Abstract
Chiral tertiary boronic esters are important precursors to bioactive compounds and versatile synthetic intermediates to molecules containing quaternary stereocenters. The development of conjugate boryl addition to α,β-unsaturated amide has been hampered by the intrinsic low electrophilicity of the amide group. Here we show the catalytic asymmetric synthesis of enantioenriched tertiary boronic esters through hydroboration of β,β-disubstituted α,β-unsaturated amides. The Rh-catalyzed hydroboration occurs with previously unattainable selectivity to provide tertiary boronic esters in high enantioselectivity. This strategy opens a door for the hydroboration of inert Michael acceptors with high stereocontrol and may provide future applications in the synthesis of biologically active molecules.
Subject terms: Asymmetric catalysis, Asymmetric synthesis, Synthetic chemistry methodology
The development of conjugate boryl addition to α,β-unsaturated amide has been hampered by the intrinsic low electrophilicity of the amide group. Here the authors show a catalytic asymmetric synthesis of enantioenriched tertiary boronic esters through hydroboration of β,β-disubstituted α,β-unsaturated amides.
Introduction
The organoboron compounds have found widespread applications in the design of functional materials and chemical sensors1,2. In addition, organoborons exhibit important biological properties, including antibacterial, anticancer, and antiviral activities3,4. These properties have spurred the development of novel therapeutic agents for drug discovery. Moreover, chiral, non-racemic organoboronates are valuable compounds in organic synthesis. A growing list of methods have been developed that enable stereospecific conversion of these compounds to a broad range of functionalized molecules5–11. Tertiary boronic esters are particularly attractive because they provide rapid access to quaternary stereocenters through subsequent transformations12–19.
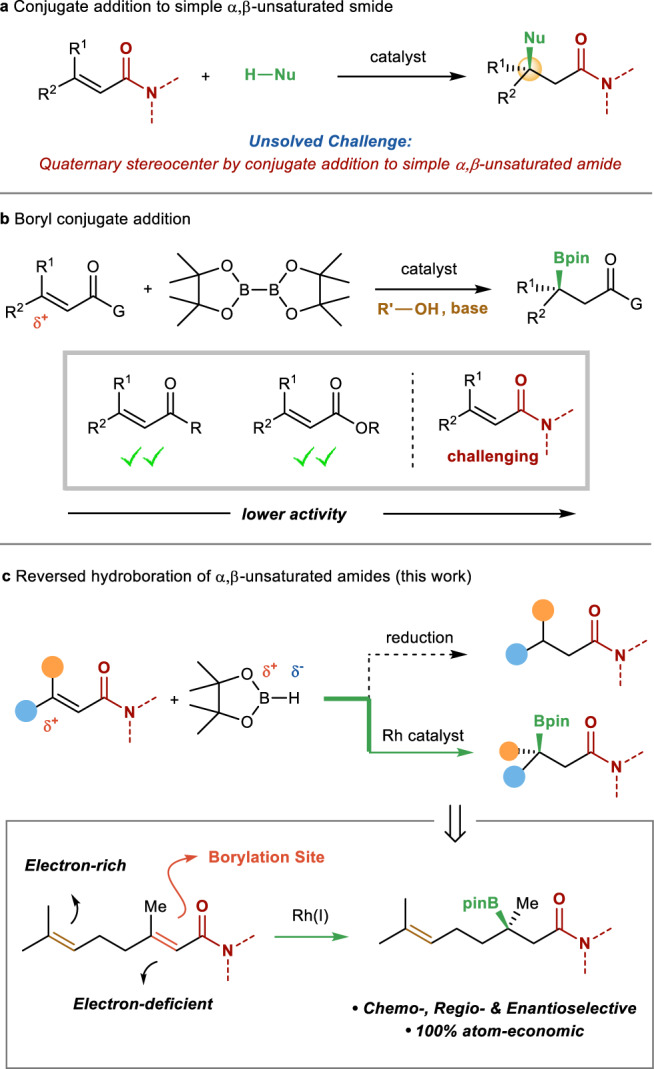
Pioneering work from Yun20,21, Shibasaki22,23, Hoveyda16,24,25, and others26–31 have established metal-catalyzed boryl conjugate addition as a powerful method for the synthesis of chiral tertiary boronic esters. Starting from α,β-unsaturated compounds, such as ketones and esters, tertiary boronic esters were generated in high enantioselectivities32–34. As one of the most important functional groups in organic chemistry, the amide group is ubiquitous in proteins, drugs, and pharmaceutically active compounds35,36. However, due to the intrinsic low electrophilicity of the amide group, the activity of α,β-unsaturated amide is significantly lower as a Michael acceptor37–39. The delocalization of the nitrogen lone pair makes carboxamide the least electron-deficient carboxylic acid derivative. As a result, despite significant advances made in catalytic conjugate additions, currently there are only limited successful examples of catalytic asymmetric conjugate additions to simple α,β-unsaturated amides40–46, and very few of them formed a quaternary stereocenter (Fig. 1a)47. In particular, metal-catalyzed boryl conjugate to simple unsaturated amides has been limited to the synthesis of secondary boronic ester30,48–51. There is one example for a copper-catalyzed enantioselective addition to acyclic β,β-disubstituted α,β-unsaturated amide to form a tertiary boronic ester, in which a β-aryl group is necessary to activate the substrate (Fig. 1b)52. Thus, catalytic asymmetric conjugate addition to inert α,β-unsaturated amides to form quaternary stereocenters remains a significant challenge in asymmetric catalysis. A general protocol for catalytic asymmetric hydroboration of β,β-disubstituted α,β-unsaturated amide to form a tertiary boronic ester remains undeveloped.
Fig. 1. Catalytic hydroboration of alkenes.
a Conjugate addition to α,β-unsaturated amides. b Boryl conjugate addition. c Reversed hydroboration of α,β-unsaturated amides.
We surmised that a metal-catalyzed reversed hydroboration of β,β-disubstituted α,β-unsaturated amide would provide an entry to address this challenge (Fig. 1c)53–56. In this strategy, a hydride is first incorporated at the α position followed by delivery of the boryl group at the β position, which is mechanistically distinct from a boryl conjugate addition (vide infra). In this type of mechanism, the migratory insertion into the metal hydride by an alkene with low polarity may occur, thus overcoming the inherent low electrophilicity of α,β-unsaturated amide. If the regioselectivity is effectively controlled and the enantioface of the alkene is successfully discriminated, this method would enable facile access to chiral tertiary boronic esters from inert α,β-unsaturated amides.
However, we are aware of substantial challenges associated with this design. First, hydroboration of electron-deficient alkenes has been notoriously problematic. Due to the inherent electronic requirement, metal-catalyzed hydroboration of an α,β-unsaturated compound affords a boron enolate57,58. This type of selectivity has long impeded the development of catalytic enantioselective hydroboration of widely existing electron-deficient alkenes. Second, in addition to the electronic requirement that favors the enolate formation, the steric hindrance at the β position also prefers the addition of a small hydride at this site. The formation of a sterically hindered tertiary boronic ester is disfavored. Third, to obtain high enantioselectivity, the catalyst must be able to differentiate between two similar alkyl groups at the β position in the acyclic system. Therefore, in order to achieve the hydroboration of β,β-disubstituted α,β-unsaturated amide, the catalyst must exert substantial control to override both the electronic and steric preferences, and to obtain high enantioselectivity.
We report here a catalytic asymmetric hydroboration of inert α,β-unsaturated amides to construct tertiary boronic esters. The catalyst system we developed is able to (1) reverse the inherent electronic requirement of a hydroboration process, (2) overcome the steric hindrance at the disubstituted β position, and (3) distinguish between the similar steric size of the two β substituents. Starting from easily available materials, this atom-economic process provides a facile method to generate tertiary boronic esters with high regio- and enantioselectivities. This strategy opens a door for the catalytic conjugate addition of inert disubstituted α,β-unsaturated amide to generate quaternary stereocenters.
Results and discussion
Reaction development
To take advantage of amide as a key designing element59–63, we began our study by testing the hydroboration of 1a and pinacolborane (HBpin; Table 1). In the presence of Rh(COD)2OTf, a series of chiral ligands were tested. However, only the reduction product (2a) was detected (entries 1–7). After substantial effort, we found that in the presence of (+)-DIOP ligand L8 (entry 8)64, the reversed hydroboration product 3a was obtained in 45% yield together with reduction product. In addition, the tertiary boronic ester was formed in good enantioselectivity. Subsequently, various solvents were tested (entries 9–12). When the reaction was conducted in 1,2-difluorobenzene, the selectivity was improved, favoring the formation of 3a. Lowering the reaction temperature to 0 °C led to an increase ee of 93% (entry 13). Further lowering the temperature to −20 °C increased the enantioselectivity and suppressed the undesired reduction product (entry 14). Interestingly, the addition of catalytic amount of tert-butanol (tBuOH) further improved the yield (entry 15), although its role in the reaction is still unclear (see the Supplementary information). Lowering the catalyst loading to 2 mol% did not affect the yield (entry 16).
Table 1.
Optimization of reaction conditionsa.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Ligand | Solvent | Temp. (°C) | 2a (yield%) | 3a (yield%) | 3a (ee%) |
| 1 | L1 | THF | 50 | 21 | <5 | — |
| 2 | L2 | THF | 50 | 27 | <5 | — |
| 3 | L3 | THF | 50 | 48 | <5 | — |
| 4 | L4 | THF | 50 | 40 | <5 | — |
| 5 | L5 | THF | 50 | 24 | <5 | — |
| 6 | L6 | THF | 50 | 38 | <5 | — |
| 7 | L7 | THF | 50 | 15 | <5 | — |
| 8 | L8 | THF | 50 | 29 | 45 | 85 |
| 9 | L8 | DCE | 50 | 15 | 7 | 82 |
| 10 | L8 | Toluene | 50 | 35 | 39 | 82 |
| 11 | L8 | 1,4-Dioxane | 50 | 29 | 51 | 86 |
| 12 | L8 | 1,2-C6H4F2 | 50 | 6 | 76 | 85 |
| 13 | L8 | 1,2-C6H4F2 | 0 | 7 | 89 | 93 |
| 14 | L8 | 1,2-C6H4F2 | −20 | <5 | 76 | 96 |
| 15b | L8 | 1,2-C6H4F2 | −20 | <5 | 91 | 96 |
| 16c | L8 | 1,2-C6H4F2 | −20 | <5 | 90 (87) | 96 |
THF tetrahydrofuran, DCE 1,2-dichloroethane.
aReaction performed on 0.20 mmol scale. Yields were determined by GC using n-dodecane as an internal standard. Isolated yield in parenthesis.
b10 mol% tBuOH added.
c2% Rh catalyst, 4 mol% tBuOH.
Reaction scope
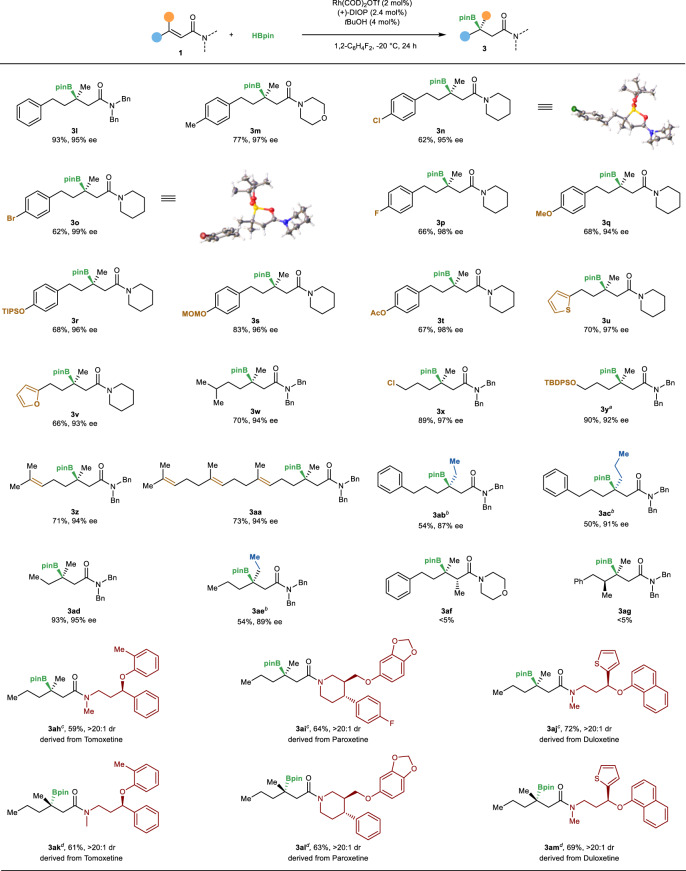
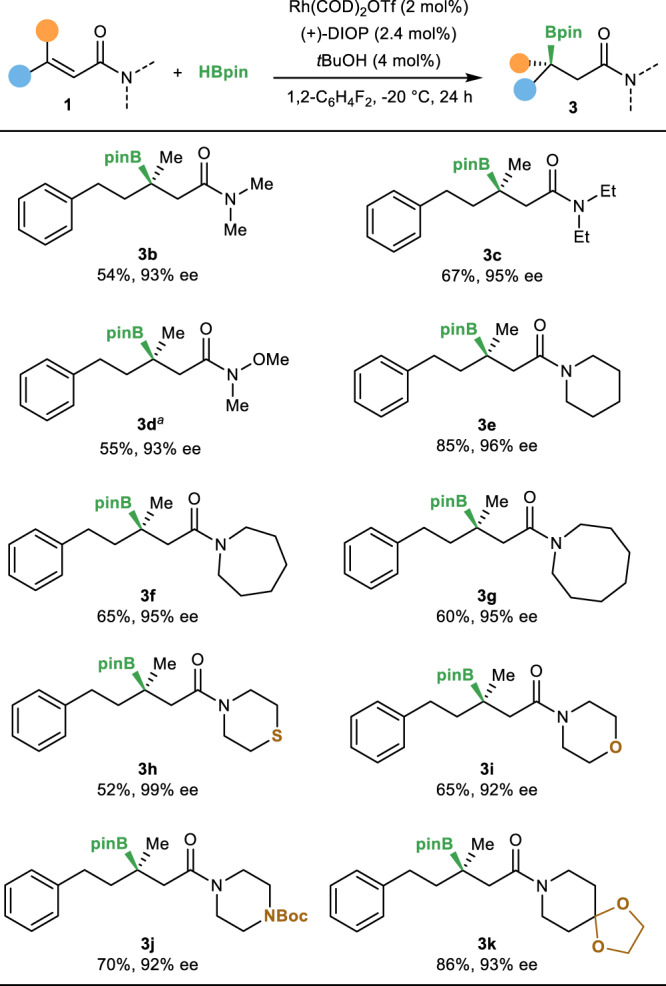
With this simple yet effective conditions in hand, we investigated the scope of various α,β-unsaturated amides. As summarized in Fig. 2, α,β-unsaturated amides derived from a variety of amines underwent the reversed hydroboration to give tertiary boronic esters in good yields and high enantioselectivities. The reaction is applicable to substrates derived from both acyclic amines (3b–3d) and cyclic amines (3e–3k). The successful hydroboration of Weinreb amide (3d) provides an opportunity to access ketone product. Functional groups, including sulfur, ether, carbamate, and acetal, were compatible with this system (3h–3k). However, hydroboration of α,β-unsaturated secondary amide with a free NH group did not provide any desired product.
Fig. 2. Amide scope.
Reversed hydroboration of N-substituted α, β-unsaturated amide. aThe product was isolated after oxidation. Boc t-butyloxy carbonyl.
Next, we sought to explore the reversed hydroborations of various β-disubstituted α,β-unsaturated amides (Fig. 3). A series of β-disubstituted α,β-unsaturated amides underwent regioselective hydroboration to afford the corresponding products in good yields and high enantioselectivities. Aryl and alkyl halide, ether, silyl ether, ester, and heteroarenes were well compatible with the catalytic system (3l–3y). The absolute configurations of compounds 3n and 3o were unambiguously confirmed by X-ray crystallography. In addition, the reaction is highly chemoselective, as demonstrated by the preferential reaction of electron-deficient alkene in the presence of electron-rich ones (3z, 3aa). Furthermore, the catalyst system tolerated not only β-methyl substituent, but also larger ethyl and propyl groups (3ab, 3ac). Importantly, the high enantioselectivities obtained by 3ad and 3ae highlighted the ability of the catalyst to differentiate quite similar groups. However, the attempts to form vicinal quaternary and tertiary stereocenters failed despite significant efforts, likely because of the increased steric hindrance (3af, 3ag).
Fig. 3. Substrate scope.
Reversed hydroboration of β,β-disubstituted α,β-unsaturated amide. a5 mol% Rh catalyst. b7.5 mol% Rh catalyst, 3.5 equivalent of HBpin, 0 °C. The product was isolated after oxidation. c5 mol% Rh catalyst. The product was isolated after oxidation. d5 mol% Rh catalyst, 6.0 mol% (−)-DIOP. The product was isolated after oxidation. TIPS triisopropylsilyl, MOM methoxymethyl, TBDPS t-butyldiphenylsilyl.
The synthetic utility of this reversed hydroboration was further probed by the reactions of amides derived from drug molecules. Hydroboration of α,β-unsaturated amides derived from tomoxetine, paroxetine, and duloxetine generated the products with high diastereoselectivities (3ah, 3ai, 3aj), no racemization occurred for the existing stereocenters. In addition, the stereoisomers of 3ah, 3ai, 3aj were also obtained with high diastereoselectivities, respectively, when we use (−)-DIOP as the ligand.
Transformation of products
The resulting reversed hydroboration products could be further transformed to a series of functional groups (Fig. 4). For example, enantioenriched tertiary alcohol 4 could be obtained by stereospecific oxidation of the boron compound 3i. Treatment of 3i with KHF2 yields the potassium trifluoroborate salt 5 in 83% yield. In addition, tertiary boronic esters underwent C–C bond formation with vinyl Grignard or aryl lithium reagent to afford vinylation and arylation compounds 6 and 7 bearing an all-carbon quaternary stereocenter in high enantioselectivities, respectively. Finally, a homologation of the tertiary boronic ester occurred without erosion of the enantioselectivity (8).
Fig. 4. Synthetic utility.
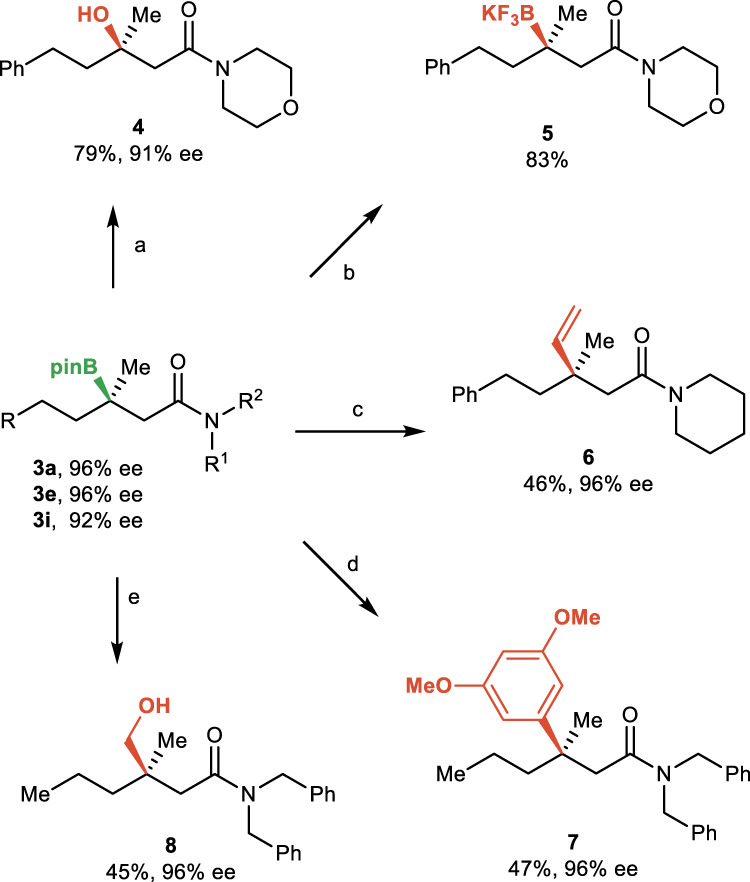
Further transformations of the hydroboration products. Reaction conditions: a NaBO3, H2O, THF; b KHF2, MeCN, H2O; c (1) vinylMgBr, THF, r.t; (2) I2, MeOH, −78 °C; (3) NaOMe, MeOH; d (1) 1-bromo-3,5-dimethoxybenzene, tBuLi, THF, −78 °C; (2) NBS, THF, −78 °C; e (1) ClCH2I, nBuLi, THF, −78 °C; (2) H2O2, NaOH, MeOH, H2O.
Mechanistic studies
To gain insight into the reaction mechanism, a series of control experiments were performed. First, when α,β-unsaturated ester 9 was used, no reversed hydroboration product 9a was observed under the standard reaction conditions (Fig. 5a). Thus, the coordinating ability of an amide group plays a crucial role in reaction system. To probe the possibility of a reaction sequence involving alkene isomerization followed by directed alkene hydroboration, catalytic hydroboration of β,γ-unsaturated amides 10 and 11 were conducted. No hydroboration product was observed when using β,γ-unsaturated amide 10 (Fig. 5b). In addition, the hydroboration of β,γ-unsaturated amide 11 occurred preferentially at the γ position (Fig. 5c). The β-boration product 3i was obtained in low yield and, more importantly, opposite sense of enantioselectivity. These results provide evidence that the reverse hydroboration occurs directly with α,β-unsaturated amides without prior isomerization.
Fig. 5. Control experiments.
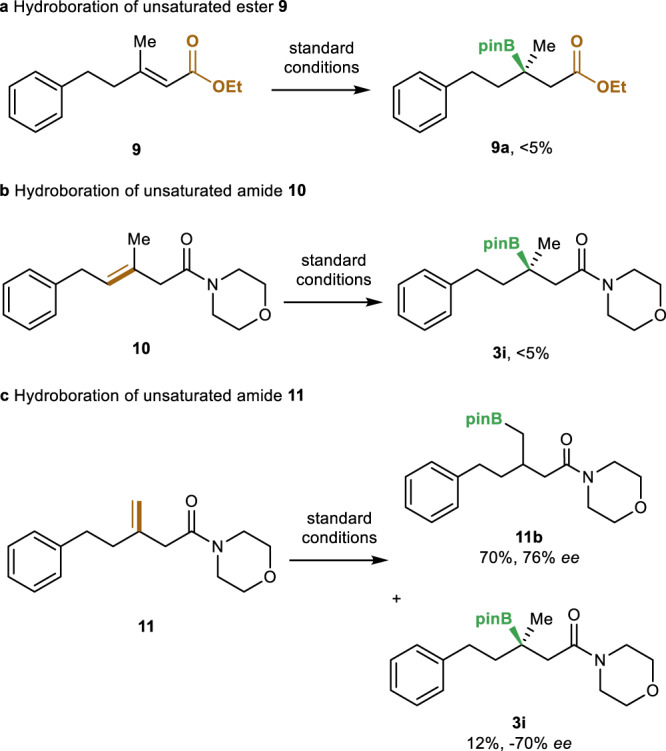
a Hydroboration of unsaturated ester 9. b Hydroboration of unsaturated amide 10. c Hydroboration of unsaturated amide 11.
Computational studies
Computational studies provided information of the energy of each step in the catalytic cycle (Fig. 6). Oxidative addition of HBpin to the amide bound rhodium complex (Int-1) generates a five-coordinated rhodium hydride (Int-2). After migratory insertion of the alkene into the rhodium hydride, an alkyl rhodium complex (Int-3) is formed, in which the amide is coordinated to the metal center. Finally, C–B forming reductive elimination delivers the hydroboration product and regenerates the catalyst through ligand exchange. The calculated energies suggest that the migratory insertion is irreversible and determines the enantioselectivity.
Fig. 6. Proposed mechanism.
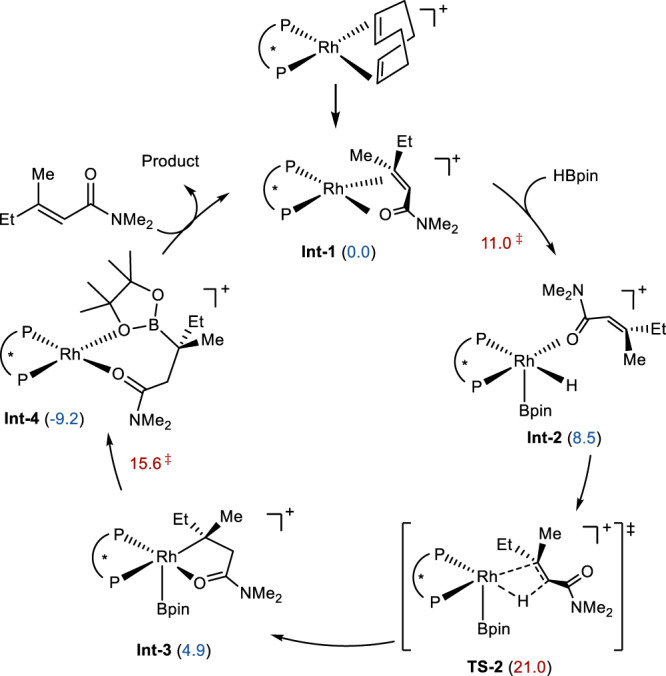
Catalytic cycle with computed free energies of transition states (red) and intermediates (blue) in kcal/mol.
To further understand the origin of enantioselectivity, the energies of different transition states for migration insertion were computed (Fig. 7). The lowest energy pathway leading to the S enantiomer has an activation barrier of 21.0 kcal/mol (TS-2), while the lowest energy pathway leading to the R enantiomer has an activation barrier of 23.4 kcal/mol (TS-2′). The energy difference between TS-2 and TS-2′ (2.4 kcal/mol) correlates with the major enantiomer observed in experiments. In TS-2′, the α,β-unsaturated amide coordinates to rhodium through the opposite enantioface to that in TS-2. In TS-2, the phenyl group on the phosphine atom of the ligand forms two attractive CH…O interaction with the carbonyl group of the substrate (CH…O distance 2.26 and 2.45 Å, respectively)65,66. In contrast, such interaction is not observed in TS-2′ due to the orientation of the carbonyl group. In addition, the phenyl group on the ligand compels the substrate in a way that the ethyl group experiences significant repulsion with the N-methyl group (TS-2′). Therefore, the CH…O interaction with the phenyl group on the ligand in TS-2 and the repulsive interaction in TS-2′ contributes to the relative energies of these transition states, and leads to the high enantioselectivity observed.
Fig. 7. Structures of competing transition states.
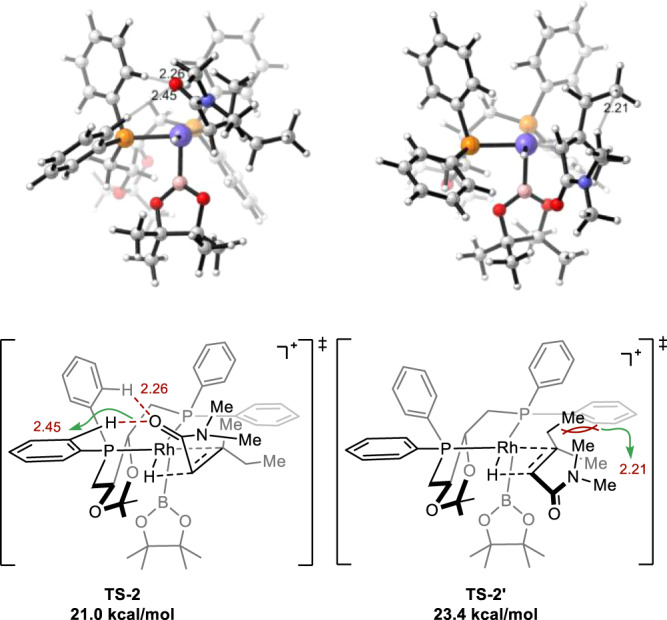
Transition state structures leading to major enantiomer (TS-2) and minor enantiomer (TS-2′).
In summary, we have developed a Rh-catalyzed asymmetric reversed hydroboration to construct chiral tertiary boronic esters. Using a catalyst formed from cationic rhodium and DIOP ligand, hydroboration of β,β-disubstituted α,β-unsaturated amides delivers chiral tertiary boronic esters with high regioselectivity and enantioselectivity. Computation studies revealed the origin of enantioselectivity. Further mechanistic studies and application of this methodology is ongoing in our laboratory.
Methods
General procedure for the catalytic hydroboration
In an Ar-filled glovebox, the α,β-unsaturated amide (0.25 mmol, 1.0 equiv.), Rh(COD)2OTf (2.3 mg, 2.0 mol%), and (+)-DIOP (3.0 mg, 2.4 mol%) were weighed into a one-dram screw-capped vial. Subsequently, 1,2-difluorobenzene (2.5 mL), tBuOH (4.0 mol%), and pinacolborane (80.0 mg, 2.5 equiv.) were added via syringes. The vial was capped with a Teflon-lined screw cap, and the reaction was then removed from the glovebox and stirred at −20 °C for 24 h. Then the solution was concentrated under reduced pressure. After removal of the solvent, the crude product was analyzed by 1H NMR and purified by column chromatography on silica gel with EtOAc/hexanes mixture as eluent.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21971139 and 22022104).
Author contributions
T.-T.G. discovered the reaction and optimized the reaction conditions. T.-T.G., H-.X.L., and P.-C.G. investigated the scope of the substrate. T.-T.G. conducted the DFT calculation. B.-J.L. directed the project and wrote the manuscript with input from all of the authors.
Data availability
The authors declare that all the data supporting the findings of this research are available within the article and its Supplementary Information. Crystallographic data of compounds 3n and 3o data have been deposited in the Cambridge Crystallographic Data Center under accession number CCDC: 2031005 and 2031762.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Jiefeng Hu and Zhuangzhi Shi for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-24012-z.
References
- 1.Mellerup SK, Wang S. Boron-based stimuli responsive materials. Chem. Soc. Rev. 2019;48:3537–3549. doi: 10.1039/C9CS00153K. [DOI] [PubMed] [Google Scholar]
- 2.Bull SD, et al. Exploiting the reversible covalent bonding of boronic acids: recognition, sensing, and assembly. Acc. Chem. Res. 2013;46:312–326. doi: 10.1021/ar300130w. [DOI] [PubMed] [Google Scholar]
- 3.Smoum R, Rubinstein A, Dembitsky VM, Srebnik M. Boron containing compounds as protease inhibitors. Chem. Rev. 2012;112:4156–4220. doi: 10.1021/cr608202m. [DOI] [PubMed] [Google Scholar]
- 4.Touchet S, Carreaux F, Carboni B, Bouillon A, Boucher J-L. Aminoboronic acids and esters: from synthetic challenges to the discovery of unique classes of enzyme inhibitors. Chem. Soc. Rev. 2011;40:3895–3914. doi: 10.1039/c0cs00154f. [DOI] [PubMed] [Google Scholar]
- 5.Leonori D, Aggarwal VK. Stereospecific couplings of secondary and tertiary boronic esters. Angew. Chem. Int. Ed. 2015;54:1082–1096. doi: 10.1002/anie.201407701. [DOI] [PubMed] [Google Scholar]
- 6.Collins BSL, Wilson CM, Myers EL, Aggarwal VK. Asymmetric synthesis of secondary and tertiary boronic esters. Angew. Chem., Int. Ed. 2017;56:11700–11733. doi: 10.1002/anie.201701963. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Shi Z. Methodologies and strategies for selective borylation of C–Het and C–C bonds. Chem. Rev. 2020;120:7348–7398. doi: 10.1021/acs.chemrev.9b00384. [DOI] [PubMed] [Google Scholar]
- 8.Reyes RL, et al. Asymmetric remote C–H borylation of aliphatic amides and esters with a modular iridium catalyst. Science. 2020;369:970. doi: 10.1126/science.abc8320. [DOI] [PubMed] [Google Scholar]
- 9.Scott HK, Aggarwal VK. Highly enantioselective synthesis of tertiary boronic esters and their stereospecific conversion to other functional groups and quaternary stereocentres. Chem. Eur. J. 2011;17:13124–13132. doi: 10.1002/chem.201102581. [DOI] [PubMed] [Google Scholar]
- 10.Matteson DS. Boronic esters in asymmetric synthesis. J. Org. Chem. 2013;78:10009–10023. doi: 10.1021/jo4013942. [DOI] [PubMed] [Google Scholar]
- 11.Sandford C, Aggarwal VK. Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun. 2017;53:5481–5494. doi: 10.1039/C7CC01254C. [DOI] [PubMed] [Google Scholar]
- 12.Stymiest JL, Bagutski V, French RM, Aggarwal VK. Enantiodivergent conversion of chiral secondary alcohols into tertiary alcohols. Nature. 2008;456:778–782. doi: 10.1038/nature07592. [DOI] [PubMed] [Google Scholar]
- 13.Guzman-Martinez A, Hoveyda AH. Enantioselective synthesis of allylboronates bearing a tertiary or quaternary B-substituted stereogenic carbon by NHC-Cu-catalyzed substitution reactions. J. Am. Chem. Soc. 2010;132:10634–10637. doi: 10.1021/ja104254d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pulis AP, Blair DJ, Torres E, Aggarwal VK. Synthesis of enantioenriched tertiary boronic esters by the lithiation/borylation of secondary alkyl benzoates. J. Am. Chem. Soc. 2013;135:16054–16057. doi: 10.1021/ja409100y. [DOI] [PubMed] [Google Scholar]
- 15.Hong K, Liu X, Morken JP. Simple access to elusive α-boryl carbanions and their alkylation: an umpolung construction for organic synthesis. J. Am. Chem. Soc. 2014;136:10581–10584. doi: 10.1021/ja505455z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radomkit S, Hoveyda AH. Enantioselective synthesis of boron-substituted quaternary carbon stereogenic centers through NHC-catalyzed conjugate additions of (pinacolato)boron units to enones. Angew. Chem. Int. Ed. 2014;53:3387–3391. doi: 10.1002/anie.201309982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu N, et al. Synthesis of chiral α-amino tertiary boronic esters by enantioselective hydroboration of α-arylenamides. J. Am. Chem. Soc. 2015;137:6746–6749. doi: 10.1021/jacs.5b03760. [DOI] [PubMed] [Google Scholar]
- 18.Shoba VM, Thacker NC, Bochat AJ, Takacs JM. Synthesis of chiral tertiary boronic esters by oxime-directed catalytic asymmetric hydroboration. Angew. Chem. Int. Ed. 2016;55:1465–1469. doi: 10.1002/anie.201509137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakrabarty S, Takacs JM. Synthesis of chiral tertiary boronic esters: phosphonate-directed catalytic asymmetric hydroboration of trisubstituted alkenes. J. Am. Chem. Soc. 2017;139:6066–6069. doi: 10.1021/jacs.7b02324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J-E, Yun J. Catalytic asymmetric boration of acyclic α,β-unsaturated esters and nitriles. Angew. Chem. Int. Ed. 2008;47:145–147. doi: 10.1002/anie.200703699. [DOI] [PubMed] [Google Scholar]
- 21.Feng X, Yun J. Conjugate boration of β,β-disubstituted unsaturated esters: asymmetric synthesis of functionalized chiral tertiary organoboronic esters. Chem. Eur. J. 2010;16:13609–13612. doi: 10.1002/chem.201002361. [DOI] [PubMed] [Google Scholar]
- 22.Chen IH, Yin L, Itano W, Kanai M, Shibasaki M. Catalytic asymmetric synthesis of chiral tertiary organoboronic esters through conjugate boration of β-substituted cyclic enones. J. Am. Chem. Soc. 2009;131:11664–11665. doi: 10.1021/ja9045839. [DOI] [PubMed] [Google Scholar]
- 23.Chen IH, Kanai M, Shibasaki M. Copper(I)−secondary diamine complex-catalyzed enantioselective conjugate boration of linear β,β-disubstituted enones. Org. Lett. 2010;12:4098–4101. doi: 10.1021/ol101691p. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien JM, Lee K-s, Hoveyda AH. Enantioselective synthesis of boron-substituted quaternary carbons by NHC−Cu-catalyzed boronate conjugate additions to unsaturated carboxylic esters, ketones, or thioesters. J. Am. Chem. Soc. 2010;132:10630–10633. doi: 10.1021/ja104777u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H, Radomkit S, O’Brien JM, Hoveyda AH. Metal-free catalytic enantioselective c–b bond formation: (pinacolato)boron conjugate additions to α,β-unsaturated ketones, esters, weinreb amides, and aldehydes promoted by chiral N-heterocyclic carbenes. J. Am. Chem. Soc. 2012;134:8277–8285. doi: 10.1021/ja302929d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawson, Y. G. et al. Platinum catalysed 1,4-diboration of α,β-unsaturated ketones. Chem. Commun. 33, 2051–2052 (1997).
- 27.Ito H, Yamanaka H, Tateiwa J-I, Hosomi A. Boration of an α,β-enone using a diboron promoted by a copper(I)–phosphine mixture catalyst. Tetrahedron Lett. 2000;41:6821–6825. doi: 10.1016/S0040-4039(00)01161-8. [DOI] [Google Scholar]
- 28.Kubota K, Hayama K, Iwamoto H, Ito H. Enantioselective borylative dearomatization of indoles through copper(I) catalysis. Angew. Chem. Int. Ed. 2015;54:8809–8813. doi: 10.1002/anie.201502964. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K, Ishiyama T, Miyaura N. Addition and coupling reactions of bis(pinacolato)diboron mediated by CuCl in the presence of potassium acetate. Chem. Lett. 2000;29:982–983. doi: 10.1246/cl.2000.982. [DOI] [Google Scholar]
- 30.Shiomi, T., Adachi, T., Toribatake, K., Zhou, L. & Nishiyama, H. Asymmetric β-boration of α,β-unsaturated carbonyl compounds promoted by chiral rhodium–bisoxazolinylphenyl catalysts. Chem. Commun. 45, 5987–5989 (2009). [DOI] [PubMed]
- 31.Lillo V, Geier MJ, Westcott SA, Fernández E. Ni and Pd mediate asymmetric organoboron synthesis with ester functionality at the β-position. Org. Biomol. Chem. 2009;7:4674–4676. doi: 10.1039/b909341a. [DOI] [PubMed] [Google Scholar]
- 32.Schiffner JA, Müther K, Oestreich M. Enantioselective conjugate borylation. Angew. Chem. Int. Ed. 2010;49:1194–1196. doi: 10.1002/anie.200906521. [DOI] [PubMed] [Google Scholar]
- 33.Calow ADJ, Whiting A. Catalytic methodologies for the β-boration of conjugated electron deficient alkenes. Org. Biomol. Chem. 2012;10:5485–5497. doi: 10.1039/c2ob25908g. [DOI] [PubMed] [Google Scholar]
- 34.Chen J-B, Whiting A. Recent advances in copper-catalyzed asymmetric hydroboration of electron-deficient alkenes: methodologies and mechanism. Synthesis. 2018;50:3843–3861. doi: 10.1055/s-0037-1609583. [DOI] [Google Scholar]
- 35.Bray BL. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat. Rev. Drug Discov. 2003;2:587–593. doi: 10.1038/nrd1133. [DOI] [PubMed] [Google Scholar]
- 36.Valeur E, Bradley M. Amide bond formation: beyond the myth of coupling reagents. Chem. Soc. Rev. 2009;38:606–631. doi: 10.1039/B701677H. [DOI] [PubMed] [Google Scholar]
- 37.Vicario, J. L. R. E, Carrillo, L & Uria, U. Organocatalytic Asymmetric Nucleophilic Addition to Electron−Deficient Alkenes, 119–188 (Wiley−VCH, 2014).
- 38.Rodríguez-Fernández M, Yan X, Collados JF, White PB, Harutyunyan SR. Lewis acid enabled copper-catalyzed asymmetric synthesis of chiral β-substituted amides. J. Am. Chem. Soc. 2017;139:14224–14231. doi: 10.1021/jacs.7b07344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y-B, Tian H, Yin L. Copper(I)-catalyzed asymmetric 1,4-conjugate hydrophosphination of α,β-unsaturated amides. J. Am. Chem. Soc. 2020;142:20098–20106. doi: 10.1021/jacs.0c09654. [DOI] [PubMed] [Google Scholar]
- 40.Mauduit, M. B. O, Clavier, H, Crévisy, C & Denicourt-Nowicki, A. Metal-Catalyzed Asymmetric Nucleophilic Addition to Electron−Deficient Alkenes, 189−341 (Wiley−VCH, 2014).
- 41.Heravi MM, Dehghani M, Zadsirjan V. Rh-catalyzed asymmetric 1,4-addition reactions to α,β-unsaturated carbonyl and related compounds: an update. Tetrahedron. Asymmetry. 2016;27:513–588. doi: 10.1016/j.tetasy.2016.05.004. [DOI] [Google Scholar]
- 42.Sakuma S, Miyaura N. Rhodium(I)-catalyzed asymmetric 1,4-addition of arylboronic acids to α,β-unsaturated amides. J. Org. Chem. 2001;66:8944–8946. doi: 10.1021/jo010747n. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki H, Sato I, Yamashita Y, Kobayashi S. Catalytic asymmetric direct-type 1,4-addition reactions of simple amides. J. Am. Chem. Soc. 2015;137:4336–4339. doi: 10.1021/jacs.5b01943. [DOI] [PubMed] [Google Scholar]
- 44.Nemoto T, et al. Catalytic asymmetric epoxidation of α,β-unsaturated amides: efficient synthesis of β-aryl α-hydroxy amides using a one-pot tandem catalytic asymmetric epoxidation−pd-catalyzed epoxide opening process. J. Am. Chem. Soc. 2002;124:14544–14545. doi: 10.1021/ja028454e. [DOI] [PubMed] [Google Scholar]
- 45.Hatano M, Horibe T, Ishihara K. Chiral magnesium(II) binaphtholates as cooperative brønsted/lewis acid–base catalysts for the highly enantioselective addition of phosphorus nucleophiles to α,β-unsaturated esters and ketones. Angew. Chem. Int. Ed. 2013;52:4549–4553. doi: 10.1002/anie.201300938. [DOI] [PubMed] [Google Scholar]
- 46.Yasukawa T, Saito Y, Miyamura H, Kobayashi S. Chiral nanoparticles/lewis acids as cooperative catalysts for asymmetric 1,4-addition of arylboronic acids to α,β-unsaturated amides. Angew. Chem. Int. Ed. 2016;55:8058–8061. doi: 10.1002/anie.201601559. [DOI] [PubMed] [Google Scholar]
- 47.Mazet C, Jacobsen EN. Dinuclear {(salen)Al} complexes display expanded scope in the conjugate cyanation of α,β-unsaturated imides. Angew. Chem. Int. Ed. 2008;47:1762–1765. doi: 10.1002/anie.200704461. [DOI] [PubMed] [Google Scholar]
- 48.Chea H, Sim H-S, Yun J. Copper-catalyzed conjugate addition of diboron reagents to α,β-unsaturated amides: highly reactive copper-1,2- bis(diphenylphosphino)benzene catalyst system. Adv. Synth. Catal. 2009;351:855–858. doi: 10.1002/adsc.200900040. [DOI] [Google Scholar]
- 49.Molander GA, Wisniewski SR, Hosseini-Sarvari M. Synthesis and Suzuki–Miyaura cross-coupling of enantioenriched secondary potassium β-trifluoroboratoamides: catalytic, asymmetric conjugate addition of bisboronic acid and tetrakis(dimethylamino)diboron to α,β-unsaturated carbonyl compounds. Adv. Synth. Catal. 2013;355:3037–3057. doi: 10.1002/adsc.201300640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hirsch-Weil D, Abboud KA, Hong S. Isoquinoline-based chiral monodentate N-heterocyclic carbenes. Chem. Commun. 2010;46:7525–7527. doi: 10.1039/c0cc02211j. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi S, Xu P, Endo T, Ueno M, Kitanosono T. Chiral copper(II)-catalyzed enantioselective boron conjugate additions to α,β-unsaturated carbonyl compounds in water. Angew. Chem. Int. Ed. 2012;51:12763–12766. doi: 10.1002/anie.201207343. [DOI] [PubMed] [Google Scholar]
- 52.Kitanosono T, Xu P, Kobayashi S. Heterogeneous versus homogeneous copper(II) catalysis in enantioselective conjugate-addition reactions of boron in water. Chem. Asian J. 2014;9:179–188. doi: 10.1002/asia.201300997. [DOI] [PubMed] [Google Scholar]
- 53.Gao T-T, Zhang W-W, Sun X, Lu H-X, Li B-J. Stereodivergent synthesis through catalytic asymmetric reversed hydroboration. J. Am. Chem. Soc. 2019;141:4670–4677. doi: 10.1021/jacs.8b13520. [DOI] [PubMed] [Google Scholar]
- 54.Bai X-Y, Zhao W, Sun X, Li B-J. Rhodium-catalyzed regiodivergent and enantioselective hydroboration of enamides. J. Am. Chem. Soc. 2019;141:19870–19878. doi: 10.1021/jacs.9b10578. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z-X, Li B-J. Construction of acyclic quaternary carbon stereocenters by catalytic asymmetric hydroalkynylation of unactivated alkenes. J. Am. Chem. Soc. 2019;141:9312–9320. doi: 10.1021/jacs.9b03027. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z-X, Bai X-Y, Li B-J. Metal-catalyzed substrate-directed enantioselective functionalization of unactivated alkenes. Chin. J. Chem. 2019;37:1174–1180. doi: 10.1002/cjoc.201900308. [DOI] [Google Scholar]
- 57.Evans DA, Fu GC. Conjugate reduction of.alpha.,.beta.-unsaturated carbonyl compounds by catecholborane. J. Org. Chem. 1990;55:5678–5680. doi: 10.1021/jo00309a007. [DOI] [Google Scholar]
- 58.Leutenegger U, Madin A, Pfaltz A. Enantioselective reduction of α,β-unsaturated carboxylates with NaBH4 and catalytic amounts of chiral cobalt semicorrin complexes. Angew. Chem. Int. Ed. Engl. 1989;28:60–61. doi: 10.1002/anie.198900601. [DOI] [Google Scholar]
- 59.Smith SM, Thacker NC, Takacs JM. Efficient amide-directed catalytic asymmetric hydroboration. J. Am. Chem. Soc. 2008;130:3734–3735. doi: 10.1021/ja710492q. [DOI] [PubMed] [Google Scholar]
- 60.Smith SM, Takacs JM. Amide-directed catalytic asymmetric hydroboration of trisubstituted alkenes. J. Am. Chem. Soc. 2010;132:1740–1741. doi: 10.1021/ja908257x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Z, Ni HQ, Zeng T, Engle KM. Catalytic carbo- and aminoboration of alkenyl carbonyl compounds via five- and six-membered palladacycles. J. Am. Chem. Soc. 2018;140:3223–3227. doi: 10.1021/jacs.8b00881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang G, et al. Iridium-catalyzed distal hydroboration of aliphatic internal alkenes. Angew. Chem. Int. Ed. 2019;58:8187–8191. doi: 10.1002/anie.201902464. [DOI] [PubMed] [Google Scholar]
- 63.Bai Z, et al. Palladium-catalyzed amide-directed enantioselective carboboration of unactivated alkenes using a chiral monodentate oxazoline ligand. ACS Catal. 2019;9:6502–6509. doi: 10.1021/acscatal.9b01350. [DOI] [PubMed] [Google Scholar]
- 64.Dang, T. P. & Kagan, H. B. The asymmetric synthesis of hydratropic acid and amino-acids by homogeneous catalytic hydrogenation. J. Chem. Soc. D. 481(1971).
- 65.Huang Z, et al. Arene CH-O hydrogen bonding: a stereocontrolling tool in palladium-catalyzed arylation and vinylation of ketones. Angew. Chem. Int. Ed. 2013;52:4906–4911. doi: 10.1002/anie.201300621. [DOI] [PubMed] [Google Scholar]
- 66.Chen L, Yang Y, Liu L, Gao Q, Xu S. Iridium-catalyzed enantioselective α-C(sp3)–H borylation of azacycles. J. Am. Chem. Soc. 2020;142:12062–12068. doi: 10.1021/jacs.0c06756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all the data supporting the findings of this research are available within the article and its Supplementary Information. Crystallographic data of compounds 3n and 3o data have been deposited in the Cambridge Crystallographic Data Center under accession number CCDC: 2031005 and 2031762.