Abstract
Background:
Inherited human aldehyde dehydrogenase 2 (ALDH-2) deficiency reduces the risk for alcoholism. Kudzu plants and extracts have been used for 1,000 years in traditional Chinese medicine to treat alcoholism. Kudzu contains daidzin, which inhibits ALDH-2 and suppresses heavy drinking in rodents. Decreased drinking due to ALDH-2 inhibition is attributed to aversive properties of acetaldehyde accumulated during alcohol consumption. However, daidzin can reduce drinking in some rodents without necessarily increasing acetaldehyde. Therefore, a selective ALDH-2 inhibitor might affect other metabolic factors involved in regulating drinking.
Methods:
Aldehyde dehydrogenase 2 inhibitors were synthesized based on the co-crystal structure of ALDH-2 and daidzin. We tested the efficacy of a highly selective reversible ALDH-2 inhibitor, CVT-10216, in models of moderate and high alcohol drinking rats. We studied 2-bottle choice and deprivation-induced drinking paradigms in Fawn Hooded (FH) rats, operant self-administration in Long Evans (LE), FH, and inbred P (iP) rats and in cue-induced reinstatement in iP rats. We also assayed blood acetaldehyde levels as well as dopamine (DA) release in the nucleus accumbens (NAc) and tested possible rewarding/aversive effects of the inhibitor in a conditioned place preference (CPP) paradigm.
Results:
CVT-10216 increases acetaldehyde after alcohol gavage and inhibits 2-bottle choice alcohol intake in heavy drinking rodents, including deprivation-induced drinking. Moreover, CVT-10216 also prevents operant self-administration and eliminates cue-induced reinstatement of alcohol seeking even when alcohol is not available (i.e., no acetaldehyde). Alcohol stimulates DA release in the NAc, which is thought to contribute to increased drinking and relapse in alcoholism. CVT-10216 prevents alcohol-induced increases in NAc DA without changing basal levels. CVT-10216 does not show rewarding or aversive properties in the CPP paradigm at therapeutic doses.
Conclusion:
Our findings suggest that selective reversible ALDH-2 inhibitors may have therapeutic potential to reduce excessive drinking and to suppress relapse in abstinent alcoholics.
Keywords: Alcohol, ALDH-2 Inhibitor, Acetaldehyde, Dopamine, Rat
EDITOR’S NOTE
[Correction added after online publication 10 August 2009.] Please see the related Commentary to this article by Dr. Ting-Kai Li, which will appear online in November 2009 and in print in issue 34:1.
Alcoholism is one of the most prevalent disorders in the world and is a major international public health problem with serious socioeconomic consequences. More is known about the neuropharmacology of alcoholism than other addictions and, as a result, new treatments are being developed (Johnson, 2008). However, none of the approved medications has achieved complete success in managing alcoholics, particularly because of a high rate of relapse to heavy drinking. In this study, we have exploited an important clue from diverse genetic and clinical observations which indicate that a selective deficiency of aldehyde dehydrogenase 2 (ALDH-2) is often associated with reduced drinking and risk for alcoholism (Eriksson, 2001). About 15 to 40% of Southeast Asians have an inactivation of ALDH-2 due to an E487K mutation (Quintanilla et al., 2005). Heterozygotes usually, but not always, drink less alcohol and homozygotes virtually never develop alcoholism (Quintanilla et al., 2005; Thomasson et al., 1991). Knockdown of ALDH-2 also confers protection against heavy drinking in rodents, validating a role for ALDH-2 in affecting drinking behavior (Quintanilla et al., 2005; Rezvani et al., 2002). When consuming alcohol, subjects with ALDH-2 deficiency often exhibit a characteristic “flushing reaction” and experience unpleasant symptoms due to the accumulation of acetaldehyde (Eriksson, 2001). Acetaldehyde is a product of hepatic alcohol dehydrogenase (ADH) and is metabolized to acetate by cytoplasmic ALDH-1 and mitochondrial ALDH-2. Current concepts suggest that elevations in acetaldehyde due to ALDH-2 deficiency or excessive activity of ADH (Isse et al., 2002) appear to discourage continued drinking. Disulfiram, a nonspecific toxic and irreversible inhibitor of both mitochondrial ALDH-2 and cytoplasmic ALDH-1, increases acetaldehyde levels during drinking. Therefore, disulfiram has been used as an aversive therapeutic agent to help alcoholics remain abstinent (Suh et al., 2006). Disulfiram has limited clinical value; however, because of poor compliance and many side effects, probably due to a range of off-target actions (Eneanya et al., 1981). This nonspecific toxicity appears to be caused by chelating metals and reacting with sulfhydryl groups to inactivate diverse proteins and other enzymes in addition to ALDH-1 and ALDH-2.
Kudzu and kudzu extracts have been used in traditional Chinese folk medicine to treat alcoholism for about 1,000 years, continuing to this day. Keung and Vallee (1993a,b) identified daidzin as an antidrinking principle in Kudzu. Daidzin is an isoflavone that selectively inhibits mitochondrial ALDH-2 and reduces alcohol intake in heavy drinking rodents. Paradoxically, daidzin reduces heavy drinking in Golden Syrian hamsters without increasing acetaldehyde levels (Keung et al., 1995). Failure to increase acetaldehyde levels appears to be due to alternative metabolism of acetaldehyde by high levels of uninhibited cytosolic ALDH-1 in these hamsters (Keung et al., 1997). Reducing drinking without elevating acetaldehyde suggests that additional pathways in the brain may be involved in the beneficial effect of a selective ALDH-2 inhibitor to suppress drinking. Based on the X-ray co-crystal structure of ALDH-2 and daidzin, we developed a small molecule inhibitor of ALDH-2 to improve therapeutic effectiveness and to investigate the role of ALDH-2 in uncontrolled heavy drinking and alcohol-seeking behavior. We first asked whether this novel, reversible, highly selective ALDH-2 inhibitor would suppress heavy drinking in rat models of alcoholism. Relapse is the most serious limitation of effective medical treatment of alcoholism. We next asked whether CVT-10216 would prevent alcohol seeking during daily operant self-administration, after weeks of abstinence, or even without alcohol to drink (i.e., no acetaldehyde). Besides oxidizing acetaldehyde, ALDH-2 is also involved in DA metabolism in brain (Eisenhofer et al., 2004). Increased DA release in nucleus accumbens (NAc) appears to play a role in driving alcohol seeking, consumption, and central nervous system (CNS) responses to alcohol (Weiss et al., 1993). Therefore, we asked whether CVT-10216 also modifies DA signaling in the NAc in addition to elevating acetaldehyde blood levels during exposure to alcohol.
MATERIALS AND METHODS
ALDH Activity—Ki
Aldehyde dehydrogenase activity was assayed in sodium phosphate buffer (50 mM, pH 7.4) containing 1.2 mM NAD+, 1 nM ALDH-2 or 10 nM ALDH-1, various concentrations of CVT-10216, and 0.3 mM formaldehyde (ALDH-2) or 0.18 mM acetaldehyde (for ALDH-1). Concentrations of formaldehyde and acetaldehyde used in the assays were their Km values for ALDH-2 and ALDH-1, respectively. Formaldehyde instead of acetaldehyde was used in ALDH-2 assays because it has a much higher Km (0.3 mM) than acetaldehyde (200 nM) and hence allows more accurate measurement of activity. Reactions were initiated by adding aldehyde substrate and rates were recorded at 25°C in a FluoroMax-2 Fluorimeter with excitation/emission wavelengths set at 340/460 nm. IC50 values were determined by fitting concentration-inhibition data to sigmoidal dose–response curves (GraphPad Prism) (experiments performed at Harvard Medical School).
Blood Acetaldehyde
Animals.
Adult male Sprague–Dawley (SD) rats from Charles River Laboratories (Wilmington, MA) weighing 300 to 350 g were used. The animals had carotid artery catheter cannulation to facilitate multiple blood drawings. They were maintained on a reverse 12 h light: 12 h dark cycle and had free access to laboratory rodent diet 50001 (PMI Feeds, Inc., St Louis, MO) and tap water for 1 day before experiments were performed (Thomas Jefferson University).
Acetaldehyde Measurements.
Acetaldehyde was measured in plasma using reverse phase high pressure liquid chromatography according to the established protocols (Garver et al., 2000) with minor modifications. CVT-10216 (suspended in 1% carboxymethylcellulose [CMC] in saline to yield a final concentration of 15 mg/ml) or vehicle (1% CMC) was injected i.p. 30 minutes before alcohol gavage (2 g/kg of 20% solution in saline v/v) and serial bleedings were performed during the next 4 hours.
Behavioral Studies
Animals.
Three strains of rats (Fawn Hooded [FH], inbred P [iP], and Long Evans [LE]) were used to test effectiveness of CVT-10216 in different models of alcohol addiction. To better understand the effect of CVT-10216, we tested our compound in 2 heavy alcohol drinking rat models (FH and iP rats) and 1 moderate alcohol drinking rat model (LE rats). These models and strains differ in sensitivity to drugs that regulate alcohol consumption (Cowen et al., 2005), Therefore, CVT-10216 dose ranges varied accordingly. FH rats at about 60 days of age were selected from breeding colonies maintained at the Bowles Center for Alcohol Studies at UNC in accordance with their IACUC approved animal protocols. FH rats were placed in standard cages that permitted drinking tubes to be placed either at the front or the top of the cage. The animal room was maintained at 22°C and 50% humidity under a reversed 12 h light:12 h dark cycle, with lights out between 10:00 and 22:00 hours. iP rats were obtained from the breeding colony at the Howard Florey Institute, University of Melbourne, Australia. Parental stock had previously been obtained from Professor T.K. Li (while at Indiana University, Indianapolis). Experiments using iP rats were performed in accordance with the Prevention of Cruelty to Animals Act, 1986 under the guidelines of the National Health and Medical Research Council Code of Practice for the Care and Use of Animals for Experimental Purposes in Australia. Male LE rats (Charles River Laboratories, Wilmington, MA) weighing approximately 250 g at the beginning of the studies were individually housed at CV Therapeutics (CVT) with food and water available ad libitum except when stated otherwise. They were maintained on a reversed 12 h light:12 h dark cycle, lights on at 18:00 hours. The experimental procedures were approved in advance by the CVT Institutional Animal Care and Use Committee. Animals received humane care according to criteria outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Alcohol 2-Bottle Choice.
FH (UNC) rats were trained to drink alcohol using standard methods (Overstreet et al., 1997). After 1 day of water only, they were forced to drink 10% (v/v) alcohol for 3 consecutive days. Thereafter, except for special conditions to be described later, they were given continuous access to water and 10% (v/v) alcohol. Administration of drugs did not begin until at least 4 weeks later, when the rats had demonstrated stable levels of alcohol drinking. FH rats received i.p. CVT-10216 (7.5, 15, and 30 mg/kg, in 0.5% methylcellulose [MC] or vehicle [0.5% MC]) in a counterbalanced within-subject design. Fluid volumes were recorded 2, 4, 6, and 24 hours after treatment. Food intake was measured at 24 hours.
Alcohol Deprivation.
FH rats exhibit increased alcohol intake following alcohol deprivation (Rezvani et al., 2002; Rodd et al., 2004). This alcohol deprivation effect appears to be an index of craving for alcohol (McBride et al., 2002). We studied the effect of CVT-10216 on deprivation-induced drinking in a new group of FH (UNC) rats which had been drinking 10% (v/v) alcohol voluntarily for at least 2 months. The alcohol tube was removed from the cage for 5 days. Approximately 30 minutes prior to the return of the alcohol tubes at the beginning of the dark cycle (10:00 h), 3 groups of rats received either i.p. vehicle (0.5% MC), 15 mg/kg CVT 10216 or no treatment. Fluid volumes recorded 2, 4, 6, and 24 hours after returning the alcohol tubes were compared to the same rat’s previously recorded baseline drinking at the same time points.
Alcohol Operant Self-Administration.
FH (UNC), LE (CVT), and iP (University of Melbourne, Australia) rats were trained in operant chambers (Med Associates, Georgia, VT) using previously published protocols (Arolfo et al., 2004; Besheer et al., 2008; Liang et al., 2006; Samson, 1986). Before beginning alcohol operant self-administration, rats were restricted to 1 hour of water per day for 2 consecutive days (LE rats were previously exposed to 10% [v/v] as the only liquid source in home cage for 4 days). On the night of the second day of water restriction, rats were placed in the operant chambers for a 12 to 15 hours overnight session on an FR1 schedule (1 reinforcer of 0.1 ml per lever press) with 10% sucrose as reinforcer and both levers active. The next day, rats began operant self-administration training according to the sucrose fading technique (Samson, 1986) with minor modifications. Animals were maintained on water restriction for the next 4 to 5 days and received one 45 minute session per day on an FR1 schedule with 10% sucrose as reinforcer. Rats were given free water in their home cages for the remainder of the experiment and were trained an additional 2 to 3 times according to the above described sessions. The next day, sessions were shortened to 30 minutes and the ratio of responding was increased to FR3. Alcohol was added to the sweet solution and rats received 3 to 4 sessions of this solution, followed by at least 20 sessions with 10% (v/v) alcohol only. Only 1 lever delivering alcohol was active. FH rats were trained as described before, but the alcohol concentration was 15% (v/v) under a FR4 schedule and a second lever delivered water. iP rats were trained as previously published (Lawrence et al., 2006; Liang et al., 2006) similar to the procedures described above but without any fluid restriction. In addition, 1 lever delivered 10% (v/v) alcohol and the other lever delivered water, both under FR3. In iP rats, availability of alcohol was conditioned by the presence of an olfactory cue (S+; 2 drops of vanilla essence, placed on the bedding of the operant chamber directly under the active lever), plus a 1-second light stimulus (CS+) that occurred when FR3 was obtained.
In all studies, a minimum average of 0.3 g/kg alcohol consumption in 8 sessions prior to the beginning of any drug treatment was required. FH rats received i.p. CVT-10216 (1, 3, 10 mg/kg in 0.5% MC) or vehicle (0.5% MC), LE rats received i.p. CVT-10216 (3.75, 7.5, or 15 mg/kg) or vehicle (0.5% MC) and iP rats received i.p. CVT-10216 (0.94, 1.88, or 3.75 mg/kg) or vehicle (0.5% MC), 30 minutes before testing in a within-subject counterbalanced design.
Cue-Induced Reinstatement of Alcohol Seeking.
Following normal operant training as detailed above, a group of iP rats that were naïve to CVT-10216 treatment, was then subjected to extinction training, during which time there were no cues present in the operant chamber and there was no programmed response subsequent to task completion (Lawrence et al., 2006). Extinction sessions continued until responding on the “active” or alcohol lever was similar to that on the water lever, and stable between trials. Reinstatement was then triggered by introducing the olfactory cue under the “active” lever and also reprogramming the software such that the stimulus light was activated (for 1 second) after every FR3 response, although there was no delivery of alcohol into the receptacle. Three different groups of rats were treated with either i.p. vehicle (0.5% MC) or CVT-10216 (1.88 or 3.75 mg/kg in 0.5% MC) 30 minutes prior to the reinstatement session. A group of LE rats trained to self-administer alcohol as described in the section above (at least 20 sessions of alcohol self-administration) received i.p. vehicle (0.5% MC) or CVT-10216 (15 mg/kg) 30 minutes prior to the operant session in which alcohol was not delivered upon lever press (within-subject study). The purpose of this study was to determine the effect of CVT-10216 on alcohol seeking after 1 day of abstinence.
Nucleus Accumbens Dopamine Microdialysis
Male LE (Medical University of South Carolina) rats were anesthetized with an isoflurane-medical breathing air mixture (1 to 3% isoflurane) and were implanted with guide cannulae equipped with obturators aimed at the shell region of the NAc (AP +2.2, ML +0.7, DV −6.2 mm from bregma and skull surface according to Paxinos and Watson (2005). The guide cannula was secured to the skull with miniature screws and dental cement, and upon successful completion of surgery, rats were given 5 days to recover in cylindrical microdialysis cages (Instech, Plymouth Meeting, PA) before microdialysis procedures. Following at least 5 days of recovery, animals were lightly re-anesthetized, implanted with microdialysis probes (2 mm cuprophane membranes; SciPro, Sanborn, NY) that were constantly perfused with artificial CSF (Olive et al., 2000). On the following day, 4 baseline samples were collected every 15 minutes and prior to administration of i.p. CVT-10216 (7.5 or 15 mg/kg) or vehicle (0.5% MC). Next, 6 additional samples every 15 minutes were collected. In a different group of rats, we examined the effects of CVT-10216 on alcohol-induced increases in extracellular dialysate DA in the NAc. In this new group of rats, following collection of the 4 baseline samples, animals were administered i.p. vehicle (0.5% MC) or CVT-10216 (7.5 and 15 mg/kg), and 2 additional samples every 15 minutes were collected. Immediately after the last sample collection, animals received alcohol i.p. (1 g/kg). Additional 6 samples were collected following alcohol administration. Microdialysis samples were collected into 3 μl of 0.75 M perchloric acid to prevent oxidation of DA, and were analyzed by HPLC with electrochemical detection.
Conditioned Place Preference (CPP)
Male LE (Medical University of South Carolina) rats were conditioned in the CPP apparatus (Med Associates) using procedures published elsewhere (Morales et al., 2007). The CPP apparatus (Med Associates) consisted of 2 conditioning adjacent conditioning compartments (20.3 cm L × 15.9 cm W × 21.3 cm H) separated by a manual guillotine-type door measuring 8.9 × 11.4 cm. One of the conditioning chambers was equipped with black and white striped acrylic walls (2.5 cm stripe width) and a steel mesh floor, and the other was equipped with plain white acrylic walls and a wire rod floor (3.2 mm rod thickness, placed on 8 mm centers). Conditioning compartments were equipped with 4 sets each of infrared photobeams that were interfaced to a PC computer to monitor the animal’s position in the apparatus. CPP chambers were located in sound-attenuating cubicles equipped with an overhead house light and exhaust fans to help mask external noise and odors. All testing was performed during the dark phase of the light–dark cycle. Animals were first allowed to habituate to the apparatus in 2 separate 20 minute habituation sessions. Following habituation, animals were subjected to a 20-minute pre-conditioning test to determine any innate preference for one of the conditioning compartments. Cocaine was used as a positive control for CPP. Based on this preconditioning test, each animal was then assigned to receive CVT-10216 or cocaine in the initially nonpreferred conditioning compartment (i.e., biased design). Next, animals underwent place conditioning in twice-daily conditioning sessions, with vehicle conditioning (0.5% MC or saline) in the morning and drug conditioning (CVT-10216 7.5 or 15 mg/kg i.p. or cocaine 10 mg/kg i.p.) in the afternoon. Conditioning sessions were 20 minutes and were separated by at least 4 hours. For conditioning with CVT-10216 or its corresponding vehicle, injections were made 30 minute prior to the conditioning sessions. For conditioning with cocaine, animals were injected with cocaine or saline immediately prior to placement in the conditioning chamber. Following sequential 4 days of conditioning, animals were tested for place preference by placing them in the CPP apparatus and allowing free access to both conditioning compartments for 20 minutes. A sample size of n = 8 was used for all groups.
Statistics
Appropriate statistical analyses were performed according to each experimental design. These included one- or two-way ANOVAs with repeated measures followed by post-hoc Fisher tests where appropriate and repeated measures t-tests. A value of p < 0.05 was considered significant.
For the microdialysis studies, the absolute concentration of DA (pg/μl) in each of the first 4 microdialysis samples from each animal was averaged to produce a baseline value for that animal, and subsequently all data points for that animal were converted to a percent of the animal’s baseline values for DA.
RESULTS
CVT-10216 Selectively Inhibits ALDH-2 In Vitro and In Vivo
CVT-10216 (3-((3-(4-(methylsulfonamido)phenyl)-4-oxo-4H-chromen-7-yloxy)methyl)benzoic acid) inhibits human ALDH-2 selectively as a function of concentration. The IC50 value for ALDH-2 is ~29 nM whereas the IC50 for ALDH-1 is 1300 nM (Fig. 1A). Detailed methods and kinetic studies of CVT-10216 inhibition of ALDH-2 are available in the online Supporting Information (Scheme S1 and S2 and Fig. S1). Unlike disulfiram which effectively and irreversibly inhibits cytoplasmic ALDH-1, as well as mitochondrial ALDH-2, CVT-10216 is a highly selective reversible inhibitor of mitochondrial ALDH-2. Again, unlike disulfiram, CVT-10216 does not inhibit tyrosine hydroxylase, monoamine oxidase A MAO-A, MAO-B, or dopamine β-hydroxylase (DBH) at concentrations up to 10 μM (data not shown). CVT-10216 also inhibits ALDH-2 in vivo. CVT-10216 dose dependently increases blood acetaldehyde levels in SD rats after alcohol gavage (Fig. 1B).
Fig. 1.
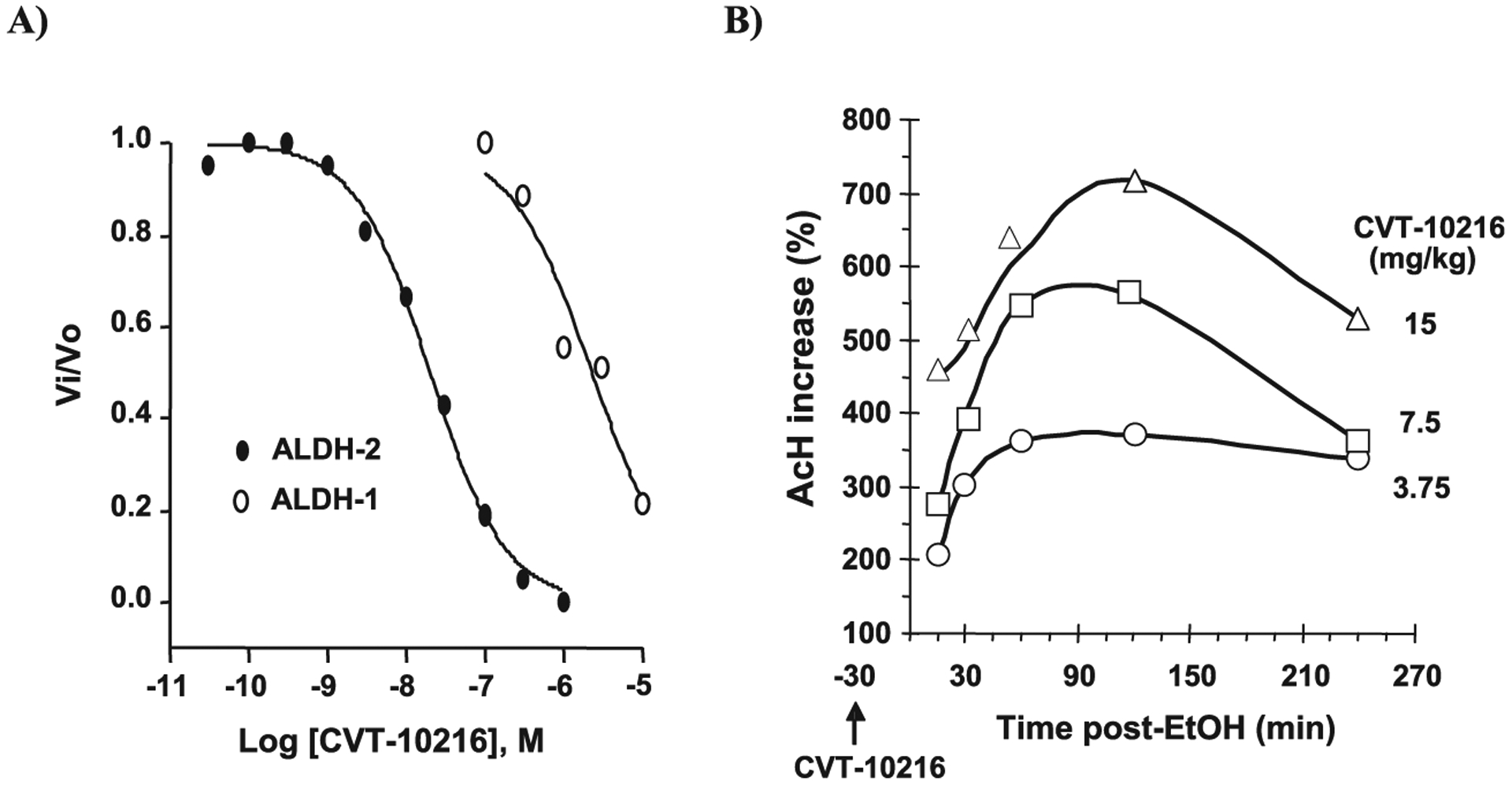
CVT-10216 inhibits human aldehyde dehydrogenase (ALDH)-2 and ALDH-1 in vitro and increases blood acetaldehyde (AcH) in vivo after alcohol (EtOH) gavage. (A) ALDH activity was assayed in sodium phosphate buffer (50 mM, pH 7.4) containing 1.2 mM NAD+, 1 nM ALDH-2, or 10 nM ALDH-1, various concentrations of CVT-10216, and 0.3 mM formaldehyde for ALDH-2 or 0.18 mM acetaldehyde for ALDH-1, substrate concentrations equal to their Km for ALDH-2 and ALDH-1, respectively. Formaldehyde was used in ALDH-2 assay because ALDH-2 has a low Km for acetaldehyde (200 nM). Reactions were initiated by adding aldehyde substrate and rates recorded at 25°C in a FluoroMax-2 Fluorimeter with excitation/emission wavelengths of 340/460 nm. IC50 values were determined by fitting concentration-inhibition data to sigmoidal dose–response curves. The IC50 is ~29 nM for ALDH-2 and 1300 nM for ALDH-1 (see Supporting Information Scheme S1 and S2 and Fig. S1 for more details) (B) SD rats with a carotid artery catheter received i.p. CVT-10216 (3.75, 7.5, or 15 mg/kg) 30 minutes before intragastric alcohol (2 g/kg) (n = 3). Timed blood samples obtained after alcohol. Plasma acetaldehyde was measured as a stable DNPH derivative using reverse phase high pressure liquid chromatography with minor modifications.
CVT-10216 Suppresses 2-Bottle Choice Alcohol Intake in Heavy Drinking FH Rats
Rodents trained to drink alcohol are commonly used to model human alcoholism (O’Brien and Gardner, 2005). In 2-bottle choice testing, both alcohol and water are continuously available in the home cage where rats have a free choice to drink either solution over a 24-hour period. We studied 2-bottle choice consumption in heavy drinking FH rats. CVT-10216 reduces alcohol intake in a dose-dependent manner in FH rats over 24 hours (Fig. 2A) (repeated measure ANOVA for alcohol intake (g/kg) shows a significant effect of CVT-10216 treatment (7.5, 15, and 30 mg/kg) [F(3,31) = 14.71, p < 0.001], a significant effect of time after treatment (2, 4, 6, and 24 hours) [F(3,93) = 260.3, p < 0.001] and no significant treatment × time after treatment interaction [F(9,93) = 1.43, NS]; with significant Fisher post-hoc test of 7.5, 15, and 30 mg/kg CVT-10216 versus vehicle at different time points. The greatest inhibition occurred in the first 2 hours with carryover effects afterward. There was little or no inhibition of water, total fluid, and food consumption (see Supporting Information Tables S5–S7). Relapse to heavy alcohol drinking after a period of abstinence is another feature of human alcoholism (Rezvani et al., 2002). We studied this behavior in heavy drinking FH rats using the 2-bottle choice method. Alcohol drinking in these rats is markedly increased after a period of abstinence due to forced deprivation (Rodd et al., 2004). We find in heavy drinking FH rats, that when compared with stable baseline drinking in each rat before a 5-day period of alcohol withdrawal, CVT-10216 abolishes a 350% increase in alcohol intake that occurs in the first 2 hours when alcohol is made available to the deprived rats (Fig. 2B) (one-way ANOVA shows a significant effect of treatment: untreated, vehicle or 15 mg/kg of CVT-10216 on alcohol intake expressed in g/kg: [F(8,24) = 4.04, p < 0.01]; Fisher post hoc test revealed significant difference between 15 mg/kg CVT-10216 and vehicle treatments at 2, 4, 6, and 24 hour time points, p < 0.01, p < 0.01, p < 0.01, and p < 0.05, respectively). Importantly, a single dose of CVT-10216 continues to suppress increased deprivation-induced drinking to below baseline levels during the entire 24 hour experiment (Fig. 2B).
Fig. 2.
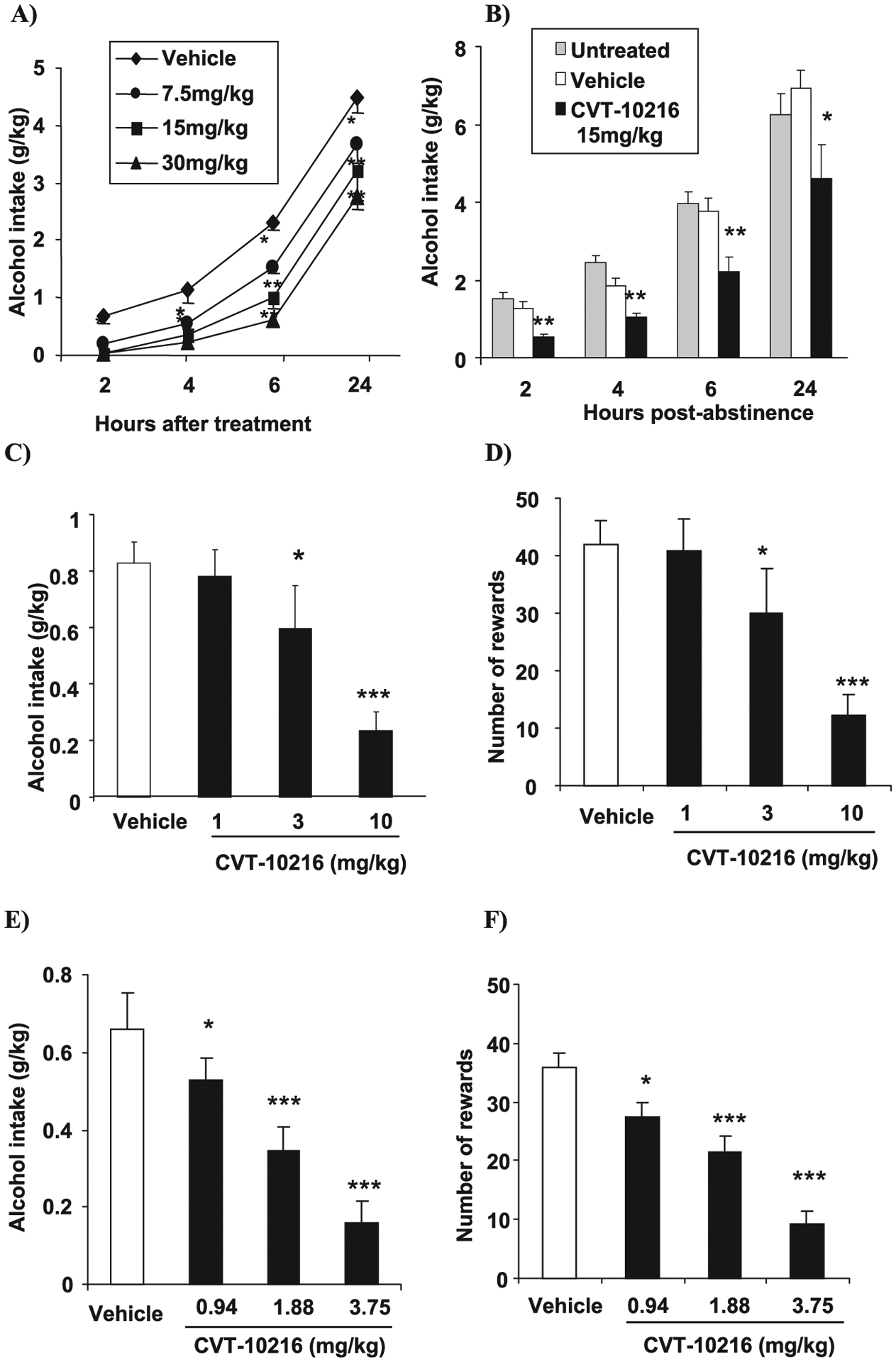
CVT-10216 (i.p.) decreases alcohol intake and seeking in high alcohol drinking rat models. All data are expressed as the mean ± SEM (A) 2-bottle choice alcohol intake (g/kg) in Fawn Hooded (FH) rats (n = 8 to 9). (B) Deprivation-induced alcohol intake (g/kg) in FH rats. Untreated, vehicle, and CVT-10216 rats were alcohol deprived for 5 days. CVT 10216 (15 mg/kg) was administered before providing alcohol on day 6 (n = 6). (C) Alcohol self-administration: alcohol intake (g/kg) and (D) number of alcohol rewards in FH rats (n = 12). (E) Alcohol self-administration: alcohol intake (g/kg) and (F) number of alcohol rewards in iP rats. *p < 0.05, **p < 0.01, ***p < 0.001, compared with vehicle [repeated measure ANOVA (Fisher post hoc) or repeated measures t-test].
CVT-10216 Suppresses Operant Self-Administration in FH, iP, and LE Rats
In operant self-administration training, alcohol is not available in the home cage, but rats will lever press to obtain alcohol in a testing cage during a 30-minute session every 24 hours during 5 d/wk. Here, CVT-10216 potently inhibits alcohol self-administration in FH, iP, and LE rats (Figs. 2C,E and 4A, respectively) (FH rats: repeated measure ANOVA for alcohol intake (g/kg): [F(3,33) = 11.65, p < 0.001] and number of alcohol rewards [F(3,33) = 10.94, p < 0.001] (Fig. 2D); 3 and 10 mg/kg versus vehicle, p < 0.05 and p < 0.001, respectively, Fisher post-hoc test; iP rats: repeated measure ANOVA for alcohol intake (g/kg): [F(3,54) = 21.94, p < 0.001] and number of alcohol rewards [F(3,54) = 19.14, p < 0.001] (Fig. 2F); 0.94, 1.88, and 3.75 mg/kg versus vehicle, p < 0.05, p < 0.001, and p < 0.001, respectively, Fisher post-hoc test; LE rats: repeated measure ANOVA for alcohol intake (g/kg): [F(3,15) = 16.38, p < 0.001] (Fig. 4A); 7.5 and 15 mg/kg vs. vehicle, p < 0.05, and p < 0.001, respectively, Fisher post-hoc test). Water lever response in iP rats was significantly decreased only at the 3.75 mg/kg dose but not affected in FH rats (data not shown).
Fig. 4.
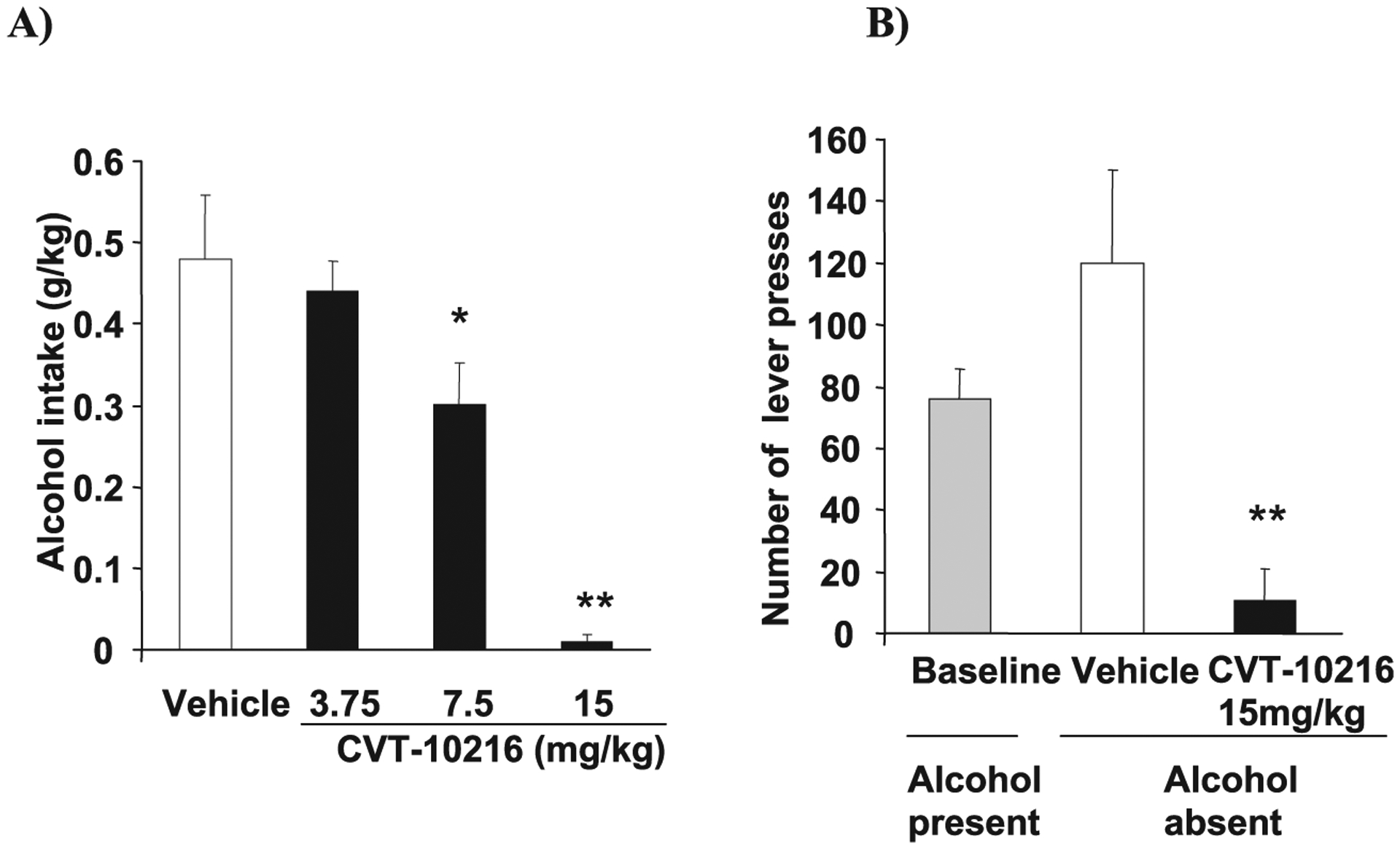
CVT-10216 (i.p.) decreases operant alcohol intake and seeking in Long Evans (LE) rats. All data are expressed as the mean ± SEM (A) Alcohol self-administration (alcohol intake in g/kg) in LE rats (n = 7 to 13). (B) Alcohol seeking (lever presses) in LE rats. Rats were trained as in (A) except no alcohol was available during testing and only 15 mg/kg dose of CVT-10216 was tested (n = 6). *p < 0.05, **p < 0.01, compared with vehicle [repeated measure ANOVA (Fisher post hoc)].
CVT-10216 Suppresses Alcohol Seeking in iP and LE Rats
CVT-10216 produces a profound reduction in cue-induced reinstatement of alcohol-seeking behavior in iP rats (Fig. 3) (repeated measures ANOVA indicated a significant main effect of paradigm: extinction versus reinstatement, [F(1,26) = 38.785, p < 0.001], a main effect of treatment [F(2,26) = 1.132, p < 0.001] and a paradigm × treatment interaction [F(2,26) = 14.74, p < 0.001]; 3.75 vs. vehicle, p < 0.001, Fisher post-hoc test). Responding on the active lever at 3.75 mg/kg was not different than extinction. Data is expressed as lever presses because alcohol was not delivered. We next studied alcohol seeking in LE rats after 24 hour of abstinence. Here, CVT-10216 also suppresses alcohol seeking when alcohol is not delivered but replaced by cues associated with alcohol (Fig. 4B) (repeated measure ANOVA for number of lever presses: [F(2,10) = 8.74, p < 0.01]; 15 mg/kg vs. vehicle, p < 0.01, Fisher post-hoc test). These results show that CVT-10216 suppresses lever pressing for alcohol whether or not alcohol is delivered at the time of testing, suggesting that nonacetaldehyde pathways appear to be involved in the response to a selective ALDH-2 inhibitor.
Fig. 3.
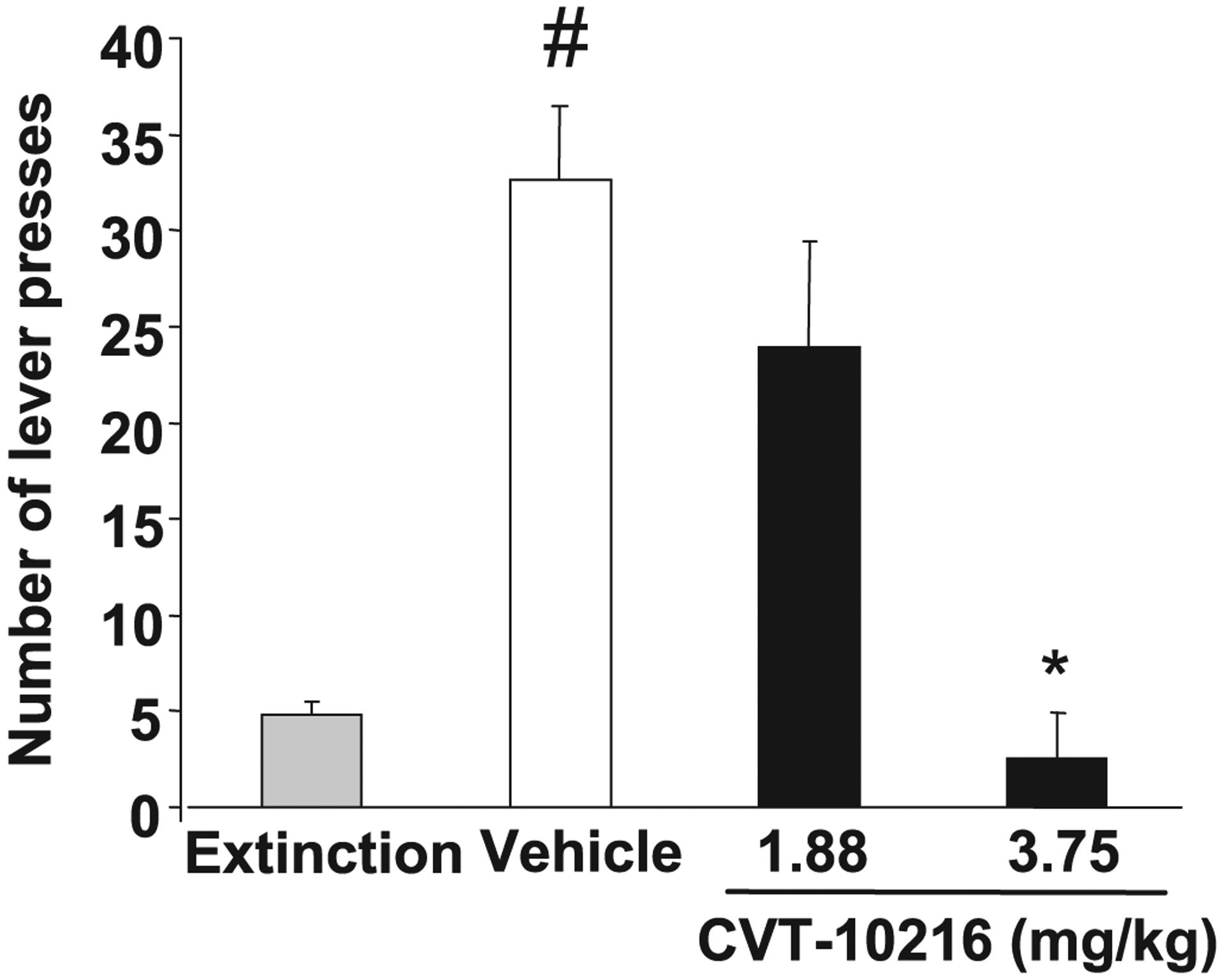
Cue-induced alcohol-seeking (lever presses) in iP rats. Rats trained to self-administer alcohol received extinction sessions (no cues/fluid), followed by cue-induced reinstatement session under vehicle or CVT-10216 (1.88 and 3.75 mg/kg, n = 10 per dose). *p < 0.05 compared with vehicle; #p < 0.05, compared with extinction [repeated measure ANOVA (Fisher post hoc)].
CVT-10216 Prevents Alcohol-Activated DA Release in the NAc
Increased DA release in the NAc activated by alcohol and addictive drugs (Di Chiara and Bassareo, 2007; Weiss et al., 1993) appears to play a role in alcohol addiction (Berridge, 2007). Moreover, operant self-administration procedures alone may be sufficient to produce an anticipatory increase in DA in the NAc (Wise, 2002). Therefore, we next asked whether the effectiveness of CVT-10216 under activating conditions could be related, in part, to preventing an increase in alcohol-stimulated DA levels in the NAc. LE rats were prepared with microdialysis probes in the NAc to collect and measure DA release after exposure to alcohol. Figure 5A shows that CVT-10216 prevents alcohol-stimulated increases in NAc DA in a dose-dependent manner. Dialysate DA concentrations were significantly lower in the 2nd through 5th samples collected postalcohol compared to vehicle treated animals (repeated measure ANOVA on dialysate levels of DA after alcohol [F(2,15) = 11.017, p = 0.001]; 7.5 and 15 mg/kg vs. vehicle, p < 0.01, and p < 0.001, respectively, Fisher post-hoc test, Fig. 5A). Importantly, CVT-10216 does not change basal levels of DA in the NAc from controls not receiving alcohol (Fig. 5B) (repeated measure ANOVA on basal dialysate levels of DA [F(2,13) = 0.367], p = 0.699, NS; 7.5 and 15 mg/kg vs. vehicle, NS). This is consistent with our observations in control animals that therapeutic doses of CVT-10216 do not inhibit normal physiologic parameters, including acquiring food and water and locomotor activity (see Supporting Information Tables S1–S7).
Fig. 5.
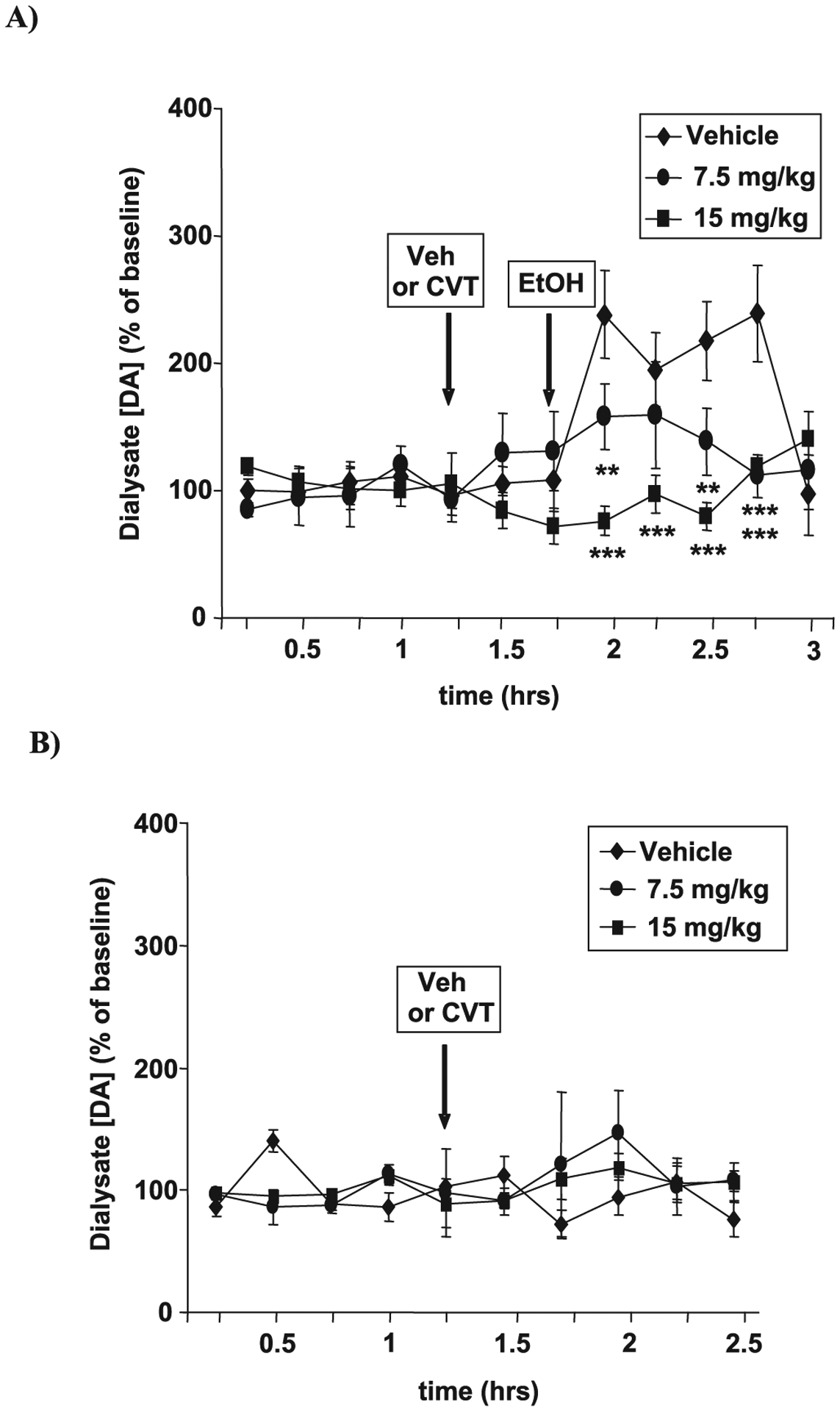
(A) CVT-10216 prevents alcohol-activated nucleus accumbens (NAc) dopamine (DA) release in vivo. Long Evans rats with NAc microdialysis probes received i.p. CVT-10216 (7.5 or 15 mg/kg) or vehicle 30 minutes before alcohol (EtOH) (1 g/kg i.p.). Data are the mean ± SEM of NAc DA concentration (% of baseline) (n = 6). (B) CVT-10216 does not affect basal NAc DA levels in vivo. Rats were treated as in (A) except without alcohol administration (n = 4 to 6). **p < 0.01, ***p < 0.001, compared with vehicle [repeated measure ANOVA (Fisher post hoc)].
Lack of Conditioned Place Preference or Aversion Produced by CVT-10216
There was no evidence of CPP when rats were conditioned with either 7.5 or 15 mg/kg CVT-10216 in the CPP paradigm. Neither group of treated rats exhibited a significant change in the percent time spent in the initially nonpreferred side (Fig. 6). These data suggest that CVT-10216 does not appear to possess any intrinsic rewarding or aversive effects. As a positive control, however, when cocaine (10 mg/kg) was used as the conditioning drug, a significant increase in the percent time spent on the initially nonpreferred side was observed (CVT-10216 at 7.5 mg/kg: [F(1,7) = 0.787], p = 0.405, NS; CVT-10216 at 15 mg/kg: [F(1,7) = 0.265], p = 0.623, NS; cocaine: [F(1,7) = 36.748], p < 0.001).
Fig. 6.
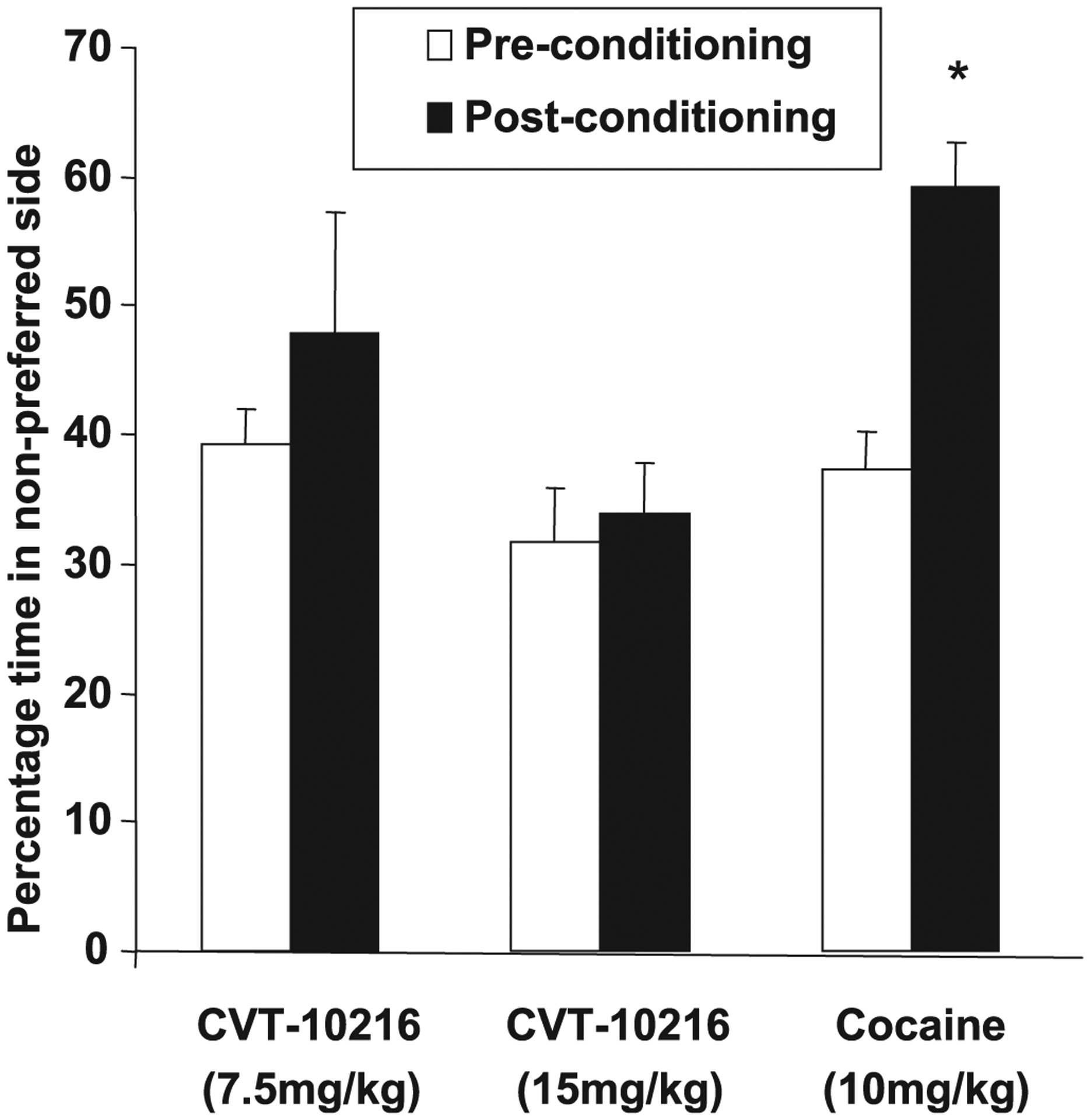
CVT-10216 does not induce conditioned place preference (CPP). Animals treated with CVT-10216 (7.5 and 15 mg/kg) spent a similar amount of time in the nonpreferred side during pre- and postconditioning sessions. Animals treated with cocaine (10 mg/kg) as a positive control showed CPP. Animals in this group spent significantly more time in the nonpreferred side during postconditioning session (n = 8). *p < 0.05 compared with preconditioning time [repeated measure ANOVA (Fisher post hoc)].
DISCUSSION
Recent epidemiologic studies indicate that the pathological consequences of excessive drinking by alcoholics have become major health problems. Here, we show that treatment with CVT-10216, a selective reversible inhibitor of ALDH-2 (Fig. 1A) reduces heavy drinking in rodents when alcohol is continuously available in a 24 hour 2-bottle choice study (Fig. 2A). A high rate of relapse among recovering abstinent alcoholics (80 to 90%) is, perhaps, one of the most serious limitations of effective medical treatment (Mann et al., 2004). Here, we also show that CVT-10216 suppresses alcohol self-administration (Figs. 2C–F and 4A), deprivation-induced drinking (Fig. 2B) and cue-induced reinstatement of alcohol seeking (Fig. 3), a rodent model of relapse. In addition, CVT-10216 attenuates the typical elevation in responding under extinction conditions during a 30-minute operant self-administration that followed 24 hour abstinence (Fig. 4B). Current concepts suggest that increases in blood acetaldehyde levels as a consequence of diminished ALDH-2 activity discourage alcohol consumption. Consistent with this view, we find that CVT-10216 increases blood acetaldehyde levels after alcohol gavage (Fig. 1B). However, an increase in acetaldehyde does not appear to be the only mechanism activated by CVT-10216 to discourage alcohol intake. CVT-10216 also prevents alcohol seeking even when alcohol is not offered for drinking during the 30-minute test and had not been available for 24 hours (Fig. 4B). Moreover, using another model of human alcohol relapse during abstinence in which the responding for alcohol in iP rats is extinguished over a period of time (and consequently also includes a period of abstinence), CVT-10216 also inhibits cue-induced reinstatement of lever pressing for alcohol, even without having alcohol to drink during this experiment (Fig. 3). These findings suggest that in addition to elevating acetaldehyde levels during alcohol intake other pathways appear to be involved in CVT-10216 suppression of alcohol-seeking behavior.
Increased levels of DA in the NAc are correlated with CNS responses to alcohol, alcohol-seeking behavior, reinstatement of alcohol intake, and craving for addictive agents (Rodd et al., 2004; Weiss et al., 1993). Because ALDH-2 plays a role in DA metabolism in the brain (Eisenhofer et al., 2004), we asked whether CVT-10216, which crosses blood–brain barrier, attenuates alcohol-stimulated increases in DA. We find that CVT-10216 dose dependently prevents an alcohol-induced increase in DA levels in the NAc (Fig. 5A). Consistent with these findings, Gonzales and Weiss (1998) have reported that naltrexone inhibition of alcohol self-administration is also associated with suppression of alcohol-induced increases in NAc DA. The inhibitory effect of CVT-10216 appears to be confined to alcohol-activated increases in NAc DA levels because CVT-10216 does not affect basal levels of DA in controls. This is consistent with our observations that at therapeutic doses, CVT-10216 does not appear to affect locomotor activity and acquiring food and water (see Supporting Information Tables S4–S7).
Although CVT-10216 prevents alcohol-stimulated increases in DA levels in the NAc, the mechanism of action remains unclear. There are conflicting reports about the role of acetaldehyde. Ward and colleagues (1997) reported that increased acetaldehyde, produced as a consequence of inhibiting ALDH-2 during exposure to alcohol, is associated with reduced DA levels in the NAc. This could explain the response to CVT-10216. By contrast, Melis and colleagues (2007) reported recently that acetaldehyde actually increases accumbal DA levels and dopaminergic cell firing in the ventral tegmental area (VTA). Moreover, a study by Foddai and colleagues (2004) showed that acetaldehyde also increases dopaminergic activity in the VTA. The Melis and Foddai studies suggest that elevated acetaldehyde cannot account for CVT-10216 dose dependently decreasing DA levels in the presence of alcohol. Other possibilities appear more likely. Eisenhofer and colleagues (2004) have discovered that ALDH-2 is one of several DA aldehyde metabolizing pathways in brain. Therefore, it seems possible that during treatment with CVT-10216, DA aldehyde or downstream products could be involved in suppressing DA synthesis and or release by feedback inhibition. This proposed mechanism of action remains to be established and is under active study. Despite this limitation, however, the results from several independent collaborating laboratories in this report suggest that a selective ALDH-2 inhibitor has anti-drinking properties with possible potential for treating heavy drinking and/or relapse. Moreover, CVT-10216 does not appear to have addictive properties in a CPP test when assayed at an effective therapeutic dose (15 mg/kg) (Fig. 6). We also determined that CVT-10216 does not appear to be a general inhibitor of CNS function. Thus, at therapeutic doses, CVT-10216 does not interfere with locomotor activity even in animals that have been drinking alcohol (data not shown); the rats appear healthy and active, with normal food and water intake, and normal blood pressure, heart rate, and body temperature (Tables S1–S7).
There is always a possibility that selective inhibition of ALDH-2 might interfere with general reinforcement or incentive. Thus, we find a minor decrease in lever pressing for water when CVT-10216 almost completely suppresses alcohol self-administration in iP rats (highest dose only, data not shown). However, such a minor effect on water intake in rodents does not appear to limit therapeutic usefulness in people. For example, although opioid receptor antagonists reduce water, sucrose and saccharin intake in rats, these opioid antagonists are approved to treat human alcoholics (Cichelli and Lewis, 2002). Moreover, millions of Southeast Asians with reduced or absent ALDH-2 activity appear to have normal behavior, motivation, and incentive.
Disulfiram also inhibits ALDH activity but there are serious limitations that make disulfiram an undesirable therapeutic option (Eneanya et al., 1981). Disulfiram is a non-specific inhibitor, inhibiting both mitochondrial ALDH-2 and cytosolic ALDH-1 irreversibly. Moreover, disulfiram and its major metabolite, diethydithiocarbamate, are reported to inhibit many other sulfhydryl containing enzymes and cofactors (Brady et al., 1991; Christensen, 1951). In addition, they chelate copper and zinc to inactivate metal containing enzymes, such as DBH (Goldstein et al., 1964). By contrast, CVT-10216 has a more favorable profile. CVT-10216 is a reversible and highly selective inhibitor of ALDH-2 (Fig. 1A). And, at therapeutic doses, CVT-10216 does not inhibit many other enzymes and neurotransmitter receptors when tested in CEREP (CEREP ®—Safety Profile and Diversity Express kinase profile) screens (see Supporting Information).
Human epidemiologic studies confirm that continued heavy drinking by alcoholics can cause serious organ pathology and must be avoided. However, sustained abstinence as a cure for alcoholism is increasingly considered to be an unrealistic goal in clinical practice (Gastfriend et al., 2007). Indeed, most abstinent alcoholics relapse within 12 months and current medical therapies like naltrexone and acamprosate, while beneficial, do not prevent the majority of treated alcoholics from resuming drinking (Johnson, 2008; Whitworth et al., 1996). Thus, many physicians now propose that a more realistic goal for treating alcoholism is to convert excessive drinking to moderate consumption, thereby avoiding serious pathologies caused by long-term heavy drinking (Gastfriend et al., 2007). Moreover, an added benefit to this approach is that moderate alcohol consumption may protect against coronary artery disease, apparently because alcohol is one of the few agents that increases HDL levels (Redmond et al., 2000). It is possible that selective reversible inhibitors of ALDH-2 might lead to the development of a novel therapeutic agent to help human alcoholics successfully reduce excessive drinking. Relapse is a major impediment to the successful treatment of alcoholism. ALDH-2 inhibition in this study also appears to abolish cue-induced reinstatement, a behavioral paradigm modeling relapse in human alcoholics. Thus, selective, reversible small molecule inhibitors of ALDH-2 might prevent relapse in human alcoholics without requiring increases in acetaldehyde levels. In this regard, our results suggest that selective reversible inhibition of ALDH-2 may open a new avenue of therapy for alcoholism and alcohol abuse.
Supplementary Material
Fig. S1. Double reciprocal plots: 1/vo against 1/[3-PCA] at different concentrations of (A) NAD+ and (B) CVT-10216. The best-fit equations for the straight lines obtained at different concentrations of CVT-10216 are shown on the left. (C) Secondary plots of the slopes and intercepts from (A). Slopes and Y-intercepts from (A) against NAD+ concentrations. (D) Secondary plots of the slopes and intercepts from (B). Slopes and Y-intercepts from (B) against CVT-10216 concentrations.
Table S1. CVT-10216 does not affect blood pressure in SD rats.
Table S2. CVT-10216 does not affect heart rate in SD rats.
Table S3. CVT-10216 does not affect body temperature in SD rats.
Table S4. CVT-10216 does not affect locomotor activity in LE rats.
Table S5. CVT-10216 does not affect water intake in FH rats.
Table S6. CVT-10216 does not affect total (water + alcohol) fluid intake in FH rats.
Table S7. CVT-10216 does not affect food consumption in FH rats.
Scheme S1. Kinetic mechanisms of mitochondrial ALDH2.
Scheme S2. CVT-10216 inhibits ALDH2 by binding to free enzyme and enzyme 3PCA complex.
ACKNOWLEDGMENTS
We thank T.K. Li for invaluable discussions and Z. Jiang, C. Ou, M. Santikul, T. Bajorek, T. Cothern, and E. Krstew for technical assistance and Z. Llamas for manuscript preparation at CVT. We thank R. Niculescu for acetaldehyde measurements in SD rats at TJU, NIAAA grant AA015421.
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article:
REFERENCES
- Arolfo MP, Yao L, Gordon AS, Diamond I, Janak PH (2004) Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol Clin Exp Res 28:1308–1316. [DOI] [PubMed] [Google Scholar]
- Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 191:391–431. [DOI] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW (2008) Effects of mGlu1-receptor blockade on ethanol self-administration in inbred alcohol-preferring rats. Alcohol 42:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JF, Xiao F, Wang MH, Li Y, Ning SM, Gapac JM, Yang CS (1991) Effects of disulfiram on hepatic P450IIE1, other microsomal enzymes, and hepatotoxicity in rats. Toxicol Appl Pharmacol 108:366–373. [DOI] [PubMed] [Google Scholar]
- Christensen JA (1951) Mechanism of the action of tetraethylthiuram disulfide in alcoholism; iron as antidote for the TETD-alcohol reaction. Q J Stud Alcohol 12:30–39. [PubMed] [Google Scholar]
- Cichelli MJ, Lewis MJ (2002) Naloxone nonselective suppression of drinking of ethanol, sucrose, saccharin, and water by rats. Pharmacol Biochem Behav 72:699–706. [DOI] [PubMed] [Google Scholar]
- Cowen MS, Djouma E, Lawrence AJ (2005) The mGlu5 antagonist MTEP reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther 315:590–600. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V (2007) Reward system and addiction: what dopamine does and doesn’t do [erratum appears in Curr Opin Pharmacol 7(2):233]. Curr Opin Pharmacol 7:69–76. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJ, Goldstein DS (2004) Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56:331–349. [DOI] [PubMed] [Google Scholar]
- Eneanya DI, Bianchine JR, Duran DO, Andresen BD (1981) The actions of metabolic fate of disulfiram. Annu Rev Pharmacol Toxicol 21:575–596. [DOI] [PubMed] [Google Scholar]
- Eriksson CJ (2001) The role of acetaldehyde in the actions of alcohol (update 2000). Alcohol Clin Exp Res 25 (5 Suppl. ISBRA):15S–32S. [DOI] [PubMed] [Google Scholar]
- Foddai M, Dosia G, Spiga S, Diana M (2004) Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology 29:530–536. [DOI] [PubMed] [Google Scholar]
- Garver E, Ross AD, Tu GC, Cao QN, Zhou F, Israel Y (2000) Paradigm to test a drug-induced aversion to ethanol. Alcohol Alcohol 35:435–438. [DOI] [PubMed] [Google Scholar]
- Gastfriend DR, Garbutt JC, Pettinati HM, Forman RF (2007) Reduction in heavy drinking as a treatment outcome in alcohol dependence. J Subst Abuse Treat 33:71–80. [DOI] [PubMed] [Google Scholar]
- Goldstein M, Anagnoste B, Lauber E, McKeregham MR (1964) Inhibition of dopamine-beta-hydroxylase by disulfiram. Life Sci 3:763–767. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F (1998) Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci 18:10663–10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isse T, Oyama T, Kitagawa K, Matsuno K, Matsumoto A, Yoshida A, Nakayama K, Nakayama K, Kawamoto T (2002) Diminished alcohol preference in transgenic mice lacking aldehyde dehydrogenase activity. Pharmacogenetics 12:621–626. [DOI] [PubMed] [Google Scholar]
- Johnson BA (2008) Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol 75:34–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung WM, Klyosov AA, Vallee BL (1997) Daidzin inhibits mitochondrial aldehyde dehydrogenase and suppresses ethanol intake of Syrian golden hamsters. Proc Natl Acad Sci U S A 94:1675–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung WM, Lazo O, Kunze L, Vallee BL (1995) Daidzin suppresses ethanol consumption by Syrian golden hamsters without blocking acetaldehyde metabolism. Proc Natl Acad Sci USA 92:8990–8993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung WM, Vallee BL (1993a) Daidzin and daidzein suppress free-choice ethanol intake by Syrian golden hamsters. Proc Natl Acad Sci USA 90:10008–10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung WM, Vallee BL (1993b) Daidzin: a potent, selective inhibitor of human mitochondrial aldehyde dehydrogenase. Proc Natl Acad Sci U S A 90:1247–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B (2006) The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 148:752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JH, Chen F, Krstew E, Cowen MS, Carroll FY, Crawford D, Beart PM, Lawrence AJ (2006) The GABA(B) receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats. Neuropharmacology 50:632–639. [DOI] [PubMed] [Google Scholar]
- Mann K, Lehert P, Morgan MY (2004) The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res 28:51–63. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Le AD, Noronha A (2002) Central nervous system mechanisms in alcohol relapse. Alcohol Clin Exp Res 26:280–286. [PubMed] [Google Scholar]
- Melis M, Enrico P, Peana AT, Diana M (2007) Acetaldehyde mediates alcohol activation of the mesolimbic dopamine system. Eur J Neurosci 26:2824–2833. [DOI] [PubMed] [Google Scholar]
- Morales L, Perez-Garcia C, Herradon G, Alguacil LF (2007) Place conditioning in a two- or three-conditioning compartment apparatus: a comparative study with morphine and U-50,488. Addict Biol 12:482–484. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Gardner EL (2005) Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther 108:18–58. [DOI] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Hodge CW (2000) Modulation of extracellular neurotransmitter levels in the nucleus accumbens by a taurine uptake inhibitor. Eur J Pharmacol 409:291–294. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, McArthur RA, Rezvani AH, Post C (1997) Selective inhibition of alcohol intake in diverse alcohol-preferring rat strains by the 5-HT2A antagonists amperozide and FG 5974. Alcohol Clin Exp Res 21:1448–1454. [PubMed] [Google Scholar]
- Paxinos G, Watson C (2005) The Rat Brain in Stereotaxic Coordinates, 5th edn. Elsevier, Burlington, MA. [Google Scholar]
- Quintanilla ME, Tampier L, Sapag A, Israel Y (2005) Polymorphisms in the mitochondrial aldehyde dehydrogenase gene (Aldh2) determine peak blood acetaldehyde levels and voluntary ethanol consumption in rats. Pharmacogenet Genomics 15:427–431. [DOI] [PubMed] [Google Scholar]
- Redmond EM, Sitzmann JV, Cahill PA (2000) Potential mechanisms for cardiovascular protective effect of ethanol. Acta Pharmacologica Sinica 21:385–390. [PubMed] [Google Scholar]
- Rezvani AH, Parsian A, Overstreet DH (2002) The Fawn-Hooded (FH/Wjd) rat: a genetic animal model of comorbid depression and alcoholism. Psychiatr Genet 12:1–16. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Sable HJ, Murphy JM, McBride WJ (2004) Recent advances in animal models of alcohol craving and relapse. Pharmacol Biochem Behav 79:439–450. [DOI] [PubMed] [Google Scholar]
- Samson HH (1986) Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res 10:436–442. [DOI] [PubMed] [Google Scholar]
- Suh JJ, Pettinati HM, Kampman KM, O’Brien CP (2006) The status of disulfiram: a half of a century later [see comment]. J Clin Psychopharmacol 26:290–302. [DOI] [PubMed] [Google Scholar]
- Thomasson HR, Edenberg HJ, Crabb DW, Mai XL, Jerome RE, Li TK, Wang SP, Lin YT, Lu RB, Yin SJ (1991) Alcohol and aldehyde dehydrogenase genotypes and alcoholism in Chinese men. Am J Hum Genet 48:677–681. [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Colantuoni C, Dahchour A, Quertemont E, De Witte P (1997) Acetaldehyde-induced changes in monoamine and amino acid extracellular microdialysate content of the nucleus accumbens. Neuropharmacology 36:225–232. [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF (1993) Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther 267:250–258. [PubMed] [Google Scholar]
- Whitworth AB, Fischer F, Lesch OM, Nimmerrichter A, Oberbauer H, Platz T, Potgieter A, Walter H, Fleischhacker WW (1996) Comparison of acamprosate and placebo in long-term treatment of alcohol dependence [see comment]. Lancet 347:1438–1442. [DOI] [PubMed] [Google Scholar]
- Wise RA (2002) Brain reward circuitry: insights from unsensed incentives. Neuron 36:229–240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Double reciprocal plots: 1/vo against 1/[3-PCA] at different concentrations of (A) NAD+ and (B) CVT-10216. The best-fit equations for the straight lines obtained at different concentrations of CVT-10216 are shown on the left. (C) Secondary plots of the slopes and intercepts from (A). Slopes and Y-intercepts from (A) against NAD+ concentrations. (D) Secondary plots of the slopes and intercepts from (B). Slopes and Y-intercepts from (B) against CVT-10216 concentrations.
Table S1. CVT-10216 does not affect blood pressure in SD rats.
Table S2. CVT-10216 does not affect heart rate in SD rats.
Table S3. CVT-10216 does not affect body temperature in SD rats.
Table S4. CVT-10216 does not affect locomotor activity in LE rats.
Table S5. CVT-10216 does not affect water intake in FH rats.
Table S6. CVT-10216 does not affect total (water + alcohol) fluid intake in FH rats.
Table S7. CVT-10216 does not affect food consumption in FH rats.
Scheme S1. Kinetic mechanisms of mitochondrial ALDH2.
Scheme S2. CVT-10216 inhibits ALDH2 by binding to free enzyme and enzyme 3PCA complex.


