Abstract
Introduction
Career firefighters experience chronic circadian rhythm disruption, increasing their risk of cardiometabolic disease. The recent discovery that eating patterns regulate circadian rhythmicity in metabolic organs has raised the hypothesis that maintaining a consistent daily cycle of eating and fasting can support circadian rhythms and reduce disease risks. Preclinical animal studies and preliminary clinical trials have shown promising effects of time-restricted eating (TRE) to reduce disease risk without compromising physical performance. However, there is a lack of research on TRE in shift workers including firefighters. This study aims to investigate the feasibility and efficacy of 10-hour TRE on health parameters that contribute to cardiometabolic disease risks among career firefighters who work on a 24-hour shift schedule.
Methods and analyses
The Healthy Heroes Study is a randomised controlled parallel open-label clinical trial with 150 firefighters over 1 year. Firefighters are randomised with a 1:1 ratio to either the control or intervention group. The control group receives Mediterranean diet nutritional counselling (standard of care, ‘SOC’). The intervention group receives the same SOC and a self-selected 10-hour TRE window. After the 2-week baseline, participants enter a 3-month monitored intervention, followed by a 9-month self-guided period with follow-up assessments. The impact of TRE on blood glucose, body weight, body composition, biomarkers (neuroendocrine, inflammatory and metabolic), sleep and mood is evaluated. These assessments occur at baseline, at the end of intervention and at 6, 9 and 12-month follow-ups. Temporal calorie intake is monitored with the smartphone application myCircadianClock throughout the study. Continuous glucose monitors, wrist-worn actigraphy device and questionnaires are used to monitor glucose levels, activity, sleep and light exposure.
Ethics and dissemination
The study was approved by the Institutional Review Boards of the University of California San Diego and the Salk Institute for Biological Studies. Results will be disseminated through peer-reviewed manuscripts, reports and presentations.
Trial registration number
Keywords: general medicine (see internal medicine), diabetes & endocrinology, cardiology, hypertension, general diabetes, physiology
Strengths and limitations of this study.
In accordance with the funding agency’s recommendation to avoid unintended health-based discrimination at work, the eligibility criteria do not exclude healthy firefighters with normal values of metabolic health (relevant data can be analysed on stratification of healthy or non-healthy parameters).
Mediterranean diet is used as the standard of care as it is known to improve metabolic health.
The career firefighters in San Diego Fire and Rescue adopt a 24-hour shift schedule that is also followed among 74% of fire departments in the USA; however, the feasibility, adoptability and efficacy of a 10-hour time-restricted eating among volunteer firefighters, and those with a shift schedule different from 24 hours may not be generalised from this study.
The study uses the myCircadianClock app to monitor and guide participants, which reduces the burden of frequent clinic visits and can be used for large-scale adoption of the results at the national and international levels.
The study uses continuous measurements of activity, sleep and interstitial glucose levels with integrative analyses of these data streams to offer deep insight into the impact of shift work on blood glucose regulation.
Introduction
Shift workers constitute up to 20% of the workforce in industrial countries and they are indispensable to the functioning of modern societies. Firefighters are shift workers who often work at night when our circadian rhythm instructs our body to sleep. Chronic disruption of circadian (~24 hours) daily rhythms among shift workers, including firefighters, increases the risk of obesity, diabetes, cardiovascular diseases, insomnia and cancer.1–9 However, pragmatic lifestyle intervention to counteract the adverse health effects of shift work is lacking. Recent progress in circadian science has raised the possibility of novel interventions for reducing the disease risk of firefighters.
While the major emphasis on reducing circadian disruption has been on restoring sleep, the impact of food timing on health has opened new avenues to lessen the adverse effects of circadian disruption. Preclinical studies have shown that restricting all food intake to a consistent 8–12 hours window—without reducing calories—can prevent and reverse obesity, diabetes, digestive disorders, liver disease and cardiovascular disease.10 Restricting the timing of food without explicitly reducing calories is called time-restricted eating (TRE). More importantly, TRE does not compromise physical fitness, but rather improves motor coordination and endurance,11 12 critical attributes for firefighters. Preclinical animal models revealed cellular and molecular changes by which TRE improves health. TRE enhances the circadian clock to optimise health by coordinating the timing of digestive hormones, metabolic enzymes and storage depots in the metabolism of sugar and fat for optimal function.13 14 Abnormal sugar and fat metabolism is implicated in numerous diseases including obesity, diabetes and cardiovascular diseases.15
The scope and feasibility of TRE in shift workers have not been studied. Random eating patterns are widespread among both non-shift and shift workers.11 16 Non-shift workers can adopt a 10-hour eating window and self-sustain the new behaviour that reduces body weight and improves sleep.11 Such an eating pattern intervention also indirectly improves nutrition quality by reducing excessive caloric intake by up to 20%, most of which comes from an energy-dense diet.
There is increasing evidence that TRE or other forms of fasting can lead to reduced cardiovascular diseases and even cancer.17 More importantly, the American Heart Association18 and the National Nutrition Task Force19 have emphasised the importance of daily eating-fasting rhythm in disease prevention.
However, research on TRE is lacking among firefighters. Nearly 75% of firefighters nationwide are overweight or obese.20–22 Comorbidities associated with obesity are prevalent among firefighters.22 Obesity jeopardises their safety and well-being as well as public safety. Obesity is also a significant risk factor for subsequent disability.23 24 Therefore, we hypothesise that TRE can improve blood glucose regulation, reduce obesity and attenuate comorbidities associated with impaired glucose homeostasis or obesity.
In a randomised controlled trial (RCT), this protocol will test the efficacy and feasibility of eating time intervention relative to Mediterranean diet behavioural counselling on firefighters’ health. The firefighters’ cardiometabolic disease risks will be tested through a series of blood tests. A customised smartphone app developed in our laboratory will be used to guide participants to adopt the new eating pattern and log their lifestyle data.
Methods
Overview
In this RCT, firefighters from San Diego County are randomly assigned to a control group of Mediterranean diet nutrition counselling (standard of care, ‘SOC’) or the intervention group of SOC with the addition of adopting a 10-hour eating window for 3 months (TRE). Participants are followed up for 1 year. Participants in the TRE group may eat outside the 10-hour window up to 2 days/week to allow social commitments that are deemed necessary for sustaining their emotional health. The research team also works with participants to help adjust for challenging schedules. The impact of TRE on blood glucose levels, nuclear magnetic resonance (NMR) lipid profile, biomarkers, body weight, body composition, sleep and mood will be evaluated. Questionnaires will be administered for self-reported health and wellness assessments. Participants use an electronic diary (smartphone myCircadianClock application, ‘mCC app’) to log their caloric intake. Sleep and activity are passively measured with actiwatches at baseline and follow-up assessments every 3 months. We hypothesise that imposing eating-fasting cycles (TRE) will restore the equilibrium between catabolic and anabolic processes, which will promote glucose and lipid homeostasis, strengthen neuroendocrine signals, improve the regulation of circadian rhythms leading to a reduction of cardiometabolic disease risks and improve sleep and subjective quality of life.
The first participant was enrolled in the study on 8 May 2018. Due to COVID-19 and severe fire seasons in California, the study timeline was delayed. The study is expected to be completed in February 2021, but we anticipate further delay due to any unforeseen natural disaster and the course of the COVID-19 pandemic.
Recruitment
Participants were recruited via flyers/pamphlets, a short informational video describing the study, emails from San Diego Fire and Rescue (SDFR) providing study information, recruitment events and speaking to firefighters in the fire stations.
Enrolment and randomisation
Up to one hundred and sixty firefighters from San Diego County will be enrolled into the study for screening, so that one hundred and fifty firefighters (75 in the SOC group and 75 in the TRE group) will be randomised to the study intervention. All participants receive a unique coded identifier to maintain patient confidentiality. Participants are screened and must meet inclusion and exclusion criteria (box 1) before study enrolment. The statistician dictated the randomisation of participants. We anticipate a 20% dropout rate (the study is ongoing).
Box 1. Study enrolment criteria.
Inclusion criteria
Firefighters who work on a 24-hour shift schedule with San Diego Fire and Rescue or other fire departments in San Diego County.
Age: 21–65 years.
Own a smartphone (Apple iOS or Android OS).
If participants are on cardiovascular medications (HMG-CoA reductase inhibitors (statins), other lipid-modifying drugs (including over-the-counter drugs such as red yeast rice and fish oil), antihypertensives, antidiabetes drugs), no dose adjustments will be allowed during the study period.
Exclusion criteria
Insulin-dependent diabetes mellitus.
Presence of acute chronic inflammatory or autoimmune disease (defined by acute symptoms or C-reactive protein >10 mg/L), malabsorption syndromes, liver disease or kidney disease (stage 3 or greater).
Uncontrolled thyroid disease.
Intake of drugs likely to interfere with study endpoints, including corticosteroids, anabolic steroids, antipsychotics, antiretroviral drugs and immunosuppressive drugs (within 3 months of starting the study).
The presence or recent history of anaemia (haematocrit <33% within 3 months of starting the study).
History of bariatric surgery.
Pregnant or breastfeeding women.
Current or recent (within 12 months of starting the study) pregnancy or breast feeding, or intention of becoming pregnant in the next 6 months.
Any cancer other than non-melanoma skin cancer in the last 3 years.
On a special or prescribed diet for other reasons (eg, coeliac disease).
Depression as determined by the Beck Depression Inventory (BDI).
Planned international travel during the study period.
Insufficient logging on the myCircadianClock (mCC) app (does not log at least two entries a day for 10 of 14 days) during baseline will exclude from being randomised into the intervention period.
Inability or unwillingness to adhere to the study protocol and instructions from study personnel.
The firefighters are assured that study participation or withdrawal from the study has no bearing on their employment or receiving any benefit from the fire department. Individual participant data and any identifiable data will not be explicitly shared with the fire department. The study participants were compensated with $100 for their time, effort and travel to the clinic for each clinic visit.
Due to the low risk of harm, a Data Safety Monitoring Board was not appointed for this study. Instead, a Data Safety Monitoring Plan is provided (online supplemental file).
bmjopen-2020-045537supp001.pdf (42.8KB, pdf)
Outcomes
The primary, secondary and other outcomes are listed in table 1. Since the inclusion/exclusion criteria do allow the recruitment of participants whose outcome measures are within the reference range, we anticipate each arm will have participants with heterogeneous health parameters. Therefore, in addition to the comparison of all participants in the SOC and TRE arms, we will also do subanalyses of outcome measures for participants who are outside the reference range at the beginning of the intervention period.
Table 1.
Study outcomes
| Primary outcome measures | 1. Evaluate the impact of TRE on glucose homeostasis. The primary endpoint will be the change in glucose levels assessed via fasting blood glucose and continuous glucose monitors (CGM). Data from CGMs will be analysed to determine changes in glucose response within individuals and a daily average for glucose value will be computed. 2. Assess the feasibility and adherence of TRE. This will be measured by the percentage of days logged that participants ate within their TRE window and end-of-study surveys. |
| Secondary outcome measures | 3. Assess changes in metabolic and neuroendocrine biomarkers in response to TRE. Cardiometabolic homeostasis will be measured with blood biochemistry (including, but not limited to, fasting glucose, HbA1c, cholesterol, triglycerides, NMR LipoProfile and hs-CRP). Neuroendocrine markers include insulin and leptin. 4. Systolic blood pressure (mm Hg) in response to TRE. 5. Diastolic blood pressure (mm Hg) in response to TRE. 6. Body weight (kg) in response to TRE. |
| Other outcomes | 7. Body mass index (kg/m2). 8. Waist and hip circumference (cm). 9. Hip (cm)/waist (cm) ratio. 10. Body composition including, but not limited to, the fat percentage (%), fat mass (kg) and lean mass (kg). 11. Questionnaires (SF-36, ESS, PSQI, BDI). |
| Subanalyses | 12. For each outcome measure (1–11), subanalyses will be done on participants in both arms who are outside the reference healthy range for the respective measures. |
BDI, Beck Depression Inventory; ESS, Epworth Sleepiness Scale; hs-CRP, high-sensitivity C-reactive protein; NMR, nuclear magnetic resonance; PSQI, Pittsburgh Sleep Quality Index; SF-36, Short Form-36 Quality of Life; TRE, time-restricted eating.
Intervention
Groups
There are two groups in this study. The SOC group is given nutritional guidelines to follow the Mediterranean diet and is advised to continue their habitual daily eating pattern. The second group will implement the 10-hour TRE intervention with SOC.
The study statistician will generate the study randomisation table before the start of the study using the SPSS (IBM. Released 2020. IBM SPSS Statistics for Windows, V.26.0) program using block sizes of 4 and 8. He will be contacted by the study coordinator when a subject is ready to be randomised. Participants will be randomised into the interventional TRE+SOC arm or the SOC-only arm. It is not possible to blind the research team from the intervention group allocation as the eating window had to be known to assess adherence throughout the study.
Time-restricted eating
Participants assigned to the TRE group are instructed to consume all foods and beverages (except water) within a consistent self-selected time window of 10 hours/day for the 3-month intervention and continue through the 12-month follow-up visit. Participants may consume caffeine (without additional nutritional content such as cream, sugar or artificial sweeteners) outside the eating window as needed, and log it in the mCC app.
Remote engagement with the participants/educational materials
Lifestyle or behavioural intervention studies typically require frequent in-person study visits to adopt the suggested intervention. Such frequent physical visits can be burdensome for firefighters. Therefore, the mCC app is used to deliver educational materials, reminders/encouragement, and answer frequently asked questions.
During the 2-week baseline period, all participants receive text notifications reminding them to log their caloric intake in the app to improve logging compliance. After the participants are randomised to the SOC or TRE group, each group receives two to three notifications/week as messages within the app. These notifications are group specific and intended to increase the participants’ understanding of the SOC or TRE treatment. After the 3-month monitored intervention, on entering the self-guided 9-month follow-up period, participants receive weekly educational materials of ~800–1000 word articles on the impact of SOC or TRE on their overall health. Participants also receive short four to five question surveys that serve the dual function of raising their awareness of general health and assessing their understanding of how shift work affects their health. The participants can use the contact section of the app to communicate with the research team.
Assessment of adherence
If participants in the control group restrict their eating window to less than their habitual ≥12 hours/day or if the eating window of participants in the TRE group deviates from their self-selected 10-hour eating window ≥4 days during the first week, the participants will be contacted via telephone to ensure that they have understood the concept of their group assignment. Participants in the TRE group choose their self-selected window at clinic visit 2 (CV2) and enter it in the app. During the intervention, the TRE participants can change their eating window if they are not satisfied with the originally selected window. These changes are only made when necessary and approved by the research team. Changes to the eating window will be documented and reported with results.
Adherence to logging will be calculated as the per cent of days that participants logged at least two calorie-containing items with a minimum of 5 hours apart. For the TRE arm, non-adherence to TRE will be assessed by the percentage of days that participants logged more than 1 hour outside (before or after) their designated eating window. The eating window will be determined by the 95% interval of all calorie-containing ingestion events during baseline, the 3-month intervention period and within the 2 weeks leading up to follow-up visits at 6, 9 and 12 months. The eating window will be calculated for both groups. The adherence criterion is not applied in the control group. Adherence analysis is based on Wilkinson et al.25
Patient and public involvement
To assess and improve the participants’ experience in the study, participants are asked about the successes and challenges they face with adherence to the intervention. These assessments were taken at clinic and fire station visits, and through short surveys via the mCC app.
Members of SDFR, including the SDFR chief, health and wellness officers and the SDFR union representative, have been involved throughout the study development including applications for funding, protocol development, participant recruitment, and are active members of an advisory committee.
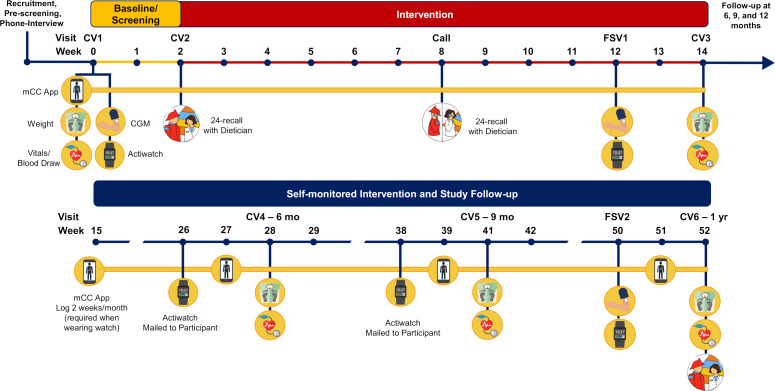
Visits
The study includes six clinic visits from the participants, two fire station visits from the research team and one phone call from the dietician (see figure 1, table 2). For clinical visits 4 and 5, participants are mailed an actiwatch to wear for 2 weeks before the clinic visit.
Figure 1.
Study design and timeline. CGM, continuous glucose monitor; CV, clinic visit; FSV, fire station visit; mCC, myCircadianClock.
Table 2.
Overview of study visits
| Measure/event | Pre | CV1 | CV2 | Call | FSV1 | CV3 | CV4 | CV5 | FSV2 | CV6 |
| Week of study | Pre | 0 | 2 | 8 | 10 | 14 | 28 | 41 | 50 | 52 |
| Inclusion/exclusion screening | X | X | ||||||||
| Medical history | X | |||||||||
| Informed consent | X | |||||||||
| Pregnancy test (fertile women only) | X | |||||||||
| mCC app instructions | X | |||||||||
| Randomised to intervention group and select 10-hour eating window if TRE | X | |||||||||
| Vitals, body weight, body composition | X | X | X | X | X | |||||
| Blood draw | X | X | X | X | X | |||||
| CGM applied | X | X | X | |||||||
| Actigraphy | X | X | X | X | X | X | X | |||
| SF-36 | X | X | X | X | X | |||||
| BDI-II | X | X | X | X | X | |||||
| PSQI | X | X | X | X | X | |||||
| ESS | X | X | X | X | X | |||||
| 24-hour dietary recall | X | X | X | |||||||
| End-of-study survey | X |
BDI-II, Beck Depression Inventory-II; CGM, continuous glucose monitor; CV, clinic visit; ESS, Epworth Sleepiness Scale; FSV, fire station visit; mCC, myCircadianClock; PSQI, Pittsburgh Sleep Quality Index; SF-36, Short Form-36 Quality of Life; TRE, time-restricted eating.
Clinic visit 1 (CV1, day 1)—After visit 1, participants enter a 2-week baseline period where they are instructed to use the mCC app to document all oral intake (logging water is optional). Vitals, body weight, body composition and fasting blood draw will be obtained. A continuous glucose monitor (CGM) is applied and an actiwatch is provided to wear for 2 weeks.
CV2 (end of week 2)—Participants are randomised into either the SOC or TRE group. Participants return to the clinic to return the CGM and actigraphy device. If randomised to the TRE group, participants will select a 10-hour eating window and set it on the app. All participants meet with the dietician who provides Mediterranean diet nutritional counselling and obtains a 24-hour dietary recall.
Monitored intervention period (weeks 3–14)—Participants enter the 3-month monitored intervention period in which they will either be engaged in the SOC or the TRE intervention starting at CV2.
Phone call (week 8)—All participants will speak with the dietician to reinforce good nutritional practices with the Mediterranean diet over the phone and provide a 24-hour dietary recall.
Fire station visit 1 (week 12)—A research team member will go to the fire station to provide the firefighters with a CGM and actigraphy device, which they will wear for 2 weeks.
Clinic visit 3 (week 14)—Participants will return the CGM and actigraphy device. Vitals, body weight, body composition and fasting blood draw will be obtained. They will be asked to complete the same questionnaires from visits 1 and 2. This will conclude the 3-month monitored intervention period, and it will also initiate the 9-month self-guided period.
During the self-guided period (months 3–12), there will be three clinic visits and one fire station visit. During these 9 months, they are asked to use the mCC app for at least two consecutive weeks every month.
Clinic visit 4 (month 6) and clinic visit 5 (month 9)—Participants will use the mCC app for at least two consecutive weeks/month. They will also complete the same questionnaires, vitals, body weight/composition and fasting blood draws as they did at clinic visits 1 and 3.
Fire station visit 2 (month 12)—The research team will go to the fire station to provide the firefighters with a CGM and an actigraphy device, which they will wear for 2 weeks.
Clinic visit 6 (end of month 12)—At this final visit, all assessments will be repeated, CGM and actigraphy device will be returned and the dietician will also conduct a 24-hour dietary recall.
COVID-19 protocol
Due to COVID-19, the Altman Clinical and Translational Research Institute (ACTRI) temporarily closed from April to July 2020, after which it remains accessible only to essential therapeutic research studies. During this period, clinic visits were conducted at the San Diego Fire-Rescue Department wellness centre, where its nurses are trained and provided supplies by the University of California San Diego (UCSD) research staff. Two weeks before these visits, participants were sent the actigraphy device and questionnaires via US mail. The actigraphy device was worn for 2 weeks and then returned to the staff at their clinic visit. Starting in June 2020, we phased out questionnaires by mail when possible and switched to administering some questionnaires online. Additionally, participants received a questionnaire in the mail asking if any changes to medications or sleeping habits, travel plans and specific dates worked over the past 2 weeks, as these were questions that we asked in person when visits were held at ACTRI.
For the 3 and 12-month visits, participants were also mailed a new CGM in the mail along with the watch and surveys 2 weeks prior. In place of fire station visits, Zoom was used to video chat with the participants and give them instructions on how to apply and activate the CGM using the reader. They remained blind to the CGM data.
Data collection and measurements
Data will be collected during clinic visit days and in free-living conditions.
Data collection during clinic visits
All clinical testing (vitals, blood draw, questionnaires) will be performed at the UCSD-ACTRI. Blood is processed at the UCSD Clinical Laboratory. All participants are advised to fast overnight and visits are scheduled between 08:00 and 12:00.
Anthropometric and vital signs. At every clinic visit the following measurements will be made by standardised and hospital-grade equipment: height, weight, body temperature, blood pressure and heart rate. Body composition will be assessed by a standardised Tanita scale (DC-430U; Tokyo, Japan).
Blood tests. All participants will have their blood drawn at the ACTRI by certified nurses. Venous blood samples are collected in the fasting state at all six clinic visits. The laboratory tests for cardiometabolic function (comprehensive metabolic panel, complete blood count, thyroid-stimulating hormone, haemoglobin A1c, high-sensitivity C-reactive protein, triglycerides, low-density lipoprotein, high-density lipoprotein, cholesterol assessed via NMR LipoProfile, insulin) are done at a certified analytical laboratory contracted by UCSD.
Deidentified blood samples (specifically, serum and plasma) obtained during this study are biobanked at UCSD or the Salk Institute for possible further biochemical testing. Samples will not be shared with researchers outside of those associated with this protocol. Samples are biobanked indefinitely.
Questionnaires. Participants are asked to complete the following questionnaires:
-
Clinic visits 1, 3, 4, 5, 6
Short Form-36 Quality of Life: to measure overall functional health score as well as separate physical and mental health dimension components.
Beck Depression Inventory-II: to screen for depression.
Epworth Sleepiness Scale (ESS): to assess daytime sleepiness (CV2 instead of CV1).
Pittsburgh Sleep Quality Index (PSQI): to evaluate sleep in seven major areas—subjective sleep quality, sleep latency, sleep duration, sleep disturbances, sleep efficiency, use of sleeping aids and daytime dysfunction (CV2 instead of CV1).
-
CV2
ESS.
PSQI.
Twenty-four-hour food recall: to assess nutrient consumption, amounts and timing based on the prior day’s intake.
-
Week 8 phone call
Twenty-four-hour food recall.
-
Clinic visit 6
End of study surveys: to obtain self-reported impressions of the study and receive feedback.
Data collection during free-living conditions (outside the clinic)
The mCC app for self-logging lifestyle parameters. The mCC app is designed to run on both Android and iOS devices that account for more than 90% of all smartphones and uses Health Insurance Portability and Accountability Act-compliant Amazon Web Services for server-side operations. During TRE, participants set their daily eating periods and receive alerts and reminders specific to TRE protocols. Participants may choose to receive an automated alert 15 or 30 min before the end of the eating interval to finish their last meal of the day. All participants can log their food, sleep and exercise. For food entries, the user can annotate the food picture with food names and other descriptors (portion size, leftovers, etc). The app is customised with push notifications, reminders and educational materials that are structured to guide the firefighters throughout the study, enable self-monitoring and improve adherence and retention.
CGM. All participants are fitted with the Abbott Freestyle Libre Pro CGM and will be instructed on its use. CGM measures interstitial fluid glucose using a subcutaneous sensor placed in the upper arm area every 15 min for up to 14 days. Participants wear the CGM for 2 weeks at a time during baseline (weeks 1–2), end of the 3-month intervention (weeks 12–14) and 1-year follow-up (weeks 50–52). CGMs estimate blood glucose levels with high accuracy that correlates with those obtained from either venous or capillary blood.26 Changes in the average daily glucose and SD will be calculated.
Wrist-worn actigraphy device. To measure habitual physical activity and sleep, participants wear a Phillips accelerometer (Spectrum Plus) on their non-dominant arm for 2 weeks at a time during the study period (baseline, and 2 weeks leading up to the end of the 3-month intervention, and follow-up visits at 6, 9 and 12 months). Wrist accelerometers have an acceptable correlation (r=0.90) between daily physical activity and activity counts reported by accelerometers27 and have been used reliably in National Health and Nutrition Examination Survey.28 The actigraphy data will be used to measure sleep onset, sleep duration and sleep efficiency.
Statistical analysis plan
Data from randomised participants will be analysed with an intention-to-treat protocol. All collected participant data will be included. A subanalysis will be performed to assess changes in participants who had health factors out of the normal range at baseline. Data will be reported with standard descriptive statistics as mean±SD, or 95% CI for normally distributed data, and as median with (Q1:Q3) for non-normally distributed data. Analysis will be performed by examination of the distribution of variables to assess their means, SDs and skewness. Continuous measures will be tested for normality and homogeneity of variance. Non-normally distributed variables will be transformed to meet the normal distribution assumption for linear models. Randomisation will be assessed by performing a series of Wilcoxon rank-sum tests, χ2 tests or Fisher’s exact tests to compare the groups on demographic and baseline clinical variables. Any variables on which the groups differ initially will be explored as covariates in subsequent analyses. The 95% eating window will be computed for the baseline and end of intervention. A mixed-effects model will be used.29–31 Data will be analysed using SPSS (V.26.0). All analyses will be two tailed, where applicable, with α=0.05, which will be considered statistically significant.
Sample size calculation
To date, there are no published RCTs assessing TRE as an intervention in firefighters. Most of the published TRE studies of comparable length of intervention are not RCT. Therefore, we used weight loss as a surrogate outcome for calculating sample size. The required sample size was calculated using G*Power software and for the mixed model approach using the RMASS program provided by Hedeker (http://tigger.uic.edu/%7Ehedeker/ml.html). We are confident that the sample size of 150 provides us with a minimum power of 80% to detect a medium effect size for the primary hypotheses. Medium effect sizes were selected based on previous studies that were reviewed earlier. For the mixed model, the medium effect size is defined as a between-group difference increasing linearly from 0 at baseline to 0.5 SD units at the last time point. The minimum power estimation is based on sample size calculation for 10% and 20% attrition, correlations of 0.2, 0.5 and 0.8 between the repeated measures, and for medium and large effect sizes.
Missing data
Missing data will be examined to assess randomness. The pattern of missing data will be examined according to the procedure recommended by Little and Rubin32 which includes comparing group differences in the primary outcomes of subjects with missing data versus without missing data. This allows inclusion of subjects with missing data or those who terminated the study early, without relying on data imputation procedures.
Ethics and dissemination
The study has been approved by the Institutional Review Boards (IRB) of the UCSD and Salk Institute for Biological Studies, La Jolla. Modifications to the protocol due to COVID-19 were allowed without full approval because there was no increased risk to the participants and the imminent and unique nature of the situation. Protocol modifications due to COVID-19 will be reported to the IRB. Any change in endpoints or analysis plan that occurs will be amended in ClinicalTrials.gov.
Informed consent will be obtained by the study coordinators or research personnel associated with this protocol. Informed consent procedures will be supervised either by the study coordinator, supervising physicians or the principal investigator. All research personnel giving informed consent will have undergone proper training and obtained the required certificates.
This paper was reported using the Standard Protocol Items: Recommendations for Interventional Trials reporting guidelines.33 Results from the study will address whether 10-hour TRE is effective and feasible in improving health outcomes among career firefighters who do 24-hour shift work. Results will be disseminated through peer-reviewed manuscripts, reports and presentations. Findings, regardless of the result, will be reported as described. Participants will receive their data at the end of the 1-year follow-up. The data include summaries of sleep, CGMs (when available), blood work and food entries from baseline, at the end of intervention and at 6, 9 and 12-month follow-up assessments. All coauthors will comply with the International Committee of Medical Journal Editors and no professional writers will be engaged.
Discussion
This study is the first workplace RCT to introduce TRE among the US career shift workers to promote healthy circadian behavioural change within the existing work schedule and culture. The RCT design with a self-guided follow-up period of the trial will demonstrate the feasibility of adopting TRE among fire service personnel without changing their work schedules. The complementary in-clinic and free-living measurements of various vital, behavioural, biochemical and physiological parameters at multiple time points throughout the study will help assess the impact of TRE on the multidimensional aspects of health. The use of a smartphone app to collect longitudinal data on nutrition and the use of the same medium for bidirectional engagement with the participants with relevant and actionable educational materials are also a unique strength of this study. If successful, this digital infrastructure will allow rapid dissemination and implementation of the TRE programme among fire service personnel.
Why a 10-hour TRE?
Although TRE with a wide range of eating windows from 6 to 12 hours has been shown to offer health benefits in preclinical animal models and humans, we chose a 10-hour window for several reasons. In both animal models and a recently completed study on patients with metabolic syndrome, 10 hours of eating and 14 hours of fasting reduced adiposity, and improved blood pressure, blood glucose and blood cholesterol.25 Therefore, we reasoned 10-hour TRE may also benefit the firefighters. A 10-hour TRE may also be a feasible and sustainable window for many firefighters. In SDFD and many other fire departments with communal eating at fire stations, breakfast and dinner are typically prepared within a 10-hour window with the dinner being served at ~18:00, which makes adopting to TRE easier. However, adopting this habit at home may be a challenge as the firefighters often want to share meals with their family during off-days, and sustained 10-hour TRE may involve the participation of the family to incorporate a TRE lifestyle.
Can it be adopted among fire services?
If successful, TRE may have a relatively lower barrier to implementation relative to other approaches to reduce circadian disruption. Circadian rhythms are disrupted by light at night, sleep disruption and erratic eating patterns. Methods to reduce the circadian disruptive impact of light or to improve sleep will likely involve both education and infrastructure investments to optimise lighting to support circadian rhythm or to improve sleep. On the other hand, if TRE is found beneficial, it can be implemented with minimal to no alterations in infrastructure.
Supplementary Material
Acknowledgments
We thank the members of San Diego Fire and Rescue (SDFR) for support and collaboration on this study, specifically: David Picone (Battalion Chief, Health and Safety), Brent Brainard (Captain/Wellness Officer, Health and Safety), Kyle O’Neill (Fire Engineer and Cancer and Health Coordinator), Jeri Miuccio (SDFD Union Representative), SDFD Chief Collin Stowell and all participants. A special thank you to David Picone for facilitating this study in collaboration with the SDFR, and Kyle O’Neill for facilitating fire station visits for participant recruitment and the necessary fire station visits as part of the study protocol. We thank the members of the advisory council for their valuable input in preparing the educational materials for the self-guided intervention portion of the study.
Footnotes
Twitter: @satchinpanda
Contributors: SP, PRT, ENCM and AZ conceived the study concept, developed the study design and protocol, applied for funding and initiated writing the manuscript. ENCM, AZ, HCL, AS, NRG, AR and AP coordinated the project. AZ, AS, HCL, AP, AR, NRG and ENCM screened/recruited participants and conducted clinic and fire station visits. AZ, AS, HCL, AP, AR, NRG, XW and ENCM collected the data. SG and JGF developed and conducted the statistical analysis plan. SP, PRT and ENCM interpreted the data. SP and PRT are coprincipal investigators and grant holders. All authors contributed and approved the final version of the manuscript.
Funding: This study is primarily funded by the Department of Homeland Security (DHS) under grant FEMA-EMW-2016-FP-00788 with some cost sharing by the Salk Institute. Additional funds were obtained from the William Doner Foundation. The myCircadianClock app is supported by a grant from the Robert Wood Johnson Foundation (grant ID 76014). ENCM is partly supported by a fellowship from the Larry Hillblom Foundation.
Competing interests: SP is the author of the book 'The Circadian Code' for which he collects nominal author royalty. PRT is a consultant for Amgen, Esperion, Boehringer Ingelheim, Novo Nordisk and Sanofi, and is a shareholder for Epirium Bio.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethical approval and oversight of this clinical study were approved and provided by the University of California San Diego (UCSD) Institutional Review Board Human Research Protections Program (IRB 172083) and the Salk Institute for Biological Studies Institutional Review Board (IRB 18-0001). The study is registered on ClinicalTrials.gov. All participants provided written informed consent.
References
- 1. Pan A, Schernhammer ES, Sun Q, et al. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med 2011;8:e1001141. 10.1371/journal.pmed.1001141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roenneberg T, Allebrandt KV, Merrow M, et al. Social jetlag and obesity. Curr Biol 2012;22:939–43. 10.1016/j.cub.2012.03.038 [DOI] [PubMed] [Google Scholar]
- 3. Jarrin DC, McGrath JJ, Drake CL. Beyond sleep duration: distinct sleep dimensions are associated with obesity in children and adolescents. Int J Obes 2013;37:552–8. 10.1038/ijo.2013.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arora T, Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. Int J Obes 2015;39:39–44. 10.1038/ijo.2014.157 [DOI] [PubMed] [Google Scholar]
- 5. Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015;161:84–92. 10.1016/j.cell.2015.03.015 [DOI] [PubMed] [Google Scholar]
- 6. Scheer FAJL, Hilton MF, Mantzoros CS, et al. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 2009;106:4453–8. 10.1073/pnas.0808180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buxton OM, Cain SW, O'Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 2012;4:129ra43. 10.1126/scitranslmed.3003200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A 2013;110:5695–700. 10.1073/pnas.1216951110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McHill AW, Melanson EL, Higgins J, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci U S A 2014;111:17302–7. 10.1073/pnas.1412021111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panda S. Circadian physiology of metabolism. Science 2016;354:1008–15. 10.1126/science.aah4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab 2015;22:789–98. 10.1016/j.cmet.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chaix A, Zarrinpar A, Miu P, et al. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 2014;20:991–1005. 10.1016/j.cmet.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qian J, Scheer FAJL. Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol Metab 2016;27:282–93. 10.1016/j.tem.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henriksson E, Lamia KA. Adipose clocks: burning the midnight oil. J Biol Rhythms 2015;30:364–73. 10.1177/0748730415581234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Efeyan A, Comb WC, Sabatini DM. Nutrient-Sensing mechanisms and pathways. Nature 2015;517:302–10. 10.1038/nature14190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta NJ, Kumar V, Panda S. A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS One 2017;12:e0172852. 10.1371/journal.pone.0172852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rothschild J, Hoddy KK, Jambazian P, et al. Time-restricted feeding and risk of metabolic disease: a review of human and animal studies. Nutr Rev 2014;72:308–18. 10.1111/nure.12104 [DOI] [PubMed] [Google Scholar]
- 18. St-Onge M-P, Ard J, Baskin ML, et al. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American heart association. Circulation 2017;135:e96–121. 10.1161/CIR.0000000000000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abbasi J. NIH charts a path for nutrition science. JAMA 2017;317:243–4. 10.1001/jama.2016.18217 [DOI] [PubMed] [Google Scholar]
- 20. Donovan R, Nelson T, Peel J, et al. Cardiorespiratory fitness and the metabolic syndrome in firefighters. Occup Med 2009;59:487–92. 10.1093/occmed/kqp095 [DOI] [PubMed] [Google Scholar]
- 21. Tsismenakis AJ, Christophi CA, Burress JW, et al. The obesity epidemic and future emergency responders. Obesity 2009;17:1648–50. 10.1038/oby.2009.63 [DOI] [PubMed] [Google Scholar]
- 22. Poston WSC, Haddock CK, Jahnke SA, et al. The prevalence of overweight, obesity, and substandard fitness in a population-based firefighter cohort. J Occup Environ Med 2011;53:266–73. 10.1097/JOM.0b013e31820af362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soteriades ES, Hauser R, Kawachi I, et al. Obesity and cardiovascular disease risk factors in firefighters: a prospective cohort study. Obes Res 2005;13:1756–63. 10.1038/oby.2005.214 [DOI] [PubMed] [Google Scholar]
- 24. Soteriades ES, Hauser R, Kawachi I, et al. Obesity and risk of job disability in male firefighters. Occup Med 2008;58:245–50. 10.1093/occmed/kqm153 [DOI] [PubMed] [Google Scholar]
- 25. Wilkinson MJ, Manoogian ENC, Zadourian A, et al. Ten-Hour Time-Restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab 2020;31:92–104. 10.1016/j.cmet.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vrolix R, Mensink RP. Variability of the glycemic response to single food products in healthy subjects. Contemp Clin Trials 2010;31:5–11. 10.1016/j.cct.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 27. Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc 2005;37:S531–43. 10.1249/01.mss.0000185657.86065.98 [DOI] [PubMed] [Google Scholar]
- 28. Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: adults compliance with the physical activity guidelines for Americans. Am J Prev Med 2011;40:454–61. 10.1016/j.amepre.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 29. Gibbons RD, Hedeker D, Waternaux C, et al. Random regression models: a comprehensive approach to the analysis of longitudinal psychiatric data. Psychopharmacol Bull 1988;24:438–43. [PubMed] [Google Scholar]
- 30. Hedeker D, Gibbons RD, Davis JM. Random regression models for multicenter clinical trials data. Psychopharmacol Bull 1991;27:73–7. [PubMed] [Google Scholar]
- 31. Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:963–74. 10.2307/2529876 [DOI] [PubMed] [Google Scholar]
- 32. Little R, Rubin D. Statistical analysis with missing data. New York: John Willey & Sons: Inc, 2002. [Google Scholar]
- 33. Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-045537supp001.pdf (42.8KB, pdf)