Summary
Background
Available therapies for myelofibrosis can exacerbate cytopenias and are not indicated for patients with severe thrombocytopenia. Pacritinib, which inhibits both JAK2 and FLT3, induced spleen responses with limited myelosuppression in phase 1/2 trials. We aimed to assess the efficacy and safety of pacritinib versus best available therapy in patients with myelofibrosis irrespective of baseline cytopenias.
Methods
This international, multicentre, randomised, phase 3 trial (PERSIST-1) was done at 67 sites in 12 countries. Patients with higher-risk myelofibrosis (with no exclusions for baseline anaemia or thrombocytopenia) were randomly assigned (2:1) to receive oral pacritinib 400 mg once daily or best available therapy (BAT) excluding JAK2 inhibitors until disease progression or unacceptable toxicity. Randomisation was stratified by risk category, platelet count, and region. Treatment assignments were known to investigators, site personnel, patients, clinical monitors, and pharmacovigilance personnel. The primary endpoint was spleen volume reduction (SVR) of 35% or more from baseline to week 24 in the intention-to-treat population as assessed by blinded, centrally reviewed MRI or CT. We did safety analyses in all randomised patients who received either treatment. Here we present the final data. This trial is registered with ClinicalTrials.gov, number NCT01773187.
Findings
Between Jan 8, 2013, and Aug 1, 2014, 327 patients were randomly assigned to pacritinib (n=220) or BAT (n=107). Median follow-up was 23·2 months (IQR 14·8–28·7). At week 24, the primary endpoint of SVR of 35% or more was achieved by 42 (19%) patients in the pacritinib group versus five (5%) patients in the BAT group (p=0·0003). 90 patients in the BAT group crossed over to receive pacritinib at a median of 6·3 months (IQR 5·8–6·7). The most common grade 3–4 adverse events through week 24 were anaemia (n=37 [17%]), thrombocytopenia (n=26 [12%]), and diarrhoea (n=11 [5%]) in the pacritinib group, and anaemia (n=16 [15%]), thrombocytopenia (n=12 [11%]), dyspnoea (n=3 [3%]), and hypotension (n=3 [3%]) in the BAT group. The most common serious adverse events that occurred through week 24 were anaemia (10 [5%]), cardiac failure (5 [2%]), pyrexia (4 [2%]), and pneumonia (4 [2%]) with pacritinib, and anaemia (5 [5%]), sepsis (2 [2%]), and dyspnoea (2 [2%]) with BAT. Deaths due to adverse events were observed in 27 (12%) patients in the pacritinib group and 14 (13%) patients in the BAT group throughout the duration of the study.
Interpretation
Pacritinib therapy was well tolerated and induced significant and sustained SVR and symptom reduction, even in patients with severe baseline cytopenias. Pacritinib could be a treatment option for patients with myelofibrosis, including those with baseline cytopenias for whom options are particularly limited.
Funding
CTI BioPharma Corp.
Introduction
Myelofibrosis can arise as primary disease or evolve as secondary myelofibrosis from other myeloproliferative neoplasms, specifically essential thrombocythaemia and polycythaemia vera.1–4 Characteristics of myelofibrosis can include debilitating constitutional symptoms, extramedullary haemopoiesis, cytopenias (anaemia and thrombocytopenia), progressive bone marrow fibrosis, and risk of transformation to acute leukaemia.2,4–6 In a retrospective analysis7 of 1000 patients with myelofibrosis, 38% presented with anaemia and 18% with thrombocytopenia; prevalence increased to 58% with anaemia and 28% with thrombocytopenia within 1 year. Severe anaemia can have a substantial negative impact on patients’ quality of life.5,8 Data from an international database of patients with myelofibrosis (n=418) showed that thrombocytopenia (<100 000 platelets per μL) was associated with significantly increased incidence of anaemia, leucopenia, and red blood cell (RBC) transfusion dependence, as well as more severe symptom burden as measured by a significantly higher total symptom score (TSS) per the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF).9 A retrospective analysis10 of 1100 patients from the MD Anderson Cancer Center (1984–2013) also showed more severe symptom burden and significantly shorter overall survival for patients with fewer than 50 000 platelets per μL compared with patients with between 50 000 and 100 000 platelets per μL, or more than 100 000 platelets per μL.
No effective non-myelosuppressive therapies have been approved for the reduction of splenomegaly and symptom burden in patients with myelofibrosis and cytopenias. The only US Food and Drug Administration (FDA)-approved agent for myelofibrosis, ruxolitinib, is not indicated for patients with fewer than 50 000 platelets per μL and is associated with clinically significant and dose-limiting anaemia and thrombocytopenia.11,12 Enrolment in both phase 3 studies of ruxolitinib (COMFORT-I11 and COMFORT-II12) in higher-risk myelofibrosis required a platelet count of at least 100 000 platelets per μL. Furthermore, patients with baseline platelet counts between 100 000 and 200 000 platelets per μL received a lower starting dose of ruxolitinib (15 mg vs 20 mg twice daily) in an attempt to minimise treatment-related cytopenias. Patients with platelet counts between 100 000 and 200 000 platelets per μL in COMFORT-I11 (ruxolitinib vs placebo in higher-risk myelofibrosis) had lower mean percentage changes in both spleen volume and TSS than did patients with platelet counts greater than 200 000 platelets per μL.13 Additionally, 33 (77%) of 43 patients with baseline platelet counts per μL of between 100 000 and 200 000 required further dose reductions (median final titrated dose of 10 mg twice daily), and final titrated doses of less than 10 mg twice daily were associated with less reduction in spleen volume and myelofibrosis-related symptoms.14 In a separate phase 2 study15 of ruxolitinib in patients with platelet counts per μL between 50 000 and 100 000, patients were initially treated with ruxolitinib 5 mg twice daily, with only 56% (23 of 41) of patients able to increase their dose to 10 mg or more twice daily. Results of a phase 3 trial16 of the immunomodulatory agent pomalidomide failed to show improvement in myelofibrosis-related anaemia, and no established agents have been shown to induce RBC transfusion independence. Pacritinib is a kinase inhibitor with specificity for JAK2, FLT3, IRAK1, and CSF1R, and minimal activity against JAK1 at pharmacologically relevant levels.17,18 In a kinome analysis of pacritinib,18 half maximal inhibitory concentration (IC50) for JAK2 (JAK2 Val617Phe), FLT3, IRAK1, CSF1R, and JAK1 were 6·0 nM (9·4 nM), 14·8 nM, 13·6 nM, 39·5 nM, and inactive (82% control), respectively. Published results19–21 of kinase inhibition profiles of other JAK inhibitors in development (ruxolitinib, momelotinib, fedratinib) indicate that all demonstrate nM inhibition of both JAK1 and JAK2. The findings of previous, non-randomised studies22 of pacritinib in myelofibrosis showed clinically significant reductions in splenomegaly, durable improvements in symptoms, and manageable toxicities, even in patients with baseline anaemia and thrombocytopenia. We describe here the results of PERSIST-1, which aimed to compare pacritinib with best available therapy (BAT) in patients with myelofibrosis with no exclusions for baseline platelet counts or haemoglobin levels.
Methods
Study design and participants
In the PERSIST-1 international, randomised, phase 3 study, we compared pacritinib with BAT in patients with myelofibrosis. Based on data from Jan 17, 2015, (median follow-up 11·5 months) the FDA placed pacritinib on a full clinical hold from Feb 8, 2016, due to concerns over interim survival results, bleeding, and cardiovascular events, and all therapy was discontinued. However, upon review of the final PERSIST-1 data, final data from the phase 3 PERSIST-2 study,23 and planned dose comparison protocol in patients with failure of prior JAK2-directed therapy, the FDA removed that clinical hold on Jan 5, 2017. Herein, final data (end of treatment due to clinical hold) with a median follow-up of 23·2 months are presented, including patients who crossed over from BAT to pacritinib.
Patients aged 18 years or older were enrolled from 67 centres in the USA, Europe (Belgium, Czech Republic, France, Germany, Hungary, Italy, the Netherlands, and the UK), Russia, Australia, and New Zealand (appendix pp 1–2). Eligible patients had primary myelofibrosis, post-essential thrombocythaemia myelofibrosis, or post-polycythaemia vera myelofibrosis (locally confirmed via bone marrow biopsy at screening), intermediate-risk or high-risk disease by the Dynamic International Prognostic Scoring System (DIPSS), and a palpable spleen at least 5 cm below the left costal margin. Other elegibility criteria were a score of at least 3 for at least two symptoms or a score over 4 for at least one symptom other than fatigue on the original MPN-SAF TSS (initial protocol), or a TSS of at least 13 on the MPN-SAF TSS 2.0 (amended protocol [Aug 15, 2013]), an Eastern Cooperative Oncology Group performance status of 0 to 3, peripheral blast count lower than 10%, absolute neutrophil count greater than 500 neutrophils per μL, adequate hepatic and renal function, and a life expectancy of 6 months or more. No prior splenectomy or allogeneic stem cell transplantation, or plans to undergo splenectomy or allogeneic stem cell transplantation were allowed. Eligible patients had also been at least 12 months without radioactive phosphorus, at least 6 months without splenic irradiation, at least 4 weeks without any experimental treatment for myelofibrosis, at least 4 weeks without erythropoietic agents, at least 2 weeks without thrombopoietic agents, at least 1 week without treatment with potent cytochrome P450 3A4 inhibitors, and at least 2 weeks without any other treatments for myelofibrosis. No prior treatment with JAK2 inhibitors was allowed. We did not exclude patients on the basis of platelet or haemoglobin levels; patients with RBC transfusion dependence were eligible. We excluded patients with inflammatory or chronic functional bowel disorders, or clinically symptomatic and uncontrolled cardiovascular disease. Other exclusion criteria were any gastrointestinal or metabolic condition that could interfere with absorption of oral medication; uncontrolled intercurrent illnesses that would limit compliance with study requirements; other malignancy within the past 3 years, other than curatively treated basal-cell or squamous-cell skin cancer, carcinoma in situ of the cervix, organ-confined or treated non-metastatic prostate cancer with negative prostate-specific antigen, in-situ breast carcinoma after complete surgical resection, or superficial transitional cell bladder carcinoma; history of any of the following within 6 months prior to randomisation: myocardial infarction, severe or unstable angina, or symptomatic congestive heart failure; New York Heart Association Class II, III, or IV congestive heart failure; ongoing cardiac dysrhythmias of National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or higher, corrected QT interval prolongation greater than 450 ms, or other factors that increase the risk for QT interval prolongation (eg, heart failure, hypokalaemia, or family history of long QT interval syndrome); known seropositivity for HIV; known active hepatitis A, B, or C virus infection; or pregnancy.
The study protocol was approved by the institutional review boards at each participating institution, and study procedures were conducted in accordance with the principles outlined in the Declaration of Helsinki. All patients provided written informed consent before any study procedures were performed.
Randomisation and masking
Patients were stratified at randomisation by DIPSS risk category (intermediate-1 or intermediate-2 vs high-risk), platelet count (<50 000 per μL vs 50 000–99 999 per μL vs ≥100 000 per μL), and geographical region, and randomised via a central interactive website or voice response system 2:1 to pacritinib or BAT. Treatment assignments were known to investigators, site personnel, patients, clinical monitors, and pharmacovigilance personnel. The funder remained blinded until database lock for the primary analysis, and independent radiographic assessors remained blinded throughout the study.
Procedures
BAT consisted of any physician-selected treatment, excluding JAK2 inhibitors, and could also include no treatment (ie, watchful waiting) or symptom-directed treatment. Pacritinib was administered orally at a dose of 400 mg once daily. Patients in both groups were treated until disease progression (increase in splenic volume ≥25% from baseline as centrally assessed every 12 weeks by MRI or CT, splenic irradiation, splenectomy, or leukaemic transformation [peripheral blood blasts ≥20% for ≥8 weeks or bone marrow blasts ≥20%, measured via week 24 bone marrow biopsy that could be evaluated centrally in addition to locally]) or unacceptable toxicity. Pacritinib dosing was interrupted for management of grade 3–4 non-haematological toxicities and in case of clinically significant worsening of myelosuppression of duration 7 days or more or associated with infection or bleeding. Pacritinib was resumed when the toxicity resolved to grade 1 or lower or baseline grade. Up to two dose reductions were allowed, first to 300 mg once daily, and then to 200 mg once daily; no dose re-escalation was allowed. Patients randomly assigned to BAT could cross over to pacritinib upon disease progression, or without progression at 24 weeks and beyond. The MPN-SAF TSS (all versions) was completed daily for 7–10 consecutive days before start of study treatment and then daily up to week 48 of the study or until the patient discontinued study treatment. The Patient Global Impression of Change (PGIC), which consists of one domain with scores ranging from 1 (very much improved) to 7 (very much worse), was completed every 8 weeks up to week 24, and then every 12 weeks until the patient discontinued study treatment.
Study visits in the first 24 weeks were on days 4 (week 1), 8 (week 2), 15 (week 3), 28 (week 4), 56 (week 8), 84 (week 12), 112 (week 16), 140 (week 20), and 168 (week 24). After the first 24 weeks, patients were assessed every 12 weeks up to termination of study treatment, with a final assessment at 30 days after treatment termination. After discontinuing treatment, patients were followed up for leukaemia-free survival and overall survival. Adverse events were assessed at each study visit, documented, and reported throughout the study in accordance with International Conference on Harmonisation Good Clinical Practice guidelines and graded according to CTCAE version 4.0. Serious adverse events were followed until the event was resolved, returned to baseline, stabilised, or the patient was lost to follow-up. Patients were called on day 4 of treatment to assess the need for modifying supportive treatments for gastrointestinal adverse events. Bleeding and cardiac events were further assessed by standardised MEDRA query (SMQ) analysis.
Outcomes
The primary endpoint was spleen volume reduction (SVR) of 35% or more from baseline to week 24 as assessed by blinded, centrally reviewed MRI or CT. The key secondary endpoint was the proportion of patients achieving a reduction of 50% or more in TSS from baseline to week 24 on the MPN-SAF TSS 2.0, which was developed at the request of regulatory authorities to more accurately reflect the symptom burden of myelofibrosis than was possible with the original MPN-SAF TSS. However, this trial was initiated using the original MPN-SAF TSS, with version 2.0 introduced following the protocol amendment on Aug 15, 2013. Due to differences between questions and recall periods implemented in the two versions, we analysed patients administered MPN-SAF TSS questionnaires at study entry separately and in combination with TSS 2.0 (using six common symptoms, appendix p 3) as supportive analyses.
Other secondary endpoints were proportions of patients with baseline thrombocytopenia (<100 000 platelets per μL) or severe thrombocytopenia (<50 000 platelets per μL) who achieved SVR of 35% or more or 50% reduction or greater in TSS from baseline to week 24. Exploratory endpoints included quality of life, overall survival, achievement of RBC transfusion independence by Gale criteria (no transfusions for 90 days),24 and improvements in platelet and haemoglobin levels. We assessed symptoms and quality of life using the MPN-SAF TSS 2.0 and the PGIC.
Statistical analysis
The primary hypothesis was that treatment with pacritinib would result in a greater proportion of patients achieving 35% SVR or more at week 24 than with treatment with BAT. We planned a sample size of 270 patients (180 randomly assigned to pacritinib, 90 randomly assigned to BAT) to provide 90% power to detect a treatment difference in the primary endpoint, with a two-sided α of 0·05. We did efficacy analyses using the intention-to-treat (ITT; all randomised patients) and evaluable populations. The evaluable population consisted of all randomised patients with baseline and follow-up assessments relevant for that endpoint. For the primary endpoint, this included patients with baseline and week 24 spleen assessments by MRI or CT.
We tested treatment differences in proportions of patients achieving 35% SVR or more using Fisher’s exact test, with 95% CIs based on the Agresti-Caffo method. We calculated percentage reduction from baseline in TSS at week 24 with:
where baseline TSS was the mean of the daily TSS over the 7 consecutive days preceding randomisation and TSS at week 24 was the mean of the daily TSS over the 28 days before the week 24 visit. For a sensitivity analysis, we redefined TSS at week 24 as the mean of the 7 daily TSS before the week 24 visit; if fewer than 4 daily TSS were available, the TSS for week 24 was considered missing. We tested the primary endpoint at α=0·05 (two-sided); we tested the secondary endpoints in succession at α=0·05 (two-sided) only if the primary endpoint had been reached. We used final end of treatment data (due to clinical hold) for these analyses. The safety population consisted of randomly assigned patients who received at least one dose of pacritinib or BAT, including all patients not receiving active drug (ie, watchful waiting approach). We used SAS version 9.4 for all statistical analyses. This study is registered with ClinicalTrials.gov, number NCT01773187.
Role of the funding source
The study was sponsored by CTI BioPharma. CTI BioPharma was involved in the analysis and interpretation of the data. The first and senior authors (RAM, CNH) prepared the first draft of the manuscript with assistance from a medical writer funded by CTI BioPharma. All authors had access to any data requested, reviewed and approved the manuscript, and vouch for the accuracy and completeness of the data. The corresponding author had full access to all the data and had final responsibility to submit for publication.
Results
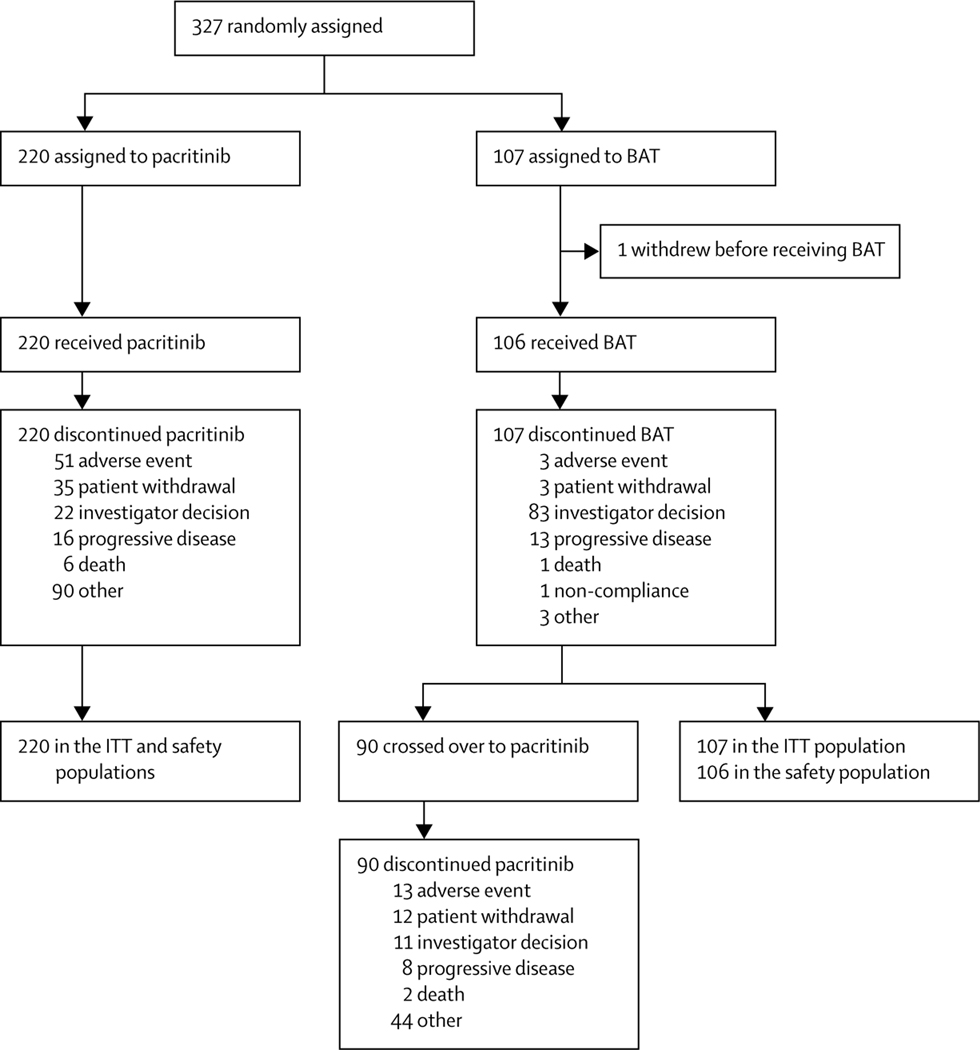
Between Jan 8, 2013, and Aug 1, 2014, 357 patients were assessed for eligibility, with 30 found ineligible. 327 patients were enrolled and randomly assigned to pacritinib (n=220, 67%) or BAT (n=107, 33%; figure 1). One patient randomly assigned to BAT withdrew before receiving treatment and was the only patient not included in the safety population. Median follow-up was 23·2 months (IQR 14·8–28·7) overall: 22·9 months (14·5–28·0) for pacritinib and 24·2 months (15·5–28·7) for BAT. Overall, 171 (78%) of 220 patients in the pacritinib group and 82 (77%) of 107 patients in the BAT group completed 24 weeks of study treatment. The median duration of pacritinib treatment was 15·6 months (IQR 5·6–23·7) and median duration of BAT treatment was 5·9 months (5·6–6·5). 90 patients randomly assigned to BAT (84%) crossed over to receive pacritinib at a median of 6·3 months (IQR 5·8–6·7). Median duration of pacritinib treatment post-crossover was 13·8 months (6·8–17·8).
Figure 1: Trial profile.
84 patients who discontinued pacritinib for other reasons did so due to the clinical hold placed by the US Food and Drug Administration in February, 2016, (hold removed in January, 2017). Reasons given for the remaining six patients were incorrect diagnosis at study entry, treatment lock of efficacy, splenectomy, patient withdrawal from treatment but not from follow-up, clinical deterioration, and terminal illness leading to withdrawal of study drug and replacement with palliative care. 77 patients who discontinued BAT due to investigator decision did so to cross over to pacritinib treatment. The remaining six patients who discontinued BAT due to investigator decision were withdrawn because of the treating physician’s decision to change treatment (this patient remained on study for the following 3 months for safety follow-up), worsening of myelofibrosis symptoms, worsening status of patient (this patient died 1 week after discontinuing BAT), clinical progression (n=2; one patient remained on study for safety follow-up for 6 months and the other for 10 months; both patients died), and heavy overall complications caused by BAT. Of the remaining 13 patients who crossed over to pacritinib, 11 patients discontinued BAT because of disease progression, one patient discontinued BAT because of adverse events, and one patient because of patient withdrawal. BAT=best available therapy. ITT=intention-to-treat.
Baseline demographic and disease characteristics generally seemed well balanced (table 1). However, despite stratification, we noted imbalances in some DIPSS risk factor components between pacritinib and BAT groups (appendix p 3). The most frequently administered treatment in the BAT group was hydroxyurea (60 [57%] of 106 patients); 27 patients (25%) received only watchful waiting and the remainder received a variety of agents typically used to treat myelofibrosis but not approved for myelofibrosis at the time (appendix p 3). Overall, patients in the BAT group who had received hydroxyurea had better prognostic features than did patients who received other agents or no therapy (appendix p 4).
Table 1:
Baseline characteristics
| Pacritinib (n=220) |
BAT (n=107) |
|
|---|---|---|
| Median age (years) | 67(60–73) | 65 (59–72) |
| ≥65 | 135 (61%) | 55 (51%) |
| Sex | ||
| Male | 125 (57%) | 60 (56%) |
| Female | 95 (43%) | 47 (44%) |
| ECOG performance status | ||
| 0–1 | 192 (87%) | 96 (90%) |
| 2–3 | 28 (13%) | 11 (10%) |
| Myelofibrosis diagnosis | ||
| Primary myelofibrosis | 144 (65%) | 59 (55%) |
| Post-polycythaemia vera myelofibrosis | 48 (22%) | 33 (31%) |
| Post-essential thrombocythaemia myelofibrosis | 27 (12%) | 15 (14%) |
| Missing | 1 (<1%) | 0 |
| DIPSS score | ||
| Intermediate-1 | 124 (56%) | 49 (46%) |
| Intermediate-2 | 63 (29%) | 43 (40%) |
| High | 32 (15%) | 15 (14%) |
| Missing | 1 (<1%) | 0 |
| Median spleen length by physical exam (cm)* | 12 (8–16) | 12 (8–17) |
| Median spleen volume by MRI/CT (cm3)† | 2005·6 (1396·6–2889·0) |
2152·7 (1545·2–3136·0) |
| JAK2 Val6l7Phe-positive | 154 (70%) | 92 (86%) |
| Bone marrow biopsy completed | 219 (>99%) | 107 (100%) |
| Reticulin and collagen fibrosis staging | ||
| MF 0–1 | 32/219 (15%) | 18 (17%) |
| MF 2–3 | 180/219 (82%) | 83 (78%) |
| Missing | 7/219 (3%) | 6 (6%) |
| Peripheral blasts | ||
| <1% | 78 (35%) | 44 (41%) |
| ≥1% | 94 (43%) | 38 (36%) |
| <5% | 159 (72%) | 74 (69%) |
| ≥5% | 13 (6%) | 8 (7%) |
| Missing | 48 (22%) | 25 (23%) |
| White blood cell count | ||
| Median×109/L | 9·9 (6·1–21·1) |
11·7 (6·3–24·5) |
| ≤25×109/L | 177 (80%) | 80 (75%) |
| >25×109/L | 43 (20%) | 26 (24%) |
| Haemoglobin | ||
| <10 g/dL | 84 (38%) | 47 (44%) |
| ≥10 g/dL | 136 (62%) | 59 (55%) |
| Red blood cell transfusion dependence‡ | ||
| Dependent | 36 (16%) | 16 (15%) |
| Independent | 156 (71%) | 75 (70%) |
| Indeterminate | 29 (13%) | 16 (15%) |
| Missing | 0 | 0 |
| Platelet count | ||
| <50 000/μL | 35 (16%) | 16 (15%) |
| 50 000–99 999/μL | 37 (17%) | 18 (17%) |
| ≥100 000/μL | 148 (67%) | 73 (68%) |
Data are n (%) or median (IQR). BAT=best available therapy. DIPSS=Dynamic International Prognostic Scoring System. ECOG=Eastern Cooperative Oncology Group.
n=219 for pacritinib, n=106 for BAT.
n=218 for pacritinib.
≥6 units per 90 days as defined as per Gale criteria.24
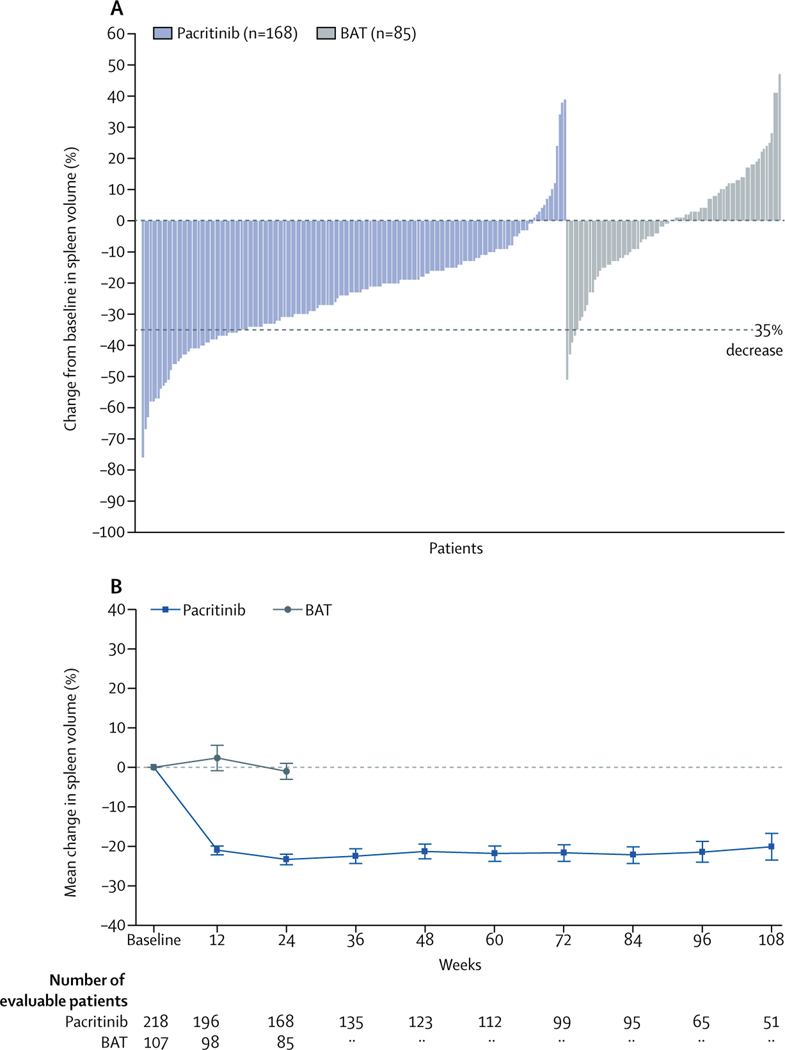
At week 24 in the ITT population, 42 (19%) patients in the pacritinib group had achieved SVR of 35% or more versus five (5%) patients in the BAT group (p=0·0003; table 2). SVR at week 24 was not dependent on baseline spleen volume (Pearson correlation coefficient 0·0163; p=0·80). For patients with primary myelofibrosis, 28 (19%) of 144 patients in the pacritinib group achieved SVR of 35% or more versus two (3%) of 59 patients in the BAT group; for patients with secondary myelofibrosis, 14 (19%) of 75 patients in the pacritinib group achieved SVR of 35% or more versus three (6%) of 48 patients in the BAT group. For patients with baseline thrombocytopenia, significantly more patients in both platelet subgroups achieved SVR of 35% or more in the pacritinib group compared with in the BAT group (table 2). The magnitude of these differences was increased in the evaluable population both overall and in the prespecified platelet subgroups (table 2, figure 2). Median duration of SVR of 35% or more was 34·3 weeks (95% CI 24·1–48·4) in the pacritinib group and not estimable in the BAT group. For evaluable patients in the pacritinib group, the number of patients with SVR of 35% or more at week 24 (25%; table 2) remained similar through the last timepoint (week 108, 13 [27%] of 49 patients). The mean absolute reduction in spleen volume was more than 20% at all timepoints in the pacritinib group (evaluable population); in the BAT group, mean spleen volume did not differ from baseline to week 24 (figure 2). Results were similar for patients with fewer than 50 000 platelets per μL and fewer than 100 000 platelets per μL (appendix p 17).
Table 2:
Patients in the intention-to-treat and evaluable populations overall and by prespecified baseline platelet subgroups who achieved ≥35% reduction in spleen volume at week 24
| Intention-to-treat |
Evaluable |
|||||
|---|---|---|---|---|---|---|
| Pacritinib | BAT | p value | Pacritinib | BAT | p value | |
| Overall | 42/220 (19%) | 5/107 (5%) | 0∙0003 | 42/168 (25%) | 5/85 (6%) | 0∙0001 |
| Platelets | ||||||
| <100 000/μL | 12/72 (17%) | 0/34 | 0∙0086 | 12/51 (24%) | 0/24 | 0∙0072 |
| <50 000/μL | 8/35 (23%) | 0/16 | 0∙045 | 8/24 (33%) | 0/11 | 0∙037 |
Data are n/N (%).
Figure 2: Spleen volume reduction according to treatment group.
(A) Best percentage change from baseline in spleen volume in the first 24 weeks of treatment for evaluable patients. (B) Mean percentage change in spleen volume over time for evaluable patients. Intervals at each timepoint indicate SEM. BAT=best available therapy.
For patients who crossed over from BAT to pacritinib (n=90, ITT population), SVR of 35% or more was achieved in 11 patients (12%) after 24 weeks of pacritinib treatment. For evaluable patients who crossed over, the proportion of patients who had SVR of 35% or more at week 24 (11 [16%] of 68 patients) remained similar through the last timepoint measured post-crossover (week 84, two [13%] of 16 patients). The mean absolute reduction in spleen volume after crossover ranged from 12% to 17% at all timepoints.
Patients in the pacritinib group also showed a greater median reduction in JAK2 Val617Phe allele burden at 24 weeks (−15·8% [IQR −41·0 to 1·4]) compared with patients in the BAT group (−7·9% [−21·3 to 2·4]; p=0·072). For patients in the pacritinib group, the median greatest reduction in allele burden from baseline at any timepoint was −31·6% (−57·5 to −7·5; appendix p 4) and decrease in allele burden was strongly correlated with SVR (p=0·0030; appendix p 4). Data for additional driver mutations of myelofibrosis were not collected.
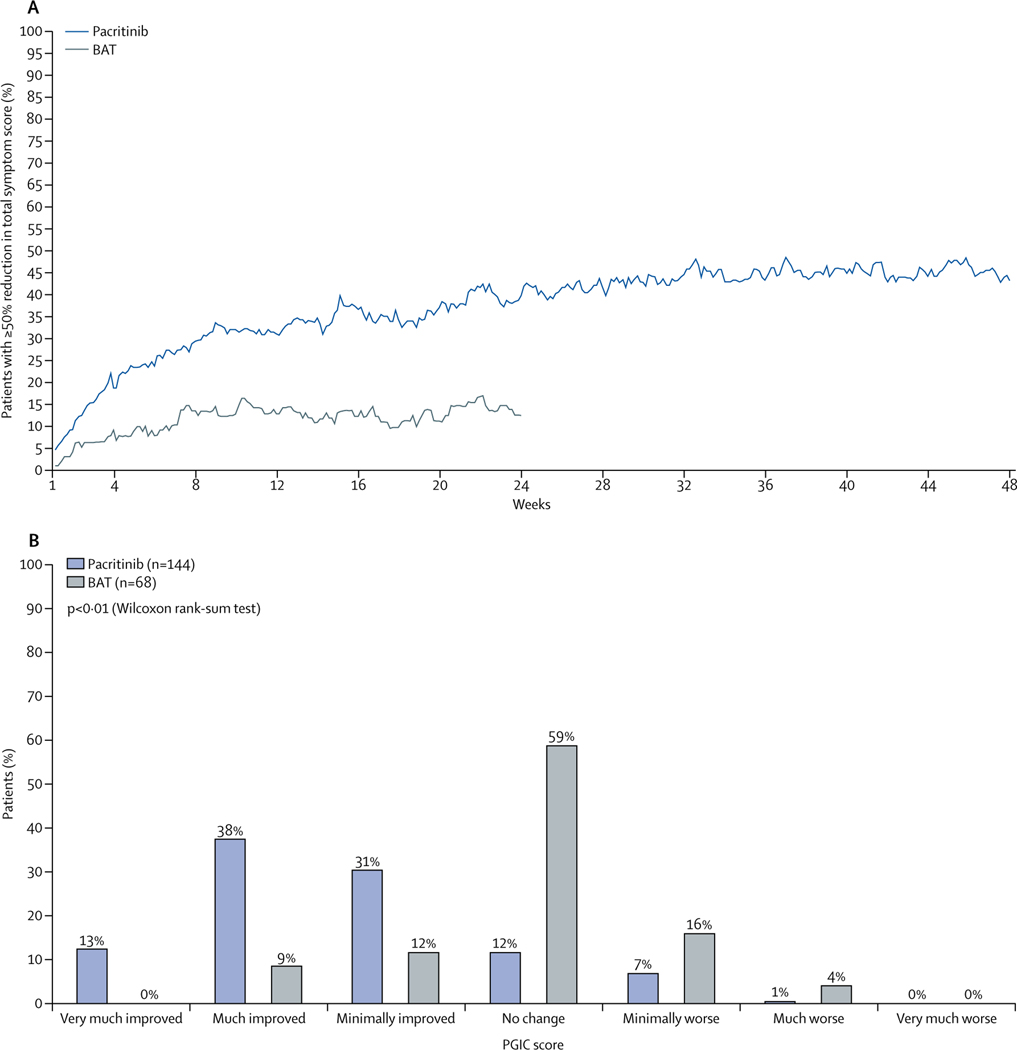
In the ITT population of patients enrolled with TSS 2.0, we observed no difference between the pacritinib and BAT groups in patients achieving 50% reduction or greater in TSS 2.0 from baseline to week 24 (table 3). However, in the evaluable population, 19 (36%) of 53 patients in the pacritnib group achieved this endpoint compared with five (14%) of 36 patients in the BAT group (p=0·029). In the ITT population, we also observed a difference between the pacritinib and BAT groups in patients achieving 50% reduction or greater in TSS 2.0 from baseline to week 48 (table 3). For patients with baseline or severe thrombocytopenia, numerically more patients in the pacritinib group achieved a 50% reduction or greater in TSS 2.0 at weeks 24 and 48 compared with patients in the BAT group, but these results did not achieve significance, probably because of limited numbers of patients in these groups (table 3). Because of the consistent results found between the MPN-SAF TSS and MPN-SAF TSS 2.0 (appendix p 4), the similar and consistent PGIC responses based on the two versions (appendix p 5), and to fully evaluate symptom reduction for all patients, we did a combined analysis using the common six symptoms between both versions (fatigue/tiredness, early satiety, abdominal discomfort, night sweats, pruritus, and bone pain). At week 24, more patients in the pacritinib group achieved 50% reduction or greater in TSS of the six common symptoms compared with patients in the BAT group (ITT: 54 (25%) patients in the pacritinib group vs seven [7%] patients in the BAT group, p<0·0001; evaluable: 54 [41%] of 132 patients in the pacritinib group vs seven [10%] of 71 patients in the BAT group, p<0·0001). Improvements with pacritinib were rapid (more than 20% of evaluable patients in the pacritinib group had at least 50% reduction in TSS by 4 weeks) and the number of patients achieving this level of improvement increased throughout the 48-week period (evaluable: 42 [46%] of 91 patients in the pacritinib group vs one [17%] of six patients in the BAT group; figure 3). Median improvements in each individual symptom score were also greater in the pacritinib group than in the BAT group at weeks 24 and 48, apart from bone pain at week 48 (appendix p 5). Improvements in TSS correlated with improvements in PGIC (figure 3). Among evaluable patients who crossed over from BAT to pacritinib, the percentage of patients who achieved 50% reduction or greater in TSS of the six common symptoms increased from 15% (seven of 47 patients) at week 24 to 30% (eight of 27 patients) at week 36 after crossover. At week 48 post-crossover, three (17%) of 18 patients had 50% reduction or greater in TSS.
Table 3:
Patients in the intention-to-treat population overall and prespecified platelet subgroups achieving ≥50% reduction in Total Symptom Score 2.0 at weeks 24 and 48
| Week 24 |
Week 48 |
|||||
|---|---|---|---|---|---|---|
| Pacritinib | BAT | p value | Pacritinib | BAT | p value | |
| Overall | 19/100 (19%) | 5/48 (10%) | 0∙24 | 15/100 (15%) | 0/48 | 0∙0027 |
| Platelets | ||||||
| <100 000/μL | 7/28 (25%) | 1/13 (8%) | 0∙40 | 3/28 (11%) | 0/13 | 0∙54 |
| <50 000/μL | 3/11 (27%) | 0/5 | 0∙51 | 2/11 (18%) | 0/5 | 1∙0 |
Data are n/N (%).
Figure 3: Change in total symptom score.
(A) Percentage of evaluable patients achieving 50% reduction or greater over time for the six common symptoms between the MPN-SAF TSS original version and TSS version 2.0. (B) Patient Global Impression of Change (PGIC) responses for evaluable patients at week 24. BAT=best available therapy. MPN-SAF=Myeloproliferative Neoplasm Symptom Assessment Form. TSS=total symptom score.
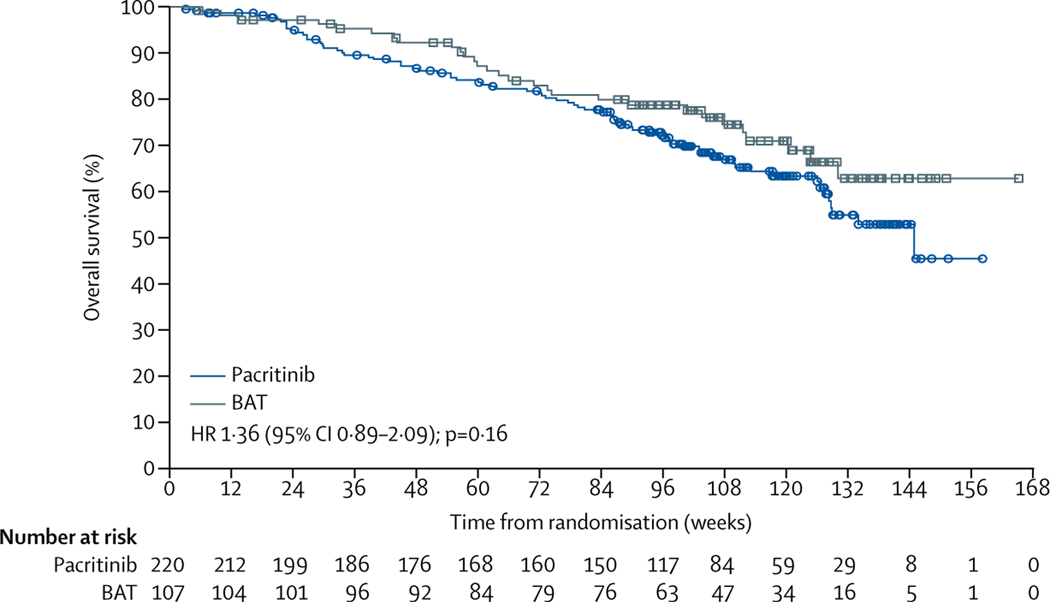
Before week 24, overall survival (in the ITT population) was similar between both treatment groups (figure 4). The probability of survival at 24 weeks did not differ: 95% (95% CI 91–97) for patients in the pacritinib group versus 97% (92–99) for patients in the BAT group. Achievement of SVR greater than 10% at week 24 correlated with improved overall survival relative to achievement of SVR of less than 10% in patients in the pacritinib group (appendix p 5). We found no correlation between SVR at week 24 and overall survival for patients in the BAT group. After week 24, results suggested improved survival for the BAT group; however, 90 (84%) BAT-treated patients crossed over to receive pacritinib (primarily at week 24). At final analysis, 76 (35%) patients in the pacritinib group and 29 (27%) patients in the BAT group had died (appendix p 6).
Figure 4: Overall survival (intention-to-treat population).
Squares and circles show censored patients. BAT=best available therapy. HR=hazard ratio.
Changes in platelet levels from baseline between patients in the pacritinib and BAT groups through week 24 were not significant (appendix p 19). In patients in the pacritinib group with a baseline platelet count of fewer than 50 000 platelets per μL, treatment with pacritinib resulted in increases in platelets up to and including week 24 (p=0·055; appendix pp 19–20). Regarding anaemia, differences in mean change in haemoglobin levels from baseline between the pacritinib and BAT groups for all patients and patients with baseline anaemia (haemoglobin levels <10 g/dL) are presented in the appendix (pp 20–21). For patients in the pacritinib group with baseline haemoglobin levels lower than 10 g/dL who did not receive transfusions, median haemoglobin on pacritinib increased from 9·1 g/dL at baseline to 10·4 g/dL at week 24 (p=0·017), whereas concentrations did not differ in the BAT group (9·2 g/dL to 9·5 g/dL; p=0·18). A greater proportion of patients in the pacritinib group who were RBC transfusion dependent at baseline achieved RBC transfusion independence during the study (nine [25%] of 36 patients) compared with none of 16 patients in the BAT group (p=0·043).
Total patient-years of exposure were 280·0 to pacritinib (excluding crossover patients) versus 60·0 to BAT. Diarrhoea, nausea, and vomiting were the most frequently reported non-haematological adverse events and were more frequent in the pacritinib group than in the BAT group (table 4, appendix p 7). More than half of these events in the pacritinib group were grade 1 in severity, both through week 24 and at any time during the study. In the pacritinib group, grade 3 diarrhoea occurred in 16 (7%) patients, nausea in three (1%) patients, and vomiting in six (3%) patients at any time during the study (appendix p 8); no grade 4 or 5 events were observed for diarrhoea, nausea, and vomiting. The most common grade 3–4 adverse events through week 24 were anaemia (n=37 [17%]), thrombocytopenia (n=26 [12%]), and diarrhoea (n=11 [5%]) in the pacritinib group, and anaemia (n=16 [15%]), thrombocytopenia (n=12 [11%]), dyspnoea (n=3 [3%]), and hypotension (n=3 [3%]) in the BAT group. The most common serious adverse events through week 24 were anaemia (n=10 [5%]), cardiac failure (n=5 [2%]), pyrexia (n=4 [2%]), and pneumonia (n=4 [2%]) in the pacritinib group, and anaemia (n=5 [5%]), sepsis (n=2 [2%]), and dyspnoea (n=2 [2%]) in the BAT group (appendix p 9).
Table 4:
Most common adverse events up to week 24 or initial treatment discontinuation
| Pacritinib (n=220) |
BAT (n=106) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1/2 | Grade 3 | Grade 4 | Grade 5 | All | Grade 1/2 | Grade 3 | Grade 4 | Grade 5 | All | |
| Diarrhoea | 109 (50%) | 11 (5%) | 0 | 0 | 120 (55%) | 11 (10%) | 0 | 0 | 0 | 11 (10%) |
| Nausea | 58 (26%) | 2 (1%) | 0 | 0 | 60 (27%) | 7 (7%) | 0 | 0 | 0 | 7 (7%) |
| Anaemia | 15 (7%) | 32 (15%) | 5 (2%) | 0 | 52 (24%) | 5 (5%) | 13 (12%) | 3 (3%) | 0 | 21 (20%) |
| Thrombocytopenia | 11 (5%) | 12 (5%) | 14 (6%) | 0 | 37 (17%) | 3 (3%) | 9 (8%) | 3 (3%) | 0 | 15 (14%) |
| Vomiting | 34 (15%) | 2 (1%) | 0 | 0 | 36 (16%) | 6 (6%) | 0 | 0 | 0 | 6 (6%) |
| Fatigue | 17 (8%) | 5 (2%) | 0 | 0 | 22 (10%) | 9 (8%) | 1 (1%) | 0 | 0 | 10 (9%) |
| Abdominal pain | 18 (8%) | 3 (1%) | 0 | 0 | 21 (10%) | 10 (9%) | 0 | 0 | 0 | 10 (9%) |
| Peripheral oedema | 16 (7%) | 1 (<1%) | 0 | 0 | 17 (8%) | 12 (11%) | 1 (1%) | 0 | 0 | 13 (12%) |
| Decreased appetite | 11 (5%) | 1 (<1%) | 0 | 0 | 12 (5%) | 3 (3%) | 0 | 0 | 0 | 3 (3%) |
| Electrocardiogram QT prolonged | 9 (4%) | 3 (1%) | 0 | 0 | 12 (5%) | 1 (1%) | 0 | 0 | 0 | 1 (1%) |
| Pyrexia | 7 (3%) | 4 (2%) | 0 | 0 | 11 (5%) | 10 (9%) | 1 (1%) | 0 | 0 | 11 (10%) |
| Pneumonia | 3 (1%) | 4 (2%) | 0 | 1 (<1%) | 8 (4%) | 0 | 0 | 0 | 0 | 0 |
| Leucopenia | 3 (1%) | 3 (1%) | 1 (<1%) | 0 | 7 (3%) | 0 | 2 (2%) | 0 | 0 | 2 (2%) |
| Hypertension | 2 (1%) | 5 (2%) | 0 | 0 | 7 (3%) | 1 (1%) | 0 | 0 | 0 | 1 (1%) |
| Cardiac failure | 1 (<1%) | 5 (2%) | 0 | 0 | 6 (4%) | 0 | 1 (1%) | 0 | 1 (1%) | 2 (2%) |
| Atrial fibrillation | 1 (<1%) | 3 (1%) | 0 | 0 | 4 (2%) | 1 (1%) | 0 | 0 | 0 | 1 (1%) |
Data are n (%). Table shows all-grade adverse events in more than 5% of patients in the pacritinib group or grade 3 in more than 1% of patients in the pacritinib group. Additional grade 4 adverse events in the pacritinib group were neutropenia (n=4), platelet count reduction (n=3), hyperuricemia (n=2), hyperkalaemia (n=1), cardiac failure congestive (n=1), thrombocytosis (n=1), sepsis (n=1), delayed haemolytic transfusion reaction (n=1), neutrophil count reduction (n=1), cerebral haemorrhage (n=1), and acute respiratory distress syndrome (n=1). Additional grade 5 adverse events in the pacritinib group were disease progression (n=1), cardiorespiratory arrest (n=1), and multiorgan failure (n=1). Additional grade 4 adverse events in the BAT group were neutropenia (n=1), colon neoplasm (n=1), hypoglycaemia (n=1), sepsis (n=1), hyponatraemia (n=1), septic shock (n=1), acute respiratory distress syndrome (n=1). Additional grade 5 adverse events in the BAT group were disease progression (n=1) and sepsis (n=1). BAT=best available therapy.
Diarrhoea was the most frequently observed gastrointestinal adverse event; median time to onset among patients in the pacritinib group who had diarrhoea was 3·1 weeks (95% CI 1·1–25·4) and median time to resolution of first event was 2·1 weeks (1·1–4·0, appendix p 9). Median time to resolution of first event was 2·1 weeks (1·4–4·3) for nausea and 0·3 weeks (0·1–0·4) for vomiting. At any time during the study, nine (4%) patients in the pacritinib group discontinued treatment due to gastrointestinal adverse events. In the pacritinib group, diarrhoea led to discontinuation in six (3%) patients and dose reductions in 11 (5%) patients. At baseline, diarrhoea, nausea, and vomiting were observed among two (<1%), two (<1%), and three (1%) of all patients, respectively. Anaemia and thrombocytopenia were the most frequently observed haematological toxicities in patients in both groups. Worsening (by grade) of pre-existing thrombocytopenia and anaemia was similar between groups (appendix p 7). Overall, for patients with baseline assessments, 129 (42%) of 307 patients had baseline grade 1–4 thrombocytopenia (50 [16%] patients had grade ≥3), and 231 (71%) of 326 patients had baseline grade 1–3 anaemia (29 [9%] patients had grade 3). Importantly, among the 90 patients in the BAT group who crossed over to receive pacritinib, incidence of any-grade anaemia and thrombocytopenia were similar after crossover (16 [18%] patients with anaemia and 11 [12%] patients with thrombocytopenia before crossover vs 19 [21%] patients with anaemia and 15 [16%] patients with thrombocytopenia after crossover), but incidence of diarrhoea increased after crossover (10 [11%] patients vs 52 [58%] patients). In the pacritinib group, adverse events and resulting dose modifications were numerically higher in patients with baseline thrombocytopenia (appendix p 10).
In the safety population, bleeding events (assessed by SMQ) of any grade occurred in 43 (20%) patients in the pacritinib group and 20 (19%) patients in the BAT group through week 24 (appendix p 11); severe bleeding events (SMQ; grade 3–4) were infrequent through week 24 (seven [3%] patients in the pacritinib group vs two [2%] patients in the BAT group). In the pacritinib group, 14 (6%) patients had grade 3–4 bleeding events at any time during the study (median duration of treatment 15·6 months; appendix pp 12–13); events reported in more than one patient in the pacritinib group were epistaxis (n=4), haematoma (n=2), and postprocedural haemorrhage (n=2). Ten (71%) of 14 patients in the pacritinib group with severe bleeding events had resolution of first event, at a median of 2·3 weeks (95% CI 1·0 to not estimable). Grade 3–4 bleeding events were reported at any time post-crossover to pacritinib in seven (8%) of 90 patients.
In the safety population, cardiac events (assessed by SMQ) of any grade occurred through week 24 in 44 (20%) patients in the pacritinib group and 22 (21%) patients in the BAT group (appendix p 14). Severe (grade 3–4) cardiac events were infrequent through week 24 (18 [8%] patients in the pacritinib group vs six [6%] patients in the BAT group). In the pacritinib group, 27 (12%) patients had grade 3–4 cardiac events at any time during the study (appendix pp 14–15); grade 3–4 events at any time reported in more than one patient in the pacritinib group were cardiac failure (n=6), atrial fibrillation (n=4), congestive cardiac failure (n=4), electrocardiogram (ECG) QT prolonged (n=3), syncope (n=2), and pulmonary oedema (n=2). 18 (69%) of 26 patients in the pacritinib group with grade 3–4 cardiac events had resolution of first event, at a median of 1·2 weeks (95% CI 0·9–1·9). Grade 3–4 cardiac events were reported at any time post-crossover to pacritinib in seven (8%) of 90 patients. ECG QTc intervals greater than 480 ms were reported in eight (4%; four [2%] >500 ms) patients in the pacritinib group and one (1%) patient in the BAT group. We found no meaningful differences in deaths due to cardiac or bleeding events between patients in the pacritinib group and patients in the BAT group (appendix p 6).
Median leucocyte and neutrophil counts decreased slightly from baseline to week 24 in patients in both groups (appendix p 15). Peripheral neuropathy was observed in two (1%) patients in the pacritinib group and four (4%) patients in the BAT group. Incidence of serious opportunistic infections was low; herpes zoster infections were observed in three (1%) patients in the pacritinib group and no patients in the BAT group. Extrapulmonary tuberculosis was reported in one (<1%) patient in the pacritinib group. Progressive multifocal leukoencephalopathy was not reported in either group. The mean relative dose intensity observed with pacritinib was 94·9%, and 104 (47%) patients in the pacritinib group required dose modifications owing to adverse events. Through week 24, dose reductions owing to adverse events occurred in 22 (10%) patients in the pacritinib group compared with nine (9%) patients in the BAT group. Through week 24, 22 (10%) patients in the pacritinib group discontinued due to adverse events compared with three (3%) patients in the BAT group (appendix p 10).
Deaths due to adverse events were observed in 27 (12%) patients in the pacritinib group (eight [4%] during the first 24 weeks of treatment) and 14 (13%) patients in the BAT group (11 [10%] after crossover to pacritinib, appendix p 6). The most frequent adverse events leading to death in the pacritinib group were disease progression (n=6) and pneumonia (n=3). Leukaemic transformation was observed at any time in 11 (5%) patients in the pacritinib group compared with two (2%) patients in the BAT group (p=0·23), with no transformations after crossover.
We did post-hoc analyses to further examine the effect of baseline imbalances in prognostic variables for overall survival. A post-hoc, exploratory multivariate Cox analysis of randomisation stratification variables, DIPSS risk factors, and other baseline characteristics that were identified as potentially affecting overall survival showed that increased age, decreased platelet count, increased white blood cell count, and decreased haemoglobin level were significantly associated with reduced overall survival (appendix p 6). After adjusting for these risk factors with Cox modeling, the hazard ratio for pacritinib compared with BAT was 1·22 (95% CI 0·79–1·88) versus 1·36 (0·89–2·09) in the primary ITT analysis. An analysis of various subgroups showed that baseline white blood cell count greater than 25 000 cells per μL was associated with reduced overall survival (appendix p 18), particularly for patients randomly assigned to pacritinib. Further examination of six key risk factors (white blood cell count >25 000/μL, peripheral blood blasts ≥1%, platelet count <100 000/μL, haemoglobin <10 g/dL, myelofibrosis grade >1, and age >65 years) showed that patients with white blood cell count greater than 25 000 cells per μL randomly assigned to pacritinib had notably higher rates and multiplicity of other adverse risk factors than did patients with baseline white blood cell count of 25 000 cells per μL or lower (appendix p 3). Curves for overall survival by treatment group, segmented by multiplicity of risk factors, are shown in the appendix (p 18).
Discussion
In this phase 3 study of pacritinib versus BAT in patients with myelofibrosis, pacritinib therapy was well tolerated and induced significant and sustained SVR and symptom reduction, even in patients with severe baseline cytopenias. Treatment options for patients with myelofibrosis and severe anaemia or thrombocytopenia are limited. Approximately 58% of patients with myelofibrosis will become anaemic and 28% of patients will become thrombocytopenic within a year from diagnosis; these percentages increase with time from diagnosis, and these cytopenias are associated with shorter survival.6,7 Patients with myelofibrosis and cytopenias are a substantially underserved population. Currently, the only disease-specific approved therapy for patients with myelofibrosis, ruxolitinib, is not indicated for initiation in patients with fewer than 50 000 platelets per μL, and its efficacy (particularly in terms of SVR) has been shown to be reduced in patients with fewer than 100 000 platelets per μL due to the need for dose reduction.15 Other JAK2 inhibitors and novel agents in development have also been associated with a relatively high incidence of grade 3–4 thrombocytopenia.25,26 In this randomised, phase 3 study, treatment with pacritinib resulted in a significantly greater proportion of patients achieving SVR of 35% or more at week 24 compared with BAT, regardless of the inclusion of patients with low baseline platelet counts and specifically in the subsets of patients with fewer than 100 000 or 50 000 platelets per μL. Missed assessments at baseline or at week 24 created a discrepancy in patient numbers between ITT and evaluable populations; however, the difference in SVR of 35% or more between patients in the pacritinib and BAT groups reached statistical significance in both populations. Responses to pacritinib were durable, with the proportion of patients achieving SVR of 35% or more maintained through week 108.
In addition, among evaluable patients, pacritinib was associated with significant and durable improvements in TSS; the proportion of patients in the pacritinib group with 50% reduction or greater in TSS increased over time through week 48 (last symptom assessment). In patients with baseline cytopenias (<50 000 platelets per μL or haemoglobin <10 g/dL) in the pacritinib group, meaningful improvements were observed in platelet and haemoglobin levels. Among patients who were RBC transfusion dependent at baseline, only patients treated with pacritinib achieved RBC transfusion independence.
The most frequently occurring adverse events with pacritinib were manageable gastrointestinal symptoms, particularly diarrhoea and nausea. Although gastrointestinal adverse events have been reported with JAK inhibitors, the mechanisms for this have not been elucidated. In this study, gastrointestinal adverse events were primarily of grades 1–2 and generally resolved with standard measures without precluding continued pacritinib therapy. Although greater incidences of cardiac and bleeding events occured in patients in the pacritinib group than in the BAT group, the longer exposure to pacritinib compared with BAT and the disproportionate number of patients with adverse risk factors in the pacritinib group might have been partially contributory. Although pacritinib is a potent inhibitor of JAK2, the absence of activity against JAK1 might contribute to the relative absence of dose-related thrombocytopenia and anaemia.18 Additionally, although inhibition of JAK1 has known anti-inflammatory effects,27 observed anti-inflammatory activity with pacritinib might instead be due to inhibition of IRAK1 and CSF1R.28,29
Final data presented herein showed similar incidence of cardiac and bleeding adverse events (assessed by SMQ) with pacritinib or BAT through week 24, and infrequent reports of severe cardiac or bleeding adverse events with pacritinib at any time on study. Overall survival did not differ significantly between the treatment groups. Confounding factors include imbalances in baseline covariates with known prognostic effects on overall survival and crossover of 90 patients (84%) from the BAT group. Crossover from BAT and the resulting differences in time on treatment and patient-years of exposure also impact safety analyses; most deaths occurred after week 24 and therefore after many BAT patients had crossed over to pacritinib. Despite these confounding factors, results of our study show that treatment with pacritinib induces significant reduction in splenomegaly and improvement in symptoms in patients with myelofibrosis, regardless of the presence of severe cytopenias, and is minimally myelosuppressive. The separate phase 3 PERSIST-2 study23 of pacritinib (400 mg once daily or 200 mg twice daily) versus BAT, including ruxolitinib, in patients with myelofibrosis and baseline thrombocytopenia (≤100 000 platelets per μL) has also shown that pacritinib was significantly more effective than BAT for SVR with an improved benefit–risk profile. Additional studies of pacritinib in patients with myelofibrosis are planned, including a dose-exploration trial in patients with primary myelofibrosis with whom prior ruxolitinib therapy has failed.
Supplementary Material
Research in context.
Evidence before this study
Myelofibrosis is a myeloproliferative neoplasm, the characteristics of which include marked splenomegaly, extramedullary haemopoiesis, bone marrow fibrosis that contributes to anaemia and thrombocytopenia, and risk of transformation to acute leukaemia. Ruxolitinib, a JAK1/2 inhibitor, is the only approved therapy for patients with myelofibrosis. Although ruxolitinib reduces splenomegaly and constitutional symptoms, it is also associated with myelosuppression and is not indicated for patients with severe thrombocytopenia, a disease feature in approximately 25% of patients with myelofibrosis. Data from phase 2 studies of pacritinib in patients with myelofibrosis showed that pacritinib was effective at reducing splenomegaly and improving symptoms in patients with myelofibrosis, including those with anaemia and severe thrombocytopenia.
Added value of this study
To our knowledge, PERSIST-1 is the first randomised study of pacritinib in patients with myelofibrosis that does not exclude patients with severe thrombocytopenia (baseline platelet count <100 000 platelets per μL).
Implications of all the available evidence
The results of this study indicate that pacritinib can induce significant reduction in splenomegaly and improvement in disease-related symptoms in patients with myelofibrosis, regardless of the presence of severe cytopenias, and is minimally myelosuppressive.
Acknowledgments
We thank Stacey Rose of Nexus Global Group Science (Chicago, IL, USA) for providing medical writing assistance.
Declaration of interests
RAM reports research support from Incyte, Gilead, CTI BioPharma, Promedior, Genentech, Lilly, and NS Pharma; and consultancy for Novartis, Galena, and Ariad. AMV reports grants and personal fees from Novartis. AP reports speaker funding from Novatis. JM reports grants, personal fees, and non-financial support from Cell Therapeutics; and grants from Novartis. JWS, HZ, and JPD are employed by CTI BioPharma and have stock in CTI BioPharma. PAtB reports personal fees from Novartis, CTI BioPharma, Alexion, BMS, and Gilead. J-JK reports grants and personal fees from Novartis. CNH reports personal fees from CTI BioPharma; research funding from Novartis; speaker funding from CTI BioPharma, Novartis, Sanofi, and Baxter; and advisor fees from CTI BioPharma, Novartis, Gilead, and Baxter.
Footnotes
The remaining authors declare no competing interests.
Contributor Information
Ruben A Mesa, Division of Hematology and Medical Oncology, Mayo Clinic, Scottsdale, AZ, USA.
Alessandro M Vannucchi, Center of Research and Innovation of Myeloproliferative Neoplasms, Department of Experimental and Clinical Medicine, AOU Careggi and University of Florence, Florence, Italy.
Adam Mead, Clinical Haematology, NIHR Biomedical Research Centre, Churchill Hospital, Oxford, UK.
Miklos Egyed, Department of Hematology, Somogy County Kaposi Mor Hospital, Kaposvar, Hungary.
Anita Szoke, Second Department of Internal Medicine and Cardiology Center, Albert Szent-Györgyi Clinical Center, University of Szeged, Szeged, Hungary.
Aleksandr Suvorov, First Republican Clinical Hospital of Udmurtia, Izhevsk, Russia.
Janos Jakucs, Békés Megyei Pándy Kálmán Kórház, Gyula, Hungary.
Andrew Perkins, ICON Cancer Care, South Brisbane, QLD, Australia.
Ritam Prasad, Royal Hobart Hospital, Hobart, Australia.
Jiri Mayer, Department of Internal Medicine, University Hospital Brno, Brno, Czech Republic.
Judit Demeter, First Department of Internal Medicine, Semmelweis University, Budapest, Hungary.
Peter Ganly, Department of Haematology, Christchurch Hospital, Christchurch, New Zealand.
Jack W Singer, CTI BioPharma Corp, Seattle, WA, USA.
Huafeng Zhou, CTI BioPharma Corp, Seattle, WA, USA.
James P Dean, CTI BioPharma Corp, Seattle, WA, USA.
Peter A te Boekhorst, Erasmus Medical Center, Rotterdam, Netherlands.
Jyoti Nangalia, Wellcome Trust Sanger Instutite, Hinxton, UK.
Jean-Jacques Kiladjian, Centre d’Investigations Cliniques, APHP, Hopital Saint-Louis, INSERM CIC 1427, Paris, France; Paris Diderot University, Paris, France.
Claire N Harrison, Department of Haematology, Guy’s and St Thomas’ NHS Foundation Trust, London, UK.
References
- 1.Stein BL, Cervantes F, Giles F, Harrison CN, Verstovsek S. Novel therapies for myelofibrosis. Leuk Lymphoma 2015; 56: 2768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geyer HL, Mesa RA. Therapy for myeloproliferative neoplasms: when, which agent, and how? Blood 2014; 124: 3529–37. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A. How I treat myelofibrosis. Blood 2011; 117: 3494–504. [DOI] [PubMed] [Google Scholar]
- 4.Cervantes F. How I treat myelofibrosis. Blood 2014; 124: 2635–42. [DOI] [PubMed] [Google Scholar]
- 5.Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood 2013; 122: 1395–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol 2011; 29: 392–97. [DOI] [PubMed] [Google Scholar]
- 7.Tefferi A, Lasho TL, Jimma T, et al. One thousand patients with primary myelofibrosis: the mayo clinic experience. Mayo Clin Proc 2012; 87: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer 2007; 109: 68–76. [DOI] [PubMed] [Google Scholar]
- 9.Geyer H, Scherber R, Kosiorek H, et al. Symptom burden profile in myelofibrosis patients with thrombocytopenia: lessons and unmet needs. Blood 2015; 126: 4080 (abstr). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alhuraiji A, Masarova L, Bose P, et al. Clinical features and outcome of patients with poor-prognosis myelofibrosis based on platelet count <50 × 10⁹/L: a single-cener experience in 1100 myelofibrosis patients. Proc Am Soc Clin Oncol 2016; 34 (suppl): 7068 (abstr). [Google Scholar]
- 11.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012; 366: 787–98. [DOI] [PubMed] [Google Scholar]
- 12.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012; 366: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verstovsek S, Mesa RA, Gotlib J, et al. The clinical benefit of ruxolitinib across patient subgroups: analysis of a placebo-controlled, Phase III study in patients with myelofibrosis. Br J Haematol 2013; 161: 508–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verstovsek S, Gotlib J, Gupta V, et al. Management of cytopenias in patients with myelofibrosis treated with ruxolitinib and effect of dose modifications on efficacy outcomes. Onco Targets Ther 2013; 7: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talpaz M, Paquette R, Afrin L, et al. Interim analysis of safety and efficacy of ruxolitinib in patients with myelofibrosis and low platelet counts. J Hematol Oncol 2013; 6: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tefferi A, Passamonti F, Barbui T, et al. Phase 3 study of pomalidomide in myeloproliferative neoplasm (MPN)-associated myelofibrosis with RBC-transfusion-dependence. Blood 2013; 122: 394.23687088 [Google Scholar]
- 17.Hatzimichael E, Tsolas E, Briasoulis E. Profile of pacritinib and its potential in the treatment of hematologic disorders. J Blood Med 2014; 5: 143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer JW, Al-Fayoumi S, Ma H, Komrokji RS, Mesa R, Verstovsek S. Comprehensive kinase profile of pacritinib, a nonmyelosuppressive Janus kinase 2 inhibitor. J Exp Pharmacol 2016; 8: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furqan M, Mukhi N, Lee B, Liu D. Dysregulation of JAK-STAT pathway in hematological malignancies and JAK inhibitors for clinical application. Biomark Res 2013; 1: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia 2009; 23: 1441–45. [DOI] [PubMed] [Google Scholar]
- 21.Zhou T, Georgeon S, Moser R, Moore DJ, Caflisch A, Hantschel O. Specificity and mechanism-of-action of the JAK2 tyrosine kinase inhibitors ruxolitinib and SAR302503 (TG101348). Leukemia 2014; 28: 404–07. [DOI] [PubMed] [Google Scholar]
- 22.Komrokji RS, Seymour JF, Roberts AW, et al. Results of a phase 2 study of pacritinib (SB1518), a JAK2/JAK2(V617F) inhibitor, in patients with myelofibrosis. Blood 2015; 125: 2649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mascarenhas J, Hoffman R, Talpaz M, et al. Results of the PERSIST-2 phase 3 study of pacritinib (PAC) versus best available therapy (BAT), including ruxolitinib (RUX), in patients (pts) with myelofibrosis (MF) and paltelet counts <100,000/μL. Blood 2016; 128: LBA-5 (abstr). [Google Scholar]
- 24.Gale RP, Barosi G, Barbui T, et al. What are RBC-transfusion-dependence and -independence? Leuk Res 2011; 35: 8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardanani A, Laborde RR, Lasho TL, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia 2013; 27: 1322–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tefferi A, Lasho TL, Begna KH, et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N Engl J Med 2015; 373: 908–19. [DOI] [PubMed] [Google Scholar]
- 27.Guschin D, Rogers N, Briscoe J, et al. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transudction pathway in response to interleukin-6. EMBO J 1995; 14: 1421–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhyasen GW, Starczynowski DT. IRAK signalling in cancer. Br J Cancer 2015; 112: 232–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol 2006; 18: 39–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.