Summary
Malignant lymphoma (ML) is a common hematological malignancy with many subtypes. Patients with ML usually undergo traditional treatment failure and become relapsed or refractory (R/R) cases. Recently, immunotherapy, such as immune checkpoint inhibitors (ICIs) and cellular treatment, has gradually emerged and used in clinical trials with encouraging achievements for ML treatment, which exerts anti‐tumor activity by blocking the immune evasion of tumor cells and enhancing the attack ability of immune cells. Targets of immune checkpoints include programmed cell death‐1 (PD‐1), programmed cell death‐ligand 1 (PD‐L1), cytotoxic T lymphocyte‐associated protein 4 (CTLA‐4), T cell immunoglobulin and ITIM domain (TIGIT), T cell immunoglobulin‐3 (TIM‐3) and lymphocyte activation gene 3 (LAG‐3). Examples of cellular treatment are chimeric antigen receptor (CAR) T cells, cytokine‐induced killer (CIK) cells and natural killer (NK) cells. This review aimed to present the current progress and future prospects of immunotherapy in lymphoma, with the focus upon ICIs and cellular treatment.
Keywords: cell therapy, clinical trials, immune checkpoint inhibitors (ICIs), immunotherapy, lymphoma
Most patients with malignant lymphoma inevitably develop into relapsed or refractory (R/R) cases after traditional treatment failure. Immunotherapy, such as immune checkpoint inhibitors (ICIs) and cellular treatment has played roles in ML treatment by blocking the immune evasion of tumor cells and enhancing the attack ability of immune cells. This review was aimed to present the current progress and future prospects of immunotherapy in lymphoma, with the focus on ICIs and cellular treatment.
Introduction
Malignant lymphoma (ML) is one of the most common hematological malignancies, accounting for 3–4% of all malignant tumors. It is a heterogeneous entity, generally divided into two main types as non‐Hodgkin’s lymphoma (NHL) and Hodgkin’s lymphoma (HL) [1, 2, 3]. Traditional treatments for lymphoma include chemotherapy, radiotherapy, surgery and bone marrow transplantation. On one hand, conventional chemotherapy and radiotherapy may lead to severe adverse events in low‐risk lymphoma patients; on the other hand, some patients underwent disease recurrence after hematopoietic stem‐cell transplantation in aggressive high‐risk lymphoma patients [4, 5]. Also, 30–40% of diffuse large B cell lymphoma (DLBCL) patients, which is the most common subtype of NHL, may relapse or become refractory (R/R) cases after standard treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R‐CHOP) [6]. Thus, to find innovative and novel strategies to solve the current dilemma is an urgent matter.
In recent years, immunotherapy has gradually emerged and applied in clinical trials, with encouraging achievements for malignant lymphoma. The mechanisms of immunotherapy can be divided into two categories. One is to block the immune evasion of tumor cells, therapies represented by immune checkpoint inhibitors (ICIs) with the focus on programmed cell death‐1 (PD‐1), programmed cell death‐ligand 1 (PD‐L1), cytotoxic T lymphocyte‐associated protein 4 (CTLA‐4), T cell immunoglobulin and ITIM domain (TIGIT), T cell immunoglobulin‐3 (TIM‐3) and lymphocyte activation gene 3 (LAG‐3) [7, 8]. The other category is to enhance the attack ability of immune cells towards tumor cells, therapies represented by cellular treatment with chimeric antigen receptor (CAR)‐T cells, cytokine‐induced killer (CIK) cells and natural killer (NK) cells [9]. This review aimed to present the current progress and future prospects of immunotherapy in lymphoma (Fig. 1).
Fig. 1.
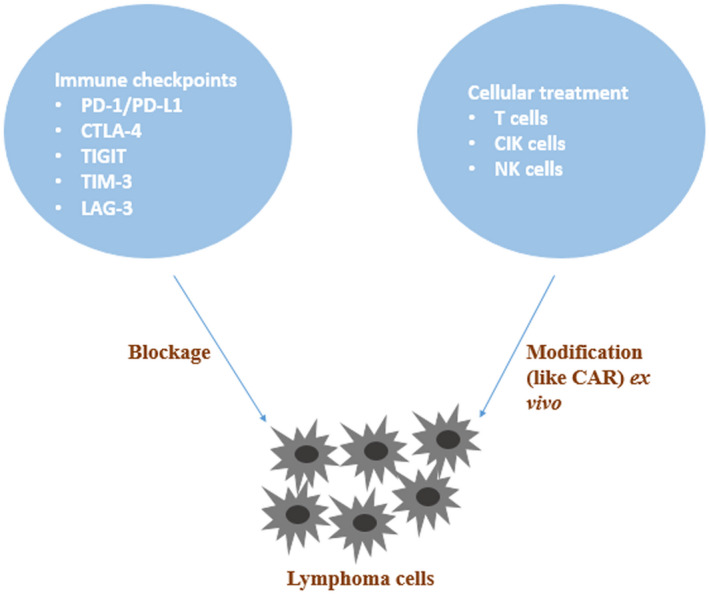
Schematic diagram of lymphoma immunotherapy.
Immune checkpoint inhibitors (Table 1)
Table 1.
Clinical trials of immune checkpoint inhibitors (ICIs) in various types of lymphoma
| Name of ICIs | Target | Clinical trial | Disease | Phase | Sample size | Efficacy | Safety | References |
|---|---|---|---|---|---|---|---|---|
| Nivolumab (Opdivo) | PD‐1 | NCT01592370 | HL | I/II | 23 | 4 patients (17%) had CR, 16 patients (70%) had PR and 3 patients (13%) had SD | 18 patients (78%) had drug‐related AEs, such as rash and a decreased platelet count | [12] |
| NCT02181738 | HL | II | 80 | 7 patients (9%) had CR and 46 patients (58%) had PR | 71 patients (89%) had drug‐related AEs, such as fatigue, infusion‐related reaction, rash, arthralgia, pyrexia, nausea, diarrhea and pruritus | [13] | ||
| NCT01592370 | NHL, MM | I | 81 | 8 patients (8/31, 26%) had OR among B cell lymphoma; 4 patients (4/23, 17%) had OR among T cell lymphoma;1 patient (1/27, 4%) had OR among MM | 53 patients (65%) had drug‐related AEs, including non‐hematological, hematological and immune‐mediated AEs | [14] | ||
| NCT02857426 | PCNSL, PTL | II | 5 | All patients had OR; PFS in 5 patients was from 13+ months to 17+ months | Drug‐related AEs occurred in one patient with grade 2 pruritus and another patient with grade 2 fatigue | [15] | ||
| NCT02572167 | HL | I/II | 61 | 37 patients (61%) had CR, 13 patients (21%) had PR, 5 patients (8%) had SD and 4 patients (7%) had PD | 60 patients (98%) underwent AEs, such as nausea, fatigue and infusion‐related reactions | [16] | ||
| Pembrolizumab (Keytruda) | PD‐1 | NCT01953692 | HL | I | 31 | 5 patients (16%) had CR, 15 patients (48%) had PR, 7 patients (23%) had SD and 4 patients (13%) had PD; The rate of PFS at 24 weeks and 52 weeks was 69% and 46%, respectively | 68% patients had treatment‐related AEs, such as hypothyroidism, diarrhea, nausea and pneumonitis | [17] |
| NCT02453594 | HL | II | 210 | 47 patients (22.4%) had CR, 98 patients (46.7%) had PR, 31 patients (14.8%) had SD, and 30 patients (14.3%) had PD | The most common treatment‐related AEs were hypothyroidism (26/210, 12.4%) and pyrexia (22/210, 10.5%) | [18] | ||
| NCT01953692 | PMBCL | I | 17 | 2 patients (11.8%) had CR, 5 patients (29.4%) had PR, 6 patients (35.3%) had SD and 3 patients (17.6%) had PD | 11 patients (61%) had drug‐related AEs, such as hypothyroidism, diarrhea, nausea, fatigue, pyrexia and decreased appetite | [19] | ||
| NCT03021057 | NK/T cell lymphoma | II | 7 | All patients had OR | 1 patient had grade 2 rash | [20] | ||
| Pidilizumab | ||||||||
| (CT‐011) | PD‐1 | – | AML, CLL, HL, NHL, MDS, MM | I | 17 | 1 patient had CR, 4 patients had SD, and 1 patient had MR | 11 patients (61%) had grade 1/2 AEs | [21] |
| NCT00532259 | DLBCL, PMBCL | II | 72 | 12 patients had CR, 6 patients had PR, 13 patients had PD and 4 patients had PD | 69 patients (96%) underwent 613 AEs, and the most common grade 3/4 AEs were neutropenia and thrombocytopenia | [22] | ||
| Atezolizumab (Tecentriq) | PD‐L1 | NCT02541604 | Solid tumors, NHL, HL | I/II | 90 | 4 patients had PR, 10 patients had PD and 63 patients had PD | 57 patients (66%) had drug‐related AEs, and the most common drug‐related AE was fatigue (17 patients, 20%) | [23] |
| Durvalumab (Imfinzi) | PD‐L1 | NCT02401048 | DLBCL, FL | Ib/II | 61 | 15 patients (25%) had OR and the median PFS and OS was 4.6 months and 18.1 months, respectively | ≥15% patients underwent treatment‐related AEs and 34 patients (56%) had grade 3/4 treatment‐related AEs | [24] |
| Ipilimumab (Yervoy) | CTLA‐4 | NCT00089076 | B cell NHL | I | 18 | 1 patient had PR for 19 months and 1 patient had 31+ months of PFS | No grade 4/5 AEs occurred and the most common treatment‐related AEs were fatigue and diarrhea | [28] |
| NCT01729806 | NHL | I | 33 | 2 patients had CR, 6 patients had SD, 11 patients had PD and the median PFS was 2.6 months | Toxicity was manageable, and the most common non‐hematological AEs was diarrhea in 10 patients | [29] |
OR = objective response; CR = complete response; PR = partial response; SD = stable disease; PD = progressive disease; MR = minimal response; AEs = adverse events; PFS = progression‐free survival; OS = overall survival; AML = acute myeloid leukemia; CLL = chronic lymphocytic leukemia; HL = Hodgkin’s lymphoma; NHL = non‐Hodgkin’s lymphoma; MDS = myelodysplastic syndrome; MM = multiple myeloma; DLBCL = diffuse large B cell lymphoma; PMBCL = primary mediastinal B cell lymphoma; FL = follicular lymphoma; PCNSL = primary central nervous system lymphoma; PTL = primary testicular lymphoma.
PD‐1/PD‐L1
As an immunosuppressive molecule, PD‐1 is mainly expressed on activated T cells, B cells, NK cells and myeloid cells. In the tumor microenvironment, PD‐1 on tumor‐infiltrating T cells binds to PD‐L1 on tumor cells, which suppresses T cell cytotoxicity. By blocking PD‐1/PD‐L1 signaling, the T cell‐mediated immune response can be restored [10, 11]. PD‐1 inhibitors as nivolumab, pembrolizumab and pidilizumab and PD‐1 inhibitors as atezolizumab, durvalumab and avelumab are exploited in clinical trials with various kinds of lymphoma patients.
Nivolumab (trade name: Opdivo), a fully human immunoglobulin (Ig)G4 anti‐PD‐1 monoclonal antibody, was first studied in 23 patients with R/R Hodgkin’s lymphoma (HL) by Ansell et al. [12]. The results showed that the objective response rate (ORR) was achieved in 87% patients, with complete remission (CR) in 17% patients and partial remission (PR) in 70% patients. A Phase II clinical trial carried out by Younes et al. further confirmed the efficacy of nivolumab in 80 patients with R/R HL [13]. The ORR was 66.3%, with 9% patients obtaining CR, and the most common grades 3/4 adverse events (AEs) were neutropenia and increased lipase in 5% of patients. Another Phase I study conducted by Lesokhin et al. evaluated the efficacy of nivolumab in 10 patients with follicular lymphoma (FL), 11 patients with diffuse large B cell lymphoma (DLBCL) and five patients with peripheral T cell lymphoma (PTCL); the corresponding ORRs were 40, 36 and 40%, respectively [14]. Moreover, Nayak et al. observed the clinical outcome of nivolumab in four cases with R/R primary central nervous system lymphoma (PCNSL) and one case with recurrent primary testicular lymphoma (PTL) [15]. The results showed that four cases reached CR and one reached PR, with a median progression‐free survival (PFS) of 9 months. Based on the encouraging results of nivolumab as a monotherapy, the clinical efficacy of nivolumab combined with other therapies were further explored. A Phase I/II clinical trial recruiting 61 patients with R/R HL assessed the tolerability and feasibility of nivolumab combined with brentuximab vedotin (BV) [16]. A total of 50 patients had objective responses and 60 patients underwent manageable AEs.
Pembrolizumab (trade name: Keytruda) is the second human IgG4 monoclonal antibody targeting PD‐1. A Phase I clinical trial investigated the efficacy and safety of pembrolizumab in 31 classical HL (cHL) patients experiencing BV treatment failure [17]. Among these patients, ORR was 65%, with 48% patients developing PR; 16% patients had grade 3 AEs and no treatment‐related deaths occurred. A subsequent Phase II clinical trial also explored the anti‐tumor activity and safety profile of pembrolizumab in 210 subjects with R/R cHL, and the clinical outcomes were similar to the above Phase I study [18]. For R/R primary mediastinal large B cell lymphoma (PMBCL), seven of 17 patients responded to pembrolizumab and survived at the end of a Phase Ib study. Also, 11 patients suffered from treatment‐related AEs and grades 1/2 AEs were mostly frequent [19]. For natural killer (NK)/T cell lymphoma, all the seven included subjects had responses to pembrolizumab and only one case underwent grade 2 AE [20].
Pidilizumab is a fully human IgG1 anti‐PD‐1 monoclonal antibody. A Phase I study was designed to estimate the toxicity and tolerability of pidilizumab in 17 patients with advanced hematological malignancies (including four patients with NHL). No drug‐related toxicities were observed and AEs independent of the treatment appeared in 11 patients. Six patients responded to the treatment, including one case with CR. The half‐life of pidilizumab in blood was approximately 9–17 days [21]. In another Phase II clinical trial, pidilizumab was evaluated in 66 patients with DLBCL after autologous hematopoietic stem‐cell transplantation. The results suggested that the 16‐month PFS in 24 high‐risk patients (0·70, 90% CI = 0·51–0·82) was comparable to that in overall patients (0·72, 90% CI = 0·60–0·82); ORR was 51% among 35 evaluable patients [22].
Atezolizumab (trade name: Tecentriq) is a human IgG1 monoclonal antibody blocking PD‐L1. A Phase I/II clinical study was executed to evaluate the safety profile and serum concentration of atezolizumab in children and young adults with R/R solid tumors, NHL and HL. Of the 90 enrolled patients, no mortal complications occurred and a high serum atezolizumab concentration was detected in all patients, while the efficacy was limited, with only four patients showing a response to the therapy [23]. Furthermore, some clinical trials are in progress to assess the anti‐tumor activity of atezolizumab combined with other inhibitors or antibodies in DLBCL (NCT03276468, NCT02926833, NCT02220842, NCT03321643, NCT03422523, NCT02596971) and FL (NCT03276468, NCT02631577, NCT02220842, NCT02596971). The efficacy of atezolizumab as monotherapy is also evaluated in DLBCL (NCT03463057), cutaneous T cell lymphoma (CTCL) (NCT03357224), PTCL (NCT03046953) and HL (NCT03120676).
Durvalumab (trade name: Imfinzi) is also a human IgG1 monoclonal antibody that inhibits PD‐L1. In a Phase Ib/II clinical trial, the safety, tolerability and clinical response of ibrutinib plus durvalumab were measured in 27 cases with R/R FL and 34 cases with R/R DLBCL [16 germinal center B cell (GCB) subtype, 16 non‐GCB subtype and two unclassified subtype]. Fifteen of 61 patients acquired an objective response (seven had CR and eight had PR), with a median response time of 11·3 months. Median progression‐free survival (PFS) and overall survival (OS) were 4·6 and 18·1 months, respectively, and FL patients both had longer survivals than DLBCL patients. A total of 34 patients had grades 3/4 adverse events (AEs) and 12 patients had immune‐related AEs, with no fatal AEs [24]. The safety and efficacy of durvalumab plus other drugs, radiotherapy or CAR‐T cells are under investigation for lymphoma treatment, including DLBCL (NCT03003520, NCT03685344, NCT03610061, NCT02706405, NCT03212807, NCT02549651), FL (NCT03685344, NCT03610061), mantle cell lymphoma (MCL) (NCT03685344), NK/T cell lymphoma (NCT03054532), PTCL (NCT03161223, NCT03011814) and CTCL (NCT03011814).
Avelumab (trade name: Bavencio) is another human IgG1 monoclonal antibody targeting PD‐L1, which has the ability to enhance antibody‐dependent cell‐mediated cytotoxicity [25]. Some clinical trials are designed to evaluate the feasibility of avelumab in DLBCL (NCT03244176, NCT02951156, NCT03440567), FL (NCT03636503), MCL (NCT03440567), PTCL (NCT03046953, NCT03905135), anaplastic large cell lymphoma (ALCL) (NCT03905135), NK/T cell lymphoma (NCT03439501) and HL (NCT03617666, NCT02603419).
CTLA‐4
CTLA‐4 is a transmembrane receptor on T cells, which can induce T cell anergy and negatively regulate immune response when binding to B7 ligand on antigen‐presenting cells (APCs) [26]. Blocking CTLA‐4 causes increased T cell proliferation and activation to attack tumor cells.
Ipilimumab (trade name: Yervoy) was first introduced as the CTLA‐4 inhibitor to treat metastatic melanoma patients [27]. The clinical efficacy of ipilimumab was studied in a Phase I clinical trial of 18 patients with R/R B cell malignant lymphoma. The study included nine cases of FL at grade 1, five cases of FL at 2 grade, three cases of DLBCL and one case of MCL. The results showed that one DLBCL achieved 31 months of continuous remission and one FL achieved 19 months of PR [28]. Another Phase I clinical trial performed by Tuscano et al. demonstrated the safety and efficacy of ipilimumab combined with rituximab in 33 patients with CD20‐positive R/R B cell lymphoma. The clinical outcome indicated that eight patients (eight of 33, 24%) had a response with a median PFS of 2·6 months, while seven patients (seven of 13, 54%) had a response with a median PFS of 5·6 months, particularly in follicular lymphoma patients. Adverse events were under control and the ratio of CD45RA– regulatory T cells (Treg : Treg) could be associated with patient response using this therapeutic strategy [29].
Tremelimumab is another CTLA‐4 inhibitor which was initially explored in malignant mesothelioma [30]. Three clinical trials are currently under investigation regarding the application of tremelimumab in lymphoma, including durvalumab combined with tremelimumab in R/R DLBCL (NCT02549651), MEDI6469 (OX40 monoclonal antibody) combined with tremelimumab in aggressive B cell lymphoma (NCT02205333) and tremelimumab combined with durvalumab and poly‐ICLC [Toll‐like receptor (TLR)‐3 agonist] in cutaneous T cell lymphoma (NCT02643303).
TIGIT, TIM‐3 and LAG‐3
TTIGIT, TIM‐3 and LAG‐3 are newly discovered immune checkpoints that regulate immune function and are associated with cancer development. TIGIT is highly expressed in Tregs, follicular helper T cells, effector T cells and NK cells as a co‐inhibitory factor mediating immunosuppression [31, 32]. TIGIT was found in FL [33], various subtypes of NHL [34] and HL [35], indicating TIGIT blockage as a major concern of immune checkpoint therapies in the field of lymphoma. TIM‐3 is a type of surface inhibitory molecule on CD4+ helper T cells and CD8+ cytotoxic T cells, which can cause T cell exhaustion during cancer progression and chronic virus infection [36, 37]. In the context of lymphoma, TIM‐3 demonstrated expression in DLBCL [38], NK/T cell lymphoma [39], PTCL [40] and FL [41], showing the potent anti‐lymphoma activity of impeding TIM‐3. LAG‐3 is a negative immune regulator mainly distributed on activated T cells and NK cells [42]. LAG‐3 expression was up‐regulated in DLBCL [38], NK/T cell lymphoma [39] and FL [43], indicating that it might be the potential target for lymphoma treatment.
Cellular treatment (Table 2)
Table 2.
Clinical trials of cellular treatment in various types of lymphoma
| Cellular treatment | Target | Clinical trial | Disease | Phase | Sample size | Efficacy | Safety | References |
|---|---|---|---|---|---|---|---|---|
| CAR‐T cells | CD19 | NCT02348216 | Large B cell lymphoma | I/II | 108 | 59 patients had CR, 25 had PR, 10 had SD, and 5 had PD | All patients had manageable AEs and 106 had ≥ grade 3 AEs | [47] |
| NCT02445248 | DLBCL | II | 93 | 37 patients (40%) had CR, 11 (12%) had PR, 14 (15%) had SD, and 24 (26%) had PD | 111 AEs occurred, and 99 were related to treatment | [48] | ||
| NCT01865617 | CD19+ B cell malignancies | I | 32 | 19 patients had OR, with 10 achieving CR | 4 patients had sCRS and 9 patients had ≥ grade 3 neurotoxicity | [49] | ||
| CD20 | NCT00012207 | CD20+ MCL or indolent NHL | – | 7 | 2 patients had CR, 1 had PR and 4 had SD | No treatment‐related AEs occurred | [50] | |
| NCT00621452 | MCL, FL | – | 4 | 2 patients had no evaluable disease and 1 achieved PR | Grades 1 or 2 AEs were most common and manageable | [51] | ||
| NCT01735604 | DLBCL | 7 | 1 patient had CR for 14+ months and 3 had PR for 3–6 months | All patients had delayed treatment‐related AEs, such as CRS, serous cavity effusion and lung dysfunction | [52] | |||
| CD30 | NCT02259556 | CD30+ R/R lymphoma | I | 18 | 7 patients had PR and 7 had SD | All patients had grade 1/2 febrile syndrome, and nearly all the patients underwent cytopenias | [53] | |
| NCT01316146 | CD30+ R/R lymphoma | – | 9 | 3 patients had CR and 3 had SD | No treatment‐related AEs occurred | [54] | ||
| CD19, CD70 | – | PCNS‐DLBCL | – | 1 | This patient achieved CR | Slight hematological toxicity was observed | [57] | |
| CIK cells | – | – | HL, NHL | I | 9 | 2 patients had PR and 2 had SD | Mild toxicities were observed and manageable | [62] |
| – | – | HL, NHL | – | 8 | 1 patients had CR and 7 had PR | All patients experienced fever and no serious complications happened | [63] | |
| – | – | HL,NHL, RCC, HCC | – | 12 | 3 patients had CR and 2 had SD | 2 patients had grade 2 fever | [64] | |
| – | – | Hematological malignancies | – | 20 | 11 patients had CR, 7 had PR and 2 had SD | 3 patients had mild malaise and low‐grade fever | [65] | |
| – | – | DLBCL | – | 9 | All patients achieved CR | 2 patients had mild fatigue and low‐grade fever | [66] | |
| NK cells | – | – | CD20+ R/R NHL | – | 6 | 2 patients had CR and 2 had PR | Non‐hematological toxicities occurred, including rigors and fevers, skin redness/swelling, fatigue, etc. | [73] |
| NCT01181258 | R/R NHL and CLL | – | 16 | 2 patients had CR, 2 had PR and 10 had PD | All patients underwent hematological toxicities, including thrombocytopenia, anemia and neutropenia | [74] | ||
| NK‐92 cells | – | NCT00990717 | HL, NHL, MM | I | 12 | 2 patients had CR, 2 had MR and 7 had PD | Acute toxicities included fever and/or chills, blurred vision, nausea and pneumonia | [75] |
| CAR‐NK cells | CD19 | NCT03056339 | CD19+ NHL and CLL | I/II | 11 | 8 patients had OR, including 7 with CR | None had CRS, neurotoxicity or GVHD | [83] |
CAR‐T cells = chimeric antigen receptor T cells; CIK cells = cytokine‐induced killer cells; NK cells = natural killer cells; OR = objective response; CR = complete response; PR = partial response; SD = stable disease; PD = progressive disease; MR = minor response; AEs = adverse events; CRS = cytokine release syndrome; sCRS = severe CRS; HL = Hodgkin’s lymphoma; NHL = non‐Hodgkin’s lymphoma; MCL = mantle cell lymphoma; FL = follicular lymphoma; R/R = refractory or relapsed; PCNS‐DLBCL = primary central nervous system diffuse large B cell lymphoma; RCC = renal cell carcinoma; HCC = hepatocellular carcinoma; CLL = chronic lymphocytic leukemia; MM = multiple myeloma; GVHD = graft‐versus‐host disease.
CAR‐T cell therapy
CAR‐T cell therapy is a novel adoptive immunotherapy by equipping T cells with ‘CAR’ to recognize specific tumor antigen. CAR consists of antigen‐binding, transmembrane and signal transduction regions. T cells are extracted from peripheral blood and manufactured as CAR‐T cells, which can enhance the anti‐tumor activity of T cells for specific targets [44, 45].
CAR‐T cell therapy was first applied to attack CD19‐positive B lineage malignancies due to CD19 expression on malignant and normal B cells [45]. Kochenderfer et al. reported the first case with FL who reached partial remission and maintained for 32 weeks after CD19‐specific CAR‐T cell therapy [46]. A Phase I/II multicenter clinical trial (NCT02348216) collected 108 patients with large B cell lymphoma who received a single dose of anti‐CD19 CAR‐T cellular treatment (axicabtagene ciloleucel) [47]. For efficacy evaluation, 84 of 101 evaluable patients had responses with a median duration of 11·1 months. For safety analysis, 52 of 108 evaluable patients suffered from at least grade 3 non‐treatment‐related AEs, including cytokine release syndrome (CRS) and neurotoxicity [47]. Subsequently, a Phase IIa single‐center clinical trial (NCT02445248) recruited 93 patients with R/R DLBCL who were administered CD19‐specific CAR‐T cell infusion (tisagenlecleucel) [48]. Objective responses appeared in 52% patients and the rate of relapse‐free survival was 65% within 1 year after response. Most patients underwent AEs‐like cytopenias and CRS, with no treatment‐related deaths [48]. Another CAR‐T cell therapy (lisocabtagene maraleucel) with a distinct 1 : 1 CD4+ : CD8+ ratio was investigated in 32 patients with R/R B cell NHL [49]. Twenty patients who had previously had lymphodepletion chemotherapy comprised of cyclophosphamide and fludarabine (Flu) achieved 72% ORR and 50% CR, with stable CAR‐T cell expansion and persistence in vivo. CRS and neurotoxicity were observed in 13 and 28% of all 32 patients [49].
Studies regarding anti‐CD20 CAR‐T cell therapy were also explored. Till et al. reported seven cases with R/R FL successfully infused anti‐CD20 CAR‐T cells for treatment (NCT00012207), and demonstrated the safety and effectiveness in these patients [50]. They subsequently used anti‐CD20 CAR‐T cells with co‐stimulatory domains of CD28 and 4‐1BB to treat three MCL and one FL (NCT00621452), and again proved the feasibility and tolerability of this method [51]. Another study assessed the anti‐tumor activity of CD20‐specific CAR‐T cells in seven patients with advanced DLBCL; these patients achieved at least 3 months of tumor regression. AEs were considered to be associated with tumor size and location [52].
CAR‐T cell therapy targeting CD30 may provide alternatives for patients with CD30 positive R/R HL or NHL. In a Phase I clinical trial, anti‐CD30 CAR‐T cell therapy was applied in 18 patients with R/R HL [53]. The results showed that seven patients had PR, two patients appeared to be grade ≥ 3 AEs, serum CAR‐T cells increased and CD30 antigens decreased, proving that anti‐CD30 CAR‐T cell therapy was tolerated and effective. Another Phase I study regarding CD30‐specific CAR‐T cell therapy was performed in seven cases with R/R HL and two cases with R/R ALCL [54]. The clinical outcome showed that three patients achieved CR with a response duration of 9 months, 2 years and > 2·5 years, respectively. Serum CAR‐T cells persisted for more than 6 weeks, and no treatment‐related toxicities occurred.
Dual‐target CAR‐T cell therapy, which means that CAR‐T cells are manufactured to recognize bispecific antigens, has become a new focus in lymphoma immunotherapy. Preclinical data illustrated the anti‐tumor activity of anti‐CD19–CD20 CAR‐T cells towards B cell malignancies [55, 56]. Tu et al. discussed a case presented with R/R PCNS‐DLBCL using CAR‐T cells against CD19 and CD70 [57]. The patient achieved CR within 1 month and disease‐free survival for more than 17 months; no neurotoxicities occurred. Additionally, CD19/CD22 bispecific CAR‐T cell therapy was investigated in patients with acute B cell lymphoblastic leukemia and considered to be safe and efficient [58].
CIK cell therapy
CIK cells originate from peripheral blood mononuclear cells with stimulation of interferon (IFN)‐γ, interleukin (IL)‐2 and anti‐CD3 monoclonal antibody [59]. CIK cells express both CD3 and CD56 markers, with strong anti‐tumor activity‐like T lymphocytes and non‐major histocompatibility complex (MHC)‐restricted tumor killing‐like NK cells [60, 61].
A Phase I clinical trial conducted by Leemhuis et al. explored the efficacy of CIK cellular treatment with different doses for relapsed B cell lymphoma after autologous transplantation. Two patients achieved partial response and two patients achieved stable disease among the nine enrolled patients, while no relationship was found between dose level and clinical outcome due to the small sample size [62]. Guo et al. also performed a retrospective study to demonstrate the feasibility of CIK treatment in eight patients with refractory lymphoma after various chemotherapy regimens. All these patients had complete response or partial response with no serious complications after CIK cell infusion, indicating the effectiveness and safety of this novel therapy [63]. Another study proved that CIK cellular treatment could improve immunity in refractory or relapsed lymphoma patients, with increased level of CD3+CD8+ and CD3+CD56+ cells in their peripheral blood [64, 65, 66]. CIK cell therapy was also safe and effective for elderly patients with malignant lymphoma [65, 66].
NK cell therapy
NK cells are important innate immune cells, which can kill tumor cells without antigen pre‐sensitization [67, 68]. NK cells can exert cytotoxicity through a series interaction of activated and inhibitory receptors and corresponding ligands. The most common mechanism is called ‘missing‐self’, meaning that low expression of human leukocyte antigen (HLA)‐I molecules on the tumor cell surface leads to the escape of tumor killing by cytotoxic T cells but activates tumor killing by NK cells [69]. Activated NK cells directly kill target cells by releasing perforin and granzymes [70]. Another important way technique for activating NK cell function is mediated by IgG, termed ‘antibody‐dependent cell‐mediated cytotoxicity’ (ADCC). Here, the Fab segment of IgG links to the antigen epitopes of tumor cells while the Fc segment of IgG links to CD16 expressed on NK cell surface; target cells are then directly killed by NK cells [71, 72].
In 2010, Bachanova et al. first investigated the clinical efficacy of adoptive haploidentical donor NK cells combined with IL‐2 and rituximab to treat six relapsed or refractory CD20+ NHL patients [73]. The addition of IL‐2 and rituximab enhanced the NK cell‐related ADCC effect. Four of six patients obtained complete response (two cases) and partial response (two cases), while NK cell survival was transient (no more than 7 days) in these patients with increased levels of Tregs. Another study was also performed by Bachanova et al. in 2018 [74], and four of 14 patients obtained complete response (two cases) and partial response (two cases). In this study, NK cells persisted in vivo for at least 7 days due to enhanced immunodepletion and Treg depletion therapy. Williams et al. evaluated a Phase I trial of irradiated NK‐92 cell therapy in 12 patients with relapsed hematological malignancies after autologous hematopoietic cell transplantation [75]. The clinical outcome showed that five patients had remission and improvement, and no serious adverse events occurred.
Similarly to CAR‐T, CAR‐NK cellular treatment against specific antigens is introduced to attack tumor cells. Currently, several preclinical researches have assessed the feasibility of CAR‐NK cells in the field of malignant lymphoma, such as anti‐CD20 CAR‐NK cells against lymphoma cells in vitro [76, 77] and a Burkitt’s lymphoma mouse model in vivo [77], anti‐CD3 CAR‐NK‐92 cells against peripheral T cell lymphoma [78], anti‐CD19 CAR‐NK‐92 cells against B cell lymphoma [79], anti‐CD4 CAR‐NK‐92 cells against T cell lymphoma [80], anti‐CD5 CAR‐NK cells against T cell malignancies [81] and anti‐38 CAR‐NK‐92 cells against Burkitt’s lymphoma cells [82]. A clinical trial investigated by Liu et al. has shown the effectiveness and safety of anti‐CD19 CAR‐NK cell therapy in 11 relapsed or refractory CD19‐positive hematological neoplasms, including six NHL patients and five chronic lymphocytic leukemia patients. A total of eight patients achieved remission response within 1 month after CAR‐NK treatment and the duration of CAR‐NK cells in vivo was at least 12 months [83].
Discussion
With the rapid development of basic and clinical research of malignant lymphoma, clinical trials of immunotherapy for malignant lymphoma are gradually emerging and bring more benefits to targeted patients. However, therapeutic efficacy is limited in certain types of lymphoma, and adverse events cannot be ignored. For PD‐1/PD‐L1 inhibitors, the most common drug‐related toxicities were fatigue, nausea and diarrhea, which were tolerated and manageable. For other immune checkpoints, such as CTLA‐4, TIGIT, TIM‐3 and LAG‐3, preclinical studies of their inhibitors have demonstrated feasibility, while related clinical trials are ongoing and efficacy and safety remain to be determined. For cellular treatment, donor source and cell infusion‐related complications should be thoroughly considered and solved. Despite some encouraging clinical results from cellular treatment, large‐scale studies need to be carried out to further support these results. In future, ICIs combined with cellular treatment, such as combination of PD/PD‐L1 inhibitors and CAR‐T cell therapy, might further enhance anti‐lymphoma activity. Extensive clinical trials are ongoing to provide optimal strategies and improve the prognosis for lymphoma patients.
Disclosures
The authors have no conflicts of interest to declare.
Author contributions
F. L. proposed the idea for this study and revised the paper. F. L. and Y. C. collected the literature and wrote the initial paper. M. P. and P. Y. reviewed the literature and provided suggestions for revision. H. J. had primary responsibility for the final content.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Beijing Municipality (7182178) and the Peking University Medical Youth Science and Technology Innovation Cultivation Fund.
Data Availability Statement
All data used in the study are available online or from the corresponding author on reasonable request.
References
- 1. Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66:115–32. [DOI] [PubMed] [Google Scholar]
- 2. Huh J. Epidemiologic overview of malignant lymphoma. Korean J Hematol 2012; 47:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armitage JO, Gascoyne RD, Lunning MA et al. Non‐Hodgkin lymphoma. Lancet 2017; 390:298–310. [DOI] [PubMed] [Google Scholar]
- 4. Radford J, Illidge T, Counsell N et al. Results of a trial of PET‐directed therapy for early‐stage Hodgkin’s lymphoma. N Engl J Med 2015; 372:1598–607. [DOI] [PubMed] [Google Scholar]
- 5. Cairo MS, Gerrard M, Sposto R et al. Results of a randomized international study of high‐risk central nervous system B non‐Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood 2007; 109:2736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang J, Medeiros LJ, Young KH. Cancer immunotherapy in diffuse large B‐cell lymphoma. Front Oncol 2018; 8:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armand P. Checkpoint blockade in lymphoma. Hematology Am Soc Hematol Educ Program 2015; 2015:69–73. [DOI] [PubMed] [Google Scholar]
- 8. Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol 2018; 29:71–83. [DOI] [PubMed] [Google Scholar]
- 9. Hansrivijit P, Gale RP, Barrett J et al. Cellular therapy for acute myeloid leukemia – current status and future prospects. Blood Rev 2019; 37:100578. [DOI] [PubMed] [Google Scholar]
- 10. Ok CY, Young KH. Targeting the programmed death‐1 pathway in lymphoid neoplasms. Cancer Treat Rev 2017; 54:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ilcus C, Bagacean C, Tempescul A et al. Immune checkpoint blockade: the role of PD‐1‐PD‐L axis in lymphoid malignancies. Onco Targets Ther 2017; 10:2349–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ansell SM, Lesokhin AM, Borrello I et al. PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 2015; 372:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Younes A, Santoro A, Shipp M et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem‐cell transplantation and brentuximab vedotin: a multicentre, multicohort, single‐arm phase 2 trial. Lancet Oncol 2016; 17:1283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lesokhin AM, Ansell SM, Armand P et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol 2016; 34:2698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nayak L, Iwamoto FM, LaCasce A et al. PD‐1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood 2017; 129:3071–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrera AF, Moskowitz AJ, Bartlett NL et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood 2018; 131:1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Armand P, Shipp MA, Ribrag V et al. Programmed death‐1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol 2016; 34:3733–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen R, Zinzani PL, Fanale MA et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic hodgkin lymphoma. J Clin Oncol 2017; 35:2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zinzani PL, Ribrag V, Moskowitz CH et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B‐cell lymphoma. Blood 2017; 130:267–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kwong YL, Chan TSY, Tan D et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T‐cell lymphoma failing l‐asparaginase. Blood 2017; 129:2437–42. [DOI] [PubMed] [Google Scholar]
- 21. Berger R, Rotem‐Yehudar R, Slama G et al. Phase I safety and pharmacokinetic study of CT‐011, a humanized antibody interacting with PD‐1, in patients with advanced hematologic malignancies. Clin Cancer Res 2008; 14:3044–51. [DOI] [PubMed] [Google Scholar]
- 22. Armand P, Nagler A, Weller EA et al. Disabling immune tolerance by programmed death‐1 blockade with pidilizumab after autologous hematopoietic stem‐cell transplantation for diffuse large B‐cell lymphoma: results of an international phase II trial. J Clin Oncol 2013; 31:4199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Geoerger B, Zwaan CM, Marshall LV et al. Atezolizumab for children and young adults with previously treated solid tumours, non‐Hodgkin lymphoma, and Hodgkin lymphoma (iMATRIX): a multicentre phase 1–2 study. Lancet Oncol 2020; 21:134–44. [DOI] [PubMed] [Google Scholar]
- 24. Herrera AF, Goy A, Mehta A et al. Safety and activity of ibrutinib in combination with durvalumab in patients with relapsed or refractory follicular lymphoma or diffuse large B‐cell lymphoma. Am J Hematol 2020; 95:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boyerinas B, Jochems C, Fantini M et al. Antibody‐dependent cellular cytotoxicity activity of a novel anti‐PD‐L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res 2015; 3:1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stamper CC, Zhang Y, Tobin JF et al. Crystal structure of the B7–1/CTLA‐4 complex that inhibits human immune responses. Nature 2001; 410:608–11. [DOI] [PubMed] [Google Scholar]
- 27. Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ansell SM, Hurvitz SA, Koenig PA et al. Phase I study of ipilimumab, an anti‐CTLA‐4 monoclonal antibody, in patients with relapsed and refractory B‐cell non‐Hodgkin lymphoma. Clin Cancer Res 2009; 15:6446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tuscano JM, Maverakis E, Groshen S et al. A Phase I study of the combination of rituximab and ipilimumab in patients with relapsed/refractory B‐cell lymphoma. Clin Cancer Res 2019; 25:7004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Calabrò L, Morra A, Fonsatti E et al. Tremelimumab for patients with chemotherapy‐resistant advanced malignant mesothelioma: an open‐label, single‐arm, phase 2 trial. Lancet Oncol 2013; 14:1104–11. [DOI] [PubMed] [Google Scholar]
- 31. Yu X, Harden K, Gonzalez LC et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol 2009; 10:48–57. [DOI] [PubMed] [Google Scholar]
- 32. Manieri NA, Chiang EY, Grogan JL. TIGIT: a key inhibitor of the cancer immunity cycle. Trends Immunol 2017; 38:20–8. [DOI] [PubMed] [Google Scholar]
- 33. Josefsson SE, Huse K, Kolstad A et al. T cells expressing checkpoint receptor TIGIT are enriched in follicular lymphoma tumors and characterized by reversible suppression of T‐cell receptor signaling. Clin Cancer Res 2018; 24:870–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Josefsson SE, Beiske K, Blaker YN et al. TIGIT and PD‐1 mark intratumoral T cells with reduced effector function in B‐cell non‐Hodgkin lymphoma. Cancer Immunol Res 2019; 7:355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li W, Blessin NC, Simon R et al. Expression of the immune checkpoint receptor TIGIT in Hodgkin’s lymphoma. BMC Cancer 2018; 18:1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anderson AC. Tim‐3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol Res 2014; 2:393–8. [DOI] [PubMed] [Google Scholar]
- 37. Anderson AC, Joller N, Kuchroo VK. Lag‐3, Tim‐3, and TIGIT: co‐inhibitory receptors with specialized functions in immune regulation. Immunity 2016; 44:989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen BJ, Dashnamoorthy R, Galera P et al. The immune checkpoint molecules PD‐1, PD‐L1, TIM‐3 and LAG‐3 in diffuse large B‐cell lymphoma. Oncotarget 2019; 10:2030–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feng Y, Zhong M, Liu Y et al. Expression of TIM‐3 and LAG‐3 in extranodal NK/T cell lymphoma, nasal type. Histol Histopathol 2018; 33:307–15. [DOI] [PubMed] [Google Scholar]
- 40. Murga‐Zamalloa CA, Brown NA, Wilcox RA. Expression of the checkpoint receptors LAG‐3, TIM‐3 and VISTA in peripheral T cell lymphomas. J Clin Pathol 2020; 73:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang ZZ, Grote DM, Ziesmer SC et al. IL‐12 upregulates TIM‐3 expression and induces T cell exhaustion in patients with follicular B cell non‐Hodgkin lymphoma. J Clin Invest 2012; 122:1271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huard B, Mastrangeli R, Prigent P et al. Characterization of the major histocompatibility complex class II binding site on LAG‐3 protein. Proc Natl Acad Sci USA 1997; 94:5744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang ZZ, Kim HJ, Villasboas JC et al. Expression of LAG‐3 defines exhaustion of intratumoral PD‐1(+) T cells and correlates with poor outcome in follicular lymphoma. Oncotarget 2017; 8:61425–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Savoldo B, Ramos CA, Liu E et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor‐modified T cells in lymphoma patients. J Clin Invest 2011; 121:1822–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cooper LJ, Ausubel L, Gutierrez M et al. Manufacturing of gene‐modified cytotoxic T lymphocytes for autologous cellular therapy for lymphoma. Cytotherapy 2006; 8:105–17. [DOI] [PubMed] [Google Scholar]
- 46. Kochenderfer JN, Wilson WH, Janik JE et al. Eradication of B‐lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010; 116:4099–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Locke FL, Ghobadi A, Jacobson CA et al. Long‐term safety and activity of axicabtagene ciloleucel in refractory large B‐cell lymphoma (ZUMA‐1): a single‐arm, multicentre, phase 1–2 trial. Lancet Oncol 2019; 20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schuster SJ, Bishop MR, Tam CS et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B‐cell lymphoma. N Engl J Med 2019; 380:45–56. [DOI] [PubMed] [Google Scholar]
- 49. Turtle CJ, Hanafi LA, Berger C et al. Immunotherapy of non‐Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19‐specific chimeric antigen receptor‐modified T cells. Sci Transl Med 2016; 8:355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Till BG, Jensen MC, Wang J et al. Adoptive immunotherapy for indolent non‐Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20‐specific T cells. Blood 2008; 112:2261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Till BG, Jensen MC, Wang J et al. CD20‐specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4‐1BB domains: pilot clinical trial results. Blood 2012; 119:3940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang Y, Zhang WY, Han QW et al. Effective response and delayed toxicities of refractory advanced diffuse large B‐cell lymphoma treated by CD20‐directed chimeric antigen receptor‐modified T cells. Clin Immunol 2014; 155:160–75. [DOI] [PubMed] [Google Scholar]
- 53. Wang CM, Wu ZQ, Wang Y et al. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory hodgkin lymphoma: an open‐label phase I trial. Clin Cancer Res 2017; 23:1156–66. [DOI] [PubMed] [Google Scholar]
- 54. Ramos CA, Ballard B, Zhang H et al. Clinical and immunological responses after CD30‐specific chimeric antigen receptor‐redirected lymphocytes. J Clin Invest 2017; 127:3462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zah E, Lin MY, Silva‐Benedict A et al. T cells expressing CD19/CD20 bispecific chimeric antigen receptors prevent antigen escape by malignant B cells. Cancer Immunol Res 2016; 4:498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martyniszyn A, Krahl AC, André MC et al. CD20–CD19 bispecific CAR‐T cells for the treatment of B‐cell malignancies. Hum Gene Ther 2017; 28:1147–57. [DOI] [PubMed] [Google Scholar]
- 57. Tu S, Zhou X, Guo Z et al. CD19 and CD70 dual‐target chimeric antigen receptor T‐cell therapy for the treatment of relapsed and refractory primary central nervous system diffuse large B‐cell lymphoma. Front Oncol 2019; 9:1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhao J, Song Y, Liu D. Clinical trials of dual‐target CAR‐T cells, donor‐derived CAR‐T cells, and universal CAR‐T cells for acute lymphoid leukemia. J Hematol Oncol 2019; 12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schmidt‐Wolf IG, Negrin RS, Kiem HP et al. Use of a SCID mouse/human lymphoma model to evaluate cytokine‐induced killer cells with potent antitumor cell activity. J Exp Med 1991; 174:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ortaldo JR, Winkler‐Pickett RT, Yagita H et al. Comparative studies of CD3– and CD3+ CD56+ cells: examination of morphology, functions, T cell receptor rearrangement, and pore‐forming protein expression. Cell Immunol 1991; 136:486–95. [DOI] [PubMed] [Google Scholar]
- 61. Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol 1994; 153:1687–96. [PubMed] [Google Scholar]
- 62. Leemhuis T, Wells S, Scheffold C et al. A phase I trial of autologous cytokine‐induced killer cells for the treatment of relapsed Hodgkin disease and non‐Hodgkin lymphoma. Biol Blood Marrow Transplant 2005; 11:181–7. [DOI] [PubMed] [Google Scholar]
- 63. Guo Z, Liu H, He XP et al. A clinical study of cytokine‐induced killer cells for the treatment of refractory lymphoma. Oncol Lett 2011; 2:531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Olioso P, Giancola R, Di Riti M et al. Immunotherapy with cytokine induced killer cells in solid and hematopoietic tumours: a pilot clinical trial. Hematol Oncol 2009; 27:130–9. [DOI] [PubMed] [Google Scholar]
- 65. Yang B, Lu XC, Yu RL et al. Repeated transfusions of autologous cytokine‐induced killer cells for treatment of haematological malignancies in elderly patients: a pilot clinical trial. Hematol Oncol 2012; 30:115–22. [DOI] [PubMed] [Google Scholar]
- 66. Lu XC, Yang B, Yu RL et al. Clinical study of autologous cytokine‐induced killer cells for the treatment of elderly patients with diffuse large B‐cell lymphoma. Cell Biochem Biophys 2012; 62:257–65. [DOI] [PubMed] [Google Scholar]
- 67. Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer 1975; 16:216–29. [DOI] [PubMed] [Google Scholar]
- 68. Kiessling R, Klein E, Wigzell H. ‘Natural’ killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 1975; 5:112–7. [DOI] [PubMed] [Google Scholar]
- 69. Ljunggren HG, Kärre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 1990; 11:237–44. [DOI] [PubMed] [Google Scholar]
- 70. Carotta S, Targeting NK. Cells for anticancer immunotherapy: clinical and preclinical approaches. Front Immunol 2016; 7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev 2006; 20:123–37. [DOI] [PubMed] [Google Scholar]
- 72. Romee R, Foley B, Lenvik T et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease‐17 (ADAM17). Blood 2013; 121:3599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bachanova V, Burns LJ, McKenna DH et al. Allogeneic natural killer cells for refractory lymphoma. Cancer Immunol Immunother 2010; 59:1739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bachanova V, Sarhan D, DeFor TE et al. Haploidentical natural killer cells induce remissions in non‐Hodgkin lymphoma patients with low levels of immune‐suppressor cells. Cancer Immunol Immunother 2018; 67:483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Williams BA, Law AD, Routy B et al. A phase I trial of NK‐92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 2017; 8:89256–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Müller T, Uherek C, Maki G et al. Expression of a CD20‐specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK‐resistance of lymphoma and leukemia cells. Cancer Immunol Immunother 2008; 57:411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chu Y, Yahr A, Huang B et al. Romidepsin alone or in combination with anti‐CD20 chimeric antigen receptor expanded natural killer cells targeting Burkitt lymphoma in vitro and in immunodeficient mice. Oncoimmunology 2017; 6:e1341031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen KH, Wada M, Firor AE et al. Novel anti‐CD3 chimeric antigen receptor targeting of aggressive T cell malignancies. Oncotarget 2016; 7:56219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Oelsner S, Friede ME, Zhang C et al. Continuously expanding CAR NK‐92 cells display selective cytotoxicity against B‐cell leukemia and lymphoma. Cytotherapy 2017; 19:235–49. [DOI] [PubMed] [Google Scholar]
- 80. Pinz KG, Yakaboski E, Jares A et al. Targeting T‐cell malignancies using anti‐CD4 CAR NK‐92 cells. Oncotarget 2017; 8:112783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xu Y, Liu Q, Zhong M et al. 2B4 costimulatory domain enhancing cytotoxic ability of anti‐CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J Hematol Oncol 2019; 12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hambach J, Riecken K, Cichutek S et al. Targeting CD38‐expressing multiple myeloma and burkitt lymphoma cells in vitro with nanobody‐based chimeric antigen receptors (Nb‐CARs). Cells 2020; 9:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Liu E, Marin D, Banerjee P et al. Use of CAR‐transduced natural killer cells in CD19‐positive lymphoid tumors. N Engl J Med 2020; 382:545‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in the study are available online or from the corresponding author on reasonable request.


