Key Points
Question
What is the updated evidence-based consistency and magnitude of neurocognitive functioning in individuals at clinical high risk for psychosis (CHR-P) compared with healthy control individuals?
Findings
In this systematic review and meta-analysis of 78 studies, the neurocognitive functioning of 5162 individuals at CHR-P was compared with that of 2865 healthy control individuals and stratified across their longitudinal risk of developing psychosis. Converging evidence suggested substantial deficits on several neurocognitive tasks, some of which were associated with the longitudinal risk of psychosis onset; these findings were controlled for biases and several moderating factors.
Meaning
This meta-analysis provides state-of-the-art updated knowledge on neurocognitive deficits that differentiate individuals at CHR-P and healthy control individuals or that are associated with to their longitudinal risk of developing psychosis, which may inform future detection strategies, the development of individualized prognostication algorithms, and the refinement of effective preventive approaches.
This systematic review and meta-analysis provides an updated synthesis of evidence on the consistency and magnitude of neurocognitive functioning in individuals at clinical high risk for psychosis.
Abstract
Importance
Neurocognitive functioning is a potential biomarker to advance detection, prognosis, and preventive care for individuals at clinical high risk for psychosis (CHR-P). The current consistency and magnitude of neurocognitive functioning in individuals at CHR-P are undetermined.
Objective
To provide an updated synthesis of evidence on the consistency and magnitude of neurocognitive functioning in individuals at CHR-P.
Data Sources
Web of Science database, Cochrane Central Register of Reviews, and Ovid/PsycINFO and trial registries up to July 1, 2020.
Study Selection
Multistep literature search compliant with Preferred Reporting Items for Systematic Reviews and Meta-analyses and Meta-analysis of Observational Studies in Epidemiology performed by independent researchers to identify original studies reporting on neurocognitive functioning in individuals at CHR-P.
Data Extraction and Synthesis
Independent researchers extracted the data, clustering the neurocognitive tasks according to 7 Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) domains and 8 CHR-P domains. Random-effect model meta-analyses, assessment of publication biases and study quality, and meta-regressions were conducted.
Main Outcomes and Measures
The primary effect size measure was Hedges g of neurocognitive functioning in individuals at CHR-P (1) compared with healthy control (HC) individuals or (2) compared with individuals with first-episode psychosis (FEP) or (3) stratified for the longitudinal transition to psychosis.
Results
A total of 78 independent studies were included, consisting of 5162 individuals at CHR-P (mean [SD; range] age, 20.2 [3.3; 12.0-29.0] years; 2529 [49.0%] were female), 2865 HC individuals (mean [SD; range] age, 21.1 [3.6; 12.6-29.2] years; 1490 [52.0%] were female), and 486 individuals with FEP (mean [SD; range] age, 23.0 [2.0; 19.1-26.4] years; 267 [55.9%] were female). Compared with HC individuals, individuals at CHR-P showed medium to large deficits on the Stroop color word reading task (g = −1.17; 95% CI, −1.86 to −0.48), Hopkins Verbal Learning Test–Revised (g = −0.86; 95% CI, −1.43 to −0.28), digit symbol coding test (g = −0.74; 95% CI, −1.19 to −0.29), Brief Assessment of Cognition Scale Symbol Coding (g = −0.67; 95% CI, −0.95 to −0.39), University of Pennsylvania Smell Identification Test (g = −0.55; 95% CI, −0.97 to −0.12), Hinting Task (g = −0.53; 95% CI, −0.77 to −0.28), Rey Auditory Verbal Learning Test (g = −0.50; 95% CI, −0.78 to −0.21), California Verbal Learning Test (CVLT) (g = −0.50; 95% CI, −0.64 to −0.36), and National Adult Reading Test (g = −0.52; 95% CI, −1.01 to −0.03). Individuals at CHR-P were less impaired than individuals with FEP. Longitudinal transition to psychosis from a CHR-P state was associated with medium to large deficits in the CVLT task (g = −0.58; 95% CI, −1.12 to −0.05). Meta-regressions found significant effects for age and education on processing speed.
Conclusions and Relevance
Findings from this meta-analysis support neurocognitive dysfunction as a potential detection and prognostic biomarker in individuals at CHR-P. These findings may advance clinical research and inform preventive approaches.
Introduction
Indicated prevention in young people at clinical high risk for psychosis (CHR-P)1 is a promising avenue for enhancing clinical outcomes.2,3,4 Neurocognitive dysfunction may represent a useful biomarker to identify individuals at CHR-P and refine their risk of developing psychosis, which is currently 30% at 4 years’ follow-up—approximately 50-fold higher than that of the general population. Furthermore, neurocognitive functioning is a moderator of outcomes in the recommended preventive cognitive behavioral therapy.5 Establishing reproducible, robust neurocognitive biomarkers serves a critical need to advance individualized clinical research knowledge6,7 and tailored interventions, which are currently lacking.8
A number of evidence syntheses have characterized neurocognitive functioning in individuals at CHR-P.9,10,11,12,13,14 Since our previous meta-analysis,15 many more studies have been released at a rapid pace, making periodic reviews essential. The main aim of the present study is to provide a meta-analytical examination of the consistency and magnitude of neurocognitive functioning in individuals at CHR-P.
Methods
The study protocol was registered on PROSPERO (CRD42020192826) and was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guideline16 (PRISMA; eTable 1 in the Supplement), the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline17 (eTable 2 in the Supplement), and the Enhancing the Quality and Transparency of Health Research (EQUATOR) reporting guideline.18
Search Strategy and Selection Criteria
A systematic, multistep literature search (search terms appended in eMethods 1 in the Supplement) was implemented by 2 independent researchers (A.C., G.S.P.), consistent with our previous study.9 Web of Science database (Clarivate Analytics), incorporating the Web of Science Core Collection, BIOSIS Citation Index, KCI-Korean Journal Database, MEDLINE, Russian Science Citation Index, and SciELO Citation Index, as well as Cochrane Central Register of Reviews, PubMed, and Ovid/PsycINFO databases were searched until July 1, 2020. Abstracts of articles identified were screened and, after excluding those not relevant, the full texts were assessed for eligibility. The references of previously published meta-analyses and systematic reviews and of the articles included were then manually searched.
Studies were included if they (1) were original articles published in a peer-reviewed journal; (2) included individuals at CHR-P, defined according to validated CHR-P psychometric interviews (eMethods 2 in the Supplement); (3) focused on neurocognitive tasks (eMethods 3 in the Supplement); (4) included a control group, preferably HC, or stratified neurocognitive functioning according to longitudinal transition to psychosis; and (5) were published in English. Studies were excluded if they (1) were reviews, clinical cases, abstracts, conference proceedings, or study protocols; (2) used nonestablished CHR-P psychometric interviews (eMethods 2 in the Supplement); (3) did not report meta-analyzable data; (4) reported only composite neurocognitive data (to avoid potentially spurious or pseudospecific results10); (5) lacked an HC group or data stratification on the transition to psychosis; or (6) overlapped on the same sample and neurocognitive task. Corresponding authors were contacted by email to retrieve additional information. To further minimize data missingness, we used WebPlotDigitizer version 4.419 to extract data that were only available in figures.20 When there were 2 or more overlapping studies, the largest one was chosen.
Outcome Measures and Data Extraction
Three researchers (C.A., S.D., V.S.) independently extracted data from all identified studies (eMethods 4 in the Supplement). The databases were then cross-checked and discrepancies were resolved through consensus under the supervision of a senior researcher (P.F-P.).
Consistent with our earlier meta-analysis,9 neurocognitive tasks were clustered into 7 Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) domains,21,22 namely (1) processing speed, (2) attention or vigilance, (3) working memory, (4) verbal learning, (5) visual learning, (6) reasoning and problem-solving, and (7) social cognition (eMethods 3 in the Supplement). To ensure the comprehensiveness of our review, we also considered additional CHR-P tasks that had been included in studies of this population and that are not included in the more limited MATRICS framework (eMethods 3 in the Supplement). These tasks were categorized by senior experts (A.G., W.S.) into the following 8 domains: (1) general intelligence, (2) premorbid IQ, (3) visuospatial ability, (4) verbal memory, (5) visual memory, (6) executive functioning, (7) motor functioning, and (8) olfaction.
Statistical Analyses
The primary meta-analytical effect size measure was Hedges g, with negative values reflecting worse functioning in individuals at CHR-P compared with HC individuals (or individuals with first-episode psychosis [FEP]), or in individuals at CHR-P transitioning to psychosis vs those not transitioning to psychosis.
For the main meta-analysis, each specific neurocognitive task (eMethods 3 in the Supplement) was analyzed separately when at least 3 independent studies were available. We conducted 2 primary comparisons of neurocognitive functioning: (1) a cross-sectional meta-analysis of individuals at CHR-P vs HC individuals and (2) a longitudinal meta-analysis of individuals at CHR-P transitioning to psychosis vs those not transitioning to psychosis. Three supplementary meta-analyses included: (1) comparing neurocognitive functioning in individuals at CHR-P with individuals with FEP (when these contrasts were reported in the articles retrieved), (2) estimating the pooled effect sizes for individuals at CHR-P vs HC individuals and individuals at CHR-P vs individuals with FEP across each of the 15 neurocognitive domains, and (3) in association with transition to psychosis (eMethods 5 in the Supplement).
We used a random-effects model,23 as heterogeneity was expected to be high. Heterogeneity was assessed using the Q statistic and I2 index.24 Publication biases were evaluated by visually inspecting funnel plots and performing the Egger test.25 When publication biases were detected, trim-and-fill25 sensitivity analyses26 were used. Study quality was assessed using a modified version of the Newcastle-Ottawa scale, previously validated in CHR-P meta-analyses27,28 (eTable 3 in the Supplement). When at least 7 studies were available, meta-regressions evaluated the effect of several factors (eMethods 6 in the Supplement). Analyses were carried out with Comprehensive Meta-Analysis version 3 (Biostat) and Stata version 16 (StataCorp). For a comprehensive glossary of terms, see eMethods 7 in the Supplement. All tests were 2-sided, and significance was set at P < .05.
Results
Characteristics of the Database
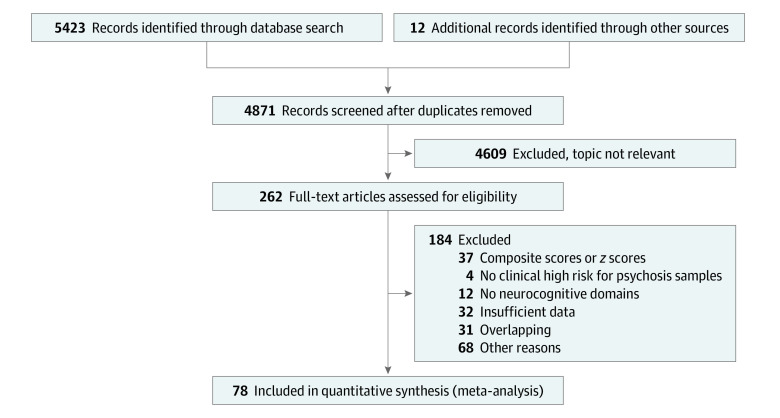
Of 262 eligible studies screened, 78 were included (Figure 1; eTable 4 in the Supplement), comprising 5162 individuals at CHR-P (mean [SD; range] age, 20.2 [3.3; 12.0-29.0] years; 2529 [49.0%] were female), 2865 HC individuals (mean [SD; range] age, 21.1 [3.6; 12.6-29.2] years; 1490 [52.0%] were female), and 486 individuals with FEP (mean [SD; range] age, 23.0 [2.0; 19.1-26.4 years; 267 [54.9%] were female). The mean (SD) education was 11.86 (1.64) years for individuals at CHR-P, 13.02 (1.69) years for HC individuals, and 11.57 (1.57) years for individuals with FEP. Of 5162 individuals at CHR-P, 3707 (71.8%) fulfilled attenuated psychotic symptoms criteria, 374 (7.2%) fulfilled brief limited intermittent psychotic symptoms criteria, 700 (13.6%) fulfilled genetic risk and deterioration syndrome criteria, and 381 (7.4%) fulfilled basic symptoms criteria. At baseline, 1027 individuals at CHR-P (19.9%) had been treated with antipsychotic medication (at any dosage).
Figure 1. PRISMA Flowchart Outlining the Study Selection Process.
Neurocognitive Functioning in Individuals at CHR-P Compared With HC Individuals
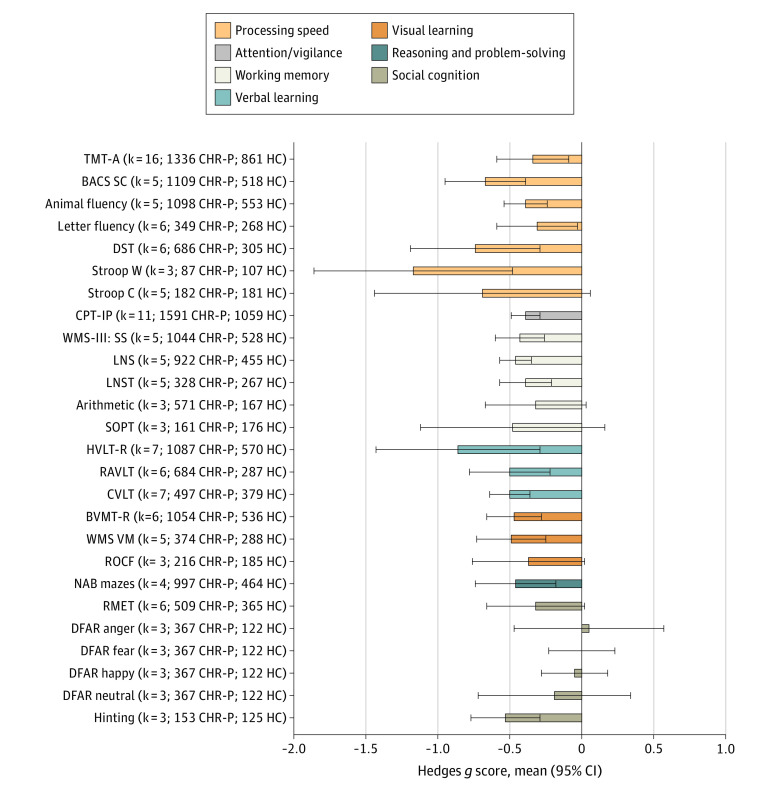
Within the 7 MATRICS domains (Figure 2), individuals at CHR-P performed worse than HC individuals in the following tasks (in descending order of magnitude): Stroop color word reading task (Stroop W) (g = −1.17; 95% CI, −1.86 to −0.48), Hopkins Verbal Learning Test–Revised (HVLT-R) (g = −0.86; 95% CI, −1.43 to −0.28), digit symbol coding test (DST) (g = −0.74; 95% CI, −1.19 to −0.29), Brief Assessment of Cognition Symbol Coding (BACS SC) (g = −0.67; 95% CI, −0.95 to −0.39), Hinting Task (g = −0.53; 95% CI, −0.77 to −0.28), Rey Auditory Verbal Learning Test (RAVLT) (g = −0.50; 95% CI, −0.78 to −0.21), California Verbal Learning Test (CVLT) (g = −0.50; 95% CI, −0.64 to −0.36), Wechsler Memory Scale Immediate Visual Memory (g = −0.49; 95% CI, −0.73 to −0.25), Brief Visuospatial Memory Test–Revised (g = −0.47; 95% CI, −0.66 to −0.28), Letter Number Span (g = −0.46; 95% CI, −0.57 to −0.34), Wechsler Memory Scale-III Spatial Span (g = −0.43; 95% CI, −0.60 to −0.27), Neuropsychological Assessment Battery Mazes (g = −0.46; 95% CI, −0.74 to −0.19), animal fluency (g = −0.39; 95% CI, −0.54 to −0.24), Continuous Performance Test–Identical Pairs version (CPT-IP) (g = −0.39; 95% CI, −0.49 to −0.29), Letter Number Sequencing Test (LNST) (g = −0.39; 95% CI, −0.57 to −0.22), Trail Making Test–Part A (TMT-A) (g = −0.34; 95% CI, −0.59 to −0.09), and letter fluency (g = −0.31; 95% CI, −0.59 to−0.04) (Figure 2; eTable 5 in the Supplement). There were no differences in the Stroop color naming task (Stroop C), arithmetic, Rey-Osterrieth Complex Figure (ROCF) Immediate Recall, Degraded Facial Affect Recognition (DFAR), Self-ordered Pointing Test, or Reading the Mind in the Eyes Test (Figure 2; eTable 5 in the Supplement).
Figure 2. Neurocognitive Task-Level Functioning of Individuals at Clinical High Risk for Psychosis (CHR-P) Compared With Healthy Control (HC) Individuals Across the 7 Measurement and Treatment Research to Improve Cognition in Schizophrenia Domains.
Hedges g scores (mean and 95% CI) are given (negative values indicate worse performance among individuals at CHR-P vs HC individuals), along with the number of studies (k) included and sample size. BACS SC indicates Brief Assessment of Cognition Scale Symbol Coding; BVMT-R, Brief Visuospatial Memory Test–Revised; CPT-IP, Continuous Performance Test–Identical Pairs; CVLT, California Verbal Learning Test; DFAR, Degraded Facial Affect Recognition; DST, digit symbol coding test; HVLT-R, Hopkins Verbal Learning Test–Revised; LNS, Letter Number Span; LNST, Letter Number Sequencing Test; NAB Mazes, Neuropsychological Assessment Battery Mazes; RAVLT, Rey Auditory Verbal Learning Test; RMET, Reading the Mind in the Eyes Test; ROCF, Rey-Osterrieth Complex Figure Immediate Recall; SOPT, Self-ordered Pointing Test; Stroop C, Stroop color naming task; Stroop W, Stroop color word reading task; TMT-A, Trail Making Test–Part A; WMS-III: SS, Wechsler Memory Scale III: Spatial Span; WMS VM, Wechsler Memory Scale Immediate Visual Memory.
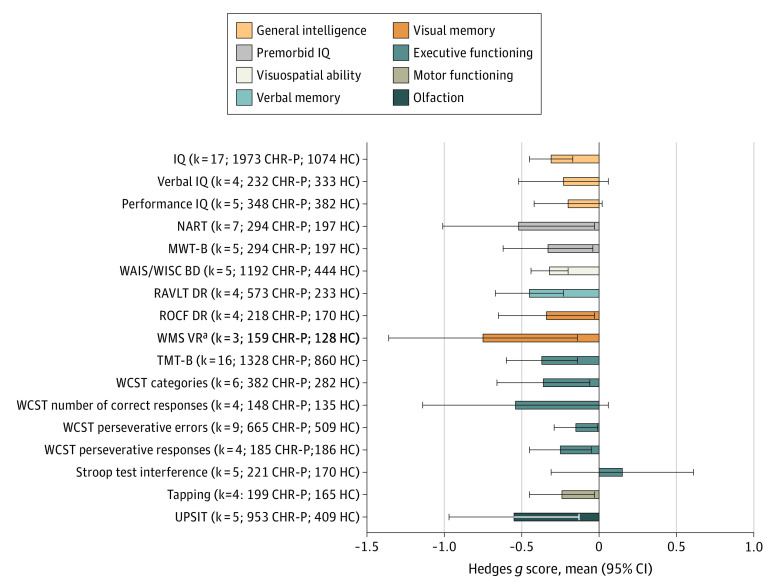
Within the 8 CHR-P domains (Figure 3; eTable 5 in the Supplement), individuals at CHR-P performed worse than HC individuals in the following tasks (in descending order of magnitude): Wechsler Memory Scale Visual Reproduction Delayed Recall (WMS VR) (g = −0.75; 95% CI, −1.36 to −0.14; uncorrected publication bias), University of Pennsylvania Smell Identification Test (UPSIT) (g = −0.55; 95% CI, −0.97 to −0.12), National Adult Reading Test (NART) (g = −0.52; 95% CI, −1.01 to −0.03), RAVLT Delayed Recall (g = −0.45; 95% CI, −0.67 to −0.22), Trail Making Test–Part B (TMT-B) (g = −0.49; 95% CI, −0.72 to −0.27), Wisconsin Card Sorting Test (WCST) categories (g = −0.36; 95% CI, −0.66 to −0.07), ROCF Delayed Recall (ROCF DR) (g = −0.34; 95% CI, −0.65 to −0.03), Mehrfach-Wortschaftz-Intelligenz Test–Part B (MWT-B) (g = −0.33; 95% CI, −0.62 to −0.03), Wechsler Adult Intelligence Scale/Wechsler Intelligence Scale for Children Block Design (g = −0.32; 95% CI, −0.44 to −0.20), IQ (g = −0.31; 95% CI, −0.45 to −0.17), and WCST perseverative responses (g = −0.25; 95% CI, −0.45 to −0.05), Tapping (g = −0.24; 95% CI, −0.45 to −0.04), WCST perseverative errors (g = −0.15; 95% CI, −0.29 to −0.01; corrected after publication bias). There were no differences in the Stroop interference, verbal IQ, performance IQ, or WSCT number of correct responses (Figure 3; eTable 5 in the Supplement).
Figure 3. Neurocognitive Task-Level Functioning of Individuals at Clinical High Risk for Psychosis (CHR-P) Compared With Healthy Control (HC) Individuals Across the CHR-P Domains.
Hedges g scores (mean and 95% CI) are given (negative values indicate worse performance in individuals at CHR-P vs HC individuals), along with the number of studies included (k) and sample size. IQ indicates Wechsler Intelligence Scales (full); Verbal IQ, Wechsler Intelligence Scales (verbal); Performance IQ, Wechsler Intelligence Scales performance; MWT-B, Mehrfach-Wortschaftz-Intelligenz Test–Part B; NART, National Adult Reading Test; RAVLT DR, Rey Auditory Verbal Learning Test Delayed Recall; ROCF DR, Rey-Osterrieth Complex Figure Delayed Recall; TMT-B, Trail Making Test–Part B; UPSIT, University of Pennsylvania Smell Identification Test; WAIS/WISC BD, Wechsler Adult Intelligence Scale/Wechsler Intelligence Scale for Children Block Design; WCST, Wisconsin Card Sorting Test; WMS VR, Wechsler Memory Scale Visual Reproduction Delayed Recall.
aAffected by publication bias.
Neurocognitive Functioning in Individuals at CHR-P Associated With Transition to Psychosis
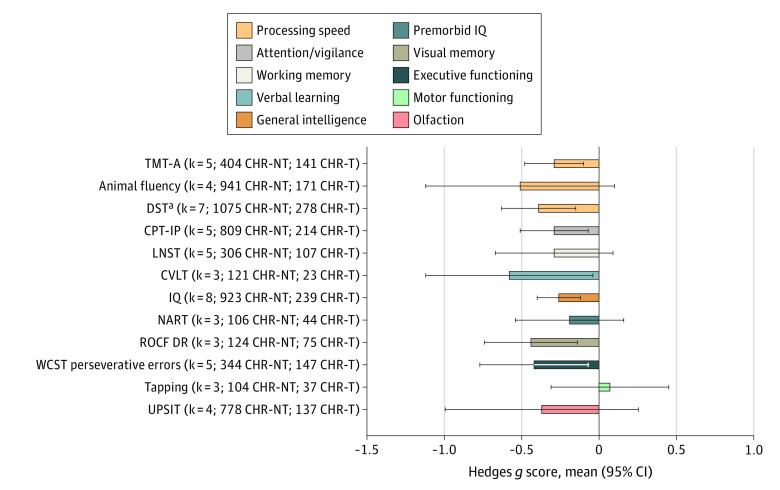
Within the subset of 22 longitudinal studies analyzing transition to psychosis, individuals at CHR-P transitioning to psychosis (Figure 4; eTable 6 in the Supplement) presented worse neurocognitive functioning than those not transitioning to psychosis (in descending order of magnitude): CVLT (g = −0.58; 95% CI, −1.12 to −0.05), ROCF DR (g = −0.44; 95% CI, −0.74 to −0.14), WCST perseverative errors (g = −0.42; 95% CI, −0.77 to −0.07; corrected after publication bias), DST (g = −0.39; 95% CI, −0.63 to −0.14; uncorrected publication bias), CPT-IP (g = −0.29; 95% CI, −0.51 to −0.08), TMT-A (g = −0.29; 95% CI, −0.48 to −0.09), and IQ (g = −0.26; 95% CI, −0.40 to −0.11). There were no differences in the animal fluency, LNST, NART, Tapping, or UPSIT tests (Figure 4; eTable 6 in the Supplement).
Figure 4. Neurocognitive Task-Level Functioning of Individuals at Clinical High Risk for Psychosis (CHR-P) Developing Psychosis Compared With Those Not Developing Psychosis Across the Measurement and Treatment Research to Improve Cognition in Schizophrenia and CHR-P Domains.
Hedges g scores (mean and 95% CI) are given (negative values indicate worse performance in individuals at CHR-P who transitioned to psychosis [CHR-T] vs individuals at CHR-P who did not transition to psychosis [CHR-NT] groups), along with the number of studies included (k) and sample size. CPT-IP indicates Continuous Performance Test–Identical Pairs; CVLT, California Verbal Learning Test; DST, digit symbol coding test; IQ, Wechsler Intelligence Scales (full); LNST, Letter Number Sequencing Test; NART, National Adult Reading Test; ROCF DR, Rey-Osterrieth Complex Figure Delayed Recall; TMT-A, Trail Making Test–Part A; UPSIT, University of Pennsylvania Smell Identification Test; WCST, Wisconsin Card Sorting Test.
aAffected by publication bias.
Supplementary Meta-analyses
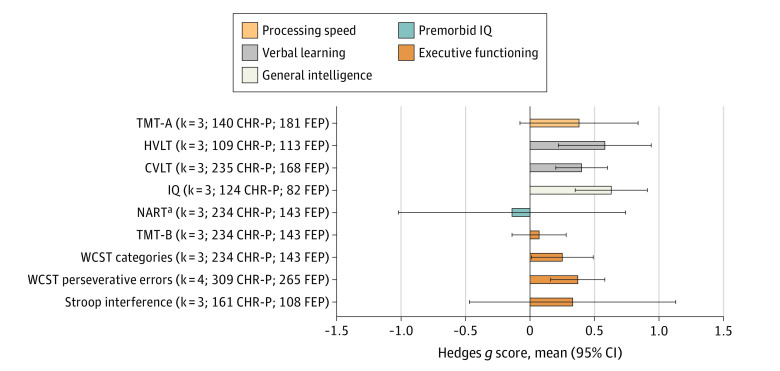
Compared with individuals with FEP (Figure 5; eTable 7 in the Supplement), individuals at CHR-P presented better IQ (g = 0.63; 95% CI, 0.35 to 0.91), HVLT-R (g = 0.58; 95% CI, 0.22 to 0.95), CVLT (g = 0.40; 95% CI, 0.20 to 0.60), WCST perseverative errors (g = 0.37; 95% CI, 0.16 to 0.57), and WCST categories (g = 0.25; 95% CI, 0.01 to 0.50), but were similarly impaired in the Stroop interference, TMT-A, NART (publication bias detected), and TMT-B tests.
Figure 5. Neurocognitive Task-Level Functioning of Individuals at Clinical High Risk for Psychosis (CHR-P) Compared With Individuals With First-Episode Psychosis (FEP) Across Measurement and Treatment Research to Improve Cognition in Schizophrenia and CHR-P Domains.
Hedges g scores (mean and 95% CI) across neurocognitive tasks are given (negative values indicate worse performance in individuals at CHR-P compared with individuals with FEP) along with the number of studies included (k) and sample size. CVLT indicates California Verbal Learning Test; HVLT-R, Hopkins Verbal Learning Test–Revised; IQ, Wechsler Intelligence Scales (full); NART, National Adult Reading Test; TMT-A, Trail Making Test–Part A; TMT-B, Trail Making Test–Part B; WCST, Wisconsin Card Sorting Test.
aAffected by publication bias.
When all neurocognitive tasks were pooled across the 15 broader neurocognitive domains, individuals at CHR-P performed more poorly than HC individuals across all domains (in decreasing order of magnitude): olfaction (g = −0.55; 95% CI, −0.97 to −0.12), verbal learning (g = −0.51; 95% CI, −0.63 to −0.39), reasoning and problem-solving (g = −0.46; 95% CI, −0.74 to −0.19), visual memory (g = −0.45; 95% CI, −0.77 to −0.13), verbal memory (g = −0.45; 95% CI, −0.67 to −0.22), working memory (g = −0.44; 95% CI, −0.57 to −0.31), visual learning (g = −0.43; 95% CI, −0.57 to −0.29), executive functioning (g = −0.42; 95% CI, −0.60 to −0.24), general intelligence (g = −0.39; 95% CI, −0.57 to −0.22), processing speed (g = −0.39; 95% CI, −0.56 to −0.21), attention or vigilance (g = −0.39; 95% CI, −0.49 to −0.29), premorbid intelligence (g = −0.38; 95% CI, −0.63 to −0.13), visuospatial ability (g = −0.32; 95% CI, −0.44 to −0.20), social cognition (g = −0.29; 95% CI, −0.50 to −0.07), and motor functioning (g = −0.24; 95% CI, −0.45 to −0.04) (eFigure 1 and eTable 8 in the Supplement). Longitudinal transition to psychosis was associated with neurocognitive deficits in the domains of verbal learning (g = −0.58; 95% CI, −1.12 to −0.05), visual memory (g = −0.44; 95% CI, −0.74 to −0.14), processing speed (g = −0.39; 95% CI, −0.59 to −0.19), attention or vigilance (g = −0.29; 95% CI, −0.51 to −0.08), and general intelligence (g = −0.26; 95% CI, −0.4 to −0.11) (eFigure 2 and eTable 9 in the Supplement). Individuals at CHR-P performed better than individuals with FEP in general intelligence (g = 0.63; 95% CI, 0.35 to 0.91), verbal learning (g = 0.46; 95% CI, 0.29 to 0.61), and executive functioning (g = 0.33; 95% CI, 0.11 to 0.56) (eFigure 3 and eTable 10 in the Supplement).
Heterogeneity, Publication Bias, and Meta-regression
Heterogeneity across studies was moderate to high (eTables 5-7 in the Supplement). The quality rating of the studies ranged from 4 to 8 (mean = 5.8; median = 6) (eTable 3 in the Supplement). Publication biases are reported in eTables 5-7 and eFigures 4-20 in the Supplement. Meta-regressions for the CHR-P vs HC analysis revealed that older age (β = −0.06; 95% CI, −0.12 to −0.01; P = .02) and fewer years of education (β = 0.17; 95% CI, 0.06 to 0.28; P = .003) were associated with greater processing speed impairments, although several meta-regressions were not feasible (eResults and eTables 11-13 in the Supplement).
Discussion
This systematic review and meta-analysis identified medium to large neurocognitive deficits in individuals at CHR-P compared with HC individuals and individuals with FEP. Some of these deficits were associated with a longitudinal transition to psychosis.
To our best knowledge, this is the largest meta-analysis characterizing neurocognitive functioning in individuals at CHR-P to date. Compared with previous meta-analyses (encompassing from 6 to 49 studies with up to 2506 individuals at CHR-P; 32 studies published from 2015 to 2020),9,10,13,14,29,30,31,32,33 we included many more studies (k = 78) and participants (5162 individuals at CHR-P, 2865 individuals in HC groups, and 486 individuals with FEP). Our larger sample size confers greater statistical power, which is essential for accurate estimates of biomarkers, in particular for relatively infrequent events, such as transition to psychosis.7,8 Compared with older meta-analyses, this study used the most comprehensive CHR-P neurocognitive classification scheme by extending the standard 7 MATRICS domains with additional domains frequently used with individuals at CHR-P. A further merit of this study is the adoption of a complementary analytic approach focusing both on specific neurocognitive tasks and on broader neurocognitive domains.
The first main finding is of a widespread impairment of neurocognitive functioning in individuals at CHR-P compared with HC individuals, encompassing all neurocognitive domains, albeit to varying degrees. Overall, these updated findings align with and elaborate previous CHR-P meta-analyses.9,10,11,13,14,15,29,30,33 Given the replication crisis in psychiatry, rapid pace of CHR-P publications, and unstable findings (eg, earlier meta-analytic efficacy of CHR-P preventive interventions34 has recently been disconfirmed35), comprehensive and confirmatory evidence is essential to consolidate reliable clinical knowledge. At the same time, domain-level differences were noted in reasoning and problem-solving, working memory,10 and processing speed.9 These discrepancies are likely because of the inclusion of more studies (eg, 4 Tapping studies in our meta-analysis compared with 3 Tapping studies in a previous meta-analysis10 and 8 reasoning and problem-solving studies included in our meta-analysis compared with 4 reasoning and problem-solving studies included in a previous meta-analysis10), more rigorous meta-analytical methods to compute pooled estimates (not previously acknowledged29,30), new tasks29,30 (eg, DFAR task had not been analyzed before), and different task categorization methods (eg, Facial Affect Labeling Test was included in social cognition in a previous meta-analysis10 but not in the present study, and WCST perseverative errors or responses were included in executive functioning in the present study but not in a previous meta-analysis10). As noted above, we observed high variability within different neurocognitive domains. For example, within the processing speed domain, performance on the Stroop W but not the Stroop C was impaired in individuals at CHR-P vs individuals in HC groups.
Our second main finding includes having analyzed and identified specific, task-level neurocognitive dysfunctions in individuals at CHR-P compared with HC individuals or individuals with FEP. This is essential to allow accurate reproducibility and implementation of neurocognitive biomarkers in clinical research. Neurocognitive tasks that are more likely to distinguish individuals at CHR-P from HC individuals (ie, those that have moderate to large effect sizes) include the Stroop W,36 HVLT-R,37 DST,38 BACS SC,39 Hinting Task,40 RVALT,41 UPSIT,42 and NART43 (see eDiscussion 1 in the Supplement). These tasks were all impaired in previous meta-analyses9,10,11,14 except the Stroop W,10,11 although some of them (HVLT-R, BACS SC, Hinting, UPSIT, and NART9,10) were not analyzed. The administration time of these tests ranges from 2 to 10 minutes (Stroop W,36 HVLT-R,37 DST,38 BACS SC,39 NART43) to 20 to 40 minutes (RVALT,41 Hinting Task, UPSIT42), and some of them can be administered via digital devices, facilitating their usability. More to this point, some of these dysfunctions have demonstrated neurobiological correlates in individuals at CHR-P.44,45 This converging evidence suggests that these neurocognitive tasks are good candidates to help distinguish individuals at CHR-P from their typically developing peers.
In supplementary analyses, we also found that some of these tasks (eg, HVLT-R and CVLT) can help distinguish individuals at CHR-P from individuals with FEP, which may inform the diagnostic assessment and associated pathways to care of young people accessing preventive services.46 Several other neurocognitive tasks can also differentiate individuals at CHR-P from individuals in HC groups but at a lower magnitude (ie, small to medium effect sizes). Notably, biomarkers with an individual small effect could still hold some value within multivariate approaches, but at the cost of complexity and logistic challenges. Overall, neurocognitive biomarkers consolidated by this meta-analysis could be further validated by future studies for improving the identification of individuals at CHR-P, a key rate-limiting step toward large-scale preventive efforts.47
The third main finding is that baseline neurocognitive impairments in verbal learning, visual memory, processing speed, attention or vigilance, and general intelligence were associated with the longitudinal risk of psychosis onset. These findings align with earlier meta-analyses,9,10 except for the working memory domain. This discrepancy may be because of the inclusion of new individual studies reporting different findings45 (eg, higher LNST scores for individuals at CHR-P who transitioned to psychosis compared with those who did not develop the disorder). The neurocognitive tasks that are more likely to predict psychosis onset among individuals at CHR-P encompass the CVLT (medium to large effect sizes) and, to a lesser extent, the TMT-A, CPT-IP, and IQ (small to medium effect sizes). These potential prognostic biomarkers are ideal candidates to refine existing individualized multivariable prediction models that integrate multimodal domains (eg, clinical, neuroimaging, electrophysiological, and neurocognitive).48,49 A further downstream clinical impact of these findings may be to present an opportunity for refining preventive interventions. At the moment, to our knowledge, no effective pharmacological or psychological interventions are available to ameliorate neurocognitive deficits in individuals at CHR-P,35,50 and recent neurocognitive remediation trials have produced negative findings.51,52
We tested several potential moderators of neurocognitive functioning. Younger age was associated with increased neurocognitive impairments between individuals at CHR-P and HC individuals, while more years of education were related to decreased differences. Age53 and education level21 are consistently associated with neurocognitive function. Importantly, we found no evidence that baseline antipsychotic exposure was associated with neurocognitive functioning. This may align with recent findings showing no evidence that current interventions have a robust effect on clinical outcomes in samples of individuals at CHR-P.35,50,54 However, several meta-regressions were underpowered or not feasible because of the lack of data.
Limitations
This meta-analysis presents some limitations. The validity of the findings is limited mostly to individuals who seek help and cannot be generalized to the general population.55 As neurocognitive functioning is a main determinant of developmental transdiagnostic psychopathology across different psychiatric disorders (and a major domain of the Research Domain Criteria initiative56), transdiagnosticity of these neurocognitive dysfunctions needs further comparative studies with other psychiatric samples.57 Furthermore, some cross-sectional estimates as well as most longitudinal (ie, transition) estimates were based on a significantly smaller data set than the cross-sectional analyses. Additionally, like any other biomarker in this field, the magnitude of the observed effect sizes was largely modest and would not likely support accurate univariate prediction. Large-scale international CHR-P consortia recently completed (eg, NAPLS-3,58 PRONIA,59 PSYSCAN,60 HARMONY61) are expected to test whether sequential assessment frameworks62,63 integrating multivariable predictors across modalities can deliver improved prediction models for clinical practice (eDiscussion 2 in the Supplement). Additionally, while the current analyses update the literature on neurocognition in individuals at CHR, our findings are constrained by heterogeneity in the CHR phenotype itself and by heterogeneity attributable to differences in neurocognitive measures selected, numbers of individuals assessed, and the nature of the study samples. In this context, effect size comparisons among specific measures should be interpreted with caution because we did not empirically compare different neurocognitive tasks or domains against each other.
Conclusions
Findings from this meta-analysis support neurocognitive dysfunction as a potential detection and prognostic biomarker in individuals at CHR-P. These findings characterize the neurocognitive features of psychosis risk states and can advance clinical research and inform multivariable prediction and preventive approaches.
eMethods 1. Search terms used in the literature search
eMethods 2. Types of CHR-P psychometric interviews included
eMethods 3. Neurocognitive domains considered in the current meta-analysis (7 MATRICS domains and 8 CHR-P domains)
eMethods 4. Extracted variables
eMethods 5. Methods for pooling non-independent neurocognitive tasks
eMethods 6. Meta regression factors
eMethods 7. Glossary of terms
eTable 1. PRISMA statement and checklist
eTable 2. MOOSE checklist
eTable 3. Risk of bias (quality) assessment using modified Newcastle-Ottawa Scale for cross-sectional and cohort studies
eTable 4. Characteristics of included studies
eTable 5. Meta-analytical comparisons: CHR-P vs HC. CHR-P
eTable 6. Meta-analytical comparisons: CHR-P not transitioning vs those transitioning
eTable 7. Meta-analytical comparisons: CHR-P vs FEP. CHR-P
eTable 8. Pooled meta-analysis across 15 neurocognitive domains: CHR-P vs HC
eFigure 1. Pooled meta-analysis across 15 neurocognitive domains: CHR-P vs HC
eTable 9. Pooled meta-analysis across neurocognitive domains: CHR-P transitioned vs CHR-P non transitioned
eFigure 2. Pooled meta-analysis across neurocognitive domains CHR-P transitioning vs CHR-P non transitioning
eTable 10. Pooled meta-analysis across neurocognitive domains: CHR-P vs FEP
eFigure 3. Pooled meta-analysis across neurocognitive domains: CHR-P vs FEP
eFigure 4. Funnel plot for processing speed tasks: CHR-P vs HC. CHR-P
eFigure 5. Funnel plot for attention/vigilance tasks: CHR-P vs HC. CHR-P
eFigure 6. Funnel plot for working memory tasks: CHR-P vs HC. CHR-P
eFigure 7. Funnel plot for verbal learning tasks: CHR-P vs HC. CHR-P
eFigure 8. Funnel plot for visual learning tasks: CHR-P vs HC. CHR-P
eFigure 9. Funnel plot for reasoning and problem-solving tasks: CHR-P vs HC
eFigure 10. Funnel plot for social cognition tasks: CHR-P vs HC
eFigure 11. Funnel plot for general intelligence tasks: CHR-P vs HC
eFigure 12. Funnel plot for premorbid IQ: CHR-P vs HC
eFigure 13. Funnel plot for visuospatial ability tasks: CHR-P vs HC
eFigure 14. Funnel plot for verbal memory tasks: CHR-P vs HC
eFigure 15. Funnel plot for visual memory tasks: CHR-P vs HC
eFigure 16. Funnel plot executive functioning tasks: CHR-P vs HC
eFigure 17. Funnel plot for motor functioning task: CHR-P vs HC
eFigure 18. Funnel plot for olfaction task: CHR-P vs HC. CHR-P
eFigure 19. Funnel plot for neurocognitive domains by tasks: CHR-P not transitioning vs those transitioning
eFigure 20. Funnel plot for neurocognitive domains by tasks: CHR-P vs FEP
eResults. Metaregressions
eDiscussion 1. Additional discussion
eTable 11. Metaregressions CHR-P vs HC
eTable 12. Metaregressions CHR-transitioning vs CHR-P non transitioning
eTable 13. Metaregressions: CHR-P vs FEP
eDiscussion 2.
References
- 1.Fusar-Poli P. The clinical high-risk state for psychosis (CHR-P), Version II. Schizophr Bull. 2017;43(1):44-47. doi: 10.1093/schbul/sbw158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catalan A, Salazar de Pablo G, Vaquerizo Serrano J, et al. Annual research review: prevention of psychosis in adolescents—systematic review and meta-analysis of advances in detection, prognosis and intervention. J Child Psychol Psychiatry. 2020;62(5):657-673. doi: 10.1111/jcpp.13322 [DOI] [PubMed] [Google Scholar]
- 3.Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755-765. doi: 10.1001/jamapsychiatry.2019.4779 [DOI] [PubMed] [Google Scholar]
- 4.Fusar-Poli P, McGorry PD, Kane JM. Improving outcomes of first-episode psychosis: an overview. World Psychiatry. 2017;16(3):251-265. doi: 10.1002/wps.20446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute for Health and Care Excellence NICE Guideline . Psychosis and schizophrenia in adults: prevention and management. Accessed December 1, 2020. https://www.nice.org.uk/guidance/cg178
- 6.Scarr E, Millan MJ, Bahn S, et al. Biomarkers for psychiatry: the journey from fantasy to fact, a report of the 2013 CINP Think Tank. Int J Neuropsychopharmacol. 2015;18(10):pyv042. doi: 10.1093/ijnp/pyv042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fusar-Poli P, Hijazi Z, Stahl D, Steyerberg EW. The science of prognosis in psychiatry: a review. JAMA Psychiatry. 2018;75(12):1289-1297. doi: 10.1001/jamapsychiatry.2018.2530 [DOI] [PubMed] [Google Scholar]
- 8.Salazar de Pablo G, Studerus E, Vaquerizo-Serrano J, et al. Implementing precision psychiatry: a systematic review of individualized prediction models for clinical practice. Schizophr Bull. 2021;47(2):284-297. doi: 10.1093/schbul/sbaa120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fusar-Poli P, Deste G, Smieskova R, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69(6):562-571. doi: 10.1001/archgenpsychiatry.2011.1592 [DOI] [PubMed] [Google Scholar]
- 10.Hauser M, Zhang J-P, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psychiatry. 2017;78(1):e28-e40. doi: 10.4088/JCP.15r10197 [DOI] [PubMed] [Google Scholar]
- 11.Bora E, Lin A, Wood SJ, Yung AR, McGorry PD, Pantelis C. Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. Acta Psychiatr Scand. 2014;130(1):1-15. doi: 10.1111/acps.12261 [DOI] [PubMed] [Google Scholar]
- 12.Seidman LJ. Identifying neurocognitive impairments prior to psychosis: new meta-analyses of neurocognition in the psychosis risk syndrome and in youth at familial risk for schizophrenia. Biol Psychiatry. 2012;71(8):85S-86S. [Google Scholar]
- 13.De Herdt A, Wampers M, Vancampfort D, et al. Neurocognition in clinical high risk young adults who did or did not convert to a first schizophrenic psychosis: a meta-analysis. Schizophr Res. 2013;149(1-3):48-55. doi: 10.1016/j.schres.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 14.Giuliano AJ, Li H, Mesholam-Gately RI, Sorenson SM, Woodberry KA, Seidman LJ. Neurocognition in the psychosis risk syndrome: a quantitative and qualitative review. Curr Pharm Des. 2012;18(4):399-415. doi: 10.2174/138161212799316019 [DOI] [PubMed] [Google Scholar]
- 15.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220-229. doi: 10.1001/archgenpsychiatry.2011.1472 [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG; Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 17.Stroup DF, Berlin JA, Morton SC, et al. ; Meta-analysis of Observational Studies in Epidemiology (MOOSE) group . Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 18.Altman DG, Simera I, Hoey J, Moher D, Schulz K. EQUATOR: reporting guidelines for health research. Lancet. 2008;371(9619):1149-1150. doi: 10.1016/S0140-6736(08)60505-X [DOI] [PubMed] [Google Scholar]
- 19.Rohatgi A. WebPlotDigitizer. Version 4.3. Automeris LLC; 2020. Accessed August 1, 2020. https://automeris.io/WebPlotDigitizer
- 20.Cramond F, O’Mara-Eves A, Doran-Constant L, Rice AS, Macleod M, Thomas J. The development and evaluation of an online application to assist in the extraction of data from graphs for use in systematic reviews. Wellcome Open Res. 2019;3(157):157. doi: 10.12688/wellcomeopenres.14738.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS Consensus Cognitive Battery, part 2: co-norming and standardization. Am J Psychiatry. 2008;165(2):214-220. doi: 10.1176/appi.ajp.2007.07010043 [DOI] [PubMed] [Google Scholar]
- 22.Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165(2):203-213. doi: 10.1176/appi.ajp.2007.07010042 [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 24.Lipsey MW, Wilson DB. Practical Meta-analysis. Sage Publications; 2000. [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Cochrane; 2011. Accessed June 1, 2020. https://training.cochrane.org/handbook
- 27.Fusar-Poli P, Tantardini M, De Simone S, et al. Deconstructing vulnerability for psychosis: meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. Eur Psychiatry. 2017;40:65-75. doi: 10.1016/j.eurpsy.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 28.Salazar de Pablo G, Catalan A, Fusar-Poli P. Clinical validity of DSM-5 attenuated psychosis syndrome: advances in diagnosis, prognosis, and treatment. JAMA Psychiatry. 2020;77(3):311-320. doi: 10.1001/jamapsychiatry.2019.3561 [DOI] [PubMed] [Google Scholar]
- 29.Lee TY, Hong SB, Shin NY, Kwon JS. Social cognitive functioning in prodromal psychosis: a meta-analysis. Schizophr Res. 2015;164(1-3):28-34. doi: 10.1016/j.schres.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 30.van Donkersgoed RJ, Wunderink L, Nieboer R, Aleman A, Pijnenborg GH. Social cognition in individuals at ultra-high risk for psychosis: a meta-analysis. PLoS One. 2015;10(10):e0141075. doi: 10.1371/journal.pone.0141075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bora E, Murray RM. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr Bull. 2014;40(4):744-755. doi: 10.1093/schbul/sbt085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Paula AL, Hallak JE, Maia-de-Oliveira JP, Bressan RA, Machado-de-Sousa JP. Cognition in at-risk mental states for psychosis. Neurosci Biobehav Rev. 2015;57:199-208. doi: 10.1016/j.neubiorev.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 33.Zheng W, Zhang Q-E, Cai D-B, et al. Neurocognitive dysfunction in subjects at clinical high risk for psychosis: a meta-analysis. J Psychiatr Res. 2018;103:38-45. doi: 10.1016/j.jpsychires.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 34.Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T. Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ. 2013;346:f185. doi: 10.1136/bmj.f185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies C, Cipriani A, Ioannidis JPA, et al. Lack of evidence to favor specific preventive interventions in psychosis: a network meta-analysis. World Psychiatry. 2018;17(2):196-209. doi: 10.1002/wps.20526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golden CJ. Stroop Color and Word Test. Stoelting Co; 1978. [Google Scholar]
- 37.Brandt J, Benedict RHB. The Hopkins Verbal Learning Test–Revised: Professional Manual. Psychological Assessment Resources, In.; 2001. [Google Scholar]
- 38.Wechsler D. Wechsler Intelligence Scale for Children. 3rd edition. Psychological Corporation; 1991. [Google Scholar]
- 39.Keefe RSE. Brief Assessment of Cognition in Schizophrenia (BACS) Manual—A: Version 2.1. Duke University Medical Center; 1999. [Google Scholar]
- 40.Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophr Res. 1995;17(1):5-13. doi: 10.1016/0920-9964(95)00024-G [DOI] [PubMed] [Google Scholar]
- 41.Lezak MD. Neuropsychological Assessment. Oxford University Press; 2004. [Google Scholar]
- 42.Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94(2, pt 1):176-178. doi: 10.1288/00005537-198402000-00004 [DOI] [PubMed] [Google Scholar]
- 43.Nelson HE. National Adult Reading Test (NART): Test Manual. NFER-Nelson; 1982. [Google Scholar]
- 44.Li RR, Lyu HL, Liu F, et al. Altered functional connectivity strength and its correlations with cognitive function in subjects with ultra-high risk for psychosis at rest. CNS Neurosci Ther. 2018;24(12):1140-1148. doi: 10.1111/cns.12865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atkinson RJ, Fulham WR, Michie PT, et al. ; MinT Consortium . Electrophysiological, cognitive and clinical profiles of at-risk mental state: the longitudinal Minds in Transition (MINT) study. PLoS One. 2017;12(2):e0171657. doi: 10.1371/journal.pone.0171657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fusar-Poli P, Díaz-Caneja CM, Patel R, et al. Services for people at high risk improve outcomes in patients with first episode psychosis. Acta Psychiatr Scand. 2016;133(1):76-85. doi: 10.1111/acps.12480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fusar-Poli P, Sullivan SA, Shah JL, Uhlhaas PJ. Improving the detection of individuals at clinical risk for psychosis in the community, primary and secondary care: an integrated evidence-based approach. Front Psychiatry. 2019;10:774. doi: 10.3389/fpsyt.2019.00774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cannon TD, Yu C, Addington J, et al. An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry. 2016;173(10):980-988. doi: 10.1176/appi.ajp.2016.15070890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee TY, Hwang WJ, Kim NS, et al. Prediction of psychosis: model development and internal validation of a personalized risk calculator. Psychol Med. 2020;1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fusar-Poli P, Davies C, Solmi M, et al. Preventive treatments for psychosis: umbrella review (just the evidence). Front Psychiatry. 2019;10:764. doi: 10.3389/fpsyt.2019.00764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glenthøj LB, Mariegaard LS, Fagerlund B, et al. Cognitive remediation plus standard treatment versus standard treatment alone for individuals at ultra-high risk of developing psychosis: results of the FOCUS randomised clinical trial. Schizophr Res. 2020;224:151-158. doi: 10.1016/j.schres.2020.08.016 [DOI] [PubMed] [Google Scholar]
- 52.Kristensen TD, Ebdrup BH, Hjorthøj C, et al. No effects of cognitive remediation on cerebral white matter in individuals at ultra-high risk for psychosis—a randomized clinical trial. Front Psychiatry. 2020;11:873. doi: 10.3389/fpsyt.2020.00873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee J, Green MF, Nuechterlein KH, et al. The effects of age and sex on cognitive impairment in schizophrenia: findings from the Consortium on the Genetics of Schizophrenia (COGS) study. PLoS One. 2020;15(5):e0232855. doi: 10.1371/journal.pone.0232855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bosnjak Kuharic D, Kekin I, Hew J, Rojnic Kuzman M, Puljak L. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;2019(11). doi: 10.1002/14651858.CD012236.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fusar-Poli P, Cappucciati M, Rutigliano G, et al. At risk or not at risk? a meta-analysis of the prognostic accuracy of psychometric interviews for psychosis prediction. World Psychiatry. 2015;14(3):322-332. doi: 10.1002/wps.20250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schweizer TH, Snyder HR, Young JF, Hankin BL. The breadth and potency of transdiagnostic cognitive risks for psychopathology in youth. J Consult Clin Psychol. 2020;88(3):196-211. doi: 10.1037/ccp0000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.East-Richard CR, Mercier A, Nadeau D, Cellard C. Transdiagnostic neurocognitive deficits in psychiatry: a review of meta-analyses. Can Psychol. 2020;61(3):190-214. doi: 10.1037/cap0000196 [DOI] [Google Scholar]
- 58.Addington J, Liu L, Brummitt K, et al. North American Prodrome Longitudinal Study (NAPLS 3): methods and baseline description. Schizophr Res. 2020;S0920-9964(20)30217-6. doi: 10.1016/j.schres.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.PRONIA. Accessed May 13, 2021. https://www.pronia.eu/the-project/about-pronia/
- 60.The PSYSCAN Project. Accessed May 13, 2021. http://psyscan.eu/the-project/
- 61.HARMONY. Accessed May 13, 2021. https://grantome.com/grant/NIH/U01-MH082004-09S1
- 62.Schmidt A, Cappucciati M, Radua J, et al. Improving prognostic accuracy in subjects at clinical high risk for psychosis: systematic review of predictive models and meta-analytical sequential testing simulation. Schizophr Bull. 2017;43(2):375-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koutsouleris N, Dwyer DB, Degenhardt F, et al. Multimodal machine learning workflows for prediction of psychosis in patients with clinical high-risk syndromes and recent-onset depression. JAMA Psychiatry. 2021;78(2):195-209. doi: 10.1001/jamapsychiatry.2020.3604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salazar de Pablo G, Radua J, Pereira J, et al. Probability of transition to psychosis in individuals at clinical high risk: an updated meta-analysis. JAMA Psychiatry. Published online 2021. doi: 10.1001/jamapsychiatry.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Search terms used in the literature search
eMethods 2. Types of CHR-P psychometric interviews included
eMethods 3. Neurocognitive domains considered in the current meta-analysis (7 MATRICS domains and 8 CHR-P domains)
eMethods 4. Extracted variables
eMethods 5. Methods for pooling non-independent neurocognitive tasks
eMethods 6. Meta regression factors
eMethods 7. Glossary of terms
eTable 1. PRISMA statement and checklist
eTable 2. MOOSE checklist
eTable 3. Risk of bias (quality) assessment using modified Newcastle-Ottawa Scale for cross-sectional and cohort studies
eTable 4. Characteristics of included studies
eTable 5. Meta-analytical comparisons: CHR-P vs HC. CHR-P
eTable 6. Meta-analytical comparisons: CHR-P not transitioning vs those transitioning
eTable 7. Meta-analytical comparisons: CHR-P vs FEP. CHR-P
eTable 8. Pooled meta-analysis across 15 neurocognitive domains: CHR-P vs HC
eFigure 1. Pooled meta-analysis across 15 neurocognitive domains: CHR-P vs HC
eTable 9. Pooled meta-analysis across neurocognitive domains: CHR-P transitioned vs CHR-P non transitioned
eFigure 2. Pooled meta-analysis across neurocognitive domains CHR-P transitioning vs CHR-P non transitioning
eTable 10. Pooled meta-analysis across neurocognitive domains: CHR-P vs FEP
eFigure 3. Pooled meta-analysis across neurocognitive domains: CHR-P vs FEP
eFigure 4. Funnel plot for processing speed tasks: CHR-P vs HC. CHR-P
eFigure 5. Funnel plot for attention/vigilance tasks: CHR-P vs HC. CHR-P
eFigure 6. Funnel plot for working memory tasks: CHR-P vs HC. CHR-P
eFigure 7. Funnel plot for verbal learning tasks: CHR-P vs HC. CHR-P
eFigure 8. Funnel plot for visual learning tasks: CHR-P vs HC. CHR-P
eFigure 9. Funnel plot for reasoning and problem-solving tasks: CHR-P vs HC
eFigure 10. Funnel plot for social cognition tasks: CHR-P vs HC
eFigure 11. Funnel plot for general intelligence tasks: CHR-P vs HC
eFigure 12. Funnel plot for premorbid IQ: CHR-P vs HC
eFigure 13. Funnel plot for visuospatial ability tasks: CHR-P vs HC
eFigure 14. Funnel plot for verbal memory tasks: CHR-P vs HC
eFigure 15. Funnel plot for visual memory tasks: CHR-P vs HC
eFigure 16. Funnel plot executive functioning tasks: CHR-P vs HC
eFigure 17. Funnel plot for motor functioning task: CHR-P vs HC
eFigure 18. Funnel plot for olfaction task: CHR-P vs HC. CHR-P
eFigure 19. Funnel plot for neurocognitive domains by tasks: CHR-P not transitioning vs those transitioning
eFigure 20. Funnel plot for neurocognitive domains by tasks: CHR-P vs FEP
eResults. Metaregressions
eDiscussion 1. Additional discussion
eTable 11. Metaregressions CHR-P vs HC
eTable 12. Metaregressions CHR-transitioning vs CHR-P non transitioning
eTable 13. Metaregressions: CHR-P vs FEP
eDiscussion 2.