Abstract
Potential roles for neutrophils in the pathophysiology of intracranial aneurysm have long been suggested by clinical observations. The presence of neutrophil enzymes in the aneurysm wall has been associated with significant increases in rupture risk. However, the mechanisms by which neutrophils may promote aneurysm rupture are not well understood. Neutrophil extracellular traps (NETs) were implicated in many diseases that involve inflammation and tissue remodeling, including atherosclerosis, vasculitis, and abdominal aortic aneurysm. Therefore, we hypothesized that NETs may promote the rupture of intracranial aneurysm, and that removal of NETs can reduce the rate of rupture.
We employed both pharmacological and genetic approaches for the disruption of NETs, and used a mouse model of intracranial aneurysm to investigate the roles of NETs in the development of intracranial aneurysm rupture.
Here we showed that NETs are detected in human intracranial aneurysms. Both global and granulocyte-specific knockout of peptidyl arginine deiminase 4 (an enzyme essential for NET formation) reduced the rate of aneurysm rupture. Pharmacological blockade of the NET formation by Cl-amidine also reduced the rate of aneurysm rupture. In addition, the resolution of already formed NETs by deoxyribonuclease was effective against aneurysm rupture. Inhibition of NETs formation with Cl-amidine decreased mRNA expression of pro-inflammatory cytokines (ICAM-1, IL-1β, MCP-1, and TNF-α) in cerebral arteries.
This data suggests that NETs promote the rupture of intracranial aneurysm. Pharmacological removal of NETs, by inhibition of peptidyl arginine deiminase 4 or resolution of already-formed NETs, may represent a potential therapeutic strategy for preventing aneurysmal rupture.
Keywords: Cerebrovascular disease, Intracranial aneurysm, Neutrophil extracellular traps, inflammation, mice
Introduction
Potential roles for neutrophils in the pathophysiology of intracranial aneurysm have long been suggested by clinical observations.1–6 Plasma levels of neutrophil elastase are elevated in patients with intracranial aneurysm, and this has been associated with vascular wall degradation.1, 2 Also, a higher degree of neutrophil infiltration has been observed in ruptured aneurysms compared to unruptured aneurysms.3–5 Furthermore, the presence of neutrophil enzymes in the aneurysm wall has been associated with significant increases in rupture risk, suggesting that neutrophils may play a causal role in aneurysm rupture.6 However, the mechanisms by which neutrophils promote aneurysm rupture are not well understood.
Neutrophils kill pathogens by multiple mechanisms, including phagocytosis and the secretion of antimicrobial granular contents. A lesser-known neutrophil defense mechanism is the release of neutrophil extracellular traps (NETs). NETs are networks of unraveled chromatin decorated with histones and antimicrobial granular enzymes.7, 8 NET formation results in neutrophil death, and may be triggered in response to stimuli such as microorganisms, activated platelets, and pro-inflammatory cytokines.9 While NETs have an antimicrobial effect, they may also cause tissue damage by degrading the extracellular matrix and inducing inflammation. Recent studies have revealed roles for NETs in a range of diseases that involve inflammation and tissue remodeling, including atherosclerosis, vasculitis, and abdominal aortic aneurysm.9, 10 NET formation leads to a vicious cycle of tissue injury, inflammatory-cytokine release, neutrophil recruitment, and recurrent production of NETs, resulting in conditions of sustained inflammation and tissue degradation.11 Importantly, chronic inflammation and degradation of vascular walls are well-established contributors in the pathophysiology of intracranial aneurysm.12–14
Blocking NET formation and resolution of NETs have been successfully used to reverse or alleviate the progression of the diseases that involve NETs-induced inflammation.15–17 Peptidyl arginine deiminase 4 (PAD4) is an enzyme that triggers the decondensation of chromatin and is essential for NET formation.9 Genetic and pharmacological manipulation of PAD4 have been used to examine the roles of NETs in various disease models.15, 18 Pharmacological inhibition of PAD4, using Cl-amidine, has been shown to disrupt NET formation in vivo and alleviate inflammatory conditions.16, 17 Also, treatment with deoxyribonuclease (DNase) has been used to reduce NET burden by degrading already-formed NETs.7, 11, 19
In this study, we hypothesized that NETs promote the rupture of intracranial aneurysm, and that removal of NETs can reduce the rate of rupture. We employed both pharmacological and genetic approaches for the disruption of NETs, and used a mouse model of intracranial aneurysm to investigate the roles of NETs in the development of intracranial aneurysm rupture.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Mouse model of intracranial aneurysm
Experiments were conducted in accordance with the guidelines approved by the Institutional Animal Care and Use Committee. PAD4flox/flox (PAD4f/f) mice, mice expressing Cre recombinase under the control of the myeloid-specific lysosome M (LysM) promoter (LysMCre), PAD4 global knockout (PAD4 KO) mice, and C57BL/6J (wild-type) mice were obtained from Jackson Laboratory (Bar Harbor, Maine). PAD4f/f mice lacking LysMCre (PAD4f/fLysMCre-) and C57BL/6J mice were used as controls. PAD4 KO and PAD4f/fLysMCre+ mice were used as global knockout and neutrophil-specific knockout, respectively. To induce aneurysm formation, we combined systemic hypertension and a single injection of elastase (35 mU) into the cerebrospinal fluid at the right basal cistern as we previously described.20, 21. To induce systemic hypertension, we used deoxycorticosterone acetate (DOCA)-salt-induced hypertension. 20, 21
To detect aneurysmal rupture, two blinded observers performed a daily neurological examination as previously described.21 Aneurysms are defined as a localized outward bulging of the vascular wall (>150% of the control artery).22 When mice develop neurological symptoms associated with aneurysmal rupture (neurological score: 1–5), we euthanize them immediately (within 4 hours). In both euthanized and dead mice, we inspect the brain samples and verify the presence of aneurysm and hematoma from subarachnoid hemorrhage by examining the Circle of Willis and its major branches under a dissecting microscope (10X).21 Our study confirmed the specificity and sensitivity of this approach in detecting aneurysmal rupture.21 All remaining mice were euthanized three weeks after aneurysm induction.21, 23, 24 Mice were transcardially perfused with phosphate-buffered saline, followed by a gelatin-containing blue dye to visualize cerebral arteries. Representative images of brain base showing ruptured, unruptured, or no aneurysm are presented in Figure 1B.
Figure 1. NETs are present in human intracranial aneurysms.
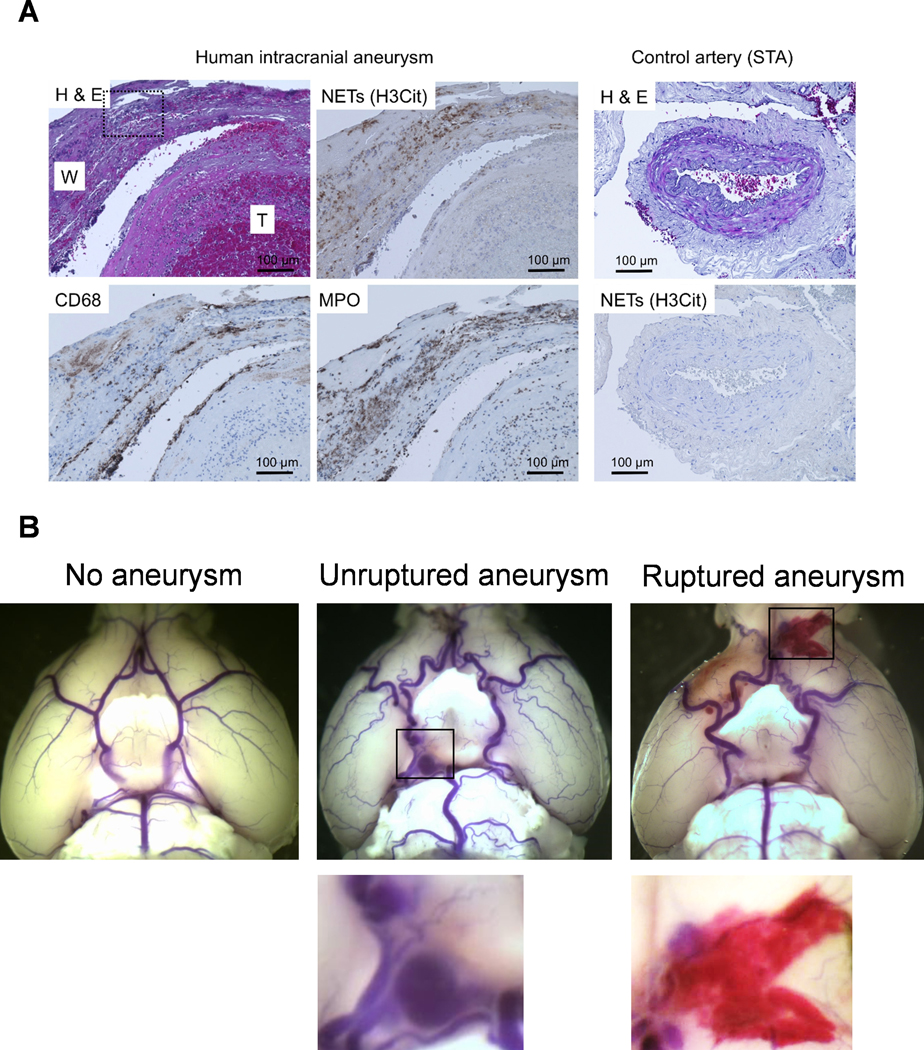
A. Representative pictures of an unruptured aneurysm with a partially organized thrombus (left and middle panels). There were abundant NET formations indicated by H3Cit positive areas in the aneurysm wall (indicated as W). There was almost no H3Cit positive area inside the partially organized thrombus (indicated as T). H3Cit was overlapping with MPO (neutrophils marker), but not with CD68 (macrophage marker). In contrast, there is no H3Cit positivity in the control tissue from the superficial temporal artery (STA, Fig. 1A, right panel). H & E, hematoxylin and eosin staining. Dashed square on the H & E image is used for the high power image in Figure S2 showing the polymorphonuclear cells. B. Representative images of brain base showing ruptured, unruptured or no aneurysm from the mouse model used in the current study.
Drug treatment
Cl-amidine (a PAD4 inhibitor, Sigma-Aldrich, St. Louis, MO) was diluted in 200 μM Lock’s solution (150 mM NaCl, 5 mM KCl, 2mM CaCl2, 0.1 % glucose, and 10 mM HEPES buffer, pH 7.3), and administered at a dose of 10 mg/kg per day intraperitoneally.16 DNase 1 (Pulmozyme; Genentech, CA, USA) was diluted in sterile saline and injected daily (50 μg intraperitoneally and 10 μg intravenously).25, 26 Both treatments were started 6 days and continued until 21 days after aneurysm induction. This timing of the treatment was found to affect aneurysmal rupture without affecting the formation of aneurysms.21, 23, 24
Histology and real-time PCR detection of pro-inflammatory cytokines
For the quantitative analysis of NETs and neutrophils, we collected the brain tissue samples from the mice treated with vehicle or Cl-amidine at 8 days after aneurysm induction (n = 5 from each group). For the real-time PCR detection of proinflammatory cytokines, we collected total RNA samples from cerebral arteries (Circle of Willis, including aneurysms) 8 days after aneurysm induction as previously described.14, 27 We chose 8 days after elastase injection for these analyses to capture the molecular and histological changes that occur before aneurysms start rupturing. Our previous studies showed that aneurysms start rupturing around 7 – 8 days after aneurysm induction (For more details, please see http://hyper.ahajournals.org).21, 23
Statistical Analysis
Fisher’s exact test was used to analyze the incidences of aneurysm formation and subarachnoid hemorrhage. Log-rank (Mantel-Cox) test was used for the analysis of survival rate. Multiple t-test was used for blood pressure analysis. RT-PCR data were analyzed by the Mann-Whitney test. P-values < 0.05 were considered statistically significant. Data are expressed as means ± standard deviation.
Results
NETs are present in human intracranial aneurysms
As a first step, we examined whether NETs were present in human aneurysm samples. We collected 4 intracranial aneurysm tissues and 3 superficial temporal artery tissues from patients who underwent aneurysm clipping. We used a combination of H3Cit (citrullinated histone H3: NET formation marker) and MPO (Myeloperoxidase: a neutrophil marker) to detect NETs in aneurysm samples. Both ruptured and unruptured aneurysms showed NET formations in the aneurysm wall. Figure 1A (left and middle panels) shows representative pictures of an unruptured aneurysm with a partially organized thrombus. There were abundant NET formations indicated by H3Cit positive areas in the aneurysm wall (indicated as W). There was almost no H3Cit positive area inside the partially organized thrombus (indicated as T). The area positively stained by H3Cit was largely overlapping with MPO staining (neutrophils marker), but not with CD68 (macrophage marker). In contrast, there is no H3Cit positivity in the control tissue from the superficial temporal artery (STA, Fig. 1A, right panel). The high-magnification picture of hematoxylin and eosin staining shows the presence of polymorphonuclear cells (Figure S2, please see http://hyper.ahajournals.org).
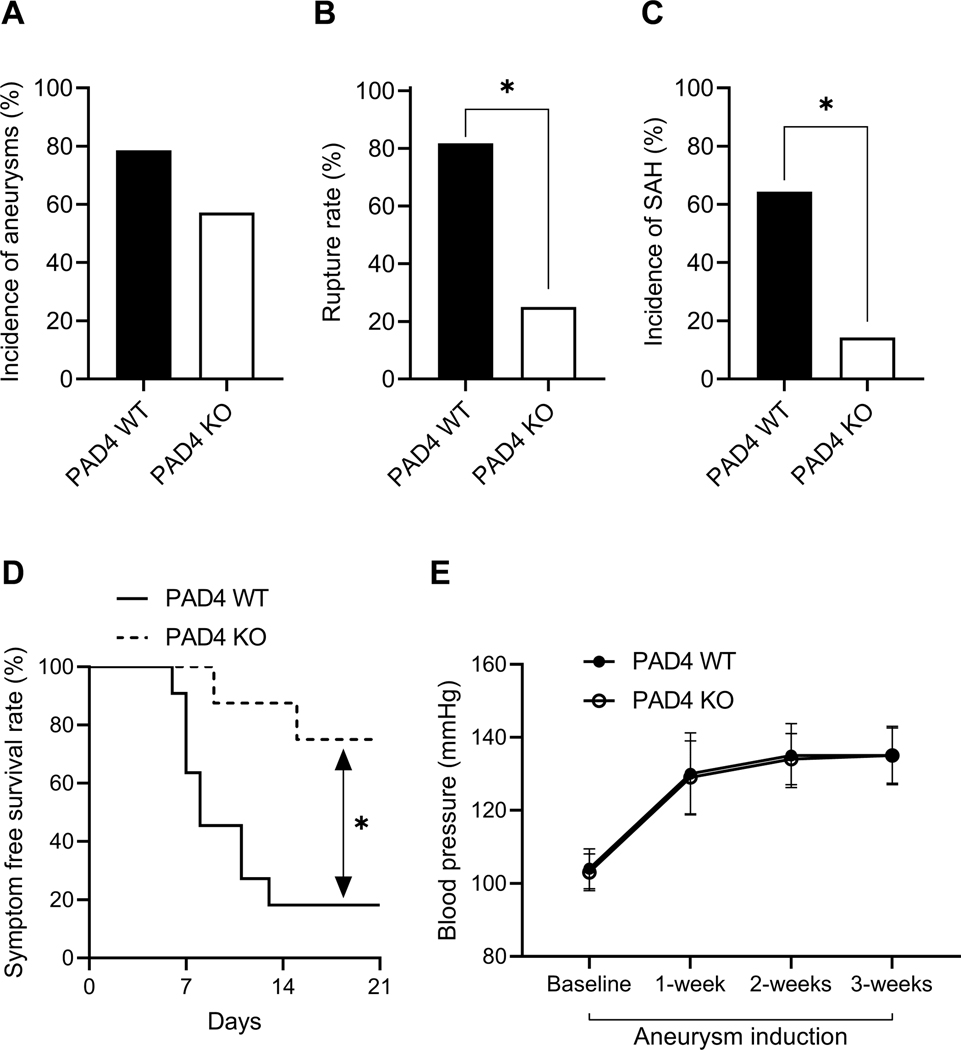
Global knockout of PAD4 reduces the rate of aneurysm rupture
We assessed the contribution of NETs to the rupture of intracranial aneurysm using PAD4-knockout (PAD4 KO) mice, mice that are incapable of forming NETs (Figure 2).9, 28, 29 There was no significant difference in the incidence of aneurysm formation between PAD4 KO mice and wild-type littermates (PAD4 WT, Figure 2A). PAD4 KO mice experienced a significant reduction in the rate of aneurysm rupture compared to wild-type (Figure 2B, 82% vs. 25%, n = 11 vs. 8, wild-type vs. knockout, P < 0.05). Similarly, PAD4 KO mice also had a significant reduction in the incidence rate of SAH (ruptured vs. unruptured + no aneurysm) compared to wild-type (Figure 2C, 64% vs. 14%, n = 14 for both, wild-type vs. knockout, P < 0.05). PAD4 KO had significantly improved symptom-free survival rate (Figure 2D, Log-rank (Mantel-Cox) test, P < 0.05). There was no difference in systolic blood pressure between the two groups (Figure 2E).
Figure 2. Global knockout of PAD4 reduces the rate of aneurysm rupture.
There is no difference in the incidence of aneurysm between wild-type and PAD4 KO mice (A). There are significantly decreased aneurysm rupture rate (B) and SAH rate (C) in PAD4 KO mice as compared to wild-type control mice. A significantly increased symptom-free survival rate (D) is seen in PAD4 KO mice as compared to wild-type control mice. There is no difference in blood pressure between the two groups (E). Fisher’s exact test was used to analyze the incidences of aneurysm formation and subarachnoid hemorrhage (B and C). Log-rank (Mantel-Cox) test was used for the analysis of survival rate (D). Multiple t-test was used for blood pressure analysis (E). Data are expressed as means ± standard deviation. ∗ P < 0.05.
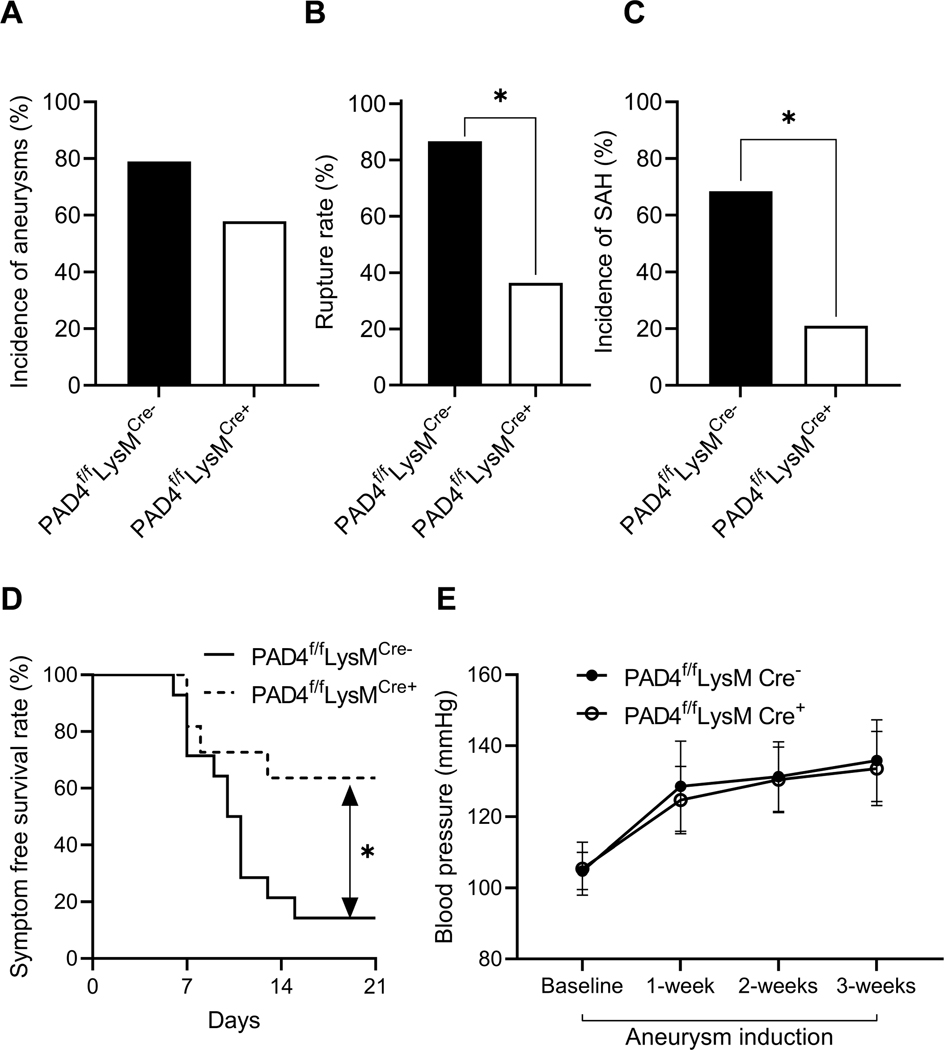
Granulocyte-specific knockout of PAD4 reduces the rate of aneurysm rupture
Since global PAD4-knockout mice experienced a significant reduction in the rate of aneurysm rupture, we investigated whether this effect was due to the loss of PAD4 expression in neutrophils. We compared the rate of aneurysm rupture between granulocyte-specific PAD4 KO mice (PAD4f/fLysMCre+) and their control littermates (PAD4f/fLysMCre-). There was no difference in the incidence of aneurysm formation between the two groups (Figure 3A). However, PAD4f/fLysMCre+ mice experienced a significant reduction in rate of aneurysm rupture compared to PAD4f/fLysMCre- littermates (Figure 3B, 87% vs. 36%, n = 15 vs. 11, PAD4f/fLysMCre- vs. PAD4f/fLysMCre+, P < 0.05). PAD4f/fLysMCre+ mice also had a significant reduction in rate of SAH compared to PAD4f/fLysMCre- littermates (Figure 3C, 68% vs. 21%, n = 19 for both, PAD4f/fLysMCre- vs. PAD4f/fLysMCre+, P < 0.05). Additionally, PAD4f/fLysMCre+ mice had significantly improved symptom-free survival rate (Figure 3D, Log-rank (Mantel-Cox) test, P < 0.05). There was no difference in systolic blood pressure between the two groups (Figure 3E).
Figure 3. Granulocyte-specific knockout of PAD4 reduces the rate of aneurysm rupture.
There is no difference in the incidence of aneurysm between flox control mice (PAD4f/fLysMCre-) and granulocyte-specific PAD4 KO (PAD4f/fLysMCre+) mice (A). There are significantly decreased aneurysm rupture rate (B) and SAH rate (C) in PAD4f/fLysMCre+ KO mice as compared to control mice. A significantly increased symptom-free survival rate (D) is seen in PAD4f/fLysMCre+ KO mice as compared to control mice. There is no difference in blood pressure between the two groups (E). Fisher’s exact test was used to analyze the incidences of aneurysm formation and subarachnoid hemorrhage (B and C). Log-rank (Mantel-Cox) test was used for the analysis of survival rate (D). Multiple t-test was used for blood pressure analysis (E). Data are expressed as means ± standard deviation. ∗ P < 0.05.
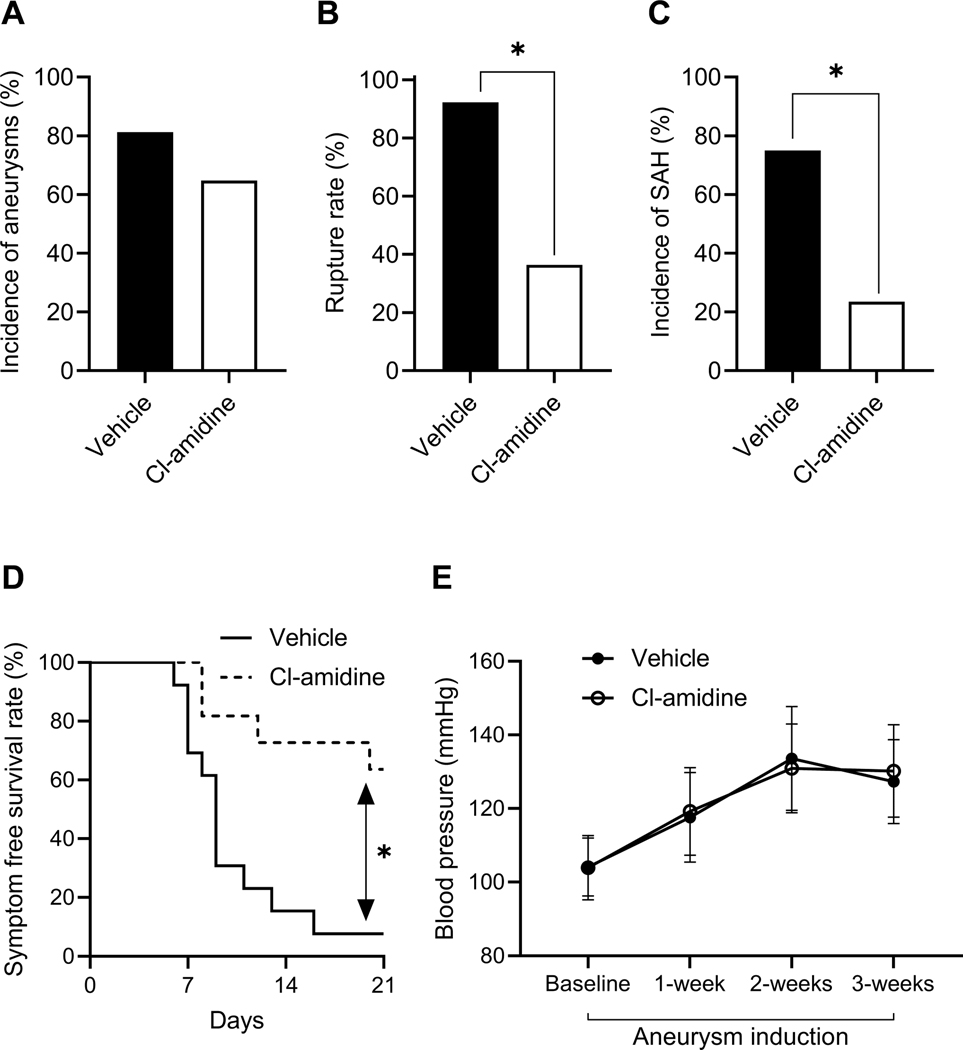
Pharmacological blockade of the NET formation by Cl-amidine reduced the rate of aneurysm rupture
As a next step, we tested the effects of pharmacological blocking of NETs on the formation and rupture of intracranial aneurysm. We used Cl-amidine to block PAD4 (an enzyme that is essential for NET formation). There was no difference in the incidence of aneurysm formation between the vehicle-treated and Cl-amidine-treated groups (Figure 4A). However, compared to vehicle-treated control mice, Cl-amidine-treated mice had a significant reduction in both the rate of aneurysm rupture (Figure 4B, 93% vs. 36%, n = 13 vs. 11, vehicle vs. Cl-amidine, P < 0.05) and the rate of SAH (Figure 4C, 75% vs. 24%, n = 16 vs. 17, vehicle vs. Cl-amidine, P < 0.05). Additionally, Cl-amidine-treated mice had significantly improved symptom-free survival rate (Figure 4D, Log-rank (Mantel-Cox) test, P < 0.05). There was no difference in systolic blood pressure between the two groups (Figure 4E)
Figure 4. Pharmacological blockade of the NET formation by Cl-amidine reduced the rate of aneurysm rupture.
There is no difference in the incidence of aneurysm between vehicle-treated control mice and CI-amidine-treated mice (A). There are significantly decreased aneurysm rupture rate (B) and SAH rate (C) in CI-amidine-treated mice as compared to vehicle-treated control mice. A significantly increased symptom-free survival rate (D) is seen in CI-amidine-treated mice as compared to control mice. There is no difference in blood pressure between the two groups (E). Fisher’s exact test was used to analyze the incidences of aneurysm formation and subarachnoid hemorrhage (B and C). Log-rank (Mantel-Cox) test was used for the analysis of survival rate (D). Multiple t-test was used for blood pressure analysis (E). Data are expressed as means ± standard deviation. ∗ P < 0.05.
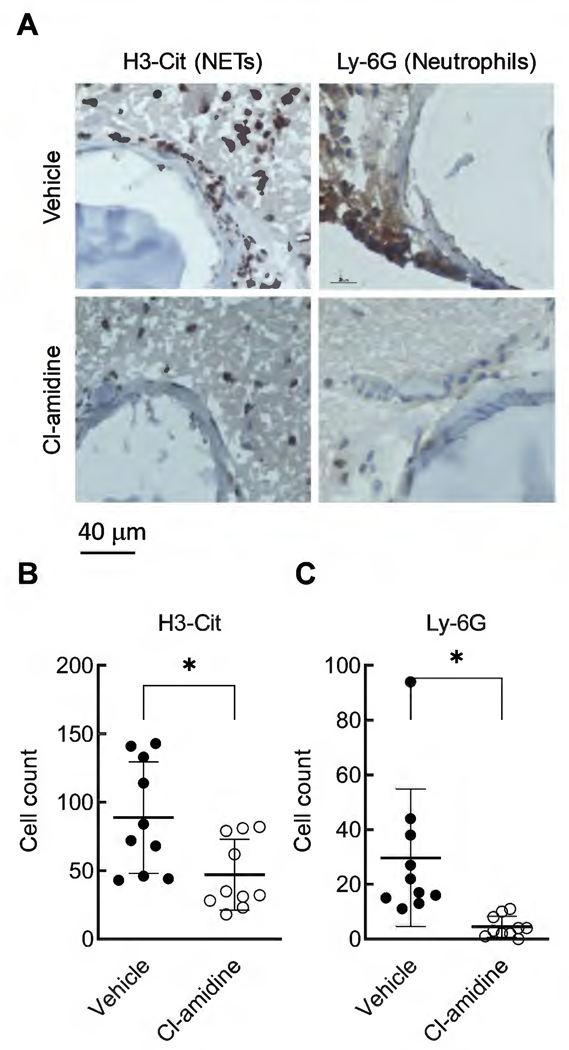
Since Cl-amidine is not specific to PAD4, we stained for H3-Cit (a NETs marker) and Ly-6G (a neutrophil marker) in cerebral arteries to confirm that Cl-amidine treatment blocked the formation of NETs. Figure 5A shows representative staining of cerebral arteries for H3-Cit and Ly-6G. We found a significant reduction in both H3-Cit- and Ly-6G-positive cells in cerebral arteries from Cl-amidine-treated mice compared to vehicle-treated mice (Figure 5B, H3-Cit: 89 ± 41 vs. 47 ± 26, n = 10 for both, vehicle vs. Cl-amidine, P < 0.05; Figure 5C, Ly-6G: 30 ± 25 vs. 4.5 ± 3.8, n = 10 for both, vehicle vs. Cl-amidine, P < 0.05)
Figure 5. Cl-amidine treatment reduced the H3-Cit and Ly-6G strained cells.
Representative images showing a clear reduction of the number of cells stained by H3-Cit (NETs marker) and Ly-6G (neutrophil marker) (A). Quantification showing that Cl-amidine treatment significantly reduced the positively stained cells in both H3-Cit (B) and Ly-6G (C) immunohistochemical staining. Data are expressed as means ± standard deviation, Student t-test, ∗ P < 0.05
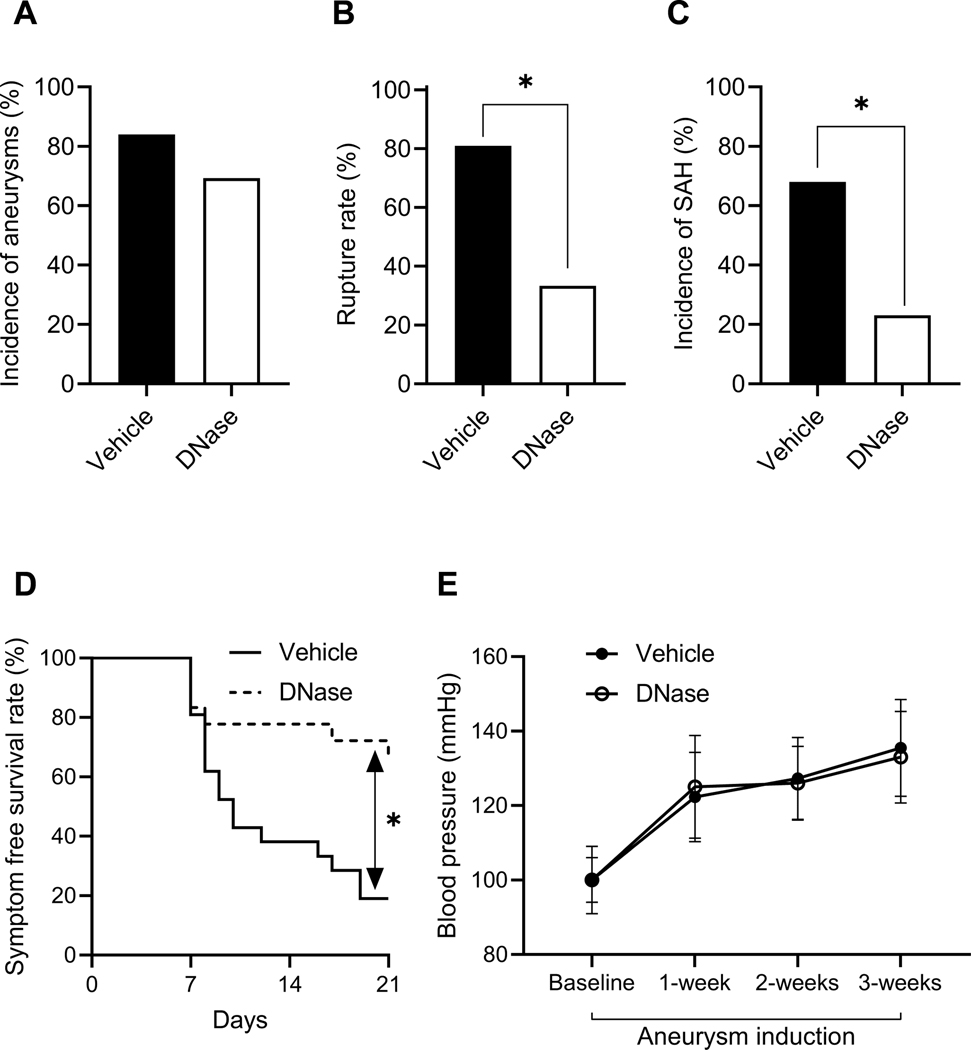
Resolution of NETs by DNase reduced the rate of aneurysm rupture
Next, we tested whether degradation of already-formed NETs would affect the rate of rupture. There was no difference in the incidence of aneurysm formation between the vehicle-treated and DNase-treated groups (Figure 6A). However, compared to vehicle-treated control mice, DNase-treated mice had a significant reduction in both the rate of aneurysm rupture (Figure 6B, 81% vs. 33%, n = 21 vs. 18, vehicle vs. DNase, P < 0.05) and the rate of SAH (Figure 6C, 68% vs. 23%, n = 25 vs. 26, vehicle vs. DNase, P < 0.05). Additionally, DNase-treated mice had significantly improved symptom-free survival rate (Figure 6D, Log-rank (Mantel-Cox) test, P < 0.05). There was no difference in systolic blood pressure between the two groups (Figure 6E).
Figure 6. The resolution of NETs by DNase reduced the rate of aneurysm rupture.
There is no difference in the incidence of aneurysm between vehicle-treated control mice and DNase-treated mice (A). However, there are significantly decreased aneurysm rupture rate (B) and SAH rate (C) in DNase-treated mice as compared to vehicle-treated control mice. A significantly increased symptom-free survival rate (D) is seen in DNase-treated mice as compared to control mice. There is no difference in blood pressure between the two groups (E). Fisher’s exact test was used to analyze the incidences of aneurysm formation and subarachnoid hemorrhage (B and C). Log-rank (Mantel-Cox) test was used for the analysis of survival rate (D). Multiple t-test was used for blood pressure analysis (E). Data are expressed as means ± standard deviation. ∗ P < 0.05.
Pharmacological inhibition of PAD4 reduced mRNA expression of pro-inflammatory cytokines in cerebral arteries
To examine the effects of blocking NET formation on inflammation in cerebral arteries, we performed real time RT-PCR to measure the mRNA expression levels of multiple genes involved in inflammation from mice treated with vehicle or Cl-amidine, a PAD4 blocker. Cl-amidine-treated mice had significantly decreased mRNA expression of ICAM-1, IL-1β, MCP-1 and TNF-α as compared to vehicle-treated controls (Figure S1, vehicle vs. Cl-amidine; ICAM-1: 1.0 ± 0.3 vs. 0.5 ± 0.16, n = 8 for both, P < 0.05; IL-1β: 1.0 ± 1.1 vs. 0.37 ± 0.23, n = 8 vs. 6, P < 0.05; MCP-1: 1.0 ± 0.51 vs. 0.44 ± 0.18, n = 7 vs. 6, P < 0.05; TNFα: 1.0 ± 0.88 vs. 0.23 ± 0.15, n = 7 for both, P < 0.05). There was no significant difference in mRNA expression of CXCL-1, E-selectin, KC or MIP-2 between vehicle-treated or Cl-amidine-treated groups (please see http://hyper.ahajournals.org).
Discussion
NETs are decorated with cytotoxic granular enzymes such as neutrophil elastase, metalloproteinases, and myeloperoxidase.7 While these proteases are essential for the antimicrobial function of NETs, they can also cause tissue damage, which leads to pro-inflammatory cytokine secretion, neutrophil recruitment, and further NET formation.11, 30 In vascular tissues, this vicious cycle may result in a state of sustained inflammation and degradation of the vascular wall, conditions which have been closely linked to the aneurysm pathophysiology.12–14 In the context of intracranial aneurysm, the formation of NETs may be triggered by endothelial damage and neutrophil activation that are triggered by hemodynamic stresses, tissue remodeling, and inflammation.
In this study, using a mouse model of intracranial aneurysm, we showed that both genetic and pharmacological disruption of NETs significantly reduced the rate of aneurysm rupture. These findings provide support for the link between NETs and aneurysm rupture that has been suggested by clinical studies.
We found that the global knockout of PAD4 significantly reduced the rate of rupture. PAD4 initiates the process of NET formation by catalyzing histone citrullination and chromatin decondensation. Genetic deletion of PAD4 genes has previously been shown to block the formation of NETs in mice.9, 28, 29 To confirm that the reduction in rupture rate was due to loss of PAD4 in neutrophils, we used granulocyte-specific PAD4 knockout mice (PAD4f/fLysMCre+), which lack PAD4 specifically in neutrophils and other myeloid lineage cells. Indeed, PAD4f/fLysMCre+ mice experienced significant reductions in rupture rate, confirming that NET formation promotes aneurysm rupture.
In addition to the genetic deletion of PAD4, we employed two distinct pharmacological approaches of eliminating NETs. First, we treated mice with Cl-amidine, which prevents NET formation by inhibition of PAD4. Treatment with Cl-amidine significantly reduced the rate of aneurysm rupture. Moreover, we confirmed that Cl-amidine treatment blocked NET formation and neutrophil infiltration in cerebral arteries. The blocking of NET formation by Cl-amidine resulting in the reduction of aneurysm wall inflammation may be the underlying mechanism that prevents further neutrophil activation and infiltration. Additionally, we examined whether degradation of NETs after their formation would have similar efficacy in the prevention of aneurysm rupture. Indeed, treatment with DNase significantly reduced the rate of rupture. DNase treatment represents a clinically-relevant means of targeting NETs for the prevention of aneurysm rupture, wherein patients would begin treatment after the diagnosis of an unruptured aneurysm. Our data show that interrupting pre-established NETs is sufficient to reduce the rate of aneurysm rupture.
In vascular tissues, NETs promote sustained inflammation and degradation of the vascular wall, conditions which are commonly associated with the pathophysiology of intracranial aneurysm.12–14 Therefore, we examined the effects of eliminating NETs on inflammation in cerebral arteries. We found that Cl-amidine-treated groups had significantly reduced expression of ICAM-1, IL-1β, MCP-1, and TNF-α in cerebral arteries. These data suggest that pharmacological prevention of NET formation may prevent the rupture of intracranial aneurysm by attenuating vascular inflammation.
This study has several limitations. First, the animal model may not completely replicate biological events that lead to aneurysm formation and growth, as aneurysms were induced, rather than spontaneously formed. While many studies indicated the critical roles of vascular inflammation in the pathophysiology of intracranial aneurysms, there may be significant differences in the triggering factors of vascular inflammation between human aneurysms and this model. In addition, the time course of aneurysmal formation and rupture in this model is shorter than that of the human aneurysm. However, the phenotypes of intracranial aneurysms in the model closely mimic that of intracranial aneurysms in humans.20, 21 More importantly, human intracranial aneurysms and aneurysms in this model share the end phenotypes, aneurysmal rupture, and associated neurological symptoms, further indicating the biological similarities between human intracranial aneurysms and this mouse model of intracranial aneurysm.21, 23
In our immunohistochemical analyses, we found MPO within the proximity of NETs. Both NET itself and MPO are pro-inflammatory and can damage the aneurysm wall. Thus, it is likely that naked DNAs of NETs and accompanying neutrophil enzymes such as MPO synergistically promote the development of aneurysmal rupture. Although the images, to a reasonable degree, showing the overlapping staining of NETs marker (H3Cit) with MPO, it would be the best option to perform additional double or even triple immunofluorescence staining of the molecular/cell markers. The high-magnification picture of hematoxylin and eosin staining does show the presence of many polymorphonuclear cells (presumably neutrophils) in the same area where NETs and MPO overlap. However, we cannot exclude the possibility that other inflammatory cells may involve in NETs formation. Indeed, recent reports show that macrophage, mast cells, and basophils can form NET-like structures.31–35 This point remains a limitation of our study.
Our previous study suggested that the gut microbiota may contribute to the disease course of intracranial aneurysms by modulating inflammation.14 While there are no reports that Cl-amidine or DNase directly impact the gut microbiota, it is possible that loss of NETs and/or drug treatment itself could affect the gut microbiota, and contribute to the observed differences in rupture rate. This may warrant further investigation in future studies
Another limitation of this study is that our genetic deletion of PAD4 is not entirely neutrophil-specific, as LysM-Cre mice express Cre recombinase in all myeloid lineage cells. Therefore, the observed effects could be influenced by the deletion of PAD4 in other cell types, such as monocytes and macrophages. Future studies using Cx3cr1-Cre mice, which have been shown to delete floxed genes in monocytes, macrophages, and mast cells, but not in neutrophils, may be employed to exclude the potential contributions of PAD4 expression in these non-neutrophil populations. It should also be considered that Cl-amidine is not specific to PAD4 and may inhibit other PADs, even though it has been used widely for PAD4 inhibition.36 A specific PAD4 inhibitor may be used in future studies to rule out the influence of other PADs.28 Furthermore, as NET formation may involve multiple neutrophil enzymes including neutrophil elastase, myeloperoxidase, and gasdermin D, future studies should explore the pharmacological manipulation of these enzymes as additional therapeutic approaches.
Finally, we used only male mice in this study. Our previous studies showed that the incidence of aneurysm formation and rupture rates are higher in ovariectomized female mice than male mice and sham-ovariectomized female mice, indicating protective effects of estrogen against the formation and rupture of intracranial aneurysms.24, 37 The baseline differences in the incidence of aneurysm formations and aneurysmal ruptures among male, non-ovariectomized female, and ovariectomized female mice make the inclusion and comparison of these groups and the experimental design far more complex. While this proof-of-concept study started with experiments using male animals, roles of sex differences should be carefully examined in future studies.
Perspectives
This study suggests that NETs promote the rupture of intracranial aneurysm. Pharmacological removal of NETs, by inhibition of PAD4 or resolution of already-formed NETs, may represent a potential therapeutic strategy for preventing aneurysmal rupture. Further studies are warranted to firmly establish the causal role of NETs in the rupture of intracranial aneurysm.
Supplementary Material
Novelty and Significance.
1. What Is New?
We discovered a molecular complex called neutrophil extracellular traps (NETs), may be a novel viable target for preventing aneurysm rupture at the base of the brain.
We proved this with both genetic and pharmacological manipulations in a mouse model of intracranial aneurysm (a berry-like structure on the arteries at the base of the brain).
2. What Is Relevant?
High blood pressure has been shown to play an important role in the formation and rupture of intracranial aneurysms.
Currently, there is no clinical treatment available for this devastating disease.
3. Summary - of the conclusions of the study.
Genetically knockout a key enzyme (PAD4) that is essential for NET formation significantly reduced the rupture rate of aneurysms.
Both pharmacological inhibition of NETs formation with Cl-amidine or disruption of already formed NETs with DNase significantly decreased the rupture rate of aneurysms.
Pharmacological removal of NETs, by inhibition of PAD4 or resolution of already-formed NETs, may represent a potential therapeutic strategy for preventing aneurysmal rupture.
Acknowledgments
Sources of funding
The project was supported by grant number R01NS082280 (TH), R01NS109382 (TH, SE), and R01NS109584 (TH) from the National Institute of Neurological Disorders and Stroke (NIH/NINDS); Cami Clark Chair of Research and Fight Like Frank Chair of Research (HS), Cami Clark Chair of Research (JP), and Shirley Dudek Demmer Chair of Research and the Taylor Richelson Chair of Research (JA) from the Brain Aneurysm Foundation; and Barrow Neurological Foundation (JA, TH). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Footnotes
Disclosures
None.
References
- 1.Baker CJ, Fiore A, Connolly ES Jr., Baker KZ, Solomon RA. Serum elastase and alpha-1-antitrypsin levels in patients with ruptured and unruptured cerebral aneurysms. Neurosurgery. 1995;37:56–61; discussion 61–52 [DOI] [PubMed] [Google Scholar]
- 2.Connolly ES Jr., , Fiore AJ, Winfree CJ, Prestigiacoma CJ, Goldman JE, Solomon RA. Elastin degradation in the superficial temporal arteries of patients with intracranial aneurysms reflects changes in plasma elastase. Neurosurgery. 1997;40:903–908; discussion 908–909 [DOI] [PubMed] [Google Scholar]
- 3.Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke. 1999;30:1396–1401 [DOI] [PubMed] [Google Scholar]
- 4.Frosen J, Piippo A, Paetau A, Kangasniemi M, Niemela M, Hernesniemi J, Jaaskelainen J. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: Histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35:2287–2293 [DOI] [PubMed] [Google Scholar]
- 5.Tulamo R, Frosen J, Junnikkala S, Paetau A, Pitkaniemi J, Kangasniemi M, Niemela M, Jaaskelainen J, Jokitalo E, Karatas A, Hernesniemi J, Meri S. Complement activation associates with saccular cerebral artery aneurysm wall degeneration and rupture. Neurosurgery. 2006;59:1069–1076; discussion 1076–1067 [DOI] [PubMed] [Google Scholar]
- 6.Gounis MJ, Vedantham S, Weaver JP, Puri AS, Brooks CS, Wakhloo AK, Bogdanov AA, Jr. Myeloperoxidase in human intracranial aneurysms: Preliminary evidence. Stroke. 2014;45:1474–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535 [DOI] [PubMed] [Google Scholar]
- 8.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: Netosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorensen OE, Borregaard N. Neutrophil extracellular traps - the dark side of neutrophils. J Clin Invest. 2016;126:1612–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120:736–743 [DOI] [PubMed] [Google Scholar]
- 11.Sayah DM, Mallavia B, Liu F, Ortiz-Munoz G, Caudrillier A, DerHovanessian A, Ross DJ, Lynch JP 3rd, Saggar R, Ardehali A, Lung Transplant Outcomes Group I, Ware LB, Christie JD, Belperio JA, Looney MR. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2015;191:455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamio Y, Miyamoto T, Kimura T, Mitsui K, Furukawa H, Zhang D, Yokosuka K, Korai M, Kudo D, Lukas RJ, Lawton MT, Hashimoto T. Roles of nicotine in the development of intracranial aneurysm rupture. Stroke. 2018;49:2445–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitsui K, Ikedo T, Kamio Y, Furukawa H, Lawton MT, Hashimoto T. Tlr4 (toll-like receptor 4) mediates the development of intracranial aneurysm rupture. Hypertension. 2019:HYPERTENSIONAHA11812595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shikata F, Shimada K, Sato H, Ikedo T, Kuwabara A, Furukawa H, Korai M, Kotoda M, Yokosuka K, Makino H, Ziegler EA, Kudo D, Lawton MT, Hashimoto T. Potential influences of gut microbiota on the formation of intracranial aneurysm. Hypertension. 2019;73:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willis VC, Banda NK, Cordova KN, Chandra PE, Robinson WH, Cooper DC, Lugo D, Mehta G, Taylor S, Tak PP, Prinjha RK, Lewis HD, Holers VM. Protein arginine deiminase 4 inhibition is sufficient for the amelioration of collagen-induced arthritis. Clin Exp Immunol. 2017;188:263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knight JS, Zhao W, Luo W, Subramanian V, O’Dell AA, Yalavarthi S, Hodgin JB, Eitzman DT, Thompson PR, Kaplan MJ. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013;123:2981–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chumanevich AA, Causey CP, Knuckley BA, Jones JE, Poudyal D, Chumanevich AP, Davis T, Matesic LE, Thompson PR, Hofseth LJ. Suppression of colitis in mice by cl-amidine: A novel peptidylarginine deiminase inhibitor. Am J Physiol Gastrointest Liver Physiol. 2011;300:G929–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinod K, Fuchs TA, Zitomersky NL, Wong SL, Demers M, Gallant M, Wang Y, Wagner DD. Pad4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood. 2015;125:1948–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, Toy P, Werb Z, Looney MR. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N, Lawton MT, Young WL, Liang EI, Nuki Y, Hashimoto T. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makino H, Tada Y, Wada K, Liang EI, Chang M, Mobashery S, Kanematsu Y, Kurihara C, Palova E, Kanematsu M, Kitazato K, Hashimoto T. Pharmacological stabilization of intracranial aneurysms in mice: A feasibility study. Stroke. 2012;43:2450–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tada Y, Wada K, Shimada K, Makino H, Liang EI, Murakami S, Kudo M, Kitazato KT, Nagahiro S, Hashimoto T. Roles of hypertension in the rupture of intracranial aneurysms. Stroke. 2014;45:579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tada Y, Wada K, Shimada K, Makino H, Liang EI, Murakami S, Kudo M, Shikata F, Pena Silva RA, Kitazato KT, Hasan DM, Kanematsu Y, Nagahiro S, Hashimoto T. Estrogen protects against intracranial aneurysm rupture in ovariectomized mice. Hypertension. 2014;63:1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savchenko AS, Borissoff JI, Martinod K, De Meyer SF, Gallant M, Erpenbeck L, Brill A, Wang Y, Wagner DD. Vwf-mediated leukocyte recruitment with chromatin decondensation by pad4 increases myocardial ischemia/reperfusion injury in mice. Blood. 2014;123:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimada K, Furukawa H, Wada K, Wei Y, Tada Y, Kuwabara A, Shikata F, Kanematsu Y, Lawton MT, Kitazato KT, Nagahiro S, Hashimoto T. Angiotensin-(1–7) protects against the development of aneurysmal subarachnoid hemorrhage in mice. J Cereb Blood Flow Metab. 2015;35:1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD, Bicker KL, Bingham RP, Campbell M, Chen YH, Chung CW, Craggs PD, Davis RP, Eberhard D, Joberty G, Lind KE, Locke K, Maller C, Martinod K, Patten C, Polyakova O, Rise CE, Rudiger M, Sheppard RJ, Slade DJ, Thomas P, Thorpe J, Yao G, Drewes G, Wagner DD, Thompson PR, Prinjha RK, Wilson DM. Inhibition of pad4 activity is sufficient to disrupt mouse and human net formation. Nat Chem Biol. 2015;11:189–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, Hu J, Wang Y, Wagner DD. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110:8674–8679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yipp BG, Kubes P. Netosis: How vital is it? Blood. 2013;122:2784–2794 [DOI] [PubMed] [Google Scholar]
- 31.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080 [DOI] [PubMed] [Google Scholar]
- 32.Chow OA, von Kockritz-Blickwede M, Bright AT, Hensler ME, Zinkernagel AS, Cogen AL, Gallo RL, Monestier M, Wang Y, Glass CK, Nizet V. Statins enhance formation of phagocyte extracellular traps. Cell host & microbe. 2010;8:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aulik NA, Hellenbrand KM, Czuprynski CJ. Mannheimia haemolytica and its leukotoxin cause macrophage extracellular trap formation by bovine macrophages. Infect Immun. 2012;80:1923–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Boer OJ, Li X, Teeling P, Mackaay C, Ploegmakers HJ, van der Loos CM, Daemen MJ, de Winter RJ, van der Wal AC. Neutrophils, neutrophil extracellular traps and interleukin-17 associate with the organisation of thrombi in acute myocardial infarction. Thromb Haemost. 2013;109:290–297 [DOI] [PubMed] [Google Scholar]
- 35.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175 [DOI] [PubMed] [Google Scholar]
- 36.Causey CP, Jones JE, Slack JL, Kamei D, Jones LE, Subramanian V, Knuckley B, Ebrahimi P, Chumanevich AA, Luo Y, Hashimoto H, Sato M, Hofseth LJ, Thompson PR. The development of n-alpha-(2-carboxyl)benzoyl-n(5)-(2-fluoro-1-iminoethyl)-l-ornithine amide (o-f-amidine) and n-alpha-(2-carboxyl)benzoyl-n(5)-(2-chloro-1-iminoethyl)-l-ornithine amide (o-cl-amidine) as second generation protein arginine deiminase (pad) inhibitors. J Med Chem. 2011;54:6919–6935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tada Y, Makino H, Furukawa H, Shimada K, Wada K, Liang EI, Murakami S, Kudo M, Kung DK, Hasan DM, Kitazato KT, Nagahiro S, Lawton MT, Hashimoto T. Roles of estrogen in the formation of intracranial aneurysms in ovariectomized female mice. Neurosurgery. 2014;75:690–695; discussion 695 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.