Abstract
Since the discovery of manifest Zn deficiency in 1961, the increasing number of studies demonstrated the association between altered Zn status and multiple diseases. In this chapter, we provide a review of the most recent advances on the role of Zn in health and disease (2010 – 20), with a special focus on the role of Zn in neurodegenerative and neurodevelopmental disorders, diabetes and obesity, male and female reproduction, as well as COVID-19. In parallel with the revealed tight association between ASD risk and severity and Zn status, the particular mechanisms linking Zn2+ and ASD pathogenesis like modulation of synaptic plasticity through ProSAP/Shank scaffold, neurotransmitter metabolism, and gut microbiota, have been elucidated. The increasing body of data indicate the potential involvement of Zn2+ metabolism in neurodegeneration. Systemic Zn levels in Alzheimer’s and Parkinson’s disease were found to be reduced, whereas its sequestration in brain may result in modulation of amyloid β and α--synuclein processing with subsequent toxic effects. Zn2+ was shown to possess adipotropic effects through the role of zinc transporters, zinc finger proteins, and Zn-α2-glycoprotein in adipose tissue physiology, underlying its particular role in pathogenesis of obesity and diabetes mellitus type 2. Recent findings also contribute to further understanding of the role of Zn2 + in spermatogenesis and sperm functioning, as well as oocyte development and fertilization. Finally, Zn2+ was shown to be the potential adjuvant therapy in management of novel coronavirus infection (COVID-19), underlining the perspectives of zinc in management of old and new threats.
1. Introduction
Zinc is a IIB group metal and is the 23rd most abundant element in the Earth’s crust. The history of investigation of Zn essentiality dates back for more than 150 years. For example, its essentiality for Aspergillus niger was demonstrated in 1869, for plants in 1926, for laboratory rodents in 1933, for pigs in 1955, and for humans in 1963 (King, 2011; Prasad, 2014a, 2014b). The essential role of Zn in living organisms is mediated by its involvement in a plethora of physiological processes. More than 300 enzymes and proteins were considered as Zn-dependent, being regulated by more than 2000 transcription factors (Prasad, 2014a, 2014b). Particularly, Zn plays a significant role in regulation of cell cycle, DNA replication and reparation, cell proliferation and differentiation, apoptosis, metabolism of lipids and carbohydrates, as well as other processes (Chasapis, Ntoupa, Spiliopoulou, & Stefanidou, 2020; Maret, 2017a).
A significant part of biological effects of Zn is mediated through by antioxidant and anti-inflammatory role ( Jarosz, Olbert, Wyszogrodzka, Młyniec, & Librowski, 2017). Zinc ions are considered as key regulators of redox homeostasis. Antioxidant activity of Zn2+ may be mediated by its direct interaction with amino acid residues preventing oxidative modification of protein molecules, its structural role in enzymatic antioxidants (Cu, Zn-superoxide dismutase) and metallothionein synthesis, as well as modulation of Nrf2 signaling. At the same time, at increasing concentrations Zn2+ may possess prooxidant activity (Maret, 2019; Oteiza, 2012). Zn-dependent modulation of redox homeostasis results in regulation of redox-sensitive transcription factors including NF-κB. Specifically, the anti-inflammatory effect of Zn2+ has been shown to be dependent on NF-κB inhibition with subsequent downregulation of target genes of proinflammatory cytokines, including TNF-α and IL-1β (Jarosz et al., 2017). In addition, Zn2+ may also modulate anti-inflammatory pathways including TGFβ, IL-2, and IL-4 downstream signaling (Gammoh & Rink, 2017). Taken together, these mechanisms result in a significant Zn-induced reduction in circulating C-reactive protein in human studies (Mousavi, Djafarian, Mojtahed, Varkaneh, & Shab-Bidar, 2018). Apart from modulation of inflammatory response, Zn is also known as an essential regulator of immune system development and functioning through regulation of proliferation and maturation of T- and B-lymphocytes, natural killers and dendritic cells, as well as B cell-mediated antibody production, phagocytosis, and antigen presentation (Wessels, Maywald, & Rink, 2017).
Due to involvement of Zn in a variety of physiological processes, deficiency in this trace element is associated with metabolic dysfunction and the resulting diseases. Zn deficiency as well as evidence of its essentiality was discovered by Prof. Ananda Prasad in the Middle East, where he described the case of Zn deficiency in 21 y.o. Iranian farmer characterized by hepatosplenomegaly, anemia, dwarfism, and hypogonadism (Prasad, Halsted, & Nadimi, 1961). Later studies by Prasad and coauthors further characterized the clinical manifestations of Zn deficiency (Prasad, 2013). Since then an increasing number of studies demonstrated the role of altered Zn status in a variety of pathological processes and diseases. Briefly, Zn deficiency was shown to be associated with neuropsychiatric and neurosensory disorders, skin lesions, acrodermatitis, hypogonadism and infertility, growth retardation, as well as thymic atrophy and immune dysfunction. In turn, excessive Zn intake may result in copper imbalance, gastrointestinal symptoms including nausea and vomiting, lymphocyte dysfunction, neurotoxicity, and respiratory symptoms in the case of Zn smoke inhalation (Plum, Rink, & Haase, 2010).
A state-of-the-art review on zinc biology and its particular health effects was described in a number of excellent reviews, summarizing the key findings in this field (Chasapis et al., 2020; King, 2011; Plum et al., 2010; Roohani, Hurrell, Kelishadi, & Schulin, 2013). Therefore, in this chapter, we provided a review of the most recent advances on the role of Zn in health and disease based on papers published in the last decade (2010–2020). In view of the high number of invaluable studies on molecular biology and pathology of Zn, we focused only on certain aspects of Zn biology that have been advanced over the last decade, providing an insight into the data obtained on the role of Zn in neurodegenerative and neurodevelopmental disorders, diabetes and obesity, male and female reproduction, as well as COVID-19.
2. Zinc defiency worldwide: A 10-year update
Although the risk of low dietary Zn intake has significantly reduced from 22% to 16% in a period of 1992–2011, the prevalence of Zn deficiency is still significant (Kumssa et al., 2015). However, the existing data on Zn status in different regions on the edge of 2020s are highly variable. Zinc status was shown to be strongly related to economic development of the regions, achieving a prevalence of more than 20% in the majority of low- and middle-income countries (Gupta, Brazier, & Lowe, 2020). Despite reports on low prevalence of Zn deficiency in a number of countries including Afghanistan, Nigeria, and Chine, these outcomes may occur from the use of uncommon cutoffs (Hess, 2017).
High rate of Zn deficiency was observed in Latin America with the highest prevalence of inadequate Zn intake in Guyana (44%), Saint Vincent and Grenadines (42.9%), Bolivia (40.1%), Guatemala (38%), Paraguay (37.5%), Panama (36.5%), Peru (34.5%), El Salvador (34.5%), Nicaragua (33%), Honduras (31.6%), Suriname (31.6%), and Haiti (31.2%) (Cediel, Olivares, Brito, Cori, & López de Romaña, 2015). In Brazilian children the prevalence of dietary Zn insufficiency varies significantly from 16.6% to 46.0% (Pedraza & Sales, 2017), although certain regions (Southern Brazil) were characterized by low incidence of Zn deficiency in children (Sangalli, Rauber, & Vitolo, 2016). In Colombian 12- to 59-months-old children, the prevalence of Zn deficiency reached 49%, especially in poor families (Pinzón-Rondón, Hoyos-Martínez, Parra-Correa, Pedraza-Flechas, & Ruiz-Sternberg, 2019). Even higher rates of inadequate Zn status were detected in pregnant women from the Andean region (Ecuador) (Narváez-Caicedo, Moreano, Sandoval, & Jara-Palacios, 2018).
Analysis of national and subnational data from African countries demonstrated that the highest prevalence of Zn deficiency is observed in Nigeria (63%) followed by South Africa (39%) and Ethiopia (32%) (Harika, Faber, Samuel, Kimiywe, et al., 2017). Data from the same region demonstrated that in pregnant and non-pregnant women of reproductive age the prevalence of Zn deficiency is 46%–76% and 34%, respectively (Harika, Faber, Samuel, Mulugeta, et al., 2017). A recent meta-analysis demonstrated the prevalence of Zn deficiency in Ethiopian children and pregnant women of 38.4% and 59.9%, respectively (Berhe, Gebrearegay, & Gebremariam, 2019).
Middle East region is also characterized by high prevalence of Zn deficiency, although the existing data are highly variable. In a study originating from Bandar Abbas (Iran) the prevalence of Zn deficiency in 6-month to 12-year old children was estimated as 17.5% with higher rate in boys (20.94%) (Rahmati, Safdarian, Zakeri, & Zare, 2017). The results of the CASPIAN-V study which included more than 3500 children and adolescents (7–18 y.o.) revealed the prevalence of subclinical Zn deficiency of 4.9% in Iran (Azemati et al., 2020). In Turkey, subclinical Zn deficiency was observed in 27.8% of children and adolescents (6–18 y.o.) (Vuralli, Tumer, & Hasanoglu, 2017). In an elderly population from Riyadh (Saudi Arabia) the rate of Zn deficiency was found to be 36% (Alqabbani & AlBadr, 2020).
The prevalence of Zn deficiency in India was shown to be highly variable due to differences in locations and the studied groups (Gonmei & Toteja, 2018). In Uttar Pradesh the prevalence of Zn deficiency in 4–6 y.o. children was estimated as 65.3 (Sharma & Yadav, 2019). In turn, 17.9% children from Ludhiana district of Punjab were characterized by low serum Zn levels (Bains et al., 2015), whereas in women from the same location the prevalence of suboptimal serum Zn was 31.4% (Bains, Kaur, & Bajwa, 2019). It has been also demonstrated that the prevalence of suboptimal Zn intake in India has significantly increased from 17.1% to 24.6% in a period of 1983–2012 (Smith, DeFries, Chhatre, Ghosh-Jerath, & Myers, 2019).
In contrast, Zn status in China was significantly improved during the last decades. Data from China Nutrition and Health Survey 2002 and 2012 demonstrated that the prevalence of Zn deficiency in schoolchildren has changed significantly from 44.4% to 10.2% ( Liu, Piao, et al., 2017). Moreover, in younger children aged 3–5 y.o. the prevalence of Zn deficiency was even lower reaching 5.5% in rural and 2.4% urban children (Bi et al., 2020). 31% of Chinese adults were considered at high risk of Zn deficiency due to low dietary Zn intake during China Nutritional Transition Cohort Survey (CNTCS) 2015 (Wang et al., 2018).
Despite a high heterogeneity, the existing data demonstrate that the prevalence of Zn deficiency in developing countries is ~20%, although higher rates may be inherent to susceptible groups, such as infants, pregnant women, elderly, or low-income groups. A recent study also demonstrated that increased CO2 production may promote the risk of zinc deficiency for additional 132–180 million predominantly in South Asia and Africa people by 2050. Particularly, the estimated CO2-associated Zn deficiency may affect up to 48 million people only in India (Myers, Wessells, Kloog, Zanobetti, & Schwartz, 2015). In contrast to developing countries, economically developed countries are characterized by higher micronutrient intake. However, systematic data on Zn status in the Western countries are less available.
The results of 2005–2012 NHANES demonstrated that 15% of adult US population are characterized by Zn inadequacy (Reider, Chung, Devarshi, Grant, & Hazels Mitmesser, 2020). More recent estimates (NHANES 2011–2014) of the prevalence of low serum Zn were 3.8%, 8.6%, and 8.2% in children, adult men and women, respectively (Hennigar, Lieberman, Fulgoni, & McClung, 2018). In Japan, the prevalence of marginal Zn status in adult men and women was estimated as 46% and 38.4%, whereas Zn deficiency was revealed in 0.4% men and 0.6% women (Yokokawa et al., 2020). The highest rate of Zn deficiency was observed in infants and elderly (Yasuda & Tsutsui, 2016). Correspondingly, high rate of Zn deficiency (10.1%) was also observed in Norwegian community-living elderly (65–87 y.o.) reaching 13.1% in men and 7.3% in women (Kvamme, Grønli, Jacobsen, & Florholmen, 2015). In 60- to 84-years-old people involved in Berlin Aging Study II low plasma Zn levels were found in 18.7% ( Jung, Spira, Steinhagen-Thiessen, Demuth, & Norman, 2017). Therefore, existing data demonstrate that even in economically developed countries the prevalence of Zn deficiency may reach 10%, especially in several risk groups.
A population-wide assessment of hair Zn levels in Russia demonstrated that increased risk of Zn deficiency may be observed in 18%–46% of the population depending on the region (Skalny & Kiselev, 2012).
Generally, the existing data on the prevalence of Zn deficiency are highly variable resulting in complicated assessment of the worldwide prevalence of low Zn status. It is proposed that Zn deficiency is observed in approximately 17% of the global population, although these values refer to 2012 (Chasapis et al., 2020).
3. Autism
3.1. Zinc status and autism
Recent studies provide evidence on significantly altered Zn stats in autism spectrum disorder (ASD). Correspondingly, systematic review and meta-analysis demonstrated lower serum Zn levels in autistic patients than those in the neurotypical children. Concomitantly, the difference in hair Zn content was found to be region-specific, being higher and lower in cases living in non-Asian and Asian regions (Saghazadeh, Ahangari, Hendi, Saleh, & Rezaei, 2017). However, the results of another meta-analysis demonstrated lack of significant group difference in hair, nail, and teeth Zn levels between autistic and neurotypical children (Babaknejad, Sayehmiri, Sayehmiri, Mohamadkhani, & Bahrami, 2016). A number of other studies failed to reveal any significant alteration of Zn levels in patients with ASD (Skalny et al., 2017; Sweetman, O’Donnell, Lalor, Grant, & Greaney, 2019; Tschinkel, Bjørklund, Conón, Chirumbolo, & Nascimento, 2018).
Despite the accumulating evidence of group difference in Zn status, its relationship to autism severity was demonstrated only in the last decade. An Italian study revealed an inverse association between hair Zn levels, defective play, and stereotype behavior in autistic children (Fiore et al., 2020). In addition, the presence of ADHD in children with ASD was characterized by a more profound decline in hair Zn (Skalny, Mazaletskaya, et al., 2020). This observation generally corroborated an earlier study which demonstrated inverse correlation between Zn concentration and hyperactivity, as well as fine motor skills severity (Russo et al., 2012). However, Priya and Geetha (2011) revealed a significant positive correlation between hair and nail Zn content and CARS values in children with ASD (Priya & Geetha, 2011). The observed contradictions may result from differences in clinical characteristics of the patients as well as background nutritional status of the studied populations.
Although an inverse association between maternal multivitamin multi-mineral supplementation and ASD risk in the offspring was proposed to be partially mediated by Zn (Guo, Li, Zhai, & Ding, 2019), the question of Zn supplementation in autism remains opened. A recent study demonstrated that 12-week Zn supplementation significantly reduced CARS values and improved locomotor and object control score in 3–8 y.o. children with ASD that may be related to modulation of copper and metallothionein levels (Meguid et al., 2019).
3.2. Zinc and autism spectrum disorder pathogenesis
Recent advances in research on the particular role of zinc in autism demonstrated a role for the interplay between Zn and synaptic dysfunction in ASD pathogenesis, amplifying earlier reports on the involvement of Zn into regulation of neuroinflammation (Grabrucker & Grabrucker, 2017). The relationship between Zn and ASD stems from observations of behavioral deficits in Zn-deficient animals, demonstrating high rate of impaired social behavior, aggression, and anxiety (Hagmeyer, Haderspeck, & Grabrucker, 2014). Correspondingly, prenatal Zn deficiency was shown to induce autism-like behavior in adulthood (Grabrucker, Boeckers, & Grabrucker, 2016). Male but not female Znt3-deficient mice were demonstrated to have autism-like behavior, increased cortical volume, as well as MMP-9 and BDNF up-regulation (Yoo, Kim, Yoon, & Koh, 2016).
Prenatal LPS exposure was shown to induce maternal and offspring Zn deficiency and autism-like behavior, which was associated with impaired striatal dopaminergic signaling evidence by decreased striatal tyrosine hydroxylase and increased mTOR levels. These effects were shown to be reversed by Zn supplementation (Kirsten, Chaves-Kirsten, et al., 2015). In turn, Zn treatment was shown to ameliorate autism-like behavior and prevented increase in BDNF production in a rat model of autism induced by prenatal LPS exposure (Kirsten, Queiroz-Hazarbassanov, Bernardi, & Felicio, 2015). In a model of valproic acid (VPA)-induced autism Zn treatment also prevented repetitive and restrictive behaviors, impaired social interaction, and cognitive inflexibility, but not prevent VPA-induced reduction in striatal tyrosine hydroxylase protein expression (Cezar et al., 2018).
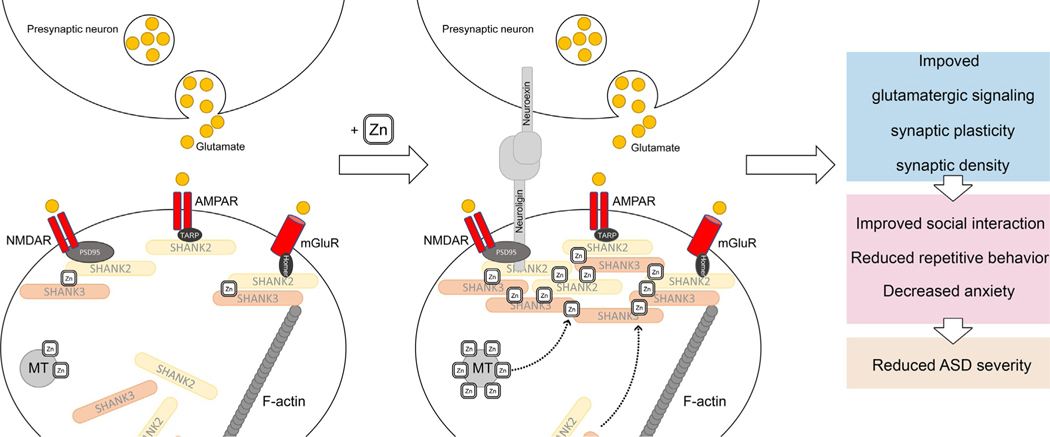
Grabrucker proposed that Zn2+ deficiency impairs synaptic ProSAP/Shank scaffold contributing to altered synapse plasticity, formation, and maturation, being associated with autism spectrum disorder-related behavior (Grabrucker, 2014; Grabrucker et al., 2014) (Fig. 1). Particularly, in a murine model of autism lacking Shank2 Zn mobilization significantly increased NMDAR signaling and improved social interaction (Lee et al., 2015). At the same time, in a Shank3 –/– mouse model of ASD, Zn supplementation prevented autism-like repetitive and anxiety behaviors, increased recruitment of Zn-sensitive SHANK2 to synapses, modulated postsynaptic NMDAR currents (Fourie et al., 2018) and presynaptic function at glutamatergic synapses (Vyas, Lee, Jung, & Montgomery, 2020). It is also notable that both models SHANK3-deficient mice and PZD mice are characterized by increased basal ganglia structures, whereas thalamus was differentially affected in genetic and non-genetic ASD models (Schoen et al., 2019). In turn, Zn supplementation was shown to significantly improve alterations in NMDAR subunits 1 and 2a, Shank gene expression, and decreased synaptic density associated with an ASD like biometal profile in a hippocampal cell culture (Hagmeyer et al., 2015).
Fig. 1.
Involvement of ProSAP/Shank scaffold Zn2+-induced modulation of synaptic (dys)function in ASD. Zn2+ supplementation was shown to increase SHANK2 recruitment to synapses, modulate postsynaptic NMDAR currents (Fourie et al., 2018), increase Shank gene expression, and improve synaptic density (Hagmeyer, Mangus, Boeckers, & Grabrucker, 2015), altogether resulting in improved social interaction and reduced autism-like repetitive and anxiety behaviors (Fourie et al., 2018; Lee et al., 2015). Furthermore, Zn deficiency is associated with altered synapse plasticity, formation, and maturation (Grabrucker, 2014; Grabrucker et al., 2014).
Of note, patients with Phelan McDermid Syndrome (PMDS) characterized by ASD-related behavioral problems and SHANK3 mutations were found to have lower Zn levels due to the potential association between SHANK3 deficiency and enterocyte zinc transporter (ZIP2, ZIP4) expression (Pfaender et al., 2017). Therefore, the relationship between SHANK family and zinc in shankopathies including autism may be possess significant effects not only in brain, but also in gut through alteration of Zn absorption and further aggravation of Zn dysregulation (Hagmeyer, Sauer, & Grabrucker, 2018).
Zinc was shown to improve dopamine uptake and amphetamine (AMPH)-induced, but not baseline dopamine efflux in ASD-associated human dopamine transporter (hDAT) mutation (Hamilton et al., 2015), suggesting a role for Zn in modulation of dopaminergic dysfunction in autism. Correspondingly, prenatally Zn deficient mice also considered as ASD model are characterized by altered brain lateralization, increased striatal volume, and impaired striatal lateralization of dopamine receptor 1 (DR1) expression (Grabrucker et al., 2018).
Several studies demonstrated the potential involvement of other Zn-dependent signaling mechanisms in ASD pathogenesis. Genetic deficiency of cytoskeleton-regulating cortactin binding protein 2 (CTTNBP2) results in altered synaptic plasticity and autism-like behavior, as well as reduced brain Zn levels, whereas Zn supplementation upregulated CTTNBP2-dependent synaptic proteins (Shih et al., 2020). Due to the role of purinergic system disturbances in ASD (Cheffer et al., 2018), downregulation of P2X7R-mediated signaling by Zn2+ may be at least partially involved in the role of Zn in ASD (Kovács et al., 2018). Modulation of gut-brain axis was also proposed as the potential mechanism of the role of Zn2+ in autism and other disorders (Vela et al., 2015).
Generally, the most recent human and laboratory data clearly demonstrate a significant role of altered Zn metabolism and autism pathogenesis. Although the potential benefits of Zn supplementation are widely discussed, further studies are required to evaluate clinical efficiency and the mechanisms of Zn supplementation in autism.
4. Zinc and Alzheimer’s disease
4.1. Zinc status and Alzheimer’s disease
Alzheimer’s disease (AD) pathogenesis is known to be tightly related to impaired metabolism of a number of toxic and essential metals including Zn2+ (González-Domínguez, García-Barrera, & Gómez-Ariza, 2014). Recent epidemiological and experimental studies further highlighted the potential role of Zn in Alzheimer’s disease. As results from systematic reviews and meta-analyses, the existing data demonstrate that circulating Zn levels were found to be reduced in patients with Alzheimer’s disease (Li, Zhang, Wang, & Zhao, 2017; Ventriglia et al., 2015). Of note, the use of acetylcholinesterase inhibitors in Alzheimer’s disease was shown to increase plasma Zn concentrations (Giacconi et al., 2019). At the same time, no significant increase in neocortical Zn levels was observed in AD patients (Schrag, Mueller, Oyoyo, Smith, & Kirsch, 2011). No significant association between dietary Zn intake and cognitive decline and/or Alzheimer’s disease risk was revealed (Loef, von Stillfried, & Walach, 2012). Moreover, in a group of AD patients with advanced age (70 y.o. and older) Zn supplementation was shown to reduce blood free Cu levels and afforded a protective effect against cognitive decline (Brewer & Kaur, 2013).
4.2. Zinc transporters in Alzheimer’s disease
The observed alterations in Zn2+ handling in AD are mediated by aberrant expression of zinc transporters (Xu, Xiao, Liu, & Lang, 2019). In post-mortem brain samples mRNA levels of zinc transporters LIV1, ZIP1, ZnT1, ZnT4, and ZnT6 were found to increase in association with AD progression (Beyer et al., 2012). Laboratory studies also demonstrated increased expression of ZnT1, ZnT3, ZnT4, ZnT6, and ZnT7 expression in APPswe/PS1dE9 transgenic mouse brains, although the localization patterns were quite different. Specifically, ZnT7 was expressed in the core of the plaque, ZnT3, ZnT5, and ZnT6 expression was localized in the peripheral part of the plaques, whereas ZnT1 and ZnT4 were expressed in all parts of the plaques (Zhang et al., 2010). It is notable that human amyloid precursor protein-transgenic (Tg2576) mice are characterized by Zn2+ and ZnT3 localization in dystrophic neurites in a proximity to amyloid plaques, as well as in activated astrocytes (Lee, Cho, Seo, Hwang, & Koh, 2012). Correspondingly, Zn ions were found to be deposited in Aβ plaques in a APP/PS1 mouse model even after adjustment for tissue density ( James et al., 2017).
Increased cerebrospinal fluid ZnT3 level was also shown to be associated with cognitive decline in AD (Enache et al., 2020). However, cerebral ZnT3 levels in AD patients with dementia were characterized by a significant reduction in association with increased tau tangle pathology and severity of delusions/agitation (Whitfield, Francis, Ballard, & Williams, 2018). In turn, in AD hippocampus, Zn2+ levels were found to be significantly increased in soluble fractions and synaptic vesicles in association with reduced ZnT3 expression (Bjorklund et al., 2012).
4.3. Direct interaction between zinc and amyloid β
Multiple studies have demonstrated direct interaction between Zn2+ cations and amyloid β, although the existing data are highly contradictory (Fig. 2). Several studies demonstrated that such interaction promotes amyloid aggregation and subsequent toxicity. Particularly, Zn2+ binding to Aβ metal-binding domain induced protein oligomerization (Istrate et al., 2016) and subsequent aggregation (Khatua et al., 2019), whereas inhibition of this interaction may prevent AD pathogenesis (Takeda & Tamano, 2015). Particularly, Zn2+ chelation by various agents was shown to prevent Zn-mediated Aβ aggregation and toxicity (Li, Xie, Dong, & Sun, 2018; (Liu, Dong, Liu, Zheng, & Sun, 2017); Tian, Wang, Wang, Li, & Wang, 2019). ZnAβ oligomers were proposed to be more neurotoxic, as well as more potent inducers of neuroinflammation through hippocampal microglia activation (Lee et al., 2018).
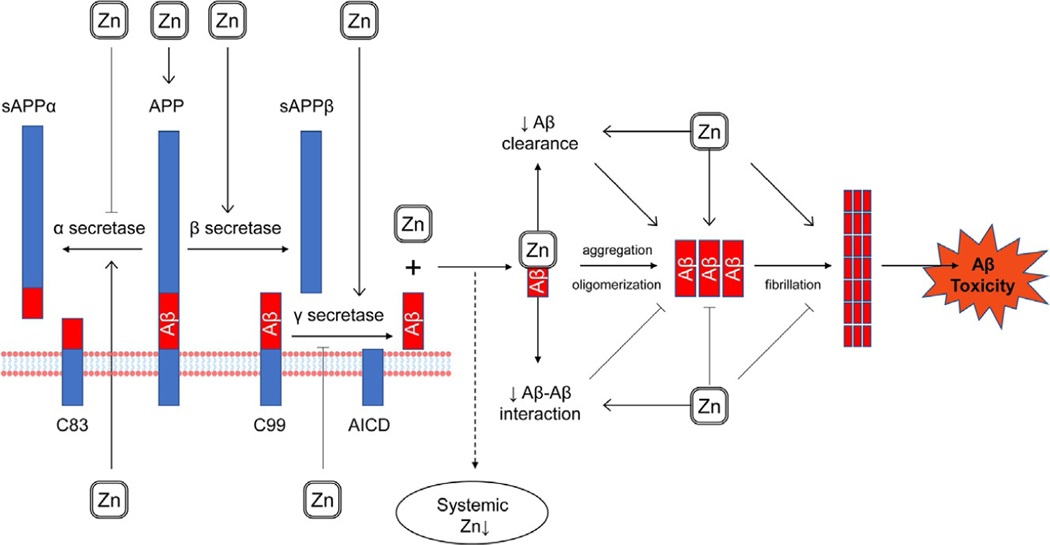
Fig. 2.
The impact of Zn2+ on amyloid production and processing. Zn2+ is capable of direct interaction with amyloid β, although data on this interaction have yet to be fully delineated. On one hand, Zn2+-Aβ binding induced protein oligomerization (Istrate et al., 2016) and subsequent aggregation (Khatua, Mondal, & Bandyopadhyay, 2019), whereas its prevention was shown to reduce Aβ toxicity (Takeda & Tamano, 2015). On another hand, certain studies reported that Zn2+ may decrease Aβ-Aβ interactions (Hane, Hayes, Lee, & Leonenko, 2016) and reduced fibril elongation (Abelein, Gräslund, & Danielsson, 2015). In turn, direct interaction between Zn2 + and Aβ results in plaque Zn sequestration ultimately leading to a decrease in systemic Zn levels (Baum et al., 2010). In addition to direct interaction with Aβ, Zn2+ was also shown to modulate amyloidogenic pathways (Kim, Lim, & Kim, 2018), although the data are highly contradictory. Particularly, certain studies demonstrated antiamyloidogenic effects of Zn2+ through inhibition of γ-secretase (Li, Liu, Xu, Li, & Xu, 2018), whereas others revealed activation of amyloidogenic pathway through inhibition of α-secretase and a concomitant activation of β- and γ-secretase (Wang et al., 2010).
On the other hand, numerous studies revealed that Zn may alter Aβ aggregation and subsequent fibril formation. Nanomolar Zn2+ concentrations were shown to decrease the affinity of Aβ-Aβ interactions, whereas Cu2+ did not possess any significant effect (Hane et al., 2016). In addition, Zn was shown to irreversibly perturb formation of Aβ(1–40) and Aβ(1–42) amyloid fibers even at concentrations lower than the affinity threshold (Matheou, Younan, & Viles, 2016). Zn-induced reduction of amyloid fibril elongation was shown to be mediated by Zn2+ binding to Aβ40 monomer with subsequent folding of N-terminus around metal atom (Abelein et al., 2015). It is proposed that targeted Zn delivery to the brain may reduce plaque size and neuroinflammation in a APP23 mouse model of AD (Vilella et al., 2018).
In addition, Zn2+ ions, unlike Cu2 + and Fe3 +, were also capable of promoting annular Aβ protofibril formation (Chen, Liao, Yu, Cheng, & Chen, 2011). In another study Zn2+ was shown to promote formation of smaller non-fibrillary Aβ42 aggregates with β-sheet structures (Zhang, Pauly, & Nagel-Steger, 2018). These findings generally corroborate earlier evidence of higher polymorphism of Aβ aggregates (Miller, Ma, & Nussinov, 2010).
It is also notable that Aβ1–42 uptake also alters intracellular Zn2+ handling that is responsible for at least of a part of the toxic effects (Tamano et al., 2020) and reduced systemic Zn levels (Baum et al., 2010). Specifically, Zn sequestration by amyloid was shown to prevent Zn2+ association with ProSAP2/Shank3 leading to impaired synapse maturation and decreased synaptic density in hippocampus (Grabrucker et al., 2011). In addition, direct interaction of extracellular Zn2+ with Aβ1–42 was shown to be essential for its uptake into rat hippocampus, whereas the uptake of both species was blocked by Ca2 +-EDTA (Takeda et al., 2017). At the same time, neither human Aβ1–40 nor rat Aβ1–42 injection (25 pmol, 1 μL) did not increase intracellular Zn2+ levels in the dentate gyrus, indicating a Zn-independent mechanism of toxicity (Tamano, Takiguchi, Shimaya, et al., 2019; Tamano, Takiguchi, Tanaka, et al., 2019).
The existing contradictions may have resulted from different methodological approaches to assess Zn-Ab interaction, as well as the animal species used in the experiments (Arena & Rizzarelli, 2019). It has also been proposed that the outcome of Zn-Aβ interaction may be a function of Zn concentrations (Viles, 2012), age and gender (Datki et al., 2020).
4.4. Zinc and (non)amyloidogenic pathways
Zinc was shown to be involved in the crossroad between amyloidogenic and non-amyloidogenic pathways of APP processing (Kim et al., 2018). Zn treatment was shown to increase APP expression and its amyloidogenic APP cleavage with subsequent Aβ deposition both in vivo (APP and presenilin 1 transgenic mice) and in vitro (SHSY-5Y) (Wang et al., 2010). Exposure to Zn-containing drinking water (10 ppm as ZnCO3) was shown to increase deposition of insoluble Aβ, whereas ZnT3 were characterized by an inverse correlation with soluble Aβ levels (Flinn, Bozzelli, Adlard, & Railey, 2014).
In contrast, Zn administration for 4–10 months at 100 mg/kg body weight/day did not increase Aβ or tau deposition in AβPP and AβPP/tau transgenic mice (Akiyama et al., 2012). Moreover, in a transgenic 3xTg-AD mouse model of AD Zn supplementation for 11–13 months (30 ppm as ZnSO4) significantly reduced Aβ and tau pathology in hippocampus, also significantly increasing BDNF expression and ameliorating memory deficits (Corona et al., 2010).
Although earlier data demonstrate the potential inhibitory effects of Zn2+ on α-secretase, in a cell model Zn exposure was shown to reduce Aβ1–40 production through inhibition of γ-secretase activity (Li, Liu, et al., 2018). It is also notable that Zn is capable of increasing APP-C99 dimerization and reducing its cleavage by γ-secretase (Gerber, Wu, Dimitrov, Osuna, & Fraering, 2017). At the same time, other studies demonstrated the potential role of Zn-metalloproteinases in Aβ and APP non-amyloidogenic cleavage (Gough, Parr-Sturgess, & Parkin, 2011). It has also been proposed that Aβ Zn binding may interfere with Aβ clearance mechanisms (Lanza, Bellia, & Rizzarelli, 2018).
4.5. Zinc and tau
In a transgenic murine model of Alzheimer’s disease expressing human gene for tau protein (P301L) Zn supplementation was shown to increase neurofibrillary tangle formation, although the level of free Zn2+ decreased due to sequestration by tangles (Craven, Kochen, Hernandez, & Flinn, 2018). Zn2+ was shown to increase Tau aggregation with subsequent cytotoxicity and apoptosis in SH-SY5Y neuroblastoma cells (Hu et al., 2017). Zn binding was also shown to increase toxicity of Tau aggregates (Zn2+-tau-R3), resulting in reduced neurite number and length (Li, Du, & Ni, 2019).
It has been also demonstrated that Zn promotes Tau phosphorylation at Ser262 through a mechanism involving GSK3β activation (Kwon et al., 2015). Release of Zn2+ in glutamatergic synapses was also shown to induce tau hyperphosphorylation through protein phosphatase 2A (PP2A) down-regulation (Sun et al., 2012) through Src-dependent enzyme phosphorylation (Xiong et al., 2013). Another study demonstrated a key role of COX-2 and its products PGI2 and F2α in tau phosphorylation (Wang, Guan, et al., 2017).
It is also notable that Zn2+-mediated increase in Tau toxicity may occur due to direct binding of metal ion to Tau without inducing its phosphorylation (Huang et al., 2014).
5. Parkinson’s disease (PD)
5.1. Zinc status in PD in epidemiological and experimental studies
In a meta-analysis including data from 822 PD patients and 777 healthy controls serum Zn levels were found to be significantly lower as compared to the control values (SMD=− 0.779, 95%CI=[−1.323, −0.234], P< 0.001) (Sun et al., 2017). Another meta-analysis revealed similar associations (SMD=− 0.59; 95% CI [−1.06, −0.12]; P = 0.014), also demonstrating a tendency to reduced CSF Zn levels (Du, Liu, Zhong, & Wei, 2017). At the same time, higher hair Zn levels were associated with depression, hallucination, illusion, paranoid ideation, and total Scales for Outcomes in Parkinson’s disease-Psychiatric Complications (SCOPA-PC) score in Brazilian PD patients (Dos Santos, Bezerra, Rocha, Barreto, & Kohlmeier, 2019). However, Zn intake was not associated with PD risk (Cheng et al., 2015). No significant PD-specific differences in Zn levels in brain regions were revealed (Genoud et al., 2017). Furthermore, patient-derived human olfactory neurosphere cultures with ATP13A2 deficiency were characterized by lower intracellular Zn2+ levels, aberrant expression of Zn transporters, and increased susceptibility to Zn2+-induced mitochondrial dysfunction and cytotoxicity (Park, Koentjoro, Veivers, Mackay-Sim, & Sue, 2014).
Intranasal exposure to ZnO nanoparticles resulted in significant accumulation of Zn in olfactory bulb, hippocampus, striatum, and cerebral cortex inducing oxidative stress and neuroinflammation. In a model of PC12 cells differentiated to dopaminergic neurons under NGF stimulation ZnO nanoparticles significantly affected cytoskeletal proteins, induced mitochondrial dysfunction and oxidative stress, leading to reduced cell viability (Liu, Yang, et al., 2020).
In turn, increased PARK9 expression improves zinc resistance increasing its transport to vesicular compartments, as well as reduces intracellular α-synuclein levels through its externalization in exosomes (Kong et al., 2014). It is also interesting that Zn supplementation to a level where control Drosophila flies demonstrate adverse effects significantly improves lifespan and motor function in parkin mutants Drosophila (Saini & Schaffner, 2010).
5.2. Zinc and α-synuclein
In male Wistar rats Zn exposure resulted in a significant Zn accumulation in substantia nigra, loss of dopaminergic neurons with striatal dopamine decline, as well as elevated α-synuclein expression and aggregation, whereas L-DOPA treatment partially restored these effects and behavioral changes (Kumar et al., 2018). Zn was found to be increased in the regions of α-synuclein aggregation in the olfactory bulb, whereas high free Zn2+ levels were noted in Lewy bodies, mitochondria, and lipofuscin, supporting the role of zinc in PD pathogenesis (Gardner et al., 2017).
Zn2+ was demonstrated to bind α-synuclein molecule due to the presence of high (Asp121) and lower (His50) affinity sites (Ramis, Ortega-Castro, Vilanova, Adrover, & Frau, 2018). Although certain studies demonstrated a significant increase in α-synuclein fibrillation in presence of high Zn2+ (Khodabandeh et al., 2020), it has been revealed that ZnO nanoparticles prevent α-synuclein fibrillation, shifting to lesser toxic flocs formation (Asthana et al., 2020). Increased Zn2+ levels were found to be associated with lysosomal dysfunction and α-synuclein accumulation in cases of ATP13A2 (PARK9) mutation characterized by juvenile-onset parkinsonism (Tsunemi & Krainc, 2014).
5.3. Zinc dopaminergic toxicity
Zn-induced alteration of dopaminergic system may be also considered as the potential Zn-dependent mechanism of PD. In an in vivo study using intraperitoneal injection of ZnSO4 (20 mg/kg) for 12 weeks, Zn-induced a decrease in striatal dopamine which was associated with NF-κB and Bax activation, whereas treatment with anti-inflammatory compounds ameliorated both behavioral and neurochemical disturbances, corroborating a significant role for neuroinflammation in Zn-induced dopaminergic neurodegeneration (Chauhan, Mittra, Kumar, Patel, & Singh, 2016). In addition, minocycline, an inhibitor of microglia activation, also alleviated Zn-induced alteration of tyrosine hydroxylase (TH), vesicular monoamine transporter-2 (VMAT-2), and dopamine transporter (DAT) expression, as well as loss of TH-positive neurons (Kumar et al., 2016). Such mechanism of Zn dopaminergic neurotoxicity resembles that of paraquat, a neurotoxic pesticide (Mittra, Chauhan, Singh, Patel, & Singh, 2020).
Zn2+ influx into nigrostriatal dopaminergic neurons due to AMPA receptor activation was considered as the key mechanism of 6-hydroxydopamine (6-OHDA)-induced PD (Tamano, Nishio, Morioka, & Takeda, 2019). Correspondingly, striatal synaptic Zn2+ release may contribute to 6-hydroxydopamine-induced behavioral, locomotor, and memory deficits in mice, whereas ZnT3-deficient mice characterized by lower synaptic Zn2+ release are more resistant to 6-hydroxydopamine-induced disorders (Sikora, Kieffer, Paoletti, & Ouagazzal, 2020). These findings corroborate earlier data from the same laboratory, indicating the association between AMPA-induced Zn2+ influx into nigrostriatal dopaminergic neurons and movement disorders in rats (Tamano, Morioka, Nishio, Takeuchi, & Takeda, 2018). Similarly, inhibition of Zn influx or toxicity using Zn chelator or antioxidant prevents paraquat-induced PD in rats (Tamano, Morioka, Nishio, et al., 2019; ). Of all redox events, increased NADPH-oxidase activity and reduced GSH pools were shown to play a key role in Zn2+-induced oxidative stress, apoptosis, and dopaminergic neurodegeneration (Kumar et al., 2012). In contrast, a recent study demonstrated that Zn2+ may possess protective effects against dopamine neurotoxicity due to modulation of Nrf2 and Bach-1 signaling (Kaufman, Salvador, Liu, & Oteiza, 2020).
It has been also demonstrated that Zn2+ exposure both activates and inhibits intrinsic excitability of nigrostriatal dopaminergic neurons through modulation of transient A-type K+ (KA) channel (Noh, Chang, Wang, & Chung, 2011). Modulation of Ca2+-channels may be also considered as the mechanism of Zn-induced modulation of dopaminergic system (Noh & Chung, 2019). Binding Zn2+ ion to Zn2+-binding site of the human dopamine transporter (DAT) was shown to possess biphasic effect with stimulation at low doses (1 μM) and inhibition at high ones (10 μM) (Li, Mayer, et al., 2017; Li, Zhang, et al., 2017).
The particular mechanisms of Zn toxicity to dopaminergic neurons are still to be elucidated. Currently, it has been revealed that Gadd45b-induced cell death pathway activation and the associated inhibition of JNK survival pathway may be considered as the potential mechanism underlying Zn and DA dopaminergic degeneration, being in agreement with the observed increase in Gadd45b mRNA levels in PD patients (Yang et al., 2016). Another mechanism of Zn2+-induced nigrostriatal dopaminergic degeneration may include inhibition of neuronal NOS activity, whereas the use of NO-donors was capable of reversal of striatal dopamine depletion, tyrosine hydroxylase expression, as well as the resulting behavioral changes (Singh et al., 2017).
Altogether, the most recent findings in the field demonstrate that Zn2+ may be involved in pathogenesis of neurodegenerative diseases through modulation of amyloid β and α-synuclein processing. Alteration of Zn homeostasis in neurodegenerative diseases was shown to be organ specific, resulting in reduced systemic Zn2+ levels in parallel with its cerebral accumulation and subsequent toxic effects.
6. Diabetes mellitus type 2
6.1. Zinc status and diabetes mellitus type 2
The results of a meta-analysis demonstrated that low Zn status as assessed by serum Zn levels or increased urinary Zn excretion is associated with poor glycemic control in patients with DM2 (da Silva Bandeira et al., 2017). We have also demonstrated that serum Zn levels are inversely associated with glucose and HbA1c levels even independently of diabetes (Skalnaya et al., 2017). Our recent data revealed an association between lower serum Zn levels and insulin resistance (HOMA-IR) in prediabetic women (Skalnaya et al., 2018). Similarly, serum Zn levels and circulating oxysterol levels were significantly associated with these parameters even after adjustment for the presence of DM1 or DM2 (Samadi et al., 2020). It is also mote worthy that serum Zn levels were significantly lower in diabetic subjects injected with insulin than those not treated with insulin ( Jansen et al., 2012). In another meta-analysis duration of DM2 was inversely associated with whole blood Zn levels, albeit being not significantly related to lower Zn intake (Fernández-Cao et al., 2018). However, a recent meta-analysis from the same research group revealed an association between elevated serum/plasma zinc concentration and increased T2DM risk in the general population (Fernández-Cao et al., 2019), being generally in agreement with earlier studies demonstrating a direct correlation between exchangeable Zn pool and fasting insulin and HOMA-IR values (Perez et al., 2018). While considering Zn status and diabetes, it is essential to note a recently estimated decrease in circulating Zinc-α2-glycoprotein in subjects with altered glucose metabolism, although this association may be highly affected by increased body weight (Pearsey et al., 2020).
Due to the role of Zn in insulin synthesis and signaling, as well as the observed inverse association between Zn status and diabetes, multiple studies were performed in order to assess the impact of Zn supplementation on glycemic control and other metabolic parameters in DM2. A systematic review and meta-analysis by Wang et al. (2019) which included data from 1700 participants in 14 countries demonstrated a significant reduction of fasting and 2-h postprandial glucose, fasting insulin and HOMA-IR, HbA1c and CRP levels (Wang et al., 2019). The results of the most recent analysis demonstrated that the effect of Zn on carbohydrate metabolism in DM2 depends on both dose and treatment duration. Briefly, short-term (<12 weeks) interventions reduced glucose and triglyceride levels as well as insulin resistance, whereas long-term treatment (>12 weeks) reduced serum glucose, triglycerides, total and LDL cholesterol. In turn, low-dose zinc supplementation (<25 mg/d) significantly reduced serum glucose, triglycerides, total and LDL cholesterol, and decreased insulin resistance. Significant reduction in insulin resistance and HbA1c was revealed in the case of high-dose zinc supplementation (≥25 mg/d). Therefore, the authors recommend long-term low-dose Zn supplementation for significant improvement of metabolic parameters in DM2 patients (Pompano & Boy, 2021). Significant improvement of lipid profile in diabetic patients in response to Zn supplementation was also demonstrated in another study (Asbaghi et al., 2020). It is also notable that the particular form of Zn supplements (Wang et al., 2019) as well as its coadministration with other nutraceuticals (Jafarnejad, Mahboobi, McFarland, Taghizadeh, & Rahimi, 2019) may have a significant impact on the efficiency of Zn treatment.
In addition to therapeutic potential of Zn treatment in diabetes, preventive effect of Zn supplementation is of particular interest, although the existing data are insufficient. A double-blind randomized placebo-controlled pilot study performed in Bangladesh an involving 55 subjects with prediabetes demonstrated that daily intake of 30 mg for 6 months significantly improved fasting glucose, beta cell function, and insulin resistance as compared to placebo (Islam et al., 2016). At the same time, systematic reviews and meta-analyses of the effect of zinc supplementation in pre-diabetes are lacking (Du et al., 2019). Indications of preventive effect of Zn intake may arise from prospective studies. Specifically, a 5-year prospective study involving 16,160 healthy Japanese adults (40–65 y.o.) revealed an inverse association between dietary Zn intake and DM2 risk, that was diagnosed in 396 cases within a 5-year period (Eshak, Iso, Maruyama, Muraki, & Tamakoshi, 2018).
Despite the rather clear indications of the beneficial effect of Zn in DM2, the results of epidemiological studies regarding the relationship between both Zn status and Zn supplementation in DM2 are still contradictory (Ruz, Carrasco, Sánchez, Perez, & Rojas, 2016). In contrast, experimental in vivo studies aimed at investigation of antidiabetic potential of Zn are less contradictory. In addition to significant improvement of Zn metabolism (Barman, Pradeep, & Srinivasan, 2017; Pathak, Sharma, Kumar, & Dhawan, 2011) and glycemic control (Cooper-Capetini et al., 2017; Wang, Li, Fan, & Liu, 2012) Zn supplementation was shown to prevent or reduce diabetes-associated disorders including osteoporosis (Qi, He, et al., 2020), nephropathy (Barman, Pradeep, & Srinivasan, 2018), cardiomyopathy (Wang, Wang, et al., 2017), lung dysfunction (Sacan et al., 2016), cataract (Barman & Srinivasan, 2019), and others.
Despite certain contradictions, both human and experimental data clearly indicates the interference between Zn status either at baseline or in response to supplementation and glycemic control in DM2. The observed association is mediated by the regulatory role of Zn2+ in carbohydrate metabolism that has been significantly highlighted in the recent years.
6.2. Zinc as a factor of β-cell development and functioning
Zinc is essential for β-cell development and functioning due to its clearly demonstrated role in insulin processing and secretion (Li, 2014). Recent studies further characterized the role of Zn2+ in β-cell development and regulation. Zn2+ levels in cellular environment has a regulatory effect on β-cell functioning. Specifically, chelation and supplementation of Zn2+ in the physiological range reduce and increase insulin content and secretion, whereas more profound decreases and increases in Zn2+ levels are associated with β-cell apoptosis and necrosis, respectively (Nygaard, Larsen, Knuhtsen, Rungby, & Smidt, 2014). Maturation of insulin-producing cells differentiated from human adipose-derived stem cells is associated with significant changes in intracellular Zn2+ levels due to modulation of ZIP4 expression (Ohta et al., 2019). Differentiation of human exfoliated deciduous tooth-derived stem cells into β cell-like stem cells was also associated with up-regulation of ZnT8 expression and Zn2+ significantly increases insulin secretion (Kim, Shin, & Pae, 2016). Zinc (ZnO) was also used as an essential factor for promotion of endometrial stem cells differentiation into insulin-producing cells (Hoveizi & Mohammadi, 2019). Secreted Zn2+ may also regulate glucose-stimulated insulin secretion by β-cells in an autocrine manner through modulation of KATP/Ca2+ channels (Slepchenko, Daniels, Guo, & Li, 2015).
6.3. Zinc and insulin signal transduction
Although the role of Zn2+ in the key mechanisms of insulin signal transduction has been clearly demonstrated previously (Maret, 2017b), the most recent studies clarified the insulin mimetic effect of Zn2+ and its influence on the mechanisms of insulin resistance (Fig. 3). Wu et al. (2016) demonstrated that in insulin-resistant L6 myotubes Zn2+ increases glucose uptake through upregulating Akt phosphorylation, GLUT4 translocation, and GSK3β phosphorylation, as well as inhibiting mTOR and S6K1 expression (Wu et al., 2016). It is also notable that Zn and insulin possessed synergistic activity in promotion of myogenic cell proliferation through phosphoinositide 3-kinase (PI3K)/Akt and ERK cascade (Ohashi et al., 2015). It is also notable that in human skeletal muscle cells Zn2+ activated ERK1/2, Akt, GSK-3β, and p38 signaling, whereas in murine cells activation of PRAS40, ERK1/2, Akt and GSK-3β was observed (Norouzi, Adulcikas, Sohal, & Myers, 2018). Insulin-mimetic effects of Bis-(hinokitiolato)-zinc complex ([Zn(hkt)2]) were also attributed to induction of Akt phosphorylation and inhibition of PTP1B and PTEN activity that were observed without activation of insulin receptor (Naito et al., 2016). A similar effect was observed for bis-(maltolato)-zinc(II) complex in adipose tissue (Naito, Yamamoto, Yoshikawa, & Yasui, 2019). The role of Zn2+ in insulin-dependent Akt activation was also supported in Znt7-KO mice (Tepaamorndech et al., 2016). Generally, the most recent data demonstrate that Akt is one of the key target pathways activated by Zn2+ and mediating its insulin-mimetic activity (Sun et al., 2018), although the antidiabetic effect involves multiple other pathways (Vardatsikos, Pandey, & Srivastava, 2013).
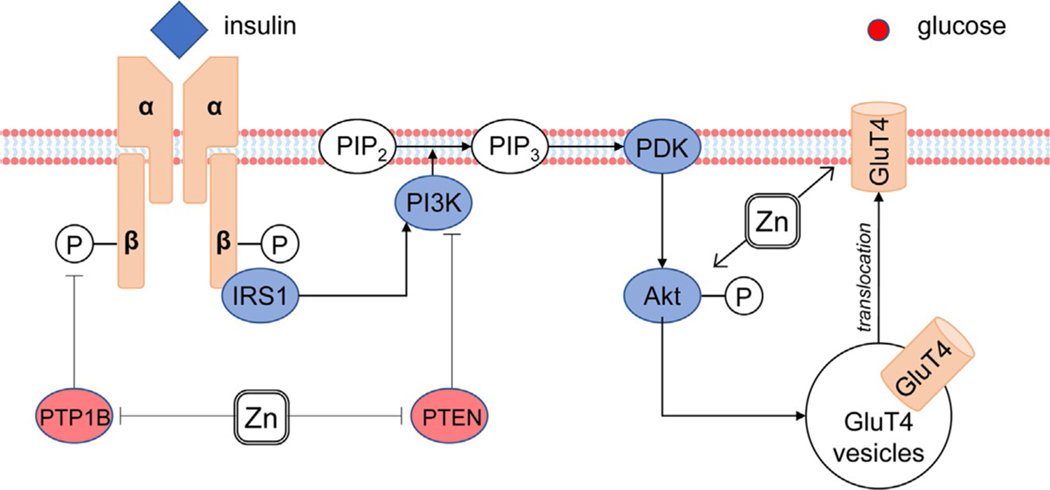
Fig. 3.
Involvement of Zn2+ in insulin signal transduction. Zn2+ increases phosphorylation of insulin receptor through inhibition of PTP1B, as well as up-regulates phosphoinositide 3-kinase (PI3K)/Akt due to PTEN down-regulation. These effects mediate stimulatory effects of Zn2+ on GluT4 translocation and glucose uptake. It is also notable that Akt phosphorylation and inhibition of PTP1B and PTEN activity that were observed without activation of insulin receptor (Naito, Yoshikawa, Masuda, & Yasui, 2016).
6.4. Zinc transporters in β-cell functioning and diabetes
In addition to the role of Zn2+ ions in pancreatic physiology and pathology, in the last decade significant progress was achieved in understanding the involvement of ZnT (Zn2+ influx) and ZIP (Zn2+ efflux) transporters in the effects of Zn. Moreover, it has been demonstrated that (patho)physiological effects of Zn2+ under certain circumstances is mediated by modulation of zinc transporters.
ZnT8 (SLC30A8) plays a key role in Zn2+ uptake by insulin secretory granules in β cells (Davidson, Wenzlau, & O’Brien, 2014). Specifically, an in vivo study using Znt8 knockout (Znt8KO) mice demonstrated that ZnT8 is essential for beta-cell zinc influx, glucose-stimulated insulin secretion, insulin processing, and formation of insulin granules (Wijesekara et al., 2010). The predominant role of ZnT8 as compared to ZnT7 in glucose-stimulated insulin secretion (Syring et al., 2016). Moreover, ZnT8 expression is also essential for regulation of adequate hypoglycemia-induced secretion of glucagon in a particular subset of α-cells (Solomou et al., 2015), whereas ZnT8-mediated Zn2+ signals from β-cells do not impact glucagon production (Hardy, Serino, Wijesekara, Chimienti, & Wheeler, 2011). Due to numerous functions associated with ZnT8, disturbances of the latter are significantly associated with impaired pancreatic function, playing a distinct role in pathogenesis of DM1 and DM2 (Yi, Huang, & Zhou, 2016).
In pediatric DM1 patients, ZnT8 is considered as a major CD8+ T-cell autoantigen proposed to play a pathogenetic role in DM1 (Éńee et al., 2012). ZnT8 autoantibody levels were found to be associated with diabetes-related antibodies to glutamic acid decarboxylase (GAD), IA2, and islet cell autoantibody in adult patients with DM1 (Rogowicz-Frontczak, Pilacinski, Wyka, Wierusz-Wysocka, & Zozulinska-Ziolkiewicz, 2018), being considered as a tool for DM1 differential diagnosis (Boudiaf et al., 2018). Correspondingly, a positive association between ZnT8 autoantibodies and risk of ketoacidosis was observed (Niechciał et al., 2018). However, ZnT8 antibody levels were shown to decrease during the first year after disease onset (Vaziri-Sani et al., 2010).
At the same time, loss of ZnT8 function in human β cells was shown to result in improved glucose responsiveness and insulin secretion (Dwivedi et al., 2019). Downregulation of ZnT8 in pancreatic β-cells was shown to reduce inflammation-induced cytotoxicity protecting the cells from apoptosis (Merriman & Fu, 2019). Correspondingly, genetic variants of human ZnT8 were found to be differentially associated with DM risk. Hyperreactive Arg-325 variant is linked to increased DM2 susceptibility, whereas loss-of--function mutations as well as Trp-325 variants of ZnT8 were associated with reduced DM2 risk (Merriman, Huang, Rutter, & Fu, 2016). The latter are related to enhanced glucose responsiveness and proinsulin processing with subsequent insulin synthesis (Zhang, Jian, He, & Wu, 2020).
ZnT3 was found to localize in insulin containing granules being functionally antagonistic to ZnT8 (Smidt et al., 2016). It has been demonstrated that siRNA-mediated knock-down of ZnT3 results in a reduction of insulin secretion in INS-1E cells, whereas ZnT8 knock-down is associated with increased intracellular insulin content (Petersen et al., 2011).
ZIP7 and ZIP6 were found to be involved in regulation of insulin excretion through modulation of cytosolic Zn2+ levels, whereas the latter may be also responsible for maintaining β cell survival from fatty acid-induced cell apoptosis (Liu et al., 2015). Myers, Nield, Chew, and Myers (2013) demonstrated that Zip7 is implicated into regulation of carbohydrate metabolism in skeletal muscle cells and its deficiency is associated with altered expression of insulin receptor, IRS-2, GluT4, impaired Akt signaling, as well as impaired expression of downstream genes of glucose metabolism (Myers et al., 2013). Moreover, ZIP7 response to glucose levels in skeletal muscles was shown to depend on insulin sensitivity. Specifically, insulin-resistant cells respond to glucose load with downregulation of ZIP7 expression, that is associated with decreased expression of Akt, GluT4, and other genes involved in carbohydrate metabolism (Norouzi et al., 2019). The involvement of ZIP7 in insulin signaling and insulin resistance may be also associated by regulation of endoplasmic reticulum stress (Adulcikas, Sonda, Norouzi, Sohal, & Myers, 2019).
ZnT7 is considered as an essential regulator of insulin secretion (Nunemaker & Benninger, 2016). ZnT7 localized predominantly to Golgi apparatus positively regulates insulin gene transcription, mRNA and protein synthesis through modulation of metal-responsive transcription factor Mtf1, also increasing glucose-induced (Huang, Yan, & Kirschke, 2010). The role of ZnT7 in glucose metabolism is also supported by the observation of increased susceptibility to diet-induced insulin resistance in ZnT7-KO mice due to reduced mRNA expression of Insr, Irs2, and Akt1 in skeletal muscles, whereas ZnT7 overexpression upregulated insulin sensitivity and improved glucose uptake (Huang et al., 2012). It is also interesting that the role of ZnT7 in carbohydrate metabolism may be at least partially mediated by its interference with fatty acid metabolism. Particularly, insulin resistance in ZnT7 knockout mice was found to be associated with increased fatty acid accumulation in skeletal muscles due to upregulation of fatty acid-binding protein 3 and other fatty acid transporters (Huang et al., 2018).
Maxel et al. (2019) demonstrated an essential role of Zip14 in INS-1E beta-cell line, that was shown to increase in response to glucose exposure. Zip14 was shown to be involved in regulation of protein biosynthesis, oxidative phosphorylation, and insulin secretion (Maxel et al., 2019). Involvement of ZIP14 into pathogenesis of diabetes was found not to be limited only to pancreatic beta-cells. Specifically, in addition to altered insulin production and secretion, ZIP14-KO was associated with increased intestinal barrier permeability, endotoxinemia (Kim et al., 2020), systemic inflammation, and hepatic insulin resistance (Aydemir, Kim, & Cousins, 2017). The latter was shown to be mediated by Zn-dependent regulation of endosomal insulin receptor activity (Aydemir, Troche, Kim, & Cousins, 2016).
6.5. Zinc and hyperglycemia
Zn was shown to be a mediator of the (patho)physiological effects of hyperglycemia, that vary significantly between β-cells and other tissues.
Glucose exposure was shown to increase cytosolic Zn2+ levels to a maximum observed at 24 h through increased expression of ZIP6, ZIP7, and ZIP8 and reduced metallothionein expression in order to stimulate insulin processing and secretion, although chronic increase in [Zn2+]i levels contributes to β-cell toxicity (Bellomo, Meur, & Rutter, 2011). At the same time, the results of another study demonstrated that prolonged stimulation of beta-cells (MIN6) with potassium chloride mimicking hyperglycemia resulted in a nearly threefold reduction in total Zn content, down-regulation of ZIP1, ZIP6, ZIP7 and ZIP14 mRNA expression, as well as altered expression of β-cell markers (Lawson, Maret, & Hogstrand, 2018).
In non-pancreatic tissues, Zn was also involved in mechanisms of glucose toxicity. Specifically, glucose-induced ROS overproduction was shown to induce mitochondrial fission and increased mitochondrial Zn2+ that promoted fission through the recruitment of the fission factor Drp-1 ultimately resulting in mitochondrial fragmentation (Abuarab, Munsey, Jiang, Li, & Sivaprasadarao, 2017). It is also noteworthy that hyperglycemia-induced alterations of intracellular Zn2+ levels with reduction of cytoplasmic and mitochondrial metal levels and increased endoplasmic reticulum Zn2+ content are mediated by opposite regulation of ZIP7 (upregulation) and ZNT7 (downregulation) in cardiomyocytes (Tuncay, Bitirim, Durak, Rutter, & Turan, 2016). Correspondingly, hyperglycemia-induced increase in ZIP7 and ZIP14, and decrease in ZIP8 and ZnT7 expression in insulin resistant cardiomyocytes was found to be inversed by sodium-glucose cotransporter 2 inhibitor (dapagliflozin) followed by improved MMP-2 and MMP-9 protein expression, and reduced oxidative stress (Olgar & Turan, 2019). Generally, the studies from the last decade provided more detail on the involvement of Zn in regulation of carbohydrate metabolism and its disturbance in diabetes mellitus type 2. In addition to further understanding of the role of Zn in β-cell development, insulin production, and insulin signaling, the particular role of Zn transporters was demonstrated. The existing data clearly demonstrate that modulation of Zn homeostasis could be considered as the potential prophylactic or therapeutic tool in management of diabetes mellitus type 2, although more systematic data on its efficiency are required.
7. Obesity
7.1. Zinc status in obesity
The existing data demonstrate a significant decrease in blood (Fan, Zhang, & Bu, 2017), serum (Rios-Lugo, Madrigal-Arellano, Gaytán-Hernández, Hernández-Mendoza, & Romero-Guzmán, 2020) and hair (Suliburska, Bogdański, Pupek-Musialik, & Krejpcio, 2011) Zn levels in patients with overweight/obesity. Correspondingly, the results of meta-analysis demonstrated lower serum Zn levels in obese children and adults (Gu, Xiang, Zhang, Sun, & Jiang, 2019). However, certain studies failed to reveal any obesity-related difference in markers of Zn status (García et al., 2012; Jaksic et al., 2019). The latter may be associated with inhomogeneous distribution of Zn in the various biosamples. Particularly, we have demonstrated reduced hair Zn levels in patients with overweight/obesity, whereas no significant group difference in hair contents was observed. In contrast, urinary Zn level was found to be higher in high-BMI group, being potentially indicative of increased Zn excretion in obesity (Tinkov et al., 2020). In turn, weight-loss in overweight/obese subjects was associated with increased plasma Zn levels (Voruganti et al., 2010) and redistribution of Zn between tissues (Freire, Fisberg, & Cozzolino, 2013).
Epidemiological studies also demonstrated an association between impaired Zn status and obesity-associated metabolic disturbances including insulin resistance (García et al., 2013), systemic inflammation and altered lipid profile (Costarelli et al., 2010). Similar associations were observed in patients with metabolic syndrome tightly associated with obesity. Particularly, serum Zn levels significantly correlated with the number of metabolic syndrome components including triglyceride levels (Seo, Song, Han, Lee, & Kim, 2014). Urinary Zn levels were increased in obese subjects characterized by higher cardiovascular risk (Severo, Morais, Beserra, Clímaco Cruz, et al., 2020). Correspondingly, higher Zn excretion was found to be associated with altered metabolic profile in obese subjects (Xu et al., 2020).
Multiple studies evaluated the impact of Zn supplementation on obesity and associated metabolic disturbances. Particularly, Zn supplementation was shown to reduce body weight and body mass index (Payahoo et al., 2013), as well as waist and hip circumference (Khorsandi et al., 2019) in obese subjects. A meta-analysis indicated that Zn supplementation in overweight/obese subjects is associated with a significant reduction in body weight, although the results were highly heterogeneous (Abdollahi et al., 2020). The results of systematic reviews and meta-analyses demonstrated that Zn supplementation in obese subjects is associated with reduced insulin resistance (Cruz, Morais, de Oliveira, Severo, & do Nascimento Marreiro, 2017) and dyslipidemia (Severo et al., 2019). In a placebo-controlled trial Zn supplementation was shown to reduce IL-6 and CRP levels, serving as markers of systemic inflammation in obesity (Kim & Ahn, 2014). In addition, it has been shown that Zn supplementation may reduce circulating leptin levels, although this effect was mainly attributable to long-term interventions (>6 weeks) performed in women (Khorshidi et al., 2019).
In vivo laboratory experiments generally corroborate to results noted in human studies, demonstrating protective effect of Zn on diet-induced obesity and associated metabolic risk. Zinc supplementation (6 mg/kg) starting from the 15th week of dietary intervention significantly reduced diet-induced increase in body weight, adipose tissue mass, circulating insulin, leptin, and triglyceride levels in high-fat/high-fructose-fed rats (Thoen et al., 2019). Combining in vivo (high-fat diet-fed mice) and in vitro (HepG2 cells) approaches, Qi, Zhang, et al., 2020 demonstrated that Zn2+ supplementation significantly improves diet-induced alterations in lipid and carbohydrate metabolism reducing gluconeogenesis, increasing glycolysis and promoting glucose uptake, as well as decreasing lipid accumulation (Qi, Zhang, et al., 2020). Our previous study also demonstrated a significant decrease in hepatic liver accumulation in Zn supplemented (227 mg/L Zn as ZnSO4 in drinking water) high-fat high-carbohydrate diet-fed rats (Gatiatulina et al., 2019).
In addition to supplementation studies, the protective role of Zn in obesity and metabolic syndrome was revealed in restriction studies. Specifically, Zn deficiency aggravated, whereas Zn supplementation ameliorated diet-induced alteration of glucose homeostasis, insulin resistance, and hypertriglyceridemia, well as cardiac inflammation and hypertrophy (Wang, Luo, et al., 2016). Similar modulatory effect of Zn status was demonstrated on obesity-induced vascular inflammation, oxidative stress, and aortic remodeling (Chen et al., 2016). Prenatal and postnatal Zn deficiency was shown to result in increased adipocyte hypertrophy, as well as elevated triglyceride levels and insulin resistance (Abregú et al., 2019), being in agreement with the observation of Zn-deficiency-induced dysglycemia ( Jou, Philipps, & Lönnerdal, 2010).
7.2. Adipose tissue as a target for zinc
In parallel with obesity-associated alteration of Zn content in various tissues (Min & Chung, 2018), a number of studies demonstrated a significant decrease in adipose tissue Zn levels (Tallman & Taylor, 2003; Tinkov et al., 2016). Adipose tissue Zn content was inversely associated with circulating leptin levels, insulin resistance, and systemic inflammation (Tinkov et al., 2016). Moreover, Zn deficiency in high-fat diet mice resulted in its decrease in adipose tissue due to altered expression of Zn transporters, as well as leptin overproduction and exacerbation of adipose tissue macrophage infiltration (Liu et al., 2013). Reduced Zn content in obese adipose tissue may be indicative of its role as the potential target of Zn physiological effects.
7.3. The impact of zinc on adipogenesis
Modulation of Zn levels has a significant impact on adipocyte differentiation. Particularly, ZnO treatment increased 3T3-L1 differentiation with subsequent lipid accumulation through upregulation of PPARγ, FABP4, C/EBPα, and SREBP1 mRNA and protein expression (Pandurangan, Jin, & Kim, 2016). These findings corroborate the results of the pioneer study by Tanaka and coauthors (Tanaka, Takahashi, Matsui, & Yano, 2001). Upregulation of PPARγ and C/EBPα in zinc ascorbate-induced adipogenesis are also associated with increased aP2 and GLUT4 expression, resulting in insulin-responsive adipocytes (Ghosh et al., 2013). Zn citrates were found to induce differentiation of pre-adipocytes into mature adipocytes accompanied by upregulation of PPARγ, adiponectin, GluTs expression (Tsave et al., 2018). It is also notable that Zn potentiated stimulatory effect of insulin treatment on PPARy expression (Tsave et al., 2015). In turn, high dose Zn supplementation significantly increased adipose tissue, adipocyte hypertrophy, and leptin production through modulation of Akt signaling (Huang et al., 2017). Therefore, Zn2+ may be considered as a potent regulator of adipogenesis. At the same time, both Zn deficiency (Pandurangan et al., 2016) or excess (Huang et al., 2017) result in adipose tissue dysfunction. The particular role of zinc transporters and Zn-containing effector molecules in adipose tissue physiology will be discussed below.
7.4. Zinc transporters in adipose tissue and obesity
The interaction between Zn metabolism and obesity may be mediated by alteration of zinc transporters (Noh, Paik, Kim, & Chung, 2014). Particularly, Psammomys obesus, being a model of obesity and diabetes, is characterized by multidirectional changes in ZIP6, ZIP8, ZIP9, and ZnT9 expression in visceral and subcutaneous adipose tissue depots (Maxel, Pold, et al., 2015).
ZIP14 is specifically expressed in white adipose tissue playing a significant role in its physiology (Aydemir & Cousins, 2018). It has been demonstrated that Zip14 deficiency is associated with adipocyte hypertrophy and upregulation of proinflammatory cytokine expression due to NF-κB activation, especially under endotoxinemia (Troche, Aydemir, & Cousins, 2016). It has been also demonstrated that patients with obesity are characterized by reduced Zip14 expression in subcutaneous adipose tissue. At the same time, Zip14 expression is up-regulated during adipogenesis, being associated with PPARγ expression (Maxel, Smidt, et al., 2015). In turn, ZIP14 expression in adipose tissue was found to be reduced in obesity, also positively correlating with PPARγ expression (Maxel et al., 2017).
In turn, ZIP13 functioning is associated with inhibition of “brite” adipocyte transformation through modulation of C/EBP-β expression (Fukunaka et al., 2017).
Another transporter, tightly involved in adipogenesis regulation is Znt7 (Tepaamorndech, Kirschke, & Huang, 2014). Znt7 is significantly upregulated during adipogenesis, reaching peak values in fully differentiated 3T3-L1 cells (Huang et al., 2016). At the same time, the results of another study demonstrate that inhibition of adipogenesis due to Znt7 deficiency is not associated with altered PPARγ and C/EBPα expression (Tepaamorndech et al., 2016).
7.5. Zinc-α2-glycoprotein (ZAG)
Zinc-α2-glycoprotein (ZAG) is an adipokine that is inhibited by obesity, high-fat intake, inflammatory response (TNFα), and glucocorticoid and β3-adreno receptor antagonists (Bing, Mracek, Gao, & Trayhurn, 2010). Given the presence of Zn-binding sites in ZAG molecule, as well as the role of Zn2+ in its polymerization (Zahid et al., 2016) it is proposed that ZAG may mediate at least a part of Zn effects in adipose tissue (patho)physiology.
The existing data demonstrate a significant decrease in ZAG mRNA expression in subcutaneous and epidydimal adipose tissue of ob/ob mice, with TNFα overproduction being considered as the leading mechanism of ZAG downregulation (Mracek et al., 2010). In addition, increased ZAG expression in high-fat fed mice resulted in a significant decrease in adipose tissue mass (Liu et al., 2018), and prevented obesity-associated non-alcoholic fatty liver disease (Xiao et al., 2018). ZAG was also responsible for improvement of glucose uptake and insulin sensitivity in adipocytes (Ceperuelo-Mallafré et al., 2015) and skeletal muscles (Gao et al., 2018), underlying the earlier mentioned association between ZAG levels and insulin resistance.
ZAG acts as autocrine and paracrine regulator of adipocyte metabolism (Severo, Morais, Beserra, dos Santos, et al., 2020) primarily regulating lipid metabolism by increasing lipolysis and reducing lipogenesis (Pelletier et al., 2013). Particularly, ZAG overexpression in obese mice significantly increased hormone-sensitive lipase and decreased fatty acid synthase mRNA expression (Gong et al., 2010). The influence of ZAG on lipid metabolism may be also mediated by modulation of SREBP-1c (Liao et al., 2016). It is also notable that ZAG-dependent changes in lipid metabolism were shown to affect response to dexamethasone treatment (Zhang, Qiao, et al., 2020). In addition to modulation of lipid metabolism, ZAG may promote browning of white adipose tissue and the corresponding increase in mitochondrial biogenesis and UCP1 expression through PKA and p38 MAPK pathways (Elattar, Dimri, & Satyanarayana, 2018; Fan et al., 2020).
The observed decrease in lipogenic genes is also associated with down-regulation of PPARγ and C/EBPα expression in ZAG-overexpressing 3T3-L1 cells (Zhu et al., 2013). On the one hand, PPARγ is considered as one of the regulators of ZAG production (McDermott, Jadoon, & Cunningham, 2012), whereas ZAG may also possess modulatory effect on PPARγ expression (Wei et al., 2019). It is also notable that the influence of ZAG on adipose tissue metabolism may be also mediated by its stimulatory effect on adiponectin expression (Balaz et al., 2014).
Human data also corroborate the results of laboratory studies, demonstrating a tight association between ZAG metabolism and obesity. It has been demonstrated that ZAG mRNA expression in subcutaneous adipose tissue, as well as its circulating levels, are significantly increased in obese subjects (Liu et al., 2018). Moreover, adipose tissue ZAG content is associated with insulin resistance, and adiponectin expression and circulating levels (Garrido-Sánchez et al., 2012). The most recent study demonstrated that low serum ZAG levels are associated with metabolically unhealthy phenotype in obese subjects and together with adiponectin levels may be successfully used for discrimination of metabolic health abnormalities (Liu, Zhang, et al., 2020). At the same time, a significant decrease in plasma ZAG levels following Roux-En-Y gastric bypass surgery (RYGB), as well as an inverse association between ZAG and reductions in BMI and body fat, may be indicative of the protective effect of ZAG during rapid weight loss (Morse, Astbury, Walczyszyn, Hashim, & Geliebter, 2017).
7.6. Zinc finger proteins in adipogenesis
The influence of Zn on adipocyte differentiation may be mediated by its structural role in zinc finger proteins, being considered as early adipogenic regulators (Wei et al., 2013). Being a rather heterogenous group of proteins, various molecules may possess both stimulatory and inhibitory effect on adipogenesis.
Specifically, it has been demonstrated that Znf638 is induced at early stages of adipocyte differentiation and stimulates adipogenesis through C/EBPα and subsequent PPARγ upregulation (Du, Ma, Meruvu, Hugendubler, & Mueller, 2014). At the same time, Znf638 deficiency inhibits adipogenesis (Meruvu, Hugendubler, & Mueller, 2011). Another protein, Zfp423 may be also considered as adipogenic regulator stimulating PPARγ expression (Gupta et al., 2010). Correspondingly, Zfp423 overexpression accompanies adipocyte differentiation, whereas its epigenetic dysregulation is associated with subcutaneous adipocyte hypertrophy (Longo et al., 2018). Zfp423 inhibition with retinoic acid in vitro results not only in reduced white adipogenesis, but also increases brown adipocyte development (Wang, Fu, et al., 2017). It is also notable that biological effects of Zfp423 strongly depend on the functional state of the cell. In particular, at early stages of development Zfp423 deficiency results in altered differentiation and adipose tissue dysfunction, whereas in mature adipocytes it is associated with a shift to brown adipocyte phenotype (Shao et al., 2016). Zfp467 also up-regulated adipogenic differentiation of the precursor cells through increased expression of regulatory PPARγ and C/EBPα with subsequent induction of adiponectin and resistin production (Quach et al., 2011).
In turn, Zfp521 was shown to be a negative regulator of adipogenesis (Chiarella et al., 2018) preventing precursor cells from adipogenic differentiation and maintaining proliferative activity, whereas Zfp521 inhibition results in increased number of adipocytes and its maturation (Gustafson, Nerstedt, & Smith, 2019). Inhibitory effect of Zfp521 on adipogenesis may be at least partially mediated by downregulation of Zfp423 expression (Kang et al., 2012).
A significant role in regulation of adipogenesis was also demonstrated for ZFP217 (Liu et al., 2019), ZFP30 (Chen, Schwalie, et al., 2019) and other zinc finger proteins (Wei et al., 2013).
Although main focus in Zn studies was aimed at β-cells for a long time followed by the role of Zn in insulin signaling, the most recent data demonstrate that Zn may be considered as “adipotropic” metal due to its specific impact on adipose tissue development and functioning. In addition, particular Zn-containing effector molecules in adipose tissue including ZAG and zinc finger proteins, as well as their role in obesity was revealed. Therefore, addressing adipotropic effects of Zn may be considered as a potential treatment of obesity and obesity-associated disorders.
8. Male reproduction
8.1. Zinc, sperm quality, and infertility
The results of meta-analysis of 2600 infertile men and 867 controls demonstrated that infertility is associated with significantly lower seminal plasma Zn levels, whereas Zn supplementation significantly increased semen volume, sperm motility, and improved sperm morphology (Zhao et al., 2016). Correspondingly, a significant correlation between seminal plasma Zn levels and reduced risk of asthenozoospermia was observed in another meta-analysis (Taravati & Tohidi, 2016). Seminal plasma (Kothari & Chaudhari, 2016) as well as hair (Chang, Choi, Kim, & Park, 2011) Zn levels were also found to be associated with free testosterone levels. It is also notable that chronic prostatitis may significantly contribute to reduced seminal plasma Zn levels (Cui et al., 2015).
In infertile patients with varicocele lower seminal Zn level was associated with higher DNA fragmentation index (Nguyen, Trieu, Tran, & Luong, 2019). In turn, in vitro treatment with Zn in combination with D-aspartic acid, and coenzyme Q10 reduced lipid peroxidation in sperm of both normozoospermic and asthenozooseprmic subjects, although no effect on sperm DNA fragmentation was observed (Giacone et al., 2017).
Correspondingly, a number of studies addressed the efficiency of Zn supplementation for improvement of semen quality and male fertility. Zn supplementation (220 mg daily) for 3 months resulted in a significant increase in semen volume, sperm motility, and morphology, and was associated with increased high- and low-molecular weight Zn binding ligands (Hadwan, Almashhedy, & Alsalman, 2012). The results of meta-analysis performed by Salas-Huetos et al. (2018) demonstrated that Zn supplementation is capable of increasing total sperm concentrations and sperm motility (Salas-Huetos et al., 2018).
8.2. Zinc in spermatogenesis and sperm functioning
The observed associations between altered Zn status and male infertility are mediated by the critical role of Zn in spermatogenesis (Foresta et al., 2014). Short-term low-dose exposure to ZnO nanoparticles also promoted spermatogenesis through stimulation of cell self-renewal and differentiation of spermatogonia ( Javadi et al., 2020). In turn, Zn deficiency was shown to result in oxidative stress, inflammatory response, and increased proapoptotic signaling (Bax, caspase-3) in germ cells, whereas antiapoptotic signals were reduced (Bcl-2) (Omu et al., 2015). Zn deficiency was associated with reduced Zip6 and Zip10 expression and altered seminiferous tubule structure with abnormal germinal epithelium irrespectively of systemic Zn and testosterone levels (Croxford, McCormick, & Kelleher, 2011). Leydig cell atrophy may also indirectly contribute to Zn deficiency-induced alterations in spermatogenesis (Kumari, Nair, & Bedwal, 2011).
Further studies also demonstrated that activation of proapoptotic and proinflammatory pathways induced by CCl4 treatment in testicular cells was aggravated by Zn deficiency (Chen, Yang, Wang, Yang, & Guo, 2019). Modulation of apoptosis, inflammation, and oxidative stress was also attributable to the protective effect of Zn against diabetes-induced testicular damage (Maremanda, Khan, & Jena, 2016). Zn2+also improved DNA methylation, chromatin integrity, testicular structure, and increased spermatogonial stem cell number in a model of testicular toxicity induced by bleomycin etoposide and cis-platin treatment (Khadivi, Razavi, & Hashemi, 2020).
Along with spermatogenesis Zn2+ regulates other aspects of sperm physiology (Fallah, Mohammad-Hasani, & Colagar, 2018). It has been demonstrated that Zn2+ stimulates sperm capacitation and acrosome reaction by epidermal growth factor receptor activation and G-protein coupled receptor (Michailov, Ickowicz, & Breitbart, 2014). Conversely, sperm capacitation was associated with Zn2+ redistribution (Kerns, Zigo, Drobnis, Sutovsky, & Sutovsky, 2018). Signaling pathways of Zn2+-induced capacitation are also responsible for hyperactivated sperm motility (Allouche-Fitoussi, Bakhshi, & Breitbart, 2018).
9. Female reproduction
9.1. Zinc and pregnancy, its complications, and outcome
Recent data demonstrate that pregnant women are characterized by lower serum Zn levels as compared to non-pregnant ones (Iqbal, Ali, Rust, Kundi, & Ekmekcioglu, 2020), being indicative of both increased Zn requirements and higher risk of Zn deficiency. A systematic review and meta-analysis demonstrated that cord blood Zn levels was significantly lower in pregnancies with complications as compared to physiological pregnancy (Akdas & Yazihan, 2020). Particularly, the risk of preeclampsia (Ma, Shen, & Zhang, 2015) and eclampsia (Zhu et al., 2016) is significantly associated with Zn deficiency. Correspondingly, the risk of pregnancy-induced hypertension also correlates with low Zn status (He, Lang, Li, Liu, & Yao, 2016). Although no association between Zn intake and circulating levels and GDM was observed (Wilson, Grieger, Bianco-Miotto, & Roberts, 2016), in women with gestational diabetes mellitus material Zn levels were inversely associated with birth weight (Luo et al., 2020). Zn status also has a significant impact on pregnancy outcome. Particularly, maternal Zn deficiency was shown to increase the risk of preterm birth by a factor of more than two (OR= 2.41) (Wang, Hu, et al., 2016 ), also being a significant risk factor for fetal growth restriction (Wang et al., 2015). In addition, lower serum Zn levels were also observed in women with miscarriage (Omeljaniuk et al., 2015). Correspondingly, increased maternal Zn level is associated with lower risk of stillbirth (Özgan Çelikel, Doğan, & Aksoy, 2018).
In view of a tight relationship between Zn status and pregnancy, the efficiency of Zn supplementation for improvement of pregnancy outcome was studied. A systematic review and meta-analysis involving 7637 women and indexed in Cochrane Database demonstrated that Zn supplementation reduced preterm birth by 14% (Ota et al., 2015).
9.2. Zinc and female infertility
Zinc is known to play a significant role in female reproductive system functioning and reproductive disorders (Nasiadek, Stragierowicz, Klimczak, & Kilanowicz, 2020). Grieger et al. (2019) demonstrated that women with lower plasma Zn levels are characterized by significantly longer time to pregnancy (Grieger et al., 2019). Our previous studies also revealed lower hair Zn content in women who underwent in vitro fertilization-induced pregnancy as compared to those with spontaneous pregnancy (Skalny et al., 2018). Certain studies unraveled the association between Zn status and causes of female infertility. Despite being rather heterogeneous, the existing data demonstrate that women with polycystic ovarian syndrome are characterized by inefficiently lower circulating Zn levels (Abedini, Ghaedi, Hadi, Mohammadi, & Amani, 2019). Similarly, Zn deficiency was observed in endometriosis (Lai et al., 2017), although patients with endometriosis who had IVF-induced pregnancy had higher follicular Zn levels as compared to cases with tubal infertility (Singh, Chattopadhyay, Chakravarty, & Chaudhury, 2013).
9.3. Zinc in oocyte, placenta, and fetal development
Physiological role of Zn2+ was demonstrated at all steps of fetal development starting from preconceptional oocyte development, its fertilization, trophoblast and placenta development, and fetal organogenesis.
Zn accumulation is essential during oocyte meiosis and may possess regulatory effect on cell cycle by modulation of cytostatic factor activity through EMI2 component (Bernhardt, Kong, Kim, O’Halloran, & Woodruff, 2012). Correspondingly, Zn deficiency results in meiotic block in oocytes altering its maturation and blastocyst formation (Jeon et al., 2015). In another study, maternal Zn deficiency before conception was shown to decrease histone and DNA methylation, lower fertilization rate and impaired blastocyst formation (Tian & Diaz, 2013). Recent study demonstrated that Zn supplementation was also capable of endoplasmic reticulum stress reduction in oocytes, thus promoting normal maturation and embryogenesis (Ridlo, Kim, Taweechaipaisankul, Kim, & Lee, 2020). Formation of Zn spark containing millions of Zn2+ ions due to exocytosis of Zn-containing vesicles is also known to be an essential mechanism of embryo formation and avoiding polyspermy (Duncan et al., 2016).
Zn is also involved in regulating of trophoblast invasion and migration through modulation of matrix metalloproteinase-2/9, thus contributing to placental development and ameliorating fetal growth restriction (Zong, Wei, Gou, Huang, & Lv, 2017). Correspondingly, Zn deficiency is associated with impaired placental morphogenesis through modulation of placental labyrinth microstructure ultimately resulting in lower blood flow and altered nutrition of the fetus (Wilson et al., 2017). These findings corroborate earlier indications of smaller fetal placenta formation and decreased key placental transcripts expression ultimately leading to higher incidence of neural tube defects in embryo (Tian, Anthony, Neuberger, & Diaz, 2014). Zn deficiency may be also responsible for increased NF-κB expression in placenta in cases of fetal growth restriction (Wang et al., 2015). In turn, Zn treatment was shown to reduce inflammatory response in decidual endothelial cells exposed to proinflammatory TNF-α (Balduit et al., 2020). Correspondingly, Zn supplementation was shown to prevent lipopolysaccharide-induced fetal growth restriction through its anti-inflammatory activity (Chen et al., 2012).
Maintenance of adequate Zn supply is essential for fetal organogenesis including development of cerebral cortex (Hasna, Bohic, Lemoine, Blugeon, & Bouron, 2019) and myocardium (Lin & Li, 2018). Correspondingly, maternal Zn deficiency and the concomitant decrease in placental metallothionein-1 and ZnT-1 mRNA expression are associated with heart malformations (Liu et al., 2014). Placental and fetal ZIP8 deficiency was shown to reduce Zn transport and adversely affect organogenesis resulting in liver, spleen, kidney, and lung hypoplasia (Gálvez-Peralta et al., 2012). Taken together, the existing data clearly demonstrate the role of zinc deficiency in fetal growth restriction and malformations, as well as complications in preterm neonates (Terrin et al., 2015).
10. Zinc and urgent challenges: Covid-19 pandemic
In December 2019, the worldwide community faced a massive outbreak of COVID-19 infection caused by SARS-CoV-2 coronavirus. During less than two years, more than 163 million people worldwide were infected by COVID-19 with more than 3.3 million patients deceased https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (World Health Organization, 2021). COVID-19 predominantly affects respiratory system causing viral pneumonia that is associated with systemic inflammation, endothelial dysfunction (Varga et al., 2020), and immune dysregulation. The latter is characterized by reduced T helper, T suppressor, and Treg cell counts in parallel with leukocytosis (Qin et al., 2020).
Zinc was proposed as a useful adjuvant for COVID-19 therapy due to its systemic anti-inflammatory, antioxidant, immunoregulatory activity, as well as its role in respiratory protection (Skalny, Rink, et al., 2020). Indirect data supporting the potential usefulness of Zn in management of COVID-19 pneumonia included indications of negative association between Zn status and pneumonia (Barnett, Hamer, & Meydani, 2010), as well as certain viral diseases (Read, Obeid, Ahlenstiel, & Ahlenstiel, 2019). However, evidence supporting this hypothesis was published only recently.
In a study involving 249 COVID-19 patients a significant inverse correlation between serum Zn levels and disease severity was demonstrated, with the highest mortality and highest severity in patients with serum Zn< 50 μg/dl. In an in vitro study using Vero E6 cells the authors also revealed higher viral expansion in SARS-CoV-2-infected cells (Vogel et al., 2020). We have also demonstrated a 8% lower serum Zn levels in COVID-19 patients as compared to healthy controls (Skalny et al., 2021). In an Indian study patients with Zn deficiency were characterized by longer hospital stay and higher frequency of complications, acute respiratory distress syndrome, and higher mortality as compared to Zn adequate patients. In an in vitro study the authors also demonstrated that Zn deficiency promotes interaction between angiotensin-converting enzyme 2 (ACE2) and SARS-CoV-2 spike protein ( Jothimani et al., 2020).
In addition to the role of zinc in immunity in general, and antiviral immunity in particular, the interest in Zn as the potential treatment agent in COVID-19 was supported by results from an in vitro study by Te Velthuis et al. (2010). Specifically, the authors have demonstrated that Zn2+ inhibits RNA-dependent RNA polymerase (RdRp) in SARS-coronavirus, resulting in reduced replication of viral particles (Te Velthuis et al., 2010). However, direct data on the efficiency of Zn compounds in COVID-19 treatment are still insufficient. Carlucci et al. (2020) demonstrated that inclusion of ZnSO4 into the protocol of COVID-19 treatment with hydroxychloroquine and azithromycin significantly reduced the need in ventilation, admission to the intensive care unit, as well as decreased mortality in COVID-19 patients, although no significant impact of Zn treatment on the length of hospitalization, ventilation, or hospital stay was observed (Carlucci et al., 2020). A number of trials on the efficacy of Zn in COVID-19 treatment protocols have been registered, although systematic data are not available to date (Doboszewska, Wlaź, Nowak, & Młyniec, 2020).
Therefore, Zn supplementation may be recommended for improvement of antiviral resistance in deficient subjects (Wessels, Rolles, & Rink, 2020). It is proposed that Zn supplementation may prevent excessive inflammatory response and cytokine storm in COVID-19 (Alexander et al., 2020). However, it is still unclear whether Zn compounds may be included in treatment protocols.
11. Conclusion
Recent findings significantly expanded the understanding of the role of Zn in neurodevelopmental and neurodegenerative disorders, diabetes and obesity, male and female infertility, and support the use of Zn in management of COVID-19. However, recent advances in Zn research biology and medicine are not limited to these diseases, and supporting the importance of Zn in human health. These findings are of extreme importance especially in view of still persisting high risk of Zn deficiency even in developed countries. Further laboratory studies in this field should focus on the particular mechanisms of the role of altered Zn homeostasis in disease pathogenesis. In turn, human studies should be designed in order to obtain high-quality systematic data on therapeutic and prophylactic efficiency of Zn to provide evidence-based support for the use of Zn in the management of various diseases.
References
- Abdollahi S, Toupchian O, Jayedi A, Meyre D, Tam V, & Soltani S. (2020). Zinc supplementation and body weight: A systematic review and dose–response meta-analysis of randomized controlled trials. Advances in Nutrition, 11(2), 398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedini M, Ghaedi E, Hadi A, Mohammadi H, & Amani R. (2019). Zinc status and polycystic ovarian syndrome: A systematic review and meta-analysis. Journal of Trace Elements in Medicine and Biology, 52, 216–221. [DOI] [PubMed] [Google Scholar]
- Abelein A, Gräslund A, & Danielsson J. (2015). Zinc as chaperone-mimicking agent for retardation of amyloid β peptide fibril formation. Proceedings of the National Academy of Sciences of the United States of America, 112(17), 5407–5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abregú FMG, Gobetto MN, Castañón A, Lucero D, Caniffi C, Elesgaray R, et al. (2019). Fetal and postnatal zinc restriction: Sex differences in metabolic alterations in adult rats. Nutrition, 65, 18–26. [DOI] [PubMed] [Google Scholar]
- Abuarab N, Munsey TS, Jiang LH, Li J, & Sivaprasadarao A. (2017). High glucose–induced ROS activates TRPM2 to trigger lysosomal membrane permeabilization and Zn2 +-mediated mitochondrial fission. Science Signaling, 10(490), eaal4161. [DOI] [PubMed] [Google Scholar]
- Adulcikas J, Sonda S, Norouzi S, Sohal SS, & Myers S. (2019). Targeting the zinc transporter ZIP7 in the treatment of insulin resistance and type 2 diabetes. Nutrients, 11(2), 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdas S, & Yazihan N. (2020). Cord blood zinc status effects on pregnancy outcomes and its relation with maternal serum zinc levels: A systematic review and meta-analysis. World Journal of Pediatrics, 16, 366–376. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Hosokawa M, Kametani F, Kondo H, Chiba M, Fukushima M, et al. (2012). Long-term oral intake of aluminium or zinc does not accelerate Alzheimer pathology in AβPP and AβPP/tau transgenic mice. Neuropathology, 32(4), 390–397. [DOI] [PubMed] [Google Scholar]
- Alexander J, Tinkov A, Strand TA, Alehagen U, Skalny A, & Aaseth J. (2020). Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients, 12(8), 2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allouche-Fitoussi D, Bakhshi D, & Breitbart H. (2018). Signaling pathways involved in human sperm hyperactivated motility stimulated by Zn2+. Molecular Reproduction and Development, 85(6), 543–556. [DOI] [PubMed] [Google Scholar]
- Alqabbani HM, & AlBadr NA (2020). Zinc status (intake and level) of healthy elderly individuals in Riyadh and its relationship to physical health and cognitive impairment. Clinical Nutrition Experimental, 29, 10–17. [Google Scholar]
- Arena G, & Rizzarelli E. (2019). Zn2+ interaction with Amyloid-B: Affinity and speciation. Molecules, 24(15), 2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbaghi O, Sadeghian M, Fouladvand F, Panahande B, Nasiri M, Khodadost M, et al. (2020). Effects of zinc supplementation on lipid profile in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Nutrition, Metabolism, and Cardiovascular Diseases, 30(8), 1260–1271. [DOI] [PubMed] [Google Scholar]
- Asthana S, Bhattacharyya D, Kumari S, Nayak PS, Saleem M, Bhunia A, et al. (2020). Interaction with zinc oxide nanoparticle kinetically traps α-synuclein fibrillation into off-pathway non-toxic intermediates. International Journal of Biological Macromolecules, 150, 68–79. [DOI] [PubMed] [Google Scholar]
- Aydemir TB, & Cousins RJ (2018). The multiple faces of the metal transporter ZIP14 (SLC39A14). The Journal of Nutrition, 148(2), 174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydemir TB, Troche C, Kim MH, & Cousins RJ (2016). Hepatic ZIP14-mediated zinc transport contributes to endosomal insulin receptor trafficking and glucose metabolism. The Journal of Biological Chemistry, 291(46), 23939–23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydemir TB, Kim MH, & Cousins RJ (2017). Zip14-mediated zinc transport contributes to regulation of glucose homeostasis in intestine, pancreas and liver. FASEB Journal, 31, 299.7. [Google Scholar]
- Azemati B, Khoramdad M, Qorbani M, Rastad H, Shafiee G, Heshmat R, et al. (2020). Percentile values of serum zinc concentration and prevalence of its deficiency in Iranian children and adolescents: The CASPIAN-V study. Journal of Pediatric Endocrinology & Metabolism, 33(4), 525–531. [DOI] [PubMed] [Google Scholar]
- Babaknejad N, Sayehmiri F, Sayehmiri K, Mohamadkhani A, & Bahrami S. (2016). The relationship between zinc levels and autism: A systematic review and meta-analysis. Iranian Journal of Child Neurology, 10(4), 1–9. [PMC free article] [PubMed] [Google Scholar]
- Bains K, Kaur H, Bajwa N, Kaur G, Kapoor S, & Singh A. (2015). Iron and zinc status of 6-month to 5-year-old children from low-income rural families of Punjab, India. Food and Nutrition Bulletin, 36(3), 254–263. [DOI] [PubMed] [Google Scholar]
- Bains K, Kaur H, & Bajwa NK (2019). Iron and zinc status of adult women from low income rural families of Punjab. India. Indian Journal of Ecology, 46(4), 933–937. [Google Scholar]
- Balaz M, Vician M, Janakova Z, Kurdiova T, Surova M, Imrich R, et al. (2014). Subcutaneous adipose tissue zinc-α2-glycoprotein is associated with adipose tissue and whole-body insulin sensitivity. Obesity, 22(8), 1821–1829. [DOI] [PubMed] [Google Scholar]
- Balduit A, Mangogna A, Agostinis C, Zito G, Romano F, Ricci G, et al. (2020). Zinc oxide exerts anti-inflammatory properties on human placental cells. Nutrients, 12(6), 1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman S, & Srinivasan K. (2019). Zinc supplementation ameliorates diabetic cataract through modulation of crystallin proteins and polyol pathway in experimental rats. Biological Trace Element Research, 187(1), 212–223. [DOI] [PubMed] [Google Scholar]
- Barman S, Pradeep SR, & Srinivasan K. (2017). Zinc supplementation mitigates its dyshomeostasis in experimental diabetic rats by regulating the expression of zinc transporters and metallothionein. Metallomics, 9(12), 1765–1777. [DOI] [PubMed] [Google Scholar]
- Barman S, Pradeep SR, & Srinivasan K. (2018). Zinc supplementation alleviates the progression of diabetic nephropathy by inhibiting the overexpression of oxidative-stress--mediated molecular markers in streptozotocin-induced experimental rats. The Journal of Nutritional Biochemistry, 54, 113–129. [DOI] [PubMed] [Google Scholar]
- Barnett JB, Hamer DH, & Meydani SN (2010). Low zinc status: A new risk factor for pneumonia in the elderly? Nutrition Reviews, 68(1), 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum L, Chan IHS, Cheung SKK, Goggins WB, Mok V, Lam L, et al. (2010). Serum zinc is decreased in Alzheimer’s disease and serum arsenic correlates positively with cognitive ability. Biometals, 23(1), 173. [DOI] [PubMed] [Google Scholar]
- Bellomo EA, Meur G, & Rutter GA (2011). Glucose regulates free cytosolic Zn2+ concentration, Slc39 (ZiP), and metallothionein gene expression in primary pancreatic islet β-cells. The Journal of Biological Chemistry, 286(29), 25778–25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhe K, Gebrearegay F, & Gebremariam H. (2019). Prevalence and associated factors of zinc deficiency among pregnant women and children in Ethiopia: A systematic review and meta-analysis. BMC Public Health, 19(1), 1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML, Kong BY, Kim AM, O’Halloran TV, & Woodruff TK (2012). A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biology of Reproduction, 86(4), 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer N, Coulson DT, Heggarty S, Ravid R, Hellemans J, Irvine GB, et al. (2012). Zinc transporter mRNA levels in Alzheimer’s disease postmortem brain. Journal of Alzheimer’s Disease, 29(4), 863–873. [DOI] [PubMed] [Google Scholar]
- Bi Y, Yang Z, Pang X, Duan Y, Lai J, Jiang S, et al. (2020). The phenomenon of zinc deficiency among children in China aged 3–5 years old. The Proceedings of the Nutrition Society, 79(OCE2), E214. [Google Scholar]
- Bing C, Mracek T, Gao D, & Trayhurn P. (2010). Zinc-α2-glycoprotein: An adipokine modulator of body fat mass? International Journal of Obesity, 34(11), 1559–1565. [DOI] [PubMed] [Google Scholar]
- Bjorklund NL, Reese LC, Sadagoparamanujam VM, Ghirardi V, Woltjer RL, & Taglialatela G. (2012). Absence of amyloid β oligomers at the postsynapse and regulated synaptic Zn 2+ in cognitively intact aged individuals with Alzheimer’s disease neuropathology. Molecular Neurodegeneration, 7(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudiaf AL, Bouziane D, Smara M, Meddour Y, Haffaf EM, Oudjit B, et al. (2018). Could ZnT8 antibodies replace ICA, GAD, IA2 and insulin antibodies in the diagnosis of type 1 diabetes? Current Research in Translational Medicine, 66(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, & Kaur S. (2013). Zinc deficiency and zinc therapy efficacy with reduction of serum free copper in Alzheimer’s disease. International Journal of Alzheimer’s Disease, 2013, 586365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlucci PM, Ahuja T, Petrilli C, Rajagopalan H, Jones S, & Rahimian J. (2020). Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. Journal of Medical Microbiology, 69(10), 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cediel G, Olivares M, Brito A, Cori H, & Lópezde Romaña D. (2015). Zinc deficiency in Latin America and the Caribbean. Food and Nutrition Bulletin, 36(2 Suppl), S129–S138. [DOI] [PubMed] [Google Scholar]
- Ceperuelo-Mallafré V, Ejarque M, Duran X, Pachón G, Vázquez-Carballo A, Roche K, et al. (2015). Zinc-α2-glycoprotein modulates AKT-dependent insulin signaling in human adipocytes by activation of the PP2A phosphatase. PLoS One, 10(6), e0129644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cezar LC, Kirsten TB, da Fonseca CCN, de Lima APN, Bernardi MM, & Felicio LF (2018). Zinc as a therapy in a rat model of autism prenatally induced by valproic acid. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 84, 173–180. [DOI] [PubMed] [Google Scholar]
- Chang CS, Choi JB, Kim HJ, & Park SB (2011). Correlation between serum testosterone level and concentrations of copper and zinc in hair tissue. Biological Trace Element Research, 144(1–3), 264–271. [DOI] [PubMed] [Google Scholar]
- Chasapis CT, Ntoupa PSA, Spiliopoulou CA, & Stefanidou ME (2020). Recent aspects of the effects of zinc on human health. Archives of Toxicology, 94, 1–18. [DOI] [PubMed] [Google Scholar]
- Chauhan AK, Mittra N, Kumar V, Patel DK, & Singh C. (2016). Inflammation and B-cell lymphoma-2 associated X protein regulate zinc-induced apoptotic degeneration of rat nigrostriatal dopaminergic neurons. Molecular Neurobiology, 53(8), 5782–5795. [DOI] [PubMed] [Google Scholar]
- Cheffer A, Castillo ARG, Corrêa-Velloso J, Gonçalves MCB, Naaldijk Y, Nascimento IC, et al. (2018). Purinergic system in psychiatric diseases. Molecular Psychiatry, 23(1), 94–106. [DOI] [PubMed] [Google Scholar]
- Chen WT, Liao YH, Yu HM, Cheng IH, & Chen YR (2011). Distinct effects of Zn2+, Cu2+, Fe3+, and Al3+ on amyloid-β stability, oligomerization, and aggregation amyloid-β destabilization promotes annular protofibril formation. The Journal of Biological Chemistry, 286(11), 9646–9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Zhao M, Chen X, Zhang Y, Wang H, Huang YY, et al. (2012). Zinc supplementation during pregnancy protects against lipopolysaccharide-induced fetal growth restriction and demise through its anti-inflammatory effect. The Journal of Immunology, 189(1), 454–463. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang S, Luo M, Zhang Z, Dai X, Kong M, et al. (2016). From the cover: Zinc deficiency worsens and supplementation prevents high-fat diet induced vascular inflammation, oxidative stress, and pathological remodeling. Toxicological Sciences, 153(1), 124–136. [DOI] [PubMed] [Google Scholar]
- Chen W, Schwalie PC, Pankevich EV, Gubelmann C, Raghav SK, Dainese R, et al. (2019). ZFP30 promotes adipogenesis through the KAP1-mediated activation of a retrotransposon-derived Pparg2 enhancer. Nature Communications, 10(1), 1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yang J, Wang Y, Yang M, & Guo M. (2019). Zinc deficiency promotes testicular cell apoptosis in mice. Biological Trace Element Research, 195, 1–8. [DOI] [PubMed] [Google Scholar]
- Cheng P, Yu J, Huang W, Bai S, Zhu X, Qi Z, et al. (2015). Dietary intake of iron, zinc, copper, and risk of Parkinson’s disease: A meta-analysis. Neurological Sciences, 36(12), 2269–2275. [DOI] [PubMed] [Google Scholar]
- Chiarella E, Aloisio A, Codispoti B, Nappo G, Scicchitano S, Lucchino V, et al. (2018). ZNF521 has an inhibitory effect on the adipogenic differentiation of human adipose-derived mesenchymal stem cells. Stem Cell Reviews and Reports, 14(6), 901–914. [DOI] [PubMed] [Google Scholar]
- Cooper-Capetini V, De Vasconcelos DAA, Martins AR, Hirabara SM, Donato J Jr., Carpinelli AR, et al. (2017). Zinc supplementation improves glucose homeostasis in high fat-fed mice by enhancing pancreatic β-cell function. Nutrients, 9(10), 1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona C, Masciopinto F, Silvestri E, Del Viscovo A, Lattanzio R, La Sorda R, et al. (2010). Dietary zinc supplementation of 3xTg-AD mice increases BDNFlevels and prevents cognitive deficits as well as mitochondrial dysfunction. Cell Death & Disease, 1(10), e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costarelli L, Muti E, Malavolta M, Cipriano C, Giacconi R, Tesei S, et al. (2010). Distinctive modulation of inflammatory and metabolic parameters in relation to zinc nutritional status in adult overweight/obese subjects. The Journal of Nutritional Biochemistry, 21(5), 432–437. [DOI] [PubMed] [Google Scholar]
- Craven KM, Kochen WR, Hernandez CM, & Flinn JM (2018). Zinc exacerbates tau pathology in a tau mouse model. Journal of Alzheimer’s Disease, 64(2), 617–630. [DOI] [PubMed] [Google Scholar]
- Croxford TP, McCormick NH, & Kelleher SL (2011). Moderate zinc deficiency reduces testicular Zip6 and Zip10 abundance and impairs spermatogenesis in mice. The Journal of Nutrition, 141(3), 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz KJC, Morais JBS, de Oliveira ARS, Severo JS, & do Nascimento Marreiro D. (2017). The effect of zinc supplementation on insulin resistance in obese subjects: A systematic review. Biological Trace Element Research, 176(2), 239–243. [DOI] [PubMed] [Google Scholar]
- Cui D, Han G, Shang Y, Mu L, Long Q, & Du Y. (2015). The effect of chronic prostatitis on zinc concentration of prostatic fluid and seminal plasma: A systematic review and meta-analysis. Current Medical Research and Opinion, 31(9), 1763–1769. [DOI] [PubMed] [Google Scholar]
- da Silva Bandeira V, Pires LV, Hashimoto LL, de Alencar LL, Almondes KGS, Lottenberg SA, et al. (2017). Association of reduced zinc status with poor glycemic control in individuals with type 2 diabetes mellitus. Journal of Trace Elements in Medicine and Biology, 44, 132–136. [DOI] [PubMed] [Google Scholar]
- Datki Z, Galik-Olah Z, Janosi-Mozes E, Szegedi V, Kalman J, Hunya ÁG, et al. (2020). Alzheimer risk factors age and female sex induce cortical Aβ aggregation by raising extracellular zinc. Molecular Psychiatry, 25(11), 2728–2741. [DOI] [PubMed] [Google Scholar]
- Davidson HW, Wenzlau JM, & O’Brien RM (2014). Zinc transporter 8 (ZnT8) and β cell function. Trends in Endocrinology and Metabolism, 25(8), 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doboszewska U, Wlaź P, Nowak G, & Młyniec K. (2020). Targeting zinc metalloenzymes in coronavirus disease 2019. British Journal of Pharmacology, 177(21), 4887–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos AB, Bezerra MA, Rocha ME, Barreto GE, & Kohlmeier KA (2019). Higher zinc concentrations in hair of Parkinson’s disease are associated with psychotic complications and depression. Journal of Neural Transmission, 126(10), 1291–1301. [DOI] [PubMed] [Google Scholar]
- Du C, Ma X, Meruvu S, Hugendubler L, & Mueller E. (2014). The adipogenic transcriptional cofactor ZNF638 interacts with splicing regulators and influences alternative splicing. Journal of Lipid Research, 55(9), 1886–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Liu MY, Zhong X, & Wei MJ (2017). Decreased circulating Zinc levels in Parkinson’s disease: A meta-analysis study. Scientific Reports, 7(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Shi L, Gao H, Fu X, Zhang X, Zhang Y, et al. (2019). The effect of zinc supplementation in pre-diabetes: A protocol for systematic review and meta-analysis. Medicine, 98(27), e16259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan FE, Que EL, Zhang N, Feinberg EC, O’Halloran TV, & Woodruff TK (2016). The zinc spark is an inorganic signature of human egg activation. Scientific Reports, 6, 24737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi OP, Lehtovirta M, Hastoy B, Chandra V, Krentz NA, Kleiner S, et al. (2019). Loss of ZnT8 function protects against diabetes by enhanced insulin secretion. Nature Genetics, 51(11), 1596–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elattar S, Dimri M, & Satyanarayana A. (2018). The tumor secretory factor ZAG promotes white adipose tissue browning and energy wasting. The FASEB Journal, 32(9), 4727–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enache D, Pereira JB, Jelic V, Winblad B, Nilsson P, Aarsland D, et al. (2020). Increased cerebrospinal fluid concentration of ZnT3 is associated with cognitive impairment in Alzheimer’s disease. Journal of Alzheimer’s Disease, 77, 1–13. [DOI] [PubMed] [Google Scholar]
- Éńee É, Kratzer R, Arnoux JB, Barilleau E, Hamel Y, Marchi C, et al. (2012). ZnT8 is a major CD8+ T cell–recognized autoantigen in pediatric type 1 diabetes. Diabetes, 61(7), 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshak ES, Iso H, Maruyama K, Muraki I, & Tamakoshi A. (2018). Associations between dietary intakes of iron, copper and zinc with risk of type 2 diabetes mellitus: A large population-based prospective cohort study. Clinical Nutrition, 37(2), 667–674. [DOI] [PubMed] [Google Scholar]
- Fallah A, Mohammad-Hasani A, & Colagar AH (2018). Zinc is an essential element for male fertility: A review of Zn roles in men’s health, germination, sperm quality, and fertilization. Journal of Reproduction & Infertility, 19(2), 69. [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Zhang C, & Bu J. (2017). Relationship between selected serum metallic elements and obesity in children and adolescent in the US. Nutrients, 9(2), 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, Dang X, Li Y, Chen J, Zhao R, & Yang X. (2020). Zinc-α2-glycoprotein promotes browning of white adipose tissue in cold-exposed male mice. Molecular and Cellular Endocrinology, 501, 110669. [DOI] [PubMed] [Google Scholar]
- Fernández-Cao JC, Warthon-Medina M, Moran VH, Arija V, Doepking C, & Lowe NM (2018). Dietary zinc intake and whole blood zinc concentration in subjects with type 2 diabetes versus healthy subjects: A systematic review, meta-analysis and meta-regression. Journal of Trace Elements in Medicine and Biology, 49, 241–251. [DOI] [PubMed] [Google Scholar]
- Fernández-Cao JC, Warthon-Medina M, H Moran V, Arija V, Doepking C, Serra-Majem L, et al. (2019). Zinc intake and status and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Nutrients, 11(5), 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M, Barone R, Copat C, Grasso A, Cristaldi A, Rizzo R, et al. (2020). Metal and essential element levels in hair and association with autism severity. Journal of Trace Elements in Medicine and Biology, 57, 126409. [DOI] [PubMed] [Google Scholar]
- Flinn JM, Bozzelli PL, Adlard PA, & Railey AM (2014). Spatial memory deficits in a mouse model of late-onset Alzheimer’s disease are caused by zinc supplementation and correlate with amyloid-beta levels. Frontiers in Aging Neuroscience, 6, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresta C, Garolla A, Cosci I, Menegazzo M, Ferigo M, Gandin V, et al. (2014). Role of zinc trafficking in male fertility: From germ to sperm. Human Reproduction, 29(6), 1134–1145. [DOI] [PubMed] [Google Scholar]
- Fourie C, Vyas Y, Lee K, Jung Y, Garner CC, & Montgomery JM (2018). Dietary zinc supplementation prevents autism related behaviors and striatal synaptic dysfunction in Shank3 exon 13–16 mutant mice. Frontiers in Cellular Neuroscience, 12, 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire SC, Fisberg M, & Cozzolino SMF (2013). Dietary intervention causes redistribution of zinc in obese adolescents. Biological Trace Element Research, 154(2), 168–177. [DOI] [PubMed] [Google Scholar]
- Fukunaka A, Fukada T, Bhin J, Suzuki L, Tsuzuki T, Takamine Y, et al. (2017). Zinc transporter ZIP13 suppresses beige adipocyte biogenesis and energy expenditure by regulating C/EBP-β expression. PLoS Genetics, 13(8), e1006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez-Peralta M, He L, Jorge-Nebert LF, Wang B, Miller ML, Eppert BL, et al. (2012). ZIP8 zinc transporter: Indispensable role for both multiple-organ organogenesis and hematopoiesis in utero. PLoS One, 7(5), e36055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammoh NZ, & Rink L. (2017). Zinc in infection and inflammation. Nutrients, 9(6), 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SX, Guo J, Fan GQ, Qiao Y, Zhao RQ, & Yang XJ (2018). ZAG alleviates HFD-induced insulin resistance accompanied with decreased lipid depot in skeletal muscle in mice. Journal of Lipid Research, 59(12), 2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García OP, Ronquillo D, del Carmen Caamaño M, Camacho M, Long KZ, & Rosado JL (2012). Zinc, vitamin A, and vitamin C status are associated with leptin concentrations and obesity in Mexican women: Results from a cross-sectional study. Nutrition and Metabolism, 9(1), 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García OP, Ronquillo D, del Carmen Caamaño M, Martínez G, Camacho M, López V, et al. (2013). Zinc, iron and vitamins A, C and E are associated with obesity, inflammation, lipid profile and insulin resistance in Mexican school-aged children. Nutrients, 5(12), 5012–5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner B, Dieriks BV, Cameron S, Mendis LH, Turner C, Faull RL, et al. (2017). Metal concentrations and distributions in the human olfactory bulb in Parkinson’s disease. Scientific Reports, 7(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Sánchez L, García-Fuentes E, Fernández-García D, Escoté X, Alcaide J, Perez-Martinez P, et al. (2012). Zinc-alpha 2-glycoprotein gene expression in adipose tissue is related with insulin resistance and lipolytic genes in morbidly obese patients. PLoS One, 7(3), e33264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatiatulina ER, Sheina EA, Nemereshina ON, Popova EV, Polyakova VS, Agletdinov EF, et al. (2019). Effect of Zn supplementation on trace element status in rats with diet-induced non-alcoholic fatty liver disease. Biological Trace Element Research, 197, 202–212. [DOI] [PubMed] [Google Scholar]
- Genoud S, Roberts BR, Gunn AP, Halliday GM, Lewis SJ, Ball HJ, et al. (2017). Subcellular compartmentalisation of copper, iron, manganese, and zinc in the Parkinson’s disease brain. Metallomics, 9(10), 1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber H, Wu F, Dimitrov M, Osuna GMG, & Fraering PC (2017). Zinc and copper differentially modulate amyloid precursor protein processing by γ-secretase and amyloid-β peptide production. Journal of Biological Chemistry, 292(9), 3751–3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh C, Yang SH, Kim JG, Jeon TI, Yoon BH, Lee JY, et al. (2013). Zinc-chelated vitamin C stimulates adipogenesis of 3T3-L1 cells. Asian-Australasian Journal of Animal Sciences, 26(8), 1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacconi R, Giuli C, Casoli T, Balietti M, Costarelli L, Provinciali M, et al. (2019). Acetylcholinesterase inhibitors in Alzheimer’s disease influence Zinc and Copper homeostasis. Journal of Trace Elements in Medicine and Biology, 55, 58–63. [DOI] [PubMed] [Google Scholar]
- Giacone F, Condorelli RA, Mongioì LM, Bullara V, La Vignera S, & Calogero AE (2017). In vitro effects of zinc, D-aspartic acid, and coenzyme-Q10 on sperm function. Endocrine, 56(2), 408–415. [DOI] [PubMed] [Google Scholar]
- Gong FY, Deng JY, Zhu HJ, Pan H, Wang LJ, & Yang HB (2010). Fatty acid synthase and hormone-sensitive lipase expression in liver are involved in zinc-α2-glycoprotein-induced body fat loss in obese mice. Chinese Medical Sciences Journal, 25(3), 169–175. [DOI] [PubMed] [Google Scholar]
- Gonmei Z, & Toteja GS (2018). Micronutrient status of Indian population. The Indian Journal of Medical Research, 148(5), 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Domínguez R, García-Barrera T, & Gómez-Ariza JL (2014). Homeostasis of metals in the progression of Alzheimer’s disease. Biometals, 27(3), 539–549. [DOI] [PubMed] [Google Scholar]
- Gough M, Parr-Sturgess C, & Parkin E. (2011). Zinc metalloproteinases and amyloid Beta-Peptide metabolism: The positive side of proteolysis in Alzheimer’s disease. Biochemistry Research International, 2011, 721463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabrucker AM (2014). A role for synaptic zinc in ProSAP/Shank PSD scaffold malformation in autism spectrum disorders. Developmental Neurobiology, 74(2), 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabrucker S, & Grabrucker AM (2017). Zinc in autism. In Biometals in neurodegenerative diseases (pp. 153–173). Academic Press. [Google Scholar]
- Grabrucker AM, Schmeisser MJ, Udvardi PT, Arons M, Schoen M, Woodling NS, et al. (2011). Amyloid beta protein-induced zinc sequestration leads to synaptic loss via dysregulation of the ProSAP2/Shank3 scaffold. Molecular Neurodegeneration, 6(1), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabrucker S, Jannetti L, Eckert M, Gaub S, Chhabra R, Pfaender S, et al. (2014). Zinc deficiency dysregulates the synaptic ProSAP/Shank scaffold and might contribute to autism spectrum disorders. Brain, 137(1), 137–152. [DOI] [PubMed] [Google Scholar]
- Grabrucker S, Boeckers TM, & Grabrucker AM (2016). Gender dependent evaluation of autism like behavior in mice exposed to prenatal zinc deficiency. Frontiers in Behavioral Neuroscience, 10, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabrucker S, Haderspeck JC, Sauer AK, Kittelberger N, Asoglu H, Abaei A, et al. (2018). Brain lateralization in mice is associated with zinc signaling and altered in prenatal zinc deficient mice that display features of autism spectrum disorder. Frontiers in Molecular Neuroscience, 10, 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger JA, Grzeskowiak LE, Wilson RL, Bianco-Miotto T, Leemaqz SY, Jankovic-Karasoulos T, et al. (2019). Maternal selenium, copper and zinc concentrations in early pregnancy, and the association with fertility. Nutrients, 11(7), 1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu K, Xiang W, Zhang Y, Sun K, & Jiang X. (2019). The association between serum zinc level and overweight/obesity: A meta-analysis. European Journal of Nutrition, 58(8), 2971–2982. [DOI] [PubMed] [Google Scholar]
- Guo BQ, Li HB, Zhai DS, & Ding SB (2019). Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: A systematic review and meta-analysis. Nutrition Research, 65, 4–16. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, et al. (2010). Transcriptional control of preadipocyte determination by Zfp423. Nature, 464(7288), 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Brazier AK, & Lowe NM (2020). Zinc deficiency in low-and middle-income countries: Prevalence and approaches for mitigation. Journal of Human Nutrition and Dietetics, 33(5), 624–643. [DOI] [PubMed] [Google Scholar]
- Gustafson B, Nerstedt A, & Smith U. (2019). Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nature Communications, 10(1), 2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwan MH, Almashhedy LA, & Alsalman ARS (2012). Oral zinc supplementation restores high molecular weight seminal zinc binding protein to normal value in Iraqi infertile men. BMC Urology, 12(1), 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmeyer S, Haderspeck JC, & Grabrucker AM (2014). Behavioral impairments in animal models for zinc deficiency. Frontiers in Behavioral Neuroscience, 8, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmeyer S, Mangus K, Boeckers TM, & Grabrucker AM (2015). Effects of trace metal profiles characteristic for autism on synapses in cultured neurons. Neural Plasticity, 2015, 985083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmeyer S, Sauer AK, & Grabrucker AM (2018). Prospects of zinc supplementation in autism spectrum disorders and Shankopathies such as Phelan McDermid Syndrome. Frontiers in Synaptic Neuroscience, 10, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PJ, Shekar A, Belovich AN, Christianson NB, Campbell NG, Sutcliffe JS, et al. (2015). Zn 2+ reverses functional deficits in a de novo dopamine transporter variant associated with autism spectrum disorder. Molecular Autism, 6(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane FT, Hayes R, Lee BY, & Leonenko Z. (2016). Effect of copper and zinc on the single molecule self-affinity of Alzheimer’s amyloid-β peptides. PLoS One, 11(1), e0147488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy AB, Serino AS, Wijesekara N, Chimienti F, & Wheeler MB (2011). Regulation of glucagon secretion by zinc: Lessons from the β cell-specific Znt8 knockout mouse model. Diabetes, Obesity & Metabolism, 13, 112–117. [DOI] [PubMed] [Google Scholar]
- Harika R, Faber M, Samuel F, Kimiywe J, Mulugeta A, & Eilander A. (2017). Micronutrient status and dietary intake of iron, vitamin A, iodine, folate and zinc in women of reproductive age and pregnant women in Ethiopia, Kenya, Nigeria and South Africa: A systematic review of data from 2005 to 2015. Nutrients, 9(10), 1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harika R, Faber M, Samuel F, Mulugeta A, Kimiywe J, & Eilander A. (2017). Are low intakes and deficiencies in iron, vitamin A, zinc, and iodine of public health concern in Ethiopian, Kenyan, Nigerian, and South African children and adolescents? Food and Nutrition Bulletin, 38(3), 405–427. [DOI] [PubMed] [Google Scholar]
- Hasna J, Bohic S, Lemoine S, Blugeon C, & Bouron A. (2019). Zinc uptake and storage during the formation of the cerebral cortex in mice. Molecular Neurobiology, 56(10), 6928–6940. [DOI] [PubMed] [Google Scholar]
- He L, Lang L, Li Y, Liu Q, & Yao Y. (2016). Comparison of serum zinc, calcium, and magnesium concentrations in women with pregnancy-induced hypertension and healthy pregnant women: A meta-analysis. Hypertension in Pregnancy, 35(2), 202–209. [DOI] [PubMed] [Google Scholar]
- Hennigar SR, Lieberman HR, Fulgoni VL III, & McClung JP (2018). Serum zinc concentrations in the US population are related to sex, age, and time of blood draw but not dietary or supplemental zinc. The Journal of Nutrition, 148(8), 1341–1351. [DOI] [PubMed] [Google Scholar]
- Hess SY (2017). National risk of zinc deficiency as estimated by national surveys. Food and Nutrition Bulletin, 38(1), 3–17. [DOI] [PubMed] [Google Scholar]
- Hoveizi E, & Mohammadi T. (2019). Differentiation of endometrial stem cells into insulin-producing cells using signaling molecules and zinc oxide nanoparticles, and three-dimensional culture on nanofibrous scaffolds. Journal of Materials Science: Materials in Medicine, 30(9), 101. [DOI] [PubMed] [Google Scholar]
- Hu JY, Zhang DL, Liu XL, Li XS, Cheng XQ, Chen J, et al. (2017). Pathological concentration of zinc dramatically accelerates abnormal aggregation of full-length human Tau and thereby significantly increases Tau toxicity in neuronal cells. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1863(2), 414–427. [DOI] [PubMed] [Google Scholar]
- Huang L, Yan M, & Kirschke CP (2010). Over-expression of ZnT7 increases insulin synthesis and secretion in pancreatic β-cells by promoting insulin gene transcription. Experimental Cell Research, 316(16), 2630–2643. [DOI] [PubMed] [Google Scholar]
- Huang L, Kirschke CP, Lay YAE, Levy LB, Lamirande DE, & Zhang PH (2012). Znt7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance. The Journal of Biological Chemistry, 287(40), 33883–33896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wu Z, Cao Y, Lang M, Lu B, & Zhou B. (2014). Zinc binding directly regulates tau toxicity independent of tau hyperphosphorylation. Cell Reports, 8(3), 831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Tepaamorndech S, Kirschke CP, Pedersen TL, Keyes WR, & Newman JW (2016). Zinc transporter 7 (Znt7) knockout in mice differentially affects lipid metabolism in adipose tissues. The FASEB Journal, 30(1_supplement), 691–694. [Google Scholar]
- Huang X, Jiang D, Zhu Y, Fang Z, Che L, Lin Y, et al. (2017). Chronic high dose zinc supplementation induces visceral adipose tissue hypertrophy without altering body weight in mice. Nutrients, 9(10), 1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Tepaamorndech S, Kirschke CP, Newman JW, Keyes WR, Pedersen TL, et al. (2018). Aberrant fatty acid metabolism in skeletal muscle contributes to insulin resistance in zinc transporter 7 (znt7)-knockout mice. The Journal of Biological Chemistry, 293(20), 7549–7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S, Ali I, Rust P, Kundi M, & Ekmekcioglu C. (2020). Selenium, zinc, and manganese status in pregnant women and its relation to maternal and child complications. Nutrients, 12(3), 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MR, Attia J, Ali L, McEvoy M, Selim S, Sibbritt D, et al. (2016). Zinc supplementation for improving glucose handling in pre-diabetes: A double-blind randomized placebo controlled pilot study. Diabetes Research and Clinical Practice, 115, 39–46. [DOI] [PubMed] [Google Scholar]
- Istrate AN, Kozin SA, Zhokhov SS, Mantsyzov AB, Kechko OI, Pastore A, et al. (2016). Interplay of histidine residues of the Alzheimer’s disease Aβ peptide governs its Zn-induced oligomerization. Scientific Reports, 6(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarnejad S, Mahboobi S, McFarland LV, Taghizadeh M, & Rahimi F. (2019). Meta-analysis: Effects of zinc supplementation alone or with multi-nutrients, on glucose control and lipid levels in patients with type 2 diabetes. Preventive Nutrition and Food Science, 24(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaksic M, Martinovic M, Gligorovic-Barhanovic N, Vujacic A, Djurovic D, & Nedovic-Vukovic M. (2019). Association between inflammation, oxidative stress, vitamin D, copper and zinc with pre-obesity and obesity in school children from the city of Podgorica, Montenegro. Journal of Pediatric Endocrinology & Metabolism, 32(9), 951–957. [DOI] [PubMed] [Google Scholar]
- James SA, Churches QI, de Jonge MD, Birchall IE, Streltsov V, McColl G, et al. (2017). Iron, copper, and zinc concentration in Aβ plaques in the APP/PS1 mouse model of Alzheimer’s disease correlates with metal levels in the surrounding neuropil. ACS Chemical Neuroscience, 8(3), 629–637. [DOI] [PubMed] [Google Scholar]
- Jansen J, Rosenkranz E, Overbeck S, Warmuth S, Mocchegiani E, Giacconi R, et al. (2012). Disturbed zinc homeostasis in diabetic patients by in vitro and in vivo analysis of insulinomimetic activity of zinc. The Journal of Nutritional Biochemistry, 23(11), 1458–1466. [DOI] [PubMed] [Google Scholar]
- Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, & Librowski T. (2017). Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology, 25(1), 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi A, Mokhtari S, Moraveji SF, Sayahpour FA, Farzaneh M, Gourabi H, et al. (2020). Short time exposure to low concentration of zinc oxide nanoparticles up-regulates self-renewal and spermatogenesis-related gene expression. The International Journal of BiochThe International Journal of Biochemistry & Cell Biologyemistry & Cell Biology., 127, 105822. [DOI] [PubMed] [Google Scholar]
- Jeon Y, Yoon JD, Cai L, Hwang SU, Kim E, Zheng Z, et al. (2015). Zinc deficiency during in vitro maturation of porcine oocytes causes meiotic block and developmental failure. Molecular Medicine Reports, 12(4), 5973–5982. [DOI] [PubMed] [Google Scholar]
- Jothimani D, Kailasam E, Danielraj S, Nallathambi B, Ramachandran H, Sekar P, et al. (2020). COVID-19: Poor outcomes in patients with zinc deficiency. International Journal of Infectious Diseases, 100, 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou MY, Philipps AF, & Lönnerdal B. (2010). Maternal zinc deficiency in rats affects growth and glucose metabolism in the offspring by inducing insulin resistance postnatally. The Journal of Nutrition, 140(9), 1621–1627. [DOI] [PubMed] [Google Scholar]
- Jung A, Spira D, Steinhagen-Thiessen E, Demuth I, & Norman K. (2017). Zinc deficiency is associated with depressive symptoms–Results from the Berlin Aging Study II. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 72(8), 1149–1154. [DOI] [PubMed] [Google Scholar]
- Kang S, Akerblad P, Kiviranta R, Gupta RK, Kajimura S, Griffin MJ, et al. (2012). Regulation of early adipose commitment by Zfp521. PLoS Biology, 10(11), e1001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman Z, Salvador GA, Liu X, & Oteiza PI (2020). Zinc and the modulation of Nrf2 in human neuroblastoma cells. Free Radical Biology & Medicine, 155, 1–9. [DOI] [PubMed] [Google Scholar]
- Kerns K, Zigo M, Drobnis EZ, Sutovsky M, & Sutovsky P. (2018). Zinc ion flux during mammalian sperm capacitation. Nature Communications, 9(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadivi F, Razavi S, & Hashemi F. (2020). Protective effects of zinc on rat sperm chromatin integrity involvement: DNA methylation, DNA fragmentation, ubiquitination and protamination after bleomycin etoposide and cis-platin treatment. Theriogenology, 142, 177–183. [DOI] [PubMed] [Google Scholar]
- Khatua P, Mondal S, & Bandyopadhyay S. (2019). Effects of metal ions on Aβ42 peptide conformations from molecular simulation studies. Journal of Chemical Information and Modeling, 59(6), 2879–2893. [DOI] [PubMed] [Google Scholar]
- Khodabandeh A, Yakhchian R, Hasan A, Paray BA, Shahi F, Rasti B, et al. (2020). Silybin as a potent inhibitor of α-synuclein aggregation and associated cytotoxicity against neuroblastoma cells induced by zinc oxide nanoparticles. Journal of Molecular Liquids, 310, 113198. [Google Scholar]
- Khorsandi H, Nikpayam O, Yousefi R, Parandoosh M, Hosseinzadeh N, Saidpour A, et al. (2019). Zinc supplementation improves body weight management, inflammatory biomarkers and insulin resistance in individuals with obesity: A randomized, placebo-controlled, double-blind trial. Diabetology & Metabolic Syndrome, 11(1), 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorshidi M, Zarezadeh M, Sadeghi A, Teymouri A, Emami MR, Kord-Varkaneh H, et al. (2019). The effect of zinc supplementation on serum leptin levels: A systematic review and meta-analysis of randomized controlled trials. Hormone and Metabolic Research, 51(08), 503–510. [DOI] [PubMed] [Google Scholar]
- Kim J, & Ahn J. (2014). Effect of zinc supplementation on inflammatory markers and adipokines in young obese women. Biological Trace Element Research, 157(2), 101–106. [DOI] [PubMed] [Google Scholar]
- Kim G, Shin KH, & Pae EK (2016). Zinc up-regulates insulin secretion from β cell-like cells derived from stem cells from human exfoliated deciduous tooth (SHED). International Journal of Molecular Sciences, 17(12), 2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AC, Lim S, & Kim YK (2018). Metal ion effects on Aβ and tau aggregation. International Journal of Molecular Sciences, 19(1), 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Aydemir TB, Jimenez-Rondan FR, Ruggiero CH, Kim MH, & Cousins RJ (2020). Deletion of metal transporter Zip14 (Slc39a14) produces skeletal muscle wasting, endotoxemia, Mef2c activation and induction of miR-675 and Hspb7. Scientific Reports, 10(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JC (2011). Zinc: An essential but elusive nutrient. The American Journal of Clinical Nutrition., 94(2), 679S–684S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsten TB, Queiroz-Hazarbassanov N, Bernardi MM, & Felicio LF (2015). Prenatal zinc prevents communication impairments and BDNF disturbance in a rat model of autism induced by prenatal lipopolysaccharide exposure. Life Sciences, 130, 12–17. [DOI] [PubMed] [Google Scholar]
- Kirsten TB, Chaves-Kirsten GP, Bernardes S, Scavone C, Sarkis JE, Bernardi MM, et al. (2015). Lipopolysaccharide exposure induces maternal hypozincemia, and prenatal zinc treatment prevents autistic-like behaviors and disturbances in the striatal dopaminergic and mTOR systems of offspring. PLoS One, 10(7), e0134565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong SM, Chan BK, Park JS, Hill KJ, Aitken JB, Cottle L, et al. (2014). Parkinson’s disease-linked human PARK9/ATP13A2 maintains zinc homeostasis and promotes α-Synuclein externalization via exosomes. Human Molecular Genetics, 23(11), 2816–2833. [DOI] [PubMed] [Google Scholar]
- Kothari RP, & Chaudhari AR (2016). Zinc levels in seminal fluid in infertile males and its relation with serum free testosterone. Journal of Clinical and Diagnostic Research: JCDR, 10(5), CC05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács G, Környei Z, Tóth K, Baranyi M, Brunner J, Neubrandt M, et al. (2018). Modulation of P2X7 purinergic receptor activity by extracellular Zn2+ in cultured mouse hippocampal astroglia. Cell Calcium, 75, 1–13. [DOI] [PubMed] [Google Scholar]
- Kumar A, Singh BK, Ahmad I, Shukla S, Patel DK, Srivastava G, et al. (2012). Involvement of NADPH oxidase and glutathione in zinc-induced dopaminergic neurodegeneration in rats: Similarity with paraquat neurotoxicity. Brain Research, 1438, 48–64. [DOI] [PubMed] [Google Scholar]
- Kumar V, Singh BK, Chauhan AK, Singh D, Patel DK, & Singh C. (2016). Minocycline rescues from zinc-induced nigrostriatal dopaminergic neurodegeneration: Biochemical and molecular interventions. Molecular Neurobiology, 53(5), 2761–2777. [DOI] [PubMed] [Google Scholar]
- Kumar V, Singh D, Singh BK, Singh S, Mittra N, Jha RR, et al. (2018). Alpha-synuclein aggregation, Ubiquitin proteasome system impairment, and L-Dopa response in zinc-induced Parkinsonism: Resemblance to sporadic Parkinson’s disease. Molecular and Cellular Biochemistry, 444(1–2), 149–160. [DOI] [PubMed] [Google Scholar]
- Kumari D, Nair N, & Bedwal RS (2011). Effect of dietary zinc deficiency on testes of Wistar rats: Morphometric and cell quantification studies. Journal of Trace Elements in Medicine and Biology, 25(1), 47–53. [DOI] [PubMed] [Google Scholar]
- Kumssa DB, Joy EJ, Ander EL, Watts MJ, Young SD, Walker S, et al. (2015). Dietary calcium and zinc deficiency risks are decreasing but remain prevalent. Scientific Reports, 5, 10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvamme JM, Grønli O, Jacobsen BK, & Florholmen J. (2015). Risk of malnutrition and zinc deficiency in community-living elderly men and women: The Tromsø Study. Public Health Nutrition, 18(11), 1907–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KJ, Lee EJ, Cho KS, Cho DH, Shin CY, & Han SH (2015). Ginkgo biloba extract (egb761) attenuates zinc-induced tau phosphorylation at ser262 by regulating gsk3β activity in rat primary cortical neurons. Food & Function, 6(6), 2058–2067. [DOI] [PubMed] [Google Scholar]
- Lai GL, Yeh CC, Yeh CY, Chen RY, Fu CL, Chen CH, et al. (2017).Decreased zinc and increased lead blood levels are associated with endometriosis in Asian Women. Reproductive Toxicology, 74, 77–84. [DOI] [PubMed] [Google Scholar]
- Lanza V, Bellia F, & Rizzarelli E. (2018). An inorganic overview of natural Aβ fragments: Copper (II) and zinc (II)-mediated pathways. Coordination Chemistry Reviews, 369, 1–14. [Google Scholar]
- Lawson R, Maret W, & Hogstrand C. (2018). Prolonged stimulation of insulin release from MIN6 cells causes zinc depletion and loss of β-cell markers. Journal of Trace Elements in Medicine and Biology, 49, 51–59. [DOI] [PubMed] [Google Scholar]
- Lee JY, Cho E, Seo JW, Hwang JJ, & Koh JY (2012). Alteration of the cerebral zinc pool in a mouse model of Alzheimer disease. Journal of Neuropathology and Experimental Neurology, 71(3), 211–222. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Lee H, Huang TN, Chung C, Shin W, Kim K, et al. (2015). Trans-synaptic zinc mobilization improves social interaction in two mouse models of autism through NMDAR activation. Nature Communications, 6(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Yu WC, Shih YH, Chen CY, Guo ZH, Huang SJ, et al. (2018). Zinc ion rapidly induces toxic, off-pathway amyloid-β oligomers distinct from amyloid-β derived diffusible ligands in Alzheimer’s disease. Scientific Reports, 8(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YV (2014). Zinc and insulin in pancreatic beta-cells. Endocrine, 45(2), 178–189. [DOI] [PubMed] [Google Scholar]
- Li Y, Mayer FP, Hasenhuetl PS, Burtscher V, Schicker K, Sitte HH, et al. (2017). Occupancy of the zinc-binding site by transition metals decreases the substrate affinity of the human dopamine transporter by an allosteric mechanism. The Journal of Biological Chemistry, 292(10), 4235–4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DD, Zhang W, Wang ZY, & Zhao P. (2017). Serum copper, zinc, and iron levels in patients with Alzheimer’s disease: A meta-analysis of case-control studies. Frontiers in Aging Neuroscience, 9, 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xie B, Dong X, & Sun Y. (2018). Bifunctionality of iminodiacetic acid-modified lysozyme on inhibiting Zn2+-mediated amyloid β-protein aggregation. Langmuir, 34(17), 5106–5115. [DOI] [PubMed] [Google Scholar]
- Li GZ, Liu F, Xu C, Li JY, & Xu YJ (2018). Selenium and zinc against Aβ 25–35--induced cytotoxicity and tau phosphorylation in PC12 cells and inhibits γ-cleavage of APP. Biological Trace Element Research, 184(2), 442–449. [DOI] [PubMed] [Google Scholar]
- Li X, Du X, & Ni J. (2019). Zn2+ aggravates tau aggregation and neurotoxicity. International Journal of Molecular Sciences, 20(3), 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Wang X, Li H, Li L, Zhang G, Yang M, et al. (2016). Sodium-glucose cotransporter 2 (SGLT2) inhibitor increases circulating zinc-Α 2-glycoprotein levels in patients with type 2 diabetes. Scientific Reports, 6, 32887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, & Li D. (2018). Zinc and zinc transporters: Novel regulators of ventricular myocardial development. Pediatric Cardiology, 39(5), 1042–1051. [DOI] [PubMed] [Google Scholar]
- Liu MJ, Bao S, Bolin ER, Burris DL, Xu X, Sun Q, et al. (2013). Zinc deficiency augments leptin production and exacerbates macrophage infiltration into adipose tissue in mice fed a high-fat diet. The Journal of Nutrition, 143(7), 1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, He X, Hong X, Kang F, Chen S, Wang Q, et al. (2014). Suppression of placental metallothionein 1 and zinc transporter 1 mRNA expressions contributes to fetal heart malformations caused by maternal zinc deficiency. Cardiovascular Toxicology, 14(4), 329–338. [DOI] [PubMed] [Google Scholar]
- Liu Y, Batchuluun B, Ho L, Zhu D, Prentice KJ, Bhattacharjee A, et al. (2015). Characterization of zinc influx transporters (ZIPs) in pancreatic β cells roles in regulating cytosolic zinc homeostasis and insulin secretion. The Journal of Biological Chemistry, 290(30), 18757–18769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Dong X, Liu F, Zheng J, & Sun Y. (2017). Iminodiacetic acid-conjugated nanoparticles as a bifunctional modulator against Zn2+-mediated amyloid β-protein aggregation and cytotoxicity. Journal of Colloid and Interface Science, 505, 973–982. [DOI] [PubMed] [Google Scholar]
- Liu X, Piao J, Zhang Y, He Y, Li W, Yang L, et al. (2017). Assessment of zinc Status in school-age children from rural areas in China nutrition and health survey 2002 and 2012. Biological Trace Element Research, 178(2), 194–200. [DOI] [PubMed] [Google Scholar]
- Liu M, Zhu H, Dai Y, Pan H, Li N, Wang L, et al. (2018). Zinc-α2-glycoprotein is associated with obesity in Chinese people and HFD-induced obese mice. Frontiers in Physiology, 9, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zhao Y, Wu R, Jiang Q, Cai M, Bi Z, et al. (2019). ZFP217 regulates adipogenesis by controlling mitotic clonal expansion in a METTL3-m6A dependent manner. RNA Biology, 16(12), 1785–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Zhang K, Wang L, Yang H, Yan K, Pan H, et al. (2020). Serum ZAG and adiponectin levels were closely related to obesity and the metabolically abnormal phenotype in Chinese population. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 13, 3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Yang H, Fang Y, Li K, Tian L, Liu X, et al. (2020). Neurotoxicity and biomarkers of zinc oxide nanoparticles in main functional brain regions and dopaminergic neurons. Science of the Total Environment, 705, 135809. [DOI] [PubMed] [Google Scholar]
- Loef M, von Stillfried N, & Walach H. (2012). Zinc diet and Alzheimer’s disease: A systematic review. Nutritional Neuroscience, 15(5), 2–12. [DOI] [PubMed] [Google Scholar]
- Longo M, Raciti GA, Zatterale F, Parrillo L, Desiderio A, Spinelli R, et al. (2018). Epigenetic modifications of the Zfp/ZNF423 gene control murine adipogenic commitment and are dysregulated in human hypertrophic obesity. Diabetologia, 61(2), 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Wu W, Zhang P, Chen X, Feng Y, Ma N, et al. (2020). Zinc levels and birth weight in pregnant women with gestational diabetes mellitus: A matched cohort study in China. The Journal of Clinical Endocrinology & Metabolism, 105(7), dgaa171. [DOI] [PubMed] [Google Scholar]
- Ma Y, Shen X, & Zhang D. (2015). The relationship between serum zinc level and preeclampsia: A meta-analysis. Nutrients, 7(9), 7806–7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maremanda KP, Khan S, & Jena GB (2016). Role of zinc supplementation in testicular and epididymal damages in diabetic rat: Involvement of Nrf2, SOD1, and GPX5. Biological Trace Element Research, 173(2), 452–464. [DOI] [PubMed] [Google Scholar]
- Maret W. (2017a). Zinc in cellular regulation: The nature and significance of “zinc signals”. International Journal of Molecular Sciences, 18(11), 2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W. (2017b). Zinc in pancreatic islet biology, insulin sensitivity, and diabetes. Preventive Nutrition and Food Science, 22(1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maret W. (2019). The redox biology of redox-inert zinc ions. Free Radical Biology and Medicine, 134, 311–326. [DOI] [PubMed] [Google Scholar]
- Matheou CJ, Younan ND, & Viles JH (2016). The rapid exchange of zinc2+ enables trace levels to profoundly influence amyloid-β misfolding and dominates assembly outcomes in Cu2+/Zn2+ mixtures. Journal of Molecular Biology, 428(14), 2832–2846. [DOI] [PubMed] [Google Scholar]
- Maxel T, Pold R, Larsen A, Pedersen SB, Carlson D, Rolin B, et al. (2015). Dysregulation of zinc and iron balance in adipose tissue from diabetic sand rats (Psammomys obesus). Journal of Diabetes and Metabolism, 6, 2. [Google Scholar]
- Maxel T, Smidt K, Larsen A, Bennetzen M, Cullberg K, Fjeldborg K, et al. (2015). Gene expression of the zinc transporter ZIP14 (SLC39a14) is affected by weight loss and metabolic status and associates with PPARγ in human adipose tissue and 3T3-L1 pre-adipocytes. BMC Obesity, 2, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxel T, Svendsen PF, Smidt K, Lauridsen JK, Brock B, Pedersen SB, et al. (2017). Expression patterns and correlations with metabolic markers of zinc transporters ZIP14 and ZNT1 in obesity and polycystic ovary syndrome. Frontiers in Endocrinology, 8, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxel T, Smidt K, Petersen CC, Honoré B, Christensen AK, Jeppesen PB, et al. (2019). The zinc transporter Zip14 (SLC39a14) affects beta-cell function: Proteomics, gene expression, and insulin secretion studies in INS-1E cells. Scientific Reports, 9(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott L, Jadoon A, & Cunningham P. (2012). ZAG and a potential role in systemic lipid homeostastis: Examining the evidence from in vitro human studies and patients with chronic illness. Clinical Lipidology, 7(4), 409–417. [Google Scholar]
- Meguid NA, Bjørklund G, Gebril OH, Doşa MD, Anwar M, Elsaeid A, et al. (2019). The role of zinc supplementation on the metallothionein system in children with autism spectrum disorder. Acta Neurologica Belgica, 119(4), 577–583. [DOI] [PubMed] [Google Scholar]
- Merriman C, & Fu D. (2019). Down-regulation of the islet-specific zinc transporter-8 (ZnT8) protects human insulinoma cells against inflammatory stress. The Journal of Biological Chemistry, 294(45), 16992–17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriman C, Huang Q, Rutter GA, & Fu D. (2016). Lipid-tuned zinc transport activity of human ZnT8 protein correlates with risk for type-2 diabetes. The Journal of Biological Chemistry, 291(53), 26950–26957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meruvu S, Hugendubler L, & Mueller E. (2011). Regulation of adipocyte differentiation by the zinc finger protein ZNF638. The Journal of Biological Chemistry, 286(30), 26516–26523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailov Y, Ickowicz D, & Breitbart H. (2014). Zn2+-stimulation of sperm capacitation and of the acrosome reaction is mediated by EGFR activation. Developmental Biology, 396(2), 246–255. [DOI] [PubMed] [Google Scholar]
- Miller Y, Ma B, & Nussinov R. (2010). Zinc ions promote Alzheimer Aβ aggregation via population shift of polymorphic states. Proceedings of the National Academy of Sciences, 107(21), 9490–9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, & Chung J. (2018). Effects of high-fat diet induced obesity on tissue zinc concentrations and zinc transporter expressions in mice. Journal of Nutrition and Health, 51(6), 489–497. [Google Scholar]
- Mittra N, Chauhan AK, Singh G, Patel DK, & Singh C. (2020). Postnatal zinc or paraquat administration increases paraquat or zinc-induced loss of dopaminergic neurons: Insight into augmented neurodegeneration. Molecular and Cellular Biochemistry, 467(1), 27–43. [DOI] [PubMed] [Google Scholar]
- Morse KW, Astbury NM, Walczyszyn A, Hashim SA, & Geliebter A. (2017). Changes in zinc-α2-glycoprotein (ZAG) plasma concentrations pre and post Roux-En-Y gastric bypass surgery (RYGB) or a very low calorie (VLCD) diet in clinically severe obese patients: Preliminary Study. Integrative Obesity and Diabetes, 3(2), 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SM, Djafarian K, Mojtahed A, Varkaneh HK, & Shab-Bidar S. (2018). The effect of zinc supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. European Journal of Pharmacology, 834, 10–16. [DOI] [PubMed] [Google Scholar]
- Mracek T, Gao D, Tzanavari T, Bao Y, Xiao X, Stocker C, et al. (2010). Downregulation of zinc-{alpha}2-glycoprotein in adipose tissue and liver of obese ob/ob mice and by tumour necrosis factor-alpha in adipocytes. The Journal of Endocrinology, 204(2), 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SA, Nield A, Chew GS, & Myers MA (2013). The zinc transporter, Slc39a7 (Zip7) is implicated in glycaemic control in skeletal muscle cells. PLoS One, 8(11), e79316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SS, Wessells KR, Kloog I, Zanobetti A, & Schwartz J. (2015). Rising atmospheric CO2 increases global threat of zinc deficiency. The Lancet Global Health, 3(10), e639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Yoshikawa Y, Masuda K, & Yasui H. (2016). Bis (hinokitiolato) zinc complex ([Zn (hkt) 2]) activates Akt/protein kinase B independent of insulin signal transduction. Journal of Biological Inorganic Chemistry, 21(4), 537–548. [DOI] [PubMed] [Google Scholar]
- Naito Y, Yamamoto H, Yoshikawa Y, & Yasui H. (2019). In vivo effect of Bis (Maltolato) zinc (II) complex on Akt phosphorylation in adipose tissues of mice. Biological Trace Element Research, 192(2), 206–213. [DOI] [PubMed] [Google Scholar]
- Narváez-Caicedo C, Moreano G, Sandoval BA, & Jara-Palacios MÁ. (2018). Zinc deficiency among lactating mothers from a peri-urban community of the ecuadorian andean region: An initial approach to the need of zinc supplementation. Nutrients, 10(7), 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiadek M, Stragierowicz J, Klimczak M, & Kilanowicz A. (2020). The role of zinc in selected female reproductive system disorders. Nutrients, 12(8), 2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Trieu TS, Tran TO, & Luong TLA (2019). Evaluation of sperm DNA fragmentation index, Zinc concentration and seminal parameters from infertile men with varicocele. Andrologia, 51(2), e13184. [DOI] [PubMed] [Google Scholar]
- Niechciał E, Rogowicz-Frontczak A, Piłaciński S, Fichna M, Skowrońska B, Fichna P, et al. (2018). Autoantibodies against zinc transporter 8 are related to age and metabolic state in patients with newly diagnosed autoimmune diabetes. Acta Diabetologica, 55(3), 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J, & Chung JM (2019). Modulation of dopaminergic neuronal excitability by zinc through the regulation of calcium-related channels. Experimental Neurobiology, 28(5), 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh J, Chang SY, Wang SY, & Chung JM (2011). Dual function of Zn2+ on the intrinsic excitability of dopaminergic neurons in rat substantia nigra. Neuroscience, 175, 85–92. [DOI] [PubMed] [Google Scholar]
- Noh H, Paik HY, Kim J, & Chung J. (2014). The alteration of zinc transporter gene expression is associated with inflammatory markers in obese women. Biological Trace Element Research, 158(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Norouzi S, Adulcikas J, Sohal SS, & Myers S. (2018). Zinc stimulates glucose oxidation and glycemic control by modulating the insulin signaling pathway in human and mouse skeletal muscle cell lines. PLoS One, 13(1), e0191727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norouzi S, Adulcikas J, Henstridge DC, Sonda S, Sohal SS, & Myers S. (2019). The zinc transporter Zip7 is downregulated in skeletal muscle of insulin-resistant cells and in mice fed a high-fat diet. Cell, 8(7), 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, & Benninger RK (2016). Zinc transport gets its zing back: Double-knockout of ZnT7 and ZnT8 reveals the importance of zinc transporters to insulin secretion. Endocrinology, 157(12), 4542–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard SB, Larsen A, Knuhtsen A, Rungby J, & Smidt K. (2014). Effects of zinc supplementation and zinc chelation on in vitro β-cell function in INS-1E cells. BMC Research Notes, 7(1), 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K, Nagata Y, Wada E, Zammit PS, Shiozuka M, & Matsuda R. (2015). Zinc promotes proliferation and activation of myogenic cells via the PI3K/Akt and ERK signaling cascade. Experimental Cell Research, 333(2), 228–237. [DOI] [PubMed] [Google Scholar]
- Ohta S, Ikemoto T, Wada Y, Saito Y, Yamada S, Imura S, et al. (2019). A change in the zinc ion concentration reflects the maturation of insulin-producing cells generated from adipose-derived mesenchymal stem cells. Scientific Reports, 9(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olgar Y, & Turan B. (2019). A sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin comparison with insulin shows important effects on Zn2+-transporters in cardiomyocytes from insulin-resistant metabolic syndrome rats through inhibition of oxidative stress. Canadian Journal of Physiology and Pharmacology, 97(6), 528–535. [DOI] [PubMed] [Google Scholar]
- Omeljaniuk WJ, Socha K, Borawska MH, Charkiewicz AE, Laudański T, Kulikowski M, et al. (2015). Antioxidant status in women who have had a miscarriage. Advances in Medical Sciences, 60(2), 329–334. [DOI] [PubMed] [Google Scholar]
- Omu AE, Al-Azemi MK, Al-Maghrebi M, Mathew CT, Omu FE, Kehinde EO, et al. (2015). Molecular basis for the effects of zinc deficiency on spermatogenesis: An experimental study in the Sprague-dawley rat model. Indian Journal of Urology, 31(1), 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, et al. (2015). Zinc supplementation for improving pregnancy and infant outcome. The Cochrane Database of Systematic Reviews, 2015(2), CD000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteiza PI (2012). Zinc and the modulation of redox homeostasis. Free Radical Biology and Medicine, 53(9), 1748–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özgan Çelikel Ö., Doğan Ö, & Aksoy N. (2018). A multilateral investigation of the effects of zinc level on pregnancy. Journal of Clinical Laboratory Analysis, 32(5), e22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandurangan M, Jin BY, & Kim DH (2016). ZnO Nanoparticles upregulates adipocyte differentiation in 3T3-L1 cells. Biological Trace Element Research, 170(1), 201–207. [DOI] [PubMed] [Google Scholar]
- Park JS, Koentjoro B, Veivers D, Mackay-Sim A, & Sue CM (2014). Parkinson’s disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Human Molecular Genetics, 23(11), 2802–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak A, Sharma V, Kumar S, & Dhawan DK (2011). Supplementation of zinc mitigates the altered uptake and turnover of 65 Zn in liver and whole body of diabetic rats. Biometals, 24(6), 1027–1034. [DOI] [PubMed] [Google Scholar]
- Payahoo L, Ostadrahimi A, Mobasseri M, Bishak YK, Farrin N, Jafarabadi MA, et al. (2013). Effects of zinc supplementation on the anthropometric measurements, lipid profiles and fasting blood glucose in the healthy obese adults. Advanced Pharmaceutical Bulletin, 3(1), 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearsey HM, Henson J, Sargeant JA, Davies MJ, Khunti K, Suzuki T, et al. (2020). Zinc-alpha2-glycoprotein, dysglycaemia and insulin resistance: A systematic review and meta-analysis. Reviews in Endocrine & Metabolic Disorders, 21, 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza DF, & Sales MC (2017). Brazilian studies on zinc deficiency and supplementation: Emphasis on children. Revista Brasileira de Saúde Materno Infantil, 17(2), 217–232. [Google Scholar]
- Pelletier CC, Koppe L, Croze ML, Kalbacher E, Vella RE, Guebre-Egziabher F, et al. (2013). White adipose tissue overproduces the lipid-mobilizing factor zinc α2-glycoprotein in chronic kidney disease. Kidney International, 83(5), 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A, Rojas P, Carrasco F, Basfi-Fer K, Perez-Bravo F, Codoceo J, et al. (2018). Association between zinc nutritional status and glycemic control in individuals with well-controlled type-2 diabetes. Journal of Trace Elements in Medicine and Biology, 50, 560–565. [DOI] [PubMed] [Google Scholar]
- Petersen AB, Smidt K, Magnusson NE, Moore F, Egefjord L, & Rungby J. (2011). siRNA-mediated knock-down of ZnT3 and ZnT8 affects production and secretion of insulin and apoptosis in INS-1E cells. APMIS, 119(2), 93–102. [DOI] [PubMed] [Google Scholar]
- Pfaender S, Sauer AK, Hagmeyer S, Mangus K, Linta L, Liebau S, et al. (2017). Zinc deficiency and low enterocyte zinc transporter expression in human patients with autism related mutations in SHANK3. Scientific Reports, 7(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzón-Rondón ÁM, Hoyos-Martínez A, Parra-Correa D, Pedraza-Flechas AM, & Ruiz-Sternberg ÁM (2019). Association of nutritional support programs with zinc deficiency in Colombian children: A cross-sectional study. BMC Nutrition, 5(1), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum LM, Rink L, & Haase H. (2010). The essential toxin: Impact of zinc on human health. International Journal of Environmental Research and Public Health, 7(4), 1342–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompano LM, & Boy E. (2021). Effects of dose and duration of zinc interventions on risk factors for type 2 diabetes and cardiovascular disease: A systematic review and meta-analysis. Advances in Nutrition, 12, 141–160. nmaa087 Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AS (2013). Discovery of human zinc deficiency: Its impact on human health and disease. Advances in Nutrition, 4(2), 176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AS (2014a). Impact of the discovery of human zinc deficiency on health. Journal of Trace Elements in Medicine and Biology, 28(4), 357–363. [DOI] [PubMed] [Google Scholar]
- Prasad AS (2014b). Zinc is an antioxidant and anti-inflammatory agent: Its role in human health. Frontiers in Nutrition, 1, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AS, Halsted JA, & Nadimi M. (1961). Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. The American Journal of Medicine, 31, 532–546. [DOI] [PubMed] [Google Scholar]
- Priya MDL, & Geetha A. (2011). Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biological Trace Element Research, 142(2), 148–158. [DOI] [PubMed] [Google Scholar]
- Qi S, He J, Zheng H, Chen C, Jiang H, & Lan S. (2020). Zinc supplementation increased bone mineral density, improves bone histomorphology, and prevents bone loss in diabetic rat. Biological Trace Element Research, 194(2), 493–501. [DOI] [PubMed] [Google Scholar]
- Qi Y, Zhang Z, Liu S, Aluo Z, Zhang L, Yu L, et al. (2020). Zinc supplementation alleviates lipid and glucose metabolic disorders induced by a high-fat diet. Journal of Agricultural and Food Chemistry, 68(18), 5189–5200. [DOI] [PubMed] [Google Scholar]
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. (2020). Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 71(15), 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach JM, Walker EC, Allan E, Solano M, Yokoyama A, Kato S, et al. (2011). Zinc finger protein 467 is a novel regulator of osteoblast and adipocyte commitment. The Journal of Biological Chemistry, 286(6), 4186–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmati M, Safdarian F, Zakeri M, & Zare S. (2017). The prevalence of zinc deficiency in 6-month to 12-year old children in Bandar Abbas in 2013. Electronic Physician, 9(8), 5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramis R, Ortega-Castro J, Vilanova B, Adrover M, & Frau J. (2018). A systematic DFT study of some plausible Zn (II) and Al (III) interaction sites in N-terminally acetylated α-synuclein. The Journal of Physical Chemistry. A, 122(2), 690–699. [DOI] [PubMed] [Google Scholar]
- Read SA, Obeid S, Ahlenstiel C, & Ahlenstiel G. (2019). The role of zinc in antiviral immunity. Advances in Nutrition, 10(4), 696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reider CA, Chung RY, Devarshi PP, Grant RW, & Hazels Mitmesser S. (2020). Inadequacy of immune health nutrients: Intakes in US adults, the 2005–2016 NHANES. Nutrients, 12(6), 1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridlo MR, Kim GA, Taweechaipaisankul A, Kim EH, & Lee BC (2020). Zinc supplementation alleviates endoplasmic reticulum stress during porcine oocyte in vitro maturation by upregulating zinc transporters. Journal of Cellular Physiology, 236(4), 2869–2880. 10.1002/jcp.30052. [DOI] [PubMed] [Google Scholar]
- Rios-Lugo MJ, Madrigal-Arellano C, Gaytán-Hernández D, Hernández-Mendoza H, & Romero-Guzmán ET (2020). Association of serum zinc levels in overweight and obesity. Biological Trace Element Research, 198, 51–57. [DOI] [PubMed] [Google Scholar]
- Rogowicz-Frontczak A, Pilacinski S, Wyka K, Wierusz-Wysocka B, & Zozulinska-Ziolkiewicz D. (2018). Zinc transporter 8 autoantibodies (ZnT8-ab) are associated with higher prevalence of multiple diabetes-related autoantibodies in adults with type 1 diabetes. Diabetes Research and Clinical Practice, 146, 313–320. [DOI] [PubMed] [Google Scholar]
- Roohani N, Hurrell R, Kelishadi R, & Schulin R. (2013). Zinc and its importance for human health: An integrative review. Journal of Research in Medical Sciences: The Official Journal of Isfahan University of Medical Sciences, 18(2), 144. [PMC free article] [PubMed] [Google Scholar]
- Russo AJ, Bazin AP, Bigega R, Carlson RS III, Cole MG, Contreras DC, et al. (2012). Plasma copper and zinc concentration in individuals with autism correlate with selected symptom severity. Nutrition and Metabolic Insights, 5, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruz M, Carrasco F, Sánchez A, Perez A, & Rojas P. (2016). Does zinc really “metal” with diabetes? The epidemiologic evidence. Current Diabetes Reports, 16(11), 111. [DOI] [PubMed] [Google Scholar]
- Sacan O, Turkyilmaz IB, Bayrak BB, Mutlu O, Akev N, & Yanardag R. (2016). Zinc supplementation ameliorates glycoprotein components and oxidative stress changes in the lung of streptozotocin diabetic rats. Biometals, 29(2), 239–248. [DOI] [PubMed] [Google Scholar]
- Saghazadeh A, Ahangari N, Hendi K, Saleh F, & Rezaei N. (2017). Status of essential elements in autism spectrum disorder: Systematic review and meta-analysis. Reviews in the Neurosciences, 28(7), 783–809. [DOI] [PubMed] [Google Scholar]
- Saini N, & Schaffner W. (2010). Zinc supplement greatly improves the condition of parkin mutant Drosophila. Biological Chemistry, 391(5), 513–518. [DOI] [PubMed] [Google Scholar]
- Salas-Huetos A, Rosique-Esteban N, Becerra-Tomás N, Vizmanos B, Bulló M, & Salas-Salvadó J. (2018). The effect of nutrients and dietary supplements on sperm quality parameters: A systematic review and meta-analysis of randomized clinical trials. Advances in Nutrition, 9(6), 833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi A, Isikhan SY, Tinkov AA, Lay I, Doşa MD, Skalny AV, et al. (2020). Zinc, copper, and oxysterol levels in patients with type 1 and type 2 diabetes mellitus. Clinical Nutrition, 39(6), 1849–1856. [DOI] [PubMed] [Google Scholar]
- Sangalli CN, Rauber F, & Vitolo MR (2016). Low prevalence of inadequate micronutrient intake in young children in the south of Brazil: A new perspective. The British Journal of Nutrition, 116(5), 890–896. [DOI] [PubMed] [Google Scholar]
- Schoen M, Asoglu H, Bauer HF, Müller HP, Abaei A, Sauer AK, et al. (2019). Shank3 transgenic and prenatal zinc-deficient autism mouse models show convergent and individual alterations of brain structures in MRI. Frontiers in Neural Circuits, 13, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag M, Mueller C, Oyoyo U, Smith MA, & Kirsch WM (2011). Iron, zinc and copper in the Alzheimer’s disease brain: A quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Progress in Neurobiology, 94(3), 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo JA, Song SW, Han K, Lee KJ, & Kim HN (2014). The associations between serum zinc levels and metabolic syndrome in the Korean population: Findings from the 2010 Korean National Health and Nutrition Examination Survey. PLoS One, 9(8), e105990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severo JS, Morais J, Beserra JB, de Farias LM, dos Santos LR, de Sousa Melo SR, et al. (2019). Effect of zinc supplementation on lipid profile in obese people: A systematic review. Current Nutrition & Food Science, 15(6), 551–556. [Google Scholar]
- Severo JS, Morais JBS, Beserra JB, dos Santos LR, de Sousa Melo SR, de Sousa GS, et al. (2020). Role of zinc in zinc-α2-glycoprotein metabolism in obesity: A review of literature. Biological Trace Element Research, 193(1), 81–88. [DOI] [PubMed] [Google Scholar]
- Severo JS, Morais J, Beserra JB, Clímaco Cruz KJ, de Oliveira AR, dos Santos LR, et al. (2020). Biomarkers of cardiovascular risk in obese women and their relationship with zinc status. Current Nutrition & Food Science, 16(5), 734–742. [Google Scholar]
- Shao M, Hepler C, Vishvanath L, MacPherson KA, Busbuso NC, & Gupta RK (2016). Fetal development of subcutaneous white adipose tissue is dependent on Zfp423. Molecular Metabolism, 6(1), 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U, & Yadav N. (2019). Prevalence and risk factors of anemia and zinc deficiency among 4–6-year-old children of Allahabad District, Uttar Pradesh. Indian Journal of Public Health, 63(1), 79. [DOI] [PubMed] [Google Scholar]
- Shih PY, Hsieh BY, Lin MH, Huang TN, Tsai CY, Pong WL, et al. (2020). CTTNBP2 controls synaptic expression of zinc-related autism-associated proteins and regulates synapse formation and autism-like behaviors. Cell Reports, 31(9), 107700. [DOI] [PubMed] [Google Scholar]
- Sikora J, Kieffer BL, Paoletti P, & Ouagazzal AM (2020). Synaptic zinc contributes to motor and cognitive deficits in 6-hydroxydopamine mouse models of Parkinson’s disease. Neurobiology of Disease, 134, 104681. [DOI] [PubMed] [Google Scholar]
- Singh AK, Chattopadhyay R, Chakravarty B, & Chaudhury K. (2013). Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reproductive Toxicology, 42, 116–124. [DOI] [PubMed] [Google Scholar]
- Singh BK, Kumar V, Chauhan AK, Dwivedi A, Singh S, Kumar A, et al. (2017). Neuronal nitric oxide synthase negatively regulates zinc-induced nigrostriatal dopaminergic neurodegeneration. Molecular Neurobiology, 54(4), 2685–2696. [DOI] [PubMed] [Google Scholar]
- Skalnaya MG, Skalny AV, Yurasov VV, Demidov VA, Grabeklis AR, Radysh IV, et al. (2017). Serum trace elements and electrolytes are associated with fasting plasma glucose and HbA 1c in postmenopausal women with type 2 diabetes mellitus. Biological Trace Element Research, 177(1), 25–32. [DOI] [PubMed] [Google Scholar]
- Skalnaya MG, Skalny AV, Serebryansky EP, Yurasov VV, Skalnaya AA, & Tinkov AA (2018). ICP-DRC-MS analysis of serum essential and toxic element levels in postmenopausal prediabetic women in relation to glycemic control markers. Journal of Trace Elements in Medicine and Biology, 50, 430–434. [DOI] [PubMed] [Google Scholar]
- Skalny AV, & Kiselev VF (2012). Element status of population of Russia. Part III. Element status of population of North-Western, Southern and North-Caucasian Federal districts. Spb: ELBI. [Google Scholar]
- Skalny AV, Simashkova NV, Skalnaya AA, Klyushnik TP, Bjørklund G, Skalnaya MG, et al. (2017). Assessment of gender and age effects on serum and hair trace element levels in children with autism spectrum disorder. Metabolic Brain Disease, 32(5), 1675–1684. [DOI] [PubMed] [Google Scholar]
- Skalny AV, Tinkov AA, Bohan TG, Shabalovskaya MB, Terekhina O, Leshchinskaia SB, et al. (2018). Toxicological and nutritional status of trace elements in hair of women with in vitro fertilization (IVF) pregnancy and their 9-month-old children. Reproductive Toxicology, 82, 50–56. [DOI] [PubMed] [Google Scholar]
- Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI, et al. (2020). Zinc and respiratory tract infections: Perspectives for COVID-19. International Journal of Molecular Medicine, 46(1), 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalny AV, Mazaletskaya AL, Ajsuvakova OP, Bjørklund G, Skalnaya MG, Notova SV, et al. (2020). Hair trace element concentrations in autism spectrum disorder (ASD) and attention deficit/hyperactivity disorder (ADHD). Journal of Trace Elements in Medicine and Biology, 61, 126539. [DOI] [PubMed] [Google Scholar]
- Skalny AV, Timashev PS, Aschner M, Aaseth J, Chernova LN, Belyaev VE, et al. (2021). Serum zinc, copper, and other biometals are associated with COVID-19 severity markers. Metabolites, 11(4), 244. 10.3390/metabo11040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepchenko KG, Daniels NA, Guo A, & Li YV (2015). Autocrine effect of Zn 2+ on the glucose-stimulated insulin secretion. Endocrine, 50(1), 110–122. [DOI] [PubMed] [Google Scholar]
- Smidt K, Larsen A, Brønden A, Sørensen KS, Nielsen JV, Praetorius J, et al. (2016). The zinc transporter ZNT3 co-localizes with insulin in INS-1E pancreatic beta cells and influences cell survival, insulin secretion capacity, and ZNT8 expression. Biometals, 29(2), 287–298. [DOI] [PubMed] [Google Scholar]
- Smith MR, DeFries R, Chhatre A, Ghosh-Jerath S, & Myers SS (2019). Inadequate zinc intake in India: Past, present, and future. Food and Nutrition Bulletin, 40(1), 26–40. [DOI] [PubMed] [Google Scholar]
- Solomou A, Meur G, Bellomo E, Hodson DJ, Tomas A, Li SM, et al. (2015). The zinc transporter Slc30a8/ZnT8 is required in a subpopulation of pancreatic α-cells for hypoglycemia-induced glucagon secretion. The Journal of Biological Chemistry, 290(35), 21432–21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suliburska J, Bogdański P, Pupek-Musialik D, & Krejpcio Z. (2011). Dietary intake and serum and hair concentrations of minerals and their relationship with serum lipids and glucose levels in hypertensive and obese patients with insulin resistance. Biological Trace Element Research, 139(2), 137–150. [DOI] [PubMed] [Google Scholar]
- Sun XY, Wei YP, Xiong Y, Wang XC, Xie AJ, Wang XL, et al. (2012). Synaptic released zinc promotes tau hyperphosphorylation by inhibition of protein phosphatase 2A (PP2A). The Journal of Biological Chemistry, 287(14), 11174–11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Liu X, Ge H, Wang T, Wang Y, & Li W. (2017). Association between serum zinc levels and the risk of Parkinson’s disease: A meta-analysis. Biological Trace Element Research, 179(1), 45–51. [DOI] [PubMed] [Google Scholar]
- Sun W, Yang J, Wang W, Hou J, Cheng Y, Fu Y, et al. (2018). The beneficial effects of Zn on Akt-mediated insulin and cell survival signaling pathways in diabetes. Journal of Trace Elements in Medicine and Biology, 46, 117–127. [DOI] [PubMed] [Google Scholar]
- Sweetman DU, O’Donnell SM, Lalor A, Grant T, & Greaney H. (2019). Zinc and vitamin A deficiency in a cohort of children with autism spectrum disorder. Child: Care, Health and Development, 45(3), 380–386. [DOI] [PubMed] [Google Scholar]
- Syring KE, Boortz KA, Oeser JK, Ustione A, Platt KA, Shadoan MK, et al. (2016). Combined deletion of Slc30a7 and Slc30a8 unmasks a critical role for ZnT8 in glucose-stimulated insulin secretion. Endocrinology, 157(12), 4534–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A, & Tamano H. (2015). Regulation of extracellular Zn2+ homeostasis in the hippocampus as a therapeutic target for Alzheimer’s disease. Expert Opinion on Therapeutic Targets, 19(8), 1051–1058. [DOI] [PubMed] [Google Scholar]
- Takeda A, Tamano H, Tempaku M, Sasaki M, Uematsu C, Sato S, et al. (2017). Extracellular Zn2+ Is essential for amyloid β1–42-induced cognitive decline in the normal brain and its rescue. The Journal of Neuroscience, 37(30), 7253–7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallman DL, & Taylor CG (2003). Effects of dietary fat and zinc on adiposity, serum leptin and adipose fatty acid composition in C57BL/6J mice. The Journal of Nutritional Biochemistry, 14(1), 17–23. [DOI] [PubMed] [Google Scholar]
- Tamano H, Morioka H, Nishio R, Takeuchi A, & Takeda A. (2018). AMPA-induced extracellular Zn2+ influx into nigral dopaminergic neurons causes movement disorder in rats. Neurotoxicology, 69, 23–28. [DOI] [PubMed] [Google Scholar]
- Tamano H, Takiguchi M, Tanaka Y, Murakami T, Adlard PA, Bush AI, et al. (2019). Preferential neurodegeneration in the dentate gyrus by amyloid β 1–42-induced intracellular Zn 2+ dysregulation and its defense strategy. Molecular Neurobiology, 57, 1–14. [DOI] [PubMed] [Google Scholar]
- Tamano H, Nishio R, Morioka H, & Takeda A. (2019). Extracellular Zn 2+ influx into nigral dopaminergic neurons plays a key role for pathogenesis of 6-hydroxydopamine-induced Parkinson’s disease in rats. Molecular Neurobiology, 56(1), 435–443. [DOI] [PubMed] [Google Scholar]
- Tamano H, Morioka H, Nishio R, Takeuchi A, & Takeda A. (2019). Blockade of rapid influx of extracellular Zn 2+ into nigral dopaminergic neurons overcomes Paraquat-induced Parkinson’s disease in rats. Molecular Neurobiology, 56(6), 4539–4548. [DOI] [PubMed] [Google Scholar]
- Tamano H, Takiguchi M, Shimaya R, Adlard PA, Bush AI, & Takeda A. (2019). Extracellular Zn2+-independently attenuated LTP by human amyloid β1–40 and rat amyloid β1–42. Biochemical and Biophysical Research Communications, 514(3), 888–892. [DOI] [PubMed] [Google Scholar]
- Tamano H, Ishikawa Y, Shioya A, Itoh R, Oneta N, Shimaya R, et al. (2020). Adrenergic β receptor activation reduces amyloid β1–42-mediated intracellular Zn2+ toxicity in dentate granule cells followed by rescuing impairment of dentate gyrus LTP. Neurotoxicology, 79, 177–183. 10.1016/j.neuro.2020.06.001. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Takahashi E, Matsui T, & Yano H. (2001). Zinc promotes adipocyte differentiation in vitro. Asian-Australasian Journal of Animal Sciences, 14(7), 966–969. [Google Scholar]
- Taravati A, & Tohidi F. (2016). Association between seminal plasma zinc level and asthenozoospermia: A meta-analysis study. Andrologia, 48(6), 646–653. [DOI] [PubMed] [Google Scholar]
- Te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, & van Hemert MJ (2010). Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathogens, 6(11), e1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepaamorndech S, Kirschke CP, & Huang L. (2014). Linking cellular zinc status to body weight and fat mass: Mapping quantitative trait loci in Znt7 knockout mice. Mammalian Genome, 25(7–8), 335–353. [DOI] [PubMed] [Google Scholar]
- Tepaamorndech S, Kirschke CP, Pedersen TL, Keyes WR, Newman JW, & Huang L. (2016). Zinc transporter 7 deficiency affects lipid synthesis in adipocytes by inhibiting insulin-dependent Akt activation and glucose uptake. The FEBS Journal, 283(2), 378–394. [DOI] [PubMed] [Google Scholar]
- Terrin G, Berni Canani R, Di Chiara M, Pietravalle A, Aleandri V, Conte F, et al. (2015). Zinc in early life: A key element in the fetus and preterm neonate. Nutrients, 7(12), 10427–10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoen RU, Barther NN, Schemitt E, Bona S, Fernandes S, Coral G, et al. (2019). Zinc supplementation reduces diet-induced obesity and improves insulin sensitivity in rats. Applied Physiology, Nutrition, and Metabolism, 44(6), 580–586. [DOI] [PubMed] [Google Scholar]
- Tian X, & Diaz FJ (2013). Acute dietary zinc deficiency before conception compromises oocyte epigenetic programming and disrupts embryonic development. Developmental Biology, 376(1), 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Anthony K, Neuberger T, & Diaz FJ (2014). Preconception zinc deficiency disrupts postimplantation fetal and placental development in mice. Biology of Reproduction, 90(4), 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian ZY, Wang CY, Wang T, Li YC, & Wang ZY (2019). Glial S100A6 degrades β-amyloid aggregation through targeting competition with zinc ions. Aging and Disease, 10(4), 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkov AA, Popova EV, Gatiatulina ER, Skalnaya AA, Yakovenko EN, Alchinova IB, et al. (2016). Decreased adipose tissue zinc content is associated with metabolic parameters in high fat fed Wistar rats. Acta Scientiarum Polonorum. Technologia Alimentaria, 15(1), 99–105. [DOI] [PubMed] [Google Scholar]
- Tinkov AA, Skalnaya MG, Ajsuvakova OP, Serebryansky EP, Chao JC, Aschner M, et al. (2020). Selenium, zinc, chromium, and vanadium levels in serum, hair, and urine samples of obese adults assessed by inductively coupled plasma mass spectrometry. Biological Trace Element Research, 199, 490–499. 10.1007/s12011-020-02177-w. [DOI] [PubMed] [Google Scholar]
- Troche C, Aydemir TB, & Cousins RJ (2016). Zinc transporter Slc39a14 regulates inflammatory signaling associated with hypertrophic adiposity. American Journal of Physiology. Endocrinology and Metabolism, 310(4), E258–E268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsave O, Halevas E, Yavropoulou MP, Papadimitriou AK, Yovos JG, Hatzidimitriou A, et al. (2015). Structure-specific adipogenic capacity of novel, well-defined ternary Zn (II)-Schiff base materials. Biomolecular correlations in zinc-induced differentiation of 3T3-L1 pre-adipocytes to adipocytes. Journal of Inorganic Biochemistry, 152, 123–137. [DOI] [PubMed] [Google Scholar]
- Tsave O, Yavropoulou MP, Kafantari M, Gabriel C, Yovos JG, & Salifoglou A. (2018). Comparative assessment of metal-specific adipogenic activity in zinc and vanadium-citrates through associated gene expression. Journal of Inorganic Biochemistry, 186, 217–227. [DOI] [PubMed] [Google Scholar]
- Tschinkel PFS, Bjørklund G, Conón LZZ, Chirumbolo S, & Nascimento VA (2018). Plasma concentrations of the trace elements copper, zinc and selenium in Brazilian children with autism spectrum disorder. Biomedicine and Pharmacotherapy, 106, 605–609. [DOI] [PubMed] [Google Scholar]
- Tsunemi T, & Krainc D. (2014). Zn2+ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Human Molecular Genetics, 23(11), 2791–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncay E, Bitirim V, Durak A, Rutter GA, & Turan B. (2016). Both reactive ROS and RNS contribute to intracellular free Zn2+ regulation in cardiomyocytes via zinc transporter ZIP7 under hyperglycemia. Free Radical Biology and Medicine, 100, S153. [Google Scholar]
- Vardatsikos G, Pandey NR, & Srivastava AK (2013). Insulino-mimetic and anti-diabetic effects of zinc. Journal of Inorganic Biochemistry, 120, 8–17. [DOI] [PubMed] [Google Scholar]
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. (2020). Endothelial cell infection and endotheliitis in COVID-19. The Lancet, 395(10234), 1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri-Sani F, Oak S, Radtke J, Lernmark Å, Lynch K, Agardh CD, et al. (2010). ZnT8 autoantibody titers in type 1 diabetes patients decline rapidly after clinical onset. Autoimmunity, 43(8), 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vela G, Stark P, Socha M, Sauer AK, Hagmeyer S, & Grabrucker AM (2015). Zinc in gut-brain interaction in autism and neurological disorders. Neural Plasticity, 2015, 972791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventriglia M, Brewer GJ, Simonelli I, Mariani S, Siotto M, Bucossi S, et al. (2015). Zinc in Alzheimer’s disease: A meta-analysis of serum, plasma, and cerebrospinal fluid studies. Journal of Alzheimer’s Disease, 46(1), 75–87. [DOI] [PubMed] [Google Scholar]
- Vilella A, Belletti D, Sauer AK, Hagmeyer S, Sarowar T, Masoni M, et al. (2018). Reduced plaque size and inflammation in the APP23 mouse model for Alzheimer’s disease after chronic application of polymeric nanoparticles for CNS targeted zinc delivery. Journal of Trace Elements in Medicine and Biology, 49, 210–221. [DOI] [PubMed] [Google Scholar]
- Viles JH (2012). Metal ions and amyloid fiber formation in neurodegenerative diseases. Copper, zinc and iron in Alzheimer’s, Parkinson’s and prion diseases. Coordination Chemistry Reviews, 256(19–20), 2271–2284. [Google Scholar]
- Vogel M, Tallo-Parra M, Herrera-Fernandez V, Perez-Vilaro G, Chillon M, Nogues X, et al. (2020). Low zinc levels at clinical admission associates with poor outcomes in COVID-19. BMJ Yale. Preprint [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voruganti VS, Cai G, Klohe DM, Jordan KC, Lane MA, & Freeland-Graves JH (2010). Short-term weight loss in overweight/obese low-income women improves plasma zinc and metabolic syndrome risk factors. Journal of Trace Elements in Medicine and Biology, 24(4), 271–276. [DOI] [PubMed] [Google Scholar]
- Vuralli D, Tumer L, & Hasanoglu A. (2017). Zinc deficiency in the pediatric age group is common but underevaluated. World Journal of Pediatrics, 13(4), 360–366. [DOI] [PubMed] [Google Scholar]
- Vyas Y, Lee K, Jung Y, & Montgomery JM (2020). Influence of maternal zinc supplementation on the development of autism-associated behavioural and synaptic deficits in offspring Shank3-knockout mice. Molecular Brain, 13(1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Wang T, Zheng W, Zhao BL, Danscher G, Chen YH, et al. (2010). Zinc overload enhances APP cleavage and Aβ deposition in the Alzheimer mouse brain. PLoS One, 5(12), e15349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li H, Fan Z, & Liu Y. (2012). Effect of zinc supplementation on type 2 diabetes parameters and liver metallothionein expressions in Wistar rats. Journal of Physiology and Biochemistry, 68(4), 563–572. [DOI] [PubMed] [Google Scholar]
- Wang H, Hu YF, Hao JH, Chen YH, Su PY, Wang Y, et al. (2015). Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction: A population-based birth cohort study. Scientific Reports, 5, 11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Luo M, Zhang Z, Gu J, Chen J, Payne KM, et al. (2016). Zinc deficiency exacerbates while zinc supplement attenuates cardiac hypertrophy in high-fat diet-induced obese mice through modulating p38 MAPK-dependent signaling. Toxicology Letters, 258, 134–146. [DOI] [PubMed] [Google Scholar]
- Wang H, Hu YF, Hao JH, Chen YH, Wang Y, Zhu P, et al. (2016). Maternal serum zinc concentration during pregnancy is inversely associated with risk of preterm birth in a Chinese population. The Journal of Nutrition, 146(3), 509–515. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang B, Wang Y, Tong Q, Liu Q, Sun J, et al. (2017). Zinc prevents the development of diabetic cardiomyopathy in db/db mice. International Journal of Molecular Sciences, 18(3), 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guan PP, Yu X, Guo YS, Zhang YJ, Wang ZY, et al. (2017). COX-2 metabolic products, the prostaglandin I2 and F2α, mediate the effects of TNF-α and Zn2 + in stimulating the phosphorylation of Tau. Oncotarget, 8(59), 99296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Fu X, Zhu MJ, & Du M. (2017). Retinoic acid inhibits white adipogenesis by disrupting GADD45A-mediated Zfp423 DNA demethylation. Journal of Molecular Cell Biology, 9(4), 338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jia XF, Zhang B, Wang ZH, Zhang JG, Huang FF, et al. (2018). Dietary zinc intake and its association with metabolic syndrome indicators among Chinese adults: An analysis of the China Nutritional Transition Cohort Survey 2015. Nutrients, 10(5), 572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wu W, Zheng W, Fang X, Chen L, Rink L, et al. (2019). Zinc supplementation improves glycemic control for diabetes prevention and management: A systematic review and meta-analysis of randomized controlled trials. The American Journal of Clinical Nutrition., 110(1), 76–90. [DOI] [PubMed] [Google Scholar]
- Wei S, Zhang L, Zhou X, Du M, Jiang Z, Hausman GJ, et al. (2013). Emerging roles of zinc finger proteins in regulating adipogenesis. Cellular and Molecular Life Sciences, 70(23), 4569–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Liu X, Tan C, Mo L, Wang H, Peng X, et al. (2019). Expression and function of zinc-α2-glycoprotein. Neuroscience Bulletin, 35, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I, Maywald M, & Rink L. (2017). Zinc as a gatekeeper of immune function. Nutrients, 9(12), 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels I, Rolles B, & Rink L. (2020). The potential impact of zinc supplementation on COVID-19 pathogenesis. Frontiers in Immunology, 11, 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield DR, Francis PT, Ballard C, & Williams G. (2018). Associations between ZnT3, tau pathology, agitation, and delusions in dementia. International Journal of Geriatric Psychiatry, 33(8), 1146–1152. [DOI] [PubMed] [Google Scholar]
- Wijesekara N, Dai FF, Hardy AB, Giglou PR, Bhattacharjee A, Koshkin V, et al. (2010). Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia, 53(8), 1656–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RL, Grieger JA, Bianco-Miotto T, & Roberts CT (2016). Association between maternal zinc status, dietary zinc intake and pregnancy complications: A systematic review. Nutrients, 8(10), 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RL, Leemaqz SY, Goh Z, McAninch D, Jankovic-Karasoulos T, Leghi GE, et al. (2017). Zinc is a critical regulator of placental morphogenesis and maternal hemodynamics during pregnancy in mice. Scientific Reports, 7(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2021. Coronavirus disease (COVID-19) pandemic, https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed 19 May, 2021). [Google Scholar]
- Wu Y, Lu H, Yang H, Li C, Sang Q, Liu X, et al. (2016). Zinc stimulates glucose consumption by modulating the insulin signaling pathway in L6 myotubes: Essential roles of Akt–GLUT4, GSK3β and mTOR–S6K1. The Journal of Nutritional Biochemistry, 34, 126–135. [DOI] [PubMed] [Google Scholar]
- Xiao XH, Wang YD, Qi XY, Wang YY, Li JY, Li H, et al. (2018). Zinc alpha2 glycoprotein protects against obesity-induced hepatic steatosis. International Journal of Obesity, 42(8), 1418–1430. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Jing XP, Zhou XW, Wang XL, Yang Y, Sun XY, et al. (2013). Zinc induces protein phosphatase 2A inactivation and tau hyperphosphorylation through Src dependent PP2A (tyrosine 307) phosphorylation. Neurobiology of Aging, 34(3), 745–756. [DOI] [PubMed] [Google Scholar]
- Xu Y, Xiao G, Liu L, & Lang M. (2019). Zinc transporters in Alzheimer’s disease. Molecular Brain, 12(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wei Y, Long T, Wang R, Li Z, Yu C, et al. (2020). Association between urinary metals levels and metabolic phenotypes in overweight and obese individuals. Chemosphere, 254, 126763. [DOI] [PubMed] [Google Scholar]
- Yang TC, Wu PC, Chung IF, Jiang JH, Fann MJ, & Kao LS (2016). Cell death caused by the synergistic effects of zinc and dopamine is mediated by a stress sensor gene Gadd45b–implication in the pathogenesis of Parkinson’s disease. Journal of Neurochemistry, 139(1), 120–133. [DOI] [PubMed] [Google Scholar]
- Yasuda H, & Tsutsui T. (2016). Infants and elderlies are susceptible to zinc deficiency. Scientific Reports, 6, 21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi B, Huang G, & Zhou Z. (2016). Different role of zinc transporter 8 between type 1 diabetes mellitus and type 2 diabetes mellitus. Journal of Diabetes Investigation, 7(4), 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokokawa H, Fukuda H, Saita M, Miyagami T, Takahashi Y, Hisaoka T, et al. (2020). Serum zinc concentrations and characteristics of zinc deficiency/marginal deficiency among Japanese subjects. Journal of General and Family Medicine, 21, 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo MH, Kim TY, Yoon YH, & Koh JY (2016). Autism phenotypes in ZnT3 null mice: Involvement of zinc dyshomeostasis, MMP-9 activation and BDNF upregulation. Scientific Reports, 6(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid H, Miah L, Lau AM, Brochard L, Hati D, Bui TT, et al. (2016). Zinc-induced oligomerization of zinc α2 glycoprotein reveals multiple fatty acid-binding sites. The Biochemical Journal, 473(1), 43–54. [DOI] [PubMed] [Google Scholar]
- Zhang LH, Wang X, Zheng ZH, Ren H, Stoltenberg M, Danscher G, et al. (2010). Altered expression and distribution of zinc transporters in APP/PS1 transgenic mouse brain. Neurobiology of Aging, 31(1), 74–87. [DOI] [PubMed] [Google Scholar]
- Zhang T, Pauly T, & Nagel-Steger L. (2018). Stoichiometric Zn2+ interferes with the self-association of Aβ42: Insights from size distribution analysis. International Journal of Biological Macromolecules, 113, 631–639. [DOI] [PubMed] [Google Scholar]
- Zhang W, Qiao Y, Qi F, Shen Q, Zhao R, & Yang X. (2020). Zinc-α2-glycoprotein knockout influenced genes expression profile in adipose tissue and decreased the lipid mobilizing after dexamethasone treatment in mice. Hormone and Metabolic Research, 52(10), 755–763. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jian W, He L, & Wu J. (2020). ZnT8 in T2D: A novel therapeutic target for maintaining insulin secretion capacity. Acta Biochimica et Biophysica Sinica, 52(9), 1050–1051. [DOI] [PubMed] [Google Scholar]
- Zhao J, Dong X, Hu X, Long Z, Wang L, Liu Q, et al. (2016). Zinc levels in seminal plasma and their correlation with male infertility: A systematic review and meta-analysis. Scientific Reports, 6(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HJ, Ding HH, Deng JY, Pan H, Wang LJ, Li NS, et al. (2013). Inhibition of preadipocyte differentiation and adipogenesis by zinc-α2-glycoprotein treatment in 3T3-L1 cells. Journal of Diabetes Investigation, 4(3), 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Zhang L, Chen X, Zhou J, Liu J, & Chen J. (2016). Association between zinc level and the risk of preeclampsia: A meta-analysis. Archives of Gynecology and Obstetrics, 293(2), 377–382. [DOI] [PubMed] [Google Scholar]
- Zong L, Wei X, Gou W, Huang P, & Lv Y. (2017). Zinc improves learning and memory abilities of fetal growth restriction rats and promotes trophoblast cell invasion and migration via enhancing STAT3-MMP-2/9 axis activity. Oncotarget, 8(70), 115190. [DOI] [PMC free article] [PubMed] [Google Scholar]