Abstract
Jujube (Ziziphus jujuba Mill.), a highly nutritious and functional fruit, is reported to have various health benefits and has been extensively planted worldwide, especially in China. Many studies have shown that bioactive components derived from jujube fruit have significant nutritional and potential biological effects. In this paper, the latest progress in research on major bioactive compounds obtained from jujube is reviewed, and the potential biological functions of jujube fruit resources are discussed. As a dietary supplement, jujube fruit is well recognized as a healthy food which contains a variety of bioactive substances, such as polysaccharides, polyphenols, amino acids, nucleotides, fatty acids, dietary fiber, alkaloids, and other nutrients. These nutrients and non-nutritive phytochemicals obtained from jujube fruit have physiological functions including anticancer, antioxidant, anti-inflammatory, anti-hyperlipidemic, anti-hyperglycemic, immunoregulatory, neuroprotective, sedative, and antiviral functions. Of note is that new constituents, including alkaloids, dietary fiber, and other bioactive substances, as well as the antiviral, hypoglycemic, lipid-lowering, and neuroprotective effects of jujube fruit, are systematically reviewed here for the first time. Meanwhile, problems affecting the exploitation of jujube fruit resources are discussed and further research directions proposed. Therefore, this review provides a useful bibliography for the future development of jujube-based products and the utilization of jujube nutritional components in functional foods.
Keywords: Jujube, Bioactive components, Biological activity, Functional foods, Health benefits
1 Introduction
In recent decades, the demand for healthier and safer food products, from cereals to vegetables, has been booming (Das et al., 2012; Ashaolu and Ashaolu, 2020; Ashaolu and Reale, 2020). Many health factors in foods can improve people's quality of life by preventing diseases (Chen et al., 2016; Xu et al., 2018; Gowd et al., 2019).A large proportion of these functional foods are fruits with a variety of nutrients and biological activity (Cai, 2019). Ziziphus jujuba Mill., also known as Chinese jujube or red date, belongs to the Rhamnaceae family. As a highly nutritious and functional fruit, jujube is distributed mainly in Europe and most of Asia (Gao et al., 2013). China is not only the center of origin, but also the main production region of jujube, where annual production accounts for more than 90% of the world's total production. Nearly 700 cultivars of jujube are widely cultivated in the areas of the Yellow River and the northwest region, including the Shandong, Hebei, Shanxi, Shannxi, and Henan provinces, and the Xinjiang Uygur Autonomous Region (Yuan et al., 2002; Li et al., 2007; Ministry of Agriculture and Rural Affairs of the People's Republic of China, 2020; Wang BN et al., 2020). Jujube fruit have been used in folk medicine for 4000 thousand years. According to Huangdi Neijing, an early classical book of ancient Chinese medicine, the jujube was regarded as one of the five most nutritious and healthy fruits. Furthermore, the jujube fruit was recorded as an excellent herbal medicine in Shennong Bencao Jing, serving to improve the quality of sleep, eliminate toxins, and beautify skin (Chen et al., 2014, 2017; Ji et al., 2017). Nowadays, with the evolution of scientific and technological methods, the nutritional components of jujube have been extensively studied and applied in the fields of functional foods and bio-medicine (Gao et al., 2013; Rodríguez Villanueva and Rodríguez Villanueva, 2017).
Most recently, phytochemistry research has indicated that jujube fruit are rich in polysaccharides, polyphenols, amino acids, triterpenic acids, fatty acids, nucleosides, and nucleobases (Gao et al., 2013; Kou et al., 2015; Hernández et al., 2016; Rashwan et al., 2020). Based on the literature, bioactive compounds extracted from jujube have various bioactivity, including antioxidant (Zhang et al., 2010; Zhao HX et al., 2014), anti-inflammatory (Yu et al., 2012), anticancer (Choi et al., 2012; Plastina et al., 2012), anti-hyperglycemic (Kawabata et al., 2017), anti-hyperlipidemic (Jeong and Kim, 2019), immunomodulatory (Dash et al., 2015), and other activity. In addition, other biologically active components obtained from jujube, especially alkaloids and saponin, have been explored for their antiviral, sedative, and neuroprotective effects (Pandey et al., 2008; Shad et al., 2014; Abdoul-Azize, 2016; Ninave and Patil, 2019; He SR et al., 2020).
As a classic prescription in traditional Chinese medicine (TCM), jujube is often used in combination with other herbs to treat a variety of diseases. Qi Fu Yin, a Ming Dynasty prescription containing seven traditional Chinese herbs, has the effect of promoting blood circulation and calming the spirit. In addition, Suanzaoren decoction (Ziziphus spinose) is the most frequently used formula for the treatment of insomnia (Singh and Zhao, 2017; Ong et al., 2018). At present, with the increasing modernization of TCM, research on the material basis and mechanisms of the jujube prescription has attracted much attention. Many new lines of research on jujube fruit have been explored or established and much knowledge gained. Hence, the aim of this review was to summarize the latest findings on the chemical constituents and biological functional activity of jujube fruit. Also, the potential problems associated with the development and utilization of jujube, and the new research directions are critically discussed and evaluated.
2 Major elements and nutrients in jujube fruit
Jujube fruit is well-known as a favored and healthy food, rich in nutritional ingredients such as carbohydrates, proteins, dietary fiber (DF), unsaturated fatty acids, vitamins, and minerals (Gao et al., 2013). The major nutrient elements contained in jujube fruit are discussed below.
2.1. Carbohydrates and proteins
Carbohydrate and protein can provide energy for organs and muscles to keep the body functioning properly (Jéquier, 1994; Wu, 2016). Jujube fruit has been identified as an excellent source of carbohydrate and protein. Li et al. (2007) studied the contents of carbohydrate and protein (percentage of dry weight (DW)) in five cultivars of Chinese jujube. They found that the carbohydrate contents among these jujube fruits were similar, ranging from 80.86% (cv. Yazao) to 85.63% (cv. Sanbianhong). In addition, the protein contents of the cultivars ranged from 4.75% (cv. Jianzao) to 6.86% (cv. Yazao). Rahman et al. (2018) compared and evaluated the nutrient contents of four cultivars of jujube, namely "Hupingzao," "Huizao," "Xiaozao," and "Junzao," grown in Northwest China. The results showed that the carbohydrate contents of these fruits ranged from (82.35±4.50) to (89.73±5.43) g/100 g DW. The protein contents ranged from (4.43±0.66) to (6.01±0.58) g/100 g DW.
2.2. Dietary fiber
DF refers to the polysaccharides, namely lignin, cellulose, and hemicellulose, which cannot be digested by the human body. It is generally classified into two categories: the water-insoluble fibers of cellulose, hemicellulose, and lignin, and the water-soluble fibers of pectin, gums, and mucilages (Cai, 2019). DF can selectively promote metabolism and the proliferation of beneficial bacteria to produce energy and nutrients for the body (Williams et al., 2017). At present, methods for extraction of DF include mainly crude separation, chemical methods, membrane separation, enzymatic methods, enzymatic chemical combination methods, and fermentation methods (Yan et al., 2019; He YY et al., 2020; Karra et al., 2020). Miklavčič Višnjevec et al. (2019) performed a modified enzymatic-gravimetric method to determine the total, soluble, and insoluble fiber contents of jujube sample Zj2. Chemical analysis indicated that the content of insoluble fiber ((6.0±0.2) g/100 g) was higher than that of soluble fiber ((3.8±0.4) g/100 g). The results of this study with regards to insoluble DF content were similar to those from the study of Li et al. (2007), but the mass fraction of soluble fiber in Zj2 was higher. Hernández et al. (2016) analyzed the chemical properties of four jujube cultivars ("GAL," "MSI," "PSI," and "DAT") from a commercial farm located in San Isidro, Spain. Their results showed that the crude fiber content ranged from 0.7 to 1.1 g/100 g DW and "GAL" had the lowest crude fiber content. Studies on the optimization of methods for extracting DF from jujube fruit have also been reported in recent years. Liu and Deng (2016) optimized the conditions for the extraction of DF from Jinsixiaozao by an acid-based treatment. An orthogonal test showed that the yield of DF could reach 5.1% when 5 g jujube fruit was hydrolyzed in 150 mL sulfuric acid (1.5%) solution for 40 min, and 100 mL potassium hydroxide (1.75%) solution for 30 min. Guo et al. (2014) also reported optimum conditions for the preparation of soluble DF from jujube by a cellulose enzymatic method, which yielded up to 6.20%.
2.3. Vitamins and minerals
Jujube fruits contain plenty of minerals and vitamins (especially vitamin C). Mineral analysis showed that jujube fruits contain 17 minerals including 6 macro-elements (K, Ca, Mg, Na, S, and P) and 11 trace elements (Fe, Zn, Cu, Mn, Ni, Se, Pb, Br, Rb, Sr, and Mo) (Li et al., 2007; Gao et al., 2013; Hernández et al., 2016; Miklavčič Višnjevec et al., 2019). Studies have shown that K is the most abundant element in jujube fruit, ranging from 12.4 to 17.3 g/kg DW. Differences in K content may be due to the cultivars or growing conditions (Li et al., 2007; Hernández et al., 2016). The major vitamins detected in jujube fruits are vitamin A, vitamin B complex, carotene, thiamine, riboflavin, and ascorbic acid (Wojdyło et al., 2016).
Ascorbic acid, also known as vitamin C, is a powerful reducing and chelating agent, with numerous biological functions in cells and tissues (Paciolla et al., 2019). Studies have shown that jujube fruits qualify as a good source of vitamin C. A wide range in the concentration of vitamin C was detected among the 15 jujube cultivars analyzed, from 1.671 (cv. Dalongzao) to 4.247 (cv. Guanyangduanzao) mg/g fresh weight (FW) (Kou et al., 2015).
2.4. Fatty acids
Fatty acids are essential nutrients, some of which must be ingested through food to maintain health. The variety and content of fatty acids in jujube fruit could satisfy people's need for nourishment (Guil-Guerrero et al., 2004). Reche et al. (2019) reported that a total of 11 fatty acid compounds, including myristic acid, myristoleic acid, palmitic acid, trans-palmitoleic acid, cis-palmitoleic acid, stearic acid, oleic acid, 11-octadecenoic acid, elaidic acid, linoleic acid, and linolenic acid, were available in four jujube cultivars. Song et al. (2019) detected capric acid (C10:0), lauric acid (C12:0), myristoleic acid (C14:1n5), palmitic acid (C16:0), palmitic acid (C16:1n7), oleic acid (C18:1n9c), and linoleic acid (C18:2n6c) from four ripening stages of jujube fruit. Palmitic acid (C16:0), palmitic acid (C16:1n7), oleic acid (C18:1n9c), and linoleic acid (C18:2n6c) were the predominant fatty acid components. Another study found a total of 16 different fatty acids in jujube fruit samples (Zj2–Zj6) (Miklavčič Višnjevec et al., 2019). The predominant fatty acids were linoleic acid (C18:2) and oleic acid (C18:1).
3 Bioactive compounds in jujube fruit
A variable number of bioactive substances are available in jujube fruit beside the carbohydrates, fatty acids, proteins, and other general nutrients (Choi et al., 2011). Jujube fruits have rich nutritional and medicinal values. In recent years, increasing attention has been paid to their polysaccharides, polyphenols, saponins, amino acids, triterpenes, alkaloids, and other bioactive compounds (Plastina et al., 2012; Tahergorabi et al., 2015).
3.1. Polysaccharides
Jujube fruit polysaccharides are very important water-soluble polysaccharides, most of which are neutral or acid polysaccharides. The extraction, separation, and purification of polysaccharides from jujube fruit are the first step in the study of biological functions. Currently, the mature extraction approaches to improve the yield and quality of plant polysaccharides are hot-water extraction (Li et al., 2008), alkali purification (Ding et al., 2012), enzymatic hydrolytic methods (Wu et al., 2014), and ultrasonic and microwave-assisted extraction (Sun et al., 2019). Generally, the identification and evaluation of jujube polysaccharides are based mainly on their content, composition, and structure. Analysis of the chemical structure of polysaccharides includes a variety of techniques (Ji et al., 2017), such as gas chromatography (GC) for determining the polysaccharide composition and high-performance liquid chromatography (HPLC) for determining the molecular weight. Infrared (IR) spectroscopy, nuclear magnetic resonance (NMR), gas chromatography-mass spectrometry (GC-MS), acid hydrolysis, and methylation analysis are also used to analyze and identify complex polysaccharide structures. In the past five years, numerous studies have discussed the chemical structure of jujube polysaccharides including their monosaccharide composition, monosaccharide sequence, molecular weight, type of glycosyl bonds, and biological activity (Table 1). Five polysaccharide fragments (PZMP1, ZMP, SAZMP3, PZMP2-2, and PZMP3-2) were successively isolated with different chemical structures and biological functions from jujube fruit using water extraction, ultrasound-assisted, alkali extraction, and other purification conditions (Ji et al., 2018a, 2018b, Ji et al., 2019a, 2020b; Lin et al., 2019). In addition, Cai et al. (2018) extracted JCS-1 and JCS-2 from cv. Jinchangzao using a hot-water extraction method. HPLC analysis indicated that JCS-1 and JCS-2 were composed mainly of galacturonic acid (GalA) (Table 1). A recent study found that SAZMP4 (a novel antioxidant pectin isolated from jujube) was composed mainly of rhamnose, arabinose, xylose, mannose, and GalA through 1,4-linked GalA (93.48%) (Lin et al., 2020).
Table 1.
Identified polysaccharides in jujube (Ziziphus jujuba Mill.) fruit
| No. | Z. jujuba cultivar | Name of component | Molecular weight (kDa) | Monosaccharide composition | Structure | Bioactivity | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Muzao | PZMP1 | 16.97 | Arabinose, galactose, glucose, mannose, and xylose in a molar ratio of 17.36:3.29:2.68:1.05:1.00 | Backbone is composed of 1,3,5-linked Araf, 1,3-linked Araf, 1,5-linked Araf, 1,4-linked Glcp, 1-linked Araf, and 1-linked Glcp; the repeating unit of PZMP1 is a linear backbone with branches at positions O-3 and O-5 | Hypolipidemic | Ji et al., 2018b |
| 2 | Jinchangzao | JCS-1 | 71.75 | Galacturonic acid (GalA), galactose, and arabinose in a molar ratio of 39.04:1.26:1.39 | Had α-configurations; typical resonances of C-6 of the carboxyl groups of GalA were observed at 174.92 and 175.05 ppm (part per million); anomeric region signals were showed in the 96‒107 ppm region | Immunomodulatory | Cai et al., 2018 |
| 3 | Jinchangzao | JCS-2 | 357.39 | GalA, mannose, rhamnose, arabinose, and galactose in a molar ratio of 19.87:2.07:1.77:1.65:1.16 | Had α-configurations; typical resonances of C-6 of the carboxyl groups of GalA were observed at 175.05 ppm; anomeric region signals showed in the 96‒107 ppm region | Immunomodulatory | Cai et al., 2018 |
| 4 | Muzao | ZMP | 89.90 | Rhamnose, arabinose, xylose, mannose, glucose, galactose, and GalA in a molar ratio of 1.46:2.47:2.27:1.12:1.00:1.57:5.40 | Stretching vibration C-CO-C stretching vibration may exist in Z. jujuba cv. Muzao polysaccharides | Antioxidant and anti-inflammatory | Ji et al., 2018a |
| 5 | Muzao | SAZMP3 | 9.37 | Rhamnose, arabinose, xylose, mannose, glucose, galactose, and GalA in a molar ratio of 10.51:6.70:0.50:0.26:0.50:6.75:74.69 | Backbone is composed of 1,4-α-D-GalAp with side chains of 1,3-β-D-Galp, 1,3,5-linked Araf, 1,2,4-α-L-Rhap, and terminals of 1-linked Araf, 1-linked Rhap, and 1-linked Galp | Antioxidant | Lin et al., 2019 |
| 6 | Muzao | PZMP2-2 | 62.73 | Rhamnose, arabinose, xylose, galactose, and GalA in a molar ratio of 1.18:5.23:0.22:2.68:2.20 | Backbone is composed of 1-linked Galp, 1,3-linked Araf, 1,2,4-linked Rhap, 1,3-linked Galp, 1,4-linked GalpA, and 1,3,5-linked Araf; a linear backbone of (1→4)-linked GlcpA and (1→2,4)-linked Rhap residues, with branches at the O-4 position, consisting of Araf and Galp residues | Ji et al., 2019a | |
| 7 | Muzao | PZMP3-2 | 58.21 | Rhamnose, arabinose, galactose, and GalA in a molar ratio of 1.74:2.00:341.00:18.69 | A→4]-GalpA-[1→backbone, with few branches at the O-2 position of some Araf and Rhap residues | Ji et al., 2020b | |
| 8 | Muzao | SAZMP4 | 28.94 | Rhamnos, arabinos, xylose, mannose, and GalA in a molar ratio of 1.00:0.90:0.05:0.07:28.90 | A pectic polysaccharide mainly containing 1,4-linked GalA (93.48%) with side chains of 1,2,4-linked Rha and 1,3,5-linked Ara and terminals of 1-linked Rha and 1-linked Ara | Antioxidant | Lin et al., 2020 |
3.2. Polyphenols
Polyphenols such as phenolic acids, tannins, flavonoids, stilbenes, and lignans are secondary metabolites of plants (Al-Dujaili, 2015; Li et al., 2016). In the past decade, many studies have focused on the role of jujube polyphenols in treating human diseases. The main aspects of current research on polyphenols from jujube fruit are extraction and purification, identification of polyphenols, and the determination of total and specific contents. The methods available for the extraction of jujube polyphenols are organic solvent extraction (Lai et al., 2011), ultrasonic-assisted extraction (Ran et al., 2013), and enzymatic hydrolysis (Kammerer et al., 2005), while the total polyphenol content is measured by the Folin-Ciocalteu colorimetric method. Kou et al. (2015) analyzed the bioactive compounds of 15 jujube cultivars from the Loess Plateau of Shanxi, China. The study indicated that the total phenolic content (bound+free polyphenols) of jujube fruit ranged from (0.558±0.043) mg gallic acid equivalent (GAE)/g FW (cv. Zanhuangzao) to (2.520±0.032) mg GAE/g FW (cv. Tengzhouchanghongzao), while the content of free polyphenols was much higher than that of bound polyphenols. These 15 jujube cultivars are also rich in flavonoids, with total contents ranging from (0.47±0.06) mg rutin equivalent (RE)/g FW (cv. Hupingzao) to (2.00±0.08) mg RE/g FW (cv. Nanjingyazao). Wojdyło et al. (2016) applied liquid chromatography (LC)-MS-quadrupole time-of-flight (QTOF), and ultra-performance liquid chromatography-photodiode array-fluorescence detector (UPLC-PDA-FL) methods for the determination of 25 phenolic compounds in Spanish jujube. Their analysis revealed that the jujube contained flavan-3-ols (one of the main phenolic acid components) comprising 89% to 94% of the total polyphenol content, which ranged from 1442 to 3432 mg/100 g dry matter (DM). Recently, another group evaluated the total phenol and flavonoid contents of 16 jujube cultivars from China (Wang BN et al., 2020). They found that the content of polyphenols ranged from 2.534 to 4.949 mg GAE/g, and flavonoids from 1.253 to 4.254 mg rutin/g. Subsequently, a total of ten phenolic acids and two flavonoids were determined in jujube, among which p-hydroxybenzoic acid, protocatechuic acid, chlorogenic acid, and rutin were the main components. However, studies also reported that the phenolic acid composition and content levels of jujube fruits depend mainly on the cultivar and the stage of fruit development ( Gao et al., 2012; Pu et al., 2018; Shi et al., 2018). To facilitate the comprehensive discussion of this review, we have summarized the advanced research on individual phenolic compounds and the range of polyphenols of jujube fruits in Table 2.
Table 2.
Identified polyphenols in jujube (Ziziphus jujuba Mill.) fruit
| Phenolic compound | Content | Reference |
|---|---|---|
| Phenolic acid | ||
| Gallic acid | 0.103‒15.700* | Gao et al., 2012; Shi et al., 2018 |
| Chlorogenic acid | 0.016‒9.480* | Gao et al., 2012; Pu et al., 2018; Shi et al., 2018 |
| Caffeic acid | 0.004‒2.270* | Gao et al., 2012; Pu et al., 2018; Shi et al., 2018 |
| Ferulic acid | 0.002‒5.000* | Gao et al., 2012; Pu et al., 2018; Shi et al., 2018 |
| Cinnamic acid | 0.008‒4.460* | Gao et al., 2012; Pu et al., 2018; Shi et al., 2018 |
| Protocatechuic acid | 0.778‒0.880* | Gao et al., 2012; Pu et al., 2018 |
| p-Cumaric acid | 0.18‒0.66* | Pu et al., 2018 |
| p-Hydroxybenzoic acid | 7.01‒12.53* | Pu et al., 2018 |
| Ellagic acid | 0.0156‒1.5800* | Gao et al., 2012; Pu et al., 2018 |
| Flavan-3-ol | ||
| (+)-Catechin | 1.30‒16.82* | Gao et al., 2012; Pu et al., 2018; Shi et al., 2018 |
| (-)-Epicatechin | 0.12‒30.41* | Gao et al., 2012; Pu et al., 2018; Shi et al., 2018 |
| Polymeric proanthocyanidin | 939‒2548# | Wojdyło et al., 2016 |
| Degree of polymerization (DP) | 2.02‒2.34 | Wojdyło et al., 2016 |
| Procyanidin B1 | 3.119‒29.030* | Shi et al., 2018 |
| Procyanidin B2 | 239‒389# | Wojdyło et al., 2016 |
| Procyanidin B3 | 3.89‒49.10* | Shi et al., 2018 |
| Flavanol | ||
| Quercetin | 0.022‒4.280* | Gao et al., 2012; Pu et al., 2018; Shi et al., 2018 |
| Rutin | 0.52‒7.33* | Gao et al., 2012; Pu et al., 2018 |
| Quercetin-3-rhamnoside | 0.8‒1.9# | Wojdyło et al., 2016 |
| Quercetin-3-galactoside | 0.2‒0.5# | Wojdyło et al., 2016 |
| Quercetin-3-O-robinobioside | 0.3‒1.1# | Wojdyło et al., 2016 |
| Quercetin-3-O-rutinoside | 41.7‒157.0# | Wojdyło et al., 2016 |
| Quercetin-3-O-robinobioside | 0.0‒2.2# | Wojdyło et al., 2016 |
| Quercetin-3-O-arabino-rhamnoside | 21.1‒70.4# | Wojdyło et al., 2016 |
| Quercetin-3-O-xyloso-rhamnoside | 0‒13# | Wojdyło et al., 2016 |
| Quercetin-3-O-rutinoside-7-O-hexoside | 0.6‒3.2# | Wojdyło et al., 2016 |
| Quercetin-3-O-rutinoside-7-O-pentoside | 0.7‒2.9# | Wojdyło et al., 2016 |
| Kaempferol-3-O-rutinoside | 0.6‒1.9# | Wojdyło et al., 2016 |
| Kaempferol-3-O-robinobioside | 7.2‒12.2# | Wojdyło et al., 2016 |
| Kaempferol-3-O-hexose-O-deoxy-hexose-O-pentoside | 7.8‒48.6# | Wojdyło et al., 2016 |
| Flavanone | ||
| Eriodictyol derivative | 1.2‒4.5# | Wojdyło et al., 2016 |
| Dihydrochalcone | ||
| Phloretin-3',5'-di-glucoside | 1.2‒2.0# | Wojdyło et al., 2016 |
* Content in mg/100 g fresh weight (FW). # Content in mg/100 g dry weight (DW).
3.3. Amino acids
Amino acids are essential nutrients for human health (Hou et al., 2019). The content and composition of amino acids in jujube fruit affect its flavor and biological activity (Pu et al., 2018). Studies found that jujube fruit contain at least a dozen major amino acids, which vary depending on the cultivar, maturity stage, and processing method (Rahman et al., 2018; Song et al., 2019; Bao et al., 2021). A study detected a total of 18 amino acids in four jujube cultivars ("Hupingzao," "Huizao," "Xiaozao," and "Junzao") grown in northwest China. Proline (Pro), aspartic acid (Asp), and glutamic acid (Glu) accounted for 64.5%–70.0% of the total amino acids (Kaeidi et al., 2015). These amino acids have about 76.5%–80.8% of the antioxidant and anti-inflammatory properties of the total amino acids. Essential amino acids (EAAs), such as lysine (Lys), threonine (Thr), tryptophan (Trp), valine (Val), leucine (Leu), isoleucine (Ile), histidine (His), phenylalanine (Phe)+tyrosine (Tyr), and methionine (Met)+cysteine (Cys), were also analyzed in the four jujube cultivars. According to the results, cv. Junzao had the highest EAA reference score among these cultivars (Rahman et al., 2018). Song et al. (2019) compared the composition and content of amino acids in jujube fruit at four stages of ripening: green maturity (GM), yellow maturity (YM), half-red maturity (HRM), and red maturity (RM). A total of 26 free amino acids were detected, and their total contents gradually decreased with the maturity of the fruit (Song et al., 2019). Pu et al. (2018) found that high temperature accelerates the loss of free amino acids in cv. Junzao fruit during the long-term storage. In contrast, the proline (Pro) content of the fruit increased, which may be related to the environmental and temperature stress response of the fruit under poor storage conditions.
3.4. Nucleosides and nucleobases
Nucleosides and nucleobases are often selected as quality control markers for functional foods and are involved in the regulation of a variety of biological processes, such as gene expression, cell proliferation, antioxidant, anti-inflammatory, cardioprotective and anticancer activities (Yamamoto et al., 1997; Yuan et al., 2008; Phan et al., 2018). Therefore, it is always important to evaluate and analyze the content of nucleosides/nucleobases to understand the effects of different jujube cultivars or processing methods. Guo et al. (2015b) analyzed the content and composition of nucleosides and nucleobases in jujube (cv. Lingwuchangzao) fruits treated by different processing methods. The results of HPLC-diode array detection (DAD) and LC-MS analyses showed that nine nucleosides and nucleobases, including uracil, hypoxanthine, guanine, cytidine, uridine, adenine, cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), and guanosine, were found in jujube. The study also found that the total content of nucleosides and nucleobases in jujube fruit increased with the extension of drying time. However, cAMP and cGMP in the jujube fruit were not stable during the steaming process. The cyclic nucleotides (mainly cAMP and cGMP) in jujube fruit act as secondary messengers, and are involved in the regulation of a variety of physiological and biochemical processes in the body (Nair et al., 2019). Kou et al. (2015) revealed that the cAMP content of 15 jujube species ranged from 17.38 to 193.93 μg/g FW. The Z. jujuba cv. Hupingzao showed promising prospects for the extraction and utilization of cAMP. The effects of different drying methods on the contents of cAMP and cGMP in fruit of Z. jujuba cv. Jinsixiaozao were described by Wang et al. (2016). The study suggested that the content of cyclic nucleotides in jujube was reduced by air drying (AD), sun drying (SD), or microwave drying (MD). After process optimization, the authors indicated that AD at 50 ℃ was a better choice to obtain product with high levels of cAMP and cGMP from cv. Jinsixiaozao.
3.5. Triterpenic acids
Triterpenoids are one of the major functional constituents of Z. jujuba. Similar to most other nutrients of jujube, the content of triterpenoids is affected by the cultivar and processing methods (Guo et al., 2015a, 2015b; Song et al., 2020). The total triterpene contents of 15 types of jujube fruit were analyzed by Kou et al. (2015). Values ranged from (7.52±0.18) to (16.57±0.11) mg ursolic acid equivalent (UAE)/g FW. The highest amount of total triterpenes was found in cv. Shenglizao (ShLZ), and the lowest in cv. Xiangfenyuanzao (XFYZ). A recent study showed that the total triterpenoid acid contents of 99 jujube samples ranged from 1.08 to 7.92 μg/g DW, with an average value of 3.73 μg/g DW. Moreover, a total of 16 triterpenoid acid peaks were detected by UPLC, among which betulinic acid, alphitolic acid, maslinic acid, oleanolic acid, and ursolic acid were the major triterpenoid acids (Song et al., 2020). Phytochemical studies have shown that most triterpenoids derived from cereals and vegetables have a variety of functions, such as antioxidant, anti-inflammatory, hepatoprotective, and anti-tumor properties (Xu et al., 2018; Yang et al., 2018; Ghante and Jamkhande, 2019). Ursonic acid, a pentacyclic triterpenoid extracted from Z. jujuba, may alleviate the deterioration caused by tumors or skin aging by inhibiting the expression of matrix metalloproteinase (MMP) genes (Son and Lee, 2020). A research group from Japan uncovered the anti-hyperglycemia effect of the triterpenoids of jujube fruit (Kawabata et al., 2017). They found that betulonic acid, betulinic acid, oleanonic acid, and ursonic acid could promote glucose uptake in rat L6 myotubes in a glucose transporter-4-dependent manner. Li et al. (2018) established a highly efficient, sensitive, and accurate UPLC-MS/MS method to detect the pharmacokinetic characteristics of seven triterpenoids in normal and immunosuppressed rats after oral administration of jujube fruit. They found that the pharmacokinetic parameters of triterpenoids were significantly different between normal and immunosuppressed rats. Thus, these kinetic characteristics may provide a reference value for the clinical application of triterpenic acids from jujube fruit (Li et al., 2018).
3.6. Alkaloids
Alkaloids are basic nitrogen-containing organic compounds, most of which have a complex nitrogen-containing heterocyclic ring. They are known to have significant physiological effects (Cushnie et al., 2014; Senchina et al., 2014). As characteristic components of jujube plants, alkaloids are found mostly in the roots, stems, leaves, and seed (Yoon et al., 2009; Kang et al., 2015; Sakna et al., 2019). Although there are many kinds of alkaloids in jujube fruit, the contents are generally low and they are difficult to separate and extract. As a result, related research has rarely been reported. Zhang et al. (2019) reported that the optimal method for the extraction of alkaloids from "Goutou" jujube fruit was as follows: solid-liquid mass ratio 1:12, ethanol concentration 70%, and extraction time 2.5 h. They also found that the alkaloids of "Goutou" jujube could effectively scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radicals, which confirmed their antioxidant activity.
4 Research advances on jujube fruit health functions
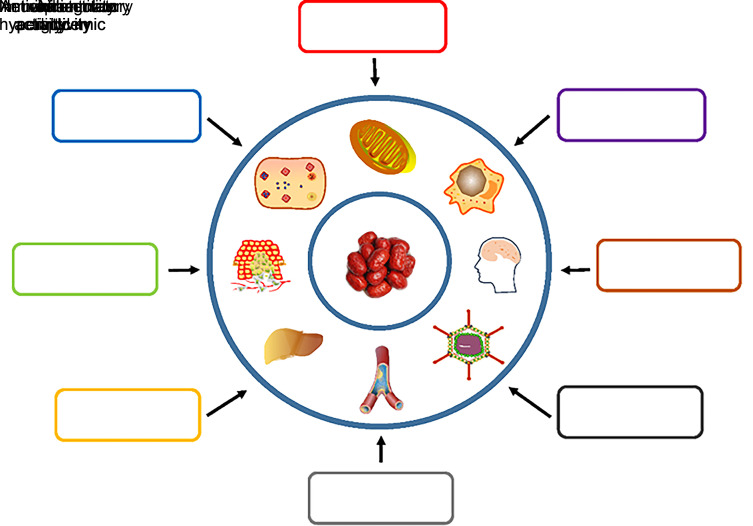
Jujube fruit is delicious and nutritious, and has always been regarded as a healthy supplement. For more than a thousand years, in China the jujube has also been a traditional herbal medicine to calm the mind and soothe the nerves (Chen et al., 2017). Now, modern scientific research has revealed that the biologically active substances in jujube have cancer prevention, antioxidant, anti-inflammatory, hepatoprotective, neuroprotective, and antiviral properties, and other healthcare effects including improving immune function (Table 3; Fig. 1) (Gao et al., 2013; Abedini et al., 2016; Ji et al., 2017).
Table 3.
Health benefits of jujube fruit
| Biological function | Jujube sample used | Experimental model | Findings | Reference |
|---|---|---|---|---|
| Anticancer activity | Jujube fruit was supplemented in feed at 5% and 10% (mass fraction) | Colitis-associated colon carcinogenesis in AOM/DSS-treated mice | Jujube fruit attenuated aberrant crypt foci and decreased the progression of hyperplasia to dysplasia. In addition, it reduced circulating white blood cells, lymphocytes, neutrophils, monocytes, eosinophils, basophils, and platelets compared to colon cancer mice. | Periasamy et al., 2015 |
| Jujube fruit diet for 70 d (5% or 10%, mass fraction) | Colitis-associated colon carcinogenesis in AOM/DSS-treated mice | Dietary jujube increased colon length and suppressed the activation of NF-κB/IL-6/JAK1/STAT3 signaling pathway. | Periasamy et al., 2020 | |
| Jujube extract (ursonic acid) | A549, H1299, and HaCaT cells | Ursonic acid inhibited ERK and CREB signaling pathways and reduced the transcriptional expression levels of gelatinase (MMP-2 and MMP-9) in non-small cell lung cancer cells, thereby exerting remarkable anticancer capabilities. | Son and Lee, 2020 | |
| Jujube fruit aqueous extract | Cervical cancer cell line (OV2008), breast cancer cell line (MCF-7), and normal cell line (MCF-10A) | Jujube fruit aqueous extract inhibited the proliferation of OV2008 and MCF-7 cancer cells. The potential mechanism is through increasing the expression of Bax gene and reducing the expression of Bcl2 gene. | Abedini et al., 2016 | |
| Jujube polysaccharides | AOM/DSS-induced colitis cancer in C57BL/6 mice | Jujube polysaccharides could significantly decrease Firmicutes/Bacteroidetes and ward off colon cancer by ameliorating colitis cancer-induced gut dysbiosis. | Ji et al., 2020a | |
| Z. jujuba cv. Muzao polysaccharides (ZMP) | AOM/DSS-induced colitis cancer in C57BL/6 mice | ZMP increased the enrichment of Bifidobacterium, Bacteroides, and Lactobacillus, reduced the expression of proinflammatory factors, increased the concentrations of short-chain fatty acids, and prevented further progression of colon cancer. | Ji et al., 2019b | |
| Jujube powder | Mouse MC38 colon tumor model | Jujube powder increased the species richness of Lachnospiraceae, decreased the abundance of Prevotellaceae, and improved both the response rate and therapeutic efficiency of anti-PD-L1. | Wang LYet al., 2020 | |
| Antioxidant activity | Two active polysaccharides (LZJP3 and LZJP4) extracted from jujube | DPPH, hydroxyl radical, superoxide anion, and hydrogen peroxide scavenging activity | LZJP3 and LZJP4 extracted from Z. jujuba cv. Linzexiaozao have strong antioxidant effects on DPPH, hydroxyl radicals, hydrogen peroxide, and superoxide radicals. | Wang et al., 2018 |
| Jujube fruit residues polysaccharide extract (SAZMP3) | Hydroxyl free radical and DPPH free radical scavenging ability | SAZMP3 could scavenge hydroxyl radicals and DPPH radicals in a concentration-dependent manner in vitro. | Lin et al.,2019 | |
| Jujube fruit powder supplementation (30 and 150 mg/mL) | Fruit flies | Jujube fruit powder supplementation increased flies’ ability to resist starvation stress and ROS stress. | Ghimire and Kim, 2017 | |
| Flavonoid extracted from Z. jujuba cv. Jinsixiaozao (ZJF) | In vitro (DPPH, ABTS, FRAP); in vivo (male BALB/c mice) | ZJF could increase the activity of SOD and GSH in the mouse liver. | Huang et al., 2017 | |
| Anti-inflammatory activity | Alcohol extract from jujube fruit (EEZJ) 800, 1200, and1600 mg/kg | Carrageenan-induced paw oedema in female Wistar rats | EEZJ eliminated the carrageenan-induced paw oedema in female Wistar rats by inhibiting inflammation. | Mesaik et al., 2018 |
| ZJF | Male BALB/c mice | ZJF decreased APAP-induced inflammatory mediator production (NO, TNF-α, IL-6, and IL-1β) and inhibited NF-κB signaling pathway to protect the mouse liver. | Huang et al., 2017 | |
| Jujube fruit diet for 70 d (5% or 10%, mass fraction) | AOM/DSS-treated mice | Jujube fruit suppressed intestinal inflammation by blocking pathway of NF-κB/IL-6/JAK1/STAT3. | Periasamy et al., 2020 | |
| Hydroalcoholic extract of Z. jujuba fruits | Rat with induced ulcerative colitis | Jujube extract could decrease the myeloperoxidase activity and stimulate SOD and GSH peroxidase activity. | Tanideh et al., 2016 | |
| Polysaccharides from Z. jujuba cv. Pozao | Cyclophosphamide-induced ICR mice for 28 d | Polysaccharides from Z. jujuba cv. Pozao could increase the levels of IL-2, IL-4, IL-10, and IFN-γ in the spleens of immunosuppressed mice. | Han et al., 2020 | |
| Anti-hyperglycemic activity | Dried jujube fruit and chokeberry dietary | Mice fed 60% high-fat and 10% fructose diet | Dried jujube and chokeberry reduced the HFFD mice body weight, attenuated blood glucose and triglyceride concentrations. | Jeong and Kim, 2019 |
| Jujube fruit active substances (betulinic acid, oleanolic acid, and ursonic acid) | Rat L6 myotube | These polycyclic triterpenoids induced glucose uptake in a glucose transporter-4-dependent manner, and finally promoted glucose uptake in rat L6 myotubes. | Kawabata et al., 2017 | |
| Jujube(cv. Shaanbeitanzao) polysaccharide (ZSP) 0, 200, and 400 mg/kg BW for four weeks | Mice fed high-fructose | ZSP significantly improved the HDL-C and HOMA-IR levels, reduced insulin resistance, and balanced blood lipid homeostasis in high-fructose diet mice. | Zhao Yet al., 2014 | |
| Jujube water extract from Z. jujuba cv. Muzao (PZMP1) | The normal human liver cell line L02 | PZMP1 reduced the activity of ALT and inhibited the oleic acid-induced triglyceride and lipid accumulation in a concentration-dependent manner in L02 cells. | Ji et al., 2018b | |
| Immunoregulatory activity | Z. jujube cv. Jinchangzao ethanol extract (JJC1 and JJC2) | Kunming male mice | JJC1 and JJC2 stimulated the NO production and phagocytic activity of RAW264.7 cells and promoted the proliferation of spleen lymphocytes. | Cai et al., 2017 |
| Z. jujuba cv. Jinchangzao polysaccharides (JCS-1 and JCS-2) | RAW264.7 cells | There was a positive dose-dependent relationship between JCS-1, JCS-2 and phagocytic indices. JCS-1 and JCS-2 showed immunological activity in a dose-dependent manner. Sulfated derivatives exhibited stronger immunological activity than native polysaccharides. | Cai et al., 2018 | |
| Z. jujuba cv. Huizao polysaccharides (HP1 and HP2) | Kunming male mice | HP1 and HP2 improved the functions of spleen and thymus, promoted the formation of serum hemolysin, increased the phagocytosis of macrophages, and alleviated the edema of foot pads of mice. | Zou et al., 2018 | |
| Neuroprotective activity | Jujube aqueous extracts | PC12 cells | Aqueous extracts promoted the expression of neuronal cell-specific cytoskeleton proteins in PC12 cells. | Chen et al., 2015 |
| Jujube fruit in traditional Chinese medicine prescriptions | PC12 cells | Jujube-containing herbal decoctions stimulated the growth of neurite and protein expression of neurofilaments afterco-incubation with PC12 cells. | Lam et al., 2016 | |
| Antiviral activity | Jujube active substance (betulinic acid) | Influenza A/PR/8 virus infected A549 cell and mice | Betulinic acid (50 μmol/L) showed satisfactory antiviral activity without significant cytotoxicity to influenza A/PR/8 virus-infected cell line A549. In vivo experiments have shown that betulinic acid can relieve the symptoms of lung necrosis and edema caused by influenza A/PR/8 virus in mice. | Hong et al., 2015 |
| Jujube flavonoids (compound 1 and compound 2) | Anti-tobacco mosaic virus (TMV) activity was tested using the half-leaf method | These two new compounds possessed significant activity against tobacco mosaic virus replication, with inhibition rates of 92.8% and 88.6%, respectively. | Li et al., 2013 | |
| Jujube fruit in Yakammaoto (a prescription of traditional Chinese medicine) | HEp-2, A549, and HK-2 cell lines | Inhibited coxsackievirus B4 (CVB4)-induced cellular damage by preventing viral attachment, internalization, and replication. | Yen et al., 2014 |
AOM: azoxymethane; DSS: dextran sodium sulphate; NF-κB: nuclear factor-κB; IL: interleukin; JAK1: Janus kinase 1; STAT3: signal transducer and activator of transcription 3; ERK: extracellular signal-regulated kinase; CREB: cyclic adenosine monophosphate (cAMP) response element-binding protein; MMP: matrix metalloproteinase; DPPH: 2,2-diphenyl-1-picrylhydrazyl; ROS: reactive oxygen species; ABTS: 2,2'-azinobis-(3-ethylbenzthiazoline-6-sulphonate); FRAP: ferric-reducing antioxidant power; SOD: superoxide dismutase; GSH: glutathione; TNF-α: tumor necrosis factor-α; IFN-γ: interferon-γ; HFFD: high-fat-fructose diet; BW: body weight; HDL-C: high-density lipoprotein-cholesterol; HOMA-IR: homeostasis model assessment-insulin resistance; APAP: acetaminophen; ALT: alanine aminotransferase.
Fig. 1. Bioactive functions of jujube. The major benefits from the bioactive functions of jujube fruit include anticancer, antioxidant, anti-inflammation, anti-hyperglycemia, anti-hyperlipidemia, antivirus, neuroprotection, and immunoregulation.
4.1. Anticancer activity
Many experimental studies have shown that jujube fruit bioactive substances can slow down the occurrence and development of certain cancers by inducing cellular apoptosis, inhibiting the related signaling pathways, and regulating gut microbes. Researchers found that consumption of jujube fruit by dextran sodium sulphate (DSS)/azoxymethane (AOM)-induced colorectal cancer model mice for 62 consecutive days could decrease colon aberrant crypt foci and delay the process of colon carcinogenesis (Periasamy et al., 2015). A recent study found that jujube fruit ultimately reduced the number of colon tumors in DSS/AOM-induced mice by inhibiting the nuclear factor-κB (NF-κB)/interleukin-6 (IL-6)/Janus kinase 1 (JAK1)/signal transducer and activator of transcription 3 (STAT3) signaling pathway (Periasamy et al., 2020). Likewise, Son and Lee (2020) found that ursonic acid (a pentacyclic triterpenoid compound extracted from jujube fruit) exhibited anticancer activity by inhibiting the extracellular signal-regulated kinase (ERK) and cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB) signaling pathways and reducing the transcriptional expression levels of gelatinase (MMP-2 and MMP-9) in non-small cell lung cancer cells. Pro-apoptosis is a classical anticancer mechanism. Jujube aqueous extract from semi-dried fruits was reported to inhibit the proliferation of OV2008 and MCF-7 cancer cells by increasing the expression of B-cell lymphoma 2 (Bcl2)-assosiated X (Bax) gene and reducing the expression of the Bcl2 gene (Abedini et al., 2016). In recent years, inhibition of tumor development by regulating intestinal microorganisms using jujube extract has attracted much attention. Ji et al. (2020a) found that an active polysaccharide purified from jujube fruit significantly reduced the abundance of Firmicutes/Bacteroidetes, and was effective in preventing and treating DSS/AOM-induced colitis cancer in a mouse model by reshaping the intestinal flora. Subsequently, the team also reported that cv. Muzao polysaccharides could significantly increase the Bifidobacterium, Bacteroides, and Lactobacillus in the intestinal tract of mice, reduce the expression of proinflammatory parameters, increase the concentration of short-chain fatty acids, and prevent further progression of colon cancer (Ji et al., 2019b). Moreover, Wang LY et al. (2020) found that jujube fruit powder could increase the species abundance of Lachnospiraceae and decrease the abundance of Prevotellaceae, improving both the response rate and treatment effect of anti-PD-L1 in an MC38 colon tumor model mouse.
4.2. Antioxidant activity
Increasing evidence shows that many diseases are closely related to the accumulation of harmful free oxygen radicals (Halliwell, 2012; Poprac et al., 2017). Reactive oxygen species (ROS), a general term for oxygen ions, free radicals, and peroxides of both inorganic and organic componds, are involved in various biological pathways such as cell proliferation, apoptosis, and cell signal transduction (Brieger et al., 2012; Diebold and Chandel, 2016; Forrester et al., 2018). Jujube fruit, as a Chinese herb, has a long history of antioxidant applications. In recent years, studies have found that the two homologous active polysaccharides (LZJP3 and LZJP4) extracted from Z. jujuba cv. Linzexiaozao have strong antioxidant effects, as evaluated by DPPH radicals, hydroxyl radicals, hydrogen peroxide, and superoxide radicals (Wang et al., 2018). Lin et al. (2019) reported that an acidic polysaccharide (SAZMP3) from Z. jujuba cv. Muzao fruit could scavenge hydroxyl and DPPH radicals in a concentration-dependent manner in vitro. They also indicated that SAZMP3 showed a strong ferric ion-reducing capacity. In in vivo antioxidant studies, it was reported that fruit flies fed with 150 mg/mL jujube fruit supplement could effectively reduce ROS stress and increase their average survival time (Ghimire and Kim, 2017). Huang et al. (2017) pointed out that flavonoid extracted from Z. jujuba cv. Jinsixiaozao (ZJF) is a natural antioxidant that can increase the activity of superoxide dismutase (SOD) and glutathione (GSH) in the mouse liver.
4.3. Anti-inflammatory activity
In general, inflammation is the body's automatic defense response to pathogens and tissue injury (Kuprash and Nedospasov, 2016). Chronic inflammation may result in diabetes, stroke, cardiovascular disease, and even cancer (Mantovani et al., 2008; Medzhitov, 2008, 2010). Therefore, anti-inflammatory therapeutics are necessary for healthy living. Ulcerative colitis (UC) is one kind of inflammatory bowel diseases (IBDs), which affects the normal functions of the descending colon and rectum. Tanideh et al. (2016) indicated that a hydroalcoholic extract of Z. jujube fruit has a healing effect in damaged colonic tissue and reduces GSH peroxidase and IL-1 levels in 3% acetic acid-induced UC rats. Mesaik et al. (2018) reported that an alcohol extract from jujube fruit (EEZJ) eliminated carrageenan-induced paw oedema in female Wistar rats by inhibiting inflammation. Huang et al. (2017) studied the anti-inflammatory properties ZJF and found that ZJF decreased acetaminophen (APAP)-induced inflammatory mediator production (NO, tumor necrosis factor-α (TNF-α), IL-6, and IL-1β) and inhibited the NF-κB signaling pathway to protect the mouse liver. Periasamy et al. (2020) demonstrated that Z. jujuba fruit suppressed intestinal inflammation by blocking the NF-κB/IL-6/JAK1/STAT3 pathway in AOM/DSS-induced colorectal mice. Han et al. (2020) found that a dietary supplement of polysaccharides from Z. jujuba cv. Pozao could increase the levels of IL-2, IL-4, IL-10, and interferon-γ (IFN-γ) in the spleen of immunosuppressed mice, which effectively improved the inflammatory response to cyclophosphamide-induced hypoimmunity.
4.4. Anti-hyperglycemic and anti-hyperlipidemic properties
The incidence of diabetes is linked to abnormal glucose metabolism and dyslipidemia (Matschinsky, 2005; Wu and Parhofer, 2014). In many in vivo experiments, the effect of jujube fruit on the regulation of glucose and lipids has been proved. Jeong and Kim (2019) reported the effects of dried jujube fruit and chokeberry dietary intervention in high-fat-fructose diet (HFFD) mice for 11 weeks. The results showed that the diet significantly reduced the mouse body weight, and attenuated blood glucose and triglyceride concentrations. The consumption of chokeberry and dried jujube activated the insulin receptor substrate-1 (IRS-1)/phosphatidylinositol-3-OH kinase (PI3K)/protein kinase B (Akt) signaling pathway and increased insulin sensitivity in the HFFD-induced obese mice. Kawabata et al. (2017) found that jujube fruit contains polycyclic triterpenoids (betulinic acid, oleanolic acid, betulinic acid, and ursonic acid). These compounds induced glucose uptake in a glucose transporter-4-dependent manner, and promoted glucose uptake in rat L6 myotubes. Zhao Y et al. (2014) fed mice with 20% high-fructose water and jujube (cv. Shaanbeitanzao) polysaccharide (ZSP) for four weeks. The results showed that the serum glucose and insulin concentrations of the mice were lowered by 6.5% and 12.5%, respectively, in the HF+LZSP (20% high-fructose water+200 mg/kg body weight (BW) ZSP) group, and by 10.0% and 38.4%, respectively, in the HF+HZSP (20% high-fructose water+400 mg/kg BW ZSP) group, compared to the HF group. The homeostasis model assessment-insulin resistance (HOMA-IR) score of the mice in the HF+LZSP and HF+HZSP groups showed a decrease of about 25.0% and 31.3%, respectively, compared to the HF group. In addition, in vitro studies showed that PZMP1 (a kind of neutral polysaccharide isolated from Z. jujuba cv. Muzao) reduced the activity of alanine aminotransferase (ALT) and inhibited oleic acid-induced triglyceride and lipid accumulation in a concentration-dependent manner in L02 cells (Ji et al., 2018b).
4.5. Immunoregulatory activity
In general, functional foods can regulate the immune system by enhancing or suppressing the immune response, providing host defenses against infection, and suppressing allergies and inflammation (Ashaolu, 2020). Cai et al. (2017) studied the immunological activity of two biological water-soluble lignins (JJC1 and JJC2) from Z. jujuba cv. Jinchangzao. They showed that both JJC1 and JJC2 could stimulate NO production and phagocytic activity of RAW264.7 cells, and promote the proliferation of spleen lymphocytes. Moreover, they found that JCS-1 and JCS-2, two active polysaccharides isolated from cv. Jinchangzao, had the ability to regulate the immune function. The immunomodulatory activity of polysaccharides was further enhanced after sulfated modification (Cai et al., 2018). Zou et al. (2018) recently demonstrated that two acidic polysaccharides (HP1 and HP2) purified from Z. jujuba cv. Huizao could significantly improve the function of the spleen and thymus, promote the formation of serum hemolysin, increase the phagocytosis of macrophages, and alleviate edema of the foot pads of mice. Furthermore, polysaccharide component HP2 had a more significant and stable effect on immune regulation than HP1.
4.6. Neuroprotection
Chen et al. (2015) compared the neuroprotective effects of ripe and unripe jujube fruit cultivated in Cangzhou, Hebei Province, China. They revealed that aqueous extracts from the two developmental stages of jujube fruit promoted the expression of the neuronal cell-specific cytoskeleton protein, neurofilament 68, in PC12 cells. The neuroprotective effect of the mature jujube fruit extract was the most significant. (Chen et al., 2015). Guizhi Tang (GZT), Neibu Dangguijianzhong Tang (NDT), and Zao Tang (ZOT) are three TCM prescriptions containing jujube fruit which have been found to stimulate the growth of neurite and the expression of neurofilament proteins after co-incubation with PC12 cells. Most importantly, jujube as a synergistic agent of these three kinds of decoctions, can significantly increase neuroprotective activity and reduce drug toxicity (Lam et al., 2016).
4.7. Antiviral activity
Effectively dealing with the damage caused by infectious viruses to animals and plants is a major challenge for the world's public health systems. In developing research work, the effective antiviral effects and active ingredients of jujube have attracted great attention in the field of medicine.
Betulinic acid, a pentacyclic triterpenoid, was first extracted from jujube fruit. Hong et al. (2015) found that betulinic acid had a special inhibitory activity against influenza A/PR/8 virus. The study indicated that 50 μmol/L of betulinic acid showed satisfactory antiviral activity without significant cytotoxicity to influenza A/PR/8 virus-infected cell line A549. In vivo experiments showed that betulinic acid can relieve the symptoms of lung necrosis and edema caused by influenza A/PR/8 virus in mice.
Jujube is a traditional herbal medicine and is frequently used in many traditional Chinese antiviral formulas. For example, Yakammaoto is a prescription of TCM containing nine components, including jujube fruit. In cellular experiments, Yakammaoto was proven to inhibit coxsackievirus B4 (CVB4)-induced cellular damage by preventing viral attachment, internalization, and replication (Yen et al., 2014). Furthermore, Kang et al. (2015) extracted eight cyclopeptide alkaloids (1–8) from dried roots of Z. jujuba using an acid-base method. Their data suggested that compounds 2 (jubanines-G), 3 (jubanines-H), and 6 (nummularine-B) had potential inhibitory effects on porcine epidemic diarrhea virus (PEDV). It was also the first report of antiviral activity from plant-derived cyclopeptide alkaloids, especially in Z. jujube. Li et al. (2013) also reported two new flavonoids, 8-formyl-3',4'-dihydroxy-6,7-dimethoxyflavone (compound 1) and 8-formyl-4'-hydroxy-3',6,7-trimethoxyflavone (compound 2), isolated from the fruits of jujube. Their results suggested that these two new compounds had significant activity against tobacco mosaic virus replication, with inhibition rates of 92.8% and 88.6%, respectively.
5 Future perspectives and conclusions
As a dietary supplement, jujube fruit is now considered to be a cheap, readily applicable, acceptable, and available product for the prevention and treatment of a variety of diseases ( Rodríguez Villanueva and Rodríguez Villanueva, 2017). When nutrients and non-nutritive phytochemicals in jujube fruit, such as polysaccharides, polyphenols, amino acids, nucleotides, fatty acids, DF, and other key components, are absorbed by human body, they coordinate and interact with each other to fulfil physiological functions such as anti-oxidative, anti-inflammatory, liver protective, antiviral, anticancer, neuroprotective, and sedative functions. However, caution is essential when attempting to extrapolate relationships between nutrients and health functions in jujube. This is because the cultivar, stage of maturation, and storage and processing conditions of the fruit can affect its nutritional value and eventually lead to an increase or loss of some active functions (Ding et al., 2017; Pu et al., 2018; Shi et al., 2018).
Overall, jujube fruit contains a large number of functional substances with a variety of physiological effects. Apart from providing nutrition for the body, there have been few clinical studies on the significant effects of jujube, which limits the application of its active components in clinical medicine. In many experiments, the biological effects of jujube nutritional components in cell models are achievable only at supraphysiological intracellular concentrations, which are usually higher than those available in the human body. Moreover, the bioavailability of most nutrients could be reduced after being digested and absorbed by the human intestinal tract. Therefore, in-depth analysis of the metabolic pathways and pharmacokinetics of the active components of jujube is crucial for future clinical applications. Currently, although many TCM prescriptions containing jujube have been used in the prevention and treatment of diseases, more studies are needed to clarify the correspondence between the content of active components and biological functions of jujube and the molecular mechanisms. These studies also will provide a more safe, scientific, and rigorous theoretical basis for applying the jujube as a functional food.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. U1703105) and the Zhejiang Provincial Key R&D Program of China (No. 2019C02074).
Author contributions
Yang LU and Wei CHEN conducted this review. Yang LU, Wei CHEN, and Tao BAO wrote and edited the manuscript. Yang LU, Jianling MO, and Jingdan NI participated in creating and editing the tables. All authors have read and approved the final manuscript and, therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Yang LU, Tao BAO, Jianling MO, Jingdan NI, and Wei CHEN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Abdoul-Azize S, 2016. Potential benefits of jujube ( Zizyphus lotus L.) bioactive compounds for nutrition and health. J Nutr Metab, 2016: 2867470. 10.1155/2016/2867470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedini MR, Erfanian N, Nazem H, et al. , 2016. Anti-proliferative and apoptotic effects of Ziziphus jujube on cervical and breast cancer cells. Avicenna J Phytomed, 6(2): 142-148. [PMC free article] [PubMed] [Google Scholar]
- Al-Dujaili EA, 2015. Natural polyphenols: potential for disease prevention. EC Nutr, 2(2): 337-345. [Google Scholar]
- Ashaolu TJ, 2020. Immune boosting functional foods and their mechanisms: a critical evaluation of probiotics and prebiotics. Biomed Pharmacother, 130: 110625. 10.1016/j.biopha.2020.110625 [DOI] [PubMed] [Google Scholar]
- Ashaolu TJ, Ashaolu JO, 2020. Perspectives on the trends, challenges and benefits of green, smart and organic (GSO) foods. Int J Gastron Food Sci, 22: 100273. 10.1016/j.ijgfs.2020.100273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashaolu TJ, Reale A, 2020. A holistic review on Euro-Asian lactic acid bacteria fermented cereals and vegetables. Microorganisms, 8(8): 1176. 10.3390/microorganisms8081176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao T, Hao X, Shishir MRI, et al. , 2021. Cold plasma: an emerging pretreatment technology for the drying of jujube slices. Food Chem, 337: 127783. 10.1016/j.foodchem.2020.127783 [DOI] [PubMed] [Google Scholar]
- Brieger K, Schiavone S, Miller FJ, et al. , 2012. Reactive oxygen species: from health to disease. Swiss Med Wkly, 142: w13659. 10.4414/smw.2012.13659 [DOI] [PubMed] [Google Scholar]
- Cai M, 2019. Fruit-based functional food. In: Galanakis CM (Ed.), The Role of Alternative and Innovative Food Ingredients and Products in Consumer Wellness. Academic Press, New York, p.35-72. 10.1016/B978-0-12-816453-2.00002-4 [DOI] [Google Scholar]
- Cai YQ, Zhou XP, Han AZ, et al. , 2017. In vitro immunological and anti-complementary activities of two water-soluble lignins from Zizyphus jujube cv. Jinchangzao. Int J Biol Macromol, 105(Pt 1): 204-212. 10.1016/j.ijbiomac.2017.07.026 [DOI] [PubMed] [Google Scholar]
- Cai YQ, Chen P, Wu CY, et al. , 2018. Sulfated modification and biological activities of polysaccharides derived from Zizyphus jujuba cv. Jinchangzao. Int J Biol Macromol, 120(Pt A): 1149-1155. 10.1016/j.ijbiomac.2018.08.141 [DOI] [PubMed] [Google Scholar]
- Chen JP, Maiwulanjiang M, Lam KYC, et al. , 2014. A standardized extract of the fruit of Ziziphus jujuba (jujube) induces neuronal differentiation of cultured PC12 cells: a signaling mediated by protein kinase A. J Agric Food Chem, 62(8): 1890-1897. 10.1021/jf405093f [DOI] [PubMed] [Google Scholar]
- Chen JP, Chan PH, Lam CTW, et al. , 2015. Fruit of Ziziphus jujuba (jujube) at two stages of maturity: distinction by metabolic profiling and biological assessment. J Agric Food Chem, 63(2): 739-744. 10.1021/jf5041564 [DOI] [PubMed] [Google Scholar]
- Chen JP, Liu XY, Li ZG, et al. , 2017. A review of dietary Ziziphus jujuba fruit (jujube): developing health food supplements for brain protection. Evid Based Complement Alternat Med, 2017: 3019568. 10.1155/2017/3019568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Xu Y, Zhang LX, et al. , 2016. Blackberry subjected to in vitro gastrointestinal digestion affords protection against Ethyl Carbamate-induced cytotoxicity. Food Chem, 212: 620-627. 10.1016/j.foodchem.2016.06.031 [DOI] [PubMed] [Google Scholar]
- Choi SH, Ahn JB, Kozukue N, et al. , 2011. Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. J Agric Food Chem, 59(12): 6594-6604. 10.1021/jf200371r [DOI] [PubMed] [Google Scholar]
- Choi SH, Ahn JB, Kim HJ, et al. , 2012. Changes in free amino acid, protein, and flavonoid content in jujube (Ziziphus jujube) fruit during eight stages of growth and antioxidative and cancer cell inhibitory effects by extracts. J Agric Food Chem, 60(41): 10245-10255. 10.1021/jf302848u [DOI] [PubMed] [Google Scholar]
- Cushnie TPT, Cushnie B, Lamb AJ, 2014. Alkaloids: an overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int J Antimicrob Agents, 44(5): 377-386. 10.1016/j.ijantimicag.2014.06.001 [DOI] [PubMed] [Google Scholar]
- Das A, Raychaudhuri U, Chakraborty R, 2012. Cereal based functional food of Indian subcontinent: a review. J Food Sci Technol, 49(6): 665-672. 10.1007/s13197-011-0474-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash SK, Chattopadhyay S, Tripathy S, et al. , 2015. Self-assembled betulinic acid augments immunomodulatory activity associates with IgG response. Biomed Pharmacother, 75: 205-217. 10.1016/j.biopha.2015.07.033 [DOI] [PubMed] [Google Scholar]
- Diebold L, Chandel NS, 2016. Mitochondrial ROS regulation of proliferating cells. Free Radic Biol Med, 100: 86-93. 10.1016/j.freeradbiomed.2016.04.198 [DOI] [PubMed] [Google Scholar]
- Ding SH, Wang RR, Shan Y, et al. , 2017. Changes in pectin characteristics during the ripening of jujube fruit. J Sci Food Agric, 97(12): 4151-4159. 10.1002/jsfa.8285 [DOI] [PubMed] [Google Scholar]
- Ding X, Zhu FS, Gao SG, 2012. Purification, antitumour and immunomodulatory activity of water-extractable and alkali-extractable polysaccharides from Solanum nigrum L. Food Chem, 131(2): 677-684. 10.1016/j.foodchem.2011.09.060 [DOI] [Google Scholar]
- Forrester SJ, Kikuchi DS, Hernandes MS, et al. , 2018. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res, 122(6): 877-902. 10.1161/CIRCRESAHA.117.311401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao QH, Wu CS, Yu JG, et al. , 2012. Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphus jujuba Mill.) selections. J Food Sci, 77(11): C1218-1225. 10.1111/j.1750-3841.2012.02946.x [DOI] [PubMed] [Google Scholar]
- Gao QH, Wu CS, Wang M, 2013. The jujube (Ziziphus jujuba Mill.) fruit: a review of current knowledge of fruit composition and health benefits. J Agric Food Chem, 61(14): 3351-3363. 10.1021/jf4007032 [DOI] [PubMed] [Google Scholar]
- Ghante MH, Jamkhande PG, 2019. Role of pentacyclic triterpenoids in chemoprevention and anticancer treatment: an overview on targets and underling mechanisms. J Pharmacopuncture, 22(2): 55-67. 10.3831/KPI.201.22.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghimire S, Kim MS, 2017. Jujube (Ziziphus Jujuba Mill.) fruit feeding extends lifespan and increases tolerance to environmental stresses by regulating aging-associated gene expression in Drosophila. Biogerontology, 18(2): 263-273. 10.1007/s10522-017-9686-8 [DOI] [PubMed] [Google Scholar]
- Gowd V, Bao T, Chen W, 2019. Antioxidant potential and phenolic profile of blackberry anthocyanin extract followed by human gut microbiota fermentation. Food Res Int, 120: 523-533. 10.1016/j.foodres.2018.11.001 [DOI] [PubMed] [Google Scholar]
- Guil-Guerrero JL, Díaz Delgado A, Matallana González MC, et al. , 2004. Fatty acids and carotenes in some ber (Ziziphus jujuba Mill) varieties. Plant Foods Hum Nutr, 59(1): 23-27. 10.1007/s11130-004-0017-2 [DOI] [PubMed] [Google Scholar]
- Guo S, Duan JA, Qian DW, et al. , 2015a. Content variations of triterpenic acid, nucleoside, nucleobase, and sugar in jujube (Ziziphus jujuba) fruit during ripening. Food Chem, 167: 468-474. 10.1016/j.foodchem.2014.07.013 [DOI] [PubMed] [Google Scholar]
- Guo S, Duan JA, Zhang Y, et al. , 2015b. Contents changes of triterpenic acids, nucleosides, nucleobases, and saccharides in jujube (Ziziphus jujuba) fruit during the drying and steaming process. Molecules, 20(12): 22329-22340. 10.3390/molecules201219852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo XX, Mu JL, Wang J, et al. , 2014. Preparation of soluble dietary fiber of jujube fruit residues with cellulase. J Agric Sci Technol, 16(5): 154-159 (in Chinese). 10.13304/j.nykjdb.2014.211 [DOI] [Google Scholar]
- Halliwell B, 2012. Free radicals and antioxidants: updating a personal view. Nutr Rev, 70(5): 257-265. 10.1111/j.1753-4887.2012.00476.x [DOI] [PubMed] [Google Scholar]
- Han X, Bai BY, Zhou Q, et al. , 2020. Dietary supplementation with polysaccharides from Ziziphus Jujuba cv. Pozao intervenes in immune response via regulating peripheral immunity and intestinal barrier function in cyclophosphamide-induced mice. Food Funct, 11(7): 5992-6006. 10.1039/D0FO00008F [DOI] [PubMed] [Google Scholar]
- He SR, Zhao CB, Zhang JX, et al. , 2020. Botanical and traditional uses and phytochemical, pharmacological, pharmacokinetic, and toxicological characteristics of Ziziphi Spinosae Semen: a review. Evid Based Complement Alternat Med, 2020: 5861821. 10.1155/2020/5861821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YY, Li W, Zhang XY, et al. , 2020. Physicochemical, functional, and microstructural properties of modified insoluble dietary fiber extracted from rose pomace. J Food Sci Technol, 57(4): 1421-1429. 10.1007/s13197-019-04177-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández F, Noguera-Artiaga L, Burló F, et al. , 2016. Physico-chemical, nutritional, and volatile composition and sensory profile of Spanish jujube (Ziziphus jujuba Mill.) fruits. J Sci Food Agric, 96(8): 2682-2691. 10.1002/jsfa.7386 [DOI] [PubMed] [Google Scholar]
- Hong EH, Song JH, Kang KB, et al. , 2015. Anti-influenza activity of betulinic acid from Zizyphus jujuba on influenza A/PR/8 virus. Biomol Ther (Seoul), 23(4): 345-349. 10.4062/biomolther.2015.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YQ, He WL, Hu SD, et al. , 2019. Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids, 51(8): 1153-1165. 10.1007/s00726-019-02751-0 [DOI] [PubMed] [Google Scholar]
- Huang WZ, Wang YJ, Jiang XY, et al. , 2017. Protective effect of flavonoids from Ziziphus jujuba cv. Jinsixiaozao against acetaminophen-induced liver injury by inhibiting oxidative stress and inflammation in mice. Molecules, 22(10): 1781. 10.3390/molecules22101781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong O, Kim HS, 2019. Dietary chokeberry and dried jujube fruit attenuates high-fat and high-fructose diet-induced dyslipidemia and insulin resistance via activation of the IRS-1/PI3K/Akt pathway in C57BL/6 J mice. Nutr Metab, 16: 38. 10.1186/s12986-019-0364-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jéquier E, 1994. Carbohydrates as a source of energy. Am J Clin Nutr, 59(3): 682S-685S. 10.1093/ajcn/59.3.682S [DOI] [PubMed] [Google Scholar]
- Ji XL, Peng Q, Yuan YP, et al. , 2017. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): a review. Food Chem, 227: 349-357. 10.1016/j.foodchem.2017.01.074 [DOI] [PubMed] [Google Scholar]
- Ji XL, Peng Q, Yuan YP, et al. , 2018a. Extraction and physicochemical properties of polysaccharides from Ziziphus Jujuba cv . Muzao by ultrasound-assisted aqueous two-phase extraction. Int J Biol Macromol, 108: 541-549. 10.1016/j.ijbiomac.2017.12.042 [DOI] [PubMed] [Google Scholar]
- Ji XL, Liu F, Peng Q, et al. , 2018b. Purification, structural characterization, and hypolipidemic effects of a neutral polysaccharide from Ziziphus Jujuba cv . Muzao. Food Chem, 245: 1124-1130. 10.1016/j.foodchem.2017.11.058 [DOI] [PubMed] [Google Scholar]
- Ji XL, Zhang F, Zhang R, et al. , 2019a. An acidic polysaccharide from Ziziphus Jujuba cv. Muzao: purification and structural characterization . Food Chem, 274: 494-499. 10.1016/j.foodchem.2018.09.037 [DOI] [PubMed] [Google Scholar]
- Ji XL, Hou CY, Zhang XL, et al. , 2019b. Microbiome-metabolomic analysis of the impact of Zizyphus jujuba cv. Muzao polysaccharides consumption on colorectal cancer mice fecal microbiota and metabolites. Int J Biol Macromol, 131: 1067-1076. 10.1016/j.ijbiomac.2019.03.175 [DOI] [PubMed] [Google Scholar]
- Ji XL, Hou CY, Gao YG, et al. , 2020a. Metagenomic analysis of gut microbiota modulatory effects of jujube (Ziziphus jujuba Mill.) polysaccharides in a colorectal cancer mouse model. Food Funct, 11(1): 163-173. 10.1039/C9FO02171J [DOI] [PubMed] [Google Scholar]
- Ji XL, Yan YZ, Hou CY, et al. , 2020b. Structural characterization of a galacturonic acid-rich polysaccharide from Ziziphus Jujuba cv. Muzao. Int J Biol Macromol, 147: 844-852. 10.1016/j.ijbiomac.2019.09.244 [DOI] [PubMed] [Google Scholar]
- Kaeidi A, Taati M, Hajializadeh Z, et al. , 2015. Aqueous extract of Zizyphus jujuba fruit attenuates glucose induced neurotoxicity in an in vitro model of diabetic neuropathy. Iran J Basic Med Sci, 18(3): 301-306. [PMC free article] [PubMed] [Google Scholar]
- Kammerer D, Claus A, Schieber A, et al. , 2005. A novel process for the recovery of polyphenols from grape (Vitis vinifera L.) pomace. J Food Sci, 70(2): C157-C163. 10.1111/j.1365-2621.2005.tb07077.x [DOI] [Google Scholar]
- Kang KB, Ming G, Kim GJ, et al. , 2015. Jubanines F‒J, cyclopeptide alkaloids from the roots of Ziziphus jujuba . Phytochemistry, 119: 90-95. 10.1016/j.phytochem.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karra S, Sebii H, Yaich H, et al. , 2020. Effect of extraction methods on the physicochemical, structural, functional, and antioxidant properties of the dietary fiber concentrates from male date palm flowers. J Food Biochem, 44(6): e13202. 10.1111/jfbc.13202 [DOI] [PubMed] [Google Scholar]
- Kawabata K, Kitamura K, Irie K, et al. , 2017. Triterpenoids isolated from Ziziphus jujuba enhance glucose uptake activity in skeletal muscle cells. J Nutr Sci Vitaminol, 63(3): 193-199. 10.3177/jnsv.63.193 [DOI] [PubMed] [Google Scholar]
- Kou XH, Chen Q, Li XH, et al. , 2015. Quantitative assessment of bioactive compounds and the antioxidant activity of 15 jujube cultivars. Food Chem, 173: 1037-1044. 10.1016/j.foodchem.2014.10.110 [DOI] [PubMed] [Google Scholar]
- Kuprash DV, Nedospasov SA, 2016. Molecular and cellular mechanisms of inflammation. Biochemistry (Moscow), 81(11): 1237-1239. 10.1134/S0006297916110018 [DOI] [PubMed] [Google Scholar]
- Lai RH, Lai XF, Zhao WX, et al. , 2011. Influence of different extraction methods of tea polyphenols on proportions of catechins. Adv Mater Res, 311-313: 2114-2120. 10.4028/www.scientific.net/AMR.311-313.2114 [DOI] [Google Scholar]
- Lam CTW, Gong AGW, Lam KYC, et al. , 2016. Jujube-containing herbal decoctions induce neuronal differentiation and the expression of anti-oxidant enzymes in cultured PC12 cells. J Ethnopharmacol, 188: 275-283. 10.1016/j.jep.2016.05.015 [DOI] [PubMed] [Google Scholar]
- Li GP, Wu LF, Wei J, et al. , 2013. Two new flavonoids from the fruits of Ziziphus jujuba . Chem Nat Comp, 49(4): 617-620. 10.1007/s10600-013-0692-z [DOI] [Google Scholar]
- Li JW, Fan LP, Ding SD, et al. , 2007. Nutritional composition of five cultivars of Chinese jujube. Food Chem, 103(2): 454-460. 10.1016/j.foodchem.2006.08.016 [DOI] [Google Scholar]
- Li SG, Wang DG, Tian W, et al. , 2008. Characterization and anti-tumor activity of a polysaccharide from Hedysarum polybotrys Hand. -Mazz. Carbohydr Polym, 73(2): 344-350. 10.1016/j.carbpol.2007.12.001 [DOI] [Google Scholar]
- Li WJ, Guo Y, Zhang CY, et al. , 2016. Dietary phytochemicals and cancer chemoprevention: a perspective on oxidative stress, inflammation, and epigenetics. Chem Res Toxicol, 29(12): 2071-2095. 10.1021/acs.chemrestox.6b00413 [DOI] [PubMed] [Google Scholar]
- Li Y, Guo S, Hua TT, et al. , 2018. Comparative pharmacokinetics of triterpenic acids in normal and immunosuppressed rats after oral administration of Jujubae Fructus extract by UPLC-MS/MS. J Chromatogr B, 1077-1078: 13-21. 10.1016/j.jchromb.2018.01.026 [DOI] [PubMed] [Google Scholar]
- Lin XM, Ji XL, Wang M, et al. , 2019. An alkali-extracted polysaccharide from Zizyphus jujuba cv. Muzao: structural characterizations and antioxidant activities . Int J Biol Macromol, 136: 607-615. 10.1016/j.ijbiomac.2019.06.117 [DOI] [PubMed] [Google Scholar]
- Lin XM, Liu KS, Yin S, et al. , 2020. A novel pectic polysaccharide of jujube pomace: structural analysis and intracellular antioxidant activities. Antioxidants, 9(2): 127. 10.3390/antiox9020127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AH, Deng P, 2016. Optimization of extraction condition of dietary fiber from red dates. Food Ferment Sci Technol, 52(4): 58-61 (in Chinese). 10.3969/j.issn.1674-506X.2016.04-013 [DOI] [Google Scholar]
- Mantovani A, Allavena P, Sica A, et al. , 2008. Cancer-related inflammation. Nature, 454(7203): 436-444. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- Matschinsky FM, 2005. Glucokinase, glucose homeostasis, and diabetes mellitus. Curr Diab Rep, 5(3): 171-176. 10.1007/s11892-005-0005-4 [DOI] [PubMed] [Google Scholar]
- Medzhitov R, 2008. Origin and physiological roles of inflammation. Nature, 454(7203): 428-435. 10.1038/nature07201 [DOI] [PubMed] [Google Scholar]
- Medzhitov R, 2010. Inflammation 2010: new adventures of an old flame. Cell, 140(6): 771-776. 10.1016/j.cell.2010.03.006 [DOI] [PubMed] [Google Scholar]
- Mesaik AM, Poh HW, Bin OY, et al. , 2018. In vivo anti-inflammatory, anti-bacterial and anti-diarrhoeal activity of Ziziphus jujuba fruit extract. Open Access Maced J Med Sci, 6(5): 757-766. 10.3889/oamjms.2018.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklavčič Višnjevec A, Baruca Arbeiter A, Hladnik M, et al. , 2019. An integrated characterization of jujube (Ziziphus jujuba Mill.) grown in the north Adriatic region. Food Technol Biotechnol, 57(1): 17-28. 10.17113/ftb.57.01.19.5910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China , 2020. Data and Statistics. http://zdscxx.moa.gov.cn: 8080/nyb/pc/index.jsp (in Chinese). [Google Scholar]
- Nair A, Chauhan P, Saha B, et al. , 2019. Conceptual evolution of cell signaling. Int J Mol Sci, 20(13): 3292. 10.3390/ijms20133292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninave PB, Patil SD, 2019. Antiasthmatic potential of Zizyphus jujuba Mill and Jujuboside B. —possible role in the treatment of asthma. Respir Physiol Neurobiol, 260: 28-36. 10.1016/j.resp.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Ong WY, Wu YJ, Farooqui T, et al. , 2018. Qi Fu Yin—a Ming dynasty prescription for the treatment of dementia. Mol Neurobiol, 55(9): 7389-7400. 10.1007/s12035-018-0908-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciolla C, Fortunato S, Dipierro N, et al. , 2019. Vitamin C in plants: from functions to biofortification. Antioxidants, 8(11): 519. 10.3390/antiox8110519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey MB, Singh AK, Singh JP, et al. , 2008. Three new cyclopeptide alkaloids from Zizyphus species. J Asian Nat Prod Res, 10(8): 709-713. 10.1080/10286020802016024 [DOI] [PubMed] [Google Scholar]
- Periasamy S, Liu CT, Wu WH, et al. , 2015. Dietary Ziziphus jujuba fruit influence on aberrant crypt formation and blood cells in colitis-associated colorectal cancer mice. Asian Pac J Cancer Prev, 16(17): 7561-7566. 10.7314/APJCP.2015.16.17.7561 [DOI] [PubMed] [Google Scholar]
- Periasamy S, Wu WH, Chien SP, et al. , 2020. Dietary Ziziphus jujuba fruit attenuates colitis-associated tumorigenesis: a pivotal role of the NF-κB/IL-6/JAK1/STAT3 pathway. Nutr Cancer, 72(1): 120-132. 10.1080/01635581.2019.1615515 [DOI] [PubMed] [Google Scholar]
- Phan CW, Wang JK, Cheah SC, et al. , 2018. A review on the nucleic acid constituents in mushrooms: nucleobases, nucleosides and nucleotides. Crit Rev Biotechnol, 38(5): 762-777. 10.1080/07388551.2017.1399102 [DOI] [PubMed] [Google Scholar]
- Plastina P, Bonofiglio D, Vizza D, et al. , 2012. Identification of bioactive constituents of Ziziphus jujube fruit extracts exerting antiproliferative and apoptotic effects in human breast cancer cells. J Ethnopharmacol, 140(2): 325-332. 10.1016/j.jep.2012.01.022 [DOI] [PubMed] [Google Scholar]
- Poprac P, Jomova K, Simunkova M, et al. , 2017. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci, 38(7): 592-607. 10.1016/j.tips.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Pu YF, Ding T, Wang WJ, et al. , 2018. Effect of harvest, drying and storage on the bitterness, moisture, sugars, free amino acids and phenolic compounds of jujube fruit (Zizyphus jujuba cv. Junzao). J Sci Food Agric, 98(2): 628-634. 10.1002/jsfa.8507 [DOI] [PubMed] [Google Scholar]
- Rahman E, Momin A, Zhao L, et al. , 2018. Bioactive, nutritional composition, heavy metal and pesticide residue of four Chinese jujube cultivars. Food Sci Biotechnol, 27(2): 323-331. 10.1007/s10068-017-0256-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran JJ, Fan MT, Li YH, et al. , 2013. Optimisation of ultrasonic-assisted extraction of polyphenols from apple peel employing cellulase enzymolysis. Int J Food Sci Technol, 48(5): 910-917. 10.1111/ijfs.12041 [DOI] [Google Scholar]
- Rashwan AK, Karim N, Shishir MRI, et al. , 2020. Jujube fruit: a potential nutritious fruit for the development of functional food products. J Funct Foods, 75: 104205. 10.1016/j.jff.2020.104205 [DOI] [Google Scholar]
- Reche J, Almansa MS, Hernández F, et al. , 2019. Fatty acid profile of peel and pulp of Spanish jujube (Ziziphus jujuba Mill.) fruit. Food Chem, 295: 247-253. 10.1016/j.foodchem.2019.05.147 [DOI] [PubMed] [Google Scholar]
- Rodríguez Villanueva J, Rodríguez Villanueva L, 2017. Experimental and clinical pharmacology of Ziziphus jujuba Mills. Phytother Res, 31(3): 347-365. 10.1002/ptr.5759 [DOI] [PubMed] [Google Scholar]
- Sakna ST, Mocan A, Sultani HN, et al. , 2019. Metabolites profiling of Ziziphus leaf taxa via UHPLC/PDA/ESI-MS in relation to their biological activities. Food Chem, 293: 233-246. 10.1016/j.foodchem.2019.04.097 [DOI] [PubMed] [Google Scholar]
- Senchina DS, Hallam JE, Kohut ML, et al. , 2014. Alkaloids and athlete immune function: caffeine, theophylline, gingerol, ephedrine, and their congeners. Exerc Immunol Rev, 20: 68-93. [PubMed] [Google Scholar]
- Shad AA, Ahmad S, Ullah R, et al. , 2014. Phytochemical and biological activities of four wild medicinal plants. Sci World J, 2014: 857363. 10.1155/2014/857363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi QQ, Zhang Z, Su JJ, et al. , 2018. Comparative analysis of pigments, phenolics, and antioxidant activity of Chinese jujube (Ziziphus jujuba Mill.) during fruit development. Molecules, 23(8): 1917. 10.3390/molecules23081917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Zhao KC, 2017. Treatment of insomnia with traditional Chinese herbal medicine. Int Rev Neurobiol, 135: 97-115. 10.1016/bs.irn.2017.02.006 [DOI] [PubMed] [Google Scholar]
- Son J, Lee SY, 2020. Ursonic acid exerts inhibitory effects on matrix metalloproteinases via ERK signaling pathway. Chem Biol Interact, 315: 108910. 10.1016/j.cbi.2019.108910 [DOI] [PubMed] [Google Scholar]
- Song JX, Bi JF, Chen QQ, et al. , 2019. Assessment of sugar content, fatty acids, free amino acids, and volatile profiles in jujube fruits at different ripening stages. Food Chem, 270: 344-352. 10.1016/j.foodchem.2018.07.102 [DOI] [PubMed] [Google Scholar]
- Song LJ, Zhang L, Xu L, et al. , 2020. Optimized extraction of total triterpenoids from jujube (Ziziphus jujuba Mill.) and comprehensive analysis of triterpenic acids in different cultivars. Plants, 9(4): 412. 10.3390/plants9040412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HY, Li CY, Ni YJ, et al. , 2019. Ultrasonic/microwave-assisted extraction of polysaccharides from Camptotheca acuminata fruits and its antitumor activity. Carbohydr Polym, 206: 557-564. 10.1016/j.carbpol.2018.11.010 [DOI] [PubMed] [Google Scholar]
- Tahergorabi Z, Abedini MR, Mitra M, et al. , 2015. “Ziziphus jujube”: a red fruit with promising anticancer activities. Pharmacogn Rev, 9(18): 99-106. 10.4103/0973-7847.162108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanideh N, Jamshidzadeh A, Ghanbari Saghesloo A, et al. , 2016. Effects of hydroalcoholic extract of Ziziphus jujuba on acetic acid induced ulcerative colitis in male rat (Rattus norvegicus). J Coloproctol, 36(4): 189-195. 10.1016/j.jcol.2016.04.007 [DOI] [Google Scholar]
- Wang BN, Liu LG, Huang QY, et al. , 2020. Quantitative assessment of phenolic acids, flavonoids and antioxidant activities of sixteen jujube cultivars from China. Plant Foods Hum Nutr, 75(2): 154-160. 10.1007/s11130-020-00796-1 [DOI] [PubMed] [Google Scholar]
- Wang LY, Jing N, Liu XR, et al. , 2020. Nurturing and modulating gut microbiota with jujube powder to enhance anti-PD-L1 efficiency against murine colon cancer. J Funct Foods, 64: 103647. 10.1016/j.jff.2019.103647 [DOI] [Google Scholar]
- Wang RR, Ding SH, Zhao DD, et al. , 2016. Effect of dehydration methods on antioxidant activities, phenolic contents, cyclic nucleotides, and volatiles of jujube fruits. Food Sci Biotechnol, 25(1): 137-143. 10.1007/s10068-016-0021-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YG, Xu Y, Ma XQ, et al. , 2018. Extraction, purification, characterization and antioxidant activities of polysaccharides from Zizyphus jujuba cv. Linzexiaozao. Int J Biol Macromol, 118: 2138-2148. 10.1016/j.ijbiomac.2018.07.059 [DOI] [PubMed] [Google Scholar]
- Williams BA, Grant LJ, Gidley MJ, et al. , 2017. Gut fermentation of dietary fibres: physico-chemistry of plant cell walls and implications for health. Int J Mol Sci, 18(10): 2203. 10.3390/ijms18102203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojdyło A, Carbonell-Barrachina ÁA, Legua P, et al. , 2016. Phenolic composition, ascorbic acid content, and antioxidant capacity of Spanish jujube (Ziziphus jujube Mill.) fruits. Food Chem, 201: 307-314. 10.1016/j.foodchem.2016.01.090 [DOI] [PubMed] [Google Scholar]
- Wu GY, 2016. Dietary protein intake and human health. Food Funct, 7(3): 1251-1265. 10.1039/C5FO01530H [DOI] [PubMed] [Google Scholar]
- Wu H, Zhu JX, Diao WC, et al. , 2014. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata). Carbohydr Polym, 113: 314-324. 10.1016/j.carbpol.2014.07.025 [DOI] [PubMed] [Google Scholar]
- Wu LY, Parhofer KG, 2014. Diabetic dyslipidemia. Metabolism, 63(12): 1469-1479. 10.1016/j.metabol.2014.08.010 [DOI] [PubMed] [Google Scholar]
- Xu H, Yuan ZZ, Ma X, et al. , 2018. Triterpenoids with antioxidant activities from Myricaria squamosa . J Asian Nat Prod Res, 20(3): 292-298. 10.1080/10286020.2017.1321636 [DOI] [PubMed] [Google Scholar]
- Xu Y, Xie LH, Xie JH, et al. , 2019. Pelargonidin-3-O-rutinoside as a novel α-glucosidase inhibitor for improving postprandial hyperglycemia. Chem Commun, 55(1): 39-42. 10.1039/C8CC07985D [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Wang MF, Adjei AA, et al. , 1997. Role of nucleosides and nucleotides in the immune system, gut reparation after injury, and brain function. Nutrition, 13(4): 372-374. 10.1016/S0899-9007(96)00376-0 [DOI] [PubMed] [Google Scholar]
- Yan JK, Wu LX, Cai WD, et al. , 2019. Subcritical water extraction-based methods affect the physicochemical and functional properties of soluble dietary fibers from wheat bran. Food Chem, 298: 124987. 10.1016/j.foodchem.2019.124987 [DOI] [PubMed] [Google Scholar]
- Yang HM, Yin ZQ, Zhao MG, et al. , 2018. Pentacyclic triterpenoids from Cyclocarya paliurus and their antioxidant activities in FFA-induced HepG2 steatosis cells. Phytochemistry, 151: 119-127. 10.1016/j.phytochem.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Yen MH, Lee JJ, Yeh CF, et al. , 2014. Yakammaoto inhibited human coxsackievirus B4 (CVB4)-induced airway and renal tubular injuries by preventing viral attachment, internalization, and replication. J Ethnopharmacol, 151(3): 1056-1063. 10.1016/j.jep.2013.11.049 [DOI] [PubMed] [Google Scholar]
- Yoon SR, Jo YJ, Yang SL, et al. , 2009. Sanjoinine A isolated from Semen Zizyphi Spinosi protects against kainic acid-induced convulsions. Arch Pharm Res, 32(11): 1515-1523. 10.1007/s12272-009-2103-3 [DOI] [PubMed] [Google Scholar]
- Yu L, Jiang BP, Luo D, et al. , 2012. Bioactive components in the fruits of Ziziphus jujuba Mill. against the inflammatory irritant action of Euphorbia plants . Phytomedicine, 19(3-4): 239-244. 10.1016/j.phymed.2011.09.071 [DOI] [PubMed] [Google Scholar]
- Yuan JP, Zhao SY, Wang JH, et al. , 2008. Distribution of nucleosides and nucleobases in edible fungi. J Agric Food Chem, 56(3): 809-815. 10.1021/jf0719205 [DOI] [PubMed] [Google Scholar]
- Yuan YH, Gao ZP, Shi YG, 2002. Industrialization of Chinese jujube. J Northwest Sci-Tech Univ Agric For (Nat Sci Ed), 30(S1): 95-98 (in Chinese). 10.3321/j.issn:1671-9387.2002.z1.023 [DOI] [Google Scholar]
- Zhang H, Jiang L, Ye S, et al. , 2010. Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem Toxicol, 48(6): 1461-1465. 10.1016/j.fct.2010.03.011 [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Qu XY, Liu C, et al. , 2019. Optimization of extraction technology and oxidation resistance analysis of alkaloids in jujube. Mol Plant Breed, 17(3): 972-977 (in Chinese). 10.13271/j.mpb.017.000972 [DOI] [Google Scholar]
- Zhao HX, Zhang HS, Yang SF, 2014. Phenolic compounds and its antioxidant activities in ethanolic extracts from seven cultivars of Chinese jujube. Food Sci Hum Well, 3(3-4): 183-190. 10.1016/j.fshw.2014.12.005 [DOI] [Google Scholar]
- Zhao Y, Yang XB, Ren DY, et al. , 2014. Preventive effects of jujube polysaccharides on fructose-induced insulin resistance and dyslipidemia in mice. Food Funct, 5(8): 1771-1778. 10.1039/C3FO60707K [DOI] [PubMed] [Google Scholar]
- Zou M, Chen YL, Sun-Waterhouse D, et al. , 2018. Immunomodulatory acidic polysaccharides from Zizyphus jujuba cv. Huizao: insights into their chemical characteristics and modes of action . Food Chem, 258: 35-42. 10.1016/j.foodchem.2018.03.052 [DOI] [PubMed] [Google Scholar]