Abstract
Metal oxide nanoparticles are known to exhibit unique properties such as catalyzing the neutralization of superoxide anions, hydroxyl radicals, hydrogen peroxides and behave as antioxidant enzymes. Oxidative stress, damage and chronic inflammation are major causes and consequences of aging and age-associated disorders. With the increasing popularity of metal oxide nanoparticles, they have been applied in various age-related pathologies using their antioxidant property. Metal oxide nanoparticles have been used as diagnostic, therapeutic, and as theranostics. This review summarizes the applications of metal oxide nanoparticles in aging and age-associated disorders such as cardiovascular diseases, diabetes, cancer, neurodegenerative disorders. Oxidative stress plays a central role in the activation of inflammatory pathways, disturbing the mitochondrial function, decreasing the telomere length and leading the cell towards senescence or death. Oxidative damage is the common pathway in the progression of aging and related diseases. Metal oxide nanoparticles scavenge or precisely detect the generated reactive oxygen species, hence applied in both diagnostics and therapeutics.
Keywords: Aging, Metal oxide nanoparticles, Nanozymes, Oxidative stress, Inflammation
Introduction
Aging is a multidimensional process. Cellular aging is a process of accumulation of harmful changes in the lifetime of a cell. The morphological and physiological changes in the lifespan of a cell ultimately lead to death. The metabolism of cell slows down, hence, the functioning also alters during cellular aging. Sometimes the manifestation of aging is a genetic process. It is a universal phenomenon for all organisms, but the rate of aging depends on various environmental factors. According to the world population aging 2019 report published by the department of economic and social affairs of the United Nations, all over the world there were 703 million persons above 65 years or over in 2019 and are projected to double by 2050. The old-age population has outnumbered the population of children under age 5 which is considered a demographic milestone. This trend will continue due to decreasing fertility rates and increasing life expectancy in developing countries like India with high populations. Therefore, the mechanisms of aging and its associated disorders become an important area for research. The vulnerability to chronic non-communicable disorders such as cancer, heart diseases, dementia increases with age, which also is a major concern. Often aging results in health and economic dependency, that is why it is one of the most daunting areas to study for the independence of older people and decrease the costs of their long-term care.
The major hurdle in understanding the symptoms of aging is their complexity. The real mechanism behind cellular aging is not known. However, many theories have been given to elucidate the process. Neither of the theories satisfactorily explained the causes of the aging process. Dr. Denham Harman in 1981 proposed a free radical theory of aging, according to which the accumulation of damages caused by superoxide and other free radicals over the life span of cells, slows down the metabolism of the cell and lead to eventual death (Harman 1981). Free radicals are highly reactive species that can lose or accept an electron, hence behave as an oxidizing or reducing agent. The oxygen-derived radicals are known as Reactive Oxygen Species (ROS) and nitrogen-derived radicals are known as Reactive Nitrogen Species (RNS). The ROS such as superoxide (O2•−), hydroxyl radicals (•OH), and hydrogen peroxide (H2O2), and RNS such as nitric oxide (•NO), nitrous oxide (NO2•), and nitrosonium ion (NO−) are generated under normal physiological metabolic reactions but their imbalance has deleterious consequences on the membrane, nucleic acids, ion channels, and other cellular structures. The electron transport chain (ETC) leakage in mitochondria, secretion by neutrophils and macrophages during inflammation, and certain enzymes such as NAPDH oxidase (NOx) and xanthine/xanthine oxidase involving reactions are the major sites of free radical productions. The free radicals oxidize proteins, lipids, nucleic acids and result in various oxidized by-products such as protein carbonyls, nitrotyrosine, disulfides, malonaldehyde (MDA), 4-hydroxynonenal (4-HNE), and 8-oxo, 7-hydroxy deoxyguanosine (8-OHDG). The accumulation of these by-products over the lifespan of a cell results in pathological conditions of age-accelerated diseases. A cell is well equipped with antioxidants to counter these oxidative radicals. The antioxidants can be categorized as enzymatic and non-enzymatic antioxidants. The major antioxidant enzymes are superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), peroxidase present in every aerobic organism. The non-enzymatic antioxidants are small, low molecular weight chemicals such as vitamins, β-carotene, and reduced glutathione (GSH) which are generally taken in diet. The imbalance of oxidative stress and antioxidants basically the redox status is the biomarker of progression of aging and related diseases. The antioxidants are promising therapeutic agents on cellular aging (Simioni et al. 2018).
Nanoparticles (NPs) are structures with a size in the range of nanometers. The nanosize provides the NPs with a large surface area which is responsible for their unique properties. The physical and chemical properties of nanoparticles such as large surface area volume, the possibility of target-specific functionalization, high strength, and flexible optical and electrical properties, explain their extensive biomedical applications. Gold nanoparticles with appropriate modifications can be applied to diagnostic, therapeutics, drug delivery, and bioimaging (Mahato et al. 2019). Nanoparticles have been applied broadly as a delivery system to macromolecules (Kumar et al. 2020) to nanozymes, that is nanoparticles showing enzyme-like activity (Singh 2019). With advances in the area of nanomedicine, nanomaterials have been proved to be assured free radical scavengers or antioxidants. In 2007, Gao et al. discovered the intrinsic peroxidase-like activity of iron oxide (Fe3O4) nanoparticles. After the discovery, it has been established that several nanoparticles show enzyme-like properties. Cerium oxide nanoparticles being famous as nanoceria, exhibit the SOD and catalase-like activity as shown in Fig. 1. It owes the ability to mimic SOD, catalase enzymes to the interchangeability of two oxidation states of cerium ion Ce3+ and Ce4+. The enzyme-like activity of cerium oxide nanoparticles has been well researched both in vitro and in animal models. The surface of cerium oxide nanoparticles can be decorated with some ligands which can alter the activity of nanoparticles. Yadav and Singh (2021a) showed that the effect of phosphomolybdic acid (PMA) and phosphotungstic acid (PTA) on SOD and CAT-like enzyme activity of cerium oxide nanoparticles. The PTA improved the enzyme-like activities of Cerium oxide NPs whereas PMA showed no effect on CAT-like activity and inhibited the SOD-like activity of Cerium oxide NPs. This review summaries the applications of nanozymes in aging and age-related diseases. The dietary effect of Fe3O4 NPs on cell longevity has been studied in Drosophila melanogaster (Zhang et al. 2016). The daily doses of 200 µg iron oxide NPs enhanced the climbing ability of 6-week-old flies and reduced the ROS levels. Fe3O4 NPs also increased the life span of flies from 49 to 57 days in Fe3O4 NPs ingesting flies. These nanoparticles mimicking the natural enzymes are known as nanozymes. In this review, we will discuss the applications of these NP oxides in the treatment of consequences of aging and age-associated disorders.
Fig. 1.
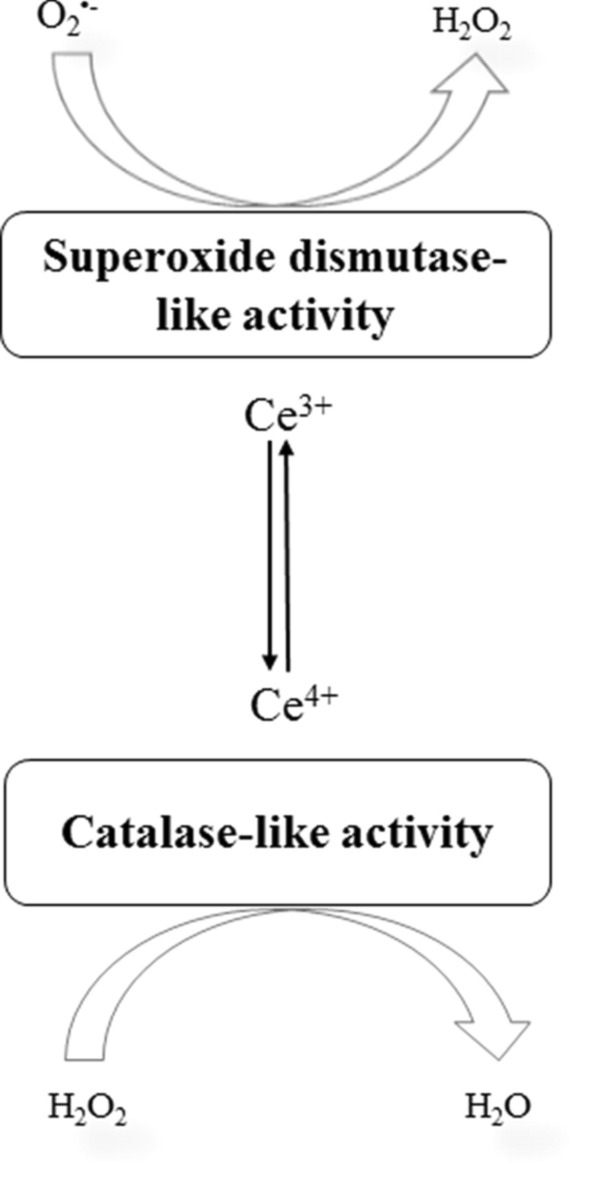
Superoxide dismutase-like and catalase-like activity of cerium ions (Ce3+ and Ce4+) in cerium oxide nanoparticles. Cerium in Ce3+ oxidation state preferentially behaves as a superoxide dismutase-like enzyme and catalyzes the conversion of superoxide (O2•−) into hydrogen peroxide (H2O2) while, cerium in Ce4+ oxidation state behaves as a catalase-like activity and converts H2O2 into water (H2O)
Complications of aging and age-associated disorders
Before the twentieth century, the average life expectancy was around 47 years and the major causes of death were infectious diseases. But in the twentieth century and onwards, the average life expectancy improved upto 77–80 years with improvement in public health management such as sanitation, better housing, and nutrition, vaccination programs, sewage management. Today, the major causes of death are chronic, non-communicable diseases such as cancer, heart diseases, diabetes, and lifestyle disorders. With the increasing aged population, it is necessary to prevent these diseases and help people age healthily. According to the oxidation-inflammatory theory of aging, the loss of homeostasis due to accumulating chronic oxidative stress affects the endocrine, nervous, and immune systems. Aging is associated with chronic and long-term accumulating diseases including Alzheimer’s disease (AD), cancer, diabetes, and heart problems. However, the association should not be misunderstood as a cause. Aging does not necessarily cause these diseases but the accumulation of deleterious effects over the life span can originate these disorders. Oxidative stress in cells stops cell proliferation and leads the cells into cellular senescence. These senescent cells release various senescence-associated secretory phenotypes (SASP) including metalloproteases (MMPs), extracellular matrix (ECM) components, chemokines, and interleukins. All these factors (oxidative stress, SASP, and senescence) have been studied in many chronic age-associated disorders.
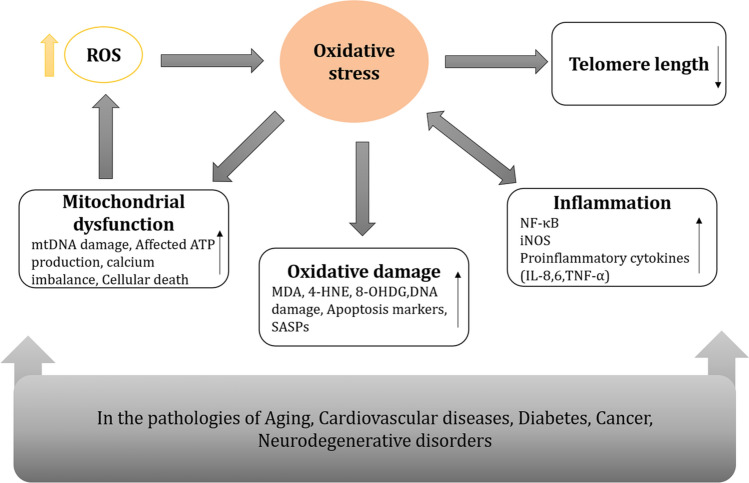
The relations between oxidative stress, damage, inflammation, and aging and age-associated diseases have been illustrated in Fig. 2.
Fig. 2.
Effect of increased ROS on the pathology of aging and its associated disorders. The increased ROS levels cause oxidative stress which shows a prominent effect on the physiology of cells by activating oxidative damage, inflammatory pathways, mitochondrial dysfunction, and decreasing the telomere length. (MDA malondialdehyde, 4-HNE 4-hydroxynonenal, 8-OHDG 8-oxo, 7-hydroxy deoxyguanosine, SASPs senescence-associated secretory phenotypes, iNOS nitric oxide synthase)
Oxidative stress and inflammation
Oxidative stress is the most studied phenomenon when it comes to aging and related diseases. The causes of oxidative stress are well elucidated. The production of ROS is a normal physiological process, but redox imbalance caused by the production of ROS leads to pathological problems in cells. The exact mechanism for the role of oxidative stress in aging is still not clear, but crosstalk and correlating pathways including the oxidative-inflammation axis are well studied.
Oxidative stress in connection with inflammation has been suggested more than once during the pathophysiology of aging and related diseases. The major signaling pathways of NF-κB, SAPK/JNK, and p38 MAPK are elevated during the progression of aging. A redox-sensitive transcription factor NF-κB regulates the expression of factors that are responsible for cell proliferation, redox status, and inflammation. Hence, the activation of the NF-κB pathway has been studied in nearly all age-related disorders. NF-κB activation induces the expression of the nitric oxide synthase (iNOS). Dietary heptadecane has been studied for its anti-inflammatory effect in the kidney of aged rats (Kim et al. 2013). This study established that NF-κB was highly expressed as well as the positively related iNOS genes expression was also high in the aged rats.
The tolerance to oxidative stress of the heart reduces over the lifespan of a human. Endothelial dysfunction has a central role in all cardiovascular diseases (CVD) such as atherosclerosis, myocardial infarction, and strokes. The endothelial impairment is closely related to oxidative stress and inflammation. The main biomarkers of oxidative stress associated with CVD risk factors are oxidized low-density lipoproteins (oxLDL). The enhanced production of oxLDL has been shown in atherosclerosis. Increased generation of oxLDL is also associated with endothelial cell damage. oxLDL induced pro-inflammatory cytokines such as IL-8 and IL-6 when incubated in human aortic endothelial cells (Sohrabi et al. 2020). It reprogrammed the metabolic and epigenetic pathways of the cells and activated the stress pathways, hence, advanced to atherosclerosis.
The hyperglycemic condition in diabetes induces oxidative stress and damage. Oxidative stress and diabetes are linked directly, shown by the increased levels of oxidative biomarkers in diabetes patients. High levels of MDA and decreased status of total antioxidants have been studied in diabetic patients (Rani and Mythili 2014). High-level glucose-induced oxidative stress enhances the secretion of inflammatory cytokines, interleukins, and results in pathological conditions (Aouacheri et al. 2015). Tahara et al. (2013) showed ipragliflozin, a sodium-glucose co-transporter 2 (SGLT2), managed the high levels of oxidative biomarkers and inflammatory markers. Oxidative biomarkers such as protein carbonyls, thiobarbituric acid reactive substances (TBARS), and inflammatory markers such as TNF-α, IL-6, CRP, and MCP-1 were increased in diabetic mice.
The oxidative damage over the life span of a cell induces many somatic mutations by DNA damage and hence increases the risk of different kinds of cancers. It is clear that with aging, accumulating oxidative damages to cell structure and functions lead to the initiation and progression of cancer. ROS generation plays a significant role in the initiation of endothelial to mesenchymal transition and angiogenesis (Andrisic et al. 2018). Oxidative stress biomarkers are shown to be increased in all types of cancers. The high levels of oxidative stress biomarkers such as NOx and Nitro oxide (NO), and low levels of antioxidant enzymes such as SOD, CAT, and GSH have been shown in bladder cancer (Islam et al. 2019). The oxidative stress parameters such as total oxidant status (TOS) and total antioxidant status (TAS) have been compared in fasting blood samples of colorectal cancer (CRC) patients and healthy controls and oxidative stress index (OSI) was measured (Wu et al. 2017). The TOS and OSI were significantly increased and the levels of TAS were notably declined in CRC patients compared to healthy subjects. Salvianolic acid B (Sal-B) showed antitumor properties by decreasing inflammation, oxidative stress biomarkers, and enhancing the levels of antioxidants (Katary et al. 2019). The levels of MDA in plasma and TNF-α were decreased by Sal-B and GSH plasma levels were increased in Ehrlich solid carcinoma cell lines injected mice.
The nervous system is highly vulnerable to oxidation due to a large amount of oxygen consumption; hence, the ROS production plays a central role in the pathogenesis of neurodegenerative disorders. Chronic inflammatory signaling pathways also influence CNS physiology. NOx-facilitated oxidative stress has been established as the main cause of neurodegenerative pathologies (Tarafdar and Pula 2018). Increased levels of oxidative stress and inflammation have been shown in rats during the early pathogenesis of neurodegenerative diseases (Snyder et al. 2017). The plasma was observed for inflammatory markers and a brain autopsy was performed to measure the levels of oxidative biomarkers in different parts of the brain.
Mitochondrial dysfunction
Mitochondria are known to be the powerhouse of the cell as they produce ATP for cell through ETC and provide cells with important metabolites including NAD+ and NADH. Fusion and fission of a large number of mitochondria of a cell maintain its redox homeostasis. The DNA damage caused by initial oxidative assault triggers a cascade of events that lead to mitochondrial dysfunction (Fakouri et al. 2019).
Mitochondrial dysfunction has been studied in aging and practically all age-related disorders. The mitochondrial DNA (mtDNA) is the most vulnerable site due to the closeness to the production site of the ROS. This affects many metabolic and signaling pathways in the cell, leading to its senescence (Vasileiou et al. 2019). Mitochondrial dysfunction in cardiomyocytes has the four most basic consequences on the cellular health of the cardiovascular system. First, the contractibility of cardiomyocytes depends on fatty acid-based ATP production which is heavily affected by the dysfunction. Second, defects in mitochondrial and endoplasmic reticulum co-ordination for calcium homeostasis can affect the central functions of the heart like electrical conduction. Third, cellular integrity disrupts due to mitochondrial dysfunction or accumulation of secondary products of this dysfunction leading to tissue loss and regulated cell death. Finally, the accumulation of damaged and permeabilized mitochondria induces inflammatory responses. Hence, several mitochondria-targeted agents such as L-carnitine, sirtuin, calcium channel inhibitors, many mitophagy pathways, fission and fusion pathways of mitochondria, and antioxidants have been observed as therapeutic tools for many CVDs including atherosclerosis, myocardial infarction, hypertension, arrhythmias, and heart failure (Bonora et al. 2019).
The consequences of mitochondrial dysfunction in type 2 diabetes mellitus (T2DM) have been explained on the basis of four mechanisms (Yaribeygi et al. 2019). First, point mutations in mtDNA are closely related to both type 1 and type 2 DM and their complications. Second, the accumulation of lipid-based secondary products of damaged mitochondria can activate protein kinase C (PKC) isoforms. PKCs phosphorylate the insulin receptor substrate at serine sites and develop insulin resistance. Third, the excess generation of free radicals in mitochondria impairs insulin signaling and other intracellular signaling pathways leading to diabetes and diabetes-induced kidney, heart, and nervous system malfunctions. Lastly, the mitochondrial impairment of pancreatic β-cells results in decreased insulin secretion.
Mitochondrial dysfunction has been seen through the prism of genetics in cancer. The mtDNA is more susceptible to mutations than nuclear DNA and does not have any proper repair mechanism. Similar to aging, this accumulation of mutations results in the development of cancer. Mitochondria directly affect the transcription and translation as well as alternate splicing of proteins, hence, regulate the nuclear gene expression. The impairment in the functions of mitochondria caused by mutations leads to altered gene expression in the nucleus which increases the risk of aging, cancer, and degenerative diseases (Moro 2019). Damaged mitochondria release several damage-associated molecular proteins (DAMPs) such as mtDNA in the cytosol and ROS generated in mitochondria. The mtDAMPs trigger innate immune responses such as inflammasomes which can act as a switch for cancer. Mitochondrial dysfunction in brain cells mimics the salient features of various neurodegenerative disorders such as AD, Parkinson’s diseases (PD), Huntington’s diseases, and amyotrophic lateral sclerosis (Wang et al. 2019c).
Telomere length
The repetitive short DNA sequences in humans (TTAGGG)n which form the ending of the chromosomes are telomeres. They do not code for any protein instead they protect the chromosome from degradation. The telomeres shorten with each cell division over the lifespan of the organism. The shortening of telomeres is a cell-specific phenomenon, hence the speed of shortening depends on the rate of proliferation of the cell. Telomere length has been a well-observed and studied biomarker of aging but the correlation between telomere length and age-related disorders is contradictory in nature.
The significantly short telomeres in cells develop cardiomyopathy due to impaired cell division or cellular senescence in mice (Zhan and Hägg 2019). Cellular senescence and accumulation of dead cells trigger inflammation pathways which result in the development of atherosclerosis (Meyer et al. 2018). In the case of T2DM, it is not clear whether telomere shortening causes T2DM or T2DM leads to the shortening of telomeres. The increased levels of glucose in the blood during diabetes cause oxidative stress. Oxidative stress triggers the shortening of telomeres in a cell. Telomere attrition in β-cells of pancreases prompts pre-mature death and hence, the impairment in their function and glucose intolerance. That is why the correlation of telomere length and diabetes is an interdependent process.
Telomere shortening in neurodegenerative disorders has been studied extensively but the results are contradictory. Telomeres of microglial cells shorten leading to microglial dystrophy which is a salient feature of amyloid deposition in AD patients. Shorter leukocyte length has been observed in mild cognitive impairment (MCI) and the attrition in telomeres is related to the advancement of MCI to AD (Nudelman et al. 2019). However, there was no significant difference in telomere length in the AD patients compared to controls. Similarly, PD studies show inconsistent results for telomere attrition (Levstek et al. 2020). It has been studied that schizophrenic (SCZ) patients have longer telomere length compared to the healthy control (Maurya et al. 2017). The leukocyte telomere length was measured by qPCR assay in patients at different stages of SCZ. The non-remitted SCZ showed a larger telomere length than remitted SCZ.
Applications of nanoparticle oxides
Metal oxide NPs are evolving nanomaterials with a vast range of chemical, physical and biological properties and that is why they are found to be applied in many biological pathologies, degradation of environmental pollutants. In the area of medicine, metal oxide nanoparticles have been used as diagnostic agents, drug carriers, and both as diagnostic and therapeutic agents. Many metal oxide NPs behave as antioxidant enzymes such as SOD, CAT, peroxidase and scavenge the ROS in pathological conditions. For example, the cerium oxide NPs, nanoceria functionalized with poly (ethylene glycol) (PEG) on their surface decreased the generation of ROS in human epidermal cells (A431 cells) (Singh et al. 2014). Pirmohamed et al. (2010) showed that the nanoceria, i.e., cerium oxide NPs (CeO2 NPs) exhibited CAT-like activity and catalyzed the conversion of H2O2 into H2O and O2. The cerium oxide nanoparticles (CeNPs) were shown to have a protective role in hepatic cell culture (Singh and Singh 2019). The CeNPs mimic the catalase enzyme and catalyze the conversion of H2O2 into H2O. The 3-Amino-1,2,4-Triazole (3-AT) toxicity causes irreversible inhibition of catalase enzyme in the cells and results in accumulation of ROS. The hepatocyte cell culture model (WRL-68) was exposed to 3-AT which led to cell death, while the pre-incubated WRL-68 with CeNPs were able to endure the toxicity very well. The elongated cellular morphology was also retained after pre-incubation, whereas during the exposure of 3-AT, the cellular morphology changed to sphere. This study showed that CeNPs helped the cells to dodge the DNA fragmentation caused by the ROS and 3-AT-induced apoptosis. Hence, cerium oxide NPs are the most well-known nanoparticles which show enzyme-like properties. Table 1 summaries the reports studying the biomedical applications of cerium oxide nanoparticles. Here, we discussed the applications of metal oxide NPs in the diagnostics and therapeutics of aging and its associated disorders. Some examples of metal oxide NP applications have been presented in Table 2.
Table 1.
The studies on applications of cerium oxide nanoparticles as an antioxidant
| Nanoparticles | Size | Results | References |
|---|---|---|---|
| Polyoxometalate, 12-phosphotungstic acid-coated nanoceria-gold nanoparticles conjugate | Nanoceria ~ 27 nm and gold NPs ~ 10 nm | The multi-enzyme complex was synthesized. The nanocomposite showed 82% efficiency in converting 4-nitrophenol into 4-aminophenol. The Peroxidase-like activity of gold NPs in nanocomposites also increased when investigated by the oxidation of 3,3’,5,5’-Tetramethylbenzidine (TMB), a peroxide substrate | (Shah et al. 2021) |
| Cerium oxide NPs coated with poly (acrylic acid) and poly (ethylene glycol) | ~ 7.8 nm | The coated cerium oxide NPs have been investigated for superoxide dismutase-like, catalase-like, oxidase-like, peroxidase-like activities. Polymer coatings inhibit the catalase-like activity, have no effect on superoxide dismutase-like activity, impair oxidase-like activity, and enhances the peroxidase-like activity of NPs | (Baldim et al. 2020) |
| Cerium vanadate (CeVO4) nanorods | ~ 50 nm– ~ 150 nm | Superoxide dismutase-like activity of nanorods was investigated in neuronal cells, SHSY5Y. The synthesized nanorods decreased the levels of superoxides generated due to the treatment of a SOD inhibitor, diethyldithiocarbamate (DDC), and maintained the mitochondrial integrity of the cell | (Singh et al. 2021) |
| Nanocomposites of sulforaphane loaded on silk fibroin NPs (SFSNPs), cerium oxide NPs and carbon dots (CDs) | ~ 365–385 nm | A multifunctional formulation with diagnostic and therapeutic ability was prepared in the form of CeNPs-CDs-SFSNPs nanocomposites. Sulforaphane is an antioxidant drug. The CeNPs showed enzyme-like activity and neutralized the ROS, whereas CDs act as a probe for fluorescence imaging | (Passi et al. 2020) |
| Cerium oxide nanorods | ~ 1.88 nm | The synthesized nanorods showed SOD-like activity. The hepatocyte model (WRL-68) was incubated with Buthionine sulfoximine (BSO) which causes the depletion of GSH in cells. Cerium oxide nanorods neutralized the free radicals generated due to BSO toxicity in cells | (Yadav and Singh 2021b) |
Table 2.
The applications of metal oxide nanoparticles in age-associated disorders
| Diseases | Nanoparticles used | Outcomes | References |
|---|---|---|---|
| Myocardial infarction | Iron oxide NPs (Fe3O4 NPs) | The connexin 43 expression was improved after the treatment of Fe3O4 NPs. Connexin 43 is a gap junction protein between cardiomyoblasts and mesenchymal cells and its increased expression improved rate of rat survival and its heart physiology | (Han et al. 2015) |
| Diabetes | Zinc oxide NPs (ZnO NPs) | Homocysteine (Hcy) and asymmetrical dimethylarginine (ADMA), the biomarkers of endothelial dysfunction and fasting blood glucose, insulin, NO levels were found to be increased in STZ-induced diabetic rats. ZnO NPs loaded with Docosahexaenoic acid (DHA) reduced these complications of diabetes in rats | (Hussein et al. 2020) |
| Hypertension | Cerium oxide nanoparticles (CeO2 NPs) | An aptasensor acting as antibody for protein epithelial sodium channel (ENaC), was prepared by the electrodepositing CeO2 NPs on carbon electrode. The CeO2 NP aptasensor detected ENaC concentrations in a linear range of 0.05–3.0 ng ml−1, and with the limits of detection of 0.012 ng ml−1 | (Hartati et al. 2021) |
| Cancer | Cerium oxide nanoparticles (CeO2 NPs) | CeO2 NPs were cytotoxic to melanoma 518A2 and colorectal adenocarcinoma HT-29 cell lines. CeO2 NPs showed prooxidant activity and induced the generation of reactive ROS which led to the hypoxic death of cancer cells | (pešić et al. 2015) |
| Alzheimer’s disease | Quercetin-conjugated superparamagnetic iron oxide nanoparticles (QT-Fe3O4 NPs) | Aluminum chloride-induced Alzheimer’s disease Wistar rats were treated with QT-Fe3O4 NPs. The treatment improved the cognitive functioning and memory also it decreased the expression of APP gene and miRNA101 in diseased rats. Hence, protecting the neurons against AD pathology | (Amanzadeh Jajin et al. 2021) |
| Amyotrophic lateral sclerosis (ALS) | Cerium oxide nanoparticles (CeO2 NPs) | The antioxidant enzyme-like behavior of CeO2 NPs improved the survival and the symptoms of ALS like improvement in muscle function in SODG93A transgenic mice. The CeO2 NPs showed oxidase and catalase-like activity in vitro | (DeCoteau et al. 2016) |
In cell longevity
The main cause of cellular aging is the accumulation of various oxidative damage products over the life span of cells. Redox imbalance activates inflammatory pathways which lead to cell senescence. In recent years, various nanoparticle oxides have been developed which show antioxidant, anti-inflammatory activities and can act as a therapeutic model in cellular aging and related disorders. For example, nanoceria, the most studied nanoparticle oxide in biomedical problems. Nanoceria has surface defects in its nanostructure creating oxygen vacancies. Hence, they are capable of scavenging the free radicals and ROS in physiological conditions. The oxygen vacancies also act as the active sites similar to the natural enzymes, which equips the nanoceria with enzyme-like properties. The Michaelis–Menten equation describing the enzyme–substrate interaction of nanoceria with hydrogen peroxide decomposition showed the interchangeability of Ce3+/Ce4+ during the scavenging process and pH dependency of the whole process (Seminko et al. 2021). The anti-oxidative and anti-inflammatory properties of CeO2 NPs were shown in Human corneal epithelial cells (HCECs) (Zheng et al. 2019). Dichlorofluorescein-diacetate (DCFDA) assay was used to measure oxidative stress intracellularly. The anti-oxidative capacity of CeO2 NPs depended on the size of nanoparticles. The smaller the size of nanoparticles, the higher the surface area and high oxygen vacancies in the lattice. The markers NO, IL-6, and TNF-α were assessed to measure the anti-inflammatory effect of CeO2 NPs. It was established that oxidative stress and inflammation are interdependent processes and CeO2 NPs act as both antioxidants and anti-inflammatory. CeONPs enhance the life span of stored RBCs (Rzigalinski et al. 2020). The morphology and number of RBCs were preserved for 42 days after the treatment of 10 nM and 100 nM CeONPs. CeONPs also preserved the function of mitochondria which was assessed by the measurement of ATP content. The NPs maintained the maximum decline of ATP content at 27% for 42 days. The effect of cerium oxide NPs on the life span of an organism was studied in Drosophila melanogaster (Cohen et al. 2008). CeONPs treatment enhanced the life span of female flies by 18 days which were fed 0–100 μm concentrations. Male flies responded poorly but motor functioning increased by 900%. The antioxidant and anti-genotoxic effect of CeO2 NPs was studied in a human epithelial lung cell line (Rubio et al. 2016). CeO2 NPs were able to quench the stress generated by KBrO3 after the 24-h treatment. The anti-genotoxicity was measured by comet assay which showed that the treatment of CeO2 NPs repressed the formation of any oxidative DNA damage. Chronic toxicity of TiO2 NPs in soil increased the life span of Caenorhabditis elegans from P0 to F3 generation (Hu et al. 2020). The genes such as daf-2, age-1, and skn-1 were expressed in the presence of TiO2 NPs and these genes maintained the levels of antioxidant enzymes.
The catalase-like activity of iron oxide nanoparticles has been applied to enhance cell proliferation in wound healing (Hu et al. 2017). The enzyme-like activity of iron oxide NPs was measured as the rate of H2O2 decomposition by a fluorometric assay. Iron oxide NPs behave as catalase, an antioxidant enzyme and convert H2O2 into water and oxygen. The lipid hydroperoxide (LHPO) content in the liver and blood increases very rapidly in 30- to 32-month-old Wistar male rats and reduces the survival rate of the organism (Nikitchenko et al. 2021a). Orthovanadate (OV) NPs were shown to reduce the generation of LHPO by 22% in old rats and have an effect on various mitochondrial biomarkers. The oxidative stress during aging inactivates the aconitase protein in mitochondria. The activity of aconitase protein in mitochondria was shown to increase by 23.3% in old rats after the treatment of orthovanadate NPs due to the antioxidant property of NPs. In addition, the OV NPs enhanced the activity of antioxidants (Glutathione peroxidase, Glucose-6-phosphate dehydrogenase, Glutathione reductase, Glutaredoxin, Glutathione-S-transferase, and Isocitrate dehydrogenase) in old-age rats. Hence, orthovanadate NPs are reliable and stable antioxidant system which can be further applied in human cell line and blood. OV NPs lowered the respiratory metabolic pathways and oxidative phosphorylation hence the ATP production in the isolated mitochondria from aged rats. Nikitchenko et al. (2021b) established that continued use of OV NPs will increase the chance of survival and lifespan of the organism by stabilizing the redox balance. Age-related macular degeneration causes blindness in old-age people due to oxidative damage. The retina of nuclear factor erythroid 2-related factor (Nrf2) knockout (Nrf2−/−) mice were more susceptible to oxidative stress. Glycol chitosan-coated cerium oxide NPs (GCCNPs) were shown to protect the retinal pigment epithelium (RPE) and photoreceptor cells (PRC) from atrophy caused by light-induced oxidative stress in Nrf2−/− mice (Wang et al. 2019a). In addition, GCCNPs restored the retinal functions in light-exposed Nrf2−/− mice. Treatment of GCCNPs maintained the levels of oxidants and pro-inflammatory proteins such as hypoxia-inducible factor (HIF-1a), AP-1, and NF-κB, p65.
The above discussion suggests that the popularity of nanoparticle oxides is increasing in the treatment of symptoms related to aging. Many NPs have been studied for increasing the lifespan of the cell and for their antioxidant, anti-inflammatory, anti-microbial effects. However, one little reminder that should be kept in mind is all these studies and observations are of preliminary nature.
In cardiovascular diseases
The pathologies of cardiovascular diseases such as atherosclerosis, myocardial infarction, and cardiomyopathy are directly linked to oxidative damage caused by ROS production. Several nanoparticle oxides have been applied as antioxidants and anti-inflammatory to treat these pathologies. Nanoparticles provide theranostics models against particular diseases by acting both as detection tools and therapeutics. For example, a theranostics model was prepared using iron oxide NPs as a diagnostic tool and cerium oxide NPs as an antioxidant. The contrasting ability of IO@CO NPs was assessed in vitro in macrophages J774A.1 (Wu et al. 2018). MRI images of cells treated with NPs showed good contrast compared to untreated cells. In addition, the cells incubated with IO@CO NPs were able to scavenge ROS generated by H2O2 exposure in vitro in macrophage cell lines. Oxidative stress and inflammation are known as major causes of heart diseases. The higher production of ROS, pro-inflammatory cytokines secretion and endoplasmic reticulum stress are major contributors to the initiation and progression of heart dysfunction. Cerium oxide NPs were applied to evaluate their effect on cardiac dysfunction in mice (Niu et al. 2007). The left ventricular (LV) dilatation and contractility impairment was observed by echocardiography with aging in mice. The CeO2-treated MCP mice showed inhibition of reduction in LV fractional shortening. The immunohistology confirmed the suppression of cellular infiltration, cardiac myocyte degeneration, and interstitial fibrosis after the treatment of CeO2 NPs in MCP mice. Treatment of CeO2 NPs reduced the production of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 in the myocardial tissue of MCP mice. MCP-1 and CRP protein expressions were also suppressed after the treatment of CeO2 NPs. The increased levels of peroxynitrite, a biomarker for protein oxidation were shown to decrease by immunohistochemistry after the treatment of CeO2 NPs. The endoplasmic reticulum stresses are measured by the expression of associated genes such as protein disulfide isomerase (PDI), glucose-regulated protein 78 (Grp78), and Heat shock proteins (HSP) 25, 40, 70. CeO2 NPs treatment suppressed the amplified expression of these endoplasmic reticulum stress-associated genes. The death of endothelial cells initiated by oxidative stress various cardiovascular complications. The percent of apoptosis decreased in endothelial cells after the treatment of CeO2 NPs (Chen et al. 2013). This study established the cell uptake nanoceria by endocytosis through caveolae- and clathrin-pathways and get evenly dispersed in the cytoplasm of the cell. Cigarette smoke generates a high amount of ROS and damages cellular function and majorly the cardiovascular system. The antioxidant ability of CeO2 NPs due to the existence of both oxidation states Ce3+ and Ce4+ and oxygen vacancies at the surface have been applied to curb the effect of cigarette smokes generated ROS and inflammation in rat H9c2 cardiomyocytes (Niu et al. 2011). CeO2 NPs treatment prevented the generation of ROS, cell death and inhibited the NF-κB associated inflammatory genes such as TNF-α, IL-6, and IL-1β. Thus, the CeO2 NPs can help the cells to overcome the cigarette smoke-induced cell damages by inhibiting iNOS and increasing the cellular concentration of SOD, glutathione. Isoproterenol causes cardiac toxicity by producing a large number of ROS and increases cellular irregularities. The isoproterenol toxicity increased the amount of all heart enzymes such as creatine kinase, alanine transaminase, aspartate transaminase, and lactate dehydrogenase tremendously and enhanced the oxygen demand which led to oxidative damage to myocardial cells. The CeO2 NPs treatment in comparison to traditional drug captopril reduced the levels of these cardiac enzymes and scavenged the ROS and free radicals which were shown through the reduction in the levels of stress biomarkers such as MDA and enhancement of antioxidants such as SOD and CAT enzymes (El Shaer et al. 2017). The isoproterenol elevated the cortisol and aldosterone levels in serum. The treatment of two different concentrations (0.5, 5 μKg/week) of CeO2 NPs significantly reduced the levels of aldosterone and cortisol. The cardiac biomarkers such as Copeptin, myoglobin (Mb), creatine kinases (CK-MB) and troponin I, which are increased in the bloodstream with the progression of CVD. The biomarkers like Mb are increased within an hour of acute myocardial infarction (AMI), hence, the Mb can be used as a clinical diagnosis during early symptoms of AMI.
NPs are potential diagnostic tools detecting electrochemically due to their high surface area to volume ratio, high thermal conductivity, and high electrical conductivity. Manganese (Mn)-doped Zinc oxide (ZnO) NPs have been applied to detect the Mb as an early biomarker of AMI upto 3–15 nM concentrations using cyclic voltammetry and electrochemical impedance spectroscopy (EIS) (Haque et al. 2020). The sensing method in this study depended on the electron transfer during reduction (Fe2+Mb) of the existing oxidized Mb (Fe3+Mb) which in exchange oxidized the Mn-doped ZnO NP-coated electrode. The equilibrium reaching when the potential was applied, Fe2+ converted into Fe3+ from the electron released from ZnO film and this reversible reduction current was measured. The ZnO NPs mitigated the oxidative damage caused by 8 Gy of gamma rays. Abdel-Magied and Shedid (2020) showed that 8 Gy gamma rays irradiated rats had increased levels of oxidative stress biomarkers such as MDA, thioredoxin‐interacting protein (TXNIP), some pro-inflammatory cytokines TNF-α, IL-18, CRP and the increased levels of total nitrite/nitrate, homocysteine, asymmetric dimethylarginine (ADMA), lactate dehydrogenase (LDH), creatine kinase (CK‐MB), intercellular adhesion molecule 1 (ICAM‐1), and cardiac troponin‐I (cTn‐I) marked the endothelial dysfunction. The treatment of ZnO NPs (10 mg/kg) lessened these symptoms in cardiovascular tissues caused by oxidative stress. The metal oxides due to their unique physical and biological properties have been applied in the field of medicine very appropriately. As we have observed, the NP oxides can be used in therapeutics, diagnostics of cardiovascular diseases. Many metal oxides acting as a multi-enzyme can also act as theranostics model for atherosclerosis, myocardial infarction.
In diabetes
Diabetes is the most prevalent non-communicable and genetic or lifestyle-generated disease in the world. Diabetes has been categorized into three types: type-I, type-II and gestational diabetes. Oxidative stress is the major cause behind the progression of diabetes. Hence, the search for a novel antioxidant to treat diabetes continues. The metal oxide NPs due to their antioxidant activities and antioxidant enzyme-like activities are the new bets. For example, the antioxidant property of CeO2 NPs has been applied to protect the β-cells from the copper ion or H2O2 generated oxidative stress (Zhai et al. 2016). The copper ion in the presence of H2O2 catalyzes the production of hydroxyl radicals in Rat insulinoma RINm5f cell lines which CeO2 NPs treatment inhibited. CeO2 NPs reduced the effect of H2O2 toxicity and protected the cells from apoptosis. As shown by Khaksar et al. (2017) CeO2 and yttrium oxide (Y2O3) NPs exhibit anti-diabetic activity and reduce the oxidative stress in the pancreas of the rats exposed to diazinon. The study showed levels of acetylcholinesterase (AChE) activity in the pancreas were decreased in diazinon-exposed rats by 49% than the control rats and the treatment of either of NPs, CeO2/Y2O3, or a combination of both increased the levels of AChE activity. The levels of proteins insulin, pro-insulin, and C-peptide were maintained by either NPs alone or the combination which otherwise were highly expressed during diazinon toxicity. The levels of these proteins directly told the physiology of the pancreas. The increased oxidative stress biomarkers such as lipid peroxidation, total thiol molecules, and decreased total antioxidant capacity and CAT activity in serum due to diazinon toxicity were mitigated by the NPs. The alone NPs and combination of two increased the cell viability upto 74% (CeO2), 82.3% (Y2O3) and 89.3% (combination) which decreased upto 61.7% during diazinon exposure. Diabetes is generally associated with endothelial dysfunction and chronic hyperglycemia promotes the expression of pro-inflammatory cytokines. The chemically stabilized ZnO NPs with biodegradable hydroxyl ethyl cellulose mitigated the diabetic complications and related symptoms of endothelial dysfunction (Hussein et al. 2018). The fasting glucose levels were very high (217.4 mg/dl) in streptozotocin (STZ)-induced diabetic rats than normal rats (89 mg/dl). This study showed that ZnO NPs treatment decreased this increased level of fasting glucose (121.6 mg/dl). The MDA levels were increased and serum antioxidant enzyme PON-1 levels were decreased in the aortic tissue of diabetic rats 117.3 nmol/g tissue and 160.0 IU/g tissue, respectively, than normal rat MDA levels 49 nmol/g tissue and PON-1 levels 272.2 IU/g tissue. The ZnO NPs treatment (10 mg/kg) of these diabetic rats for 30 days reduced the MDA levels upto 67.4 nmol/g tissue and increased the PON-1 levels upto 208.0 IU/g tissue. The high levels of IL-1α, CRP, elevated ADMA levels and reduced NO production were shown as the markers of endothelial dysfunction in diabetic rats and the proof of association of diabetes with CVD. The treatment of ZnO NPs maintained the regular levels of these biomarkers in the tissue of diabetic rats. This is assertive that metal oxides can be applied to treat symptoms of diabetes. The anti-diabetic activity of green-synthesized ZnO, CeO2, silver (Ag) NPs and Momordica charantia (MC) were compared in STZ-induced diabetic rats (Shanker et al. 2017). The levels of glucose were high in STZ-induced diabetic rats than the normal rats. The ZnO and Ag NPs treatment brought down the high glucose levels to normal levels than CeO2 NPs. The reduced levels of fasting insulin in diabetic rats were reinstated to normal by ZnO and Ag NPs than CeO2 NPs and MC. Histopathological studies showed the regeneration of decreasing β-cells in islets of Langerhans of diabetic rats after the treatment of ZnO and CeO2 NPs. This study directly studied the effect of metal oxide NPs in diabetic tissues.
The wound healing impairment and ulcer formation are the major complications of diabetes. The microRNA, miR-146a regulates the production of pro-inflammatory cytokines at optimal levels. The levels of this miR-146a were decreased in impaired wound healing response in diabetic rats than non-diabetic rats. Zgheib et al. (2019) delivered the miR-146a in conjugation with antioxidant CeO2 NPs (CNP-miR-146a) which decreased the inflammation and oxidative damage hence, the improved healing ability of diabetic rats. The diabetic wounds were treated with 100 ng of CeO2 NPs and 100 ng of CNP-miR-146a. The wounds treated with CNP-miR-146a showed rapid healing than CeO2 NPs treatment and control diabetic wounds. The biomechanical properties of wounded skin of diabetic rats improved after 4 weeks of wounding. In addition, the CNP-miR-146a treatment increased the angiogenesis in a diabetic wound which was studied by the histochemistry for endothelial marker CD31 at 14 days. The immunohistochemistry for CD45-positive cells showed that CNP-miR-146a treatment decreased the amount of CD45-positive cells in diabetic wounds which showed that CNP-miR-146a inhibited the inflammation in the wounds. The metal oxide NPs have been applied to diagnostics of diabetes. The Na-doped p-type ZnO (Na:ZnO) nanoflowers were prepared and sensing layers were fabricated (Jaisutti et al. 2017). The synthesized nanoflowers were used to detect acetone and other alcohol-based gases. Diabetic patients have acetone in their breath which can be detected using these nanoflowers. The metal oxide NPs have been extensively applied in diabetic pathophysiology and related complications. The antioxidant, anti-inflammatory, and hypoglycemic activities of the NPs have been proved to be the most useful when treating the symptoms of diabetes.
In cancer
The chemotherapy of cancer has side effects as they kill normal cells with cancer cells. The nanoparticles provide a targeted therapeutic system against cancer cells. Oxidative stress and chronic inflammation are some of the major causes of cancers. The metal oxide nanoparticles behaving as antioxidants and anti-inflammatory have been applied in the therapeutics of cancer. The antitumorigenic effect of CeO2 NPs was studied in N‐methyl‐N‐nitrosourea (NMU) and benzo(a)pyrene (BaP) induced breast cancer in female Wistar rats (Adebayo et al. 2020). The activity of myeloperoxidase (MPO) and the amount of NO were increased by 2.3 and 3.2 times in the serum of NMU-BaP exposed rats which were attenuated by CeO2 NPs treatment. Similarly, the oxidative stress marker MDA, NO, and MPO levels were increased by 30%, 24%, and 39%, respectively, and levels of antioxidant GSH and total sulfhydryl were decreased by 35% and 18%, respectively, in the mammary tissue of NMU-BaP administered rats. The CeO2 NPs treatment decreased the levels of oxidative stress biomarkers and increased the non-enzymatic antioxidants in the tissue. The CeO2 NPs treatment of diseased rats enhanced the activity of antioxidant enzymes such as SOD, CAT, and GST. The mild expression of proteins such as Bax and caspase‐3 were studied in diseased mammary tissue of rats by histochemical staining and the treatment of CeO2 NPs led to the moderate expression of these proteins. The CeO2 NPs also reduced the increased weight of mammary tissue in NMU-BaP-administered female rats. This study established the antitumorigenic effect by enhancing apoptosis and maintaining the redox balance of the cells. A novel nanohybrid was prepared by mobilizing a natural enzyme glucose oxidase (GOx) on enzyme-like activity showing superparamagnetic iron oxide-based nanozyme (MIONzyme) and carboxymethyl cellulose biopolymer stabilizing the nanocomposite (Mansur et al. 2020). This nanocomposite was applied to U-87 MG glioblastoma cells to study its antitumor effect. The nanocomposites reduced cell viability and led to cancer cell death by a two-step reaction. First, the glucose oxidase depleted the glucose and produced H2O2; second, the MIONzyme catalyzed the H2O2 into hydroxyl radicals which induced severe oxidative damage and subsequent cell death. Wang et al. (2019b) synthesized the cobalt (Co)-doped iron oxide (Fe3O4) nanozymes with higher peroxidase-like activity and a stronger affinity for H2O2 than Fe3O4 nanozymes. The Co-Fe3O4 nanozymes were employed in human renal cancer cells to switch on the oxidative stress burst to kill the cancer cells. The 0.2 mg/ml nanozymes killed at least 60% of the cancer cells in the presence of H2O2 in 24 h of incubation. The cells incubated with Co–Fe3O4 nanozymes showed a particular apoptosis pattern that is, increased ROS levels. To study the effect of these nanozymes, the renal tumor model was administered with Co–Fe3O4 nanozymes and the tumor volume was monitored thrice in a week. The nanozymes showed extraordinary antitumor activity in vivo in the presence of H2O2. A multifunctional cerium oxide nanocarrier has been synthesized to carry a PTEN plasmid which is a tumor suppressor gene and a siRNA to knockout AKT3 protein kinase in prostate cancer cells (Bhagat and Singh 2020). During the normal cellular process, PTEN regulates the expression of AKT3 while in the carcinogenic condition the loss of PTEN causes overexpression of phosphatidylinositol 3-kinase (PI3K)/AKT pathway resulting in enhanced cellular proliferation. PTEN plasmid and AKT3 siRNA incorporated cerium oxide nanoliposomes with ~ 100 nm diameter internalized in prostate cancer cell culture model (PC-3) by endocytosis and induced DNA fragmentation, apoptosis, and cell death while inhibiting further proliferation and metastasis. The cerium oxide nanoparticles mimicked the SOD enzyme in this formulation and helped to reduce the free radical overload from prostate cancer cells.
The CeO2 NPs behave both as antioxidants and prooxidant depending on the pH of the medium. They behave as a prooxidant in an acidic environment and this prooxidant ability of CeO2 NPs has been exploited to target the cancers (Datta et al. 2020). The CeO2 NPs were incubated with different concentrations 5, 10, 20, 40, 60, 80, and 100 μg/mL in human colorectal carcinoma cell lines (HCT 116) and normal human embryonic kidney (HEK 293) cells. The three concentrations 30, 50, and 70 μg/mL of CeO2 NPs showed cytotoxicity in HCT 116 whereas no cytotoxicity at 30 μg/mL and very little at 50 and 70 μg/mL in HEK 293 cells. It has been established that ROS production induced by CeO2 NPs treatment is the molecular mechanism behind apoptosis-dependent cell death. Further, the apoptosis markers such as increased levels of external phosphatidylserine, modifications in mitochondrial membrane potential (MMP), increased apoptotic proteins Bax, Bak, cyt-c, decreased anti-apoptotic protein Bcl2, apoptosis induced DNA damage, suppressed mdm2, and increased fragmentation of nucleus were studied in CeO2-treated cells. This study skillfully explained the mechanism underlying the ROS-generated apoptosis of CeO2 NP-treated cancer cells. The iron oxide nanoparticles (IONPs) such as Fe2O3 and Fe3O4 have been well researched and used as a probe in near-infrared fluorescence (NIRF) imaging, magnetic resonance imaging (MRI), Positron emission tomography (PET), and as a biosensor of molecules like glucose in the diagnosis of cancer (Vallabani and Singh 2018). IONPs showed peroxidase-like activity in the presence of peroxide. ATP enhances the catalytic property of IONPs, and this increased activity was used to detect glucose in human blood serum (Vallabani et al. 2017). This single-step detection can be applied to sense increased levels of glucose from diabetes to cancer. All the above studies review the applications of metal oxide NPs in treating the symptoms of cancer.
In neurodegenerative diseases
Neurodegenerative disorders are directly associated with the aging of the organism. Oxidative stress has been established as a common cause of nearly all neurocognitive disorders. The metal oxide NPs have been shown to reduce neurotoxicity by maintaining the redox balance. Tau aggregation is known to be the major biomarker of Alzheimer’s disease. Oxidative stress leads to the hyperphosphorylation of tau protein and further mitochondrial dysfunction leading to apoptosis of neuronal cells and causing cognitive pathology. A nanocomposite with many functions was prepared with Ceria (cerium oxide) nanocrystals as antioxidant and iron oxide NPs as MRI contrasting agent, methylene blue as tau aggregation inhibitor, and T807 as a tau tracer all conjugated on the surface of mesoporous silica NPs (Chen et al. 2018). The CeO2 NC/Fe3O4 NC/MSN-T807 nanocomposites targeted the hyperphosphorylated tau protein in Okadaic acid (OA)-induced tau pathology in SHSY5Y cells. In vivo, tauopathy induced by OA administration to rats and the treatment of CeO2 NC/Fe3O4 NC/MSN-T807 nanocomposites enhanced the MRI signaling for tau. The treatment of CeO2 NC/Fe3O4 NC/MSN-T807 nanocomposites in OA-exposed SHSY5Y cells reduced the levels of mitochondrial ROS accumulation, activated the Akt/GSK-3β pathways and maintained the levels of pro-apoptotic proteins (Bax, caspase-3), hence, the nanocomposites saved the neuronal cells from apoptosis. The study also showed that nanocomposites improved memory in OA-induced AD rats by reducing inflammation and reduction in microglia and astrocytes activation. Thus, the combining effect of all nanoparticles and other components of CeO2 NC/Fe3O4 NC/MSN-T807 nanocomposites proved to be an excellent theranostics model for AD pathology.
The CuxO nanoclusters were developed and applied as an antioxidant-like enzyme against the pathology of Parkinson’s disease (Hao et al. 2019). The oxidant scavenging effect of CuxO NCs was studied in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mice. The levels of HNE were reduced in CuxO NC-treated mice groups than PD group. Tyrosine hydroxylase (TH), a crucial enzyme in dopamine synthesis was diminished and increased levels of ionized calcium-binding adapter molecule 1 (IBA-1) in striata of PD mice, showed neuroinflammation and dopamine depletion in PD pathology. The CuxO NCs treatment enhanced the TH levels and reduced the levels of IBA-1. This study established that CuxO NCs treatment improved the memory and cognitive behavior of PD mice by scavenging the ROS and protecting the neuronal tissue from inflammation. The oxidative stress generated by dopaminergic neuron loss is the central feature of PD. Mn3O4 NPs having flower-like structure (Mnf) were applied to neutralize the increased ROS and free radicals acting like antioxidant enzymes SOD, CAT, GPx in SHSY5Y cells with MPTP-induced PD pathology (Singh et al. 2017).
Conclusion
The increase in the aging population requires the exploration of the possible therapeutic system for aging and its associated disease. Oxidative stress plays a central role in the pathologies of all age-associated diseases. It aggravates the situation by activating inflammatory pathways, mitochondrial dysfunction, and decreasing the telomere length. Several therapies have been devised targeting these pathways. Nanoparticles having nanosize possess unique physical properties. Some NPs exhibit enzyme-like activities due to large surface area and defects or vacancies in their lattice acting as the active site of enzymes. Cerium oxide NPs have SOD-like and CAT-like properties which enable the NPs to scavenge free radicals in an oxidative stressed cell. Iron oxide NPs exhibiting peroxidase enzyme-like properties have been applied in diagnostics of a broad range of diseases. The intrinsic property of metal oxide NPs to behave as an antioxidant, anti-inflammatory, anti-diabetic, and anti-cancer has been applied in treating the symptoms of age-associated diseases and aging. The metal oxide NPs have been applied to increase the cell life. Numerous metal oxide NPs have been studied in vitro as well as in vivo to establish their use in diagnostics, drug delivery systems, therapeutics, and artificial enzyme complexes. In this review, the studies we discussed are in a preliminary stage. Further studies are required for confirmatory results and solid inference. Clinical trials of synthesized and well-studied NPs are also necessary.
Acknowledgements
This study was supported by Fellowship from the Council of Scientific and Industrial Research (CSIR), Government of India to Somu Yadav (09/1152(0013)/2019-EMR-I). This agency had no role in the interpretation, or writing the manuscript.
Declarations
Conflict of interest
The authors declare no conflict of interest.
References
- Abdel-Magied N, Shedid SM. Impact of zinc oxide nanoparticles on thioredoxin-interacting protein and asymmetric dimethylarginine as biochemical indicators of cardiovascular disorders in gamma-irradiated rats. Environ Toxicol. 2020;35(4):430–442. doi: 10.1002/tox.22879. [DOI] [PubMed] [Google Scholar]
- Adebayo OA, Akinloye O, Adaramoye OA. Cerium oxide nanoparticles elicit antitumourigenic effect in experimental breast cancer induced by N-methyl-N-nitrosourea and benzo(a)pyrene in female Wistar rats. J Biochem Mole Toxicol. 2020;35(4):e22687. doi: 10.1002/jbt.22687. [DOI] [PubMed] [Google Scholar]
- Amanzadeh Jajin E, Esmaeili Soheila A, Noorbakhshnia RM. Quercetin-conjugated superparamagnetic iron oxide nanoparticles protect AlCl3-induced neurotoxicity in a rat model of alzheimer’s disease via antioxidant genes APP gene and miRNA-101. Front Neurosci. 2021 doi: 10.3389/fnins.2020.598617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrisic L, Dudzik D, Barbas C, Milkovic L, Grune T, Zarkovic N. Short overview on metabolomics approach to study pathophysiology of oxidative stress in cancer. Redox Biol. 2018;14:47–58. doi: 10.1016/j.redox.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouacheri O, Saka S, Krim M, Messaadia A, Maidi I. The investigation of the oxidative stress-related parameters in type 2 diabetes mellitus. Can J Diabetes. 2015;39(1):44–49. doi: 10.1016/j.jcjd.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Baldim V, Yadav N, Bia N, Graillot A, Loubat C, Singh S, Karakoti AS, Berret J-F. Polymer-coated cerium oxide nanoparticles as oxidoreductase-like catalysts. ACS Appl Mater Interf. 2020;12(37):42056–42066. doi: 10.1021/acsami.0c08778. [DOI] [PubMed] [Google Scholar]
- Bhagat S, Singh S. Co-delivery of AKT3 siRNA and PTEN plasmid by antioxidant nanoliposomes for enhanced antiproliferation of prostate cancer cells. ACS Appl Bio Mater. 2020;3(7):3999–4011. doi: 10.1021/acsabm.9b01016. [DOI] [PubMed] [Google Scholar]
- Bonora M, Wieckowski MR, Sinclair DA, Kroemer G, Pinton P, Galluzzi L. Targeting mitochondria for cardiovascular disorders: therapeutic potential and obstacles. Nat Rev Cardiol. 2019;16(1):33–55. doi: 10.1038/s41569-018-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Hou Y, Cheng G, Zhang C, Wang S, Zhang J. Cerium oxide nanoparticles protect endothelial cells from apoptosis induced by oxidative stress. Biol Trace Elem Res. 2013;154(1):156–166. doi: 10.1007/s12011-013-9678-8. [DOI] [PubMed] [Google Scholar]
- Chen Q, Du Y, Zhang K, Liang Z, Li J, Yu H, Ren R, Feng J, Jin Z, Li F, Sun J, Zhou M, He Q, Sun X, Zhang H, Tian M, Ling D. Tau-targeted multifunctional nanocomposite for combinational therapy of Alzheimer’s disease. ACS Nano. 2018;12(2):1321–1338. doi: 10.1021/acsnano.7b07625. [DOI] [PubMed] [Google Scholar]
- Cohen CA, Karfakis JA, Kurnick MD, Rzigalinski B. Cerium oxide nanoparticles reduce free radical-mediated toxicity in drosophila melanogaster. FASEB J. 2008;22(S1):624.621–624.621. doi: 10.1096/fasebj.22.1_supplement.624.1. [DOI] [Google Scholar]
- Datta A, Mishra S, Manna K, Saha KD, Mukherjee S, Roy S. Pro-oxidant therapeutic activities of cerium oxide nanoparticles in colorectal carcinoma cells. ACS Omega. 2020;5(17):9714–9723. doi: 10.1021/acsomega.9b04006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoteau W, Heckman KL, Estevez AY, Reed KJ, Costanzo W, Sandford D, Studlack P, Clauss J, Nichols E, Lipps J, Parker M, Hays-Erlichman B, Leiter JC, Erlichman JS. Cerium oxide nanoparticles with antioxidant properties ameliorate strength and prolong life in mouse model of amyotrophic lateral sclerosis. Nanomed Nanotechnol Biol Med. 2016;12(8):2311–2320. doi: 10.1016/j.nano.2016.06.009. [DOI] [PubMed] [Google Scholar]
- El Shaer SS, Salaheldin TA, Saied NM, Abdelazim SM. In vivo ameliorative effect of cerium oxide nanoparticles in isoproterenol-induced cardiac toxicity. Exp Toxicol Pathol. 2017;69(7):435–441. doi: 10.1016/j.etp.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Fakouri NB, Hou Y, Demarest TG, Christiansen LS, Okur MN, Mohanty JG, Croteau DL, Bohr VA. Toward understanding genomic instability, mitochondrial dysfunction and aging. FEBS J. 2019;286(6):1058–1073. doi: 10.1111/febs.14663. [DOI] [PubMed] [Google Scholar]
- Hao C, Qu A, Xu L, Sun M, Zhang H, Xu C, Kuang H. Chiral molecule-mediated porous CuxO nanoparticle clusters with antioxidation activity for ameliorating Parkinson’s disease. J Am Chem Soc. 2019;141(2):1091–1099. doi: 10.1021/jacs.8b11856. [DOI] [PubMed] [Google Scholar]
- Han J, Kim B, Shin JY, Ryu S, Noh M, Woo J, Park JS, Lee Y, Lee N, Hyeon T, Choi D, Kim BS. Iron oxide nanoparticle-mediated development of cellular gap junction crosstalk to improve mesenchymal stem cells’ therapeutic efficacy for myocardial infarction. ACS Nano. 2015;9(3):2805–2819. doi: 10.1021/nn506732n. [DOI] [PubMed] [Google Scholar]
- Hartati YW, Komala DR, Hendrati D, Gaffar S, Hardianto A, Sofiatin Y, Bahti HH. An aptasensor using ceria electrodeposited-screen-printed carbon electrode for detection of epithelial sodium channel protein as a hypertension biomarker. Royal Soc Open Sci. 2021 doi: 10.1098/rsos.202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M, Fouad H, Seo HK, Othman AY, Kulkarni A, Ansari ZA. Investigation of Mn doped ZnO nanoparticles towards ascertaining myocardial infarction through an electrochemical detection of myoglobin. IEEE Access. 2020;8:164678–164692. doi: 10.1109/ACCESS.2020.3021458. [DOI] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci. 1981;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Korschelt K, Daniel P, Landfester K, Tremel W, Bannwarth MB. Fibrous nanozyme dressings with catalase-like activity for H2O2 reduction to promote wound healing. ACS Appl Mater Interf. 2017;9(43):38024–38031. doi: 10.1021/acsami.7b12212. [DOI] [PubMed] [Google Scholar]
- Hu C, Hou J, Zhu Y, Lin D. Multigenerational exposure to TiO2 nanoparticles in soil stimulates stress resistance and longevity of survived C. elegans via activating insulin/IGF-like signaling. Environ Poll. 2020;263:114376. doi: 10.1016/j.envpol.2020.114376. [DOI] [PubMed] [Google Scholar]
- Hussein J, El-Banna M, Razik TA, El-Naggar ME. Biocompatible zinc oxide nanocrystals stabilized via hydroxyethyl cellulose for mitigation of diabetic complications. Int J Biol Macromol. 2018;107:748–754. doi: 10.1016/j.ijbiomac.2017.09.056. [DOI] [PubMed] [Google Scholar]
- Hussein J, El-Naggar M, Badawy E, El-laithy N, El-Waseef M, Hassan H, Abdel-Latif Y. Homocysteine and asymmetrical dimethylarginine in diabetic rats treated with docosahexaenoic acid–loaded zinc oxide nanoparticles. Appl Biochem Biotechnol. 2020;191(3):1127–1139. doi: 10.1007/s12010-020-03230-z. [DOI] [PubMed] [Google Scholar]
- Islam MO, Bacchetti T, Ferretti G. Alterations of antioxidant enzymes and biomarkers of nitro-oxidative stress in tissues of bladder cancer. Oxid Med Cell Longev. 2019;2019:2730896. doi: 10.1155/2019/2730896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaisutti R, Lee M, Kim J, Choi S, Ha T-J, Kim J, Kim H, Park SK, Kim Y-H. Ultrasensitive room-temperature operable gas sensors using p-type Na:ZnO nanoflowers for diabetes detection. ACS Appl Mater Interf. 2017;9(10):8796–8804. doi: 10.1021/acsami.7b00673. [DOI] [PubMed] [Google Scholar]
- Katary MA, Abdelsayed R, Alhashim A, Abdelhasib M, Elmarakby AA. Salvianolic acid B slows the progression of breast cancer cell growth via enhancement of apoptosis and reduction of oxidative stress, inflammation, and angiogenesis. Int J Mol Sci. 2019;20(22):5653. doi: 10.3390/ijms20225653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaksar MR, Rahimifard M, Baeeri M, Maqbool F, Navaei-Nigjeh M, Hassani S, Moeini-Nodeh S, Kebriaeezadeh A, Abdollahi M. Protective effects of cerium oxide and yttrium oxide nanoparticles on reduction of oxidative stress induced by sub-acute exposure to diazinon in the rat pancreas. J Trace Elem Med Biol. 2017;41:79–90. doi: 10.1016/j.jtemb.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Kim DH, Park MH, Choi YJ, Chung KW, Park CH, Jang EJ, An HJ, Yu BP, Chung HY. Molecular study of dietary heptadecane for the anti-inflammatory modulation of NF-kB in the aged kidney. PLoS ONE. 2013;8(3):e59316. doi: 10.1371/journal.pone.0059316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Gautam V, Kumar V, Maurya PK. Chapter 11 - Nanoparticle-based macromolecule drug delivery to lungs. In: Dua K, Hansbro PM, Wadhwa R, Haghi M, Pont LG, Williams KA, editors. Targeting chronic inflammatory lung diseases using advanced drug delivery systems. Academic Press; 2020. pp. 227–259. [Google Scholar]
- Levstek T, Kozjek E, Dolžan V, Trebušak Podkrajšek K. Telomere attrition in neurodegenerative disorders. Front Cell Neurosci. 2020;14:219–219. doi: 10.3389/fncel.2020.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahato K, Nagpal S, Shah MA, Srivastava A, Maurya PK, Roy S, Jaiswal A, Singh R, Chandra P. Gold nanoparticle surface engineering strategies and their applications in biomedicine and diagnostics. 3 Biotech. 2019;9(2):57. doi: 10.1007/s13205-019-1577-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur AAP, Mansur HS, Carvalho SM. Engineered hybrid nanozyme catalyst cascade based on polysaccharide-enzyme-magnetic iron oxide nanostructures for potential application in cancer therapy. Catal Today. 2020 doi: 10.1016/j.cattod.2020.06.083. [DOI] [Google Scholar]
- Maurya Pawan K, Rizzo Lucas B, Xavier G, Tempaku PF, Zeni-Graiff M, Santoro ML, Mazzotti DR, Zugman A, Pan P, Noto C, Maes M, Asevedo E, Mansur RB, Cunha GR, Gadelha A, Bressan RA, Belangero Sintia I, Brietzke E. Shorter leukocyte telomere length in patients at ultra high risk for psychosis. Eur Neuropsychopharmacol. 2017;27(5):538–542. doi: 10.1016/j.euroneuro.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Meyer TD, Nawrot T, Bekaert S, Buyzere MLD, Rietzschel ER, Andrés V. Telomere length as cardiovascular aging biomarker: JACC review topic of the week. J Am Coll Cardiol. 2018;72(7):805–813. doi: 10.1016/j.jacc.2018.06.014. [DOI] [PubMed] [Google Scholar]
- Moro L. Mitochondrial dysfunction in aging and cancer. J Clin Med. 2019;8(11):1983. doi: 10.3390/jcm8111983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitchenko YV, Klochkov VK, Kavok NS, Averchenko KA, Karpenko NA, Nikitchenko IV, Yefimova SL, Bozhkov AI. Anti-aging effects of antioxidant rare-earth orthovanadate nanoparticles in wistar rats. Biol Trace Elem Res. 2021 doi: 10.1007/s12011-020-02531-y. [DOI] [PubMed] [Google Scholar]
- Nikitchenko YV, Klochkov VK, Kavok NS, Karpenko NA, Yefimova SL, Nikitchenko IV, Bozhkov AI. Age-related effects of orthovanadate nanoparticles involve activation of GSH-dependent antioxidant system in liver mitochondria. Biol Trace Elem Res. 2021;199(2):649–659. doi: 10.1007/s12011-020-02196-7. [DOI] [PubMed] [Google Scholar]
- Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res. 2007;73(3):549–559. doi: 10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Wang K, Kolattukudy PE. Cerium oxide nanoparticles inhibits oxidative stress and nuclear factor-κB activation in H9c2 cardiomyocytes exposed to cigarette smoke extract. J Pharmacol Exp Ther. 2011;338(1):53–61. doi: 10.1124/jpet.111.179978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman KNH, Lin J, Lane KA, Nho K, Kim S, Faber KM, Risacher SL, Foroud TM, Gao S, Davis JW, Weiner MW, Saykin AJ, Alzheimer’s Disease Neuroimaging I Telomere shortening in the Alzheimer's disease neuroimaging initiative cohort. J Alzheimers Dis. 2019;71(1):33–43. doi: 10.3233/JAD-190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passi M, Kumar V, Packirisamy G. Theranostic nanozyme: Silk fibroin based multifunctional nanocomposites to combat oxidative stress. Mater Sci Eng C. 2020;107:110255. doi: 10.1016/j.msec.2019.110255. [DOI] [PubMed] [Google Scholar]
- Pešić M, Podolski-Renić A, Stojković S, Matović B, Zmejkoski D, Kojić V, Bogdanović G, Pavićević A, Mojović M, Savić A, Milenković I, Kalauzi Ksenija A, Radotić K. Anti-cancer effects of cerium oxide nanoparticles and its intracellular redox activity. Chem Biol Interact. 2015;232:85–93. doi: 10.1016/j.cbi.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Pirmohamed T, Dowding JM, Singh S, Wasserman B, Heckert E, Karakoti AS, King JES, Seal S, Self WT. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun. 2010;46(16):2736–2738. doi: 10.1039/B922024K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani AJ, Mythili SV. Study on total antioxidant status in relation to oxidative stress in type 2 diabetes mellitus. J Clin Diagn Res. 2014;8(3):108–110. doi: 10.7860/JCDR/2014/7603.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio L, Annangi B, Vila L, Hernández A, Marcos R. Antioxidant and anti-genotoxic properties of cerium oxide nanoparticles in a pulmonary-like cell system. Arch Toxicol. 2016;90(2):269–278. doi: 10.1007/s00204-015-1468-y. [DOI] [PubMed] [Google Scholar]
- Rzigalinski BA, Giovinco HM, Cheatham BJ. Cerium oxide nanoparticles improve lifespan of stored blood. Military Med. 2020;185(Supplement_1):103–109. doi: 10.1093/milmed/usz210. [DOI] [PubMed] [Google Scholar]
- Seminko V, Maksimchuk P, Grygorova G, Okrushko E, Avrunin O, Semenets V, Malyukin Y. Mechanism and dynamics of fast redox cycling in cerium oxide nanoparticles at high oxidant concentration. J Physical Chem C. 2021;125(8):4743–4749. doi: 10.1021/acs.jpcc.1c00382. [DOI] [Google Scholar]
- Shah F, Yadav N, Singh S. Phosphotungstate-sandwiched between cerium oxide and gold nanoparticles exhibit enhanced catalytic reduction of 4-nitrophenol and peroxidase enzyme-like activity. Colloids Surf B. 2021;198:111478. doi: 10.1016/j.colsurfb.2020.111478. [DOI] [PubMed] [Google Scholar]
- Shanker K, Naradala J, Mohan GK, Kumar G, Pravallika PJRa, A sub-acute oral toxicity analysis and comparative in vivo anti-diabetic activity of zinc oxide, cerium oxide, silver nanoparticles, and Momordica charantia in streptozotocin-induced diabetic Wistar rats. RSC Adv. 2017;7(59):37158–37167. doi: 10.1039/C7RA05693A. [DOI] [Google Scholar]
- Simioni C, Zauli G, Martelli AM, Vitale M, Sacchetti G, Gonelli A, Neri LM. Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget. 2018;9(24):17181–17198. doi: 10.18632/oncotarget.24729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. Nanomaterials exhibiting enzyme-like properties (Nanozymes): current advances and future perspectives. Front Chem. 2019 doi: 10.3389/fchem.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Singh S. Redox-dependent catalase mimetic cerium oxide-based nanozyme protect human hepatic cells from 3-AT induced acatalasemia. Colloids Surf B. 2019;175:625–635. doi: 10.1016/j.colsurfb.2018.12.042. [DOI] [PubMed] [Google Scholar]
- Singh R, Shukla RK, Kumar A, Dhawan A, Singh S. PEGylated nanoceria protect human epidermal cells from reactive oxygen species. Mol Cytogenet. 2014;7(1):P78. doi: 10.1186/1755-8166-7-S1-P78. [DOI] [Google Scholar]
- Singh N, Savanur MA, Srivastava S, D'Silva P, Mugesh G. A redox modulatory Mn3O4 nanozyme with Multi-Enzyme activity provides efficient cytoprotection to human cells in a Parkinson's disease model. Angew Chem Int Ed. 2017;56(45):14267–14271. doi: 10.1002/anie.201708573. [DOI] [PubMed] [Google Scholar]
- Singh N, NaveenKumar SK, Geethika M, Mugesh G. A cerium vanadate nanozyme with specific superoxide dismutase activity regulates mitochondrial function and ATP synthesis in neuronal cells. Angew Chem Int Ed. 2021;60(6):3121–3130. doi: 10.1002/anie.202011711. [DOI] [PubMed] [Google Scholar]
- Snyder B, Shell B, Cunningham JT, Cunningham RL. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol Rep. 2017;5(9):e13258. doi: 10.14814/phy2.13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabi Y, Lagache SMM, Voges VC, Semo D, Sonntag G, Hanemann I, Kahles F, Waltenberger J, Findeisen HM. OxLDL-mediated immunologic memory in endothelial cells. J Mol Cell Cardiol. 2020;146:121–132. doi: 10.1016/j.yjmcc.2020.07.006. [DOI] [PubMed] [Google Scholar]
- Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Li Q, Tomiyama H, Kobayashi Y, Noda A, Sasamata M, Shibasaki M. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715(1):246–255. doi: 10.1016/j.ejphar.2013.05.014. [DOI] [PubMed] [Google Scholar]
- Tarafdar A, Pula G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int J Mol Sci. 2018;19(12):3824. doi: 10.3390/ijms19123824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabani NVS, Singh S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech. 2018;8(6):279. doi: 10.1007/s13205-018-1286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabani NVS, Karakoti AS, Singh S. ATP-mediated intrinsic peroxidase-like activity of Fe3O4-based nanozyme: one step detection of blood glucose at physiological pH. Colloids Surf B. 2017;153:52–60. doi: 10.1016/j.colsurfb.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Vasileiou PVS, Evangelou K, Vlasis K, Fildisis G, Panayiotidis MI, Chronopoulos E, Passias P-G, Kouloukoussa M, Gorgoulis VG, Havaki S. Mitochondrial Home Cell Senescence Cells. 2019;8(7):686. doi: 10.3390/cells8070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Zheng M, Lester KL, Han Z. Light-induced Nrf2−/− mice as atrophic age-related macular degeneration model and treatment with nanoceria laden injectable hydrogel. Sci Rep. 2019;9(1):14573. doi: 10.1038/s41598-019-51151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li H, Guo L, Jiang Q, Liu FJRA. A cobalt-doped iron oxide nanozyme as a highly active peroxidase for renal tumor catalytic therapy. RSC Adv. 2019;9(33):18815–18822. doi: 10.1039/C8RA05487H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xu E, Musich PR, Lin F. Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci Ther. 2019;25(7):816–824. doi: 10.1111/cns.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Feng J, Yang Y, Dai C, Lu A, Li J, Liao Y, Xiang M, Huang Q, Wang D, Du X-B. Significance of serum total oxidant/antioxidant status in patients with colorectal cancer. PLoS ONE. 2017;12(1):e0170003. doi: 10.1371/journal.pone.0170003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Yang Y, Zhao W, Xu ZP, Little PJ, Whittaker AK, Zhang R, Ta HT. Novel iron oxide–cerium oxide core–shell nanoparticles as a potential theranostic material for ROS related inflammatory diseases. J Mater Chem B. 2018;6(30):4937–4951. doi: 10.1039/C8TB00022K. [DOI] [PubMed] [Google Scholar]
- Yadav N, Singh S. Polyoxometalate-mediated vacancy-engineered cerium oxide nanoparticles exhibiting controlled biological enzyme-mimicking activities. Inorg Chem. 2021;60(10):7475–7489. doi: 10.1021/acs.inorgchem.1c00766. [DOI] [PubMed] [Google Scholar]
- Yadav N, Singh S. SOD mimetic cerium oxide nanorods protect human hepatocytes from oxidative stress. Emergent Mater. 2021 doi: 10.1007/s42247-021-00220-7. [DOI] [Google Scholar]
- Yaribeygi H, Atkin SL, Sahebkar A. Mitochondrial dysfunction in diabetes and the regulatory roles of antidiabetic agents on the mitochondrial function. J Cell Physiol. 2019;234(6):8402–8410. doi: 10.1002/jcp.27754. [DOI] [PubMed] [Google Scholar]
- Zgheib C, Hilton SA, Dewberry LC, Hodges MM, Ghatak S, Xu J, Singh S, Roy S, Sen CK, Seal S, Liechty KW. Use of cerium oxide nanoparticles conjugated with MicroRNA-146a to correct the diabetic wound healing impairment. J Am Coll Surg. 2019;228(1):107–115. doi: 10.1016/j.jamcollsurg.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J-H, Wu Y, Wang X-Y, Cao Y, Xu K, Xu L, Guo Y. Antioxidation of cerium oxide nanoparticles to several series of oxidative damage related to Type II diabetes mellitus in vitro. Med Sci Monit. 2016;22:3792–3797. doi: 10.12659/msm.901068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Hägg S. Telomere length and cardiovascular disease risk. Curr Opin Cardiol. 2019;34(3):270–274. doi: 10.1097/hco.0000000000000613. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Z, Li X, Wang L, Yin M, Wang L, Chen N, Fan C, Song H. Dietary iron oxide nanoparticles delay aging and ameliorate neurodegeneration in drosophila. Adv Mater. 2016;28(7):1387–1393. doi: 10.1002/adma.201503893. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Fang Y, Zeng L, Li X, Chen H, Song H, Huang J, Shi S. Cytocompatible cerium oxide-mediated antioxidative stress in inhibiting ocular inflammation-associated corneal neovascularization. J Mater Chem B. 2019;7(43):6759–6769. doi: 10.1039/C9TB01066A. [DOI] [PubMed] [Google Scholar]