Abstract
Background
Even when low-density lipoprotein-cholesterol (LDL-C) levels are lower than guideline thresholds, a residual risk of atherosclerosis remains. It is unknown whether triglyceride (TG) levels are associated with subclinical atherosclerosis and vascular inflammation regardless of LDL-C.
Objectives
This study sought to assess the association between serum TG levels and early atherosclerosis and vascular inflammation in apparently healthy individuals.
Methods
An observational, longitudinal, and prospective cohort study, including 3,754 middle-aged individuals with low to moderate cardiovascular risk from the PESA (Progression of Early Subclinical Atherosclerosis) study who were consecutively recruited between June 2010 and February 2014, was conducted. Peripheral atherosclerotic plaques were assessed by 2-dimensional vascular ultrasound, and coronary artery calcification (CAC) was assessed by noncontrast computed tomography, whereas vascular inflammation was assessed by fluorine-18 fluorodeoxyglucose uptake on positron emission tomography.
Results
Atherosclerotic plaques and CAC were observed in 58.0% and 16.8% of participants, respectively, whereas vascular inflammation was evident in 46.7% of evaluated participants. After multivariate adjustment, TG levels ≥150 mg/dl showed an association with subclinical noncoronary atherosclerosis (odds ratio [OR]: 1.35; 95% confidence interval [CI]: 1.08 to 1.68; p = 0.008). This association was significant for groups with high LDL-C (OR: 1.42; 95% CI: 1.11 to 1.80; p = 0.005) and normal LDL-C (OR: 1.85; 95% CI: 1.08 to 3.18; p = 0.008). No association was found between TG level and CAC score. TG levels ≥150 mg/dl were significantly associated with the presence of arterial inflammation (OR: 2.09; 95% CI: 1.29 to 3.40; p = 0.003).
Conclusions
In individuals with low to moderate cardiovascular risk, hypertriglyceridemia was associated with subclinical atherosclerosis and vascular inflammation, even in participants with normal LDL-C levels. (Progression of Early Subclinical Atherosclerosis [PESA]; NCT01410318)
Key Words: arterial inflammation, CACS, coronary calcification, subclinical atherosclerosis, triglycerides
Abbreviations and Acronyms: 18F-FDG, fluorine-18 fluorodeoxyglucose; CACS, coronary artery calcium score; CT, computed tomography; CV, cardiovascular; CVRF, cardiovascular risk factor; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; ESC, European Society of Cardiology; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PET, positron emission tomography; SCORE, Systematic Coronary Risk Evaluation; TG, triglyceride
Central Illustration
Dyslipidemia includes a wide variety of biochemical disorders that have been linked to adverse cardiovascular (CV) events (1). Accumulated evidence shows that lifetime cumulative exposure to lipids has a clear impact on the risk of CV events (2). Early identification of atherogenic dyslipidemias, particularly in individuals at low to moderate CV risk (a large subset of patients), could guide interventions to improve primary prevention (3). Risk stratification can be further refined by characterizing identified plaques as inflamed or noninflamed (4).
Dyslipidemia leads to CV events by atherosclerotic plaque development and progression (5,6). Therefore, screening tools are needed to detect and characterize subclinical atherosclerosis and to design strategies to restratify CV risk and guide lifestyle and drug interventions (7). Among dyslipidemia factors, the main cause of atherosclerotic plaque development and subsequent CV events is considered to be low-density lipoprotein cholesterol (LDL-C) (8). Although the evidence is less clear, other lipids are thought to contribute to atherosclerosis development, especially in the absence of high LDL-C levels (9). Adverse CV events have been linked to high levels of triglycerides (TGs) (10,11). Current clinical practice guidelines for the treatment of dyslipidemia recommend initiation of statins for serum TG levels >200 mg/dl, but only in patients at high CV risk (12). More solid evidence is needed to guide clinical management of hypertriglyceridemia, particularly in individuals who are not at high CV risk according to current scales.
In this study, we investigated the association between serum TG and subclinical atherosclerosis in individuals without an indication for lipid-lowering interventions according to current guidelines (individuals at low to moderate CV risk and normal LDL-C levels).
Methods
Study design
This study was conducted in a subset of participants from the PESA (Progression of Early Subclinical Atherosclerosis) study, an observational prospective cohort study using multiterritory imaging to assess determinants of atherosclerosis presence and progression. The study rationale and design have been reported previously (13). Briefly, between June 2010 and February 2014, PESA enrolled 4,184 volunteers between the ages of 40 and 54 years without previous CV disease. All participants were employees of Santander Bank headquarters in Madrid, Spain. The PESA exclusion criteria were previous CV disease, extreme obesity (body mass index ≥40 kg/m2), chronic kidney disease (estimated glomerular filtration rate <60 ml/min/m2), active treatment for cancer, or any condition reducing life expectancy or affecting study adherence. The study protocol was approved by the Carlos III Health Institute Ethics Committee, and all participants provided written informed consent.
CV mortality risk was assessed with the European Society of Cardiology (ESC) CV disease risk assessment tool, the Systematic Coronary Risk Evaluation (SCORE) (14). Participants were classified according to 10-year risk as having a low (<1%) or moderate (≥1% and <5%) CV risk. Participants with high CV risk (≥5%) were excluded from this study (n = 3). A further 149 participants were excluded because of noninterpretable images or missing laboratory, questionnaire, or imaging data, and 278 individuals were excluded because they were receiving statin therapy. The final study group thus consisted of 3,754 PESA participants (Supplemental Figure 1).
Assessment of cardiovascular risk and lipid profile
Anthropometric, clinical, and laboratory data were collected according to the PESA study protocol. The assessed CV risk factors (CVRFs) were dyslipidemia, smoking, hypertension, diabetes, obesity, and a family history of premature CV disease, as reported previously (13). Venous blood was collected after 8 h of fasting. Serum TG, total cholesterol, high-density lipoprotein cholesterol (HDL-C), and lipoprotein(a) were measured by spectrophotometric assays in an Architect-Ci8200 analyzer (Abbott Laboratories, Abbott Park, Illinois) and using the manufacturer’s kits. LDL-C was calculated by the Friedewald method, except for participants with TG levels >300 mg/dl, in which case it was measured directly. Participants were stratified into 3 groups according to the fourth Adult Treatment Panel guidelines for fasting serum TG (11): hypertriglyceridemia was defined as TG ≥150 mg/dl, and values <150 mg/dl were further classified as either low normal (<100 mg/dl) or high normal (100 to 149 mg/dl). Participants were classified as having normal or high serum LDL-C according to the ESC guideline thresholds of 116 mg/dl for individuals at low CV risk and 100 mg/dl for those at moderate CV risk (12). The inflammatory marker high-sensitivity C-reactive protein was assessed in all patients.
Assessment of subclinical atherosclerosis
The presence of subclinical atherosclerotic plaques was assessed in all participants by 2-dimensional vascular ultrasound (Philips iU22 ultrasound, Philips Healthcare, Bothell, Washington). Plaques were defined according to the Mannheim criteria as focal protrusions into the arterial lumen of thickness >0.5 mm or >50% of the surrounding intima-media thickness or as a diffuse intima-media thickness >1.5 mm (15). Cross-sectional sweeps were made at 7 vascular locations: the bilateral carotid arteries, the infrarenal aorta, and the bilateral iliac and femoral arteries. All images were analyzed at the National Center for Cardiovascular Research (CNIC) core imaging laboratory in Madrid, Spain by experienced, blinded operators.
The coronary artery calcium score (CACS) was determined in all patients by noncontrast 16-slice computed tomography (CT, Philips Brilliance, Philips Healthcare, Andover, Massachusetts), as previously described (16,17). After electrocardiography-gated prospective CT acquisition, the CACS was calculated from CT images by the Agatston method (18).
Assessment of vascular inflammation
As described elsewhere (4), a subgroup of 755 PESA participants with evidence of atherosclerosis on 2-dimensional vascular ultrasound or CACS was examined by hybrid fluorine-18 fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) to assess vascular inflammation. Of this subgroup, 141 participants were excluded because of previous statin therapy, lack of a SCORE assessment, or incomplete imaging studies. Thus, the final sample assessed in this substudy included 614 subjects. The 18F-FDG PET scan covered 10 vascular territories per participant: the right and left carotid arteries; the ascending, arch, and descending thoracic aorta; the infrarenal abdominal aorta; the left and right iliac arteries; and the left and right femoral arteries. Qualitative PET analysis in each vessel was performed after adjustment of the color scale to between 0 and 2 standardized uptake value (SUV) units. Arterial 18F-FDG uptake was defined as a localized area of increased signal intensity encompassing an artery that could not be explained by adjacent structures such as lymph nodes or ureters.
Statistical analysis
Study participants’ baseline characteristics are presented as number (%) for categorical variables and as mean ± SD or median (interquartile range) for normally and non-normally distributed continuous variables. Participants’ characteristics across TG groups were compared by analysis of variance with multiple-testing correction (Bonferroni) for continuous variables or by the chi-square test for categorical variables. Adjusted ordinal regression models were used to assess the relationship between TG and the number of non-coronary atherosclerotic territories (0, 1, 2, 3, ≥4). Results were expressed as the odds ratio (OR) with its 95% confidence interval (CI). Potential confounders included to assess the association between subclinical atherosclerosis and TG categories were age, sex, systolic blood pressure, LDL-C, HDL-C, smoking, glycated hemoglobin (hemoglobin A1c), body mass index, family history of CV disease, moderate to vigorous physical activity, ethanol consumption (g/day), and eating pattern (17). The relationship between TG levels as a continuous variable and the risk of subclinical atherosclerosis was estimated using a quadratic fit. The association between TG and vascular inflammation was assessed by logistic regression, with adjustment for variables previously reported to predict vascular inflammation in the PESA study (4). Statistical analyses were conducted using Stata software version 15 (StataCorp, College Station, Texas). Differences were considered statistically significant at a p value <0.05.
Results
A total of 3,754 PESA participants with low to moderate CV risk were included (45.5 ± 4.2 years of age; 61.2% men). Most individuals (84.9%) had a low 10-year CV risk, and the remaining 568 (15.1%) had a moderate CV risk. Mean LDL-C was 133.0 ± 29.4 g/dl. Of the participants, 1,044 (27.8%) had serum LDL-C levels within the ESC-defined normal range (12): <100 and <116 mg/dl for moderate– and low–CV risk individuals, respectively. Mean serum TG was 92.2 ± 52.3 mg/dl; 393 individuals (10.5%) had TG ≥150 mg/dl, 781 (20.8%) had TG between 100 and 150 mg/dl, and 2,580 (68.7%) had TG <100 mg/dl. Compared with participants with TG <100 mg/dl, those with TG >150 mg/dl were older, they were more frequently men, and they had a worse CVRF profile and a higher SCORE value (Table 1).
Table 1.
Baseline Characteristics of Low to Moderate Cardiovascular Risk PESA Study Participants Stratified by Serum TG Level
| Total (N = 3,754) | TG <100 (n = 2,580) | TG 100–150 (n = 781) | TG ≥150 (n = 393) | p Value | |
|---|---|---|---|---|---|
| Age, yrs | 45.5 ± 4.2 | 45.2 ± 4.1 | 46.2 ± 4.4 | 46.0 ± 4.1 | <0.001 |
| Women | 1,456 (38.8) | 1,290 (50.0) | 132 (16.9) | 34 (8.7) | <0.001 |
| Hypertension | 367 (9.8) | 165 (6.4) | 123 (15.7) | 79 (20.1) | <0.001 |
| Diabetes | 12 (0.3) | 1 (0.0) | 1 (0.1) | 10 (2.5) | <0.001 |
| Dyslipidemia | 1,376 (36.7) | 661 (25.6) | 421 (53.9) | 294 (74.8) | <0.001 |
| Smoking | 753 (20.3) | 451 (17.7) | 195 (25.3) | 107 (27.4) | <0.001 |
| BMI ≥30 kg/m2 | 486 (12.9) | 204 (7.9) | 170 (21.8) | 112 (28.5) | <0.001 |
| Family history of CVD | 562 (15.0) | 346 (13.4) | 152 (19.5) | 64 (16.3) | 0.107 |
| Total cholesterol, mg/dl | 201 ± 33.0 | 193 ± 30.1 | 214 ± 30.6 | 226 ± 35.4 | <0.001 |
| LDL-C, mg/dL | 133 ± 29.4 | 127 ± 27.1 | 145 ± 29.5 | 146 ± 32.4 | <0.001 |
| HDL-C, mg/dl | 49.5 ± 12.2 | 52.7 ± 12.2 | 44.2 ± 9.3 | 39.5 ± 7.8 | <0.001 |
| SBP, mm Hg | 116 ± 12.4 | 113 ± 11.9 | 120 ± 11.8 | 123 ± 11.9 | <0.001 |
| DBP, mm Hg | 72.1 ± 9.3 | 70.3 ± 8.8 | 75.3 ± 9.1 | 77.8 ± 9.1 | <0.001 |
| BMI, kg/m2 | 25.9 ± 3.7 | 25.0 ± 3.5 | 27.6 ± 3.5 | 28.4 ± 3.5 | <0.001 |
| HbA1c, % | 5.4 (5.2–5.6) | 5.4 (5.1–5.6) | 5.4 (5.2–5.7) | 5.5 (5.3–5.8) | <0.001 |
| hs-CRP, mg/ml | 0.09 (0.05–0.18) | 0.08 (0.04–0.16) | 0.13 (0.07–0.23) | 0.14 (0.08–0.27) | <0.001 |
| Lp(a), mg/dl | 17.4 (6.8–41.9) | 17.1 (7.0–42.0) | 18.3 (6.3–42.8) | 17.3 (5.7–40.1) | 0.183 |
| SCORE risk | 0.34 (0.14–0.72) | 0.24 (0.09–0.54) | 0.60 (0.31–1.03) | 0.69 (0.40–1.18) | <0.001 |
| MVPA, % | 34.3 (22.7–49.7) | 34.8 (22.8–50.5) | 32.7 (22.4–48.4) | 33.7 (21.8–48.5) | 0.008 |
| Ethanol, g/day | 6.5 (1.6–14.2) | 5.5 (1.2–12.6) | 8.8 (2.8–18.2) | 10.2 (3.1–19.0) | <0.001 |
| Eating pattern | |||||
| Mediterranean | 1,457 (39.6) | 1,133 (44.9) | 229 (29.8) | 95 (24.7) | <0.001 |
| Western | 1,546 (42.1) | 1,018 (40.4) | 343 (44.7) | 185 (48.2) | <0.001 |
| Social-business | 672 (18.3) | 372 (14.7) | 196 (25.5) | 104 (27.1) | <0.001 |
Values are mean ± SD, n (%), or median (interquartile range).
BMI = body mass index; CVD = cardiovascular disease; DBP = diastolic blood pressure; HbA1c = glycated hemoglobin; HDL-C = high-density lipoprotein cholesterol; hs-CRP = high-sensitivity C-reactive protein; LDL-C = low-density lipoprotein cholesterol; Lp(a) = lipoprotein(a); MVPA = moderate to vigorous physical activity; PESA = Progression of Early Subclinical Atherosclerosis; SBP = systolic blood pressure; SCORE = Systematic Coronary Risk Evaluation; TG = triglyceride.
TG and subclinical atherosclerosis
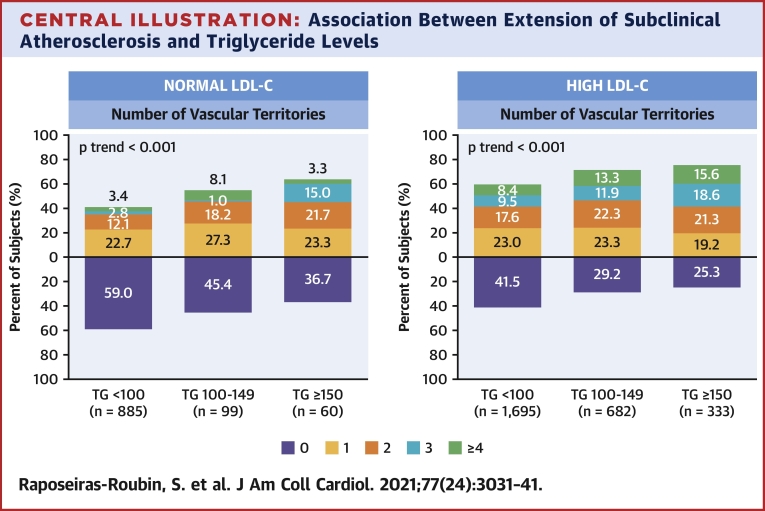
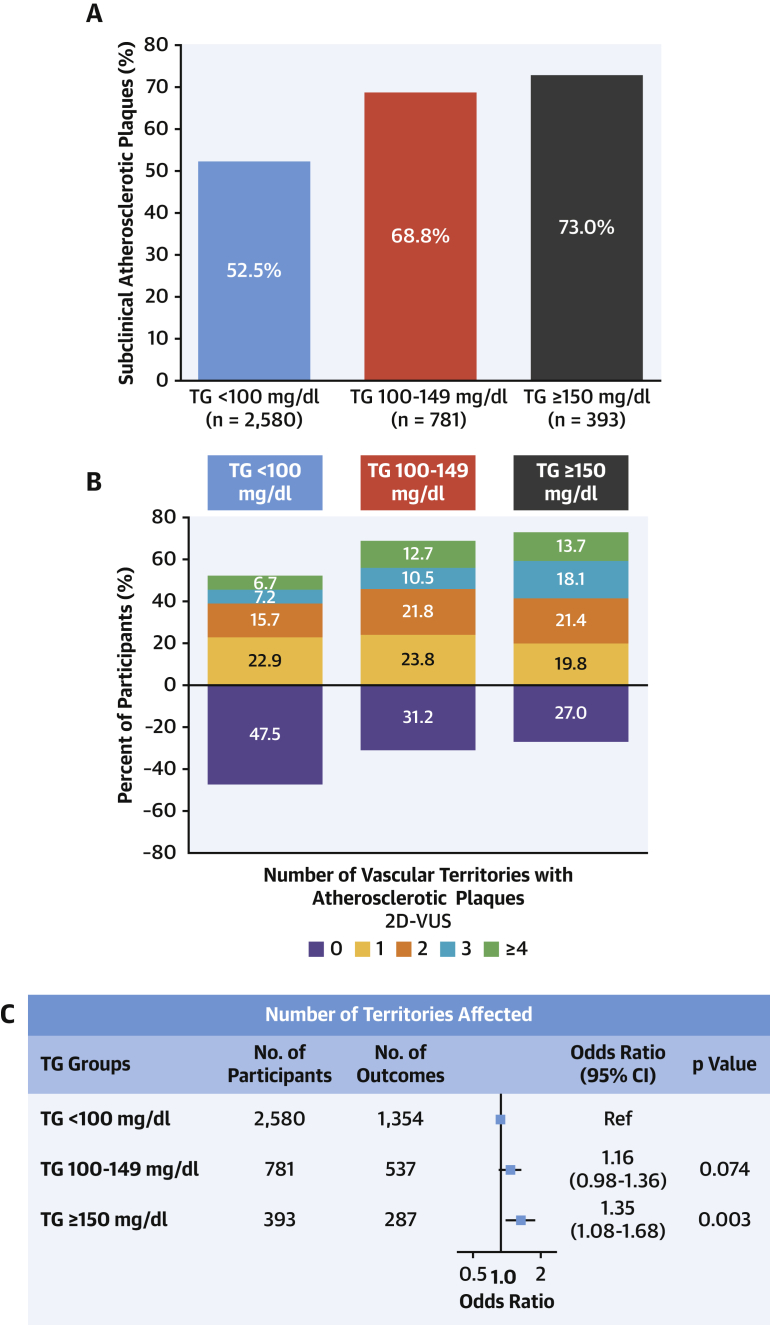
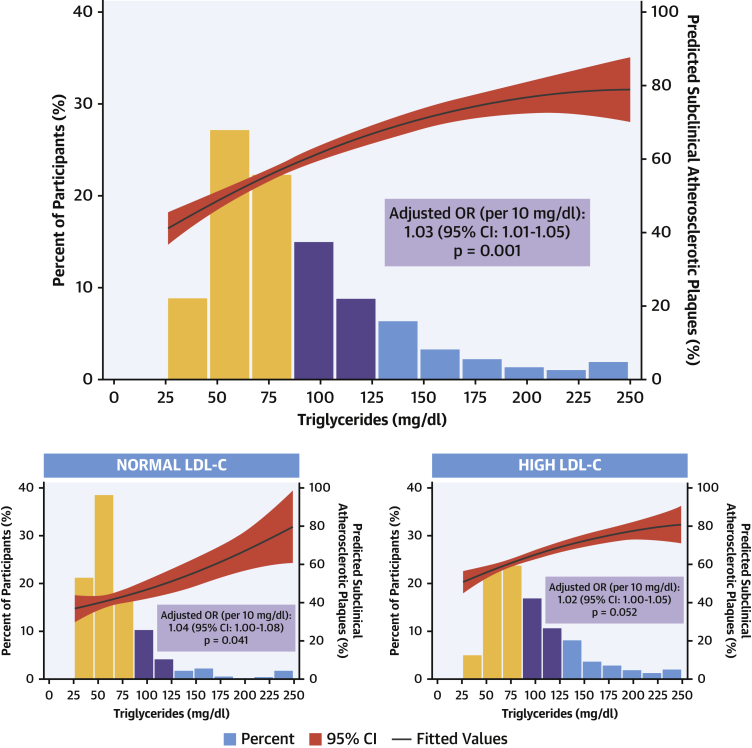
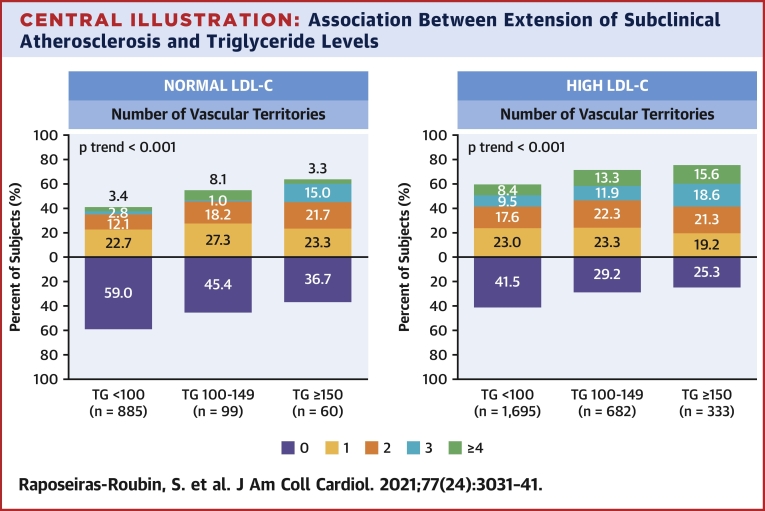
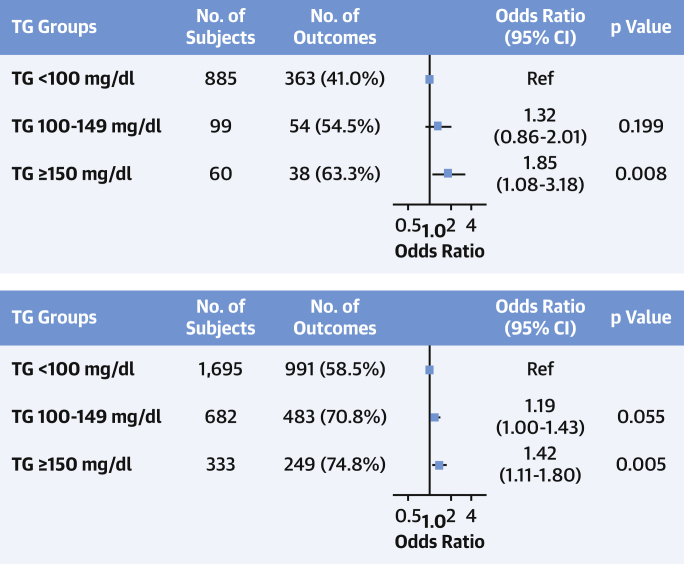
Imaging analysis detected subclinical atherosclerosis in 52.5%, 68.8%, and 73.0% of participants with serum TG <100 mg/dl, 100 to 149 mg/dl, and ≥150 mg/dl, respectively; the stepwise increase across TG groups was also evident for the number of vascular territories affected (Figures 1A to 1C). After multivariate adjustment, serum TG ≥150 mg/dl was associated with a significantly higher prevalence of noncoronary atherosclerosis (OR: 1.35; 95% CI: 1.08 to 1.68; p = 0.008) than TG <100 mg/dl. Regardless of LDL-C concentration, lower serum TG was associated with a lower risk of subclinical atherosclerosis (Figure 2). The number of vascular territories affected with subclinical atherosclerosis was higher as TG levels increased for individuals with both normal and high LDL-C (Central Illustration). The association between TG ≥150 mg/dl and the presence of noncoronary atherosclerotic plaques was observed both in individuals with high LDL-C (OR: 1.42; 95% CI: 1.11 to 1.80) and in those with normal LDL-C (OR: 1.85; 95% CI: 1.08 to 3.18) (Figure 3). CVRFs and TG showed no significant interaction in the prevalence of subclinical atherosclerosis (Supplemental Figure 2). TG showed no association with CACS (OR per 10 mg/dl increase: 1.01; 95% CI: 0.99 to 1.03; p = 0.405) (Supplemental Figures 3 and 4).
Figure 1.
Prevalence and Extension of Subclinical Atherosclerosis According to TG Levels
(A) Percentage of subjects with atherosclerotic plaques in the different groups according to triglyceride (TG) levels. (B) Distribution of subclinical atherosclerosis evaluated with number of noncoronary vascular territories affected according to triglyceride levels. (C) Ordinal regression model to assess the relationship between triglyceride levels and the number of noncoronary atherosclerotic territories (0, 1, 2, 3, ≥4). Results were adjusted for potential confounders, including age, sex, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, smoking, glycated hemoglobin, body mass index, family history of cardiovascular disease, moderate to vigorous physical activity, ethanol consumption, and eating pattern. CI = confidence interval; 2D-VUS = 2-dimensional vascular ultrasound.
Figure 2.
Association Between TG Levels and Presence of Subclinical Atherosclerotic Plaques
(Top) The relationship between triglyceride (TG) levels as a continuous variable and the risk of subclinical atherosclerosis was graphically represented using a quadratic fit. Triglyceride levels were truncated at 250 mg/dl because only 58 participants had values >250 mg/dl. The predicted percentage of subclinical atherosclerotic plaques is shown by the black line with its 95% confidence interval (CI) in red, and it is represented in the right y-axis. A binary regression model was used to assess the relationship between triglyceride levels and the presence of atherosclerotic plaques, after adjusting for age, sex, systolic blood pressure, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol, smoking, glycated hemoglobin, body mass index, family history of cardiovascular disease, moderate to vigorous physical activity, ethanol consumption, and eating pattern. (Bottom) Results after repeating analysis by groups of low-density lipoprotein cholesterol (normal: <116 mg/dl for individuals at low cardiovascular risk and <100 mg/dl for those at moderate cardiovascular risk). OR = odds ratio.
Central Illustration.
Association Between Extension of Subclinical Atherosclerosis and Triglyceride Levels
Distribution of number of vascular territories affected with subclinical atherosclerosis according to triglyceride levels in patients with normal and high low-density lipoprotein cholesterol. Normal low-density lipoprotein cholesterol levels were defined as values within recommended targets by European Society of Cardiology guidelines (<116 mg/dl for individuals at low cardiovascular risk and <100 mg/dl for those at moderate cardiovascular risk). High low-density lipoprotein cholesterol levels were defined as those higher than recommended values. LDL-C = low-density lipoprotein cholesterol; TG = triglycerides.
Figure 3.
Subclinical Atherosclerosis and TGs According to LDL-C Levels
Forest plot showing the relationship between triglyceride (TG) levels and the number of noncoronary atherosclerotic territories (0, 1, 2, 3, ≥4) according to low-density lipoprotein cholesterol (LDL-C) levels. Results were assessed by ordinal regression analysis adjusted for potential confounders, including age, sex, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, smoking, glycated hemoglobin, body mass index, family history of cardiovascular disease, moderate to vigorous physical activity, ethanol consumption, and eating pattern. CI = confidence interval.
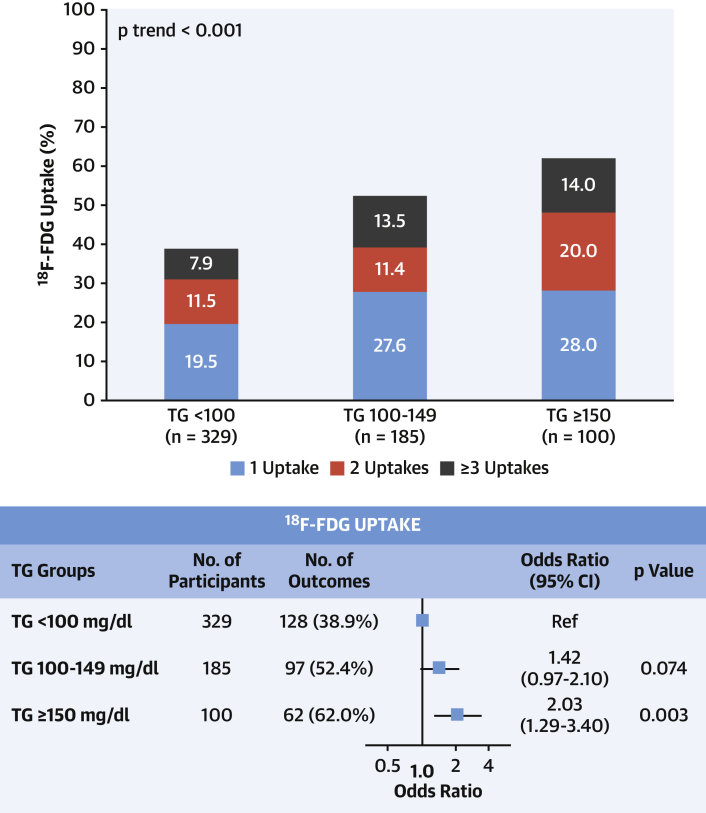
TG and vascular inflammation
Serum TG showed a weak but significant correlation with high-sensitivity C-reactive protein (R = 0.298; 95% CI: 0.269 to 0.327; p < 0.001). Arterial 18F-FDG uptake and the number of plaques with 18F-FDG uptake increased with increasing TG level (Figure 4). Individuals with TG ≥150 mg/dl had a 2-fold higher risk of arterial 18F-FDG uptake (adjusted OR: 2.09; 95% CI: 1.29 to 3.40; p = 0.003) than those with TG <100 mg/dl.
Figure 4.
Relationship Between Arterial Inflammation and TG Levels
(Top) Distribution of arterial inflammation evaluated with number of fluorine-18 fluorodeoxyglucose (18F-FDG) uptakes assessed by positron emission tomography according to levels triglyceride (TG) levels. (Bottom) Binary logistic regression model to assess the relationship between triglyceride levels and arterial fluorine-18 fluorodeoxyglucose uptake. Results were adjusted for potential confounders, including age, sex, smoking, and obesity. CI = confidence interval.
Discussion
In this study, we assessed the association between serum TG and atherosclerosis in a group of apparently healthy middle-aged participants in the PESA study. We reported 3 main findings: 1) serum TG is associated with the presence of noncoronary atherosclerotic plaques in individuals at low to moderate CV risk; 2) this association is present even in persons with normal LDL-C levels according to ESC guidelines and with no indication for lipid-lowering therapy; 3) serum TG is associated with general arterial inflammation and with inflamed atherosclerotic plaques. These findings are likely to be clinically relevant. TG is easy and inexpensive to measure, thus making TG testing as an index of underlying atherosclerosis available to most laboratories. Moreover, our results revealed hypertriglyceridemia as a potential target for therapies to reduce the risk of atherosclerosis and future clinical events.
To our knowledge, this is the first large cross-sectional study reporting an association between serum TG and both subclinical atherosclerosis and elevated vascular inflammation in individuals at low to moderate CV risk. ESC guidelines recommend treating hypertriglyceridemia as a primary prevention measure to reduce CV risk only in high-risk individuals with TG >200 mg/dl (Class I recommendation, Level of Evidence: B) or with TG between 135 and 499 mg/dl despite statin treatment (Class IIa recommendation, Level of Evidence: B) (12). Our data suggest that lipid-lowering intervention may be beneficial even in individuals at low to moderate CV risk with TG >150 mg/dl.
Numerous studies have shown an association between high TG values and CV risk. A meta-analysis of data from 262,525 participants in 29 prospective studies showed that serum TG is a strong and independent predictor of CV risk (19). In that study, the association between high serum TG and CV risk was attenuated but not eliminated by adjustment for HDL-C concentration. Data from the secondary prevention PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction 22) trial revealed that serum TG, regardless of LDL-C concentration, had a substantial impact on CV outcomes in patients with acute coronary syndromes (20). Notably, among statin-treated patients, on-treatment TG <150 mg/dl was associated with reduced CV risk independently of LDL-C concentration. Furthermore, the beneficial effect of reducing LDL-C to <70 mg/dl was highest in individuals with TG <150 mg/dl (20). Recently, Lee et al. (3) compared the incidence and risk of CV events according to each lipid parameter in a cohort of 30,330 young, statin-naïve individuals 20 to 39 years of age who were included in the South Korean National Health Insurance Service database. These investigators found that serum TG was strongly associated with clinical events regardless of HDL-C and LDL-C concentrations (3).
In our study, we focused on subclinical atherosclerosis and vascular inflammation as early markers of the risk of future CV events. The development of atherosclerotic plaques—especially vulnerable plaques, such as those with a high metabolic rate or inflammation—is the mechanistic link between dyslipidemia and CV events. The association between serum TG and subclinical atherosclerosis can be used to identify those individuals in the greatest need of primary prevention measures to halt atherosclerosis and thus prevent transition to manifest disease (21). The detection of subclinical atherosclerosis at a very early stage is critical to improving CV prevention and outcomes by allowing early implementation of primary prevention strategies (22).
This is the first study to evaluate the association between TG and subclinical atherosclerosis; however, a link between TG and low-grade vascular inflammation has been reported previously. A multidirectional mendelian randomization study including 60,608 individuals demonstrated that elevated remnant cholesterol (directly related to serum TG) was associated with both low-grade inflammation and ischemic heart disease, whereas elevated LDL-C was associated with ischemic heart disease without inflammation (23). Unlike LDL-C, TGs do not accumulate in foam cells (24), and the hydrolysis of remnant TGs by lipoprotein lipase at the endothelial surface or within the subendothelial space generates a host of proinflammatory mediators (25). Inflammation alters endothelial function and contributes to the vulnerability of plaques regardless of LDL-C levels (26). Recent insights into inflammation as a major component of plaque development, erosion, and rupture have greatly expanded our understanding of atherosclerosis. Many atherosclerotic CV events occur in individuals with normal LDL-C (27), and this has turned attention to other pathophysiological drivers of atherosclerosis. In an intriguing post hoc analysis of the AFCAPS/TexCAPS (Air Force/Texas Coronary Atherosclerosis Prevention Study), investigators observed that statin therapy remained effective at mitigating risk in individuals with elevated high-sensitivity C-reactive protein but low LDL-C (28), results consistent with the findings of JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) of statin therapy in primary prevention (29). The intimate involvement of inflammation in all stages of atherosclerosis makes intervention to modulate systemic or local inflammatory responses an attractive route to reducing CV risk (30). Whether strategies to decrease TG levels can reduce arterial and plaque inflammation remains to be investigated.
We observed a significant association between TG ≥150 mg/dl and the presence both of atherosclerotic plaques and of arterial inflammation, even in study participants with LDL-C levels within recommended limits. Hypertriglyceridemia and its association with arterial inflammation could partially explain the residual risk in persons with low to moderate CV risk and normal LDL-C. Our findings indicate that controlling and lowering serum TG may become key measures for reducing the residual risk of CV events in individuals who have already achieved guideline-recommended LDL-C targets, even if they are at low to moderate CV risk. Recently, 2 trials assessed the effect of omega-3 fatty acids in high–CV risk patients with hypertriglyceridemia with discordant results. In REDUCE-IT (Reduction of Cardiovascular Events With EPA–Intervention Trial), treatment with a purified form of eicosapentaenoic acid (EPA) significantly reduced the risk of ischemic events, including CV death, in patients with established CV disease or high CV risk with elevated TG levels (135 to 499 mg/dl) and low LDL-C (<100 mg/dl) (31). However, in the STRENGTH (STatin Residual Risk Reduction With EpaNova in HiGh CV Risk PatienTs With Hypertriglyceridemia) trial, in patients at high CV risk with TG levels between 180 and 500 mg/dl and LDL-C < 100 mg/dl, a mixture of EPA and docosahexaenoic acid (DHA) did not result in a reduction of CV events (32). A possible explanation is based on the different biochemical and physiochemical properties of omega-3 fatty acids; research has shown that EPA stabilizes cell membranes and DHA could destabilize them (33), meaning that the DHA could have counteracted the beneficial effects of the EPA in the STRENGTH trial. Another possible explanation would lie in the effect of the resolvins, which are specialized proresolving mediators derived from omega-3 fatty acids that actively facilitate resolution of acute inflammation (34). It has recently been suggested that the anti-inflammatory effects of EPA or DHA partly a result of the resolvins (35). However, the resolvins generated from EPA and DHA are different: resolvins derived from EPA are termed resolvin E, whereas those derived from DHA are termed resolvin D (34). Moreover, the anti-inflammatory and proresolving effects of both resolvins are different. Therefore, it is expected that the effects of both EPA and DHA are not equal, and controlling these lipid mediators will possibly prevent and/or provide treatment options for diseases related to inflammation, such as atherosclerosis.
Regarding coronary atherosclerotic risk, we have not found an association between TG and CACS. Vascular calcification is a late event in the atherosclerotic process (36), and the prevalence of CACS >0 in the relatively young PESA study group is low compared with that of noncoronary atherosclerotic plaques. A lack of association between TG levels and CACS was also reported in the CARDIA (Coronary Artery Risk Development in Young Adults) study (37).
Study limitations
The data presented in this study are observational, and therefore the clear association between TG and subclinical atherosclerosis should not be interpreted as indicating a causal relationship. A second limitation is that, despite optimal multivariate analysis, it remains possible that the observed associations could be partly explained by residual confounders. Third, TG measurements were performed with participants in the fasting state, in line with traditional recommendations for blood sampling for lipid analysis. Recent studies have used nonfasting sampling, which generally increases serum TG by 27 mg/dl (38). However, for most individuals, the mean increase in recorded TG is clinically irrelevant. Fourth, coronary atherosclerosis was assessed by CACS, and we therefore cannot rule out an association between serum TG and the prevalence or progression of noncalcified coronary plaques. Fifth, the study group was essentially a nondiabetic cohort, with <1% with diabetes mellitus (n = 12). Finally, the PESA study cohort is a relatively homogeneous occupational cohort that may not be representative of the general population.
Conclusions
A significant association between hypertriglyceridemia (TG ≥150 mg/dl) and noncoronary atherosclerosis and vascular inflammation was identified in a group of apparently healthy persons with low to moderate CV risk, even in those persons with normal LDL-C levels. Our findings identify TG determination as a potential screening tool with therapeutic implications. These data reinforce the role of targeting hypertriglyceridemia in primary CV disease prevention. In light of these data, clinical guidelines could revisit their recommendations and consider a TG target of 150 mg/dl or lower for primary prevention for individuals at any level of CV risk, regardless of LDL-C concentration.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Serum TG levels are associated with subclinical atherosclerosis and vascular inflammation in persons with low to intermediate CV risk, irrespective of LDL-C level.
TRANSLATIONAL OUTLOOK: Further studies are needed to assess the impact of lowering blood TGs across a range of LDL-C levels on atherosclerosis progression and long-term clinical outcomes.
Funding Support and Author Disclosures
The PESA study is funded by the National Center for Cardiovascular Research (CNIC) and Santander Bank. The study also has received funding from the Carlos III Health Institute (ISCIII; PI15/02019, PI17/00590, and PI20/00819) and the European Regional Development Fund. The CNIC is supported by the ISCIII, the Ministry of Science and Innovation, and the Pro CNIC Foundation. CNIC is a Severo Ochoa Center of Excellence (SEV-2015-0505). The funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. Dr. Ibáñez is the recipient of a European Research Council grant MATRIX (ERC-COG-2018-ID: 819775). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank Simon Bartlett for providing English editing.
Footnotes
Matthew Budoff, MD, served as Guest Associate Editor for this paper. Athena Poppas, MD, served as the Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental figures, please see the online version of this paper.
Contributor Information
Borja Ibáñez, Email: bibanez@cnic.es.
Valentin Fuster, Email: vfuster@cnic.es.
Appendix
References
- 1.Ference B.A., Kastelein J.J.P., Ray K.K. Association of triglyceride-lowering LPL variants and LDL-C-lowering LDLR variants with risk of coronary heart disease. JAMA. 2019;321:364–373. doi: 10.1001/jama.2018.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borén J., Chapman M.J., Krauss R.M. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H., Park J.B., Hwang I.C. Association of four lipid components with mortality, myocardial infarction, and stroke in statin-naive young adults: a nationwide cohort study. Eur J Prev Cardiol. 2020;27:870–881. doi: 10.1177/2047487319898571. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Friera L., Fuster V., Lopez-Melgar B. Vascular inflammation in subclinical atherosclerosis detected by hybrid PET/MRI. J Am Coll Cardiol. 2019;73:1371–1382. doi: 10.1016/j.jacc.2018.12.075. [DOI] [PubMed] [Google Scholar]
- 5.Budoff M.J., Young R., Lopez V.A. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61:1231–1239. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Melgar B., Fernandez-Friera L., Oliva B. Short-term progression of multiterritorial subclinical atherosclerosis. J Am Coll Cardiol. 2020;75:1617–1627. doi: 10.1016/j.jacc.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Alvira J.M., Fuster V., Pocock S. Predicting subclinical atherosclerosis in low-risk individuals: ideal cardiovascular health score and Fuster-BEWAT score. J Am Coll Cardiol. 2017;70:2463–2473. doi: 10.1016/j.jacc.2017.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Friera L., Fuster V., Lopez-Melgar B. Normal LDL-cholesterol levels are associated with subclinical atherosclerosis in the absence of risk factors. J Am Coll Cardiol. 2017;70:2979–2991. doi: 10.1016/j.jacc.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Ingelsson E., Schaefer E.J., Contois J.H. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776–785. doi: 10.1001/jama.298.7.776. [DOI] [PubMed] [Google Scholar]
- 10.Nordestgaard B.G., Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384:626–635. doi: 10.1016/S0140-6736(14)61177-6. [DOI] [PubMed] [Google Scholar]
- 11.Klempfner R., Erez A., Sagit B.Z. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes. 2016;9:100–108. doi: 10.1161/CIRCOUTCOMES.115.002104. [DOI] [PubMed] [Google Scholar]
- 12.Mach F., Baigent C., Catapano A.L. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Ortiz A., Jimenez-Borreguero L.J., Penalvo J.L. The Progression and Early detection of Subclinical Atherosclerosis (PESA) study: rationale and design. Am Heart J. 2013;166:990–998. doi: 10.1016/j.ahj.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Rossello X., Dorresteijn J.A., Janssen A. Risk prediction tools in cardiovascular disease prevention: a report from the ESC Prevention of CVD Programme led by the European Association of Preventive Cardiology (EAPC) in collaboration with the Acute Cardiovascular Care Association (ACCA) and the Association of Cardiovascular Nursing and Allied Professions (ACNAP) Eur J Prev Cardiol. 2019;26:1534–1544. doi: 10.1177/2047487319846715. [DOI] [PubMed] [Google Scholar]
- 15.Touboul P.J., Hennerici M.G., Meairs S. Mannheim intima-media thickness consensus. Cerebrovasc Dis. 2004;18:346–349. doi: 10.1159/000081812. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Friera L., Penalvo J.L., Fernandez-Ortiz A. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: the PESA (Progression of Early Subclinical Atherosclerosis) study. Circulation. 2015;131:2104–2113. doi: 10.1161/CIRCULATIONAHA.114.014310. [DOI] [PubMed] [Google Scholar]
- 17.Rossello X., Fuster V., Oliva B. Association between body size phenotypes and subclinical atherosclerosis. J Clin Endocrinol Metab. 2020;105:3734–3744. doi: 10.1210/clinem/dgaa620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 19.Sarwar N., Danesh J., Eiriksdottir G. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 20.Miller M., Cannon C.P., Murphy S.A. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51:724–730. doi: 10.1016/j.jacc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Singh S.S., Pilkerton C.S., Shrader C.D., Jr., Frisbee S.J. Subclinical atherosclerosis, cardiovascular health, and disease risk: is there a case for the Cardiovascular Health Index in the primary prevention population? BMC Public Health. 2018;18:429. doi: 10.1186/s12889-018-5263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatto L., Prati F. Subclinical atherosclerosis: how and when to treat it? Eur Heart J Suppl. 2020;22(Suppl E):E87–E90. doi: 10.1093/eurheartj/suaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varbo A., Benn M., Tybjaerg-Hansen A., Nordestgaard B.G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128:1298–1309. doi: 10.1161/CIRCULATIONAHA.113.003008. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro M.D., Fazio S. From lipids to inflammation: new approaches to reducing atherosclerotic risk. Circ Res. 2016;118:732–749. doi: 10.1161/CIRCRESAHA.115.306471. [DOI] [PubMed] [Google Scholar]
- 25.Saraswathi V., Hasty A.H. The role of lipolysis in mediating the proinflammatory effects of very low density lipoproteins in mouse peritoneal macrophages. J Lipid Res. 2006;47:1406–1415. doi: 10.1194/jlr.M600159-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Lüscher T.F. Inflammation and features of the vulnerable plaque: from mechanisms and imaging to outcomes. Eur Heart J. 2020;41:2923–2927. doi: 10.1093/eurheartj/ehaa686. [DOI] [PubMed] [Google Scholar]
- 27.Blake G.J., Ridker P.M., Kuntz K.M. Potential cost-effectiveness of C-reactive protein screening followed by targeted statin therapy for the primary prevention of cardiovascular disease among patients without overt hyperlipidemia. Am J Med. 2003;114:485–494. doi: 10.1016/s0002-9343(03)00074-3. [DOI] [PubMed] [Google Scholar]
- 28.Ridker P.M., Rifai N., Clearfield M. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 29.Ridker P.M., Danielson E., Fonseca F.A. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 30.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 31.Bhatt D.L., Steg P.G., Miller M. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 32.Nicholls S.J., Lincoff A.M., Garcia M. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324:2268–2280. doi: 10.1001/jama.2020.22258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorjao R., Azevedo-Martins A.K., Rodrigues H.G. Comparative effects of DHA and EPA on cell function. Pharmacol Ther. 2009;122:56–64. doi: 10.1016/j.pharmthera.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serhan C.N., Levy B.D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128:2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Criqui M.H., Kamineni A., Allison M.A. Risk factor differences for aortic versus coronary calcified atherosclerosis: the multiethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2289–2296. doi: 10.1161/ATVBAHA.110.208181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pletcher M.J., Bibbins-Domingo K., Liu K. Nonoptimal lipids commonly present in young adults and coronary calcium later in life: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Ann Intern Med. 2010;153:137–146. doi: 10.1059/0003-4819-153-3-201008030-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapman M.J., Ginsberg H.N., Amarenco P. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Heart J. 2011;32:1345–1361. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.