Abstract
The world is currently facing a frightening coronavirus disease-2019 (COVID-19) epidemic. Severity of COVID-19 presentation is highly variable among infected individuals with increasingly recognized risk factors. Although observational studies suggested lower COVID-19 severity in populations consuming fermented foods, no controlled study investigated the role of diet. Yogurt, a fermented dairy product, exhibits interesting properties related to the presence of bioactive peptides and probiotics that may play a beneficial role in COVID-19 presentation and outcome. Peptides contained in yogurt are responsible for angiotensin-converting enzyme-inhibitory, bradykinin potentiating, antiviral, anti-inflammatory, antithrombotic, and antioxidant effects. The types and activity of these peptides vary widely depending on their amino acid sequence, on the probiotics used in yogurt production and on intestinal digestion. Additionally, probiotics used in yogurt exhibit direct angiotensin-converting enzyme-inhibitory, antiviral and immune boosting activities. Since COVID-19 pathogenesis involves angiotensin II accumulation and bradykinin deficiency, yogurt bioactive peptides appear as potentially beneficial. Therefore, epidemiological investigations and randomized controlled clinical trials to evaluate the exact role of yogurt consumption on COVID-19 manifestations and outcome should be encouraged.
Keywords: Angiotensin-converting enzyme, Bioactive peptide, Bradykinin, COVID-19, Yogurt
1. Introduction
Since December 2019, the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has spread worldwide causing life-threatening manifestations and fatalities (Phan et al., 2020). In the beginning of 2021, the coronavirus disease-2019 (COVID-19) pandemic has been responsible for more than 82 million reported contaminations and 1.2 million deaths. In parallel, an extraordinary number of researches has been launched to investigate risk factors, clinical manifestations, preventative options and possible anti-COVID-19 therapies. SARS-CoV-2-infected patient presentations have been reported to include pneumonia (Gattinoni et al., 2020), venous thromboembolic events related to endothelial injury and hypercoagulability (Voicu et al., 2020), excessive inflammatory conditions with cytokine storm (Jamilloux et al., 2020), immune dysregulation (Giamarellos-Bourboulis et al., 2020), oxidative stress (Delgado-Roche and Mesta, 2020), hypertension (Ruocco et al., 2020) and new onset diabetes (Yang et al., 2010).
COVID-19 presentation highly varies between individuals suggesting the presence of underlying individual risk factors that affect the disease severity (Rahman and Sathi, 2020). Some have been extensively investigated factors such as age (Kang and Jung, 2020), gender (Jin et al., 2020) and various other demographic characteristics (Nepomuceno et al., 2020). Studies have reported a higher risk for severity in adults than children (Clark et al., 2020, Zimmermann and Curtis, 2020). Additionally, health conditions associated with increased COVID-19 severity have been reported to include obesity (Hamer et al., 2020), diabetes, prior morbidities at risk of immunodeficiency and chronic cardiovascular, kidney and respiratory diseases (Centers for Disease Control and Prevention, 2020).
Surprisingly, studies investigating dietary habits as risk factor for COVID-19 variability are scarce. The US Centers for Disease Control and Prevention (CDC) stated that dietary supplementation has no direct role in COVID-19 prevention or treatment, thus only recommending special diet due to possible modular effects of vitamins and minerals on the immune system (Centers for Disease Control and Prevention (CDC), 2020). Interestingly, differences in dietary habits have been hypothesized as playing a potential role in COVID-19 geographical (Jayawardena and Misra, 2020) and fatality rate variability (Bousquet et al., 2020). Cabbage and fermented milk consumption in certain countries such as Bulgaria, Greece, Romania and Turkey, have been associated with lower mortality rates (Bousquet et al., 2020, Fonseca et al., 2020). Such findings justified the hypothesis of probable protective effects of antioxidants and angiotensin-converting enzyme (ACE)-inhibitory peptides present in fermented foods that could affect COVID-19 presentation and outcome. More recently, a case–control study including 505 participants suggested that dietary intake of dough and yogurt is able to play a significant protective role by limiting COVID-19 incidence (Odds ratios 0.62, 95%-confidence [0.44–0.87] and 0.74 [0.56–0.98], respectively) (Mohseni et al., 2021).
Many nutrients contain bioactive peptides with ACE-inhibitory effects. Such peptides are effective alternatives to the pharmaceutical ACE inhibitors (Fan et al., 2019). Most fermented dairy products exhibit ACE-inhibitory activities with various potency according to the product type (Hernández-Ledesma et al., 2004). Yogurt being a fermented dairy product is uniquely rich in bioactive peptides with potent effective multi-functions useful to beneficially influence COVID-19 manifestations (del Contreras et al., 2009, Donkor et al., 2007, Guerin-Danan et al., 1998). Multiple ACE-inhibitory peptides are present in probiotic yogurts with effective effects comparable to those of synthetic ACE-inhibitors (Donkor et al., 2007). Additionally, probiotics used in yogurt industry have beneficial immunomodulatory and antiviral effects with a possible role in the prevention and alleviation of COVID-19 infection. Since yogurt is more widely studied than any other dairy product, we aimed to review its properties that may beneficially alter COVID-19 pathogenesis, expression and outcome, reporting the characteristics, potency and stability of the different bioactive peptides involved.
The three main electronic databases (Pubmed, Embase and Google Scholar) were searched for the 1990/01–2020/12 period using the following keywords (“Yogurt” OR “Probiotics”) AND (“COVID-19” OR “SARS-Cov-2” OR “Renin-angiotensin” OR “Kinin-kallikreine”). Our research was limited to the available English reports (abstracts or full texts). We selected all reports (original and review articles) focusing on the pathophysiological mechanisms and potential benefits in COVID-19 patients.
2. Reported health effects of yogurt
Yogurt, a dairy product produced by milk fermentation, contains lactic acid bacteria (LAB) which ferment lactose (producing lactic acid) and affects milk peptides and proteins (Algaron et al., 2004). Being a dairy product, yogurt is rich in variable minerals such as calcium, magnesium, potassium, and zinc, and vitamins such as vitamin B. Yogurt is also a good source of various other nutriments and energy. Interestingly, higher levels of proteins, vitamins and minerals have been reported in yogurt than milk, supporting its role in improving nutritional status and health of older adults and possibly healthy and active aging (El-Abbadi et al., 2014).
The fermentation process in yogurt includes complex reactions resulting in different fermentation by-products including enzymes. They activate inert milk proteins (e.g., albumins and casein) to bioactive peptides providing yogurt with functionalities that lack in non-fermented dairy products (Donkor et al., 2007). Multiple nutritional and health benefits of yogurt consumption have been attributed to these bioactive peptides including antihypertensive (del Contreras et al., 2009, Hata et al., 1996), ACE-inhibitory (Donkor et al., 2007, Nielsen et al., 2009), immunomodulatory (Coste et al., 1992), anti-inflammatory (Guerin-Danan et al., 1998, Yoon et al., 2019), antioxidant (Gjorgievski et al., 2014, Lin and Yen, 1999), antithrombotic (Rojas-Ronquillo et al., 2012), platelet aggregation-inhibitory (Jolles et al., 1986) antimicrobial (López-Expósito et al., 2007), antiviral (Farnaud and Evans, 2003), anticancer (Rea et al., 2018), antidiabetic (Barengolts et al., 2019) and dietary nitrogen absorption regulatory effects (Gaudichon et al., 1994). Interestingly, yogurt has been reported to enhance resistance to upper respiratory tract infections (de Araujo et al., 2015, Fujita et al., 2013, Guillemard et al., 2010, Pu et al., 2017; Y. Wang et al., 2016), prevent common cold and influenza (Lehtoranta et al., 2014, Makino et al., 2010; Y. Yamamoto et al., 2019) and treat acute gastroenteritis and diarrhea (Dinleyici et al., 2012, Szajewska et al., 2013).
Many of these reported effects could appear of interest in COVID-19 patient management. However, each peptide from yogurt have multifunctional properties and activities depending on its amino acid sequence (Meisel and FitzGerald, 2003, Tagliazucchi et al., 2015). One supplemental issue is that health benefits have been reported based on different types of yogurt. Therefore, separating and identifying the useful peptides and studying the molecular mechanisms of their bioactivity and pharmacodynamics are crucial to consider potential clinical applications.
3. Yogurt bioactive ACE-inhibitory peptides
Various bioactive peptides derived from different yogurt types have been separated and sequenced (Table 1). Antihypertensive ACE-inhibitory peptides exhibit variable amino acid sequences including Val-Pro-Pro (VPP), Ile-Pro-Pro (IPP), Tyr-Pro, Lys-Val-Leu-Pro-Val-Pro-Gln, Thr–Tyr–Lys–Glu–Glu, Tyr–Gln–Glu–Pro–Val–Leu, Ser–Leu–Pro–Gln–Asn, Arg–Ile–Asn–Lys–Lys, Ala–Arg–His–Pro–His, Phe-Phe-Val-Ala-Pro (CEI5), Ala-Val-Pro-Tyr-Pro-Gln-Arg (CElβ7) and Phe-Phe-Va1-A1a-Pro-Phe-Pro-G1u-Va1-Phe-G1y-Lys (CEI12) (Bousquet et al., 2020, del Contreras et al., 2009, Donkor et al., 2007, Gobbetti et al., 2000, Maeno et al., 1996, Maruyama et al., 1985, Mohanty et al., 2016, Nagpal et al., 2011, Nakamura et al., 1995, Seppo et al., 2003, Tuomilehto et al., 2004; J. Wang et al., 2015; N. Yamamoto et al., 1999). All these bioactive peptides originate from the enzymatic degradation of casein during food processing and gastrointestinal digestion (Lebrun et al., 1995, Maruyama et al., 1985). They exhibit variable ACE-inhibitory potency with additional alternative antihypertensive mechanisms such as bradykinin-potentiating effects as suggested by the onset of hypotensive effects at concentrations lower than their effective ACE-inhibitory concentrations determined in vitro (Foltz et al., 2007).
Table 1.
The main milk-derived bioactive peptides and their reported activities.
| Milk proteins | Bioactive peptide sequence | Enzymes and LAB involved in their production | Reported activities | Reference |
|---|---|---|---|---|
| β-casein | Val-Pro-Pro |
Lactobacillus helveticus Lactobacillus delbrueckii ssp. bulgaricus Streptococcus thermophilus |
ACE inhibition | (Donkor et al., 2007, Mohanty et al., 2016) |
| Ile-Pro-Pro | Lactobacillus helveticus | ACE inhibition | (Seppo et al., 2003) | |
| Lys-Val-Leu-Pro-Val-Pro-(Glu) | Lactobacillus helveticus | ACE inhibition | (Mohanty et al., 2016) | |
| Asp-Lys-Ile-His-Pro-Phe, Tyr-Gln-Glu-Pro- Val-Leu |
Lactobacillus rhamnosus Pepsin |
ACE inhibition | (Mohanty et al., 2016) | |
| Tyr-Gln-Glu-Pro-Val-Leu-Gly-Pro-Val-Arg-Gly-Pro-Phe-Pro-Ile-Ile-Val | Lactobacillus casei Shirota | ACE-inhibition Thrombin inhibition | (Rojas-Ronquillo et al., 2012) | |
| Leu-Asn-Val-Pro-Gly-Glu-Ile-Val-Glu | Lactobacillus delbrueckii subsp. bulgaricus | ACE inhibition | (Gobbetti et al., 2000) | |
| Asn-Ile-Pro-Pro-Leu-Thr-Gln-Thr-Pro-Val | ND | ACE inhibition | (Gobbetti et al., 2000) | |
| Leu-Asn-Val-pro-Gly-Glu-IleVal-Glu | ND | ACE inhibition | (Gobbetti et al., 2000) | |
| Pro-Pro-Leu-Thr-Gln-Thr-Pro-Val | ND | ACE inhibition | (Gobbetti et al., 2000) | |
| Tyr–Gln–Glu–Pro–Val–Leu |
Lactobacillus delbrueckii ssp. bulgaricus Streptococcus thermophilus |
ACE inhibition | (Donkor et al., 2007) | |
| Phe-Phe-Va1-A1a-Pro-Phe-ProG1u-Va1-Phe-G1y-Lys (CEI12) | Trypsin and proline-specific endopeptidase | ACE inhibition Bradykinin potentiation | (Donkor et al., 2007) | |
| Phe-Phe-Val-Ala-Pro (CEl5) | Trypsin and proline-specific endopeptidase | ACE inhibition Bradykinin potentiation | (Donkor et al., 2007) | |
| Ala-Val-Pro-Tyr-Pro-Gln-Arg (CElβ7)' | Trypsin and proline-specific endopeptidase | ACE inhibition Bradykinin potentiation | (Donkor et al., 2007) | |
| Tyr-Pro-Phe-Pro-Gly-Pro-Ile | ND | Opioid agonist activity ACE inhibition Immunomodulation |
(Nagpal et al., 2011) | |
| Tyr-Gln-Gln-Pro-Val-Leu-Gly-Pro-Val-Arg | ND | ACE inhibition Immunomodulation |
(Nagpal et al., 2011) | |
| Tyr-Pro-Phe-Pro, Ala-Val-Pro-Tyr-Pro-Gln Arg,Thr-Thr-Met-Pro-Leu-Trp |
Lactobacillus GG Pepsin and trypsin |
Opioid agonist activity ACE inhibition Immunomodulation |
(Mohanty et al., 2016) | |
| k-casein | Ala–Arg–His–Pro–His |
Lactobacillus delbrueckii ssp. bulgaricus Streptococcus thermophilus |
ACE inhibition | (Donkor et al., 2007) |
| Val-Ile-Gly-Ser-Pro-Pro-Glu-Ile-Asn | Lactobacillus lactis ssp. cremoris | ACE inhibition | (Gobbetti et al., 2000) | |
| Ser-Pro-Pro-Glu-Ile-Asn | Lactobacillus lactis ssp. cremoris | ACE inhibition | (Gobbetti et al., 2000) | |
| Ala-Arg-His-Pro-His-Pro-His-Leu-Ser-Phe-met | Lactobacillus delbrueckii ssp. bulgaricus | Anti-oxidative activity | (Mohanty et al., 2016) | |
| Met-Ala-Ile-Pro-Pro-Lys-Lys-Asn-Gln-Asp-Lys | ND | Antithrombotic activity | (Gobbetti et al., 2000) | |
| Ser-Arg-Tyr-Pro-Ser-Tyr-OH | ND | Opioid antagonist activity | (Gobbetti et al., 2000) | |
| αS1-casein | Thr-Thr-Met-Pro-Leu-Trp | ND | ACE inhibition Immunomodulation |
(Gobbetti et al., 2000) |
| Tyr-Lys-Val-Pro-Gln-Leu | ND | ACE inhibition | (Gobbetti et al., 2000) | |
| Arg-Tyr-Leu-Gly-Tyr-Leu | ND | Opioid agonist activity | (Gobbetti et al., 2000) | |
| Tyr-Leu-Gly-Tyr-Leu-Glu | ND | Opioid agonist activity | (Gobbetti et al., 2000) | |
| Val-Ala-Pro-Phe-Pro-Glu-Val-Phe | Pepsin | ACE inhibition Anti-oxidative activity |
(Contreras et al., 2009) | |
| Arg-Tyr-Leu-Gly-Tyr | Pepsin | ACE inhibition Anti-oxidative activity |
(Contreras et al., 2009) | |
| αS2-casein | Tyr-Gln-Lys-Phe-Pro-Gln-Tyr | Pepsin | ACE inhibition Anti-oxidative activity |
(Contreras et al., 2009) |
ACE, angiotensin-converting enzyme, LAB, lactic acid bacteria, ND, not determined.
Originally, the first ACE-inhibitory peptides were snake venom-derived bradykinin-potentiating peptides (BBPs) (Ondetti et al., 1971). Thereafter, such peptides were separated from bovine caseins and among plants and other food proteins (Yamamoto, 1997). Interestingly, amino acid sequences of snake venom-derived BBPs have been identified in the casein protein chains as inactive forms. They have been shown to typically contain 5 to 13 proline-rich peptides with a pyroglutamic acid residue at the N-terminus and a proline residue at the C-terminus. BPPs with lengths of more than seven amino acids show a high content of proline residues and a specific tripeptide sequence (Ile-Pro-Pro) at the C-terminus (Ianzer et al., 2004). The presence of proline-rich peptides within the sequenced casein-derived peptides supports the hypothesis stating that intestinal or peptic hydrolysis gives bradykinin potentiation characteristics to these peptides. Subsequently, enhanced yogurt proteolysis exposes the formed peptides to further modifications as the formation of N-terminal pyroglutamic acids (Pinto et al., 2020). Bovine casein-derived ACE-inhibitory peptides which share the common amino acid sequence at C-terminal (Pro-Pro or Ala-Pro) with snake venom-derived BBPs, exhibit almost equal effects on ACE and bradykinin (Maruyama et al., 1985).
Bioavailability, mechanisms and potency of ACE-inhibitory peptides are mainly determined by their size and sequence (Table 1). The amino acid sequence of the bioactive peptide controls its effects (Hernández-Ledesma et al., 2004). The abundance of proline residues, as observed in the most potent ACE-inhibitory peptides generated from casein fractions, enhances ACE-inhibitory effects of the bioactive peptides (Otte et al., 2007). Generally, potent ACE-inhibitory peptides are composed of hydrophobic, positively charged and aromatic or cyclic amino acid residues at the third, second, and first position from the C-terminus, respectively (Hernández-Ledesma et al., 2004). The presence of hydrophobic amino acids such as leucine, isoleucine, phenylalanine or proline at the C-terminal position of a peptide is predictive of its ACE-inhibitory activity (Tagliazucchi et al., 2015). The C-terminal tripeptide residues play an important role in determining its potency. The aromatic amino acids and imino acid proline have been shown to be the most effective C-terminal amino acids in binding to ACE, while other amino acids (e.g., dicarboxylic residues) exhibit weak binding characteristics (Cheung et al., 1980). Of note, bioactive peptides also vary in sequence and effects according to the LAB involved in yogurt fermentation (Hernández-Ledesma et al., 2004). The main LAB involved are Lactobacillus bulgaricus and Streptococcus thermophiles (Dellaglio, 1988). Other LAB such as other Lactobacillus, Streptococcus, Leuconostoc, and Bifidobacterium are added to produce the particularities of each given yogurt. These probiotics may have direct antioxidant activities and even improve inflammatory conditions and regulate innate immunity.
The other main factor that controls the bioavailability and potency of ACE-inhibitor peptides is the intestinal and peptic digestion. ACE-inhibitory peptides may be hydrolysed by cellular peptidases prior to their transport across the intestinal epithelium (Quirós et al., 2008). The presence of proline residue in these peptides increases their resistance to enzymatic proteolysis (Tauzin et al., 2002). Small peptides containing N-terminal Tyr and/or C-terminal Pro have shown improved stability against enterocyte peptidases, thus increasing their bioavailability (Fan et al., 2019). Also, digestion may modulate yogurt-related beneficial activity by promoting the formation of more active peptides (Hernández-Ledesma et al., 2004). In vivo peptide activation or deactivation is controlled by endogenous enzymatic proteolysis. Some peptides may be potent in vivo and weak in vitro and vice-versa (Yamamoto et al., 1999). Generation of ACE-inhibiting peptides from intestinal peptidase-mediated digestion of meals containing protein precursors has been investigated in vitro using simulated gastrointestinal fluids (Manso and Lopez-Fandino, 2003, Savoie et al., 2005), and in vivo. This was observed with the increase in plasma Ile-Pro-Pro and Leu-ProPro peptides after lactotripeptide-enriched yogurt ingestion (Lebrun et al., 1995). Probiotic strains involved in milk fermentation produce oligopeptides that may generate bioactive peptides following further digestion by pepsin and trypsin (Rokka et al., 1997). Finally, in vivo deactivation of ACE-inhibitory peptides obtained from casein occurs in case of intestinal breakdown, thus at risk of blood pressure lowering in such situations (FitzGerald et al., 2004).
4. COVID-19 pathogenesis and relation to yogurt bioactive peptides
SARS-CoV-2 cell entry is due to its interaction mediated by the spike receptor binding domain with ACE2 protein at the host cell membrane (Walls et al., 2020). This interaction induces ACE2 down-regulation (Hoffmann et al., 2020, Silhol et al., 2020). ACE2 is a monocarboxypeptidase homologue of ACE that converts angiotensin II into angiotensin 1–7. Most studies support that ACE2 down-regulation contributes to COVID-19 manifestations through its critical counter-regulatory effects on the renin-angiotensin system which dysfunction results in the accumulation of angiotensin II and deficiency in angiotensin 1–7 (Gurwitz, 2020, Miesbach, 2020).
By contrast, ACE inhibition leads to a decrease in angiotensin II synthesis and angiotensin 1–7 breakdown (Deddish et al., 1998, Kuba et al., 2005). In heart failure patients, ACE inhibitors have been shown to reduce inflammatory cytokine production thus resulting in beneficial effects on the immune system (Gage et al., 2004). Using a population-based cohort study with multivariable propensity score-based regression, prior ACE inhibitors have been shown to substantially lower short-term mortality after sepsis (Hsu et al., 2020). Recently, a meta-analysis reported that prior ACE inhibitor use may have similarly reduced mortality in COVID-19 patients (Ghosal et al., 2020).
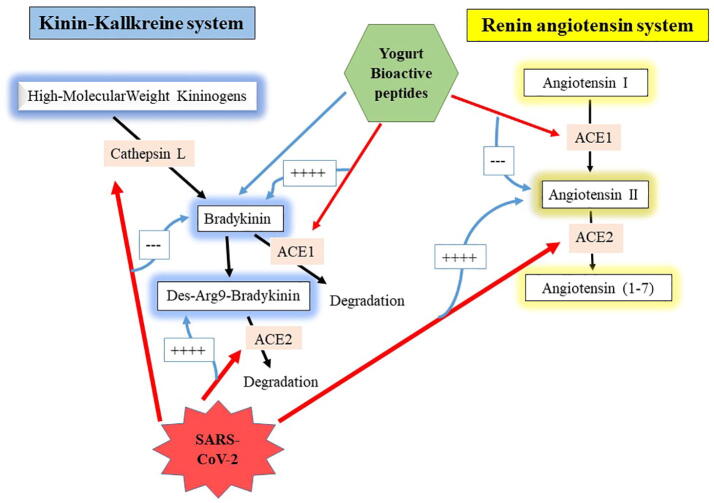
ACE2 plays a crucial role in affecting both renin-angiotensin and kinin-kallikrein systems, although this latter effect has been generally omitted (Sodhi et al., 2018, van de Veerdonk et al., 2020). We recently reviewed COVID-19-related effects on these systems, showing that snake-derived BPPs, if available and safe as a pharmaceutical drug, could act as an optimal therapy in COVID-19 patients due to its ACE-inhibitory and bradykinin-potentiating effects (Gouda and Mégarbane, 2020). As mentioned above, the reported potent ACE-inhibitory effects of yogurt-derived peptides together with their proposed bradykinin-potentiating effects may render these bioactive peptides effective to counteract COVID-19 pathogenesis and its deleterious health consequences (Fig. 1).
Fig. 1.
Pathological consequences of SARS-CoV-2 effects on the renin-angiotensin and kinin-kallikrein systems and the presumed beneficial activities of yogurt-derived bioactive peptides on both systems. The red arrows refer to inhibition and the blue arrows refer to potentiation. ACE, angiotensin-converting enzyme.
5. Yogurt probiotics and possible benefits in COVID-19 patients
Aside from its role in producing bioactive peptides during food fermentation, the daily consumption of probiotics was suggested to be beneficial to human health by inhibiting allergy mechanisms, boosting the immune response and stimulating the antimicrobial and anti-viral defense (Bustamante et al., 2020). Debris of dead probiotic cells additionally plays a direct ACE inhibitor effect (Miremadi et al., 2014). All these benefits have been attributed to the ability of probiotics to regulate the gut bacterial ecosystem and subsequently modulate the immune system (Dargahi et al., 2019). For instance, probiotics interact with macrophages to facilitate the production of interleukin-12 which stimulates the production of interferon-γ, a major antiviral cytokine (de Roock et al., 2011, Kitazawa et al., 1994, Kudva et al., 2011). Moreover, some probiotic strains including Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. Bulgaricus and Bifidobacterium bifidum enhance interferon-α production by monocytes (Kitazawa et al., 1994). Interestingly, the reported immunomodulatory effects of probiotics are strain-specific (Wu et al., 2019).
Probiotics exhibit potent antimicrobial activity against viruses and bacteria causing respiratory tract infections (Kassaa, 2016). A meta-analysis of 52 published studies strongly supported the evidence that probiotics effectively contributed to prevent respiratory tract infections (Liu et al., 2018). Another meta-analysis of 23 randomized controlled trials supported that probiotics significantly reduced the severity of respiratory tract infections in children (de Araujo et al., 2015). Probiotics have been demonstrated useful for preventing and treating influenza A H1N1 and respiratory syncytial viruses in experimental models (Eguchi et al., 2019, Kawase et al., 2010). Consumption of milk sources of probiotics significantly reduced the incidence of respiratory tract infections (Makino et al., 2010, Merenstein et al., 2010, Shida et al., 2017, Taipale et al., 2011).
SARS-CoV-2 may affect the intestinal and lung microbiota (Kopel et al., 2020, Xiao et al., 2020). Dysbiosis of beneficial bacteria and growth of opportunistic pathogens have been shown to correlate with the severity of COVID-19 (Tang et al., 2020, Zuo et al., 2020). COVID-19 patients may present intestinal microbial dysbiosis characterized by low numbers of various probiotic species such as Bifidobacterium and Lactobacillus, thus possibly requiring probiotic administration to restore the intestinal flora balance and decrease the risks related to SARS-CoV-2 infection (Xu et al., 2020).
Probiotics may help preventing and treating COVID-19 by preserving the gastrointestinal tract and lung microbiota since dysbiosis plays a major role in susceptibility to infections (Olaimat et al., 2020). Diet containing probiotics may alleviate SARS-CoV-2 infection or at least the onset of complications by preserving the GI microbiota including its structure, diversity and function (Gasmi et al., 2020). The use of fermented foods as dietary sources of probiotics to prevent or alleviate SARS-CoV-2 infection has been proposed (Olaimat et al., 2020). Several trials to investigate probiotics-related efficacy to treat or prevent COVID-19 are currently ongoing (Infusino et al., 2020).
6. Direct antiviral effects of probiotics and bioactive peptides
Of SARS-CoV-2 proteins, the spike glycoprotein and 3-chymotrypsin-like cysteine protease (3CLpro) are the most critical proteins needed for viral cell entry and replication, respectively (Hall and Ji, 2020). The spike glycoprotein facilitates viral cell entry through the interaction of its receptor-binding domain (RBD) with human ACE2. The 3CLpro catalytically cleaves the coronavirus polyprotein at 11 conserved sites including a peptide bond between a glutamine at position P1 and a small amino acid (serine, alanine, or glycine) at position P1′. These two glycoproteins represent the major potential drug targets for coronavirus infections and were thus investigated in silico as possibly targeted by probiotics and bioactive peptides contained in yogurt (Table 2).
Table 2.
Established antiviral activities of probiotics and bioactive peptides present in yogurt.
| Investigated probiotics and bioactive peptides | Antiviral activity against | Methodology | References |
|---|---|---|---|
| Bioactive peptides produced by Lactobacillus delbrueckii WS4 | SARS-CoV, SARS-CoV-2, MERS-CoV, HCoV-HKU1 | In silico | (Chourasia et al., 2020) |
| Bioactive peptides derived from beta-lactoglobulin | SARS-CoV-2 | In silico | (Çakır et al., 2021) |
| Bioactive peptides derived from Lactobacillus plantarum and Bifidobacterium bifidum | Enterovirus 71 | In vitro | (Choi et al., 2010) |
| Lactobacillus reuteri Protectis | Coxsackievirus A, Enterovirus 71 | In vitro | (Ang et al., 2016) |
| Probiotic metabolites of Lactobacillus casei and Bifidobacterium adolescentis | Rotavirus | In vitro | (Olaya Galán et al., 2016) |
| P18 peptide of Bacillus subtilis | Influenza virus | In vitro and in vivo (mice) | (Starosila et al., 2017) |
| Lactobacillus gasseri SBT2055 | Respiratory sentential virus | In vivo (mice) | (Eguchi et al., 2019) |
SARS-CoV-2, severe acute respiratory syndrome coronavirus; MERS-CoV, Middle-East respiratory syndrome-related coronavirus; HCoV-HKU1, human coronavirus HKU1.
Recently, a study screened the possible inhibition of these two SARS-CoV-2 glycoproteins by 1420 bioactive peptides identified from the soy cheese peptidome produced using Lactobacillus delbrueckii WS4 for antiviral activity by employing the web tools, AVPpred, and meta-iAVP (Chourasia et al., 2020). Molecular docking analyses revealed that one of these peptides, the “KFVPKQPNMIL” demonstrated a strong affinity to Spike-RBD and 3CLpro of SARS-CoV-2. This peptide exhibited also the ability to interact with the Spike-RBD and 3CLpro of other β-coronaviruses including SARS-CoV, Middle-East respiratory syndrome-related coronavirus (MERS-CoV) and human coronavirus HKU1. Another computer-based study investigated two bioactive peptides derived from beta-lactoglobulin obtained by the treatment of goat milk whey fraction with trypsin (Ala-Leu-Pro-Met-His-Ile-Arg and Ile-Pro-Ala-Val-Phe-Lys) and showed their ability to inactivate both SARS-CoV-2 and ACE (Cakir et al., 2021).
Table 2 reviews additional yogurt bioactive peptides and probiotics tested in vitro and in vivo with direct antiviral activities including against viruses sharing some mechanistic similarities with SARS-CoV-2. Yogurt fermented with Lactobacillus plantarum and Bifidobacterium bifidum exhibited high anti-enterovirus 71 activity (Choi et al., 2010). Lactobacillus reuteri Protectis displayed a dose-dependent antiviral activity against Enterovirus 71 and Coxsackievirus type A strains 6 and 16 but not against Coxsackievirus type B strain 2 (Ang et al., 2016). Probiotic metabolites of Lactobacillus casei, and Bifidobacterium adolescentis were shown able to reduce protein liberation and calcium release in an intracellular model on MA104 cells, suggesting an effective antiviral activity against rotavirus infection (Olaya Galán et al., 2016). P18 peptide produced by the probiotic strain Bacillus subtilis showed complete inhibition of influenza virus in vitro and similar protective effect in mice to that of oseltamivir phosphate (Starosila et al., 2017). Finally, Lactobacillus gasseri SBT2055, a probiotic lactic acid bacterium, was shown able to prevent influenza A and respiratory syncytial virus infections in mice (Eguchi et al., 2019).
7. Conclusions and perspectives
Yogurt contains several bioactive peptides with reported benefits including antiviral, antioxidant, anti-inflammatory, antithrombotic and chest infection preventive effects. These peptides are effective through their ACE-inhibitory and possible bradykinin potentiating activities. These peptide activity and potency vary widely depending on their amino acid sequence, the prebiotics used in yogurt fermentation, and the effect of intestinal digestion. As per our review, these effects are beneficial in COVID-19. We hypothesized that yogurt consumption may influence COVID-19 presentation and outcome. Further epidemiological studies evaluating the exact role of yogurt consumption on COVID-19 severity should be encouraged. Similarly, randomized controlled clinical studies evaluating the effects of special yogurt-enriched diet protocols on moderate-to-severe COVID-19 patients admitted to the hospital should be evaluated.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Algaron F., Miranda G., Le Bars D., Monnet V. Milk fermentation by Lactococcus lactis with modified proteolytic systems to accumulate potentially bio-active peptides. Le Lait. 2004;84(1–2):115–123. doi: 10.1051/lait:2003034. [DOI] [Google Scholar]
- Ang L.Y.E., Too H.K.I., Tan E.L., Chow T.K., Shek L.P., Tham E.H., Alonso S. Antiviral activity of Lactobacillus reuteri Protectis against Coxsackievirus A and Enterovirus 71 infection in human skeletal muscle and colon cell lines. Virol. J. 2016;13:111. doi: 10.1186/s12985-016-0567-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barengolts E., Smith E., Reutrakul S., Tonucci L., Anothaisintawee T. The Effect of Probiotic Yogurt on Glycemic Control in Type 2 Diabetes or Obesity: A Meta-Analysis of Nine Randomized Controlled Trials. Nutrients. 2019;11(3):671. doi: 10.3390/nu11030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet J., Anto J.M., Iaccarino G., Czarlewski W., Haahtela T., Anto A., Akdis C.A., Blain H., Canonica G.W., Cardona V., Cruz A.A., Illario M., Ivancevich J.C., Jutel M., Klimek L., Kuna P., Laune D., Larenas-Linnemann D., Mullol J., Zuberbier T. Is diet partly responsible for differences in COVID-19 death rates between and within countries? Clin. Transl. Allergy. 2020;10(1):16. doi: 10.1186/s13601-020-00323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante M., Oomah B.D., Oliveira W.P., Burgos-Díaz C., Rubilar M., Shene C. Probiotics and prebiotics potential for the care of skin, female urogenital tract, and respiratory tract. Folia Microbiol. (Praha) 2020;65(2):245–264. doi: 10.1007/s12223-019-00759-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çakır B., Okuyan B., Şener G., Tunali-Akbay T. Investigation of beta-lactoglobulin derived bioactive peptides against SARS-CoV-2 (COVID-19): In silico analysis. Eur. J. Pharmacol. 2021;891 doi: 10.1016/j.ejphar.2020.173781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2020. People who are at higher risk for severe illness. Coronavirus Disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/need-extraprecautions/people-at-higher-risk.html. [PubMed]
- Centers for Disease Control and Prevention (CDC), 2020. Food and Coronavirus Disease 2019 (COVID-19). Coronavirus Disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/food-and-COVID-19.html.
- Cheung, H. S., Wang, F. L., Ondetti, M. A., Sabo, E. F., Cushman, D. W., 1980. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 255(2), 401–407. http://www.ncbi.nlm.nih.gov/pubmed/6243277. [PubMed]
- Choi H.-J., Song J.-H., Park K.-S., Baek S.-H., Lee E.-S., Kwon D.-H. Antiviral activity of yogurt against enterovirus 71 in vero cells. Food Sci. Biotechnol. 2010;19(2):289–295. doi: 10.1007/s10068-010-0042-x. [DOI] [Google Scholar]
- Chourasia R., Padhi S., Chiring Phukon L., Abedin M.M., Singh S.P., Rai A.K. A potential peptide from soy cheese produced using lactobacillus delbrueckii WS4 for effective inhibition of SARS-CoV-2 main protease and S1 glycoprotein. Front. Mol. Biosci. 2020;7 doi: 10.3389/fmolb.2020.601753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A., Jit M., Warren-Gash C., Guthrie B., Wang H.H.X., Mercer S.W., Sanderson C., McKee M., Troeger C., Ong K.L., Checchi F., Perel P., Joseph S., Gibbs H.P., Banerjee A., Eggo R.M., Nightingale E.S., O’Reilly K., Jombart T., Jarvis C.I. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob. Health. 2020;8(8):e1003–e1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras, M. del M., Carrón, R., Montero, M. J., Ramos, M., Recio, I., 2009. Novel casein-derived peptides with antihypertensive activity. Int. Dairy J. 19(10), 566–573. https://doi.org/10.1016/j.idairyj.2009.05.004
- Coste M., Rochet V., Léonil J., Mollé D., Bouhallab S., Tomé D. Identification of C-terminal peptides of bovine β-casein that enhance proliferation of rat lymphocytes. Immunol. Lett. 1992;33(1):41–46. doi: 10.1016/0165-2478(92)90091-2. [DOI] [PubMed] [Google Scholar]
- Dargahi N., Johnson J., Donkor O., Vasiljevic T., Apostolopoulos V. Immunomodulatory effects of probiotics: Can they be used to treat allergies and autoimmune diseases? Maturitas. 2019;119:25–38. doi: 10.1016/j.maturitas.2018.11.002. [DOI] [PubMed] [Google Scholar]
- de Araujo G.V., de Oliveira Junior M.H., Peixoto D.M., Sarinho E.S.C. Probiotics for the treatment of upper and lower respiratory-tract infections in children: systematic review based on randomized clinical trials. J. Pediatr. (Rio J) 2015;91(5):413–427. doi: 10.1016/j.jped.2015.03.002. [DOI] [PubMed] [Google Scholar]
- de Roock S., van Elk M., Hoekstra M.O., Prakken B.J., Rijkers G.T., de Kleer I.M. Gut derived lactic acid bacteria induce strain specific CD4+ T cell responses in human PBMC. Clin. Nutr. 2011;30(6):845–851. doi: 10.1016/j.clnu.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Deddish P.A., Marcic B., Jackman H.L., Wang H.-Z., Skidgel R.A., Erdös E.G. N-Domain–Specific Substrate and C-Domain Inhibitors of Angiotensin-Converting Enzyme. Hypertension. 1998;31(4):912–917. doi: 10.1161/01.HYP.31.4.912. [DOI] [PubMed] [Google Scholar]
- Delgado-Roche L., Mesta F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch. Med. Res. 2020;51(5):384–387. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellaglio F. Starters for fermented milks section: taxonomy and metabolism. Bull. Int. Dairy Fed. 1988;277:7–18. https://agris.fao.org/agris-search/search.do?recordID=BE19890031498 [Google Scholar]
- Dinleyici E.C., Eren M., Ozen M., Yargic Z.A., Vandenplas Y. Effectiveness and safety of Saccharomyces boulardii for acute infectious diarrhea. Expert Opin. Biol. Ther. 2012;12(4):395–410. doi: 10.1517/14712598.2012.664129. [DOI] [PubMed] [Google Scholar]
- Donkor O.N., Henriksson A., Singh T.K., Vasiljevic T., Shah N.P. ACE-inhibitory activity of probiotic yoghurt. Int. Dairy J. 2007;17(11):1321–1331. doi: 10.1016/j.idairyj.2007.02.009. [DOI] [Google Scholar]
- Eguchi K., Fujitani N., Nakagawa H., Miyazaki T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019;9(1):4812. doi: 10.1038/s41598-019-39602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Abbadi N.H., Dao M.C., Meydani S.N. Yogurt: role in healthy and active aging. The Am. J. Clin. Nutr. 2014;99(5):1263S–1270S. doi: 10.3945/ajcn.113.073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Liao W., Wu J. Molecular interactions, bioavailability, and cellular mechanisms of angiotensin-converting enzyme inhibitory peptides. J. Food Biochem. 2019;43(1) doi: 10.1111/jfbc.12572. [DOI] [PubMed] [Google Scholar]
- Farnaud S., Evans R.W. Lactoferrin—a multifunctional protein with antimicrobial properties. Mol. Immunol. 2003;40(7):395–405. doi: 10.1016/S0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- FitzGerald R.J., Murray B.A., Walsh D.J. Hypotensive Peptides from Milk Proteins. J. Nutr. 2004;134(4):980S–988S. doi: 10.1093/jn/134.4.980S. [DOI] [PubMed] [Google Scholar]
- Foltz M., Meynen E.E., Bianco V., van Platerink C., Koning T.M.M.G., Kloek J. Angiotensin Converting Enzyme Inhibitory Peptides from a Lactotripeptide-Enriched Milk Beverage Are Absorbed Intact into the Circulation. J. Nutr. 2007;137(4):953–958. doi: 10.1093/jn/137.4.953. [DOI] [PubMed] [Google Scholar]
- Fonseca S., Rivas I., Romaguera D., Quijal M., Czarlewski W., Vidal A., Fonseca J., Ballester J., Anto J., Basagana X., Cunha L.M., Bousquet J. Association between consumption of fermented vegetables and COVID-19 mortality at a country level in Europe. MedRxiv Preprint, Preprint. 2020 doi: 10.1101/2020.07.06.20147025. [DOI] [Google Scholar]
- Fujita R., Iimuro S., Shinozaki T., Sakamaki K., Uemura Y., Takeuchi A., Matsuyama Y., Ohashi Y. Decreased duration of acute upper respiratory tract infections with daily intake of fermented milk: A multicenter, double-blinded, randomized comparative study in users of day care facilities for the elderly population. Am. J. Infect. Control. 2013;41(12):1231–1235. doi: 10.1016/j.ajic.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Gage J.R., Fonarow G., Hamilton M., Widawski M., Martínez-Maza O., Vredevoe D.L. Beta Blocker and Angiotensin-Converting Enzyme Inhibitor Therapy Is Associated with Decreased Th1/Th2 Cytokine Ratios and Inflammatory Cytokine Production in Patients with Chronic Heart Failure. NeuroImmunoModulation. 2004;11(3):173–180. doi: 10.1159/000076766. [DOI] [PubMed] [Google Scholar]
- Gasmi A., Noor S., Tippairote T., Dadar M., Menzel A., Bjørklund G. Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Chiumello D., Rossi S. COVID-19 pneumonia: ARDS or not? Crit. Care. 2020;24(1):154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudichon, C., Roos, N., Mahe, S., Sick, H., Bouley, C., Tome, D., 1994. Gastric emptying regulates the kinetics of nitrogen absorption from 15N-labeled milk and 15N-labeled yogurt in miniature pigs. J. Nutr. 124, 1970–7. https://doi.org/10.1093/jn/124.10.1970 [DOI] [PubMed]
- Ghosal S., Mukherjee J.J., Sinha B., Gangopadhyay K.K. The effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on death and severity of disease in patients with coronavirus disease 2019 (COVID-19): A meta-analysis. MedRxiv Preprint. 2020 doi: 10.1101/2020.04.23.20076661. [DOI] [Google Scholar]
- Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.-E., Katsaounou P., Ntaganou M., Kyriakopoulou M., Dimopoulos G., Koutsodimitropoulos I., Velissaris D., Koufargyris P., Karageorgos A., Katrini K., Lekakis V., Koutsoukou A. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorgievski N., Tomovska J., Dimitrovska G., Makarijoski B., Shariati M.A. Determination of antioxidant activity in Yogourt. J. Hyg. Eng. Des. 2014;8:88–92. https://www.cabdirect.org/cabdirect/abstract/20153070783 [Google Scholar]
- Gobbetti M., Ferranti P., Smacchi E., Goffredi F., Addeo F. Production of Angiotensin-I-Converting-Enzyme-Inhibitory Peptides in Fermented Milks Started by Lactobacillus delbrueckiisubsp. bulgaricus SS1 and Lactococcus lactissubsp. cremoris FT4. Appl. Environ. Microbiol. 2000;66(9):3898–3904. doi: 10.1128/AEM.66.9.3898-3904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouda A.S., Mégarbane B. Snake venom-derived bradykinin-potentiating peptides: A promising therapy for COVID-19? Drug Dev. Res. 2020;82(1):38–48. doi: 10.1002/ddr.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin-Danan C., Chabanet C., Pedone C., Popot F., Vaissade P., Bouley C., Szylit O., Andrieux C. Milk fermented with yogurt cultures and Lactobacillus casei compared with yogurt and gelled milk: influence on intestinal microflora in healthy infants. Am. J. Clin. Nutr. 1998;67(1):111–117. doi: 10.1093/ajcn/67.1.111. [DOI] [PubMed] [Google Scholar]
- Guillemard E., Tondu F., Lacoin F., Schrezenmeir J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114 001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Brit. J. Nutr. 2010;103(1):58–68. doi: 10.1017/S0007114509991395. [DOI] [PubMed] [Google Scholar]
- Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020;81(5):537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall D.C., Ji H.-F. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Travel Med. Infect. Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M., Gale C.R., Kivimäki M., Batty G.D. Overweight, obesity, and risk of hospitalization for COVID-19: A community-based cohort study of adults in the United Kingdom. Proc. Natl. Acad. Sci. U. S. A. 2020;117(35):21011–21013. doi: 10.1073/pnas.2011086117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y., Yamamoto M., Ohni M., Nakajima K., Nakamura Y., Takano T. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am. J. Clin. Nutr. 1996;64(5):767–771. doi: 10.1093/ajcn/64.5.767. [DOI] [PubMed] [Google Scholar]
- Hernández-Ledesma B., Amigo L., Ramos M., Recio I. Angiotensin Converting Enzyme Inhibitory Activity in Commercial Fermented Products. Formation of Peptides under Simulated Gastrointestinal Digestion. J. Agric. Food Chem. 2004;52(6):1504–1510. doi: 10.1021/jf034997b. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W.-T., Galm B.P., Schrank G., Hsu T.-C., Lee S.-H., Park J.Y., Lee C.-C. Effect of Renin-Angiotensin-Aldosterone System Inhibitors on Short-Term Mortality After Sepsis. Hypertension. 2020;75(2):483–491. doi: 10.1161/HYPERTENSIONAHA.119.13197. [DOI] [PubMed] [Google Scholar]
- Ianzer D., Konno K., Marques-Porto R., Vieira Portaro F.C., Stöcklin R., Martins de Camargo A.C., Pimenta D.C. Identification of five new bradykinin potentiating peptides (BPPs) from Bothrops jararaca crude venom by using electrospray ionization tandem mass spectrometry after a two-step liquid chromatography. Peptides. 2004;25(7):1085–1092. doi: 10.1016/j.peptides.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Infusino F., Marazzato M., Mancone M., Fedele F., Mastroianni C.M., Severino P., Ceccarelli G., Santinelli L., Cavarretta E., Marullo A.G.M., Miraldi F., Carnevale R., Nocella C., Biondi-Zoccai G., Pagnini C., Schiavon S., Pugliese F., Frati G., D’Ettorre G. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients. 2020;12(6):1718. doi: 10.3390/nu12061718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamilloux Y., Henry T., Belot A., Viel S., Fauter M., El Jammal T., Walzer T., François B., Sève P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020;102567 doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena R., Misra A. Balanced diet is a major casualty in COVID-19. Diabetes & Metabolic Syndrome: Clin. Res. Rev. 2020;14(5):1085–1086. doi: 10.1016/j.dsx.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.-M., Bai P., He W., Wu F., Liu X.-F., Han D.-M., Liu S., Yang J.-K. Gender Differences in Patients With COVID-19: Focus on Severity and Mortality. Front. Public Health. 2020;8 doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles P., Levy-Toledano S., Fiat A.-M., Soria C., Gillessen D., Thomaidis A., Dunn F.W., Caen J.P. Analogy between fibrinogen and casein. Effect of an undecapeptide isolated from k-casein on platelet function. Eur. J. Biochem. 1986;158(2):379–382. doi: 10.1111/j.1432-1033.1986.tb09764.x. [DOI] [PubMed] [Google Scholar]
- Kang S.-J., Jung S.I. Age-Related Morbidity and Mortality among Patients with COVID-19. Infect. Chemother. 2020;52(2):154. doi: 10.3947/ic.2020.52.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassaa, I. Al., 2016. New Insights on Antiviral Probiotics: From Research to Applications (I. Al Kassaa (ed.); 1st ed.). Springer US.
- Kawase M., He F., Kubota A., Harata G., Hiramatsu M. Oral administration of lactobacilli from human intestinal tract protects mice against influenza virus infection. Lett. Appl. Microbiol. 2010;51(1):6–10. doi: 10.1111/j.1472-765X.2010.02849.x. [DOI] [PubMed] [Google Scholar]
- Kitazawa H., Tomioka Y., Matsumura K., Aso H., Mizugaki M., Itoh T., Yamaguchi T. Expression of mRNA encoding IFN α in macrophages stimulated with Lactobacillus gasseri. F. E. M. S. Microbiol. Lett. 1994;120(3):315–321. doi: 10.1111/j.1574-6968.1994.tb07052.x. [DOI] [PubMed] [Google Scholar]
- Kopel J., Perisetti A., Gajendran M., Boregowda U., Goyal H. Clinical Insights into the Gastrointestinal Manifestations of COVID-19. Dig. Dis. Sci. 2020;65(7):1932–1939. doi: 10.1007/s10620-020-06362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nature Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudva A., Scheller E.V., Robinson K.M., Crowe C.R., Choi S.M., Slight S.R., Khader S.A., Dubin P.J., Enelow R.I., Kolls J.K., Alcorn J.F. Influenza A Inhibits Th17-Mediated Host Defense against Bacterial Pneumonia in Mice. J. Immunol. 2011;186(3):1666–1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun I., Lebrun F.L.A.S., Henriques O.B., Carmona A.K., Juliano L., Camargo A.C.M. Isolation and characterization of a new bradykinin potentiating octapeptide from casein. Can. J. Physiol. Pharmacol. 1995;73:85–91. doi: 10.1139/y95-012. [DOI] [PubMed] [Google Scholar]
- Lehtoranta L., Pitkäranta A., Korpela R. Probiotics in respiratory virus infections. European J. Clin. Microbiol. Infect. Dis. 2014;33(8):1289–1302. doi: 10.1007/s10096-014-2086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M.Y., Yen C.L. Reactive Oxygen Species and Lipid Peroxidation Product-Scavenging Ability of Yogurt Organisms. J. Dairy Sci. 1999;82(8):1629–1634. doi: 10.3168/jds.S0022-0302(99)75391-9. [DOI] [PubMed] [Google Scholar]
- Liu Y., Tran D.Q., Rhoads J.M. Probiotics in Disease Prevention and Treatment. J. Clin. Pharmacol. 2018;58:S164–S179. doi: 10.1002/jcph.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Expósito I., Quirós A., Amigo L., Recio I. Casein hydrolysates as a source of antimicrobial, antioxidant and antihypertensive peptides. Le Lait. 2007;87(4–5):241–249. doi: 10.1051/lait:2007019. [DOI] [Google Scholar]
- Maeno M., Yamamoto N., Takano T. Identification of an Antihypertensive Peptide from Casein Hydrolysate Produced by a Proteinase from Lactobacillus helveticus CP790. J. Dairy Sci. 1996;79(8):1316–1321. doi: 10.3168/jds.S0022-0302(96)76487-1. [DOI] [PubMed] [Google Scholar]
- Makino S., Ikegami S., Kume A., Horiuchi H., Sasaki H., Orii N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Brit. J. Nutr. 2010;104(7):998–1006. doi: 10.1017/S000711451000173X. [DOI] [PubMed] [Google Scholar]
- Manso M.A., Lopez-Fandino R. Angiotensin I Converting Enzyme-Inhibitory Activity of Bovine, Ovine, and Caprine κ-Casein Macropeptides and Their Tryptic Hydrolysates. J. Food Protect. 2003;66(9):1686–1692. doi: 10.4315/0362-028X-66.9.1686. [DOI] [PubMed] [Google Scholar]
- Maruyama S., Nakagomi K., Tomizuka N., Suzuki H. Angiotensin I-Converting Enzyme Inhibitor Derived from an Enzymatic Hydrolysate of Casein. II. Isolation and Bradykinin-potentiating Activity on the Uterus and the Ileum of Rats. Agric. Biol. Chem. 1985;49(5):1405–1409. doi: 10.1080/00021369.1985.10866901. [DOI] [Google Scholar]
- Meisel H., FitzGerald J. Biofunctional Peptides from Milk Proteins: Mineral Binding and Cytomodulatory Effects. Curr. Pharm. Des. 2003;9(16):1289–1295. doi: 10.2174/1381612033454847. [DOI] [PubMed] [Google Scholar]
- Merenstein D., Murphy M., Fokar A., Hernandez R.K., Park H., Nsouli H., Sanders M.E., Davis B.A., Niborski V., Tondu F., Shara N.M. Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114 001) to decrease the rate of illness in kids: the DRINK study A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial. Eur. J. Clin. Nutr. 2010;64(7):669–677. doi: 10.1038/ejcn.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesbach W. Pathological Role of Angiotensin II in Severe COVID-19. T.H. Open. 2020;04(02):e138–e144. doi: 10.1055/s-0040-1713678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miremadi F., Ayyash M., Sherkat F., Stojanovska L. Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic Lactobacilli and Bifidobacteria. J. Funct. Foods. 2014;9:295–305. doi: 10.1016/j.jff.2014.05.002. [DOI] [Google Scholar]
- Mohanty D.P., Mohapatra S., Misra S., Sahu P.S. Milk derived bioactive peptides and their impact on human health – A review. Saudi J. Biol. Sci. 2016;23(5):577–583. doi: 10.1016/j.sjbs.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni H., Amini S., Abiri B., Kalantar M., Kaydani M., Barati B., Pirabbasi E., Bahrami F. Are history of dietary intake and food habits of patients with clinical symptoms of COVID 19 different from healthy controls? A case–control study. Clin. Nutr. ESPEN. 2021;42:280–285. doi: 10.1016/j.clnesp.2021.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal R., Behare P., Rana R., Kumar A., Kumar M., Arora S., Morotta F., Jain S., Yadav H. Bioactive peptides derived from milk proteins and their health beneficial potentials: an update. Food Funct. 2011;2(1):18–27. doi: 10.1039/C0FO00016G. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yamamoto N., Sakai K., Okubo A., Yamazaki S., Takano T. Purification and Characterization of Angiotensin I-Converting Enzyme Inhibitors from Sour Milk. J. Dairy Sci. 1995;78(4):777–783. doi: 10.3168/jds.S0022-0302(95)76689-9. [DOI] [PubMed] [Google Scholar]
- Nepomuceno M.R., Acosta E., Alburez-Gutierrez D., Aburto J.M., Gagnon A., Turra C.M. Besides population age structure, health and other demographic factors can contribute to understanding the COVID-19 burden. Proc. Natl. Acad. Sci. U. S. A. 2020;117(25):13881–13883. doi: 10.1073/pnas.2008760117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M.S., Martinussen T., Flambard B., Sørensen K.I., Otte J. Peptide profiles and angiotensin-I-converting enzyme inhibitory activity of fermented milk products: Effect of bacterial strain, fermentation pH, and storage time. Int. Dairy J. 2009;19(3):155–165. doi: 10.1016/j.idairyj.2008.10.003. [DOI] [Google Scholar]
- Olaimat A.N., Aolymat I., Al-Holy M., Ayyash M., Abu Ghoush M., Al-Nabulsi A.A., Osaili T., Apostolopoulos V., Liu S.-Q., Shah N.P. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. Npj. Sci. Food. 2020;4(1):17. doi: 10.1038/s41538-020-00078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaya Galán N.N., Ulloa Rubiano J.C., Velez Reyes F.A., Fernandez Duarte K.P., Salas Cárdenas S.P., Gutierrez Fernandez M.F. In vitro antiviral activity of Lactobacillus casei and Bifidobacterium adolescentis against rotavirus infection monitored by NSP 4 protein production. J. Appl. Microbiol. 2016;120(4):1041–1051. doi: 10.1111/jam.13069. [DOI] [PubMed] [Google Scholar]
- Ondetti M.A., Williams N.J., Sabo E., Pluscec J., Weaver E.R., Kocy O. Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochem. 1971;10(22):4033–4039. doi: 10.1021/bi00798a004. [DOI] [PubMed] [Google Scholar]
- Otte J., Shalaby S.M., Zakora M., Pripp A.H., El-Shabrawy S.A. Angiotensin-converting enzyme inhibitory activity of milk protein hydrolysates: Effect of substrate, enzyme and time of hydrolysis. Int. Dairy J. 2007;17(5):488–503. doi: 10.1016/j.idairyj.2006.05.011. [DOI] [Google Scholar]
- Phan L.T., Nguyen T.V., Luong Q.C., Nguyen T.V., Nguyen H.T., Le H.Q., Nguyen T.T., Cao T.M., Pham Q.D. Importation and Human-to-Human Transmission of a Novel Coronavirus in Vietnam. New Engl. J. Med. 2020;382(9):872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto G., Picariello G., Addeo F., Chianese L., Scaloni A., Caira S. Proteolysis and Process-Induced Modifications in Synbiotic Yogurt Investigated by Peptidomics and Phosphopeptidomics. J. Agric. Food Chem. 2020;68(32):8744–8754. doi: 10.1021/acs.jafc.0c02603. [DOI] [PubMed] [Google Scholar]
- Pu F., Guo Y., Li M., Zhu H., Wang S., Shen X., He M., Huang C., He F. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: A randomized controlled open-label trial. Clin. Interv. Aging. 2017;12:1223–1231. doi: 10.2147/CIA.S141518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirós, A., Dávalos, A., Lasunción, M. A., Ramos, M., Recio, I., 2008. Bioavailability of the antihypertensive peptide LHLPLP: Transepithelial flux of HLPLP. Int. Dairy J. 18(3), 279–286. https://doi.org/10.1016/j.idairyj.2007.09.006.
- Rahman, A., Sathi, N. J., 2020. Risk Factors of the Severity of COVID-19: a Meta-Analysis. MedRxiv Preprint. https://doi.org/0.1101/2020.04.30.20086744.
- Rea D., Coppola G., Palma G., Barbieri A., Luciano A., Del Prete P., Rossetti S., Berretta M., Facchini G., Perdonà S., Turco M.C., Arra C. Microbiota effects on cancer: from risks to therapies. Oncotarget. 2018;9(25):17915–17927. doi: 10.18632/oncotarget.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Ronquillo R., Cruz-Guerrero A., Flores-Nájera A., Rodríguez-Serrano G., Gómez-Ruiz L., Reyes-Grajeda J.P., Jiménez-Guzmán J., García-Garibay M. Antithrombotic and angiotensin-converting enzyme inhibitory properties of peptides released from bovine casein by Lactobacillus casei Shirota. Int. Dairy J. 2012;26(2):147–154. doi: 10.1016/j.idairyj.2012.05.002. [DOI] [Google Scholar]
- Rokka T., Syväoja E.-L., Tuominen J., Korhonen H.J.T. Release of bioactive peptides by enzymatic proteolysis of Lactobacillus GG fermented UHT milk. Milchwissenschaft - Milk Sci. Int. 1997;52:675–678. https://jukuri.luke.fi/handle/10024/472839 [Google Scholar]
- Ruocco G., Feola M., Palazzuoli A. Hypertension prevalence in human coronavirus disease: the role of ACE system in infection spread and severity. Int. J. Infect. Dis. 2020;95:373–375. doi: 10.1016/j.ijid.2020.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoie L., Agudelo R.A., Gauthier S.F., Marin J., Pouliot Y. In vitro determination of the release kinetics of peptides and free amino acids during the digestion of food proteins. J. A. O. A. C. Int. 2005;88(3):935–948. http://www.ncbi.nlm.nih.gov/pubmed/16001871 [PubMed] [Google Scholar]
- Seppo L., Jauhiainen T., Poussa T., Korpela R. A fermented milk high in bioactive peptides has a blood pressure–lowering effect in hypertensive subjects. Am. J. Clin. Nut. 2003;77(2):326–330. doi: 10.1093/ajcn/77.2.326. [DOI] [PubMed] [Google Scholar]
- Shida K., Sato T., Iizuka R., Hoshi R., Watanabe O., Igarashi T., Miyazaki K., Nanno M., Ishikawa F. Daily intake of fermented milk with Lactobacillus casei strain Shirota reduces the incidence and duration of upper respiratory tract infections in healthy middle-aged office workers. Eur. J. Nut. 2017;56(1):45–53. doi: 10.1007/s00394-015-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhol F., Sarlon G., Deharo J.-C., Vaïsse B. Downregulation of ACE2 induces overstimulation of the renin–angiotensin system in COVID-19: should we block the renin–angiotensin system? Hypertens. Res. 2020;43(8):854–856. doi: 10.1038/s41440-020-0476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi C.P., Wohlford-Lenane C., Yamaguchi Y., Prindle T., Fulton W.B., Wang S., McCray P.B., Chappell M., Hackam D.J., Jia H. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg 9 bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;314(1):L17–L31. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starosila D., Rybalko S., Varbanetz L., Ivanskaya N., Sorokulova I. Anti-influenza Activity of a Bacillus subtilis Probiotic Strain. Antimicrob. Agents Chemother. 2017;61(7):e00539–17. doi: 10.1128/AAC.00539-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szajewska H., Skórka A., Ruszczyński M., Gieruszczak-Białek D. Meta-analysis: Lactobacillus GG for treating acute gastroenteritis in children - updated analysis of randomised controlled trials. Alim. Pharmacol. Therap. 2013;38(5):467–476. doi: 10.1111/apt.12403. [DOI] [PubMed] [Google Scholar]
- Tagliazucchi D., Martini S., Bellesia A., Conte A. Identification of ACE-inhibitory peptides from Phaseolus vulgaris after in vitro gastrointestinal digestion. Int. J. Food Sci. Nutr. 2015;66(7):774–782. doi: 10.3109/09637486.2015.1088940. [DOI] [PubMed] [Google Scholar]
- Taipale T., Pienihäkkinen K., Isolauri E., Larsen C., Brockmann E., Alanen P., Jokela J., Söderling E. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in infancy. Brit. J. Nut. 2011;105(3):409–416. doi: 10.1017/S0007114510003685. [DOI] [PubMed] [Google Scholar]
- Tang L., Gu S., Gong Y., Li B., Lu H., Li Q., Zhang R., Gao X., Wu Z., Zhang J., Zhang Y., Li L. Clinical Significance of the Correlation between Changes in the Major Intestinal Bacteria Species and COVID-19 Severity. Engineering (Beijing) 2020;6(10):1178–1184. doi: 10.1016/j.eng.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauzin J., Miclo L., Gaillard J.-L. Angiotensin-I-converting enzyme inhibitory peptides from tryptic hydrolysate of bovine α S2 -casein. F. E. B. S. Lett. 2002;531(2):369–374. doi: 10.1016/S0014-5793(02)03576-7. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J., Lindström J., Hyyrynen J., Korpela R., Karhunen M.-L., Mikkola L., Jauhiainen T., Seppo L., Nissinen A. Effect of ingesting sour milk fermented using Lactobacillus helveticus bacteria producing tripeptides on blood pressure in subjects with mild hypertension. J. Hum. Hypertens. 2004;18(11):795–802. doi: 10.1038/sj.jhh.1001745. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk F.L., Netea M.G., van Deuren M., van der Meer J.W., de Mast Q., Brüggemann R.J., van der Hoeven H. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. ELife. 2020;9 doi: 10.7554/eLife.57555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voicu S., Bonnin P., Stépanian A., Chousterman B.G., Le Gall A., Malissin I., Deye N., Siguret V., Mebazaa A., Mégarbane B. High Prevalence of Deep Vein Thrombosis in Mechanically Ventilated COVID-19 Patients. J. Am. Coll. Cardiol. 2020;76(4):480–482. doi: 10.1016/j.jacc.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li C., Xue J., Yang J., Zhang Q., Zhang H., Chen Y. Fermentation characteristics and angiotensin I-converting enzyme–inhibitory activity of Lactobacillus helveticus isolate H9 in cow milk, soy milk, and mare milk. J. Dairy Sci. 2015;98(6):3655–3664. doi: 10.3168/jds.2015-9336. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li X., Ge T., Xiao Y., Liao Y., Cui Y., Zhang Y., Ho W., Yu G., Zhang T. Probiotics for prevention and treatment of respiratory tract infections in children. Medicine. 2016;95(31) doi: 10.1097/MD.0000000000004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Lewis E.D., Pae M., Meydani S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2019;9 doi: 10.3389/fimmu.2018.03160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterol. 2020;158(6):1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Cai H., Shen Y., Ni Q., Chen Y., Hu S., Li J., Wang H., Yu L., Huang H., Qiu Y., Wei G., Fang Q., Zhou J., Sheng J., Liang T., Li L. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(1):147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N. Antihypertensive peptides derived from food proteins. Biopolymers. 1997;43(2):129–134. doi: 10.1002/(SICI)1097-0282(1997)43:2<129::AID-BIP5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Maeno M., Takano T. Purification and Characterization of an Antihypertensive Peptide from a Yogurt-Like Product Fermented by Lactobacillus helveticus CPN4. J. Dairy Sci. 1999;82(7):1388–1393. doi: 10.3168/jds.S0022-0302(99)75364-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Saruta J., Takahashi T., To M., Shimizu T., Hayashi T., Morozumi T., Kubota N., Kamata Y., Makino S., Kano H., Hemmi J., Asami Y., Nagai T., Misawa K., Kato S., Tsukinoki K. Effect of ingesting yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 on influenza virus-bound salivary IgA in elderly residents of nursing homes: a randomized controlled trial. Acta Odontol. Scand. 2019;77(7):517–524. doi: 10.1080/00016357.2019.1609697. [DOI] [PubMed] [Google Scholar]
- Yang J.-K., Lin S.-S., Ji X.-J., Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47(3):193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.-W., Ahn S.-I., Jhoo J.-W., Kim G.-Y. Antioxidant Activity of Yogurt Fermented at Low Temperature and Its Anti-inflammatory Effect on DSS-induced Colitis in Mice. Food Sci. Anim. Resour. 2019;39(1):162–176. doi: 10.5851/kosfa.2019.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Curtis N. Coronavirus Infections in Children Including COVID-19. Pediatr. Infect. Dis. J. 2020;39(5):355–368. doi: 10.1097/INF.0000000000002660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H., Wan Y., Chung A.C.K., Cheung C.P., Chen N., Lai C.K.C., Chen Z., Tso E.Y.K., Fung K.S.C., Chan V., Ling L., Joynt G., Hui D.S.C., Chan F.K.L., Ng S.C. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterol. 2020;159(3):944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]