Highlights
-
•
Knockdown of CDK5 down-regulates PD-L1 in lung adenocarcinoma and improves tumor immunity.
-
•
Interference of CDK5 leads to ubiquitination and degradation of PD-L1 protein.
-
•
TRIM21 mediates the ubiquitination and degradation process of PD-L1.
-
•
Combination of CDK5 disruption and anti-PD-L1 therapy has a stronger effect on inhibiting tumor formation, compared with CDK5 knockdown alone.
Keywords: CDK5, PD-L1, Ubiquitination, Immunotherapy, Lung cancer
Abstract
Although immunotherapy (anti-PD-1/PD-L1 antibodies) has been approved for clinical treatment of lung cancer, only a small proportion of patients respond to monotherapy. Hence, understanding the regulatory mechanism of PD-L1 is particularly important to identify optimal combinations. In this study, we found that inhibition of CDK5 induced by shRNA or CDK5 inhibitor leads to reduced expression of PD-L1 protein in human lung adenocarcinoma cells, while the mRNA level is not substantially altered. The PD-L1 protein degradation is mediated by E3 ligase TRIM21 via ubiquitination-proteasome pathway. Subsequently, we studied the function of CDK5/PD-L1 axis in LUAD. In vitro, the absence of CDK5 in mouse Lewis lung cancer cell (LLC) has no effect on cell proliferation. However, the attenuation of CDK5 or combined with anti-PD-L1 greatly suppresses tumor growth in LLC implanted mouse models in vivo. Disruption of CDK5 elicits a higher level of CD3+, CD4+ and CD8+ T cells in spleens and lower PD-1 expression in CD4+ and CD8+ T cells. Our findings highlight a role for CDK5 in promoting antitumor immunity, which provide a potential therapeutic target for combined immunotherapy in LUAD.
Introduction
Lung cancer remains the leading cause of cancer-related deaths in the world [1]. According to histologic subtypes, lung cancer is classified into non-small cell lung cancer (adenocarcinoma, squamous carcinoma and large cell carcinoma) and small cell lung cancer [2]. For lung adenocarcinoma (LUAD), the treatment is stage specific and surgical resection is the main treatment for early-stage LUAD patients. However, most patients are at advanced stage once diagnosed. Hence, they can only receive chemotherapy, radiotherapy, targeted therapy and immunotherapy [3].
The PD-1/PD-L1 axis is a key determinant of physiological immune homeostasis [4]. Suppression of PD-1/PD-L1 is a promising therapeutic alternative which restores the immune system in tumor patients. The PD-1/PD-L1 blockade therapy shows broad prospects in the treatment of various tumors, including colorectal cancer, melanoma, renal cell carcinoma and lung cancer [5]. Despite recent development in immunotherapy for LUAD, there are still many patients cannot benefit from it [6]. Considering the heterogeneity of tumors, which often develop resistance to monotherapy and limit clinical efficacy, combination immunotherapy has become a hot topic in LUAD [7].
Cyclin-dependent kinases (CDKs) are members of the serine/threonine protein kinases family, which function in regulating cell cycle and transcription [8]. As an atypical CDK, CDK5 mainly expressed in neuros and predominantly play its role in neurodegenerative diseases rather than in the cell cycle [9]. Nevertheless, recent studies have uncovered that CDK5 plays a critical role in the molecular mechanisms driving tumor formation and development, including medullary thyroid carcinoma, neuroblastoma, melanoma and lung cancer [10]. CDK5 is highly expressed in a variety of lung cancer compared with normal lung tissues and positively associated with poor prognosis [11,12]. In vitro and in vivo experiments, CDK5 promotes cell growth [13,14], migration [14], invasion [13,15], epithelial mesenchymal transition [16] and angiogenesis [17] in lung cancer.
Strikingly, a novel function of CDK5 in cancer immunity has been reported in recent years. Some researchers found that loss of CDK5 results in reduced PD-L1 expression on tumor cells due to the upregulation of interferon regulatory factor-2 (IRF2) and interferon regulatory factor 2-binding protein 2 (IRF2BP2). In a mouse model of medulloblastoma, attenuation of CDK5 expression leads to potent CD4+ T cell-mediated tumor rejection [18]. In melanoma and breast cancers of mouse models, CRISPR-Cas9 genome editing system of CDK5 downregulates PD-L1 expression on tumor cells and elicits strong CD8+T cell-mediated immune responses in tumor microenvironment with decreased regulatory T cells (Tregs) [19]. However, it is still unknown whether CDK5 affects the immune status of lung cancer. In this study, we found that RNA interference of CDK5 expression down-regulates PD-L1 protein on tumor cells and further promotes antitumor immunity.
Materials and methods
Antibodies and regents
Anti-PD-L1 (E1L3N) rabbit mAb (13,684), anti-CDK5 (2506S) and anti-ubiquitin (3933S) were purchased from Cell Signaling Technology. Mouse PD-L1 antibody (MAB90781-100) was purchased from R&D systems. Anti-TRIM21 (12,108-1-AP) was purchased from Proteintech. Fluorochrome-conjugated monoclonal antibodies including anti-CD3, anti-CD4, anti-CD8, anti-CD45, anti-PD-1 and live/dead were purchased from BD Pharmingen. In vivo mab anti-mouse PD-L1 was purchased from BioXcell. Cycloheximide (CHX) was purchased from Selleck. MG132 was purchased from MedChemExpress. Silver stain kit was purchased from Solarbio LIFE SCIENCES.
Cell lines and cell culture
The NCI-H1975 cell lines, A549 cell lines, LTEP-a-2 cell lines, 293T cell lines and LLC cell lines were purchased from the American Type Culture Collection (ATCC). NCI-H1975 cells were cultured in RPMI 1640 (Hyclone) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 IU/ml of penicillin and 100 µg/ml of streptomycin (Gibco). A549, LTEP-a-2 and LLC cells were maintained in Dulbecco's Modified Eagle's Medium (DMEM, Hyclone) containing 10% FBS, 100 mg/ml of penicillin and 100 mg/ml of streptomycin.
Quantitative real time polymerase chain reaction (qRT-PCR)
Total RNAs were extracted from cells with Trizol Reagent (Invitrogen, Carlsbad, CA). 1000 ng of total RNAs were reverse transcribed into cDNA with PrimeScript™ RT Reagent Kit (Perfect Real Time) (TaKaRa Biotechnology, Shiga, Japan). The cDNA was quantified by SYBR gene expression assays on an Applied Biosystems 7900 sequence detection system (Applied Biosystems, Foster City, CA). PCR analysis was performed using specific primers for CDK5 or PD-L1 gene (Table S1).
Western blot
Proteins were extracted from ice-cold cell lysates containing RIPA lysis buffer (Invitrogen), protease inhibitor (Cocktail), phosphatase inhibitor (Cocktail) and phenylmethylsulfonyl fluoride (Sigma). The protein lysates were dissolved in 1 × loading buffer and boiled at 95 °C for 5–10 min, separated by 10% SDS-PAGE gel and transferred to NC membranes (Millipore, Billerica, MA, USA). After being blocked in 5% non-fat milk in TBS+0.1% Tween-20 for 1 h, the membranes incubated overnight at 4 °C with primary antibodies. Then the membranes were washed with TBST and incubated with HRP conjugated secondary antibodies for 1 h at RT. The reactive bands were visualized by using High-sig ECL Western Blot Substrate and Imaging LabTM software (Bio-Rad).
Lentivirus transduction and establish of stable cell lines
The short hairpin RNAs (shRNA) targeting human or mouse CDK5 and human TRIM21 were from GeneChem (Shanghai, China). The PCDH—CDK5 was constructed by inserting the CDK5 ORF sequence into the lentiviral vector PCDH. These sequences were cloned into the lentiviral particles by using 293T cells. 48 h after cells infected with viruses, cells were selected with puromycin (1–2 μg/ml) for 7–14 days. Western blot analysis and qRT-PCR were used to verify the effect of gene silencing or overexpressing. The shRNA fragment sequences of human and mouse CDK5 were presented in Table S2. The shRNA fragment sequences of human TRIM21 were presented in Table S3.
Immunoprecipitation mass spectrometry (IP-MS)
Firstly, all the candidate proteins were chosen according to the molecular weight in silver stain. Gel pieces were cut from SDS PAGE, destained with 30% ACN/100 mM NH4HCO3. The gels were dried in a vacuum centrifuge. The in-gel proteins were reduced with dithiothreitol (10 mM DTT/ 100 mM NH4HCO3) for 30 min at 56 °C, then alkylated with iodoacetamide (200 mM IAA/100 mM NH4HCO3) in the dark at room temperature for 30 min. Gel pieces were briefly rinsed with 100 mM NH4HCO3 and ACN, respectively. Gel pieces were digested overnight in 12.5 ng/μl trypsin in 25 mM NH4HCO3. The peptides were extracted three times with 60% ACN/0.1% TFA. The extracts were pooled and dried completely by a vacuum centrifuge. The peptide mixture was loaded onto a reverse phase trap column connected to the C18-reversed phase analytical column in buffer A (0.1% Formic acid) and sep Each fraction arated with a linear gradient of buffer B (84% acetonitrile and 0.1% Formic acid) at a flow rate of 300 nl/min controlled by IntelliFlow technology. Each fraction was injected for nanoLC-MS/MS analysis. LC-MS/MS analysis was performed on a Q Exactive mass spectrometer (Thermo Scientific). And then ranked them by the percentage of the protein sequence covered by identified peptides. Finally, verified candidate interaction protein by performing IP and western blotting.
Plasmids transfection
The wild-type TRIM21 plasmid and B-box mutated TRIM21 plasmid were purchased from GENEWIZ (China). Plasmids were transfected into 293T cells by Lipofectamine 3000 (Invitrogen) with P3000 (Invitrogen) following the manufacturers’ protocol. After 72 h, cells were harvested for the following experiments.
CO-immunoprecipitation
Cells were homogenized in IP lysis buffer (Beyotime, supplemented with protease and phosphates inhibitors) at 4 °C for 1 h. The cell lysates were incubated with 50 μl Protein A/G beads (Santa Cruz) and specific antibodies overnight. The beads were then washed 3 times with IP lysis buffer. After centrifugation, the mixture was denatured in 50 μl 2 × sample loading buffer. Finally, the sample was measured by western blotting.
CCK-8 cell proliferation assay
4000 cells in 100 μl DMEM or RPMI 1640 per well were planted into 96-well plates for 24 h, 48 h and 72 h. 10 μl of CCK8 reagent (biosharp) was then dispensed to each well and incubated at 37 °C for 1–3 h. The OD value was detected at 450 nm wavelength.
Tumor models
Female C57BL/6 mice aged 6–8 weeks were selected to establish the mouse lung cancer model. 5 × 105 LLC-shNC or LLC-shCDK5 in 100 μl PBS were subcutaneously injected into the right flanks of mice. Ten days after implantation, mice were randomized into several groups and the treatment groups were intraperitoneal injected with 200 µg of anti-PD-L1 every three days. The formula of measuring tumor volume was (width2 × length)/2. All experiments were performed in compliance with the regulations of Chinese law and the local Ethical Committee Quantita.
Single cell generation from tumor or spleen tissue and flow cytometry analysis
Tumor tissues were collected immediately following surgery and minced with 2.5 ml of 0.2 mg/ml liberase (Roche) in DMEM for 1 h at 37 °C. Spleens were dissociated by using grinding rod in PBS. Single cell suspensions were then obtained by centrifuge and filtered through a 70 μm strainer. Red blood cells were lysised for 10 min. The cells were filtered again through a 70 μm strainer in FACS (1 × PBS with 2% FBS). Subsequently, 5 million cells were incubated with antibodies at 4 °C for 40 min in the dark, fixed by 1 ml 1% PFA for 15 min and resuspended with 200 μl 1% PFA before analyzed by flow cytometry. The flow analysis process was showed in Fig. S2.
Immunofluorescence microscopy
After being rehydrated, the tissue sections were incubated with primary antibody overnight. The secondary antibody was goat anti-rabbit immunoglobulin G conjugated with CY3 590 (Servicebio). The cell nuclei were stained with 4′,6-diamidino-2-phenylindole (Servicebio). Images were collected by an immunofluorescence microscope (NIKON ECLIPSE C1).
Immunohistochemistry
IHC staining for CD4 was performed and evaluated by two IHC experts from the research laboratory who scored the percentage of stained tumor cell nuclei stained (–, no staining; +, 10%; ++, 10–50%; and +++, >50%).
Statistical analysis
All the data were showed as the mean ± SEM using the GraphPad Prism software (version8.0, GraphPad Software Inc.) and compared using Student's t-test. P <0.05 was considered statistically significant.
Results
CDK5 disruption down-regulates PD-L1 protein in LUAD cells
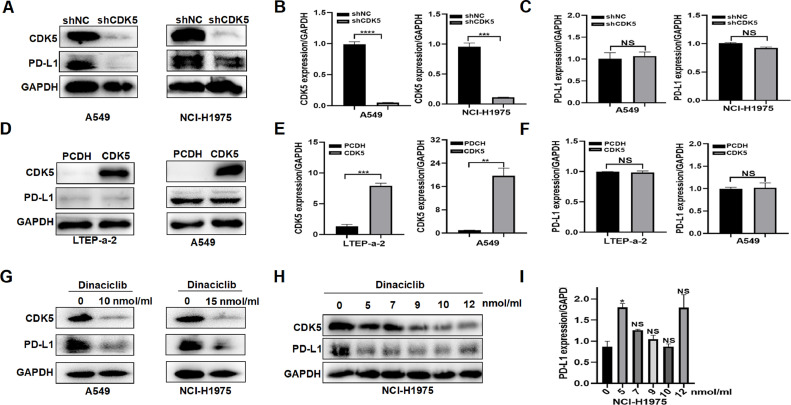
In order to interrogate the role of CDK5 in LUAD, we infected human LUAD cells with shRNA and overexpression lentiviral particles to construct stable cell lines. Western blot and qRT-PCR experiments showed that the interference (Fig. 1, A and B) or overexpression (Fig. 1, D and E) of CDK5 in LUAD cells was effective, indicating that the stable cell lines were successfully constructed. Subsequently, we used the stable cell lines to conduct following assays. Given that CDK5 regulates PD-L1 in other tumors [18,19], we speculated that it might also affect PD-L1 in lung cancer. By western blot analysis, the protein level of PD-L1 was obviously lower in shCDK5-transfected cells compared to control shNC-transfected cells (Fig. 1A). Surprisingly, mRNA levels of PD-L1 were not obviously changed by CDK5 knockdown (Fig. 1C). Moreover, CDK5 inhibitor dinaciclib also significantly inhibited PD-L1 protein (Fig. 1G) without mRNA reduction (Fig. 1I) and the pharmacologic inhibition was concentration-independent (Fig. 1H). However, the overexpression of CDK5 did not affect PD-L1 protein and mRNA expression (Fig. 1, D and F). Therefore, CDK5 may regulate PD-L1 protein through post-translational modification (PTM).
Fig. 1.
The effect of CDK5 disruption or overexpression on PD-L1 expression in LUAD cells. A. The expression levels of CDK5 and PD-L1 protein detected by western blot in A549 and NCI-H1975 cells after CDK5 knockdown. B. The expression levels of CDK5 mRNA detected by qPCR in A549 and NCI-H1975 cells after CDK5 knockdown. *** p < 0.005, **** p < 0.0005. C. qRT-PCR was used to evaluate the PD-L1 mRNA expression in CDK5 deficient cells. NS: not significant. D. The expression levels of CDK5 protein detected by western blot in LTEP-a-2 and A549 cells after CDK5 overexpression. E. The expression levels of CDK5 mRNA detected by qPCR in LTEP-a-2 and A549 cells after CDK5 overexpression. ** p < 0.01, *** p < 0.005. F. The expression levels of PD-L1 mRNA detected by qPCR in LTEP-a-2 and A549 cells after CDK5 overexpression. NS: not significant. G. Western blot analysis of PD-L1 protein levels in dinaciclib treated cells. H. Western blot measurement of PD-L1 after cells treated with different concentration of dinaciclib in NCI-H1975. I. Quantification of PD-L1 mRNA expression by using qRT-PCR in dinaciclib treated cells. NS: not significant. *p < 0.05.
CDK5 depletion results in PD-L1 protein reduction through ubiquitin degradation via TRIM21
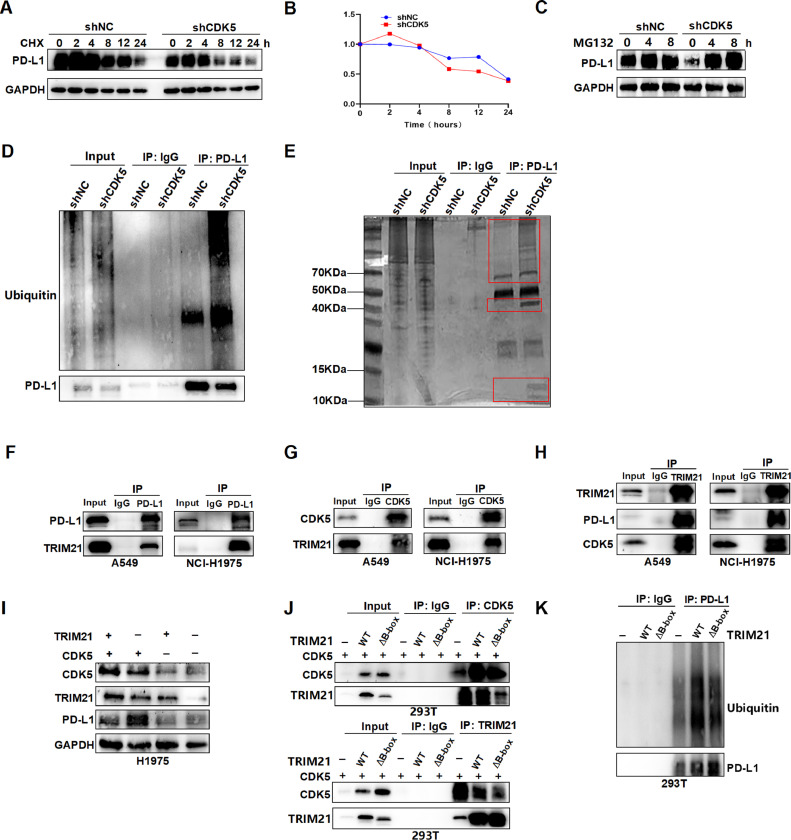
To investigate the mechanism of CDK5 regulating PD-L1 protein, we incubated the LUAD cells with cycloheximide (CHX) to suppress protein synthesis. The results showed that CDK5 depletion induced a significantly shortened half-life of PD-L1 protein (Fig. 2, A and B), suggesting that CDK5 mediates the degradation of PD-L1 protein in LUAD cells. Furthermore, the cells incubated with proteasome inhibitor MG132 could block the PD-L1 degradation (Fig. 2C). After treated with MG132 for 4 h, immunoprecipitation assay was conducted to verify the PD-L1 protein successfully precipitated. Then we found that inhibition of CDK5 increased the ubiquitination level of PD-L1 protein (Fig. 2D). These results indicated that CDK5 mediates the degradation of PD-L1 protein via a ubiquitination-proteasome mechanism in LUAD cells. In order to further explore how CDK5 regulates PD-L1 protein ubiquitination degradation, the NCI-H1975 cells knocking down CDK5 were detected by silver stained SDS-PAGE after MG132 treatment and immunoprecipitation of PD-L1 (Fig. 2E). Immunoprecipitation mass spectrometry (IP-MS) was conducted to identify the three different bands between shNC and shCDK5. From the protein analysis of IP-MS, we selected one candidate proteins to verify: TRIM21 (Table S4). CO-IP assay showed that the interactions between TRIM21 and PD-L1 or CDK5 and TRIM21 were obviously enhanced (Fig. 2F–H). TRIM21 is a member of the TRIM family of proteins with ubiquitin E3 ligase activity, which contains RING, B-box, coiled-coil (CC), and SPRY domains [20]. TRIM21 signaling is constitutively repressed by its B-box domain and activated by phosphorylation, which involves proteasomal degradation and activation of immune [21]. Next, we studied whether TRIM21 could directly mediate PD-L1 ubiquitination. After knockdown TRIM21, the PD-L1 protein level notably increased in control group without obvious change in CDK5 interference group (Fig. 2I). Then TRIM21 wild-type and B-box deletion mutant plasmids were constructed and transfected with 293T. The CO-IP results showed that the interaction with CDK5 was partially blocked by TRIM21 mutation (Fig. 2J). The ubiquitin level of PD-L1 in TRIM21 WT and mutant cells was also detected, the results showed that TRIM21 mutation decreased the ubiquitin binding with PD-L1(Fig. 2K). These above data indicated that intervention of CDK5 leads to ubiquitin degradation of PD- L1 protein via E3 ligase TRIM21.
Fig. 2.
CDK5 depletion promotes PD-L1 ubiquitination and degradation through TRIM21. A. NCI-H1975-shNC and NCI-H1975-shCDK5 were treated with CHX (10 mmol/ml) for 0, 2, 4, 8, 12 and 24 h, and immunoblotted for PD-L1 and GAPDH. B. Quantification of PD-L1 in CHX treated cells used by Image J software. C.H1975-shNC and H1975-shCDK5 cells were treated with MG132 (10 mmol/ml) for 0, 4 and 8 h, total protein was extracted and subjected to western blotting using anti-PD-L1, and anti-GAPDH antibodies. D. H1975-shNC and H1975-shCDK5 cells were treated with MG132 (10 mmol/ml) for 4 h, then lysed with IP lysis buffer. PD-L1 was immunoprecipitated with the anti-PD-L1 antibody. Western blot was conducted to detect the level of PD-L1 ubiquitination after immunoprecipitation of PD-L1. E. The PAGE gel of proteins was silver-stained. The proteins derived from H1975-shNC and H1975-shCDK5 cells that were incubated in MG132 for 4 h and then immunoprecipitated with anti-PD-L1. The different bands between the two groups were indicated by red box. F. The pulldown of PD-L1 was immunoblotted with TRIM21. G. The pulldown of CDK5 was immunoblotted with TRIM21. H. The pulldown of TRIM21 was immunoblotted with CDK5 and PD-L1. I. Western blot was used to detect the PD-L1 protein in cells of TRIM21 intervention by shRNA. +: shNC group, –: shRNA group. J. The CO-IP results of TRIM21 and CDK5 after TRIM21 B-box mutation. +: CDK5 overexpression, –: PCDH, WT: TRIM21 wild type, ΔB-box: B-box mutated TRIM21. K. The ubiquitin level of PD-L1 after transfected by TRIM21 and B-box mutated TRIM21 plasmids. –: PCDH, WT: TRIM21 wild type, ΔB-box: B-box mutated TRIM21. The percentage of input was about 3.5% in Co-IP figures. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Disruption of CDK5 mediated PD-L1 attenuation suppressed tumor growth of lung cancer
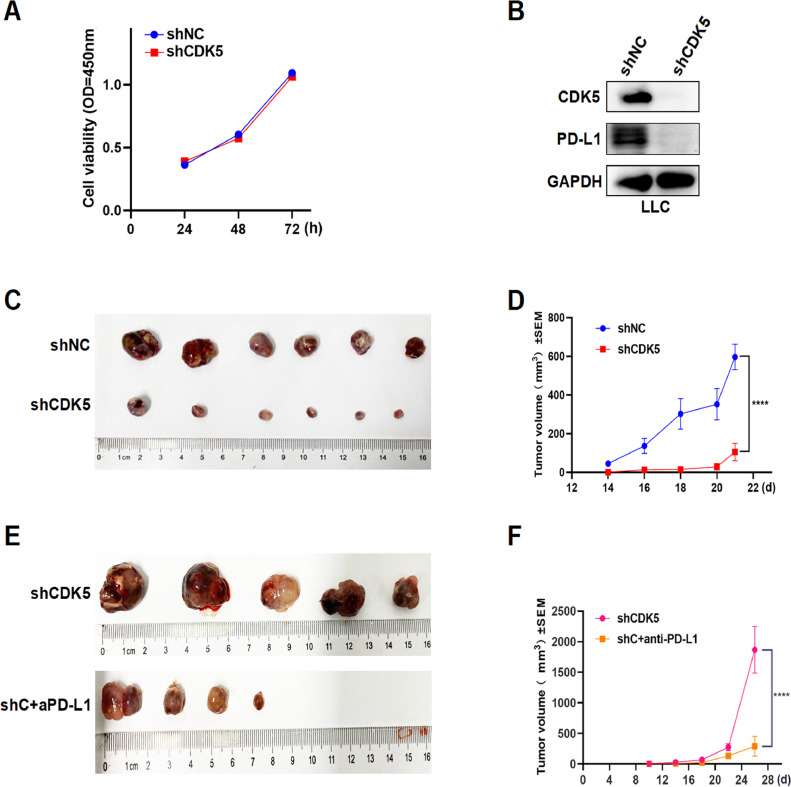
Since CDK5 was reported to promote tumor growth in human lung cancer [22], we performed CCK-8 assays to explore its role in LUAD cells. Consistently, CDK5 knockdown inhibited cell proliferation of A549 and H1975 in vitro (Fig. S1). However, CDK5 did not affect proliferation of mouse lung cancer cell (LLC) in vitro (Fig. 3A). Interference of CDK5 in LLC also decreased PD-L1 protein as predicted (Fig. 3B). Subsequently, we constructed LLC cell lines with stably interfering CDK5 (shCDK5) and analyzed tumor growth based on the tumor bearing mouse model in vivo. LLC-shCDK5 cells (5 × 105 cells) were subcutaneously injected into the right side of female C57BL/6 mice and mice injected with cells carrying empty vector were used as a control (shNC). We observed that CDK5 disruption significantly suppressed tumor growth (Fig. 3, C and D), while the combination of shCDK5 and anti-PD-L1 group showed a more significant inhibitory effect (Fig. 3, E and F). We believe that CDK5 can affect the immune status by changing the expression of PD-L1 in vivo, thereby affecting tumor growth.
Fig. 3.
Antitumor effect of shCDK5 in vitro and in vivo. A. CCK-8 assays of LLC cells stably transfected with shCDK5 or shNC were performed and analyzed. B. The interference effect of CDK5 and PD-L1 protein level in LLC was verified by western blot. C. Images showing tumor formation of LLC-shNC and LLC-shCDK5 cells in the C57BL/6 mice. D. The volume of tumors was measured every 3–4 days since LLC-shNC and LLC-shCDK5 cells were planted. E. Images showing tumor formation of LLC-shCDK5 cells in the C57BL/6 mice treated with or without PD-L1 inhibitor. F. The volume of tumors was measured every 3–4 days since LLC-shCDK5 cells were planted with or without anti-PD-L1 treatment. shC+aPD-L1: shCDK5+anti-PD-L1, **** p < 0.0005.
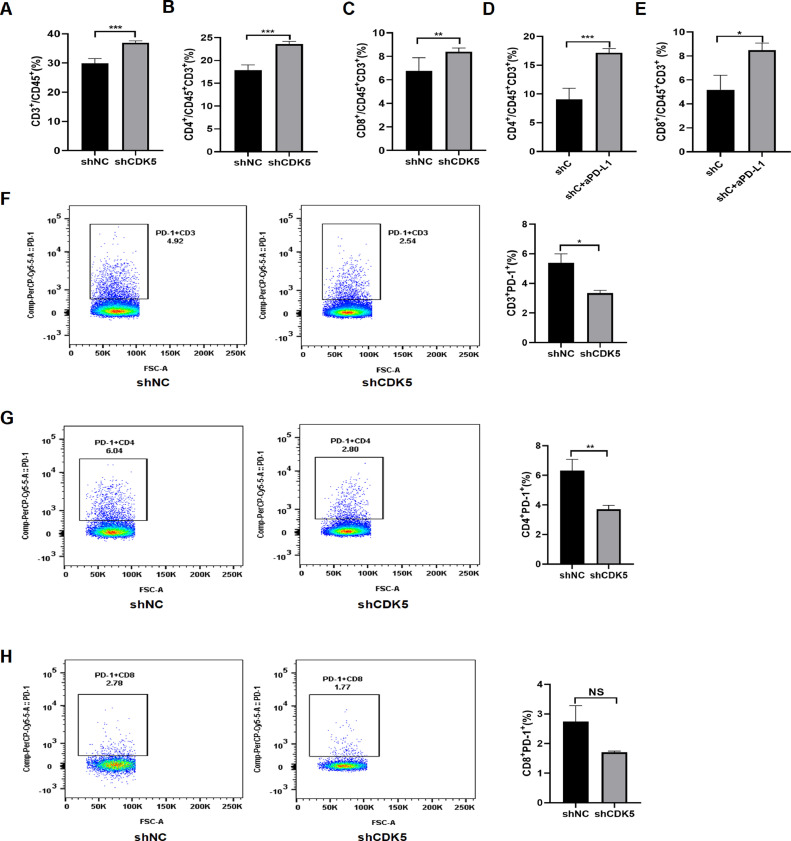
Interference of CDK5 promotes antitumor immune response
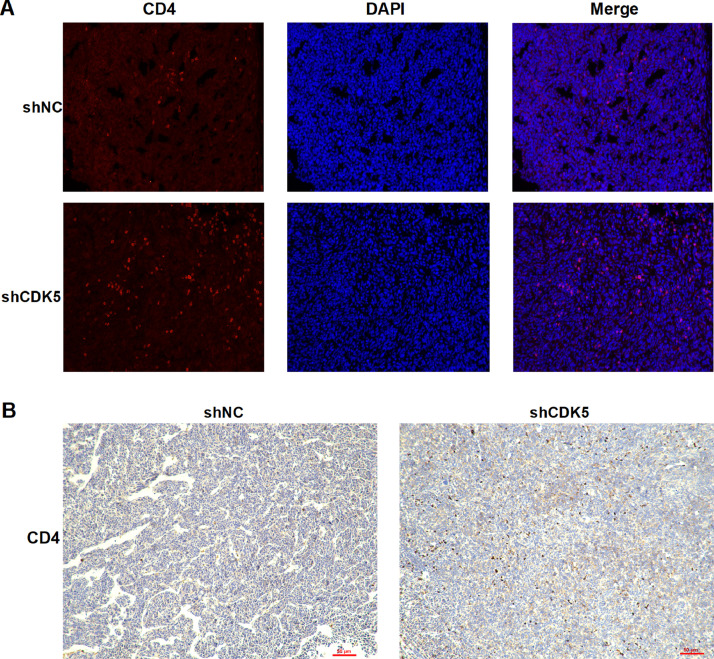
PD-L1 has been confirmed to be related to tumor immunity, therefore we conducted flow cytometry analysis to explore the immune status after CDK5 inhibition. The data showed that intervention of CDK5 improved the level of CD3+ T, CD4+ T and CD8+ T cells in spleens (Fig. 4, A–C). Furthermore, reduced CDK5 decreased the expression of PD-1 in CD3+ T, CD4+ T and CD8+ T cells in spleens compared to shNC group (Fig. 4, F–H). Importantly, flow cytometry analysis revealed that shCDK5 combined with anti-mouse PD-L1 could effectively increase the level of CD4+ and CD8+ T cells in spleens compared with CDK5 knockdown alone (Fig. 4, D and E). Immunofluorescence staining and immunohistochemistry were used to detect tumor tissue, which showed that the number of CD4+ cells increased after CDK5 depletion (Fig. 5). In summary, these data above suggested that CDK5 knockdown can improve the peripheral immune responses of LUAD.
Fig. 4.
CDK5 gene silencing improves the peripheral immune response. A, B and C. Flow cytometry analysis was performed to detect CD3+ T, CD4+ T and CD8+ T cells from spleens of control group and CDK5 knockdown group. **p < 0.01, ***p < 0.005. D and E. CD4+ T and CD8+ T cells from spleens of shCDK5 group and shCDK5+anti-PD-L1 group were detected by flow cytometry analysis. shC: shCDK5, shC+aPD-L1:shCDK5+anti-PD-L1, *p < 0.05, ***p < 0.005. F, G and H. Flow cytometry analysis was used to detect CD3+ PD-1+ T, CD4+ PD-1+ T and CD8+ PD-1+ T cells from spleens of control group and CDK5 knockdown group. All the antibody concentration used in flow cytometry is 1:100. *p < 0.05, **p < 0.01, NS: not significant.
Fig. 5.
The CD4+ cells in tumor tissue detected by immunofluorescence and immunohistochemistry. A. The expression of CD4 proteins in tumor tissue were detected by immunofluorescence from shNC group and shCDK5 group (100ⅹ). Red fluorescence was CD4 protein and blue fluorescence was nucleus. The pink fluorescence was where red and blue overlap. B. The expression of CD4 proteins in tumor tissue were detected by immunohistochemistry from shNC group and shCDK5 group (100ⅹ). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Discussion
The Programmed cell death protein 1 (PD‐1) and programmed cell death protein ligand 1 (PD‐L1) axis is a key determinant of physiological immune homeostasis. In the presence of chronic infections and tumors, target cells continue to express antigens, then T cells gradually lose function and eventually lead to T exhaustion. One of the characteristics of T exhaustion is the high expression of PD-1 [23]. PD‐1 and PD‐L1 play a critical role in immune evasion of various tumors [24], making the PD‐1/PD‐L1 axis the most promising therapeutic target [25]. Considering the limited efficacy of immune monotherapy, combined immunotherapy has become a hot spot to enhance immune response in clinical trials of various tumors [26].
It was reported that CDK5 deficiency down-regulates PD-L1 by prolonging half-life of IRF2/IRF2BP2 repressor complex and promote T cells response in medulloblastoma, melanoma and breast cancers. More importantly, immunotherapy combined with CDK5 depletion had a stronger anti-tumor immunity [18,19]. In addition, CDK5 and PD-L1 mRNA were co-occurrence and upregulated in LUAD from TCGA provisional data sets [18]. Thereby we chose CDK5 to explore its effect in regulating PD-L1 and tumor immune in LUAD. In this study, we found that inhibition of CDK5 by shRNA or drug reduced PD-L1 protein in LUAD cells without affecting PD-L1 mRNA, while CDK5 overexpression did not affect both the mRNA and protein expression of PD-L1. On the basis of the above results, we speculated that CDK5 may regulate PD-L1 through protein post-translational modification.
The PTM of PD-L1 has become an important mechanism for regulating tumor immunosuppression. Glycosylation, phosphorylation, ubiquitination and acetylation play crucial roles in the stability, translocation and protein-protein interaction of PD-L1 protein [27]. There exist two main mechanisms of protein degradation in eukaryotic cells: ubiquitination-proteasome pathway and autophagy-lysosome pathway [28]. Ubiquitin (UB) is a conserved protein which is covalently attached to substrate lysines in a three-enzyme cascade consisting of the E1 Ub-activating enzyme, the E2 Ub-conjugating enzyme, and the E3 Ub-protein ligase [29]. Owing to the strict control of E3s on ubiquitination reaction, E3s are the vital components of the cascade [30]. The E3s have been divided into three classes: the RING family, the RBR family and the HECT family [31]. TRIM21 belongs to RING family which largely participants in innate immune [32]. However, it was rarely reported about the role of TRIM21 in tumor immunity. Here we firstly discovered that disruption of CDK5 inhibit PD-L1 protein levels through E3 ubiquitin ligase TRIM21 mediated ubiquitylated degradation process.
In line with our discoveries, CDK5 was reported to enhance the cell proliferative ability of human LUAD [13,14]. However, we found that the growth of LLC cells with reduced CDK5 parallels with control cells in vitro verified by CCK-8 assays. LLC tumor model is poorly immunogenic. In many studies, it has been found that PD-L1 inhibitor alone has poor efficacy on LLC cells [33,34], so we have demonstrated the synergistic effect of the combination therapy. Here, in vivo experiments of LLC subcutaneous tumor-bearing mice, knockdown of CDK5 significantly inhibited tumor growth and combined with anti-PD-L1 therapy enhanced tumor suppression.
The immune response involves coordination between cells and tissues. The current researches mainly focus on the local immune response in the tumor microenvironment. However, the peripheral immune response is also an important part of tumor immunity that cannot be ignored [35]. In our study, more CD3+ T, CD4+ T and CD8+ T cells were found in the spleens of shCDK5 group. Moreover, PD-1 expression was declined in CD3+ T, CD4+ T and CD8+ T cells from spleens of shCDK5 group. In addition, CD4+ T and CD8+ T cells also raised in spleens of shCDK5+anti-PD-L1 group, compared with shCDK5 group. Here, we uncovered for the first time that inhibition CDK5 promotes LUAD peripheral immune response through TRIM21-mediated PD-L1 degradation.
In the past 20 years, many CDK inhibitors have been developed and successfully used in clinical trials for several tumors. In particular, CDK4/6 inhibitors have been approved clinical treatment of advanced breast cancer patients [8]. Dinaciclib is a potent inhibitor of CDK1, 2, 5 and 9, which can induce apoptosis of tumor cells and have clinical activity in refractory chronic lymphocytic leukemia [36]. However, CDK5 specific inhibitors have not yet been developed. In this study, we preliminary explored the effect of dinaciclib and found that it can also down-regulate the PD-L1 protein in LUAD cells. We intended to further study its role in LUAD tumor immune in the future.
In conclusion, our results have shown that CDK5 inhibition can significantly inhibit LLC growth in vivo through activating antitumor immunity. Thus, it provides evidence that CDK5 can act not only as a prognostic marker [11,12], but also as a potential target combined with immunotherapy for advanced lung adenocarcinoma.
CRediT authorship contribution statement
Lin Gao: Conceptualization, Visualization, Writing – original draft, Formal analysis, Validation, Software, Methodology. Liliang Xia: Conceptualization, Project administration, Supervision, Writing – review & editing, Methodology. Wenxiang Ji: Investigation. Yanshuang Zhang: Formal analysis, Validation, Software. Weiliang Xia: Conceptualization, Project administration, Supervision, Writing – review & editing, Data curtion, Resources, Methodology. Shun Lu: Funding acquisition, Project administration, Supervision, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Ethics approval and consent to participate
The present study was approved by the Institutional Review Boards of Shanghai Chest Hospital (Ethical review approval number: KS1732)
Acknowledgments
The study was supported by grants from the National Key R&D Program of China (2016YFC1303300 to S.L), the National Natural Science Foundation of China Grants (81672272 to S.L), the Science and Technology Innovation program of Shanghai (19411950500 to S.L), Shanghai Municipal Science and Technology Commission Research Project (17431906103 to S.L.), Shanghai Chest Hospital Project of Collaborative Innovation (YJXT20190105 to S.L).and Program for Outstanding Medical Academic Leader (to S.L).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2021.101148.
Contributor Information
Weiliang Xia, Email: wlxia@sjtu.edu.cn.
Shun Lu, Email: shunlu@sjtu.edu.cn.
Appendix. Supplementary materials
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Travis W.D., Brambilla E., Burke A.P. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J. Thorac. Oncol. 2015;10(9):1240–1242. doi: 10.1097/JTO.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 3.Duma N., Santana-Davila R., Molina J R. Vol. 94. 2019. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment; pp. 1623–1640. (Mayo Clin. Proc.). [DOI] [PubMed] [Google Scholar]
- 4.Nurieva R.I., Liu X., Dong C. Yin-Yang of costimulation: crucial controls of immune tolerance and function. Immunol. Rev. 2009;229(1):88–100. doi: 10.1111/j.1600-065X.2009.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou W., Wolchok J.D., Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016;8(328):328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia L., Liu Y., Wang Y. PD-1/PD-L1 blockade therapy in advanced non-small-cell lung cancer: current status and future directions. Oncologist. 2019;24(Suppl 1):S31–s41. doi: 10.1634/theoncologist.2019-IO-S1-s05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Donnell J S., Teng M W L., Smyth M J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019;16(3):151–167. doi: 10.1038/s41571-018-0142-8. [DOI] [PubMed] [Google Scholar]
- 8.Asghar U., Witkiewicz A.K., Turner N.C. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat. Rev. Drug Discov. 2015;14(2):130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung Z.H., Ip N.Y. Cdk5: a multifaceted kinase in neurodegenerative diseases. Trends Cell Biol. 2012;22(3):169–175. doi: 10.1016/j.tcb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Pozo K., Bibb J.A. The emerging role of Cdk5 in cancer. Trends Cancer. 2016;2(10):606–618. doi: 10.1016/j.trecan.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J.L., Wang X.Y., Huang B.X. Expression of CDK5/p35 in resected patients with non-small cell lung cancer: relation to prognosis. Med. Oncol. 2011;28(3):673–678. doi: 10.1007/s12032-010-9510-7. [DOI] [PubMed] [Google Scholar]
- 12.Wei K., Ye Z., Li Z. An immunohistochemical study of cyclin-dependent kinase 5 (CDK5) expression in non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC): a possible prognostic biomarker. World J. Surg. Oncol. 2016;14(1):34. doi: 10.1186/s12957-016-0787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J.L., Gu R.X., Zhou X.S. Cyclin-dependent kinase 5 regulates the proliferation, motility and invasiveness of lung cancer cells through its effects on cytoskeletal remodeling. Mol. Med. Rep. 2015;12(3):3979–3985. doi: 10.3892/mmr.2015.3868. [DOI] [PubMed] [Google Scholar]
- 14.Zeng J., Xie S., Liu Y. CDK5 functions as a tumor promoter in human lung cancer. J Cancer. 2018;9(21):3950–3961. doi: 10.7150/jca.25967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demelash A., Rudrabhatla P., Pant H.C. Achaete-scute homologue-1 (ASH1) stimulates migration of lung cancer cells through Cdk5/p35 pathway. Mol. Biol. Cell. 2012;23(15):2856–2866. doi: 10.1091/mbc.E10-12-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia Y., Duan Y., Liu T. LncRNA TTN-AS1 promotes migration, invasion, and epithelial mesenchymal transition of lung adenocarcinoma via sponging miR-142-5p to regulate CDK5. Cell Death Dis. 2019;10(8):573. doi: 10.1038/s41419-019-1811-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou X., Gu R., Han X. Cyclin-dependent kinase 5 controls vasculogenic mimicry formation in non-small cell lung cancer via the FAK-AKT signaling pathway. Biochem. Biophys. Res. Commun. 2017;492(3):447–452. doi: 10.1016/j.bbrc.2017.08.076. [DOI] [PubMed] [Google Scholar]
- 18.Dorand R.D., Nthale J., Myers J.T. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science. 2016;353(6297):399–403. doi: 10.1126/science.aae0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng H., Tan S., Gao X. Cdk5 knocking out mediated by CRISPR-Cas9 genome editing for PD-L1 attenuation and enhanced antitumor immunity. Acta Pharm. Sin. B. 2020;10(2):358–373. doi: 10.1016/j.apsb.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickson C., Fletcher A.J., Vaysburd M. Intracellular antibody signaling is regulated by phosphorylation of the Fc receptor TRIM21. eLife. 2018:7. doi: 10.7554/eLife.32660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foss S., Bottermann M., Jonsson A. TRIM21-from intracellular immunity to therapy. Front. Immunol. 2019;10:2049. doi: 10.3389/fimmu.2019.02049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tripathi B.K., Qian X., Mertins P. CDK5 is a major regulator of the tumor suppressor DLC1. J. Cell Biol. 2014;207(5):627–642. doi: 10.1083/jcb.201405105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015;15(8):486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Constantinidou A., Alifieris C., Trafalis D T. Targeting programmed cell death -1 (PD-1) and Ligand (PD-L1): a new era in cancer active immunotherapy. Pharmacol. Ther. 2019;194:84–106. doi: 10.1016/j.pharmthera.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Liu B., Song Y., Liu D. Recent development in clinical applications of PD-1 and PD-L1 antibodies for cancer immunotherapy. J. Hematol. Oncol. 2017;10(1):174. doi: 10.1186/s13045-017-0541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang Y., Chen M., Nie H. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum. Vaccin. Immunother. 2019;15(5):1111–1122. doi: 10.1080/21645515.2019.1571892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu J.M., Li C.W., Lai Y.J. Posttranslational modifications of PD-L1 and their applications in cancer therapy. Cancer Res. 2018;78(22):6349–6353. doi: 10.1158/0008-5472.CAN-18-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Le W.D. Autophagy and ubiquitin-proteasome system. Adv. Exp. Med. Biol. 2019;1206:527–550. doi: 10.1007/978-981-15-0602-4_25. [DOI] [PubMed] [Google Scholar]
- 29.Berndsen C.E., Wolberger C. New insights into ubiquitin E3 ligase mechanism. Nat. Struct. Mol. Biol. 2014;21(4):301–307. doi: 10.1038/nsmb.2780. [DOI] [PubMed] [Google Scholar]
- 30.Zheng N., Shabek N. Ubiquitin ligases: structure, function, and regulation. Annu. Rev. Biochem. 2017;86:129–157. doi: 10.1146/annurev-biochem-060815-014922. [DOI] [PubMed] [Google Scholar]
- 31.Deshaies R.J., Joazeiro C.A. RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y., Zhu Y., Liu Z.J. The emerging roles of the DDX41 protein in immunity and diseases. Protein Cell. 2017;8(2):83–89. doi: 10.1007/s13238-016-0303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin H., Wei S., Hurt E.M. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J. Clin. Invest. 2018;128(2):805–815. doi: 10.1172/JCI96113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosely S.I., Prime J.E., Sainson R.C. Rational selection of syngeneic preclinical tumor models for immunotherapeutic drug discovery. Cancer Immunol. Res. 2017;5(1):29–41. doi: 10.1158/2326-6066.CIR-16-0114. [DOI] [PubMed] [Google Scholar]
- 35.Spitzer M.H., Carmi Y., Reticker-Flynn N.E. Systemic immunity is required for effective cancer immunotherapy. Cell. 2017;168(3):487–502. doi: 10.1016/j.cell.2016.12.022. e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flynn J., Jones J., Johnson A.J. Dinaciclib is a novel cyclin-dependent kinase inhibitor with significant clinical activity in relapsed and refractory chronic lymphocytic leukemia. Leukemia. 2015;29(7):1524–1529. doi: 10.1038/leu.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.