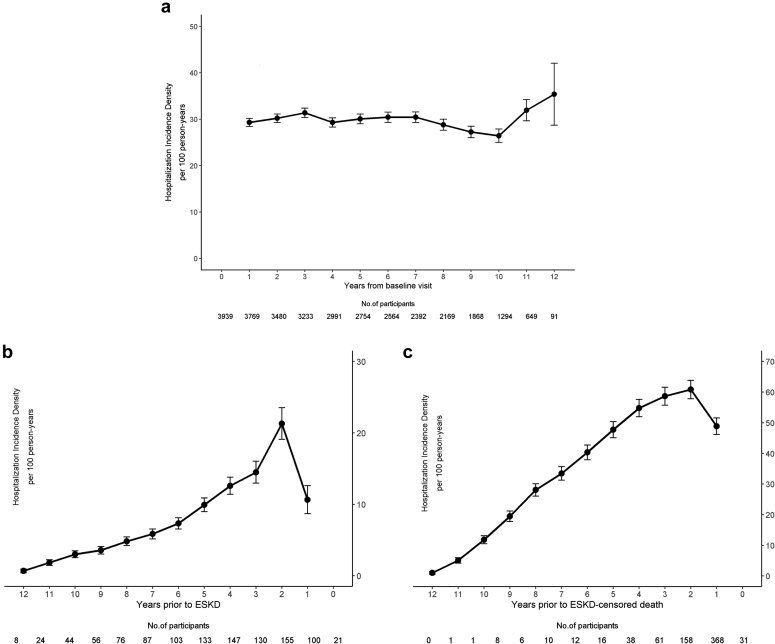
Hospitalization incidence densities remained stable over time in all CRIC Study participants (N = 3939). However, participants who progressed to ESKD (n = 1084) and ESKD-censored death (n = 710) had rising hospitalization incidence densities in the years prior to each respective outcome (Figure 1).
Figure 1.
Hospitalization incidence densities. Hospitalizations incidence densities over time in (a) all CRIC Study participants (N = 3939), (b) participants who experienced ESKD (n = 1084), and (c) ESKD-censored death (n = 710). In panel (a), time 0 is the baseline visit in the CRIC Study. In panels (b) and (c), time 0 is the onset of the outcome. For each panel, incidence densities are calculated based on the individuals at risk for hospitalization in the period prior to the point estimate. For instance, estimates for year 1 are based on individuals at risk from 0 to 1 year in each panel, respectively. CRIC, Chronic Renal Insufficiency Cohort; ESKD, end-stage kidney disease.
Cumulative All-Cause Hospitalization Trajectories
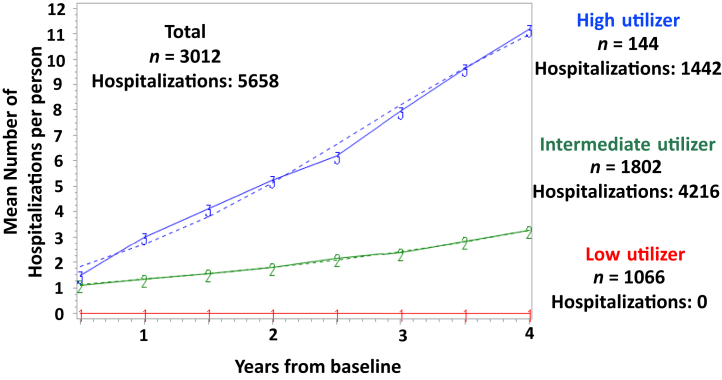
We labeled the identified 4-year hospitalization trajectory groups based on their hospital utilization pattern: low-utilizer, intermediate-utilizer, and high-utilizer (Figure 2). Table 1 shows characteristics of the 3 hospitalization trajectory groups at the year 4 study visit. Compared with low-utilizers, intermediate- and high-utilizers were more likely to be female, black, have lower household income, have a greater prevalence of diabetes mellitus and cardiovascular disease, have lower serum albumin and hemoglobin, have higher body mass index and proteinuria, and lower eGFR. Within the first 4 years of study entry used to form the trajectory groups, the mean number of cumulative all-cause hospitalizations were 6.3 ± 4.2, 2.2 ± 1.5, and zero hospitalizations in the high-, intermediate-, and low-utilizer groups, respectively (P < 0.001). Participants in the high-utilizer group had the longest hospital lengths of stay (high: 1.6 [0.7–3.3], intermediate: 0.5 [0–2.0], low: 0 days; P < 0.001). Participants in the high-utilizer group were more likely to be rehospitalized within 30 days (high: 31.4%, intermediate: 12.9%, low: 0%; P < 0.001).
Figure 2.
Cumulative all-cause hospitalization trajectories. There were 5658 hospitalizations among 3012 participants who did not progress to ESKD and survived to their year 4 study visit. The low-utilizer group was composed of 1066 participants who were not hospitalized through their year 4 study visit. The intermediate-utilizer group had 1802 participants with 4216 hospitalizations, and the high-utilizer group had 144 participants with 1442 hospitalizations. ESKD, end-stage kidney disease.
Table 1.
Year 4 characteristics of hospitalization trajectory groups (N = 3012)
| Characteristics | Low-utilizer (n = 1066) | Intermediate-utilizer (n = 1802) | High-utilizer (n = 144) | P |
|---|---|---|---|---|
| Demographics and clinical | ||||
| Age, yr, mean ± SD | 60.2 ± 11.3 | 62.9 ± 10.5 | 60.9 ± 10.3 | <0.001 |
| Female | 468 (43.9) | 873 (48.5) | 73 (50.7) | 0.04 |
| Race | ||||
| Black | 365 (34.2) | 761 (42.2) | 79 (54.9) | <0.001 |
| Hispanic | 133 (12.5) | 170 (9.4) | 15 (10.4) | 0.04 |
| Household income | <0.001 | |||
| ≤$20,000 | 227 (21.3) | 512 (28.4) | 64 (44.4) | |
| $20,001–$50,000 | 247 (23.1) | 497 (27.6) | 34 (23.9) | |
| $50,001–$100,000 | 242 (22.7) | 346 (19.2) | 15 (10.1) | |
| >$100,000 | 172 (16.1) | 182 (10.1) | 8 (5.3) | |
| No answer | 179 (16.8) | 265 (14.7) | 23 (16.3) | |
| Education level | <0.001 | |||
| <High school | 165 (15.4) | 334 (18.5) | 32 (21.9) | |
| High school graduate | 164 (15.4) | 348 (19.3) | 30 (20.7) | |
| Some college | 271 (25.4) | 551 (30.6) | 53 (36.8) | |
| ≥College graduate | 466 (43.8) | 569 (31.6) | 30 (20.6) | |
| Health insurance | 0.14 | |||
| Yes | 873 (81.9) | 1572 (87.3) | 120 (83.3) | |
| No | 193 (18.1) | 228 (12.7) | 24 (16.7) | |
| Health insurance groups | <0.001 | |||
| None | 154 (14.4) | 191 (10.6) | 20 (13.6) | |
| Medicaid/public aid | 115 (10.8) | 284 (15.7) | 42 (29.2) | |
| Medicare | 256 (24.0) | 623 (34.6) | 46 (31.8) | |
| VA/military/CHAMPVA | 46 (4.4) | 97 (5.4) | 10 (7.2) | |
| Private/commercial | 221 (20.8) | 257 (14.3) | 15 (10.4) | |
| Unknown/incomplete | 274 (25.7) | 350 (19.4) | 11 (7.8) | |
| Current smoking | 99 (9.3) | 178 (9.9) | 14 (9.6) | 1.0 |
| Body mass index, kg/m2, mean ± SD | 31.2 ± 7.5 | 32.5 ± 7.9 | 34.1 ± 8.2 | <0.001 |
| Systolic blood pressure, mmHg, mean ± SD | 125.3 ± 19.6 | 128.4 ± 21.4 | 126.7 ± 20.0 | 0.005 |
| Comorbid conditions | ||||
| Diabetes mellitus | 430 (40.4) | 968 (53.7) | 88 (61.1) | <0.001 |
| Any cardiovascular disease | 256 (24.0) | 832 (46.2) | 87 (60.3) | <0.001 |
| Medications | ||||
| ACE inhibitors or ARB | 696 (65.3) | 1191 (66.1) | 83 (57.4) | 0.71 |
| Antiplatelet drugs | 510 (47.8) | 975 (54.1) | 87 (60.1) | 0.04 |
| β-blockers | 441 (41.4) | 1007 (55.9) | 99 (69.0) | <0.001 |
| Statins | 630 (59.1) | 1147 (63.7) | 87 (60.1) | 0.33 |
| Laboratory data | ||||
| eGFR, ml/min/1.73m2, mean ± SD | 44.9 ± 16.4 | 41.8 ± 17.1 | 39.4 ± 17.3 | <0.001 |
| Proteinuria, g/g, median (IQR) | 0.33 (0.08–1.54) | 0.43 (0.10–1.61) | 0.85 (0.15–2.19) | 0.02 |
| Serum albumin, g/dl, mean ± SD | 4.0 ± 0.4 | 3.9 ± 0.4 | 3.8 ± 0.4 | <0.001 |
| Hemoglobin, g/dl, mean ± SD | 13.0 ± 1.7 | 12.5 ± 1.8 | 12.1 ± 1.6 | <0.001 |
| Hospitalization data through year 4 visit | ||||
| No. of hospitalizations, mean ± SD | 0 | 2.2 ± 1.5 | 6.3 ± 4.2 | <0.001 |
| Length of hospital stay, d, median (IQR) | 0 | 0.5 (0.0–2.0) | 1.6 (0.7–3.3) | <0.001 |
| No. of readmissions within 30 d | 0 | 542 (12.9) | 452 (31.4) | <0.001 |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; CHAMPVA, Civilian Health and Medical Program of the Department of Veterans Affairs; eGFR, estimated glomerular filtration rate; IQR, interquartile range; SD, standard deviation.
Unless otherwise noted, values are presented as n (%).
Primary Causes for Hospitalizations
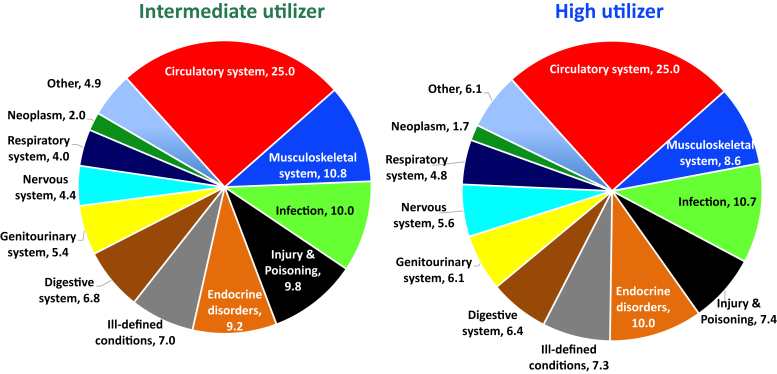
Among the 5658 hospitalizations, 5404 (96%) hospitalizations had ICD-9 codes available to determine the primary cause for hospitalization. For participants in the high-utilizer group, the top 5 reasons for hospitalization were due to circulatory system disorders (25%), infectious diseases (10.7%), and endocrine disorders (10%), musculoskeletal system disorders (8.6%), and injury and poisoning (7.4%) (Figure 3). Compared with the participants in the high-utilizer group, the intermediate-utilizer group had the same top 5 reasons for hospitalization.
Figure 3.
Primary causes of hospitalization. The top 5 causes of hospitalization were for complaints related to the circulatory system, infection, endocrine disorders, musculoskeletal system, or injury and poisoning for both intermediate- and high-utilizers.
All-Cause Hospitalization Trajectories and Risks of ESKD and Death
During a median follow-up time of 5.1 years, 544 participants progressed to ESKD and 437 participants experienced ESKD-censored death. Table 2 shows the unadjusted and multivariable-adjusted associations between hospitalization trajectory groups and each outcome. Compared with the low-utilizer group, the multivariable-adjusted hazard ratio of ESKD was 1.49 (95% CI 1.22–1.84) for the intermediate-utilizer group and 1.75 (95% CI 1.20–2.56) for the high-utilizer group. Compared with the low-utilizer group, the multivariable-adjusted hazard ratio of ESKD-censored death was 1.48 (95% CI 1.17–1.87) for the intermediate-utilizer group and 2.58 (95% CI 1.74–3.83) for the high-utilizer group. The estimates remained independent of multiple covariates, including proteinuria and eGFR, which were the strongest determinants of risk. There was no evidence of statistical interaction between hospitalization trajectory groups and eGFR for either ESKD (P for interaction: 0.70) or ESKD-censored death (P for interaction: 0.52).
Table 2.
Risks of ESKD and death by hospitalization trajectory (N = 3012)
| Trajectory Groups | n | No. of Events | Events per 1000 person-years | Model 1, HR (95% CI) | Model 2, HR (95% CI) | Model 3, HR (95% CI) | Model 4, HR (95% CI) |
|---|---|---|---|---|---|---|---|
| ESKD | |||||||
| Low-utilizer | 1066 | 137 | 23.7 | Reference | Reference | Reference | Reference |
| Intermediate-utilizer | 1802 | 368 | 41.4 | 1.73 (1.42–2.11) | 1.74 (1.42–2.13) | 1.59 (1.30–1.95) | 1.49 (1.22–1.84) |
| High-utilizer | 144 | 39 | 64.8 | 2.64 (1.85–3.77) | 2.34 (1.63–3.37) | 2.05 (1.42–2.97) | 1.75 (1.20–2.56) |
| ESKD-censored death | |||||||
| Low-utilizer | 1066 | 100 | 17.3 | Reference | Reference | Reference | Reference |
| Intermediate-utilizer | 1802 | 299 | 33.6 | 1.95 (1.56–2.45) | 1.64 (1.30–2.07) | 1.51 (1.19–1.91) | 1.48 (1.17–1.87) |
| High-utilizer | 144 | 38 | 63.2 | 3.69 (2.54–5.36) | 3.29 (2.24–4.83) | 2.82 (1.90–4.19) | 2.58 (1.74–3.83) |
CI, confidence interval; HR, hazards ratio.
Model 1 is unadjusted.
Model 2 is stratified by center and adjusts for age, sex, race, ethnicity, income level, education level, and health insurance.
Model 3 is Model 2 with further adjustment for systolic blood pressure, body mass index, smoking status, diabetes mellitus, any cardiovascular disease, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, beta-blockers, statins, and antiplatelet drugs.
Model 4 is Model 3 with further adjustment for hemoglobin, serum albumin, natural log transformed proteinuria, and eGFR.
Sensitivity Analyses
Because 2051 (36.2%) hospitalizations from baseline to the year 4 visit among the 3012 participants had a length of stay ≤1 day, we repeated the primary analysis only including hospitalizations >1 day (Supplementary Figure S4). After multivariable adjustment, there was a 1.33- and 1.66-fold increased risk for progression to ESKD in the intermediate- and high-utilizer groups compared with the low-utilizer group, respectively. There was a 1.83- and 3.46-fold increased risk for ESKD-censored death in the intermediate- and high-utilizer groups compared with the low-utilizer group, respectively (Supplementary Table S1).
After introducing a 1-year lag, 2774 participants with 4984 hospitalizations were eligible for creation of the hospitalization trajectory groups because they did not progress to ESKD and survived to their year 5 study visit. After multivariable adjustment, there was a 1.65- and 1.90-fold increased risk for progression to ESKD in the intermediate- and high-utilizer groups compared with the low-utilizer group, respectively. There was a 1.47- and 2.91-fold increased risk for ESKD-censored death in the intermediate- and high-utilizer groups compared with the low-utilizer group, respectively (Supplementary Table S2).
There were 3497 participants with 3387 hospitalizations eligible for creation of the hospitalization trajectory groups because they did not progress to ESKD and survived to their year 2 visit (Supplementary Figure S5). After multivariable adjustment, there was a 1.33- and 2.29-fold increased risk for progression to ESKD in the intermediate- and high-utilizer groups compared with the low-utilizer group, respectively. There was a 1.44- and 4.04-fold increased risk for ESKD-censored death in the intermediate- and high-utilizer groups compared with the low-utilizer group, respectively (Supplementary Table S3).
To account for baseline hospitalization status, we adjusted for hospitalization from the baseline to year 1 visit, which did not qualitatively change the primary results (Supplementary Table S4).
There were 3775 participants who did not progress to ESKD and survived to their year 1 visit with 1827 hospitalizations. There was a nominally higher risk for ESKD in individuals who had 1 or >1 hospitalization compared with individuals who had no hospitalizations, which was no longer statistically significant after multivariable adjustment. Individuals who had 1 or >1 hospitalization had a 1.24-fold and 1.81-fold higher risk for ESKD-censored death compared with individuals who had no hospitalizations, respectively (Supplementary Table S5).
Discussion
In this prospective study of more than 3000 participants with CKD stages 2 to 4, we identified distinct subgroups of hospital utilization in individuals with CKD based on their trajectories of cumulative all-cause hospitalization over 4 years. We found that participants with increased hospital utilization represented a patient population of lower socioeconomic status who had a greater prevalence of comorbid conditions, higher proteinuria, and lower kidney function compared with participants with less hospital utilization. High-utilizers were hospitalized more often than intermediate-utilizers despite similar primary causes for hospitalization. After multivariable adjustment, intermediate- and high-utilizers had significantly higher risks of subsequent ESKD and death compared with low-utilizers. Our findings suggest that cumulative all-cause hospitalization trajectories are able to identify subgroups of individuals with CKD who are at high risk of ESKD and death.
Population-level data reported that hospitalization rates are slowly declining over time among individuals with CKD.6 We found stable hospitalization incidence densities among all participants with CKD stages 2 to 4. However, consistent with prior reports, among the extreme phenotypes of CRIC Study participants who progressed to ESKD or died during follow-up, hospital utilization increased in the years prior to ESKD and death.3,6,13 These findings provide evidence that there are heterogeneous patterns of hospital utilization among individuals with CKD, which could be missed when limiting analyses to the population level. Our analytic approach, which deployed group-based trajectory modeling,23 allowed us to identify 3 subgroups of participants who had significantly different hospital utilization patterns over time. Although the low- (n = 1066) and intermediate-utilizer groups (n = 1802) represented 95% of the study cohort, a smaller high-utilizer group (n = 144) was responsible for more than 25% of the hospitalizations in the first 4 years of the study. Collectively, our results suggest that trajectories of cumulative all-cause hospitalization identify high-risk individuals with CKD who have rapidly declining health, as suggested by their need for increased health care resource utilization.
Prior data identified older age, female sex, black race, multiple comorbid conditions, and worse kidney function as risk factors for hospitalization among individuals with CKD.3,6,26, 27, 28, 29 Our results confirm the findings from prior studies as our study participants who had increasing hospital utilization over time had lower socioeconomic status, a greater prevalence of comorbid conditions, higher proteinuria, and lower eGFR. Our data suggest that compared with other subgroups, the high-utilizer phenotype possesses more medical and social complexities, which along with declining health status may lead to more hospitalizations over time.
A number of studies have suggested that the primary causes for hospitalization among individuals with CKD are related to cardiovascular or infectious diseases.3,13,30 Similar to the prior published data, our study participants were most likely to be hospitalized for circulatory system or infectious reasons. Interestingly, we found similar primary causes for hospitalization across trajectory groups. However, compared with intermediate-utilizers, high-utilizers had slightly longer lengths of stay and a higher likelihood of rehospitalization within 30 days, which may be attributable to the combination of increased medical complexity and low socioeconomic status that we observed in the high-utilizer group. We speculate that social disadvantage of the high-utilizer group may have limited access to high-quality outpatient preventive and postdischarge follow-up care.31 Certain comorbid conditions, such as anemia, may lead to hospitalizations if not well managed, but strategies to effectively manage anemia may reduce hospitalizations in individuals with advanced CKD.32 Our findings warrant additional research to investigate whether specific primary causes of hospitalization that are representative of ambulatory care–sensitive conditions22,33,34 may be preventable by interventions such as telehealth monitoring,35,36 home hospital services,15,37,38 or enhanced ambulatory care.11,16,17
Although it has been long understood that CKD increases the risks for hospitalization,3,6,13,14,26,27,29 most studies analyze time to first hospitalization and do not fully capture the global burden of hospitalizations. Few studies have evaluated the association of hospital utilization over time with adverse clinical outcomes in individuals with CKD. One study found that higher numbers of predialysis hospitalizations increased the risk for 1-year mortality,30 and another found that higher numbers of hospitalizations for heart failure had a graded association with CKD progression and death in individuals with CKD stages 2 to 4.39 We found that intermediate and high all-cause hospital utilization provided a stepwise increase in the risks of subsequent ESKD and death independent of known risk factors including proteinuria and kidney function. The magnitude of association between high hospital utilization and subsequent ESKD and death remained robust in our sensitivity analyses, where we adjusted for baseline hospitalization status, excluded short-stay hospitalizations (≤1 day), introduced a lag time period, or used less time to form trajectory groups. Our results support the associations found in prior studies that focus on cause-specific hospitalizations and expand the evidence base for the impact of the global burden of hospitalizations over time on the health of individuals with CKD. If our findings are confirmed, all-cause hospitalizations could help enrich clinical trial populations to identify high-risk individuals with CKD or be considered a surrogate outcome in future studies of individuals with CKD.
Strengths of this study include use of a large and well-characterized cohort of individuals with CKD, incorporation of analytic methods that are able to subphenotype individuals based on hospitalization trajectories over time, and detailed covariate data that allowed for comprehensive multivariable adjustment. This study has limitations. Our finding of lower hospital utilization in the year preceding ESKD or death may have been due to the low number of participants and the resulting unstable estimates of hospitalization incidence densities. Because our study design required participants to survive 4 years without development of ESKD, our study population was not identical to the entire CRIC Study population. We cannot account for severity of illness during hospitalizations, which could lead to worsening health over time. Although the CRIC Study was able to capture >90% of hospitalizations, it was possible that some hospitalizations were missed. Although we required 4 years of hospitalization data to generate trajectories that may not always be available to treating providers, our results were similar when using 2 years of hospitalization data, which more robustly informed risks of adverse outcomes than 1 year of hospitalization data. We adjusted for socioeconomic and insurance status to account for the intersection of health care access and delivery, but we were unable to determine whether repeated hospitalizations over time were due to issues surrounding health care access or quality.
In conclusion, our study demonstrates that cumulative all-cause hospitalization trajectories identify subgroups of individuals with CKD at high risk of subsequent ESKD and death independent of known risk factors. Although increasing hospitalizations over time may capture inadequacies of the health care system, they may represent another available, but potentially overlooked, severity of illness marker that could be incorporated into a patient’s electronic medical record for review by the treating physician. Changing the trajectory of a patient’s health is difficult even with enhanced care,40 but our study provides empirical evidence for the associations of increasing hospital utilization over time with adverse outcomes and suggests the need for design and evaluation of innovative care delivery models to reduce the burden of hospital utilization among individuals with CKD.
Acknowledgments
This work was supported by the George M. O’Brien Kidney Research Center at Northwestern University (NU-GoKIDNEY; P30DK114857) from the National Institutes of Health (NIH) / National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). AS is supported by NIH grant K23DK120811, the Dixon Translational Research Grants Initiative at Northwestern Medicine and the Northwestern University Clinical and Translational Sciences Institute (UL1TR001422), and NIDDK Kidney Precision Medicine Project Opportunity Pool grant under U2CDK114886. RM is supported by grant KL2TR001424 from the NIH’s National Center for Advancing Translational Sciences. DIC is supported by NIH grant K23DK125670. KTM is supported, in part, by grant P20GM109036 from the National Institute of General Medical Sciences. TI is supported by NIH grants R01DK102438, R01DK110087, and U01DK099930 from the NIDDK and K24HL150235 from the National Heart, Lung, and Blood Institute. Funding for the Chronic Renal Insufficiency Cohort study was obtained from grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902 under a cooperative agreement from NIDDK. In addition, this study was supported, in part, by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/National Center for Advancing Translational Sciences (UL1TR000003), the Johns Hopkins Institute for Clinical and Translational Research (UL1TR000424), University of Maryland General Clinical Research Center (M01RR-16500), Clinical and Translational Science Collaborative of Cleveland (UL1TR000439), Michigan Institute for Clinical and Health Research (UL1TR000433), University of Illinois at Chicago Clinical and Translational Science Awards (UL1RR029879), Tulane Center of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases (P20GM109036), and Kaiser Permanente National Institutes of Health/National Center for Research Resources University of California San Francisco Clinical and Translational Science Institute (UL1RR024131).
Part of this work was presented as an oral presentation at the American Society of Nephrology Scientific Session on October 25, 2018, in San Diego, California.
Author Contributions
Concept and design: AS, JPL, TI; acquisition, analysis or interpretation of data: AS, XC, RM, JPL, TI; drafting of manuscript: AS, TI; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: XC, AS, JL, JYH, TI; obtained funding: TI; administrative, technical, or material support: AS, XC, JPL, TI; supervision: AS, TI.
Footnotes
Figure S1. Flowchart describing participants included to characterize longitudinal evolution of hospital use.
Figure S2. Flowchart describing study cohort to derive hospitalization trajectories.
Figure S3. Posterior probability of membership to each hospitalization trajectory group.
Figure S4. Cumulative all-cause hospitalization trajectories for hospitalizations with length of stay greater than 1 day.
Figure S5. Cumulative all-cause hospitalization trajectories through the year 2 visit.
Table S1. Risks of ESKD and death by hospitalization trajectory for hospitalization with length of stay greater than 1 day.
Table S2. Risks of ESKD and death by hospitalization trajectory after introduction of 1-year lag period.
Table S3. Risks of ESKD and death by hospitalization trajectory formed through the year 2 visit.
Table S4. Risks of ESKD and death by hospitalization trajectory after adjustment for baseline hospitalization status.
Table S5. Risks of ESKD and death by number of hospitalizations in the first year of the CRIC Study.
Contributor Information
Anand Srivastava, Email: anand.srivastava@northwestern.edu.
CRIC Study Investigators:
Lawrence J. Appel, Harold I. Feldman, Alan S. Go, Jiang He, Robert G. Nelson, Mahboob Rahman, Panduranga S. Rao, Vallabh O. Shah, Raymond R. Townsend, and Mark L. Unruh
Appendix
List of CRIC Study Investigators
Lawrence J. Appel, Harold I. Feldman, Alan S. Go, Jiang He, Robert G. Nelson, Mahboob Rahman, Panduranga S. Rao, Vallabh O. Shah, Raymond R. Townsend, and Mark L. Unruh.
Disclosure
AS received honoraria from Horizon Therapeutics PLC and AstraZeneca, and consulting fees from CVS Caremark. RM has interest in Abbot Laboratories, AbbVie Inc, and Teva Pharmaceuticals Industries Ltd and has received honoraria from Akebia/Otsuka. TI received grant support from Shire, honoraria from Bayer and Eli Lilly, and consulting fees from Kyowa Kirin and LifeSci Capital LLC. All the other authors declared no competing interests.
Supplementary Material
Figure S1. Flowchart describing participants included to characterize longitudinal evolution of hospital use.
Figure S2. Flowchart describing study cohort to derive hospitalization trajectories.
Figure S3. Posterior probability of membership to each hospitalization trajectory group.
Figure S4. Cumulative all-cause hospitalization trajectories for hospitalizations with length of stay greater than 1 day.
Figure S5. Cumulative all-cause hospitalization trajectories through the year 2 visit.
Table S1. Risks of ESKD and death by hospitalization trajectory for hospitalization with length of stay greater than 1 day.
Table S2. Risks of ESKD and death by hospitalization trajectory after introduction of 1-year lag period.
Table S3. Risks of ESKD and death by hospitalization trajectory formed through the year 2 visit.
Table S4. Risks of ESKD and death by hospitalization trajectory after adjustment for baseline hospitalization status.
Table S5. Risks of ESKD and death by number of hospitalizations in the first year of the CRIC Study.
References
- 1.Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance System website. https://nccd.cdc.gov/CKD Available at:
- 2.Anavekar N., McMurrary J., Velazquez E. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 3.Go A., Chertow G., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Levey A., Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 5.Chen T.K., Knicely D.H., Grams M.E. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322:1294–1304. doi: 10.1001/jama.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Renal Data System: United States Renal Data System 2018 annual data report. https://www.usrds.org/2017/view/Default.aspx Available at:
- 7.Tonelli M., Wiebe N., Manns B.J. Comparison of the complexity of patients seen by different medical subspecialists in a universal health care system. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Healthcare Cost and Utilization Project: National inpatient hospital costs: the most expensive conditions by payer, 2013. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.jsp Available at: [PubMed]
- 9.Martin A.B., Hartman M., Benson J., Catlin A., National Health Expenditure Accounts Team National health spending in 2014: faster growth driven by coverage expansion and prescription drug spending. Health Aff (Millwood) 2016;35:150–160. doi: 10.1377/hlthaff.2015.1194. [DOI] [PubMed] [Google Scholar]
- 10.Nichols G.A., Ustyugova A., Deruaz-Luyet A. Health care costs by type of expenditure across eGFR stages among patients with and without diabetes, cardiovascular disease, and heart failure. J Am Soc Nephrol. 2020;31:1594–1601. doi: 10.1681/ASN.2019121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doshi S., Wish J. Strategies to reduce rehospitalization in patients with CKD and kidney failure. Clin J Am Soc Nephrol. 2021;16:328–334. doi: 10.2215/CJN.02300220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tonelli M., Wiebe N., Guthrie B. Comorbidity as a driver of adverse outcomes in people with chronic kidney disease. Kidney Int. 2015;88:859–866. doi: 10.1038/ki.2015.228. [DOI] [PubMed] [Google Scholar]
- 13.Mix T., St Peter W., Ebben J. Hospitalization during advancing chronic kidney disease. Am J Kidney Dis. 2003;42:972–981. doi: 10.1016/j.ajkd.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Daratha K.B., Short R.A., Corbett C.F. Risks of subsequent hospitalization and death in patients with kidney disease. Clin J Am Soc Nephrol. 2012;7:409–416. doi: 10.2215/CJN.05070511. [DOI] [PubMed] [Google Scholar]
- 15.Levine D.M., Ouchi K., Blanchfield B. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172:77–85. doi: 10.7326/M19-0600. [DOI] [PubMed] [Google Scholar]
- 16.Vassalotti J., DeVinney R., Lukasik S. CKD quality improvement intervention with PCMH integration: health plan results. Am J Manag Care. 2019;25:e326–e333. [PubMed] [Google Scholar]
- 17.Conley J., O'Brien C.W., Leff B.A. Alternative strategies to inpatient hospitalization for acute medical conditions: a systematic review. JAMA Intern Med. 2016;176:1693–1702. doi: 10.1001/jamainternmed.2016.5974. [DOI] [PubMed] [Google Scholar]
- 18.Feldman H., Appel L., Chertow G. Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 19.Lash J.P., Go A.S., Appel L.J. Chronic Renal Insufficiency Cohort Study Group. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denker M., Boyle S., Anderson A.H. Chronic Renal Insufficiency Cohort Study Investigators. Chronic Renal Insufficiency Cohort Study (CRIC): overview and summary of selected findings. Clin J Am Soc Nephrol. 2015;10:2073–2083. doi: 10.2215/CJN.04260415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parsa A., Kao W.H., Xie D. AASK Study Investigators; CRIC Study Investigators. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figueroa J.F., Burke L.G., Zheng J. Trends in hospitalization vs observation stay for ambulatory care-sensitive conditions. JAMA Intern Med. 2019;179:1714–1716. doi: 10.1001/jamainternmed.2019.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagin D., Odgers C. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 24.Jones B., Nagin D., Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29:374–393. [Google Scholar]
- 25.Nagin D., Odgers C. Group-based trajectory modeling (nearly) two decades later. J Quant Criminol. 2010;26:445–453. doi: 10.1007/s10940-010-9113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland D., Lam M. Predictors of hospitalization and death among pre-dialysis patients: a retrospective cohort study. Nephrol Dial Transplant. 2000;15:650–658. doi: 10.1093/ndt/15.5.650. [DOI] [PubMed] [Google Scholar]
- 27.Xie Y., Bowe B., Xian H. Rate of kidney function decline and risk of hospitalizations in stage 3A CKD. Clin J Am Soc Nephrol. 2015;10:1946–1955. doi: 10.2215/CJN.04480415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitsch D., Nonyane B.A., Smeeth L. CKD and hospitalization in the elderly: a community-based cohort study in the United Kingdom. Am J Kidney Dis. 2011;57:664–672. doi: 10.1053/j.ajkd.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan S.S., Kazmi W.H., Abichandani R. Health care utilization among patients with chronic kidney disease. Kidney Int. 2002;62:229–236. doi: 10.1046/j.1523-1755.2002.00432.x. [DOI] [PubMed] [Google Scholar]
- 30.Shah S., Meganathan K., Christianson A.L. Pre-dialysis acute care hospitalizations and clinical outcomes in dialysis patients. PLoS One. 2019;14 doi: 10.1371/journal.pone.0209578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniel H., Bornstein S.S., Kane G.C. Addressing social determinants to improve patient care and promote health equity: an American College of Physicians Position Paper. Ann Intern Med. 2018;168:577–578. doi: 10.7326/M17-2441. [DOI] [PubMed] [Google Scholar]
- 32.Block G.A., Block M.S., Smits G. A pilot randomized trial of ferric citrate coordination complex for the treatment of advanced CKD. J Am Soc Nephrol. 2019;30:1495–1504. doi: 10.1681/ASN.2018101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiebe N., Klarenbach S.W., Allan G.M. Alberta Kidney Disease Network. Potentially preventable hospitalization as a complication of CKD: a cohort study. Am J Kidney Dis. 2014;64:230–238. doi: 10.1053/j.ajkd.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Ronksley P.E., Hemmelgarn B.R., Manns B.J. Potentially preventable hospitalization among patients with CKD and high inpatient use. Clin J Am Soc Nephrol. 2016;11:2022–2031. doi: 10.2215/CJN.04690416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishani A., Christopher J., Palmer D. Center for Innovative Kidney Care. Telehealth by an interprofessional team in patients with CKD: a randomized controlled trial. Am J Kidney Dis. 2016;68:41–49. doi: 10.1053/j.ajkd.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Thilly N., Chanliau J., Frimat L. Cost-effectiveness of home telemonitoring in chronic kidney disease patients at different stages by a pragmatic randomized controlled trial (eNephro): rationale and study design. BMC Nephrol. 2017;18:126. doi: 10.1186/s12882-017-0529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gravil J.H., Al-Rawas O.A., Cotton M.M. Home treatment of exacerbations of chronic obstructive pulmonary disease by an acute respiratory assessment service. Lancet. 1998;351:1853–1855. doi: 10.1016/s0140-6736(97)11048-0. [DOI] [PubMed] [Google Scholar]
- 38.Davies L., Wilkinson M., Bonner S. "Hospital at home" versus hospital care in patients with exacerbations of chronic obstructive pulmonary disease: prospective randomised controlled trial. BMJ. 2000;321:1265–1268. doi: 10.1136/bmj.321.7271.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bansal N., Zelnick L., Bhat Z. CRIC Study Investigators. Burden and outcomes of heart failure hospitalizations in adults with chronic kidney disease. J Am Coll Cardiol. 2019;73:2691–2700. doi: 10.1016/j.jacc.2019.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finkelstein A., Zhou A., Taubman S., Doyle J. Health care hotspotting—a randomized, controlled trial. N Engl J Med. 2020;382:152–162. doi: 10.1056/NEJMsa1906848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.