Abstract
The GL261 cell line, syngeneic on the C57BL/6 background, has, since its establishment half a century ago in 1970, become the most commonly used immunocompetent murine model of glioblastoma. As immunotherapy has entered the mainstream of clinical discourse in the past decade, this model has proved its worth as a formidable opponent against various immunotherapeutic combinations. Although advances in surgical, radiological, and chemotherapeutic interventions have extended mean glioblastoma patient survival by several months, 5-year survival postdiagnosis remains below 5%. Immunotherapeutic interventions, such as the ones explored in the murine GL261 model, may prove beneficial for patients with glioblastoma. However, even common immunotherapeutic interventions in the GL261 model still have unclear efficacy, with wildly discrepant conclusions being made in the literature regarding this topic. Here, we focus on anti-PD-1 checkpoint blockade monotherapy as an example of this pattern. We contend that a fine-grained analysis of how biological variables (age, sex, tumor location, etc.) predict treatment responsiveness in this preclinical model will better enable researchers to identify glioblastoma patients most likely to benefit from checkpoint blockade immunotherapy moving forward.
Keywords: Anti-PD-1, GBM, GL261
Key Points.
Anti-PD-1 monotherapy has equivocal efficacy in the GL261 model.
Known but under-referenced factors impact murine survival in the GL261 model.
Glioblastoma is a devastating malignancy with a median survival of 12–18 months postdiagnosis.1–3 Even this brief window of survival is hard-won, requiring a full standard of care upon diagnosis, this includes maximum safe surgical resection, radiotherapy, and chemotherapy by temozolomide.4 While this combination of treatments (the “Stupp Protocol”) is a significant improvement over previous expectations for glioblastoma patients,5,6 the prognosis remains bleak and there exists a clear need for improved therapeutic options.
Increasing numbers of immunotherapy trials are entering the clinic for various cancers,7 including multiple different immunotherapy regimens attempted for cancers of the CNS.8,9 Immunotherapy is a promising avenue for the treatment of brain tumors, as immune cells can cross into the brain and occupy tumors therein10 whereas many conventional treatment strategies are confounded by the protective blood–brain barrier.11 The development of these immunotherapies for GBM has benefited enormously from the abundance of immunocompetent murine models of glioblastoma.12 Among the most commonly used of these is the GL261 model13 that shares a number of significant parallels with human glioblastoma.14–21 The extensively characterized12,13,22 GL261 cell line has been repeatedly used in murine survival studies for various immunotherapeutic interventions, but the efficacy of many of these therapies remains controversial. The present work will focus on a single example of this, unpacking the literature to determine why anti-PD-1 checkpoint blockade immunotherapy (CBI) monotherapy, a highly published, flagship immunotherapy agent, has unclear efficacy in the GL261 model in which it has been so repeatedly tested.
We contend that disparate outcomes in publications treating the GL261 model glioma with anti-PD-1 monotherapy are driven by the broad spread of experimental parameters between studies. Furthermore, we will argue that the influence of many of these factors is already known but underappreciated. The systematic study of how preclinical biological variables influence the survival of these animals is a potentially untapped resource that could impact the field’s ability to better predict patient outcomes in future immunotherapy trials. Minimally, these variables ought to be duly considered during the experimental design process and, where necessary, controlled for. In summary, this review will lay forth and evaluate the evidence supporting each of a number of experimental parameters as potential drivers of the discord in reported survival times of GL261-bearing mice treated with anti-PD-1 monotherapy.
Rationale
The necessity of this report can be appreciated in comparing these 2 sentences from research publications in 2019:
PD-1 antibody therapy in GL261 resulted in almost half of the animals with long-term survival, which is consistent with previously published findings.—Jahan et al.23
GL261 tumors are intrinsically unresponsive to anti-PD-1 therapy & All untreated mice succumbed to their disease prior to Day 21, and no animals survived beyond Day 24 in the groups receiving [anti-PD-1 therapy] indicating no significant survival benefit was conferred.—Kim et al.24
Obviously, these absolutist statements on anti-PD-1 antibody’s therapeutic efficacy cannot both be true. Some nuance must exist that would permit such discrepant outcomes in the same model system. The jarring dissonance in the literature regarding anti-PD-1 therapy in the GL261 model, which stretches far beyond the 2 publications quoted above, has been previously noted.25 In that review, the differences in outcome were largely attributed to varying frequency and dose of anti-PD-1 administration.25 We view this attribution as insufficiently broad, given the wealth of published knowledge implicating many other experimental variables as well. We aim to expand this conversation and show that anti-PD-1 CBI monotherapy is not simply effective or simply ineffective against the GL261 glioma model but that its efficacy is influenced by myriad factors, far beyond just dosing, which need to be both appropriately accounted for and clearly communicated with the research community. With a solidified understanding of why study outcomes are so disparate, the abundance of preclinical data in the GL261 model can help guide the way to more nuanced personalization of immunotherapies for patients.
Approach
We have observed a wide range of published survival times for anti-PD-1-treated GL261-bearing mice and wanted to obtain an unbiased sample of such studies to examine more deeply. At the time of writing, 29 publications were retrieved by the PubMed “All Fields” search term “(PD-1) AND (GL261).” One of these was excluded for being a Review. Our focus is survival outcomes of anti-PD-1 CBI monotherapy for C57BL/6 mice bearing orthotopic GL261 gliomas, so studies that lacked such trial arms (eg, those only using combination therapies26 or not conducting GL261 survival studies27) were also excluded. The remaining 16 research papers are explored in detail below23,24,28–41 (Table 1). This search engine-based sample of the literature does not include all studies that explore outcomes of GL261-bearing mice treated with anti-PD-1 monotherapy42 but, rather, provides an approachable number of studies in order to showcase common experimental methods in the field, preserve brevity, and prevent the incorporation of author bias. This scope has prevented us from recognizing the work of many of our talented colleagues and we apologize to those whose relevant studies were unable to include. The variables selected for Table 1 are the ones that have been linked, in a direct or indirect fashion, to the survival of GL261-bearing mice treated with anti-PD-1 monotherapy and each variable will be discussed in greater detail below. In the interest of full transparency, the nature of the control arms of each study has also been included in Table 1. While the sham exposure of the control arm cannot change the absolute survival time or percentage of the anti-PD-1-treated experimental arm, different control set-ups could potentially impact relative statements of treatment efficacy. It is worth noting, however, that variations in the survival of control-treated animals between studies are more likely reflective of the differences in other biological variables like age or tumor inoculum than differences between the types of control interventions. Two of the most common control interventions among our studies (phosphate buffered saline [PBS] and isotype IgG) have been shown to have an indistinguishable effect on murine survival.33 The strength of evidence linking each variable to the modulation of survival outcomes in this context is color-coded in Figure 1 for easy reference. We hope that assessing the contribution of each variable in a systematic way demystifies some of the enormous spread of murine survival appreciable in Table 1 and in the literature more broadly.
Table 1.
Significant Characteristics of Selected Manuscripts
| Group | First Author; Year | Anti-PD-1 (µg/dose); Route | Dosing (days after tumor innoculation) | Anti-PD-1 Clone; Vendor | Tumor Inoculation (# cells; volume; depth) | Mice (sex; weeks old; vendor) | % LTS | Median Survival (Sham; anti-PD1) (days) | Ctrl. |
|---|---|---|---|---|---|---|---|---|---|
| A | Belcaid; 2020 | 200 µg/dose; I.P. | 7, 9, 11 | G4; PIH | 50 000 GL261; NA; 3 mm | F; 6–8; Harlan | 0% | (NA; 22.5) | IgG |
| A | Dai; 2018 | 200 µg/dose; I.P. | 2, 4, 6b | RMP1-14; BioXCell | 50 000 GL261; 5 µL; 1.5 mm | M; 6–8; CMCYU | 0% | NA | Saline |
| A | Galstyan; 2019 | 200 µga/dose; I.V. | 8, 13, 16, 20, 23, 27 | J43; BioXCell | 20 000 GL261; 2 µL; NA | F; 8; JL | 0% | NA | PBS |
| A | Kim; 2019 | 200 µg/dose; I.P. | 8, 13, 16, 29, 23, 26 | RMP1-14; BioXCell | 200 000 GL261; 3 µL; 3.5 mm | F; 6; CRL | 0% | (19; 21) | Untreated |
| B | Hardcastle; 2017 | 200 µg/dose; I.P. | 6, 8, 14 | RMP1-14; BioLegend | 300 000 GL261; NA; NA | NA; NA; CRL | 0% | NA | Untreated |
| B | Lamano; 2019 | 250 µg/dose; I.P. | 7, 10, 13, 16, 19, 22, 25, 28 | RMP1-14; BioXCell | 300 000 Gl261; 2 µL; 3 mm | NA; 7–8; JL | 0% | (19; 30.5) | IgG |
| B | Shevtsov; 2019 | 250 µg/dose; I.P. | 6, 9, 12, 15 | RMP1-30; eBioscience | 100 000 GL261; 2 µL; 3 mm | M; 8–10; RAMS | 0% | NA | PBS/IgG |
| B | Zeng; 2013 | 200 µga/dose; I.P. | 10, 12, 14 | G4; PIH | 130 000 Gl261-Luc; 1 µL; 3 mm | F; 6–8; JL | 0% | (26; 30) | IgG |
| C | Dejaegher; 2017 | 200 µg/dose; I.P. | 5, 10, 15 | RMP1-14c; PIH | 500 000 GL261; 10 µL; 3m m | F; 8–10; Envigo | 55% | (24.5; NA) | IgG |
| C | Hung; 2018 | 200 µg/dose; I.P. | 10, 12, 14 | 4 H2; Bristol-Myers Squibb | 130 000 GL261-Luc; 1 µL; 3 mm | F; 6–8; JL | 14.3% | (25; 34) | Untreated |
| C | Jahan; 2019 | 200 µg/dose; I.P. | 3, 6, 9 | RMP1-14; BioXCell | 75 000 GL261; 3 µL; 2.5 mm | F; 6–7; NCI | 20% | NA | IgG |
| C | Karachi; 2019 | 200 µga/dose; I.P. | 7, 12, 17, 22b | RMP1-14; BioXCell | NA; NA; 4 mm | NA; NA; JL | 50% | NA | Untreated |
| C | Kim; 2017 | 200 µg/dose; NA | 10, 12, 14 | G4; PIH | 130 000 GL261-Luc; 1 µL; 3 mm | F; 6–8; JHUAF | 27.8% | (22; 33) | Unspec. |
| C | Mathios; 2016 | 200 µg/dose; I.P. | 10, 12, 14 | G4; PIH | 130 000 GL261-Luc; 1 µL; 3 mm | F; 6–8; JL | 20% | (16; 25.5) | PBS |
| C | Speranza; 2018 | 200 µg/dose; I.P. | 10, 13, 16, 19 | 29F.1A12; PIH | 100 000 GL261-Luc; 5 µL; 3 mm | F; 6–8; Envigo | 50% | NA | Unspec. |
| C | Wu; 2019 | 200 µg/dose; I.P. | 10, 12, 14 | NA; Bristol-Myers Squibb | 130 000 GL261-Luc; 1 µL; 3 mm | F; 6–8; JL | 30% | (24; 30) | Untreated |
I.P., intraperitoneal; I.V., intravenous; NA, not applicable/data not provided in manuscript; PIH, antibodies that are “Produced In House” from a cultured hybridoma; CMCYU, Comparative Medical Center of Yangzhou University; JL, The Jackson Laboratory; CRL, Charles River Laboratories; RAMS, Russian Academy of Medical Sciences; JHUAF, Johns Hopkins University Animal Facility; LTS, long-term survivors; Ctrl., nature of control group; Unspec., the control group of unspecified nature.
Experimental parameters of the selected studies are listed. For ease of comparison, each publication has been assigned to a group based on the survival benefit the authors declared anti-PD-1 CBI monotherapy to provide for GL261 glioma-bearing animals. The groups are as follows: (A) No increase in survival as a result of therapeutic intervention, (B) some increase in survival but no long-term survivors, and (C) a survival benefit and some number of long-term survivors following therapy. Within groups, manuscripts are arranged alphabetically by the last name of the first author.
aDemarcates doses of anti-PD-1 that were written as 10 mg/kg in the study in question. For ease of comparison with studies that administered a consistent amount (µg) of anti-PD-1 regardless of mouse weight, these scaled doses have been standardized assuming a weight of 20 g, which would not be unusual for a young female C57BL/6 mouse.
bDemarcates a dosing timecourse where significant ambiguity existed in the original manuscript and we were forced to draw an inference regarding the methods.
cThis manuscript listed the clone used as “RPMI-14,” but, as no corroborating evidence as to the existence of this clone could be found, we have made the assumption that the authors intended to write “RMP1-14” which is a commonly used clone of anti-PD-1 antibody.
Figure 1.
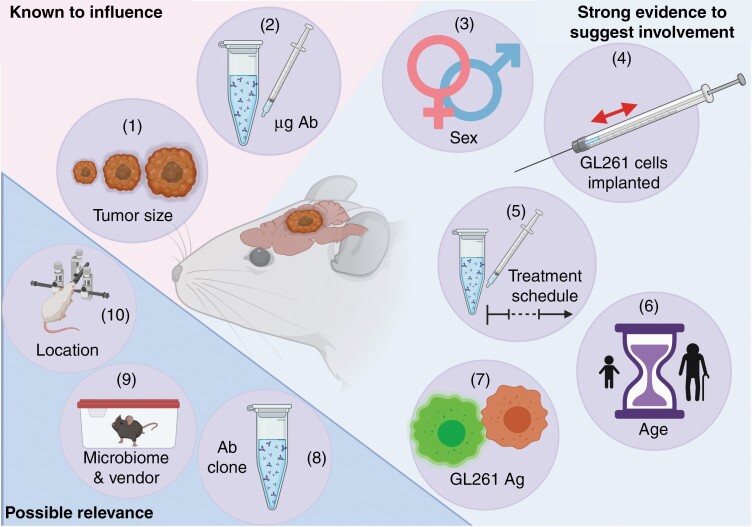
Factors known to, or likely to, impact survival of GL261-bearing mice treated with anti-PD-1 monotherapy categorized by strength of supporting evidence. Many experimental variables that fluctuate across experiments investigating the survival benefit of anti-PD-1 monotherapy in the GL261 model are known to, or suspected of, modulating murine survival time. The factors for which there is evidence of direct modulation of survival time in anti-PD-1-treated GL261-bearing animals include the tumor size at the time of treatment (1) and the dosage of delivered antibody (2). This direct evidential support is indicated by the pink background. The pale blue background includes variables that are known to alter the survival of GL261-bearing animals and could, presumably, also do so in the context of anti-PD-1 monotherapy. These include the sex of the treated animals (3), the number of GL261 cells inoculated (4), the schedule with which the checkpoint blockade agent is administered (5), the age of mice at the time of tumor inoculation (6), and the antigenic potential of the GL261 cell line used (7). Experimental variables for which indirect evidence in related models suggests a possible relation to the survival of anti-PD-1-treated GL261-bearing animals are on a darker blue background. These factors include the clone of anti-PD-1 antibody used (8), the source of the mice and their accompanying microbiome (9), the coordinates at which the tumor is implanted (10).
Variables Relating to the Anti-PD-1 Antibody
The dosing strategy of anti-PD-1 antibody has been suggested as a pivotal determinant in the variations in survival outcomes observed in the GL261 model25 and so we chose to begin our investigations there (Table 1). More specifically, that review stated based on examining 2 manuscripts34,43 that “In the preclinical GL261 model, the success of anti-PD-1 monotherapy is dependent on antibody dosage levels.” 25 With Table 1 we can assess this statement in light of our broad selection of preclinical GL261 studies and observe that, with few exceptions,30,38 the anti-PD-1 antibody was delivered intraperitoneally and the doses, after those given in mg/kg,30,34,37 are put in the context of an average mouse’s weight, all hovered around 200 µg (Table 1). The success of the monotherapeutic intervention clearly varied dramatically between studies but the antibody dosage did not, suggesting that other variables are contributing to differential murine survival. That being said, antibody dose certainly has some role to play. It is known that in the GL261 model, all else held equal, very low doses of anti-PD-1 monotherapy (eg, 100 µg/day) have minimal impact on mouse survival in conditions where a very high dose (eg, 500 µg/day) has therapeutic efficacy.44 Thus, the notion that the success of anti-PD-1 monotherapy is dosage dependent25 appears to be a necessary but not a sufficient relationship to ensure therapeutic success in the GL261 model. Dose alone (µg antibody delivered), which hardly changes between studies, fails to explain the variability in Table 1.
While the amount of anti-PD-1 antibody delivered per dose may vary little across Table 1, significant variation between studies was observed in the schedule with which anti-PD-1 is administered (Table 1). The most prevalent day for initiation of therapy is day 10 after tumor inoculation (6/16; 37.5%), with the range spreading from day 2 to day 10. Other immunotherapeutic interventions in the GL261 model have shown great dependency upon time of therapy initiation45–47; anti-CTLA-4 monotherapy, for instance, had indistinguishable efficacy from a control IgG when given to GL261-bearing mice 12 days postinoculation, while it had a statistically significant impact on murine survival when given 3 days postinoculation.45 To the best of our knowledge, the direct association between earlier treatment and better outcomes in the GL261 model has yet to be demonstrated for anti-PD-1 CBI. What has been demonstrated, however, is the link between GL261 tumor volume at the time of anti-PD-1 administration and the survival outcome.44 Knowing that GL261 tumor volume is a function of time,48 it seems prudent to assume that increased time after tumor inoculation could influence survival outcome following anti-PD-1 CBI monotherapy until the contrary has been established. This presumed association is impossible to determine from a retrospective literature review because many other variables beyond the altered date of therapy initiation fluctuate between these studies (Table 1). Accordingly, there is no stark delineation between early treatment (eg, day 2 postinoculation29) and late treatment (eg, day 10 postinoculation34) clearly driving differential survival (Table 1). That being said, the links that have been forged between GL261 tumor volume and time, and between tumor volume and treatment resistance, lead us to suggest that CBI studies, anti-PD-1 therapy included, in the GL261 model attempt both an “early” and a “late” time-point therapy initiation. This would not only be enormously beneficial in determining the extent to which early treatment of this model glioblastoma is a determinant of survival outcome, but also do significant good in moving preclinical practices closer to clinical reality, as many of the presenting symptoms leading to the diagnosis and treatment of glioblastoma are those accompanying late-stage disease.1
Another antibody-related factor beyond dosing strategy that could contribute to the variable outcomes of anti-PD-1-treated GL261-bearing animals is the potential for non-equivalencies in various anti-PD-1 clones used for preclinical work, a possibility which has remained underexplored. Intriguing new data demonstrates the variable impact of anti-PD-1 clones routinely utilized in murine preclinical studies.49 Particularly, this work shows the Armenian Hamster IgG G4 clone to promote depletion of PD-1+ T cells in comparison to the Rat IgG2a RMP1-14 clone49 (these clones were used in 4/16 and 7/16 of the studies discussed here, respectively; Table 1). Furthermore, there is evidence to suggest that the RMP1-30 clone, used in one of the 16 studies explored here,33 does not actually block the interaction of the PD-1 molecule with PD-L1 and PD-L2 like the J43 and RMP1-14 clones do.50 While we do not know the impact of either of those findings in the context of CBI for the GL261 glioma model, the potential intrinsic differences between the various clones are certainly worth further investigation as a potential contributor to the mixed preclinical data on CBI in GL261, along with dose differences, timing of administration, and route of administration.
Variables Relating to the Intracranial Inoculation of GL261 Cells
When considering disparate outcomes of CBI in an implantable tumor model, there are multitudinous variables that contribute to the actual process of inoculation. Some of these factors related to GL261 inoculation are already known to contribute to varying survival in the context of anti-PD-1 CBI and some have a more indirect connection that merits further investigation. It has been established that higher cell counts for the initial tumor inoculum can shorten the survival of mice bearing GL261 gliomas.15 Accordingly, comparing the efficacy of anti-PD-1 CBI in the GL261 model when starting tumor load can vary by an order of magnitude or more across studies is potentially confounded. In the 16 papers examined, a range of GL261 inoculum from 20 000 cells30 to 500 000 cells35 was observed, with a plurality (5/16; 31.25%) using 130 000 cells (Table 1). One publication failed to list the number of cells inoculated at all.37 Like with anti-PD-1 dosing, no clear trend exists to separate groups A, B, and C by starting cell count alone (Table 1). It is possible that this variable is either insufficient to change the outcome of a study by itself, or that the impact of increasing tumor cellularity has a nonlinear relationship with anti-PD-1 resistance. The relationship between absolute GL261-tumor volume and the efficacy of anti-PD-1 monotherapy44 likely has interplay with this variable as well, as different initial tumor inoculums could—even at equivalent days postinoculation—vary dramatically in size from one another and therefore display different therapeutic outcomes even were all other variables to be held constant.
Other tumor-related factors beyond the starting cell count may interface with murine survival in underappreciated manners, such as tumor antigenicity and location. First, the relative immunogenicity of wild-type GL261 compared to the firefly luciferase-expressing GL261 cell line (GL261-Luc), while somewhat conflicted in the literature, is certainly worth noting. While an older study failed to observe any gross changes in overall growth kinetics or T-cell infiltration between GL261 and GL261-Luc tumors,51 a more recent study found that wild-type GL261-bearing mice had shorter survival than mice bearing either of 2 types of Luciferase-expressing GL261.52 The ability of each of these 3 cell lines to replicate in vitro was comparable and the difference in survival was instead linked to a more inflammatory state within the Luciferase-expressing tumors as shown by a cytokine microarray revealing elevated IFN-y and IL-1α levels.52 Whether the differential cytokine levels in the tumor microenvironment are sufficient to dictate a differential response to anti-PD-1 therapy is yet unknown, but the equivalence of GL261 and GL261-Luciferase cell lines should not be taken for granted without further study. Another minimally explored, but potentially significant, factor in influencing murine survival between studies is the location of tumor inoculation. Some orthotopic glioma models have been shown to have a strongly location-dependent impact on survival.53 This trend was hypothesized to be due to each tumor’s relative proximity to the ventricles increasing its odds of growing into the ventricles and causing obstructive hydrocephalus, leading to endpoint-associated symptoms even without significant tumor mass.53 A similar finding with ventricle-contacting tumor growth has been observed to limit survival in glioblastoma patients,54 but we have yet to find a study investigating the impact of varying depths or locations of GL261 inoculation on murine survival. If tumor size, antigen composition, and location do systematically alter tumor susceptibility to anti-PD-1 CBI, methodical study of these factors in the GL261 model could help both to clear up existing confusion in the literature and to identify key hallmarks of susceptible tumors in order to focus future CBI clinical trials on patients likely to receive the maximal benefit.
Variables Relating to Mice Used
Glioblastoma is primarily diagnosed in the older adult population and is more common among men than women.55,56 Despite these facts, the vast majority of the 16 studies explored here (11/16; 68.75%) were conducted in 6- to 12-week-old female mice (Table 1). By one approximation, 6-week-old mice, which are used in at least 10 of the 16 studies discussed above, have been compared to 12-year-old human adolescents.57 This is far removed from the 64 years of life the average glioblastoma patient has accumulated before their diagnosis.1 This discrepancy matters as increased age is known to diminish expected survival times both in human GBM patients58 and in the CT-2A and GL261 murine glioma models.57 This increased age was also a negative prognostic factor for response to an anti-PD-1 inclusive combination immunotherapy in both murine models.57 The fact that heterochronic bone marrow transplant was unable to reverse this trend suggests that non-hematopoietic factors were involved in the age-associated immunosuppression preventing therapeutic success.57 While we are unaware of any studies specifically examining anti-PD-1 monotherapy in the context of aging in the GL261 model, the weight of evidence suggests that there is likely to be a strong age-associated decline in therapeutic efficacy (Figure 1) which makes the justification of choosing young mice an important task if the preclinical work is meant to have clinical relevance. Furthermore, these facts make the complete omission of mouse age in some of the studies examined in Table 1,31,37 a point of concern in regards to the generalizability of their findings.
Pertaining to biological sex, it has been established that males are not only more commonly diagnosed with glioblastoma59 but also have shorter survival after diagnosis than do females.59,60 This has been shown to hold true with the implantable GL261 and SB28 murine glioma models61 as well. Interestingly, this survival benefit for female mice has been linked to the hematopoietic compartment, with elegant bone marrow transfers from male donors into female hosts completely abrogating the sex-specific survival benefit in both murine glioma models.61 This difference was determined to be driven by sex-specific differences in myeloid-derived suppressor cell (MDSC) accumulation at the tumor site,61 which is of particular interest in the context of anti-PD-1 treatment given the increasingly appreciated effect of this intervention on sculpting myelopoiesis.62 Despite the sex-associated immunologic and clinical differences of both glioblastoma and murine glioma models, only 2 of the murine studies explored here29,33 use males as experimental animals for their immunotherapy regimen. Three of the studies fail to list the sex of their animals at all31,32,37 and not a single paper used both sexes of animals for their survival studies. It has not been established that sex influences the efficacy of anti-PD-1 monotherapy in the GL261 model, but the fact that sex has an impact on survival alone61 (Figure 1) provides a significant barrier to interstudy comparison and perhaps contributes to why neither of the studies using male mice falls into Group C of Table 1. Vetting a therapeutic strategy that will largely be utilized in older male humans on young/adolescent female mice, while certainly not invalidating the work, raises questions of applicability and generalizability that will be addressed at length below.
Another potential issue related to variations in mice used for these studies is the vendor from which the mice are obtained. While this facet has received significant attention in recent years in the context of gut microbiota differences, particularly between C57BL/6 animals from Taconic versus The Jackson Laboratory,63 this only accounts for part of the variance among C57BL/6 mice. Not only the vendor-associated gut microbiome varies, but also the subspecies of C57BL/6 that have arisen through separate inbreeding at discreet centers. C57BL/6 animals from multiple different vendors have been shown to have differential susceptibility to various pathogens,64–66 to have differential behaviors,67,68 and to have various genetic differences that have accumulated over time.69 While these vendor-specific effects have been underexplored in the context of the glioma models, the gut microbiome has been implicated both in responsiveness to anti-PD-1 CBI in humans70–72 and in controlling glioma progression73 and anti-PD-1 responsiveness in the GL261 model.74 It could certainly be possible for C57BL/6 mice from different vendors to respond differently both to glioma inoculation and to treatment with anti-PD-1 on the grounds of either slight genetic alterations or shifts in gut microbiome, and these potential sources of variability should be explored further.
Discussion
The lack of consensus in the literature pertaining to the therapeutic efficacy of checkpoint blockade strategies in the GL261 model should give us pause. This is especially true in light of the reproducibility crisis observed in other disciplines.75–77 Overcoming, and even benefiting from, the significant deviation that exists within our field will require being methodical in the choosing and reporting of our methods. Even analysis of a single measure of a single experiment type—survival time of GL261-bearing C57BL/6 mice when treated with anti-PD-1 CBI monotherapy—we observed widely discrepant outcomes (Table 1). Were we to broaden our scope to other immunotherapies and other preclinical glioma models, we would observe similar broad variability. For instance, some studies find that anti-CTLA-4 antibody monotherapy leads to 50% long-term survival of GL261-bearing mice,78 while others show the intervention to not lead to any survival extension at all.79 Relatedly, in the CT-2A glioma model, some researchers have observed up to 60% long-term survival following anti-PD-1 monotherapy80 while most observe no survival benefit whatsoever.28,81 We do not intend to cast doubt on the scientific practices or the honest work of our colleagues, but rather to contend that common and innocuous variations in the execution of a simple experiment can lead to drastically different conclusions. Obviously, the complete standardization of experimental practices across the scientific community is an unrealistic expectation. Given the heterogeneous nature of any human patient population, such standardization might not even be desirable. Thus, the goal of our article is not to argue for homogeneity in approach but rather to advocate for intentionality in the selection and reporting of experimental parameters.
In Figure 1, we arrange all 10 discussed variables by the degree of published data supporting their involvement in the modulation of animal survival in orthotopic GL261 experiments. These factors, covered at length in the sections above and enumerated in the figure legend, all have direct or circumstantial evidence tying them to murine survival in glioma studies. The effort put into addressing these individual variables should be commensurate with the degree to which they are likely to play an important role in affecting the study outcome of murine survival. By that reasoning, the least supported variables of anti-PD-1 clone, mouse origin, and coordinates of tumor inoculation could all be the subjects of interesting studies in the future but likely do not need to be widely addressed by individual research programs. Without more supportive evidence that these variables are sufficient to drive survival study outcomes, the effort for individual research programs to test their immunotherapies against tumors inoculated at varying coordinates or with varying anti-PD-1 clones may not be warranted.
The variables which have a greater deal of evidence supporting their modulation of GL261-bearing mouse survival should, minimally, be considered during the process of experimental design. Just as increased knowledge about the mutational burden of GL261 tumors has led increasingly to them being viewed as a model of hypermutated glioblastoma,26,82 increased knowledge of other experimental variables should change our practices moving forward. For instance, knowing that modification of the GL261 cell line with bioluminescent reporters enhances its antigenicity52 should temper the enthusiasm of successful immunotherapeutic strategies vetted in that model and perhaps encourage the utilization of a second model system like CT-2A, SB28, or wild-type GL261 for verification. Other sets of experimental factors should inform our practices as well. Human glioblastoma patients are often first diagnosed on account of symptoms associated with late-stage disease1 and a number of experimental variables in the murine GL261 setting go into recapitulating that scenario. This interweaving of variables, including cell count of tumor inoculum, the time allowed for tumor engraftment prior to therapy, and the stochastic differences in tumor size and growth all make it difficult to determine exactly what qualifies as “late stage.” One way to encapsulate all these variables and to minimize the difficulties in interstudy comparisons is by encouraging the reporting of measurements of tumor size at the time of treatment, for example, by the use of T2-weighted MRI imaging. Knowing this absolute value would make the comparison of a study inoculating 20 000 cells and treating 8 days later30 versus a study inoculating 500 000 cells and treating 5 days later35 more easily comprehensible. This may not be within every research program’s ability to perform. Therefore, an alternative approach could be to initiate therapy at the first sign of murine behavioral changes under that laboratory’s particular inoculation protocol. A move toward either of these practices would increase the interpretability of future immunotherapy regimens in preclinical glioma models and facilitate an easier recapitulation of the observed clinical trend of glioblastoma diagnosis at the time of advanced symptoms.
In considering how choices in experimental design change the nature of what is being modeled, it is also worth considering the experimental variables of murine sex and age. Increased age negatively impacts the survival of both CT-2A and GL261-bearing mice.57 Meanwhile, male sex is a negative prognostic factor for the survival of both SB28 and GL261-bearing mice.61 Both these trends have been observed in human glioblastoma patients as well.58–60 If preclinical immunotherapy studies properly report these variables, or even more rigorously assess the effect of murine sex and age groups in their studies, we could maximize the utility of our preclinical data and gain a better understanding of human GBM patients most likely to benefit from the tested intervention. Being more cognizant of which comparable human population is being modeled will ensure the most accurate interpretation and translation of preclinical study results.
Reflecting on the broadly discrepant outcomes of anti-PD-1 antibody monotherapy in the GL261 model gives us an opportunity to reassess our common assumptions regarding our experimental practices. Acknowledging interstudy variability will allow therapeutic success achieved in the GL261 model to be considered more rigorously against competing therapeutic approaches. Furthermore, systematically assessing the contribution of initial conditions like age, sex, and tumor size on ultimate survival outcomes will help researchers both to better understand the limits of their model and add additional nuance to our preclinical understanding before presumptive therapies are translated to the clinic. This degree of heightened experimental rigor will give the best chance of capitalizing on the enormous potential that immunotherapeutic interventions carry to revolutionize the life expectancy of patients diagnosed with the grim malignancy of glioblastoma.
Acknowledgments
Figure 1 was created with Biorender.com software.
Funding
Z.P.T. was supported by the National Institutes of Health (T32 AI07425-25).
Conflict of interest statement. The authors have no conflicts of interest to report.
References
- 1. Alphandéry E. Glioblastoma treatments: an account of recent industrial developments. Front Pharmacol. 2018;9:879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tykocki T, Eltayeb M. Ten-year survival in glioblastoma. A systematic review. J Clin Neurosci. 2018;54:7–13. [DOI] [PubMed] [Google Scholar]
- 3. Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee ShU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017;18(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shergalis A, Bankhead A 3rd, Luesakul U, Muangsin N, Neamati N. Current challenges and opportunities in treating glioblastoma. Pharmacol Rev. 2018;70(3):412–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eriksson M, Kahari J, Vestman A, et al. Improved treatment of glioblastoma—changes in survival over two decades at a single regional Centre. Acta Oncol. 2019;58(3):334–341. [DOI] [PubMed] [Google Scholar]
- 6. deSouza RM, Shaweis H, Han C, et al. Has the survival of patients with glioblastoma changed over the years? Br J Cancer. 2016;114(12):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang J, Pearce L, O’Donnell-Tormey J, Hubbard-Lucey VM. Trends in the global immuno-oncology landscape. Nat Rev Drug Discov. 2018;17(11):783–784. [DOI] [PubMed] [Google Scholar]
- 8. Fecci PE, Heimberger AB, Sampson JH. Immunotherapy for primary brain tumors: no longer a matter of privilege. Clin Cancer Res. 2014;20(22):5620–5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hodges TR, Ferguson SD, Heimberger AB. Immunotherapy in glioblastoma: emerging options in precision medicine. CNS Oncol. 2016;5(3):175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ratnam NM, Gilbert MR, Giles AJ. Immunotherapy in CNS cancers: the role of immune cell trafficking. Neuro Oncol. 2019;21(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oh T, Fakurnejad S, Sayegh ET, et al. Immunocompetent murine models for the study of glioblastoma immunotherapy. J Transl Med. 2014;12:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maes W, Van Gool SW. Experimental immunotherapy for malignant glioma: lessons from two decades of research in the GL261 model. Cancer Immunol Immunother. 2011;60(2):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018;24(9):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Szatmári T, Lumniczky K, Désaknai S, et al. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. 2006;97(6):546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trent J, Meltzer P, Rosenblum M, et al. Evidence for rearrangement, amplification, and expression of c-myc in a human glioblastoma. Proc Natl Acad Sci U S A. 1986;83(2):470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zagzag D, Amirnovin R, Greco MA, et al. Vascular apoptosis and involution in gliomas precede neovascularization: a novel concept for glioma growth and angiogenesis. Lab Invest. 2000;80(6):837–849. [DOI] [PubMed] [Google Scholar]
- 18. Zagzag D, Esencay M, Mendez O, et al. Hypoxia- and vascular endothelial growth factor-induced stromal cell-derived factor-1alpha/CXCR4 expression in glioblastomas: one plausible explanation of Scherer’s structures. Am J Pathol. 2008;173(2):545–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Dube C, GibertM, Jr., et al. The p53 pathway in glioblastoma. Cancers (Basel). 2018;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ayasoufi K, Pfaller CK, Evgin L, et al. Brain cancer induces systemic immunosuppression through release of non-steroid soluble mediators. Brain. 2020;143(12):3629–3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gustafson MP, Lin Y, New KC, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12(7):631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacobs VL, Valdes PA, Hickey WF, De Leo JA. Current review of in vivo GBM rodent models: emphasis on the CNS-1 tumour model. ASN Neuro. 2011;3(3):e00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jahan N, Talat H, Alonso A, Saha D, Curry WT. Triple combination immunotherapy with GVAX, anti-PD-1 monoclonal antibody, and agonist anti-OX40 monoclonal antibody is highly effective against murine intracranial glioma. Oncoimmunology. 2019;8(5):e1577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim SS, Harford JB, Moghe M, Slaughter T, Doherty C, Chang EH. A tumor-targeting nanomedicine carrying the p53 gene crosses the blood-brain barrier and enhances anti-PD-1 immunotherapy in mouse models of glioblastoma. Int J Cancer. 2019;145(9):2535–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeo AT, Charest A. Immune checkpoint blockade biology in mouse models of Glioblastoma. J Cell Biochem. 2017;118(9):2516–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Genoud V, Marinari E, Nikolaev SI, et al. Responsiveness to anti-PD-1 and anti-CTLA-4 immune checkpoint blockade in SB28 and GL261 mouse glioma models. Oncoimmunology. 2018;7(12):e1501137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qian J, Wang C, Wang B, et al. The IFN-γ/PD-L1 axis between T cells and tumor microenvironment: hints for glioma anti-PD-1/PD-L1 therapy. J Neuroinflammation. 2018;15(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Belcaid Z, Berrevoets C, Choi J, et al. Low-dose oncolytic adenovirus therapy overcomes tumor-induced immune suppression and sensitizes intracranial gliomas to anti-PD-1 therapy. Neurooncol Adv. 2020;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dai B, Qi N, Li J, Zhang G. Temozolomide combined with PD-1 antibody therapy for mouse orthotopic glioma model. Biochem Biophys Res Commun. 2018;501(4):871–876. [DOI] [PubMed] [Google Scholar]
- 30. Galstyan A, Markman JL, Shatalova ES, et al. Blood-brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat Commun. 2019;10(1):3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hardcastle J, Mills L, Malo CS, et al. Immunovirotherapy with measles virus strains in combination with anti-PD-1 antibody blockade enhances antitumor activity in glioblastoma treatment. Neuro Oncol. 2017;19(4):493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lamano JB, Lamano JB, Li YD, et al. Glioblastoma-derived IL6 induces immunosuppressive peripheral myeloid cell PD-L1 and promotes tumor growth. Clin Cancer Res. 2019;25(12):3643–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shevtsov M, Pitkin E, Ischenko A, et al. Ex vivo Hsp70-activated NK cells in combination with PD-1 inhibition significantly increase overall survival in preclinical models of glioblastoma and lung cancer. Front Immunol. 2019;10:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dejaegher J, Verschuere T, Vercalsteren E, et al. Characterization of PD-1 upregulation on tumor-infiltrating lymphocytes in human and murine gliomas and preclinical therapeutic blockade. Int J Cancer. 2017;141(9):1891–1900. [DOI] [PubMed] [Google Scholar]
- 36. Hung AL, Maxwell R, Theodros D, et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology. 2018;7(8):e1466769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karachi A, Yang C, Dastmalchi F, et al. Modulation of temozolomide dose differentially affects T-cell response to immune checkpoint inhibition. Neuro Oncol. 2019;21(6):730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim JE, Patel MA, Mangraviti A, et al. Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res. 2017;23(1):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mathios D, Kim JE, Mangraviti A, et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci Transl Med. 2016;8(370):370ra180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Speranza MC, Passaro C, Ricklefs F, et al. Preclinical investigation of combined gene-mediated cytotoxic immunotherapy and immune checkpoint blockade in glioblastoma. Neuro Oncol. 2018;20(2):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu A, Maxwell R, Xia Y, et al. Combination anti-CXCR4 and anti-PD-1 immunotherapy provides survival benefit in glioblastoma through immune cell modulation of tumor microenvironment. J Neurooncol. 2019;143(2):241–249. [DOI] [PubMed] [Google Scholar]
- 42. Park J, Kim CG, Shim JK, et al. Effect of combined anti-PD-1 and temozolomide therapy in glioblastoma. Oncoimmunology. 2019;8(1):e1525243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol Res. 2016;4(2):124–135. [DOI] [PubMed] [Google Scholar]
- 44. Wu S, Calero-Perez P, Arus C, Candiota AP. Anti-PD-1 immunotherapy in preclinical GL261 glioblastoma: influence of therapeutic parameters and non-invasive response biomarker assessment with MRSI-based approaches. Int J Mol Sci. 2020;21(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Agarwalla P, Barnard Z, Fecci P, Dranoff G, Curry WT Jr. Sequential immunotherapy by vaccination with GM-CSF-expressing glioma cells and CTLA-4 blockade effectively treats established murine intracranial tumors. J Immunother. 2012;35(5):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dong B, Wang L, Nie S, et al. Anti-glioma effect of intracranial vaccination with tumor cell lysate plus flagellin in mice. Vaccine. 2018;36(52):8148–8157. [DOI] [PubMed] [Google Scholar]
- 47. Xu M, Yao Y, Hua W, et al. Mouse glioma immunotherapy mediated by A2B5+ GL261 cell lysate-pulsed dendritic cells. J Neurooncol. 2014;116(3):497–504. [DOI] [PubMed] [Google Scholar]
- 48. Rutter EM, Stepien TL, Anderies BJ, et al. Mathematical analysis of glioma growth in a murine model. Sci Rep. 2017;7(1):2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Polesso F, Munks MW, Rott KH, Smart S, Hill AB, Moran AE. PD-1-specific “blocking” antibodies that deplete PD-1(+) T cells present an inconvenient variable in preclinical immunotherapy experiments. Eur J Immunol. 2021. doi: 10.1101/2020.04.14.041608 [DOI] [PubMed] [Google Scholar]
- 50. Matsumoto K, Inoue H, Nakano T, et al. B7-DC regulates asthmatic response by an IFN-gamma-dependent mechanism. J Immunol. 2004;172(4):2530–2541. [DOI] [PubMed] [Google Scholar]
- 51. Clark AJ, Safaee M, Oh T, et al. Stable luciferase expression does not alter immunologic or in vivo growth properties of GL261 murine glioma cells. J Transl Med. 2014;12:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sanchez VE, Lynes JP, Walbridge S, et al. GL261 luciferase-expressing cells elicit an anti-tumor immune response: an evaluation of murine glioma models. Sci Rep. 2020;10(1):11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Irtenkauf SM, Sobiechowski S, Hasselbach LA, et al. Optimization of glioblastoma mouse orthotopic xenograft models for translational research. Comp Med. 2017;67(4):300–314. [PMC free article] [PubMed] [Google Scholar]
- 54. van Dijken BRJ, Jan van Laar P, Li C, et al. ; FCRS . Ventricle contact is associated with lower survival and increased peritumoral perfusion in glioblastoma. J Neurosurg. 2018;131(3):717–723. [DOI] [PubMed] [Google Scholar]
- 55. Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. 2016;20(5 suppl):S2–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lukas RV, Wainwright DA, Ladomersky E, Sachdev S, Sonabend AM, Stupp R. Newly diagnosed glioblastoma: a review on clinical management. Oncology (Williston Park). 2019;33(3):91–100. [PMC free article] [PubMed] [Google Scholar]
- 57. Ladomersky E, Zhai L, Lauing KL, et al. Advanced age increases immunosuppression in the brain and decreases immunotherapeutic efficacy in subjects with glioblastoma. Clin Cancer Res. 2020;26(19):5232–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Minniti G, Lombardi G, Paolini S. Glioblastoma in elderly patients: current management and future perspectives. Cancers (Basel). 2019;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yang W, Warrington NM, Taylor SJ, et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci Transl Med. 2019;11(473). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ostrom QT, Rubin JB, Lathia JD, Berens ME, Barnholtz-Sloan JS. Females have the survival advantage in glioblastoma. Neuro Oncol. 2018;20(4):576–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bayik D, Zhou Y, Park C, et al. Myeloid-derived suppressor cell subsets drive glioblastoma growth in a sex-specific manner. Cancer Discov. 2020;10(8):1210–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Strauss L, Mahmoud MAA, Weaver JD, et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol. 2020;5(43). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ivanov II, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stough JM, Dearth SP, Denny JE, et al. Functional characteristics of the gut microbiome in C57BL/6 mice differentially susceptible to Plasmodium yoelii. Front Microbiol. 2016;7:1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Villarino NF, LeCleir GR, Denny JE, et al. Composition of the gut microbiota modulates the severity of malaria. Proc Natl Acad Sci U S A. 2016;113(8):2235–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gauguet S, D’Ortona S, Ahnger-Pier K, et al. Intestinal microbiota of mice influences resistance to Staphylococcus aureus pneumonia. Infect Immun. 2015;83(10):4003–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Khisti RT, Wolstenholme J, Shelton KL, Miles MF. Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol. 2006;40(2):119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Simon MM, Greenaway S, White JK, et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol. 2013;14(7):R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mekada K, Abe K, Murakami A, et al. Genetic differences among C57BL/6 substrains. Exp Anim. 2009;58(2):141–149. [DOI] [PubMed] [Google Scholar]
- 70. Gong J, Chehrazi-Raffle A, Placencio-Hickok V, Guan M, Hendifar A, Salgia R. The gut microbiome and response to immune checkpoint inhibitors: preclinical and clinical strategies. Clin Transl Med. 2019;8(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zheng Y, Wang T, Tu X, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7(1):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. D’Alessandro G, Antonangeli F, Marrocco F, et al. Gut microbiota alterations affect glioma growth and innate immune cells involved in tumor immunosurveillance in mice. Eur J Immunol. 2020;50(5):705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dees KJ, Koo H, Humphreys JF, et al. Human gut microbial communities dictate efficacy of anti-PD-1 therapy in a humanized microbiome mouse model of glioma. Neurooncol Adv. 2021;3(1):vdab023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Baker M. 1,500 scientists lift the lid on reproducibility. Nature. 2016;533(7604):452–454. [DOI] [PubMed] [Google Scholar]
- 76. Yong E. Replication studies: bad copy. Nature. 2012;485(7398):298–300. [DOI] [PubMed] [Google Scholar]
- 77. Freedman LP, Cockburn IM, Simcoe TS. The economics of reproducibility in preclinical research. PLoS Biol. 2015;13(6):e1002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Grauer OM, Nierkens S, Bennink E, et al. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007;121(1):95–105. [DOI] [PubMed] [Google Scholar]
- 79. Belcaid Z, Phallen JA, Zeng J, et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One. 2014;9(7):e101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nair S, Mazzoccoli L, Jash A, et al. Zika virus oncolytic activity requires CD8+ T cells and is boosted by immune checkpoint blockade. JCI Insight. 2021;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Passaro C, Alayo Q, De Laura I, et al. Arming an oncolytic herpes simplex virus type 1 with a single-chain fragment variable antibody against PD-1 for experimental glioblastoma therapy. Clin Cancer Res. 2019;25(1):290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Aslan K, Turco V, Blobner J, et al. Heterogeneity of response to immune checkpoint blockade in hypermutated experimental gliomas. Nat Commun. 2020;11(1):931. [DOI] [PMC free article] [PubMed] [Google Scholar]