Abstract
Background
WHO’s Global Action Plan on Antimicrobial Resistance includes as a priority to increase public education surrounding antibiotic use and resistance. Monitoring population-level antibiotic behaviours is crucial for informing intervention strategies, but data from a broad range of settings, particularly lower-resourced countries, are lacking.
Objectives
We measured public knowledge, attitudes and practices regarding antibiotics and antibiotic resistance in Cambodia, providing baseline information against which to monitor the progress of future interventions.
Methods
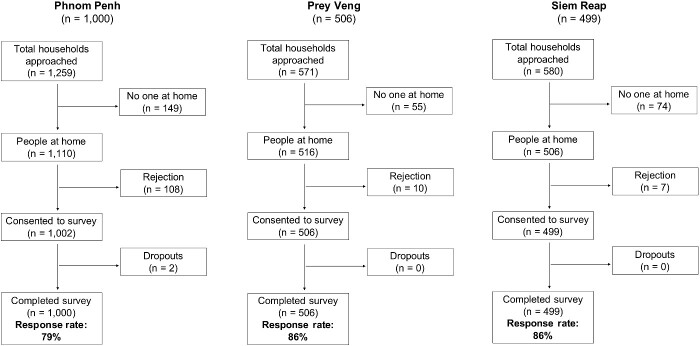
Between September and October 2018, we conducted a household survey of knowledge, attitudes and practices related to antibiotic use in urban and rural populations of three Cambodian provinces: Phnom Penh, Siem Reap and Prey Veng. Response rates were respectively 79%, 86% and 86%.
Results
Among the 2005 participants, we found high levels of awareness of terms relating to antibiotics (86.5%) and antibiotic resistance; most participants also recognized that antibiotic resistance is a problem (58.4%). However, few understood that antibiotics are effective only against bacterial infections (1.2%). We also found province-specific differences in participants’ sources of antibiotics and their sources of AMR-related information. In regression analyses, more favourable antibiotic practice scores were associated with higher knowledge (β = 0.18; 95% CI: 0.14–0.22) and attitude (β = 0.16; 95% CI: 0.11–0.22) scores, as well as trust in healthcare sources to obtain antibiotics and antibiotic information.
Conclusions
This study highlights the importance of interventions and public communication on antibiotic use and resistance that is effectively targeted to the local context through trusted healthcare providers.
Introduction
Antibiotic resistance is a global public health threat1–3 fuelled in large part by rising antibiotic use in both healthcare and community settings.4,5 To address the rising per capita antibiotic consumption, countries are committed to adopting national action plans on antimicrobial resistance (AMR) in line with WHO’s Global Action Plan (GAP) on Antimicrobial Resistance.6 The WHO GAP highlights the importance of understanding public perceptions of antibiotic usage and drivers in order to inform future responsible use campaigns and interventions.
While numerous such studies have been conducted in high-income settings, data from lower-resourced settings are lacking.7 Many such settings face additional challenges8–11 related to unrestricted access to antibiotics and access through informal sources. In Cambodia, major contributors to overuse of antibiotics in the community include non-prescription use, particularly for respiratory tract infections8 and diarrhoea in children, uncontrolled antibiotic sales and unregulated antibiotic use in the agricultural sector.12–14
Inappropriate antibiotic prescribing is also common – more than half of all visits to primary healthcare providers in Cambodia receive an antibiotic.15 In tertiary healthcare settings, data available from a small number of studies suggest widespread antibiotic resistance among pathogens of particular concern, challenging the provision of effective health care.16–20
Here, we present findings from a community-based survey of public knowledge, attitudes and practices relating to antibiotic use and resistance in Cambodia that can be used as baseline data to monitor progress and to inform effective and targeted future interventions for AMR.
Methods
Study population
Between September and October 2018, we conducted a household survey of knowledge, attitudes and practices related to antibiotic use in three Cambodian provinces: Phnom Penh, Siem Reap and Prey Veng. These provinces were purposively selected as they represent a mix of urban and rural settings. Participants were selected using a stratified, multi-stage cluster-sample design using households as the primary sampling unit. Within each household a randomly selected adult (>18 years old) was interviewed.
Sample size
We aimed to recruit 2000 households, 1000 in urban and 1000 in rural settings. The sample size of 1000 per urban/rural stratum was based on the ability to detect a prevalence of a binary knowledge, attitude or practice outcome between 40%–60% with 95% precision. Assuming a design effect of 2.5 (intra-class correlation coefficient, ρ = 0.166), a sample comprising 99 clusters and 10 observations per cluster is required, for a total sample size of 990 households.
Study sites
We first enumerated all villages in urban communes within Phnom Penh from official statistics. From this list, we randomly selected 10 communes using sampling probabilities proportional to population size. From each commune, we selected 10 villages at random in which to conduct the survey. In each village, field workers created a census of all households in consultation with village leaders. A sampling interval (n = N/10, where N is the total number of households) was determined and field workers started from the village leader’s house and sampled every nth house. For the rural provinces of Siem Reap and Prey Veng, we recruited 500 households per province. From each province, we selected five communes at random based on probability proportional to population size, subsequently selecting 10 villages at random from these communes. Households in each selected village were enumerated and 10 households selected by interval sampling as described above.
Survey instrument
An interviewer-administered questionnaire was used to collect the data on electronic tablets using Open Data Kit (ODK) software21 (the questionnaire is available as Supplementary data at JAC-AMR Online). The interviews lasted 15–20 min and were conducted by field staff hired through KHANA, a non-governmental organization based in Cambodia. The interviews were conducted in Khmer, the national language of Cambodia.
Participants were asked about their demographic and socioeconomic information, as well as knowledge, attitudes and practices surrounding antibiotic use. The knowledge section included questions about what conditions can be effectively treated with antibiotics, appropriate usage of antibiotics, and consequences of inappropriate antibiotic use. The attitudes section explored individual perceptions of and attitudes towards antibiotic use and resistance. The practices section explored actual usage and purchasing of antibiotics, types of antibiotics used, compliance with prescriptions, and self-medication with antibiotics. The questionnaire was adapted from various sources, including the WHO multi-country public awareness survey on antibiotic resistance,22 the Cambodia Demographic and Health Survey,23 and previous studies conducted in other contexts.14,24–26 We also asked participants whether they had leftover antibiotics at home at the time of interview. For those who did, we requested permission to take photographs, which were subsequently coded to record what types of antibiotics participants had at home.
Data analysis
We assessed the representativeness of the survey sample by comparing participants’ demographic and socioeconomic characteristics with province-specific data from the Cambodia Demographic Health Survey (2014).23 These include household assets that were used as a proxy for participants’ socioeconomic status, modes of transportation and overall household characteristics. We applied sampling weights for prevalence estimation, to obtain estimates representative of the population in the three provinces.
Knowledge, attitude and practice scores
We assigned each participant an antibiotic knowledge score, an antibiotic attitude score and a practice score (variables scored are shown in Table S2). Participants’ antibiotic knowledge scores were based on the number of correct responses in the respective sections. Each correct answer was assigned 1 point and incorrect responses were assigned 0 points. Favourable attitudes and appropriate practice responses were similarly assigned 1 point each, while unfavourable responses were assigned 0 points. The maximum knowledge, attitude and practice scores that participants could obtain for each section were respectively 15, 6 and 10 points.
Descriptive analysis
In addition to participants’ sociodemographic characteristics, we tabulated variables related to participants’ antibiotic knowledge, attitudes, practices and their level of awareness surrounding antibiotic resistance. Additionally, we tabulated variables related to participants’ most recent antibiotic use behaviours as well as their trusted sources of information regarding antibiotics.
Multivariable analysis
In a first set of multivariable analyses, we fitted three separate multivariable linear (Gaussian) regression models to investigate the relationship between participants’ sociodemographic characteristics and their antibiotic knowledge, attitude and practice scores. We used each score as a continuous outcome variable. Explanatory variables included sociodemographic characteristics such as province (Phnom Penh, Prey Veng, Siem Reap), age, gender, education level (No education, Some primary, Completed primary, Some secondary, Completed secondary, More than secondary) and number of household assets. Regression coefficients with 95% CI were derived from the regression model, representing the average change in the outcome score per unit change in the respective explanatory variable.
In a second multivariable analysis, we investigated whether participants’ antibiotic practice score was associated with antibiotic knowledge and attitude scores, common sources of antibiotics and who they trusted to get information relating to antibiotics, controlling for participants’ sociodemographic characteristics. Residual plots can be found in Figure S1.
All data were analysed using R version 3.6.1.27
Ethics
This study was approved by the National Ethics Committee for Health Research, Cambodia (ref no: 117NECHR) and the institutional review board of the National University of Singapore (ref no: S-18-161).
Results
A total of 2005 participants completed the survey: 1000 in Phnom Penh, 499 in Siem Reap and 506 in Prey Veng. Response rates for each province were respectively 79%, 86% and 86% (Figure 1). The median age of participants was 46 years (range: 18–86 years). There were higher proportions of female than male participants in all three provinces, and a higher proportion of participants with no formal education in the rural provinces compared with Phnom Penh. Almost all participants were of Khmer ethnicity. A comparison of participant characteristics with province-specific data from the Cambodian Demographic Health Survey (2014) can be found in Table 1.
Figure 1.
Participant recruitment flow chart.
Table 1.
Demographic and socioeconomic characteristics of survey participants by province, Cambodia, 2018
| Phnom Penh (%) |
Prey Veng (%) |
Siem Reap (%) |
||||
|---|---|---|---|---|---|---|
| Characteristic | KAP (n = 1000) | DHSa (2014) | KAP (n = 506) | DHS (2014) | KAP (n = 499) | DHS (2014) |
| Sex | ||||||
| Female | 70.5 | 53.1 | 71.7 | 52.3 | 78.4 | 52.3 |
| Male | 29.5 | 46.9 | 28.3 | 47.7 | 21.6 | 47.7 |
| Education | ||||||
| No education | 10.9 | 7.0 | 20.2 | 16.0 | 29.5 | 23.2 |
| Some primary | 29.1 | 30.1 | 51.6 | 47.7 | 39.1 | 47.9 |
| Completed primary | 8.4 | 4.8 | 8.3 | 6.1 | 7.0 | 5.0 |
| Some secondary | 25.2 | 34.2 | 14.6 | 27.4 | 13.6 | 18.2 |
| Completed secondary | 15.0 | 7.3 | 3.6 | 1.7 | 7.8 | 3.3 |
| More than secondary | 11.4 | 16.8 | 1.8 | 2.5 | 3.0 | 2.6 |
| Ethnicity | ||||||
| Khmer | 92.8 | – | 96.8 | – | 99.8 | – |
| Other (Cham/Chinese/Vietnamese) | 7.2 | – | 3.2 | – | 0.2 | – |
| Age (years)b | ||||||
| 18–49 | 56.2 | 54.4 | 50.6 | 47.2 | 61.5 | 47.2 |
| 50–64 | 30.4 | 12.2 | 35.0 | 11.5 | 27.3 | 11.5 |
| 65+ | 13.4 | 5.2 | 14.4 | 5.7 | 11.2 | 5.7 |
| Household possessions | ||||||
| Radio | 34.1 | 52.1 | 31.5 | 31.7 | ||
| Television | 95.6 | 93.8 | 77.6 | 63.9 | ||
| Mobile phone | 98.9 | 97.1 | 93.1 | 85.7 | ||
| Non-mobile phone | 1.5 | 10.2 | 0.23 | 0.9 | ||
| Refrigerator | 65.5 | 48 | 11.4 | 9.2 | ||
| Watch | 60.1 | 49.9 | 23 | 28.7 | ||
| Electricity | 99.1 | 99.6 | 79.4 | 50.6 | 73.6 | 50.4 |
| Main mode of transport | ||||||
| Motorbike | 87.2 | – | 76.3 | – | 71.8 | – |
| Car | 5.1 | – | 0.5 | – | 1.7 | – |
| Bicycle | 2.4 | – | 14.2 | – | 21 | – |
| Walk | 4.3 | – | 8.6 | – | 5.4 | – |
| Boat | 0.1 | – | 0 | – | 0 | – |
| Other | 0.9 | – | 0.3 | – | 0.2 | – |
| Cook source | ||||||
| Electricity | 2.5 | 0.8 | 0.5 | 1.5 | 0.5 | 1.2 |
| LPG | 83.1 | 72.7 | 16.8 | 14.7 | 10.7 | 18.8 |
| Wood | 14.4 | 16.1 | 82.5 | 79 | 88.8 | 66.4 |
| Floor material | ||||||
| Ceramic tile | 78.9 | 44.1 | 8.7 | 8.7 | 23.6 | 5.5 |
| Wood plank | 15.9 | 19.4 | 30.3 | 14.8 | 69.5 | 58.6 |
| Otherc | 5.2 | 36.5 | 61 | 76.5 | 6.9 | 35.9 |
DHS, Cambodia Demographic and Health Survey (2014).
Age groups provided in DHS are as follows: <15 years, 15–49 years, 50–64 years, >65 years.
Other options in DHS include earth/sand, palm/bamboo, dung, parquet/polished wood, vinyl/asphalt strips, cement tiles, cement, floating house.
Knowledge
With regard to the aetiology of common upper respiratory tract infections, only a minority of participants (6.3%) were able to correctly identify that viruses cause the common cold and influenza. More frequent responses were rain (75%), changing temperatures (68.5%), ice water (33%), dust (24.8%) and bacteria (10.2%).
Antibiotics
Most of the participants (86.5%) were aware of the term ‘antibiotic’. Participants were also familiar with specific antibiotic types, including ‘penicillin’ (87.5%), ‘amoxicillin’ (82.6%), ‘tetracycline’ (77%), and ‘ampicillin’ (75%).
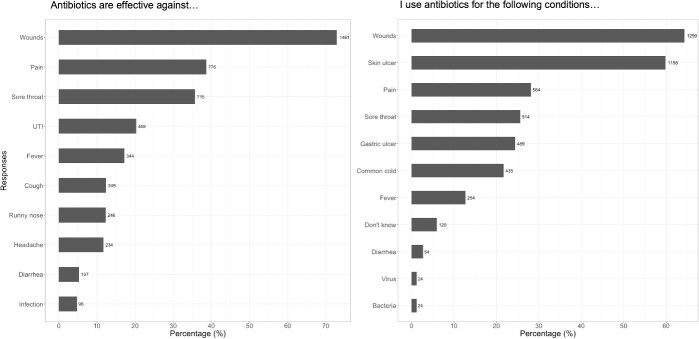
More than half of the participants stated that antibiotics are effective against wounds (64.3%) and skin ulcers (59.8%), referring to any injuries that break the skin or other body tissue. Less common responses were pain (28.1%), sore throat (25.6%), gastric ulcer (24.4%), the common cold (21.7%) and fever (12.7%). Only a small proportion of participants correctly identified that antibiotics are effective specifically against bacteria (1.2%). Correspondingly, common conditions that participants used antibiotics for were wounds (72.9%), pain (38.7%), urinary tract infections (20.3%), fever (17.2%), cough (12.4%), runny nose (12.3%), headache (11.7%) and diarrhoea (5.3%) (Figure 2).
Figure 2.
Participants’ knowledge of antibiotic effectiveness versus antibiotic use for common conditions.
Antibiotic resistance
Around half of participants (58.4%) had heard of the term ‘drug resistance’. Other terms related to antimicrobial resistance were less familiar but included ‘antibiotic resistance’ (42.6%), ‘AMR’ (22.9%) and ‘antibiotic-resistant bacteria’ (16.5%). A third of participants (35.4%) had not heard of any of these terms.
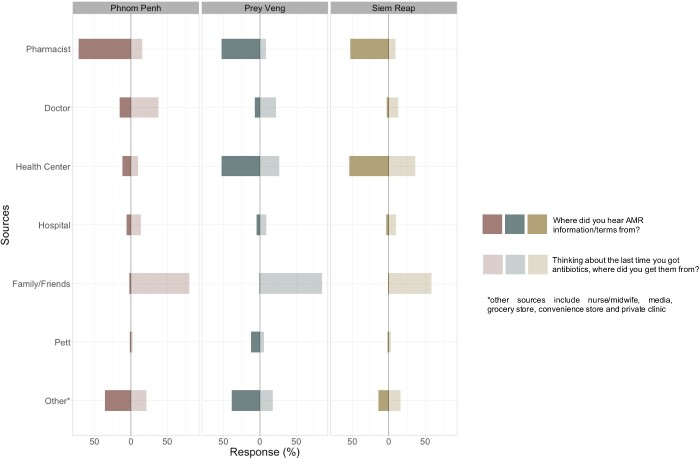
For participants who had heard of at least one of the above terms, most had heard it from friends and family or healthcare professionals – participants from Phnom Penh were more likely to have heard these terms from private doctors, while participants in Prey Veng and Siem Reap were more likely to have heard them from public health centres (Figure 3).
Figure 3.
Participants’ sources of antibiotics versus sources of AMR-related information.
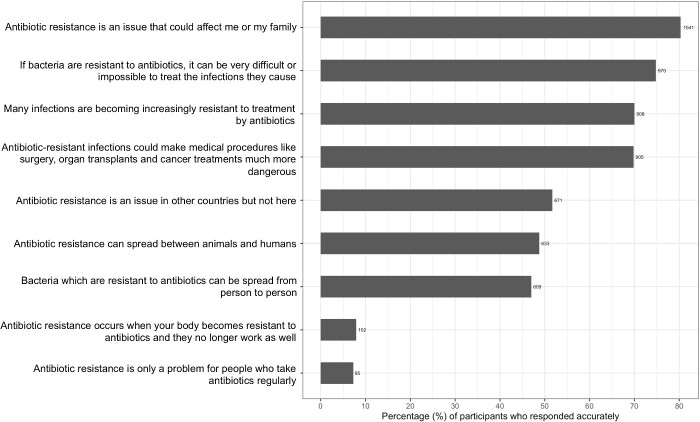
Most participants agreed that antibiotic resistance could make medical procedures more dangerous (69.7%), could make infections more difficult to treat (74.8%), could affect them or their family (80.3%) and that many infections are becoming increasingly resistant to antibiotics (70%), as shown in Figure 4. However, misconceptions persisted surrounding the mechanisms of resistance. Almost all the participants thought that antibiotic resistance occurs when our bodies become resistant to antibiotics (93.2%) and is only a problem for people who take antibiotics regularly (93.1%).
Figure 4.
Participants’ AMR knowledge.
Attitudes
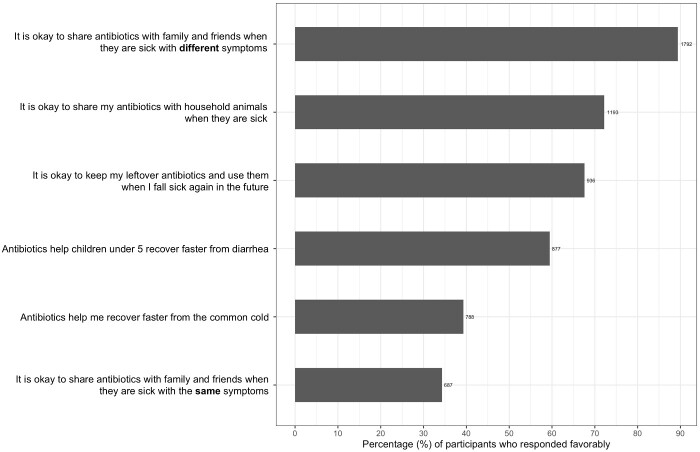
Almost half of participants felt that antibiotics could help them to recover faster from the common cold (44%) (see Figure 5). In addition, more than half of participants across the three provinces felt comfortable keeping left over antibiotics for future use (52.3%). Participants indicated that they were more likely to share antibiotics with friends and family if they were sick with the same symptoms (60.3%) as opposed to different symptoms (6.3%). A smaller proportion of participants from Phnom Penh (329/1000; 32.9%) were comfortable sharing antibiotics with household animals compared with both Prey Veng (300/506; 59.3%) and Siem Reap (237/499; 47.5%).
Practices related to antibiotic use
When asked about the last time they obtained antibiotics, most participants in Phnom Penh (640/1000; 64%), Prey Veng (296/506; 58.5%) and Siem Reap (332/499; 66.5%) said they got a prescription first. Around two-thirds (63.6%) indicated that they finished the antibiotics they got, while the rest of the participants either took some but had some leftovers (35.3%) or did not take them at all (1.1%). Of the 729 participants who did not complete their course of antibiotics, almost all stopped taking or did not take their antibiotics because they felt better (89.8%), and they most commonly discarded them (50.9%), kept them for future use (40.3%), or gave them to someone else (6.7%).
Antibiotic-seeking behaviours
Figure 3 and Table S3 highlight province-specific differences in antibiotic-seeking behaviours. Overall, participants in all three provinces trusted healthcare professionals most to get information about antibiotics; in Phnom Penh private doctors were commonly nominated as a trusted source, while in the rural provinces of Siem Reap and Prey Veng, health centres were more common trusted information sources.
Similarly, when asked about the last time they obtained antibiotics, participants in all three provinces most commonly said that they got them from a healthcare source – either from private doctors and pharmacies in Phnom Penh, or health centres and pharmacists in Prey Veng and Siem Reap. However, when asked where they obtained information about antibiotic resistance, most participants in all three provinces said that they obtained this information from family and friends.
Prevalence of leftover antibiotics
Across the three provinces, 297 participants said that they had leftover antibiotics at home. Of the 297, 115 were verified to actually have antibiotics based on photographs, corresponding to 5.7% of the households surveyed. Common leftover antibiotics across all three provinces were amoxicillin, ampicillin, penicillin, tetracycline, cephalosporins and lincomycin (Figure 6).
Figure 5.
Participants’ antibiotic attitudes.
Figure 6.
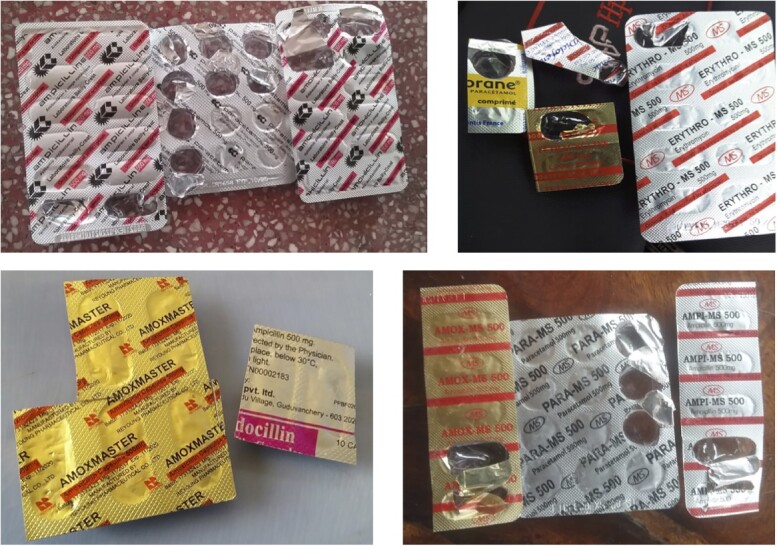
Photographs of antibiotics found in participants’ homes.
Regression analysis
Sociodemographic characteristics and scores
Participants’ median scores were 6.82 (range: 0–14) for antibiotic knowledge, 3.71 (range: 0–6) for attitude and 5.09 (range: 0–10) for practices (higher scores are more favourable). Table 2 shows the associations between participants’ scores and their sociodemographic characteristics.
Table 2.
Univariate analysis: participant characteristics associated with knowledge, attitude and practice scores
| Knowledge score |
Attitude score |
Practice score |
||||
|---|---|---|---|---|---|---|
| Characteristic | Coefficient | 95% CI | Coefficient | 95% CI | Coefficient | 95% CI |
| Estimate | 5.06 | 4.21–5.78 | 2.52 | 2.16–2.87 | 4.53 | 4.14–4.92 |
| Sex | ||||||
| Female (ref) | – | – | – | – | – | – |
| Male | 0.43** | 0.14–0.73 | 0.07 | −0.09 to 0.23 | 0.03 | −0.14 to 0.21 |
| Province | ||||||
| Phnom Penh (ref) | – | – | – | – | – | – |
| Prey Veng | 1.05*** | 0.70 to 1.40 | −0.50*** | −0.69 to 0.32 | 0.12 | −0.09 to 0.32 |
| Siem Reap | 0.01 | −0.38 to 0.39 | −0.17 | −0.36 to 0.02 | −0.27* | −0.48 to −0.06 |
| Education | ||||||
| No formal education (ref) | – | – | – | – | – | – |
| Some primary education | 0.31 | −0.13 to 0.76 | 0.32** | 0.12–0.52 | 0.55*** | 0.33–0.78 |
| Completed primary education | −0.06 | −0.68 to 0.55 | 0.55*** | 0.25–0.85 | 0.23 | −0.10 to 0.56 |
| Some secondary education | 0.39 | −0.12 to 0.89 | 0.66*** | 0.41–0.91 | 0.54*** | 0.27–0.82 |
| Completed secondary education | 0.26 | −0.31 to 0.84 | 0.64*** | 0.34–0.94 | 0.26 | −0.07 to 0.59 |
| More than secondary education | 0.75* | 0.10–1.40 | 0.56** | 0.21–0.91 | 0.62** | 0.22–1.01 |
| Age group (years) | ||||||
| 20–29 (ref) | – | – | – | – | – | – |
| 30–39 | 0.33* | −0.10 to 0.76 | 0.29* | 0.06–0.53 | 0.16 | −0.10 to 0.42 |
| 40–49 | 0.55* | 0.08–1.02 | −0.04 | −0.29 to 0.21 | 0.12 | −0.16 to 0.40 |
| 50–59 | 0.62*** | 0.15–1.10 | −0.10 | −0.35 to 0.15 | 0.31* | 0.03–0.59 |
| 60–69 | 0.88 | 0.38–1.39 | −0.01 | −0.28 to 0.25 | 0.12 | −0.18 to 0.41 |
| 70–79 | 0.20* | −0.53 to 0.92 | −0.44* | −0.79 to −0.09 | 0.05 | −0.34 to 0.44 |
| 80–89 | −1.95** | −3.88 to −0.01 | −1.72*** | −2.54 to −0.91 | −0.30 | −1.20 to 0.61 |
| Household possessionsa | ||||||
| Number of household possessions | 0.12 ** | 0.03–0.21 | 0.07** | 0.02 | 0.01 | −0.04 to 0.06 |
| Adjusted R-squared: 0.06 | Adjusted R-squared: 0.09 | Adjusted R-squared: 0.02 | ||||
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001; ‘ref’ indicates reference category.
Number of household possessions the participant owns is used as proxy for socioeconomic status. This includes electricity, backup generator/battery/solar panels, radio, television, mobile phone, non-mobile phone, refrigerator, wardrobe, sewing machine, CD/DVD player, watch. Items are taken from Cambodia’s Demographic Health Survey (2014).
Males (β = 0.43, 95% CI: 0.16–0.75) and those with higher household asset ownership (β = 0.12, 95% CI: 0.03–0.20) had higher knowledge scores. Participants with more than secondary education also scored higher than those with no formal education (β = 0.75, 95% CI: 0.09–1.36). Compared with participants in Phnom Penh, participants in Prey Veng (β = 1.05, 95% CI: 0.69–1.40) had higher knowledge scores.
More favourable attitude scores were associated with at least some primary education, while less favourable attitude scores were seen in Prey Veng (β=−0.51; 95% CI: −0.70 to −0.33) and among older participants aged 80–89 years (β=−1.72; 95% CI: −2.54 to 0.91, compared with participants aged 20–29 years).
Compared with having no formal education, participants with some primary education (β = 0.55; 95% CI: 0.33–0.78), some secondary (β = 0.54; 95% CI: 0.27–0.82) and more than secondary education (β = 0.62; 95% CI: 0.22–1.01) had higher practice scores. Participants from Siem Reap had lower practice scores (β=−0.27, 95% CI: −0.48 to −0.06) than their counterparts in Phnom Penh.
Knowledge, attitude and practice scores
After adjusting for sociodemographic characteristics, we found that participants’ attitude scores were 0.04 units higher (95% CI: 0.01–0.08) for every unit increase in their antibiotic knowledge scores (Table 3).
Table 3.
Multivariable regression analysis: factors influencing antibiotic attitude and practice scores in Phnom Penh, Prey Veng and Siem Reap
| Attitude score |
Practice score |
|||
|---|---|---|---|---|
| Factor | Coefficient | 95% CI | Coefficient | 95% CI |
| Estimate | 2.66 | 2.22–3.19 | 2.83 | 2.22–3.28 |
| Knowledge score | 0.04* | 0.01–0.07 | 0.17*** | 0.13–0.20 |
| Attitude score | – | – | 0.16*** | 0.11–0.22 |
| Trust in information sources | ||||
| Doctor | – | – | 0.62*** | 0.42–0.82 |
| Pharmacist | – | – | 0.16 | −0.04 to 0.34 |
| Health centre | – | – | 0.31** | 0.10–0.49 |
| Friends | – | – | −0.08 | −0.39 to 0.22 |
| Family | – | – | 0.62*** | 0.36–0.87 |
| Personal experience | – | – | −0.06 | −0.31 to 0.18 |
| Media | – | – | 0.82*** | 0.54–1.10 |
| Thinking about the last time you got antibiotics, where did you get your antibiotics from? | ||||
| Family/friend | – | – | −0.76* | −1.45 to −0.06 |
| Grocery store | – | – | 0.14 | −0.34 to 0.67 |
| Convenience store | – | – | −0.28 | −0.91 to 0.34 |
| Doctor | – | – | 0.34* | 0.08–0.62 |
| Pharmacist | – | – | −0.09 | −0.29 to 0.11 |
| Private clinic | – | – | 0.42*** | 0.23–0.62 |
| Health centre | – | – | 0.48*** | 0.26–0.70 |
| Hospital | – | – | 0.17 | −0.17 to 0.49 |
| Adjusted R-squared: 0.08 | Adjusted R-squared: 0.27 | |||
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001.
All regression results are adjusted for sociodemographic variables (i.e. province, sex, education, age group, number of household possessions).
Participants’ practice score increased by 0.16 (95% CI: 0.11–0.22) for every unit increase in attitude score and 0.18 (95% CI: 0.14–0.22) for every unit increase in knowledge score. In addition, trust in information from private doctors (β = 0.58, 95% CI: 0.39–0.77) and health centres (β = 0.25, 95% CI: 0.04–0.42) as well as the media (β = 0.81, 95% CI: 0.53–1.09) were associated with higher practice scores. Similarly, using doctors (β = 0.45, 95% CI: 0.24–0.62) and health centres (β = 0.52, 95% CI: 0.27–0.71) as sources of antibiotics were associated with higher practice scores, while obtaining antibiotics from friends or family (β=−0.79, 95% CI: −1.45 to −0.06) was associated with lower practice scores.
Discussion
This study, based on a large sample with good urban and rural representation, provides an in-depth understanding of the prevailing population perceptions and awareness surrounding antibiotic use and resistance in Cambodia. Findings from this study provide baseline information to monitor progress with the national action plan and evidence to inform effective and targeted interventions in the future. Our results indicated relatively high levels of awareness of terms relating to antibiotics and antibiotic resistance; most participants also recognized that antibiotic resistance is a problem.
However, misconceptions surrounding antibiotic use and resistance were prevalent in all three sampled provinces, and there were province-specific differences in antibiotic-seeking behaviours. Common misconceptions included causes of common respiratory illnesses and the effectiveness of antibiotics for these illnesses. Similar to previous studies in similar contexts, our findings show that antibiotic familiarity is not synonymous with accurate antibiotic knowledge – only a small percentage of participants were able to correctly discern that antibiotics were only effective against bacterial infections.13 Use of antibiotics for management of respiratory symptoms and wound care was also particularly common. Future communication strategies should therefore prioritize public understanding of disease causation, as well as the ineffective antibiotic use for symptoms such as general pain, sore throats, fever and runny nose.
Awareness of terms relating to AMR was not a good sole indicator of knowledge. Participants generally had misconceptions around the mechanisms of antibiotic resistance. These responses were not unique to our study – in WHO’s multicountry antibiotic resistance public awareness survey,22 a majority of participants surveyed also thought that antibiotic resistance occurs when humans (rather than bacteria) become resistant to antibiotics or that AMR is only a problem for people who regularly take antibiotics. Knowledge surrounding the mechanisms of antibiotic resistance is a proposed indicator in the monitoring and evaluation framework of the global action plan on AMR,6 suggesting the need for continued evaluation of population-level knowledge and perceptions to monitor awareness of AMR and behaviour changes.
Univariable analysis indicated that antibiotic knowledge, attitudes and practice scores were individually associated with participants’ education levels. However, the effect of education and other sociodemographic variables such as province and sex diminished after including other variables in the multivariable regression model. These included participants’ knowledge and attitude scores, their sources of antibiotics and who they trusted to get health-related information, indicating the importance of healthcare providers in positively influencing antibiotic behaviours across both urban and rural contexts.
In addition to variables that were used to compute participants’ antibiotic practice scores, other practice variables included in the survey gave us further insight into the prevalence of leftover antibiotics kept at home, as well as common sources of antibiotics and antibiotic information that participants turned to. Although a relatively small number of households kept leftover antibiotics at home, participants had access to antibiotics especially through pharmacies in urban provinces and health centres in rural provinces, indicating a need to push for greater standardization of guidelines for drug sales and prescribing in both public and private sectors. This also highlights the need to align sources of antibiotics with trusted sources of information about antibiotic resistance. In both urban and rural areas, healthcare providers including doctors, pharmacies and health centres were the most common sources of antibiotics. However, most participants heard about terms relating to antibiotic resistance from either family or friends, and obtaining antibiotics from family and friends was also associated with less-favourable practices.
These insights into participants’ antibiotic practices also highlight the varying points of healthcare access across urban and rural provinces and the importance of targeted antibiotic interventions such as antibiotic and antibiotic resistance education across different types of providers and settings. This corresponds with previous evidence indicating that targeted interventions may be more effective especially in low and lower-middle income countries (LMICs),5 where there are multiple formal and informal sources of healthcare provision and drug distributors.11 Future antibiotic interventions should focus on communicating the effectiveness of antibiotics for common conditions as well as the mechanisms of resistance. Additionally, subsequent research should focus on exploring antibiotic attitudes and practices among community healthcare providers, where evidence can be used to inform antibiotic stewardship and patient education programmes.8,28
A potential limitation with studies looking at population-level knowledge, attitudes and practices is the possibility of social desirability bias, as participants may feel pressure to give answers that they perceive to be more desirable or correct, especially with regard to their antibiotic practices. In our study, we verified participants’ responses on leftover antibiotics with information from photographs; less than half of these contained antibiotics, indicating both that respondents were unlikely to under-report unfavourable practices and that antibiotics are not clearly distinguished from other types of medication in this population. A further limitation was the over-representation of female respondents, as women were more likely to be at home at the time of the survey. However, although males had higher average knowledge scores than women in our regression analysis, we found no difference in attitude or practice scores.
Conclusions
Data from a broad range of settings are crucial for tailoring effective, targeted and context-relevant interventions against antibiotic resistance, as well as informing global strategies to address AMR. Results from this study highlight the importance of effective antibiotic communication at province-specific trusted healthcare sources and the need to engage with both public and private sector providers in AMR policy strategies. Stronger alignment between sources of antibiotics and sources of public education and information on antibiotic resistance is likely to be a key factor in improving antibiotic use practices in lower-resource settings.
Supplementary Material
Acknowledgements
We thank the KHANA administrative team and data collectors for their assistance in data collection.
Funding
This work was funded by the UHS-SPH Integrated Research Programme (USIRP) at the Saw Swee Hock School of Public Health, National University of Singapore (NUS) and the University of Health Sciences, Cambodia (UHS).
Transparency declarations
None to declare.
Author contributions
All authors attest they meet the ICMJE criteria for authorship. All authors contributed to the design and implementation of the research and provided critical feedback on the manuscript.
Supplementary data
The questionnaire (Table S1), Tables S2 and S3, and Figure S1 are available as Supplementary data at JAC-AMR Online.
References
- 1. Tacconelli E, Pezzani MD. Public health burden of antimicrobial resistance in Europe. Lancet Infect Dis 2019; 19: 4–6. [DOI] [PubMed] [Google Scholar]
- 2. Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PloS One 2017; 12: e0189621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hofer U. The cost of antimicrobial resistance. Nat Rev Microbiol 2019; 17: 3. [DOI] [PubMed] [Google Scholar]
- 4. Klein EY, Van Boeckel TP, Martinez EM et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA 2018; 115: E3463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilkinson A, Ebata A, MacGregor H. Interventions to reduce antibiotic prescribing in LMICs: a scoping review of evidence from human and animal health systems. Antibiotics 2019; 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO. Monitoring and evaluation of the global action plan on antimicrobial resistance: framework and recommended indicators. 2019. https://apps.who.int/iris/handle/10665/325006.
- 7. Reed TA, Krang S, Miliya T et al. Antimicrobial resistance in Cambodia: a review. Int J Infect Dis 2019; 85: 98–107. [DOI] [PubMed] [Google Scholar]
- 8. Godman B, Haque M, McKimm J et al. Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: findings and implications for the future. Curr Med Res Opin 2020; 36: 301–327. [DOI] [PubMed] [Google Scholar]
- 9. Auta A, Hadi MA, Oga E et al. Global access to antibiotics without prescription in community pharmacies: a systematic review and meta-analysis. J Infect 2019; 78: 8–18. [DOI] [PubMed] [Google Scholar]
- 10. Nepal G, Bhatta S. Self-medication with antibiotics in WHO Southeast Asian Region: a systematic review. Cureus 2018; 10: e2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suy S, Rego S, Bory S et al. Invisible medicine sellers and their use of antibiotics: a qualitative study in Cambodia. BMJ Glob Health 2019; 4: e001787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Om C, McLaws ML, Vlieghe E et al. Cambodia: the first national study of antibiotic prescribing and resistance using mixed methods approach. Antimicrob Resist Infect Control 2015; 4: 183. [Google Scholar]
- 13. Om C, Daily F, Vlieghe E et al. Pervasive antibiotic misuse in the Cambodian community: antibiotic-seeking behaviour with unrestricted access. Antimicrob Resist Infect Control 2017; 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Om C, Vlieghe E, McLaughlin JC et al. Antibiotic prescribing practices: A national survey of Cambodian physicians. Am J Infect Control 2016; 44: 1144–8. [DOI] [PubMed] [Google Scholar]
- 15. Chareonkul C, Khun VL, Boonshuyar C. Rational drug use in Cambodia: study of three pilot health centers in Kampong Thom Province. Southeast Asian J Trop Med Public Health 2002; 33: 418–24. [PubMed] [Google Scholar]
- 16. Stoesser N, Moore CE, Pocock JM et al. Pediatric bloodstream infections in Cambodia, 2007 to 2011. Pediatr Infect Dis J 2013; 32: e272–6. [DOI] [PubMed] [Google Scholar]
- 17. Emary KR, Carter MJ, Pol S et al. Urinary antibiotic activity in paediatric patients attending an outpatient department in north‐western Cambodia. Trop Med Int Health 2015; 20: 24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramage I. Assessment of Official Private Providers and Delivery of Health Care Services to Children under Five. WHO, 2001. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.548.3332&rep=rep1&type=pdf. [Google Scholar]
- 19. Vlieghe ER, Phe T, De Smet B et al. Bloodstream infection among adults in Phnom Penh, Cambodia: key pathogens and resistance patterns. PLoS One 2013; 8: e59775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hout B, Oum C, Men P et al. Drug resistance in bacteria isolated from patients presenting with wounds at a non-profit Surgical Center in Phnom Penh, Cambodia from 2011–2013. Trop Dis Travel Med Vaccines 2015; 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hartung C, Lerer A, Anokwa Y et al. Open data kit: tools to build information services for developing regions. In: Proceedings of the 4th ACM/IEEE international conference on information and communication technologies and development. 2010: 1–12.
- 22. WHO. Antibiotic resistance: Multi-country public awareness survey. 2015. https://apps.who.int/iris/bitstream/handle/10665/194460/9789241509817_eng.pdf.
- 23. National Institute of Statistics. Cambodia Demographic and Health Survey 2014.
- 24. Huang Y, Gu J, Zhang M et al. Knowledge, attitude and practice of antibiotics: a questionnaire study among 2500 Chinese students. BMC Med Educ 2013; 13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ahmad A, Khan MU, Patel I et al. Knowledge, attitude and practice of B. Sc. Pharmacy students about antibiotics in Trinidad and Tobago. J Res Pharm Pract 2015; 4: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. André M, Vernby Å, Berg J et al. A survey of public knowledge and awareness related to antibiotic use and resistance in Sweden. J Antimicrob Chemother 2010; 65: 1292–6. [DOI] [PubMed] [Google Scholar]
- 27. RStudio Team. RStudio: Integrated Development for R. 2015. http://www.rstudio.com/.
- 28. Khan MS, Bory S, Rego S et al. Is enhancing the professionalism of healthcare providers critical to tackling antimicrobial resistance in low-and middle-income countries? Hum Resour Health 2020; 18: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.