Abstract
Background:
The use of natural killer (NK) cells as a cellular immunotherapy has increased over the past decade, specifically their use in patients with hematologic malignancies. NK cells have been used at our institution for over 15 years. Most patients have a reaction with NK cell infusion. We retrospectively analyzed the reactions associated with NK cell infusions to characterize the types of reactions and to investigate why some patients have higher grade reactions than others.
Methods:
A retrospective chart review of NK cell infusions was performed at our institution from 9 clinical protocols from 2008 to 2016. An infusion reaction was defined as any symptom from the time of NK cell infusion up to 4 hours after infusion completion. The severity of the infusion reactions was graded based on Common Terminology Criteria for Adverse Events (CTCAE version 4). Two major endpoints of interest were: 1) infusion reaction with any symptom and 2) grade 3 or higher infusion reactions. Multivariable logistic regression models were used to investigate the association between variables of interest and the outcomes. Odds ratios and 95% CIs were obtained for each variable.
Results:
There was a total of 130 patients receiving NK cell infusions at our institution. The most common reported symptom was chills (n=110, 85%), which was mostly grade 1 and 2, with only half of the patients requiring an intervention. There were 118 (91%) patients with infusion reactions and only 36 (28%) were grade 3. There was one life-threatening grade 4 reaction and no death was reported due to infusion reaction. Among grade 3 or higher reactions, cardiovascular (mainly hypertension) was the most common and less than half of those with hypertension required intervention. NK cell dose was not associated with any of the grade 3 infusion reactions, while monocyte dose was associated with headache (grade ≤ 3; odds ratio (OR) 2.17: 95% CI [1.19, 3.97]) and cardiovascular reaction (grade ≥ 3; OR 2.13: 95% CI [1.13, 3.99]). Cardiovascular reaction (grade ≥ 3) was also associated with in vitro IL-2 incubation and storage time. Additionally, there was no association between grade 3 or higher infusion reactions and overall response rate (OR 0.75: 95% CI [0.29, 1.95]).
Conclusion:
The majority of patients who receive NK cell therapy experience grade 1 or 2 infusion reactions. Some patients experience grade 3 reactions which are mainly cardiovascular, suggesting close monitoring within the first 4 hours is beneficial. The association of monocytes with NK cell infusion reaction relates to toxicities seen in adoptive T cell therapy and needs further exploration.
Keywords: NK cells, cell therapy, immunotherapy, infusion reaction, toxicity, adverse events
Introduction
Interest in the manufacture and immunobiology of natural killer (NK) cell immunotherapies for cancer has grown substantially since the discovery of lymphokine-activating killer (LAK) cells in the 1980s [1]. While T cell and NK cell fractions of the lymphocyte compartment both exhibit cytotoxic subsets that kill transformed and virally-infected cells by cytotoxic (perforin/granzyme) or apoptotic (FAS ligand, TRAIL) pathways [2], there exists several functional differences that mediate their antigenic potential. As CD3−/CD56+ lymphocytes of the innate immune system, NK cells secrete cytokines and destroy target cells absent the prerequisite for prior sensitization [3]. They do not rearrange antigen-specific surface receptors or require antigen presentation by the major histocompatibility complex (MHC). Rather, NK cell function is inhibited by interaction of killer immunoglobulin-like receptors (KIRs) with MHC class I ligands [4]. Signaling via a complex repertoire of inhibiting and activating surface receptors regulate the disinhibition of NK cell function by sensing for alterations in expression of MHC-I and stress ligands by mechanisms that are not completely understood.
Allogeneic NK cells are capable of inducing a graft-versus-tumor effect without inciting graft-versus-host disease (GVHD) [5], making them an attractive option for therapeutic manipulation. The manufacture of NK cell immunotherapies is not standardized and a variety of methods to isolate, expand, and enhance their cytotoxic potential is under intense investigation [6]. For over the past 15 years, the University of Minnesota has infused haploidentical NK cells as part of adoptive immunotherapy clinical trials for chemotherapy-resistant hematologic malignancies and solid tumors in both hematopoietic stem cell transplant (HSCT) and non-transplant settings. In our early trials, apheresis mononuclear cells (MNC) were T cell-depleted and IL-2 activated in vitro, and this process was later modified for T cell- and B cell-depletion with either IL-2 or IL-15 activation. We have observed a range of infusion-related reactions in most patients and it has not been clear why some patients have grade 3 or higher reactions or whether higher grade could be predictive of clinical efficacy.
Here, we present the first comprehensive review of infusion reactions associated with CD3 cell-depleted or CD3/CD19 cell-depleted, haploidentical NK cells that were manufactured and administered within our institution. The goals of this study were to characterize the type, frequency, and severity of infusion reactions, as well as to evaluate for associations between grade 3 or higher infusion reactions and patient, manufacturing, or infusion-related variables.
Methods
We performed a retrospective chart review of patients who received NK cell infusions between 2008 and 2016 at our institution under 9 clinical trial protocols (Table 1). Enrolled patients had failed at least one salvage chemotherapy regimen for recurrent solid tumors or relapsed/refractory hematologic malignancies. Investigational new drug status approval from the Food and Drug Administration (FDA) and Institutional Review Board approval were obtained for all clinical trials supported by NK cell products manufactured within the Molecular and Cellular Therapeutics cGMP facility at the University of Minnesota [7].
Table 1.
Summary of clinical trial protocols from which patients included in this study were enrolled
| # of evaluable NK cell infusions |
Protocol (clinicaltirals.gov) |
Disease | NK cell product |
Preparative regimen(s) | In vivo cytokine* |
|---|---|---|---|---|---|
| 6 | MT2005-08 (NCT00376805) | Metastatic breast cancer | CD3 depleted, IL-2 activated | Cy-flu, TBI | IL-2, subcutaneous |
| 14 | MT2007-19R (NCT00652899) | Recurrent ovarian, fallopian tube, or primary peritoneal cancer | CD3 depleted, IL-2 activated | Cy-flu, TBI | IL2, subcutaneous |
| 13 | MT2009-30 (NCT01105650) | Metastatic breast cancer Recurrent ovarian, fallopian tube, or primary peritoneal cancer | CD3/CD19 depleted, IL-2 activated | Cy-flu, cyclosporine, methylprednisolone | IL-2, subcutaneous |
| 6 | MT2007-12 (NCT00625729) | Relapsed/refractory NHL or CLL | CD3 depleted, IL-2 activated | Cy-flu, rituximab | IL-2, subcutaneous |
| 15 | MT2009-15 (NCT01181258) | Relapsed/refractory NHL or CLL | CD3/CD19 depleted, IL-2 activated | Cy, pentostatin, rituximab, denileukin difitox, or Cy-flu, methylprednisolone, rituximab | IL-2, subcutaneous |
| 17 | MT2010-02 (NCT01106950) | Relapsed/refractory AML | CD3/CD19 depleted, IL-2 activated | Cy-flu, denileukin diftitox | IL-2, subcutaneous |
| 17 | MT2011-05 (NCT01370213) | Relapsed/refractory AML or high-risk MDS or CMML | CD3/CD19 depleted, IL-2 activated | Cy-flu, TBI | IL-2, subcutaneous |
| 26 | MT2010-10 (NCT01385423) | Relapsed/refractory AML | CD3/CD19 depleted, IL-15 activated | Cy-flu | IL-15, intravenous |
| 16 | MT2014-25 (NCT02395822) | Refractory/relapsed AML | CD3/CD19 depleted, IL-15 activated | Cy-flu | IL-15, subcutaneous |
AML=acute myelogenous leukemia; CLL=chronic lymphocytic leukemia; Cy=cyclophosphamide; Flu=fludarabine; MDS=myelodysplastic syndrome; NHL=non-Hodgkin lymphoma; CMML= Chronic myelomonocytic leukemia; TBI=total body irradiation
Administered at least 4 hours after NK cell infusion
NK Cell Processing
Allogeneic NK cells were derived from peripheral blood of nonmobilized, HLA haploidentical related donors and manufactured under cGMP conditions as previously described [8, 9]. Briefly, mononuclear cells collected by a 15-L leukapheresis (COBE Spectra Apheresis System, TerumoBCT, Lakewood, CO) on the day prior to infusion were manipulated for CD3 cell depletion (3 protocols) or CD3/CD19 cell depletion (6 protocols) using the automated, immunomagnetic CliniMACS Cell Selection System and reagents (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s specification. The NK cell enriched products were activated overnight by incubation in media supplemented with IL-2 (7 protocols) or IL-15 (2 protocols). Incubation of cells was done in (VueLife Teflon (FEP)) bags (American Fluoroseal) at 37°C and 5% CO2. Prior to infusion, the cells were washed twice and resuspended in 5% human serum albumin (Baxter Healthcare); our washing procedure removes cytokines from the media, and we used validation criteria of >95% reduction in IL-2 in the final wash media compared to pre-wash media. After the wash and before infusion, the cells were kept at room temperature while still suspended in the 5% human serum albumin. The cells were also subjected to lot release testing for nucleated cell (NC) count, purity, viability, Gram stain, and endotoxin.
Product characterization for NK cell (CD56+/CD3−), NKT cell (CD56+/CD3+), T cell (CD3+), and B cell (CD19+/CD20+) content was determined by immunophenotyping in our clinical flow cytometry laboratory. Monocyte content by flow cytometric analysis was not consistently available and instead was calculated by subtracting the total percentage of the other cells (T-, B-, NK and NKT cells) from 100% on products that were CD3/CD19 cell depleted. For the CD3 cell depleted products, the B cell (CD19+/CD20+) content was not determined; therefore, the monocyte content was not calculated for this group. While the study included 130 NK cell infusions, complete data was obtained only for the 100 infusions. A summary of the contents of the final NK cell product is provided in Figure 1 for the CD3/CD19 cell depleted products (N = 100). This data shows that the final product mainly contains NK cells (Mean 46.2 %, SD: 17.4) and monocytes (Mean 53.4 %, SD: 17.5).
Figure 1.
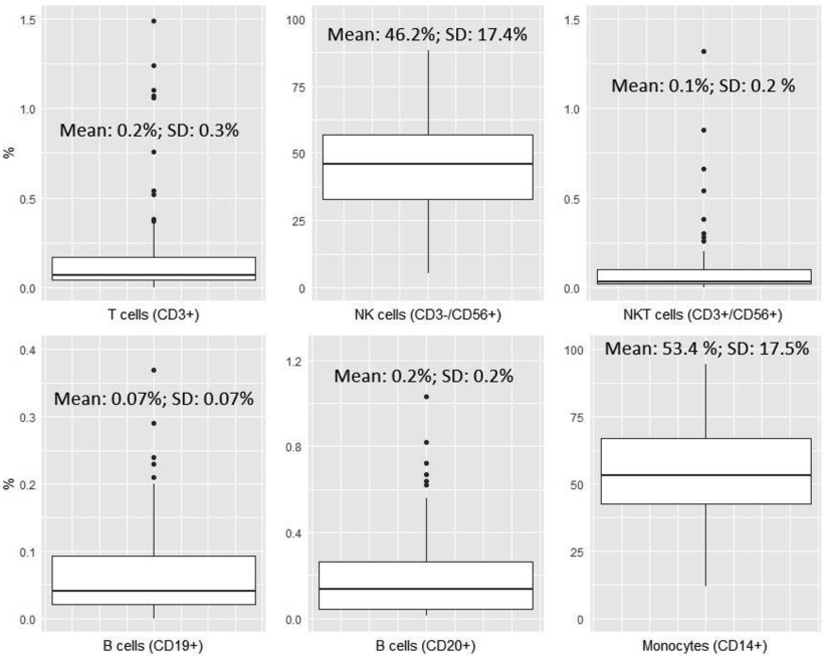
Boxplots summarizing the contents of the final NK cell therapy product for CD3/CD19 cell depleted products (N=100).
NK Cell Infusion
All patients received preparative lymphodepleting chemotherapy according to the clinical trial for which they were enrolled (Table 1). Patients were premedicated with acetaminophen 650 mg PO (pediatric dose 15 mg/kg) and/or diphenhydramine 25 mg PO (pediatric dose 0.5 mg/kg) before and 4 hours after the NK cell infusion. NK cells were administered by intravenous (IV) infusion at a dosage of up to 8 x107 NC/kg. Patients were monitored for any infusion-related symptoms during and for at least 4 hours after the NK cell infusion, prior to initiating subcutaneous IL-2 (7 protocols), subcutaneous IL-15 (1 protocol), or intravenous IL-15 (1 protocol).
Data Collection
Chart review included patient demographics (age, sex and diagnosis), NK cell product characteristics (manufacturing method, viability and cellular content, post-manufacture storage time), infusion characteristics (infusion rate and cell dosages), infusion-related symptoms, and best response to NK cell therapy (stable disease, progressive disease, complete response/remission, partial response/remission). An infusion reaction was defined as any sign or symptom from the time of NK cell infusion up to 4 hours afterwards. Severity of infusion reactions was graded based on Common Terminology Criteria for Adverse Events (CTCAE, version 4, published on June 14, 2010) [10]. CTCAE was not adopted until 2012 and therefore, some of the grading was assigned retrospectively.
The major endpoints of interest were: infusion reaction with any symptom (grade ≥ 3), respiratory reaction (grade 3), febrile reaction (grade 3), cardiovascular reaction (grade ≥ 3), headache (grade 3) and headache (grade ≤ 3). Variables analyzed for association with the major endpoints included the infusion rate, NK cell dose, monocyte dose , in vitro cytokine used for NK cell activation (IL-2 versus IL-15), product (cell) viability, product storage time (which refers to the time between the completion of product manufacturing, i.e. the final washing procedure/suspension in albumin, and the start of infusion), patient diagnosis and age. For patient diagnosis, acute myelogenous leukemia (AML), AML/myelofibrosis, myelodysplastic syndrome (MDS) and chronic myelomonocytic leukemia (CMML) were grouped together; breast cancer (BrCa), ovarian cancer, and peritoneal adenocarcinoma (PAC) were grouped together; and chronic lymphocytic leukemia (CLL), and non-Hodgkin lymphoma (NHL) were grouped together.
Statistical Analysis
Demographic variables, infusion-related variables, manufacturing-related variables, and the outcomes were summarized using descriptive statistics for the entire cohort. Multivariable logistic regression models were performed to investigate the association between variables of interest and the outcomes. Odds ratios and 95% CIs were obtained for each variable. Statistical analyses were performed using R (version 3.6.0) and SAS (version 9.4, SAS Institute, Inc., Cary, North Carolina).
Results
1. Study Population
The study population involved patients who received NK cell infusions between 2008 and 2016 at our institution under 9 clinical trial protocols (Table 1). We analyzed 130 NK cell infusions that were administered to 63 female (51%) and 61 male (49%) patients (6 with missing data for sex) with relapsed/refractory AML or AML/myelofibrosis (n=73), MDS (n=2), CMML (n=1), ovarian cancer (n=25), BrCa (n=7), PAC, (n=1), NHL (n=20) and CLL (n=1). AML, AML/myelofibrosis, MDS and CMML comprised most of the study population at 59%, BrCa, ovarian cancer and PAC comprised 25%, and CLL and NHL comprised 16% (Table 2). The mean age at the time of NK infusion was 52 years (range, 4-78).
Table 2.
Summary of demographics
| Variable | All subjects (N=130) |
|---|---|
| Diagnosis, n (%) | |
| AML, AML/myelofibrosis, MDS & CMML | 76 (58.5%) |
| BrCa, Ovarian Cancer & PAC | 33 (25.4%) |
| CLL & NHL | 21 (16.2%) |
| Patient age (decades) | |
| Mean (SD) | 5.26 (1.55) |
| Median (Range) | 5.60 (0.40, 7.80) |
| Patient sex | |
| Number missing | 6 |
| Female | 63 (50.8%) |
| Male | 61 (49.2%) |
| Patient weight (kg) | |
| Mean (SD) | 77.51 (17.61) |
| Median (Range) | 76.30 (14.80, 119.50) |
2. Summary of Infusion Reactions
Infusion-related signs and symptoms that were possibly, probably, or definitely attributed to the NK cell infusion are summarized in Table 3, while signs and symptoms that were probably or definitely due to another cause were excluded from evaluation. The most reported symptoms were chills (n=110, 85%), hypertension (n=39, 30%), fever (n=38, 29%), and headache (n=29, 22%). The majority of the chills (n=109, 84%) were grade 1 and 2, and about half these (n=56, 43%) required intervention with Meperidine (Demerol). After constitutional symptoms (chills and fever), cardiovascular reaction (mainly hypertension, grade ≤ 3) was the most common. Anecdotally, some patients experienced mild nausea and emesis; however, the tracking and grading of these episodes was incomplete and could not be enumerated. Likewise, there were 2 episodes of ungraded tachycardia (which was not part of routine reporting for CTCAE). Local reactions at the infusion site were not tracked or observed as part of this study.
Table 3.
Summary of all signs and symptoms occurring during or within 4 hours after NK cell infusion that were either definitely, probably or possibly related to the infusion
| Sign/symptom | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total, n (%) |
|---|---|---|---|---|---|
| Constitutional | |||||
| Chills | 29 | 80 | 1 | - | 110 (84.6%) |
| Fever (no infection) | 17 | 6 | 15 | - | 38 (29.2%) |
| Respiratory | |||||
| Dyspnea | 4 | 3 | 8 | - | 15 (11.5%) |
| Hypoxia | 1 | 4 | 8 | - | 13 (10.0%) |
| Bronchospasm | 0 | 3 | 3 | - | 6 (4.6%) |
| Pneumonitis | 1 | 1 (0.8%) | |||
| Cardiovascular | |||||
| Hypertension | 6 | 16 | 17 | - | 39 (30.0%) |
| Hypotension | 3 | 4 | 2 | 1 | 10 (7.7%) |
| Tachycardia* | - | - | - | - | 2 (1.5%) |
| Chest pain | 1 | 1 (0.8%) | |||
| CNS | |||||
| Headache | 12 | 9 | 8 | 29 (22.3%) | |
| Gastrointestinal | |||||
| Nausea** | - | - | - | - | - |
| Emesis** | - | - | - | - | - |
| Abdominal cramps | - | 1 | - | - | 1 (0.8%) |
| Ascites | 2 | - | - | - | 2 (1.5%) |
| Musculoskeletal | |||||
| Myalgia | 1 | 4 | 1 | - | 6 (4.6%) |
| Skin/subcutaneous | |||||
| Rash | 2 | - | - | - | 2 (1.5%) |
| Other | |||||
| Localized edema | 8 | 4 | - | - | 12 (9.2%) |
| Sweating | 1 | - | - | - | 1 (0.8%) |
| Dry eye | 1 | - | - | - | 1 (0.8%) |
Tachycardia was not part of routine reporting for CTCAE; two episodes of tachycardia were noted in the record that were not graded
Reports of nausea/vomiting were not graded - anecdotally they appeared to mostly be mild and readily treatable
The majority of patients (n=118, 91%) experienced an infusion reaction (Table 4), the most common symptoms being chills and fever as mentioned above. An infusion reaction of grade 3 or higher was observed in smaller number of patients (n=37, 29%), the majority (n=36, 28%) being just grade 3. Only one life-threatening grade 4 reaction was seen in a patient with a hypotensive reaction that necessitated transfer to the medical intensive care unit for pressor support. This patient also had chills and dyspnea requiring 1-2 L of oxygen support due to hypoxia. No deaths were attributed to an NK cell infusion.
Table 4.
Summary of infusion reactions organized by toxicity endpoints of interest
| Outcome | All subjects (N=130) |
|---|---|
| Infusion reaction with any symptom, n (%) | |
| Yes | 118 (90.8%) |
| No | 12 (9.2%) |
| Infusion reaction with any symptom, grade ≥ 3, n (%) | |
| Yes | 37 (28.5%) |
| No | 93 (71.5%) |
| Cardiovascular reaction, grade ≥ 3, n (%) | |
| Yes | 20 (15.4%) |
| No | 110 (84.6%) |
| Febrile reaction, grade 3, n (%) | |
| Yes | 15 (11.5%) |
| No | 115 (88.5%) |
| Respiratory reaction, grade 3, n (%) | |
| Yes | 8 (6.2%) |
| No | 122 (93.8%) |
| Headache, grade 3, n (%) | |
| Yes | 8 (6.2%) |
| No | 122 (93.8%) |
| Headache, grade ≤ 3, n (%) | |
| Yes | 29 (22.3%) |
| No | 101 (77.7%) |
Among those with grade 3 or higher reaction, cardiovascular reaction (n=20, 15%) was the most common, followed by febrile reaction (n=15, 12%), respiratory reaction (n=8, 6%), and headache (n=8, 6%). Headache with grade ≤ 3 (n=29, 22%) was also included in Table 4 as it was part of subsequent analysis. The majority of the grade 3 cardiovascular reactions (n=17, 13%) were hypertension, with less than half of these (n=6, 4.6%) requiring intervention with hydralazine. The patients with grade 3 hypotension did not require any intervention.
3. Summary of Variables of Interest
To understand why some patients may have higher grade reactions than others, we postulated some key variables of interest that may be risk factors. Given the unique nature of cell therapy product preparation, these included manufacturing-related variables, infusion-related variables, and patient characteristics. Infusion-related variables and manufacturing-related variables are summarized for the entire cohort in Table 5.
Table 5.
Summary of variables of interest
| Variable | All subjects (N=130) |
|---|---|
| Infusion-related variables | |
| Infusion rate (mL/min/kg) | |
| Number missing | 3 |
| Mean (SD) | 0.04 (0.02) |
| Median (Range) | 0.04 (0.01, 0.14) |
| Monocyte dose (cells/kg, divided by 10,000,000) | |
| Number missing | 26 |
| Mean (SD) | 2.18 (1.01) |
| Median (Range) | 2.05 (0.06, 4.94) |
| NK cell dose (cells/kg, divided by 10,000,000) | |
| Mean (SD) | 1.94 (1.05) |
| Median (Range) | 1.79 (0.11, 6.27) |
| Manufacturing-related variables | |
| In vitro IL-2 vs IL-15, n (%) | |
| Incubation with IL-2 | 88 (67.7%) |
| Incubation with IL-15 | 42 (32.3%) |
| % viability (7-AAD) | |
| Mean (SD) | 95.64 (4.10) |
| Median (Range) | 96.73 (71.13, 99.69) |
| Storage time (hours) | |
| Number missing | 7 |
| Mean (SD) | 5.09 (1.27) |
| Median (Range) | 4.83 (2.87, 9.28) |
4. Association between Infusion Reactions and Variables of Interest
The results of the multivariable logistic regression models to investigate the association between several variables of interest and the main outcomes are summarized in Table 6. Out of the 130 patients, only 123 were available for full analysis due to missing data. Additionally, due to missing data in earlier clinical trial protocols (for the CD3 cell depleted products) that limited the calculation of monocyte content, the number of patients included in the analysis was reduced to 100 (including only CD3/CD19 cell depleted products).
Table 6.
Association between toxicity endpoints and variables of interest (N=100)2.
| Variable | Infusion reaction with any symptom, grade ≥ 3, OR (95% CI) |
Respiratory reaction, grade 3, OR (95% CI) |
Febrile reaction, grade 3, OR (95% CI) |
Cardiovascular reaction, grade ≥3, OR (95% CI) |
Headache, grade ≤ 3, OR (95% CI) |
Headache, grade 3, OR (95% CI) |
|---|---|---|---|---|---|---|
| NK cell dose (cells/kg, divided by 10,000,000) | 0.89 (0.57, 1.38) | 0.42 (0.13, 1.35) | 0.60 (0.29, 1.27) | 0.78 (0.43, 1.41) | 0.97 (0.55, 1.72) | 1.34 (0.63, 2.85) |
| Natural log of infusion rate (mL/min/kg) | 1.11 (0.43, 2.90) | 0.99 (0.12, 8.35) | 13.07 (1.81, 94.51) | 0.88 (0.28, 2.78) | 0.39 (0.12, 1.27) | 0.62 (0.11, 3.60) |
| Monocyte dose (cells/kg, divided by 10,000,000) | 1.60 (0.98, 2.61) | 2.37 (0.93, 6.02) | 0.69 (0.31, 1.54) | 2.13 (1.13, 3.99) | 2.17 (1.19, 3.97) | 1.49 (0.70, 3.18) |
| IL-2 vs IL-15 incubation | ||||||
| IL-2 | 2.63 (0.84, 8.23) | 6.18 (0.62, 61.77) | 3.65 (0.79, 16.90) | 5.89 (1.24, 27.95) | 0.25 (0.06, 1.11) | 0.64 (0.07, 5.78) |
| IL-15 | Reference | Reference | Reference | Reference | Reference | Reference |
| % viability (7-AAD) | 0.92 (0.80, 1.06) | 0.87 (0.72, 1.05) | 0.94 (0.78, 1.12) | 1.01 (0.86, 1.17) | 0.88 (0.74, 1.06) | 0.88 (0.69, 1.12) |
| Storage time (hours) | 1.27 (0.85, 1.89) | 1.54 (0.70, 3.37) | 1.41 (0.77, 2.59) | 1.76 (1.09, 2.85) | 0.70 (0.41, 1.20) | 0.70 (0.31, 1.58) |
| Diagnosis | ||||||
| AML, AML/myelofibrosis MDS & CMML | 1.47 (0.34, 6.34) | 1.03 (0.06, 16.87) | N.E.3 | 1.05 (0.22, 4.98) | 0.33 (0.05, 2.09) | 0.62 (0.04, 10.35) |
| BrCa, Ovarian Cancer & PAC | 1.08 (0.19, 6.02) | 0.67 (0.03, 14.38) | N.E.3 | 0.31 (0.04, 2.36) | 0.71 (0.11, 4.66) | 2.82 (0.21, 38.70) |
| CLL & NHL | Reference | Reference | Reference | Reference | Reference | Reference |
| Patient age (decades) | 0.92 (0.69, 1.22) | 1.26 (0.67, 2.39) | 1.39 (0.86, 2.24) | 1.43 (0.93, 2.20) | 0.66 (0.47, 0.93) | 0.69 (0.43, 1.10) |
Multivariable logistic regression was performed for six outcomes to investigate the association between these outcomes and variables of interest. Odds ratios and 95% CIs are presented for each variable. CIs are bolded if they do not include 1.0.
Final sample size for all models is 100 due to missing independent variables.
N.E. indicates not estimable due to cell counts of 0.
Based on the analysis with N=100, some of the variables were associated with infusion reactions (Table 6). The odds of grade 3 febrile reaction increased with an increase in infusion rate, with OR of 13.07: 95% CI [1.81, 94.51]. The odds of cardiovascular reaction (grade ≥ 3) and headache (grade ≤ 3) increased with an increase in monocyte dose, with OR of 2.13: 95% CI [1.13, 3.99] and 2.17: 95% CI [1.19, 3.97], respectively. Incubation with IL-2 (OR of 5.89: 95% CI [1.24, 27.95]) and an increase in post-manufacture storage time (OR of 1.76: 95% CI [1.09, 2.85]) also increased the odds of cardiovascular reaction (grade ≥ 3). Interestingly, older age was associated with a decrease in the odds of headache, grade ≤ 3, (OR of 0.66, 95% CI [0.47, 0.93]). The rest of the variables and outcomes showed no statistically significant association as the confidence interval included 1. It is important to note here that there was no association between NK cell dose and any of the toxicity endpoints of interest in this analysis, suggesting that the NK cell content may not be associated with infusion reactions.
As mentioned, the association analysis above included CD3/CD19 cell depleted products (N=100) only since we were not able to calculate monocyte content for CD3 cell depleted products. However, we performed a logistic regression analysis to investigate if severe infusion reactions vary based on whether the NK products were CD3 cell depleted versus CD3/CD19 cell depleted. We discovered that there was no statistically significant difference in infusion reactions between the CD3 cell depleted NK cell products versus the CD3/CD19 cell depleted NK products (95% CI did not include 1 and p-values were > 0.05).
5. Association between Infusion Reactions and Overall Response Rate (ORR)
To investigate whether higher grade of infusion reactions could be predictive of clinical efficacy, we explored if ORR was associated with grade 3 or higher infusion reactions. For the total study population, the ORR was 27.6% (Table 7). Those with grade 3 or higher infusion reaction had ORR of 26.5% compared to 28.1% for those who did not have these reactions. Grade 3 or higher infusion reaction did not lead to improved overall response, with OR of 0.75, 95% CI [0.29, 1.95].
Table 7.
Association between overall response and grade 3 or higher infusion reaction (N=123).
| Variable | N with overall response (%) | OR (95% CI) |
|---|---|---|
| Total number of patients (N = 123) | 34 (27.6%) | N/A |
| Infusion reaction with any symptom, grade ≥ 3 | ||
| Yes | 9 (26.5%) | 0.75 (0.29, 1.95) |
| No | 25 (28.1%) | Reference |
| IL-2 vs IL-15 incubation | ||
| IL-2 | 25 (30.5%) | 2.56 (0.89, 7.38) |
| IL-15 | 9 (22.0%) | Reference |
| Diagnosis | ||
| AML, AML/myelofibrosis, MDS & CMML | 22 (30.1%) | 4.03 (1.17, 13.84) |
| CLL & NHL | 7 (35.0%) | 2.79 (0.72, 10.79) |
| BrCa, Ovarian Cancer & PAC | 5 (16.7%) | Reference |
| Patient age | - | 1.03 (1.0, 1.06) |
Multivariable logistic regression was performed to investigate the association between overall response and variables of interest. Odds ratios and 95% CIs are presented for each variable.
In addition to severity of infusion reaction, we were also interested to investigate if other relevant variables such as incubation with IL-2 vs IL-15 or patient diagnosis had any association with ORR. Incubation with IL-2, compared to IL-15, did not lead to any association with ORR. However, the odds of having a better overall response is higher in patients with a diagnosis of AML, AML/myelofibrosis, MDS and CMML compared to patients with BrCa, ovarian cancer and PAC, with OR of 4.03; 95% CI [1.17, 13.84].
Discussion
NK cells are part of the growing armamentarium of cell-based immunotherapies in cancer and HSCT as well as infectious disease [11-13]. The safety and feasibility of adoptively-transferred NK cells (generated by a variety of manufacturing methods) has been demonstrated in several early phase clinical trials [14]. Clinical benefit has been observed in patients with AML who were pretreated with lymphodepleting chemotherapy [15-17], and the bone marrow density of adoptively-transferred NK cells was shown to correlate with disease control [18]. However, the anti-leukemia effect is transient and efforts to improve the expansion and persistence of NK cells in vivo are underway.
While some early clinical studies reported no adverse reactions or toxicity during NK cell infusions [19-21], our group and others have reported that patients receiving NK cells could have infusion reactions [22-24]. In the setting of lymphodepleting chemotherapy (Hi-Cy/Flu regimen) and total body irradiation (TBI) as well as IL-2 or IL-15 administration, standard toxicities are expected. Nonetheless, there is a lack of studies investigating whether the NK cell therapy product by itself leads to adverse events. Therefore, this study was focused in identifying adverse events that could be classified as NK cell infusion reactions by retrospectively reviewing patient charts for symptoms observed immediately and up to 4 hours after NK cell infusion. To our knowledge, this is the first comprehensive report of the type and characteristics of infusion reactions in NK cell immunotherapy.
Chills, hypertension, fever, and headache were the most common NK cell infusion reactions observed in our study population, which are symptoms previously reported by other studies [25-27]. These symptoms have also been reported as common reactions to HSC and adoptive T cell infusions [28-30]. Nausea and vomiting, one of the most common reactions in other cell therapies, was anecdotally observed to be mostly mild and readily treatable but has not been graded or quantified as part of this study. While the percentage of patients experiencing any infusion reaction (91%) is higher than that reported for T cells (6.55%) [28] or HSCs (57%) [30], most of these reactions were grade 1 or 2. Only 28% of patients experienced grade 3 reactions, with just one life-threatening grade 4 reaction reported. Cardiovascular reaction was the most common grade 3 reaction, raising another similarity with HSC infusion [30]. Although no deaths were attributed to an NK cell infusion in our study population, the one life-threatening reaction was a grade 4 hypotensive reaction, accompanied by less severe chills and dyspnea. These findings reinforce the value of monitoring patients for at least the first 4 hours after NK cell infusions. While most patients experienced grade 1 or 2 chills, those who experienced a grade 3 reaction are more likely to have hypertension, suggesting frequent vital sign checks will be useful.
To identify risk factors of several toxicity endpoints, multivariable logistic regression was performed. While some of the variables were associated with the infusion reactions, an important finding is that NK cell dose did not have any association with any of the grade 3 or higher infusion reactions. This agrees with one of our recent studies that reported there was no association between higher NK cell dose and the development of cytokine release syndrome (CRS) [31]. CRS is a severe life-threatening toxicity due to a systemic inflammatory response that has mainly been observed after infusion of antibodies and adoptive T cell therapies [32]. While it is common in T cell therapies, the presence of CRS and neurotoxicity after NK cell therapy has only been reported recently by our group [31] and Björklund et al [27]. In adoptive T cell therapy, including chimeric antigen receptor T cell (CAR-T) therapy, toxicities such as CRS have been found to be T cell dose dependent [33]. The lack of association between grade 3 infusion reactions and NK cell dose in our current study implies that NK cells themselves may not be responsible for these reactions and other components of the NK cell therapy product may play a more significant role.
A component of the NK cell therapy product that was found to be associated with the infusion reactions was the monocyte dose. Interestingly, headache (grade ≤ 3) and cardiovascular reaction (grade ≥ 3) were associated with monocyte dose. This association is fascinating in the setting of previous reports that suggested monocytes contribute to the development of CRS and neurotoxicity after adoptive T cell therapy [34]. Norelli et al [35] and others [36] have shown that monocyte-derived IL-1 and IL-6 are required for CRS and neurotoxicity due to CAR-T cell therapy. These findings have provided the rationale for using tocilizumab, an IL-6 receptor antagonist, for treatment of CRS [37]. This suggests that monocytes could act through a similar mechanism in NK cell infusion reactions. Therefore, reducing or inhibiting the effects of monocytes maybe a way to decrease grade 3 or higher infusion reactions. Our group previously attempted to purify NK cell products by reducing the amount of monocytes, but the manufacturing process led to reduced NK-cell recovery and the final cell therapy product failed to induce hematologic remissions [9, 16]. Hence, further studies are required to investigate the role of monocytes in NK cell therapy infusion reactions and to explore strategies to target their effects.
Additionally, there were other variables that were associated with grade 3 infusion reactions. Cardiovascular reaction (grade ≥ 3) was associated with storage time and IL-2 incubation. The association with storage time suggests that delaying infusion of the NK cell product after completion of manufacturing may lead to a higher likelihood of these infusion reactions. We hypothesize that this may be due to a cytokine build-up as the cells await infusion. On the other hand, the fact that IL-2 incubation, compared to IL-15, led to an increased likelihood of cardiovascular reaction may provide another incentive for the use of IL-15 as the preferred cytokine in NK cell therapy [31]. Moreover, our analysis showed that increase in infusion rate increased the odds of grade 3 febrile reaction, while older age decreased the odds of headache (grade ≤ 3). The association with infusion rate further emphasizes the need for close monitoring of patients in the immediate hours after NK cell infusion. The explanation for the surprising result that older patients had decreased odds of headache is not clear, but interestingly, a similar association is observed in CAR-T therapy where younger age was associated with more severe neurotoxicity [38]. Overall, the above results indicate that all types of variables, including infusion-related variables (infusion rate), manufacturing-related variables (storage time) and patient characteristics (age), may be important risk factors for infusion reactions in NK cell therapy.
Another important finding of this study is that grade 3 or higher infusion reaction was not associated with improved ORR. This agrees with our recent study that showed development of CRS in NK cell therapy, in the context of IL-15 accumulation after subcutaneous dosing and delayed elimination from lymphodepletion, had no impact on disease response [31]. This is different than what has been observed in CAR-T therapy where it has been established that CRS is associated with efficacy [39]. This distinction between the two different cell therapy modalities should be considered in future strategies that attempt to reduce toxicity and infusion reactions in NK cell therapy. Moreover, we found that ORR did not vary between incubation with IL-2 versus IL-15, further validating our previous findings that IL-15 is as effective as IL-2 in inducing remission [31]. The use of IL-15 in monotherapy or with NK cells and other chemotherapy has recently shown encouraging clinical results [40]. Our results add further evidence that IL-15 maybe the preferred cytokine in NK cell therapy since it is as effective IL-2 but has better toxicity profile and avoids Treg stimulation [31, 40-42]. Finally, this study further strengthened previous observations that NK cell therapy leads to better overall response in myeloid hematological malignancies (particularly AML) compared to solid tumors such as breast and ovarian cancer. This emphasizes the need for continued efforts in exploring strategies to improve NK cell therapy for solid tumors.
The main limitation of our study is that it is a retrospective review at a single institution. This has limited the number of patients included in the study. The number of patients was further limited by missing data in earlier clinical trials. Another limitation of the study is that the monocyte dose used in our analysis is a calculated value due to variations in measuring monocyte content among the various protocols. Given the clinical implications of the role of monocytes as discussed above, future studies with measured monocyte dose may be valuable. Regardless of these limitations, our study is still the most comprehensive study on infusion reactions in NK cell therapy to date, and our findings provide a baseline for future studies.
Conclusion
Overall, our findings highlight that while infusions reactions do occur in NK cell therapy, the majority of these are grade 1 and 2. The grade 3 or higher reactions are mostly cardiovascular (mainly hypertension) suggesting that close monitoring in the first 4 hours post-infusion will be valuable. The association of monocyte dose with grade 3 infusion reactions has raised the possibility of similarities to CRS and neurotoxicity observed in CAR-T therapy, although NK cell infusion reactions were not associated with improved efficacy as is the case with CRS. With newer NK cell immunotherapy strategies emerging, our comprehensive study of NK cell therapy-related infusion reactions may provide a baseline with which to compare for future studies. Several new products in the pipeline such as CAR-NK cells could have similar infusion reactions with the associations described in this study. Therefore, as we continue to optimize NK cell immunotherapy, it will be essential to keep in mind these infusion reactions and the risk factors associated with them.
Highlights:
NK cell immunotherapy leads to grade 1 or 2 infusion reactions such as chills
Grade 3 cardiovascular reactions such as hypertension occur in some patients
Monocytes are associated with NK cell infusion reactions like in CAR-T therapy
Unlike CAR-T, overall response rate is not associated with infusion reactions
Acknowledgements
This work was supported by the Production Assistance for Cellular Therapies (PACT) program from NIH/NHLBI at University of Minnesota Molecular and Cellular Therapeutics Facility, PACT Contract # HHSN268201000008C (D.H.M, J.S.M, S.C.). Research reported in this publication was also supported by NIH grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank staff at the Molecular & Cellular Therapeutics facility, particularly Diane Kadidlo and Darin Sumstad, as well as Dixie Lewis for her role in study coordination and patient care.
Footnotes
Declaration of interest: S.C. is an employee of Fate Therapeutics. The rest of the authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Veluchamy JP, Kok N, van der Vliet HJ, Verheul HMW, de Gruijl TD, Spanholtz J, The Rise of Allogeneic Natural Killer Cells As a Platform for Cancer Immunotherapy: Recent Innovations and Future Developments, Front Immunol 8 (2017) 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bald T, Krummel MF, Smyth MJ, Barry KC, The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies, Nat Immunol 21(8) (2020) 835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Caligiuri MA, Human natural killer cells, Blood 112(3) (2008) 461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ljunggren HG, Karre K, In search of the 'missing self': MHC molecules and NK cell recognition, Immunol Today 11(7) (1990) 237–44. [DOI] [PubMed] [Google Scholar]
- [5].Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA, Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect, Blood 100(6) (2002) 1935–47. [DOI] [PubMed] [Google Scholar]
- [6].Becker PS, Suck G, Nowakowska P, Ullrich E, Seifried E, Bader P, Tonn T, Seidl C, Selection and expansion of natural killer cells for NK cell-based immunotherapy, Cancer Immunol Immunother 65(4) (2016) 477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McKenna DH, Kadidlo DM, Miller JS, Orchard PJ, Wagner JE, McCullough J, The Minnesota Molecular and Cellular Therapeutics Facility: a state-of-the-art biotherapeutics engineering laboratory, Transfus Med Rev 19(3) (2005) 217–28. [DOI] [PubMed] [Google Scholar]
- [8].McKenna DH Jr., Sumstad D, Bostrom N, Kadidlo DM, Fautsch S, McNearney S, Dewaard R, McGlave PB, Weisdorf DJ, Wagner JE, McCullough J, Miller JS, Good manufacturing practices production of natural killer cells for immunotherapy: a six-year single-institution experience, Transfusion 47(3) (2007) 520–8. [DOI] [PubMed] [Google Scholar]
- [9].Williams SM, Sumstad D, Kadidlo D, Curtsinger J, Luo X, Miller JS, McKenna DH Jr., Clinical-scale production of cGMP compliant CD3/CD19 cell-depleted NK cells in the evolution of NK cell immunotherapy at a single institution, Transfusion 58(6) (2018) 1458–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].National Cancer Institute. Cancer therapy evaluation program, common terminology criteria for adverse events, version 4.0, DCTD, NCI, NIH, DHHS. (2010). [Google Scholar]
- [11].Schmidt S, Tramsen L, Rais B, Ullrich E, Lehrnbecher T, Natural killer cells as a therapeutic tool for infectious diseases - current status and future perspectives, Oncotarget 9(29) (2018) 20891–20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rezvani K, Rouce RH, The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer, Front Immunol 6 (2015) 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Knorr DA, Bachanova V, Verneris MR, Miller JS, Clinical utility of natural killer cells in cancer therapy and transplantation, Semin Immunol 26(2) (2014) 161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Koepsell SA, Miller JS, McKenna DH Jr., Natural killer cells: a review of manufacturing and clinical utility, Transfusion 53(2) (2013) 404–10. [DOI] [PubMed] [Google Scholar]
- [15].Curti A, Ruggeri L, D'Addio A, Bontadini A, Dan E, Motta MR, Trabanelli S, Giudice V, Urbani E, Martinelli G, Paolini S, Fruet F, Isidori A, Parisi S, Bandini G, Baccarani M, Velardi A, Lemoli RM, Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high risk acute myeloid leukemia patients, Blood 118(12) (2011) 3273–9. [DOI] [PubMed] [Google Scholar]
- [16].Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB, Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer, Blood 105(8) (2005) 3051–7. [DOI] [PubMed] [Google Scholar]
- [17].Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W, NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia, J Clin Oncol 28(6) (2010) 955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grzywacz B, Moench L, McKenna D Jr., Tessier KM, Bachanova V, Cooley S, Miller JS, Courville EL, Natural Killer Cell Homing and Persistence in the Bone Marrow After Adoptive Immunotherapy Correlates With Better Leukemia Control, J Immunother 42(2) (2019) 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG, Bug G, Treatment of patients with advanced cancer with the natural killer cell line NK-92, Cytotherapy 15(12) (2013) 1563–70. [DOI] [PubMed] [Google Scholar]
- [20].Yoon SR, Lee YS, Yang SH, Ahn KH, Lee JH, Lee JH, Kim DY, Kang YA, Jeon M, Seol M, Ryu SG, Chung JW, Choi I, Lee KH, Generation of donor natural killer cells from CD34(+) progenitor cells and subsequent infusion after HLA-mismatched allogeneic hematopoietic cell transplantation: a feasibility study, Bone Marrow Transplant 45(6) (2010) 1038–46. [DOI] [PubMed] [Google Scholar]
- [21].Iliopoulou EG, Kountourakis P, Karamouzis MV, Doufexis D, Ardavanis A, Baxevanis CN, Rigatos G, Papamichail M, Perez SA, A phase I trial of adoptive transfer of allogeneic natural killer cells in patients with advanced non-small cell lung cancer, Cancer Immunol Immunother 59(12) (2010) 1781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, McGlave P, Weisdorf DJ, Blazar BR, Miller JS, Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein, Blood 123(25) (2014) 3855–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D, Dusenbery K, Bliss R, Downs LS, Miller JS, A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer, Cytotherapy 13(1) (2011) 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Curti A, Ruggeri L, Parisi S, Bontadini A, Dan E, Motta MR, Rizzi S, Trabanelli S, Ocadlikova D, Lecciso M, Giudice V, Fruet F, Urbani E, Papayannidis C, Martinelli G, Bandini G, Bonifazi F, Lewis RE, Cavo M, Velardi A, Lemoli RM, Larger Size of Donor Alloreactive NK Cell Repertoire Correlates with Better Response to NK Cell Immunotherapy in Elderly Acute Myeloid Leukemia Patients, Clin Cancer Res 22(8) (2016) 1914–21. [DOI] [PubMed] [Google Scholar]
- [25].Bachanova V, Burns LJ, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lindgren BR, Cooley S, Weisdorf D, Miller JS, Allogeneic natural killer cells for refractory lymphoma, Cancer Immunol Immunother 59(11) (2010) 1739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yang Y, Lim O, Kim TM, Ahn YO, Choi H, Chung H, Min B, Her JH, Cho SY, Keam B, Lee SH, Kim DW, Hwang YK, Heo DS, Phase I Study of Random Healthy Donor-Derived Allogeneic Natural Killer Cell Therapy in Patients with Malignant Lymphoma or Advanced Solid Tumors, Cancer Immunol Res 4(3) (2016) 215–24. [DOI] [PubMed] [Google Scholar]
- [27].Bjorklund AT, Carlsten M, Sohlberg E, Liu LL, Clancy T, Karimi M, Cooley S, Miller JS, Klimkowska M, Schaffer M, Watz E, Wikstrom K, Blomberg P, Wahlin BE, Palma M, Hansson L, Ljungman P, Hellstrom-Lindberg E, Ljunggren HG, Malmberg KJ, Complete Remission with Reduction of High-Risk Clones following Haploidentical NK-Cell Therapy against MDS and AML, Clin Cancer Res 24(8) (2018) 1834–1844. [DOI] [PubMed] [Google Scholar]
- [28].Cruz CR, Hanley PJ, Liu H, Torrano V, Lin YF, Arce JA, Gottschalk S, Savoldo B, Dotti G, Louis CU, Leen AM, Gee AP, Rooney CM, Brenner MK, Bollard CM, Heslop HE, Adverse events following infusion of T cells for adoptive immunotherapy: a 10-year experience, Cytotherapy 12(6) (2010) 743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Truong TH, Moorjani R, Dewey D, Guilcher GM, Prokopishyn NL, Lewis VA, Adverse reactions during stem cell infusion in children treated with autologous and allogeneic stem cell transplantation, Bone Marrow Transplant 51(5) (2016) 680–6. [DOI] [PubMed] [Google Scholar]
- [30].Vidula N, Villa M, Helenowski IB, Merchant M, Jovanovic BD, Meagher R, Mehta J, Singhal S, Winter JN, Frankfurt O, Altman JK, Williams SF, Gordon LI, Adverse Events During Hematopoietic Stem Cell Infusion: Analysis of the Infusion Product, Clin Lymphoma Myeloma Leuk 15(11) (2015) e157–62. [DOI] [PubMed] [Google Scholar]
- [31].Cooley S, He F, Bachanova V, Vercellotti GM, DeFor TE, Curtsinger JM, Robertson P, Grzywacz B, Conlon KC, Waldmann TA, McKenna DH, Blazar BR, Weisdorf DJ, Miller JS, First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia, Blood Adv 3(13) (2019) 1970–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, Grupp SA, Mackall CL, Current concepts in the diagnosis and management of cytokine release syndrome, Blood 124(2) (2014) 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M, Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial, Mol Ther 18(4) (2010) 666–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sterner RM, Sakemura R, Cox MJ, Yang N, Khadka RH, Forsman CL, Hansen MJ, Jin F, Ayasoufi K, Hefazi M, Schick KJ, Walters DK, Ahmed O, Chappell D, Sahmoud T, Durrant C, Nevala WK, Patnaik MM, Pease LR, Hedin KE, Kay NE, Johnson AJ, Kenderian SS, GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts, Blood 133(7) (2019) 697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C, Cristofori P, Traversari C, Bordignon C, Ciceri F, Ostuni R, Bonini C, Casucci M, Bondanza A, Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells, Nat Med 24(6) (2018) 739–748. [DOI] [PubMed] [Google Scholar]
- [36].Singh N, Hofmann TJ, Gershenson Z, Levine BL, Grupp SA, Teachey DT, Barrett DM, Monocyte lineage-derived IL-6 does not affect chimeric antigen receptor T-cell function, Cytotherapy 19(7) (2017) 867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA, Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia, N Engl J Med 378(5) (2018) 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, Halton E, Wang X, Senechal B, Purdon T, Cross JR, Liu H, Vachha B, Chen X, DeAngelis LM, Li D, Bernal Y, Gonen M, Wendel HG, Sadelain M, Brentjens RJ, Clinical and Biological Correlates of Neurotoxicity Associated with CAR T-cell Therapy in Patients with B-cell Acute Lymphoblastic Leukemia, Cancer Discov 8(8) (2018) 958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Maude SL, Barrett D, Teachey DT, Grupp SA, Managing cytokine release syndrome associated with novel T cell-engaging therapies, Cancer J 20(2) (2014) 119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Robinson TO, Schluns KS, The potential and promise of IL-15 in immuno-oncogenic therapies, Immunol Lett 190 (2017) 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sordo-Bahamonde C, Vitale M, Lorenzo-Herrero S, Lopez-Soto A, Gonzalez S, Mechanisms of Resistance to NK Cell Immunotherapy, Cancers (Basel) 12(4) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rodella L, Zamai L, Rezzani R, Artico M, Peri G, Falconi M, Facchini A, Pelusi G, Vitale M, Interleukin 2 and interleukin 15 differentially predispose natural killer cells to apoptosis mediated by endothelial and tumour cells, Br J Haematol 115(2) (2001) 442–50. [DOI] [PubMed] [Google Scholar]


