Abstract
Purpose
The purpose of this study was to perform a scoping review of published literature reporting on surgical management of tibial cysts which developed after ACLR.
Methods
A scoping review was conducted following the Arksey and O’Malley framework for scoping studies and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) extension for scoping reviews (PRISMA-ScR) guidelines. A search strategy using the terms [“Tibial Cyst” AND “ACL”], [“Pretibial Cyst” AND “ACL”] was applied to the PUBMED database.
Results
Thirty-seven studies published between 1990 and 2019 were a part of this scoping review. Non-absorbable implants for tibial graft fixation were used in 10 studies (comprising a total 21 patients), while bio-absorbable implants were used in 27 studies (comprising a total 115 patients). Incidence of tibial cyst was reported in 3 studies (434 primary ACLRs) from whom 3.9% (n = 17) developed tibial cyst. Tibial cyst development in relation to use of bio-absorbable screws for tibial ACL graft fixation was reported in 16 studies (42.1%). Use of bio-absorbable screws with another factor was found to be related to tibial cyst development in another 1 study (2.6%). Most common symptoms were presence of mass or swelling, pain, tenderness, drainage, instability and effusion.
Conclusion
This scoping review demonstrated that tibial cysts is more frequently related to bioabsorbable screws, however it can also occur due to other causes. Current literature on tibial cyst after ACLR is of low-quality evidence. Future research is required to better understand aetiology, risk factors for cyst formation and the best possible mode of management.
Level of evidence
IV
Supplementary Information
The online version contains supplementary material available at 10.1186/s40634-021-00356-9.
Keywords: ACL, Tibial cyst, Pretibial cyst, Interference screw
Background
Anterior Cruciate Ligament Reconstruction (ACLR) has been associated with significantly improved patient reported outcomes with respect to quality of life, knee symptoms and sports function when compared to non-operative treatment for patients with anterior cruciate ligament (ACL) tears [5]. Development of tibial cyst following ACLR is a rare but known complication of ACLR. To our knowledge Sgaglione was the first to report a tibial cyst related to ACLR [57].
Tibial graft fixation in ACLR was initially attained with staples, screws, washer posts and sutures tied directly to bone. Significant improvements have been witnessed in the make and design of implants for tibial graft fixation. Bio-absorbable screws have been developed and their use has facilitated surgeons to overcome some complications related to non-absorbable implants [50]. Bio-absorbable materials are a popular method of tibial fixation due to advantages like the absence of artefacts on postoperative magnetic resonance imaging (MRI), simpler revision surgery and less graft damage compared to metallic implants [2, 16, 30, 44]. Unfortunately, bio-absorbable screws aren't exempt of complications, and several authors have related them to tibial cyst development after ACLR and ghost screws formation [16, 25, 53].
Furthermore, available literature about surgical treatment of tibial cysts following.
ACLR is scarce. For these reasons, a scoping review, was conducted in order to map the extent, range and quality of literature associated with development of tibial cysts after ACLR, giving an overview that further helps clinicians. A scoping review methodology was selected because this approach is considered to be superior when addressing an exploratory research question [27, 47].
Review
Study selection
A scoping review of the literature was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) extension for scoping reviews (PRISMA-ScR) guidelines [63] and the methodological framework of Arksey and O´Malley [6]. The study protocol was registered with the open science framework study registry prior to commencing data collection – OSF [73] database (reference blinded for review). The five-stage methodological framework in a scoping review of Arksey and O’Malley [6] were followed: as (1) the identification of a research question; (2) identifying the relevant studies; (3) the selection of studies to be included in the review; (4) data extraction from the included studies; and (5) collating, summarizing, and reporting the results of the review.
-
Identification of research questions
The research question was “What is known from the existing literature regarding development and management of tibial cyst after ACLR?”.
-
Identifying Relevant Studies
Studies were identified by applying the search strategy to the PubMed database. The following keywords were included [“Tibial Cyst” AND “Anterior Cruciate Ligament”], [“Pretibial Cyst” AND Anterior Cruciate Ligament”] with automatic mapping to Medical Subject Headings terms. The search was conducted on May 16, 2020 (search date last executed), by 2 independent investigators (XX. and YY) (Table 1). Limits were applied to retrieve English-language, Spanish-Language and Portuguese-Language articles published. Both investigators reviewed the titles and abstracts of all identified records and potentially eligible studies were retrieved for full-text review. Reference lists of these articles were also reviewed, and any further potentially eligible studies were identified.
-
Study selection
All identified studies reporting clinical outcomes of tibial cyst surgery after ACLR were included. The following article types were excluded: non-clinical studies such as cadaveric and animal studies. The senior author resolved any disagreements between investigators regarding whether a study met the eligibility criteria.
Data Extraction
Table 1.
Literature search sequence on Pubmed—Tibial cysts after ACLR (last performed on March 27, 2020)
| 1 | Tibial Cyst ACL | 94 items |
| 2 | Tibial Cyst | 1218 items |
| 3 | Pretibial cyst ACL | 24 items |
| 4 | Pretibial Cyst | 32 items |
The included studies were analysed in details and data from each was recorded in Excel 2013 (Microsoft Corp., Redmond, WA) and then subjected to a stepwise analysis. The recorded data from each study included patients’ demographic and clinical information, imaging findings and peri-operative findings. With demographics, patients’ clinical information consisted of the symptoms at presentation, their duration and their effect on activities of daily living. The imaging findings recorded from the pre-operative MRI were presence of tibial tunnel enlargement and presence of tibial communication with the knee joint. Recorded peri-operative findings included details of surgical technique for managing tibial cyst, status of bio-absorbable screws, and intra operative testing of joint communication with tibial cyst. Findings of tissue sample screening by a microbiologist, and histopathologist were recorded. Complications including failure (defined as recurrence of tibial cyst after surgical excision) were recorded and evaluated.
Collating, summarizing and reporting the results
Due to a small number of published studies and heterogeneity between them, no statistical analyses were performed. Instead, the findings were summarized through a narrative analysis of the included published literature. The risk of bias in included case series was assessed using the Methodological Index for Non-Randomized Studies (MINORS) [60]. Overall quality of evidence for each of the potential risk factors studied was assessed using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) working group criteria [28].
Results
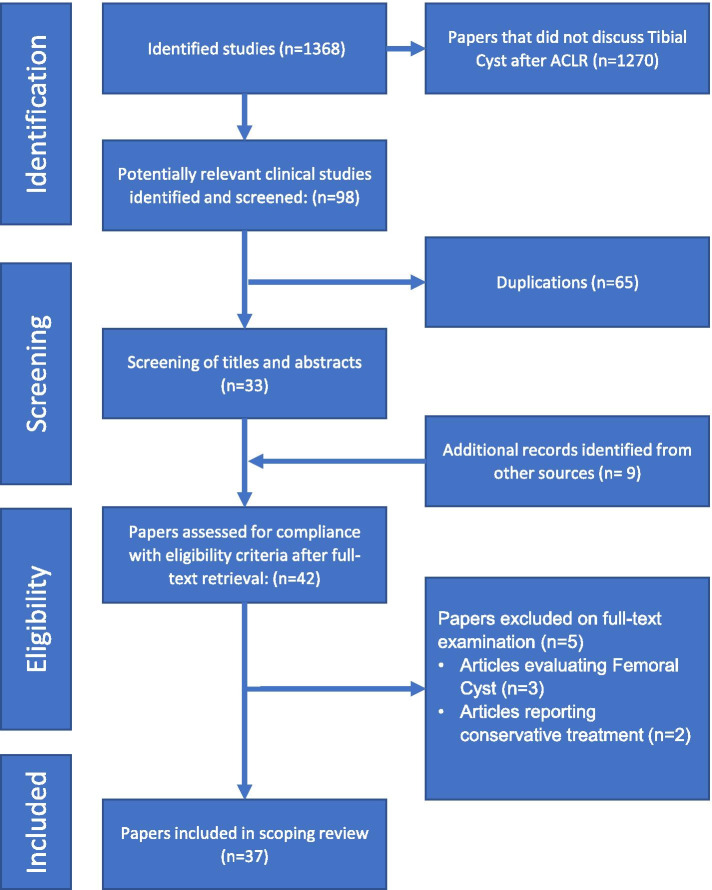
Application of the search strategy identified 1368 records from the searched databases. With title and abstract screening, 98 potentially relevant studies were isolated. 65 studies were removed as they were duplicates, 9 additional records identified from another source (33 studies references review) and 5 papers were excluded on full text examination. Thirty-seven studies were eligible for inclusion in the systematic review. The flow-chart of studies is represented in Fig. 1. The publication dates of the included studies ranged from 1999 to 2019. Using the adjusted Oxford Center For Evidence-Based Medicine criteria [74, 75] for the level of evidence we found that 1 study was Level I [11], 1 Level II [25], 1 Level III [57] and 34 studies were level IV [1, 3, 10, 12–17, 19–21, 23, 29, 33, 36, 42, 43, 46, 49, 51, 53, 54, 56, 58, 59, 62, 64–66, 69, 71, 72] case series or case reports.
Fig. 1.
Flow-chart of identification, screening, and selection of studies
Basic characteristics of included studies
From all the included studies, 136 patients were evaluated with mean age of 31.0 (14 – 57) years. Main symptoms following tibial cyst development after ACLR were mass or swelling in the area of tibial tunnel, pain, instability, and fluid discharge from the earlier surgical incision. Mean time to surgery was 40.2 (0.2 – 240) months. Incidence was calculated in 3 studies comprising a total 434 patients from whom 3.9% (n = 17) developed tibial cyst. Follow-up after the surgery for tibial cysts was reported in 28 studies comprising a total 122 patients. The mean duration of reported follow-up was 37.7 (2 – 70) months. All the data of interest from the included studies have been illustrated in Table 2.
Table 2.
Demographic, radiological and surgical data from included studies
| Authors | Date | Patients | Age | ACL surgery | Fixation method | Bioabsorbable interference screw | |
| Sgaglione NA | 1990 | 1 (NA) | NA | repair +—semitendinous augmentation | NR | ||
| Brettler D | 1995 | 1 (M) | 41 | autograft bptb | metal interference screw | ||
| Victoroff BN | 1995 | 4 (3 M) | 31 (17–47) | allograft achilles | staples / screw washer | ||
| Simonian PT | 1998 | 3 (M) | 29,3 (25–35) |
2 autograft hamstring 1 autograft iliotibial band |
2 staple; 1 in situ |
||
| Martinek V | 1999 | 1 (M) | 32 | autograft bptb | PDLLA interference screw | Sysorb, Sulzer Orthopedics | |
| Deie M | 2000 | 2 (F) | 26,3 (20, 33) | autograft hamstring | 2 staples | ||
| Brager MA | 2002 | 3 (2 M) | 39,7 (36–47) | autograft hamstring |
1 screw washer 2 in situ |
||
| Malhan K | 2002 | 1 (F) | 22 | autograft hamstring | PLLA + β-TCP interference screw | BioLok, Atlantech | |
| Ilahi OA | 2003 | 1 (F) | 26 | autograft bptb | metal interference screw | ||
| Sekiya JK | 2004 | 1 (F) | 16 | autograft hamstring | over a post Ethibond | ||
| Tsuda E | 2006 | 1 (M) | 18 | autograft hamstring | PLLA interference screw + over a post Ethibond | Fixsorb, Depuy Mitek | |
| Busfield BT | 2007 | 2 (F) | 34 (28, 39) |
1 allograft achilles 1 autograft hamstring |
1 PLLA interference screw + screw washer; 1 PLLA interference screw + over a post |
1 PLLA Delta, Arthrex 1 Bioscrew, Arthrex |
|
| Thaunat M | 2007 | 1 (F) | 47 | autograft hamstring | PLLA interference screw | Phusiline, Phusis | |
| Dujardin J | 2008 | 1 (M) | 32 | autograft hamstring | PDLG + CA interference screw | Calaxo, Smith and Nephew | |
| Gaweda K | 2009 | 2 (NA) | NA | autograft hamstring | PLLA interference screw | NR, Arthrex | |
| Umar M | 2009 | 1 (M) | 28 | autograft hamstring | PLLA + β-TCP interference screw | BiLok, Arthocare | |
| Sadat-Ali M | 2010 | 1 (M) | 23 | autograft hamstring | PLLA interference screw | NR, Bionix Implants | |
| Oh HL | 2010 | 1 (F) | 15 | autograft hamstring | PLLA + β-TCP interference screw | Bio-INTRAFIX, Depuy Mitek | |
| Gonzalez-Lomas G | 2011 | 7 (NA) | 39,3 (22–57) |
4 autograft hamstring 3 allograft |
PLLA interference screw | PLLA Delta, Arthrex | |
| Quatman CE | 2011 | 1 (F) | 28 | allograft tibialis anterior | PLLA + HA interference screw + staple | BioRCI, Smith and Nephew | |
| Apostolopoulos A | 2012 | 1 (M) | 26 | autograft hamstring | PLLA interference screw | NR | |
| Bernard JA | 2013 | 3 (2 M) | 28 (25–32) |
2 autograft hamstring 1 allograft tibialis anterior |
2 PDLLA + β-TCP interference screw + PLLA SwiveLock 1 PLLA interference screw |
2 BioComposite, Arthrex 1 Bio-Interference, Arthrex |
|
| Bourke HE | 2013 | 1 (NA) | NA | autograft hamstring | PDLG + CA interference screw | Calaxo, Smith and Nephew | |
| Shen MX | 2013 | 1 (M) | 21 | autograft hamstring | PLLA interference screw | Bio-Interference, Arthrex | |
| Bulisani LEP | 2014 | 1 (M) | 40 | autograft hamstring | PLLA + HA interference screw | NR | |
| Díaz Heredia J | 2014 | 3 (1 M) | 25 (23–27) | autograft hamstring | PLLA + HA interference screw | Biosure, Smith and Nephew | |
| Ramsingh V | 2014 | 14 (9 M) | 27,1 (14–39) |
12 autograft hamstring 1 autograft bptb 1 allograft achilles |
PLLA + β-TCP interference screw | BiLok, Arthocare | |
| Zabala IL | 2014 | 1 (M) | 29 | autograft hamstring | PLLA interference screw | NR | |
| Haragus H | 2015 | 1 (M) | 20 | autograft hamstring | PLLA interference screw | NR | |
| Joshi YV | 2015 | 1 (M) | 27 | autograft bptb | metal interference screw | ||
| Metcalf K | 2015 | 2 (1 M) | 39,5 (25–54) | allograft tibialis anterior | PDLLA + β-TCP interference screw | Bio-INTRAFIX, Depuy Mitek | |
| Alonso B | 2016 | 1 (M) | 17 | autograft bptb | PDLLA + β-TCP interference screw | Megafix C, Karl Storz | |
| Zicaro JP | 2017 | 13 (9 M) | 35,6 (NA) | autograft hamstring |
6 bioabsorbable interference screw 3 bioabsorbable interference screw + staple 4 in situ + Ethibond |
NR | |
| Weiss KS | 2017 | 1 (F) | 20 | autograft hamstring | PDLLA + β-TCP interference screw | ComposiTCP; Biomet | |
| Christodoulidis A | 2018 | 2 (1 M) | 50,5 (49, 52) | autograft hamstring | PLLA interference screw + PLLA cross pin | Bio-Interference, Arthrex | |
| Chevallier R | 2019 | 53 (20 M) | 30,8 (NA) |
44 autograft hamstring 8 autograft bptb 1 autograft iliotibial band |
28 PLLA + HA 10 PLLA + β-TCP 7 PLLA 5 bioabsorbable interference screw 2 PDLG + CA 1 PLGA |
21 BioRCI, Smith and Nephew 7 Biosure, Smith and Nephew 6 Bio-INTRAFIX, Depuy Mitek 3 Milagro, Depuy Synthes 1 TLS, FH orthopedics 7 GTS, Smith and Nephew 5 NR 2 Calaxo, Smith and Nephew 1 CentraLoc, Biomet |
|
| Dockry A | 2019 | 1 (M) | 33 | allograft NR | bioabsorbable interference screw | NR | |
| Authors | Time to diagnosis/surgery | Presentation | Tunnel enlargment | MRI joint communication | Surgery joint communication | ||
| Sgaglione NA | 44 | NR | NR | NR | no | ||
| Brettler D | 22 | mass tenderness | yes | NR | yes | ||
| Victoroff BN | 15,8 (7–29) |
2 mass 2 mass tenderness |
NR | NR |
3 yes 1 NR |
||
| Simonian PT | 46,3 (25–72) | mass tenderness | no |
1 yes 2 NR |
yes | ||
| Martinek V | 8 | mass tenderness | yes | no | NR | ||
| Deie M | 16 (15–17) | Mass | yes | NR | no | ||
| Brager MA | 46,3 (21–76) | mass tenderness |
1 yes 2 NR |
1 yes 2 NR |
1 yes 1 no 1 NR |
||
| Malhan K | 12 | mass tenderness | yes | no | NR | ||
| Ilahi OA | 12 | mass tenderness | NR | NR | yes | ||
| Sekiya JK | 60 | mass tenderness | yes | no | no | ||
| Tsuda E | 24 | mass tenderness | yes | yes | yes | ||
| Busfield BT | 27 (18–36) | mass tenderness | NR | NR | no | ||
| Thaunat M | 60 | mass tenderness | yes | no | NR | ||
| Dujardin J | 8 | effusion | yes | no | no | ||
| Gaweda K | 18 (16–20) |
1 drainage 1 mass tenderness |
NR | NR | NR | ||
| Umar M | 30 | mass tenderness | NR | NR | no | ||
| Sadat-Ali M | 36 | mass tenderness | no | NR | no | ||
| Oh HL | 4 | drainage | NR | no | no | ||
| Gonzalez-Lomas G | 29,1 (24–36) |
4 mass 2 mass tenderness 1 drainage |
yes |
4 yes 3 no |
NR | ||
| Quatman CE | 78 | mass tenderness | yes | yes | no | ||
| Apostolopoulos A | 48 | Mass | yes | NR | no | ||
| Bernard JA | 20,7 (16–24) |
1 mass 2 mass tenderness |
NR | NR | NR | ||
| Bourke HE | 9 | Mass | NR | no | NR | ||
| Shen MX | 24 | mass tenderness | no | no | no | ||
| Bulisani LEP | 36 | Mass | no | yes | yes | ||
| Díaz Heredia J | 0,3 (0,2–0,5) | drainage | no | yes |
2 yes 1 no |
||
| Ramsingh V | 27,8 (11,5–38,5) |
12 mass tenderness 2 mass tenderness effusion |
NR | NR |
2 yes 12 no |
||
| Zabala IL | 24 | mass tenderness | yes | no | no | ||
| Haragus H | 42 | mass tenderness | yes | no | no | ||
| Joshi YV | 240 | instability | no | no | no | ||
| Metcalf K | 22 (8–36) | mass tenderness | no |
1 no 1 NR |
no | ||
| Alonso B | 24 | mass tenderness | NR | NR | no | ||
| Zicaro JP | 29 (NA) | Mass | yes | no | NR | ||
| Weiss KS | 15 | Mass | no | no | no | ||
| Christodoulidis A | 84 (84–84) |
1 mass; 1 mass tenderness |
no | no | no | ||
| Chevallier R | 55,2 (3,1–228) |
22 mass tenderness 31 tenderness |
4 yes 49 NR |
2 yes 51 no |
4 yes 49 no |
||
| Dockry A | 16 | mass tenderness | NR | NR | NR | ||
| Authors | Surgery | Screw degradation | Microbiology | Histology | Follow-up (M) | Recorrence | |
| Sgaglione NA | excision | NR | NR | NR | NR | NR | |
| Brettler D | excision | NR | NR | NR | 18 | no | |
| Victoroff BN |
1 excision + curetage 3 excision + curetage + bone graft |
NR | NR |
1 no 3 yes |
24,8 (22–29) | 1 | |
| Simonian PT |
1 excision + curetage; 2 excision + curetage + bone graft |
NR | NR |
1 no 2 yes |
39,3 (24–70) | no | |
| Martinek V | excision | breakdown | NR | yes | 2 | no | |
| Deie M | excision | NR | NR | yes | 10,5 (9–12) | no | |
| Brager MA |
2 excision + curetage 1 excision + curetage + bone graft |
NR | NR | yes | 30 (6–54) | no | |
| Malhan K | excision + curetage | breakdown | negative | yes | 3 | no | |
| Ilahi OA | excision + curetage + bone graft | intact | NR | yes | 2 | no | |
| Sekiya JK | excision + curetage | NR | NR | no | NR | 1 | |
| Tsuda E | excision + curetage + bone graft | breakdown | negative | yes | 12 | no | |
| Busfield BT | excision + curetage | breakdown | negative | yes | 6 | no | |
| Thaunat M | excision + curetage + bone graft | absorption | NR | yes | 2 | no | |
| Dujardin J | excision + curetage + bone graft | breakdown | negative | yes | 3 | no | |
| Gaweda K | excision + curetage | intact | NR | yes | NR | no | |
| Umar M | excision + curetage | breakdown | NR | yes | NR | no | |
| Sadat-Ali M | excision + curetage | breakdown | NR | yes | 9 | no | |
| Oh HL | excision + curetage | intact | M. fortuitum | no | NR | no | |
| Gonzalez-Lomas G | excision | NR | negative | yes | 5,3 (5–6) | no | |
| Quatman CE | excision + curetage + bone graft | NR | NR | no | NR | no | |
| Apostolopoulos A | excision + curetage + bone graft | absorption | NR | yes | 12 | no | |
| Bernard JA |
2 excision 1 excision + curetage |
breakdown | negative | no | 4 (2–6) | no | |
| Bourke HE | excision | NR | NR | no | NR | 1 | |
| Shen MX | excision | absorption | negative | yes | NR | no | |
| Bulisani LEP | excision + curetage + bone graft | absorption | NR | yes | 6 | no | |
| Díaz Heredia J | excision + curetage | intact | negative | yes | 24 | no | |
| Ramsingh V | excision + curetage |
13 breakdown 11 absorption |
negative | yes | 12 | no | |
| Zabala IL | excision + curetage | breakdown | NR | yes | 3 | no | |
| Haragus H | excision | intact | NR | yes | 3 | no | |
| Joshi YV | excision + curetage + bone graft | NR | NR | yes | 6 | no | |
| Metcalf K | excision + curetage |
1 intact 1 breakdown |
P. Acnes none |
yes | 12 | no | |
| Alonso B | excision + curetage | breakdown | negative | yes | 2 | no | |
| Zicaro JP |
6 excision + curetage 7 excision + curetage + bone graft |
NR | negative | yes | 35 (NR) | 1 | |
| Weiss KS | excision + curetage + bone graft | absorption | S. epidermidis | yes | 4 | no | |
| Christodoulidis A | excision + curetage | absorption | NR | yes | 6, NR | no | |
| Chevallier R | excision + curetage + bone graft |
12 intact 32 breakdown 9 absorption |
negative |
12 no 41 yes |
64,8 (7–146) | 1 | |
| Dockry A | excision + curetage + bone graft | NR | negative | no | NR | no | |
Non-absorbable implants for tibial graft fixation were used in 10 studies (comprising a total 21 patients), while bio-absorbable implants were used in 27 studies (comprising a total 115 patients). Composition of bio-absorbable screws and frequency of development of tibial ACL cysts with their use are described in Table 3.
Table 3.
Method of fixation, interference screw composition and auxiliar fixation frequency
| Fixation method | Frequency |
| NR | 1 (0,7%) |
| None | 3 (2,2%) |
| Screw washer | 1 (0,7%) |
| Screw washer + metal interference screw | 1 (0,7%) |
| Screw washer + staple (removed before cyst development) | 1 (0,7%) |
| Screw washer + 2 staples (removed before cyst development) | 1 (0,7%) |
| Screw washer + poly-L-lactide (PLLA) interference screw | 1 (0,7%) |
| Staple | 2 (1,5%) |
| Staples 2 | 3 (2,2%) |
| Staples 2 (removed before cyst development) | 1 (0,7%) |
| Staple + not reported bioabsorbable interference screw | 3 (2,2%) |
| Staple + poly-L-lactide (PLLA) + hydroxyapatite (HA) interference screw | 1 (0,7%) |
| Ethibond | 4 (2,9%) |
| Over a post Ethibond | 1 (0,7%) |
| Over a post Ethibond + poly-L-lactide (PLLA) interference screw | 1 (0,7%) |
| Over a post + poly-L-lactide (PLLA) interference screw | 1 (0,7%) |
| Bioabsorbable cross pin in PLLA + poly-L-lactide (PLLA) interference screw | 2 (1,5%) |
| PLLA SwiveLock + poly-D,L-lactide (PDLLA) + β-tricalcium phosphate (β-TCP) Interference screw | 2 (1,5%) |
| Metal interference screw | 2 (1,5%) |
| Not reported bioabsorbable interference screw | 12 (8,8%) |
| Poly(lactic-co-glycolic) acid (PLGA) interference screw | 1 (0,7%) |
| Poly-L-lactide (PLLA) + hydroxyapatite (HA) interference screw | 32 (23,5%) |
| Poly-L-lactide (PLLA) + β-tricalcium phosphate (β-TCP) interference screw | 27 (19,9%) |
| Poly-L-lactide (PLLA) interference screw | 23 (16,9%) |
| Poly-D,L-lactide (PDLLA) + β-tricalcium phosphate (β-TCP) interference screw | 4 (2,9%) |
| Poly-D,L-lactide (PDLLA) interference screw | 1 (0,7%) |
| Poly(lactic-co-glycolic) acid (PLGA) interference screw | 1 (0,7%) |
| Poly(D,L-lactideecoglycolide) (PDLG) + calcium carbonate interference screw | 4 (2,9%) |
| Interference screw composition | Frequency |
| NR | 1 (0,7%) |
| None | 17 (12,5%) |
| Metal | 3 (2,2%) |
| Not reported bioabsorbable | 15 (11%) |
| Poly-L-lactide (PLLA) | 28 (20,6%) |
| Poly-L-lactide (PLLA) + hydroxyapatite (HA) | 33 (24,3%) |
| Poly-L-lactide (PLLA) + β-tricalcium phosphate (β-TCP) | 27 (19,9%) |
| Poly-D,L-lactide (PDLLA) | 1 (0,7%) |
| Poly-D,L-lactide (PDLLA) + β-tricalcium phosphate (β-TCP) | 6 (4,4%) |
| Poly(lactic-co-glycolic) acid (PLGA) | 1 (0,7%) |
| Poly(D,L-lactideecoglycolide) (PDLG) + calcium carbonate | 4 (2,9%) |
| Auxiliar fixation | Frequency |
| NR | 1 (0,7%) |
| None | 109 (80,1%) |
| Removed before cyst | 3 (2,2%) |
| Screw washer | 3 (2,2%) |
| Staple | 6 (4,4%) |
| Staples 2 | 3 (2,2%) |
| Ethibond | 4 (2,9%) |
| over a post Ethibond | 2 (1,5%) |
| over a post | 1 (0,7%) |
| Bioabsorbable cross pin in PLLA | 2 (1,5%) |
| PLLA SwiveLock | 2 (1,5%) |
The methodological quality of included case series evaluated by the MINORS tool varied between 5 and 8 indicating a high risk of bias (Additional file 1).
The overall strength of the evidence available in the scoping review using GRADE recommendations (Table 4) was very low.
Table 4.
Quality of evidence of literature on Tibial cyst development after ACLR
| Risk Factor | Risk of Bias | Inconsistency | Indirectness | Imprecision | Grade |
|---|---|---|---|---|---|
| Bioabsorbable screw | likely | unexplained heterogeneity | indirect | imprecision | very low |
| Tibial Comunication | unlikely | unexplained heterogeneity | indirect | imprecision | very low |
| Graft Type | unlikely | unexplained heterogeneity | indirect | imprecision | very low |
| Infection | unlikely | unexplained heterogeneity | indirect | imprecision | very low |
Tibial cyst development
Tibial cyst development in relation to use of bio-absorbable screws for tibial ACL graft fixation was reported in 16 studies (42.1%). Use of bio-absorbable screws and reaction to suture material was found to be related to tibial cyst development in one study (2.6%) [64]. Development of tibial cyst was also related to communication between the tibial tunnel and knee joint in 8 studies (21.1%), other causes were appointed in 9 articles (21.1%): increased synovial fluid production [13], tendon necrosis [19], suture fragments reaction [56], allograft tendon [10], graft micro-motion [36], infection [46, 49, 69] and multifactorial aetiology [72]. Also, 3 studies did not provide any information on the reason for development of tibial cysts.
Imaging findings
Tibial tunnel enlargement was assessed in 25 studies comprising of 53 patients. Thirty-eight (71.7%) of them were found to have ACL tibial tunnel enlargement in either pre-operative x-ray or MRI scan done before the surgery for tibial cyst.
Communication of the ACL tibial tunnel with the knee joint was evaluated in preoperative MRI scans in 23 studies (comprising a total 91 patients).
Communication could be identified in 14 patients and was not present in 85.4% (n = 80) patients.
Surgical findings
Surgical procedure technique was reported in 37 articles (comprising a total 136 patients) in 55.9% (n = 76) of them, cyst excision was associated with curettage and bone (allo or auto) grafting. Also, in 12,5% (n = 17) isolated cyst excision was performed and in 31.6% (n = 43) curettage and excision were performed.
Screw absorption status at time of surgery was reported in 24 articles comprising a total 97 patients, 21.6% (n = 21) of them reported an intact screw implant, 60.8% (n = 59) presented a partially resorbed screw and in 17.9% (n = 17) screw was completely resorbed at the time of tibial cyst surgery.
In 90% of patients autograft was used (n = 122, 106 hamstring, 14 patellar tendon, 2 iliotibial band). The remaining used allograft (n = 14, 6 Achilles tendon, 4 tibialis anterior tendon, 4 NR).
Tissue processing
Samples from the cyst were sent for processing either to the microbiologist and/or to the histopathologist. Presence of infection was reported in 3 patients from 16 studies (comprising a total 102 patients) in which the tissue sample was sent to the microbiologist for evaluation. Organism isolated in these 3 patients was different in each. Staphylococcus epidermidis, Propionibacterium acnes and Mycobacterium fortuitum were the organisms isolated in the three patients.
Tissue sample was sent for analyses to a histopathologist in 29 studies, comprising a total 112 patients. Foreign body reaction was found to be present in 10 patients (9%).
Complications
The only reported complications of Tibial cyst excision after ACLR were recurrences of tibial cyst after surgical management reported in 4 patients in 4 different studies.
Discussion
The most important finding of this study is that tibial cyst in ACLR, is more frequently related to bio-absorbable implants, however it also has been related to other causes.
Clinical presentation and aetiology
Our scope identified tibial cysts occurring with several types of fixation methods, screw composition and auxiliar fixation methods as described in Table 3. Typically, tibial cyst after ACLR presents with mass or tenderness over the distal tibial aperture within 40.2 months after the primary procedure, although immediate or late-term presentations have also been reported.
This scoping review reveals that tibial cyst development after ACLR is a rather uncommon condition. Incidence of tibial cysts was reported by Ramsingh et al. being up to 5% at 2–3 years [53]. Overall in this review, incidence could be calculated in 3 articles totalling a total 434 patients, 3.9% of them (n = 17) developed tibial cyst after ACLR.
Bioabsorbable implants were used in 27 studies and non-absorbable implants for tibial graft fixation were used in 10 studies. The biggest frequencies of tibial cysts were associated to bioabsorbable screws – 23.5% were poly(L-lactic) acid (PLLA) + hydroxyapatite (HA), 19.9% were PLLA + B-tricalcium phosphate (B-TCP) and 16.9% were PLLA interference screws (Table 3).
Tibial cyst formation has been linked to several causes, such has foreign body reaction [53], leakage of joint fluid through the tunnel [62], intraosseous graft necrosis with incomplete graft incorporation [66] and graft micro-motion [59, 64, 66], among other causes. Development of tibial ACL cysts has also been controversially linked to the tibial graft fixation methods. [26, 59, 62, 64, 66]. In our scoping review, almost half (42.1%) of the studies related tibial cyst development to the use of bio-absorbable implants.
Bio-absorbable implants
Bio-absorbable implants were developed in order to address the limitations with the use of non-absorbable implant. Some of the concerns with the use of non-absorbable implants include screw breakage, artefact in MRI, and hardware interference in ACL Revision and subsequent need for hardware removal [44]. The natural history of the bio-absorbable implant is that it will be absorbed and replaced by bone in the tibial tunnel, however this isn´t consistently seen in vivo [7, 52, 67]. Through our review we found complete absorption of the screw evident in only 17 (17.9%) patients. Others either remain partially resorbed or un-resorbed. Also, though bio-absorbable address some of the limitations encountered with the use of non-bioabsorbable screws, their use is not without complications. Complications in ACLR, [38] related to the use of bio-absorbable tibial ACL screws include foreign body reaction [26], breakdown [64], migration and tibial cyst formation.
Degradation of bioabsorbable materials occurs over five stages: hydration, depolymerization, loss of mass integrity, absorption and elimination [52]. During hydrolysis, the screw may release acid products (resultant from screw composition degradation) harmful to surrounding tissues. As so, different materials result in different degradation products, with different effects on surrounding tissues, and different timings of degradation which may lead to fluid collection on the bone tunnel and progress to tibial cysts [68, 70].
Bone tunnel fluid collections are common in ACLR, however not all fluid collections in the bone tunnel mature into tibial cysts [67]. Moreover, fluid collection can resolve [55]. Chevallier et al. present the biggest series of reported tibial cysts after ACLR in a retrospective clinical study that included 53 patients with an average 4.6 years (+ -3.1 months) after primary ACLR. The authors found that bio-absorbable interference screws absorption can be symptomatic independent of screw composition and correlated tibial cysts to bio-absorbable screw absorption [16]. Unfortunately, the authors didn´t provide individual results database.
However, some prospective imaging studies following up bio-absorbable implants fail to report on tibial cysts. Tecklenburg et al. despite a short follow-up of 24 months after ACLR, reported no inflammatory response in the tibial tunnels in a prospective imaging study of patients with bio-absorbable and allograft screws [61]. Furthermore, Barber et al. in a long-term study of bio-absorbable screws degradation, demonstrated no tibial cysts and complete degradation with no screw remnant at 3 years after BPTB (Bone patella tendon bone) graft ACLR in 14 patients [8]. Also, Jonhston et al. in a computed tomography study of 65 patients after ACLR with bioabsorbable screw showed no tunnel enlargement, osteolysis or reported tibial cysts at long term [35]. Thus, other causes may also be related to tibial cyst development.
Non-absorbable implants and other tibial cyst causes
Tibial cysts development was already described in early ALCR articles with non-absorbable methods of fixation. Our scoping review included 10 articles in which nonabsorbable implants were used for graft fixation. These authors related tibial cysts with several causes such as drainage from the joint through the tibial tunnel, which could be caused by a tunnel with difference in diameter in relation to the graft, eccentric positioning of the tendon in the bone tunnel, intraosseous tendon necrosis during graft incorporation [19], incomplete allograft incorporation [15, 33, 59, 66], graft micro-motion [36, 59, 64, 66], synovitis [13] and foreign body reaction due to non-absorbable suture [56].
Victoroff et al. and Simonian et al. described tibial cyst after ACLR with non-absorbable implants, the authors associated incomplete graft tissue incorporation in the bone tunnel to tibial cysts. Accordingly, graft necrosis led to synovialization that allowed synovial fluid to be transmitted through the tibial tunnel [59, 66]. As so, hydrostatic pressure within the knee joint would drive synovial fluid allowing accumulation and development of tibial cyst [41, 64, 66].
Furthermore, prospective imaging studies have failed to show difference in tibial cyst formation between bio-absorbable and non-absorbable fixation implants.
In a systematic review by Debieux et al. [18] on bio-absorbable versus metallic screws for graft fixation in anterior cruciate ligament reconstruction, the authors chose to include 12 randomised controlled trial published between 1995 and 2015 [4, 9, 22, 24, 31, 32, 34, 37, 39, 40, 45, 48]. Of the included studies only Arama et al. reported tibial cyst formation, and according to the authors there were no differences between bio-absorbable (4 of 17 pts PLLA-HA) and non-bioabsorbable (3 of 19 pts Titanium) groups in cyst formation or graft integration [4].
Surgical preference
In our scoping review surgical resection and bone grafting was the most preferred surgical approach in 84 patients (61.76%). Tibial cyst recurrence was reported in only 4 patients [11, 56, 66, 72].
Communicating vs non-communicating tibial cyst
Distinguishing between communicating and non-communicating cysts might be helpful in further understanding the cause of tibial cyst development as described by Zicaro [72]. Communication between joint and tibial tunnel is in theory always possible after ACLR procedure. Depending on the amount of communication, hydrostatic pressure in the tibial tunnel may lead to tibial cyst formation at early, medium or long-term [66]. Thus more than one factor may be responsible for formation of tibial ACL cysts as pointed out by Zicaro [72] and other authors.
This review identified when using bio-absorbable implants (28 articles), 19 (67.8%) articles evaluated tibial communication with the joint with MRI and communication was found in 12 (13.6%) patients. During surgical procedure 19 (67.8%) articles evaluated tibial communication with the joint communication – it was found in 10 (11.2%) patients. However, probing the tibial tunnel with an arthroscopic probe may not be enough to rule out tibial tunnel communication. Noteworthy, in our review only one article performed a fistulogram with radiographic contrast dye in order to confirm communication of tibial tunnel with the joint [66].
Histopathology
In our scope we found 10 patients with histopathology report of foreign body reaction, overall, we encountered great variability among the reports (Table 2).
Study limitations and strengths
The limitation of this of this scoping review is the inclusion of mostly level IV studies. However, it is worthy to include them as the incidence of occurrence and reporting of ACL tibial cyst is low. Thus, every piece of information will contribute to better understanding of incidence, natural history, pathology, and best possible management of tibial cysts after ACLR.
The strength of this scoping review is that the authors have managed to create an up-to-date evidence-based resource on tibial cysts after ACLR. Though the level of evidence is low, all the evidence consolidated will certainly help the authors of future studies to better understand the patient characteristics, preoperative imaging findings, surgical findings and biopsy related to the tibial cysts after ACLR. The resource will also facilitate clinicians who encounter this complication to be equipped with evidence-based knowledge related to tibial cysts after ACLR.
Conclusions
In our understanding, the major finding of this scope is that tibial cyst in ACLR, is more frequently related to bio-absorbable implants, however it also has been related to other causes. The natural history behind the development of these cysts and their best possible management is still controversial. More standardised reporting on patients who develop tibial cysts is needed to further add to the existing knowledge and understanding related to the tibial cysts after ACLR in the published literature.
Supplementary Information
Additional file 1. Quality assessment of included articles using the Methodological Index for Non-Randomized Studies (MINORS).
Acknowledgements
No acknowledgments
Abbreviations
- ALCR
Anterior Cruciate Ligament Reconstruction
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- PRISMA-ScR
PRISMA extension for scoping reviews
- ACL
Anterior cruciate ligament
- MRI
Magnetic resonance imaging
- MINORS
Methodological Index for Non-Randomized Studies
- GRADE
Grading of Recommendations Assessment, Development and Evaluation)
- PLLA
Poly(L-lactic) acid
- HA
Hydroxyapatite
- B-TCP
B-tricalcium phosphate
Authors’ contributions
Each authors’ contributions to the manuscript is detailed using the criteria below: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND Drafting the work or revising it critically for important intellectual content; AND Final approval of the version to be published; AND Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
No funding sources.
Declarations
Competing interests
One or more of the authors has declared the following potential conflict of interest or source of funding: BSC is a paid consultant, receives royalties and research support, and has made presentations for Arthrex. NCB is a paid consultant and has made presentations for Arthrex.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alonso B, Sobrón FB, Vidal C, Vaquero J, Alonso B, Sobrón FB, Vidal C, Vaquero J. Seudoquiste pretibial tras la reconstrucción del ligamento cruzado anterior con tornillo biocomposite. Acta Ortopédica Mex. 2016;30:150–153. [PubMed] [Google Scholar]
- 2.Ambrose CG, Clanton TO. Bioabsorbable implants: review of clinical experience in orthopedic surgery. Ann Biomed Eng. 2004;32:171–177. doi: 10.1023/B:ABME.0000007802.59936.fc. [DOI] [PubMed] [Google Scholar]
- 3.Apostolopoulos A, Nikolopoulos D, Polyzois I, Liarokapis S, Rossas C, Michos I. Pretibial cyst formation after anterior cruciate ligament reconstruction with poly-L acid screw fixation: a case report presentation and review of the literature. J Surg Orthop Adv. 2012;21:151–156. doi: 10.3113/JSOA.2012.0151. [DOI] [PubMed] [Google Scholar]
- 4.Arama Y, Salmon LJ, Sri-Ram K, Linklater J, Roe JP, Pinczewski LA. Bioabsorbable Versus Titanium Screws in Anterior Cruciate Ligament Reconstruction Using Hamstring Autograft: A Prospective, Blinded, Randomized Controlled Trial With 5-Year Follow-up. Am J Sports Med. 2015;43:1893–1901. doi: 10.1177/0363546515588926. [DOI] [PubMed] [Google Scholar]
- 5.Ardern CL, Kvist J (2016) What is the evidence to support a psychological component to rehabilitation programs after anterior cruciate ligament reconstruction?: Curr Orthop Pract. 27:263–268
- 6.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 7.Bach FD, Carlier RY, Elis JB, Mompoint DM, Feydy A, Judet O, Beaufils P, Vallée C. Anterior cruciate ligament reconstruction with bioabsorbable polyglycolic acid interference screws: MR imaging follow-up. Radiology. 2002;225:541–550. doi: 10.1148/radiol.2252010357. [DOI] [PubMed] [Google Scholar]
- 8.Barber FA, Dockery WD, Hrnack SA. Long-term degradation of a poly-lactide co-glycolide/β-tricalcium phosphate biocomposite interference screw. Arthrosc J Arthrosc Relat Surg. 2011;27:637–643. doi: 10.1016/j.arthro.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 9.Benedetto KP, Fellinger M, Lim TE, Passler JM, Schoen JL, Willems WJ. A new bioabsorbable interference screw: preliminary results of a prospective, multicenter, randomized clinical trial. Arthrosc J Arthrosc Relat Surg. 2000;16:41–48. doi: 10.1016/S0749-8063(00)90126-9. [DOI] [PubMed] [Google Scholar]
- 10.Bernard JA, Riguad J, Tan E, Naziri Q, Zikria B. Sterile pretibial cyst formation following anterior cruciate ligament reconstruction with a bioabsorbable screw. J Long Term Eff Med Implants. 2013;23:61–65. doi: 10.1615/JLongTermEffMedImplants.2013007846. [DOI] [PubMed] [Google Scholar]
- 11.Bourke HE, Salmon LJ, Waller A, Winalski CS, Williams HA, Linklater JM, Vasanji A, Roe JP, Pinczewski LA. Randomized controlled trial of osteoconductive fixation screws for anterior cruciate ligament reconstruction: a comparison of the Calaxo and Milagro screws. Arthrosc J Arthrosc Relat Surg. 2013;29:74–82. doi: 10.1016/j.arthro.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Brager MA, Traina SM, Parker AW. Pretibial cyst following anterior cruciate ligament reconstruction using hamstring autografts. Orthopedics. 2002;25:79–82. doi: 10.3928/0147-7447-20020101-22. [DOI] [PubMed] [Google Scholar]
- 13.Brettler D, Soudry M. Tibial bone plug resorption with extra-articular cyst: a rare complication of anterior cruciate ligament reconstruction. Arthrosc J Arthrosc Relat Surg. 1995;11:478–481. doi: 10.1016/0749-8063(95)90204-X. [DOI] [PubMed] [Google Scholar]
- 14.Bulisani LEP, Bulisani E. Pre-tibial synovial cyst after reconstruction of the anterior cruciate ligament: case report. Rev Bras Ortop Engl Ed Elsevier BV. 2014;49:671–674. doi: 10.1016/j.rbo.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busfield BT, Anderson LJ. Sterile Pretibial Abscess After Anterior Cruciate Reconstruction From Bioabsorbable Interference Screws: A Report of 2 Cases. Arthroscopy Elsevier. 2007;23:911.e1–911.e4. doi: 10.1016/j.arthro.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Chevallier R, Klouche S, Gerometta A, Bohu Y, Herman S, Lefevre N. Bioabsorbable screws, whatever the composition, can result in symptomatic intra-osseous tibial tunnel cysts after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27:76–85. doi: 10.1007/s00167-018-5037-9. [DOI] [PubMed] [Google Scholar]
- 17.Christodoulidis A, Barbareschi M, Molinari M. A Delayed Formation of Pretibial Pseudocyst After Anterior Cruciate Reconstruction from Bioabsorbable Interference Screws: A Report of Two Cases and Short Review of the Literature. J Long Term Eff Med Implants. 2018;28:215–221. doi: 10.1615/JLongTermEffMedImplants.2018026903. [DOI] [PubMed] [Google Scholar]
- 18.Debieux P, Franciozi CES, Lenza M, Tamaoki MJ, Magnussen RA, Faloppa F, Belloti JC (2016) Bioabsorbable versus metallic interference screws for graft fixation in anterior cruciate ligament reconstruction. Cochrane Database Syst Rev 7:CD009772 [DOI] [PMC free article] [PubMed]
- 19.Deie M, Sumen Y, Ochi M, Murakami Y, Fujimoto E, Ikuta Y. Pretibial cyst formation after anterior cruciate ligament reconstruction using auto hamstring grafts: two case reports in a prospective study of 89 cases. Magn Reson Imaging. 2000;18:973–977. doi: 10.1016/S0730-725X(00)00207-1. [DOI] [PubMed] [Google Scholar]
- 20.Díaz Heredia J, Ruiz Iban MA, Cuéllar Gutiérrez R, Ruiz Diaz R, Martinez C, del Val I, Turrion Merino L. Early postoperative transtibial articular fistula formation after anterior cruciate ligament reconstruction: a review of three cases. Arch Orthop Trauma Surg. 2014;134:829–834. doi: 10.1007/s00402-014-1988-6. [DOI] [PubMed] [Google Scholar]
- 21.Dockry A, Magnussen RA, Baria MR. Tibial Tunnel Cyst After Anterior Cruciate Ligament Reconstruction. Am J Phys Med Rehabil. 2020;99:e101–e102. doi: 10.1097/PHM.0000000000001336. [DOI] [PubMed] [Google Scholar]
- 22.Drogset JO, Grøntvedt T, Tegnander A. Endoscopic reconstruction of the anterior cruciate ligament using bone-patellar tendon-bone grafts fixed with bioabsorbable or metal interference screws: a prospective randomized study of the clinical outcome. Am J Sports Med. 2005;33:1160–1165. doi: 10.1177/0363546504272264. [DOI] [PubMed] [Google Scholar]
- 23.Dujardin J, Vandenneucker H, Bellemans J. Tibial cyst and intra-articular granuloma formation after anterior cruciate ligament reconstruction using polylactide carbonate osteoconductive interference screws. Arthrosc J Arthrosc Relat Surg. 2008;24:238–242. doi: 10.1016/j.arthro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Fink C, Benedetto KP, Hackl W, Hoser C, Freund MC, Rieger M. Bioabsorbable polyglyconate interference screw fixation in anterior cruciate ligament reconstruction: a prospective computed tomography-controlled study. Arthrosc J Arthrosc Relat Surg. 2000;16:491–498. doi: 10.1053/jars.2000.4633. [DOI] [PubMed] [Google Scholar]
- 25.Gaweda K, Walawski J, Wegłowski R, Drelich M, Mazurkiewicz T. Early results of one-stage knee extensor realignment and autologous osteochondral grafting. Int Orthop. 2006;30:39–42. doi: 10.1007/s00264-005-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez-Lomas G, Cassilly RT, Remotti F, Levine WN. Is the etiology of pretibial cyst formation after absorbable interference screw use related to a foreign body reaction? Clin Orthop. 2011;469:1082–1088. doi: 10.1007/s11999-010-1580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf Libr J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 28.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haragus H, Lăzureanu VE, Prejbeanu R, Mioc L, Boscu A, Radu DA, Babeș V. Painful Pretibial Cyst with Bioabsorbable Poly-L-lactic Acid Screw Migration 4 Years after Anterior Cruciate Ligament Reconstruction. REVCHIM. 2015;66:1655–1658. [Google Scholar]
- 30.Harilainen A, Sandelin J. A prospective comparison of 3 hamstring ACL fixation devices–Rigidfix, BioScrew, and Intrafix–randomized into 4 groups with 2 years of follow-up. Am J Sports Med. 2009;37:699–706. doi: 10.1177/0363546508328109. [DOI] [PubMed] [Google Scholar]
- 31.Hegde AS, Rai DK, Kannampilly AJ (2014) A Comparison of Functional Outcomes After Metallic and Bioabsorbable Interference Screw Fixations in Arthroscopic ACL Reconstructions. J Clin Diagn Res JCDR 8:LC01–LC03 [DOI] [PMC free article] [PubMed]
- 32.Hofmann GO, Wagner FD, Beickert R, Gonschorek O, Bühren V. Anterior Cruciate Ligament Reconstruction Using Patellar Tendon Autograft and Bioresorbable Interference Screws. Eur J Trauma. 2001;27:241–249. doi: 10.1007/s00068-001-1089-4. [DOI] [Google Scholar]
- 33.Ilahi OA, Younas SA, Sahni IK. Pretibial cyst formation after arthroscopic anterior cruciate ligament reconstruction. Arthrosc J Arthrosc Relat Surg. 2003;19:1–3. doi: 10.1016/S0749-8063(03)70040-1. [DOI] [PubMed] [Google Scholar]
- 34.Järvelä T, Moisala A-S, Paakkala T, Paakkala A. Tunnel enlargement after double-bundle anterior cruciate ligament reconstruction: a prospective, randomized study. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 2008;24:1349–1357. doi: 10.1016/j.arthro.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Johnston M, Morse A, Arrington J, Pliner M, Gasser S. Resorption and remodeling of hydroxyapatite-poly-L-lactic acid composite anterior cruciate ligament interference screws. Arthrosc J Arthrosc Relat Surg Off Publ Arthrosc Assoc N Am Int Arthrosc Assoc. 2011;27:1671–1678. doi: 10.1016/j.arthro.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 36.Joshi YV, Bhaskar D, Phaltankar PM, Charalambous CP. Tibial Tunnel Cyst Formation after Anterior Cruciate Ligament Reconstruction Using a Non-Bioabsorbable Interference Screw. Knee Surg Relat Res. 2015;27:269–273. doi: 10.5792/ksrr.2015.27.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaeding C, Farr J, Kavanaugh T, Pedroza A. A prospective randomized comparison of bioabsorbable and titanium anterior cruciate ligament interference screws. Arthrosc J Arthrosc Relat Surg. 2005;21:147–151. doi: 10.1016/j.arthro.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Konan S, Haddad FS. A clinical review of bioabsorbable interference screws and their adverse effects in anterior cruciate ligament reconstruction surgery. Knee. 2009;16:6–13. doi: 10.1016/j.knee.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Kotani A, Ishii Y. Reconstruction of the anterior cruciate ligament using poly-L-lactide interference screws or titanium screws: a comparative study. Knee. 2001;8:311–315. doi: 10.1016/S0968-0160(01)00087-4. [DOI] [PubMed] [Google Scholar]
- 40.Laxdal G, Kartus J, Eriksson BI, Faxén E, Sernert N, Karlsson J. Biodegradable and metallic interference screws in anterior cruciate ligament reconstruction surgery using hamstring tendon grafts: prospective randomized study of radiographic results and clinical outcome. Am J Sports Med. 2006;34:1574–1580. doi: 10.1177/0363546506288014. [DOI] [PubMed] [Google Scholar]
- 41.Linn RM, Fischer DA, Smith JP, Burstein DB, Quick DC. Achilles tendon allograft reconstruction of the anterior cruciate ligament-deficient knee. Am J Sports Med. 1993;21:825–831. doi: 10.1177/036354659302100611. [DOI] [PubMed] [Google Scholar]
- 42.Malhan K, Kumar A, Rees D. Tibial cyst formation after anterior cruciate ligament reconstruction using a new bioabsorbable screw. Knee. 2002;9:73–75. doi: 10.1016/S0968-0160(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 43.Martinek V, Friederich NF. Tibial and Pretibial Cyst Formation After Anterior Cruciate Ligament Reconstruction With Bioabsorbable Interference Screw Fixation. Arthroscopy Elsevier. 1999;15:317–320. doi: 10.1016/S0749-8063(99)70042-3. [DOI] [PubMed] [Google Scholar]
- 44.Mascarenhas R, Saltzman BM, Sayegh ET, Verma NN, Cole BJ, Bush-Joseph C, Bach BR. Bioabsorbable versus metallic interference screws in anterior cruciate ligament reconstruction: a systematic review of overlapping meta-analyses. Arthrosc J Arthrosc Relat Surg. 2015;31:561–568. doi: 10.1016/j.arthro.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 45.McGuire DA, Barber FA, Elrod BF, Paulos LE. Bioabsorbable Interference Screws for Graft Fixation in Anterior Cruciate Ligament Reconstruction. Arthrosc J Arthrosc Relat Surg. 1999;15:463–473. doi: 10.1053/ar.1999.v15.015046001. [DOI] [PubMed] [Google Scholar]
- 46.Metcalf K, Ko J-WK, Quilici S, Barnes P, Crawford DC. Differentiating Occult Propionibacterium acnes Infection From Aseptic “Biologic” Interference Screw Hydrolysis After Anterior Cruciate Ligament Reconstruction: Introducing a Novel Culture Protocol for Detecting Low-Virulence Organisms. Orthop J Sports Med. 2015;3:2325967115611872. doi: 10.1177/2325967115611872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Myers P, Logan M, Stokes A, Boyd K, Watts M. Bioabsorbable versus titanium interference screws with hamstring autograft in anterior cruciate ligament reconstruction: a prospective randomized trial with 2-year follow-up. Arthrosc J Arthrosc Relat Surg. 2008;24:817–823. doi: 10.1016/j.arthro.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Oh HL, Chen DB, Seeto BG, Macdessi SJ. Mycobacterium fortuitum infection after anterior cruciate ligament reconstruction using a polylactic acid bioabsorbable screw: Case report. Knee. 2010;17:176–178. doi: 10.1016/j.knee.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Papoutsidakis A, Drosos GI, Koukou OI, Piskopakis N, Verettas D-A. Peroneal nerve damage by bicortical tibial screw in ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2010;18:794–796. doi: 10.1007/s00167-009-0980-0. [DOI] [PubMed] [Google Scholar]
- 51.Quatman CE, Paterno MV, Wordeman SC, Kaeding CC. Longitudinal Anterior Knee Laxity Related to Substantial Tibial Tunnel Enlargement after Anterior Cruciate Ligament Revision. Arthrosc J Arthrosc Relat Surg. 2011;27:1160–1163. doi: 10.1016/j.arthro.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radford MJ, Noakes J, Read J, Wood DG. The natural history of a bioabsorbable interference screw used for anterior cruciate ligament reconstruction with a 4-strand hamstring technique. Arthrosc J Arthrosc Relat Surg. 2005;21:707–710. doi: 10.1016/j.arthro.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 53.Ramsingh V, Prasad N, Lewis M. Pre-tibial reaction to biointerference screw in anterior cruciate ligament reconstruction. Knee. 2014;21:91–94. doi: 10.1016/j.knee.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 54.Sadat-Ali M, Azzam Q, Bluwi M, Al-Umran AS. Case report: Fibroxanthoma: a complication of a biodegradable screw. Clin Orthop. 2010;468:2284–2287. doi: 10.1007/s11999-009-1170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanders TG, Tall MA, Mulloy JP, Leis HT. Fluid collections in the osseous tunnel during the first year after anterior cruciate ligament repair using an autologous hamstring graft: natural history and clinical correlation. J Comput Assist Tomogr. 2002;26:617–621. doi: 10.1097/00004728-200207000-00025. [DOI] [PubMed] [Google Scholar]
- 56.Sekiya JK, Elkousy HA, Fu FH. Recurrent pretibial ganglion cyst formation over 5 years after anterior cruciate ligament reconstruction. Arthrosc J Arthrosc Relat Surg. 2004;20:317–321. doi: 10.1016/j.arthro.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 57.Sgaglione NA, Warren RF, Wickiewicz TL, Gold DA, Panariello RA. Primary repair with semitendinosus tendon augmentation of acute anterior cruciate ligament injuries. Am J Sports Med. 1990;18:64–73. doi: 10.1177/036354659001800111. [DOI] [PubMed] [Google Scholar]
- 58.Shen MX, Sathappan SS. Painful pretibial pseudocyst at bioabsorbable interference screw aperture two years after anterior cruciate ligament reconstruction. Singapore Med J. 2013;54:e211–214. doi: 10.11622/smedj.2013195. [DOI] [PubMed] [Google Scholar]
- 59.Simonian PT, Wickiewicz TL, O’Brien SJ, Dines JS, Schatz JA, Warren RF. Pretibial cyst formation after anterior cruciate ligament surgery with soft tissue autografts. Arthrosc J Arthrosc Relat Surg. 1998;14:215–220. doi: 10.1016/S0749-8063(98)70044-1. [DOI] [PubMed] [Google Scholar]
- 60.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 61.Tecklenburg K, Burkart P, Hoser C, Rieger M, Fink C. Prospective evaluation of patellar tendon graft fixation in anterior cruciate ligament reconstruction comparing composite bioabsorbable and allograft interference screws. Arthrosc J Arthrosc Relat Surg. 2006;22:993–999. doi: 10.1016/j.arthro.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 62.Thaunat M, Chambat P. Pretibial ganglion-like cyst formation after anterior cruciate ligament reconstruction: a consequence of the incomplete bony integration of the graft? Knee Surg Sports Traumatol Arthrosc. 2007;15:522–524. doi: 10.1007/s00167-006-0218-3. [DOI] [PubMed] [Google Scholar]
- 63.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 64.Tsuda E, Ishibashi Y, Tazawa K, Sato H, Kusumi T, Toh S. Pretibial cyst formation after anterior cruciate ligament reconstruction with a hamstring tendon autograft. Arthrosc J Arthrosc Relat Surg. 2006;22:691.e1–6. doi: 10.1016/j.arthro.2005.04.115. [DOI] [PubMed] [Google Scholar]
- 65.Umar M, Baqai N, Peck C (2009) Foreign body reaction to a bioabsorbable interference screw after anterior cruciate ligament reconstruction. BMJ Case Rep. bcr0920081007 [DOI] [PMC free article] [PubMed]
- 66.Victoroff BN, Paulos L, Beck C, Goodfellow DB. Subcutaneous pretibial cyst formation associated with anterior cruciate ligament allografts: a report of four cases and literature review. Arthrosc J Arthrosc Relat Surg. 1995;11:486–494. doi: 10.1016/0749-8063(95)90206-6. [DOI] [PubMed] [Google Scholar]
- 67.Warden WH, Chooljian D, Jackson DW. Ten-year magnetic resonance imaging follow-up of bioabsorbable poly-L-lactic acid interference screws after anterior cruciate ligament reconstruction. Arthrosc J Arthrosc Relat Surg. 2008;24:370.e1–3. doi: 10.1016/j.arthro.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 68.Weiler A, Hoffmann RF, Stähelin AC, Helling HJ, Südkamp NP. Biodegradable implants in sports medicine: the biological base. Arthrosc J Arthrosc Relat Surg. 2000;16:305–321. doi: 10.1016/S0749-8063(00)90055-0. [DOI] [PubMed] [Google Scholar]
- 69.Weiss KS, Weatherall JM, Eick J, Ross JR. Delayed Tibial Osteomyelitis after Anterior Cruciate Ligament Reconstruction with Hamstrings Autograft and Bioabsorbable Interference Screw: A Case Report and Review of the Literature. Case Rep Orthop. 2017;2017:6383526. doi: 10.1155/2017/6383526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Williams R, Johnson D, editors. Controversies in Knee Surgery. Oxford, New York: Oxford University Press; 2004. [Google Scholar]
- 71.Zabala IL, Solsona SS. Tibial cyst formation following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2014;44:839. doi: 10.2519/jospt.2014.0411. [DOI] [PubMed] [Google Scholar]
- 72.Zicaro JP, Ranalletta M, Avila CG, Yacuzzi C, Costa-Paz M (2017) Pretibial Cyst Formation after ACL Reconstruction. A series of 14 cases with different etiologies. Orthop J Sports Med 5 (1 Suppl):2325967117S00034.
- 73.OSF. https://osf.io/
- 74.OCEBM Levels of Evidence Working Group*. The Oxford Levels of Evidence 2. Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
- 75.Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, Moschetti I, Phillips B, Thornton H. The 2011 Oxford CEBM Levels of Evidence (Introductory Document). Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Quality assessment of included articles using the Methodological Index for Non-Randomized Studies (MINORS).