Abstract
The phytohormone production hypothesis suggests that organisms, including insects, induce galls by producing and secreting plant growth hormones. Auxins and cytokinins are classes of phytohormones that induce cell growth and cell division, which could contribute to the plant tissue proliferation which constitutes the covering gall. Bacteria, symbiotic with insects, may also play a part in gall induction by insects through the synthesis of phytohormones or other effectors. Past studies have shown that concentrations of cytokinins and auxins in gall-inducing insects are higher than in their host plants. However, these analyses have involved whole-body extractions. Using immunolocalization of cytokinin and auxin, in the gall inducing stage of Eurosta solidaginis, we found both phytohormones to localize almost exclusively to the salivary glands. Co-localization of phytohormone label with a nucleic acid stain in the salivary glands revealed the absence of Wolbachia sp., the bacterial symbiont of E. solidaginis, which suggests that phytohormone production is symbiont independent. Our findings are consistent with the hypothesis that phytohormones are synthesized in and secreted from the salivary glands of E. solidaginis into host-plant tissues for the purpose of manipulating the host plant.
Keywords: Cytokinin, Auxin, Phytohormones, Insects and Gall-induction
INTRODUCTION
Plant galls are tumor-like growths of plant tissue induced by an invading organism. A wide variety of organisms are known to induce plant galls including: protists, nematodes, mites, fungi, bacteria and insects. The mechanism of induction of bacterial galls has been established (MacDonald et al. 1986; Lichter et al. 1995; Jameson 2000; Zhu et al. 2000; Barash and Manulis-Sasson 2007), but how insects induce plant galls remains unknown.
There are two predominant hypotheses concerning how insects induce plant galls. The phytohormone production hypothesis suggests that insects and other gall-inducing organisms induce galls by producing and secreting plant growth hormones, which lead to cell division and cell expansion forming the gall (Zhu et al. 2000; Mapes and Davies 2001a, b; Reineke et al. 2008, Dorchin et al. 2009, Straka et al. 2010, Bruce et al. 2011, Connor et al. 2012, Yamaguchi et al. 2012; Tanaka et al. 2013; Bartlett and Connor 2014; Takei et al. 2015; Chanclud et al. 2016; Kai et al. 2017; Andreas et al. 2020). Alternatively, the effector protein hypothesis suggests that gall-inducing insects and other organisms might secrete effector proteins that stimulate production of phytohormones or have effects elsewhere in the cell cycle of the host plant (Wu and Baldwin 2010; Zhao et al. 2015; Cambier et al. 2019; Zhao et al. 2019). Specific examples from bacteria and fungi illustrate that phytohormone production alone or in combination with secreted effector proteins can contribute to gall induction (Zhu et al. 2000; Barash and Manulis-Sasson 2007; Doehlemann et al. 2008; Redkar et al. 2015).
Auxins and cytokinins are classes of phytohormones that induce cell growth and cell division which could contribute to the proliferation of the plant tissues formed in interactions between insects, fungi, microbial pathogens, and plants (Bruce et al. 2011; Connor et al. 2012; Erb et al. 2012; Giron et al. 2013; Bartlett and Connor 2014; Tooker and Helms 2014; Giron and Glevarec 2014; Naseem et al. 2014; Sugio et al. 2015; Robischon 2015; Dodueva et al. 2020). Auxin, also often associated with gall-inducing insects, affects the host-plant by promoting cell elongation and maintaining apical dominance by inhibiting the formation of lateral buds (Tooker and DeMoraes 2011). Cytokinin in the presence of auxin leads to cell division and promotes the growth of plant tissues that constitute the enclosing gall (Galuszka et al. 2008; Tooker and Helms 2014).
In past studies, cytokinin and auxin have been found not only in galled plant tissue, but also in the associated gall-inducing insects at concentrations much higher than in the plant (Mapes and Davies 2001a, b; Dorchin et al. 2009; Straka et al. 2010; Yamaguchi et al. 2012; Tanaka et al. 2013; Takei et al. 2015; Kai et al. 2017; Andreas et al. 2020). The high concentrations of cytokinin and auxin in gall-inducing insect species further implicates phytohormones as playing key roles in gall induction. However, most studies have used analytical chemistry to determine whole body concentrations of cytokinins and auxin in insects, so that little is known about their distribution within the bodies of gall-inducing insects. In only one instance have the concentrations of auxin and cytokinin been estimated in the organ responsible for gall-induction. In Pontania sp. (Hymenoptera: Tenthredinidae), where the ovipositing female induces the gall, the accessory gland associated with the ovipositor of adult females was specifically examined for concentrations of auxin and cytokinin rather than using whole body estimates (Yamaguchi et al. 2012). Furthermore, few studies allow time for larvae that induce galls to purge their gut prior to chemical analysis which makes it difficult to separate levels of phytohormones in insect tissues from those in the plant material that remains in the gut. Therefore, it is possible for the observed levels of cytokinin and auxin in gall-inducing insects to be at least partly the result of the consumption of plant tissues containing these phytohormones, rather than to their production by the gall-inducing insect.
Immunolocalization with commercially available antibodies has been used to examine the distribution of cytokinins and other phytohormones within galled-plant tissues (Zavala and Brandon 1983; Eberle et al. 1987; Sossountov et al. 1988; Dewitte et al. 1999; Dewitte and Van Onckelen 2001; Witiak 2006). Gall-inducing insect systems are difficult to manipulate due to insect development being closely tied to their plant host. If harm is caused to the host during experimental procedures, larval and gall development may cease. Additionally, phytohormones can also be challenging to study due their small size (~200–500 Da), relative to proteins, which prevents them from being assayed using dot blots or related molecular techniques. These aspects of phytohormones severely limit the tools available to study them, especially in a closed insect-gall system, but gives promise to immunolocalization as a viable tool for further studies. Antibodies are available for one form of auxin [indole-3-acetic acid (IAA)] and two forms of cytokinin [trans-zeatin riboside (tZR) and N-(6)-isopentenyladenosine (iPR)]. Antibodies have highly specific structural requirements for binding which renders the detection of plant hormones in tissues by immunolocalization a powerful method to study the distribution of these signaling molecules (Dewitte et al. 1999). Both tZR and iPR and their free base, nucleotide, and some methylthiol and glucoside forms, all occur at high concentrations in the gall-inducing stages of a variety of insect species, including green-island inducing moths (Mapes and Davies 2001b; Straka et al. 2010; Yamaguchi et al. 2012; Zhang et al. 2017; Andreas et al. 2020). Auxin (IAA) also occurs at high concentrations in gall-inducing insects (Mapes and Davies 2001a; Yamaguchi et al. 2012; Tanaka et al. 2013; Takei et al. 2015). Thus far, however, antibodies to cytokinin and auxin have only been used to localize these compounds in plant tissue, and not insect tissue. To our knowledge, this paper offers the first example of localization of cytokinin and auxin in the tissues of a gall-inducing insect.
Bacterial symbionts of insects may also play a role in gall induction through synthesis of phytohormones. Phyllonorycter blancardella, a leaf-mining caterpillar that feeds inside leaves, secretes high levels of cytokinins (Giron et al. 2007; Zhang et al. 2017) which prevents leaf tissues surrounding the leaf mine from senescing, thus forming “green islands” where the larvae feed and develop as the remainder of the leaf senesces (Angra-Sharma and Sharma 1999; Walters et al. 2008). P. blancardella is known to have a symbiotic Wolbachia sp., and experiments with exposure of adults to antibiotics have prevented “green island” formation by their offspring (Kaiser et al. 2010; Body et al. 2013). Although Wolbachia is not a bacterium previously characterized to produce phytohormones, it has been implicated as the source of cytokinin production in P. blancardella.
To determine if phytohormones are present in insect tissues that would suggest they are delivered into the host-plant, we examined dissected tissues using immunohistochemical methods to detect cytokinins and auxins in the larvae of Eurosta solidaginis. Eurosta solidaginis is a gall-inducing fly that attacks the apical meristem of Solidago altissima forming a ball-shaped gall. A single bacterial symbiont, identified as a strain of Wolbachia sp., has also been identified in E. solidaginis using 16s rRNA amplicon screening (Hammer et al. 2020). To determine if Wolbachia sp. in E. solidaginis could be responsible for these phytohormones, we used a generic nucleic acid stain, DAPI (4′,6-diamidino-2-phenylindole), to determine if Wolbachia sp. and phytohormones co-localize.
METHODS
Insect and Host Plant System
We examined the distribution of phytohormones in Eurosta solidaginis Fitch (Diptera: Tephritidae). The biology of E. solidaginis and its relationship with the host plant Solidago altissima L. (Asterales: Asteraceae) has been extensively researched and can be considered a model system for gall induction in insects (Abrahamson and Weis 1997). Eurosta solidaginis is univoltine and widely distributed in eastern and mid-western North America, producing ball-shaped galls (1–4cm diameter) in the stems of its host plant S. altissima. Chemical analyses of E. solidaginis and tissues of S. altissima indicate that E. solidaginis has much higher concentrations of cytokinins and auxins than in galled or un-galled plant tissues (Mapes and Davies 2001a, b; Andreas et al. 2020). Early larval stages of E. solidaginis induce the gall during feeding, so the salivary glands are the most likely source of phytohormone transfer to plant tissues through herbivory (Miles 1968).
Insect Sample Collection
Galls containing Eurosta solidaginis (Diptera: Tephritidae) larvae were collected in Duluth, Minnesota (N 46.675871, W −92.229560) on July 24th, 2017 and July 23rd, 2018 from S. altissima and shipped to San Francisco on dry ice. At that time of year, 2nd instar larvae are most abundant, but only slightly larger in size than 1st instars. Galls are still actively growing at this stage of larval development (McCrea et al. 1985; Mapes and Davies 2001a, b). Galls were then stored at 4°C, until we removed larvae from the galled plant-tissue using a sterile paring knife. After we removed larvae from the gall, larvae where immediately put aside in sterile micro centrifuge tubes for dissection with full gut contents.
Insect Tissue Collection and Storage
We dissected E. solidaginis larvae for their primary body parts at the second instar stage. We harvested body wall (musculature and integument including exoskeleton), gut (including Malpighian tubules), and salivary glands. We placed these tissues directly into sterile phosphate buffered saline x1M (PBS). We stored the separated tissue types in micro-centrifuge tubes with small amounts of PBS and fixed tissues within two hours. Our protocol for tissue fixation and antibody staining, described below, was adapted from Barbosa et al. (2014) and Thermo Fisher Scientific Protocols (2021).
Fixation
We fixed the tissues under sterile conditions using 3% paraformaldehyde (PFA) in PBS for 30 min, followed by a second fixation step with fresh fixative solution on ice for 2.5 h. We then washed samples 3 times in PBS for 45 min for each wash and stored in PBS at 4°C until used for antibody staining.
Immunohistochemical Staining of E. solidaginis Tissues
We stained the dissected tissues under sterile procedures using one of three different primary antibodies: Agrisera® rabbit-anti-N-(6)-isopentenyladenosine [Agrisera (Vännäs, SWEDEN): Cat. No. AS09 434] (iPR), Agrisera® rabbit-anti-trans-zeatin riboside [Agrisera: Cat. No. AS09 428] (tZR), and Agrisera® rabbit anti-indole-3-acetic acid [Agrisera: Cat. No. AS06 193] (IAA). To prepare tissues for antibody staining, samples were first washed with blocking buffer PBSB (PBS + 1% Bovine Serum Albumin (BSA)) 3 times, 10 minutes each wash, followed by a 30 minute wash with PBT (PBS + Triton 0.1%). The primary antibodies (anti-iPR, anti-tZR and anti-IAA) were added to the samples in blocking buffer composed of PBSB at 4°C rotating for 48 h. All antibodies were diluted in PBSB at a concentration of 1:250. The specificity of these antibodies has previously been established (Dewitte et al. 1999). The cytokinin antibodies are known to be cross reactive with freebase, riboside, nucleotide, and glucoside forms of the respective hormone, but not cross reactive with forms of cis-zeatin or dihydrozeatin. The antibody for IAA is very specific to the antigen (Dewitte et al. 1999). After washing, the antibody-stained tissues were subsequently stained with a secondary goat anti-rabbit polyclonal antibody conjugated with FITC [Vector Labs (Burlingame, CA, USA): Cat. No. BA-1000] diluted 1:1000 in PBS and incubated for 18 h at 4°C rotating in the dark. After the tissues were stained with the secondary antibody, the tissues were washed five times with PBSBT (PBS + 0.1% Triton and 1% BSA) for 10 min each, to ensure removal of excess antibodies that were unattached to antigens.
Co-localization with DAPI
After washing off secondary antibody, we added DAPI (4′,6-diamidino-2-phenylindole) to antibody-stained tissues to visualize the bacterial symbiont of E. solidaginis, Wolbachia sp. (Hammer et al. 2020). DAPI is a generic nucleic acid which attaches itself to the AT regions of dsDNA, which will stain both the nucleus of the insect’s cells and the chromosomes of any bacterial symbiont. DAPI diluted 1:1000 in PBS was added to the same tube in which tissues were stained and incubated for 8 min. Tissues were then washed in PBS 3 times for 10 mins each wash to ensure the removal of excess DAPI stain. We then mounted all tissues onto glass slides using VECTASHIELD® [Vector Labs (Burlingame, CA, USA): Cat. No. H-1000–10] and sealed with nail polish. After preparation, we placed slides in slide boxes at −20°C for long term storage.
Controls
We examined various controls to rule out autofluorescence and non-specific staining. To check for autofluorescence from the insect tissues, we performed a negative control which consisted of just the three insect tissues, salivary glands, gut, and body wall with buffer and washes and no antibodies, as well as a sample with primary antibody only. To test for non-specific staining, we followed the same protocol steps with only the secondary antibody. Autofluorescence was detected in the exoskeleton of the body wall, but non-specific staining with secondary antibody alone was not noted (See Supplementary Information).
Wolbachia sp. are known to exist within ovarian tissues (Werren 1997), thus we also examined ovarian tissues as a positive control for the presence of Wolbachia sp. using our method. Ovaries from laboratory reared adult females were dissected in July 2017 and stored at 4°C. We fixed ovarian tissue as outlined above, but with the addition of heptane in a 1:1 ratio with PFA to help remove the chorion from ovules to facilitate penetration of the stain throughout the sample. We applied the staining protocol described above for DAPI, but with no prior antibody staining to ovarian issues. Wolbachia sp. have been successfully localized in other insect species in non-ovarian tissues using DAPI without the use of heptane (Karr et al. 1998; Alberston et al. 2013). However, heptane has been commonly utilized in ovarian tissues and ovules to de-chorionate ovules to allow better permeabilization to enhance visualization of Wolbachia sp. (Limbourg and Zalokar 1973; Müller 2008; Rand et al. 2010).
Replication
Four biological replicates of experimental tissues (primary and secondary antibody, and DAPI treated) of each tissue type and each antibody were stained and visualized with three technical replicates mounted on each slide. Controls were performed on two biological replicates with three technical replicates mounted on each slide.
Visualization/Imaging
We visualized prepared slides using a Zeiss LSM 710 Confocal Microscope (Zeiss: Oberkochen, Germany). We assessed images for localizing phytohormones within the sample tissues. To visualize co-localization of DAPI and FITC stains to the cellular level, images were acquired by taking confocal sections of each tissue sample with an EC Plan-Neofluar 40x/1.3 oil lens. All co-localized images were taken while the lasers were set for DAPI (405nm laser, 410–497 nm emission) and FITC green (488 nm laser, 493–634nm emission). Singular DAPI stained images were taken with a 405nm laser (410–585 nm emission). Images were processed using Image J (Schneider et al. 2012), and images are shown with the fluorescent label used for tagging FITC depicted in green and DAPI depicted in blue. FITC-tagged phytohormones were not manipulated for brightness or contrast, but DAPI labelled nucleic acids were enhanced with both brightness and contrast filters applied to the whole images to maximize the probability of detecting bacterial chromosomes if present.
PCR of Wolbachia WSP gene
Whole genomic DNA was extracted from fresh ovaries of E. solidaginis using the Quick-gDNA MiniPrep Kit (Zymo Research, Irvine, CA, USA). A 200bp fragment of the Wolbachia surface protein locus (WSP) of the Wolbachia strain associated with E. solidaginis was amplified using PCR with primers 264f (5′ - GGGCTTTATTCGCAGCTAAGC - 3′) and 463r (5′ - TGACTACTCACAGCGGTTGC - 3′). Primers were designed based on a preliminary assembly of Illumina 100bp single-end cDNA derived from a meta-transcriptomic study and assembled using ABySS (Birol et al. 2009). The target locus was amplified with 20μl PCR reactions using AccuPower PyroHotStart Taq PCR PreMix (Bioneer, Alameda, CA, USA). Each PCR reaction included 18μl PCR water, 0.5μl of each primer, and 1μl of the genomic DNA. The PCR involved an initial denaturation at 94 °C for 4 minutes, 25 cycles of denaturation (94 °C for 30 seconds), annealing (53 °C for 35 seconds), and extension (72 °C for 50 seconds), and were followed by a final extension at 72 °C for 1 minute.
RESULTS
Localization of phytohormones
All images we present are representative of the set of biological and technical replicates for each antibody and tissue type. Variation among images for each antibody/tissue type combination was minimal.
Overall, immunolocalization indicated that cytokinin tZR-like and iPR-like immunoreactivity was found almost exclusively in the salivary glands of E. solidaginis (Fig. 1). Given the cross reactivity of the antibodies, staining may have included tZR and iPR, and their free base, nucleotide and glucoside forms. iPR-like immunoreactivity localized and aggregated to the cell membrane of salivary gland cells (Fig. 1b) which is similar to its location in plants (Eberle et al. 1987), while tZR-like immunoreactivity localized intracellularly within the cytoplasm of the salivary glands (Fig. 1e). The high intensity of fluorescence at each point suggests possible localization of tZR to vacuoles. tZR-like immunoreactivity also localized intracellularly in small quantities in gut tissue samples (see arrow in Fig. 1f). Although the stained gut tissue samples were full gut specimens, the staining was consistent with intracellular staining. iPR-like immunoreactivity was absent from full gut tissue samples (Fig. 1c).
Fig 1.
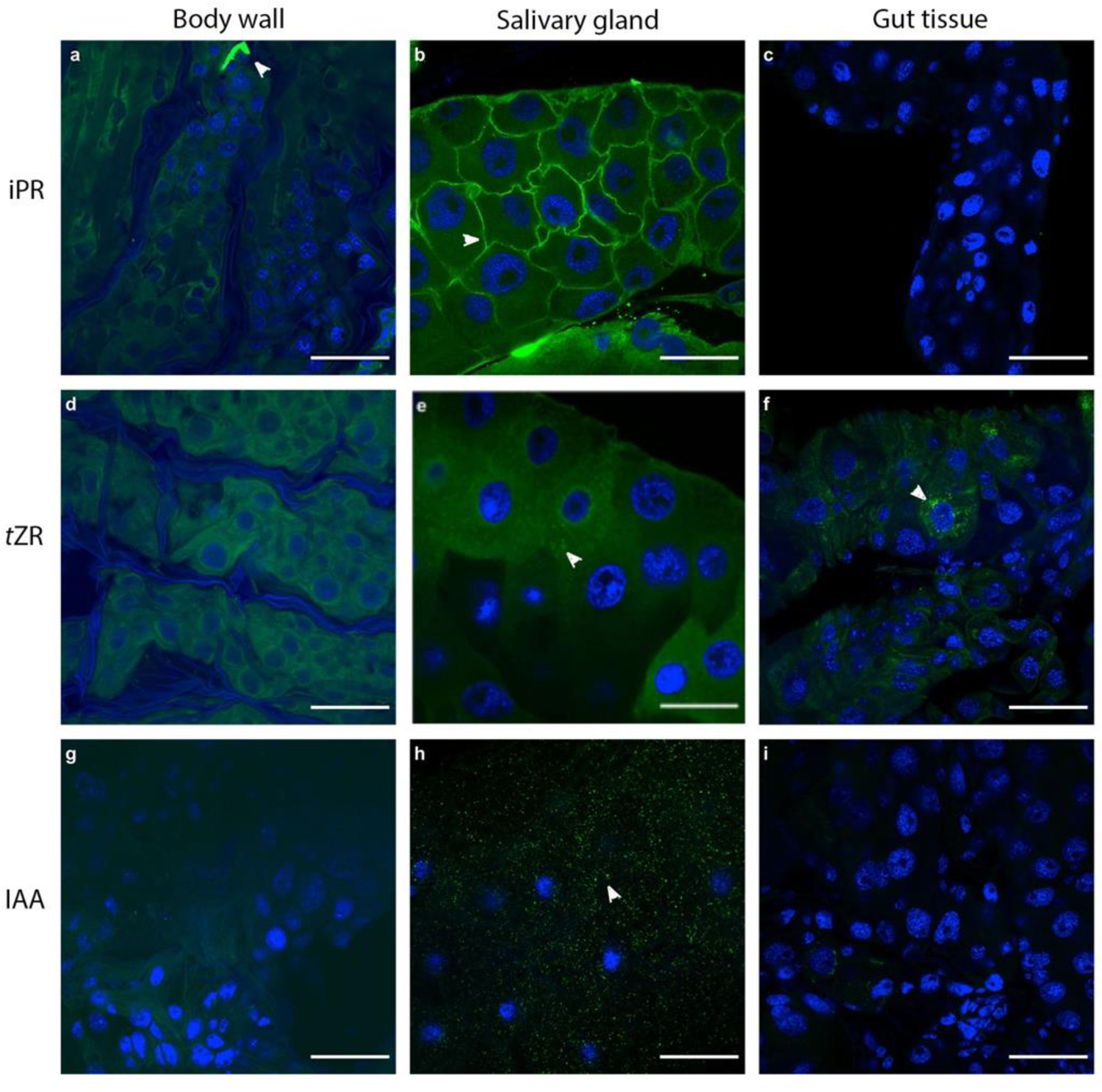
Localization of cytokinin and auxin antibodies, and co-localization of Wolbachia sp. with DAPI nuclear stain. Immunolocalization using rabbit anti-iPR, tZR and IAA antibodies in E. solidaginis tissues with FITC-conjugated secondary polyclonal goat anti-rabbit antibodies (green). Localization of iPR in a) body wall, b) salivary gland tissue, and c) gut tissue. Localization of tZR in d) body wall, e) salivary gland tissue, and f) gut tissue. Localization of IAA in g) body wall, h) salivary gland tissue, and i) gut tissue. DAPI stain for cell nuclei and for all nucleic acids (blue) showed a lack of bacteria in these tissues based on the size of stained particles. DAPI fluorescence has been enhanced with brightness and contrast using ImageJ to identify all DAPI stain. All images were taken under 40x oil magnification. Scale bar for all images is 50 μm.
Similar to tZR, immunolocalization also suggested that auxin (IAA) localized to the cytoplasm in the salivary glands (Fig. 1h), but was mostly absent from other tissues. Salivary gland tissue showed high amounts of specific IAA-like staining intracellularly in the cytoplasm (Fig. 1h). The gut tissue showed only trace amounts of IAA-like fluorescence (arrowhead) intracellularly within the cytoplasm (Fig. 1i).
Staining was absent in the body wall for iPR (Fig. 1a), tZR (Fig. 1d) and IAA (Fig. 1g), although autofluorescence was obvious in these larval body wall tissue samples. This was likely due to the chitinous composition of the outer layer of insect integument, because similar autofluorescence was absent in the gut (Fig. 1c and i), and because autofluorescence was especially noticeable in the denticles (Fig. 1a; arrowhead) and some cuticle.
Co-localization of Wolbachia sp.
DAPI stained the insect cell nuclei of the salivary glands, body wall, and gut tissues, as indicated by consistently large stained nuclei (blue). The lack of smaller stained components suggested that the smaller chromosomes of the bacterial symbiont, Wolbachia sp., and therefore Wolbachia itself, were not present in these tissue types. The lack of staining of Wolbachia sp. chromosomes, particularly in the salivary glands where tZR, iPR, and IAA localize suggests that these phytohormones do not co-localize with Wolbachia sp. (Fig. 1).
PCR analysis of a marker of the Wolbachia sp. WSP gene, indicated that the ovaries of E. solidaginis contain Wolbachia sp. Thus, we established the validity of the DAPI staining technique for detection of Wolbachia sp. within E. solidaginis tissue by staining ovary tissue. DAPI staining in the ovarian tissue is consistent with intracellular localization of a bacterium (Fig. 2), where both ovarian nuclei (large arrowheads) and smaller flecks (small arrowheads), consistent with bacterial chromosomes, were detected, confirming presence of the strain of Wolbachia sp. in E. solidaginis by PCR. The sister strain, Wolbachia pipientis, has rod-like cells (0.5–1.3 μm) and coccoid forms that range widely in size (0.25–1.8 μm) (Stouthamer et al. 1999) which are similar in size to those we detected in the ovarian tissue (Fig. 2). Measurements made on the small flecks stained with DAPI (Fig. 2b), which we presume to be the chromosomes of Wolbachia sp., are similar in size to those reported for W. pipientis (mean = 0.998, se±0.05 μm, n=30). DAPI staining of the ovary was compared to that of a salivary gland sample that was stained with DAPI. There were some specks present, but they were not consistent with the size range of Wolbachia sp. (mean=2.06, se±0.702 μm, n=30), and Hammer et al. (2020) report no other resident bacterial symbionts for E. solidaginis.
Fig 2.
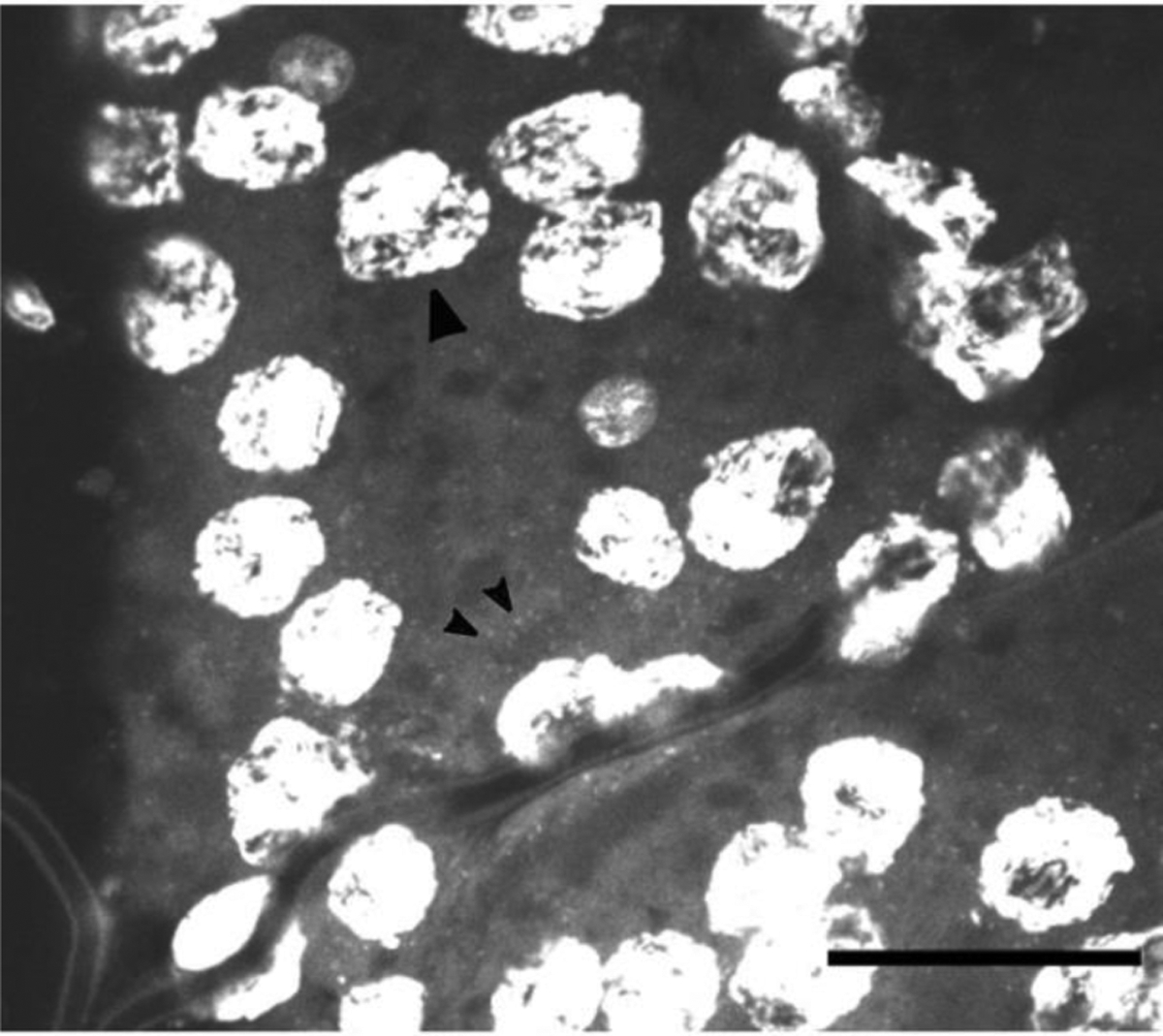
DAPI stained E. solidaginis ovaries show cell nuclei (large arrowhead) and smaller flecks consistent with Wolbachia sp. chromosomes (small arrowheads). Image taken with a 40x oil-immersion lens. DAPI fluorescence has been enhanced with brightness and contrast in ImageJ to emphasize presence of small fleck staining. Scale bar for image is 50 μm.
DISCUSSION
Our experiments have shown that cytokinins (tZR, iPR and their free base, nucleotide, and glucoside forms) and auxin (IAA) within E. solidaginis larvae localize predominantly to the salivary glands. Because E. solidaginis larvae induce plant galls, localization of phytohormones to the salivary glands is consistent with the idea that they are produced in the salivary glands and secreted into host-plant tissues for the purpose of manipulating the host plant. The antibody staining in the salivary glands implies that in E. solidaginis, the reported high whole-body concentrations of auxin and cytokinins (Mapes and Davies 2001a, b, Andreas et al. 2020) are almost entirely found in the salivary glands. A wide range of glandular secretions are known to be produced by insects, so that galls induced by feeding are likely to arise because of salivary secretions (Miles 1968; Doyle and Laufer 1969) and those induced by oviposition via glandular secretions associated with the ovipositor (Yamaguchi et al. 2012).
Localization of tZR and IAA in gut tissue was much less prevalent than in salivary glands. The amount of staining of tZR and IAA intracellularly in gut tissues was visibly much less than in the salivary glands (Fig.1f, i). While it is possible that some absorption and sequestration of tZR and IAA from dietary sources occurs, the high concentrations in the insect relative to the host plant (up to 90 fold difference) suggest that this could only be a minor contribution to the overall pool of IAA and tZR (Mapes and Davies 2001a, b; Straka et al. 2010, Bartlett and Connor 2014; Andreas et al. 2020). Phytohormone localization was performed on early instar larvae with full guts to gage the impact of dietary sources of phytohormones. We observed no extracellular staining of any phytohormones in the gut, implying that gut contents contributed little to the whole-body estimates of cytokinin or auxin concentrations.
It is possible that some phytohormone synthesis occurs in gut tissues. The intima of the foregut of insects is chitinous and non-secretory. However, in the midgut, digestive enzymes are synthesized and secreted, so conceivably phytohormone synthesis could occur in the lumen of the midgut. Alternatively, the cellular material in gut samples could have been of plant origin, or tissues from the salivary glands could have contaminated our whole mounts of gut and Malphigian tubules, giving the false impression that auxin and cytokinins are synthesized there as well. Therefore, we conclude that cytokinins and auxins in gut tissues are at most a very small part of the overall pool of phytohormones in E. solidaginis.
Our results are consistent with those reported for other members of Insecta. The gall-inducing wasp Pontania sp. accessory glands associated with the ovipositor of Pontania sp., had the highest concentrations of cytokinins ever reported for a gall-inducing insect, along with substantial concentrations of auxin (Yamaguchi et al. 2012). Stable isotope labeling has shown that nymphs of the mirid bug Tupiocoris notatus, which does not induce a gall, deliver cytokinin, specifically iP (isopentenyl adenine), into host-plant tissues resulting in manipulation of the host plant (Brütting et al. 2018). The presence of auxin and cytokinins in glands where they can be delivered to the host plant implies that they are produced in these glands for secretion into host-plant tissues to manipulate the host plant.
The hypothesis that a bacterial symbiont could contribute to gall induction by insects stems from two pieces of evidence. First, there are known bacterial species that induce plant galls that have biosynthetic pathways to produce auxin and cytokinin (MacDonald et al. 1986; Lichter et al. 1995; Jameson 2000; Barash and Malulis-Sasson 2007, 2009), bolstered by experiments involving Wolbachia sp. in the “green island” inducing P. blancardella (Giron et al. 2007; Kaiser et al. 2010; Body et al. 2013; Zhang et al. 2017). Secondly, by the apparent lack of biosynthetic pathways for auxin and cytokinin production in insects (Frébort et al. 2011). Our attempt to co-localize phytohormones and the Wolbachia sp. symbiont of E. solidaginis using a nucleic acid stain (DAPI) did not show staining of bacterial chromosomes in any of the larval tissues we examined, despite enhancing both the brightness and contrast for the blue wavelengths to increase the chances of detecting a signal (Fig. 1). However, we did detect bacterial chromosomes in the ovaries of adult females, indicating that our assay was effective (Fig. 2). Wolbachia sp. is known to reside in the reproductive organs of its host, so we interpret the absence of Wolbachia sp. from the salivary glands, where the phytohormones localize, to indicate that Wolbachia sp. does not contribute to phytohormone production in E. solidaginis. Although Wolbachia sp. in the past was implicated in cytokinin production, recent evidence further suggests that symbionts may not be contributing to phytohormone production in insects or to gall induction. An analysis of six species of gall-inducing cynipid wasps using transcriptome and whole genome sequencing, found that only two species had Wolbachia sp. symbionts present (Hearn et al. 2019). Hou et al. (2020) also reported multiple populations of the chestnut gall wasp, Dryocosmus kuriphilus (Hymenoptera: Cynipidae) that induce galls while lacking a Wolbachia sp. symbiont. Finally, examination of twelve insect species including gall-inducing and non-gall-inducing species using 16s rRNA amplicon sequencing yielded no evidence of a specific bacterial symbiont or a community of symbionts associated with gall induction (Hammer et al. 2020).
So, if a bacterial symbiont is not involved in the provisioning of auxin and cytokinins for gall-inducing insects, how do insects synthesize these compounds? Virtually all the research on cytokinin and auxin biosynthesis focuses on plants. Among plants, the consensus for cytokinin is that the de novo-synthesis-ipt pathway (adenylate dimethylallyl transferase, EC: 2.5.1.27 and EC: 2.5.1.112) accounts for the bulk of cytokinins found in plants, and furthermore the tRNA-ipt pathway leads largely to the production of cis-zeatins rather than to tZ or iP, which are the forms that are in high concentration in gall-inducing insects (Mapes and Davies 2001b; Straka et al. 2010; Yamaguchi et al. 2012; Tanaka et al. 2013; Takei et al. 2015; Kai et al. 2017; Andreas et al. 2020). However, the tRNA-ipt pathway (tRNA dimethylallyl transferase, EC: 2.5.1.75) for cytokinin biosynthesis is found in insects and in all organisms, although previously thought be absent from Archaea (Frébort et al. 2011; Nishii et al. 2018) is has recently been detected in this domain (Wang et al. 2020). Additionally, distantly related taxa from gall-inducing fungi to dogs, have been found to contain high concentrations of cytokinins, including tZ and iP, yet only possess the tRNA-ipt pathway (Morrison et al. 2015 a, b and Seegobin et al. 2018). Cytokinins have recently been reported to be widespread and abundant in plant-feeding insects, not just in gall-inducing species, and the tRNA-ipt pathway is likely the source of these cytokinins (Andreas et al. 2020). We note that the concentrations of cytokinins in gall-inducing insects such as E. solidaginis are much higher than in plants, and mostly in the form of tZ and iP and their ribosides, nucleotides, and glucosides. Such a pattern would seem to undermine the notion that the tRNA-ipt pathway is unproductive and only yields cis-zeatins as claimed in the literature on cytokinin biosynthesis in plants (Sakakibara 2006; Galuszka et al. 2008; Stirk and van Staden 2010; Frébort et al. 2011; Spichal 2012). We concur with the suggestion that the tRNA-ipt pathway functions differently and considerably more efficiently in insects for the production of cytokinins than in plants (Andreas et al. 2020).
Both Eurosta solidaginis and Wolbachia sp. have the tRNA-ipt pathway, so it is still conceivable that Wolbachia sp. could contribute to gall induction for E. solidaginis. This could occur either via production of cytokinins in tissues that we did not survey, such as the fat body, and their subsequent translocation to the salivary glands, simply by their production of HMBDP which can serve as the prenyl group donor necessary for cytokinin synthesis, or by secretion of other effectors. Sorting out this possibility will require either targeted gene expression analysis or transcriptomic studies. However, the lack of any evidence of Wolbachia sp. in the salivary glands suggests that it is unlikely that Wolbachia sp. contribute to gall induction in E. solidaginis.
The literature on auxins reveals several pathways for biosynthesis that are found in plants and/or bacteria (Woodward and Bartel 2007; Spaepen et al. 2007; Masaguichi et al. 2011; Patten et al. 2013; Tivendale et al. 2014; Yue et al. 2014), and partial pathways defined for some fungi (Reineke et al. 2008). However, despite a few older studies reporting auxins in animals (Weissbach et al. 1959; Gordon and Buess 1967), only recent work on silkworms has attempted to elucidate an auxin pathway in animals (Suzuki et al. 2014; Yokoyama et al. 2017; Takei et al. 2018). All identified pathways for the biosynthesis of auxin involve tryptophan as the precursor, which insects freely obtain from their diet or from bacterial symbionts. However, Wolbachia sp. lack the ability to synthesize tryptophan so cannot be a source of tryptophan for their insect hosts (Xie et al. 2003). Therefore, auxin production by E. solidaginis is likely to arise from a pathway that is potentially widespread among animals.
We have built a firm case for a contribution of secreted auxin and cytokinin in the stimulation of the tissue growth in the host plant that constitutes the covering gall – the phytohormone production hypothesis. We provide strong evidence that the Wolbachia sp. symbiont of E. solidaginis is unlikely to be involved in phytohormone production and gall induction. However, the evidence we provide here for localization of auxin and cytokinin to a glandular structure primed to deliver secretions to the host plant does not rule out the possibility that secreted effector proteins also could contribute to gall induction by insects – the effector protein hypothesis. Continued inquiry into the mechanism of gall-induction in insects, and into the production and use of phytohormones in the manipulation of plants by phytophagous insects will be required to determine the relative merits of these hypotheses to gall induction by insects.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Lisa Galli, Dr. Sarah Witiak, Dr. Blake Riggs, and Dr. Mark Chan for technical advice and guidance, Dr. Tim Craig for collecting and shipping samples, Stephannie Seng and Natalie Fiutek for their assistance with lab work, and Dr. John Tooker for comments on an earlier version of this manuscript. The authors wish to thank the reviewers for their constructive and insightful remarks improving our manuscript. This research was supported by the ARCS Foundation: Northern California Chapter to GEP, National Science Foundation Grant DEB 0943263 to EFC, and National Institute of Health MS/PhD Bridges: R25-GM048972 to GEP.
Funding:
The ARCS Foundation: Northern California Chapter to GEP, National Science Foundation Grant DEB 0943263 to EFC and National Institute H MS/PhD Bridges: R25-GM048972 to GEP
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Data Availability: Negative Control Images, Single and Dual Channel Images, and Protocol for Immunolocalization of Phytohormones are available through the Figshare repository (Figshare link will be available after publication).
Conflicts of interest/Competing interests: Not applicable
REFERENCES
- Abrahamson WG, Weis AE (1997) Evolutionary ecology across three trophic levels: goldenrods, gallmakers, and natural enemies. Princeton University Press, Princeton, NJ, 10.12987/9780691209432 [DOI] [Google Scholar]
- Albertson R, Tan V, Leads RR, Reyes M, Sullivan W, Casper-Lindley C (2013) Mapping Wolbachia distributions in the adult Drosophila brain. Cell Microbiol 15:1527–1544, 10.1111/cmi.12136 [DOI] [PubMed] [Google Scholar]
- Andreas P, Kisiala A, Emery RJN, De Clerck-Floate R, Tooker JF, Price PW, Miller DG III, Chen MS, Connor EF (2020) Cytokinins are abundant and widespread among insect species. Plants 9(2), 208, 10.3390/plants9020208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angra-Sharma R, Sharma DK (1999) Cytokinins in pathogenesis and disease resistance of Pyrenophora teres-barley and Dreschslera maydis-maize interactions during early stages of infection. Mycopathologia 148: 87–95, 10.1023/a:1007126025955 [DOI] [PubMed] [Google Scholar]
- Barbosa P, Berry D, Kary CK (2014) Insect Histology: Practical Laboratory Techniques. Oxford, UK: John Wiley & Sons. 10.1002/9781118876114. [DOI] [Google Scholar]
- Barash I, Manulis-Sasson S (2007) Virulence mechanisms and host-specificity of gall forming Pantoea agglomerans. Trends Microbiol 15: 538–545, 10.1016/j.tim.2007.10.0090 [DOI] [PubMed] [Google Scholar]
- Barash I, Manulis-Sasson S (2009) Recent evolution of bacterial pathogens: The gall-forming Pantoea agglomerans case. Ann Rev Phytopath 47:133–152, 10.1146/annurev-phyto-080508-081803 [DOI] [PubMed] [Google Scholar]
- Bartlett L, Connor EF (2014) Exogenous phytohormones and the induction of plant galls by insects. Arthropod-Plant Inter 8:339–348, 10.1146/annurev-phyto-080508-081803 [DOI] [Google Scholar]
- Birol I, Jackman SD, Nielsen CB, Qian JQ,Varhol1 R, Stazyk G, Morin RD, Zhao Y, Hirst M, Schein JE, Horsman E, Connors JM, Gascoyne RD, Marra MA, Jones SJM (2009) De novo transcriptome assembly with ABySS. Bioinformatics 25: 2872–2877, 10.1093/bioinformatics/btp367 [DOI] [PubMed] [Google Scholar]
- Body M, Kaiser W, Dubreuil G, Casas J, Giron D (2013) Leaf-miners co-opt microorganisms to enhance their nutritional environment. J Chem Ecol 39:969–977, 10.1007/s10886-013-0307-y [DOI] [PubMed] [Google Scholar]
- Bruce SA, Saville BJ, Emery RJN (2011) Ustilago maydis produces cytokinins and abscisic acid for potential regulation of tumor formation in maize. J Plant Growth Regul 30:51–63, 10.1007/s00344-010-9166-8 [DOI] [Google Scholar]
- Brütting C, Crava CM, Schäfer M, Schuman MC, Meldau S, Adam N, Baldwin IT (2018) Cytokinin transfer by a free-living mirid to Nicotiana attenuate recapitulates a strategy of endophytic insects. eLife 7:e36268, 10.7554/eLife.36268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier S, Ginis O, Moreau SJM, Gayral P, Hearn J, Stone GN, Giron D, Huguet E, Drezen JM (2019) Gall wasp transcriptomes unravel potential effectors involved in molecular dialogues with oak and rose. Front Physiol 10:926, 10.3389/fphys.2019.00926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanclud E, Kisiala A, Emery RJN, Chalvon V, Ducasse A, Romiti-Michel C, Gravot A, Kroj T, Morel J (2016) Cytokinin production by the rice blast fungus is a pivotal requirement for full virulence. PloS Pathog 12(2):e1005457, 10.1371/journal.ppat.1005457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor EF, Bartlett L, O’Toole S, Byrd S, Biskar K, and Orozco J (2012) The mechanism of gall induction makes galls red. Arthropod-Plant Inter 6:489–495, 10.1007/s11829-012-9210-7 [DOI] [Google Scholar]
- Dewitte W, Chiappetta A, Azmi A, Witters A, Strnad M, Rembur J, Noin M, Chriqui D, van Onckelen HA (1999) Dynamics of cytokinins in apical shoot meristems of a day neutral tobacco during floral transition and flower formation. Plant Physiol 119:111–121, 10.1104/pp.119.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitte W, van Onckelen H (2001) Probing the distribution of plant hormones by immunocytochemistry. Plant Growth Regul 33:67–74, 10.1023/A:1010729703354 [DOI] [Google Scholar]
- Dodueva IE, Lebedeva MA, Kuznetsova KA, Gancheva MS, Papononva SS, Lutova LL (2020) Plant tumors: a hundred years of study. Planta 251:82, 10.1007/s00425-020-03375-5 [DOI] [PubMed] [Google Scholar]
- Doehlemann G, Wahl R, Horst RJ, Voll LM, Usadel B, Poree F, Stitt M, Pons-Kühnemann J, Sonnewald U, Kahmann R, Kämper J (2008) Reprogramming a maize plant: transcriptional and metabolic changes induced by the fungal biotroph Ustilago maydis. Plant J 56:181–195, 10.1111/j.1365-313X.2008.03590.x [DOI] [PubMed] [Google Scholar]
- Dorchin N, Hoffman J, Stirk W, Novak O, Strnad M, van Staden J (2009) Sexually dimorphic gall structures correspond to differential phytohormone contents in male and female wasps. Physiol Entomol 34:359–369, 10.1111/j.1365-3032.2009.00702.x [DOI] [Google Scholar]
- Doyle D, Laufer H (1969) Sources of larval salivary gland secretion in the dipteran Chironomus tentans. J Cell Biol 40:61–78, 10.1083/jcb.40.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle J, Wang TL, Cook S, Wells B, Weiler EW (1987) Immunoassay and ultrastructural localization of isopentenyladenine and related cytokinins using monoclonal antibodies. Planta 172:289–297, 10.1007/BF00398657 [DOI] [PubMed] [Google Scholar]
- Erb M, Meldau S, Howe GA (2012) Role of phytohormones in insect-specific plant reactions. Trends Plant Sci 17:250–259, 10.1016/j.tplants.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frébort I, Kowalska M, Hluska T, Frébortová J, Galuszka T (2011) Evolution of cytokinin biosynthesis and degradation. J Exp Bot 62:2431–2452, 10.1093/jxb/err004 [DOI] [PubMed] [Google Scholar]
- Galuszka P, Spíchal L, Kopečný D, Tarkowski P, Frébortová J, Šebela M, Frébort I (2008) Metabolism of plant hormones cytokinins and their function in signaling, cell differentiation and plant development. In: In Rahman AU (ed) Studies in Natural Products Chemistry, Vol. 34. New York: Elsevier, pp 203–264, 10.1016/S1572-5995(08)80024-5 [DOI] [Google Scholar]
- Giron D, Kaiser W, Imbault N, Casas J (2007) Cytokinin-mediated leaf manipulation by a leafminer caterpillar. Biol Lett 3:340–343, 10.1098/rsbl.2007.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron D, Frago E, Glevarec G, Pieterse CMJ, Dicke M (2013) Cytokinins as key regulators in plant–microbe–insect interactions: connecting plant growth and defense. Funct Ecol 27:599–609, 10.1111/1365-2435.12042 [DOI] [Google Scholar]
- Giron D, Glevarec GJ (2014) Cytokinin-Induced phenotypes in plant-insect interactions: learning from the bacterial world. J Chem Ecol 40: 826–835, 10.1007/s10886-014-0466-5 [DOI] [PubMed] [Google Scholar]
- Gordon SA, Buess E (1967) Observations on the physiology and radiation response of auxin Ann NY Acad Sci. 144:136–145, 10.1111/j.1749-6632.1967.tb34008.x [DOI] [PubMed] [Google Scholar]
- Hammer TJ, De Clerck-Floate R, Tooker JF, Price PW, Miller DG III, Connor EF (2020) Are bacterial symbionts associated with gall induction in insects? Arthropod-Plant Inter 15: 1–12, 10.1007/s11829-020-09800-6 [DOI] [Google Scholar]
- Hearn J, Blaxter M, Schonrögge K, Nieves-Aldrey JL, Pujade-Villar J, Huguet E, Drezen JM, Shorthouse JD, Stone GN (2019) Genomic dissection of an extended phenotype: Oak galling by a cynipid gall wasp. PLoS Genet 15(11): e1008398, 10.1371/journal.pgen.1008398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou HQ, Zhao GZ, Su CY, Zhu DH (2020) Wolbachia prevalence patterns: horizontal transmission, recombination, and multiple infections in chestnut gall wasp-parasitoid communities. Entomol Exp Appl 168: 752–765, 10.1111/eea.12962 [DOI] [Google Scholar]
- Jameson P (2000) Cytokinins and auxins in plant pathogen interactions – an overview. Plant Growth Regul 32:369–380, 10.1023/A:1010733617543 [DOI] [Google Scholar]
- Kai S, Kumashiro S, Adachi S, Suzuki Y, Shiomi Y, Matsunaga K, Gyoutoku N, Asami T, Tokuda M (2017) Life history of Stenopsylla nigricornis (Hemiptera: Psylloidea: Triozidae) and phytohormones involved in gall induction. Arthropod-Plant Inter 11:99–108, 10.1007/s11829-016-9470-8 [DOI] [Google Scholar]
- Kaiser W, Huguet E, Casas J, Commin C, Commin C, Giron D (2010) Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts. P Roy Soc Lond B 277:2311–2319, 10.1098/rspb.2010.0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr TL, Yang W, Feder ME (1998) Overcoming cytoplasmic incompatibility in Drosophila. Proc Roy Soc. Lond. B 265: 391–395, 10.1098/rspb.1998.0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichter A, Barash I, Valinsky L, Manulis-Sasson S (1995) The genes involved in cytokinin biosynthesis in Erwinia herbicola pv. gypsophilae: Characterization and role in gall formation. J Bacteriol 177:4457–4465, 10.1128/jb.177.15.4457-4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbourg B, Zalokar M (1973) Permeabilization of Drosophila eggs. Developmental Biol 35: 382–387, 10.1016/0012-1606(73)90034-1 [DOI] [PubMed] [Google Scholar]
- MacDonald EMS, Powell GK, Regier DA, Glass NL, Roberto F, Kosuge T, Morris RO (1986) Secretion of zeatin, ribosylzeatin, and ribosyl-1”-methylzeatin by Pseudomonas savastanoi. Plant Physiol 82:742–747, 10.1104/pp.82.3.742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea KD, Abrahamson WG, Weis AE (1985) Goldenrod ball gall effects on Solidago altissima: 14C translocation and growth. Ecology 66:1902–1907, 10.2307/2937386 [DOI] [Google Scholar]
- Mapes CC, Davies PJ (2001) Indole-3-acetic acid and ball gall development on Solidago altissima. New Phytol 151:195–202, 10.1046/j.1469-8137.2001.00161.x [DOI] [PubMed] [Google Scholar]
- Mapes CC, Davies PJ (2001) Cytokinins in the ball gall of Solidago altissima and in the gall forming larvae of Eurosta solidaginis. New Phytol 151:203–212, 10.1046/j.1469-8137.2001.00158.x [DOI] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sajai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, McSteen P, Zhao Y, Hayashi K, Kamiya Y, Kasahara H (2011) The main auxin biosynthetic pathway in Arabidopsis. P Natl Acad Sci USA 108:18513–18517, 10.1073/pnas.1108434108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles PW (1968) Insect secretions in plants. Annu Rev Phytopathol 6:137–164, 10.1146/annurev.py.06.090168.001033 [DOI] [Google Scholar]
- Morrison EN, Emery RJN, Saville BJ (2015) Phytohormone involvement in the Ustilago maydis-Zea mays pathosystem: relationships between abscisic acid and cytokinin levels and strain virulence in infected cob tissue. PLoS ONE 10(6):e0130945, 10.1371/journal.pone.0130945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrsion EN, Knowles S, Thorn RG, Saville BJ, Emery RJN (2015) Detection of phytohormones in temperate forest fungi predicts consistent abscisic acid production and a common pathway for cytokinin biosynthesis. Mycologia 107:245–257, 10.3852/14-157 [DOI] [PubMed] [Google Scholar]
- Müller H-AJ (2008) Immunolabeling of embryos. In Dahmann C (Ed.), Drosophila: Methods and Protocols (pp. 207–218). Humana Press. 10.1007/978-1-59745-583-1_12 [DOI] [Google Scholar]
- Naseem M, Wölfling M, Dandekar T (2014) Cytokinins for immunity beyond growth, galls and green islands. Trends Plant Sci 19:481–484, 10.1016/j.tplants.2014.04.001 [DOI] [PubMed] [Google Scholar]
- Nishii K, Wright F, Chen YY, Möller M (2018) Tangled history of a multigene family: the evolution of isopentenyltransferase genes. PLoS ONE 13(8):e0201198, 10.1371/journal.pone.0201198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten CL, Blakney AJC, Coulson TJD (2013) Activity, distribution and function of indole-3-acetic acid biosynthetic pathways in bacteria. Crit Rev Microbiol 39:395–415, 10.3109/1040841X.2012.716819 [DOI] [PubMed] [Google Scholar]
- Rand MD, Kearney AL, Dao J, Clason T (2010). Permeabilization of Drosophila embryos for introduction of small molecules. Insect Biochem Molec Biol 40: 792–804. 10.1016/j.ibmb.2010.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redkar A, Hoser R, Schilling L, Zechmann B, Krzymowska M, Walbot V, Doehlemann G (2015) A secreted effector protein of Ustilago maydis guides maize leaf cells to form tumors. Plant Cell 27:1332–1351, 10.1105/tpc.114.131086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke G, Heinze B, Schiraski J, Buettner H, Kahmann R, Basse CW (2008) Indole-3-acetic acid (IAA) biosynthesis in the smut fungus Ustilago maydis and its relevance for increased IAA levels in infected tissue and host tumor formation. Mol Plant Pathol 9: 339–355, 10.1111/j.1364-3703.2008.00470.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robischon M (2015) Do cytokinins function as two-way signals between plants and animals? BioEssays 37:356–363, 10.1002/bies.201400099 [DOI] [PubMed] [Google Scholar]
- Sakakibara H (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57:431–449, 10.1146/annurev.arplant.57.032905.105231 [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nature Meth 9, 671–675, 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegobin M, Kisiala A, Noble A, Kaplan D, Brunetti C, Emery RJN (2018) Canis familiaris tissues are characterized by different profiles of cytokinins typical of the tRNA degradation pathway. FASEB J 10.1096/fj.201800347 [DOI] [PubMed] [Google Scholar]
- Sossountov L, Maldiney R, Sotta B, Sabbagh I, Habricot Y, Bonnet M, Miginiac E (1988) Immunocytochemical localization of cytokinins in Craigella tomato and a sideshootless mutant. Planta 175:291–304, 10.1007/BF00396334 [DOI] [PubMed] [Google Scholar]
- Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448, 10.1111/j.1574-6976.2007.00072.x [DOI] [PubMed] [Google Scholar]
- Spíchal L (2012) Cytokinins – recent news and views of evolutionally old molecules. Funct Plant Biol 39:267–284, 10.1071/FP11276 [DOI] [PubMed] [Google Scholar]
- Straka JR, Hayward AR, Emery RJN (2010) Gall-inducing Pachypsylla celtidis (Psyllidae) infiltrate hackberry trees with high concentrations of phytohormones. J Plant Inter 5:197–203, 10.1080/17429145.2010.484552 [DOI] [Google Scholar]
- Stirk WA, van Staden J (2010) Flow of cytokinins through the environment. Plant Growth Regul 62:101–116, 10.1007/s10725-010-9481-x [DOI] [Google Scholar]
- Stouthamer R, Breeuwer JAJ, Hurst GDD (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53:71–102, 10.1146/annurev.micro.53.1.71 [DOI] [PubMed] [Google Scholar]
- Sugio A, Dubreuil G, Giron D, Simon JC (2015) Plant–insect interactions under bacterial influence: ecological implications and underlying mechanisms. J Exp Bot 66:467–478, 10.1093/jxb/eru435 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Yokokura J, Ito T, Arai R, Yokoyama C, Toshima H, Nagata S, Asami T, Suzuki Y (2014) Biosynthetic pathway of the phytohormone auxin in insects and screening of its inhibitors. Insect Biochem Molec 53:66–72, 10.1016/j.ibmb.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Takei M, Yoshida S, Kawai T, Hasegawa M, Suzuki Y (2015) Adaptive significance of gall formation for a gall-inducing aphids on Japanese elm trees. J Insect Phys 72:43–51, 10.1016/j.jinsphys.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Takei M, Kogure S, Yokoyama C, Kouzuma Y, Suzuki Y (2018) Identification of an aldehyde oxidase involved in indole-3-acetic acid synthesis in Bombyx mori silk gland. Biosci Biotechnol Biochem 83:129–136, 10.1080/09168451.2018.1525275 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Okada K, Asami T, Suzuki Y (2013) Phytohormones in Japanese mugwort gall induction by a gall-inducing gall midge. Biosci Biotechnol Biochem 9:1942–1948, 10.1271/bbb.130406 [DOI] [PubMed] [Google Scholar]
- Tivendale ND, Ross JJ, Cohen JD (2014) The shifting paradigms of auxin biosynthesis. Trends Plant Sci 19:44–51, 10.1016/j.tplants.2013.09.012 [DOI] [PubMed] [Google Scholar]
- Thermo Fisher Scientific (2021) DAPI counterstaining protocols. https://www.thermofisher.com/us/en/home/references/protocols/cell-and-tissue-analysis/dapi-protocol/basic-dapi-counterstaining-protocols.html
- Tooker JF, De Moraes CM (2011) Feeding by Hessian fly (Mayetiola destructor [Say]) larvae on wheat increases levels of fatty acids and indole-3-acetic acid but not hormones involved in plant defense signaling. J Plant Growth Regul 30:158–165, 10.1007/s00344-010-9177-5 [DOI] [Google Scholar]
- Tooker JF, Helms AM (2014) Phytohormone dynamics associated with gall insects, and their potential role in the evolution of the gall-inducing habit. J Chem Ecol 40:742753, 10.1007/s10886-014-0457-6 [DOI] [PubMed] [Google Scholar]
- Walters DR, McRoberts N, Fitt BDL (2008) Are green islands red herrings? significance of green islands in plant interactions with pathogens and pests. Biol Rev 83:79–102, 10.1111/j.1469-185X.2007.00033.x [DOI] [PubMed] [Google Scholar]
- Wang X, Lin S, Liu D, Gan L, McAvoy R, Ding J, Li Y (2020) Evolution and roles of cytokinin genes in angiosperms 1: Do ancient IPTs play housekeeping while non-ancient play regulatory roles? Horticulture Research 7:28, 10.1038/s41438-019-0211-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach H, King W, Sjoerdsma A, Udenfriend S (1959) Formation of indole-3-acetic acid from tryptamine in animals: a method for the estimation of indole-3-acetic acid in tissues. J Biol Chem 234:81–86 [PubMed] [Google Scholar]
- Werren JH (1997) Biology of Wolbachia. Annu Rev Entomol 42: 587–609, 10.1146/annurev.ento.42.1.587 [DOI] [PubMed] [Google Scholar]
- Witiak SM (2006) Hormonal and Molecular Investigations of Phylloxera Leaf Gall Development. Dissertation, Pennsylvania State University, http://etda.libraries.psu.edu/theses/approved/WorldWideIndex/ETD-1564/index.html [Google Scholar]
- Woodward A, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot 95:707–735, 10.1093/aob/mci083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Baldwin IT (2010) New insights into plant responses to the attack from insect herbivores. Annu Rev Genet 44:1–24, 10.1146/annurev-genet-102209-163500 [DOI] [PubMed] [Google Scholar]
- Xie G, Keyhani NO, Bonner CA, Jensen RA (2003) Ancient origin of the tryptophan operon and the dynamics of evolutionary change. Microbiol Mol Biol R 67:303–342, 10.1128/mmbr.67.3.303-342.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Tanaka H, Hasegawa M, Tokuda M, Asami T, Suzuki Y (2012) Phytohormones and willow gall induction by a gall-inducing sawfly. New Phytol 196:586–595, 10.1111/j.1469-8137.2012.04264.x [DOI] [PubMed] [Google Scholar]
- Yokoyama C, Takei M, Kouzuma Y, Nagata S, Suzuki Y (2017) Novel tryptophan metabolic pathways in auxin biosynthesis in silkworm. J Insect Physiol 101:91–96, 10.1016/j.jinsphys.2017.07.006 [DOI] [PubMed] [Google Scholar]
- Yue J, Hu X, Huang J (2014) Origin of plant auxin biosynthesis. Trends Plant Sci 19:764–770, 10.1016/j.tplants.2014.07.004 [DOI] [PubMed] [Google Scholar]
- Zavala ME, Brandon DL (1983) Localization of a phytohormone using immunocytochemistry. J Cell Biol 97:1235–1239, 10.1083/jcb.97.4.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Guiget A, Dubreuil G, Kisiala A, Andreas P, Emery RJN, Huguet E, Body M, Giron D (2017) Dynamics and origin of cytokinins involved in plant manipulation by a leaf-mining insect. Insect Sci 24:1065–1078, 10.1111/eea.12681 [DOI] [PubMed] [Google Scholar]
- Zhao C, Navarro Escalante L, Chen H, Benatti TR, Qu J, Chellapilla S, Waterhouse RM, Wheeler D, Andersson MN, Bao R, Batterton M, Behura SK, Blankenburg KP, Caragea D, Carolan JC, Coyle M, El-Bouhssini M, Francisco L, Friedrich M, Gill N, Grace T, Grimmelikhuijzen CJP, Han Y, Hauser F, Herndon N, Holder M, Ioannidis P, Jackson L, Javaid M, Jhangiani SN, Johnson AJ, Kalra D, Korchina V, Kovar CL, Lara F, Lee SL, Liu X, Lofstedt C, Mata R, Mathew T, Muzny DM, Nagar S, Nazareth LV, Okwuonu G, Ongeri F, Perales L, Peterson BF, Pu LL, Robertson HM, Schemerhorn BJ, Scherer SE, Shreve JT, Simmons D, Subramanyam S, Thornton RL, Xue K, Weissenberger JM, Williams CE, Worley KC, Zhu D, Zhu Y, Harris MO, Shukle RH, Werren JH, Zdobnov EM, Chen MS, Brown SJ, Stuart JJ, Richards S (2015) A massive expansion of effector genes underlies gall-formation in the wheat pest Mayetiola destructor. Curr Biol 25:613–620, 10.1016/j.cub.2014.12.057 [DOI] [PubMed] [Google Scholar]
- Zhao C, Rispe C, Nabity PD (2019) Secretory RING finger proteins function as effectors in a grapevine galling insect. BMC Genomics 20:923, 10.1186/s12864-019-6313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Oger PM, Schrammeijer B, Hooykaas PJJ, Farrand SK, Winans SC (2000) The bases of crown gall tumorigenesis. J Bacteriol 182:3885–3895, 10.1128/jb.182.14.3885-3895.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


