Abstract
Detachment is the initial and critical step for cancer metastasis. Only the cells that survive from detachment can develop metastases. Following the disruption of cell–extracellular matrix (ECM) interactions, cells are exposed to a totally different chemical and mechanical environment. During which, cells inevitably suffer from multiple stresses, including loss of growth stimuli from ECM, altered mechanical force, cytoskeletal reorganization, reduced nutrient uptake, and increased reactive oxygen species generation. Here we review the impact of these stresses on the anchorage-independent survival and the underlying molecular signaling pathways. Furthermore, its implications in cancer metastasis and treatment are also discussed.
Subject terms: Metastasis, Cell death
Facts
There are four different forms of anchorage-independent survival, such as anoikis, autophagy, entosis, and cell cycle arrest, reported in the literature.
After detaching from the extracellular matrix (ECM), both the nonmalignant and malignant cells will be exposed to multiple stresses, including loss of growth stimuli from ECM, altered mechanical force, cytoskeletal reorganization, reduced nutrient uptake, and increased reactive oxygen species (ROS) generation.
Multiple signaling pathways, including integrin transduction and its downstream signaling pathways, such as paxillin/p130CAS, Ras-ERK, PI3K/AKT, Rho/ROCK, and YAP/TAZ pathway, are activated during detachment and contribute to anchorage-independent survival.
As the initial step of cancer metastasis, anchorage-independent survival shares many similarities with cancer metastasis, especially in regulation and activation of integrin transduction and its downstream signaling pathways.
Blocking integrin transduction and its downstream signaling pathways suppresses cancer metastasis; however, there emerges clinical treatment resistance.
Open questions
Is there any other form of anchorage-independent survival for detached cells? What is the regulation and mechanism in cancer metastasis?
How to establish in vitro models to mimic anchorage-independent survival, in vivo, and to study the regulation and mechanism of anchorage-independent survival?
How can we overcome treatment resistance of targeting therapy in cancer metastasis?
Introduction
Attachment, which mainly depends on cell–ECM interactions, is one of the most important factors regulating cellular morphology, dynamic, behavior, and finally, cell fate in both normal and malignant cells. In normal cells and tissues, there is a dynamic balance between attachment and detachment in maintaining cell survival and homeostasis [1], and disruption of this balance could contribute to malignant transformation [2]. In malignant cells and tissues, both attachment and detachment contribute to cancer progress. Attachment promotes the growth of cancer cells; however, detachment initiates cancer metastasis, which causes 90% of human cancer deaths [3]. Once detached from the ECM, both the normal and transformed cells present a round cell shape and are exposed to a completely different chemical and mechanical environment. In turn, the environmental chemical and mechanical stresses will challenge the cell fate. Regarding that, we first review different forms of anchorage-independent survival reported in the literature. Then we discuss the impact of environmental stresses on anchorage-independent survival during detachment. The underlying molecular signaling pathways involved in anchorage-independent survival are also reviewed. Given their critical role in metastasis, we finally discuss the implications of anchorage-independent survival in cancer metastasis and treatment.
Different forms of anchorage-independent survival
The correlation of cell adherence and growth was first unveiled by MacPherson and Montagnier in 1964 [4]. In 1975, Folkman and Greenspan demonstrated the importance of anchorage for cell growth control [5]. The following researchers proved the importance of anchorage-independent survival in normal tissue dynamics and cancer progress. Four different forms of anchorage-independent survival, including apoptotic cell death (anoikis), nonapoptotic cell death (including autophagy and entosis), and cell cycle arrest, are reported in the literature (Fig. 1).
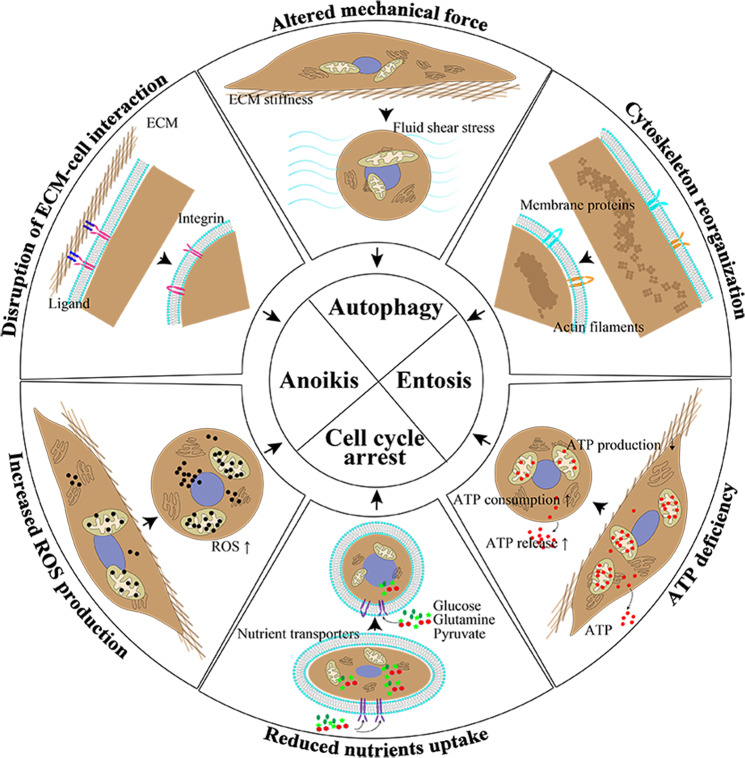
Fig. 1. The anchorage-independent survival and cellular and environmental changes during detachment.
After losing anchorage to ECM, the cells are exposed to a totally different environment and present multiple cellular changes, including disruption of extracellular matrix (ECM)–cell interaction, altered mechanical force, cytoskeleton reorganization, ATP deficiency, reduced nutrient uptake, and increased reactive oxygen species (ROS) production. A Disruption of extracellular matrix (ECM)–cell interaction. Integrins lose the binding of ligands and stimulation from the ECM. B Altered mechanical force. The main mechanical force shifts from ECM stiffness to fluid shear stress. C Cytoskeleton reorganization. The cells present a round cell morphology and the membrane proteins undergo structure deformation and activation. D ATP deficiency. ATP deficiency is a result of reduced ATP production, increased ATP release, and enhanced ATP consumption. E Reduced nutrient uptake. The uptake of glucose, glutamine, and pyruvate is reduced in detached cells due to various reasons. F Increased ROS production. The ROS generation is increased during detachment. As a result, the detached cells undergo anoikis, autophagy, entosis, and cell cycle arrest.
Apoptotic cell death: anoikis
In 1993, Meredith and colleagues found that loss of attachment to ECM could induce cell death in several cell types [6]. Once detached, the endothelial cells and gut epithelial cells presented the typical apoptotic morphology indicated by cell morphological changes and nuclear fragmentation; however, ureteral epithelial cells exhibited a distinct morphology [6]. The apoptotic phenotype in suspended epithelial cells was also observed by Frisch and Francis, and they termed this phenomenon “anoikis”–the ancient Greek word for “homelessness” [7]. The following studies proved its critical role in the homeostasis of skin [8], digestive tract [9], and mammary gland [10], as well as in physiological processes, such as fibrinolysis [11], aortic valves [12, 13], and vascular remodeling [14]. Intrinsic cell death molecules such as Bcl-2 family molecules and cytochrome c, extrinsic cell death molecules such as TNFR, DR5, or Fas, and other signaling molecules such as integrins and EGF reportedly modulate anoikis, see reviews for further information [15, 16].
Nonapoptotic cell death
The results that ureteral epithelial cells exhibited a nonapoptotic morphology from Meredith’s study [6] and that blocking proapoptotic signaling pathway conferred partial but not complete resistance to anoikis [7] imply the presence of nonapoptotic cell death in detached cells.
Autophagy
Autophagy is an adaptive response to a variety of integrated cellular and microenvironmental stresses, such as deprivation of nutrients, oxygen, and growth factor [17, 18]. The presence of autophagy in detached normal human mammary epithelial cells (hMECs) was proved by the observation of cytoplasmic vacuoles in the dying central cells in 3D suspension culture in vitro [1]. Induction of autophagy promoted cell survival in detached nontransformed epithelial cells and primary fibroblasts [19, 20]. Researchers also identified the protective role of autophagy in suspension or spheroid-cultured malignant cells, such as breast cancer, fibrosarcoma, glioma, ovarian cancer, and lung cancer [21–25]. Molecules and signaling pathways regulating redox metabolism and cell growth are critical regulators in autophagy during detachment, so are those involved in cell detachment and cytoskeleton organization, such as ERK/AMPK, mTOR, integrins, and GTPase [17, 26, 27].
Entosis
Entosis is a process involving cell engulfment first observed in detached cells [28]. Overholtzer and colleagues documented cell-in-cell structure and termed as “entosis” in several suspension-cultured nontumorigenic cells and tumor cells. Cell internalization increased with the elongation of cell detachment independent of apoptosis. Moreover, the internalized cells might undergo cell death by lysosomal digestion, division, or release [28]. Once documented, entosis was found in multiple malignant disease, including breast cancer, colon carcinoma, stomach carcinoma, cervical carcinoma, liver carcinoma, melanoma, lung small cell carcinoma, prostate cancer, and pancreatic cancer, in vivo and in vitro [28–34]. Interestingly, the cell-in-cell structure is much more common in fluid-derived cancer samples, in vivo [35–37]. By now, E-cadherin, α-catenin, and RhoA GTPase are necessary and sufficient to induce the formation of cell-in-cell structure, and autophagy pathway proteins are required for entotic cell death [38]. In addition, recent report finds that genetic features are significantly associated with entosis, such as TP53 mutation, KRAS amplification, and c-myc amplification [38, 39].
Cell cycle arrest
Cell cycle arrest with the cease of cell growth and DNA synthesis was also observed in normal and transformed epithelial cells during detachment, which could be reversed by cell reattachment [40]. Similarly, fibroblast cells underwent reversible cell growth withdrawal and arrest of mRNA production and protein synthesis when exposed to suspension condition [41, 42]. Further studies confirmed its presence in normal and transformed epithelial and endothelial cells, fibroblasts, and smooth muscle cells under suspension condition, and it is noteworthy that cells are arrested in G1 phase during detachment [7, 43–46]. It is also worth mentioning that some groups propose cell cycle arrest as one of the mechanisms to acquire anoikis resistance [44, 46, 47]. These studies proved that integrins and cell cycle inhibitors, such as p27 and p57, could induce cell cycle arrest in suspended cells [43–46, 48].
The environmental and cellular stresses during detachment
Disruption of cell–ECM interactions
Cell–ECM interactions mainly depend on the architecture of focal adhesions (FAs) and cytoskeletal proteins. FAs are integrin-based multiprotein complexes composed of ~160 distinct components including activation and inhibition molecules of integrins, signaling molecules (kinase, phosphatases, and G proteins and their regulators), and actin filaments. Cytoskeletal proteins are actin-based structures and regulate cell shape and motility by changing cytoskeleton organization. Physically, FAs interconnect with cytoskeletal proteins via the ends of actin filaments. Functionally, there are feedback networks between cytoskeleton reorganization and integrin activation. For further information, see reviews [49, 50] and Box 1.
It is well established that integrins protect cells against anoikis [15, 16], and worthy to note that the protecting role of different integrins differs in different cell types. For example, integrin αVβ3 is required for angiogenic vascular cell survival during detachment [51]. However, it is dispensable for the survival of suspended MG-63 human osteosarcoma cells [52] and melanoma cells [53]. Moreover, acinar morphogenesis of human breast epithelial cells could be blocked by anti-β1 or anti-α2 integrin antibody but not by anti-α3 integrin antibody [54]. These studies showed that the expression, translation, degradation, and function of integrins in different cell types might account for these differences [54, 55].
As mentioned above, autophagy is increased during detachment and protects cells against stresses from ECM detachment [17, 26]. In suspension conditions, the function of integrin is downregulated because of lacking ligand binding; hence, integrin inhibition by a specific antibody or cilengitide, an αv integrin antagonist, could induce autophagy [56, 57].
Similarly, disregulation of integrin signaling induces cell cycle arrest of suspended cells [44, 47, 58–60]. Deletion of β4 integrin cytoplasmic domain leads to epithelial cells detachment and cell cycle defects, in vivo [60]. Vice versa, overexpression or activation of downstream kinases of integrin pathway, such as protein kinase C, ERK, and ILK, protects cells from cell cycle withdrawal [47, 58, 59].
Altered mechanical forces and cytoskeleton reorganization
Besides reciprocal relations between integrin and cytoskeletal organization, mechanical force is another major factor regulating cytoskeleton dynamics and cell survival [61]. The major source of mechanical force for the attached cells comes from the biophysical property of ECM (e.g. stiffness) and interstitial fluid pressure; however, the main mechanical stress for the detached cells derives from fluid-based mechanics, such as fluid shear flow [62]. Environmental mechanical force induces biochemical signaling cascades through modulating the activation of mechanosensitive proteins and cytoskeletal dynamics, which were termed as “mechanical transduction”. Please refer the reviews for further information [61, 63, 64]. Additionally, mechanical force and cytoskeleton reorganization could directly modulate the protein activity of integrin and its adaptor proteins by regulating their 3D structure and binding affinity [61, 65, 66]. For example, force applied to Notch-ligand bond could also expose a cleavage site of Notch to initiate Notch and integrin signaling activation [66]. Cell membrane deformation induces opening and activation of mechanosensitive PANX1 channels, which permits cell recovery from traumatic deformation [67].
A body of studies have proven that increased mechanical force, either from contracted ECM or increased fluid shear flow, in vitro and in vivo, promotes apoptosis [68], autophagy flux [69], and G1–S cell cycle transition [70, 71]. Despite that the cells in these studies are cultured in attached conditions, it is possible that altered mechanical force and cytoskeleton organization could also modulate anoikis, autophagy, entosis, and cell cycle in a similar manner under detached conditions. Further efforts are needed to establish effective models to investigate the impact of altered fluid shear stress on cell survival in suspension culture.
ATP deficiency, reduced nutrient uptake, and increased reactive oxygen species generation
ATP deficiency, reduced nutrient uptake, and increased reactive oxygen species (ROS) generation are ubiquitous in cells deprived of ECM. ATP deficiency usually results from enhanced ATP releasing, increased ATP consumption, or reduced ATP production. Increased ATP releasing is found in cells activated by shear stress, which could augment mitochondrial ATP generation [72]. Membrane deformation also induces increased ATP releasing, and the released ATP in turn suppresses deformation-induced apoptosis of vascular metastatic breast cancer cells [67]. Under the altered mechanical force, ATP consumption is also enhanced to maintain the dynamics of actin cytoskeleton [73].
Remarkably, ATP production is diminished as the result of decreased nutrient uptake during detachment [74]. Enhanced glucose uptake by ERBB2 overexpression restores ATP production and facilitates cell survival [74]. Restricted uptake of three key carbon sources (glucose, glutamine, and pyruvate) during detachment is also recorded [48]. Thus, the flux through glycolysis, the pentose phosphate pathway, and the TCA cycle is reduced, all of which could be reversed by downregulation of PDK4, an important PDH inhibitor. In addition, the authors find that PDK4 expression is increased in detached cells and correlates with the expression of cell cycle inhibitors (p27 and p57) in 3D suspension culture system, which results in cell growth arrest [48]. The enhanced activity of PDK4 is also found in detached hMECs, and depletion of PDK4 increases glucose oxidation and ROS production, and hence results in heightened anoikis. However, PDK4 is overexpressed in human cancer cells and contributes to anoikis resistance [75]. Similar trends for glutamine metabolism that increased glutaminolytic enzyme GDH1 expression promote ATP production and anoikis resistance has been unveiled as well in detached lung cancer cells [76].
Until now, the precise reasons for reduced nutrient uptake during detachment are largely unknown. Glucose transporter (GLUT) 1 and 4, two members of the major glucose-uptake protein family, are generally stored in cytoplasmatic vesicles and can be transported to the plasma membrane along the actin cytoskeleton [77, 78]. Disrupting actin cytoskeleton assembly results in reduced glucose uptake [78]. Moreover, cytoskeleton reorganization might also impair the activity of nutrient-uptake channels or receptors on the membrane [61, 65]. These results indicate that cytoskeleton reorganization may account for nutrient starvation during detachment.
Despite the above elegant studies, the precise manner in which ROS is modulated during ECM detachment remains incompletely understood. However, the antioxidant defense is enhanced to promote anchorage-independent survival and inhibition of antioxidant defense causes cell death during detachment. For example, the expression of antioxidant enzymes, including catalase and superoxide dismutase (SOD2), is upregulated in detached hMECs, and both antioxidant compounds and overexpression of these antioxidant enzymes decrease the ROS level, enhance ATP generation and promote survival of detached hMECs [79]. What is more, silencing antioxidant gene expression in breast cancer cells results in compromised ATP production and limited anchorage-independent growth [79]. Endoplasmic reticulum (ER) stress signaling pathway is also activated under suspension, and inhibition of PERK and eIF2α, two important regulators in ER stress signaling pathway, decreases anchorage-independent cell survival [21, 80, 81]. More specifically, ATF4, another master transcription factor of ER stress signaling, activates the coordinated program of cytoprotective autophagy and antioxidant response through upregulation of the major antioxidant enzyme heme oxygenase 1 (HO-1), and hence protects detached colorectal fibrosarcoma cells from anoikis and promotes their lung colonization in nude mice [22].
Important signaling pathways contributing to anchorage-independent survival
Integrin transduction and its downstream signaling pathway
Integrin signaling is the major signaling pathway connecting the extracellular and intracellular environment. After losing anchorage to ECM, the environmental factors and intracellular changes, as reviewed above (Fig. 1), directly or indirectly modulate the activation of integrins and their downstream signaling pathways (Fig. 2). The main downstream molecules of integrins are FAK and SFKs. Worthy mentioning, only specific integrins (β1, β3, β5, and α11) were able to stimulate FAK/SFK phosphorylation [82, 83]. Additionally, there are cooperative interactions between integrin/FAK/SFKs pathway and growth receptor pathways. With or without cooperation with growth receptor pathways, FAK/SFK provoke downstream signaling pathways, including paxillin/p130CAS, Ras-ERK, PI3K/AKT, Rho/ROCK, and YAP/TAZ pathways [64, 84, 85].
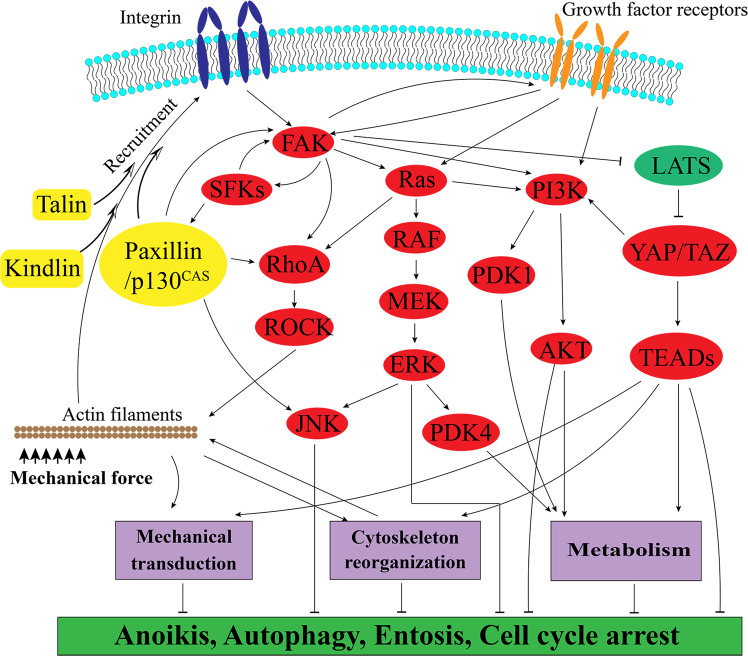
Fig. 2. Molecular pathways sustaining anchorage-independent survival.
During detachment, the ligand–integrin interaction between cells and ECM is disrupted and the cells lose the growth stimuli from ECM. However, the integrin can be also stimulated by mechanical force and cytoskeleton reorganization that actin filaments recruit integrin adaptor proteins, such as talin, kindlin, paxillin, and p130CAS to integrin, hence inducing integrin clustering and activation. The activated integrin induces FAK/SFK activation and its downstream signaling proteins. There is also crosstalk between FAK/SFK and growth receptor signaling, such as EGFR, PDGFR, VEGFR, and IGFR signaling pathway. In cooperation with growth receptor signaling, integrin/FAK/SFKs induces Paxillin/p130CAS/JNK, Ras/ERK, PI3K/AKT, YAP/TAZ, and RhoA/ROCK signaling activation, and hence regulate mechanical transduction, cytoskeleton reorganization, and metabolism. Noteworthy, there is also crosstalk between these downstream signaling pathways. Eventually, activated integrin signaling and its downstream signaling inhibits various forms of cell death, including anoikis, autophagy, cell cycle arrest and entosis.
FAK/SFK-paxillin/ p130CAS bidirectional signaling pathway
Activated by integrins, FAK/SFKs recruit and activate integrin adaptor proteins (IAPs), including talin, kindling, paxillin, and p130CAS (also known as BCAR1) [63, 64, 86, 87]. On the other hand, mechanical force induces conformational changes of actin cytoskeleton and hence triggers recruitment and activation of those IAPs, which in turn phosphorylate and activate FAK and integrin [63, 86, 87]. Overall, it is a bidirectional pathway between FAK/SFKs and paxillin/p130CAS regulating cell survival. As reported, FAK overexpression rescues detached hMECs and fibroblast cells from anoikis [88], while FAK inhibition reverses anoikis resistance and blocks protective autophagy in multiple cancers [89, 90]. The similar role of SFKs in anchorage-independent survival has been identified as well [89–91]. Consistently, paxillin and p130CAS are upregulated in detached cancer cells and involved in FAK/SFK induced cell survival during detachment [92–94]. Notably, it is demonstrated that paxillin is essentially required for facilitating anchorage-independent survival via phosphorylating FAK [95].
Cooperative growth receptor and death receptor signaling
During detachment, integrin/FAK/SFK pathway regulates the expression and activity of growth factor receptors, including EGFR, PDGFR, VEGFR, HGFR, and IGFR, see review [96]. It is also reported that activated growth factor receptor signaling pathways involve in integrin activation and recycling [97–99]. Hence, integrin/ FAK/SFKs and growth receptor pathways are closely cooperated to ensure anchorage-independent survival [24, 96, 100, 101].
On the other hand, there is a crosstalk between integrin/FAK/SFKs and death receptor pathways. For example, receptor-interacting protein (RIP) acts as a key shuttling protein between integrin/FAK signals and Fas/FasL signals. After dissociating from FAK, RIP binds to Fas and forms a death-inducing signaling complex, which activates caspase-3 and eventually results in anoikis [102]. In addition, death receptors such as FasL, DR5, and TNFR, are downregulated or inactivated in suspended cells against anoikis [102, 103].
Ras/ERK signaling pathway
Early study reveals that Ras overexpression induces malignant transformation and protects epithelial cells from anoikis [7]. Ras/ERK upregulation and the de novo Kras mutation are detected in anoikis-resistant endothelial cells and cancer cells [2, 104]. Overexpression of key molecules in Ras/ERK signaling pathway attenuates cellular stress and promotes anchorage-independent survival [48], while Ras/ERK pathway inhibitors reverse anoikis resistance [2, 105]. Moreover, ERK activation, independent of serum and FAK or PAK activity, during detachment is sustained longer than growth factors induced activation [106]. These results indicate the protective and essential role of Ras/ERK signaling in response to detachment Box 1.
Box 1 Integrin activation and its role in cell survival.
The integrins comprise a family of 24 different heterodimers and they are assembled by 18 α and 8 β subunits with distinct ligand-binding specificities and signaling properties [84, 85]. Proteins from ECM, such as laminin, fibronectin, vitronectin, and collagen, are the major ligands of integrins [84]. Ligand-binding triggers integrin activation and initiates integrin-binding adaptor proteins binding to integrin cytoplasmic domain, and hence leads to actin cytoskeleton reorganization, integrin clustering, and fully activation. Consequently, fully activated integrin induces activation of focal adhesion kinase (FAK) and SRC family kinases (SFKs) and their downstream signaling pathways, which in turn control survival, proliferation, autophagy, and other cell fate transitions [64, 84, 85]. Thus, detachment will challenge cellular dynamics, behavior, and cell fate via modulating integrin signaling pathway.
PI3K/AKT signaling pathway
Similarly, PI3K/AKT signaling pathway can be activated by detachment and protects cells from death during detachment [74, 105]. Previous studies demonstrate that PI3K/AKT downstream proapoptotic and antiapoptotic molecules, including Bcl-2, Bak, Bcl-X(L), and Bax, modulate anoikis in transformed and nontransformed cells [7, 15, 16]. The following studies prove that PI3K/AKT signaling pathway, cooperating with or without Ras/ERK signaling, enhances the entry of glucose carbons into the TCA cycle, promotes ATP production, and cell survival during detachment [48, 74]. Interestingly, the finding that overexpression active forms of AKT in PDK1-knockdown breast cancer cells are unable to rescue anchorage-independent growth indicates that PI3K could also be activated by PDK1, which is the downstream target of Ras/ERK signaling [107].
Rho signaling pathway
Rho family small GTPases are key molecules regulating remodeling of the actin cytoskeleton. The central molecules in Rho signaling are RhoA, Rac1, and Cdc42, which can be activated by multiple signaling molecules, such as growth factor receptors, and mechanical forces [64, 108]. Active Cdc42 and Rac1 protect epithelial cells and fibroblasts from anoikis via activation of AKT and ERK signaling pathway [109, 110]. Rho-associated kinase (ROCK), the main effector in Rho signaling pathway, protects anchorage-independent survival, while ROCK inhibitor Y27632 reverses anoikis resistance [111, 112]. Y27632 also reduces entosis and protects cells from lysosomal cell death [28]. Furthermore, a report that ROCK inhibitors reduce damages and improve outcomes in retinal detachment, in vivo [113], also supports the critical role of Rho signaling pathway in anchorage-independent survival.
Hippo signaling pathway
Hippo signaling plays a critical role in mechanical transduction in multiple cancers, for further information, see [114, 115]. YAP and TAZ, two key cotranscription factors in hippo signaling pathway, could be activated by αvβ3 integrin, while the activated YAP/TAZ transcriptionally upregulate GLUT3 expression and increase glucose uptake to support anchorage-independent survival of glioblastoma cells [116]. During detachment, cytoskeleton reorganization activates Lats1/2 and leads to YAP phosphorylation and inactivation, then induces anoikis in nontransformed cells [117]. Additionally, YAP could activate PI3K/AKT signaling pathway via transcriptional regulation of PI3Kcb, a catalytic subunit of PI3K/AKT signaling [118].
The implication of anchorage-independent survival in cancer metastasis
Cancer metastasis is a process that cancer cells detach from the primary site, enter the vascular or lymphatic vessel, localize, and reproduce at remote sites [96, 119]. Thus, anchorage-independent survival is critical for the success of metastasis. While tumor heterogeneity endows tumor cells the potential to survive from various stresses, tumor cells successfully adapt to a stressed environment via activation of the above key signaling pathways that will take the priority of colonization and develop metastasis. Thus, therapies against the above processes or signaling pathways hold the potential to prevent or cure cancer metastasis (Table 1).
Table 1.
The role of signaling pathways in anchorage-independent survival and their implication in the treatment of cancer metastasis.
| Active signaling pathway | Functions during detachment | Activated in cancer metastasis | Efficacy of targeting inhibitors in metastatic patient or animal |
|---|---|---|---|
| Integrin/FAK/SFKs pathway | Protecting cells from anoikis and activating protective autophagy | Activated in a variety of metastasis, including breast cancer, lung cancer, melanoma, colorectal cancer, prostate cancer, glioblastoma, liver cancer [96] | Melanoma [176], breast cancer [177], colorectal cancer [178], ovarian cancer [179], hepatocellular carcinoma [180], non-small cell lung cancer [181], etc. |
| Ras/ERK signaling pathway | Attenuating cellular stress and enhancing anoikis resistance | Colorectal cancer [132, 135], germ-cell tumors [136], multiple myeloma [137], skin cancer [138, 139], melanoma [140] and lung cancer [141] | Thyroid cancer [142, 143], non-small cell lung cancer [144, 182], melanoma [145, 183, 184], colorectal cancer [185], biliary tract cancer [186] |
| PI3K/ATK signaling pathway | Enhancing nutrients uptake, decreasing ROS production and inhibiting anoikis | Colorectal cancer [135, 146], melanoma [147, 148], prostate cancer [149], lung cancer, breast cancer and renal cell carcinomas [150] | Endometrial cancer [155], breast cancer [156, 187–189], gastric cancer [190], cervical carcinoma [191], prostate cancer [192, 193], non-small cell lung cancer [194] and other cancers [195, 196] |
| Rho signaling pathway | Regulating cytoskeleton reorganization and mechanical transduction, protecting cells from anoikis and entosis | Lymphoma [157], colorectal cancer [158, 159], breast cancer [197, 198], liver cancer [199, 200], melanoma [201, 202], pancreatic cancer [203] | Nasopharyngeal cancer [160], pancreatic cancer [161], breast cancer [204], melanoma and colorectal cancer [162] |
| Hippo signaling pathway | Regulating mechanical transduction, enhancing nutrients uptake, protecting from anoikis and autophagy | Breast cancer [165, 205, 206], prostate cancer [117], lung cancer [207], colorectal cancer [208], melanoma [165], gastric cancer [163] | Breast cancer [165, 209] and melanoma [165] |
Integrin/FAK/SFK signaling in metastasis
Numerous studies prove that integrin/FAK/SFK signaling pathway plays a critical role in cancer metastasis [64, 96]. Hence, inhibition of integrin/FAK/SFK signaling seems prospective to prevent metastasis. However, integrin inhibitors failed to show monotherapy efficacy in patients with advanced or metastatic cancer in several clinical trials [120–124]. Given that, researchers explore the combination therapy with other drugs in different cancers and some report combinational efficacy and acceptable toxicity in advanced lung cancer [125–127]. Same as integrin inhibitors, limited clinical efficacy is documented in advanced cancer patients receiving a single FAK/SFK inhibitor [128–131]. Collectively, integrin/FAK/SFK targeting therapy still holds the prospect in metastatic cancer treatment but needs further investigation. It is important to keep in mind that there is more to integrin/FAK/SFK signaling inhibition therapy. First, most integrins play a redundant role in both adhesion and signaling transduction, and there is also compensatory upregulation of a nontargeted integrin. Both of which make it difficult to block these processes with a single drug. Moreover, it is extremely hard to achieve acceptable toxicity in metastasis treatment due to the critical function of integrins in normal tissue homeostasis.
Ras/ERK signaling in metastasis
Genetic extinction of oncogenic Kras signaling results in specific elimination of invasive and metastatic disease while allowing for sustained primary tumor growth [132]. Moreover, oncogenic transformation of Ras in NIH/3T3 generates a metastasis phenotype [133, 134]. Recently, it is clear that Ras/ERK signaling is functionally required for cancer metastasis in colorectal cancer [132, 135], germ-cell tumors [136], multiple myeloma [137], skin cancer [138, 139], melanoma [140], and lung cancer [141]. Clinically, Ras/ERK signaling pathway inhibitors show antitumor activities in untreated BRAF mutant unresectable or metastatic cancers [142–145] and prolong the patients’ survival. Due to increasing application in primary or metastatic cancers, however, treatment resistance of Ras/ERK pathway inhibitors turns to be an inevitable and frustrating issue.
PI3K/AKT signaling in metastasis
Genomic profiling reveals that metastasis specific genetic mutation or activation are enriched in PI3K/AKT signaling pathway in multiple types of cancer, including colorectal cancer [135, 146], melanoma [147, 148], prostate cancer [149], lung cancer, breast cancer, and renal cell carcinomas [150]. Moreover, kinases in PI3K/AKT signaling pathway are activated in circulating tumor cells (CTCs), which are derived from primary sites and developed several years before metastasis [151, 152]. Animal experiments report the efficacy of PI3K/AKT signaling inhibitors in reducing metastasis [153, 154]. Clinically, PI3K/AKT inhibitors demonstrate combinational therapeutic efficacy with other therapies in metastasis or advanced cancers [155, 156]. However, same as Ras/ERK pathway inhibitors, drug resistance is the major obstacle for PI3K/AKT signaling inhibitors in metastasis.
Rho signaling in metastasis
Accumulating evidences indicate that increased activity or expression of Rac1, Cdc42, and ROCK enhances metastatic potential of cancer cells, in vitro and in vivo [157–159]. Several preclinical studies report the efficacy of Rho/ROCK inhibitors in treating metastatic nasopharyngeal, pancreatic carcinoma, and breast cancer [160, 161]. Furthermore, Huang et al. demonstrate that Fasudil, an FDA-approved RhoA/ROCK inhibitor, could reduce metastasis through facilitating the arrest of CTCs [162]. These results imply the potential of Rho signaling inhibitors in metastasis treatment.
YAP/TAZ signaling in metastasis
An abundance of studies demonstrate the high activation of YAP/TAZ signaling pathway in metastatic tumor [115, 117]. In CTCs, YAP transcriptionally upregulates Rho GTPase activation protein 29 (ARHGAP29) and hence promotes metastasis of gastric cancer [163]. Moreover, YAP-dependent metabolic adaptation promotes lymph node metastasis in melanoma patients [164]. Currently, Verteporfin, a YAP/TAZ–TEAD interaction inhibitor, suppresses the prometastasis effect of YAP in breast cancer and melanoma [165]. However, the clinical application of verteporfin in cancer patients is restricted because of global toxicity and low solubility. Also, very slight penetration into the brain, which is one of the most common metastatic sites, is another challenge for verteporfin-treating metastasis.
Targeting mechanical transduction and adaptation
During metastasis, cancer cells suffer from multiple mechanical forces, including fluid shear stress when circulating within the vessel systems and cell deformation when passaging through the microvasculature [62, 67, 166]. More than 90% of cancer cells died due to these mechanical forces [166, 167]. The range of fluid shear stress varies from vessels that are 0.64–12 dyn/cm2 in the lymphatic system, 4–30 dyn/cm2 in arteries, and 1–4 dyn/cm2 in veins [62]. It is well established that high mechanical force leads to cell cycle arrest and even death [62, 168]. In addition, cancer cells undergo mechanical transduction as reviewed above against such restraints [62, 168]. There are also mechanical adapted strategies against mechanical stresses. For example, membrane stretch induced by microvascular deformation induces PANX1 opening and activation, leading to increased ATP releasing, in turn, the released ATP supports cell viability by activating P2Y receptors in microvascular metastatic breast cancer [67]. Hence, inhibition of mechanical transduction and adaptation would decrease the burden of CTCs and hence prevent metastasis.
Suppression of metabolism and antioxidant response
The expression of nutrient transporters such as lipid transporter and antioxidant defense-related genes, is increased in metastatic cancer cells, which promote colonization at lipid-rich tissue, in vivo [169]. Tasdogan and colleagues report that inhibition of MCT1, which transports lactate to maintain pentose phosphate pathway and redox balance, depletes CTCs in melanoma and reduces metastatic burden in patient-derived xenografts [170]. These results imply that inhibition of metabolism and antioxidant response poses the potential in treating cancer metastasis. However, controversary conclusions are found in antioxidant inhibition therapy. Some others report that both systemic antioxidant dosing and activation of cell-intrinsic antioxidant pathways promote metastasis in animal models of melanoma [171], breast cancer [172], and lung cancer [173, 174].
Entosis-targeted therapy in metastatic cancer
It is well documented in various types of cancer that the presence of entosis in metastatic cancers is much common compared with primary cancers [28, 39, 175]. Given that entosis can generate distinct functional cellular entities by division or releasing from cell-in-cell structure, it is reasonable that entosis not only promotes cell viability during metastasis, but also contributes to its heterogeneity and malignancy after colonization. Entosis-targeting therapy presents a high possibility fortifying metastasis treatment. Except Rho/ROCK signaling pathway [28], however, the molecular mechanism of cell-in-cell structure formation and entotic cell death is largely unknown. Recently, Hayashi group reports that genetic features, including TP53 mutation, Kras amplification, and MYC amplification, are significantly associated with entosis in pancreatic cancer [39]. Without doubt, effective in vitro and in vivo models will boost the understanding of the role and regulation of entosis in metastasis, and help metastasis treatment.
Conclusion
Detachment, the initial step of metastasis, is a stressed event and during which cells suffer from multiple stresses, including loss of growth stimuli, altered mechanical force, cytoskeletal reorganization, diminished nutrient uptake, and increased ROS production. Those failed to adapt to these stresses will undergo various forms of cell death, such as anoikis, autophagy, cell cycle arrest, and entosis. Consequently, the majority of cells die; however, a very small number of cells survive, in vitro and in vivo. The survived cells colonize and develop metastasis at the remote sites. Hence, detachment acts as selection and evolution power that imposes cancer cells metastatic potential and promotes malignancy during cancer development.
Previous studies demonstrate that a variety of signaling pathways are upregulated during detachment and required for anchorage-independent survival, such as integrin/FAK/SFKs, Ras/ERK, PI3K/AKT, Rho, YAP/TAZ, and other cooperative signaling pathways. Those pathways are also found to be highly activated in metastatic cancer samples. Thus, the mechanism study of cellular and genetic adaptation to anchorage-independent survival will shed light on the understanding of metastasis and implications for metastasis treatment. Indeed, therapies targeting metabolism, antioxidant response, mechanical transduction, and the related signaling pathways show impressive efficacy in various metastasis models. Clinically, some specific inhibitors against integrin/FAK/SFKs, Ras/ERK and PI3K/AKT signaling pathways could improve the outcome of patients with metastasis. However, apart from limited clinical efficacy and potential toxicity in metastasis treatment, treatment resistance turns to be a critical challenge and obstacle for cancer treatment. Therefore, mechanism and regulation study of anchorage-independent survival will help reveal the mechanism of drug resistance, explore the combinational efficacy, and improve metastasis patient’s survival. Worthy nothing, the efforts to explore the appropriate models, in vitro and in vivo, will accelerate and broaden our understanding of anchorage-independent survival and cancer metastasis.
Acknowledgements
The authors would like to thank Dr. Hai Yu at the Department of Neurosurgery, the First Affiliated Hospital of Xi’an Jiaotong University for editing the manuscript.
Author contributions
Z.D. and N.Z. designed the review. Z.D., H.X.W., J.L.L., and Y.D. undertook the initial research. J.L.L. contributed to financial support. Z.D., H.X.W., and Y.D. designed and drafted the figures. Z.D., Y.D., and N.Z. drafted the manuscript and all authors contributed to the final version. All authors read and approved the final manuscript.
Funding
This work was supported by Guangdong Science and Technology Project, No. 2014A020209025.
Conflict of interest
The authors declare no competing interests.
Footnotes
Edited by G. Blandino
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Consent for publication All authors consent for publication
These authors contributed equally: Zhong Deng, Huixue Wang.
Contributor Information
Yuan Deng, Email: ophden@163.com.
Nu Zhang, Email: zhangnu2@mail.sysu.edu.cn.
References
- 1.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/S0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 2.Derouet M, Wu X, May L, Hoon Yoo B, Sasazuki T, Shirasawa S, et al. Acquisition of anoikis resistance promotes the emergence of oncogenic K-ras mutations in colorectal cancer cells and stimulates their tumorigenicity in vivo. Neoplasia. 2007;9:536–45. doi: 10.1593/neo.07217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christofori G. New signals from the invasive front. Nature. 2006;441:444–50. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 4.Macpherson I, Montagnier L. Agar suspension culture for the selective assay of cells transformed by polyoma virus. Virology. 1964;23:291–4. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J, Greenspan HP. Influence of geometry on control of cell growth. Biochimica Biophys Acta. 1975;417:211–36. doi: 10.1016/0304-419x(75)90011-6. [DOI] [PubMed] [Google Scholar]
- 6.Meredith JE, Jr., Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–61. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polakowska RR, Haake AR. Apoptosis: the skin from a new perspective. Cell Death Differ. 1994;1:19–31. [PubMed] [Google Scholar]
- 9.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107:3569–77. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 10.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–3. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho-Tin-Noé B, Rojas G, Vranckx R, Lijnen HR, Anglés-Cano E. Functional hierarchy of plasminogen kringles 1 and 4 in fibrinolysis and plasmin-induced cell detachment and apoptosis. FEBS J. 2005;272:3387–3400. doi: 10.1111/j.1742-4658.2005.04754.x. [DOI] [PubMed] [Google Scholar]
- 12.Kochtebane N, Choqueux C, Passefort S, Nataf P, Messika-Zeitoun D, Bartagi A, et al. Plasmin induces apoptosis of aortic valvular myofibroblasts. J Pathol. 2010;221:37–48. doi: 10.1002/path.2681. [DOI] [PubMed] [Google Scholar]
- 13.Kochtebane N, Choqueux C, Michel JB, Jacob MP. [Aortic stenosis and extracellular matrix remodeling] Biol Aujourd’hui. 2012;206:135–43. doi: 10.1051/jbio/2012015. [DOI] [PubMed] [Google Scholar]
- 14.Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thrombosis Haemost. 2001;86:324–33. doi: 10.1055/s-0037-1616230. [DOI] [PubMed] [Google Scholar]
- 15.Gilmore AP. Anoikis. Cell Death Differ. 2005;12:1473–7. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 16.Adeshakin FO, Adeshakin AO, Afolabi LO, Yan D, Zhang G, Wan X. Mechanisms for Modulating Anoikis Resistance in Cancer and the Relevance of Metabolic Reprogramming. Front Oncol. 2021;11:626577. doi: 10.3389/fonc.2021.626577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lock R, Debnath J. Extracellular matrix regulation of autophagy. Curr Opin Cell Biol. 2008;20:583–8. doi: 10.1016/j.ceb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroemer G, Mariño G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–93. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishikawa F, Ushida K, Mori K, Shibanuma M. Loss of anchorage primarily induces non-apoptotic cell death in a human mammary epithelial cell line under atypical focal adhesion kinase signaling. Cell Death Dis. 2015;6:e1619. doi: 10.1038/cddis.2014.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell. 2008;19:797–806. doi: 10.1091/mbc.e07-10-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, et al. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011;31:3616–29. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dey S, Sayers CM. Verginadis, II, Lehman SL, Cheng Y, Cerniglia GJ, et al. ATF4-dependent induction of heme oxygenase 1 prevents anoikis and promotes metastasis. J Clin Investig. 2015;125:2592–608. doi: 10.1172/JCI78031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talukdar S, Pradhan AK, Bhoopathi P, Shen XN, August LA, Windle JJ, et al. MDA-9/Syntenin regulates protective autophagy in anoikis-resistant glioma stem cells. Proc Natl Acad Sci USA. 2018;115:5768–73. doi: 10.1073/pnas.1721650115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z, Liu Y, Wei X, Zhou X, Gong C, Zhang T, et al. Co-targeting EGFR and autophagy impairs ovarian cancer cell survival during detachment from the ECM. Curr Cancer Drug Targets. 2015;15:215–26. doi: 10.2174/1568009615666150126161939. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Wang G, Zhou R, Li X, Sun Y, Li Y, et al. SPIB promotes anoikis resistance via elevated autolysosomal process in lung cancer cells. FEBS J. 2020;287:4696–4709. doi: 10.1111/febs.15272. [DOI] [PubMed] [Google Scholar]
- 26.Hawk MA, Schafer ZT. Mechanisms of redox metabolism and cancer cell survival during extracellular matrix detachment. J Biol Chem. 2018;293:7531–7. doi: 10.1074/jbc.TM117.000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kast DJ, Dominguez R. The cytoskeleton-autophagy connection. Curr Biol. 2017;27:R318–R326. doi: 10.1016/j.cub.2017.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131:966–79. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 29.Brouwer M, de Ley L, Feltkamp CA, Elema J, Jongsma AP. Serum-dependent “cannibalism” and autodestruction in cultures of human small cell carcinoma of the lung. Cancer Res. 1984;44:2947–51. [PubMed] [Google Scholar]
- 30.Abodief WT, Dey P, Al-Hattab O. Cell cannibalism in ductal carcinoma of breast. Cytopathology: Off J Br Soc Clin Cytol. 2006;17:304–5. doi: 10.1111/j.1365-2303.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- 31.Krajcovic M, Johnson NB, Sun Q, Normand G, Hoover N, Yao E, et al. A non-genetic route to aneuploidy in human cancers. Nat Cell Biol. 2011;13:324–30. doi: 10.1038/ncb2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwegler M, Wirsing AM, Schenker HM, Ott L, Ries JM, Büttner-Herold M, et al. Prognostic value of homotypic cell internalization by nonprofessional phagocytic cancer cells. Bio Med Res Int. 2015;2015:359392. doi: 10.1155/2015/359392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durgan J, Tseng YY, Hamann JC, Domart MC, Collinson L, Hall A, et al. Mitosis can drive cell cannibalism through entosis. Elife. 2017;6:e27134. doi: 10.7554/eLife.27134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khalkar P, Díaz-Argelich N, Antonio Palop J, Sanmartín C, Fernandes AP. Novel methylselenoesters induce programed cell death via entosis in pancreatic cancer cells. Int J Mol Sci. 2018;19:2849. doi: 10.3390/ijms19102849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta K, Dey P. Cell cannibalism: diagnostic marker of malignancy. Diagn Cytopathol. 2003;28:86–87. doi: 10.1002/dc.10234. [DOI] [PubMed] [Google Scholar]
- 36.Kojima S, Sekine H, Fukui I, Ohshima H. Clinical significance of “cannibalism” in urinary cytology of bladder cancer. Acta Cytol. 1998;42:1365–9. doi: 10.1159/000332169. [DOI] [PubMed] [Google Scholar]
- 37.Hattori M, Nishino Y, Kakinuma H, Matsumoto K, Ohbu M, Okayasu I. Cell cannibalism and nucleus-fragmented cells in voided urine: useful parameters for cytologic diagnosis of low-grade urothelial carcinoma. Acta Cytol. 2007;51:547–51. doi: 10.1159/000325792. [DOI] [PubMed] [Google Scholar]
- 38.Fais S, Overholtzer M. Cell-in-cell phenomena in cancer. Nat Rev Cancer. 2018;18:758–66. doi: 10.1038/s41568-018-0073-9. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi A, Yavas A, McIntyre CA, Ho YJ, Erakky A, Wong W, et al. Genetic and clinical correlates of entosis in pancreatic ductal adenocarcinoma. Mod Pathol: Off J US Can Acad Pathol, Inc. 2020;33:1822–31. doi: 10.1038/s41379-020-0549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoker M, O’Neill C, Berryman S, Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968;3:683–93. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- 41.Otsuka H, Moskowitz M. Arrest of 3T3 cells in G1 phase in suspension culture. J Cell Physiol. 1975;87:213–9. doi: 10.1002/jcp.1040870209. [DOI] [PubMed] [Google Scholar]
- 42.Benecke BJ, Ben-Ze’ev A, Penman S. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell. 1978;14:931–9. doi: 10.1016/0092-8674(78)90347-1. [DOI] [PubMed] [Google Scholar]
- 43.Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–43. doi: 10.1016/S0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- 44.Varner JA, Emerson DA, Juliano RL. Integrin alpha 5 beta 1 expression negatively regulates cell growth: reversal by attachment to fibronectin. Mol Biol Cell. 1995;6:725–40. doi: 10.1091/mbc.6.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–78. doi: 10.1016/S0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 46.Yoo BH, Wu X, Li Y, Haniff M, Sasazuki T, Shirasawa S, et al. Oncogenic ras-induced down-regulation of autophagy mediator Beclin-1 is required for malignant transformation of intestinal epithelial cells. J Biol Chem. 2010;285:5438–49. doi: 10.1074/jbc.M109.046789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins NL, Reginato MJ, Paulus JK, Sgroi DC, Labaer J, Brugge JS. G1/S cell cycle arrest provides anoikis resistance through Erk-mediated bim suppression. Mol Cell Biol. 2005;25:5282–91. doi: 10.1128/MCB.25.12.5282-5291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grassian AR, Metallo CM, Coloff JL, Stephanopoulos G, Brugge JS. Erk regulation of pyruvate dehydrogenase flux through PDK4 modulates cell proliferation. Genes Dev. 2011;25:1716–33. doi: 10.1101/gad.16771811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 50.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–67. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, et al. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–64. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92:6161–5. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montgomery AM, Reisfeld RA, Cheresh DA. Integrin alpha v beta 3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci USA. 1994;91:8856–60. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by beta 1 integrins in normal human breast epithelium but not in breast carcinoma. J Cell Sci. 1995;108:1945–57. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- 55.Alanko J, Mai A, Jacquemet G, Schauer K, Kaukonen R, Saari M, et al. Integrin endosomal signalling suppresses anoikis. Nat Cell Biol. 2015;17:1412–21. doi: 10.1038/ncb3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen N, Debnath J. IκB kinase complex (IKK) triggers detachment-induced autophagy in mammary epithelial cells independently of the PI3K-AKT-MTORC1 pathway. Autophagy. 2013;9:1214–27. doi: 10.4161/auto.24870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lomonaco SL, Finniss S, Xiang C, Lee HK, Jiang W, Lemke N, et al. Cilengitide induces autophagy-mediated cell death in glioma cells. Neuro Oncol. 2011;13:857–65. doi: 10.1093/neuonc/nor073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tibudan SS, Wang Y, Denning MF. Activation of protein kinase C triggers irreversible cell cycle withdrawal in human keratinocytes. J Invest Dermatol. 2002;119:1282–9. doi: 10.1046/j.1523-1747.2002.19625.x. [DOI] [PubMed] [Google Scholar]
- 59.Cho HJ, Youn SW, Cheon SI, Kim TY, Hur J, Zhang SY, et al. Regulation of endothelial cell and endothelial progenitor cell survival and vasculogenesis by integrin-linked kinase. Arterioscler Thromb Vasc Biol. 2005;25:1154–60. doi: 10.1161/01.ATV.0000164312.20008.93. [DOI] [PubMed] [Google Scholar]
- 60.Murgia C, Blaikie P, Kim N, Dans M, Petrie HT, Giancotti FG. Cell cycle and adhesion defects in mice carrying a targeted deletion of the integrin beta4 cytoplasmic domain. EMBO J. 1998;17:3940–51. doi: 10.1093/emboj/17.14.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Case LB, Waterman CM. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat Cell Biol. 2015;17:955–63. doi: 10.1038/ncb3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Follain G, Herrmann D, Harlepp S, Hyenne V, Osmani N, Warren SC, et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat Rev Cancer. 2020;20:107–24. doi: 10.1038/s41568-019-0221-x. [DOI] [PubMed] [Google Scholar]
- 63.Sun Z, Costell M, Fässler R. Integrin activation by talin, kindlin and mechanical forces. Nat Cell Biol. 2019;21:25–31. doi: 10.1038/s41556-018-0234-9. [DOI] [PubMed] [Google Scholar]
- 64.Cooper J, Giancotti FG. Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell. 2019;35:347–67. doi: 10.1016/j.ccell.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–6. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X, Ha T. Defining single molecular forces required to activate integrin and notch signaling. Science. 2013;340:991–4. doi: 10.1126/science.1231041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furlow PW, Zhang S, Soong TD, Halberg N, Goodarzi H, Mangrum C, et al. Mechanosensitive pannexin-1 channels mediate microvascular metastatic cell survival. Nat Cell Biol. 2015;17:943–52. doi: 10.1038/ncb3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shifrin Y, Pinto VI, Hassanali A, Arora PD, McCulloch CA. Force-induced apoptosis mediated by the Rac/Pak/p38 signalling pathway is regulated by filamin A. Biochem J. 2012;445:57–67. doi: 10.1042/BJ20112119. [DOI] [PubMed] [Google Scholar]
- 69.Das J, Chakraborty S, Maiti TK. Mechanical stress-induced autophagic response: a cancer-enabling characteristic? Semin Cancer Biol. 2019;66:101–109. doi: 10.1016/j.semcancer.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 70.Uroz M, Wistorf S, Serra-Picamal X, Conte V, Sales-Pardo M, Roca-Cusachs P, et al. Regulation of cell cycle progression by cell-cell and cell-matrix forces. Nat Cell Biol. 2018;20:646–54. doi: 10.1038/s41556-018-0107-2. [DOI] [PubMed] [Google Scholar]
- 71.Forth S, Kapoor TM. The mechanics of microtubule networks in cell division. J Cell Biol. 2017;216:1525–31. doi: 10.1083/jcb.201612064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamamoto K, Imamura H, Ando J. Shear stress augments mitochondrial ATP generation that triggers ATP release and Ca(2+) signaling in vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2018;315:H1477–h1485. doi: 10.1152/ajpheart.00204.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mehrafrooz B, Shamloo A. Mechanical differences between ATP and ADP actin states: a molecular dynamics study. J Theor Biol. 2018;448:94–103. doi: 10.1016/j.jtbi.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 74.Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, et al. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–13. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kamarajugadda S, Stemboroski L, Cai Q, Simpson NE, Nayak S, Tan M, et al. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol. 2012;32:1893–907. doi: 10.1128/MCB.06248-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jin L, Chun J, Pan C, Kumar A, Zhang G, Ha Y, et al. The PLAG1-GDH1 axis promotes anoikis resistance and tumor metastasis through CamKK2-AMPK signaling in LKB1-deficient lung cancer. Mol Cell. 2018;69:87–99.e87. doi: 10.1016/j.molcel.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanzaki M, Furukawa M, Raab W, Pessin JE. Phosphatidylinositol 4,5-bisphosphate regulates adipocyte actin dynamics and GLUT4 vesicle recycling. J Biol Chem. 2004;279:30622–33. doi: 10.1074/jbc.M401443200. [DOI] [PubMed] [Google Scholar]
- 78.Kim JI, Park J, Ji Y, Jo K, Han SM, Sohn JH, et al. During adipocyte remodeling, lipid droplet configurations regulate insulin sensitivity through F-actin and G-actin reorganization. Mole Cell Biol. 2019;39:e00210–19. doi: 10.1128/MCB.00210-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davison CA, Durbin SM, Thau MR, Zellmer VR, Chapman SE, Diener J, et al. Antioxidant enzymes mediate survival of breast cancer cells deprived of extracellular matrix. Cancer Res. 2013;73:3704–15. doi: 10.1158/0008-5472.CAN-12-2482. [DOI] [PubMed] [Google Scholar]
- 80.Sequeira SJ, Ranganathan AC, Adam AP, Iglesias BV, Farias EF, Aguirre-Ghiso JA. Inhibition of proliferation by PERK regulates mammary acinar morphogenesis and tumor formation. PloS one. 2007;2:e615. doi: 10.1371/journal.pone.0000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sequeira SJ, Wen HC, Avivar-Valderas A, Farias EF, Aguirre-Ghiso JA. Inhibition of eIF2alpha dephosphorylation inhibits ErbB2-induced deregulation of mammary acinar morphogenesis. BMC Cell Biol. 2009;10:64. doi: 10.1186/1471-2121-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Otey CA. pp125FAK in the focal adhesion. Int Rev Cytol. 1996;167:161–83. doi: 10.1016/S0074-7696(08)61347-9. [DOI] [PubMed] [Google Scholar]
- 83.Akiyama SK, Yamada SS, Yamada KM, LaFlamme SE. Transmembrane signal transduction by integrin cytoplasmic domains expressed in single-subunit chimeras. J Biol Chem. 1994;269:15961–4. doi: 10.1016/S0021-9258(17)33955-8. [DOI] [PubMed] [Google Scholar]
- 84.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 85.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 86.Moser M, Legate KR, Zent R, Fässler R. The tail of integrins, talin, and kindlins. Science. 2009;324:895–9. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 87.Goult BT, Yan J, Schwartz MA. Talin as a mechanosensitive signaling hub. J Cell Biol. 2018;217:3776–84. doi: 10.1083/jcb.201808061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–9. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li K, Zhao G, Ao J, Gong D, Zhang J, Chen Y, et al. ZNF32 induces anoikis resistance through maintaining redox homeostasis and activating Src/FAK signaling in hepatocellular carcinoma. Cancer Lett. 2019;442:271–8. doi: 10.1016/j.canlet.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 90.Sandilands E, Serrels B, McEwan DG, Morton JP, Macagno JP, McLeod K, et al. Autophagic targeting of Src promotes cancer cell survival following reduced FAK signalling. Nat Cell Biol. 2011;14:51–60. doi: 10.1038/ncb2386. [DOI] [PubMed] [Google Scholar]
- 91.Aslan B, Monroig P, Hsu MC, Pena GA, Rodriguez-Aguayo C, Gonzalez-Villasana V, et al. The ZNF304-integrin axis protects against anoikis in cancer. Nat Commun. 2015;6:7351. doi: 10.1038/ncomms8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wei L, Yang Y, Zhang X, Yu Q. Anchorage-independent phosphorylation of p130(Cas) protects lung adenocarcinoma cells from anoikis. J Cell Biochem. 2002;87:439–49. doi: 10.1002/jcb.10322. [DOI] [PubMed] [Google Scholar]
- 93.Konstantinovsky S, Davidson B. Reich R. Ezrin and BCAR1/p130Cas mediate breast cancer growth as 3-D spheroids. Clin Exp Metastasis. 2012;29:527–40. doi: 10.1007/s10585-012-9468-2. [DOI] [PubMed] [Google Scholar]
- 94.Zouq NK, Keeble JA, Lindsay J, Valentijn AJ, Zhang L, Mills D, et al. FAK engages multiple pathways to maintain survival of fibroblasts and epithelia: differential roles for paxillin and p130Cas. J Cell Sci. 2009;122:357–67. doi: 10.1242/jcs.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wade R, Brimer N, Lyons C, Vande Pol S. Paxillin enables attachment-independent tyrosine phosphorylation of focal adhesion kinase and transformation by RAS. J Biol Chem. 2011;286:37932–44. doi: 10.1074/jbc.M111.294504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18:533–48. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Onodera Y, Nam JM, Hashimoto A, Norman JC, Shirato H, Hashimoto S, et al. Rab5c promotes AMAP1-PRKD2 complex formation to enhance β1 integrin recycling in EGF-induced cancer invasion. J Cell Biol. 2012;197:983–96. doi: 10.1083/jcb.201201065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, et al. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol Cell. 2000;6:851–60. [PubMed] [Google Scholar]
- 99.De S, Razorenova O, McCabe NP, O’Toole T, Qin J, Byzova TV. VEGF-integrin interplay controls tumor growth and vascularization. Proc Natl Acad Sci USA. 2005;102:7589–94. doi: 10.1073/pnas.0502935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guha D, Saha T, Bose S, Chakraborty S, Dhar S, Khan P, et al. Integrin-EGFR interaction regulates anoikis resistance in colon cancer cells. Apoptosis: Int J Progr. Cell death. 2019;24:958–71. doi: 10.1007/s10495-019-01573-5. [DOI] [PubMed] [Google Scholar]
- 101.Luey BC, May FE. Insulin-like growth factors are essential to prevent anoikis in oestrogen-responsive breast cancer cells: importance of the type I IGF receptor and PI3-kinase/Akt pathway. Mol Cancer. 2016;15:8. doi: 10.1186/s12943-015-0482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kamarajan P, Bunek J, Lin Y, Nunez G, Kapila YL. Receptor-interacting protein shuttles between cell death and survival signaling pathways. Mol Biol Cell. 2010;21:481–8. doi: 10.1091/mbc.e09-06-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aoudjit F, Vuori K. Matrix attachment regulates Fas-induced apoptosis in endothelial cells: a role for c-flip and implications for anoikis. J Cell Biol. 2001;152:633–43. doi: 10.1083/jcb.152.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Patankar M, Eskelinen S, Tuomisto A, Mäkinen MJ, Karttunen TJ. KRAS and BRAF mutations induce anoikis resistance and characteristic 3D phenotypes in Caco‑2 cells. Mol Med Rep. 2019;20:4634–44. doi: 10.3892/mmr.2019.10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Sousa Mesquita AP, de Araújo, Lopes S. Pernambuco Filho PCA, Nader HB, Lopes CC. Acquisition of anoikis resistance promotes alterations in the Ras/ERK and PI3K/Akt signaling pathways and matrix remodeling in endothelial cells. Apoptosis: Int J Progr Cell Death. 2017;22:1116–37. doi: 10.1007/s10495-017-1392-0. [DOI] [PubMed] [Google Scholar]
- 106.Al-Ayoubi A, Tarcsafalvi A, Zheng H, Sakati W, Eblen ST. ERK activation and nuclear signaling induced by the loss of cell/matrix adhesion stimulates anchorage-independent growth of ovarian cancer cells. J Cell Biochem. 2008;105:875–84. doi: 10.1002/jcb.21889. [DOI] [PubMed] [Google Scholar]
- 107.Gagliardi PA, di Blasio L, Orso F, Seano G, Sessa R, Taverna D, et al. 3-phosphoinositide-dependent kinase 1 controls breast tumor growth in a kinase-dependent but Akt-independent manner. Neoplasia. 2012;14:719–31. doi: 10.1593/neo.12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ohashi K, Fujiwara S, Mizuno K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J Biochem. 2017;161:245–54. doi: 10.1093/jb/mvw082. [DOI] [PubMed] [Google Scholar]
- 109.Zugasti O, Rul W, Roux P, Peyssonnaux C, Eychene A, Franke TF, et al. Raf-MEK-Erk cascade in anoikis is controlled by Rac1 and Cdc42 via Akt. Mol Cell Biol. 2001;21:6706–17. doi: 10.1128/MCB.21.19.6706-6717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rul W, Zugasti O, Roux P, Peyssonnaux C, Eychene A, Franke TF, et al. Activation of ERK, controlled by Rac1 and Cdc42 via Akt, is required for anoikis. Ann N Y Acad Sci. 2002;973:145–8. doi: 10.1111/j.1749-6632.2002.tb04624.x. [DOI] [PubMed] [Google Scholar]
- 111.Tanaka AR, Noguchi K, Fukazawa H, Igarashi Y, Arai H, Uehara Y. p38MAPK and Rho-dependent kinase are involved in anoikis induced by anicequol or 25-hydroxycholesterol in DLD-1 colon cancer cells. Biochem Biophys Res Commun. 2013;430:1240–5. doi: 10.1016/j.bbrc.2012.12.067. [DOI] [PubMed] [Google Scholar]
- 112.Bharadwaj S, Thanawala R, Bon G, Falcioni R, Prasad GL. Resensitization of breast cancer cells to anoikis by tropomyosin-1: role of Rho kinase-dependent cytoskeleton and adhesion. Oncogene. 2005;24:8291–303. doi: 10.1038/sj.onc.1208993. [DOI] [PubMed] [Google Scholar]
- 113.Townes-Anderson E, Sugino I, Zarbin M. Using Rho kinase inhibitors for retinal detachment. JAMA Ophthalmol. 2017;135:895. doi: 10.1001/jamaophthalmol.2017.2276. [DOI] [PubMed] [Google Scholar]
- 114.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 115.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cosset É, Ilmjärv S, Dutoit V, Elliott K, von Schalscha T, Camargo MF, et al. Glut3 addiction is a druggable vulnerability for a molecularly defined subpopulation of glioblastoma. Cancer Cell. 2017;32:856–868.e5. doi: 10.1016/j.ccell.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin Z, Zhou P, von Gise A, Gu F, Ma Q, Chen J, et al. Pi3kcb links Hippo-YAP and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ Res. 2015;116:35–45. doi: 10.1161/CIRCRESAHA.115.304457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Strilic B, Offermanns S. Intravascular survival and extravasation of tumor cells. Cancer Cell. 2017;32:282–93. doi: 10.1016/j.ccell.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 120.Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100–8. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 121.Khasraw M, Lee A, McCowatt S, Kerestes Z, Buyse ME, Back M, et al. Cilengitide with metronomic temozolomide, procarbazine, and standard radiotherapy in patients with glioblastoma and unmethylated MGMT gene promoter in ExCentric, an open-label phase II trial. J Neuro-Oncol. 2016;128:163–71. doi: 10.1007/s11060-016-2094-0. [DOI] [PubMed] [Google Scholar]
- 122.Hussain M, Le Moulec S, Gimmi C, Bruns R, Straub J, Miller K. Differential effect on bone lesions of targeting integrins: randomized phase II trial of abituzumab in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res: Off J Am Assoc Cancer Res. 2016;22:3192–3200. doi: 10.1158/1078-0432.CCR-15-2512. [DOI] [PubMed] [Google Scholar]
- 123.Cirkel GA, Kerklaan BM, Vanhoutte F, Van der Aa A, Lorenzon G, Namour F, et al. A dose escalating phase I study of GLPG0187, a broad spectrum integrin receptor antagonist, in adult patients with progressive high-grade glioma and other advanced solid malignancies. Investig. N Drugs. 2016;34:184–92. doi: 10.1007/s10637-015-0320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim KB, Prieto V, Joseph RW, Diwan AH, Gallick GE, Papadopoulos NE, et al. A randomized phase II study of cilengitide (EMD 121974) in patients with metastatic melanoma. Melanoma Res. 2012;22:294–301. doi: 10.1097/CMR.0b013e32835312e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haddad T, Qin R, Lupu R, Satele D, Eadens M, Goetz MP, et al. A phase I study of cilengitide and paclitaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2017;79:1221–7. doi: 10.1007/s00280-017-3322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Massabeau C, Khalifa J, Filleron T, Modesto A, Bigay-Gamé L, Plat G, et al. Continuous infusion of cilengitide plus chemoradiotherapy for patients with stage III non-small-cell lung cancer: a phase I study. Clin Lung Cancer. 2018;19:e277–e285. doi: 10.1016/j.cllc.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 127.Vansteenkiste J, Barlesi F, Waller CF, Bennouna J, Gridelli C, Goekkurt E, et al. Cilengitide combined with cetuximab and platinum-based chemotherapy as first-line treatment in advanced non-small-cell lung cancer (NSCLC) patients: results of an open-label, randomized, controlled phase II study (CERTO) Ann Oncol: Off J Eur Soc Med Oncol. 2015;26:1734–40. doi: 10.1093/annonc/mdv219. [DOI] [PubMed] [Google Scholar]
- 128.Mayer EL, Baurain JF, Sparano J, Strauss L, Campone M, Fumoleau P, et al. A phase 2 trial of dasatinib in patients with advanced HER2-positive and/or hormone receptor-positive breast cancer. Clin Cancer Res: Off J Am Assoc Cancer Res. 2011;17:6897–904. doi: 10.1158/1078-0432.CCR-11-0070. [DOI] [PubMed] [Google Scholar]
- 129.Johnson FM, Bekele BN, Feng L, Wistuba I, Tang XM, Tran HT, et al. Phase II study of dasatinib in patients with advanced non-small-cell lung cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28:4609–15. doi: 10.1200/JCO.2010.30.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shimizu T, Fukuoka K, Takeda M, Iwasa T, Yoshida T, Horobin J, et al. A first-in-Asian phase 1 study to evaluate safety, pharmacokinetics and clinical activity of VS-6063, a focal adhesion kinase (FAK) inhibitor in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2016;77:997–1003. doi: 10.1007/s00280-016-3010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Jonge MJA, Steeghs N, Lolkema MP, Hotte SJ, Hirte HW, van der Biessen DAJ, et al. Phase I study of BI 853520, an inhibitor of focal adhesion kinase, in patients with advanced or metastatic nonhematologic malignancies. Target Oncol. 2019;14:43–55. doi: 10.1007/s11523-018-00617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boutin AT, Liao WT, Wang M, Hwang SS, Karpinets TV, Cheung H, et al. Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev. 2017;31:370–82. doi: 10.1101/gad.293449.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bradley MO, Kraynak AR, Storer RD, Gibbs JB. Experimental metastasis in nude mice of NIH 3T3 cells containing various ras genes. Proc Natl Acad Sci USA. 1986;83:5277–81. doi: 10.1073/pnas.83.14.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Greig RG, Koestler TP, Trainer DL, Corwin SP, Miles L, Kline T, et al. Tumorigenic and metastatic properties of “normal” and ras-transfected NIH/3T3 cells. Proc Natl Acad Sci USA. 1985;82:3698–701. doi: 10.1073/pnas.82.11.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vermaat JS, Nijman IJ, Koudijs MJ, Gerritse FL, Scherer SJ, Mokry M, et al. Primary colorectal cancers and their subsequent hepatic metastases are genetically different: implications for selection of patients for targeted treatment. Clin Cancer Res: Off J Am Assoc Cancer Res. 2012;18:688–99. doi: 10.1158/1078-0432.CCR-11-1965. [DOI] [PubMed] [Google Scholar]
- 136.Taylor-Weiner A, Zack T, O’Donnell E, Guerriero JL, Bernard B, Reddy A, et al. Genomic evolution and chemoresistance in germ-cell tumours. Nature. 2016;540:114–8. doi: 10.1038/nature20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mishima Y, Paiva B, Shi J, Park J, Manier S, Takagi S, et al. The mutational landscape of circulating tumor cells in multiple myeloma. Cell Rep. 2017;19:218–24. doi: 10.1016/j.celrep.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McCreery MQ, Halliwill KD, Chin D, Delrosario R, Hirst G, Vuong P, et al. Evolution of metastasis revealed by mutational landscapes of chemically induced skin cancers. Nat Med. 2015;21:1514–20. doi: 10.1038/nm.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nassar D, Latil M, Boeckx B, Lambrechts D, Blanpain C. Genomic landscape of carcinogen-induced and genetically induced mouse skin squamous cell carcinoma. Nat Med. 2015;21:946–54. doi: 10.1038/nm.3878. [DOI] [PubMed] [Google Scholar]
- 140.Krepler C, Sproesser K, Brafford P, Beqiri M, Garman B, Xiao M, et al. A comprehensive patient-derived xenograft collection representing the heterogeneity of melanoma. Cell Rep. 2017;21:1953–67. doi: 10.1016/j.celrep.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cui R, Meng W, Sun HL, Kim T, Ye Z, Fassan M, et al. MicroRNA-224 promotes tumor progression in nonsmall cell lung cancer. Proc Natl Acad Sci USA. 2015;112:E4288–4297. doi: 10.1073/pnas.1502068112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, et al. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:1272–82. doi: 10.1016/S1470-2045(16)30166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2018;36:7–13. doi: 10.1200/JCO.2017.73.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland Å, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–16. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 145.Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381:626–36. doi: 10.1056/NEJMoa1904059. [DOI] [PubMed] [Google Scholar]
- 146.Ishaque N, Abba ML, Hauser C, Patil N, Paramasivam N, Huebschmann D, et al. Whole genome sequencing puts forward hypotheses on metastasis evolution and therapy in colorectal cancer. Nat Commun. 2018;9:4782. doi: 10.1038/s41467-018-07041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shain AH, Joseph NM, Yu R, Benhamida J, Liu S, Prow T, et al. Genomic and transcriptomic analysis reveals incremental disruption of key signaling pathways during melanoma evolution. Cancer Cell. 2018;34:45–55.e4. doi: 10.1016/j.ccell.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE, et al. β-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell. 2011;20:741–54. doi: 10.1016/j.ccr.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wyatt AW, Annala M, Aggarwal R, Beja K, Feng F, Youngren J, et al. Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J Natl Cancer Inst. 2017;109:djx118. doi: 10.1093/jnci/djx118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5:1164–77. doi: 10.1158/2159-8290.CD-15-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kallergi G, Agelaki S, Kalykaki A, Stournaras C, Mavroudis D, Georgoulias V. Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients. Breast Cancer Res. 2008;10:R80. doi: 10.1186/bcr2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Keup C, Mach P, Aktas B, Tewes M, Kolberg HC, Hauch S, et al. RNA profiles of circulating tumor cells and extracellular vesicles for therapy stratification of metastatic breast cancer patients. Clin Chem. 2018;64:1054–62. doi: 10.1373/clinchem.2017.283531. [DOI] [PubMed] [Google Scholar]
- 153.Ooms LM, Binge LC, Davies EM, Rahman P, Conway JR, Gurung R, et al. The inositol Polyphosphate 5-Phosphatase PIPP regulates AKT1-dependent breast cancer growth and metastasis. Cancer Cell. 2015;28:155–69. doi: 10.1016/j.ccell.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 154.Tenbaum SP, Ordóñez-Morán P, Puig I, Chicote I, Arqués O, Landolfi S, et al. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med. 2012;18:892–901. doi: 10.1038/nm.2772. [DOI] [PubMed] [Google Scholar]
- 155.Oza AM, Elit L, Tsao MS, Kamel-Reid S, Biagi J, Provencher DM, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC clinical trials group. J Clin Oncol: Off J Am Soc Clin Oncol. 2011;29:3278–85. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Baselga J, Im SA, Iwata H, Cortés J, De Laurentiis M, Jiang Z, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:904–16. doi: 10.1016/S1470-2045(17)30376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Campbell H, Fleming N, Roth I, Mehta S, Wiles A, Williams G, et al. 133p53 isoform promotes tumour invasion and metastasis via interleukin-6 activation of JAK-STAT and RhoA-ROCK signalling. Nat Commun. 2018;9:254. doi: 10.1038/s41467-017-02408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Anderson S, Poudel KR, Roh-Johnson M, Brabletz T, Yu M, Borenstein-Auerbach N, et al. MYC-nick promotes cell migration by inducing fascin expression and Cdc42 activation. Proc Natl Acad Sci USA. 2016;113:E5481–5490. doi: 10.1073/pnas.1610994113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhu GF, Xu YW, Li J, Niu HL, Ma WX, Xu J, et al. Mir20a/106a-WTX axis regulates RhoGDIa/CDC42 signaling and colon cancer progression. Nat Commun. 2019;10:112. doi: 10.1038/s41467-018-07998-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 160.Yuan J, Chen L, Xiao J, Qi XK, Zhang J, Li X, et al. SHROOM2 inhibits tumor metastasis through RhoA-ROCK pathway-dependent and -independent mechanisms in nasopharyngeal carcinoma. Cell Death Dis. 2019;10:58. doi: 10.1038/s41419-019-1325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vennin C, Chin VT, Warren SC, Lucas MC, Herrmann D, Magenau A. Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci Transl Med. 2017;9:eaai8504. doi: 10.1126/scitranslmed.aai8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Huang X, Yang Y, Zhao Y, Cao D, Ai X, Zeng A, et al. RhoA-stimulated intra-capillary morphology switch facilitates the arrest of individual circulating tumor cells. Int J Cancer. 2018;142:2094–105. doi: 10.1002/ijc.31238. [DOI] [PubMed] [Google Scholar]
- 163.Qiao Y, Chen J, Lim YB, Finch-Edmondson ML, Seshachalam VP, Qin L, et al. YAP regulates actin dynamics through ARHGAP29 and promotes metastasis. Cell Rep. 2017;19:1495–502. doi: 10.1016/j.celrep.2017.04.075. [DOI] [PubMed] [Google Scholar]
- 164.Lee CK, Jeong SH, Jang C, Bae H, Kim YH, Park I, et al. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science. 2019;363:644–9. doi: 10.1126/science.aav0173. [DOI] [PubMed] [Google Scholar]
- 165.Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA. 2012;109:E2441–2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–22. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 167.Weiss L, Nannmark U, Johansson BR, Bagge U. Lethal deformation of cancer cells in the microcirculation: a potential rate regulator of hematogenous metastasis. Int J Cancer. 1992;50:103–7. doi: 10.1002/ijc.2910500121. [DOI] [PubMed] [Google Scholar]
- 168.Chaudhuri PK, Low BC, Lim CT. Mechanobiology of tumor growth. Chem Rev. 2018;118:6499–515. doi: 10.1021/acs.chemrev.8b00042. [DOI] [PubMed] [Google Scholar]
- 169.Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368:eaaw5473. doi: 10.1126/science.aaw5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tasdogan A, Faubert B, Ramesh V, Ubellacker JM, Shen B, Solmonson A, et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature. 2020;577:115–20. doi: 10.1038/s41586-019-1847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–91. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16:992–1003. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wiel C, Le Gal K, Ibrahim MX, Jahangir CA, Kashif M, Yao H, et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell. 2019;178:330–345.e22. doi: 10.1016/j.cell.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 174.Lignitto L, LeBoeuf SE, Homer H, Jiang S, Askenazi M, Karakousi TR, et al. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of bach1. Cell. 2019;178:316–329.e18. doi: 10.1016/j.cell.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guadamillas MC, Cerezo A, Del Pozo MA. Overcoming anoikis-pathways to anchorage-independent growth in cancer. J Cell Sci. 2011;124:3189–97. doi: 10.1242/jcs.072165. [DOI] [PubMed] [Google Scholar]
- 176.Pickarski M, Gleason A, Bednar B, Duong LT. Orally active αvβ3 integrin inhibitor MK-0429 reduces melanoma metastasis. Oncol Rep. 2015;33:2737–45. doi: 10.3892/or.2015.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cheng C, Komljenovic D, Pan L, Dimitrakopoulou-Strauss A, Strauss L, Bäuerle T. Evaluation of treatment response of cilengitide in an experimental model of breast cancer bone metastasis using dynamic PET with 18F-FDG. Hell J Nucl Med. 2011;14:15–20. [PubMed] [Google Scholar]
- 178.Élez E, Kocáková I, Höhler T, Martens UM, Bokemeyer C, Van Cutsem E, et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial. Ann Oncol: Off J Eur Soc Med Oncol. 2015;26:132–40. doi: 10.1093/annonc/mdu474. [DOI] [PubMed] [Google Scholar]
- 179.Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, et al. Loss of E-cadherin promotes ovarian cancer metastasis via alpha 5-integrin, which is a therapeutic target. Cancer Res. 2008;68:2329–39. doi: 10.1158/0008-5472.CAN-07-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Qian J, Oppermann E, Tran A, Imlau U, Qian K, Vogl TJ. Transarterial administration of integrin inhibitor loaded nanoparticles combined with transarterial chemoembolization for treating hepatocellular carcinoma in a rat model. World J Gastroenterol. 2016;22:5042–9. doi: 10.3748/wjg.v22.i21.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Eberlein C, Kendrew J, McDaid K, Alfred A, Kang JS, Jacobs VN, et al. A human monoclonal antibody 264RAD targeting αvβ6 integrin reduces tumour growth and metastasis, and modulates key biomarkers in vivo. Oncogene. 2013;32:4406–16. doi: 10.1038/onc.2012.460. [DOI] [PubMed] [Google Scholar]
- 182.Melosky B, Bradbury P, Tu D, Florescu M, Reiman A, Nicholas G, et al. Selumetinib in patients receiving standard pemetrexed and platinum-based chemotherapy for advanced or metastatic KRAS wildtype or unknown non-squamous non-small cell lung cancer: a randomized, multicenter, phase II study. Canadian cancer trials group (CCTG) IND.219. Lung Cancer. 2019;133:48–55. doi: 10.1016/j.lungcan.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 183.Harding JJ, Catalanotti F, Munhoz RR, Cheng DT, Yaqubie A, Kelly N, et al. A retrospective evaluation of vemurafenib as treatment for BRAF-mutant melanoma brain metastases. Oncologist. 2015;20:789–97. doi: 10.1634/theoncologist.2014-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Robert C, Dummer R, Gutzmer R, Lorigan P, Kim KB, Nyakas M, et al. Selumetinib plus dacarbazine versus placebo plus dacarbazine as first-line treatment for BRAF-mutant metastatic melanoma: a phase 2 double-blind randomised study. Lancet Oncol. 2013;14:733–40. doi: 10.1016/S1470-2045(13)70237-7. [DOI] [PubMed] [Google Scholar]
- 185.Krishnamurthy A, Dasari A, Noonan AM, Mehnert JM, Lockhart AC, Leong S, et al. Phase Ib results of the rational combination of selumetinib and cyclosporin A in advanced solid tumors with an expansion cohort in metastatic colorectal cancer. Cancer Res. 2018;78:5398–407. doi: 10.1158/0008-5472.CAN-18-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bridgewater J, Lopes A, Beare S, Duggan M, Lee D, Ricamara M, et al. A phase 1b study of Selumetinib in combination with Cisplatin and Gemcitabine in advanced or metastatic biliary tract cancer: the ABC-04 study. BMC Cancer. 2016;16:153. doi: 10.1186/s12885-016-2174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Morrow PK, Wulf GM, Ensor J, Booser DJ, Moore JA, Flores PR, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol: Off J Am Soc Clin Oncol. 2011;29:3126–32. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mayer IA, Abramson VG, Formisano L, Balko JM, Estrada MV, Sanders ME, et al. A phase Ib study of alpelisib (BYL719), a PI3Kα-specific inhibitor, with letrozole in ER+/HER2- metastatic breast cancer. Clin Cancer Res: Off J Am Assoc Cancer Res. 2017;23:26–34. doi: 10.1158/1078-0432.CCR-16-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ippen FM, Grosch JK, Subramanian M, Kuter BM, Liederer BM, Plise EG, et al. Targeting the PI3K/Akt/mTOR pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases. Neuro-Oncol. 2019;21:1401–11. doi: 10.1093/neuonc/noz105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Peng X, Zhou J, Li B, Zhang T, Zuo Y. Gu X. Notch1 and PI3K/Akt signaling blockers DAPT and LY294002 coordinately inhibit metastasis of gastric cancer through mutual enhancement. Cancer Chemother Pharmacol. 2020;85:309–20. doi: 10.1007/s00280-019-03990-4. [DOI] [PubMed] [Google Scholar]
- 191.Hou MM, Liu X, Wheler J, Naing A, Hong D, Coleman RL, et al. Targeted PI3K/AKT/mTOR therapy for metastatic carcinomas of the cervix: A phase I clinical experience. Oncotarget. 2014;5:11168–79. doi: 10.18632/oncotarget.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hotte SJ, Chi KN, Joshua AM, Tu D, Macfarlane RJ, Gregg RW, et al. A phase II study of PX-866 in patients with recurrent or metastatic castration-resistant prostate cancer: Canadian cancer trials group study IND205. Clin. Genitourin. cancer. 2019;17:201–208.e1. doi: 10.1016/j.clgc.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 193.de Bono JS, De Giorgi U, Rodrigues DN, Massard C, Bracarda S, Font A, et al. Randomized phase II study evaluating akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin Cancer Res: Off J Am Assoc Cancer Res. 2019;25:928–36. doi: 10.1158/1078-0432.CCR-18-0981. [DOI] [PubMed] [Google Scholar]
- 194.Zhang Q, Zhou L, Guan Y, Cheng Y. Han X. BENC-511, a novel PI3K inhibitor, suppresses metastasis of non-small cell lung cancer cells by modulating β-catenin/ZEB1 regulatory loop. Chem Biol Interact. 2018;294:18–27. doi: 10.1016/j.cbi.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 195.Ando Y, Inada-Inoue M, Mitsuma A, Yoshino T, Ohtsu A, Suenaga N, et al. Phase I dose-escalation study of buparlisib (BKM120), an oral pan-class I PI3K inhibitor, in Japanese patients with advanced solid tumors. Cancer Sci. 2014;105:347–53. doi: 10.1111/cas.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Blagden S, Omlin A, Josephs D, Stavraka C, Zivi A, Pinato DJ, et al. First-in-human study of CH5132799, an oral class I PI3K inhibitor, studying toxicity, pharmacokinetics, and pharmacodynamics, in patients with metastatic cancer. Clin Cancer Res: Off J Am Assoc Cancer Res. 2014;20:5908–17. doi: 10.1158/1078-0432.CCR-14-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Donnelly SK, Cabrera R, Mao SPH, Christin JR, Wu B, Guo W, et al. Rac3 regulates breast cancer invasion and metastasis by controlling adhesion and matrix degradation. J Cell Biol. 2017;216:4331–49. doi: 10.1083/jcb.201704048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.van Golen KL, Davies S, Wu ZF, Wang Y, Bucana CD, Root H, et al. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res: Off J Am Assoc Cancer Res. 1999;5:2511–9. [PubMed] [Google Scholar]
- 199.Wong CM, Yam JW, Ching YP, Yau TO, Leung TH, Jin DY, et al. Rho GTPase-activating protein deleted in liver cancer suppresses cell proliferation and invasion in hepatocellular carcinoma. Cancer Res. 2005;65:8861–8. doi: 10.1158/0008-5472.CAN-05-1318. [DOI] [PubMed] [Google Scholar]
- 200.Ko FC, Chan LK, Sze KM, Yeung YS, Tse EY, Lu P, et al. PKA-induced dimerization of the RhoGAP DLC1 promotes its inhibition of tumorigenesis and metastasis. Nat Commun. 2013;4:1618. doi: 10.1038/ncomms2604. [DOI] [PubMed] [Google Scholar]
- 201.Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–5. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 202.Hakem A, Sanchez-Sweatman O, You-Ten A, Duncan G, Wakeham A, Khokha R, et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 2005;19:1974–9. doi: 10.1101/gad.1310805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Suwa H, Ohshio G, Imamura T, Watanabe G, Arii S, Imamura M, et al. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br J Cancer. 1998;77:147–52. doi: 10.1038/bjc.1998.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ying H, Biroc SL, Li WW, Alicke B, Xuan JA, Pagila R, et al. The Rho kinase inhibitor fasudil inhibits tumor progression in human and rat tumor models. Mol Cancer therapeutics. 2006;5:2158–64. doi: 10.1158/1535-7163.MCT-05-0440. [DOI] [PubMed] [Google Scholar]
- 205.Li C, Wang S, Xing Z, Lin A, Liang K, Song J, et al. A ROR1-HER3-lncRNA signalling axis modulates the Hippo-YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017;19:106–19. doi: 10.1038/ncb3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med. 2012;18:1511–7. doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lau AN, Curtis SJ, Fillmore CM, Rowbotham SP, Mohseni M, Wagner DE, et al. Tumor-propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis. EMBO J. 2014;33:468–81. doi: 10.1002/embj.201386082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cheung P, Xiol J, Dill MT, Yuan WC, Panero R, Roper J, et al. Regenerative reprogramming of the intestinal stem cell state via hippo signaling suppresses metastatic colorectal cancer. Cell Stem Cell. 2020;24:590–604.e9. doi: 10.1016/j.stem.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Akens MK, Hardisty MR, Wilson BC, Schwock J, Whyne CM, Burch S, et al. Defining the therapeutic window of vertebral photodynamic therapy in a murine pre-clinical model of breast cancer metastasis using the photosensitizer BPD-MA (Verteporfin) Breast Cancer Res Treat. 2010;119:325–33. doi: 10.1007/s10549-009-0356-7. [DOI] [PubMed] [Google Scholar]