Graphical abstract
Adoptive immunotherapy options currently under trial for COVID-19.
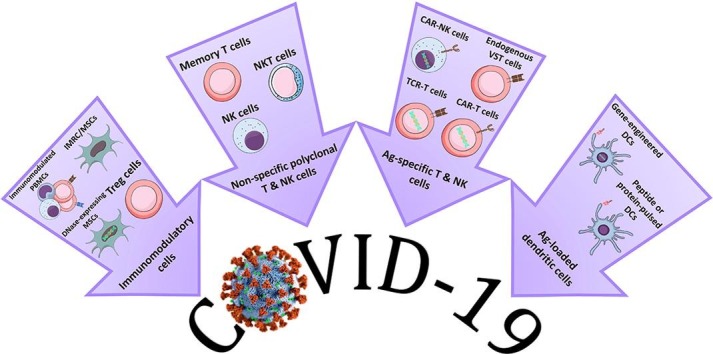
Keywords: Adoptive immunotherapy, Antigen-specific immune cells, COVID-19, SARS-CoV-2, Virus diseases
Abstract
Although not a standard-of-care yet, adoptive immunotherapeutic approaches have gradually earned a place within the list of antiviral therapies for some of fatal and hard-to-treat viral diseases. To maintain robust antiviral immunity and to effectively target the viral particles and virally-infected cells, immune cells capable of recognizing the viral antigens are required. While conventional vaccination can induce these cells in vivo; another option is to prime and generate antigen-specific immune cells ex vivo. This approach has been successfully trialed for virulent opportunistic viral infections after bone marrow transplantation. Amid the crisis of SARS-CoV2 pandemic, which has been followed by the success of certain early-authorized vaccines; some institutions and companies have explored the effects of viral-specific adoptive cell transfers (ACTs) in trials, as alternative treatments. Aimed at outlining a perspective on antigen-specific adoptive immunotherapy for viral infections, this review article specifically provides an appraisal of ACT-based studies/trials on SARS-CoV2 infection.
1. Introduction
Antigen-specific adoptive immunotherapy, with the ability of targeting and eliminating the host cells via recognition of specific antigens (Ag), has been a targeted therapeutic choice for certain viral infections and cancers [1]. Although not a standard-of-care yet, and not at the top of the treatment options list; adoptive transfer of Ag-specific effector immune cells has a successful history for viral diseases, especially for opportunistic viral infections after allogeneic bone marrow transplantation (allo-BMT) and some of hard-to-treat chronic viral infections lacking a vaccine such as HIV infection [1], [2], [3]. In fact, for the intractable viral infections lacking effective treatments and vaccine, the adoptive transfer of antigen-specific immune cells has been advocated as one of the few remaining therapeutic options, with a high efficacy profile [1], [2]. On this basis and with the advancement of platforms for generation of Ag-specific cells, a prospect can be envisioned that the use of these cellular products may acquire a role in easing the burden of epidemics of fast-spreading infectious viruses.
The outbreak of new disease-causing viruses, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), can plague the populations due to failure of human immune system to recognize the unfamiliar antigens and delayed response to the pathogen, thereby leaving the body vulnerable against the overpowering enemy. In the absence of virus-reactive central memory lymphocytes or effective neutralizing antibodies, many humans are defenseless against the viral replication and spread, and subsequent life-threatening complications, as revealed in our tangible experience of the recent coronavirus disease pandemic (COVID-19) [4]. With that being said, studies have shown that the factor that imparts an overwhelming risk for severe COVID-19 cases might be inadequate interferon responses [5], [6]; though, the results of systemic administration of interferons for such patients were controversial [7], [8].
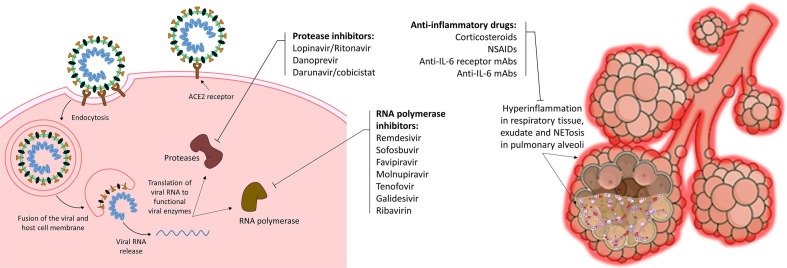
Despite progress in clinical trials, there is still no fully-approved treatment for COVID-19, which necessitates the synergistic collaboration of researchers, academic institutions, pharmaceutical companies and countries to fulfil this unmet urgent medical need [9]. Besides, there are some antiviral medications capable of curbing viral replication, viral assembly and systemic spread of infection in the body, as well as some anti-inflammatory treatments capable of suppressing the post-infectious hyperinflammation, which have been trialed on COVID-19 patients (Fig. 1 ). Although many trials supported the efficacy of these treatments for COVID-19 patients [10], [11], [12], [13], [14], [15], especially in terms of decrease in hospitalization, improvement of hospitalized patients’ condition, and reduced risk of ICU admission and mortality; further evidence failed to prove significant clinical benefit in use of some of the mentioned treatments (e.g. remdesivir and IL-6 receptor antagonists) [8], [16], and so regional guidelines for COVID-19 management have remained to be largely varied [17], [18].
Fig. 1.
Repurposed antiviral and anti-inflammatory treatments for COVID-19.
In order to empower the body's immune system to effectively tackle the virus, the most common approach is vaccination. While some trialed SARS-CoV2 vaccines have demonstrated promising efficacy [19], there have been safety concerns and doubts on durability of the post-vaccination immune response in the recipients [20], [21]. In the recent months, suspected vaccine-induced coagulopathy was on the agenda of regulatory agencies [22], [23]. At the same time, questions on the vaccine efficacy dynamics have been raised that if the developing vaccines can effectively pulse antigen presenting cells (APCs) and consequently neutralizing antibodies can be produced; this may be true for how many of the recipients and for how long [24], [25], [26].
While these uncertainties remain to be resolved, the use of one-step-forward Ag-loaded dendritic cell vaccines, can be looked as a research-worthy subject. Moreover, use of the convalescent donor-derived ex-vivo expanded endogenous virus-specific T cells (VSTs) or alternatively the genetically modified T cells expressing viral-antigen-specific T cell receptor (TCR) or chimeric antigen receptor (CAR) may be considered as the potential therapeutic options. These adoptive immunotherapies, however, require tailor-made research and carefully-designed trials for a new disease entity like COVID-19.
It is noteworthy that many adoptive immunotherapy approaches have been investigated in parallel with the vaccine development for COVID-19 during the past year. Although compared with common vaccines and antiviral medicines, adoptive cell transfers (ACT) are more costly and lower-throughput approach [27], they may be applied in case of vaccine failure as well as for the patients who are in emergency state of the disease. Hence, in this paper, an overview on antigen-specific adoptive immunotherapy for viral infections is given, and additionally an appraisal of adoptive immunotherapy-based studies and trials for SARS-CoV2 infection is provided.
2. A brief look at conventional (acellular) anti-viral vaccines
The majority of current cell-free antiviral vaccines fell into 4 common categories of live-attenuated/inactivated whole-pathogen, protein-based, viral vector-based and nucleic acid-based vaccines. The first category consists of entire virion that have been inactivated or weakened (live-attenuated) and may elicit strong multi-specific immune responses in the recipients [28], [29]. The other category that is also known as “subunit vaccines” include the immunogenic protein components or antigens of the virus. These components are either derived from the viral cultures or produced via recombinant DNA technology. The next category, the vector-based vaccines, are mainly composed of a modified non-pathogenic viral vector (e.g., adenovirus) containing the encoding gene of the antigens of the intended virus (e.g., SARS-CoV2 antigen). The relatively new fashion of vaccines is based on nucleic acids either in the form of the DNA plasmids containing the encoding genes or the mRNA of the intended viral antigens. All of the mentioned vaccine platforms are; however, involved with safety and efficacy concerns such as [29], [30], [31]: (1) induction of the disease (mostly in case of live-attenuated vaccines), (2) lack of immune response induction in case of vector-based vaccines due to pre-immunity to the vector, (3) inherent instability, limited cellular uptake and rapid clearance (mostly in case of protein-based vaccines), (4) rapid degradation of vector-based and nucleic acid-based vaccines via nucleases, (5) potential autoimmunity, unpredicted adverse and off-target effects.
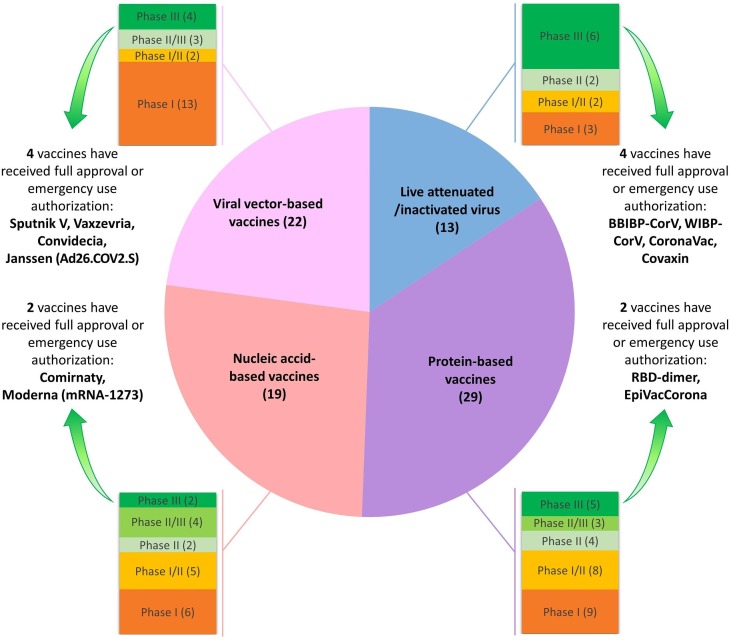
As of the onset of SARS-CoV2 pandemic, many research institutes, big pharma companies and biotechnology firms have synergized their capacities to develop efficient vaccines, and over 80 candidate vaccines have progressed into clinical trials, so far (Fig. 2 and Supplementary material 1), meanwhile some of them have led to promising results [19], [32]. To date, 12 vaccines have received emergency use authorization or full approval in different countries (Fig. 2), and early appraisals of the efficacy of some of them have unveiled rates above 70% [19], [33]. Furthermore, the recent statistics from the United States and the United Kingdom (the two countries with the earliest public vaccination program), which displayed marked drop in COVID-19 case numbers, hospitalizations and death toll [34], [35], revealed the success of the efficacious vaccines in the pipeline.
Fig. 2.
Types and trial phases of developing cell-free vaccines for SARS-CoV2.
When an immunogenic vaccine against SARS-CoV2 infection is produced, i.e. a vaccine capable of triggering a robust neutralizing antibody response; the durability of the protection elicited by the vaccine, i.e. the persistence of antibodies above the protective thresholds and/or the maintenance of immune memory cells [29], would be a matter of question. Although yet to be clarified for SARS-CoV2, previous experiences with the outbreaks of similar betacoronaviruses including SARS-CoV1 (2002–2004) and MERS-CoV (2012), have shown that the infection rarely induces long-term immunity and that the specific immunoglobulin G antibody wanes over a relatively short time (typically in less than a year or two) [36], [37]. This implies that although the role of B cells and their secreted immunoglobulins are fundamental to mount an effective prevention for prospective microbial exposures, the impact of T-cell responses should not be overlooked as they are essential to induce high-affinity antibodies and immune memory [29]. The importance of this issue is doubled when the immune evasion strategies of SARS CoV2, i.e. subversion of immediate immune response, suppression of dendritic cells and functional impairment of T cells, have been revealed [20], [31], [38]. Since the long-term reactivity to a virus depends on activation of antigen-specific B and T cells via reactive APCs, especially DCs [29], a progressive strategy might be ex vivo generation of DCs reactivated to SARS-CoV2.
3. Antigen-loaded DC vaccines
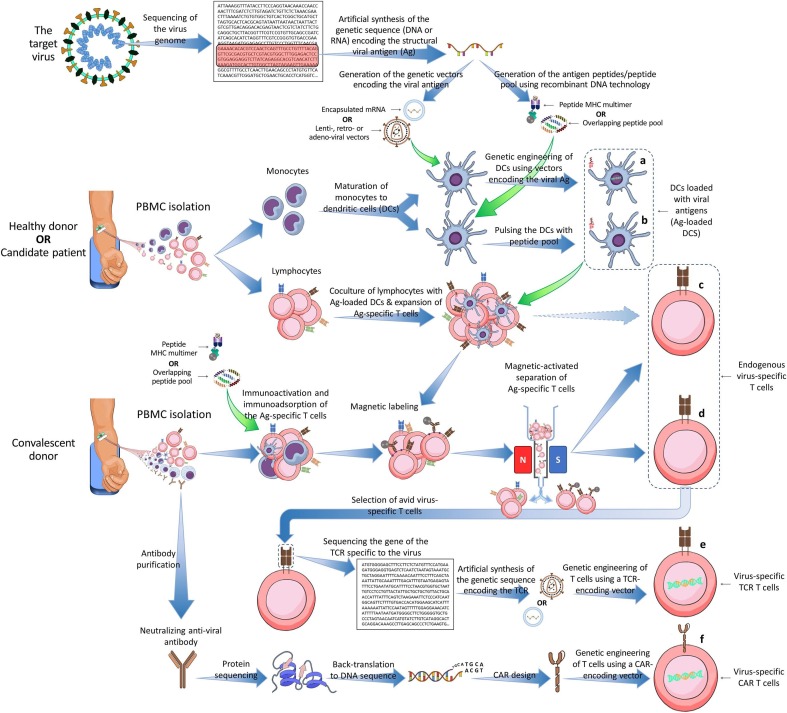
DCs are versatile APCs that are specialized in pathogen recognition at the sites of infection, transfer of Ag to the secondary lymphoid organs, priming the protective Ag-specific B and T cells, and consequently elicitation of immune effector functions [29], [39], [40]. In this sense, DCs are central in inducing the vaccine responses, especially as they are proficient in delivery of antigen-specific costimulatory signals to T cells, known as the “danger signals” required for activation of naïve T cells [29], [40], [41]. However, with the relatively suppressed capacity of DCs in priming T cell responses against SARS-CoV2 or its subunits [20], [38], some scientists have sought to develop ready-to-function Ag-loaded DCs against the virus. In this regard, five clinical trials have been registered, so far in China and the USA (Table 1 ). In these trials, the DC vaccines developed either through (1) genetic engineering of the autologous DCs using gene vectors encoding the SARS-CoV2 surface antigens or (2) ex vivo expansion of autologous DCs pulsed with synthetic overlapping peptides of SARS-CoV2 surface antigens, are employed (Fig. 3 a, b).
Table 1.
Registered trials on antigen-reactive adoptive immunotherapies for COVID-19 (Up to March 31, 2021).
| Company/Institution | Country | Composition | Trial phase (Reg. code) |
|---|---|---|---|
| Shenzhen Ruipuxun Academy for Stem Cell & Regenerative Medicine, Shenzhen Third People's Hospital | China | Genetically modified DCs to express recombinant chimeric COVID-19 epitope | I/II (ChiCTR2000030750) |
| Shenzhen Geno-Immune Medical Institute | China | Genetically-modified dendritic cells to express SARS-COV2 antigens | I/II (NCT04276896) |
| Shenzhen Geno-Immune Medical Institute | China | Genetically-modified antigen presenting cells to express SARS-COV2 antigens | I (NCT04299724) |
| Aivita Biomedical Inc. | USA | SARS-COV2 antigen loaded autologous dendritic cells | I/II (NCT04386252) |
| Indonesia-MoH, Aivita Biomedical Inc. | Indonesia | SARS-COV2 antigen loaded autologous dendritic cells | I (NCT04685603) |
| Beijing TIL Therapeutics, Liaocheng People's Hospital | China | SARS-COV2 antigen loaded autologous dendritic cells | 0 (ChiCTR2000034076) |
| Cincinnati Children's Hospital Medical Center* | USA | SARS-COV2-specific T cells | II (NCT04406064) |
| KK Women's and Children's Hospital | Singapore | SARS-COV2-specific T cells | I/II (NCT04457726) |
| Baylor College of Medicine | USA | SARS-COV2-specific T cells | I (NCT04401410) |
| M.D. Anderson Cancer Center | USA | SARS-COV2-specific T cells | I (NCT04742595) |
| University Hospital Cologne, Miltenyi Biomedicine GmbH | Germany | SARS-COV2-specific T cells | I (NCT04762186) |
| TC Erciyes University | Turkey | SARS-COV2-specific T cell-derived exosomes | I (NCT04389385) |
| Chongqing Public Health Medical Center, Chongqing Sidemu Biotech, Zhejiang Qixin Biotech | China | NKG2D CAR-, ACE2 CAR-, or NKG2D-ACE2 CAR-NK cells | I/II (NCT04324996) |
*Withdrawn (Steroid therapy for COVID-19 patients, that is according to current management guidelines, interferes with and suppresses the injected donor-derived VSTs. In addition, the principal investigator of the trial did not expect enrollment of potential participants, as the treatment options for COVID-19 have evolved).
Fig. 3.
Methods of generating antigen-specific adoptive immunotherapies for viral infections. a, Genetic engineering of the autologous dendritic cells (DCs) using gene vectors encoding the virus surface antigens. b, Ex vivo pulsing of DCs with synthetic overlapping peptides of viral surface antigens and expansion of the resultant antigen-loaded DCs. c, Coculture of seroneagative donor-derived (or candidate patient-derived) T cells with the virus-antigen-loaded DCs and subsequent enrichment of virus-specific T (VST) cells using immunomagnetic cell sorting. d, Immunoactivation and immunoadsorption of seropositive donor-derived peripheral blood mononuclear cells (PBMCs) using viral-peptide multimers or peptide pools and subsequent enrichment of VST cells via immunomagnetic cell sorting. e, Genetic engineering of autologous T cells using gene vectors encoding the viral-antigen-specific T cell receptor (TCR). f, Genetic engineering of autologous T cells using gene vectors encoding the viral-antigen-specific chimeric antigen receptor (CAR).
Shenzhen Ruipuxun Academy for Stem Cell & Regenerative Medicine has developed genetically modified DCs using a recombinant chimeric COVID-19 epitope gene (ChiCTR2000030750). Another institution, Shenzhen Geno-Immune Medical Institute, similarly engineered the genome of DCs by using an efficient lentiviral vector system (NHP/TYF) encoding the minigenes of SARS-CoV2 antigen epitopes as well as immunomodulatory genes (NCT04276896). The minigenes were based on conserved domains of the viral structural proteins and a polyprotein protease. Using the same lentiviral cassette by the same institution in another trial, volunteers undergo vaccination with the genetically modified SARS-CoV2 Ag-loaded APCs (including DCs) (NCT04299724). Aivita Biomedical Inc. has developed a platform of monocyte-derived DCs incubated with synthetized overlapping peptides of SARS-CoV2 antigens, followed by activation with granulocyte-macrophage colony-stimulating factor (NCT04386252). The company has recently started a collaboration with Indonesian ministry of health to test this investigational product (NCT04685603). Likewise, the Beijing TIL Therapeutics has run a trial employing the ex vivo expanded monocyte-derived DCs loaded with spike protein of SARS-CoV2 (ChiCTR2000034076)http://www.chictr.org.cn/showprojen.aspx?proj=55582.
Upon subcutaneous vaccination, the Ag-loaded DCs can prime the antiviral T cell responses specific to SARS-CoV2. Hence and via priming of CD4+ T helper cells (both Th1 and Th2), DCs not only can activate cytotoxic T cells, but also can orchestrate activation of naïve and memory B cells [29], [41]. In the long run, follicular DCs that are resident in germinal centers of secondary lymphoid organs may provide sustainable anti-SARS-CoV2-specific B cell memory via generation of antigen-antibody complexes and continuous B cell stimulation [39]. It has been argued that comparing to inactivated/live-attenuated virus and vector-based vaccines, the Ag-loaded DC vaccines may reduce the risk of virulence recovery, off-target effects, pre-existing anti-vector immunity, and incomplete immune response induction [20], [28], [29]. It is of note that this approach, i.e. harnessing DCs to specifically reactivate immune system against a pathogen, has been previously trialed for some challenging viral infections such as CMV, HCV and HIV, though did not lead into perfectly robust and permanent immunity [42], [43], [44].
4. Virus-specific effector lymphoid cells
Use of adoptively transferred Ag-specific effector lymphoid cells (T and NK cells) serves as a potent therapeutic measure for cancer [45], [46], [47], [48], which is based on targeted recognition and elimination of cancer cells without (or with limited) impact on the healthy tissues. This approach can also be practiced for viral infections, as the virus may alter the antigenic display of the infected cells (i.e. viral antigen peptides can be presented on MHC class I molecules) making them vulnerable to be recognized by VST cells [49]. Hence, adoptive transfer of VST cells, either CD4+ or CD8+ subsets or both (rarely called T cell vaccines), to patients with viral infections may facilitate the control, reduction, and clearance of the intracellular pathogen, though this approach may not be used as a preventive measure in pandemics [29], [50], [51]. The main mechanisms of action of VSTs to eliminate virally infected cells and viremia include [29]: (1) secretion of interferon (IFN)-γ, tumor necrosis factor (TNF)-α/β via CD4+ Th1 subsets and CD8+ T subsets, (2) induction of B cells to produce anti-viral antibodies via CD4+ Th2 subsets, (3) secretion of IL-17, IL-22, and IL-26 contributing to mucosal defense via Th17 effector cells, (4) direct killing of the infected cells by release of perforin and granzyme and Fas/FasL interactions via CD8+ T cells.
The use of VST cells for viral infections has a promising history, especially for the prevention or treatment of post-transplant opportunistic infections in the immunocompromised allogeneic bone marrow (allo-BM) recipients [2], [51], [52], [53], [54], [55], [56]. Many trials have shown that CMV-, EBV- and/or AdV-specific T cells were adequately immunoprophylactic to render the allo-BM transplanted patients viral-free and protected for long-term. These VSTs were generated via 3 general strategies [2], [51], [53], [54], [55], [56], [57]: (1) coculture of seronegative donor-derived naïve T cells with the virus-specific Ag-loaded APCs (Fig. 3c), (2) activation and immunoadsorption of seropositive donor-derived PBMCs using viral protein-derived multimers or peptide pools and subsequent isolation of VST cells from the PBMCs using multimeter-based or IFN-γ capture-based immunomagnetic cell sorting (Fig. 3d), (3) genetic engineering of autologous T cells using gene vectors encoding the TCR specific to essential surface antigens of the virus (Fig. 3e). Additionally, there have been some efforts to engineer the CAR-bearing T cells for CMV and EBV that are effectual in an MHC-independent manner (Fig. 3f), though such CAR T cells merit further research and fine tunes in order to enter the clinical protocols [2], [50]. For SARS-CoV2 infection, all of the above-mentioned strategies of production of Ag-specific immune effector cells may be taken into account.
Shenzhen Geno-Immune Medical Institute has established a platform to develop SARS-CoV2-specific T cells through priming with genetically-modified DCs expressing the conserved domain of viral structural proteins. In an ongoing trial sponsored by this institution (NCT04276896), the COVID-19 patients undergo subcutaneous injections of the 5 × 106 genetically-engineered DCs and intravenous infusion of 1 × 108 activated SARS-CoV2-specific T cells. Acute and convalescent COVID-19 patients and even unexposed healthy donors have shown to possess SARS-CoV2-reactive CD4+ and CD8+ T cells [58], [59], [60], [61]. These cells can shape the long-term immunological memory to this virus, and potentially prevent or reduce the clinical severity of the subsequent infections [58]. To activate and isolate such cells from a donor, peptide-MHC class I multimers and different peptide pools covering the SARS-CoV2 spike, nucleocapsid and membrane antigens have been generated [58], [59], [60], [61], [62], [63], [64]. In this respect, Cooper et al. formulated a GMP-compliant process for isolation and expansion of SARS-CoV2-specific T cells [62]. In their design, a GMP-compliant peptide pool was used to trigger the IFN-γ secretion from SARS-CoV2 VSTs, and then the activated cells were enriched using an IFN-γ capture complex, when they were finally magnetically sorted out of the donor-derived PBMCs. They have postulated that this allogeneic SARS-CoV2-specific T cell product can be readily separated from convalescent donors and may be used for the treatment of COVID-19 HLA-compatible patients, or can be deposited in HLA-typed banks for rapid deployment to future COVID-19 patients [62]. Using a similar approach, Keller et al demonstrated that SARS-CoV2-specific T cells can be produced from 70% of the COVID-19 convalescent donors, which can be potentially used for antiviral control in immunocompromised allo-BM recipients after transplantation [63]. Employing the similar methodology of generating endogenous SARS-CoV2 VSTs, five trials sponsored by Cincinnati Children's Hospital Medical Center (NCT04406064), KK Women's and Children's Hospital (NCT04457726), Baylor College of Medicine (NCT04401410), M.D. Anderson Cancer Center (NCT04742595) and University Hospital Cologne (NCT04762186) have been registered to investigate the effect of such cells in HLA-matched or partially HLA-matched COVID-19 patients (Table 1). Nonetheless, owing to the partial success of COVID-19 management protocols that include steroid therapy as a standard treatment module, capable of reducing the hyperinflammation but additionally suppressing VSTs; the Cincinnati trial has been withdrawn by the principal investigator. Besides, HLA mismatching and the likelihood of graft-versus-host disease (GvHD) is a legitimate concern for the enrolled patients in the mentioned trials [55], [65]. Moreover, considering the lack of data on the causes of maladaptive responses to SARS-CoV2, the VST cells derived from convalescent donors may have an over/auto-reactive behavior [66], [67], and thus, the adoptive transfer of such potentially aberrant lymphocytes to a COVID-19 patient may put them at a serious risk and jeopardize the primary intent (i.e., treating a COVID-19 patient). Hence and as a further step, the TCRs of the avid SARS-CoV2-specific T cells derived from the convalescent donors can be sequenced, so that the relevant encoding genes can be used for engineering the autologous naïve t cells to forced express SARS-CoV2-specific TCRs. This approach has been successful in generation of transgenic TCR T cells redirected against SARS-CoV1 infection as well as some opportunistic post-BMT viral infections [57], [68], however, for SARS-CoV2, further research is required. Given the important limitation of endogenous VSTs and TCR T cells, i.e. functioning in an HLA-dependent manner, TC Erciyes University has registered a clinical study to make the benefits of these cells more universally available (NCT04389385). In this trial, that is not yet recruiting, the effects of an off-the-shelf inhaler containing HLA-independent SARS-CoV2-specific T cell-derived exosomes (2 × 108 nano-vesicles/3 ml in 5 days) on COVID-19 patients are going to be investigated. In a more progressive approach, CAR-bearing immune cells, which recognize SARS-CoV2 antigens independent of HLA system, can be developed using the sequence of high affinity anti-SARS-CoV2 neutralizing/blocking monoclonal (mAb) antibodies obtained from the convalescent plasma of COVID-19 subjects, such as the two recently approved casirivimab/imdevimab and bamlanivimab [69], [70], [71]. In this context, a recently preprint publication has reported the in vitro results of CAR-bearing NK cells generated from the scFv domain of a SARS-CoV1/SARS-CoV2 specific mAb (so-called CR3022). The CR3022-CAR NK cells were shown to specifically kill pseudo-SARS-CoV2 infected cells [72]. Using the antigen-recognition domain of the same mAb (CR3022), a CAR macrophage has also been developed, which showed high affinity to receptor-binding domain of the spike protein of SARS-CoV2 and was able to considerably reduce the virion load in vitro [73]. In the circumstances that the CARs harboring the sequences of the mentioned mAbs are yet to be optimized, two Chinese biotech companies have started a collaborative trial investigating the effects of already-developed CAR NK cells on COVID-19 patients (NCT04324996). In this ongoing trial, three investigational CAR NK cells, including (1) NKG2D-CAR NK cells with an activated phenotype capable of targeting the NKG2D-expressing stressed cells (i.e., virally-infected cells), (2) ACE2-CAR NK cells capable of targeting the virus binding site on the SARS-CoV2-infected cells and competitive prevention of the relevant infection through ACE-2 expression, and (3) bi-specific NKG2D-ACE2 CAR-NK cells, are given to 3 arms of COVID-19 patients.
Altogether, it is important to note that administration of virus-specific effector lymphoid cells is not a first-line treatment for viral infections, and may be placed at the bottom of the list of therapeutic options for COVID-19 patients, provided that they are proven to be safe and efficient in trials; and this means that they may be used when all other antiviral and anti-inflammatory treatments have failed or they may be given to certain patients (such as cancer patients) based on the treating clinician’s decision.
5. Other non-specific adoptive cell therapies for COVID-19
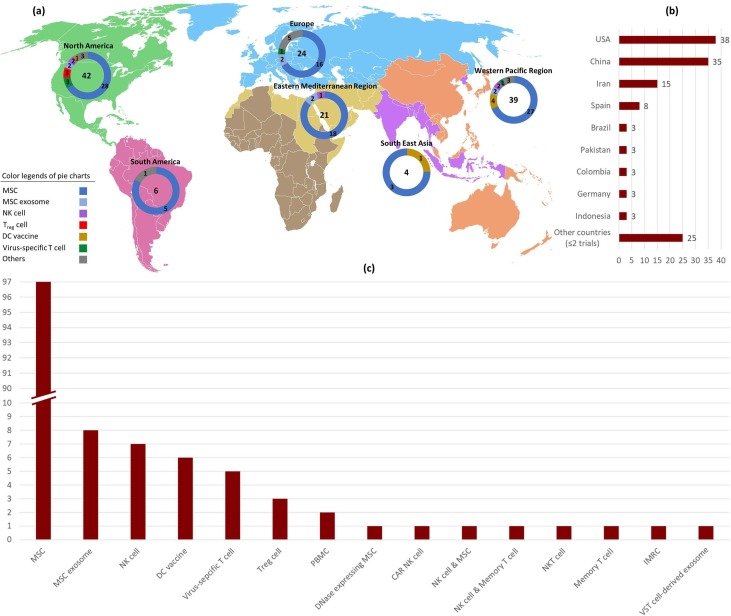
Since the severe cases of COVID-19 are characterized with idiosyncratic hyperinflammatory syndrome and cytokine storm, some scientists have advocated the use of cells with immunomodulatory effects. In this context, mesenchymal stem cells (MSCs) and regulatory T (Treg) cells are the two candidate cells that are being used in a number of clinical studies on COVID-19 patients (Supplementary material 2) [74], [75]. In addition to immunomodulation, MSCs are also identified with their regenerative effects especially on the pulmonary tissue, which makes them potentially better options than Treg cells in treating COVID-19 patients, as in one hand they can normalize the exaggerated immune response and additionally they may repair the damaged lungs [75], [76]. The MSCs are the most common cells (>70%) used in clinical trials on cell-based immunotherapy for COVID-19 patients (Fig. 4 ). Some of the completed trials using MSCs have shown promising results by diminishing the hyperinflammation, shortening the duration of hospitalization and lowering the death rate [76]. An MSC-like cell derived from human embryonic stem cells, also known as immunity-and-matrix-regulatory cell (IMRC), that has been proven to inhibit pulmonary inflammation and fibrosis in the preclinical setting with superior expression levels of proliferative, immunomodulatory and anti-fibrotic genes compared with MSCs [77], is now being trialed for COVID-19 patients (NCT04331613). Systemic injections or use of off-the-shelf inhalers of MSC exosomes are also under trial in Russia, Iran and China. As the reversal of the SARS-CoV2 induced hyperinflammatory state has been placed at the top of research agenda, immunomodulatory PBMCs are considered as another option for COVID-19 patients. In a forthcoming trial (NCT04299152), the PBMCs of COVID-19 patients are planned to be apheresed and cocultured with cord blood-derived MSCs in order to make them immunomodulated, and then the patients receive their educated PBMCs back. In an ongoing trial (NCT04513470), an irradiated PBMC-based product containing at least 40% early apoptotic cells, is given to COVID-19 patients to potentially restore immuno-homeostasis, provided that the apoptotic cells (being inherently immunomodulatory) are able to send immunotolerant signals to the macrophages and DCs [78].
Fig. 4.
Registered clinical trials investigating adoptive immunotherapies on COVID-19 patients (Up to March 31, 2021; n = 136). a, Frequency distribution of trials in geographical regions based on the cellular product. b, Frequency distribution of trials based on the country. c, Frequency distribution of trials based on the cellular product.
Inasmuch as the SARS-CoV2 infection is associated with elevated neutrophil to lymphocyte ratio and is additionally able to stimulate the excess formation of neutrophil extracellular traps (NETs) [79], [80], one revolutionary approach to disrupt this pro-inflammatory and thrombogenic web of DNA fibers would be to deliver DNase(s) in situ. Thus, and considering the great capacity of MSCs to home into lungs, a company has recently started a trial investigating the effects of intravenous infusion of RNA-engineered MSCs overexpressing a combination of DNases on patients with moderate to severe COVID-19 pneumonia (NCT04524962).
Some studies have provided evidence on the reduced numbers of circulating natural killer (NK) cells and/or an exhausted phenotype of these cells following SARS-CoV2 infection, a situation that predisposes the infected individuals to attenuated NK cell responses against early SARS-CoV2 infection [81], [82]. In this context, delivery of ex vivo expanded NK cells to the COVID-19 patients in the early stages of the infection, may seem to be rationally beneficial and has been under investigation in 9 trials (NK cell only (7 trials), NK cells + MSCs (1 trial) and NK cells + memory T cells (1 trial)). The use of NKT cells, with an effector phenotype similar to NK cells, in another trial, can be interpreted in the context of a similar reasoning (Fig. 4).
One other investigational approach using non-specific immune cells is the subcutaneous injection of allogenic memory T cells in order to amend the immunosenescence (age-related functional weakness of cellular immunity) of elderly COVID-19 patients [83]. In this regard, donor-derived CD4+ T cells, ex vivo expanded and immunoactivated with anti-CD3 and anti-CD28-coated paramagnetic beads, are now being trialed for elderly COVID-19 patients (NCT04441047). Upon multiple subcutaneous vaccination of these cells, it is claimed that priming of allo-specific Th1/CD8+ T cytotoxic memory cells (with robust capability of IFN-γ secretion to control viral infection) occurs and that these cells gradually replace the exhausted memory cells of the aged immune system [83].
6. Limitations of antigen-specific cell-based immunotherapies for viral infections
Employment of Ag-specific cell-based immunotherapies for viral infections has not gained widespread attention, since personal protective measures and conventional vaccination can effectively restrain the disease spreading with much lower costs. Nonetheless, medical researchers should pursue parallel avenues, e.g., cellular immunotherapies, as alternative treatments or in case of failure of the mentioned approaches. Despite the promise of some cellular immunotherapies for viral infections; time, cost, technical complexities and safety concerns are the top reasons that may limit their use [27]. The manufacturing of cell-based products requires GMP-compliant high-tech facilities, trained personnel and costly clinical-grade materials for cell culture/processing [27], [52]. Moreover, the process of generating adequate number of cells is time-consuming and technically demanding. These obstacles, however, can be overcome with the help of automated closed-system machines of isolating, processing and engineering PBMCs with various proteins and genetic vectors [84], [85].
A strong and long-lasting protection against a viral infection requires both antibody- and cell-mediated immunity, especially via development of memory B and T cells [86]. In this sense, it has been emphasized that in case of inadequacy of antibody-mediated protection, Ag-specific T cells are vital for viral clearance [20]. Accordingly, emerging evidence has shown that COVID-19 patients may only develop a short-term protection against SARS-CoV2 reinfection, particularly because of the absence of specific cell-mediated immunity to the virus [20], [87], [88]. This fact may undermine the current efficacy assessments of cell-free vaccines that are generally based on induction of humoral immune responses and rise in antibodies. Thus, recent position papers have argued that in elderly individuals, T cell-mediated immunity should be considered as a more reliable correlate of vaccine protection than antibody titers, and so the inclusion of T cell responses in COVID-19 vaccine design is inevitable [20], [89]. Perhaps, a balanced optimal immune response to SARS-CoV2 should comprise high titers of neutralizing antibodies and memory B and T cells [20], [90]. While loading ex vivo expanded autologous DCs with a viral antigen and administration of this cellular vaccine may elicit a more prolific immune response (at both cellular and humoral level), this approach may have limited applicability considering the constraints of large-scale production [91]. The same hurdle can be attributed to manufacturing of T-cell based products; however, these products themselves are additionally compromised with safety concerns: First, unlike convalescent plasma therapy, allogenic VSTs derived from convalescent donors cannot be considered as an off-the-shelf treatment, since HLA matching is required. Use of syngeneic VSTs, banking of HLA-typed allogeneic convalescent donor-derived VSTs or generation of gene-engineered autologous VSTs may be seen as solutions for the mentioned limitation. Second, for COVID-19 patients involved with maladaptive immune response, adoptive transfer of immune cells may appear as adding fuel to the fire. This is a crucial concern, especially when the VST cells may cause GvHD or selectively destroy the SARS-COV2-infected cells of the lung of COVID-19 patients who are simultaneously dealing with virus-induced respiratory injuries. Likewise, cytokine release syndrome is potentially expectable after application of genetically and non-genetically modified VSTs, and that may exacerbate the situation of COVID-19 patients who struggle with SARS-CoV2 induced cytokine storm [20], [31], [92]. Third, the use of gene-engineered VSTs (TCR- and CAR-VSTs) with a high profile of proliferation and overactivity to the antigen may carry the risk of uncontrolled clearance of the infected cells and consequently extensive destruction of the infected organ (particularly lungs). The two latter concerns are the reasons that some scientists have favored mRNA electroporation to generate TCR/CAR-VSTs with shortened functional activity, and probably lacking the mentioned potential hazards [92]. Fourth, for many of the moderate to severe COVID-19 patients who enroll in trials, systemic steroids may be given as a standard treatment according to the majority of guidelines. This suppresses and inactivates the immune cell-based therapies (the reason behind the withdrawal of Cincinnati trial), and thus a careful design and scrutiny for such trials are necessary. Finally, an Ag-specific cellular product generated using a given antigen plasmid or derived from a convalescent donor using a peptide library is static, whereas circulating strains of the virus in the society are not. Hence and although the same potential problem applies to the conventional vaccines, similar to worries of ineffectiveness of current COVID-19 vaccines against new variants [93], Ag-specific cellular therapy trials for SARS-CoV2 may be subjected to early termination.
7. Conclusions
Cell-based immunotherapies that have been commonly used for malignancies and some hard-to-treat diseases, have also been successful to cure chronic and opportunistic viral infections. They may additionally open new avenues for treatment of deadly outbreaks of novel viral infections lacking effective ad-hoc treatments. For the COVID-19 pandemic, which has emerged as a formidable challenge to humanity, cellular immunotherapies are under trial with regards to two main strategies: (1) to suppress the hyperinflammatory syndrome using MSCs, DNase-expressing MSCs, MSC exosomes, Treg cells, immunomodulated PBMCs, immunomodulatory (apoptotic) PBMCs and IMRCs; (2) to empower the immune system and to facilitate the viral clearance via administration of non-specific immune cells (i.e., NK cells, NKT cells and memory T cells) or viral-specific immune cells (i.e., Ag-loaded DC vaccines, endogenous VSTs, VST-derived exosomes and CAR-NK cells). In this perspective, Ag-specific DC vaccines are of particular interest, as they are able to render the clearance of the virus and to induce long-term viral-specific immunity. Still at the trial level, which requires thoughtful design, appropriate ethical considerations and rigorous analysis; these immunotherapies may help fight against COVID-19, though their safety and cost-effectiveness should be carefully evaluated.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Some of the graphical elements used in Graphical abstract and Fig. 1, Fig. 3 were produced via mindthegraph.com, which are made available freely by the website under a free culture Creative Commons license (CC BY-SA 4.0).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cellimm.2021.104398.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Registered trials on conventional (acellular) vaccines for COVID-19 (Up to March 31, 2021).
Registered trials on cell-based immunotherapies for COVID-19 (Up to March 31, 2021).
References
- 1.Maus M.V., Fraietta J.A., Levine B.L., Kalos M., Zhao Y., June C.H. Adoptive immunotherapy for cancer or viruses. Annu. Rev. Immunol. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaeuferle T., Krauss R., Blaeschke F., Willier S., Feuchtinger T. Strategies of adoptive T-cell transfer to treat refractory viral infections post allogeneic stem cell transplantation. J. Hematol. Oncol. 2019;12(1):13. doi: 10.1186/s13045-019-0701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitazono T., Okazaki T., Araya N., Yamano Y., Yamada Y., Nakamura T., et al. Advantage of higher-avidity CTL specific for Tax against human T-lymphotropic virus-1 infected cells and tumors. Cell. Immunol. 2011;272(1):11–17. doi: 10.1016/j.cellimm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Chaibakhsh S., Pourhoseingholi A., Vahedi M. Global incidence and mortality rate of COVID-19; special focus on Iran, Italy and China. Arch. Iran. Med. 2020;23(7):455–461. doi: 10.34172/aim.2020.42. [DOI] [PubMed] [Google Scholar]
- 5.Meyts I., Bucciol G., Quinti I., Neven B., Fischer A., Seoane E., et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J. Allerg. Clin. Immunol. 2021;147(2):520–531. doi: 10.1016/j.jaci.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contoli M., Papi A., Tomassetti L., Rizzo P., Vieceli Dalla Sega F., Fortini F., et al. Blood interferon-alpha levels and severity, outcomes, and inflammatory profiles in hospitalized COVID-19 patients. Front. Immunol. 2021;12:648004. doi: 10.3389/fimmu.2021.648004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabrese L.H., Lenfant T., Calabrese C. Interferon therapy for COVID-19 and emerging infections: prospects and concerns. Cleve Clin. J. Med. 2020 doi: 10.3949/ccjm.87a.ccc066. [DOI] [PubMed] [Google Scholar]
- 8.Consortium W.H.O.S.T, Pan H., Peto R., Henao-Restrepo A.M., Preziosi M.P., Sathiyamoorthy V., et al. Repurposed antiviral drugs for covid-19 – interim WHO solidarity trial results. N. Engl. J. Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lythgoe M.P., Middleton P. Ongoing clinical trials for the management of the COVID-19 pandemic. Trends Pharmacol. Sci. 2020;41(6):363–382. doi: 10.1016/j.tips.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghanbari R., Teimoori A., Sadeghi A., Mohamadkhani A., Rezasoltani S., Asadi E., et al. Existing antiviral options against SARS-CoV-2 replication in COVID-19 patients. Future Microbiol. 2020;15:1747–1758. doi: 10.2217/fmb-2020-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vicenti I., Zazzi M., Saladini F. SARS-CoV-2 RNA-dependent RNA polymerase as a therapeutic target for COVID-19. Expert Opin. Ther. Pat. 2021;1–13 doi: 10.1080/13543776.2021.1880568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahebnasagh A., Avan R., Saghafi F., Mojtahedzadeh M., Sadremomtaz A., Arasteh O., et al. Pharmacological treatments of COVID-19. Pharmacol. Rep. 2020;72(6):1446–1478. doi: 10.1007/s43440-020-00152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albani F., Fusina F., Granato E., Capotosto C., Ceracchi C., Gargaruti R., et al. Corticosteroid treatment has no effect on hospital mortality in COVID-19 patients. Sci. Rep. 2021;11(1):1015. doi: 10.1038/s41598-020-80654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Group R.C., Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., et al. Dexamethasone in hospitalized patients with covid-19. N. Engl. J. Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Q., Guo M., Zheng Y., Zhang Y., De Y., Xu C., et al. Current evidence of interleukin-6 signaling inhibitors in patients with COVID-19: a systematic review and meta-analysis. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.615972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosas I.O., Brau N., Waters M., Go R.C., Hunter B.D., Bhagani S., et al. Tocilizumab in hospitalized patients with severe covid-19 pneumonia. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo X., Liu Y., Ren M., Zhang X., Janne E., Lv M., et al. Consistency of recommendations and methodological quality of guidelines for the diagnosis and treatment of COVID-19. J. Evid. Based Med. 2021;14(1):40–55. doi: 10.1111/jebm.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kow C.S., Capstick T., Zaidi S.T.R., Hasan S.S. Consistency of recommendations from clinical practice guidelines for the management of critically ill COVID-19 patients. Eur. J. Hosp. Pharm. 2021;28(1):42–46. doi: 10.1136/ejhpharm-2020-002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashte S., Gulbake A., El-Amin Iii S.F., Gupta A. COVID-19 vaccines: rapid development, implications, challenges and future prospects. Hum. Cell. 2021 doi: 10.1007/s13577-021-00512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kostoff R.N., Briggs M.B., Porter A.L., Spandidos D.A., Tsatsakis A. COVID19 vaccine safety. Int. J. Mol. Med. 2020;46(5):1599–1602. doi: 10.3892/ijmm.2020.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merchant H.A. CoViD vaccines and thrombotic events: EMA issued warning to patients and healthcare professionals. J. Pharm. Policy Pract. 2021;14(1):32. doi: 10.1186/s40545-021-00315-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahase E. Covid-19: US suspends Johnson and Johnson vaccine rollout over blood clots. BMJ. 2021;373 doi: 10.1136/bmj.n970. [DOI] [PubMed] [Google Scholar]
- 24.Bar-Zeev N., Inglesby T. COVID-19 vaccines: early success and remaining challenges. Lancet. 2020;396(10255):868–869. doi: 10.1016/S0140-6736(20)31867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pregelj L., Hine D.C., Oyola-Lozada M.G., Munro T.P. Working hard or hardly working? Regulatory bottlenecks in developing a COVID-19 vaccine. Trends Biotechnol. 2020;38(9):943–947. doi: 10.1016/j.tibtech.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melgaco J.G., Azamor T., Ano Bom A.P.D. Protective immunity after COVID-19 has been questioned: what can we do without SARS-CoV-2-IgG detection? Cell. Immunol. 2020;353 doi: 10.1016/j.cellimm.2020.104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu J., Melenhorst J.J., Fraietta J.A. Toward precision manufacturing of immunogene T-cell therapies. Cytotherapy. 2018;20(5):623–638. doi: 10.1016/j.jcyt.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Frederiksen L.S.F., Zhang Y., Foged C., Thakur A. The long road toward COVID-19 herd immunity: vaccine platform technologies and mass immunization strategies. Front. Immunol. 2020;11:1817. doi: 10.3389/fimmu.2020.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegrist C.-A. In: Plotkin's Vaccines. sevent ed. Plotkin S., Orenstein W., Offit P., Edwards K.M., editors. Elsevier; Philadelphia, PA: 2018. Vaccine immunology; pp. 16–34.e7. [Google Scholar]
- 30.DeStefano F., Bodenstab H.M., Offit P.A. Principal controversies in vaccine safety in the United States. Clin. Infect. Dis. 2019;69(4):726–731. doi: 10.1093/cid/ciz135. [DOI] [PubMed] [Google Scholar]
- 31.Florindo H.F., Kleiner R., Vaskovich-Koubi D., Acurcio R.C., Carreira B., Yeini E., et al. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020;15(8):630–645. doi: 10.1038/s41565-020-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.COVID-19 vaccines: no time for complacency. Lancet 396(10263) (2020) 1607. [DOI] [PMC free article] [PubMed]
- 33.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moghadas S.M., Vilches T.N., Zhang K., Wells C.R., Shoukat A., Singer B.H., et al. The impact of vaccination on COVID-19 outbreaks in the United States. medRxiv. 2020 [Google Scholar]
- 35.Bernal J.L., Andrews N., Gower C., Stowe J., Robertson C., Tessier E., et al. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. medRxiv. 2021 [Google Scholar]
- 36.Cao W.C., Liu W., Zhang P.H., Zhang F., Richardus J.H. Disappearance of antibodies to SARS-associated coronavirus after recovery. N. Engl. J. Med. 2007;357(11):1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 37.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M., et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat. Commun. 2020;11(1):4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou R., To K.K., Wong Y.C., Liu L., Zhou B., Li X., et al. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity. 2020;53(4):864–77 e5. doi: 10.1016/j.immuni.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabado R.L., Balan S., Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27(1):74–95. doi: 10.1038/cr.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckwalter M.R., Albert M.L. Orchestration of the immune response by dendritic cells. Curr. Biol. 2009;19(9):R355–R361. doi: 10.1016/j.cub.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. 2020;580(7805):576–577. doi: 10.1038/d41586-020-01221-y. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y., Zhang Y., Yao Z., Moorman J.P., Jia Z. Dendritic cell-based immunity and vaccination against hepatitis C virus infection. Immunology. 2012;136(4):385–396. doi: 10.1111/j.1365-2567.2012.03590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.da Silva L.T., Santillo B.T., de Almeida A., Duarte A., Oshiro T.M. Using dendritic cell-based immunotherapy to treat HIV: how can this strategy be improved? Front. Immunol. 2018;9:2993. doi: 10.3389/fimmu.2018.02993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Craenenbroeck A.H., Smits E.L., Anguille S., Van de Velde A., Stein B., Braeckman T., et al. Induction of cytomegalovirus-specific T cell responses in healthy volunteers and allogeneic stem cell recipients using vaccination with messenger RNA-transfected dendritic cells. Transplantation. 2015;99(1):120–127. doi: 10.1097/TP.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arabi F., Torabi-Rahvar M., Shariati A., Ahmadbeigi N., Naderi M. Antigenic targets of CAR T cell therapy. A retrospective view on clinical trials. Exp. Cell Res. 2018;369(1):1–10. doi: 10.1016/j.yexcr.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Kiani J., Naderi M., Torabi-Rahvar M., Ranjbar A., Aghayan H.R., Janzamin E., et al. Generation of CD19-targeted chimeric antigen receptor T cells. Arch Iran Med. 2019;22(1):7–10. [PubMed] [Google Scholar]
- 47.Yee C., Lizee G., Schueneman A.J. Endogenous T-cell therapy: clinical experience. Cancer J. 2015;21(6):492–500. doi: 10.1097/PPO.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 48.Lim W.A., June C.H. The principles of engineering immune cells to treat cancer. Cell. 2017;168(4):724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosendahl Huber S., van Beek J., de Jonge J., Luytjes W., van Baarle D. T cell responses to viral infections – opportunities for peptide vaccination. Front. Immunol. 2014;5:171. doi: 10.3389/fimmu.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seif M., Einsele H., Loffler J. CAR T cells beyond cancer: hope for immunomodulatory therapy of infectious diseases. Front. Immunol. 2019;10:2711. doi: 10.3389/fimmu.2019.02711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blyth E., Withers B., Clancy L., Gottlieb D. CMV-specific immune reconstitution following allogeneic stem cell transplantation. Virulence. 2016;7(8):967–980. doi: 10.1080/21505594.2016.1221022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cancio M., Ciccocioppo R., Rocco P.R.M., Levine B.L., Bronte V., Bollard C.M., et al. Emerging trends in COVID-19 treatment: learning from inflammatory conditions associated with cellular therapies. Cytotherapy. 2020;22(9):474–481. doi: 10.1016/j.jcyt.2020.04.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leen A.M., Myers G.D., Sili U., Huls M.H., Weiss H., Leung K.S., et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat. Med. 2006;12(10):1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 54.Liu L., Zhang X., Feng S. Epstein-Barr virus-related post-transplantation lymphoproliferative disorders after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2018;24(7):1341–1349. doi: 10.1016/j.bbmt.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 55.Bollard C.M., Heslop H.E. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood. 2016;127(26):3331–3340. doi: 10.1182/blood-2016-01-628982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanley P.J., Melenhorst J.J., Nikiforow S., Scheinberg P., Blaney J.W., Demmler-Harrison G., et al. CMV-specific T cells generated from naive T cells recognize atypical epitopes and may be protective in vivo. Sci. Transl. Med. 2015;7(285) doi: 10.1126/scitranslmed.aaa2546. 285ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O'Reilly R.J., Prockop S., Hasan A.N., Koehne G., Doubrovina E. Virus-specific T-cell banks for 'off the shelf' adoptive therapy of refractory infections. Bone Marrow Transplant. 2016;51(9):1163–1172. doi: 10.1038/bmt.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 59.Weiskopf D., Schmitz K.S., Raadsen M.P., Grifoni A., Okba N.M.A., Endeman H., et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020;5(48) doi: 10.1126/sciimmunol.abd2071. eabd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587(7833):270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 61.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012 e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooper R.S., Fraser A.R., Smith L., Burgoyne P., Imlach S.N., Jarvis L.M., et al. Rapid GMP-compliant expansion of SARS-CoV-2-specific T cells from convalescent donors for use as an allogeneic cell therapy for COVID-19. Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.598402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keller M.D., Harris K.M., Jensen-Wachspress M.A., Kankate V., Lang H., Lazarski C.A., et al. SARS-CoV-2 specific T-cells are rapidly expanded for therapeutic use and target conserved regions of membrane protein. Blood. 2020;136(25):2905–2917. doi: 10.1182/blood.2020008488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., et al. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020;21(11):1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qian C., Wang Y., Reppel L., D'Aveni M., Campidelli A., Decot V., et al. Viral-specific T-cell transfer from HSCT donor for the treatment of viral infections or diseases after HSCT. Bone Marrow Transplant. 2018;53(2):114–122. doi: 10.1038/bmt.2017.232. [DOI] [PubMed] [Google Scholar]
- 66.Chen Z., John W.E. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020;20(9):529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buszko M., Nita-Lazar A., Park J.H., Schwartzberg P.L., Verthelyi D., Young H.A., et al. Lessons learned: new insights on the role of cytokines in COVID-19. Nat. Immunol. 2021;22(4):404–411. doi: 10.1038/s41590-021-00901-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oh H.L., Chia A., Chang C.X., Leong H.N., Ling K.L., Grotenbreg G.M., et al. Engineering T cells specific for a dominant severe acute respiratory syndrome coronavirus CD8 T cell epitope. J. Virol. 2011;85(20):10464–10471. doi: 10.1128/JVI.05039-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dotti G., Gottschalk S., Savoldo B., Brenner M.K. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol. Rev. 2014;257(1):107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41(5):355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W.T., et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369(6506):956–963. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma M., Badeti S., Geng K., Liu D. Efficacy of targeting SARS-CoV-2 by CAR-NK Cells. bioRxiv. 2020 [Google Scholar]
- 73.Fu W., Lei C., Qian K., Ma Z., Li T., Lin F., et al. CAR macrophages for SARS-CoV-2 immunotherapy. bioRxiv. 2020 doi: 10.3389/fimmu.2021.669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khoury M., Rocco P.R.M., Phinney D.G., Krampera M., Martin I., Viswanathan S., et al. Cell-based therapies for coronavirus disease 2019: proper clinical investigations are essential. Cytotherapy. 2020;22(11):602–605. doi: 10.1016/j.jcyt.2020.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Durand N., Mallea J., Zubair A.C. Insights into the use of mesenchymal stem cells in COVID-19 mediated acute respiratory failure. NPJ Regener. Med. 2020;5(1) doi: 10.1038/s41536-020-00105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shetty R., Murugeswari P., Chakrabarty K., Jayadev C., Matalia H., Ghosh A., et al. Stem cell therapy in coronavirus disease 2019: current evidence and future potential. Cytotherapy. 2020 doi: 10.1016/j.jcyt.2020.11.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu J., Song D., Li Z., Guo B., Xiao Y., Liu W., et al. Immunity-and-matrix-regulatory cells derived from human embryonic stem cells safely and effectively treat mouse lung injury and fibrosis. Cell Res. 2020;30(9):794–809. doi: 10.1038/s41422-020-0354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trahtemberg U., Mevorach D. Apoptotic cells induced signaling for immune homeostasis in macrophages and dendritic cells. Front. Immunol. 2017;8:1356. doi: 10.3389/fimmu.2017.01356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arcanjo A., Logullo J., Menezes C.C.B., de Souza Carvalho Giangiarulo T.C., Dos Reis M.C., de Castro G.M.M., et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19) Sci. Rep. 2020;10(1):19630. doi: 10.1038/s41598-020-76781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Market M., Angka L., Martel A.B., Bastin D., Olanubi O., Tennakoon G., et al. Flattening the COVID-19 curve with natural killer cell based immunotherapies. Front. Immunol. 2020;11:1512. doi: 10.3389/fimmu.2020.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pashaei M., Rezaei N. Immunotherapy for SARS-CoV-2: potential opportunities. Expert Opin. Biol. Ther. 2020;20(10):1111–1116. doi: 10.1080/14712598.2020.1807933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Har-Noy M., Or R. Allo-priming as a universal anti-viral vaccine: protecting elderly from current COVID-19 and any future unknown viral outbreak. J. Transl. Med. 2020;18(1):196. doi: 10.1186/s12967-020-02363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu F., Shah N., Xu H., Schneider D., Orentas R., Dropulic B., et al. Closed-system manufacturing of CD19 and dual-targeted CD20/19 chimeric antigen receptor T cells using the CliniMACS Prodigy device at an academic medical center. Cytotherapy. 2018;20(3):394–406. doi: 10.1016/j.jcyt.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 85.Priesner C., Aleksandrova K., Esser R., Mockel-Tenbrinck N., Leise J., Drechsel K., et al. Automated enrichment, transduction, and expansion of clinical-scale CD62L(+) T cells for manufacturing of gene therapy medicinal products. Hum. Gene Ther. 2016;27(10):860–869. doi: 10.1089/hum.2016.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amanna I.J., Slifka M.K. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology. 2011;411(2):206–215. doi: 10.1016/j.virol.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stephens D.S., McElrath M.J. COVID-19 and the path to immunity. JAMA. 2020;324(13):1279–1281. doi: 10.1001/jama.2020.16656. [DOI] [PubMed] [Google Scholar]
- 88.Poland G.A., Ovsyannikova I.G., Kennedy R.B. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sariol A., Perlman S. Lessons for COVID-19 immunity from other coronavirus infections. Immunity. 2020;53(2):248–263. doi: 10.1016/j.immuni.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ludewig B. Dendritic cell vaccination and viral infection–animal models. Curr. Top. Microbiol. Immunol. 2003;276:199–214. doi: 10.1007/978-3-662-06508-2_9. [DOI] [PubMed] [Google Scholar]
- 92.Bertoletti A., Tan A.T. Challenges of CAR- and TCR-T cell-based therapy for chronic infections. J. Exp. Med. 2020;217(5) doi: 10.1084/jem.20191663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang J.W., Toovey O.T.R., Harvey K.N., Hui D.D.S. Introduction of the South African SARS-CoV-2 variant 501Y.V2 into the UK. J. Infect. 2021;82(4):e8–e10. doi: 10.1016/j.jinf.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Registered trials on conventional (acellular) vaccines for COVID-19 (Up to March 31, 2021).
Registered trials on cell-based immunotherapies for COVID-19 (Up to March 31, 2021).