Abstract
Background
Similarities exist in behavioral expression of autism spectrum disorder (ASD) and Alzheimer’s disease and related dementias (ADRD) The purpose of this study was to assess presence of behavioral and psychiatric symptoms of dementia (BPSD) and ASD-like behaviors in adults with ADRD.
Methods
Using a cross-sectional design, data from University of Kentucky Alzheimer’s Disease Center participant cohort were used. Hierarchical linear regression was used to assess (1) the relationship between ASD-like behaviors (measured by the Gilliam Autism Rating Scale-Second Edition, GARS-2) and BPSD measured by the Neuropsychiatric Inventory (NPI), and (2) the relationship between ASD-like behaviors and dementia severity (measured by the Clinical Dementia Rating [CDR] sum of boxes), when controlling for BPSD.
Results
Complete data were available for 142 participants. Using α of 0.05, analyses identified ASD behaviors were significantly associated with BPSD severity ratings (r=0.47; p<0.001) and dementia severity (r=0.46; p<0.001). GARS-2 explained 6.1% (p < 0.001) of variance in CDR sum of boxes when controlling for NPI and other covariates.
Discussion
There is significant overlap in behaviors characteristic of ASD and BPSD as assessed by the NPI and GARS-2, despite the use of these instruments in disparate developmental vs. aging settings. ASD behaviors appear to not be solely present in early childhood as a manifestation of ASD but are also present in older adults with neurodegenerative cognitive impairment. Such associations warrant additional research into causation, assessment, and behavioral interventions to further enable new therapeutic approaches targeting ASD behaviors across the lifespan.
Keywords: Alzheimer’s disease, Autism Spectrum Disorder, behavioral assessment
Introduction
People with autism spectrum disorder (ASD) or Alzheimer’s disease and related dementias (ADRD) commonly experience a range of psychiatric and behavioral symptoms (Caselli, Langlais, Dueck, Locke, & Woodruff, 2018; Hornberger, Piguet, Kipps, & Hodges, 2008; Rossignol & Frye, 2014). Shared psychiatric symptoms include depression, agitation, apathy, and aberrant motor movement, and difficulty performing social interactions (Cerejeira, Lagarto, & Mukaetova-Ladinska, 2012; Cloak & Al Khalili, 2020). In persons with ADRD (Cloak & Al Khalili, 2020), such behaviors are commonly diagnosed as behavioral and psychiatric symptoms of dementia (BPSD). Estimated prevalence of BPSD in ADRD is high, with some studies reporting BPSD in over 80% of older adults with ADRD (Cerejeira et al., 2012). Presence of these behaviors in those with ASD and ADRD are strongly associated with patient and caregiver quality of life (Hurt et al., 2008).
To meet diagnostic criteria for ASD (DSM-V; American Psychiatric Association, 2013), symptoms demonstrating pattern, severity, and duration (i.e., deficits in social communication, interactions, and restricted, repetitive behavior, interests, or activities) must be observed in childhood or early adolescence and not due to developmental or intellectual disability (American Psychiatric Association, 2013). In prior work, Rhodus and colleagues (2020) reported presence of ASD-like behaviors in older adults without known diagnosis of ASD but with cognitive impairment using the Gilliam Autism Rating Scale, 2nd edition (GARS-2; Gilliam, 2006). This prior work, however, did not consider BPSD.
Thus, it is not clear whether the GARS-2 simply detects BPSD, or if the ASD-like behaviors are additional, unaccounted symptoms in persons with ADRD (Gerlach & Kales, 2020; Kales, Gitlin, & Lyketsos, 2015). Comparison of BPSD and ASD symptoms in persons with ADRD may identify an ASD-like behavioral phenotype in late adulthood associated with the onset of cognitive impairment. Given there exist efficacious behavioral interventions for children with ASD (Pfeiffer, Koenig, Kinnealey, Sheppard, & Henderson, 2011), this may create opportunity for exploring novel behavioral interventions for these challenging symptoms in ADRD (Maseda et al., 2018).
The purpose of this study was to evaluate the presence of BPSD and behaviors of ASD in ADRD. Here, we evaluated the association between BPSD (as measured by the Neuropsychiatric Inventory, [NPI; Cummings, 1997]) and ASD behaviors (as measured by the GARS-2) in older adult research volunteers enrolled in a longitudinal study of aging and dementia. Such comparison may provide insights into causal factors and possible shared treatment modalities (Khan et al., 2016; Rossignol & Frye, 2014; Wink, Pedapati, Horn, McDougle, & Erickson, 2015).
Materials and Methods
Participants
Participants for the current study were drawn from the University of Kentucky Alzheimer’s Disease Center (UK ADC) cohort (Schmitt et al., 2012). Data were extracted from the cohort database to assess the relationship between caregiver-reported ASD behaviors (GARS-2) and BPSD (NPI at UK ADC annual assessment closest to GARS-2 completion time). UK ADC participants received annual cognitive and clinical examinations. Full details of annual assessments and inclusion/exclusion criteria have been described elsewhere (Schmitt et al., 2012). In addition to enrollment in the UK ADC cohort, inclusion criteria consisted of diagnosis of mild cognitive impairment (MCI) or dementia, caregiver willingness to participate, GARS-2 completion, and UK ADC annual assessment within 24 months of GARS-2 completion. At the time of GARS-2 completion, no participants reported known diagnosis of ASD. This study was approved by the UK Institutional Review Board.
Diagnostic criteria
Consensus guidelines developed by the Second International Working Group on MCI (Winblad et al., 2004) and further adopted by the National Institute on Aging-Alzheimer’s Association Workgroup on Diagnostic Guidelines for Alzheimer’s Disease defined MCI as follows: (1) a cognitive complaint by the subject or informant, or evidence for longitudinal decline on cognitive test performance (at least 1.5 standard deviation decline); (2) generally intact global cognition; (3) no or minimal functional impairment; (4) not demented according to Diagnostic and Statistical Manual of Mental Disorders Fourth edition (DSM-IV; American Psychiatric Association, 1994). These guidelines were used in diagnosis of MCI.
The diagnosis of dementia was based on the criteria set forth by the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) (American Psychiatric Association, 1994). Additional guidance established by the National Institute for Aging and the Alzheimer’s Association (Albert et al., 2011) for MCI and ADRD diagnoses.
Measures
The GARS-2 measures frequency of behaviors characteristic of ASD. The tool has established validity and reliability in screening and diagnosis of autism in pediatric and adolescent populations (Gilliam, 2006; Montgomery, Newton, & Smith, 2008). The GARS-2 includes 42 objective statements of characteristic ASD behaviors based on observable frequency, and each are ranked by the caregiver. Objective statements are presented in three subscales: behaviors, communication, and social interaction (Table 1).
Table 1.
GAR-2 item examples, by Subscale.
| Behavior Subscale |
| Avoids establishing eye contact |
| Stares at hands, objects, or items |
| Communication Subscale |
| Inappropriately answers questions about a statement or brief story |
| Responds inappropriately to simple commands |
| Social Interaction Subscale |
| Withdrawn in group settings |
| Engages in ritualistic behaviors |
| Responds negatively to routine changes |
| Responds negatively when given requests or directions |
Each item is ranked on an integer scale from 0 to 3 (0 indicates the behavior is never observed, 1 is seldom observed, 2 is sometimes observed, and 3 is frequently observed). Items on each subscale are summed and converted to a standard subscale. Standard scores are summed to calculate the Autism Index Score (AIS), which has a mean of 100 and SD of 15 (Gilliam, 2006). Scoring instructions of the assessment indicate GARS-2 AIS of > 69 to classify participants as ASD Possible/Very Likely (i.e., AIS > 69) or ASD Unlikely (i.e., AIS ≤ 69).
Prior work used the GARS-2 due to the instrument’s breadth of ASD behaviors assessed which allowed for participants to be classified as ASD possible/very likely or ASD unlikely based on the measure’s established cutoff scores, and this classification was applied in the present study. To better differentiate ASD-like behaviors and BPSD, ASD unlikely participants were further classified as having higher or lower reports of BPSD (using in-sample median NPI scores). The relationship between the GARS-2 and dementia severity, controlling for NPI ratings, was also examined to evaluate the extent to which ASD-like behaviors are a unique manifestation of BPSD.
The NPI is commonly used as an assessment of BPSD in older adults diagnosed with MCI or dementia, internationally, with high reliability and validity (Cummings, 1997; Johnson, Watts, Chapin, Anderson, & Burns, 2011; Sheehan, 2012). A trained interviewer asks caregivers to indicate the presence of 12 behavioral and psychiatric domains (see Table 1). Each domain is rated as present or absent (yes/no). When caregivers indicate the presence of a symptom, they are then asked to rate the severity of the symptom as mild, moderate, or severe. For the current analysis, symptom severity was operationalized by assigning an integer rating of 0 to 3, with a ‘not present’ response = 0, mild = 1, moderate = 2, and severe = 3. The 12 domain ratings were summed to yield a total NPI severity score, with greater values indicating greater BPSD burden.
Table 2 summarizes items from GARS-2 highlighting ASD-like behaviors and items from the NPI depicting presence and severity of BPSD in persons with ADRD.
Table 2.
Comparison of Behaviors: BPSD in ADRD and ASD.
| Common BPSD in ADRD (Measured by NPI)* | Common Behaviors in ASD (Measured by GARS-2)** |
|---|---|
|
| |
| Delusions and Hallucinations | Sensory processing deficits |
| Agitation | Inflexibility in routines |
| Depression | Depression |
| Anxiety | Anxiety |
| Elation | Atypical excitation and emotion, self-stimulation |
| Apathy | Deficits in communication, social interaction, and self-care |
| Disinhibition | Lack of theory of mind; disinhibition |
| Irritability | Ritualized patterns of behavior; Fixed interests |
| Psychomotor disturbance | Repetitive movements, use of objects, or speech |
| Sleep disturbance | Sleep disturbance |
| Eating disturbance | Eats specific foods, inappropriate licking, mouthing |
In addition to behaviors, we investigated involvement of dementia severity, cognitive function and co-morbid depression. The Clinical Dementia Rating (CDR) sum of boxes, a valid and reliable measure (Rockwood, Strang, MacKnight, Downer, & Morris, 2000), was used to determine dementia severity. The CDR uses semi-structured interviews by trained clinicians with primary informants (e.g., caregivers) to determine severity of cognitive impairment (Hughes, Berg, Danziger, Coben, & Martin, 1982; Morris, 1993). Six cognitive domains are assessed in the CDR (memory, orientation, judgement and problem solving, community affairs, home and hobbies, and personal care). Following the interview, the clinician rates each domain on a 5-point scale indicating the level of impairment (0=none, 0.5=questionable, 1=mild, 2=moderate, 3=severe) based on standard rules. Ratings from all six domains are summed to create the CDR sum of boxes score, which has a range of 0 to 18, with larger values indicating increased impairment. The Mini Mental State Exam (MMSE; Folstein, Folstein, & McHugh, 1975) was included to measure objective global cognitive function. And, the 15-item Geriatric Depression Scale (GDS; Yesavage et al., 1983) was used to assess comorbid depression (Perneczky et al., 2006).
Analysis
This study used secondary analysis of UK-ADC cohort study data (Rhodus et al., 2020; Schmitt et al., 2012). T-tests and chi-square tests were used to compare covariates between respondents and non-respondents. ANOVA and chi-squared tests were used to compare group characteristics. Tukey’s HSD was used for pairwise ANOVA post-hoc comparisons. Pearson correlations were used to examine relationships between GARS-2 AIS and subscales with NPI items, NPI severity sum, and CDR sum of boxes with Holm’s adjustment for multiple comparisons. Hierarchical linear regressions were used to evaluate the association of GARS-2 AIS and subscale scores with dementia severity (CDR sum of boxes).
Because it remains to be determined whether ASD-like behaviors in ADRD are a distinct manifestation of BPSD or simply the sequelae of more advanced disease, we split the ASD Unlikely group into groups with lower and higher BPSD (i.e., using NPI severity sum). Given that BPSD are known to increase with disease progression (Bränsvik, Granvik, Minthon, Nordström, & Nägga, 2020), if ASD-like behaviors are simply part of more advanced disease, then it would be expected that the ASD Unlikely subgroup with lower BPSD would have the lowest mean GARS-2 AIS and the ASD Unlikely group with higher BPSD would have a mean GARS-2 AIS closer to the cut-off of > 69. For the ASD Unlikely group, the NPI severity median was 2, with an IQR = [1,5]. The 3rd quartile was used to classify only participants in the ASD Unlikely group as either ASD- with lower BPSD (i.e., NPI severity sum ≤ 5) or ASD Unlikely with higher BPSD (i.e., NPI severity sum > 5). We refer to these, respectively, as the Higher BPSD and Lower BPSD groups hereafter.
In all models, age, sex, education, and years since first diagnosis of MCI or dementia were included as covariates. Model 1 added NPI severity sum, and Model 2 added GARS-2 AIS to evaluate whether it contributed to the severity of impairment beyond the BPSD accounted for by the NPI. To explore which GARS-2 item(s) best differentiated the ASD Possible/Very Likely group from the Higher BPSD group, we plotted means and standard deviations for all GARS-2 items where the ASD Possible/Very Likely group mean was > 1SD higher than the Higher BPSD group mean. Analyses were completed with R (R Core Team, 2013; Revelle, 2017; Wickham, 2009) version 3.3.4.
Results
Analysis included 146 participants, for a response rate of 45.5%. Participants had a mean (SD) of 79.8 (8.38) years of age, 16.34 (3.42) years of education, and 5.09 (5.05) CDR sum of boxes. Description of participant characteristics are displayed in Table 3 and arranged in group assignment.
Table 3.
Participant Characteristics
| ASD Possible or Very Likely |
ASD Unlikely Higher BPSD |
ASD Unlikely Lower BPSD |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Continuous Variables | Mean | SD | Mean | SD | Mean | SD | p | η2 | 95% CI |
|
| |||||||||
| Age | 76.96 | 9.87 | 78.15 | 8.21 | 80.96 | 7.87 | 0.066 | 0.04 | [−0.02, 0.12] |
| Years Since Diagnosisa | 5.65 | 4.80 | 4.50 | 2.90 | 3.71 | 3.18 | 0.049 | 0.04 | [−0.02, 0.14] |
| Years of Education | 16.70 | 3.97 | 16.38 | 2.70 | 16.25 | 3.48 | 0.853 | 0.00 | [−0.02, 0.05] |
| MMSEa | 16.50 | 10.41 | 20.46 | 7.96 | 23.67 | 5.62 | < 0.001 | 0.12 | [ 0.01, 0.27] |
| GDS | 2.54 | 1.27 | 2.14 | 1.35 | 2.13 | 2.29 | 0.800 | 0.00 | [−0.02, 0.05] |
| CDR Sum of Boxesa,b | 9.50 | 6.38 | 6.67 | 5.26 | 3.55 | 3.71 | < 0.001 | 0.20 | [0.08, 0.36] |
| Years from Visit to GARS-2 | 0.52 | 0.79 | 0.42 | 0.70 | 0.40 | 0.57 | 0.706 | 0.00 | [−0.02, 0.07] |
| GARS-2 AISa,b,c | 81.96 | 8.56 | 56.77 | 8.13 | 50.26 | 8.09 | < 0.001 | 0.67 | [0.58, 0.76] |
| NPI Severity Suma,b | 9.17 | 6.98 | 9.69 | 3.33 | 1.72 | 1.58 | < 0.001 | 0.55 | [0.45, 0.68] |
| Discrete Variables | n | % | n | % | n | % | p | V | 95% CI |
| Female | 11 | 0.48 | 15 | 0.58 | 41 | 0.44 | 0.469 | 0.10 | [0.00, 0.24] |
| Nonwhite | 2 | 0.09 | 1 | 0.04 | 6 | 0.06 | 0.783 | 0.06 | [0.00, 0.19] |
| Demented | 21 | 0.91 | 20 | 0.77 | 54 | 0.58 | 0.005 | 0.27 | [0.09, 0.43] |
| ≥1 Copy of ApoE4d | 10 | 0.45 | 5 | 0.21 | 25 | 0.29 | 0.172 | 0.16 | [0.00, 0.32] |
Note: Continuous variables compared using ANOVAs with η2 for main effects and Tukey’s HSD for pairwise comparisons. Discrete variables compared using χ2-tests with Cramer’s V. ASD = Autism Spectrum Disorder. AIS = Autism Index Score. BPSD = Behavioral and psychiatric symptoms of dementia. CDR = Clinical Dementia Rating. GARS-2 = Gilliam Autism Rating Score 2. GDS = Geriatric Depression Scale. MCI = Mild Cognitive Impairment. MMSE = Mini-mental status exam. NPI = Neuropsychiatric Inventory.
Lower BPSD group ≠ ASD Possible/Very Likely group, p < 0.05
Lower BPSD group ≠ Higher BPSD group, p < 0.05
ASD Possible/Very Likely group ≠ Higher BPSD group, p < 0.05
ApoE data available for 22 in ASD Possible/Very Likely group, 24 in the Higher BPSD group, and 87 in the Lower BPSD group.
Compared to respondents, non-respondents had a longer duration of disease, completed slightly less education, had a longer span between visit and GARS-2 mailing, and were more likely to be non-white (see Supplementary Table 1). There were no significant differences between non-respondents and respondents on the CDR sum of boxes (non-respondents M = 4.87 versus respondents M = 5.14) and NPI severity sum (M = 3.8 versus M = 4.2), or any other covariates. Data from four respondents were excluded from the analysis because more than two years had elapsed since their most recent study visit. There were not significant differences in covariates between the four excluded participants and those who were included (not shown). This left 142 participants for analysis; 23 were ASD Possible/Very Likely, 26 were Higher BPSD, and 93 were Lower BPSD. Participant characteristics are shown in Table 3.
Compared to the Lower BPSD group, the ASD Possible/Very Likely group had a longer duration of impairment (p = 0.04), poorer global cognition (p < 0.001), greater CDR sum of boxes (p < 0.001), and higher NPI severity sum (p < 0.004). The Higher BPSD group had greater CDR sum of boxes (p = 0.006), increased GARS-2 AIS (p = 0.001), and higher NPI severity sum (p < 0.001) compared to the Lower BPSD group. The difference in CDR sum of boxes between the BPSD groups trended toward significance (p = 0.07). The ASD Possible/Very Likely group had significantly higher GARS-2 AIS than the Higher BPSD group, but the two groups were statistically equivalent on the NPI severity sum (p = 0.85). If the BPSD groups had been derived using their median NPI severity (instead of the 75th percentile), the only meaningful departure from these findings would be the ASD Possible/Very Likely group having a higher NPI severity sum than the Higher BPSD group (Mean = 6.5, SD = 3.8, p = 0.014; data not shown).
GARS-2 AIS was correlated with the NPI severity sum (r = 0.466; p < 0.001) and the CDR sum of boxes (r = 0.458; p < 0.001) (Table 4). There were moderate correlations between the NPI and GARS-2 behavior, communication, and social interaction subscales, as well (rs > 0.36, ps < 0.001). The social interaction subscale of the GARS-2 significantly correlates with six items on the NPI. The communication subscale of the GARS-2 correlates with four items on the NPI, and behavior subscale of the GARS-2 correlates with three items on the NPI. These moderate correlations observed in similar behaviors characteristic of ASD and in adults with MCI or dementia are clinically relevant and may illustrate potential for similar neurological involvement.
Table 4.
GARS-2 Correlations with CDRSUM, NPI Severity Sum, and NPI Item Severity
| GARS-2 Subscales |
||||
|---|---|---|---|---|
| Variable | Behavior | Communication | Social | AIS |
|
| ||||
| CDR Sum of Boxes | 0.313* | 0.466*** | 0.424*** | 0.458*** |
| NPI Severity Sum | 0.364*** | 0.383*** | 0.501*** | 0.466*** |
| NPI Delusions | 0.205 | 0.31* | 0.259 | 0.293* |
| NPI Hallucinations | 0.142 | 0.231 | 0.147 | 0.199 |
| NPI Agitation | 0.32* | 0.322* | 0.398*** | 0.384*** |
| NPI Depression | 0.189 | 0.149 | 0.194 | 0.195 |
| NPI Anxiety | 0.311* | 0.26 | 0.298* | 0.319* |
| NPI Elation | 0.217 | 0.332** | 0.238 | 0.298* |
| NPI Apathy | 0.233 | 0.306* | 0.386*** | 0.352** |
| NPI Disinhibition | 0.31* | 0.208 | 0.439*** | 0.352** |
| NPI Irritability | 0.235 | 0.209 | 0.319* | 0.284 |
| NPI Psychmotor Disturbance | 0.256 | 0.24 | 0.316* | 0.301* |
| NPI Sleep Disturbance | 0.076 | 0.185 | 0.257 | 0.207 |
| NPI Eating Disturbance | 0.209 | 0.205 | 0.26 | 0.249 |
Note: Pearson correlations with Holm’s adjustment for multiple tests. AIS = Autism index score. CDR = Clinical Dementia Rating scale. GARS-2 = Gilliam Autism Rating Scale, 2nd Edition. NPI = Neuropsychiatric Inventory.
p < 0.05
p < 0.01
p < 0.001.
Age, sex, education, and age of onset explained 22.2% of the variance in CDR sum of boxes, R2 = 0.222, F(4, 136) = 9.7, p < 0.001, R2adjusted = 0.20 (Table 5). Introducing the sum of NPI severity scores into the model accounted for an additional 9.9% of variance in the CDR sum of boxes, R2 = 0.321, F(5, 135) = 12.77, p < 0.001, R2adjusted = 0.30. Finally, the GARS-2 AIS score explained additional 6.1% of variance in the CDR sum of boxes, R2 = 0.382, F(6, 134) = 13.81, p < 0.001, R2adjusted = 0.35. That is, ASD-like behaviors explain 6.1% additional variance in dementia severity above and beyond the BPSD accounted for on the NPI.
Table 5.
Hierarchical linear regression of NPI and GARS-2 predicting CDR Sum of Boxes controlling for age, sex, education, and years since diagnosis.
| Block | Variable | R2 | ΔR2 | β | SE |
|---|---|---|---|---|---|
|
| |||||
| Base Model | 0.222 | ||||
| 1 | NPI Severity Sum | 0.321 | 0.099 | 0.335 | 0.075 |
| 2 | GARS-2 AIS | 0.382 | 0.061 | 0.102 | 0.028 |
| NPI Severity Sum | 0.208 | 0.080 | |||
Note: All model R2s and βs significant at p < 0.001 level. In all Blocks, age was significant (ps < 0.001), sex trended (ps = 0.07), education was nonsignificant (ps > 0.20), and years since diagnosis was significant (p < 0.001). CDR = Clinical Dementia Rating. GARS-2 = Gilliam Autism Rating Scale 2. NPI = Neuropsychiatric Inventory.
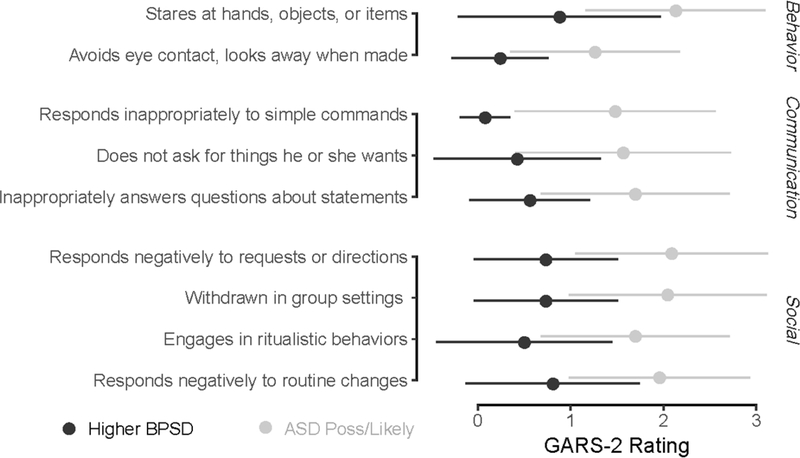
Lastly, eight GARS-2 items emerged from the exploratory descriptive analysis of GARS-2 items. A forest plot with GARS-2 item means and standard deviations were created to inspect differences between the ASD Possible/Very Likely and High BPSD groups (Figure 1).
Figure 1.
Means and SDs for GARS-2 items where the ASD Possible/Very Likely group mean is >1 SD higher than the High BPSD group mean. Items grouped by GARS-2 subscale. High BPSD in black, ASD Possible/Very Likely in grey.
Specifically, four items within social interaction category of the GARS-2 included withdrawal from group settings, engagement of ritualistic behavior, and negative response or resistance to routine changes and requests or demands. Communication deficits including inappropriate answers to questions and simple commands were observed but not measured by the NPI. Staring at hands, objects, or items and avoidance of eye contact were specific behaviors observed but not accounted for on the NPI.
Discussion
The present data provide insights into the relationship of behaviors in older adults with cognitive impairment who exhibit ASD behaviors. Results suggest BPSD in persons with ADRD may comprise a broader range of behaviors than that measured on the NPI, encompassing some typical ASD behaviors as well. In support of this contention, the present data demonstrate that the NPI and GARS-2 are uniquely associated with the measured variance in dementia severity (CDR sum of box score). These data further identify objective behaviors described on the GARS-2 that are associated with ADRD.
Assessment and treatment of behaviors observed in older adults with cognitive impairment can be challenging due to heterogenous factors influencing behavior, such as neurological function, environment, and medical status (Gitlin, Marx, Stanley, & Hodgson, 2015; Gitlin et al., 2008). Understanding the interrelationships of ASD and BPSD may provide insights into causal factors, shared anatomical and/or pathological involvement and possibly shared treatment modalities, yet there are few reports exploring cognitive impairment and ASD experiences in late adulthood (Caselli et al., 2018).
The present study adds to the growing body of literature documenting the presence of ASD-like behaviors in dementia due to ADRD (Caselli et al., 2018; Hornberger et al., 2008; Rhodus et al., 2020; Rossignol & Frye, 2014). The ASD Possible/Very Likely group had been impaired approximately one year longer than the Higher BPSD group and their mean CDRSUM was 2.83 points higher than the Higher BPSD group. Thus, the ASD Possible/Very Likely group was more impaired than we might expect the High BPSD group to be after an additional year, based on the annualized change in CDRSUM ranging from 1.44 to 1.91 reported in longitudinal studies (Eldholm et al., 2018; Li, Bilen-Green, Farahmand, & Langley, 2018; Tschanz et al., 2011; Williams, Storandt, Roe, & Morris, 2013). This difference in CDRSUM does not appear related to the time elapsed between study visit and GARS-2 completion, because this span was similar across groups (indeed, for all participants, r = 0.007, p = 0.92). However, given the cross-sectional design, we cannot conclusively say whether ASD-like symptoms are a distinct manifestation of BPSD or a general progression of disease. Nonetheless, the results suggest that the clinical course (and disease process) is different for the ASD Possible/Very Likely group.
Shared neuroanatomical pathology may help explain similarity of behaviors between ASD and ADRD (Midorikawa & Kawamura, 2012; Paulsen et al., 2000; Rossignol & Frye, 2014; Sokol, Maloney, Westmark, & Lahiri, 2019; Tai et al., 2020). As we observed here, frequency and severity of BPSDs tend to increase with dementia severity (Ecerejeira, Lagarto, & Mukaetova-Ladinska, 2012; Hashimoto et al., 2015; Srikanth, Nagaraja, & Ratnavalli, 2005), duration of disease (Paulsen et al., 2000), and are associated with increased mortality (Bränsvik et al., 2020). Increased global cortical involvement with worsening BPSD in people with ADRD may be comparable to cortical impairment seen in persons with ASD from childhood (Minshew & Williams, 2007; Raznahan et al., 2010). While literature describing aging experiences for adults with an ASD diagnosis is limited (Croen et al., 2015; Geurts & Vissers, 2012; Happe & Charlton, 2012; Lai & Baron-Cohen, 2015), recent studies have identified impaired cognitive function associated with the aging in those diagnosed with ASD (Powell, Klinger, & Klinger, 2017).
Additionally, the present study found that ASD-like behaviors captured on the GARS-2 can explain a small but appreciable amount of unique variance beyond that captured by the NPI. It is hypothesized that patterns of BPSD may have underlying, undiagnosed syndromes (Caselli et al., 2018; Jeste & Finkel, 2000). Individuals with ADRD and ASD-like behaviors may have subclinical ASD which only manifests with onset of neurodegenerative cognitive impairment (Caselli et al., 2018; Midorikawa & Kawamura, 2012). Historically, limited diagnostic measures for ASD were used when today’s older adult population were in adolescence. Some of the participants in this study may be considered high functioning on the spectrum of ASD without proper diagnosis (Caselli et al., 2018); however, typical clinical and healthcare practices for older adults with ADRD do not include screening for ASD. ASD symptoms in aging persons without diagnosis are challenging to identify due to co-morbid psychiatric conditions, the lack of awareness of healthcare professionals, and limited validated measures for ASD diagnosis in older adults (Au-Yeung et al., 2019; Bastiaansen et al., 2011; Ducharme, Price, Larvie, Dougherty, & Dickerson, 2015). Late-life expression of ASD behaviors in persons with and without a prior formal diagnosis of ASD requires further exploration that may facilitate treatment approaches for BPSD in older adults with cognitive impairment.
Items measured on the GARS-2 that are not assessed on the NPI captured ASD behaviors in this sample. Evidence supports deficits in emotional recognition, theory of mind, (Sabbagh, 2004; Yeh, 2013) and empathy (Bailey, Henry, & Von Hippel, 2008; Hsieh, Irish, Daveson, Hodges, & Piguet, 2013; Ze, Thoma, & Suchan, 2014) in ASD and in persons with ADRD. Presence of apathy also contributes to the emergence of these behaviors and interactions (van der Linde et al., 2016). These findings suggest that the NPI does not capture all of the diverse ASD-like behaviors that can be clinically expressed in ADRD (Teipel et al., 2017). Psychosocial and behavioral interventions, including sensory-based interventions, have been shown to improve apathy (Staal et al., 2007), resistance to communication and care (van Weert, van Dulmen, Spreeuwenberg, Ribbe, & Bensing, 2005), and empathy (Schneider, 2018) in people with ASD and ADRD (O’Donnell, Deitz, Kartin, Nalty, & Dawson, 2012).
BPSD can be troubling for both the patient and their caregivers (Afram et al., 2014; Hansen, Hodgson, Budhathoki, & Gitlin, 2018). Challenges with BPSD management in community-dwelling adults with ADRD is significantly correlated with caregiver burden, lowered quality of life for both the care partner and person with dementia, and heightened rates of institutional care (Finkel, Costa e Silva, Cohen, Miller, & Sartorius, 1996; Risco et al., 2015; van der Wolf, van Hooren, Waterink, & Lechner, 2019). Accurate assessment of these behaviors can improve care options to help alleviate caregiver burden (Cheng et al., 2020; El Mrayyan, Bökberg, Eberhard, & Ahlström, 2020; Gitlin et al., 2015; Teipel et al., 2017). Current clinical pharmacological interventions for BPSD have potential side effects and limited effectiveness in management of behaviors in persons with ADRD (Kales et al., 2015). Increasing evidence supports early non-pharmacological intervention for BPSD, but widespread implementation of such approaches has yet to be realized and implemented broadly (Gitlin, Kales, & Lyketsos, 2012). The present data suggest that behavioral strategies used in ASD interventions that are not dependent on high-functioning memory capacity may warrant exploration in the treatment of BPSD due to ADRD. At a time when innovative intervention development is needed, enhanced recognition of patterns within BPSD is crucial to enhance evidence-based practice and treatment planning. An ASD-like behavioral pattern may be one such avenue for exploration.
Limitations
Several limitations inherent in the current study deserve comment. Limitations include reporting biases given the nature of the GARS-2 and the mail-survey nature of the present study. Only 45.5% of the UK ADC participants who were engaged in the mailing of the survey, completed the GARS-2, indicating a possible response bias. Further, the GARS-2, while validated in childhood autism, has not been validated in a geriatric population with cognitive impairment. We aimed to compare behaviors listed on the GARS and NPI, however these instruments were not designed for decomposition of item analysis. Because of the exploratory nature of this analysis, item analysis was warranted to gain insights into the behaviors each instrument assessed. Exploration of new or revised measures for older adults with onset of ASD behavioral phenotype is needed. Lastly, the study participants were predominantly White, with high educational attainment, and in one geographical region, limiting generalizability of the study findings. Future research with more diverse samples will be needed to more fully understand the relationship between ASD and BPSD among individuals with cognitive impairment.
Despite these weaknesses, there are several strengths of this study. It is, to our knowledge, the first comparison of traditional assessments of BPSD and ASD in MCI and ADRD. Utilization of caregiver reporting and symptom assessment has been established as an effective and reliable tool for symptom reporting in dementia care (Jicha, 2011) and ASD (Bangerter et al., 2019). Despite the lack of a validated measure of ASD-like behaviors in late-life dementia, the use of a validated tool (GARS-2) specific to autism strengthens the present findings.
Conclusion
ASD in late life is poorly understood and BPSD reminiscent of ASD are not identified as related to ASD in current practice. Although the direct relationship between ASD and ADRD is yet to be determined, some individuals with MCI or dementia exhibit behaviors similar to ASD. Increased recognition of an ASD behavioral phenotype in late adulthood associated with the onset of cognitive impairment creates the potential for exploring novel behavioral interventions to help ADRD patients and their caregivers effectively cope with BPSD. Conversely, therapeutic interventions for BPSD, may also prove utility in the treatment of shared behaviors with ASD in younger populations experiencing developmental delay. Further studies aimed at clarifying the underlying substrates for such shared behavioral phenotypes clearly warrant further exploration.
Supplementary Material
Acknowledgements
This study utilized University of Kentucky Alzheimer’s Disease Cohort participants. Funding for the longitudinal cohort is provided by NIH/NIA P30 AG028383. Authors would like to acknowledge Dr. Frederick A. Schmitt for commenting on the development of this manuscript. The first author is funded by NIH T32 AG057461: “Training in Translational Research in Alzheimer’s and Related Dementias (TRIAD)”. Researchers acknowledge and thank all participants and their caregivers.
Footnotes
Disclosure of Interest
The authors have no conflicts of interest to report.
References
- Afram B, Stephan A, Verbeek H, Bleijlevens MH, Suhonen R, Sutcliffe C, … Hamers JP (2014). Reasons for institutionalization of people with dementia: informal caregiver reports from 8 European countries. J Am Med Dir Assoc, 15(2), 108–116. doi: 10.1016/j.jamda.2013.09.012 [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, … Phelps CH (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association, 7(3), 270–279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders: DSM-4. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Washington, DC. [Google Scholar]
- Au-Yeung SK, Bradley L, Robertson AE, Shaw R, Baron-Cohen S, & Cassidy S (2019). Experience of mental health diagnosis and perceived misdiagnosis in autistic, possibly autistic and non-autistic adults. Autism, 23(6), 1508–1518. doi: 10.1177/1362361318818167 [DOI] [PubMed] [Google Scholar]
- Bailey PE, Henry JD, & Von Hippel W (2008). Empathy and social functioning in late adulthood. Aging Ment Health, 12(4), 499–503. doi: 10.1080/13607860802224243 [DOI] [PubMed] [Google Scholar]
- Bangerter A, Manyakov NV, Lewin D, Boice M, Skalkin A, Jagannatha S, … Pandina G (2019). Caregiver Daily Reporting of Symptoms in Autism Spectrum Disorder: Observational Study Using Web and Mobile Apps. JMIR mental health, 6(3), e11365-e11365. doi: 10.2196/11365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen JA, Meffert H, Hein S, Huizinga P, Ketelaars C, Pijnenborg M, … de Bildt A (2011). Diagnosing autism spectrum disorders in adults: the use of Autism Diagnostic Observation Schedule (ADOS) module 4. J Autism Dev Disord, 41(9), 1256–1266. doi: 10.1007/s10803-010-1157-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bränsvik V, Granvik E, Minthon L, Nordström P, & Nägga K (2020). Mortality in patients with behavioural and psychological symptoms of dementia: a registry-based study. Aging Ment Health, 1–9. doi: 10.1080/13607863.2020.1727848 [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Langlais BT, Dueck AC, Locke DEC, & Woodruff BK (2018). Subjective Cognitive Impairment and the Broad Autism Phenotype. Alzheimer Disease & Associated Disorders, 32(4), 284–290. doi: 10.1097/wad.0000000000000273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerejeira J, Lagarto L, & Mukaetova-Ladinska E (2012). Behavioral and Psychological Symptoms of Dementia. Frontiers in Neurology, 3(73). doi: 10.3389/fneur.2012.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ST, Li KK, Losada A, Zhang F, Au A, Thompson LW, & Gallagher-Thompson D (2020). The effectiveness of nonpharmacological interventions for informal dementia caregivers: An updated systematic review and meta-analysis. Psychol Aging, 35(1), 55–77. doi: 10.1037/pag0000401 [DOI] [PubMed] [Google Scholar]
- Chung JA, & Cummings JL (2000). Neurobehavioral and neuropsychiatric symptoms in Alzheimer’s disease: characteristics and treatment. Neurol Clin, 18(4), 829–846. [DOI] [PubMed] [Google Scholar]
- Cloak N, & Al Khalili Y (2020). Behavioral And Psychological Symptoms In Dementia (BPSD). In StatPearls. Treasure Island (FL): StatPearls Publishing; Copyright © 2020, StatPearls Publishing pLLC. [PubMed] [Google Scholar]
- Croen LA, Zerbo O, Qian Y, Massolo ML, Rich S, Sidney S, & Kripke C (2015). The health status of adults on the autism spectrum. Autism, 19(7), 814–823. doi: 10.1177/1362361315577517 [DOI] [PubMed] [Google Scholar]
- Cummings JL (1997). The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology, 48(5 Suppl 6), S10–16. doi: 10.1212/wnl.48.5_suppl_6.10s [DOI] [PubMed] [Google Scholar]
- Cunningham AB, & Schreibman L (2008). Stereotypy in Autism: The Importance of Function. Research in Autism Spectrum Disorders, 2(3), 469–479. doi: 10.1016/j.rasd.2007.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme S, Price BH, Larvie M, Dougherty DD, & Dickerson BC (2015). Clinical Approach to the Differential Diagnosis Between Behavioral Variant Frontotemporal Dementia and Primary Psychiatric Disorders. American Journal of Psychiatry, 172(9), 827–837. doi: 10.1176/appi.ajp.2015.14101248 [DOI] [PubMed] [Google Scholar]
- Ecerejeira J, Lagarto L, & Mukaetova-Ladinska EB (2012). Behavioral and Psychological Symptoms of Dementia. Frontiers in Neurology, 3, 73. doi: 10.3389/fneur.2012.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mrayyan N, Bökberg C, Eberhard J, & Ahlström G (2020). Healthcare utilisation patterns among older people with intellectual disability and with affective and anxiety diagnoses in comparison with the general population. Aging Ment Health, 1–10. doi: 10.1080/13607863.2020.1742657 [DOI] [PubMed] [Google Scholar]
- Eldholm RS, Barca ML, Persson K, Knapskog A-B, Kersten H, Engedal K, … Saltvedt I (2018). Progression of Alzheimer’s Disease: A Longitudinal Study in Norwegian Memory Clinics. Journal of Alzheimer’s Disease, 61, 1221–1232. doi: 10.3233/JAD-170436 [DOI] [PubMed] [Google Scholar]
- Finkel SI, Costa e Silva J, Cohen G, Miller S, & Sartorius N (1996). Behavioral and psychological signs and symptoms of dementia: a consensus statement on current knowledge and implications for research and treatment. Int Psychogeriatr, 8 Suppl 3, 497–500. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Gerlach LB, & Kales HC (2020). Managing Behavioral and Psychological Symptoms of Dementia. Clinics in Geriatric Medicine, 36(2), 315–327. doi: 10.1016/j.cger.2019.11.010 [DOI] [PubMed] [Google Scholar]
- Geurts HM, & Vissers ME (2012). Elderly with Autism: Executive Functions and Memory. Journal of Autism and Developmental Disorders, 42(5), 665–675. doi: 10.1007/s10803-011-1291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam J (2006). GARS-2: Gilliam Autism Rating Scale–Second Edition. Austin, TX: PRO-ED. [Google Scholar]
- Gitlin LN, Kales HC, & Lyketsos CG (2012). Nonpharmacologic management of behavioral symptoms in dementia. Jama, 308(19), 2020–2029. doi: 10.1001/jama.2012.36918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Marx K, Stanley IH, & Hodgson N (2015). Translating Evidence-Based Dementia Caregiving Interventions into Practice: State-of-the-Science and Next Steps. The Gerontologist, 55(2), 210–226. doi: 10.1093/geront/gnu123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Burke J, Chernett N, Dennis MP, & Hauck WW (2008). Tailored activities to manage neuropsychiatric behaviors in persons with dementia and reduce caregiver burden: a randomized pilot study. Am J Geriatr Psychiatry, 16(3), 229–239. doi: 10.1097/JGP.0b013e318160da72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen BR, Hodgson NA, Budhathoki C, & Gitlin LN (2018). Caregiver Reactions to Aggressive Behaviors in Persons With Dementia in a Diverse, Community-Dwelling Sample. Journal of Applied Gerontology, <xocs:firstpage xmlns:xocs=““/>. doi: 10.1177/0733464818756999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe F, & Charlton RA (2012). Aging in autism spectrum disorders: a mini-review. Gerontology, 58(1), 70–78. doi: 10.1159/000329720 [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Yatabe Y, Ishikawa T, Fukuhara R, Kaneda K, Honda K, … Ikeda M (2015). Relationship between Dementia Severity and Behavioral and Psychological Symptoms of Dementia in Dementia with Lewy Bodies and Alzheimer’s Disease Patients. Dement Geriatr Cogn Dis Extra, 5(2), 244–252. doi: 10.1159/000381800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger M, Piguet O, Kipps C, & Hodges JR (2008). Executive function in progressive and nonprogressive behavioral variant frontotemporal dementia. Neurology, 71(19), 1481–1488. doi: 10.1212/01.wnl.0000334299.72023.c8 [DOI] [PubMed] [Google Scholar]
- Hsieh S, Irish M, Daveson N, Hodges JR, & Piguet O (2013). When one loses empathy: its effect on carers of patients with dementia. J Geriatr Psychiatry Neurol, 26(3), 174–184. doi: 10.1177/0891988713495448 [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, & Martin RL (1982). A new clinical scale for the staging of dementia. Br J Psychiatry, 140, 566–572. [DOI] [PubMed] [Google Scholar]
- Hurt C, Bhattacharyya S, Burns A, Camus V, Liperoti R, Marriott A, …Byrne EJ (2008). Patient and caregiver perspectives of quality of life in dementia. An investigation of the relationship to behavioural and psychological symptoms in dementia. Dement Geriatr Cogn Disord, 26(2), 138–146. doi: 10.1159/000149584 [DOI] [PubMed] [Google Scholar]
- Jeste DV, & Finkel SI (2000). Psychosis of Alzheimer’s disease and related dementias. Diagnostic criteria for a distinct syndrome. Am J Geriatr Psychiatry, 8(1), 29–34. doi: 10.1097/00019442-200002000-00004 [DOI] [PubMed] [Google Scholar]
- Jicha GA (2011). Medical management of frontotemporal dementias: the importance of the caregiver in symptom assessment and guidance of treatment strategies. J Mol Neurosci, 45(3), 713–723. doi: 10.1007/s12031-011-9558-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DK, Watts AS, Chapin BA, Anderson R, & Burns JM (2011). Neuropsychiatric profiles in dementia. Alzheimer disease and associated disorders, 25(4), 326–332. doi: 10.1097/WAD.0b013e31820d89b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales HC, Gitlin LN, & Lyketsos CG (2015). Assessment and management of behavioral and psychological symptoms of dementia. BMJ : British Medical Journal, 350, h369. doi: 10.1136/bmj.h369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Khan SA, Narendra AR, Mushtaq G, Zahran SA, Khan S, & Kamal MA (2016). Alzheimer’s Disease and Autistic Spectrum Disorder: Is there any Association? CNS Neurol Disord Drug Targets, 15(4), 390–402. doi: 10.2174/1871527315666160321104303 [DOI] [PubMed] [Google Scholar]
- Kim SH, & Lord C (2013). Chapter 1.2 - The Behavioral Manifestations of Autism Spectrum Disorders. In Buxbaum JD & Hof PR (Eds.), The Neuroscience of Autism Spectrum Disorders (pp. 25–37). San Diego: Academic Press. [Google Scholar]
- Lai MC, & Baron-Cohen S (2015). Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry, 2(11), 1013–1027. doi: 10.1016/s2215-0366(15)00277-1 [DOI] [PubMed] [Google Scholar]
- Li X, Bilen-Green C, Farahmand K, & Langley L (2018). A semiparametric method for estimating the progression of cognitive decline in dementia. IISE Transactions on Healthcare Systems Engineering, 8(4), 303–314. doi: 10.1080/24725579.2018.1455247 [DOI] [Google Scholar]
- Maseda A, Cibeira N, Lorenzo-López L, González-Abraldes I, Buján A, de Labra C, & Millán-Calenti JC (2018). Multisensory Stimulation and Individualized Music Sessions on Older Adults with Severe Dementia: Effects on Mood, Behavior, and Biomedical Parameters. J Alzheimers Dis, 63(4), 1415–1425. doi: 10.3233/jad-180109 [DOI] [PubMed] [Google Scholar]
- Midorikawa A, & Kawamura M (2012). The Relationship between Subclinical Asperger’s Syndrome and Frontotemporal Lobar Degeneration. Dement Geriatr Cogn Dis Extra, 2, 180–186. doi: 10.1159/000338174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshew NJ, & Williams DL (2007). The New Neurobiology of Autism: Cortex, Connectivity, and Neuronal Organization. Archives of Neurology, 64(7), 945–950. doi: 10.1001/archneur.64.7.945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J, Newton B, & Smith CD (2008). Test reviews: Gilliam J (2006). GARS-2: Gilliam Autism Rating Scale-Second Edition. Austin, TX: PRO-ED. Journal of Psychoeducational Assessment, 26(4), 395–401. [Google Scholar]
- Morris JC (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology, 43(11), 2412–2414. [DOI] [PubMed] [Google Scholar]
- O’Donnell S, Deitz J, Kartin D, Nalty T, & Dawson G (2012). Sensory Processing, Problem Behavior, Adaptive Behavior, and Cognition in Preschool Children With Autism Spectrum Disorders. American Journal of Occupational Therapy, 66(5), 586–594. doi: 10.5014/ajot.2012.004168 [DOI] [PubMed] [Google Scholar]
- Øyane NMF, & Bjorvatn B (2005). Sleep disturbances in adolescents and young adults with autism and Asperger syndrome. Autism, 9(1), 83–94. doi: 10.1177/1362361305049031 [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Ready RE, Stout JC, Salmon DP, Thal LJ, Grant I, & Jeste DV (2000). Neurobehaviors and psychotic symptoms in Alzheimer’s disease. J Int Neuropsychol Soc, 6(7), 815–820. doi: 10.1017/s1355617700677081 [DOI] [PubMed] [Google Scholar]
- Perneczky R, Wagenpfeil S, Komossa K, Grimmer T, Diehl J, & Kurz A (2006). Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am J Geriatr Psychiatry, 14(2), 139–144. doi: 10.1097/01.JGP.0000192478.82189.a8 [DOI] [PubMed] [Google Scholar]
- Pfeiffer BA, Koenig K, Kinnealey M, Sheppard M, & Henderson L (2011). Effectiveness of Sensory Integration Interventions in Children With Autism Spectrum Disorders: A Pilot Study. The American journal of occupational therapy : official publication of the American Occupational Therapy Association, 65(1), 76–85. doi: 10.5014/ajot.2011.09205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell PS, Klinger LG, & Klinger MR (2017). Patterns of Age-Related Cognitive Differences in Adults with Autism Spectrum Disorder. J Autism Dev Disord, 47(10), 3204–3219. doi: 10.1007/s10803-017-3238-6 [DOI] [PubMed] [Google Scholar]
- R Core Team. (2013). R: A Language and Environment for Statistical Computing. Retrieved from http://www.R-project.org/
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, … Murphy DG (2010). Cortical anatomy in autism spectrum disorder: an in vivo MRI study on the effect of age. Cereb Cortex, 20(6), 1332–1340. doi: 10.1093/cercor/bhp198 [DOI] [PubMed] [Google Scholar]
- Revelle W (2017). Procedures for Personality and Psychological Research. psych. [Google Scholar]
- Rhodus EK, Barber J, Abner EL, Duff DMC, Bardach SH, Caban-Holt A, … Jicha GA (2020). Behaviors Characteristic of Autism Spectrum Disorder in a Geriatric Cohort With Mild Cognitive Impairment or Early Dementia. Alzheimer Dis Assoc Disord, 34(1), 66–71. doi: 10.1097/wad.0000000000000345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risco E, Cabrera E, Jolley D, Stephan A, Karlsson S, Verbeek H, … Zabalegui A (2015). The association between physical dependency and the presence of neuropsychiatric symptoms, with the admission of people with dementia to a long-term care institution: a prospective observational cohort study. Int J Nurs Stud, 52(5), 980–987. doi: 10.1016/j.ijnurstu.2015.02.013 [DOI] [PubMed] [Google Scholar]
- Rockwood K, Strang D, MacKnight C, Downer R, & Morris JC (2000). Interrater reliability of the Clinical Dementia Rating in a multicenter trial. J Am Geriatr Soc, 48(5), 558–559. doi: 10.1111/j.1532-5415.2000.tb05004.x [DOI] [PubMed] [Google Scholar]
- Rossignol DA, & Frye RE (2014). The Use of Medications Approved for Alzheimer’s Disease in Autism Spectrum Disorder: A Systematic Review. Frontiers in Pediatrics, 2, 87. doi: 10.3389/fped.2014.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh MA (2004). Understanding orbitofrontal contributions to theory-of-mind reasoning: implications for autism. Brain Cogn, 55(1), 209–219. doi: 10.1016/j.bandc.2003.04.002 [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Nelson PT, Abner E, Scheff S, Jicha GA, Smith C, … Kryscio RJ (2012). University of Kentucky Sanders-Brown healthy brain aging volunteers: donor characteristics, procedures and neuropathology. Curr Alzheimer Res, 9(6), 724–733. doi: 10.2174/156720512801322591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J (2018). The Arts as a Medium for Care and Self-Care in Dementia: Arguments and Evidence. International journal of environmental research and public health, 15(6), 1151. doi: 10.3390/ijerph15061151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan B (2012). Assessment scales in dementia. Therapeutic advances in neurological disorders, 5(6), 349–358. doi: 10.1177/1756285612455733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol DK, Maloney B, Westmark CJ, & Lahiri DK (2019). Novel Contribution of Secreted Amyloid-β Precursor Protein to White Matter Brain Enlargement in Autism Spectrum Disorder. Frontiers in psychiatry, 10, 165–165. doi: 10.3389/fpsyt.2019.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S, Nagaraja AV, & Ratnavalli E (2005). Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison in Alzheimer’s disease, vascular dementia and frontotemporal dementia. J Neurol Sci, 236(1–2), 43–48. doi: 10.1016/j.jns.2005.04.014 [DOI] [PubMed] [Google Scholar]
- Staal JA, Sacks A, Matheis R, Collier L, Calia T, Hanif H, & Kofman ES (2007). The effects of Snoezelen (multi-sensory behavior therapy) and psychiatric care on agitation, apathy, and activities of daily living in dementia patients on a short term geriatric psychiatric inpatient unit. Int J Psychiatry Med, 37(4), 357–370. doi: 10.2190/PM.37.4.a [DOI] [PubMed] [Google Scholar]
- Tai C, Chang C-W, Yu G-Q, Lopez I, Yu X, Wang X, … Mucke L (2020). Tau Reduction Prevents Key Features of Autism in Mouse Models. Neuron. doi: 10.1016/j.neuron.2020.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel S, Heine C, Hein A, Krüger F, Kutschke A, Kernebeck S, … Kirste T (2017). Multidimensional assessment of challenging behaviors in advanced stages of dementia in nursing homes—The insideDEM framework. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 8, 36–44. doi: 10.1016/j.dadm.2017.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschanz JT, Corcoran CD, Schwartz S, Treiber K, Green RC, Norton MC, … Lyketsos CG (2011). Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the Cache County Dementia Progression study. Am J Geriatr Psychiatry, 19(6), 532–542. doi: 10.1097/JGP.0b013e3181faec23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linde RM, Dening T, Stephan BC, Prina AM, Evans E, & Brayne C (2016). Longitudinal course of behavioural and psychological symptoms of dementia: systematic review. Br J Psychiatry, 209(5), 366–377. doi: 10.1192/bjp.bp.114.148403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wolf E, van Hooren SAH, Waterink W, & Lechner L (2019). Well-being in elderly long-term care residents with chronic mental disorder: a systematic review. Aging Ment Health, 23(3), 287–296. doi: 10.1080/13607863.2017.1408773 [DOI] [PubMed] [Google Scholar]
- van Weert JC, van Dulmen AM, Spreeuwenberg PM, Ribbe MW, & Bensing JM (2005). Behavioral and mood effects of snoezelen integrated into 24-hour dementia care. J Am Geriatr Soc, 53(1), 24–33. doi: 10.1111/j.1532-5415.2005.53006.x [DOI] [PubMed] [Google Scholar]
- Wickham H (2009). ggplot2: Elegant Graphics for Data Analysis: Springer Publishing Company, Incorporated. [Google Scholar]
- Williams MM, Storandt M, Roe CM, & Morris JC (2013). Progression of Alzheimer’s disease as measured by Clinical Dementia Rating Sum of Boxes scores. Alzheimers Dement, 9(1 Suppl), S39–44. doi: 10.1016/j.jalz.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund L-O, … Petersen R (2004). Mild cognitive impairment - Beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment (Vol. 256). [DOI] [PubMed] [Google Scholar]
- Wink LK, Pedapati EV, Horn PS, McDougle CJ, & Erickson CA (2015). Multiple Antipsychotic Medication Use in Autism Spectrum Disorder. Journal of Child and Adolescent Psychopharmacology, 27(1), 91–94. doi: 10.1089/cap.2015.0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Z-T (2013). Role of theory of mind and executive function in explaining social intelligence: A structural equation modeling approach. Aging Ment Health, 17(5), 527–534. doi: 10.1080/13607863.2012.758235 [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, & Leirer VO (1983). Development and validation of a geriatric depression screening scale: a preliminary report. Journal of psychiatric research, 17(1), 37–49. [DOI] [PubMed] [Google Scholar]
- Ze O, Thoma P, & Suchan B (2014). Cognitive and affective empathy in younger and older individuals. Aging Ment Health, 18(7), 929–935. doi: 10.1080/13607863.2014.899973 [DOI] [PubMed] [Google Scholar]
- Zhao QF, Tan L, Wang HF, Jiang T, Tan MS, Tan L, … Yu JT (2016). The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J Affect Disord, 190, 264–271. doi: 10.1016/j.jad.2015.09.069 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.