Abstract
Background
Hospital-associated infection (HAI) and antimicrobial resistance (AMR) are major health threats in low- and middle-income countries (LMICs). Because diagnostic capacity is lacking throughout most of Africa, patients are commonly managed with prolonged empirical antibiotic therapy. Our goal was to assess mortality in relation to HAI and empirical therapy in Ethiopia’s largest referral hospital.
Methods
Cohort study of patients with suspected HAI at Tikur Anbessa Specialized Hospital from October 2016 to October 2018. Blood culture testing was performed on an automated platform. Primary outcomes were proportion of patients with bloodstream infection (BSI), antibiotic resistance patterns and 14 day mortality. We also assessed days of therapy (DOT) pre- and post-blood culture testing.
Results
Of 978 enrolled patients, 777 had blood culture testing; 237 (30%) had a BSI. Enterobacteriaceae were isolated in 49%; 81% of these were cephalosporin resistant and 23% were also carbapenem resistant. Mortality at 14 days was 31% and 21% in those with and without BSI, respectively. Ceftriaxone resistance was strongly correlated with mortality. Patients with BSI had longer DOT pre-blood culture testing compared with those without BSI (median DOT 12 versus 3 days, respectively, P < 0.0001). After testing, DOT were comparable between the two groups (20 versus 18 days, respectively).
Conclusions
BSI are frequent and fatal among patients with suspected HAI in Ethiopia. Highly resistant blood isolates are alarmingly common. This study provides evidence that investing in systematic blood culture testing in LMICs identifies patients at highest risk of death and that empirical management is frequently inappropriate. Major investments in laboratory development are critical to achieve better outcomes.
Introduction
Antimicrobial resistance (AMR) is a major public health threat for low- and middle-income countries (LMICs).1 Economic development, rising rates of hospitalizations and their attendant risk of hospital-associated infection (HAI) are correlated with increases in antibiotic consumption, particularly ‘last-resort’ drugs such as carbapenems.2
Several studies have shown increasing AMR in sub-Saharan Africa,3–7 but inadequate bacteriology laboratory capacity limits surveillance at the national level and precludes aetiological diagnosis of infections for individual patients.8–10 While the need to implement bacteriology services in LMICs is now acknowledged,11 these have yet to be prioritized at the hospital level—in large part because of poor advocacy and lack of secure funding.12,13
We recently implemented a laboratory intervention aimed at improving clinical bacteriology testing in Ethiopia’s largest referral hospital.14 This setting is considered to have low resources but moderate infrastructure and is very similar to other referral hospitals in sub-Saharan Africa.15 In the first 18 months of implementation, the number of blood cultures tested increased from an average of 2 per day to over 45 per day for this 800 bed tertiary hospital. Here, we report a prospective cohort study, the primary objective being to assess the prevalence and clinical outcomes of bloodstream infections (BSIs) and characterize patterns of AMR among hospitalized patients. Our secondary objective was to assess antibiotic usage in association with timing of blood culture collection in this setting.
Methods
Setting
The study was conducted in Tikur Anbessa Specialized Hospital (TASH), the largest medical training and tertiary referral centre in Ethiopia. The hospital is situated in Addis Ababa and provides paediatric, medical, surgical, obstetrical and critical (ICU) care to 20 000 inpatients and 330 000 outpatients per year. The average length of stay is 9.3 days and inpatient mortality for the year preceding this study was 5.8%.
Ethics
The study was approved by the Institutional Review Boards of Addis Ababa University (protocol no 045/15/IM) and the Research Institute of the McGill University Health Centre (study 15-178 MUHC).
Design
This was a prospective cohort study of BSI among patients hospitalized at TASH from October 2016 to October 2018. Treating teams were notified through educational sessions and posters that blood culture testing was available, at no cost, for patients meeting eligibility criteria: fever (body temperature >38°C) in the preceding 24 h or suspected new infection. Those with a history of fever that had resolved more than 24 h earlier or whose discharge was imminent (within 24 h) were excluded.
Bacteriology testing
The laboratory-strengthening intervention, which included training, protocols and quality assurance measures has been described elsewhere.14 Laboratory consumables for this project [blood culture bottles, reagents for identification and antibiotic susceptibility testing (AST)] were provided to the laboratory through study funds.
Treating teams collected 7–10 mL of blood from adults (older than 16 years of age) with new fever or signs of infection. Blood was inoculated into at least one aerobic bottle and incubated in the BacT/ALERT system (bioMérieux, France) for up to 5 days. Blood cultures that had not flagged positive after 5 days were reported as negative. From positive bottles, a Gram stain and bacterial identification were done as per protocol. Bacteria representing normal skin flora, such as CoNS, were considered to be contaminants unless the same organism was recovered from two or more blood cultures from the same patient within the same episode.
AST was performed by the disc diffusion method and interpreted according to CLSI breakpoints.16 AST panels were selected based on antibiotics available in Ethiopia at the time of the study: amikacin, ampicillin, ceftriaxone, ceftazidime, ciprofloxacin, gentamicin, tobramycin, trimethoprim/sulfamethoxazole and meropenem for Gram-negative organisms; and cefoxitin, oxacillin, clindamycin, erythromycin, penicillin and trimethoprim/sulfamethoxazole for Gram-positive organisms. Final blood culture results were transcribed onto a form that had to be collected from the laboratory by treating teams.
Data collection
Using a standardized case report form (CRF) for each participant, trained research nurses collected demographic information, known prior illnesses, reason for admission, microbiological data and antibiotic use (dose and duration) during the 4 weeks preceding enrolment. All enrolled patients were followed for a minimum of 14 days, or until death or hospital discharge, whichever occurred first. When the 14 day follow-up date coincided with a weekend, the assessment would be done at the earliest possible time after that date. Once all data were collected, the CRF was reviewed with a study team physician to determine probable source of infection and whether the current infection was hospital associated. We defined HAI as an infection with onset of symptoms 48 h or more after initial presentation to hospital (including the institution from which patients were transferred). Patients for whom onset of symptoms in relation to hospitalization could not be ascertained were defined as non-HAI. For patients who died, a study team physician reviewed the medical chart to determine whether death was directly attributable to the infection (e.g. death from septic shock) or indirectly attributable to the infection (e.g. patients with comorbidities whose symptoms of infection had improved but whose death occurred within 2 weeks after the onset of infection).
Outcome measures
Outcomes of interest were proportion of patients with BSI, aetiology of BSI, AST profiles of bloodstream isolates and death within 14 days. We measured antibiotic consumption using days of therapy (DOT), defined as the aggregated sum of all the days during which a patient received any individual antibiotic; the DOT for a given patient on multiple antibiotics was calculated as the sum of DOT for each antibiotic. We compared DOT pre- and post-blood culture testing for four priority antibiotics: ceftriaxone, meropenem, piperacillin/tazobactam and vancomycin. These were selected based on usage, according to hospital pharmacy data, and their perceived importance for empirical treatment among clinicians.
Statistical analysis
Patients could have multiple blood culture tests during their hospital stay. We classified patients with a blood culture showing growth of any organism other than a contaminant in any blood tests defined above as having at least one BSI.
Patients who did not get tested, whose blood cultures showed only growth of a contaminant, or whose results were lost were included in the final mortality analysis, but not in the microbiological (proportion of patients with BSI) and antibiotic use analyses.
Patients’ baseline and hospital stay (including antibiotic utilization) characteristics with and without BSI were compared using χ2 test for categorical variables and t-test or Wilcoxon test for continuous variables, depending on the parametric fit of the data.
Patterns of antibiotic susceptibility were summarized using the proportion of bacterial organisms susceptible to each antibiotic. Univariate and multivariable logistic regressions were performed to assess the association of certain variables with the pre-specified outcomes (presence of BSI and death status at 14 days). Adjustments were made for age and known comorbidities. Further subgroup mortality analyses were performed for those with BSI due to Enterobacteriaceae based on resistance to ceftriaxone and carbapenem.
All tests were conducted at two-sided 0.05 level of significance. Statistical software SAS version 9.4 was used for all calculations (SAS Institute, Cary, NC, USA).
Results
Study population
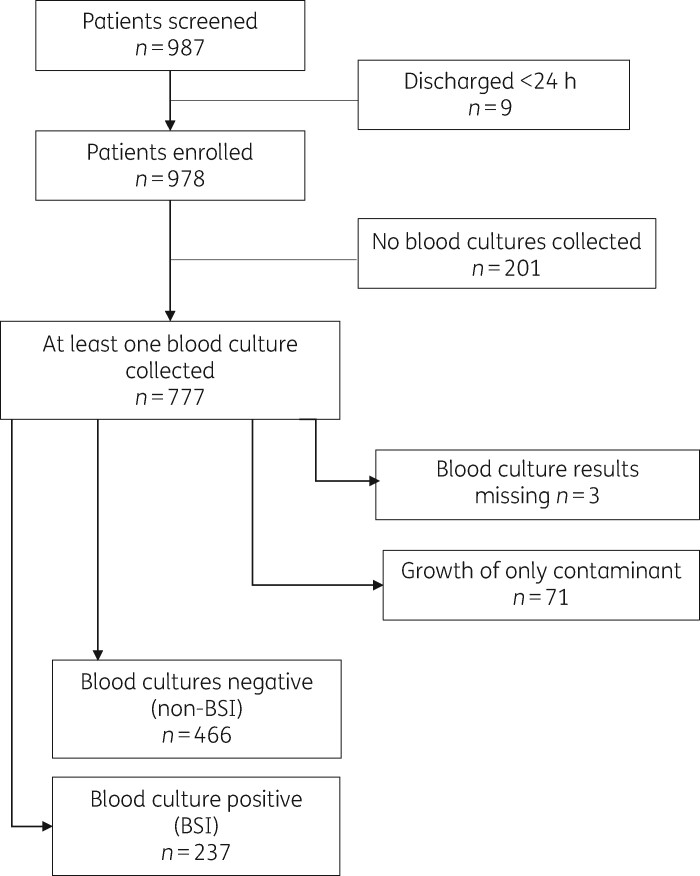
Between October 2016 and October 2018, we identified 987 hospitalized adult patients with new fever or symptoms suggestive of HAI; 9 were excluded because they had been discharged or signs of infection had resolved within 24 h after enrolment (Figure 1). Of the 978, 777 had blood culture testing with a range of 1–4 tests/patient. Of the 777 patients, 237 (30.50%) had growth of at least one pathogenic organism and were categorized as BSI; 466 (59.97%) had no growth in all blood tests and 71 (9.1%) had growth of only a contaminant and were categorized as non-BSI. Results were missing for three patients. A total of 703 patients (237 with BSI and 466 without BSI) were included in the analysis of microbiological outcomes and antibiotic use, while all enrolled patients were included in the final outcome analysis.
Figure 1.
Flow diagram for inclusion of study patients.
Baseline characteristics are summarized in Table 1. Patients with BSI had been in hospital for longer at the time of blood culture testing compared with the non-BSI group (15.5 days versus 10.2 days, P < 0.0001). Nearly all patients had received antibiotics in the preceding 4 weeks, but 107/237 (45.1%) of those with a BSI had received ≥4 antibiotics versus 134/466 (28.75%) of those without BSI. Almost all BSIs were hospital associated (224/237; 94.5%) while onset of symptoms in relation to hospitalization could not be ascertained for 13/237 (5.5%); 76/237 (32.1%) died within 14 days, compared with 98/466 (21%) of those without a BSI. Virtually all deaths in hospital were either directly or indirectly attributable to the current infection (Table 1). Admission to ICU or a medicine ward and having received four or more courses of antibiotics in the preceding 4 weeks were strong predictors of BSI (Table S1, available as Supplementary data at JAC-AMR Online).
Table 1.
Demographic and clinical characteristics of patients tested by blood culture
| Variable | BSI (n = 237) | No BSI (n = 466) | P value |
|---|---|---|---|
| Age (years), mean (SD) | 38.4 (16.1) | 36.1 (16.1) | 0.0758 |
| Number of prior comorbidities, mean (SD) | 1.05 (0.9) | 0.94 (0.9) | 0.1255 |
| Time between admission and blood culture testing (days), mean (SD) | 15.5 (19.3) | 10.2 (25) | <0.001 |
| Gender: male | 130 (54.8) | 263 (56.4) | 0.6748 |
| Resident of Addis Ababa (urban) | 91 (38.4) | 172 (36.9) | 0.7000 |
| Prior hospitalizations (last 6 months) | 88 (37.1) | 207 (44.4) | 0.0634 |
| Admission ward | |||
| emergency | 13 (5.5) | 65 (13.9) | 0.0003 |
| ICU | 54 (22.8) | 69 (14.8) | — |
| surgery | 13 (5.5) | 45 (9.6) | — |
| medicine | 162 (68.3) | 296 (63.5) | — |
| Known prior illnesses (chronic) | |||
| malignancy | 67 (28.2) | 129 (27.7) | 0.8807 |
| cardiac illness | 33 (13.9) | 72 (15.4) | 0.5914 |
| vascular illness | 33 (13.9) | 48 (10.3) | 0.1549 |
| renal disease | 27 (11.4) | 54 (1.6) | 0.9388 |
| diabetes | 32 (13.5) | 32 (6.9) | 0.004 |
| active TB | 18 (7.6) | 38 (8.1) | 0.525 |
| pulmonary disease | 7 (2.9) | 6 (1.3) | 0.269 |
| chronic hepatic disease | 7 (2.9) | 17 (3.6) | 0.629 |
| HIV status | |||
| positive | 18 (7.6) | 38 (8.1) | 0.357 |
| negative | 92 (38.8) | 155 (33.3) | — |
| unknown | 132 (55.7) | 282 (60.5) | — |
| Reason for admission | |||
| presumed infection | 141 (59) | 299 (64) | 0.2265 |
| medical cause | 72 (30.4) | 221 (47.4) | <0.0001 |
| surgical cause | 30 (12.7) | 67 (13.4) | 0.5320 |
| oncological cause | 76 (32.1) | 140 (30.0) | 0.5823 |
| Use of antibiotics in previous 4 weeks | 236 (99.6) | 451 (96.8) | 0.1044 |
| Number of antibiotic courses (past 4 weeks) | |||
| 0 | 6 (2.5) | 24 (5.1) | 0.0001 |
| 1 | 15 (6.2) | 54 (11.6) | — |
| 2 | 57 (24.1) | 152 (32.6) | — |
| 3 | 57 (24.1) | 111 (23.8) | — |
| 4+ | 107 (45.1) | 134 (28.7) | — |
| Final diagnosis of HAI | 224 (94.5) | 309 (66.3) | <0.0001 |
| 14 day status | |||
| discharged from hospital | 59 (24.9) | 169 (36.3) | 0.0012 |
| alive but not discharged | 102 (43.0) | 199 (42.7) | — |
| death | 76 (32.1) | 98 (21.0) | — |
| Cause of death | |||
| directly attributable to infection | 61 (80.3) | 57 (58.2) | 0.006 |
| indirectly attributable to infection | 13 (17.1) | 35 (35.7) | — |
| unrelated to current infection | 1 (1.3) | 5 (5) | — |
Values are shown as n (%) unless otherwise specified. Significant P values are shown in bold.
Aetiological spectrum of BSI and prevalence of drug resistance
The organisms most frequently isolated from blood cultures were Enterobacteriaceae (49.4% of BSI cases). Staphylococcus aureus, Pseudomonas spp. and Acinetobacter spp. were isolated in 34.6%, 12.2% and 4.6% respectively. Other organisms were identified in a total of 32 (13.5%) cases.
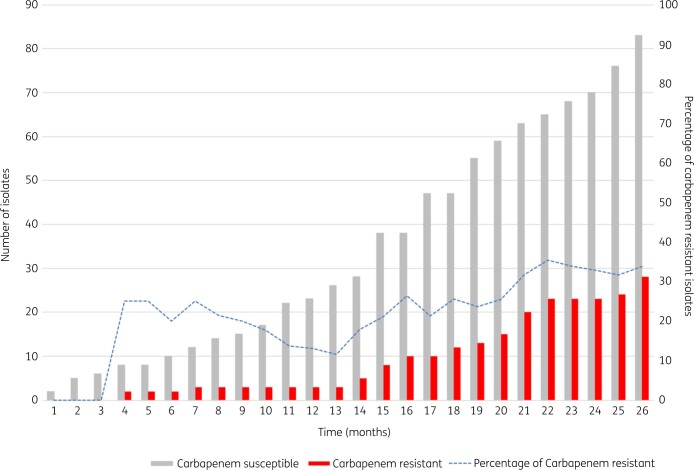
The cumulative antibiogram of blood culture isolates is presented in Table 2. Amikacin and meropenem were the only two antibiotics to which more than 70% of blood isolates remained susceptible. Resistance to carbapenems was noted in 18% of Klebsiella spp. and 10% of Escherichia coli blood isolates overall. Trends in resistance to carbapenems over the 2 year study period are shown in Figure 2.
Table 2.
Patterns of antibiotic susceptibility of blood culture isolates among patients hospitalized at TASH
| Organism | n | Susceptibility (%) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMK | AMP | CRO | CAZ | CIP | GEN | TOB | SXT | MEM | FOX | OXA | CLI | ERY | PEN | ||
| Enterobacteriaceae (n = 118 isolates) | |||||||||||||||
| E. coli | 33 | 88 | 3 | 29 | 38 | 48 | 50 | 71 | 0 | 90 | |||||
| Klebsiella spp. | 60 | 81 | 4 | 18 | 18 | 44 | 28 | 37 | 0 | 82 | |||||
| Enterobacter spp. | 11 | 86 | 0 | 28 | 71 | 57 | 33 | 40 | 0 | 80 | |||||
| Citrobacter spp. | 5 | 75 | 10 | 0 | 50 | 50 | 40 | 0 | 0 | 100 | |||||
| Other | 9 | 100 | 0 | 0 | 67 | 67 | 57 | N/A | 20 | 88 | |||||
| Pseudomonas spp. and Acinetobacter spp. (n = 40 isolates) | |||||||||||||||
| Pseudomonas spp. | 29 | 73 | 46 | 80 | 47 | 50 | N/A | 75 | |||||||
| Acinetobacter spp. | 11 | 73 | N/A | 27 | 22 | 50 | 13 | 39 | |||||||
| S. aureus (n = 82 isolates) | |||||||||||||||
| S. aureus | 82 | 36 | 59 | 62 | 69 | 59 | 13 | ||||||||
Susceptibility results were available for a total of 240 blood culture isolates from 226 patients. More than one organism was identified in 14 patients and organisms other than the ones listed in the table were identified in 11 patients, but susceptibility results are not presented.
AMK, amikacin; AMP, ampicillin; CRO, ceftriaxone; CAZ, ceftazidime; CIP, ciprofloxacin; GEN, gentamicin; TOB, tobramycin; SXT, trimethoprim/sulfamethoxazole; MEM, meropenem; FOX, cefoxitin; OXA, oxacillin; CLI, clindamycin; ERY, erythromycin; PEN, penicillin; N/A, not applicable.
Figure 2.
Trends in cumulative carbapenem resistance among Enterobacteriaceae isolated from blood cultures in patients hospitalized at TASH and with suspected HAI from 2016 to 2018. The x-axis represents time (months) during the study period; the y-axis represents the number of blood Enterobacteriaceae isolates for which full susceptibility data were available.
Thirty-eight percent of S. aureus bloodstream isolates were methicillin resistant.
Use of priority antibiotics before and after blood culture collection
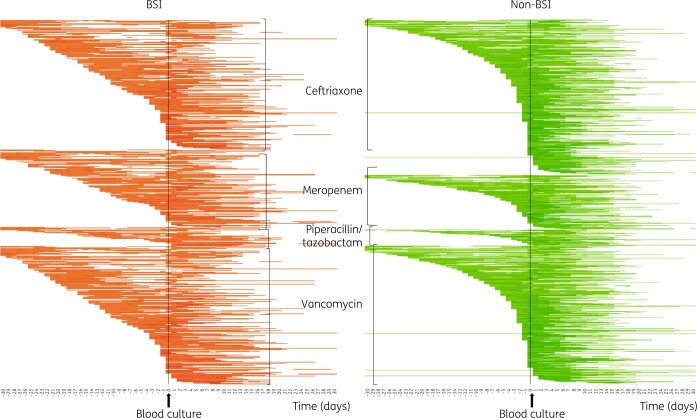
Temporal patterns of antibiotic use (for the four priority antibiotics) with respect to timing of blood cultures are depicted in heatmaps in Figure 3. This graphical representation of antibiotic use suggests that patients with a BSI had received ceftriaxone and vancomycin for a longer duration pre-blood culture testing than those without a BSI.
Figure 3.
Heatmap (density array) of days on antibiotics for four selected antibiotics, in patients with and without BSI. Each line represents a patient over time (before and after blood culture collection), with coloured cells depicting days that an individual patient was taking antibiotics. The shading of each square is proportional to the relative intensity of the exposure, with darker shades representing days on multiple antibiotics.
This is further supported by measures of antibiotic consumption. Of the 703 patients tested with blood cultures, ceftriaxone was given to 602 (86%) patients, vancomycin to 571 (81%), meropenem to 260 (37%) and piperacillin/tazobactam to 71 (10%) (Table S2). DOT pre-testing was four times higher among those with BSI than those without BSI (median DOT 12 days versus 3 days, P < 0.0001) (Table S2). Within the pre-specified follow-up, median total DOT post-culture was similar for both groups of patients (22 days versus 20 days in the BSI and non-BSI groups, respectively, P = 0.99) (Table S2). In a univariate analysis of risk of BSI in relation to specific antibiotics taken pre-blood culture, there appeared to be a correlation between BSI and having received any of the three most commonly used antibiotics [ceftriaxone, OR 1.53 (95% CI 1.12–2.10); vancomycin, OR 1.97 (95% CI 1.42–2.72); and meropenem OR 2.37 (95% CI 1.68–3.35)] (Table S3).
Mortality
A total of 215 patients (22% of study patients) died in hospital within 14 days. Patients with a hospital-associated BSI were twice as likely to die as those with other types of HAI (OR 2.45, 95% CI 1.59–3.77). This risk remained significant after adjusting for comorbidities (malignancy, chronic liver disease and HIV), as well as admission to ICU as a proxy for severity of illness (OR 1.99, 95% CI 1.26–3.15) (Table 3). Use of ≥4 courses of antibiotics in the preceding 4 weeks appeared to be correlated with death in the univariate but not in the multivariate analysis, suggesting that intense antibiotic exposure was a marker of severe illness but not an independent risk factor for death. Enterobacteriaceae accounted for most of the mortality, with 41/76 (53.9%) of the fatal BSI cases attributed to this family. Resistance to ceftriaxone appeared to increase the odds of death compared with susceptibility to this agent (OR 15.3; 95% CI 2.0–118.3). Because of the small sample size of ceftriaxone-susceptible Enterobacteriaceae (22 cases, 1 death), the CI is wide but the true risk of death remains significant (greater than 2-fold increase). Carbapenem resistance did not further increase the already very elevated risk of death.
Table 3.
Predictors of death (within 14 days) for patients with suspected HAI (n = 973)a
| Variable | Alive (N = 758)b | Dead (N = 215) | Univariate analysis, OR (95% CI) | Multivariate analysis,c OR (95% CI) |
|---|---|---|---|---|
| Age: mean (SD) | 36.91 (15.86) | 40.48 (16.87) | 0.99 (0.98–1.00) | 1.01 (1.00–1.02) |
| Gender: male, n (%) | 429 (56.6) | 115 (53.5) | 0.88 (0.65–1.20) | |
| Prior hospitalizations (6 months), n (%) | 315 (41.6) | 86 (40.0) | 0.94 (0.69–1.28) | |
| Antibiotics in prior 4 weeks, n (%) | 724 (95.5) | 210 (97.7) | 1.97 (0.76–5.11) | |
| Number of prior antibiotic courses, n (%) | ||||
| 0 | 34 (4.5) | 5 (2.3) | 1 | 1 |
| 1 | 102 (13.5) | 17 (7.9) | 1.13 (0.39–3.31) | 1.04 (0.35–3.12) |
| 2 | 237 (31.3) | 51 (23.7) | 1.46 (0.55–3.93) | 1.18 (0.43–3.24) |
| 3 | 181 (23.9) | 51 (23.7) | 1.92 (0.71–5.16) | 1.38 (0.50–3.81) |
| 4+ | 204 (26.9) | 91 (42.3) | 3.03 (1.15–8.02) | 1.99 (0.73–5.41) |
| HIV status, n (%) | ||||
| positive | 47 (6.2) | 25 (11.6) | 1.94 (1.11–3.39) | 1.98 (1.10–3.57) |
| unknown | 474 (62.5) | 125 (58.1) | 0.96 (0.69–1.35) | 1.05 (0.73–1.51) |
| Malignancy, n (%) | 161 (21.2) | 69 (32.1) | 1.75 (1.25–2.45) | 2.15 (1.49–3.11) |
| Hepatic disease, n (%) | 19 (2.5) | 14 (6.5) | 2.71 (1.33–5.50) | 3.00 (1.42–6.35) |
| Admission to ICU, n (%) | 108 (14.2) | 53 (24.7) | 1.97 (1.36–2.85) | 2.21 (1.46–3.33) |
| Blood culture result, n (%) | ||||
| not tested or no result | 218 (28.8) | 41 (19.1) | 1 | 1 |
| negative (non-BSI) | 375 (49.5) | 98 (45.6) | 1.39 (0.93–2.08) | 1.30 (0.85–1.98) |
| positive (BSI) | 165 (21.8) | 76 (35.3) | 2.45 (1.59–3.77) | 1.99 (1.26–3.15) |
| Subgroup: BSI, n (%) | ||||
| with Enterobacteriaceae | 76 (46.1) | 41 (53.9) | 1.37 (0.79–2.37) | |
| without Enterobacteriaceae | 89 (53.9) | 35 (46.1) | 1 | |
| Subgroup: BSI, Enterobacteriaceae, n (%) | ||||
| ceftriaxone results | ||||
| resistant | 55 (72.4) | 40 (97.6) | 15.27 (1.97–118.29) | |
| susceptible | 21 (27.6) | 1 (2.4) | 1 | |
| carbapenem results | ||||
| resistantd | 19 (29.2) | 8 (21.6) | 0.67 (0.27–1.72) | |
| susceptible | 46 (70.8) | 29 (78.4) | 1 |
A total of 5 patients with missing 14 day status were excluded from this comparison.
Includes patients who were either discharged or alive and still hospitalized at 14 days.
Variables with 0.05 significance level were selected from the univariate model.
Carbapenem susceptibility results were available for 65 (of the 76) blood culture isolates in the ‘alive’ group and for 37 (of the 41) isolates in the ‘dead’ group.
Discussion
This large prospective cohort study quantifies the high burden of AMR in HAI and correlates it with patterns of antibiotic use and mortality in Ethiopia’s largest tertiary care referral hospital.
We report several interesting findings. First, the overall mortality of patients with HAI was 22%, higher than previous estimates of 5%–15% mortality from HAI in LMICs17,18 and four times higher than the overall mortality rate reported for our hospital in the year preceding this study (internal data from the hospital administrative database). This suggests appropriate treatment of HAI could potentially have had a significant beneficial effect on mortality. Second, patients with a suspected HAI had a very high rate of positive blood cultures, with clinically significant pathogens isolated from 31%. Given the widespread practice of empirical antibiotic treatment for hospitalized patients, detecting such a high rate of bacteraemia was quite unexpected. This rate of bacteraemia is comparable to that of critically ill patients with sepsis in high-income countries. In our setting, where devices such as central lines and urinary catheters are rarely used, the most common focus of BSI was hospital-associated pneumonia.
One-third of patients with BSI died within 14 days of their infection, a fatality rate three times higher than that reported for bacteraemia in high-income settings, where patients are significantly older and have more comorbidities.19,20 Specifically, bacteraemia due to Enterobacteriaceae was an independent risk factor for death.
Fundamentals of clinical practice assume that timely and appropriate antibiotic therapy improves survival of BSI.21,22 We found that resistance to a broad range of antibiotics was significantly higher than was reported a few years ago in the same hospital.23 Ceftriaxone is the most commonly prescribed antibiotic in Ethiopian hospitals, a practice that has led to over 80% of Enterobacteriaceae now being resistant to third-generation cephalosporins. We found ceftriaxone resistance to be a critical determinant of poor outcomes, with resistance to this agent increasing the odds of death 15-fold. Carbapenem resistance did not additionally contribute to the already very high mortality rates observed, an expected finding considering patients were predominantly treated with ceftriaxone. Earlier use of carbapenems among patients with BSI would likely have saved lives, as 80% of Enterobacteriaceae isolates were susceptible to these agents. By the same token, earlier discontinuation and more judicious use of carbapenems in those without a BSI might have limited the emergence of carbapenem resistance a mere 3 years after introduction of this class of antibiotics in the country.
Indeed, our analysis of antibiotic use in relation to hospitalization and microbiological testing paints a concerning portrait of clinical practices in a setting that is comparable to other referral hospitals in sub-Saharan Africa. Studies in LMICs have shown that three out of four hospitalized patients are receiving empirical antibiotics at any given time.24 Our data extend this finding by demonstrating a surprising dose-dependent relationship between quantity of antibiotics received prior to testing and blood culture positivity. Repeatedly escalating empirical treatments before attempting aetiological diagnosis may be a marker of severe illness, but likely also indicates significant delays in implementing effective therapy.
Inappropriate empirical treatment and prolonging therapy in those with negative results, combined with gaps in effective infection control measures, are likely fuelling emergence of drug resistance, which we observed in a very rapid time frame. This highlights the importance of sensitizing clinicians to the high clinical relevance of bacteriology diagnostics; it further points to the importance of combining blood culture testing with clinical expertise in infectious disease and effective antimicrobial stewardship if we hope to reduce mortality from HAI and contain the emergence of AMR in LMICs.25,26
Our study must be interpreted in context. First, this was not a controlled trial comparing blood culture testing versus no testing to assess the impact of this intervention on antibiotic use and clinical outcomes. Rather, we used test results (positive versus negative) to estimate impact, because this was the most ethical and feasible study design.
We followed our study population for a limited period of time rather than the duration of hospitalization. This made it difficult to measure rates of antibiotic use per patient-days, which would have enabled more robust comparisons of antibiotic usage pre- and post-blood culture testing. Further, because it was not possible to know when clinicians became aware of the blood culture test results, we did not assess how knowledge of results for individual patients might have impacted prescription behaviour. However, the important conclusion is that blood culture testing, which is currently not available in most Ethiopian hospitals nor indeed in many hospitals in sub-Saharan Africa, enables identification of those at highest risk of death. Blind escalation of empirical antibiotic therapy, contrary to the perception of many clinicians in LMICs, appears to increase rather than decrease mortality and promotes the isolation of drug-resistant organisms.
This study provides strong evidence that investing in systematic and quality-assured blood culture testing is crucial to identify patients with HAI who are at lower risk of death and in whom antibiotics could be discontinued earlier than is current practice. We also demonstrate that spiralling empiricism is not the ‘safer’ option in LMICs—rather, by delaying aetiological diagnosis and appropriate therapy it actually drives patients with HAI to an earlier death.
Supplementary Material
Acknowledgements
We thank the HAI study team in Addis Ababa (Sr Tigist Nemera, Sr Woyneshet Seyoum, Sr Semegn Abebe, W/o Eyerusalem Teshome and W/o Emebet Bogale) for data collection and entry and the laboratory technologists at TASH (Getachew, Mequanint, Frey, Mane, Teshale, Eleni and Solomon) for enthusiastically embracing quality-assured bacteriologic diagnostics. Finally, we extend our deepest appreciation to Alina Dyachenko for statistical analysis and Barbara Ann Jardin for overall project management.
Funding
This work was supported by investigator-initiated research grant from the Research Institute of the McGill University Health Centre (GRANT #974) under the auspices of the Addis Ababa University (AAU) and McGill Partnership for Infectious Diseases (AMP-ID) http://amp-id.org/. In-kind support from bioMérieux Inc. (France) was provided via an investigator-initiated grant (donation of a blood culture instrument). C.P.Y. holds a ‘Chercheur-boursier clinicien’ career award from the Fonds de recherche du Québec – Santé (FRQS).
Transparency declarations
None to declare.
References
- 1. O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Wellcome Trust and the Department of Health, UK Government. 2016. https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf.
- 2. Klein EY, Van Boeckel TP, Martinez EM. et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A 2018; 115: E3463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Musicha P, Cornick JE, Bar-Zeev N. et al. Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998-2016): a surveillance study. Lancet Infect Dis 2017; 17: 1042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leopold SJ, van Leth F, Tarekegn H. et al. Antimicrobial drug resistance among clinically relevant bacterial isolates in sub-Saharan Africa: a systematic review. J Antimicrob Chemother 2014; 69: 2337–53. [DOI] [PubMed] [Google Scholar]
- 5. Tadesse BT, Ashley EA, Ongarello S. et al. Antimicrobial resistance in Africa: a systematic review. BMC Infect Dis 2017; 17: 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maina D, Makau P, Nyerere A. et al. Antimicrobial resistance patterns in extended-spectrum β-lactamase producing Escherichia coli and Klebsiella pneumoniae isolates in a private tertiary hospital, Kenya. Microbiology Discovery 2013; 1: 5. [Google Scholar]
- 7. Okoche D, Asiimwe BB, Katabazi FA. et al. Prevalence and characterization of carbapenem-resistant Enterobacteriaceae isolated from Mulago National Referral Hospital, Uganda. PLoS One 2015; 10: e0135745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ombelet S, Ronat JB, Walsh T. et al. Clinical bacteriology in low-resource settings: today’s solutions. Lancet Infect Dis 2018; 18: e248–58. [DOI] [PubMed] [Google Scholar]
- 9. Barbe B, Yansouni CP, Affolabi D. et al. Implementation of quality management for clinical bacteriology in low-resource settings. Clin Microbiol Infect 2017; 23: 426–33. [DOI] [PubMed] [Google Scholar]
- 10. Semret M, Ndao M, Jacobs J. et al. Point-of-care and point-of-‘can’: leveraging reference-laboratory capacity for integrated diagnosis of fever syndromes in the tropics. Clin Microbiol Infect 2018; 24: 836–44. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Second WHO Model List of Essential In Vitro Diagnostics. 2019; https://www.who.int/medical_devices/publications/Standalone_document_v8.pdf?ua=1.
- 12. Jacobs J, Hardy L, Semret M. et al. Diagnostic bacteriology in district hospitals in sub-Saharan Africa: at the forefront of the containment of antimicrobial resistance. Front Med (Lausanne) 2019; 6: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Group B-LW. Montreal Declaration on Clinical Bacteriology Diagnostics for Antimicrobial Resistance. 2019. https://docs.google.com/forms/d/e/1FAIpQLSdpJQkzY1YtrFjBlbLJN4taAfsgXrULVZYyNIuMCP_VOAda2w/viewform.
- 14. Yansouni CP, Seifu D, Libman M. et al. A feasible laboratory-strengthening intervention yielding a sustainable clinical bacteriology sector within 18-months of implementation in a large referral hospital in Ethiopia. Front Public Health 2020; doi:10.3389/fpubh.2020.00258. [DOI] [PMC free article] [PubMed]
- 15. Urdea M, Penny LA, Olmsted SS. et al. Requirements for high impact diagnostics in the developing world. Nature 2006; 444 Suppl 1: 73–9. [DOI] [PubMed] [Google Scholar]
- 16.CLSI. Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Sixth Edition: M100. 2016.
- 17. Allegranzi B, Bagheri Nejad S, Combescure C. et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet 2011; 377: 228–41. [DOI] [PubMed] [Google Scholar]
- 18. Bagheri Nejad S, Allegranzi B, Syed SB. et al. Health-care-associated infection in Africa: a systematic review. Bull World Health Organ 2011; 89: 757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng MP, Stenstrom R, Paquette K. et al. Blood culture results before and after antimicrobial administration in patients with severe manifestations of sepsis: a diagnostic study. Ann Intern Med 2019; doi:10.7326/M19-1696. [DOI] [PubMed] [Google Scholar]
- 20. Goto M, Al-Hasan MN.. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect 2013; 19: 501–9. [DOI] [PubMed] [Google Scholar]
- 21. Kollef MH. Broad-spectrum antimicrobials and the treatment of serious bacterial infections: getting it right up front. Clin Infect Dis 2008; 47 Suppl 1: S3–13. [DOI] [PubMed] [Google Scholar]
- 22. Fraser A, Paul M, Almanasreh N. et al. Benefit of appropriate empirical antibiotic treatment: thirty-day mortality and duration of hospital stay. Am J Med 2006; 119: 970–6. [DOI] [PubMed] [Google Scholar]
- 23. Seboxa T, Amogne W, Abebe W. et al. High mortality from blood stream infection in Addis Ababa, Ethiopia, is due to antimicrobial resistance. PLoS One 2015; 10: e0144944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gutema G, Håkonsen H, Engidawork E. et al. Multiple challenges of antibiotic use in a large hospital in Ethiopia - a ward-specific study showing high rates of hospital-acquired infections and ineffective prophylaxis. BMC Health Serv Res 2018; 18: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cox JA, Vlieghe E, Mendelson M. et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect 2017; 23: 812–8. [DOI] [PubMed] [Google Scholar]
- 26. Van Dijck C, Vlieghe E, Cox JA.. Antibiotic stewardship interventions in hospitals in low-and middle-income countries: a systematic review. Bull World Health Organ 2018; 96: 266–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.