Abstract
The impact of COVID-19 has been largely described after symptom development. Although the SARS-CoV-2 virus elevates heart rate (HR) prior to symptom onset, whether this virus evokes other presymptomatic alterations is unknown. This case study details the presymptomatic impact of COVID-19 on vascular and skeletal muscle function in a young woman [24 yr, 173.5 cm, 89 kg, body mass index (BMI): 29.6 kg·m–2]. Vascular and skeletal muscle function were assessed as part of a separate study with the first and second visits separated by 2 wk. On the evening following the second visit, the participant developed a fever and a rapid antigen test confirmed a positive COVID-19 diagnosis. Compared with the first visit, the participant presented with a markedly elevated HR (∼30 beats/min) and a lower mean blood pressure (∼8 mmHg) at the second visit. Vascular function measured by brachial artery flow-mediated dilation, reactive hyperemia, and passive leg movement were all noticeably attenuated (25%–65%) as was leg blood flow during knee extension exercise. Muscle strength was diminished as was ADP-stimulated respiration (30%), assessed in vitro, whereas there was a 25% increase in the apparent Km. Lastly, an elevation in IL-10 was observed prior to symptom onset. Notably, 2.5 mo after diagnosis symptoms of fatigue and cough were still present. Together, these findings provide unique insight into the physiological responses immediately prior to onset of COVID-19 symptoms; they suggest that SARS-CoV-2 negatively impacts vascular and skeletal muscle function prior to the onset of common symptoms and may set the stage for the widespread sequelae observed following COVID-19 diagnosis.
NEW & NOTEWORTHY This unique case study details the impact of SARS-CoV-2 infection on vascular and skeletal muscle function in a young predominantly presymptomatic woman. Prior to COVID-19 diagnosis, substantial reductions in vascular, skeletal muscle, and mitochondrial function were observed along with an elevation in IL-10. This integrative case study indicates that the presymptomatic impact of COVID-19 is widespread and may help elucidate the acute and long-term sequelae of this disease.
Keywords: blood flow, endothelium, mitochondria, muscle strength, SARS-CoV-2 virus
INTRODUCTION
The impact of COVID-19 on health and physiology has been well described and is characterized by systemic inflammation, endothelial damage and injury, vascular thrombosis, and end-organ damage (1–7). However, the time course over which pathophysiological alterations occur following SARS-CoV-2 exposure remains less clear. Acute symptoms of COVID-19 including fever, dry cough, fatigue, and body aches typically manifest within 5 to 7 days after exposure (6–9). Interestingly, the physiological impact of COVID-19 actually likely occurs days before symptom development. Indeed, several reports using wearable technology indicate substantial changes in heart rate (HR), sleep pattern, and physical activity before symptom onset (10, 11). In fact, retrospective analysis of HR confirmed the presence of COVID-19 in the majority of participants (10). These findings provide unique insight into the presymptomatic impact of COVID-19, but are limited by the capability of a wearable device. Understanding the presymptomatic impact of COVID-19 on multiple physiological systems may help elucidate the organ system(s) that initiate and contribute to acute and long-term COVID-19 sequelae.
Guidelines for quarantining following positive confirmation of COVID-19 require patients to remain isolated for up to 14 days or until symptoms subside (12). Similarly, based on current CDC guidelines, asymptomatic patients and those with a known exposure to COVID-19 are to remain quarantined for a minimum of 7 days (12). These precautions are critical to limit the spread of the highly contagious virus. However, as a consequence of these actions, unless being monitored in an inpatient setting, the physiological impact of the virus before symptom onset has remained largely undescribed. The current case study, in a young woman, provides a unique opportunity to examine the vascular and skeletal muscle impact of COVID-19 infection before the development of symptoms.
It should be noted that although this young woman did not require hospitalization, she reported being bedridden for 4 days after her diagnosis due to fever, body aches, and fatigue. Moreover, 2.5 mo post diagnosis and at the time of documenting this case study, she was still experiencing persistent symptoms, including intermittent coughing and fatigue indicating a likely case of postacute sequelae of COVID-19 (PASC).
METHODS
The study was approved by the Institutional Review Board at the University of Utah and the Salt Lake City Veterans Affairs Medical Center (SLC VAMC), and written informed consent was obtained from the participant. All testing reported herein was performed at the Utah Vascular Research Laboratory located at the SLC VAMC, with the exception of the muscle strength assessments, which were performed at the Skeletal Muscle Exercise Rehabilitation Facility located at the University of Utah.
The participant (female, 24 yr, 173.5 cm, 89 kg, BMI = 29.6 kg·−2) volunteered and enrolled in an ongoing investigation examining the impact of limb immobilization on vascular and skeletal muscle function. A health history performed during the consent by standard questionnaire indicated that the participant was generally healthy, free from overt disease, and occasionally active (exercising 1–2 times per week at a mild effort (walking/hiking) for 15 to 30 min). In terms of medications, the participant only reported taking sertraline, a selective serotonin reuptake inhibitor, for general anxiety. This double-blinded placebo-controlled study involves four laboratory visits: a preliminary visit and three experimental visits (V1, V2, and V3), each separated by 2 wk, with limb immobilization occurring between V2 and V3. The participant completed V1 and V2, which were the baseline and placebo condition, respectively, for this participant. During the preliminary visit, a health history was attained by a questionnaire and height and body mass were measured. In addition, the participant was familiarized with knee extension exercise for 5 min, and following a brief rest period (5 min), performed a graded maximal knee extension exercise test until exhaustion. During this test, work rate (WR) was increased every minute by 4 watts starting with unloaded (0 watts) exercise. Verbal encouragement occurred throughout the test and the end-point was determined by an inability to maintain the required cadence (60 rpm) and power output. WRmax for this participant was documented to be 40 watts. Subsequent knee extension exercise during V1 and V2 were performed at 40%, 60%, and 80% of WRmax.
On the evening following V2, the participant developed fever and body aches. That same evening, a rapid COVID-19 antigen test, performed at a community clinic, confirmed a positive COVID-19 infection. It should be noted that on the morning of V2 the participant reported being in good health (i.e., asymptomatic) during the baseline hemodynamic measures, blood collection, vascular function, and exercise blood flow assessments. During the isometric and isokinetic muscle strength testing, the participant reported an underlying feeling of mild fatigue, which, in retrospect, may have been the first indication of illness.
Experimental Protocol and Measurements
Resting blood pressure and heart rate.
During V1 and V2, following an overnight fast, the participant reported to the laboratory at 0800 and comprehensive assessments of vascular and skeletal muscle function were performed. Timing of all assessments was consistent across visits. Upon arrival at the laboratory, body mass was, again, assessed and the participant was instrumented in the seated position. Blood pressure (BP) was then measured following 5 min of uninterrupted sitting in triplicate using an automated sphygmomanometer (Tango M2, SunTech) and heart rate (HR) was assessed by a 3-lead ECG (AcqKnowledge, BIOPAC Systems).
Vascular function.
Passive leg movement (PLM) and single PLM (sPLM) were performed as previously described (13–15). PLM and sPLM were performed in an upright seated posture and consisted of passive knee flexion extension through ∼90° range of motion at 1 Hz for 60 s for PLM and 1 s for sPLM. Baseline hemodynamic measurements were recorded for 60 s with the experimental leg (left) supported at a 180° knee joint angle. Passive movement-induced hemodynamic measurements were recorded for 60 s following the onset of PLM and sPLM. The participant was instructed to remain relaxed during the protocol, and the contralateral leg was supported at 180° knee joint angle and remained motionless during PLM and sPLM. To avoid a startle reflex and active resistance to passive movement, the participant was informed that the movement would take place in ∼1 min, but was not informed of the exact timing of the movement to minimize the chance of an anticipatory response. The same member of the research team moved the leg for V1 and V2 and verified the absence of resistance (or muscle contraction) during the maneuvers. PLM and sPLM were performed in duplicates and the average was reported. The repeated PLM and sPLM trials were separated by 5 and 3 min of rest, respectively.
Brachial artery flow-mediated dilation (FMD) was performed in the supine position according to guidelines published by our group (16). Briefly, FMD was performed with a blood pressure cuff placed on the right arm, just distal to the elbow. Cuff inflation and deflation was achieved using a rapid inflation system (D.E. Hokanson, Inc.). The FMD protocol consisted of 30 s of baseline data acquisition before inflation of the pressure cuff (to 250 mmHg for 5 min) and subsequent data acquisition for 2 min after cuff deflation.
Blood analyses.
Venous blood samples were drawn from the antecubital region for blood assays and processed to isolated serum and plasma. Serum and plasma were stored at −80°C until analysis. Standard blood chemistry (CHEM 14 and lipid panel) was analyzed by the Veteran Affairs blood laboratory and a complete cytokine panel was analyzed by ARUP Laboratories.
Muscle biopsy.
A muscle sample from the vastus lateralis of the left leg, 15 cm proximal to the knee at a depth of ∼3.5 cm, was obtained using a Bergstrom needle with suction (17), under sterile conditions, following local anesthesia (1% xylocaine), on both V1 and V2. Immediately after sample collection, ∼15 mg of the muscle was immersed in ice-cold biopsy preservation fluid (BIOPS) [containing (in mM) 2.77 CaK2EGTA, 7.23 K2EGTA, 6.56 MgCl2, 0.5 DTT, 50 K-MES, 20 imidazole, 20 taurine, 5.77 Na2ATP, and 15 phosphocreatine, pH 7.1 at 4°C], before commencing the permeabilization procedure.
Mitochondrial bioenergetics.
Muscle samples were prepared and permeabilized as described by Pesta and Gnaiger (18). Briefly, the BIOPS-immersed samples were carefully separated with fine-tip forceps and subsequently bathed and shaken in a BIOPS with saponin solution (50 mg/mL) for 30 min. Following saponin treatment, samples were rinsed twice in mitochondrial respiration medium (MiR05) [containing (in mM) 110 sucrose, 60 lactobionic acid, 0.5 EGTA, 3 MgCl2, 20 Taurine, 10 KH2PO4, 20 HEPES, 1 g/L BSA, pH 7.1)] for a total of 20 min. Analysis of mitochondrial bioenergetics was performed in duplicate, as previously described (18–20). Briefly, O2 consumption was determined using an Oxygraph-2K (Oroboros Instruments, Innsbruck, Austria) in 2 mL MiR05 solution during 1) an ADP titration [substrates: succinate, pyruvate, malate, and ADP (sequential doses: 12.5, 25, 175, 250, 500, 1,000, 2,000, and 4000 µM)] and 2) a maximal respiration protocol (substrates: malate, glutamate, ADP, succinate). Cytochrome C was used to verify membrane integrity. Bioenergetic assessments were performed at 37°C following mild hyperoxygenation. Respiration was normalized to sample wet weight.
Knee extension exercise and Doppler ultrasound assessments.
Knee extension exercise was performed on a custom-built ergometer. Baseline hemodynamics were recorded for 2 min and knee extension exercise was performed at 40%, 60%, and 80% of WRmax. Each exercise intensity was maintained for 3 min and 1 min of recovery was provided between exercise bouts. Mean arterial pressure (MAP) during exercise was measured beat-by-beat by finger photoplethysmography (Finapres Medical Systems, Amsterdam, The Netherlands) and by automated plethysmography in the upper arm. The reported MAP is the average of these two methods.
Leg blood flow was measured according to recently published guidelines (21) with Doppler ultrasound (Logic e9, General Electric Medical Systems) operating in duplex mode equipped with a linear array transducer functioning at an imaging frequency of 15 MHz. The measurement of internal artery diameter was performed during the peak of the R-wave and averaged over five measurements. Blood velocity was assessed with the same probe operating at a Doppler frequency of 5 MHz operating in the high-pulse repetition frequency mode (2–25 mHz). Blood velocity was assessed with an insonation angle of 60° and sample volume was maximized and centered according to vessel size. Leg blood flow was calculated as [(mean blood velocity) × π (vessel radius)2 60 s] and the average of the final minute of each workload is reported.
Isometric and isokinetic muscle strength.
Following 5 min of warm up (semirecumbent cycling at 25 watts, 60 rpm), isometric strength was assessed with a maximal 3-s voluntary contraction (MVC) of the knee extensors (at a 60° knee angle) on a HUMAC NORM (CSMi) dynamometer. Three MVCs were performed, separated by 1 min of rest. Maximal isokinetic torque production during knee extension and knee flexion was assessed at 180°·s−1, 120°·s−1, and 60°·s−1. A set of three contractions per angular velocity was performed, each separated by 1 min of rest. The repetition yielding the highest torque value from both isometric and isokinetic tests were identified and reported.
RESULTS
Baseline Hemodynamics
Blood pressure (systolic/diastolic) and MAP, following 5 min of seated rest, were 117/78, and 91 mmHg, respectively, at V1, and was consistently lower 108/71, and 83 mmHg, respectively, at V2. Baseline HR was 67 beats/min at V1 and was elevated to 96 beats/min at V2. Leg blood flow through the common femoral artery was 492 mL/min at V1 and was elevated to 591 mL/min at V2. Arm blood flow, measured in the brachial artery, was 44 mL/min at V1 and 55 mL/min at V2.
Vascular Function
Baseline brachial artery diameter was 4.08 mm at V1 and 4.04 mm at V2. Brachial artery FMD was diminished by 57% from V1 to V2 (Table 1). In addition, reactive hyperemia following occlusion cuff release was decreased by 25% from V1 to V2. The FMD normalized for shear stress was 33% lower from V1 to V2.
Table 1.
Vascular function for a young woman during a control visit (V1) and 2 wk later immediately preceding a positive COVID test (V2)
| Assessment of Vascular Function | V1 | V2 |
|---|---|---|
| Brachial artery flow-mediated dilation (FMD) | ||
| FMD, % | 3.0 | 1.3 |
| Reactive hypermia, mL | 362 | 270 |
| FMD/shear rate, au | 0.066 | 0.044 |
| Single passive leg movement (sPLM) | ||
| Peak Δ leg blood flow, mL/min | 123 | 43 |
| Leg blood flow AUC, mL | −70 | −115 |
| Passive leg movement (PLM) | ||
| Peak Δ leg blood flow, mL/min | 441 | 189 |
| Leg blood flow AUC, mL | −31 | −202 |
AUC, area under the curve.
The peak change in hyperemia evoked by PLM and sPLM was reduced by 57% and 65%, respectively, from V1 to V2 (Table 1). In line with these changes, the overall hyperemic response to both PLM and sPLM was markedly diminished from V1 to V2 (Table 1).
Skeletal Muscle Mitochondrial Function
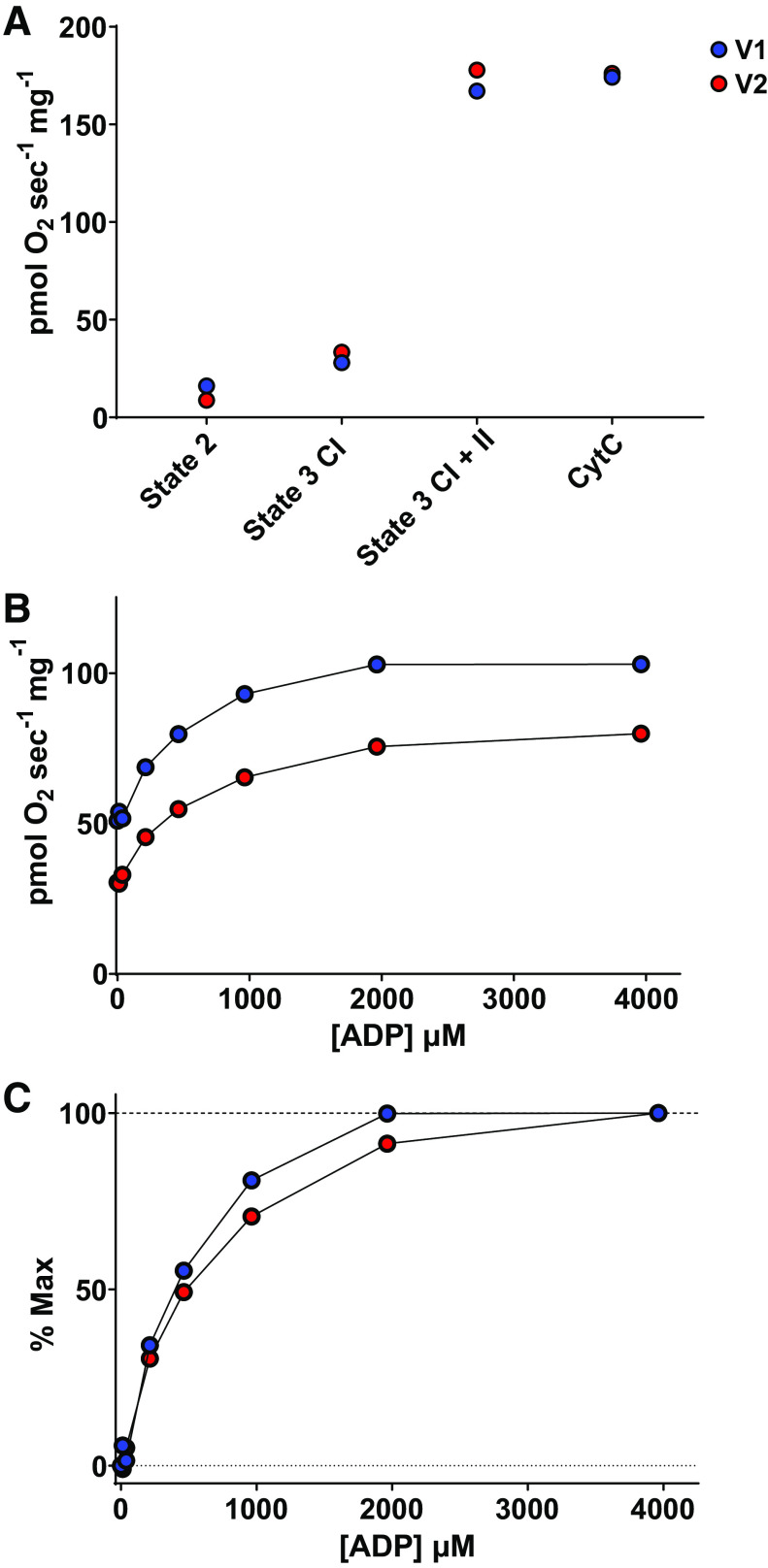
Mitochondrial respiration rates at state 2 (leak respiration), state 3 complex I, and state 3 complex I + II, were not noticeably altered from V1 to V2 (Fig. 1A). However, compared with V1, mitochondrial respiration during the ADP titration protocol was 30% lower at each ADP concentration and the apparent Km was 25% greater during V2 (502 vs. 630 μM) (Fig. 1, B and C).
Figure 1.
Mitochondrial bioenergetics. O2 kinetics were assessed in permeabilized skeletal muscle fibers during a control visit (visit 1, blue) and 2 wk later, immediately preceding a positive COVID-19 diagnosis (visit 2, red). A: mitochondrial respiration rates during states 2 and 3, and in the presence of cytochrome C. B: mitochondrial respiration rates during ADP titration. C: mitochondrial respiration rates during ADP titration expressed as a percentage of maximal respiration rates.
Central Hemodynamics and Blood Flow during Knee Extension Exercise
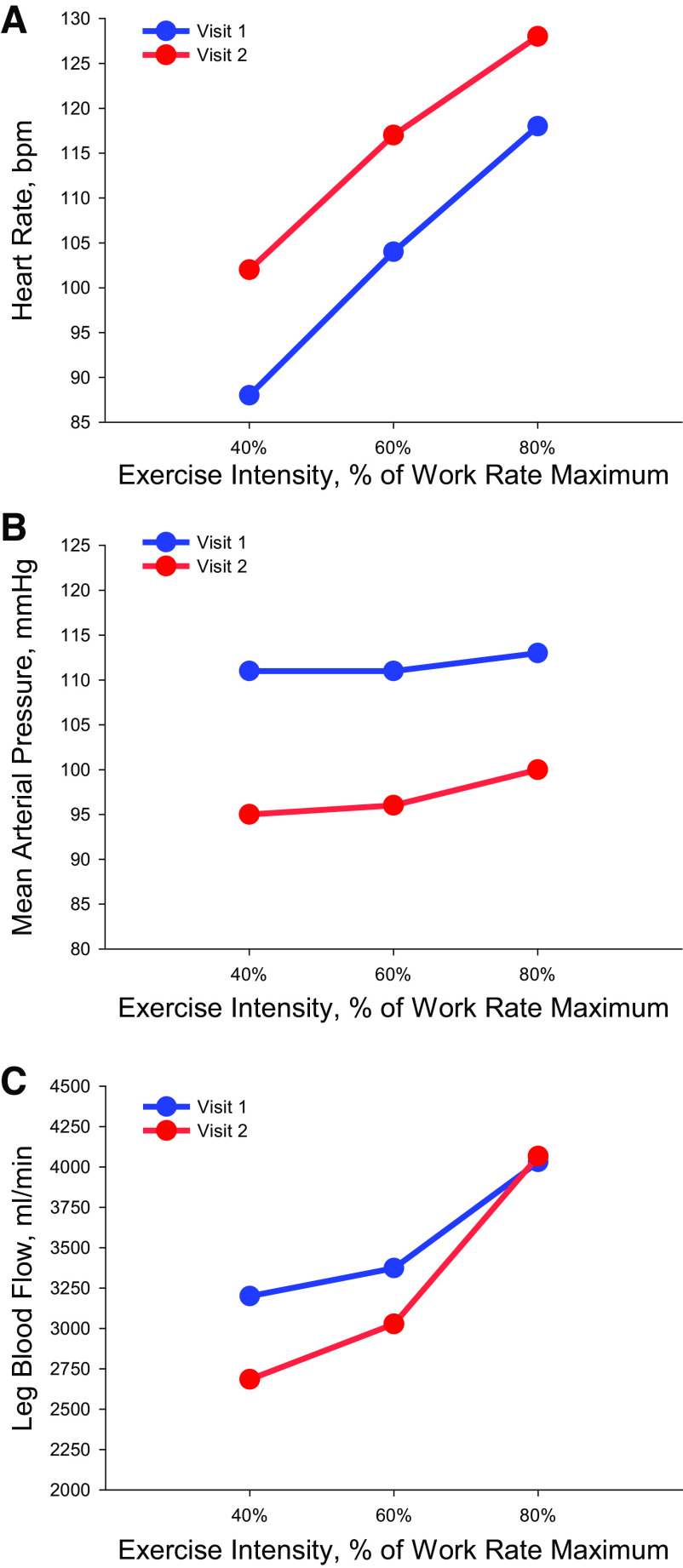
Compared with V1, HR during knee extension exercise at V2 was elevated at all intensities (range: 10–14 beats/min) and MAP was, also, lower (range: −13 to −16 mmHg) (Fig. 2). Furthermore, compared with V1, leg blood flow was lower during 40% (−516 mL/min) and 60% (−346 mL/min) of WRmax, whereas leg blood flow appeared similar between V1 and V2 at 80% WRmax (Fig. 2).
Figure 2.
Central and peripheral hemodynamics during knee extension exercise. Knee extension was performed at 40%, 60%, and 80% of work rate maximum during a control visit (visit 1, blue) and 2 wk later, immediately preceding a positive COVID-19 diagnosis (visit 2, red). Heart rate (A), mean arterial pressure (B), and leg blood flow (C) during knee extension exercise.
Skeletal Muscle Function
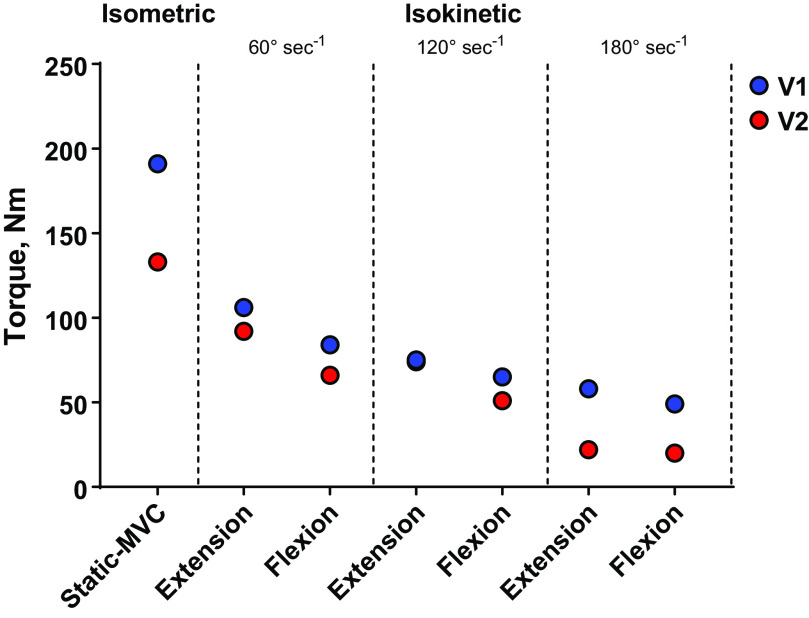
Maximal muscle strength assessed during a 3-s isometric maximal voluntary contraction was decreased by 30% from V1 to V2 (Fig. 3). Reductions in isokinetic torque during knee extension and knee flexion were most evident at the fastest contraction velocity, 180°·s−1, whereas smaller changes in isokinetic strength were observed at 60°·s−1 and 120°·s−1 (Fig. 3).
Figure 3.
Isometric and isokinetic strength during a control visit (visit 1, blue) and 2 wk later, immediately preceding a positive COVID-19 diagnosis (visit 2, red). Isometric strength was assessed during a static maximal voluntary contraction (MVC). Isokinetic tests were performed at 60°·s−1, 120°·s−1, and 180°·s−1. Knee extension and flexion assessments are presented.
Blood Characteristics and Cytokines
Results from the standard blood chemistry assessment, complete metabolic panel, and cytokine panel are presented in Table 2. Of note, the participant presented with total cholesterol (V1) and triglycerides (V1 and V2) levels outside of the general reference range. Slight reductions were also observed for hemoglobin and hematocrit from V1 to V2 and aspartate transaminase (AST) and mean corpuscular hemoglobin concentration (MCHC) levels were slightly below the reference range at V2. TNF-α was elevated above the general reference range at both V1 and V2. IL-10 increased from V1 (<2.8 pg/mL) to V2 (11.8 pg/mL). No other cytokines were appreciably altered between visits.
Table 2.
Blood chemistry for a young woman during a control visit (V1) and 2 wk later immediately preceding a positive COVID test (V2)
| V1 | V2 | Reference Range | |
|---|---|---|---|
| Metabolic panel | |||
| Glucose, mg/dL | 100 | 84 | (74–106) |
| Sodium, mmol/L | 139 | 137 | (137–145) |
| Potassium, mmol/L | 4.3 | 3.9 | (3.8–5.2) |
| Chloride, mmol/L | 105 | 104 | (100–108) |
| CO2, mmol/L | 24 | 24 | (22–32) |
| Creatinine, mg/dL | 0.77 | 0.8 | (0.57–1.25) |
| Urea nitrogen, mg/dL | 7 | 10 | (7–18) |
| Calcium, mg/dL | 9.3 | 9.1 | (8.2–10.3) |
| Total protein, g/dL | 7.6 | 7.3 | (6.4–8.3) |
| EGFR | 92 | 88 | (>60) |
| Albumin, g/dL | 4.7 | 4.5 | (3.2–5.5) |
| Total bilirubin, mg/dL | 0.8 | 1 | (0.2–1.0) |
| Alkaline phosphate, IU/L | 78 | 76 | (50–136) |
| AST, IU/L | 33 | 13* | (15–37) |
| ALT, IU/L | 38 | 12 | (9–77) |
| Lipid panel | |||
| Cholesterol, mg/dL | 210* | 188 | (118–200) |
| Triglycerides, mg/dL | 228* | 191* | (30–150) |
| HDL, mg/dL | 39 | 41 | (35–72) |
| LDL, mg/dL | 149 | 141 | (<160) |
| Cholesterol:HDL | 5.4 | 4.6 | |
| Anion gap, mmol/L | 10 | 9 | (7–15) |
| Complete blood count | |||
| White blood cell, K/µL | 4.85 | 4.32 | (3.7–8.4) |
| Red blood cell, M/µL | 4.59 | 4.17 | (4.0–5.6) |
| Hemoglobin, g/dL | 12.7 | 11.7* | (12.1–52.5) |
| Hematocrit, % | 39.8 | 36.3* | (37.1–52.5) |
| MCV, fL | 86.7 | 87.1 | (81–101) |
| MCH, pg | 27.7 | 21.8 | (27–36) |
| MCHC, g/dL | 31.9* | 32.2* | (32.9–97) |
| PLT, K/µL | 254 | 218 | (145–439) |
| RDW-CV, % | 14.4 | 14.2 | (11.2–14.5) |
| MPV, fL | 9.5 | 9.4 | (8.9–10.9) |
| Cytokine and inflammatory panel | |||
| TNF-α, pg/mL | 10.3* | 7.9* | (<7.2) |
| IL-2, pg/mL | <2.1 | <2.1 | (<2.1) |
| IL-12, pg/mL | <1.9 | <1.9 | (<1.9) |
| INFγ, pg/mL | <4.2 | <4.2 | (<4.2) |
| IL-4, pg/mL | <2.2 | <2.2 | (<2.2) |
| IL-5, pg/mL | <2.1 | <2.1 | (<2.1) |
| IL-10, pg/mL | <2.8 | 11.8* | (<2.8) |
| IL-13, pg/mL | <1.7 | <1.7 | (<1.7) |
| IL-17, pg/mL | <1.4 | <1.4 | (<1.4) |
| IL-1β, pg/mL | <6.5 | <6.5 | (<6.5) |
| IL-6, pg/mL | <2 | <2 | (<2) |
| IL-8, pg/mL | <3 | <3 | (<3) |
ALT, alanine transaminase; AST, aspartate transaminase; EGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; IL, interluekin; INF, interferon; LDL, low-density lipoprotein; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; MPV, mean platelet volume; PLT, platelets; RDW-CV, red blood cell distribution width; TNF, tumor necrosis factor. *indicates values outside the reference range.
DISCUSSION
This case study includes several interesting observations regarding the presymptomatic impact of COVID-19 on vascular and skeletal muscle function. Just before the onset of COVID-19 symptoms, the participant had an elevated HR, decreased blood pressure, attenuated leg blood flow during submaximal knee extension exercise, impaired macrovascular and microvascular function, diminished submaximal mitochondrial respiration, diminished muscle strength, and a substantial elevation in IL-10. Overall, the magnitude of these widespread alterations in vascular and skeletal muscle function are noteworthy and provide unique insight into the early pathophysiological impact of COVID-19.
Baseline Hemodynamics
An elevated HR during hospitalization and convalescence from COVID-19 appears to be common (8, 9, 22). In contrast, hypotension has, generally, only been reported in severe COVID-19 cases (22). However, it should be noted that blood pressure before hospital admission is often unknown and, therefore, the diagnosis of hypotension is based on absolute, not relative, blood pressure in such a clinical setting. Therefore, patients with preexisting hypertension may present with normal BP upon admission, which would exclude a hypotension diagnosis. In the current case, the participant’s HR was elevated by ∼30 beats/min and MAP reduced by ∼8 mmHg from V1 to V2 suggesting that these substantial physiological changes occur early following SARS-CoV-2 exposure. Several investigations using wearable technology (smartwatches with HR assessment) have reported similar presymptomatic elevations in HR (10, 11). Interestingly, retrospective analysis of these data indicated that the presymptomatic elevation in HR accurately predicted the diagnosis of COVID-19 in 81% of participants (10). A presymptomatic elevation in HR does not appear to be specific to COVID-19, but rather a general sign of respiratory illness (10, 23). The factors contributing to these presymptomatic changes in HR and BP are not entirely clear, but may be related to the early inflammatory response, alterations in autonomic function, and the interplay between the immune and parasympathetic nervous systems (24).
Vascular Function and Blood Flow
Compelling evidence indicates that vascular endothelial dysfunction mediates the widespread organ damage/dysfunction associated with COVID-19 (2, 3, 5, 25, 26). The SARS-CoV-2 virus enters tissues through the angiotensin-converting enzyme 2 (ACE2) receptor, which is robustly expressed by endothelial cells (2, 26–30), and induces free radical and cytokine storms that markedly increase oxidative stress and inflammation (31–33). This pathophysiological activation of the endothelium leads to morphological and biochemical changes that contribute to a procoagulant, proinflammatory, and prooxidant state resulting in endothelial injury and evoking alterations in systemic perfusion and oxygen delivery (5, 34–37). In the current case study, multiple assessments of vascular endothelial function, including FMD, reactive hyperemia, and PLM-induced hyperemia were lower at V2, when the young woman was predominantly presymptomatic. Recent work by Ratchford et al. (38) reported similar and marked reductions in FMD and PLM in young healthy men and women 3–4 wk after mild-to-moderate infection. Together, these findings suggest that vascular dysfunction is likely an early pathophysiological response to COVID-19, evident across macrovascular and microvascular compartments, and a critical component of COVID-19 sequelae that persists for weeks after infection. Whether these effects last for months (or even years) after COVID-19 is unknown, but this is currently the focus of a number of ongoing studies.
The precise mechanism for diminished vascular function in this predominantly presymptomatic patient is unknown, but may be related to the elevated IL-10 level. Increased IL-10 has been linked to attenuated FMD and an elevated risk of cardiovascular events (39). Of note, the current participant exhibited a rather low FMD at V1, which may be partially explained by her BMI, as well as her elevated cholesterol, triglyceride, and TNF-α levels (40, 41). Despite this low FMD, the impact of SARS-CoV-2 infection appears to be quite impactful as demonstrated by the ∼50% attenuation in FMD response observed during the second visit.
The observed reduction in leg blood flow during knee extension further highlights the critical involvement and impact of COVID-19 on the vasculature. Interestingly, leg blood flow was only altered during 40% and 60% of WRmax. As the metabolic and oxygen demand of exercise increases, the regulation of blood flow to the active skeletal muscle is progressively more dominated by metabolically derived vasodilatory mechanisms acting to match oxygen delivery and demand (42). In contrast, a greater variety of mechanisms may be in play and contributing to the decreased blood flow during lower intensity exercise (43). COVID-19 is associated with increased sympathetic activity, which results in peripheral vasoconstriction. We, and others, have consistently reported that age-related and disease-related increases in sympathetic activity are associated with diminished blood flow, especially at lower exercise intensities (44–46). As exercise intensity increases, local factors overcome this sympathetically mediated vasoconstriction, a process termed as functional sympatholysis (47), potentially resulting in preserved blood flow at higher exercise intensities, even with COVID-19. In addition, COVID-19 is associated with elevated angiotensin II (6), which we have previously reported to be involved with the potentiation of the α-adrenergic pathway that leads to augmented vasoconstriction and impaired blood flow during knee extension exercise (48). It should be noted that these proposed mechanisms, although supported by previous research, are speculatory and require additional investigation in the context of COVID-19.
Mitochondrial Respiration
Mitochondria have garnered increased attention as a potential target of SARS-CoV-2, due to the role of mitochondria in the immune response to viral infections and their potential role as the source of oxidative stress during infection (49, 50). Interestingly, alterations in mitochondrial function from peripheral blood mononuclear cells in patients admitted to the ICU following COVID-19 have been reported. Specifically, the respiratory capacity of these cells was attenuated and there was an increased reliance on glycolysis (51). To the best of our knowledge, the current direct assessment of skeletal muscle mitochondrial respiration is the first to be reported in a patient with a predominantly presymptomatic SARS-CoV-2 infection, and, potentially, for any patient with COVID-19. There was a 30% attenuation in ADP-stimulated respiration during the ADP titration protocol, which was accompanied by a 25% increase in the apparent Km, with the latter likely being indicative of an attenuated sensitivity to ADP, although alterations in the phosphate to oxygen ratio (P:O) cannot be assured. These observations may also indicate an increased reliance on glycolysis for skeletal muscle mitochondria, in line with the findings in peripheral mononuclear cells (51). However, it must be acknowledged that we did not explicitly test this hypothesis and did not directly assess the rate of ATP synthesis. Of note, there were no differences in oxidative phosphorylation during the maximal respiration protocol suggesting that structural changes and/or a loss of mitochondrial respiratory complexes are not involved in the observed decrease in submaximal ADP-stimulated respiration. The current findings could indicate that the early onset of SARS-CoV-2 infection induces rapid changes in skeletal mitochondrial function and substrate utilization, but is insufficient to change the maximal capacity of the mitochondria (with supraphysiological substrate concentrations). This observation highlights an important consideration for future COVID-19 research, as the skeletal muscle represents the largest metabolically active tissue in the body and has the greatest mitochondrial mass.
Muscle Strength
The expression of ACE2 and transmembrane protease serine 2 (TMPRSS2) in cells present in skeletal muscle indicates that SARS-CoV-2 may enter and impact skeletal muscle cells (1). The functional outcome of SARS-CoV-2 on the skeletal muscle remains largely unexplored and is difficult to dissociate from activity-induced alterations in skeletal muscle and indirect effects from systemic inflammation and oxidative stress. Generalized muscle pain and weakness have been reported in symptomatic patients with COVID-19, but, again, this may be attributed to indirect effects or inactivity (52–54). Following severe SARS infections, a sustained attenuation in muscle strength (32% loss in grip strength) and endurance capacity (13% loss in 6-min walk distance), associated with deconditioning and sustained inflammation, have been reported (55). Post COVID-19, a fall in handgrip MVC has also been reported in older patients with severe cases of the disease (56). The markedly lower muscle strength reported in this case study (∼30% reduction in MVC) is rather surprising considering the timing of this assessment (predominantly presymptomatic) and overall lack of notable inflammation. It should be noted that the participant stated feeling generally fatigued during the strength measurements, possibly the first indication of symptom onset, which may explain some of the observed decrements in muscle force production. Indeed, our understanding of the neuropathogenesis of COVID-19 is evolving and early evidence suggest that COVID-19 may impact both central and peripheral processes involved in muscle recruitment, activation, and ultimately fatigue.
Presymptomatic Immune Response
SARS-CoV-2 infection, leading to COVID-19, evokes a robust innate immune response characterized by proinflammatory cytokine release and concomitant widespread and systemic impact (6, 7, 57). In the current study, blood was collected before symptom onset and the well-characterized increase in proinflammatory cytokines was not observed, likely a result of the timing of the blood collection and/or the more mild case of COVID-19, as hospitalization was not required. Interestingly, an increase in IL-10 levels was observed. IL-10 has routinely been considered an anti-inflammatory cytokine (58); however, a rapid increase in IL-10 appears to be a distinguishing feature of COVID-19 (7, 59–62). Although not completely understood, the rapid increase in IL-10 may serve as a negative feedback mechanism to counter inflammation associated with other proinflammatory mediators. As the level of IL-10 increases, it may act as a proinflammatory immune activating signal that stimulates the production of other cytokines (60). Several reports support a pathological role of IL-10 in COVID-19 disease severity (59, 61). Based on these previous reports, the IL-10 level observed in this research participant would place her in the severe to critical range; however, these previous reports were obtained from hospitalized patients with IL-10 levels measured during hospitalization. As already noted, although the participant did not require hospitalization, she reported not being able to get out of bed for 4 days after her diagnosis due to fever, body aches, and fatigue. Moreover, at the time of writing this case study she was 2.5 mo post diagnosis and still experiencing persistent symptoms, including intermittent coughing and fatigue, indicating a likely case of PASC.
Limitations
Several important limitations warrant discussion. The physiological assessments employed possess inherent variability. For instance, the coefficient of variation for FMD averages ∼15% (16, 63–66), which is greatly improved when strict guidelines, as performed in our study, are followed (67). Likewise, the assessment of MVC over multiple testing sessions has a similar, albeit, slightly lower coefficient of variation (5%–11%) (68). The magnitude of change in our vascular function assessments, coupled with consistent changes across several complementary tests (FMD, reactive hyperemia, and PLM), lend credence to the findings reported in this case study. In addition, the observed changes in resting HR and vascular function agree with previous reports and the pathophysiological manifestation of COVID-19 (10, 11). Circulating markers of endothelial function and endotheliopathy were not assessed in this case study, which limits direct comparison with recent studies reporting elevated markers of endothelial injury and endotheliopathy in patients with critical COVID-19 infection (69, 70). Further investigation examining endothelial responses to COVID-19 are certainly warranted, as current data indicates that functional measures of vascular endothelial dysfunction (FMD, hyperemia, and PLM) are impaired in mild cases of COVID-19 (38), whereas elevated markers of endothelial injury and endotheliopathy may be reserved for critical cases of COVID-19 (69, 70). Lastly, due to significant fatigue and intermittent bouts of vigorous coughing, the participant opted out of additional laboratory-based follow up, which precluded the collection of additional, potentially very insightful data regarding COVID-19 and PASC.
Conclusions
This case study provides unique insight into the predominantly presymptomatic impact of SARS-CoV-2 on vascular and skeletal muscle function. Prior to the development of overt COVID-19-related symptoms, the participant presented with substantial alterations in baseline hemodynamics, including an elevated heart rate and attenuated blood pressure. Notable impairments were observed in macrovascular and microvascular functions, mitochondrial respiration, and muscle strength. In addition, IL-10 was elevated before the participant developing fever and body aches. Together, the findings of this case study suggest that SARS-CoV-2 infection imparts widespread physiological alterations before the development of common COVID-19 symptoms that may contribute to the future systemic sequelae of the virus.
GRANTS
This study was supported, in part, by the National Institutes of Health National Heart, Lung, and Blood Institute Grants R01HL142603 (to J.D.T.), R01HL142804, R35HL145237, and R01HL103541 (to M.T.R.), by the US Department of Veterans Affairs Clinical Science Research and Development Merit Awards I01CX001999 (to J.D.T) and I01CX001696 (to M.T.R), and by the US Department of Veterans Affairs Senior Research Career Scientist Award E9275-L (to R.S.R).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.D.T. conceived and designed research; J.D.T., J.C.C., C.C.F., A.I.M., M.T.L., and S.H.P. performed experiments; J.D.T., J.C.C., C.C.F., A.I.M., M.T.L., and S.H.P. analyzed data; J.D.T., J.C.C., C.C.F., A.I.M., M.T.L., S.H.P., M.T.R., and R.S.R. interpreted results of experiments; J.D.T., J.C.C., and A.I.M. prepared figures; J.D.T. drafted manuscript; J.D.T., J.C.C., C.C.F., A.I.M., M.T.L., S.H.P., M.T.R., and R.S.R. edited and revised manuscript; J.D.T., J.C.C., C.C.F., A.I.M., M.T.L., S.H.P., M.T.R., and R.S.R. approved final version of manuscript.
REFERENCES
- 1.Disser NP, De Micheli AJ, Schonk MM, Konnaris MA, Piacentini AN, Edon DL, Toresdahl BG, Rodeo SA, Casey EK, Mendias CL. Musculoskeletal consequences of COVID-19. J Bone Joint Surg Am 102: 1197–1204, 2020. doi: 10.2106/JBJS.20.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans PC, Rainger GE, Mason JC, Guzik TJ, Osto E, Stamataki Z, Neil D, Hoefer IE, Fragiadaki M, Waltenberger J, Weber C, Bochaton-Piallat ML, Back M. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res 116: 2177–2184, 2020. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavriilaki E, Anyfanti P, Gavriilaki M, Lazaridis A, Douma S, Gkaliagkousi E. Endothelial dysfunction in COVID-19: lessons learned from coronaviruses. Curr Hypertens Rep 22: 63, 2020. doi: 10.1007/s11906-020-01078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res 69: 1181–1189, 2020. doi: 10.1007/s00011-020-01401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P, Luscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J 41: 3038–3044, 2020. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med 26: 1017–1032, 2020. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, Dong W. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 27: 89–95, 2021. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J 41: 1798–1800, 2020. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra T, Wang M, Metwally AA, Bogu GK, Brooks AW, Bahmani A, Alavi A, Celli A, Higgs E, Dagan-Rosenfeld O, Fay B, Kirkpatrick S, Kellogg R, Gibson M, Wang T, Hunting EM, Mamic P, Ganz AB, Rolnik B, Li X, Snyder MP. Pre-symptomatic detection of COVID-19 from smartwatch data. Nat Biomed Eng 4: 1208–1220, 2020. doi: 10.1038/s41551-020-00640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Natarajan A, Su H-W, Heneghan C. Assessment of physiological signs associated with COVID-19 measured using wearable devices. NPJ Digit Med 3: 156, 2020. doi: 10.1038/s41746-020-00363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. When to quarantine. [2021 Mar 12]. COVID-19: When to Quarantine | CDC.
- 13.Broxterman RM, Trinity JD, Gifford JR, Kwon OS, Kithas AC, Hydren JR, Nelson AD, Morgan DE, Jessop JE, Bledsoe AD, Richardson RS. Single passive leg movement assessment of vascular function: contribution of nitric oxide. J Appl Physiol (1985) 123: 1468–1476, 2017. doi: 10.1152/japplphysiol.00533.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, Gmelch B, Bledsoe A, Richardson RS. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol 590: 1413–1425, 2012. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinity JD, Richardson RS. Physiological impact and clinical relevance of passive exercise/movement. Sports Med 49: 1365–1381, 2019. doi: 10.1007/s40279-019-01146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergström J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35: 609–616, 1975. doi: 10.3109/00365517509095787. [DOI] [PubMed] [Google Scholar]
- 18.Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810: 25–58, 2012. doi: 10.1007/978-1-61779-382-0_3. [DOI] [PubMed] [Google Scholar]
- 19.Holloway GP, Holwerda AM, Miotto PM, Dirks ML, Verdijk LB, van Loon LJ. Age-associated impairments in mitochondrial ADP sensitivity contribute to redox stress in senescent human skeletal muscle. Cell Rep 22: 2837–2848, 2018. doi: 10.1016/j.celrep.2018.02.069. [DOI] [PubMed] [Google Scholar]
- 20.Gnaiger E. Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41: 1837–1845, 2009. doi: 10.1016/j.biocel.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Limberg JK, Casey DP, Trinity JD, Nicholson WT, Wray DW, Tschakovsky ME, Green DJ, Hellsten Y, Fadel PJ, Joyner MJ, Padilla J. Assessment of resistance vessel function in human skeletal muscle: guidelines for experimental design, Doppler ultrasound, and pharmacology. Am J Physiol Heart Circ Physiol 318: H301–H325, 2020. doi: 10.1152/ajpheart.00649.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q, Xu L, Dai Y, Ling Y, Mao J, Qian J, Zhu W, Di W, Ge J. Cardiovascular manifestations in severe and critical patients with COVID-19. Clin Cardiol 43: 796–802, 2020. doi: 10.1002/clc.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Dunn J, Salins D, Zhou G, Zhou W, Schüssler-Fiorenza Rose SM, Perelman D, Colbert E, Runge R, Rego S. Digital health: tracking physiomes and activity using wearable biosensors reveals useful health-related information. PLoS Biol 15: e2001402, 2017. doi: 10.1371/journal.pbio.2001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fudim M, Qadri YJ, Ghadimi K, MacLeod DB, Molinger J, Piccini JP, Whittle J, Wischmeyer PE, Patel MR, Ulloa L. Implications for neuromodulation therapy to control inflammation and related organ dysfunction in COVID-19. J Cardiovasc Trans Res 13: 894–896, 2020. doi: 10.1007/s12265-020-10031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med 9: 1417, 2020. doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395: 1417–1418, 2020. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med 383: 120–128, 2020. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colmenero I, Santonja C, Alonso-Riano M, Noguera-Morel L, Hernandez-Martin A, Andina D, Wiesner T, Rodriguez-Peralto JL, Requena L, Torrelo A. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol 183: 729–737, 2020. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 77: 198–209, 2020. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395: 565–574, 2020. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Hu Y, Yu J, Ma T. Retrospective analysis of laboratory testing in 54 patients with severe- or critical-type 2019 novel coronavirus pneumonia. Lab Invest 100: 794–800, 2020. doi: 10.1038/s41374-020-0431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol 38: 1–9, 2020. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 33.Wu J. Tackle the free radicals damage in COVID-19. Nitric Oxide 102: 39–41, 2020. doi: 10.1016/j.niox.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care 24: 353, 2020. doi: 10.1186/s13054-020-03062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 78: 539–552, 2009. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell 26: 441–454, 2013. doi: 10.1016/j.devcel.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 37.Coate KC, Cha J, Shrestha S, Wang W, Gonçalves LM, Almaça J, Kapp ME, Fasolino M, Morgan A, Dai C, Saunders DC, Bottino R, Aramandla R, Jenkins R, Stein R, Kaestner KH, Vahedi G, Brissova M, Powers AC. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β cells. Cell Metab 32: 1028–1040.e4, 2020. doi: 10.1016/j.cmet.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ratchford SM, Stickford JL, Province VM, Stute N, Augenreich MA, Koontz LK, Bobo LK, Stickford ASL. Vascular alterations among young adults with SARS-CoV-2. Am J Physiol Heart Circ Physiol 320: H404–H410, 2021. doi: 10.1152/ajpheart.00897.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yilmaz MI, Solak Y, Saglam M, Cayci T, Acikel C, Unal HU, Eyileten T, Oguz Y, Sari S, Carrero JJ, Stenvinkel P, Covic A, Kanbay M. The relationship between IL-10 levels and cardiovascular events in patients with CKD. Clin J Am Soc Nephrol 9: 1207–1216, 2014. doi: 10.2215/CJN.08660813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, Deanfield JE, MacAllister RJ. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res 88: 145–151, 2001. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- 41.Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein (a) level. J Clin Invest 93: 50–55, 1994. doi: 10.1172/JCI116983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95: 549–601, 2015. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porzionato A, Emmi A, Barbon S, Boscolo-Berto R, Stecco C, Stocco E, Macchi V, De Caro R. Sympathetic activation: a potential link between comorbidities and COVID-19. FEBS J 287: 3681–3688, 2020. doi: 10.1111/febs.15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrett-O’Keefe Z, Lee JF, Ives SJ, Trinity JD, Witman MAH, Rossman MJ, Groot HJ, Sorensen JR, Morgan DE, Nelson AD, Stehlik J, Richardson RS, Wray DW. α-adrenergic receptor regulation of skeletal muscle blood flow during exercise in heart failure patients with reduced ejection fraction. Am J Physiol Regul, Integr Comp Physiol 316: R512–R524, 2019. doi: 10.1152/ajpregu.00345.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans. Circulation 100: 164–170, 1999. doi: 10.1161/01.CIR.100.2.164. [DOI] [PubMed] [Google Scholar]
- 46.Wray DW, Nishiyama SK, Richardson RS. Role of α1-adrenergic vasoconstriction in the regulation of skeletal muscle blood flow with advancing age. Am J Physiol Heart Circ Physiol 296: H497–H504, 2009. doi: 10.1152/ajpheart.01016.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity: observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- 48.Barrett-O’Keefe Z, Witman MA, McDaniel J, Fjeldstad AS, Trinity JD, Ives SJ, Conklin JD, Reese V, Runnels S, Morgan DE. Angiotensin II potentiates α-adrenergic vasoconstriction in the elderly. Clin Sci (Lond) 124: 413–422, 2013. doi: 10.1042/CS20120424. [DOI] [PubMed] [Google Scholar]
- 49.Singh KK, Chaubey G, Chen JY, Suravajhala P. Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am J Physiol Cell Physiol 319: C258–C267, 2020. doi: 10.1152/ajpcell.00224.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burtscher J, Cappellano G, Omori A, Koshiba T, Millet GP. Mitochondria: in the cross fire of SARS-CoV-2 and immunity. iScience 23: 101631, 2020. doi: 10.1016/j.isci.2020.101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ajaz S, McPhail MJ, Singh KK, Mujib S, Trovato FM, Napoli S, Agarwal K. Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am J Physiol Cell Physiol 320: C57–C65, 2021. doi: 10.1152/ajpcell.00426.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heydari K, Rismantab S, Shamshirian A, Lotfi P, Shadmehri N, Houshmand P, Zahedi M, Shamshirian D, Bathaeian S, Alizadeh-Navaei R. Clinical and paraclinical characteristics of COVID-19 patients: a systematic review and meta-analysis. medRxiv, 2020. doi: 10.1101/2020.03.26.20044057. [DOI] [Google Scholar]
- 53.Nasiri MJ, Haddadi S, Tahvildari A, Farsi Y, Arbabi M, Hasanzadeh S, Jamshidi P, Murthi M, Mirsaeidi M. COVID-19 clinical characteristics, and sex-specific risk of mortality: systematic review and meta-analysis. Front Med (Lausanne) 7: 459, 2020. doi: 10.3389/fmed.2020.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu P, Sun G-D, Li Z-Z. Clinical characteristics of two human to human transmitted coronaviruses: Corona Virus Disease 2019 versus Middle East Respiratory Syndrome Coronavirus. Eur Rev Med Pharmacol Sci 24: 5797–5809, 2020. doi: 10.26355/eurrev_202005_21374. [DOI] [PubMed] [Google Scholar]
- 55.Lau HM-C, Lee EW-C, Wong CN-C, Ng GY-F, Jones AY-M, Hui DS-C. The impact of severe acute respiratory syndrome on the physical profile and quality of life. Arch Phys Med Rehabil 86: 1134–1140, 2005. doi: 10.1016/j.apmr.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paneroni M, Simonelli C, Saleri M, Bertacchini L, Venturelli M, Troosters T, Ambrosino N, Vitacca M. Muscle strength and physical performance in patients without previous disabilities recovering from COVID-19 pneumonia. Am J Phys Med Rehabil 100: 105–109, 2021. doi: 10.1097/PHM.0000000000001641. [DOI] [PubMed] [Google Scholar]
- 57.Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 55: 102763, 2020. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol 32: 23–63, 2012. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, Zhang P, Liu X, Gao G, Liu F, Jiang Y, Cheng X, Zhu C, Xia Y. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect 9: 1123–1130, 2020. doi: 10.1080/22221751.2020.1770129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu L, Zhang H, Dauphars DJ, He Y-W. A potential role of interleukin 10 in COVID-19 pathogenesis. Trends Immunol 42: 3–5, 2021. doi: 10.1016/j.it.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y, Qin L, Zhang P, Li K, Liang L, Sun J, Xu B, Dai Y, Li X, Zhang C, Peng Y, Feng Y, Li A, Hu Z, Xiang H, Ogg G, Ho L-P, McMichael A, Jin R, Knight JC, Dong T, Zhang Y. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight 5: e139834, 2020. doi: 10.1172/jci.insight.139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang F, Hou H, Luo Y, Tang G, Wu S, Huang M, Liu W, Zhu Y, Lin Q, Mao L, Fang M, Zhang H, Sun Z. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight 5: e137799, 2020. doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hijmering ML, Stroes ESG, Pasterkamp G, Sierevogel M, Banga JD, Rabelink TJ. Variability of flow mediated dilation: consequences for clinical application. Atherosclerosis 157: 369–373, 2001. doi: 10.1016/s0021-9150(00)00748-6. [DOI] [PubMed] [Google Scholar]
- 64.Peretz A, Leotta DF, Sullivan JH, Trenga CA, Sands FN, Aulet MR, Paun M, Gill EA, Kaufman JD. Flow mediated dilation of the brachial artery: an investigation of methods requiring further standardization. BMC Cardiovasc Disord 7: 11, 2007. doi: 10.1186/1471-2261-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 300: H2–H12, 2011. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thijssen DH, Bruno RM, van Mil AC, Holder SM, Faita F, Greyling A, Zock PL, Taddei S, Deanfield JE, Luscher T. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J 40: 2534–2547, 2019. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 67.Greyling A, van Mil AC, Zock PL, Green DJ, Ghiadoni L, Thijssen DH; TIFN International Working Group on Flow Mediated Dilation. Adherence to guidelines strongly improves reproducibility of brachial artery flow-mediated dilation. Atherosclerosis 248: 196–202, 2016. doi: 10.1016/j.atherosclerosis.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 68.Todd G, Gorman RB, Gandevia SC. Measurement and reproducibility of strength and voluntary activation of lower-limb muscles. Muscle Nerve 29: 834–842, 2004. doi: 10.1002/mus.20027. [DOI] [PubMed] [Google Scholar]
- 69.Goshua G, Pine AB, Meizlish ML, Chang C-H, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, Dela Cruz CS, Dumont A, Halene S, Hwa J, Koff J, Menninger H, Neparidze N, Price C, Siner JM, Tormey C, Rinder HM, Chun HJ, Lee AI. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol 7: e575–e582, 2020. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pine AB, Meizlish ML, Goshua G, Chang C-H, Zhang H, Bishai J, Bahel P, Patel A, Gbyli R, Kwan JM. Circulating markers of angiogenesis and endotheliopathy in COVID-19. Pulm Circ 10: 2045894020966547, 2020. doi: 10.1177/2045894020966547. [DOI] [PMC free article] [PubMed] [Google Scholar]