Abstract
Background
A significant proportion of adults have normal weight obesity (NWO), defined as a normal body mass index (BMI) but disproportionately high body fat percentage. Individuals with NWO may have increased risk of cardiometabolic disorders and lower exercise tolerance, but it is unclear if this obesity phenotype is linked with dysregulated production of adipokines or myokines such as adiponectin and apelin, respectively.
Methods
This cross-sectional, secondary analysis included 177 working adults (mean age 49.6 ± 9.9 yrs, 64% female). Plasma high-molecular weight adiponectin and apelin levels were measured by ELISA. Body composition and fat distribution were assessed using dual energy X-ray absorptiometry. Exercise tolerance (VO2 maximum) was determined by treadmill testing. NWO was defined as a BMI <25 kg/m2 and body fat >30% for women or >23% for men. Participants were categorized as lean, NWO, or overweight-obese.
Results
A total of 14.7% of subjects were categorized as lean, 23.7% as having NWO, and 61.6% as having overweight-obesity. Plasma adiponectin levels were elevated in the overweight-obesity group (P < 0.05) compared to the lean and NWO groups, which did not differ from each other (P > 0.05). Adiponectin concentrations were inversely associated with BMI, fat mass, fat mass percent, visceral fat, and trunk to leg fat ratio and positively associated with leg fat mass (all P < 0.001). Plasma apelin levels were similar between the three body composition groups (P < 0.05) and were not significantly associated with any body composition indices. Apelin concentrations were inversely related to VO2 maximum (β = −0.03 ± 0.01, p = 0.002).
Conclusion
Plasma adiponectin and apelin levels did not distinguish between lean and NWO groups. Positive relationships with leg fat mass and adiponectin suggest the importance of assessing body composition and fat distribution when studying adipokines and cardiometabolic disorders. Further investigations are needed to understand relationships between exercise, body composition, and apelin secretion.
Keywords: Adipose, Cytokines, Fat distribution, Fitness, Metabolic, Obesity phenotype
Introduction
Obesity results from complex interactions between factors such as genetics, dietary intake, and physical activity [1]. Clinically relevant comorbidities such as type 2 diabetes, hypertension, and cardiovascular disease are associated with obesity [2]. In general, obesity is defined as a body mass index (BMI) >30 kg/m2. However, normal weight obesity (NWO) has been recognized as an obesity phenotype that commonly exhibits metabolic disturbances in individuals that are not determined to be obese by BMI [3], [4], as BMI does not assess a person’s relative proportions of fat and fat free mass. Individuals with NWO have excess adiposity, despite a normal BMI, partly owing to a decrease in lean mass [5]. A growing literature shows these individuals have increased cardiometabolic disease and mortality risk [3], [4], [6], [7], [8], [9], [10], [11], [12], but it is unknown if drivers of metabolic dysfunction in individuals with NWO are similar to individuals with overt obesity as assessed by BMI.
A mechanism through which excess fat mass causes metabolic changes involves adipokine secretion [13]. As an endocrine organ, cells present in adipose tissue secrete chemicals that exert metabolic effects both locally and systemically. These hormones are collectively termed adipokines. Obesity is associated with dysregulated adipokine secretion, and fat tissue distribution may also influence adipokine production [13], [14]. Given the widespread interest in determining the role of adipokines in contributing to disease onset, many groups have investigated the extent to which adipokines are involved in the metabolic changes observed with obesity. Due to the relatively high level of adiposity in individuals with NWO, they may exhibit altered adipokine secretion, though few studies have investigated these relationships [15], [16].
Adiponectin and apelin are cytokines with important roles in biological processes. Adiponectin, primarily secreted by adipose tissue (an adipokine) has been extensively studied for its anti-diabetic, anti-inflammatory, and anti-atherogenic characteristics [13]. Apelin is a more recently discovered cytokine with insulin-like functions that has roles in macronutrient metabolism and energy balance [17]. Multiple tissues, including adipose tissue and skeletal muscle, secrete apelin [18]. Some, but not all, studies have shown positive relationships of plasma apelin with obesity [19], [20], [21]. In contrast, apelin has also been identified as an exerkine whose mRNA expression in skeletal muscle increases following endurance training [22]. Additionally, lower levels of apelin or loss of the apelin receptor (APJ) resulted in loss of muscle function in mice [23]. Thus, relationships of plasma apelin with health outcomes is not clear. Given the combination of high body fat mass, low lean mass, and decreased levels of physical fitness in individuals with NWO [24], apelin may be particularly relevant to study in a population with NWO.
The current study was a secondary analysis of previously collected data from a large Atlanta-based cohort, the Emory-Georgia Tech Predictive Health Institute (PHI) cohort, where nearly a quarter of the population was categorized as having NWO [5]. The study objective was to compare plasma adiponectin and apelin concentrations between adults categorized as being lean, having NWO, or having overweight-obesity. A secondary aim was to investigate the relationships of the cytokines with body composition, fat distribution, and exercise capacity in all participants regardless of body composition group.
Subjects and methods
Participants
Emory University and Emory Healthcare employees were invited to join the PHI study from 2007 to 2013 at the Emory-Georgia Tech Center for Health Discovery and Well Being [24], [25]. The study was approved by the Emory University Institutional Review Board and informed consent was obtained from all subjects. Participants completed a variety of assessments including behavioral, biochemical, and physical measures. Exclusion criteria were having a poorly controlled chronic disease, acute illness, currently pregnant or breastfeeding, hospitalization within the previous year, severe psychosocial disorder, substance abuse or alcoholism, or current active malignant neoplasm. The present analysis was conducted in a subset of the PHI cohort. A random sample of participants from the cohort were chosen for analysis of the cytokines and included in this study.
Body composition analysis
Body composition and fat distribution was measured by dual energy x-ray absorptiometry (DXA, GE Lunar iDXA Densitometer, GE Healthcare, Madison, WI, USA). Height and weight were measured using a digital scale and stadiometer (Tanita TBF-25, Tanita Health Management, Arlington Heights, IL). BMI was calculated as weight in kilograms divided by height in meters squared. Fat mass index (FMI) was calculated as fat mass (kg) divided by height in meters squared, and lean mass index (LMI) was calculated as lean mass (kg) divided by height in meters squared. The study population was categorized into three body composition groups: lean, NWO, or overweight-obese. Lean was defined as a BMI below 25 kg/m2 and a body fat below 23% for males and below 30% for females. NWO was defined as a BMI less than 25 kg/m2 and body fat >30% for women or >23% for men [26]. Overweight-obesity was defined as a BMI greater than or equal to 25 kg/m2 and a body fat above the sex-specific cut points.
Cytokine assessments
Fasting plasma apelin concentrations were measured using an enzyme-linked immunosorbent assay (ELISA) following manufacturer’s instructions (RayBiotech, #EIA-APC-1, intra-assay CV = 4.7%). Fasting plasma high-molecular weight adiponectin concentrations were also quantified using an ELISA kit, and assay procedures followed the manufacturer’s instructions (R&D Systems, #DHWAD0, intra-assay CV = 2.8%).
Exercise testing
Participants (n = 167) underwent a submaximal volume of oxygen consumed (VO2 maximum) test following a modified Balke protocol [27] under the supervision of a trained technician. This assessment provides an objective measure of cardiorespiratory fitness. All exercise tests were performed on a GE T2100 Treadmill (GE Healthcare, Waukesha, WI). Of the ten subjects with missing treadmill data, five were missing at random, three had an injury or medical/surgical history that was a contraindication to perform the test, and data from two participants were deemed inaccurate due to highly elevated heart rates. There was no difference in mean apelin levels between the ten participants with missing VO2 maximum data and the remaining participants (P > 0.05).
Statistical analyses
Descriptive statistics were performed for all variables, and continuous variables were summarized as mean ± SD and categorical variables were summarized as count, percent (n, %). Variables that did not have a normal distribution were natural log-transformed for analyses and back-transformed as adjusted geometric mean (95% confidence interval). The data were analyzed by demographic characteristics using chi-squared (χ2) or analysis of variance (ANOVA) tests. For groups with a significant (P < 0.05) χ2 result, post-hoc pairwise χ2 analyses were conducted to determine which groups were significantly different using a Bonferroni correction with an alpha value set to 0.017. To test for differences in the cytokine levels between the body composition groups, two-way analysis of covariance (ANCOVA) was utilized, with multiple comparison correction via Tukey’s honest significant difference method. ANCOVA analyses were adjusted for age, race, and sex for both cytokines, and apelin was additionally adjusted for VO2 maximum because of its role as an exerkine [22]. To address the secondary study aim, multiple linear regression models, adjusting for age, race, and sex, were used to test for relationships between cytokine concentrations and body composition variables. In multiple linear regression analyses investigating the relationship between lean mass variables and cytokine levels, fat mass was also included as a covariate to test for the independent relationship of the cytokines with lean mass. Similarly, to evaluate the independent relationship of the fat distribution variables (leg fat and visceral adipose tissue) with cytokine levels, body fat excluding the variable of interest was included as a covariate in the model. Additional covariates considered for analysis were income and education level, but these variables were not included in the final analyses because they were not associated with apelin or adiponectin concentrations. Analyses were conducted in JMP Pro (version 15, SAS Institute Inc., Cary, NC, USA) with two-sided tests and an alpha significance value of 0.05.
Results
A total of 177 individuals were included in this study (Table 1). The majority of the population was female (n = 116, 66%), and there was a greater percentage of women in the NWO compared to the overweight-obesity group (P = 0.008). There were 41 women classified as post-menopausal, and a higher proportion of women with NWO were classified as post-menopausal than lean women (P = 0.01). The overall study population predominantly included Caucasians (76.5%), with African Americans comprising most of the remaining participants. The distribution of race between the three groups was similar (P > 0.05). The mean age of all participants was 49.7 years (SD, ± 10.0 y), and average age was similar across the three groups (P > 0.05). There were 26 participants categorized as lean (15%), 42 as having NWO (24%), and 109 as having overweight-obesity (62%). According to the definitions for lean and NWO, the mean BMI for the lean and NWO groups were similar (P > 0.05) and both groups had an average BMI significantly lower than the group with overweight-obesity (P < 0.05). Fat mass was also similar between the lean and NWO groups and significantly highest in the group with overweight-obesity (P < 0.05). Fat mass percent, fat mass index, and lean mass index differed significantly between each group (P < 0.05), while lean mass was similar between the lean and overweight-obesity groups and significantly lowest in the NWO group (P < 0.05). Among fat distribution variables, leg fat mass, trunk to leg fat ratio, and visceral fat mass were all significantly different between groups (P < 0.05). As previously reported [5], the NWO and overweight-obesity groups had lower VO2 maximum levels compared to the lean group (P < 0.05).
Table 1.
Demographic, clinical, and body composition characteristics.
| Variable | Lean (n = 26) |
Normal Weight Obesity (n = 42) |
Overweight-Obesity (n = 109) |
All participants (N = 177) |
|---|---|---|---|---|
| Age (yr) | 47.2 ± 11.4 | 47.8 ± 9.3 | 50.9 ± 9.6 | 49.6 ± 9.9 |
| Female n (%) | 15 (58) | 35 (83)* | 66 (59.5)* | 116 (66) |
| Post-menopausal n (%) | 2 (8)* | 14 (33)* | 25 (23) | 41 (23) |
| Caucasian n (%) | 23 (88) | 35 (83) | 77 (71) | 135 (76) |
| BMI (kg/m2) | 23.1 ± 0.8a | 23.0 ± 0.7a | 30.5 ± 0.4b | 27.3 ± 5.5 |
| Fat mass (%) | 22.5 ± 0.9a | 30.4 ± 0.8b | 37.3 ± 0.5c | 35.1 ± 8.3 |
| Fat mass (kg) | 15.2 ± 1.7a | 19.3 ± 1.4a | 33.1 ± 0.8b | 27.9 ± 11.1 |
| Fat mass index | 5.2 ± 0.6a | 6.8 ± 0.5b | 11.6 ± 0.3c | 9.8 ± 4.0 |
| Lean mass (kg) | 50.4 ± 1.4a | 44.0 ± 1.2b | 52.1 ± 0.7a | 47.3 ± 10.5 |
| Lean mass index (kg/m2) | 17.0 ± 0.3a | 15.3 ± 0.3b | 17.9 ± 0.2c | 16.5 ± 2.4 |
| Leg fat mass (kg) | 5.7 (5.2, 6.3)a | 6.7 (6.2, 7.3)b | 9.9 (9.5, 10.4)c | 8.9 (8.5, 9.4) |
| Trunk to leg fat ratio | 1.2 ± 0.08a | 1.5 ± 0.07b | 1.8 ± 0.04c | 1.5 ± 0.57 |
| Visceral fat mass (kg) | 0.18 (0.13, 0.25)a | 0.44 (0.34, 0.57)b | 1.18 (1.01, 1.37)c | 0.62 (0.52, 0.73) |
| VO2 maximum (mL/min/kg)+ | 44.0 ± 2.0a | 36.1 ± 1.7b | 35.3 ± 1.0b | 36.9 ± 10.7 |
Demographic characteristics are presented as mean ± SD or count (percent).
For body composition and clinical variables by body composition subtype, data are presented as adjusted mean ± SE or, for variables that were log-transformed, geometric mean (95% confidence interval). Estimates are adjusted for age, sex, and race. Variables within rows that are connected by the same letter are statistically similar (P > 0.05) while those with different letters are significantly different via Tukey’s comparisons at P < 0.05.
* Groups within rows are significantly different at P < 0.017 following χ2 post-hoc results using a Bonferroni correction.
+ N = 167; n = 25 lean, n = 40 NWO, n = 102 overweight-obesity.
Abbreviations: NWO, normal weight obesity; BMI, body mass index; VO2, volume of oxygen consumption.
Analyzed by demographic variables, females had higher adiponectin levels compared to males [5.92 (5.08, 6.91) vs 3.17 (2.56, 3.92) μg/mL, P < 0.001], this difference between males and females remained significant after adjusting for menopausal status (P < 0.001). Apelin levels were similar between males and females [69.71 (59.86, 81.18) vs 67.99 (54.01, 85.58) ng/mL, P = 0.85] and did not vary by menopausal status (P = 0.57). Caucasian participants had significantly higher adiponectin concentrations compared to other participants [5.34 (6.16, 4.63) vs. 3.34 (4.49, 2.49), P = 0.003], but apelin did not vary by race [70.44 (81.55, 60.85) vs. 65.0 (84.15, 50.20), P = 0.59]. Neither adiponectin nor apelin concentrations were related to participant age (β = 0.006 ± 0.007, P = 0.37 and β = 0.002 ± 0.006, P = 0.74, respectively).
Adiponectin
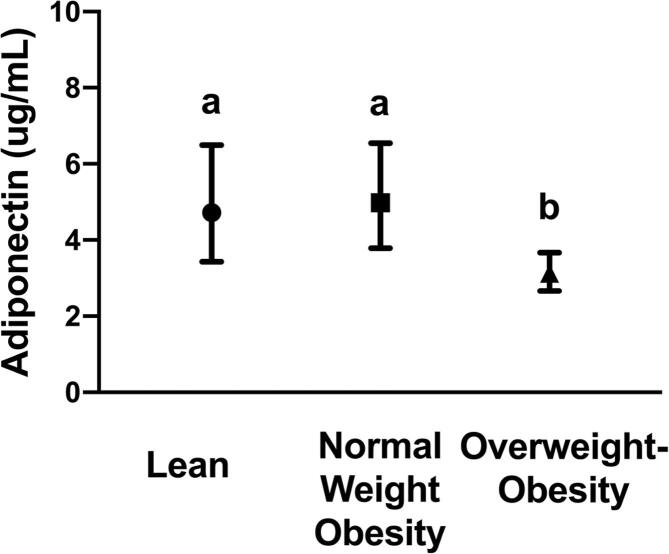
Adiponectin levels were similar between the lean and NWO groups and significantly lowest in the group with overweight-obesity (Fig. 1). In multiple linear regression analyses, adiponectin was significantly, inversely associated with measures of whole-body adiposity including BMI, fat mass percent, fat mass, and fat mass index (Table 2, P < 0.001 for all). Adiponectin concentrations were positively associated with leg fat mass (P < 0.001) and inversely related to the measures of abdominal adiposity, trunk to leg fat ratio and visceral fat mass (P < 0.001 and P = 0.03, respectively). There were no statistically significant relationships between indices of lean mass and adiponectin levels (all P > 0.05).
Fig. 1.
Levels of plasma adiponectin between lean, normal weight obesity, and overweight-obesity groups. Geometric mean and 95% confidence interval are shown for each group. Groups connected with the same letters are statistically similar (P > 0.05), while groups with different letters are significantly different at P < 0.05 according to Tukey post-hoc comparisons.
Table 2.
Cross-sectional associations between body composition measures (independent variable) and adipokine concentrations (dependent variable) [β ± SE (p-value)].a
| Body Composition Measure | Plasma adiponectinb | Plasma apelinb, c |
|---|---|---|
| Body mass index (kg/m2) |
−0.06 ± 0.01 (<0.001) |
−0.007 ± 0.01 (0.62) |
| Fat mass (%) |
−0.04 ± 0.008 (<0.001) |
−0.01 ± 0.01 (0.29) |
| Fat mass (kg) |
−0.03 ± 0.005 (<0.001) |
−0.003 ± 0.006 (0.60) |
| Fat mass index |
−0.09 ± 0.01 (<0.001) |
−0.01 ± 0.02 (0.52) |
| Lean mass (kg) | 0.01 ± 0.01 (0.32) |
0.003 ± 0.01 (0.83) |
| Lean mass index (kg/m2) | −0.0009 ± 0.04 (0.98) |
0.01 ± 0.05 (0.82) |
| Leg fat mass (kg)b |
1.05 ± 0.29 (<0.001) |
−0.07 ± 0.37 (0.84) |
| Trunk to leg fat ratio |
−0.83 ± 0.12 (<0.001) |
−0.11 ± 0.15 (0.47) |
| Visceral fat mass (kg)b |
−0.20 ± 0.09 (0.03) |
−0.02 ± 0.11 (0.88) |
R2 values for adiponectin analyses ranged from 0.33 to 0.41 with leg fat mass having the highest R2.
R2 values for apelin analyses ranged from 0.08 to 0.09, indicating low goodness of fit across all analyses.
All coefficient estimates are from multiple linear regression analyses with body composition measures as a continuous variable. Analyses were conducted individually for each measure of body composition. All estimates for adiponectin analyses are adjusted for age, race, and sex. Estimates for lean mass and lean mass index are adjusted for age, race, sex, and fat mass. Estimates for variables of fat distribution (leg fat mass, trunk to leg fat ratio, and visceral fat mass) were adjusted for age, race, sex, and fat mass excluding the variable of interest. In addition, all estimates for apelin analyses are adjusted for VO2 maximum. Bolded values indicate significant findings.
Variable was natural log-transformed for analyses.
n = 167.
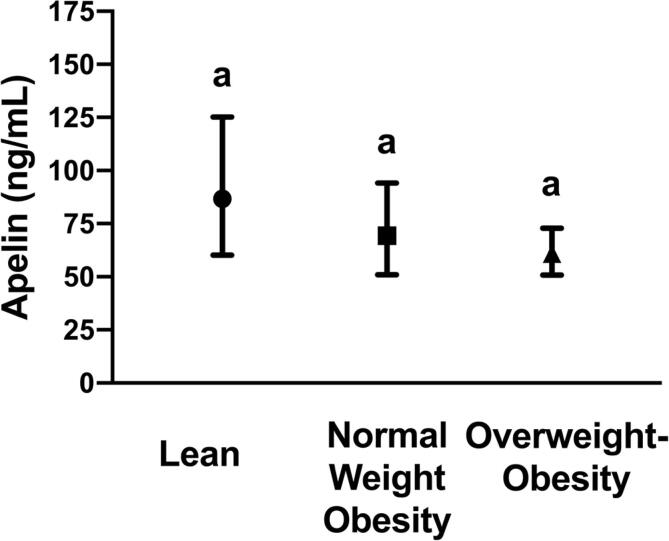
Apelin
Apelin concentrations were not significantly different between the body composition groups (Fig. 2, P = 0.2). Likewise, apelin was not significantly associated with any measures of body composition or fat distribution (Table 2, P > 0.05 for all). However, VO2 maximum was significantly, inversely associated with apelin concentrations, independent of the body composition groups, age, race, and sex (β = −0.03 ± 0.007, Fig. 3, P < 0.001). This relationship indicates that for every one unit increase in VO2 maximum, plasma apelin decreases by about 2.85%.
Fig. 2.
Levels of plasma apelin between lean, normal weight obesity, and overweight-obesity groups. Geometric mean and 95% confidence interval are shown for each group. Groups connected with the same letters are statistically similar (P > 0.05), while groups with different letters are significantly different at P < 0.05 according to Tukey post-hoc comparisons.
Fig. 3.
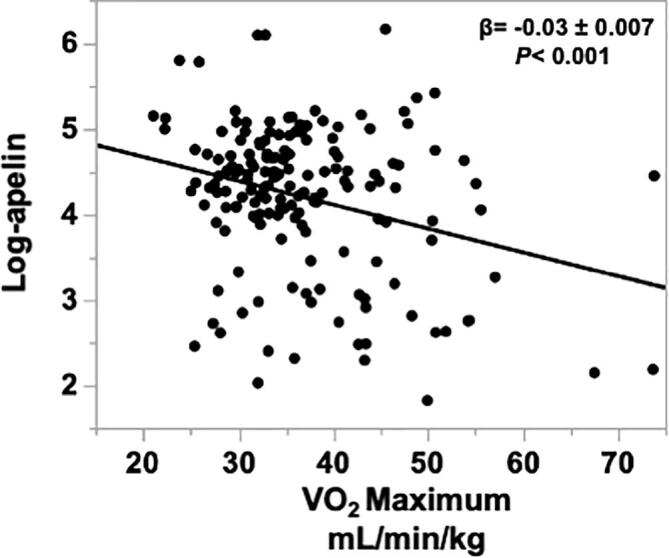
Relationship between VO2 maximum and log-transformed plasma apelin concentrations [−0.03 ± 0.01, p < 0.001, mean square error (MSE) = 0.699] adjusting for age, race, sex, and fat mass percent. A multiple linear regression model was fit to test the association between VO2 maximum and log-transformed plasma apelin.
Discussion
Obesity causes chronic low-grade inflammation, which may be linked to metabolic dysfunction and adverse health outcomes [28]. The altered release of adipokines by adipose tissue may contribute, in part, to metabolic dysfunction commonly observed in individuals with obesity. While BMI remains a popular anthropometric tool as it is simple to calculate, there is a growing body of evidence suggesting that adiposity and fat distribution [29], rather than BMI, may be more strongly correlated to circulating adipokine levels. For individuals with NWO, who have a normal BMI but high fat mass and low lean mass, it is important to determine if altered adipose tissue function is leading to increased disease and mortality risk. In the current study, findings indicated that adiponectin concentrations were similar in adults with NWO compared to lean adults and lowest in adults with overweight-obesity. Adiponectin levels were also inversely related to whole body and abdominal adiposity but were positively related to leg fat mass. Apelin concentrations were not related to the body composition groups or measures of body composition but were inversely related to cardiorespiratory fitness.
Adiponectin is expressed by adipocytes and has anti-inflammatory effects, as well as acting as an insulin sensitizer [30]. Several studies have shown a decrease in adiponectin concentrations with obesity [31], and reciprocal association of adiponectin with altered metabolic markers such as elevated C-reactive protein (CRP) and insulin resistance [32]. In the current study, adiponectin concentrations were similar between the NWO and lean groups and significantly lower only in the overweight-obesity group, which corroborates findings of two other studies in adults with NWO [16], [15]. Consistent with previous studies [14], [33], [34], adiponectin levels were inversely related to measures of total adiposity and abdominal adiposity. As further evidence linking low adiponectin levels to worsening outcomes, a five-year prospective study showed that decreased plasma adiponectin levels predicted increased visceral fat and insulin resistance in a cohort of Asian American adults [35].
We also found an independent, positive association between leg fat mass and adiponectin levels. While most studies have focused on the inverse relationship between total adiposity and adiponectin levels, few studies have investigated the specific relationship between lower body fat mass accumulation and adiponectin concentrations. In line with findings reported here, another study also reported a significant, positive association between adiponectin levels and leg fat mass, independent of trunk fat mass [36]. Quantification of adiponectin expression in human adipose tissue depots has suggested that omental or visceral fat adiponectin mRNA expression is low compared to subcutaneous abdominal or thigh adipose tissue [37], [38]. Adipose tissue that accumulates in the lower body area, such as the thigh, is less contributory to the obesity phenotype of metabolic dysregulation than android or visceral fat and is often considered protective to metabolic health [39]. Measures of leg fat mass, independent of total body fat, are associated with greater insulin sensitivity, improved lipid panels, lower inflammation, and lower blood pressure [36], [40], [41]. In addition, pre-menopausal women who store fat peripherally (i.e. gluteal-femoral fat) consistently have lower metabolic disease risk than males or women who store more abdominal fat [36], [39]. Thus, while BMI remains an easy tool to screen for health and disease risk, measurement of body fat distribution is essential when considering metabolic risk.
Apelin is a ligand of the APJ receptor [42] with several different variants based on cleavage sites, including apelin-36, apelin-17, and apelin-13. While it is expressed in adipocytes [19], it is also expressed by several other tissues, including skeletal muscle, the stomach, lung, brain, and heart [18], [19], [43]. Here, apelin concentrations were not significantly different between the three body composition groups. This finding is likely indicative of the breadth of physiological processes involving apelin [44] rather than its secretion as an adipokine specifically indicating dysfunctional adipose tissue. The apparently discordant findings reported between increased apelin levels in patients with type 1 and type 2 diabetes [45], [46] but lack of association with apelin concentrations in some populations with obesity [21] may be an effort to counteract the absence of insulin or proper insulin signaling and improve glucose utilization through increased apelin secretion [47]. There remains no consensus on the relationship between circulating apelin levels and obesity. Our data do not indicate a strong link between adiposity and circulating apelin concentrations.
On the other hand, apelin was significantly, inversely associated with VO2 maximum levels in this population. In line with this finding, a supervised 4-week aerobic exercise intervention resulted in decreased serum apelin concentrations in adults with normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes [46]. Besse-Patin et al. reported no changes in circulating apelin concentrations following an 8-week cycling and running exercise program despite a significant increase in apelin mRNA expression in skeletal muscle [22]. The authors indicated that apelin may act locally to enhance muscle metabolism [22]. In contrast, animal studies have shown acute increases in serum apelin levels following an exercise bout [23], [48], implicating this as an exerkine secreted to promote muscle function and lower inflammation following exercise [23]. Apelin has been proposed as a therapeutic agent to prevent age-related muscle loss [23], although it was not associated with measures of lean mass in this cohort. The discrepancies in findings regarding apelin with health and physical functioning outcomes may be due to differences in human and animal models or the age of the populations studied. Alternatively, apelin is a vasoactive peptide [49], and it is possible that higher circulating apelin reflects a compensatory mechanism to promote vasodilation in individuals with lower cardiorespiratory fitness. Further studies are warranted to understand the relationships between exercise, body composition, and apelin secretion.
This study contributes new data regarding concentrations of adiponectin and apelin in a population with NWO and in association with body fat distribution and cardiorespiratory fitness. Additional strengths include the detailed body composition phenotyping in a well-characterized cohort. Limitations of this work are related to its cross-sectional nature and inability to infer causality. Measurement of tissue mRNA and protein expression in mechanistic studies, as well as longitudinal studies of these cytokines will be needed to determine the directionality of relationship with adiposity and fitness. Furthermore, sample sizes were relatively small between groups, particularly in analyses stratified by sex, which may have limited power to detect differences in cytokine concentrations. Finally, the cohort used in this study is mostly Caucasian adults, and a more heterogenous cohort may demonstrate different findings.
Conclusions
Plasma adiponectin and apelin levels did not differentiate individuals with NWO from lean adults. Plasma adiponectin levels decreased with increasing central adiposity but were positively associated with leg fat mass, thus adding to the body of evidence that the pattern of body fat deposition is an important determinant of metabolic health. Plasma apelin levels were not associated with body composition but were inversely related to physical fitness, highlighting a research need for further mechanistic understanding of the physiologic processes involved in circulating apelin and its implications for metabolic health.
Funding
This work is based on information from the Emory/Georgia Tech Predictive Health Institute and Center for Health Discovery and Well Being database, supported by the National Center for Advancing Translational Sciences of the NIH under award UL1 TR002378 (Georgia Clinical and Translational Science Alliance). Additional support was received from T32 CA093423 (MPB), R01 FD-003440 (GSM), U54 AG062334 (Emory Specialized Center of Research Excellence on Sex Differences, JAA), K01 DK102851 (JAA), K24 DK096574 (TRZ).
CRediT authorship contribution statement
Moriah P. Bellissimo: Conceptualization, Formal analysis, Methodology, Writing - original draft. Emory Hsu: Conceptualization, Formal analysis, Methodology, Writing - original draft. Li Hao: Data curation, Methodology. Kirk Easley: Formal analysis. Greg S. Martin: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Supervision, Writing - review & editing. Thomas R. Ziegler: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Supervision, Writing - review & editing. Jessica A. Alvarez: Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Longo D.L., Heymsfield S.B., Wadden T.A. Mechanisms, pathophysiology, and management of obesity. N Engl J Med. 2017;376:254–266. doi: 10.1056/NEJMra1514009. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-term Trends in Health. Hyattsville (MD): National Center for Health Statistics (US); 2017. [PubMed]
- 3.Jean N., Somers V.K., Sochor O., Medina-Inojosa J., Llano E.M., Lopez-Jimenez F. Normal-weight obesity: Implications for cardiovascular health. Curr Atheroscler Rep. 2014;16:464. doi: 10.1007/s11883-014-0464-7. [DOI] [PubMed] [Google Scholar]
- 4.Oliveros E., Somers V.K., Sochor O., Goel K., Lopez-Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis. 2014;56:426–433. doi: 10.1016/j.pcad.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Bellissimo M.P., Cai Q., Ziegler T.R., Liu K.H., Tran P.H., Vos M.B. Plasma high-resolution metabolomics differentiates adults with normal weight obesity from lean individuals. Obesity. 2019;27:1729–1737. doi: 10.1002/oby.22654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Lorenzo A., Martinoli R., Vaia F., Di Renzo L. Normal weight obese (NWO) women: An evaluation of a candidate new syndrome. Nutr Metab Cardiovasc Dis. 2006;16:513–523. doi: 10.1016/j.numecd.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Kim M., Paik J.K., Kang R., Kim S.Y., Lee S.-H., Lee J.H. Increased oxidative stress in normal-weight postmenopausal women with metabolic syndrome compared with metabolically healthy overweight/obese individuals. Metab Clin Exp. 2013;62:554–560. doi: 10.1016/j.metabol.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Romero-Corral A., Somers V.K., Sierra-Johnson J., Thomas R.J., Collazo-Clavell M.L., Korinek J. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32:959–966. doi: 10.1038/ijo.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Renzo L, Galvano F, Orlandi C, Bianchi A, Di Giacomo C, La Fauci L, et al. Oxidative stress in normal-weight obese syndrome. Obesity 2010;18:2125-30. [DOI] [PubMed]
- 10.Jia A., Xu S., Xing Y., Zhang W., Yu X., Zhao Y. Prevalence and cardiometabolic risks of normal weight obesity in Chinese population: A nationwide study. Nutr Metab Cardiovasc Dis. 2018;28:1045–1053. doi: 10.1016/j.numecd.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J 2010;31:737-46. [DOI] [PMC free article] [PubMed]
- 12.Sahakyan K.R., Somers V.K., Rodriguez-Escudero J.P., Hodge D.O., Carter R.E., Sochor O. Normal-weight central obesity: Implications for total and cardiovascular mortality. Ann Intern Med. 2015;163:827–835. doi: 10.7326/M14-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adamczak M., Wiecek A. The adipose tissue as an endocrine organ. Semin Nephrol. 2013;33:2–13. doi: 10.1016/j.semnephrol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Tchernof A., Després J.P. Pathophysiology of human visceral obesity: An update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 15.Miazgowski T., Safranow K., Krzyżanowska-Świniarska B., Iskierska K., Widecka K. Adiponectin, visfatin and regional fat depots in normal weight obese premenopausal women. Eur J Clin Invest. 2013;43:783–790. doi: 10.1111/eci.12106. [DOI] [PubMed] [Google Scholar]
- 16.Marques-Vidal P., Pécoud A., Hayoz D., Paccaud F., Mooser V., Waeber G. Normal weight obesity: relationship with lipids, glycaemic status, liver enzymes and inflammation. Nutr Metab Cardiovasc Dis. 2010;20:669–675. doi: 10.1016/j.numecd.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Fève B., Bastard C., Fellahi S., Bastard J.-P., Capeau J. New adipokines. Ann Endocrinol. 2016;77:49–56. doi: 10.1016/j.ando.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Antushevich H., Wójcik M. Review: Apelin in disease. Clin Chim Acta. 2018;483:241–248. doi: 10.1016/j.cca.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinol 2005;146:1764-71. [DOI] [PubMed]
- 20.Bertrand C., Valet P., Castan-Laurell I. Apelin and energy metabolism. Front Physiol. 2015;6 doi: 10.3389/fphys.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castan-Laurell I., Dray C., Attané C., Duparc T., Knauf C., Valet P. Apelin, diabetes, and obesity. Endocrine. 2011;40:1–9. doi: 10.1007/s12020-011-9507-9. [DOI] [PubMed] [Google Scholar]
- 22.Besse-Patin A., Montastier E., Vinel C., Castan-Laurell I., Louche K., Dray C. Effect of endurance training on skeletal muscle myokine expression in obese men: Identification of apelin as a novel myokine. Int J Obes. 2014;38:707–713. doi: 10.1038/ijo.2013.158. [DOI] [PubMed] [Google Scholar]
- 23.Vinel C., Lukjanenko L., Batut A., Deleruyelle S., Pradère J.-P., Le Gonidec S. The exerkine apelin reverses age-associated sarcopenia. Nat Med. 2018;24:1360–1371. doi: 10.1038/s41591-018-0131-6. [DOI] [PubMed] [Google Scholar]
- 24.Bellissimo M.P., Bettermann E.L., Tran P.H., Crain B.H., Ferranti E.P., Binongo J.N. Physical fitness but not diet quality distinguishes lean and normal weight obese adults. J Acad Nutr Diet. 2020;120:1963–1973.e2. doi: 10.1016/j.jand.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brigham K.L. Predictive health: The imminent revolution in health care. J Am Geriatr Soc. 2010;58:S298–S302. doi: 10.1111/j.1532-5415.2010.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madeira F.B., Silva A.A., Veloso H.F., Goldani M.Z., Kac G., Cardoso V.C. Normal weight obesity is associated with metabolic syndrome and insulin resistance in young adults from a middle-income country. PLoS ONE. 2013;8:e60673. doi: 10.1371/journal.pone.0060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balke B., Ware R.W. An experimental study of physical fitness of Air Force personnel. US Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 28.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez J.A., Ziegler T.R., Millson E.C., Stecenko A.A. Body composition and lung function in cystic fibrosis: Association with adiposity and normal weight obesity. Nutrition. 2016;32:447–452. doi: 10.1016/j.nut.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achari A.E., Jain S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryo M., Nakamura T., Kihara S., Kumada M., Shibazaki S., Takahashi M. Adiponectin as a biomarker of the metabolic syndrome. Circ J. 2004;68:975–981. doi: 10.1253/circj.68.975. [DOI] [PubMed] [Google Scholar]
- 32.Ouchi N., Kihara S., Funahashi T., Nakamura T., Nishida M., Kumada M. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 33.Fontana L., Eagon J.C., Trujillo M.E., Scherer P.E., Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 34.Ryan A.S., Berman D.M., Nicklas B.J., Sinha M., Gingerich R.L., Meneilly G.S. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26:2383–2388. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 35.Han SJ, Boyko EJ, Fujimoto WY, Kahn SE, Leonetti DL. Low plasma adiponectin concentrations predict increases in visceral adiposity and insulin resistance. J Clin Endocrinol Metab 2017;102:4626-33. [DOI] [PMC free article] [PubMed]
- 36.Zhang X., Hu E.A., Wu H., Malik V., Sun Q.i. Associations of leg fat accumulation with adiposity-related biological factors and risk of metabolic syndrome. Obesity. 2013;21:824–830. doi: 10.1002/oby.20028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lihn A.S., Bruun J.M., He G., Pedersen S.B., Jensen P.F., Richelsen B. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Mol Cell Endocrinol. 2004;219:9–15. doi: 10.1016/j.mce.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Fisher F.M., McTernan P.G., Valsamakis G., Chetty R., Harte A.L., Anwar A.J. Differences in adiponectin protein expression: Effect of fat depots and type 2 diabetic status. Horm Metab Res. 2002;34:650–654. doi: 10.1055/s-2002-38246. [DOI] [PubMed] [Google Scholar]
- 39.Karastergiou K., Smith S.R., Greenberg A.S., Fried S.K. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3:13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasan S.K., Osmond C., Canoy D., Christodoulides C., Neville M.J., Di Gravio C. Comparison of regional fat measurements by dual-energy X-ray absorptiometry and conventional anthropometry and their association with markers of diabetes and cardiovascular disease risk. Int J Obes. 2018;42:850–857. doi: 10.1038/ijo.2017.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goss A.M., Gower B.A. Insulin sensitivity is associated with thigh adipose tissue distribution in healthy postmenopausal women. Metab Clin Exp. 2012;61:1817–1823. doi: 10.1016/j.metabol.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tatemoto K., Hosoya M., Habata Y., Fujii R., Kakegawa T., Zou M.-X. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 43.O'Carroll AM, Lolait SJ, Harris LE, Pope GR. The apelin receptor APJ: Journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol 2013;219:R13-35. [DOI] [PubMed]
- 44.Wysocka M.B., Pietraszek-Gremplewicz K., Nowak D. The role of apelin in cardiovascular diseases, obesity and cancer. Front Physiol. 2018;9:557. doi: 10.3389/fphys.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Habchi M., Duvillard L., Cottet V., Brindisi M.-C., Bouillet B., Beacco M. Circulating apelin is increased in patients with type 1 or type 2 diabetes and is associated with better glycaemic control. Clin Endocrinol. 2014;81:696–701. doi: 10.1111/cen.12404. [DOI] [PubMed] [Google Scholar]
- 46.Krist J., Wieder K., Klöting N., Oberbach A., Kralisch S., Wiesner T. Effects of weight loss and exercise on apelin serum concentrations and adipose tissue expression in human obesity. Obes Facts. 2013;6:57–69. doi: 10.1159/000348667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Renzo L., Bigioni M., Bottini F.G., Del Gobbo V., Premrov M.G., Cianci R. Normal weight obese syndrome: Role of single nucleotide polymorphism of IL-1 5Ralpha and MTHFR 677C–>T genes in the relationship between body composition and resting metabolic rate. Euro Rev Med and Pharmacol Sci. 2006;10:235–245. [PubMed] [Google Scholar]
- 48.Son J.S., Kim H.-J., Son Y., Lee H., Chae S.A., Seong J.K. Effects of exercise-induced apelin levels on skeletal muscle and their capillarization in type 2 diabetic rats. Muscle Nerve. 2017;56:1155–1163. doi: 10.1002/mus.25596. [DOI] [PubMed] [Google Scholar]
- 49.Mughal A., O'Rourke S.T. Vascular effects of apelin: Mechanisms and therapeutic potential. Pharmacol Ther. 2018;190:139–147. doi: 10.1016/j.pharmthera.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]