Abstract
Purpose
Diabetic nephropathy (DN) and Cardiovascular Dysfunctioning (CVD) are interlinked with each other and one of the leading causes of irreversible renal damage and cardiovascular disease. Micronutrients play an effective role in type-2 diabetes (T2D) and its related complications. Our work aimed to elucidate the effect of micronutrients alone and in combination with standard anti-diabetic drug metformin on DN and CVD using streptozotocin induced diabetes in rats.
Methods
T2D was induced with a single intraperitoneal (i.p.) injection of freshly prepared streptozotocin (55 mg/kg), 15 min after intraperitoneal injection of nicotinamide (230 mg/kg). Commercially available kits were used to measure kidney parameters and cardiac marker level. Creatinine clearance was calculated by using formula and heart rate was recorded using powerlab software.
Results
Significant decrease in blood glucose levels were observed 14 days after initial administration in metformin treated, micronutrients treated and metformin with micronutrients treated groups compared with diabetic group. After 6 weeks of metformin and micronutrients treatment, serum creatinine, blood urea nitrogen (BUN) and lactate dehydrogenase (LDH) levels were significantly decreased as compared to diabetic group. Moreover, urine creatinine level, creatinine clearance and heart rate (HR) was increased significantly in metformin and micronutrients treated group compared with a diabetic group. Micronutrients therapy also normalised the general symptoms of diabetes.
Conclusion
The results obtained from this study indicate the synergistic effect of metformin and micronutrients against diabetic heart and kidney. Therefore, micronutrients may be used as an effective add-on therapy for DN and CVD.
Keywords: Diabetes mellitus, Diabetic nephropathy, Cardiovascular dysfunctioning, Micronutrients
Introduction
Type 2 diabetes mellitus is a pandemic endocrine disease due to insulin resistance, impaired insulin secretion and modern life styles of people. Diabetes is responsible for disturbing structure and functions of neuroendocrine system which ultimately leads to dysfunctioning of vital organs of body like kidney and heart. Micro-vascular complications of diabetes includes diabetic nephropathy (DN), diabetic retinopathy and diabetic neuropathy and macro-vascular complications includes cardiovascular diseases. Molecular and cellular mechanisms behind all these complications are complex and overlapping [1]. There are multiple metabolic pathways activated in response to hyperglycaemia and leads to DN via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-Reactive oxygen species (ROS) mediated endothelial and mitochondrial injury and increased level of extracellular matrix, sodium-glucose co-transporter 2 (SGLT-2) mediated hyper-filtration and hyper-perfusion, fibroblast growth factor 23 (FGF23)-renin mediated podocyte apoptosis and the mammalian target of rapamycin (m-TOR)-Monocyte chemoattractant protein (MCP) mediated renal fibrosis [2].
Diabetes also leads to cardiovascular dysfunctioning (CVD) by transition through the several cellular and molecular mechanisms and pathways including ROS-induced oxidative stress, mitochondrial dysfunction and excessive production of advanced glycation end products (AGEs), inflammation, apoptosis, autophagy, lipotoxicity, fibrosis, endoplasmic reticulum-stress, DNA damage and renin angiotensin aldosterone system (RAAS) activation [3, 4]. By transition through some common mechanistic pathways like ROS-induced oxidative stress, RAAS activation; diabetes leads to DN and CVD which reveal that both complications cross-talk with each other. Some cross sectional study concluded that there is a close association between DN and CVD [5] which shows that diabetes is not a single disorder as it leads to DN, CVD and multiple micronutrients deficiency disorders.
Regular consumption of various multi-vitamin and multi-mineral preparations as supplements or nutraceuticals for treatment of diabetics is widespread in various countries. Micronutrients plays an important role as a co-factor and co-enzymes in major metabolic pathways. Many studies has been performed by using single or combination of two fat and water soluble vitamins or macro-minerals or trace elements for ameliorating renal dysfunction, oxidative stress, inflammation and diabetic cardiomyopathy [6, 7]. Some vitamins and minerals act as a hypoglycaemic, anti-oxidant, reno-protective, cardio-protective, anti-inflammatory and insulin-mimetic agents in many scientific researches [8, 9]. Yet, shockingly preclinical or clinical evaluation of these preparations is unavailable in the literature for many constituents. Consequently, in our study we evaluated the effect of micronutrient preparation consisting of three vitamins and four minerals alone and in combination with standard anti-diabetic drug metformin in experimental diabetic rats. Some vitamins and minerals have proven as effective nutrients in T2D but effect of their combination was not studied earlier particularly in context to treatment of DN and CVD. Moreover, in diverse experimental diabetic models and clinical studies, a number of data exists on the effect of individual vitamins, minerals, trace elements, or the mixture of few of them. The individual components of the micronutrient preparation explored in the present study were selected on the basis of compelling preclinical and clinical evidences in different diabetic animal models or human populations [10].
Metformin, which is an oral hypoglycaemic biguanide prototype drug of anti-diabetic class has been widely used for several decades in the treatment of diabetic complications because of its action on overall carbohydrate metabolism. Metformin supresses hepatic gluconeogenesis, intestinal absorption of glucose and improve insulin sensitivity by increasing peripheral glucose uptake and utilization and act as AMPK agonist through its cellular and molecular mechanisms [11]. Hence this study was undertaken to evaluate the synergistic effect of micronutrients and metformin in alleviating DN and CVD using STZ- induced diabetes in rats. This is the first confirmation in the literature which shows that micronutrients which are used for human consumption significantly attenuated the progression of diabetes and complications of diabetes viz. DN and CVD in an animal model.
Materials and methods
Experimental animals
Thirty male Wistar rats, weighing 180–200 g were purchased from National Institute of Bioscience, Pune-412,205 and maintained at a constant temperature of 24 ± 2 °C with a fixed 12:12-h light-dark cycle. High fat diet and water were supplied ad libitum throughout the experimental period and cages were cleaned twice a week. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) of Smt. Kashibai Navale College of Pharmacy, Kondhwa, Pune (Protocol approval no. IAEC-125-21/2019).
Induction of diabetes mellitus
The rats were fasted for 12 h and FBG levels were determined. STZ (55 mg/kg, i.p., Sigma-Aldrich, USA) in citrate buffer (0.1 mol/L, pH 4.5) was administered 15 min after nicotinamide (230 mg/kg, i.p., Arati Dye Chem, Gujarat) administration in rats. After 72 h of STZ injection, those animals showed FBG more than 200 mg/dl, were considered as hyperglycaemic and all rats were divided into 5 groups as follows:
Group 1 (Control): Control rats treated with vehicle alone.
Group 2 (Diabetic group): Diabetic rats without treatment.
Group 3 (Diabetic group with Metformin): Diabetic rats received metformin (50 mg/kg, i.p., Emcure Pharmaceuticals Ltd., Pune) once a day for 6 weeks.
Group 4 (Diabetic group with Micronutrients): Diabetic rats received micronutrients orally once a day for 6 weeks.
Group 5 (Diabetic group with metformin and micronutrients): Diabetic rats received metformin with micronutrients once a day for 6 weeks.
Micronutrients dose
Combination of three vitamins (viz. Vitamin B3, Vitamin D and Vitamin E) and four minerals (viz. Selenium, Chromium picolinate, Vanadium pentoxide and Magnesium sulphate) were used as the micronutrient preparation for administration in animals. The water soluble vitamins and minerals (selenium, chromium picolinate from Influx healthcare, Mumbai; vanadium pentoxide from Yogi Dye Chem Industries, Mumbai; magnesium sulphate and vitamin B3 from authorized institutional supplier) were dissolved in distilled water, while the fat soluble vitamins (vitamin D and vitamin E from authorized institutional supplier) were dissolved in coconut oil. The dose was calculated according to formula, HED (mg/kg) = Animal dose (mg/kg) x (Animal Km: Human Km). Where Km is the ratio of body weight in kg to surface area in m2. The control rats received equivalent volume of coconut oil and distilled water as well by oral gavage.
Biochemical analysis
Blood glucose was measured weekly by using Accu-check glucometer. Under light ether anaesthesia, blood sample was collected by using retro-orbital method to perform bioassay. Serum creatinine and urine creatinine level was measured by Modified Jaffe’s method, BUN levels by colorimetric detection method and Modified IFCC method was followed to measure LDH activity in serum using LDH commercial kit according to the manufacturer’s instructions.
Statistical analysis
Graph pad prism 8.4.3 software was used to perform statistical analysis using one way ANOVA for serum creatinine, urine creatinine, creatinine clearance, BUN, HR and LDH level and two way ANOVA for blood glucose level, body weight and urinary output. After ANOVA, Tukey’s multiple comparison test was performed. Results were expressed as mean ± SD. Statistical significance was achieved if the P < 0.05.
Results
Effect of metformin and micronutrient treatment on blood glucose level and body weight
From day 7 to till day 42 of study, blood glucose level was increased and sharp decrease was recorded in the body weight of diabetic rats as compared to control rats (aP < 0.05). All treated groups (group 3, 4 and 5) showed significant decrease in blood glucose level after day 14 and indicated recovery in body weight as compared to diabetic rats (bP < 0.05) after day 21 of study. Moreover, rats in group 4 and 5 showed significant decrease in blood glucose level and improvement in body weight as compared to group 3 (cP < 0.05) (Table 1).
Table 1.
Effect of six weeks repeated dose treatment of metformin and micronutrients on blood glucose level and body weight
| Blood glucose level (mg/dl) | |||||
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
| Day 0 | 67 ± 4.13 | 78 ± 6.67 | 85 ± 3.76 | 93 ± 4.46 | 83 ± 4.76 |
| Day 7 | 85 ± 6.34 | 236 ± 7.21a | 221 ± 5.56 a | 215 ± 4.53 a | 200 ± 7.43 ab |
| Day 14 | 77 ± 4.77 | 245 ± 5.56 a | 230 ± 6.45 a | 222 ± 5.67 ab | 205 ± 6.34 abc |
| Day 21 | 84 ± 6.38 | 256 ± 5.45 a | 210 ± 7.76 ab | 191 ± 5.58 ab | 183 ± 3.65 abc |
| Day 28 | 92 ± 5.49 | 238 ± 4.87 a | 192 ± 4.54 a | 178 ± 6.21 abc | 162 ± 4.46 abc |
| Day 35 | 69 ± 7.22 | 267 ± 6.57 a | 158 ± 5.35 ab | 148 ± 3.45 ab | 121 ± 5.65 abc |
| Day 42 | 89 ± 4.68 | 256 ± 5.44 a | 95 ± 6.56b | 83 ± 7.35b | 72 ± 6.37 bc |
| Body weight (g) | |||||
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
| Day 0 | 180 ± 4.52 | 200 ± 5.56 | 210 ± 6.54 | 220 ± 4.99 | 200 ± 6.54 |
| Day 7 | 190 ± 5.09 | 180 ± 6.33 a | 195 ± 6.45 ab | 185 ± 5.67 abc | 192 ± 4.77 bd |
| Day 14 | 200 ± 6.08 | 165 ± 7.65 a | 185 ± 5.34 ab | 160 ± 6.54 abc | 180 ± 6.87 abc |
| Day 21 | 195 ± 6.32 | 152 ± 4.67 a | 163 ± 4.69 ab | 157 ± 5.67 abc | 176 ± 7.32 abc |
| Day 28 | 205 ± 6.96 | 149 ± 5.43 a | 167 ± 5.55 ab | 177 ± 4.87 abc | 186 ± 4.89 abc |
| Day 35 | 210 ± 6.67 | 120 ± 6.31 a | 172 ± 7.21 ab | 180 ± 5.78 abc | 190 ± 5.32 abc |
| Day 42 | 225 ± 7.64 | 100 ± 4.78 a | 180 ± 6.05 ab | 185 ± 6.77 abc | 210 ± 5.34 abc |
Data expressed as mean ± SD, n=6. aP<0.05 vs control group, bP<0.05 vs diabetic group, cP<0.05 vs group 3.
Data expressed as mean ± SD, n = 6. aP < 0.05 vs control group, bP < 0.05 vs diabetic group, cP < 0.05 vs group 3.
Effect of metformin and micronutrient treatment on urinary output
From day 7 to till day 42 of study, urinary output was increased significantly in diabetic group as compared to control group (aP < 0.001). All treated groups (group 3, 4 and 5) showed significant decrease in urinary output after day 14 of study as compared to diabetic group (bP < 0.05). Rats treated with metformin and micronutrients showed significant decrease in urinary output as compared to group 3 (cP < 0.05) (Table 2).
Table 2.
Effect of six week repeated dose treatment of metformin and micronutrients on urinary output
| Urinary output (ml/24 h) | |||||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
| Day 0 | 8.9 ± 0.56 | 8.5 ± 0.58 | 9.4 ± 0.44 | 7.4 ± 0.47 | 9.2 ± 1.13 |
| Day 7 | 9.7 ± 1.11 | 22.4 ± 0.67 a | 20.4 ± 0.57 a | 18.3 ± 0.57 ab | 15.2 ± 0.87 abc |
| Day 14 | 8.4 ± 1.23 | 25.6 ± 0.87 a | 22.5 ± 0.65 a | 20.6 ± 0.76 ab | 18.4 ± 0.78 abc |
| Day 21 | 7.3 ± 0.89 | 24.8 ± 0.68 a | 19.2 ± 0.94 ab | 17.5 ± 0.76 ab | 15.9 ± 1.12 ab |
| Day 28 | 8.1 ± 0.94 | 23.9 ± 0.79 a | 17.8 ± 0.67 ab | 14.4 ± 0.68 ab | 9.5 ± 0.95 bcd |
| Day 35 | 7.7 ± 0.88 | 22.6 ± 0.86 a | 15.3 ± 0.59 ab | 12.6 ± 0.86 ab | 8.4 ± 0.57 bcd |
| Day 42 | 9.9 ± 0.76 | 21.7 ± 0.81 a | 12.5 ± 0.75 b | 9.6 ± 0.75 b | 6.7 ± 0.67 bc |
Data expressed as mean ± SD, n=6. aP<0.05 vs control group, bP<0.05 vs diabetic group, cP<0.05 vs. group 3, dP<0.05 vs. group 4,
Data expressed as mean ± SD, n = 6. aP < 0.05 vs. control group, bP < 0.05 vs. diabetic group, cP < 0.05 vs. group 3, dP < 0.05 vs group 4.
Effect of metformin and micronutrients on serum creatinine and BUN level
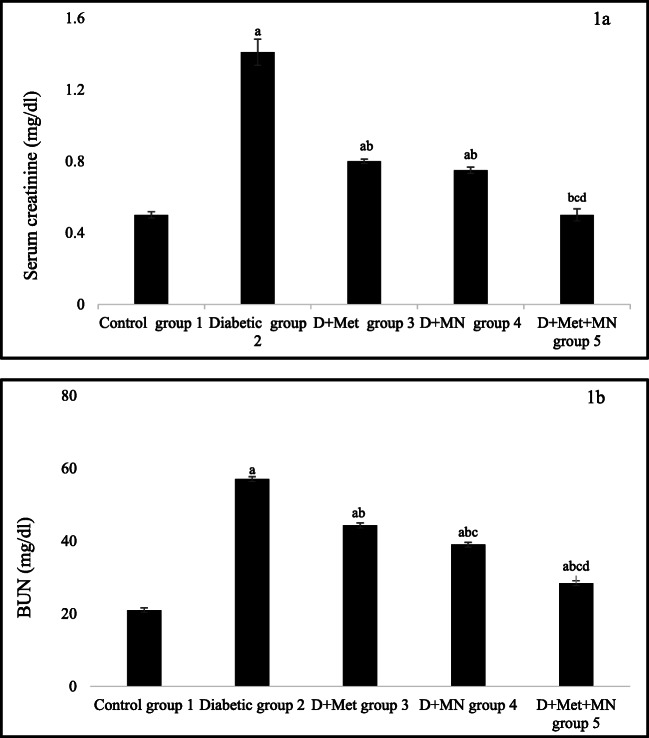
Serum creatinine and BUN level was significantly increased in diabetic group as compared to control group (aP < 0.05). After 6 weeks of metformin and micronutrients treatment, all treated groups (group 3,4 and 5) showed significant decrease in serum creatinine and BUN level as compared to diabetic group (bP < 0.05). Rats treated with metformin and micronutrients showed significant decrease in serum creatinine level as compared to group 3 (cP < 0.05) and group 4 (dP < 0.05), but no significant difference was found between group 3 and 4. However, in case of BUN level, rats in group 4 and 5 showed significant decrease as compared to group 3 (cP < 0.05) and rats in group 5 decreases BUN level as compared to group 4 (dP < 0.05) (Fig. 1 A and B).
Fig. 1.
Effect of metformin and micronutrients on a. Serum creatinine and b. BUN level after 6 weeks. Data expressed as mean ± SD, n = 6. aP < 0.05 vs. control group, bP < 0.05 vs. diabetic group, cP < 0.05 vs. group 3, dP < 0.05 vs. group 4
Effect of metformin and micronutrients on urine creatinine and creatinine clearance
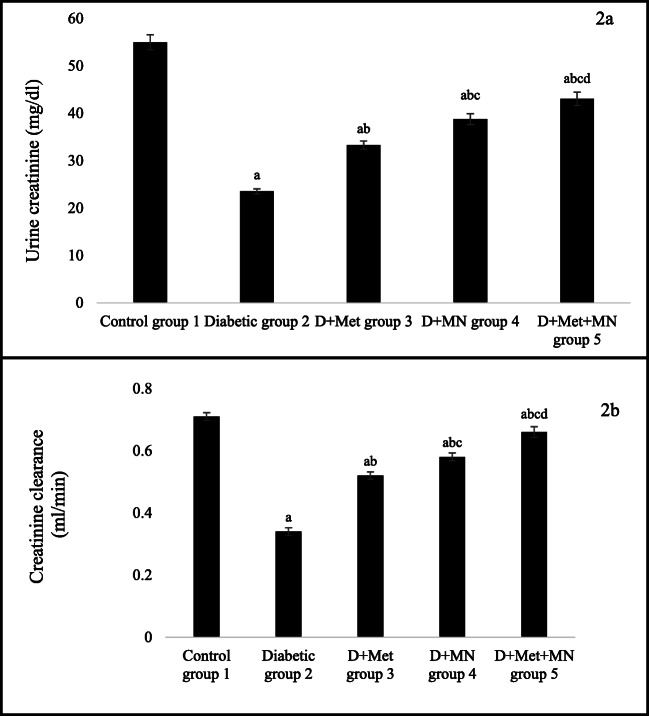
Urine creatinine level and creatinine clearance was significantly decreased in diabetic group as compared to control group (aP < 0.05). After 6 weeks of metformin and micronutrients treatment, all treated groups (group 3,4 and 5) showed significant increase in urine creatinine level and creatinine clearance as compared to diabetic group (bP < 0.05). Moreover, rats in group 4 and 5 showed significant increase in urine creatinine level and creatinine clearance as compared to group 3 (cP < 0.05). However, rats treated with metformin with micronutrients increased urine creatinine level and creatinine clearance as compared to group 4 (dP < 0.05) at the end of study (Fig. 2 A and B).
Fig. 2.
Effect of metformin and micronutrients on a. Urine creatinine and b. Creatinine clearance after 6 weeks. Data expressed as mean ± SD, n = 6. aP < 0.05 vs. control group, bP < 0.05 vs. diabetic group, cP < 0.05 vs. group 3, dP < 0.05 vs group 4
Effect of metformin and micronutrients on LDH and HR
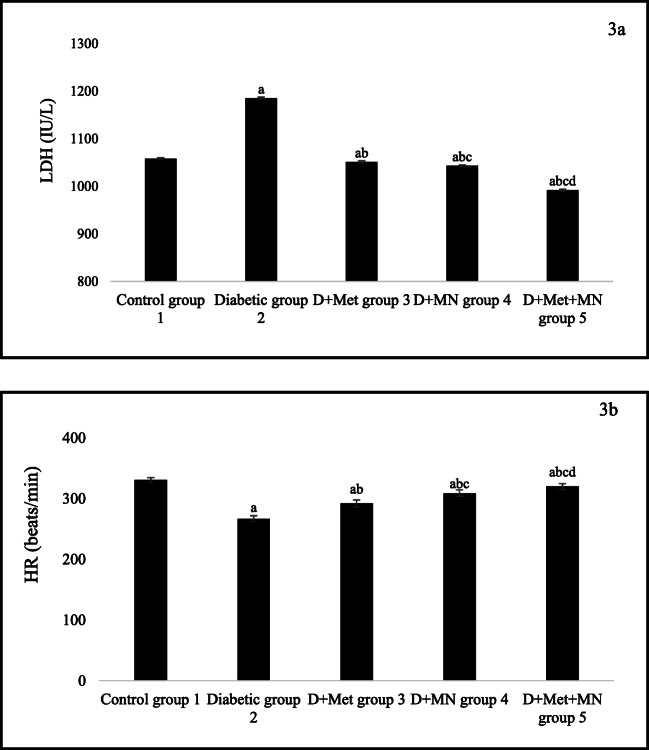
The LDH level was increased and HR was decreased significantly in diabetic rats as compared to control rats (aP < 0.05). Upon 6 weeks of metformin and micronutrients therapy, all treated rats decreased LDH level and improved HR as compared to diabetic rats (bP < 0.05). Moreover, rats in group 4 and 5 significantly decrease LDH level and increase HR as compared to group 3 (cP < 0.05). Furthermore, rats treated with metformin with micronutrients decreased LDH level and increased HR as compared to group 4 (dP < 0.05) at the end of study (Fig. 3 A and B).
Fig. 3.
Effect of metformin and micronutrients on a. LDH level and b. HR after 6 weeks. Data expressed as mean ± SD, n = 6. aP < 0.05 vs control group, bP < 0.05 vs diabetic group, cP < 0.05 vs group 3, dP < 0.05 vs group 4
Discussion
Diabetes mellitus can lead to both micro-vascular and macro-vascular complications by transition through multiple metabolic and hemodynamic pathways [2, 4] and thereby increases the tremendous burden as well as challenge for the healthcare system. Current diabetic conventional therapy have shown a limited role because of their potential for serious adverse effects [12]. According to some study, diabetes and its related complications also leads to micronutrients deficiency disorders. Therefore this study was conducted to elucidate the effect of micronutrients on DN and CVD using STZ induced diabetes in rats.
The pharmacological effect of individual vitamins on diabetes and/or its complications have been explored in many preclinical and clinical trials. A study has confirmed that a two-week treatment with vitamin B1 at a dose of 0.2% thiamin in drinking water averted diabetes-induced cardiac fibrosis without a considerable effect on blood glucose level in male diabetic rats [13]. In a randomized double-blind vehicle-controlled clinical pilot study in forty type 2 diabetic patients with microalbuminuria verified the effect of vitamin B1 therapy at the dose of 300 mg/day for a period of three months produced a drop in urinary albumin excretion with no effect on plasma glucose or HbA1c levels [14]. In yet another double-blind parallel group vehicle-controlled randomized trial, conducted in eighty seven type 2 diabetic volunteers demonstrated that a single large dose of vitamin D2 (100,000 IU) enhanced endothelial function 8 weeks post administration [15] thus confirming attenuating action of vitamin D2 in the diabetic complication. Furthermore, in a randomized, vehicle-controlled clinical trial which enrolled eight one participants confirmed that vitamin D3 supplementation (4000 IU) for a period of six months significantly improved insulin sensitivity as well as fasting insulin level in type 2 diabetic women [16].
Moreover, apart from vitamins, many preclinical and clinical trials have probed into the effects of individual minerals on diabetes and/or its complications. In a randomized double-blind vehicle-controlled trial conducted in sixty three type 2 diabetic volunteers with hypomagnesemia receiving glibenclamide, showed improvement in HbA1c, fasting glucose and insulin levels on supplementation with magnesium at the dose of 2.5 g MgCl2/day for 4 months [17]. Oral magnesium supplementation in a double-blind vehicle-controlled clinical trial conducted in eight two diabetic hypertensive adults with hypomagnesemia receiving captopril at the same dose and duration also showed notable reduction not only in fasting plasma glucose and HbA1c levels, but also systolic and diastolic blood pressure [18]. Moreover, a prospective double-blind vehicle-controlled crossover study enrolling thirty volunteers confirmed that chromium supplementation for a period of two months significantly decreased serum triglyceride level in type 2 diabetic patients but no effect on blood glucose level [19]. In yet another randomized clinical study in 180 diabetic patients, chromium administration (1000 μg/day) for four months showed favourable effects on HbA1c, glucose, insulin, and cholesterol levels [10, 20].
In the current study, metformin with micronutrients treatment were shown to reduce high blood glucose level in diabetic rats with recovery in body weight as compared to metformin and micronutrients alone treated rats. This significant difference obtained could be due to synergistic effect of micronutrients and metformin. The major cause of T2D that is insulin resistance is associated not only with pancreatic beta cell dysfunctioning but also with deficiency of micronutrients. Deficiency of several micronutrients namely magnesium, selenium, chromium, zinc, vitamin D, vitamin E, vitamin C etc. plays an important role in insulin resistance which ultimately leads to diabetes, renal and cardiovascular disease [21].
Many studies has been performed to alleviate the DN and CVD by using single or two fat and/or water soluble vitamin or macro-minerals or trace elements. In the study by Parvizi, et al. magnesium showed their reno-protective and cardio-protective effect by decreasing oxidative stress biomarkers with decrease in BUN as compared to diabetic rats [22]. Magnesium also improved hyperglycaemia via inhibition of gluconeogenesis through down-regulation of forkhead box protein O1 (FOXO1) expression [23]. Another study conducted by Selcuk, et al. chromium compounds protect the kidneys from the attack of ROS-induced oxidative stress via modulation of nuclear factor kappa light chain enhancer of activated β cells (NF-κβ) pathway [24]. High glucose and micronutrients deficiency induced-insulin resistance was decreased on supplementation of vitamins or minerals [25, 26].
Blood urea nitrogen is considered as the biomarkers of kidney degeneration and the rise in BUN after induction of diabetes is chiefly because of amplified amount of urea nitrogen in blood [27, 28]. Creatinine clearance is an indicator of glomerular filtration rate and attenuation in it is mainly due to glomerular hyperfilteration, increased glomerular capillary pressure, leaky glomerular capillaries and impaired tubuloglomeruli. The progression of glomerular damage, leads to decrease in the rate of diffusion by the thickened capillary basement membrane, eventually resulting in a decrease in GFR [29, 30].
In this study, the renal dysfunction was confirmed in diabetic rats with high serum creatinine, BUN level as well as decreased creatinine clearance and urine creatinine level as compared to control rats. Six weeks of repeated micronutrients and metformin treatment correct the abnormal condition of kidneys by decreasing level of serum creatinine, BUN and increasing level of urine creatinine and creatinine clearance as compared to diabetic rats. In this study, the rats treated with metformin with micronutrients showed significant difference as compared to metformin and micronutrients alone treated rats. Moreover, micronutrients treated rats showed more positive reno-protective effect as compared to metformin treated rats. This effects is may be due to anti-inflammatory, antioxidant, insulin like activity of micronutrients.
With the support of the other studies having used vitamins and minerals against diabetic complications, it was demonstrated that the reno- and cardio-protective effect of vitamin E and vitamin D may be due to ROS induced-NF-κβ down-regulation, phosphatidylinositol 3 kinase (PI3K)-AKT activation or increase the glucose transporter (GLUT4) expression and their translocation to plasma membrane and thereby reducing BUN, serum creatinine level [31]. Selenium and vanadium showed anti-hyperglycaemic activity may be through glutathione peroxidase 1 expression and regulate insulin signalling cascade by activating protein kinase B (PKB), P70S6 kinase and mitogen activated protein kinase (MAPK) [32]. In the study by Singh S, et al. combination of trace elements have shown antidiabetic activity by supressing lipid peroxidation via enhancing antioxidant mechanism [33]. Metformin along with vitamin D have been reported to increase the expression of vitamin D receptor, sarcoendoplasmic reticulum calcium ATPase (SERCA2) and beclin 1 and thereby proved its synergistic action in diabetic cardiomyopathy [34].
CVD was further confirmed by significant increase of LDH level in diabetic heart with abnormal heart rate. Our results reported a significant decrease of cardiac marker and increase in HR after six weeks continuous administration of metformin with micronutrients as compared to diabetic rats as well as metformin alone and micronutrients alone treated rats.
Vitamin B act as a PARP inhibitor and can restore the islet cell content of NAD towards normality, thereby preventing cellular NAD depletion and showed free radical scavenging activity by inhibiting production of nitric oxide and prevent β-cell apoptosis [35–37]. Vitamin B showed its anti-hyperglycaemic effect by increasing antioxidant enzymes and decreasing gluconeogenesis in the study by Abdullah KM and colleagues [38]. Vanadium and chromium showed their insulin-mimetic action may be by increasing tyrosine phosphorylation of insulin receptor substrate 1 (IRS-1) and PKB/AKT, AMP- activated protein kinase (AMPK) activity and act as a protein-tyrosine phosphatase 1B (PTP-1B) inhibitor, negative regulator of insulin signalling cascades [39]. The protective effect of micronutrients on diabetic heart may be due to down-regulation of RAAS and thereby correcting HR and LDH level in this study.
The findings of some studies further strengthen the notion that insulin signaling pathway may represent potential targets for both standard drug Metformin and selected micronutrients. For example, both metformin and chromium increases the glucose uptake and insulin sensitivity via AMPK pathway [40, 41] i.e. both may act as AMPK agonist for regulation of overall glucose metabolism. Another selected micronutrient magnesium and metformin reduces gluconeogenesis via inhibition of phosphoenolpyruvate carboxykinase (PEPCK; a key enzyme in gluconeogenesis pathway) gene expression in the study by Barooti A, et al. and Yuan Li, et al. which provide an evidence that PEPCK enzyme may act as one of the common target for both magnesium and metformin [23, 42]. Another important signalling pathway called Ras-Raf-MEK-ERK is also regulated by trace elements selenium and vanadium to produce insulin mimetic action like metformin [43]. Like vitamin B and Vitamin E, metformin also act as a PARP inhibitor and ROS scavenger and thereby plays a protective role in renal failure and cardiovascular dysfunctioning via AMPK-PARP1 cascade and oxidative stress pathway in the study by Shang F et al. and randomised controlled clinical trial by El-Aal AA, et al. respectively [44, 45]. Nevertheless, the findings of these studies revealed that both metformin and selected micronutrients protect the diabetic kidney and heart may be via some similar mechanistic pathways like AMPK pathway, gluconeogenesis pathway, oxidative stress pathway, MAPK pathway which are few common pathways involved in complex insulin signalling cascade. Hence prototype drug metformin and selected micronutrients were taken for this study by considering the similarity in their possible mechanism of actions.
The constraint of the present study is the inability to provide evidence on the mechanism of the effect of the micronutrient preparation and the individual impact of the all the components to the anti-diabetic effect of the preparation. However, the prospective interfaces of these components and their combined effect rather than a single component and synergistic effect with metformin could be responsible for the beneficial effects of the micronutrient preparation on the severity of diabetes and its complications.
Conclusion
Our study demonstrated potential of micronutrients as an add-on therapy for DN and CVD. Micronutrients decreased blood glucose level with reduction of general symptoms of diabetes may be because of their insulin-mimetic effect. Micronutrients protected the diabetic kidney and heart by lowering serum creatinine, BUN and LDH level with increase in urine creatinine, creatinine clearance and heart rate may be because of anti-oxidant, anti-inflammatory, reno-protective, cardio-protective and hypoglycaemic effect of micronutrients against DN and CVD. Thus findings suggest a positive effects of combination therapy on functional abnormalities in diabetes induced nephropathy and cardiac dysfunctioning and may be an approach to counterpoise early pathophysiologic damages of diabetes.
Declarations
Statement of human and animal rights
Yes
Conflict of interest
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 2.Sharaf El Din UAA, Salem MM, Abdulazim DO. Diabetic nephropathy: Time to withhold development and progression -A review. J Adv Res. 2017;8:363–73. 10.1016/j.jare.2017.04.004. [DOI] [PMC free article] [PubMed]
- 3.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57(4):660–671. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardi S, Michelli A, Zuolo G, Candido R, Fabris B. Update on RAAS modulation for the treatment of diabetic cardiovascular disease. J Diab Res. 2016;2016:1–17. 10.1155/2016/8917578. [DOI] [PMC free article] [PubMed]
- 5.Chandy A, Pawar B, John M, Isaac R. Association between diabetic nephropathy and other diabetic microvascular and macrovascular complications. Saudi J Kidney Dis Transplant. 2008;19(6):924–928. [PubMed] [Google Scholar]
- 6.Al-Harbi MS, Hamza RZ. Potential ameliorative effects of selenium and chromium supplementation against toxicity and oxidative stress in Streptozotocin diabetic rats. Int J Pharmacol. 2016;12(5):483–495. doi: 10.3923/ijp.2016.483.495. [DOI] [Google Scholar]
- 7.Vijay K, Suresh R, Loganathasamy K, Narayanan V, Pandiyan V, Satheesh Kumar T, et al. Anti-diabetic effects of vanadium Pentoxide Nanopartices in STZ induced diabetic rats. Int J Pure App Biosci. 2008;6(3):460–467. doi: 10.18782/2320-7051.6203. [DOI] [Google Scholar]
- 8.Uslu H, Uslu GA. Effects of chromium picolinate on oxidative stress and hyperglycemia in experimental type 2 diabetic rats. Asian J Pharm Clin Res. 2018;11(10):532–535. doi: 10.22159/ajpcr.2018.v11i10.28608. [DOI] [Google Scholar]
- 9.Morakinyo AO, Samuel TA, Adekunbi DA. Magnesium upregulates insulin receptor and glucose transporter-4 in streptozotocin-nicotinamide-induced type-2 diabetic rats. Endocr Regul. 2018;52:6–16. doi: 10.2478/enr-2018-0002. [DOI] [PubMed] [Google Scholar]
- 10.Sarkozy M, Fekete V, Szucs G, Torok S, Szucs C, Barkanyi J, et al. Anti-diabetic effect of a preparation of vitamins, minerals and trace elements in diabetic rats:a gender difference. BMC Endocr Disord. 2014;14(72):1–11. doi: 10.1186/1472-6823-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendez CE, Umpierrez GE. Pharmacotherapy for hyperglycemia in noncritically ill hospitalized patients. Diabetes Spectr. 2014;27(3):180–188. doi: 10.2337/diaspect.27.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohda Y, Shirakawa H, Yamane K, Otsuka K, Kono T, Terasaki F, Tanaka T. Prevention of incipient diabetic cardiomyopathy by high-dose thiamine. J Toxicol Sci. 2008;33(4):459–472. doi: 10.2131/jts.33.459. [DOI] [PubMed] [Google Scholar]
- 14.Rabbani N, Alam SS, Riaz S, Larkin JR, Akhtar MW, Shafi T, Thornalley PJ. High-dose thiamine therapy for patients with type 2 diabetes and microalbuminuria: a randomised, double-blind placebo-controlled pilot study. Diabetologia. 2009;52:208–212. doi: 10.1007/s00125-008-1224-4. [DOI] [PubMed] [Google Scholar]
- 15.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2007;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 16.Hurst PR, Stonehouse W, Jane CJ. Vitamin D supplementation reduces insulin resistance in south Asian women living in New Zealand who are insulin resistant and vitamin D deficient – a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Moran M, Guerrero-Romero F. Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects A randomized double-blind controlled trial. Diabetes Care. 2003;26(4):1147–1152. doi: 10.2337/diacare.26.4.1147. [DOI] [PubMed] [Google Scholar]
- 18.Guerrero-Romero F, Rodriguez-Moran M. The effect of lowering blood pressure by magnesium supplementation in diabetic hypertensive adults with low serum magnesium levels: a randomized, doubleblind, placebo-controlled clinical trial. J Hum Hypertens. 2009;23:245–251. doi: 10.1038/jhh.2008.129. [DOI] [PubMed] [Google Scholar]
- 19.Lee NA, Reasner CA. Beneficial effect of chromium supplementation on serum triglyceride levels in NIDDM. Diabetes Care. 1994;17(12):1449–1452. doi: 10.2337/diacare.17.12.1449. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RA, Cheng N, Bryden NA, Polansky MM, Cheng N, Chi J, Feng J. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 21.Ekpenyong CE. Micronutrient deficiency, a novel nutritional risk factor for insulin resistance and Syndrom X. Arch Food Nutr Sci. 2018;2:16–30. doi: 10.29328/journal.afns.1001013. [DOI] [Google Scholar]
- 22.Parvizi MR, Parviz M, Tavangar SM, Soltani N, Kadkhodaee M, Seifi B, et al. Protective effect of magnesium on renal function in STZ-induced diabetic rats. J Diab Metab Disord. 2014;13(84):1–9. doi: 10.1186/s40200-014-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barooti A, Kamran M, Kharazmi F, Eftakhar E, Malekzadeh K, Talebi A, Soltani N. Effect of oral magnesium sulfate administration on blood glucose hemostasis via inhibition of gluconeogenesis and FOXO1 gene expression in liver and muscle in diabetic rats. Biomed Pharmacother. 2019;109:1819–1825. doi: 10.1016/j.biopha.2018.10.164. [DOI] [PubMed] [Google Scholar]
- 24.Selcuk MY, Aygen B, Dogukan A, Tuzcu Z, Akdemir F, Komorowski JR, et al. Chromium picolinate and chromium histidinate protects against renal dysfunction by modulation of NF-κB pathway in high-fat diet fed and Streptozotocin-induced diabetic rats. Nutr Metab. 2012;9(30):1–7. doi: 10.1186/1743-7075-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddi AS, Bollineni JS. Selenium-deficient diet induces renal oxidative stress and injury via TGF-b1 in normal and diabetic rats. Kidney Int. 2001;59:1342–1353. doi: 10.1046/j.1523-1755.2001.0590041342.x. [DOI] [PubMed] [Google Scholar]
- 26.Kostov K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: focusing on the processes of insulin secretion and signaling. Int J Mol Sci. 2019;20:1–15. doi: 10.3390/ijms20061351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadkhodaee M, Mikaeili S, Zahmatkesh M, Golab F, Seifi B, Arab HA, Shams S, Mahdavi-Mazdeh M. Alteration of renal functional, oxidative stress andinflammatory indices following hepatic ischemia-reperfusion. Gen PhysiolBiophys. 2012;31:195–202. doi: 10.4149/gpb_2012_024. [DOI] [PubMed] [Google Scholar]
- 28.Trujillo J, Chirino YI, Molina-Jijon E, Anderica-Romero AC, Tapia E, Pedraza-Chaverrí J. Renprotective effect of the antioxidant curcumin. Recent findings. Redox Biol. 2013;1(1):448–456. doi: 10.1016/j.redox.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman DJ, Price CP. Tietz Textbook of Clinical Chemistry. 3. 1999. Renal Function and Nitrogen Metabolism; pp. 1204–1264. [Google Scholar]
- 30.Ganesha KR, Razdan R and Minaz N. Allantoin and micronutrients restores renal dysfunction in streptozotocin induced diabetic rats. J Diabetes Metab Manag. 2019;JDMM-10004.
- 31.Zaulkffali AS, Md Razip NN, Syed Alwi SS, Abd Jalil A, Abd Mutalib MS, Gopalsamy B, et al. Vitamins D and E stimulate the PI3K-AKT Signalling pathway in insulin-resistant SK-N-SH neuronal cells. Nutrients. 2019;11:1–21. doi: 10.3390/nu11102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Quraishy S, Dkhil MA, Abdel AE, Moneim. Anti-hyperglycemic activity of selenium nanoparticles in streptozotocin-induced diabetic rats. Int J Nanomedicine. 2015;10(1):6741–56. 10.2147/IJN.S91377. [DOI] [PMC free article] [PubMed]
- 33.Singh S, Chandel HS, Kushwaha S, Malik J. Evaluation of Antidiabetic activity of combination of trace elements. Am Chem Sci J. 2015;6(1):25–37. doi: 10.9734/ACSJ/2015/13479. [DOI] [Google Scholar]
- 34.El Sayed DM, Amin SN, Yassa HD, Rashed LA, El Tablawy N, Elattar S. Effects of vitamin D and metformin on diabetic cardiomyopathy in rats with type 2 diabetes mellitus. IJAPB. 2017;4(4):1–13. doi: 10.14445/23500301/IJAP-V4I4P101. [DOI] [Google Scholar]
- 35.Alenzi FQ. Effect of Nicotinamide on experimental induced diabetes. Iran J Allergy Asthma Immunol. 2009;8(1):11–18. [PubMed] [Google Scholar]
- 36.Suarez-Pinzon WL, Mabley JG, Power R. Szabo’C, Rabinovitch A. poly (ADPribose) polymerase inhibition prevents spontaneous and recurrent autoimmune diabetes in NOD mice by inducing apoptosis of islet-infiltrating leukocytes. Diabetes. 2003;52(7):1683–1688. doi: 10.2337/diabetes.52.7.1683. [DOI] [PubMed] [Google Scholar]
- 37.Virág L. Structure and function of poly (ADP-ribose) polymerase- 1: role in oxidative stress-related pathologies. Curr Vasc Pharmacol. 2005;3(3):209–214. doi: 10.2174/1570161054368625. [DOI] [PubMed] [Google Scholar]
- 38.Abdullah KM, Alam Md M, Iqbal Z, Naseem I. Therapeutic effect of vitamin B3 on hyperglycemia, oxidative stress and DNA damage in alloxan induced diabetic rat model. Biomed Pharmacother. 2018;105:1223–1231. doi: 10.1016/j.biopha.2018.06.085. [DOI] [PubMed] [Google Scholar]
- 39.Hua Y, Clark S, Ren J, Sreejayan N. Molecular mechanisms of chromium in alleviating insulin resistance. J Nutr Biochem. 2012;23(4):313–319. doi: 10.1016/j.jnutbio.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao P, Wang J, Ma H, Xiao Y, He L, Tong C, Wang Z, Zheng Q, Dolence EK, Nair S, Ren J, Li J. A newly synthetic chromium complex—chromium (D-phenylalanine) 3 activates AMP-activated protein kinase and stimulates glucose transport. Biochem Pharmacol. 2009;77:1002–1010. doi: 10.1016/j.bcp.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Ziegler R, Hamann A. Inhibition of phosphoenolpyruvate carboxykinase gene expression by metformin in cultured hepatocytes. Chin Med J. 2002;115(12):1843–1848. [PubMed] [Google Scholar]
- 43.Hei YJ, Farahbakhshian S, Chen X, Battell ML, McNeill JH. Stimulation of MAP kinase and S6 kinase by vanadium and selenium in rat adipocytes. Mol Cell Biochem. 1998;178:367–375. doi: 10.1023/A:1006819906820. [DOI] [PubMed] [Google Scholar]
- 44.Shang F, Zhang J, Zhao Li Z, Zhang J, Yin Y, Wang Y, et al. Cardiovascular protective effect of metformin and Telmisartan: reduction of PARP1 activity via the AMPK-PARP1 Cascade. PLoS One. 2016;11(3):1–16. doi: 10.1371/journal.pone.0151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El-Aal AA, Abd El-Ghffar EA, Ghali AA, Zughbur MR, Sirdah MM. The effect of vitamin C and/or E supplementations on type 2 diabetic adult males under metformin treatment: A single-blinded randomized controlled clinical trial. Diabetes Metab Syndr. 2018;12(4):483–489. doi: 10.1016/j.dsx.2018.03.013. [DOI] [PubMed] [Google Scholar]