Abstract
Background:
Air pollution exposure during pregnancy has been associated with adverse pregnancy and birth outcomes. Inflammation has been proposed as a potential link. We estimated associations between air pollution exposure during pregnancy and inflammatory biomarkers in maternal and cord blood. We evaluated whether maternal inflammation was associated with infant outcomes.
Methods:
Among 515 mother-infant dyads in the Healthy Start study (2009-2014), trimester-long, 7- and 30-day average concentrations of particulate matter ≤2.5 micrometers (PM2.5) and ozone (O3) during pregnancy were estimated, using inverse-distance-weighted interpolation. Inflammatory biomarkers were measured in maternal blood in mid-pregnancy (C-reactive protein [CRP], Interleukin [IL]-6, and tumor necrosis factor-α [TNFα]) and in cord blood at delivery (CRP, IL-6, IL-8, IL-10, monocyte chemoattractant protein-1 [MCP-1], and TNFα). We used linear regression to estimate associations between pollutants and inflammatory biomarkers and maternal inflammatory biomarkers and infant weight and body composition.
Results:
There were positive associations between PM2.5 during certain exposure periods and maternal IL-6 and TNFα. There were negative associations between recent O3 and maternal CRP, IL-6, and TNFα and positive associations between trimester-long O3 exposure and maternal inflammatory biomarkers, though some 95% confidence intervals included the null. Patterns were inconsistent for associations between PM2.5 and O3 and cord blood inflammatory biomarkers. No consistent associations between maternal inflammatory biomarkers and infant outcomes were identified.
Conclusions:
Air pollution exposure during pregnancy may impact maternal inflammation. Further investigations should examine the health consequences for women and infants of elevated inflammatory biomarkers associated with air pollution exposure during pregnancy.
Keywords: Air pollution, Inflammation, Pregnancy, Infant
1. Introduction
There is a growing body of evidence linking prenatal ambient air pollution exposure with fetal, infant, and child health outcomes.1–3 Several previous epidemiologic studies have reported associations between pregnancy exposure to air pollutants, such as particulate matter ≤2.5 micrometers (PM2.5) and ozone (O3), and adverse birth outcomes, including low birth weight, small for gestational age (SGA), and preterm birth.4–9 Evidence from a limited number of animal10, 11 and human studies12, 13 shows that prenatal exposure to components of air pollution may also adversely affect offspring cardio-metabolic health during childhood. These findings suggest that the prenatal time may represent a critical period of fetal susceptibility to environmental stressors, such as air pollution.14, 15
Prenatal air pollution exposure has been linked to inflammatory airway-related conditions in infancy and childhood, including decreased lung function, wheezing, and asthma.16–19 Further, differences in the levels of inflammatory biomarkers in cord blood at delivery have been identified between SGA and appropriate for gestational age infants,20–22 suggesting a role for inflammation in this outcome. Since low birth weight and subsequent rapid growth during infancy are associated with greater risk of cardio-metabolic disease during childhood,23–26 and inflammatory processes are key components of cardio-metabolic disease pathophysiology,27–29 systemic inflammation may be a plausible biological pathway through which air pollution may lead to cardio-metabolic disease later in life.
Several previous studies from the Netherlands,30 Germany,31 France,32 the Czech Republic,33–35 Canada,36 and the United States (US)37 have reported that exposure to air pollution during pregnancy was associated with levels of immune biomarkers in the mother and the neonate. However, the Dutch study was the only to investigate the relationship between the air pollutants and an inflammatory marker, C-reactive protein (CRP), in both maternal and cord blood.30 Greater PM10 exposure in the prior 1-2 weeks was associated with elevations in maternal CRP levels during trimester 1, and higher full pregnancy maternal PM10 and nitrogen dioxide (NO2) exposure were associated with higher levels of CRP in cord blood at delivery.30 Given that the inflammatory response is regulated by several different cytokines with diverse and context-dependent roles, further characterization of the relationship between air pollution and pro- and anti- inflammatory biomarkers is warranted.
We investigated the associations between ambient air pollution exposure (PM2.5 and O3) during pregnancy and maternal and cord blood inflammatory biomarkers. In both maternal and cord blood, we assessed these relationships in a core group of three pro-inflammatory biomarkers, CRP, Interleukin (IL)-6, and tumor necrosis factor-α (TNFα) that have been associated with adverse pregnancy and birth outcomes.38–41 We investigated these relationships with an expanded panel in cord blood, with the addition of IL-8, IL-10, and monocyte chemoattractant protein 1 (MCP-1). We hypothesized that greater exposure to ambient air pollution would be associated with higher concentrations of pro-inflammatory biomarkers in maternal mid-pregnancy serum, and these relationships would differ according to timing of exposure to air pollution during pregnancy. We also hypothesized that greater exposure to air pollution during pregnancy would be associated with alterations in concentrations of inflammatory biomarkers in cord blood at delivery. Additionally, we examined whether maternal inflammatory biomarkers during pregnancy were associated with offspring birth weight and body composition.
2. Methods
2.1. Study sample
We included participants from the Healthy Start study, which is an ongoing longitudinal cohort study of 1,410 ethnically diverse mother-infant dyads. Briefly, pregnant women who had not yet reached 24 completed weeks of gestation were recruited at obstetrics clinics at the University of Colorado Hospital between 2009 and 2014. Participants attended follow-up research visits through pregnancy, delivery, and now into childhood for the offspring. Eligibility criteria included singleton pregnancy, age ≥16 years, and no history of chronic disease (diabetes, cancer, asthma treated with steroids, or medication-dependent psychiatric illness) or a birth <25 weeks gestation. The participation rate for the Healthy Start study was approximately 50%. The median gestational age at time of enrollment was 17 weeks (range, 11–20). Study participants provided written informed consent. The Colorado Multiple Institutional Review Board approved all study protocols.
Of the 1,410 participants enrolled in the Healthy Start study, 11 withdrew from the study prior to delivery and 17 experienced fetal demise. Of the 1,382 eligible participants, we measured inflammatory biomarkers in 536 dyads with sufficient volume of both maternal and cord blood stored serum samples, and we excluded participants who resided outside of the Denver, Colorado metropolitan area or with no O3 and/or PM2.5 monitors within 50 km of the residence8, 42 (n=21), ultimately resulting in a sample size of 515 for the present analysis (Supplementary Figures 1 and 2). For the analysis of the offspring outcomes, which consisted of birth weight, gestational age, and body composition (% fat mass [adiposity], fat mass, and fat free mass) at birth and at 5 months of age, some participants had missing data. There were 497 and 334 mother infant-pairs included in the analysis of body composition at birth and at five months, respectively.
2.2. Assessment of exposure
We estimated average ambient air pollution exposure levels (PM2.5 and O3) for trimester-long and 7- and 30- day exposure windows during pregnancy using measurements obtained from stationary monitors in the United States Environmental Protection Agency Air Quality System. Participants reported their place of residence via questionnaire completed at the time of enrollment in the Healthy Start study. Details have been described previously.42
Briefly, there were 10 stationary monitors that measured average 24-hour PM2.5 and 19 stationary monitors that measured hourly O3, which were located within 50 km of at least one study participant. The 50 km distance was selected based on previous literature.43, 44 Most of the monitors for PM2.5 recorded measurements every three days, with some measuring daily or every six days. All of the monitors for O3 recorded measurements hourly each day. We calculated trimester-specific average and full-pregnancy average PM2.5 levels by averaging the daily values from each monitor within 50 km over the relevant exposure period for each participant. Additionally, we calculated average PM2.5 levels for different time windows (30 days and 7 days) preceding the maternal blood sample collection and cord blood collection at delivery, to capture both recent and longer term exposure and to enhance comparability with prior literature that has included similar exposure windows.30, 31, 37 For O3, the maximum 8-hour average for each 24-hour period was determined, and daily 8-hour maximum values were averaged over the same time-periods that were estimated for PM2.5. For both air pollutants, we used inverse-distance-weighting (1/(distance2)) to calculate the average exposure at each participant’s 6residence using the period-specific averages from all monitors within 50 km.45 We included averages from each monitor if the number of non-missing values was at least 75% of the expected values.44, 45
2.3. Assessment of outcomes
Fasting maternal blood samples were collected at a median gestational age of 27 weeks, and immediately separated and stored at −80°C. Inflammatory biomarkers in maternal serum were analyzed by the University of Colorado Clinical and Translational Sciences Institute Core Laboratories. Maternal CRP was measured using immunoturbidimetric methodology (Beckman Coulter, Inc, Indianapolis, IN). Maternal IL-6 was measured using Luminex MAP technology (R&D Systems, Inc, Minneapolis, MN, USA). Maternal TNFα was measured using enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Inc, Minneapolis, MN, USA).
Infant inflammatory biomarkers were measured in cord blood drawn at delivery. Samples were stored on ice for up to 20 minutes and then centrifuged for processing. They were next stored at 4 °C for up to 24 hours, before being transferred for long-term storage in a freezer kept at −80 °C. Cord blood CRP levels were determined by an ELISA (Alpco, Salem, New Hampshire, USA). Cord blood inflammatory biomarkers, IL-10, IL-1/β, IL-4, IL-6, IL-8, MCP-1, and TNFα were processed by the University of Colorado Cancer Center Flow Cytometry Shared Resource. Cord blood cytokine levels were determined by multiplex panel immunoassay, according to manufacturer’s instructions (EMD Millipore Corporation, Billerica, MA, USA). We restricted analyses to those cytokines present in at least 70% of the study sample (IL-6, IL-8, IL-10, MCP-1, TFNα). The limit of detection of the assays varied by panel. There were no values below the minimum limit of detection for the five cord blood cytokines included. For cord blood cytokine values that were above the maximum detectable concentration (IL-6, n=1; MCP-1, n=3), we used the value of the maximum detectable concentration for that panel.
Additional infant outcomes included: birth weight, gestational age at birth, and body composition (fat mass, fat free mass, and fat mass as percent of body mass, hereafter referred to as adiposity) at birth and at approximately five months of age (mean ± standard deviation: 5.1 ± 1.2 months). Birth weight and gestational age at birth were obtained from medical records. Body composition at birth and at approximately five months was measured using whole body air displacement plethysmography (PEA POD, COSMED, Rome, Italy)46 by trained research staff. Body composition was measured up to three times for each infant, and the two closest measurements were averaged.
2.4. Other variables
Participants reported their age, race/ethnicity, education completed, and the number of previous pregnancies at the time of their study enrollment. We calculated maternal pre-pregnancy body mass index (BMI) using height measured at the first study visit and weight obtained from the medical record (87%) or self-report at study enrollment (12%) if unavailable in the medical record. Participants self-reported their smoking status at two study visits during pregnancy and at delivery, and we created a binary variable to indicate any smoking during pregnancy. Census tract-level median income was obtained from the 2012-2016 American Community Survey and linked to the participant’s geocoded enrollment address. Infant sex, season of birth, and mode of delivery were obtained from the medical record.
2.5. Statistical analysis
Because ambient air pollution exposure was estimated using inverse-distance-weighted interpolation of data obtained from stationary monitors, and not directly measured, there is the possibility of measurement error due to spatial heterogeneity. We calculated period-specific intra-class correlation coefficients (ICC) for the three monitors closest to the participant’s address that contributed to the average exposure estimate. The ICC was interpreted as the degree of spatial homogeneity in the study area. We calculated Spearman correlations between period-specific air pollution exposures and between maternal and cord blood inflammatory biomarkers.
We used separate multivariable linear regression models to estimate the associations between the period-specific air pollution exposures and each of the maternal and cord blood inflammatory marker outcomes. For maternal inflammatory marker outcomes, which were measured at a median of 27 weeks of gestation, we only examined the 30- and 7-day periods preceding the sample collection and the first and second trimester exposures. For cord blood inflammatory biomarkers, which were measured at delivery, we considered all period-specific average exposures. We natural log-transformed all maternal and cord blood inflammatory biomarkers to minimize the effect of potential outliers. Results are presented as the percent change and 95% confidence intervals (CI) for an IQR increase in the pollutant. We also used multiple linear regression to investigate the association between maternal inflammatory biomarkers and infant birth weight and body composition outcomes.
We included potential confounders identified via a directed acyclic graph that represented hypothesized causal relationships and associations reported in previous literature (Supplementary Figure 3). All models were adjusted for the corresponding co-pollutant (PM2.5 or O3) during the specified pregnancy period, maternal age at delivery (years), pre-pregnancy BMI (kg/m2), race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, all others), smoking during pregnancy (any, none), median income in the Census tract (dollars), maternal education (less than 12th grade, high school degree or GED, some college or associate’s degree, four years of college, graduate degree), any previous pregnancies (yes, no), and infant sex. Models with maternal inflammatory biomarkers were additionally adjusted for gestational age at maternal blood sample collection and season of conception (winter, spring, summer, fall), and models with cord blood inflammatory biomarkers were additionally adjusted for mode of delivery (vaginal, Caesarean) and season of delivery (winter, spring, summer, fall). Models for associations between maternal inflammatory biomarkers and birth and body composition outcomes were also adjusted for gestational age at maternal blood sample collection, and the models for body composition at five months were additionally adjusted for infant age at the time of the body composition measurement. We performed a sensitivity analysis excluding infants who were born preterm (<37 weeks gestation) (n=21).47 We hypothesized that gestational age was a causal intermediate between maternal inflammatory biomarkers and birth weight and body composition, therefore, the models for the primary analyses did not include gestational age. However, we performed a sensitivity analysis adjusting for gestational age in models for these outcomes. We evaluated model assumptions by visual inspection of scatter plots between exposures and outcomes to detect departures from linearity and the presence of outliers and residual plots to identify violations of homoscedasticity.
Because there is some evidence that the maternal immune response may differ according to fetal sex, we investigated whether infant sex was a potential effect modifier by including product interaction terms between each air pollutant and infant sex, in adjusted models. We considered the corresponding co-pollutant as a potential confounder, due to the inverse relationship between PM2.5 and O3. We conducted a sensitivity analysis with models that only included a single pollutant. We performed two additional sensitivity analyses: the first in which we excluded participants who smoked during pregnancy, and the second, for cord blood only, in which we excluded values above the maximum detectable concentration for that panel.
We conducted all analyses in SAS (Version 9.4, The SAS Institute, Cary, NC, USA) and in R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
The characteristics of the participants in this study sample were comparable to those not included in this study sample and to those of the entire Healthy Start cohort (Supplementary Table 1). The average maternal age at delivery was 28 ± 6 years, and the average pre-pregnancy maternal BMI was 26 ± 7 kg/m2. The study sample was racially and ethnically diverse (Table 1).
Table 1:
Characteristics of 515 eligible mother-infant pairs in the Healthy Start Study.
| Characteristic | Mean ± SD, Median (IQR), or N (%) |
|---|---|
| Covariates | |
| Maternal age at delivery (years) | 27.6 ± 6.2 |
| Pre-pregnancy body mass index (kg/m2) | 25.9 ± 6.5 |
| Race/ethnicity | |
| Hispanic | 128 (25) |
| Non-Hispanic white | 273 (53) |
| Non-Hispanic black | 79 (15) |
| All others | 35 (7) |
| Maternal smoking during pregnancy (any) | 49 (10) |
| Median annual income in the Census tract (per $1,000) | 64.1 ± 26.7 |
| Maternal education completed | |
| Less than 12th grade | 79 (15) |
| High school degree or GED | 95 (18) |
| Some college or Associate’s degree | 106 (21) |
| Four years of college (BA, BS) | 115 (22) |
| Graduate degree (Master’s, Ph.D.) | 120 (23) |
| Season of conception | |
| Winter | 163 (32) |
| Spring | 97 (19) |
| Summer | 90 (18) |
| Fall | 165 (32) |
| Mode of delivery= Caesarean | 110 (21) |
| Previous pregnancy | 323 (63) |
| Season of birth | |
| Winter | 109 (21) |
| Spring | 136 (26) |
| Summer | 148 (29) |
| Fall | 122 (24) |
| Infant sex = male | 276 (54) |
| Gestational age at sample collection (days) | 191 ± 17 |
| Gestational age at birth (days) | 276 ± 9 |
| Age at follow-up visit (months) a | 5.1 ± 1.2 |
| Exposures | |
| Average PM2.5 (μg/m3) a | |
| Prior 30 days from sample collection | 7.13 (6.08-8.26) |
| Prior 7 days from sample collection | 6.92 (5.53-8.61) |
| Trimester 1 | 7.52 (6.89-8.14) |
| Trimester 2 | 7.39 (6.78-8.09) |
| Trimester 3 | 7.48 (6.84-8.29) |
| Full Pregnancy | 7.51 (7.13-7.86) |
| Prior 30 days to delivery | 7.24 (6.33-8.42) |
| Prior 7 days to delivery | 6.73 (5.58-8.20) |
| Average 8-hr max O3 (ppb) | |
| Prior 30 days to sample collection | 45.1 (32.7-54.2) |
| Prior 7 days to sample collection | 44.1 (33.4-54.6) |
| Trimester 1 | 42.1 (33.8-53.7) |
| Trimester 2 | 42.6 (33.9-52.3) |
| Trimester 3 | 46.1 (35.0-54.6) |
| Full Pregnancy | 44.0 (40.9-47.1) |
| Prior 30 days to delivery | 48.0 (34.1-55.9) |
| Prior 7 days to delivery | 47.0 (35.4-55.3) |
| Outcomes | |
| Birth weight (g) | 3275 ± 427 |
| Fat mass at birth (g) a | 285.3 ± 143.1 |
| Fat free mass at birth (g) a | 2843.4 ± 333.9 |
| Adiposity at birth (%) a | 8.9 ± 3.8 |
| Fat mass at postnatal follow-up visit (g) a | 1708.6 ± 526.8 |
| Fat free mass at postnatal follow-up visit (g) a | 5167 ± 643.4 |
| Adiposity at postnatal follow-up visit (%) a | 24.5 ± 5.6 |
Abbreviations: SD, standard deviation; IQR, interquartile range; PM2.5, particulate matter with diameter ≤ 2.5 micrometers; O3, average 8-hour maximum ozone concentration in parts per billion.
Missing data for the following variables (n=number missing): age at postnatal follow-up, n=181; average PM2.5 Prior 30 days to sample collection, n=31; average PM2.5 Prior 7 days to sample collection, n=30; average PM2.5 Trimester 1, n=46; average PM2.5 Trimester 2, n=44; average PM2.5 Trimester 3, n=17; average PM2.5 Prior 30 days to delivery, n=31; average PM2.5 Prior 7 days to delivery, n=32; average PM2.5 Full pregnancy, n=4; fat mass at birth, n=18; fat free mass at birth, n=18; adiposity at birth, n=18; fat mass at poshiatal follow-up, n=181; fat free mass at postnatal follow-up, n=181; adiposity at postnatal follow-up, n=181.
The median percent of stationary monitors within 50 km of the participants’ residences with less than 25% missing observations was ≥75% for PM2.5 and 78.6% for O3 (Supplementary Table 2). The median full pregnancy average PM2.5 and average 8-hour O3 maximum levels were 7.51 μg/m3 (range 5.41-9.39 μg/m3) and 44.0 parts per billion (ppb) (range 30.6-54.0 ppb), respectively (Table 1). Air pollution exposures varied seasonally according to the timing of the pregnancy and were generally weakly or inversely correlated between trimesters, and more highly correlated between shorter periods (Supplementary Figure 4).
The ICCs for period-specific average levels at the three nearest monitors were moderate to high, ranging from 0.56-0.80 for PM2.5 and 0.73-0.86 for O3, suggesting reasonable levels of spatial homogeneity in the study area.
There was a moderately high Spearman correlation (rs) between maternal CRP (CRPm) and IL-6m (rs=0.50), with weak correlations observed between CRPm and TNFαm (rs=0.02) and IL-6m and TNFαm (rs=0.10) (Table 2).
Table 2:
Concentrations and pairwise spearman correlations of cord blood and maternal inflammatory biomarkers among 515 eligible mother-infant pairs in the Healthy Start Study.
| Characteristic | Median (IQR) | Spearman Correlation Coefficient | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cord blood | Maternal blood | |||||||||
| CRP (mg/L)a | IL-6 (pg/mL) | IL-8 (pg/mL) | IL-10 (pg/mL) | MCP-1 (pg/mL) | TNFα (pg/mL) | CRP (mg/L) | IL-6 (pg/mL) | TNFα (pg/mL) | ||
| Cord blood | ||||||||||
| CRP (mg/L)a | 0.09 (0.04, 0.18) | 1.00 | 0.28 | 0.28 | 0.27 | 0.05 | 0.02 | 0.26 | 0.15 | −0.02 |
| IL-6 (pg/mL) | 9.85 (4.80, 24.46) | 1.00 | 0.64 | 0.53 | 0.32 | 0.24 | 0.02 | −0.02 | 0.00 | |
| IL-8 (pg/mL) | 16.32 (8.20, 33.64) | 1.00 | 0.56 | 0.42 | 0.33 | 0.04 | 0.02 | 0.03 | ||
| IL-10 (pg/mL) | 12.85 (8.54, 22.85) | 1.00 | 0.26 | 0.31 | 0.05 | 0.00 | 0.04 | |||
| MCP-1 (pg/mL) | 665.44 (484.24, 909.20) | 1.00 | 0.64 | 0.02 | −0.03 | −0.08 | ||||
| TNFα (pg/mL) | 32.33 (24.84, 41.59) | 1.00 | 0.02 | −0.06 | −0.05 | |||||
| Maternal blood | ||||||||||
| CRP (mg/L) | 4.48 (2.49, 8.16) | 1.00 | 0.50 | 0.02 | ||||||
| IL-6 (pg/mL) | 1.36 (0.97, 1.99) | 1.00 | 0.10 | |||||||
| TNFα (pg/mL) | 1.11 (0.69, 1.81) | 1.00 | ||||||||
Abbreviations: IQR, interquartile range; CRP, C-reactive protein; IL-6 interleukin-6; IL-8 interleukin-8; IL-10 interleukin-10; MCP-1 monocyte chemoattractant protein 1; TNFα, tumor necrosis factor-α.
Missing data for cord blood CRP (n=1).
Median concentrations of cord blood biomarkers varied by several orders of magnitude, with the lowest median concentration observed for cord blood IL-6 (IL-6cb) (9.85 pg/mL, IQR 4.80-24.46 pg/mL) and the highest median concentration observed for CRPcb (0.09 mg/L, IQR 0.04-0.18 mg/L). The pairwise Spearman correlations between the cord blood inflammatory biomarkers ranged from 0.02 to 0.64, with the highest correlations observed between IL-6cb and IL-8cb (rs=0.64) and between MCP-1cb and TNFαcb (rs=0.64) (Table 2).
For the three inflammatory biomarkers that were measured in both cord blood and maternal blood, the observed median concentrations of CRP were lower in cord blood compared to maternal blood (0.09 mg/L cord versus 4.48 mg/L maternal), whereas the median levels of both IL-6 and TNFα were higher in cord blood compared to maternal blood (IL-6: 9.85 pg/mL cord versus 1.36 pg/mL maternal; TNFα: 32.33 pg/mL cord versus 1.11 pg/mL maternal). Cord blood and maternal inflammatory biomarkers were largely uncorrelated with each other (Spearman correlation range: −0.02, 0.15), except CRP which was moderately correlated between maternal and cord blood samples (rs=0.26) (Table 2).
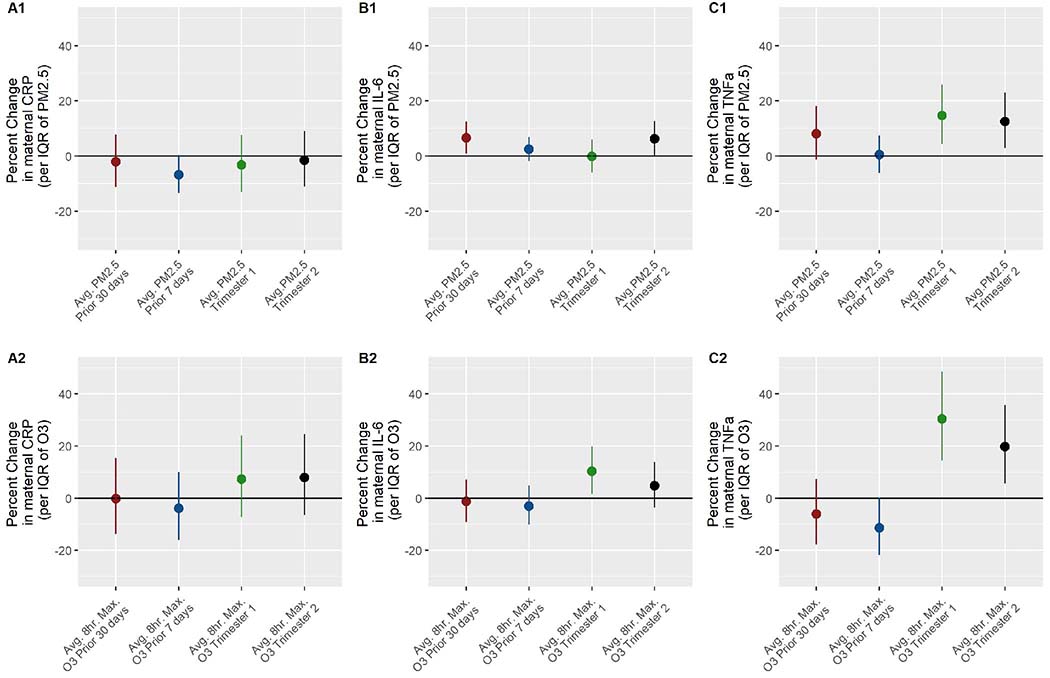
In adjusted models including the other co-pollutant, we observed higher TNFαm with higher trimester-long concentrations of PM2.5 and O3. For example, an IQR increase in trimester 1 O3 was associated with 30.39% (95% CI; 14.46, 48.54) higher TNFαm (Figure 1 and Supplementary Table 3). Higher trimester 1 and 2 PM2.5 was associated with higher TNFαm, as well as prior 30-day PM2.5 though the 95% CI included the null for this time period. PM2.5 was generally positively associated with IL-6m, notably for the prior 30- days to sample collection and trimester 2. For example, in the prior 30- days to sample collection, an IQR increase in PM2.5 was associated with 6.54% higher IL-6m (95% CI; 0.86, 12.53). PM2.5 in the 7-days prior had a non-significant negative association with CRPm. Higher trimester 1 and 2 O3 exposure was associated with higher IL-6m, though the 95% CI for trimester 2 included the null. Higher prior 30- and 7- day O3 exposure was non-significantly associated with lower TNFαm. For example, in the prior 7-days to sample collection, an IQR increase in O3 was associated with 11.43% (95% CI; −21.83, 0.34) lower TNFαm. There were non-significant positive associations between trimester 1 and 2 O3 exposure and CRPm. Results from unadjusted models were generally similar in direction and magnitude to adjusted results, except that associations between trimester 1 and 2 PM2.5 and TNFαm were attenuated and no longer significant (Supplementary Table 4). Results from single pollutant models were comparable (Supplementary Table 6).
Figure 1:
A1 and A2. Associations between average PM2.5 and 8-hour maximum O3 at the residential address during pregnancy and maternal CRP, respectively; B1 and B2. Maternal IL-6; C1 and C2. Maternal TNFα. Adjusted for maternal age, pre-pregnancy BMI, race, smoking status, median income in the Census tract, education, parity, infant sex, gestational age at sample collection, and the corresponding co-pollutant during the specified pregnancy period. Sample sizes differ by model due to missing PM2.5 exposure data (n=number missing): prior 30 days to sample collection, n=31; prior 7 days to sample collection, n=30; trimester 1, n=46; trimester 2, n=44.
Abbreviations: IQR, interquartile range; PM2.5, particulate matter with diameter ≤ 2.5 micrometers; O3, average 8-hour maximum ozone concentration in parts per billion; CRP, C-reactive protein; IL-6 interleukin-6; TNFa, tumor necrosis factor-α.
PM2.5 exposure was not consistently significantly associated with any cord blood inflammatory biomarker, however, there were several notable trends. Specifically, there were non-significant positive relationships between trimester 1, 2, and full-pregnancy PM2.5 and CRPcb. Additionally, PM2.5 exposure in trimester 1 and trimester 3 was associated with higher and lower IL-8cb levels, respectively. Finally, trimester 2, 3, and full-pregnancy PM2.5 was generally associated with lower MCP-1cb and TNFαcb, but higher levels of these inflammatory biomarkers when considering prior 30-day PM2.5 exposure (Table 3 and Supplementary Figure 5).
Table 3:
Associations between average PM2.5 and 8-hour maximum O3, at the residential address during pregnancy and cord blood inflammatory biomarkers.
| CRP (mg/L) | IL-6 (pg/mL) | IL-8 (pg/mL) | IL-10 (pg/mL) | MCP-1 (pg/mL) | TNFα (pg/mL) | |
|---|---|---|---|---|---|---|
| % change (95% CI)a per IQR | % change (95% CI)a per IQR | % change (95% CI)a per IQR | % change (95% CI)a per IQR | % change (95% CI)a per IQR | % change (95% CI)a per IQR | |
| Trimester 1 (n=469) | ||||||
| Avg. PM2.5 (μg/m3) | 4.98 (−11.26, 24.19) | 3.24 (−13.43, 23.11) | 12.19 (−2.42, 28.98) | −2.76 (−12.49, 8.04) | −0.16 (−6.27, 6.34) | −0.03 (−5.22, 5.45) |
| Avg. 8-hr max O3 (ppb) | 3.46 (−33.48, 60.92) | −16.88 (−47.59, 31.82) | −19.02 (−43.80, 16.70) | −9.70 (−31.48, 19.01) | −2.86 (−17.66, 14.60) | −0.30 (−13.30, 14.64) |
| Trimester 2 (n=471) | ||||||
| Avg. PM2.5 (μg/m3) | 7.57 (−8.03, 25.81) | 4.81 (−11.37, 23.94) | 2.67 (−10.10, 17.25) | 2.95 (−6.82, 13.76) | −0.70 (−6.50, 5.47) | −4.62 (−9.48, 0.50) |
| Avg. 8-hr max O3 (ppb) | 44.94 (−2.43, 115.31) | 55.68 (1.68, 138.35) | 39.78 (−0.24, 95.86) | 6.07 (−17.68,36.69) | 14.89 (−1.42, 33.90) | 1.65 (−11.00, 16.10) |
| Trimester 3 (n=498) | ||||||
| Avg. PM2.5 (μg/m3) | −1.04 (−16.02, 16.61) | −3.96 (−19.35, 14.38) | −7.11 (−19.31, 6.94) | −3.74 (−13.30, 6.87) | −5.46 (−11.25, 0.71) | −4.82 (−9.91, 0.56) |
| Avg. 8-hr max O3 (ppb) | 24.37 (−18.24, 89.19) | −7.09 (−40.59, 45.31) | 4.63 (−27.04, 50.05) | 12.99 (−13.55, 47.67) | 0.79 (−14.26, 18.49) | −2.77 (−15.53, 11.92) |
| Full pregnancy (n=511) | ||||||
| Avg. PM2.5 (μg/m3) | 6.89 (−8.53, 24.90) | 0.07 (−15.38, 18.34) | 3.45 (−9.60, 18.40) | −2.17 (−11.50, 8.15) | −3.84 (−9.51, 2.18) | −6.04 (−10.82, −1.01) |
| Avg. 8-hr max O3 (ppb) | 20.71 (−5.89, 54.83) | 6.52 (−18.51, 39.26) | 6.53 (−14.13, 32.16) | −0.67 (−15.38, 16.59) | 2.63 (−6.86, 13.09) | −4.60 (−12.23, 3.70) |
| Prior 30 days to delivery (n=483) | ||||||
| Avg. PM2.5 (μg/m3) | −2.69 (−16.99, 14.07) | 2.61 (−13.22, 21.32) | 1.27 (−11.54, 15.93) | −0.29 (−9.85, 10.30) | 3.49 (−2.64, 10.00) | 1.86 (−3.25, 7.23) |
| Avg. 8-hr max O3 (ppb) | 12.32 (−23.03, 63.91) | −32.19 (−54.47, 0.99) | −16.67 (−39.58, 14.93) | 12.34 (−11.62, 42.80) | −8.85 (−21.16, 5.37) | −6.01 (−16.82, 6.21) |
| Prior 7 days to delivery (n=488) | ||||||
| Avg. PM2.5 (μg/m3) | −0.30 (−9.21, 9.48) | 2.37 (−7.23, 12.97) | 4.06 (−3.88, 12.66) | 0.63 (−5.10, 6.71) | 0.31 (−3.23, 3.98) | 1.03 (−2.05, 4.21) |
| Avg. 8-hr max O3 (ppb) | 5.64 (−24.57, 47.96) | −20.89 (−44.52, 12.79) | −5.51 (−28.99, 25.75) | 7.63 (−12.86, 32.94) | 4.50 (−8.18 18.94) | 2.75 (−8.10, 14.87) |
Abbreviations: 95% CI, 95% Confidence Interval; PM2.5, particulate matter with diameter ≤ 2.5 micrometers; O3, average 8-hour maximum ozone concentration in parts per billion; IQR, interquartile range; CRP, C-reactive protein; IL-6 interleukin-6; IL-8 interleukin-8; IL-10 interleukin-10; MCP-1, monocyte chemoattractant protein 1; TNFα, tumor necrosis factor-α.
All cord blood inflammatory biomarker outcomes were natural-log transformed.
Adjusted for maternal age, pre-pregnancy BMI, race, smoking status, median income in the Census tract, education, mode of delivery, parity, season of birth, infant sex, and the corresponding co-pollutant during tire specified pregnancy period.
Similarly, O3 was not consistently significantly associated with any cord blood inflammatory marker, but there were some trends. There were non-significant positive associations between O3 during all of the examined periods of pregnancy and CRPcb. Trimester 2 O3 was positively associated with IL-6cb, IL-8cb, and MCP-1cb, and trimester 1, and prior 7- and 30-day O3 was negatively associated with IL-6cb and IL-8cb (Table 3 and Supplementary Figure 6).
We did not find evidence for exposure-by-sex interaction in cord blood at alpha of 0.05. Results from unadjusted models are presented in Supplementary Table 5. In single pollutant models, results were similar (Supplementary Table 7).
In the sensitivity analysis excluding participants who smoked during pregnancy, the results for the associations between the air pollutants and cord blood and maternal inflammatory biomarkers did not differ appreciably (Supplementary Tables 8 and 9). Similarly, the associations between the air pollutants and cord blood cytokines, excluding values that were above the maximum detectable concentration (n=4 total), were comparable to the results of models in which we substituted the maximum detected value (Supplementary Table 10).
CRPm was positively associated with infant gestational age at birth (β; 1.30, 95% CI; 0.40-2.21). There were no associations between CRPm, IL-6m, and TNFαm and birthweight, adiposity at birth, and adiposity at five-month follow-up (Table 4). We did not observe associations between the maternal inflammatory biomarkers and fat mass and fat free mass at birth and at five-month follow-up (Supplementary Table 11). Results remained null, after excluding preterm births and adjusting for gestational age (Supplementary Tables 12, 13, 14, and 15).
Table 4:
Associations between maternal inflammatory biomarkers during pregnancy and infant birth and adiposity outcomes.
| Birth weight (g) (n=515) | Gestational age (days) (n=515) | Adiposity at birth (%) (n=497) | Adiposity at postnatal (~5 months) follow-up (%) (n=334) | |
|---|---|---|---|---|
| β (95% CI) per log-unita | β (95% CI) per log-unita | β (95% CI) per log-unita | β (95% CI) per log-unitb | |
| CRP (mg/L) | 12.48 (−31.31, 56.27) | 1.30 (0.40, 2.21) | 0.06 (−0.34, 0.45) | 0.43 (−0.26, 1.12) |
| IL-6 (pg/mL) | 10.51 (−66.94, 87.95) | 0.24 (−1.37, 1.86) | 0.23 (−0.47, 0.93) | −0.04 (−1.30, 1.21) |
| TNFα (pg/mL) | 23.30 (−22.72, 69.31) | 0.29 (−0.67, 1.25) | −0.11 (−0.53, 0.31) | −0.14 (−0.91, 0.63) |
Abbreviations: 95% CI, 95% Confidence Interval; CRP, C-reactive protein; IL-6 interleukin-6; TNFα, tumor necrosis factor-α
All maternal inflammatory biomarker outcomes were natural log transformed.
Adjusted for race, maternal age, median income in the Census tract, pre-pregnancy BMI, education, smoking status, gestational age at sample collection, infant sex, and parity.
Additionally adjusted for infant age at the time of adiposity measurement.
4. Discussion
In this racially and ethnically diverse sample of mother-infant dyads, we found associations between exposure to PM2.5 and O3 during several time windows and some maternal inflammatory biomarkers during mid-pregnancy. We also observed associations between air pollution during some time windows in pregnancy and inflammatory biomarkers in cord blood at delivery, though patterns were inconsistent.
Dysregulation of the maternal immune response has been identified in pregnancy-related diseases, including pre-eclampsia, gestational diabetes (GDM),38, 39 and adverse birth outcomes, including fetal growth restriction and preterm birth.40, 41 Given the impact of maternal inflammation on both maternal and fetal health, a greater understanding of potentially preventable sources of alterations in the immune profile, such as air pollution, is pertinent. A prior study investigated the relationship between air pollution and the maternal lymphocyte immune profile.33 In a cross-sectional study in the Czech Republic, Hertz-Picciotto and colleagues found mothers who gave birth in a city with high levels of air pollution had lower percentages of T cells and a lower CD4+/CD8+ ratio compared to those who lived in a less polluted city.33 However, this study did not examine associations between ambient air pollution with cytokines. Cytokines are pleiotropic factors responsible for mediating much of T-lymphocyte function,48 as well as several other functions, and they may be mediators in the relationship between pregnancy environmental exposures and adverse offspring outcomes.
Dysregulation of IL-6,49 TNFα,50 and CRP40, 51 has been linked to pregnancy complications associated with the cardio-metabolic health of the mother and the neonate, including preeclampsia and preterm birth. During healthy pregnancy, upregulation of cytokines may promote the typical increase in maternal insulin resistance necessary for normal fetal growth; however, an excessive inflammatory response may induce abnormal insulin resistance and in some cases, the development of GDM.52–54 Higher CRP during pregnancy is found in women with obesity, which is associated with decreased insulin sensitivity during pregnancy.55 Higher levels of IL-6 and in some studies TNFα have also been associated with insulin resistance56 and GDM, respectively.49
We found positive associations between PM2.5 and IL-6m levels during mid-pregnancy. We also observed positive associations for trimester-long O3 exposure and IL-6m. While there is a lack of literature exploring this relationship in pregnant women, this finding is consistent with epidemiologic evidence suggesting an association between acute exposure to particulate matter and IL-6 in adult cohorts.57–59 However, other studies have reported null results.60, 61 IL-6 is a pro-inflammatory cytokine that has roles in both acute and chronic inflammatory responses, and in rodents, elevated levels of IL-6 and lower levels of its inhibitor (soluble gp130) have been associated with fetal loss, preterm birth, and infertility, and they have also been observed in pregnant women with preeclampsia and gestational diabetes.49 In early pregnancy, IL-6 is involved in placental development, in mid-pregnancy IL-6 levels are highly regulated and its production and activity are suppressed, and finally during labor, IL-6 levels raise to facilitate parturition.49 Disruptions in the tightly regulated and temporally-controlled levels of maternal IL-6 during pregnancy, may represent a pathophysiological rationale for the adverse pregnancy and birth outcomes observed in association with environmental exposures, such as air pollution.
We also observed positive associations between PM2.5 and O3 exposure and maternal mid-pregnancy TNFα. Elevated TNFα levels have been observed following acute exposure to particulate matter57 and diesel exhaust62 in healthy adults, however, we only observed associations with trimester-average, and not more short-term, exposure. The placenta and adipose cells are two of the main sources of TNFα production during pregnancy, and higher TNFα levels are observed during the pregravid and late pregnancy periods, with a decline in serum levels in early pregnancy.56, 63 TNFα is a known inhibitor of the tyrosine phosphorylation cascade of the insulin receptor, and as a result, it is associated with the normal insulin resistance that is characteristic of pregnancy.63 However, further elevated levels of TNFα have been identified in GDM, and consequently, it has been hypothesized to be a mediator of the growth-related birth outcomes associated with GDM and environmental exposures, such as tobacco smoke.63, 64 The present study suggests air pollution exposure during pregnancy may also disturb regulation of TNFα and given the between link between TNFα and metabolic homeostasis, this may have health implications for both the mother and the offspring.
We noted non-significant positive associations for trimester-long O3 exposure and maternal CRP levels. Previous findings from 5,067 mothers in a population-based prospective cohort in the Netherlands identified higher PM10 exposure during the prior 1-2 weeks to be associated with elevated concentrations of maternal CRP (>8 ng/mL) in trimester 1.30 Further, a study of 1,696 mothers in Pennsylvania found PM2.5 in the 28 days prior to the blood sample to be associated with higher odds of elevated maternal CRP (≥8 ng/mL) in early pregnancy for non-smokers, along with positive, but non-significant, associations with PM10 and O3 exposure.37 One potential explanation for this discrepancy is the variation in air pollution exposure levels by geographic location, as average particulate matter concentrations in Colorado65 are much lower than those found in major cities in Europe66 and the northeastern US.67
Previous literature examining associations between maternal air pollution exposure and changes in the cord blood immune profile has noted alterations in absolute numbers and percentages of lymphocyte populations.32–35 Among those that included cord blood cytokines that overlap with the set included in our study, none included PM2.5 and O3 as exposures. It is therefore difficult to directly compare findings. Latzin and colleagues found PM10 exposure late in pregnancy to be associated with lower IL-10 and higher IL-1β levels, but no associations with MCP-1 and IL-6, in cord blood.31 Van den Hooven and colleagues noted positive associations between full pregnancy PM10 and NO2 exposure and cord blood CRP levels.30 Future investigations are necessary to confirm our findings.
Interestingly, levels of cytokines in maternal blood were not highly correlated with levels of the same cytokines in cord blood. Samples of maternal blood that were used for cytokine measurement were collected in mid-pregnancy, whereas the cord blood samples were collected at delivery, and thus the difference in the timing of sample collection may be a potential explanation for the discordance. Additionally, maternal and cord blood inflammatory biomarker levels were measured using different assays and labs and were sampled from different tissues, which may explain the large differences in concentrations we observed. It is also possible that this contributed to the low correlations, however, by using the Spearman correlation coefficient, which is calculated based on rank order, we expect the correlations to be somewhat robust to measurement error. There is scarce evidence regarding concordance between maternal and cord blood immune profiles,68, 69 and a handful of ex vivo studies reported conflicting results regarding the degree of transplacental transfer of different cytokines.70–72 Studies comparing inflammatory biomarkers in maternal blood with those in amniotic fluid during mid-pregnancy have found some, but not strong, concordance.73, 74
Changes in the maternal immune response during pregnancy have been linked to preterm birth41, 75, 76 and changes in body composition at birth,77–80 though not consistently.81 Our finding that elevated levels of maternal CRP were associated with modestly older gestational age at birth was unexpected, in light of some previous studies that have found positive associations between levels of CRP in amniotic fluid and plasma and preterm birth, albeit a weaker association in plasma compared to amniotic fluid.76 Epidemiologic studies have found inverse associations between maternal CRP and birthweight,40, 80 length,78 percent body fat,80 and sum of skin folds.78, 79 While associations between maternal inflammation during pregnancy and adiposity have been noted in mid-childhood,82 we did not observe associations between maternal inflammatory biomarkers and body composition outcomes at five months postnatally. Since growth patterns in the first year differ from those later in life, it is possible that five months may be too early to observe alterations in body composition associated with maternal inflammation during pregnancy.
A recent study from the Healthy Start cohort found limited evidence for associations between the air pollution measures used in the present study and birth outcomes.42 We did not find associations between maternal inflammatory biomarkers and birth weight and infant body composition. Nevertheless, inflammation has been posited as a potential mechanistic link between in utero air pollution exposure and birth and outcomes in offspring.83 Findings from an animal study84 and a case-control study64 have begun to elucidate this relationship; future prospective observational studies are necessary.
Our study has several strengths and limitations. We utilized data from a prospective, pre-birth cohort, which allowed for adjustment of several potential confounders. The air pollution profile of the Denver metropolitan area is unique, as it is characterized by relatively low levels of PM2.5 compared to other major urban areas, but comparatively high levels of O3, which differs from other more highly studied geographic locations in the US.65 Even with the relatively low levels of particulate matter and limited variability, we observed associations with maternal pro-inflammatory cytokines. Few studies have investigated this relationship with a comprehensive panel of cytokines in maternal and cord blood concomitantly. The findings from this study contribute to existing literature characterizing the poorly understood relationship between maternal and fetal immune profiles.
We estimated exposure to air pollution using inverse-distance-weighted interpolation from stationary monitoring stations and were therefore unable to capture smaller-scale spatial variability of living close to roads and other sources of air pollution. Participants self-reported their residential address at enrollment, thus, there is potential for misclassification due to participants moving during pregnancy, as well as indoor air pollution exposures85 and exposures occurring outside of the area of residence, such as exposure during commuting and work. A prior study found pregnant women in New York did not move often, and when they did, it was over short distances.86 Thus, large changes in exposure estimates due to residential mobility, were not observed.86 While this information is unavailable for the Colorado-based Healthy Start study, it provides evidence that residential mobility may not result in substantial measurement error. Further, the air pollution measurement error is not likely to be related to the inflammatory biomarkers, and thus, may result in an underestimation of associations or a reduction in power.87 Our analysis was limited to PM2.5 and O3, however, other components of air pollution and mixtures of air pollutants may be of additional interest.
For some women, the blood sample collection occurred prior to the end of the first trimester. While this does not introduce an issue of reverse causality, it is possible that it may lead to a small amount of measurement error. Further, the levels of maternal inflammatory biomarkers fluctuate throughout pregnancy,88 and the samples in our study were collected at a single mid-pregnancy timepoint. Therefore, the conclusions based on findings from our study cannot be generalized to levels of inflammatory biomarkers in other time periods during pregnancy. Despite this limitation, there was little variation in the gestational ages at maternal blood sample collection (mean; 191, SD; 17 days), and thus, our data consistently captured the mid-pregnancy time-period; future studies are necessary to characterize these relationships with levels of early- and late- pregnancy inflammatory biomarkers. Additionally, our study was limited to six inflammatory biomarkers, three in maternal blood, and six in cord blood, and high throughput immune profile platforms are now available, which allow for the analysis of several hundred immune biomarkers simultaneously. Finally, we included only pro-inflammatory biomarkers from the mother, which did not allow for the investigation of composite indices with relative measures of pro- to anti-inflammatory measures.69
5. Conclusions
We observed estimated ambient concentrations of PM2.5 and O3 in early pregnancy to be associated with maternal levels of pro-inflammatory cytokines, during mid-pregnancy. However, levels of these maternal inflammatory biomarkers were not associated with infant birth weight, nor body composition at birth and at five months. There was some evidence supporting relationships between PM2.5 and O3 exposure and inflammatory biomarkers in cord blood at delivery, but patterns were inconsistent, and generally 95% confidence intervals included the null. Our findings highlight the potential for environmental pollutants during pregnancy to alter the maternal immune response. Future research may investigate the health implications of a heightened inflammatory response associated with air pollution exposure during pregnancy.
Supplementary Material
Highlights.
Exposure to particulate matter ≤2.5 and ozone was estimated for 515 pregnant women
Inflammatory biomarkers were measured in maternal and cord blood
Early pregnancy air pollution associated with mid-pregnancy maternal inflammation
Inconsistent associations between prenatal air pollution and cord blood inflammation
Maternal inflammatory markers did not predict infant outcomes
Acknowledgements
This work was supported in part by grants from the National Institute of Environmental Health Sciences (R00ES025817), the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK076648), and the National Institutes of Health Office of the Director (UH3OD023248). The University of Colorado Cancer Center laboratory received support from the National Cancer Institute (P30CA046934). Funders had no involvement in the data collection, analysis, or interpretation of results, and were not involved in the writing of the article or the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The Colorado Multiple Institutional Review Board approved all study protocols.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CRP
C-reactive protein
- ELISA
enzyme-linked immunosorbent assay
- EPA
Environmental Protection Agency
- GDM
gestational diabetes mellitus
- ICC
intra-class correlation coefficient
- IL
interleukin
- IQR
interquartile range
- MCP-1
monocyte chemoattractant protein 1
- NO2
nitrogen dioxide
- O3
ozone
- PM2.5
particulate matter ≤2.5 micrometers
- PM10
particulate matter ≥10 micrometers
- ppb
part per billion
- SGA
small for gestational age
- TNFα
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Works Cited
- 1.Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. Environmental pollutants and child health—A review of recent concerns. International Journal of Hygiene and Environmental Health. 2016;219(4):331–42. [DOI] [PubMed] [Google Scholar]
- 2.Russ K, Howard S. Developmental Exposure to Environmental Chemicals and Metabolic Changes in Children. Current Problems in Pediatric and Adolescent Health Care. 2016;46(8):255–85. [DOI] [PubMed] [Google Scholar]
- 3.Holstius DM, Reid CE, Jesdale BM, Morello-Frosch R. Birth weight following pregnancy during the 2003 Southern California wildfires. Environ Health Perspect. 2012;120(9):1340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environmental research. 2012;117:100–11. [DOI] [PubMed] [Google Scholar]
- 5.Shah PS, Balkhair T. Air pollution and birth outcomes: a systematic review. Environ Int. 2011;37(2):498–516. [DOI] [PubMed] [Google Scholar]
- 6.Sapkota A, Chelikowsky AP, Nachman KE, Cohen AJ, Ritz B. Exposure to particulate matter and adverse birth outcomes: a comprehensive review and meta-analysis. Air Quality, Atmosphere & Health. 2012;5(4):369–81. [Google Scholar]
- 7.Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA, et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013;121(3):267–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environmental health perspectives. 2008;116(5):680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.šrám RJ, Binková B, Dejmek J, Bobak M. Ambient air pollution and pregnancy outcomes: a review of the literature. Environmental health perspectives. 2005;113(4):375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei Y, Zhang J, Li Z, Gow A, Chung KF, Hu M, et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: findings from a natural experiment in Beijing. The FASEB Journal. 2016;30(6):2115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL, et al. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. The FASEB Journal. 2012;26(11):4743–54. [DOI] [PubMed] [Google Scholar]
- 12.Chiu YM, Hsu HL, Wilson A, Coull BA, Pendo MP, Baccarelli A, et al. Prenatal particulate air pollution exposure and body composition in urban preschool children: Examining sensitive windows and sex-specific associations. Environ Res. 2017;158:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rundle A, Hoepner L, Hassoun A, Oberfield S, Freyer G, Holmes D, et al. Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol. 2012;175(11):1163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawlor DA, Chaturvedi N. Treatment and prevention of obesity—are there critical periods for intervention? International Journal of Epidemiology. 2006;35(1):3–9. [DOI] [PubMed] [Google Scholar]
- 15.Grün F, Blumberg B. Minireview: the case for obesogens. Mol Endocrinol. 2009;23(8):1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hehua Z, Qing C, Shanyan G, Qijun W, Yuhong Z. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: A systematic review. Environmental Research. 2017;159:519–30. [DOI] [PubMed] [Google Scholar]
- 17.Leon Hsu H-H, Mathilda Chiu Y-H, Coull BA, Kloog I, Schwartz J, Lee A, et al. Prenatal particulate air pollution and asthma onset in urban children. Identifying sensitive windows and sex differences. American journal of respiratory and critical care medicine. 2015;192(9):1052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korten I, Ramsey K, Latzin P. Air pollution during pregnancy and lung development in the child. Paediatric Respiratory Reviews. 2017;21:38–46. [DOI] [PubMed] [Google Scholar]
- 19.Latzin P, Röösli M, Huss A, Kuehni CE, Frey U. Air pollution during pregnancy and lung function in newborns: a birth cohort study. European Respiratory Journal. 2009;33(3):594–603. [DOI] [PubMed] [Google Scholar]
- 20.Amarilyo G, Oren A, Mimouni FB, Ochshorn Y, Deutsch V, Mandel D. Increased cord serum inflammatory markers in small-for-gestational-age neonates. Journal of Perinatology. 2011;31(1):30–2. [DOI] [PubMed] [Google Scholar]
- 21.Matoba N, Ouyang F, Mestan KKL, Porta NFM, Pearson CM, Ortiz KM, et al. Cord blood immune biomarkers in small for gestational age births. J Dev Orig Health Dis. 2011;2(2):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duijts L, Bakker-Jonges LE, Labout JA, Jaddoe VW, Hofman A, Steegers EA, et al. Fetal growth influences lymphocyte subset counts at birth: the Generation R Study. Neonatology. 2009;95(2):149–56. [DOI] [PubMed] [Google Scholar]
- 23.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. Bmj. 2005;331(7522):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelishadi R, Poursafa P. A Review on the Genetic, Environmental, and Lifestyle Aspects of the Early-Life Origins of Cardiovascular Disease. Current Problems in Pediatric and Adolescent Health Care. 2014;44(3):54–72. [DOI] [PubMed] [Google Scholar]
- 25.Rogers I The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord. 2003;27(7):755–77. [DOI] [PubMed] [Google Scholar]
- 26.Perng W, Hajj H, Belfort MB, Rifas-Shiman SL, Kramer MS, Gillman MW, et al. Birth Size, Early Life Weight Gain, and Midchildhood Cardiometabolic Health. J Pediatr. 2016;173:122–30.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–8. [DOI] [PubMed] [Google Scholar]
- 28.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators of inflammation. 2010;2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochemical Society Transactions. 2005;33(5):1078–81. [DOI] [PubMed] [Google Scholar]
- 30.Van Den Hooven EH, De Kluizenaar Y, Pierik FH, Hofman A, Van Ratingen SW, Zandveld PYJ, et al. Chronic Air Pollution Exposure during Pregnancy and Maternal and Fetal C-Reactive Protein Levels: The Generation R Study. Environmental Health Perspectives. 2012;120(5):746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latzin P, Frey U, Armann J, Kieninger E, Fuchs O, Röösli M, et al. Exposure to moderate air pollution during late pregnancy and cord blood cytokine secretion in healthy neonates. PloS one. 2011;6(8):e23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baïz N, Slama R, Béné M-C, Charles M-A, Kolopp-Sarda M-N, Magnan A, et al. Maternal exposure to air pollution before and during pregnancy related to changes in newborn’s cord blood lymphocyte subpopulations. The EDEN study cohort. BMC Pregnancy and Childbirth. 2011;11(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hertz-Picciotto I, Dostal M, Dejmek J, Selevan SG, Wegienka G, Gomez-Caminero A, et al. Air pollution and distributions of lymphocyte immunophenotypes in cord and maternal blood at delivery. Epidemiology. 2002;13(2):172–83. [DOI] [PubMed] [Google Scholar]
- 34.Hertz-Picciotto I, Herr CE, Yap P-S, Dostál M, Shumway RH, Ashwood P, et al. Air pollution and lymphocyte phenotype proportions in cord blood. Environmental health perspectives. 2005;113(10):1391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herr CEW, Dostal M, Ghosh R, Ashwood P, Lipsett M, Pinkerton KE, et al. Air pollution exposure during critical time periods in gestation and alterations in cord blood lymphocyte distribution: a cohort of livebirths. Environmental Health. 2010;9(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ashley-Martin J, Lavigne E, Arbuckle TE, Johnson M, Hystad P, Crouse DL, et al. Air pollution during pregnancy and cord blood immune system biomarkers. Journal of occupational and environmental medicine. 2016;58(10):979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee P-C, Talbott EO, Roberts JM, Catov JM, Sharma RK, Ritz B. Particulate air pollution exposure and C-reactive protein during early pregnancy. Epidemiology (Cambridge, Mass). 2011;22(4):524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pantham P, Aye ILH, Powell TL. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta. 2015;36(7):709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harmon AC, Cornelius DC, Amaral LM, Faulkner JL, Cunningham MW, Wallace K, et al. The role of inflammation in the pathology of preeclampsia. Clinical science. 2016;130(6):409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tjoa ML, van Vugt JMG, Go ATJJ, Blankenstein MA, Oudejans CBM, van Wijk IJ. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. Journal of Reproductive Immunology. 2003;59(1):29–37. [DOI] [PubMed] [Google Scholar]
- 41.Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D, Divanovic S. Inflammation and preterm birth. J Leukoc Biol. 2016;99(1):67–78. [DOI] [PubMed] [Google Scholar]
- 42.Starling AP, Moore BF, Thomas DSK, Peel JL, Zhang W, Adgate JL, et al. Prenatal exposure to traffic and ambient air pollution and infant weight and adiposity: The Healthy Start study. Environmental Research. 2020;182:109130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padula AM, Mortimer KM, Tager IB, Hammond SK, Lurmann FW, Yang W, et al. Traffic-related air pollution and risk of preterm birth in the San Joaquin Valley of California. Ann Epidemiol. 2014;24(12):888–95e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mobasher Z, Salam MT, Goodwin TM, Lurmann F, Ingles SA, Wilson ML. Associations between ambient air pollution and Hypertensive Disorders of Pregnancy. Environ Res. 2013;123:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rivera-Gonzalez LO, Zhang Z, Sanchez BN, Zhang K, Brown DG, Rojas-Bracho L, et al. An assessment of air pollutant exposure methods in Mexico City, Mexico. J Air Waste Manag Assoc. 2015;65(5):581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urlando A, Dempster P, Aitkens S. A New Air Displacement Plethysmograph for the Measurement of Body Composition in Infants. Pediatric Research. 2003;53(3):486–92. [DOI] [PubMed] [Google Scholar]
- 47.Johnson MJ, Wootton SA, Leaf AA, Jackson AA. Preterm birth and body composition at term equivalent age: a systematic review and meta-analysis. Pediatrics. 2012;130(3):e640–e9. [DOI] [PubMed] [Google Scholar]
- 48.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76(2):241–51. [DOI] [PubMed] [Google Scholar]
- 49.Prins JR, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol. 2012;95(1-2):1–14. [DOI] [PubMed] [Google Scholar]
- 50.Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, Llurba E, Gris JM. Tumor Necrosis Factor-Alpha and Pregnancy: Focus on Biologies. An Updated and Comprehensive Review. Clin Rev Allergy Immunol. 2017;53(1):40–53. [DOI] [PubMed] [Google Scholar]
- 51.Grgic G, Skokie F, Bogdanovic G. C-reactive protein as a biochemical marker of idiopathic preterm delivery. Med Arh. 2010;64(3):132–4. [PubMed] [Google Scholar]
- 52.Richardson AC, Carpenter MW. Inflammatory mediators in gestational diabetes mellitus. Obstetrics and gynecology clinics of North America. 2007;34(2):213–24. [DOI] [PubMed] [Google Scholar]
- 53.Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes care. 2007;30(Supplement 2):S112–S9. [DOI] [PubMed] [Google Scholar]
- 54.Hauguel-de Mouzon S, Guerre-Millo M. The Placenta Cytokine Network and Inflammatory Signals. Placenta. 2006;27(8):794–8. [DOI] [PubMed] [Google Scholar]
- 55.Retnakaran R, Hanley AJG, Raif N, Connelly PW, Sermer M, Zinman B. C-Reactive Protein and Gestational Diabetes: The Central Role of Maternal Obesity. The Journal of Clinical Endocrinology & Metabolism. 2003;88(8):3507–12. [DOI] [PubMed] [Google Scholar]
- 56.Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51(7):2207–13. [DOI] [PubMed] [Google Scholar]
- 57.Pope CA III, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, O’toole T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circulation research. 2016;119(11):1204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsai D-H, Amyai N, Marques-Vidal P, Wang J-L, Riediker M, Mooser V, et al. Effects of particulate matter on inflammatory markers in the general adult population. Particle and Fibre Toxicology. 2012;9(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med. 2001;164(5):826–30. [DOI] [PubMed] [Google Scholar]
- 60.Dobreva ZG, Kostadinova GS, Popov BN, Petkov GS, Stanilova SA. Proinflammatory and anti-inflammatory cytokines in adolescents from Southeast Bulgarian cities with different levels of air pollution. Toxicology and Industrial Health. 2013;31(12):1210–7. [DOI] [PubMed] [Google Scholar]
- 61.Seaton A, Soutar A, Crawford V, Elton R, McNerlan S, Cherrie J, et al. Particulate air pollution and the blood. Thorax. 1999;54(11):1027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007; 176(4):395–400. [DOI] [PubMed] [Google Scholar]
- 63.Brogin Moreli J, Cirino Ruocco AM, Vernini JM, Rudge MVC, Calderon IMP. Interleukin 10 and Tumor Necrosis Factor-Alpha in Pregnancy: Aspects of Interest in Clinical Obstetrics. ISRN Obstetrics and Gynecology. 2012;2012:230742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niu Z, Xie C, Wen X, Tian F, Ding P, Fie Y, et al. Mediating role of maternal serum interleukin-1beta and tumor necrosis factor-alpha in the association between environmental tobacco smoke exposure in pregnancy and low birth weight at term. The Journal of Maternal-Fetal & Neonatal Medicine. 2018;31(10):1251–8. [DOI] [PubMed] [Google Scholar]
- 65.EPA, U. AQS Data Mart [Available from: https://aqs.epa.gov/aqsweb/documents/data_mart_welcome.html.
- 66.Eeftens M, Tsai M-Y, Ampe C, Anwander B, Beelen R, Bellander T, et al. Spatial variation of PM2.5, PM10, PM2.5 absorbance and PMcoarse concentrations between and within 20 European study areas and the relationship with NO2–results of the ESCAPE project. Atmospheric Environment. 2012;62:303–17. [Google Scholar]
- 67.Ebisu K, Bell ML. Airborne PM2.5 chemical components and low birth weight in the northeastern and mid-Atlantic regions of the United States. Environmental health perspectives. 2012; 120(12): 1746–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cicarelli LM, Perroni AG, Zugaib M, De Albuquerque PB, Campa A. Maternal and cord blood levels of serum amyloid A, C-reactive protein, tumor necrosis factor-α, interleukin-1β, and interleukin-8 during and after delivery. Mediators of inflammation. 2005;2005(2):96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ross KM, Miller G, Culhane J, Grobman W, Simhan HN, Wadhwa PD, et al. Patterns of peripheral cytokine expression during pregnancy in two cohorts and associations with inflammatory markers in cord blood. Am J Reprod Immunol. 2016;76(5):406–14. [DOI] [PubMed] [Google Scholar]
- 70.Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A. Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol. 2005;106(4):802–7. [DOI] [PubMed] [Google Scholar]
- 71.Reisenberger K, Egarter C, Vogl S, Sternberger B, Kiss H, Husslein P. The transfer of interleukin-8 across the human placenta perfused in vitro. Obstet Gynecol. 1996;87(4):613–6. [DOI] [PubMed] [Google Scholar]
- 72.Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004;103(3):546–50. [DOI] [PubMed] [Google Scholar]
- 73.Chow SSW, Craig ME, Jones CA, Hall B, Catteau J, Lloyd AR, et al. Differences in amniotic fluid and maternal serum cytokine levels in early midtrimester women without evidence of infection. Cytokine. 2008;44(1):78–84. [DOI] [PubMed] [Google Scholar]
- 74.Shobokshi A, Shaarawy M. Maternal serum and amniotic fluid cytokines in patients with preterm premature rupture of membranes with and without intrauterine infection. International Journal of Gynecology & Obstetrics. 2002;79(3):209–15. [DOI] [PubMed] [Google Scholar]
- 75.Huang L, Hou Q, Huang Y, Ye J, Huang S, Tian J, et al. Serum multiple cytokines for the prediction of spontaneous preterm birth in asymptomatic women: A nested case-control study. Cytokine. 2019;117:91–7. [DOI] [PubMed] [Google Scholar]
- 76.Wei S-Q, Fraser W, Luo Z-C. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstetrics & Gynecology. 2010;116(2):393–401. [DOI] [PubMed] [Google Scholar]
- 77.Radaelli T, Uvena-Celebrezze J, Minium J, Huston-Presley L, Catalano P, Hauguel-de Mouzon S. Maternal Interleukin-6: Marker of Fetal Growth and Adiposity. Journal of the Society for Gynecologic Investigation. 2006;13(1):53–7. [DOI] [PubMed] [Google Scholar]
- 78.Kuzawa CW, Fried RL, Borja JB, McDade TW. Maternal pregnancy C-reactive protein predicts offspring birth size and body composition in metropolitan Cebu, Philippines. J Dev Orig Health Dis. 2017;8(6):674–81. [DOI] [PubMed] [Google Scholar]
- 79.Lowe LP, Metzger BE, Lowe WL Jr., Dyer AR, McDade TW, McIntyre HD. Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab. 2010;95(12):5427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Oliveira LC, Franco-Sena AB, Farias DR, Rebelo F, Kac G. Maternal C-reactive protein concentrations during pregnancy and birth weight in a prospective cohort in Rio de Janeiro, Brazil. J Matern Fetal Neonatal Med. 2017;30(19):2346–53. [DOI] [PubMed] [Google Scholar]
- 81.Farah N, Hogan AE, O’Connor N, Kennelly MM, O’Shea D, Turner MJ. Correlation between maternal inflammatory markers and fetomaternal adiposity. Cytokine. 2012;60(1):96–9. [DOI] [PubMed] [Google Scholar]
- 82.Gaillard R, Rifas-Shiman SL, Perng W, Oken E, Gillman MW. Maternal inflammation during pregnancy and childhood adiposity. Obesity (Silver Spring). 2016;24(6):1320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vadillo-Ortega F, Osornio-Vargas A, Buxton MA, Sanchez BN, Rojas-Bracho L, Viveros-Alcaraz M, et al. Air pollution, inflammation and preterm birth: a potential mechanistic link. Med Hypotheses. 2014;82(2):219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang J, Zeng X, Du X, Pan K, Song L, Song W, et al. Parental PM2.5 Exposure-Promoted Development of Metabolic Syndrome in Offspring Is Associated With the Changes of Immune Microenvironment. Toxicol Sci. 2019;170(2):415–26. [DOI] [PubMed] [Google Scholar]
- 85.Shrestha PM, Humphrey JL, Carlton EJ, Adgate JL, Barton KE, Root ED, et al. Impact of Outdoor Air Pollution on Indoor Air Quality in Low-Income Homes during Wildfire Seasons. International journal of environmental research and public health. 2019;16(19):3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen L, Bell EM, Caton AR, Druschel CM, Lin S. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environmental Research. 2010; 110(2):162–8. [DOI] [PubMed] [Google Scholar]
- 87.Armstrong BG. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med. 1998;55(10):651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.