Abstract
Background
Pregnant women with HIV (PWWH) have high postpartum morbidity and mortality from infections like tuberculosis. Immunologic changes during pregnancy and postpartum periods may contribute to these risks, particularly the immunoregulatory kynurenine pathway of tryptophan catabolism, which contributes to both HIV and tuberculosis pathogenesis and increases in the early postpartum period.
Methods
Women with HIV initiating antiretroviral therapy (ART) in the Uganda AIDS Rural Treatment Outcomes (UARTO) cohort who were pregnant at enrollment or became pregnant during observation were studied (n = 54). Plasma kynurenine/tryptophan (KT) ratio, soluble CD14 (sCD14), sCD163, sCD27, interferon-inducible protein 10 (IP-10), D-dimer, interleukin-6, and intestinal fatty-acid binding protein levels were assessed through the first year of ART and at 3-month intervals throughout pregnancy and 1 year postpartum. Biomarker changes were assessed with linear mixed models adjusted for ART duration. Hemoglobin concentration changes were used to estimate pregnancy-related changes in plasma volume.
Results
The median pre-ART CD4 count was 134. D-dimer increased through the third trimester before returning to baseline postpartum, while most other biomarkers declined significantly during pregnancy, beyond what would be expected from pregnancy-associated plasma volume expansion. IP-10 and sCD14 remained suppressed for at least 12 months postpartum. KT ratio was the only biomarker that increased above prepregnancy baseline postpartum (mean + 30%; P < .001) and remained higher than baseline for ≥9 months (P ≤ .045 for all time points).
Conclusions
Several immune activation markers decline during pregnancy and remain suppressed postpartum, but the kynurenine pathway of tryptophan catabolism increases above baseline for ≥9 months postpartum. The mechanisms underlying postpartum kynurenine pathway activity are incompletely understood but may contribute to increased tuberculosis risk in this setting.
Keywords: HIV, indoleamine 2, 3-dioxygenase-1, inflammation, kynurenine/tryptophan ratio, pregnancy
Pregnant women with HIV (PWWH) have a higher risk of pregnancy-related or postpartum mortality—particularly from infections—compared with women without HIV in both high- and low-income countries [1–5]. Earlier antiretroviral therapy (ART) initiation and continuation postpartum in PWWH lowers infection rates and mortality, but pregnancy remains an important risk factor for morbidity and mortality even during suppressive ART [3, 5–10]. In people with HIV (PWH) who are not pregnant, innate and adaptive immune activation persist despite suppressive ART and are predictive of morbidity and mortality [11–21], but the degree to which the pregnancy and postpartum periods alter these immunologic pathways remains incompletely defined.
The physiologic state of pregnancy, independent of HIV, leads to many immunoregulatory changes that help maintain the fetal allograft but may increase susceptibility to infections, including tuberculosis (TB) [22–25]. Multiple studies have demonstrated this increased risk of TB in PWWH, particularly postpartum [26, 27]. Women without HIV also have an increased TB risk during pregnancy, and an even greater risk postpartum [28, 29]. How pregnancy- and postpartum-induced biomarker changes affect immune activation pathways known to predict morbidity and mortality in HIV remains incompletely understood [30]. Two studies have reported increases in C-reactive protein (CRP), interleukin-6 (IL-6), and D-dimer in the third trimester followed by declines postpartum, but it was unclear how these changes compared with prepregnancy baseline; neither study controlled for ART duration [31, 32]. Additionally, the kynurenine pathway of tryptophan catabolism, which appears to play an important immunoregulatory role including maintaining fetal allograft tolerance, also contributes to HIV and TB pathogenesis by conferring multiple adaptive immune defects, while being one of the strongest predictors of mortality in treated HIV [20, 33–38]. While a study of 20 healthy pregnant women without HIV demonstrated increases in the third trimester through 6 weeks postpartum, no data exist beyond that narrow range or in treated HIV in general [39].
To address these knowledge gaps, we leveraged a cohort of Ugandan women initiating their first ART regimen to assess changes in immunologic biomarkers every 3 months from prepregnancy baseline through pregnancy and the first year postpartum. We assessed multiple biomarkers that predict increased morbidity and mortality in treated HIV and reflect discrete but overlapping immunologic pathways, adjusting for ART duration to ensure appropriate attribution of biomarker changes to pregnancy.
METHODS
Study Design and Participants
Women aged ≥18 years initiating ART in the Uganda AIDS Rural Treatment Outcomes (UARTO) cohort who were pregnant at enrollment or became pregnant during observation (confirmed by urine human chorionic gonadotropin [hCG]), resulting in a live birth, were included. The UARTO cohort includes 759 PWH initiating their first ART regimen at Mbarara University of Science and Technology Immune Suppression Syndrome clinic between 2005 and 2014 [36]. Participants were followed every 3 months with questionnaires and biospecimen archiving. Timing of gestation and the postpartum period was estimated based on self-reported delivery dates. ART regimens reflected the local standard of care at the time and included either zidovudine/lamivudine, stavudine/lamivudine, or tenofovir/emtricitabine in combination with either nevirapine or efavirenz. Exclusion criteria included lack of viral suppression (plasma HIV RNA level >400 copies/mL) any time after 6 months of ART or known acute illness.
Biomarker Measurements
Plasma biomarkers were assessed on cryopreserved plasma obtained in the fasting state pre–ART initiation, at months 6 and 12 of ART, and from these time points when available: prepregnancy, first trimester, second trimester, third trimester, and postpartum months 0–3, 3–6, 6–9, and 9–12. As some women were already pregnant at the time of ART initiation, not all women contributed plasma for all time points. In addition to the kynurenine-to-tryptophan (KT) ratio, a ratio representing the activity of the kynurenine pathway’s rate-limiting enzyme indoleamine 2,3-dioxygenase (IDO) described above, plasma biomarkers representing discrete but related inflammatory pathways in treated HIV were assessed. D-dimer and IL-6, markers of coagulation and generalized inflammation, respectively, are both predictors of morbidity and mortality in treated HIV [11, 18, 20, 40]. Interferon-inducible protein 10 (IP-10 or CXCL10), an interferon response cytokine, is persistently abnormal despite early HIV treatment and predicts morbidity and mortality in nonpregnant people with HIV [40–42]. Soluble CD14 (sCD14) is a marker of microbial translocation and, in addition to soluble CD163 (sCD163), is a marker of monocyte and macrophage activation and predictive of morbidity and mortality [15, 18, 20, 40, 43–45]. Soluble CDC27 is a T-cell activation marker, part of the tumor necrosis factor superfamily, and a strong predictor of late mortality in treated HIV [12, 20]. Intestinal fatty-acid binding protein (I-FABP) is a marker of intestinal barrier integrity and associated with mortality in HIV [18]. KT ratio was assessed by liquid chromatography–mass spectrometry [36]. All others were assessed via commercial immunoassay kits: IP-10, sCD14, and I-FABP (R&D Systems); sCD27 (eBioscience), sCD163 (Trillium Diagnostics, now IQ products), IL-6 (Meso Scale Diagnostics), and D-dimer (Diagnostico Stago). Approximately 84% of plasma samples were obtained from blood drawn in ACD tubes (the remainder from EDTA-anticoagulated tubes). To account for differences in diluent volume, values obtained from ACD tubes were multiplied by an adjustment factor of 1.276 [20]. Expected dilutional effects with increased plasma volume in pregnancy were estimated by evaluating changes in serum hemoglobin levels [46].
Statistical Analysis
Pregnancy-related changes in biomarkers were assessed via linear mixed models with random intercepts, log-transforming outcome variables to satisfy model assumptions and adjusting for ART duration. This statistical approach accounts for clustering by participant, allowing for the inclusion of more than 1 pregnancy per participant, and also mitigates against biased inferences from missing data at any particular time point. Given the rapid and nonlinear decrease in biomarkers (and increases in CD4 counts) following ART initiation, time on ART was modeled as a linear spline with demarcations (knots) at 0–3, 3–6, 6–12, 12–24, and >24 months, which allowed for the necessary adjustment for background ART-mediated changes in biomarkers so that we could isolate the effect of pregnancy and the postpartum period. Estimated mean relative changes in biomarkers from prepregnancy baseline with 95% CIs are reported, with marginal mean estimates with 95% CIs in the Supplementary Data. Several sensitivity analyses were performed, including (1) excluding women who were pregnant at the time of ART initiation, (2) modeling ART duration with a less complex linear spline (ie, slope before and after 6 months), and (3) modeling ART duration as a linear predictor (without a spline) and excluding observations earlier than month 8 of ART (ie, when most of the major ART-related biomarker reductions occur). All statistical tests were 2-tailed, with an alpha level of .05 considered statistically significant. All analyses were performed with Stata, version 15.
Patient Consent
This study received approval of the institutional review boards of Mbarara University, the Uganda National Council of Science and Technology, the University of California, San Francisco, and Partners HealthCare. All participants provided written informed consent.
RESULTS
Patient Demographics and Characteristics
Fifty-four participants contributed 412 biomarker (and 1230 T-cell count) time points. Seven participants contributed observations from more than 1 pregnancy. The median age (interquartile range [IQR]) was 29 (25–33) years, and 30% of women were pregnant at ART initiation (Table 1). The median ART duration before pregnancy (IQR) was 11 (3–24) months. Reflective of the initial rollout of ART in rural Uganda, most participants started ART later in their HIV infection, with a median CD4+ T-cell count (IQR) of 134 (81–216) cells/mm3.
Table 1.
Characteristics of 54 Ugandan Women With HIV at ART Initiation
| Characteristic | Median (IQR) |
|---|---|
| Age, y | 29 (25–33) |
| Plasma HIV RNA level, log10 copies/mL | 5.0 (4.5–5.5) |
| CD4 count, cells/mm3 | 134 (81–216) |
| CD8 count, cells/mm3 | 639 (447–848) |
| CD4/CD8 ratio | 0.20 (0.13–0.37) |
| Pregnant at ART initiation, No. (%) | 16 (30)a |
| ≥2 pregnancy episodes during observation, No. (%) | 7 (13)b |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range.
aFirst trimester, n = 4; second trimester, n = 5; third trimester, n = 7.
bTwo pregnancies, n = 6; 3 pregnancies, n = 1.
CD4+ and CD8+ T-Cell Count Changes During Pregnancy and Postpartum
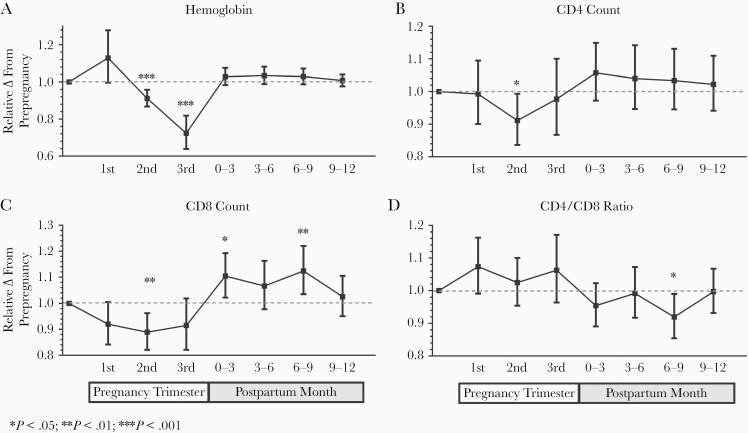
Expected ART-mediated increases in CD4 counts and CD4/CD8 ratios were observed, and subsequent models were adjusted for ART duration as a linear spline (Supplementary Figure 1). As plasma volume expansion during pregnancy might be expected to lower cell counts and plasma cytokine concentrations in the absence of real immunologic changes in the tissues, we first assessed changes in peripheral blood hemoglobin levels as an estimate for the degree to which plasma volume expansion may have occurred during pregnancy [46]. After adjustment for ART duration, blood hemoglobin concentrations tended to increase in the first trimester before declining below prepregnancy values by a mean of 9% in the second trimester and 28% in the third trimester (P < .001 for both) (Figure 1; Supplementary Table 1). After adjustment for ART duration, we observed a 9% and 11% decrease in both CD4 and CD8 counts during the second trimester compared with the prepregnancy baseline (P < .05 for both, respectively, which was comparable to the reduction in hemoglobin levels at the same time point (Figure 1; Supplementary Table 1 and Supplementary Figure 2). Mean CD4 counts declined from baseline to the third trimester to a far lesser degree (–2%; 95% CI, –13% to 10%) than hemoglobin levels (–28%; 95% CI, –36% to –18%). CD8 counts tended to increase above prepregnancy levels in the first 6–9 months postpartum.
Figure 1.
Relative changes in hemoglobin levels and T-cell counts throughout pregnancy and postpartum. Changes in peripheral blood hemoglobin (A), CD4+ (B), and CD8+ (C) T-cell counts and CD4/CD8 ratio (D) are presented as mean fold changes relative to prepregnancy baseline with 95% CIs. Changes were assessed with linear mixed models adjusted for time on ART modeled as a linear spline (0–3, 3–6, 6–12, 12–24, and >24 months). Abbreviation: ART, antiretroviral therapy.
D-dimer Increased Significantly During Pregnancy
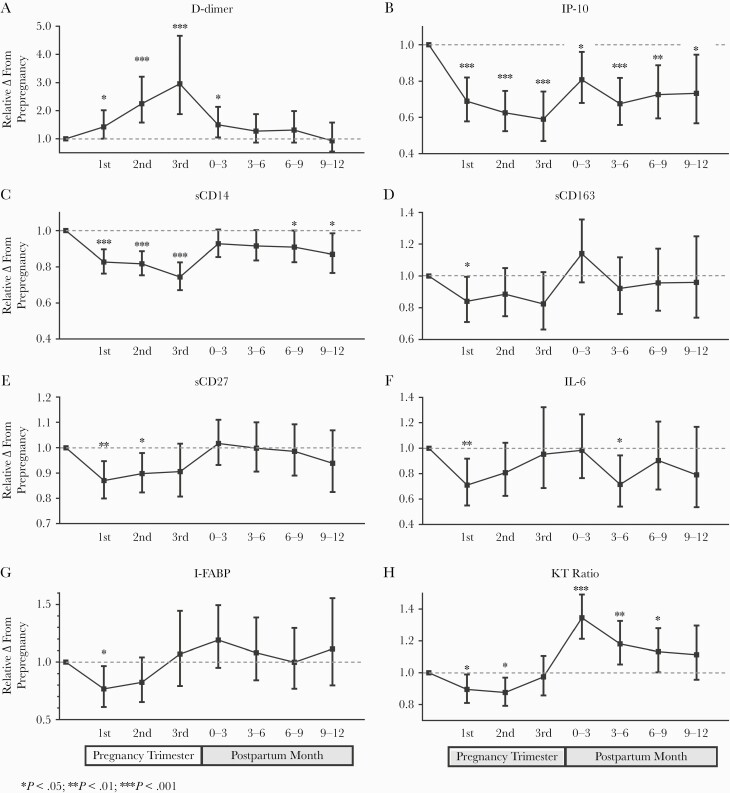
Similarly, we adjusted all plasma biomarker changes during pregnancy for concurrent changes in ART duration using linear splines (Supplementary Figures 3–4). Consistent with prior reports of hypercoagulability during pregnancy, D-dimer levels increased steadily throughout all 3 trimesters, reaching a mean 2.2-fold and 3-fold increase above baseline by the second and third trimesters (P < .001 for both) (Figure 2; Supplementary Table 1 and Supplementary Figure 5) [47, 48]. In the early postpartum period, D-dimer levels decreased significantly and approached prepregnancy levels by 3–6 months.
Figure 2.
Relative changes in biomarkers throughout pregnancy and postpartum. Changes in plasma D-dimer (A), IP-10 (B), sCD14 (C), sCD163 (D), sCD27 (E), IL-6 (F), I-FABP (G), and KT ratio (H) are presented as mean fold changes relative to prepregnancy baseline with 95% CIs. Changes were assessed with linear mixed models adjusted for time on ART modeled as a linear spline (0–3, 3–6, 6–12, 12–24, and >24 months). Abbreviations: ART, antiretroviral therapy; I-FABP, intestinal fatty-acid binding protein; IL, interleukin; IP-10, interferon-inducible protein 10; KT, kynurenine/tryptophan; sCD14, soluble CD14; sCD27, soluble CD27; sCD163, soluble CD163.
Most Inflammatory Biomarkers Declined During Pregnancy
As expected, most inflammatory biomarkers declined during ART, with the greatest changes occurring in the first few months of viral suppression (Supplementary Figures 3–4). Of all plasma biomarkers, IP-10 levels declined to the greatest degree during pregnancy—by a mean of 30%–40% in the 3 trimesters (P < .001 for all)—far exceeding concurrent hemoglobin declines (Figure 2; Supplementary Table 1 and Supplementary Figure 5). Plasma sCD14 levels also declined by a mean of 17%–18% in the first 2 trimesters (P < .001 for both), respectively, exceeding associated reductions in hemoglobin. Both sCD27 and sCD163 declined significantly in the first trimester, though more modestly than other biomarkers. IL-6 and I-FABP also declined by a mean of 29% and 23% (P < .03 for both) in the first trimester, but then increased toward prepregnancy baseline by the third trimester. Similarly, KT ratio, which should be unaffected by the degree of plasma volume expansion in pregnancy (as it reflects the ratio of 2 metabolite concentrations), declined by a mean of 10%–12% in the first 2 trimesters (P < .03 for both) before increasing back to the baseline in the third trimester (Figure 2; Supplementary Figures 5–6).
Several Biomarkers Remained Suppressed Postpartum
Multiple biomarkers were suppressed during pregnancy and remained below prepregnancy values during the postpartum period, while hemoglobin levels returned to the prepregnancy baseline (Figures 1 and 2; Supplementary Table 1 and Supplementary Figure 5). IP-10 and to a lesser degree sCD14 remained significantly below baseline for at least 12 months postpartum. Postpartum IL-6 levels fluctuated postpartum, but tended to remain lower than prepregnancy baseline.
KT Ratio Was the Only Plasma Biomarker to Increase Significantly Postpartum
After initial declines in the first 2 trimesters and a return to baseline in the third trimester, KT ratio increased by 35% in the first 3 months postpartum (P < .001) and remained elevated above the prepregnancy baseline for 6–9 months (P ≤ .045 for all). This pattern coincided with the relative increase in CD8 counts postpartum.
Sensitivity Analyses
All major inferences related to biomarker changes during pregnancy and postpartum were robust to several sensitivity analyses. These included (1) models restricted to women who had incident pregnancies after initiating ART, (2) modeling ART duration in splines with 2 (instead of 5) segments (before and after month 6 of ART), and (3) models restricted to observations after more than 8 months of ART (beyond the major ART-mediated changes).
DISCUSSION
While numerous studies have demonstrated increased morbidity and mortality largely from infections during pregnancy and postpartum in PWWH (and those without HIV), none have comprehensively assessed the immunologic changes that occur in this setting that might contribute to these risks [1–6]. Leveraging a cohort of ART-treated PWH from Uganda, we assessed how biomarkers of immune activation change in PWWH during pregnancy and postpartum. We made several observations. First, plasma levels of most of the measured inflammatory biomarkers declined significantly during early pregnancy, beyond what might be expected from pregnancy-related plasma volume expansion [46]. Second, D-dimer increased substantially during pregnancy and returned to baseline postpartum. Third, some inflammatory biomarkers remained durably suppressed for at least 9–12 months postpartum. Perhaps most importantly, KT ratio, a biomarker of the immunoregulatory kynurenine pathway of tryptophan catabolism, was the only biomarker to increase significantly postpartum, remaining elevated above prepregnancy baseline for at least 6–9 months postpartum. While the clinical implications of these findings remain unclear, they raise testable hypotheses to explain the increased risk of infectious complications postpartum.
Consistent with well-described immunoregulatory changes during pregnancy in women without HIV, most inflammatory biomarkers declined during pregnancy in treated PWWH [49, 50]. IP-10, sCD14, sCD163, and sCD27 all declined significantly throughout pregnancy, while KT ratio, I-FABP, and IL-6 transiently declined during early pregnancy before returning to baseline by parturition. CD4 and CD8 counts, however, appeared fairly stable throughout pregnancy, with the exception of modest declines in the second trimester. As these T-cell declines in the second trimester were comparable in magnitude to concurrent hemoglobin declines, it is likely that the declines are related to the well-characterized dilutional effects of pregnancy-mediated volume expansion [46]. Nevertheless, most of the plasma biomarker declines we observed occurred before significant volume expansion, suggesting that the declines are unlikely to be explained by dilution. As CD4 count, I-FABP, and IL-6 all appeared to increase back toward prepregnancy baseline in the third trimester despite concurrent volume expansion, these increases might be even greater when accounting for dilution. Collectively, these observations support the notion of relative immunosuppression during pregnancy, which partially reverses in the third trimester [30]. The magnitude of these relative changes in immune activation among PWWH compared with those seen in pregnant women without HIV is currently unknown, particularly given that microbial translocation—which is significantly greater in treated HIV compared with the HIV-negative population—may be a key effect modifier in the relationship between immune activation and stage of pregnancy [41, 49].
Like prior studies in women without HIV, we confirmed that D-dimer levels increase steadily throughout pregnancy, remain slightly elevated early postpartum, and then decrease to baseline thereafter. Compared with the HIV-negative population, however, PWWH likely have a higher baseline D-dimer, reach a higher peak, and decrease back to a prepregnancy baseline more slowly [47, 48]. A study in pregnant women without HIV demonstrated an elevated risk of venous thromboembolism beginning in the third trimester through the third postpartum month [51]. These findings are fairly compatible with the only prior study of D-dimer in PWWH, where levels increased to delivery and then returned to baseline several weeks postpartum [31, 32]. In 1 study in PWWH, 6-month postpartum D-dimer levels predicted subsequent morbidity [31]. As our study indicates that D-dimer levels have returned to prepregnancy baseline by 6 months postpartum, it remains unclear whether pregnancy-mediated increases in D-dimer are associated with increased morbidity and mortality risk beyond the well-described early thromboembolic complications [11, 17, 20, 40].
Our study also demonstrates that several biomarkers remain suppressed for at least 12 months postpartum. IP-10 and sCD14, both predictors of morbidity and mortality in treated HIV, declined within the first trimester of pregnancy and remained below baseline for at least 1 year postpartum [15, 18, 42]. While few studies have investigated postpartum changes in inflammatory biomarkers, 1 prior study found significant differences in several biomarkers among women who exclusively breastfed for 5 months vs formula-fed their infants [31]. The sustained suppression of inflammation we observed may be hormonally driven in our cohort as well; while most women likely did not breastfeed for 12 months (or exclusively for 6 months) from a qualitative study performed in a subset of the UARTO cohort, the degree of breastfeeding that may be required in prolonging these immunologic changes is unknown [52, 53]. These biomarkers, however, are mechanistically linked to the kynurenine pathway of tryptophan catabolism, which increased significantly postpartum. IP-10, like IDO, is induced by type I and II interferons. Similarly, kynurenine pathway–mediated suppression of Th17 cells may also contribute to microbial translocation, increasing sCD14 directly [54, 55], while lipopolysaccharides augment IDO activity [33]. These findings suggest a postpartum “de-linking” of these pathways.
The changes in KT ratio throughout pregnancy and postpartum represent the most significant finding in this study. We observed that the KT ratio decreased in the first and second trimesters compared with prepregnancy and then increased significantly early postpartum, with sustained elevation for at least 6–9 months. A study of HIV-uninfected pregnant women observed increases in KT ratio in the third trimester through 6 weeks postpartum with similar relative increases but with lower absolute KT ratio values compared with the present study. Ours is the first study in humans to demonstrate reductions in kynurenine pathway activity during early pregnancy and the duration of its elevation through at least 9 months postpartum [39]. Pregnancy-related changes in kynurenine pathway activity may be mediated by changes in tryptophan 2,3-dioxygenase (TDO; found in the liver and placenta), IDO (found in mucosal, lymphoid, and placental tissues), gut bacteria, or a combination [56, 57]. The reduction in KT ratio we observed in early pregnancy in humans parallels the estrogen- and progesterone-driven inhibition of liver TDO activity observed in pregnant rats and IDO-1 expression in humans at the materno-fetal interface [56–60]. Nevertheless, rat liver TDO activity does not increase postpartum despite nursing [58], raising the possibility that other enzymes (eg, IDO or microbiome-derived enzymes) are responsible for the postpartum increases in KT ratio we observed in PWWH. As ~90% of total tryptophan (as assessed in our study) is albumin-bound and unavailable to kynurenine pathway enzymes, fluctuations in albumin and free fatty acids (which displace tryptophan from albumin) might plausibly lead to an over- or underestimation of kynurenine pathway activity in vivo [61]. While serum triglycerides are known to increase in the third trimester, they tend to return to prepregnancy levels by 6 weeks postpartum [62]. Thus, changes in albumin or triglycerides are unlikely to explain the sustained increased KT ratio for 6–9 months postpartum that we observed.
The clinical significance of postpartum increases in kynurenine pathway activity is only partially understood but, in the context of HIV, is likely to be highest with regard to TB risk. Indeed, TB risk is known to be increased for at least 9 months postpartum in women with and without HIV, strikingly paralleling the prolonged postpartum kynurenine pathway elevations we observed [26–29]. Furthermore, the kynurenine pathway has been directly linked to TB pathogenesis. In animal models, increased IDO expression (and higher KT ratio) correlates with higher mycobacterial burden, and IDO inhibition increases TB killing, decreases disease progression, and reorganizes granulomas, allowing lymphocyte trafficking to infected macrophages [33, 35, 38]. KT ratio also strongly predicts incident and prevalent active and latent TB [35, 37, 38]. KT ratio also strongly predicts mortality in PWH, particularly in settings where TB is prevalent [18, 20, 36, 40]. The APPRISE trial demonstrated isoniazid preventive therapy (IPT) in PWWH to be particularly beneficial in those with low CD4+ T-cell counts [63]. As higher KT ratios have been associated with slower CD4+ T-cell recovery, the benefit of IPT in this setting could be in part a surrogate for those with increased kynurenine pathway activity and thus heightened TB risk. Given the public health importance of TB in PWWH, IDO activity may be a plausible putative driver in this setting and a viable therapeutic target for TB prevention efforts [63–65]. Postpartum kynurenine pathway activity has also been previously linked to postpartum depression, as tryptophan depletion may decrease serotonin synthesis in the brain [66, 67]. While we observed a link between KT ratio and depressive symptoms in UARTO previously [68], we did not observe a further increase in depressive symptoms postpartum among PWWH [69].
Our study had several limitations. First, our relatively small sample size may have limited our ability to detect clinically significant changes in some biomarkers. Additionally, we did not measure gestational age by last menstrual period, but rather by reported delivery dates, which could induce bias based on differential carriage to term. Third, we were unable to adjust for other confounders beyond ART duration that are known to impact markers of inflammation. Fourth, we did not measure free (ie, protein-unbound) tryptophan or its mediators. Finally, the study was too small to evaluate the association between biomarker changes and clinical outcomes, and we specifically excluded women with acute illnesses to avoid confounding by these factors (ie, acute infections are likely to increase inflammatory markers), so the clinical relevance of our findings remains unclear.
In summary, we found that most immune activation markers decline during pregnancy and several stay suppressed for up to 1 year postpartum, but only the kynurenine pathway of tryptophan catabolism increases in the postpartum period, remaining elevated above prepregnancy baseline for at least 9 months. Peripartum and postpartum kynurenine pathway induction could plausibly explain the increased risk of infection-related complications, particularly TB, in PWWH and even pregnant women without HIV. These data further support the kynurenine pathway as a relevant interventional target in future studies.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the study participants, who made this work possible; the capable UARTO study administrators, research assistants, data managers, laboratory staff, participant trackers, drivers, and volunteers, who were critical to conducting this multidisciplinary research; the Mbarara University of Science and Technology (MUST) administration and the MUST Immune Suppression Syndrome Clinic medical staff, nurses, pharmacists, and counselors for their helpful assistance; and the members of the Cleveland Immunopathogenesis Consortium for helpful scientific discussions. We also thank Dr. Abdulla Badawy, PhD, for his thoughtful review of the manuscript as well as his interpretations of the kynurenine pathway data.
Financial support. This work was supported by the following National Institutes of Health grants: K24-AI145806, R01-MH54907, R56-AI100765, R21-HD069194, P01-AI076174, P30-AI027763, and R38-HL143581.
Potential conflicts of interest. P.W.H. has received research funding from Gilead Sciences; honoraria from Gilead, Viiv, and Janssen; and consulting fees from Biotron and Viiv. L.T.M. and M.M.L. have received research funding from Gilead. T.H.B. has received options from Excision BioTherapeutics, unrelated to this work. All other authors have no conflicts of interest to declare. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentation. This work was presented in part at the 2015 Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, Abstract #899.
Author contributions. All authors contributed to the interpretation of the data and the editing of the manuscript. S.R.S. performed data analysis and wrote the first draft of the manuscript. P.W.H. conceived of and designed the study, obtained funding, coordinated the study team, assisted with biospecimen quality assurance procedures, performed the primary data analysis, and mentored S.R.S. on the analysis, interpretation, and writing of the manuscript. H.B. organized the selection and shipment of plasma samples and contributed to the interpretation of the data. Y.B., J.E.H., A.K., and D.R.B. oversaw the operations of the UARTO cohort from which participant specimens were sampled, including the recruitment and retention of participants, clinical and questionnaire data, and specimen biobanking. D.R.B. obtained funding for the UARTO cohort. J.N.M. oversaw data management and quality assurance procedures for clinical, questionnaire, and biospecimens in the UARTO cohort, helped design the study instruments, and oversaw specimen shipping and distribution. J.K., L.T.M., and A.K. oversaw the reproductive health questionnaires and testing procedures and helped characterize the timing of pregnancy for the participants. T.H.B., Y.H., R.P.T., D.M., and M.M.L. oversaw biomarker measurements and quality assurance procedures in their laboratories.
References
- 1. Calvert C, Ronsmans C. The contribution of HIV to pregnancy-related mortality: a systematic review and meta-analysis. AIDS 2013; 27:1631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis J, Landon MB, Gersnoviez RJ, et al. ; Maternal-Fetal Medicine Units Network, National Institute of Child Health and Human Development . Perioperative morbidity and mortality among human immunodeficiency virus infected women undergoing cesarean delivery. Obstet Gynecol 2007; 110:385–90. [DOI] [PubMed] [Google Scholar]
- 3. Matthews LT, Kaida A, Kanters S, et al. HIV-infected women on antiretroviral treatment have increased mortality during pregnant and postpartum periods. AIDS 2013; 27(Suppl 1):S105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. French R, Brocklehurst P. The effect of pregnancy on survival in women infected with HIV: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol 1998; 105:827–35. [DOI] [PubMed] [Google Scholar]
- 5. Zash RM, Souda S, Leidner J, et al. High proportion of deaths attributable to HIV among postpartum women in Botswana despite widespread uptake of antiretroviral therapy. AIDS Patient Care STDS 2017; 31:14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bebell LM, Ngonzi J, Siedner MJ, et al. HIV Infection and risk of postpartum infection, complications and mortality in rural Uganda. AIDS Care 2018; 30:943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lundgren JD, Babiker AG, Gordin F, et al. ; INSIGHT START Study Group . Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Danel C, Moh R, Gabillard D, et al. ; TEMPRANO ANRS 12136 Study Group . A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373:808–22. [DOI] [PubMed] [Google Scholar]
- 9. Garcia Garrido HM, Mak AMR, Wit FWNM, et al. Incidence and risk factors for invasive pneumococcal disease and community-acquired pneumonia in human immunodeficiency virus-infected individuals in a high-income setting. Clin Infect Dis 2020; 71:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffman RM, Angelidou KN, Brummel SS, et al. ; IMPAACT PROMISE 1077BF/FF team . Maternal health outcomes among HIV-infected breastfeeding women with high CD4 counts: results of a treatment strategy trial. HIV Clin Trials 2018; 19:209–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuller LH, Tracy R, Belloso W, et al. ; INSIGHT SMART Study Group . Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sunil M, Nigalye M, Somasunderam A, et al. Unchanged levels of soluble CD14 and IL-6 over time predict serious non-AIDS events in HIV-1-infected people. AIDS Res Hum Retroviruses 2016; 32:1205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lyons JL, Uno H, Ancuta P, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr 2011; 57:371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sandler NG, Wand H, Roque A, et al. ; INSIGHT SMART Study Group . Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelesidis T, Kendall MA, Yang OO, et al. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012; 206:1558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boulware DR, Hullsiek KH, Puronen CE, et al. ; INSIGHT Study Group . Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 2011; 203:1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunt PW, Sinclair E, Rodriguez B, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKibben RA, Margolick JB, Grinspoon S, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis 2015; 211:1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee S, Byakwaga H, Boum Y, et al. Immunologic pathways that predict mortality in HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis 2017; 215:1270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Angelidou K, Hunt PW, Landay AL, et al. Changes in inflammation but not in T-cell activation precede non-AIDS-defining events in a case-control study of patients on long-term antiretroviral therapy. J Infect Dis 2018; 218:239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Piccinni MP. T cell tolerance towards the fetal allograft. J Reprod Immunol 2010; 85:71–5. [DOI] [PubMed] [Google Scholar]
- 23. Poole JA, Claman HN. Immunology of pregnancy. Implications for the mother. Clin Rev Allergy Immunol 2004; 26:161–70. [DOI] [PubMed] [Google Scholar]
- 24. Wilsher ML, Hagan C, Prestidge R, et al. Human in vitro immune responses to Mycobacterium tuberculosis. Tuber Lung Dis 1999; 79:371–7. [DOI] [PubMed] [Google Scholar]
- 25. Gaunt G, Ramin K. Immunological tolerance of the human fetus. Am J Perinatol 2001; 18:299–312. [DOI] [PubMed] [Google Scholar]
- 26. Gupta A, Nayak U, Ram M, et al. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis 2007; 45: 241–9. [DOI] [PubMed] [Google Scholar]
- 27. Jana N, Vasishta K, Saha SC, Ghosh K. Obstetrical outcomes among women with extrapulmonary tuberculosis. N Engl J Med 1999; 341:645–9. [DOI] [PubMed] [Google Scholar]
- 28. Zenner D, Kruijshaar ME, Andrews N, Abubakar I. Risk of tuberculosis in pregnancy: a national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med 2012; 185:779–84. [DOI] [PubMed] [Google Scholar]
- 29. Jonsson J, Kuhlmann-Berenzon S, Berggren I, Bruchfeld J. Increased risk of active tuberculosis during pregnancy and postpartum: a register-based cohort study in Sweden. Eur Respir J 2020; 55:1901886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Singh N, Perfect JR. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin Infect Dis 2007; 45:1192–9. [DOI] [PubMed] [Google Scholar]
- 31. Russell ES, Mohammed T, Smeaton L, et al. Immune activation markers in peripartum women in Botswana: association with feeding strategy and maternal morbidity. PLoS One 2014; 9:e89928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoffman RM, Leister E, Kacanek D, et al. Biomarkers from late pregnancy to 6 weeks postpartum in HIV-infected women who continue versus discontinue antiretroviral therapy after delivery. J Acquir Immune Defic Syndr 2013; 63:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med 2010; 2:32ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998; 281:1191–3. [DOI] [PubMed] [Google Scholar]
- 35. Gautam US, Foreman TW, Bucsan AN, et al. In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 2018; 115:E62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Byakwaga H, Boum Y 2nd, Huang Y, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis 2014; 210:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Adu-Gyamfi CG, Snyman T, Hoffmann CJ, et al. Plasma indoleamine 2, 3-dioxygenase, a biomarker for tuberculosis in human immunodeficiency virus-infected patients. Clin Infect Dis 2017; 65:1356–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Collins JM, Siddiqa A, Jones DP, et al. Tryptophan catabolism reflects disease activity in human tuberculosis. JCI Insight 2020; 5:e137131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schröcksnadel K, Widner B, Bergant A, et al. Longitudinal study of tryptophan degradation during and after pregnancy. Life Sci 2003; 72:785–93. [DOI] [PubMed] [Google Scholar]
- 40. Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schnittman SR, Deitchman AN, Beck-Engeser G, et al. Beck-Engeser G, et al. Abnormal Levels of Some Biomarkers of Immune Activation Despite Very Early Treatment of Human Immunodeficiency Virus. J Infect Dis. 2021; 223:1621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hellmuth J, Slike BM, Sacdalan C, et al. Very early initiation of antiretroviral therapy during acute HIV infection is associated with normalized levels of immune activation markers in cerebrospinal fluid but not in plasma. J Infect Dis 2019; 220:1885–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knudsen TB, Ertner G, Petersen J, et al. Plasma soluble CD163 level independently predicts all-cause mortality in HIV-1-infected individuals. J Infect Dis 2016; 214:1198–204. [DOI] [PubMed] [Google Scholar]
- 44. Kirkegaard-Klitbo DM, Mejer N, Knudsen TB, et al. Soluble CD163 predicts incident chronic lung, kidney and liver disease in HIV infection. AIDS 2017; 31:981–8. [DOI] [PubMed] [Google Scholar]
- 45. Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vricella LK. Emerging understanding and measurement of plasma volume expansion in pregnancy. Am J Clin Nutr 2017; 106:1620–5S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kawaguchi S, Yamada T, Takeda M, et al. Changes in d-dimer levels in pregnant women according to gestational week. Pregnancy Hypertens 2013; 3:172–7. [DOI] [PubMed] [Google Scholar]
- 48. Murphy N, Broadhurst DI, Khashan AS, et al. Gestation-specific D-dimer reference ranges: a cross-sectional study. BJOG 2015; 122:395–400. [DOI] [PubMed] [Google Scholar]
- 49. Denney JM, Nelson EL, Wadhwa PD, et al. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine 2011; 53:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Denney JM, Nelson E, Wadhwa P, et al. Cytokine profiling: variation in immune modulation with preterm birth vs. uncomplicated term birth identifies pivotal signals in pathogenesis of preterm birth. J Perinat Med 2021; 49:299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Costagliola D, Potard V, Lang S, et al. Is the risk of myocardial infarction in people with human immunodeficiency virus (HIV) associated with atazanavir or darunavir? A nested case-control study within the French hospital database on HIV. J Infect Dis 2020; 221:516–22. [DOI] [PubMed] [Google Scholar]
- 52. Dunkley E, Ashaba S, Burns B, et al. “I beg you…breastfeed the baby, things changed”: infant feeding experiences among Ugandan mothers living with HIV in the context of evolving guidelines to prevent postnatal transmission. BMC Public Health 2018; 18:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lanktree E, Ssebuko A, Alibhai A, et al. Breastfeeding practices of HIV-positive and HIV-negative women in Kabarole district, Uganda. Matern Child Nutr 2011; 7:378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pallotta MT, Orabona C, Volpi C, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 2011; 12:870–8. [DOI] [PubMed] [Google Scholar]
- 55. Baban B, Chandler PR, Sharma MD, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol 2009; 183:2475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Badawy AA. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep 2015; 35:e00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Badawy AA. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res 2017; 10:1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Badawy AA. Effects of pregnancy on tryptophan metabolism and disposition in the rat. Biochem J 1988; 255:369–72. [PMC free article] [PubMed] [Google Scholar]
- 59. Edwards SM, Cunningham SA, Dunlop AL, Corwin EJ. The maternal gut microbiome during pregnancy. MCN Am J Matern Child Nurs 2017; 42:310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang M, Huang G, Qian H, et al. Progesterone decreased indoleamine 2,3-dioxygenase 1(IDO1) expression in early pregnancy chorionic villi and decidua. Autoimmunity. 2021; 54:156–62. [DOI] [PubMed] [Google Scholar]
- 61. Badawy AA, Guillemin G. The plasma [kynurenine]/[tryptophan] ratio and indoleamine 2,3-dioxygenase: time for appraisal. Int J Tryptophan Res 2019; 12:1178646919868978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Montes A, Walden CE, Knopp RH, et al. Physiologic and supraphysiologic increases in lipoprotein lipids and apoproteins in late pregnancy and postpartum. Possible markers for the diagnosis of “prelipemia.” Arteriosclerosis 1984; 4:407–17. [DOI] [PubMed] [Google Scholar]
- 63. Gupta A, Montepiedra G, Aaron L, et al. ; IMPAACT P1078 TB APPRISE Study Team . Isoniazid preventive therapy in HIV-infected pregnant and postpartum women. N Engl J Med 2019; 381:1333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Salazar-Austin N, Cohn S, Lala S, et al. Isoniazid preventive therapy and pregnancy outcomes in women living with human immunodeficiency virus in the Tshepiso cohort. Clin Infect Dis 2020; 71:1419–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kalk E, Heekes A, Mehta U, et al. Safety and effectiveness of isoniazid preventive therapy in pregnant women living with human immunodeficiency virus on antiretroviral therapy: an observational study using linked population data. Clin Infect Dis 2020; 71:e351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Capuron L, Neurauter G, Musselman DL, et al. Interferon-alpha-induced changes in tryptophan metabolism. Relationship to depression and paroxetine treatment. Biol Psychiatry 2003; 54:906–14. [DOI] [PubMed] [Google Scholar]
- 67. Kohl C, Walch T, Huber R, et al. Measurement of tryptophan, kynurenine and neopterin in women with and without postpartum blues. J Affect Disord 2005; 86:135–42. [DOI] [PubMed] [Google Scholar]
- 68. Martinez P, Tsai AC, Muzoora C, et al. Reversal of the kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. J Acquir Immune Defic Syndr 2014; 65:456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kaida A, Matthews LT, Ashaba S, et al. Depression during pregnancy and the postpartum among HIV-infected women on antiretroviral therapy in Uganda. J Acquir Immune Defic Syndr 2014; 67(Suppl 4):S179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.