Abstract
Extracellular vesicles (EVs) are membranous nanovesicles secreted from living cells, shuttling macromolecules in intercellular communication and potentially possessing intrinsic therapeutic activity. Due to their stability, low immunogenicity, and inherent interaction with recipient cells, EVs also hold great promise as drug delivery vehicles. Indeed, they have been used to deliver nucleic acids, proteins, and small molecules in preclinical investigations. Furthermore, EV-based drugs have entered early clinical trials for cancer or neurodegenerative diseases. Despite their appeal as delivery vectors, however, EV-based drug delivery progress has been hampered by heterogeneity of sample types and methods as well as a persistent lack of standardization, validation, and comprehensive reporting. This review highlights specific requirements for EVs in drug delivery and describes the most pertinent approaches for separation and characterization. Despite residual uncertainties related to pharmacodynamics, pharmacokinetics, and potential off-target effects, clinical-grade, high-potency EV drugs might be achievable through GMP-compliant workflows in a highly standardized environment.
Keywords: Pharmacology, clinical pharmacology, drug development, exosomes
Graphical abstract
1. Introduction
The concept of medication has evolved over time, for example from unrefined plant materials to crude extracts of those plants to purer extracts of active ingredients to synthetic active ingredients to pharmaceutically optimized synthetic compounds [1]. Recent years have given rise to a different thread of drug development, the ‘biologics’, complex macromolecules bioengineered to be produced by cells [2]. Examples include peptides, proteins, and vaccines [3].
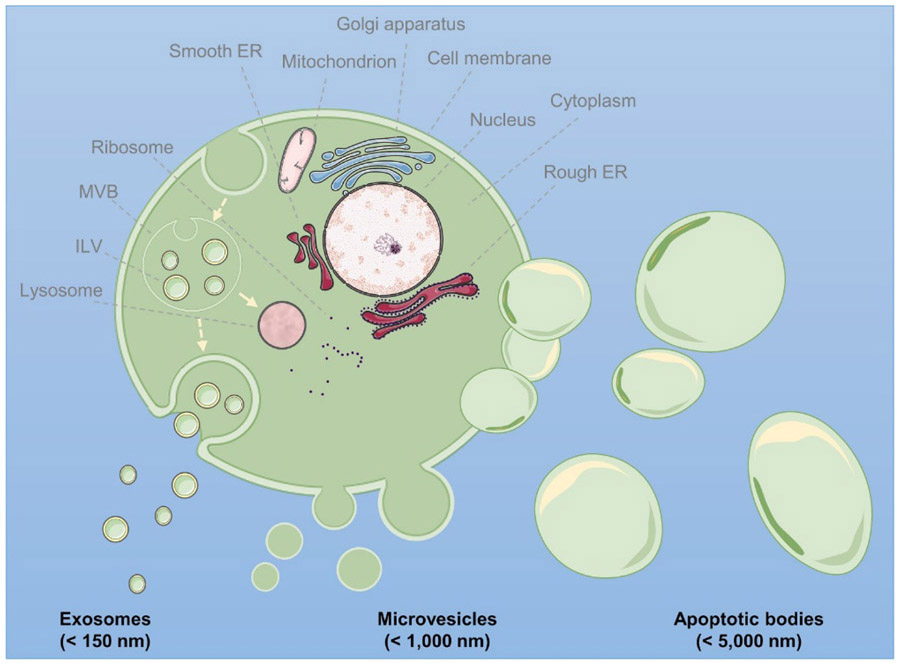
The complexity of biologics varies. Peptides are simple in structure compared with the novel category of extracellular vesicle (EV)-based drugs, a type of biologic of unparalleled complexity. In this sense, EV-based drugs might be said to share more in common with the earlier, less purified botanically-based drugs described above. EVs are lipid bilayer-enclosed vesicles secreted by all or virtually all cell types [4]. Several types of EVs have been described, varying both in how they are produced by the cell (their ‘biogenesis’) and their typical size (Figure 1). EVs have the capacity to participate in intercellular signaling, shuttling molecules from their cell of origin (‘donor cell’) to a recipient cell, affecting the phenotype of that recipient cell [5]. EVs’ exterior surface exhibits proteins that can serve to interact specifically with proteins on recipient cells, allowing a degree of specificity in their interactions with recipient cells.
Figure 1.
Schematic representation of the biogenesis of exosomes, microvesicles, and apoptotic bodies. Exosomes are produced as intraluminal vesicles (ILV) by inward budding of early endosomes, forming a multivesicular body (MVB). MVBs can either fuse with lysosomes or with the cell membrane, thereby releasing exosomes into the extracellular milieu. Microvesicles are generated by outward budding of the plasma membrane, while apoptotic bodies are released during programmed cell death via plasma membrane blebbing. ER: endoplasmic reticulum. This figure as well as the graphical abstract were created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; www.https://smart.servier.com.
EVs could be loaded with a drug (e.g., a small interfering RNA (siRNA) or small molecule or other type of drug) in one of two ways [6]. It is possible to load EVs with a drug after the EVs have been separated from non-EV impurities such as soluble proteins. This approach is called exogenous (or post-separation) loading. Alternatively, one can incubate cells with the drug, resulting in the production of EVs already containing the drug, an approach known as endogenous (or pre-separation) loading. For some drug categories including peptides and RNAs, genetically engineering producer cells to produce and subsequently encapsulate therapeutic molecules is another endogenous loading approach.
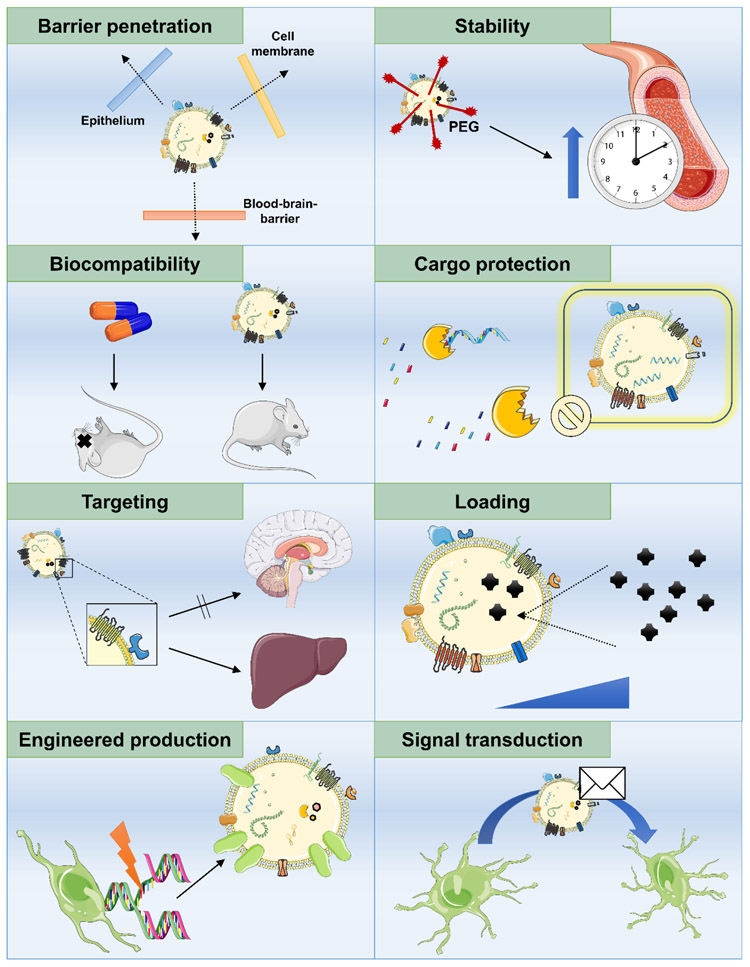
The advantages of EV-based drug delivery systems (DDS) are largely theoretical at this time, but are potentially powerful if they can be realized (Figure 2). The key theoretical advantages are enhanced in vivo stability of oral peptides and proteins or RNA enclosed in lipid carriers, EVs’ low hepatotoxicity and immunogenicity, and the possibility of ‘addressing’ the cargo of the EV to target recipient cells expressing certain surface receptors [6-8]. It is also possible that the beneficial biological effects of EVs extend beyond those of the delivered drug, although this is currently a speculative hypothetical proposition [9]. Whether the low oral bioavailability and poor epithelial penetration of peptide and protein drugs will be overcome by EV-based delivery also remains to be seen [7]. To the extent that EVs might be a newly-appreciated endogenous endocrine signaling system, their use in drug delivery could be of the greatest importance.
Figure 2.
Advantageous features of EVs that can be exploited for drug delivery purposes.
Nonetheless, achieving these advantages will be non-trivial. Loading EVs with microRNAs for drug delivery has technical pitfalls [10]. An EV has an unknown but potentially large number of different proteins on its surface, presenting opportunities for interaction with cells other than the intended target cell type. Even as the complexity of EV-based drugs could enhance their beneficial effects, it could also contribute to adverse effects. What happens to EV cargo inside cells that endocytose the EV is poorly understood [11, 12]. Moreover, the general understanding of the absorption, distribution, metabolism, and excretion of EVs-based drugs is in its infancy.
Despite these challenges, progress is being made, and EV-based drugs have begun early clinical trials [13]. The optimization of drug delivery will be essential for EV-based drugs to achieve their envisioned potential. This review focuses on the special opportunities -- and challenges -- EV-based drugs present for drug delivery.
2. Separation of EVs for drug delivery applications
Before an EV-based drug can be delivered, its EV carrier must be separated in sufficient quantity for administration. In general terms, separation refers to the process of extracting EVs from their original matrix, which might be a biofluid or a solid tissue. The past decade brought about a wide array of commercially available and homebrew methods to separate EVs from different sources. While all these and older methods have their strengths and weaknesses, some of them seem uniquely suited for drug delivery applications and will be discussed in detail in this section.
2.1. EV separation methods and their limitations
Irrespective of the specific clinical context in which therapeutic EVs are to be used, it is crucial to establish optimal source material and production systems. As EVs from a wide variety of sources have been previously studied for therapeutic purposes in preclinical models including for drug delivery, upstream parameters such as source material, culture conditions, and pre-processing will vary accordingly. For instance, EVs could be sourced from plants, milk, and even bacteria, or harvested from conditioned cell culture supernatant [14-17]. For the latter, mesenchymal stem cells (MSCs) are a commonly used production system, but other cells including red blood cells, dendritic cells, macrophages and tumor cells have been used as well [18-21]. Some of the culture conditions to be rigorously tested and optimized include type of culture (two-dimensional vs. three-dimensional, adherent vs. suspension), cell density, type and volume of media, essential and beneficial additives, as well as potential stressors (hypoxia, low pH) to trigger EV release, all of which might, however, alter the molecular makeup of secreted vesicles [22-28]. Obviously, any suitable EV-DDS production system should be highly scalable without altering relevant characteristics of producer cells and needs to meet regulatory guidelines for clinical-grade production. As these important considerations are beyond the scope of this manuscript, we will focus on the separation process, assuming that EV production has been properly optimized.
Despite a multitude of available options and some controversy in the EV separation literature, there is little doubt about one conclusion: there is no single best method to be used. Each method has its limitations, and selecting the most appropriate choice for a specific purpose, e.g. separating therapeutic EVs from a defined cell type grown under defined conditions, demands matching these limitations against the requirements of the sample type and application at hand. Some of the most important parameters that differ between separation methods include throughput, cost, ease of use, requirements in time and instrumentation, gentleness, vesicle loss during separation, and purity of the final preparation. Commonly used methods target the general physicochemical characteristics of EVs (i.e. size, density, charge) or focus on specific properties (e.g. expression of a surface marker). Depending on the respective separation principle, methods might thus be rather non-specific, enriching a broad range of EVs and, potentially, non-EV material, or highly specific, separating only defined vesicle populations. A useful framework to think about separation methods has been introduced by the 2018 Minimal Information for Studies of Extracellular Vesicles (MISEV2018) guidelines, which suggest arranging each method on a ‘recovery vs. specificity’ grid [29]. Each quadrant of this grid (e.g. high recovery, low specificity) describes procedures’ efficiency of recovering extracellular material as well as their ability to separate EVs to the exclusion of non-EV impurities. Note that the ‘high recovery, high specificity’ quadrant remains unpopulated since highly specific separation of pure vesicles without sacrificing recovery to some extent is not yet feasible using present-day methods; nor is it necessarily a requirement for all purposes. Applicable to the manufacturing of all biopharmaceuticals, ‘The Process is the Product’ is particularly true for EVs due to the heterogeneity of vesicle populations and the variety of available separation methods. By selecting highly scalable separation methods early on, changes in prematurely optimized small-scale methods -- and thus the resulting vesicle product -- can be avoided. For therapeutic EVs and EVs to be used for drug delivery, it might be extremely challenging to fully characterize the final product in order to establish its potency and its mechanism(s) of action. Because the production and separation procedures and the properties of the resulting product are inextricably connected, it is crucial to rigorously characterize, standardize, and document the entire process and to execute each step accordingly to ensure minimal batch-to-batch variability [30]. In a young field that struggles with seemingly basic issues, such as vesicle nomenclature, and still lacks rigorously standardized workflows spanning from sample collection to analytical output, using EVs therapeutically is among the EV field’s most aspirational goals [31, 32]. With sections of the EV community already striving towards expertly calibrated instruments and standardized analytics, developing best practices is one of the most pressing and well-recognized needs [33-35]. While studies on the most basic and least glamorous aspects of EV methodology, including repeatability, reproducibility, and operator-dependent biases, are still scarce, initial reports demonstrate non-trivial qualitative and quantitative effects of method differences on final analytical results [36, 37].
2.2. Separating the most relevant EV populations
Separating EVs to be used as DDS poses specific requirements compared to other downstream uses, particularly those outside of clinical settings. Most importantly, a suitable separation procedure needs to capture those EVs that are therapeutically relevant. As discussed above, EVs are heterogeneous in their biogenesis, size, and composition, even the EVs derived from a single cell type [38]. It is thus conceivable, if not likely, that not all vesicles in a preparation are equally suited for drug delivery. Some of the most relevant characteristics that might differ between vesicle types include their half-life in circulation vs. clearance rate, uptake in tissues of interest, and suitability for effective drug loading and delivery. To assess potential differences in drug delivery, Saari and coworkers loaded distinct populations of vesicles pelleted at 20,000 x g (20k EVs) or 110,000 x g (110k EVs) with paclitaxel and tracked their uptake in prostate cancer cells using fluorescence lifetime imaging microscopy (FLIM) [39]. Despite highly similar size distributions, uptake mechanisms and drug release profiles differed between the preparations, with 110k EVs delivering their payload mostly via endocytosis and 20k EVs employing both endocytosis and fusion with the plasma membrane of recipient cells. For endogenous loading, larger EVs might contain more of the respective therapeutic agent but still be inferior to smaller vesicles if the latter display more favorable biodistribution and pharmacokinetics or higher efficiency of cargo delivery. As the requirements regarding target tissue, minimal exposure time or the need to load large quantities of a therapeutic molecule differ for different applications, so might the characteristics of a vesicle that optimally meets these requirements, thus highlighting the importance of selecting a separation strategy that captures these vesicles, potentially to the exclusion of other types.
2.3. Separation methods need to preserve EV integrity and activity
While various applications might call for prioritizing different characteristics when selecting the most appropriate vesicles, a common requirement is that their structure, membrane topology, and biological activity remain unaltered after separation. Suitable methods should thus neither alter or damage vesicles nor introduce exogenous material that might interfere with their surface functionality. These requirements favor gentle separation methods and most likely disqualify those that utilize high mechanical forces, harsh reagents, or chemicals that cannot be removed in subsequent purification steps. Ultracentrifugation, for instance, which remains the most commonly used EV separation method in non-clinical settings, has been shown to damage vesicles and lead to aggregation due to high mechanical forces [40, 41]. Crucially, these changes in biophysical properties have been linked to reduced biological activity, while gentler methods preserve the functionality of the EVs after separation [42, 43].
2.4. Separation methods should not introduce exogenous material
Similarly, separation methods for EVs that are to be used as DDS ought not introduce exogenous reagents that end up as impurities in the final vesicle preparation. Should such separation reagents be impossible or extremely costly to remove, care must be taken that they do not interfere with the biological activity of EVs or have unwanted biological effects of their own. Enriching EVs by precipitation with polymers such as polyethylene glycol (PEG), for instance, inevitably leads to polymer remnants in EV preparations, which can interfere with downstream characterization and functionality of vesicles [44, 45]. What’s more, preparations derived from precipitation were shown to have lower biological activity than those separated by density gradients, which might be due to masking of important EV surface molecules by residual precipitation reagents [46]. When introducing an additional purification step such as washing by ultracentrifugation, however, residual PEG can be reduced significantly [47]. Another method that might lead to separation-related reagent impurities is density gradient centrifugation (DGC). This approach is very effective at separating EVs from non-EV material by flotation or centrifugation into gradients made of, for instance, sucrose or iodixanol and concentrates vesicles in a discrete density band [48, 49]. However, since high concentrations of these chemicals are required to achieve sufficient separation, some residual material could end up in the final EV preparations. After extraction of individual density fractions, size-exclusion chromatography (SEC) is an effective method to remove gradient material, as demonstrated for recombinant adenovirus particles as well as EVs purified by iodixanol or iohexol gradient centrifugation [50-52]. In addition to the activity of vesicles themselves, none of the relevant properties of an EV formulation to be used for drug delivery, e.g. clearance from the bloodstream, partitioning between different tissues, should be altered by the EV separation method. Likewise, any modifications made to any step of the production and purification process need to prompt rigorous validation experiments to confirm constant product properties.
2.5. EV purity for drug delivery: where to draw the line?
As discussed in section 2.1 within the framework of a ‘recovery vs. specificity’ grid, another separation method-dependent feature of EV preparations is their purity. Although there tends to be some intuition for the concept of EV purity, consensus definitions and quality metrics remain to be established. A useful way to define a high-purity EV separation method is its ability to minimize capturing non-EV material, while preferably being highly effective at capturing EVs. Compared to the source material, preparations derived from such a method would thus be enriched in EVs but depleted of non-EV components such as soluble protein. Measuring multiple different characteristics of EV preparations and calculating their relative proportions has been suggested to assess overall purity and define subpopulations. To this end, protein-to-lipid ratios, RNA-to-particle ratios, and particle-to-protein ratios have been successfully implemented [53-55]. The latter of these approaches is particularly commonly used because impure preparations are expected to be characterized by non-EV protein, reducing their particle-to-protein ratio. With the caveat that such ratios might be specific to a given biofluid or type of EV producer cell and could depend heavily on the respective separation method, they are still useful to assess batch-to-batch purity in a defined production system. Based on these suggested criteria, it has been clearly demonstrated that distinct separation principles differ in their propensity to co-separate non-EV material and thus produce EV preparations of substantially different purity [49, 56, 57]. Methods with low specificity for EVs perform particularly poorly with complex, ill-defined, and protein-rich biofluids, which bear high risk of non-vesicular protein impurities. Still, while a degree of purity is certainly required for therapeutic applications, extraordinarily pure EV preparations are not necessarily more effective than less stringently prepared ones. Damage to EV components that mediate function is a consideration when using extended purification procedures required to achieve very high purity levels, as is loss of loosely associated factors that act in conjunction with EVs to drive biological effects. In fact, it was recently shown that functional effects attributed to MSC EVs were actually mediated by paracrine factors or required co-separation of non-EV material by less stringent methods to take full effect [58]. Through the lens of potency, a well-characterized EV preparation of lower purity might thus be more efficacious than a genuinely pure preparation of vesicles. This could also improve cost efficiency since high purity levels are usually achieved at the expense of yield, scalability and increasing demands on instrumentation and processing times. At the same time, this observation reinforces the challenges of discerning EV-specific contributions to efficacy -- or safety signals -- when delivering drugs using EVs.
2.6. Additional considerations when separating EVs for drug delivery
In addition to these crucial requirements, there are more factors to consider when selecting strategies to separate EVs for drug delivery. First of all, appropriate separation methods need to be compatible with the sample material at hand. Different biofluids present their own unique peculiarities (such as low vesicle concentration, high viscosity, high protein concentration) that pose challenges for EV separation. Methods that work well for one biofluid might thus not be ideal for a different one, and pre-processing of samples (such as dilution or removal of specific components) might be required. Similarly, some methods might require additional post-separation steps such as concentrating samples that were substantially diluted during separation. While these considerations are more biological in nature, there are also technical aspects to be considered when selecting EV separation methods. These include repeatability and reproducibility, which are crucial to ensure constant purity, concentration, and efficacy of final EV formulations. For cost-effective production of vesicle-based drug carriers, EV separation should also be scalable and automatable, allowing large volumes of biofluid to be processed without proportional increases in cost. While closed systems that prohibit any contact between the manufactured product and the room environment are certainly recommended for EV production, this might not be a strict requirement for EV separation itself as vesicle preparations can be sterilized by filtration and tested for microbial contamination post-separation.
2.7. Separation methods that might be most suitable for drug delivery
Given these complex requirements for an EV separation method that lends itself to drug delivery applications, it is obvious that some approaches are more suitable than others. In light of the abundance of excellent method comparisons for a variety of biofluids, we will herein focus on those that are most promising for drug delivery [49, 56, 59]. For a brief overview of these methods and their advantages as well as disadvantages, see Table 1. No matter which method is selected, rigorous standardization and frequent quality control are crucial in order to ensure a reproducibly pure, stable, and potent product.
Table 1.
Summary of EV separation methods and examples of their utilization in in vivo drug delivery studies whenever available.
| Method | Principle | Advantages | Disadvantages |
In vivo drug delivery examples |
|---|---|---|---|---|
| Tangential flow filtration | Size | Highly scalable, automatable, gentle, choice of membrane, disposable devices | Unable to remove EV-sized impurities, non-specific interactions with membranes | [23] |
| Ultrafiltration | Size | Rapid, scalable, choice of membrane, no dedicated major equipment, disposable devices | Clogging, sample loss, EV damage, unable to remove EV-sized impurities | UF, UC, sucrose cushion [103] |
| Size-exclusion chromatography | Size | Gentle, scalable, good separation, removes soluble proteins and small molecules | Sample dilution might require post-separation concentration, low yield, limited sample capacity, unable to remove EV-sized impurities | NA |
| Bind-elute chromatography | Size/affinity | Rapid, scalable, gentle, one-step elution, removes soluble proteins and small molecules | Low yield, limited sample capacity | NA |
| Ion-exchange chromatography | Charge | Rapid, scalable, one-step elution | Low specificity for EVs, separation conditions need to be optimized, might require post-separation buffer exchange and concentration | NA |
| Affinity chromatography | Affinity | Rapid, scalable | Unclear purity, elution might damage vesicles | [104] |
| (Immuno)affinity | Affinity | Rapid, high purity, no specialized equipment, specific capture of engineered EVs | Costly, low throughput, low yield, unclear scalability, a priori knowledge of surface markers is necessary, affinity reagents need to be removed without damaging EVs | NA |
| Differential ultracentrifugation | Sedimentation | Inexpensive, easy to use | Low throughput, low scalability, needs specialized equipment, unable to remove EV-sized impurities, potential EV damage and aggregation | [105-107] |
| Density gradient centrifugation | Density | Commonly used method, inexpensive, high purity, often used in combination with other methods | Low throughput, low scalability, needs specialized equipment, low yield, lengthy and cumbersome procedure | Density gradient [108] Density cushion [109, 110] |
| Precipitation | Solubility | Highly scalable, rapid, no specialized equipment, easy to use, inexpensive | Low purity, co-separates soluble non-EV material, precipitation reagent needs to be removed | [111-113] |
2.7.1. Tangential flow filtration
Tangential flow filtration (TFF, also called cross flow filtration) is a gentle, size-based fractionation method that has well-established, Good Manufacturing Practice (GMP)-compliant uses in purifying therapeutic proteins or virus particles [60, 61]. As with so-called ‘dead-end’ ultrafiltration devices, small particles and molecules are depleted from the starting material using a membrane with a defined average pore size, on which particles above the selected size cutoff are retained. In TFF ultrafiltration, however, the EV-containing biofluid contacts the permeable membrane in a tangential, rather than direct flow, allowing for easier scaling up of the filter’s surface area using parallel, long tubules, and reducing the risk of clogging. A recent study on cell culture supernatant-derived EVs demonstrated that TFF achieved higher vesicle yields and more effective removal of soluble proteins than separation by ultracentrifugation while also improving processing time [62]. Additionally, EVs separated by TFF were morphologically intact and demonstrated superior batch-to-batch consistency. In a report by Lee and coworkers, TFF was used to separate EVs from multiple liters of adipose tissue-derived MSC supernatant [63]. Separation was highly reproducible and effective at removing waste products from cell culture. Moreover, this approach yielded EVs with therapeutic effects in a rat model of acute kidney injury. Similarly, EV yield from TFF separation was 7-fold higher compared to separation by ultracentrifugation in a recent study that implemented a novel three-dimensional culture of umbilical cord-derived MSCs [23]. Beyond these advantages, EVs separated by TFF were highly effective at delivering exogenously loaded siRNA to neurons, thus demonstrating the preservation of biological activity throughout the separation process. Several groups also combined TFF-based EV concentration with an additional size-exclusion chromatography step, thus producing highly pure and functional EVs from large starting volumes while minimizing separation-associated vesicle loss [64]. These features, combined with the potential for industrial-scale, sterile production using disposable filters and compliance with regulatory guidelines make TFF a highly promising method to separate EVs for drug delivery.
2.7.2. Ultrafiltration
Similar to TFF, dead-end ultrafiltration (often referred to simply as ultrafiltration, UF) separates particles by size via retention on a porous membrane. Given an appropriate choice of size cutoff, EVs can be separated from larger or smaller particles, often in sequential filtration steps with increasingly smaller pore sizes. Because UF is time-efficient, scalable, and does not require dedicated equipment, it has become a widely used tool to enrich EVs or to concentrate EV suspensions from separation methods that introduce considerable sample dilution [41]. In contrast to TFF, UF usually employs dead-end devices, in which sample flow is orthogonal to the filter membrane. This setup calls for pressurization, centrifugal forces, or vacuum to drive sample material through the pores, which increases the risk of both clogging membranes and damaging the vesicles. This type of separation works well for spherical and rigid particles, but flexible or non-spherical particles that should not pass through a particular membrane might be pushed through small pores by external forces. As is true of all purely size-based methods, UF is unable to separate EVs from similarly-sized non-vesicular particles, which is particularly problematic for complex biofluids with potential impurities across different size ranges (e.g. lipoprotein particles in blood-based biofluids). Additionally, as size cutoffs of membranes used for UF as well as TFF are not absolute but reflect the median cutoff across a distribution of pore sizes, particle separation might lack the desired resolution. Loss of sample via non-specific retention on filter membranes could be another downside of UF-based EV separation, although some types of membrane are less prone to non-specific binding than others [65]. Indeed, a 2017 publication by Vergauwen and coworkers highlighted the impact of membrane type and size cutoff, both of which can lead to substantial loss when concentrating EVs [51]. Still, UF can be successfully used as a standalone method or in combination with other EV separation methods such as ultracentrifugation or density-based separation [66]. Highly relevant to industrial-scale EV production, Heinemann et al. recently presented a sequential filtration protocol based on UF and TFF, which was used to separate functional vesicles from large volumes of conditioned cell culture supernatant [67]. This method demonstrated higher recovery and purity than separation by ultracentrifugation and effectively depleted soluble protein from final EV samples. Similarly, Nordin and coworkers combined UF with liquid chromatography (LC) to separate EVs from cell culture supernatant [43]. This combination resulted in improved EV yield compared to ultracentrifugation while also preserving biophysical properties of the resulting vesicles, which is crucial for therapeutic applications and drug delivery.
2.7.3. Chromatography
Column-based chromatography has been used in industry and academia for decades to selectively purify small molecules, proteins, polymers, or virus particles [68, 69]. Like many tools with long-standing use in other fields, chromatographic methods are now also extensively used to separate or purify EVs. Depending on the respective setup, researchers might use SEC, affinity chromatography or charge-based chromatography to purify their vesicles. Since the sample volume of many such methods is proportional to the bed volume of the column, pre-chromatography enrichment steps such as TFF or UF are commonly used to apply a more concentrated sample. Similarly, the purpose of chromatography in this context is separating EVs from impurities rather than enriching vesicles. Many methods thus lead to dilution of EVs, which might call for subsequent concentration steps.
2.7.3.1. Size-exclusion chromatography
Like TFF and UF, SEC is based on separation by particle size. Sample material is applied to a resin composed of porous beads, most commonly Sepharose CL-4B or Sepharose CL-2B, in an aqueous mobile phase [70, 71]. During migration through the stationary phase, small molecules, which are able to enter the pores, are retained on the column while larger particles pass by the pores and elute quickly. This simple separation principle is particularly useful to separate large particles such as EVs from free protein and has been implemented in a wide range of commercially available or homebrew column-based methods. SEC has been used as a one-step method or in combination with other methods to separate EVs from a variety of biofluids [43, 72]. In comparison with other separation methods, SEC is commonly found to be highly effective at removing soluble protein and other small impurities, although at the cost of sample dilution and reduced EV yield [71, 73]. While some reports claim SEC-derived EVs to be equal in purity to those separated by the laborious and highly pure DGC method, it is important to note that SEC alone is unable to separate EVs from other EV-sized particles such as low-density lipoproteins and chylomicrons since fractionation is strictly based on particle size [74]. Depending on sample concentration and column size, SEC can be used to process large volumes of biofluids, making it highly scalable. Additionally, separation can be implemented as an automated, continuous procedure with one or multiple columns, thus avoiding common limitations related to capacity and processing time [75]. Another advantage of SEC-based separation is that it does not utilize high mechanical forces or exogenous reagents, thus preserving the structure and biological activity of vesicles [42, 45]. These features, combined with a relative ease of use and low requirements for dedicated equipment, make SEC a very promising candidate to separate large numbers of functionally active vesicles.
2.7.3.2. Bind-elute chromatography
A close relative of SEC, bind-elute chromatography (BEC) combines the principles of size-based separation and affinity chromatography. Here, separation beads are characterized by an inert shell and pores functionalized with charged and hydrophobic ligands that bind a wide range of contaminants. Since small molecules are permanently trapped and not merely retained on the column, elution of EVs in one fraction, rather than in sequential, highly diluted fractions as in SEC, might be possible. BEC has been used to purify virus particles as well as EVs, proving to be a high-recovery, time-efficient method with promising scalability [76]. Importantly, EVs separated by BEC from cell culture supernatant were readily taken up by recipient cells, indicating preserved functionality of vesicles throughout the process. Like SEC, BEC is often used in combination with other purification methods. Onódi and coworkers used BEC to remove albumin and lipoproteins from plasma EVs previously separated by iodixanol gradient centrifugation, and reported these impurities to be below the limit of detection albeit at the cost of slight vesicle loss [77]. In a large-scale separation experiment, McNamara and colleagues enriched EVs from hundreds of milliliters of cell culture supernatant by TFF prior to BEC-based purification [64]. Compared to separation by ultracentrifugation or precipitation, this protocol provided several hundred-fold higher vesicle yield, and resulted in a homogenous population of functionally active EVs with high purity. Although BEC is not among the most frequently used methods, several reports on its utility for EV separation, as well as detailed protocols were published in the past few years [78]. In an alternative to conventional chromatography, purification could be achieved without having to use a column by simply incubating sample material with BEC resin and eluting EVs by centrifugation once sufficient depletion of impurities via binding to the functionalized core has been achieved. Such an ‘inslurry’ approach was successfully used to purify virus particles by James and coworkers, potentially paving the way for applications in EV purification [79].
2.7.3.3. Ion-exchange chromatography
Unlike the chromatographic methods described above, ion-exchange chromatography (IEC) separates EVs using their charge, rather than their size. A standard method for protein and virus particle purification, IEC uses resins with positively- (anion exchange) or negatively- (cation exchange) charged residues that bind oppositely charged molecules via non-covalent, ionic interactions [80]. Because EVs carry a net negative charge, they can be captured on anion exchange resins while uncharged or positively charged material elutes from the column without binding. Bound material is next released from the resin by altering the ionic strength or pH of the buffer in a gradient or one-step elution, often followed by buffer exchange and concentration procedures. In a publication by Heath and colleagues, EVs were separated from 1 liter of cell culture supernatant by IEC, using an NaCl gradient for elution [81]. The presented method allowed for improved flow rates and increased vesicle purity when separating EVs secreted by adherent cells or cells in suspension. Although biological activity of vesicles was not assessed in this study, IEC was found to be a rapid one-step method for large-scale EV separation. Similarly, Kim and coworkers utilized IEC to separate EVs from 1.2 liters of MSC supernatant [82]. In these experiments, the functionality of EVs was assessed in a mouse model of traumatic brain injury. Indeed, EVs injected intravenously were shown to reduce inflammation and rescue experimentally-induced cognitive impairment, suggesting that their biological activity was preserved. Although highly scalable, anion exchange purification is a more involved method than SEC and BEC since it calls for careful optimization of counterions, buffer system, and elution conditions. As with most chromatographic methods, it is not truly specific to EVs but captures other negatively charged molecules such as most proteins. Additionally, uneven distribution of surface charges and differences in net charge between EV populations might complicate efficient purification.
2.7.3.4. Affinity chromatography
While IEC solely relies on overall membrane charge to capture vesicles, affinity chromatography targets more specific EV properties such as surface proteins. Following reports that heparin blocks EV uptake, heparin affinity chromatography, which has long been used to purify proteins or virus particles, was recently applied to EV separation [83]. In this approach, heparin covalently immobilized on a chromatography resin captures proteins with heparin binding ability while other sample components are washed away with the liquid phase. Reiter and colleagues successfully used heparin affinity chromatography to separate EVs from HEK293 cell culture supernatant with a preceding BEC step to deplete heparin binding proteins that would otherwise compete with EVs for binding sites [84]. Similarly, Balaj and coworkers combined UF and heparin affinity chromatography to separate EVs from cell culture supernatant and plasma [85]. High purity with respect to soluble protein and ready EV uptake into recipient cells were reported, highlighting the potential utility of affinity-based separation. Heparin affinity chromatography seems promisingly scalable, but there are also disadvantages for specific EV separation. Depending on their molecular makeup, it might not capture all EV populations in a biofluid while the broad binding affinity of heparin to various proteins, on the other hand, might lead to non-EV impurities in the final preparation. Furthermore, the elution conditions need to be optimized to strike a balance between efficient release and preserved biological activity of EVs. Additional affinity principles that could be implemented into a chromatographic setup include binding to annexin V, shiga toxin, or antibodies against common EV surface markers, although their utility for GMP-compliant large-scale vesicle separation remains unexplored [86, 87]. As an alternative to exploiting the ability to bind endogenously expressed vesicle proteins, therapeutic EVs might be engineered to display an affinity tag for efficient downstream purification. Provided captured EVs can be released without impeding their biological activity, this approach could prove very promising for specific large-scale purification of engineered vesicles [88].
2.7.4. (Immuno)affinity
The principle of affinity-based EV separation is not limited to chromatography, but can be implemented in a variety of additional methods. Immunoaffinity, in which EVs are captured by antibodies immobilized on a solid surface, has been used for more than two decades and can be a particularly useful technique to target specific EV populations based on surface markers [89]. Capture antibodies are commonly conjugated to magnetic beads, allowing convenient retention of bound vesicles, which are subsequently washed and eluted or lyzed for downstream applications. Given the molecular heterogeneity of EV types and subpopulations, it will be challenging to establish universal surface markers to capture a broad range of vesicles [90]. Furthermore, capture efficiency depends on the density and accessibility of the respective epitope as well as the availability of high-affinity antibodies. Still, immunoaffinity methods can be a rapid and convenient tool to selectively separate engineered EVs that express a particular surface protein or affinity tag, and these methods tend to yield relatively pure vesicle preparations. Effective release of vesicles from beads, however, usually requires harsh conditions such as acidic elution buffers [91]. As these are likely to damage surface proteins and thereby reduce EV activity, immunoaffinity might not be among the most suitable separation methods for drug delivery applications. Additionally, low throughput, costly reagents, potential masking of functionally relevant epitopes by non-specific antibody binding, and restricted scalability are disadvantages of current methods. As affinity reagents are not limited to antibodies, novel approaches might be able to overcome these challenges. Membrane-sensing peptides, phospholipid-binding proteins, and synthetic peptides with high affinity to heat shock proteins have been used to successfully capture EVs from biofluids [92-94]. Furthermore, DNA aptamers were recently suggested as another intriguing type of affinity reagent for EV separation. Monovalent or multivalent aptamer constructs that bind to EV surface proteins with high affinity might be a valuable alternative to immunoaffinity reagents. Since EVs can be released nondestructively by degrading the bound aptamer or adding complementary sequences to change its secondary structure, it seems feasible to recover biologically functional vesicles, potentially paving the way for applications of EVs as DDS [95].
2.7.5. Ultracentrifugation
Differential ultracentrifugation (dUC) and variations thereof have been used to separate particles for decades and are still among the most commonly used methods in the EV field [96]. Classical protocols employ sequential centrifugation steps with increasing speed to deplete large and medium-sized particles based on their respective sedimentation rates. EVs are then pelleted from the pre-cleared supernatant at speeds of > 100,000 x g, typically for more than 70 minutes. While dUC is an effective tool to enrich EVs, it is rather unlikely to succeed as a separation method for therapeutic vesicles given its low throughput and lengthy run times, and propensity to co-pellet large amounts of soluble protein [49].
In an attempt to increase purity by adding an orthogonal separation principle, dUC is oftentimes followed by DGC. Here, EVs in the crude dUC pellet are subsequently further separated according to their densities in a gradient made of sucrose or iodixanol, among other possible substances [90, 97]. While separation might be achieved via top-down gradients or bottom-up floatation into a gradient, the common principle of partitioning EVs away from impurities with a different density remains. After DGC, which usually requires run times of at least 16 hours, specific density fractions are retrieved and re-pelleted, juxtaposing this method’s ability to yield exceedingly high-purity samples with its prohibitive demands on time and reliance on skilled operators [98]. EVs from substantial volumes of fluid can be enriched by dUC or other means of concentration prior to gradient loading, but the low throughput of DGC is unlikely to meet the scalability needs of industrial vesicle production.
2.7.6. Precipitation
Precipitation using synthetic water-excluding polymers such as PEG has been used to concentrate biological particles by altering their solubility for decades [99]. Here, samples are incubated with commercially available or in-house polymer preparations for short periods of time and particles are subsequently retrieved via low-speed centrifugation. Given that it is highly scalable, easy to use, affordable, and does not require specialized equipment, precipitation seems an appealing candidate to process large volumes of EV-containing biofluids. Due to its low specificity, however, it is clear that precipitation is merely a tool to enrich EVs rather than an effective separation method. Resulting samples are a crude mixture of vesicles and co-precipitated non-vesicular material including soluble protein and lipoprotein particles, most likely yielding EV preparations too impure for therapeutic applications without additional separation steps. Indeed, precipitation has been shown to co-enrich non-vesicular RNA from cell-free blood and led to artifactually high activity of EV samples due to non-specific capture of soluble paracrine factors in functional experiments [58, 100, 101]. Still, it seems feasible to remove both co-precipitated impurities and residual precipitation reagent by additional washing steps and use the resulting preparations in clinical settings [47, 102].
3. EV characterization: the what, why, and how
Due to the heterogeneity of EVs and the above-mentioned challenges in their separation, the need for a thorough characterization of EV preparations is significant. In particular, the development of EVs as DDS requires multiple steps of in-process quality control to achieve reproducibly safe and targeted therapeutics that follow GMP regulations. In the following section, we highlight EV characteristics that are of high relevance in studying and engineering vesicles for the specific purpose of drug delivery. In this context, pertinent EV characterization techniques will be discussed, some of which might be more suitable than others, depending on the attributes to be tested.
3.1. Relevance of EV characteristics in pharmacokinetics
3.1.1. Basic EV-DDS characteristics to be evaluated
Once a manufacturing process and EV separation method have been selected, the most important objective that needs to be addressed is setting a production standard, calling for a rigorous initial characterization of the resulting EV preparation. Currently available methods permit determination of EV size, concentration, morphology, and specific cargo. These methods can be applied irrespective of which biofluid or cell type has been selected as starting material. Due to the limitations of instrument sensitivities and high sample complexity, a combination of multiple orthogonal techniques could be of benefit in setting a quality benchmark. This standard should then be reached with high batch-to-batch consistency, requiring perpetual quality control efforts and, ideally, independent replication of these tests in a different laboratory.
Depending on which drug-loading strategy (endogenous or exogenous) and which payload (small molecules, nucleic acids, peptides, or proteins) are chosen in the therapeutic approach, the separated EV preparations have to be checked for their initial composition before as well as after cargo loading. This step-wise checking ensures that the drug component of interest has been incorporated in the EVs. In parallel, the integrity of EV preparations must be confirmed to avoid unintended reactions in vivo, early degradation and the ensuing loss of potency, or untargeted drug delivery, all of which could potentially result in adverse reactions [35]. In this regard, the intactness of EV membranes, which was previously found to be important in other contexts, may be a suitable proxy for EV-DDS integrity [114]. Moreover, adverse effects could, in principle, be minimized by applying only EV preparations with a high purity. However, ideal purity, namely the sole presence of a desired substance or molecule, is hard to reach for EV-DDS due to the above-described challenges in their separation and might not always be desirable as discussed in section 2.5. Assessing for the presence of microbial contaminants such as endotoxins, mycoplasma and fungal material, which is a common cause of drug recalls, on the other hand, is mandatory for clinical-grade DDS manufacturing. It should also be considered that viruses, which share many of the physiochemical properties of EVs, could have been similarly enriched during EV separation, resulting in a higher risk for adverse reactions. Radiation could be used to manage this type of contamination, but little is known about its impact on EV functionality [115]. Furthermore, it might be important to assess for the absence of specific EV-shuttled cargo such as miR-410, the presence of which was shown to boost carcinoma cell growth [116]. When using tumor-derived EVs for drug delivery, consideration must also be given to their potential ability to transfer oncogenic or prometastatic molecules. Moreover, according to the MISEV2018 guidelines, it is recommended to check for both the presence of EV-specific protein markers and the absence of potential impurities [29].
Prior to application in clinical trials, treatment doses of adequately characterized EVs need to be prepared and characterized. The dosage of pharmacological substances is usually ascertained based on single units sufficient to execute the mechanism of action. In the case of EV-DDS, however, it seems more reasonable to estimate an averaged drug concentration across all EVs and use EV numbers, total protein or lipid content, or sample volumes as the basis for dose-response studies. Subsequently, further experiments assessing dosing rates (single vs. multiple dosing), potency, and toxicity levels are needed.
3.1.2. Routes of administration and barriers to cross
Besides the potency and frequency of dosing, one should also consider the route of EV-DDS administration. There are many options from which to choose, such as intranasal, oral, subcutaneous, intradermal, intramuscular, and intravenous amongst others, with systemic application most likely requiring higher doses than local application. Any route bears different challenges that need to be addressed, and the loaded EVs have to be selected or engineered to withstand surrounding conditions that could lead to early degradation before EV-DDSs reach their target tissues and cells, thus resulting in low bioavailability and short half-life.
The most convenient way for patients to administer EV-DDSs would surely be orally. Administration of bovine milk EVs via the drinking water or oral gavage already demonstrated therapeutic effects on the onset of arthritis in two different mouse models, particularly when applied in high doses [117]. Although this suggests that EVs could somehow withstand the harsh gastric environment even without cocooning them in capsules or tablets, it is unclear which characteristics EVs must have to resist early degradation by digestive enzymes and low pH in the stomach, both of which could dramatically reduce bioavailability. However, more information on the intestinal behavior of nanoparticles and EVs is available. In this context, size and surface charge of nanoparticles were recently shown to be key factors enabling the penetration from the gastrointestinal tract to the circulation in mice [118]. Moreover, recent in vitro studies provided further evidence towards functional drug delivery via orally administered EVs with increased persistence in an acidic environment and intestinal mucus penetration when EVs were coated with PEG [119].
Another rather convenient route of administering EV-DDS would be inhalation, which was already examined in the context of pulmonary fibrosis [120]. Remarkably, several clinical trials that apply MSC-derived EVs administered via inhalation and investigate their therapeutic potential in respiratory disorders, including symptoms caused by SARS-CoV-2, are currently ongoing [121, 122] (NCT04276987). Furthermore, topical application of EVs also represents a very straightforward approach that has already demonstrated great success in wound healing experiments [120, 123]. Subcutaneous injections near a wound seem to be equally effective in transmitting healing properties, probably by paracrine signaling of the EVs’ microRNA and protein cargo, which contribute to reduced scar formation, improved epithelization and vascularization [124]. General characteristics that EVs should exhibit in these types of application are not yet known but could be discovered with ongoing research. For instance, a recent investigation revealed prolonged vesicle stability in the wound bed by linking EVs to a light-triggerable hydrogel [125].
Intranasal application seems to be promising in the treatment of brain-associated disorders due to the physical proximity of the nares to the brain, but systemic injections are also commonly applied for this purpose in preclinical models. For instance, EVs loaded with catalase were detected in substantial amounts in mice brains with Parkinson’s disease after intranasal application [126]. Similarly, mice suffering from different types of brain inflammation were treated with EVs loaded with curcumin or Stat3 inhibitors, both of which showed a protective effect, with EV size being identified as a critical factor for brain targeting [127]. Furthermore, siRNA-loaded EVs were shown to reach the brain within 24 hours after intravenous administration and induced a reduction of protein aggregation in Parkinson’s disease [128]. These studies highlight the apparently intrinsic ability of EVs to cross the blood-brain-barrier (BBB), even though the underlying mechanisms are not yet fully understood [129]. Surface charge, morphology, and size of EVs may play an essential role in BBB penetration [130], but the investigation of artificially modified EVs could support stronger conclusions. Moreover, specific glycoprotein expression on the surface of engineered small EVs enabled successful siRNA delivery to the brain by targeted receptor recognition at the BBB [21]. Additionally, coating the EV surface with PEG could also increase the likelihood of BBB penetration [130]. Interestingly, the rate at which EVs are able to cross the BBB appears to be higher in inflamed compared to healthy conditions [131]. Another route of administration for EV-DDS of potential value for life-threatening central nervous system disorders is intrathecal administration. For instance, both MSCs and EVs appeared equally effective in regenerating spinal cord injuries when applied via lumbar puncture in dogs [132].
The immune system might be the first barrier EVs have to get over after reaching circulation upon intravenous injection or any other route of administration. The mononuclear phagocytic system is mainly responsible for the opsonization of foreign substances, including therapeutic EVs, putatively leading to early clearance and confined treatment efficacy [133]. Therefore, the addition and control of immune-evasive properties would yield an advantage. EVs exhibiting CD47, the most prominent self-peptide signal, could counteract early clearance, thus contributing to enhanced biodistribution [134]. Similarly, PEGylation of EVs leads to reduced recognition by the immune system, which could probably be traced back to the fact that PEGylation shields nanoparticles from interactions with plasma proteins and thus also hampers their identification by cells corresponding to the reticuloendothelial system, finally leading to an enhanced circulation time [135-138]. Additionally, particle size and charge were also shown to be relevant since larger particles seem to be engulfed by macrophages more frequently, and neutrally charged particles are not [139].
When EVs reach their target cells, they have to cross the cell membrane as an additional barrier to deliver their drug cargo for intracellular functionalization. While very small and medium-sized EVs might enter the cell via membrane fusion and endocytosis, larger EVs may instead be predominantly internalized by phagocytosis [140, 141]. Various ligand-receptor interactions are also conceivable, requiring the appropriate lipid or protein composition on both sides. In this regard, numerous uptake mechanisms have already been reviewed, including phagocytosis, macropinocytosis, membrane fusion, and differently-mediated endocytosis (lipid-raft, calveolin, clathrin) [140]. There is more and more evidence that EV internalization is a highly active and energy-dependent event rather than a passive process, thus yielding more relevance to the endocytic pathways. However, it might be hard to generate a ranking of uptake mechanisms as one EV may be able to enter the cell via different routes rather than only one specific way [140]. The surface exposure of tetraspanins, integrins, and lectins appears to be particularly important in this active cellular EV uptake. In case EV-delivered drug cargo is not directly internalized into the cytosol via membrane fusion, it will most likely be enclosed in endosomes targeted for lysosomal degradation. In such instances in which efficacy can be demonstrated, an endolysosomal escape event for efficient drug release has to be assumed, as shown for cationic particles that were able to penetrate to the cytosol by the induction of a flip-flop mechanism [142].
3.1.3. Characteristics determining EV-DDS distribution and clearance
The route of administration appears to influence EV biodistribution, with intravenously administered EVs mainly accumulating in liver and spleen, and intraperitoneal injection leading to EV enrichment in the pancreas and, to a lesser extent, in the gastro-intestinal tract and liver, comparable with subcutaneous EV administration [143]. Moreover, tissue distribution, functionality, and clearance profiles of EV-DDSs are heavily dependent on the surface lipid and protein composition of EVs, such as the repertoire of integrins, proteoglycans, and tetraspanins, as already reviewed elsewhere [140, 144]. In addition, Hoshino et al. revealed that tumor EVs mirror the organotropism of their secreting cells via their surface integrin patterns [145], emphasizing that the EV origin does indeed matter. Beyond the origin of EVs, their size might also be a determinant of tissue targeting. Even though the majority of exogenously administered EVs are directed to the liver, where they are mainly scavenged by macrophages [137], EVs larger than 90 nm also displayed lymph node tropism not observed for smaller EV [146]. Apart from the presence of CD47 on the EV surface and their generally high stability in colloidal liquids due to their negative charge [134, 147], several other modifications such as protease treatment before administration, or PEGylation have been found to increase overall circulation time [138, 148]. By contrast, the presence of phosphatidylserine can lead to enhanced clearance because this phospholipid is identified by macrophages as a phagocytosis signal [149].
With most EVs accumulating in the liver and rapid clearance from the circulation within minutes up to 24 hours [143, 150], one might assume that this could be a result of an active reticuloendothelial system with liver and spleen being amongst the major involved organs [138]. However, information on this topic as well as on general EV elimination mechanisms is still limited, which might be a consequence of insufficient EV-labeling capacities. Even though EVs have been already dye-labeled with lipophilic dyes such as DiR (1,1-dioctadecyl-3,3,3,3-tetramethy-lindotricarbocyanine iodide) or PKH (Paul Karl Horan dye) to study biodistribution patterns, [137, 143], there are crucial limitations to such an approach, and novel labeling strategies have recently been developed. When using lipophilic and lipid-anchored fluorophores, the absence of free dye, dye aggregates, and unintentionally labeled non-EV material is crucial to interpretation of experimental results. This standard might be hard to meet even in apparently pure EV preparations, and biodistribution studies could be misinterpreted if dye aggregates are mistaken for labelled EVs or fluorophores are transferred from EV membranes to competing lipophilic surfaces such as lipoproteins [151]. Novel tools to improve biodistribution studies include bioluminescent, magnetic, and multimodal EV labeling and are excellently reviewed elsewhere [152, 153].
Although more and more information is available on EVs’ pharmacokinetics and the underlying characteristics of their fate in recipient organisms, many questions such as the potential toxicity and excretion mechanisms of EV-DDS remain unanswered. Cheng et al. were able to show that labeled EVs intravenously injected into rats were recovered in kidney tissue and urine [154], although the underlying mechanisms remain to be elucidated. The understanding of these factors for a standardized and safe production of highly efficient DDS requires further development of EV-labeling strategies and the implementation of adequate characterization measurements.
3.2. Overview of techniques for in-process quality control
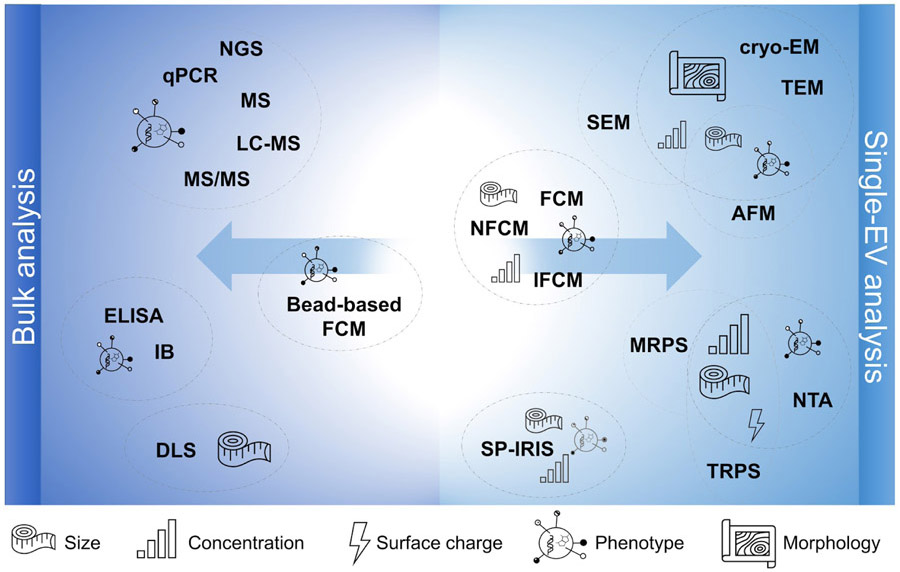
After having highlighted pertinent characteristics that are highly relevant in the context of using EVs as DDSs and should therefore be controlled rigorously, we would like to provide a summary of techniques that could be applied to accomplish said in-process controls with respect to both bulk and single-EV analyses (Figure 3). Additionally, method-specific advantages and disadvantages are summarized in Table 2, highlighting their utilization in current EV-DDS therapeutic research whenever available.
Figure 3.
Overview of EV characterization techniques and their capabilities. AFM: atomic force microscopy, cryo-EM: cryogenic electron microscopy; DLS: dynamic light scattering, ELISA: enzyme-linked immunosorbent assay, FCM: flow cytometry; IB: immunoblotting; IFCM: imaging flow cytometry; LC-MS: liquid chromatography mass spectrometry; MRPS: microfluidic resistive pulse sensing; MS: mass spectrometry; MS/MS: tandem mass spectrometry; NFCM: nanoflow cytometry; NGS: next-generation sequencing; NTA: nanoparticle tracking analysis; qPCR: quantitative polymerase chain reaction; SEM: scanning electron microscopy; SP-IRIS: Single-particle interferometric reflectance imaging sensing; TEM: transmission electron microscopy; TRPS: tunable resistive pulse sensing.
Table 2.
Summary of EV characterization methods and examples of their utilization in in vivo drug delivery studies whenever available.
| Method | Principle | Advantages | Disadvantages |
In vivo drug delivery examples |
|---|---|---|---|---|
| Scanning electron microscopy | Capturing emitted electrons | Low sample volumes, single-EV analysis, surface topography | Little informative on surface structure and membrane features, risk of agglomeration and dehydration during sample preparation | [104] |
| Transmission electron microscopy | Detecting diffracted electrons | Low sample volumes, single EV analysis, visualizing membranous makeup and intra-vesicular structures | Potentially biased by sample preparation, toxic chemicals | [117, 155-157] |
| Cryogenic electron microscopy | Detecting scattered electrons at extremely low temperature | Most native EV morphology, single EV analysis, low sample volume | Specialized sample preparation with dedicated equipment | [158, 159] |
| Atomic force microscopy | Scanning interaction forces between sample and detector tip | Label-free sample preparation, single-EV analysis, three-dimensional topography | Morphological changes by immobilization and by tip in contact mode possible, specialized equipment | [126, 157] |
| Tunable resistive pulse sensing | Measuring resistance pulses | No sample preparation required, fast | Challenging for samples with unknown size ranges, risk of pore clogging, not EV-specific | [156] |
| Microfluidic resistive pulse sensing | Measuring resistance pulses | No sample preparation required, quick, low sample volumes | Challenging for samples with unknown size ranges, risk of pore clogging, not EV-specific | NA |
| Dynamic light scattering | Detecting fluctuations in light intensities scattered by moving particles | No sample preparation required, quick | Bulk measurement, approximate size distribution, not EV-specific, heavily hampered by polydisperse samples, limited resolution | [117, 126] |
| Nanoparticle tracking analysis | Recording the displacement of particles that scatter light | Single particle analysis, fast, no sample preparation required, fluorescent labelling possible to increase specificity | Not EV specific, slightly hampered by polydisperse samples, limited resolution | [123, 126, 155, 157-159] |
| Flow cytometry | Detecting scattered light | Fast, single EV analysis and multiparametric measurement possible | Limited resolution, confounded by swarming effect and refractory index | [160] |
| Bead-based flow cytometry | Detecting scattered light | Quick, multiparametric measurements | Bulk analysis only, risk of aggregation, limited information on size and concentration | [123, 158] |
| Nanoflow cytometry | Detecting scattered light | Single-EV analysis, improved resolution, multiparametric measurement possible | Confounded by refractory index | [161] |
| Imaging flow cytometry | Detecting scattered light with subsequent microscopic imaging | Single-EV analysis, improved resolution, multiparametric measurement possible | Dedicated equipment | NA |
| Single-particle interferometric reflectance imaging sensing | Recording interferometric reflectance | Single-EV analysis, multiparametric measurement | Dedicated equipment | [159] |
| Immunoblotting | Detecting antibody-labelled signals | Established method, simple contamination check | Bulk analysis, high sample volume, requires pure preparations | [123, 126, 127, 155-158] |
| Enzyme-linked immunosorbent assay | Detecting antibody-labelled signals | Established method, commercial kits available | Bulk analysis, cross-reactivity possible | [162] |
| Mass spectrometry | Separation of ionized molecules by their mass to charge ratio | High resolution, high-throughput, comprehensive data output | Bulk analysis, time consuming, requires pure preparations, sophisticated data analysis | [155, 156] |
| Next-generation sequencing | Transcript identification with single-nucleotide resolution | High sensitivity, high-throughput, comprehensive data output | Bulk analysis, time consuming, requires pure preparations, sophisticated data analysis | [155] |
| Quantitative polymerase chain reaction | Real-time detection of sequences selected a priori | Established protocols, high sensitivity | Bulk analysis, requires pure preparations and a priori knowledge | [117] |
3.2.1. Imaging techniques
After separation, a first impression of general morphology and intactness of the native or preloaded EVs can be gathered by electron microscopy with resolutions down to 0.4 nm [163]. The most commonly applied strategies are scanning or transmission electron microscopy (SEM or TEM, respectively) and cryogenic electron microscopy (cryo-EM) [164-166]. Besides the need for very specialized and expensive instruments, which is common to all these microscopic techniques, there are differences in the measuring principles, leading to the generation of distinct results and requiring bespoke sample preparation.
SEM creates a picture of the topography of EV surfaces by capturing emitted electrons [167]. The sample preparation usually follows a simple protocol requiring low sample volumes and can include an additional sputtering with gold, for instance, to generate more secondary electrons for detection [168]. Unfortunately, this coating could also mask the EVs’ surface structure; however, the use of low-voltage SEM can make the sputtering dispensable [169]. Additionally, SEM only allows detection of particulate objects without the ability to distinguish between EVs and non-EV particles. In contrast, TEM images are based on the detection of electrons passing through the sample to be analyzed and thus provide additional information of the interior of the vesicles [170]. The results obtained by subsequent measurements can be strongly affected by the sample preparation procedures [165]. For example, it is now well-known that negative staining using uranyl acetate and other heavy metals tends to desiccate sample material and thus causes a cup-shaped appearance of vesicles. Even though cup-shaped EVs are now recognized as artifacts, this staining procedure is straightforward and widely accepted in the EV community as long as no morphologic conclusions about the vesicles are made. Carrying an increased likelihood of operator-dependent biases during sample preparation and image acquisition, a more suitable protocol conserving the bilayered vesicle morphology includes an additional embedding in methylcellulose [165, 171].
Cryo-EM seems to best preserve the actual morphology of hydrated vesicles, as there is no need for special preparation of the material prior to fixation in vitrified liquid [164]. Likewise, a combination of conventional TEM with cryogenic techniques, also referred to as freeze-fracture TEM (FF-TEM), can provide information on the 3D-topology of EVs [172]. However, cryo-EM and FF-TEM require accurate on-site sample preparation at ultra-low temperatures by experienced users, which might not be achievable in many laboratory settings outside of specialized core facilities.
Atomic force microscopy (AFM), as another microscopy-based technique, is equally able to capture native EV structure since samples do not require any preparation or labelling. Here, EVs are immobilized on a solid surface prior to scanning interaction forces between the sample and the detector tip. Depending on the chosen surface, EVs could get flattened and might be detected as such, calling for careful interpretation of structural results. Moreover, morphological modifications can be induced by the detector tip itself when using the contact mode. Alternatively, the tapping mode can be applied to reduce the time and intensity of the tip’s contact with the sample, thereby causing less damage to the EV surface [168]. In addition, one can selectively analyze populations of vesicles that display a specific surface marker by utilizing surfaces coated with antibodies, as exemplarily shown by Yuana et al. [173]. Remarkably, AFM even allows for the determination of the vesicles’ stiffness [174]. With the requirement of even more specialized equipment and user experience, however, the utility of AFM in investigating EV topography appears rather restricted.
All of the mentioned practices also allow for further conclusions on EV size and the presence of certain proteins when combined with immunogold staining or immobilization of EVs on antibody-coated surfaces [173, 175]. In addition, there is a chance to identify impurities on the microscopic images by visual inspection, a finding which, of course, has to be confirmed by other techniques [56, 164]. Regardless of the method, only a minor portion of an EV sample can be examined via microscopy. Given the huge heterogeneity of EVs, the conclusions that can be drawn might only hold true for the examined portion of the EV preparation, thus requiring the addition of other techniques.
3.2.2. Sizing and counting techniques
More precise information on the size distribution as well as concentration of EVs can be obtained by other techniques such as tunable resistive pulse sensing (TRPS), microfluidic resistive pulse sensing (MRPS), nanoparticle tracking analysis (NTA), or dynamic light scattering (DLS). All of these methods found widespread application in EV research as they are easy to use, at least much easier than electron microscopy instruments, and allow for fast measurements [166, 176, 177]. Without utilizing fluorescence staining, which is a feature of most current NTA devices, however, these techniques cannot differentiate between EVs and non-EV particles. Moreover, polydisperse and size heterogenous samples are known to produce inaccurate results [178].
The measurement principle of TRPS is based on an electrical current run across a pore and the resistance pulses that are caused by particles passing through and thereby blocking this pore [179]. The higher the blockage magnitude, the higher the particle volume and thus the larger its size. By measuring the frequency with which the pore is blocked, one can furthermore make conclusions about the particle concentration. Even the surface charge, which is also called zeta potential -- discussed as a measure of colloidal stability and intactness of vesicles [147] -- can be measured based on the blockage duration. However, depending on the chosen nanopore, the minimum detectable particle size is around 40 nm [180]. The maximum detectable size is also limited by the chosen nanopore, as it can only be crossed by particles smaller than its diameter, while larger particles can lead to clogging [181]. It bears mention that the pore can be blocked by any particle crossing it, be it an EV or any other particle. The principle of MRPS is quite similar, but relies on a microfluidic environment of just a few microliters of sample in which resistance signals are generated by particles passing a nanoscale constriction and thereby causing a shift in voltage [182]. In contrast to TRPS, MRPS is not able to determine a particle’s zeta potential; however, sizes down to 46 nm can be measured easily. Moreover, both TRPS and MRPS allow the detection of distinctly sized vesicle populations present in the sample, making them superior to DLS and NTA, which, by contrast, provide lower resolution and might only indicate one size peak with broader size distributions [177, 183].
The underlying theory of both DLS and NTA is based on the Stokes-Einstein equation, which describes the Brownian motion of particles in solution. In practice, these techniques make use of the light scattering properties of hydrodynamic particles and the calculable relationship between particle size and the speed of its movement at a given temperature. DLS analyzes the intensity fluctuations of light scattered by moving particles, which leads to an averaged size distribution even down to 10 nm [183]. However, one only gets information on the size distribution pattern of a bulk EV sample, while there is no reliable information on concentration, as DLS is not able to monitor single vesicles. In NTA, by contrast, the displacement of single particles over time is tracked by analyzing their light scattering patterns with a detection limit around 40 to 50 nm, although the measuring sensitivity considerably decreases below ~ 70 nm [176, 184]. Though single particles are tracked, their movement trajectory in and out of focus may lead to uncertainties when calculating their sizes, resulting in skewed overall size distributions that might appear broader than expected based on the actual particle sizes. Nevertheless, and in contrast to DLS, NTA has the great advantage of also measuring the particle concentration, which calls for adequate sample dilution prior to measurement, and surface charge, which could give additional and important information on the vesicle integrity [147]. Of note, non-vesicular structures (such as nanobubbles, lipoproteins, or protein aggregates) also scatter light. Hence, both DLS and NTA measurements are not specific for EVs per se and tend to overestimate vesicle concentrations. NTA in particular is so widely used to characterize EVs that some details are worth discussing. Many NTA devices allow the analysis of vesicles labeled with fluorescence dyes, which could increase the specificity for vesicle measurement significantly. This specificity largely depends on the chosen dye, which thus has to be comprehensively evaluated for its stability and specificity before starting any experiment [151]. Fluorophores are often coupled to antibodies recognizing EV-specific surface proteins. By that approach, the measurement might become more accurate; however, only a subset of EVs will be investigated, as no surface protein that is expressed on every EV has been identified yet [90]. It is therefore very important to report in detail the antibodies used as well as the dyes to assure reproducibility.
One issue that should be considered when applying light scattering for size determination is the fact that larger particles scatter more light and, when present in polydisperse suspensions even in low abundance, can thereby cover the less intense scattering of smaller particles, leading to a shift in detected size ranges towards larger diameters [183]. This phenomenon is particularly problematic in DLS measurements where large particles dominate scattered light fluctuations and thus inordinately elevate the average diameter, whereas the possibility to track single particles in NTA measurements reduces the overall effect of a few large particles that are present in the suspension and scatter light more strongly [178]. One solution to this problem could be separating EVs prior to analysis in order to generate sequential monodisperse fractions. Asymmetric-flow field-flow fractionation (AF4) is a gentle, flow-based method for high-resolution separation of particles in solution that has also been used to separate and characterize EV populations and other extracellular particles [146, 185]. When coupled to in-line multi-angle light scattering (MALS) and DLS detectors, AF4-MALS-DLS is a powerful setup to characterize EVs in various settings including drug delivery. A routine tool to extensively characterize the physical properties, stability, and loading efficiency of liposomes and other nanopharmaceuticals liposomal drug carriers, AF4-MALS-DLS could be extremely useful in the early phases of EV-DDS development as well as for batch-to-batch quality control during production [186-188].
While the general biases of light scattering methods might pose an obstacle in an initial size and concentration assessment intended to set quality standards for separated, loaded, or engineered EVs, the combination with other techniques that are not based on scattered light could circumvent this limitation. Furthermore, once appropriate quality standards are set using orthogonal methods, it might be the case that a single approach is sufficient to ensure batch-to-batch reproducibility.
3.2.3. Phenotyping techniques
Besides counting and sizing, one might also be interested in evaluating the presence or absence of specific features that determine an EV’s phenotype and might be of particular importance for EV targeting capabilities, for instance. Appropriate instruments that are able to examine single particles in high resolution are often highly specialized and require skilled operators. For a general and initial sample characterization, bulk analyses such as bead-based systems or standard blotting procedures might be sufficient and can be conducted in a fast and easy way. In most cases, a combination of different phenotyping techniques is advisable to take advantage of each method’s unique strengths and thereby generate reliable data.
Flow cytometry (FCM) is a powerful tool for EV characterization aimed at enumeration and size determination, but with additional phenotypic information. The flow cytometer detects particles that scatter light after being illuminated by a laser, but both the accuracy and validity of any (semi-)quantitative conclusions are highly dependent on the chosen instrumentation and measurement settings [189]. As a consequence, a general reporting checklist was generated to avoid artifacts and misleading data interpretation of flow cytometric EV experiments [34]. Conventional FCM possesses a lower detection limit around 300 nm [190], which is not suitable for measuring small EVs. Thus, the single-EV analysis in principle enabled by FCM would only be possible for larger EVs. With the refractory index of EVs being very low and therefore close to that of most fluids, their discrimination from the background scattering signal might also be challenging when not using fluorescent labeling [190]. Furthermore, the resolution of individual particles could be difficult if input concentrations are too high, resulting in a phenomenon called swarm effect [190]. At the same time, clusters of too-small EVs could be detected as a single large EV. To enable single-EV analysis though, the use of wide-angle forward scatter detection and/or fluorescence can be applied [191]. Of note, using fluorescent dyes requires proper removal of excess or aggregated dye, with DGC appearing to perform adequately for this purpose, while the sole use of ultracentrifugation was not efficient [191]. Even though the measurement of fluorescently labeled vesicles allows for phenotyping, only the subset of total vesicles that exposes the characteristic of interest will be investigated, while unlabeled vesicles are disregarded. The same caveat applies to multiplexed analyses with vesicles usually coupled to magnetic beads by immunoaffinity, which indeed increases the number of characteristics examined in parallel, but would still miss an unknown proportion of vesicles [192]. Furthermore, the potential formation of aggregates and bead conjugates could introduce challenges for correct sizing and single-EV counting.
Given the heterogeneity of EVs and the limitations of established methods that assess vesicles in bulk, such as bead-based FCM, the implementation of new EV characterization technologies is increasingly focused on single-particle analysis. Nanoflow cytometry measurement (NFCM or NanoFCM), which can be performed with and without fluorescent EV labeling, is closely related to FCM and is likewise used for sizing, counting, and phenotyping [57]. By the use of a smaller flow channel and lower pressure, the particles’ dwell time in the fully illuminated sample stream is improved. According to Tian et al., single-EV sizing down to 40 nm was possible [193].
Furthermore, imaging flow cytometry (IFCM) combines the specifications of FCM with subsequent multispectral microscopic imaging [194]. By applying fluorescently labeled EVs, Gorgens et al. have shown that IFCM is able to detect single EVs smaller than 200 nm. Moreover, antibody staining of EVs allows the investigation of co-expressed markers as well as distinct subpopulations in a quantitative manner. However, the use of appropriate controls and titration experiments is crucial to assure the robust detection of sample-specific signals. Single-particle interferometric reflectance imaging sensing (SP-IRIS) is a dedicated single-particle characterization platform [195]. Biological particles, such as EVs, are usually immunocaptured on a surface and the interference of light reflected from the surface and the molecules is recorded for sizing and counting. With the adapted fluorescence mode, one can currently phenotype up to four surface antigens. However, while differently sized subpopulations within one given sample can be easily distinguished, the absolute concentration can only be estimated, as the vesicles to be measured will be preselected by the antibodies chosen for immunoaffinity-based immobilization on the surface [177]. In the future, simultaneous profiling of internal vesicle molecules will be possible after vesicle fixation and permeabilization [177].
The presence of individual proteins, be they membrane-associated or intra-vesicular, is most often ascertained by proteomic bulk analyses such as immunoblotting (IB) with western blotting being arguably the most applied [166]. This is a quick and rather simple method to detect multiple antigens in parallel, but only in a semi-quantitative manner and usually with high sample volumes required. IB does not discriminate between vesicular and non-vesicular antigens but visualizes the total presence of individual proteins in a bulk sample. EV preparations should therefore already be in a highly pure condition, and the inclusion of appropriate controls has to be ensured because the presence of putative impurities as well as non-EV associated proteins could affect the outcome. The MISEV 2018 guidelines list markers thought to be associated with EVs and other proteins considered to be frequently-occurring impurities, the latter useful when assessing purity. For example, albumin in blood samples and uromodulin in urine are expected impurities [29].
By applying immunosorbent assays (ISA) or standard enzyme-linked immunosorbent assays (ELISA), EVs can be captured on an antibody-coated surface enabling the enrichment of specific EV subpopulations and even the possibility of multiplexing and simple upscaling to 96-well format assays [196]. Further, the possibility to conduct additional washing steps could decrease the presence of undesired proteins, thus, leading to low interference during analyses. Meanwhile, various new detection variants have been developed, and many commercial kits are already available [197]. Additionally, research on artificial vesicles as potential standard material is commenced [198]. Nevertheless, both IB and ISA require a restricted number of proteins to be preselected prior to phenotyping.
3.2.4. Total cargo profiling techniques
Another and very different approach to conduct in-process controls of EV preparations is to screen their entire molecular composition. Depending on what small molecules are of special interest in the respective therapeutic question, either proteomic, lipidomic, genomic analyses, or a combination thereof might be performed using already well-established techniques including mass spectrometry (MS), LC, and next-generation sequencing (NGS). However, all of these analyze vesicular cargo and potential sample impurities alike, which demands appropriate upstream EV separation steps.
Total protein amounts can be measured by fluorometric or colorimetric methods, such as the bicinchonic acid or Bradford assay, and are usually used for downstream normalization purposes and to assess sample purity [29, 53], as described in section 2.5. Beyond IB, MS allows the large-scale and high-throughput bulk analysis of proteins previously digested into peptides and has found great use in EV proteomics, as reviewed by Pocsfalvi et al. [199]. Apart from being less sensitive than antibody-based strategies, MS has the limitation of assessing only the average protein distribution of an EV preparation. While the overall proteomic pattern is of great interest in postulating functional networks, its relevance in pharmacokinetics might be limited, since respective markers should be equally distributed on all EVs in a stoichiometric manner.
In combination with liquid chromatography (LC-MS) or as a tandem method (MS/MS), MS is also increasingly applied for EV lipidomics, even though reproducibility of results might only be achieved rarely and needs further optimization [200]. So far, little is known about the lipid composition of EVs, but it has already been demonstrated that different EV fractions separated based on their buoyancy are enriched in some lipid classes, such as ceramides or phospholipids [200]. The fact that different EV subpopulations expose different sets of lipids was also shown by Osteikoetxea et al. [55]. Moreover, they introduced the total protein to lipid ratio as an additional parameter in EV quality control and normalization, applying a colorimetric sulfophosphovanilin assay to quantify total lipid content.
The total nucleic acid cargo of EVs is composed of DNA and RNA with various types of RNA, especially non-coding, being predominantly present [201]. Protease-nuclease treatment of EV preparations is highly recommended when definite assignment of nucleic acids to the EV lumen is of interest [202]. After EV lysis and nucleic acid isolation, typically performed by phenol-chloroform phase separation in combination with subsequent solid phase extraction [203], bulk DNA and/or RNA distribution and concentration can be accessed by capillary gel electrophoresis or instruments whose detection principle is based on UV-absorbance [202]. However, the latter systems might be less suitable, since nucleic acid concentrations in EVs are usually very low, falling below the detection limit of most UV-absorbance instruments. While quantitative PCR (qPCR) allows for the relative and absolute quantification of preselected target sequences, which could be of relevance in assessing overall loading success, NGS represents an un-targeted approach, enabling the comprehensive characterization of bulk nucleic acids present in EV preparations [56, 204].
4. Conclusions
Despite tremendous progress on methods to separate and characterize EVs as well as knowledge of EV biology and the existence of potential clinical applications, many open questions about potential EV-based DDSs remain. Are autologous or allogeneic source materials to be preferred? Is removal of endogenous EV cargo prior to loading feasible and, if so, beneficial? What are the most suitable methods to process source materials and to separate, characterize, and load their vesicles? As discussed above, EVs are highly heterogeneous and it is likely that disparate populations differ in their drug loading ability, stability, half-life, biodistribution patterns, and efficiency of intracellular payload delivery. Settling on the optimal constellation of source material and most suitable EV type for delivering a particular drug against a particular disease might therefore require a compromise in which the most important of these factors will be prioritized. For instance, EVs that very effectively release their cargo into the cytosol of recipient cells but are cleared from the circulation quickly might be superior to vesicles with longer half-life but poor intracellular delivery. The optimal delivery vector may well vary depending on the intended target tissue and properties of the respective drug molecule, making a generic one-size-fits-all solution unlikely. Once these issues have been addressed, additional technical questions including formulation, stability, storage conditions, choice of suitably inert plasticware and storage vials and buffers, as well as route of administration need to be answered. The notion of standardization as a prerequisite for effectively developing and translating EV-based drugs thus extends beyond mere separation and characterization. Promising investigations on the stability of EVs during storage and the impact of different storage buffers and additives, as well as a growing appreciation of the importance of inter-laboratory, multi-site method benchmarking, are therefore important parts of an interdisciplinary effort towards manufacturing a reproducibly stable and effective product [10, 205, 206].
The field of EVs and drug delivery abounds with enthusiasm and optimism, which might be warranted, but needs to be met with a healthy degree of caution. While we await a critical mass of safety data on the administration of EVs -- and, someday, EV-DDS -- in clinical trials, there are still many challenges on the road to approval of novel EV-based nanomedicines [13, 35]. Chief among them is the relatively short half-life of injected vesicles, with many studies reporting rapid clearance, often within less than 30 minutes [136, 137, 207]. Additionally, prospective EV therapeutics may have to compete with established drug carriers including synthetic nanoparticles used in applications such as mRNA COVID-19 vaccines, and head-to-head comparisons will be important [208]. It is clear that to overcome these challenges, each step along the process of producing any EV therapeutic, from source material to administration, needs to be carefully selected and optimized. Without rigorous quality control and standardization, the potential of EV-based drug delivery is bound to remain unrealized.
Given the unique properties of EVs and the limitations of characterization techniques as discussed in section 3.2, suitable reference materials to calibrate instruments and validate EV assays would be extremely valuable [194]. However, an agreed-upon vesicle standard or vesicle mimetic with a defined, traceable size, concentration, and composition that closely reflects the most important EV properties remains to be established. Conventional reference materials for instrument calibration are of limited utility because some of their characteristics including their refractive index are too different from those of biological particles. Even liposomes, which have been suggested as reference particles for EV measurements but lack the complexity, surface markers, and luminal cargo of EVs, are not an ideal solution to this problem [209]. Geeurickx and coworkers recently developed recombinant EVs with defined properties and traceable markers as a promising alternative to fully synthetic reference particles and provided detailed protocols for their production and use as reference materials in EV assays [210, 211]. Although of great importance, the development and in-depth characterization of suitable reference materials are cumbersome and challenging, as excellently reviewed elsewhere [212].
To summarize, EVs harbor great potential as vehicles to deliver drugs to specific tissues. Despite tremendous advances, the field still seems to be in its infancy with crucial questions, particularly about safety, biodistribution, and optimal routes of administration, only partially answered. Propelled by enthusiasm about promising translational applications as well as lessons learned from the liposome and nanomedicine field, EV-DDS have quickly moved into early clinical trials. In order to succeed on a broad scale, though, critical challenges related to EV production, separation, loading, administration, and pharmacokinetics need to be overcome. As a result, improved methods and devices are under development and an increasing number of partnerships between academic institutes and biopharmaceutical companies is formed to move therapeutic EVs from bench to bedside. Close collaboration with metrology institutes such as the US National Institute of Standards and Technology (NIST) will foster development and utilization of reference materials to adequately calibrate instruments and optimize separation methods. Additionally, a better dialogue between the EV, nanomedicine, and drug delivery communities as well as regulatory bodies is called for in order to develop tailored methods and to advance clinical applications. Despite current and prospective challenges as well as a still incomplete understanding of the biology and pharmacological properties of EVs, we foresee a bright future for EVs in drug delivery.
Extracellular vesicles are biological nanoparticles suitable for drug delivery.
Separation and characterization of extracellular vesicles are crucial but challenging.
Current methods are differentially suited for industrial-scale vesicle manufacturing.
Combining standardized separation and characterization methods may be expedient.
Reference materials and active collaboration will accelerate clinical translation.
Acknowledgments
Funding
JBB is supported by National Institutes of Health grant K23HL128909. The funding source had no involvement in the conception, writing, and decision to submit this article for publication.
Abbreviations
- AFM
atomic force microscopy
- BBB
blood-brain-barrier
- BEC
bind-elute chromatography
- cryo-EM
cryogenic electron microscopy
- DDS
drug delivery system
- DGC
density gradient centrifugation
- DLS
dynamic light scattering
- dUC
differential ultracentrifugation
- ELISA
enzyme-linked immunosorbent assay
- ER
endoplasmic reticulum
- EV
extracellular vesicle
- FCM
flow cytometry
- FF-TEM
freeze-fracture transmission electron microscopy
- FLIM
fluorescence lifetime imaging microscopy
- GMP
Good Manufacturing Practice
- IB
immunoblotting
- IEC
ion-exchange chromatography
- IFCM
imaging flow cytometry
- ILV
intraluminal vesicle
- ISA
immunosorbent assay
- LC
liquid chromatography
- LC-MS
liquid chromatography mass spectrometry
- MISEV
Minimal Information for Studies of Extracellular Vesicles
- MRPS
microfluidic resistive pulse sensing
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- MSC
mesenchymal stem cell
- MVB
multivesicular body
- NFCM
nanoflow cytometry
- NGS
next-generation sequencing
- NIST
National Institute of Standards and Technology
- NTA
nanoparticle tracking analysis
- PEG
polyethylene glycol
- qPCR
quantitative polymerase chain reaction
- SEC
size-exclusion chromatography
- SEM
scanning electron microscopy
- siRNA
small interfering RNA
- SP-IRIS
single-particle interferometric reflectance imaging sensing
- TEM
transmission electron microscopy
- TFF
tangential flow filtration
- TRPS
tunable resistive pulse sensing
- UF
ultrafiltration
Footnotes
Declaration of Competing Interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Steinhagen H, The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. By Raviña Enrique, ChemMedChem, 6 (2011) 1746–1747. [Google Scholar]
- [2].Beall RF, Hwang TJ, Kesselheim AS, Pre-market development times for biologic versus small-molecule drugs, Nat Biotechnol, 37 (2019) 708–711. [DOI] [PubMed] [Google Scholar]
- [3].Andrews L, Ralston S, Blomme E, Barnhart K, A snapshot of biologic drug development: Challenges and opportunities, Hum Exp Toxicol, 34 (2015) 1279–1285. [DOI] [PubMed] [Google Scholar]
- [4].van Niel G, D'Angelo G, Raposo G, Shedding light on the cell biology of extracellular vesicles, Nat Rev Mol Cell Biol, 19 (2018) 213–228. [DOI] [PubMed] [Google Scholar]
- [5].Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO, Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells, Nat Cell Biol, 9 (2007) 654–659. [DOI] [PubMed] [Google Scholar]
- [6].Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, Vader P, Extracellular vesicles as drug delivery systems: Why and how?, Adv Drug Deliv Rev, 159 (2020) 332–343. [DOI] [PubMed] [Google Scholar]
- [7].Han Y, Gao Z, Chen L, Kang L, Huang W, Jin M, Wang Q, Bae YH, Multifunctional oral delivery systems for enhanced bioavailability of therapeutic peptides/proteins, Acta Pharm Sin B, 9 (2019) 902–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Saleh AF, Lazaro-Ibanez E, Forsgard MA, Shatnyeva O, Osteikoetxea X, Karlsson F, Heath N, Ingelsten M, Rose J, Harris J, Mairesse M, Bates SM, Clausen M, Etal D, Leonard E, Fellows MD, Dekker N, Edmunds N, Extracellular vesicles induce minimal hepatotoxicity and immunogenicity, Nanoscale, 11 (2019) 6990–7001. [DOI] [PubMed] [Google Scholar]
- [9].Elia CA, Losurdo M, Malosio ML, Coco S, Extracellular Vesicles from Mesenchymal Stem Cells Exert Pleiotropic Effects on Amyloid-beta, Inflammation, and Regeneration: A Spark of Hope for Alzheimer's Disease from Tiny Structures?, Bioessays, 41 (2019) e1800199. [DOI] [PubMed] [Google Scholar]
- [10].Jeyaram A, Jay SM, Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications, AAPS J, 20 (2017) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mathieu M, Martin-Jaular L, Lavieu G, Thery C, Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication, Nat Cell Biol, 21 (2019) 9–17. [DOI] [PubMed] [Google Scholar]
- [12].Somiya M, Where does the cargo go?: Solutions to provide experimental support for the "extracellular vesicle cargo transfer hypothesis", J Cell Commun Signal, 14 (2020) 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Klyachko NL, Arzt CJ, Li SM, Gololobova OA, Batrakova EV, Extracellular Vesicle-Based Therapeutics: Preclinical and Clinical Investigations, Pharmaceutics, 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Agrawal AK, Aqil F, Jeyabalan J, Spencer WA, Beck J, Gachuki BW, Alhakeem SS, Oben K, Munagala R, Bondada S, Gupta RC, Milk-derived exosomes for oral delivery of paclitaxel, Nanomedicine, 13 (2017) 1627–1636. [DOI] [PubMed] [Google Scholar]
- [15].Wang B, Zhuang X, Deng ZB, Jiang H, Mu J, Wang Q, Xiang X, Guo H, Zhang L, Dryden G, Yan J, Miller D, Zhang HG, Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit, Mol Ther, 22 (2014) 522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jain S, Pillai J, Bacterial membrane vesicles as novel nanosystems for drug delivery, Int J Nanomedicine, 12 (2017) 6329–6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li R, Liu Q, Engineered Bacterial Outer Membrane Vesicles as Multifunctional Delivery Platforms, Frontiers in Materials, 7 (2020). [Google Scholar]
- [18].Guo S, Perets N, Betzer O, Ben-Shaul S, Sheinin A, Michaelevski I, Popovtzer R, Offen D, Levenberg S, Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury, ACS Nano, 13 (2019) 10015–10028. [DOI] [PubMed] [Google Scholar]
- [19].Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL, Kabanov AV, Batrakova EV, Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations, Nanomedicine, 14 (2018) 195–204. [DOI] [PubMed] [Google Scholar]
- [20].Usman WM, Pham TC, Kwok YY, Vu LT, Ma V, Peng B, Chan YS, Wei L, Chin SM, Azad A, He AB, Leung AYH, Yang M, Shyh-Chang N, Cho WC, Shi J, Le MTN, Efficient RNA drug delivery using red blood cell extracellular vesicles, Nat Commun, 9 (2018) 2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ, Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes, Nat Biotechnol, 29 (2011) 341–345. [DOI] [PubMed] [Google Scholar]
- [22].de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, Schiffelers RM, Gucek M, van Balkom BW, Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes, J Extracell Vesicles, 1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Haraszti RA, Miller R, Stoppato M, Sere YY, Coles A, Didiot MC, Wollacott R, Sapp E, Dubuke ML, Li X, Shaffer SA, DiFiglia M, Wang Y, Aronin N, Khvorova A, Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity, Mol Ther, 26 (2018) 2838–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang Y, Chopp M, Zhang ZG, Katakowski M, Xin H, Qu C, Ali M, Mahmood A, Xiong Y, Systemic administration of cell-free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury, Neurochem Int, 111 (2017) 69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Almeria C, Weiss R, Roy M, Tripisciano C, Kasper C, Weber V, Egger D, Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation in vitro, Front Bioeng Biotechnol, 7 (2019) 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Abramowicz A, Widlak P, Pietrowska M, Different Types of Cellular Stress Affect the Proteome Composition of Small Extracellular Vesicles: A Mini Review, Proteomes, 7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Garcia NA, Ontoria-Oviedo I, Gonzalez-King H, Diez-Juan A, Sepulveda P, Glucose Starvation in Cardiomyocytes Enhances Exosome Secretion and Promotes Angiogenesis in Endothelial Cells, PLoS One, 10 (2015) e0138849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li J, Lee Y, Johansson HJ, Mager I, Vader P, Nordin JZ, Wiklander OP, Lehtio J, Wood MJ, Andaloussi SE, Serum-free culture alters the quantity and protein composition of neuroblastoma-derived extracellular vesicles, J Extracell Vesicles, 4 (2015) 26883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gamez-Valero A, Gardiner C, Gartner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Gorgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanovic MM, Kovacs AF, Kramer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lasser C, Laurent LC, Lavieu G, Lazaro-Ibanez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Line A, Linnemannstons K, Llorente A, Lombard CA, Lorenowicz MJ, Lorincz AM, Lotvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr., Meehan KL, Mertens I, Minciacchi VR, Moller A, Moller Jorgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saa P, Sahoo S, Salas-Huenuleo E, Sanchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schoyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sodar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr., Veit TD, Vella LJ, Velot E, Verweij FJ, Vestad B, Vinas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yanez-Mo M, Yin H, Yuana Y, Zappulli V, Zarubova J, Zekas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK, Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines, J Extracell Vesicles, 7 (2018) 1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gimona M, Pachler K, Laner-Plamberger S, Schallmoser K, Rohde E, Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use, Int J Mol Sci, 18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Witwer KW, Thery C, Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature, J Extracell Vesicles, 8 (2019) 1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, Nolte-'t Hoen EN, Piper MG, Sivaraman S, Skog J, Thery C, Wauben MH, Hochberg F, Standardization of sample collection, isolation and analysis methods in extracellular vesicle research, J Extracell Vesicles, 2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].van der Pol E, Sturk A, van Leeuwen T, Nieuwland R, Coumans F, group I-S-VW, Standardization of extracellular vesicle measurements by flow cytometry through vesicle diameter approximation, J Thromb Haemost, 16 (2018) 1236–1245. [DOI] [PubMed] [Google Scholar]
- [34].Welsh JA, Van Der Pol E, Arkesteijn GJA, Bremer M, Brisson A, Coumans F, Dignat-George F, Duggan E, Ghiran I, Giebel B, Gorgens A, Hendrix A, Lacroix R, Lannigan J, Libregts S, Lozano-Andres E, Morales-Kastresana A, Robert S, De Rond L, Tertel T, Tigges J, De Wever O, Yan X, Nieuwland R, Wauben MHM, Nolan JP, Jones JC, MIFlowCyt-EV: a framework for standardized reporting of extracellular vesicle flow cytometry experiments, J Extracell Vesicles, 9 (2020) 1713526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, O'Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Gorgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Kramer-Albers EM, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lotvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BW, Wauben M, Andaloussi SE, Thery C, Rohde E, Giebel B, Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper, J Extracell Vesicles, 4 (2015) 30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Torres Crigna A, Fricke F, Nitschke K, Worst T, Erb U, Karremann M, Buschmann D, Elvers-Hornung S, Tucher C, Schiller M, Hausser I, Gebert J, Bieback K, Inter-Laboratory Comparison of Extracellular Vesicle Isolation Based on Ultracentrifugation, Transfus Med Hemother, 48 (2021) 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tiruvayipati S, Wolfgeher D, Yue M, Duan F, Andrade J, Jiang H, Schuger L, Variability in protein cargo detection in technical and biological replicates of exosome-enriched extracellular vesicles, PLoS One, 15 (2020) e0228871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zabeo D, Cvjetkovic A, Lasser C, Schorb M, Lotvall J, Hoog JL, Exosomes purified from a single cell type have diverse morphology, J Extracell Vesicles, 6 (2017) 1329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Saari H, Lisitsyna E, Rautaniemi K, Rojalin T, Niemi L, Nivaro O, Laaksonen T, Yliperttula M, Vuorimaa-Laukkanen E, FLIM reveals alternative EV-mediated cellular up-take pathways of paclitaxel, J Control Release, 284 (2018) 133–143. [DOI] [PubMed] [Google Scholar]
- [40].Linares R, Tan S, Gounou C, Arraud N, Brisson AR, High-speed centrifugation induces aggregation of extracellular vesicles, J Extracell Vesicles, 4 (2015) 29509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gardiner C, Di Vizio D, Sahoo S, Thery C, Witwer KW, Wauben M, Hill AF, Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey, J Extracell Vesicles, 5 (2016) 32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mol EA, Goumans MJ, Doevendans PA, Sluijter JPG, Vader P, Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation, Nanomedicine, 13 (2017) 2061–2065. [DOI] [PubMed] [Google Scholar]
- [43].Nordin JZ, Lee Y, Vader P, Mager I, Johansson HJ, Heusermann W, Wiklander OP, Hallbrink M, Seow Y, Bultema JJ, Gilthorpe J, Davies T, Fairchild PJ, Gabrielsson S, Meisner-Kober NC, Lehtio J, Smith CI, Wood MJ, El Andaloussi S, Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties, Nanomedicine, 11 (2015) 879–883. [DOI] [PubMed] [Google Scholar]
- [44].Scheerlinck E, Dhaenens M, Van Soom A, Peelman L, De Sutter P, Van Steendam K, Deforce D, Minimizing technical variation during sample preparation prior to label-free quantitative mass spectrometry, Anal Biochem, 490 (2015) 14–19. [DOI] [PubMed] [Google Scholar]
- [45].Gamez-Valero A, Monguio-Tortajada M, Carreras-Planella L, Franquesa M, Beyer K, Borras FE, Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles' characteristics compared to precipitating agents, Sci Rep, 6 (2016) 33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Paolini L, Zendrini A, Di Noto G, Busatto S, Lottini E, Radeghieri A, Dossi A, Caneschi A, Ricotta D, Bergese P, Residual matrix from different separation techniques impacts exosome biological activity, Sci Rep, 6 (2016) 23550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ludwig AK, De Miroschedji K, Doeppner TR, Borger V, Ruesing J, Rebmann V, Durst S, Jansen S, Bremer M, Behrmann E, Singer BB, Jastrow H, Kuhlmann JD, El Magraoui F, Meyer HE, Hermann DM, Opalka B, Raunser S, Epple M, Horn PA, Giebel B, Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales, J Extracell Vesicles, 7 (2018) 1528109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Driedonks TAP, Mol S, de Bruin S, Peters AL, Zhang X, Lindenbergh MFS, Beuger BM, van Stalborch AD, Spaan T, de Jong EC, van der Vries E, Margadant C, van Bruggen R, Vlaar APJ, Groot Kormelink T, Nolte-'t Hoen ENM, Y-RNA subtype ratios in plasma extracellular vesicles are cell type- specific and are candidate biomarkers for inflammatory diseases, J Extracell Vesicles, 9 (2020) 1764213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Buschmann D, Kirchner B, Hermann S, Marte M, Wurmser C, Brandes F, Kotschote S, Bonin M, Steinlein OK, Pfaffl MW, Schelling G, Reithmair M, Evaluation of serum extracellular vesicle isolation methods for profiling miRNAs by next-generation sequencing, J Extracell Vesicles, 7 (2018) 1481321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Peng HH, Wu S, Davis JJ, Wang L, Roth JA, Marini FC 3rd, Fang B, A rapid and efficient method for purification of recombinant adenovirus with arginine-glycine-aspartic acid-modified fibers, Anal Biochem, 354 (2006) 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Vergauwen G, Dhondt B, Van Deun J, De Smedt E, Berx G, Timmerman E, Gevaert K, Miinalainen I, Cocquyt V, Braems G, Van den Broecke R, Denys H, De Wever O, Hendrix A, Confounding factors of ultrafiltration and protein analysis in extracellular vesicle research, Sci Rep, 7 (2017) 2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang X, Borg EGF, Liaci AM, Vos HR, Stoorvogel W, A novel three step protocol to isolate extracellular vesicles from plasma or cell culture medium with both high yield and purity, J Extracell Vesicles, 9 (2020) 1791450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Webber J, Clayton A, How pure are your vesicles?, J Extracell Vesicles, 2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cvjetkovic A, Lotvall J, Lasser C, The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles, J Extracell Vesicles, 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Osteikoetxea X, Balogh A, Szabo-Taylor K, Nemeth A, Szabo TG, Paloczi K, Sodar B, Kittel A, Gyorgy B, Pallinger E, Matko J, Buzas EI, Improved characterization of EV preparations based on protein to lipid ratio and lipid properties, PLoS One, 10 (2015) e0121184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mussack V, Wittmann G, Pfaffl MW, Comparing small urinary extracellular vesicle purification methods with a view to RNA sequencing-Enabling robust and non-invasive biomarker research, Biomol Detect Quantif, 17 (2019) 100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tian Y, Gong M, Hu Y, Liu H, Zhang W, Zhang M, Hu X, Aubert D, Zhu S, Wu L, Yan X, Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry, J Extracell Vesicles, 9 (2020) 1697028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Whittaker TE, Nagelkerke A, Nele V, Kauscher U, Stevens MM, Experimental artefacts can lead to misattribution of bioactivity from soluble mesenchymal stem cell paracrine factors to extracellular vesicles, J Extracell Vesicles, 9 (2020) 1807674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, Bracke M, De Wever O, Hendrix A, The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling, J Extracell Vesicles, 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dizon-Maspat J, Bourret J, D'Agostini A, Li F, Single pass tangential flow filtration to debottleneck downstream processing for therapeutic antibody production, Biotechnol Bioeng, 109 (2012) 962–970. [DOI] [PubMed] [Google Scholar]
- [61].Potter M, Lins B, Mietzsch M, Heilbronn R, Van Vliet K, Chipman P, Agbandje-McKenna M, Cleaver BD, Clement N, Byrne BJ, Zolotukhin S, A simplified purification protocol for recombinant adeno-associated virus vectors, Mol Ther Methods Clin Dev, 1 (2014) 14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Busatto S, Vilanilam G, Ticer T, Lin WL, Dickson DW, Shapiro S, Bergese P, Wolfram J, Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid, Cells, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lee JH, Ha DH, Go HK, Youn J, Kim HK, Jin RC, Miller RB, Kim DH, Cho BS, Yi YW, Reproducible Large-Scale Isolation of Exosomes from Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Application in Acute Kidney Injury, Int J Mol Sci, 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].McNamara RP, Caro-Vegas CP, Costantini LM, Landis JT, Griffith JD, Damania BA, Dittmer DP, Large-scale, cross-flow based isolation of highly pure and endocytosis-competent extracellular vesicles, J Extracell Vesicles, 7 (2018) 1541396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lee KJ, Mower R, Hollenbeck T, Castelo J, Johnson N, Gordon P, Sinko PJ, Holme K, Lee YH, Modulation of nonspecific binding in ultrafiltration protein binding studies, Pharm Res, 20 (2003) 1015–1021. [DOI] [PubMed] [Google Scholar]
- [66].Yamashita T, Takahashi Y, Nishikawa M, Takakura Y, Effect of exosome isolation methods on physicochemical properties of exosomes and clearance of exosomes from the blood circulation, Eur J Pharm Biopharm, 98 (2016) 1–8. [DOI] [PubMed] [Google Scholar]
- [67].Heinemann ML, Ilmer M, Silva LP, Hawke DH, Recio A, Vorontsova MA, Alt E, Vykoukal J, Benchtop isolation and characterization of functional exosomes by sequential filtration, J Chromatogr A, 1371 (2014) 125–135. [DOI] [PubMed] [Google Scholar]
- [68].Burova E, Ioffe E, Chromatographic purification of recombinant adenoviral and adeno-associated viral vectors: methods and implications, Gene Ther, 12 Suppl 1 (2005) S5–17. [DOI] [PubMed] [Google Scholar]
- [69].Burden DW, Whitney DB, Protein Purification by Column Chromatography, BiotechnologyProteins to PCR: A Course in Strategies and Lab Techniques, Birkhäuser Boston, Boston, MA, 1995, pp. 93–124. [Google Scholar]
- [70].de Menezes-Neto A, Saez MJ, Lozano-Ramos I, Segui-Barber J, Martin-Jaular L, Ullate JM, Fernandez-Becerra C, Borras FE, Del Portillo HA, Size-exclusion chromatography as a stand-alone methodology identifies novel markers in mass spectrometry analyses of plasma-derived vesicles from healthy individuals, J Extracell Vesicles, 4 (2015) 27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Baranyai T, Herczeg K, Onodi Z, Voszka I, Modos K, Marton N, Nagy G, Mager I, Wood MJ, El Andaloussi S, Palinkas Z, Kumar V, Nagy P, Kittel A, Buzas EI, Ferdinandy P, Giricz Z, Isolation of Exosomes from Blood Plasma: Qualitative and Quantitative Comparison of Ultracentrifugation and Size Exclusion Chromatography Methods, PLoS One, 10 (2015) e0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Boing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R, Single-step isolation of extracellular vesicles by size-exclusion chromatography, J Extracell Vesicles, 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Stranska R, Gysbrechts L, Wouters J, Vermeersch P, Bloch K, Dierickx D, Andrei G, Snoeck R, Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma, J Transl Med, 16 (2018) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, Moller A, Optimized exosome isolation protocol for cell culture supernatant and human plasma, J Extracell Vesicles, 4 (2015) 27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Moleirinho MG, Silva RJS, Carrondo MJT, Alves PM, Peixoto C, Exosome-based therapeutics: Purification using semi-continuous multi-column chromatography, Separation and Purification Technology, 224 (2019) 515–523. [Google Scholar]
- [76].Corso G, Mager I, Lee Y, Gorgens A, Bultema J, Giebel B, Wood MJA, Nordin JZ, Andaloussi SE, Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography, Sci Rep, 7 (2017) 11561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Onodi Z, Pelyhe C, Terezia Nagy C, Brenner GB, Almasi L, Kittel A, Mancek-Keber M, Ferdinandy P, Buzas EI, Giricz Z, Isolation of High-Purity Extracellular Vesicles by the Combination of Iodixanol Density Gradient Ultracentrifugation and Bind-Elute Chromatography From Blood Plasma, Front Physiol, 9 (2018) 1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Nordin JZ, Bostancioglu RB, Corso G, El Andaloussi S, Tangential Flow Filtration with or Without Subsequent Bind-Elute Size Exclusion Chromatography for Purification of Extracellular Vesicles, Methods Mol Biol, 1953 (2019) 287–299. [DOI] [PubMed] [Google Scholar]
- [79].James KT, Cooney B, Agopsowicz K, Trevors MA, Mohamed A, Stoltz D, Hitt M, Shmulevitz M, Novel High-throughput Approach for Purification of Infectious Virions, Sci Rep, 6 (2016) 36826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Saraswat M, Musante L, Ravida A, Shortt B, Byrne B, Holthofer H, Preparative purification of recombinant proteins: current status and future trends, Biomed Res Int, 2013 (2013) 312709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Heath N, Grant L, De Oliveira TM, Rowlinson R, Osteikoetxea X, Dekker N, Overman R, Rapid isolation and enrichment of extracellular vesicle preparations using anion exchange chromatography, Sci Rep, 8 (2018) 5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ, Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI, Proc Natl Acad Sci U S A, 113 (2016) 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Nasimuzzaman M, Lynn D, van der Loo JC, Malik P, Purification of baculovirus vectors using heparin affinity chromatography, Mol Ther Methods Clin Dev, 3 (2016) 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Reiter K, Aguilar PP, Wetter V, Steppert P, Tover A, Jungbauer A, Separation of virus-like particles and extracellular vesicles by flow-through and heparin affinity chromatography, J Chromatogr A, 1588 (2019) 77–84. [DOI] [PubMed] [Google Scholar]
- [85].Balaj L, Atai NA, Chen W, Mu D, Tannous BA, Breakefield XO, Skog J, Maguire CA, Heparin affinity purification of extracellular vesicles, Sci Rep, 5 (2015) 10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lai RC, Tan SS, Yeo RW, Choo AB, Reiner AT, Su Y, Shen Y, Fu Z, Alexander L, Sze SK, Lim SK, MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA, J Extracell Vesicles, 5 (2016) 29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Burkova EE, Dmitrenok PS, Bulgakov DV, Vlassov VV, Ryabchikova EI, Nevinsky GA, Exosomes from human placenta purified by affinity chromatography on sepharose bearing immobilized antibodies against CD81 tetraspanin contain many peptides and small proteins, IUBMB Life, 70 (2018) 1144–1155. [DOI] [PubMed] [Google Scholar]
- [88].Hung ME, Lenzini SB, Stranford DM, Leonard JN, Enrichment of Extracellular Vesicle Subpopulations Via Affinity Chromatography, Methods Mol Biol, 1740 (2018) 109–124. [DOI] [PubMed] [Google Scholar]
- [89].Clayton A, Court J, Navabi H, Adams M, Mason MD, Hobot JA, Newman GR, Jasani B, Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry, J Immunol Methods, 247 (2001) 163–174. [DOI] [PubMed] [Google Scholar]
- [90].Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C, Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes, Proc Natl Acad Sci U S A, 113 (2016) E968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ, Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes, Methods, 56 (2012) 293–304. [DOI] [PubMed] [Google Scholar]
- [92].Gori A, Romanato A, Greta B, Strada A, Gagni P, Frigerio R, Brambilla D, Vago R, Galbiati S, Picciolini S, Bedoni M, Daaboul GG, Chiari M, Cretich M, Membrane-binding peptides for extracellular vesicles on-chip analysis, J Extracell Vesicles, 9 (2020) 1751428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Nakai W, Yoshida T, Diez D, Miyatake Y, Nishibu T, Imawaka N, Naruse K, Sadamura Y, Hanayama R, A novel affinity-based method for the isolation of highly purified extracellular vesicles, Sci Rep, 6 (2016) 33935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ghosh A, Davey M, Chute IC, Griffiths SG, Lewis S, Chacko S, Barnett D, Crapoulet N, Fournier S, Joy A, Caissie MC, Ferguson AD, Daigle M, Meli MV, Lewis SM, Ouellette RJ, Rapid isolation of extracellular vesicles from cell culture and biological fluids using a synthetic peptide with specific affinity for heat shock proteins, PLoS One, 9 (2014) e110443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang K, Yue Y, Wu S, Liu W, Shi J, Zhang Z, Rapid Capture and Nondestructive Release of Extracellular Vesicles Using Aptamer-Based Magnetic Isolation, ACS Sens, 4 (2019) 1245–1251. [DOI] [PubMed] [Google Scholar]
- [96].Royo F, Thery C, Falcon-Perez JM, Nieuwland R, Witwer KW, Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee, Cells, 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Willms E, Johansson HJ, Mager I, Lee Y, Blomberg KE, Sadik M, Alaarg A, Smith CI, Lehtio J, El Andaloussi S, Wood MJ, Vader P, Cells release subpopulations of exosomes with distinct molecular and biological properties, Sci Rep, 6 (2016) 22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Reiner AT, Witwer KW, van Balkom BWM, de Beer J, Brodie C, Corteling RL, Gabrielsson S, Gimona M, Ibrahim AG, de Kleijn D, Lai CP, Lotvall J, Del Portillo HA, Reischl IG, Riazifar M, Salomon C, Tahara H, Toh WS, Wauben MHM, Yang VK, Yang Y, Yeo RWY, Yin H, Giebel B, Rohde E, Lim SK, Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles, Stem Cells Transl Med, 6 (2017) 1730–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Yamamoto KR, Alberts BM, Benzinger R, Lawhorne L, Treiber G, Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification, Virology, 40 (1970) 734–744. [DOI] [PubMed] [Google Scholar]
- [100].Karttunen J, Heiskanen M, Navarro-Ferrandis V, Das Gupta S, Lipponen A, Puhakka N, Rilla K, Koistinen A, Pitkanen A, Precipitation-based extracellular vesicle isolation from rat plasma co-precipitate vesicle-free microRNAs, J Extracell Vesicles, 8 (2019) 1555410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hermann S, Buschmann D, Kirchner B, Borrmann M, Brandes F, Kotschote S, Bonin M, Lindemann A, Reithmair M, Schelling G, Pfaffl MW, Transcriptomic profiling of cell-free and vesicular microRNAs from matched arterial and venous sera, J Extracell Vesicles, 8 (2019) 1670935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B, MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease, Leukemia, 28 (2014) 970–973. [DOI] [PubMed] [Google Scholar]
- [103].Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, Ploix S, Vimond N, Peguillet I, Thery C, Lacroix L, Zoernig I, Dhodapkar K, Dhodapkar M, Viaud S, Soria JC, Reiners KS, Pogge von Strandmann E, Vely F, Rusakiewicz S, Eggermont A, Pitt JM, Zitvogel L, Chaput N, Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC, Oncoimmunology, 5 (2016) e1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kim G, Kim M, Lee Y, Byun JW, Hwang DW, Lee M, Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes, J Control Release, 317 (2020) 273–281. [DOI] [PubMed] [Google Scholar]
- [105].Guo M, Wu F, Hu G, Chen L, Xu J, Xu P, Wang X, Li Y, Liu S, Zhang S, Huang Q, Fan J, Lv Z, Zhou M, Duan L, Liao T, Yang G, Tang K, Liu B, Liao X, Tao X, Jin Y, Autologous tumor cell-derived microparticle-based targeted chemotherapy in lung cancer patients with malignant pleural effusion, Sci Transl Med, 11 (2019). [DOI] [PubMed] [Google Scholar]
- [106].Haney MJ, Zhao Y, Jin YS, Li SM, Bago JR, Klyachko NL, Kabanov AV, Batrakova EV, Macrophage-Derived Extracellular Vesicles as Drug Delivery Systems for Triple Negative Breast Cancer (TNBC) Therapy, J Neuroimmune Pharmacol, 15 (2020) 487–500. [DOI] [PubMed] [Google Scholar]
- [107].Zhang H, Wu J, Wu J, Fan Q, Zhou J, Wu J, Liu S, Zang J, Ye J, Xiao M, Tian T, Gao J, Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice, J Nanobiotechnology, 17 (2019) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG, A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes, Mol Ther, 18 (2010) 1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK, A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer, J Transl Med, 3 (2005) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G, A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy, Biomaterials, 35 (2014)2383–2390. [DOI] [PubMed] [Google Scholar]
- [111].Yang X, Shi G, Guo J, Wang C, He Y, Exosome-encapsulated antibiotic against intracellular infections of methicillin-resistant Staphylococcus aureus, Int J Nanomedicine, 13 (2018) 8095–8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, Inskoe E, Piroyan A, Sokolsky M, Okolie O, Hingtgen SD, Kabanov AV, Batrakova EV, Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells, Nanomedicine, 12 (2016) 655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wang Y, Chen X, Tian B, Liu J, Yang L, Zeng L, Chen T, Hong A, Wang X, Nucleolin-targeted Extracellular Vesicles as a Versatile Platform for Biologics Delivery to Breast Cancer, Theranostics, 7 (2017)1360–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ugalde CL, Gordon SE, Shambrook M, Nasiri Kenari A, Coleman BM, Perugini MA, Lawson VA, Finkelstein DI, Hill AF, An intact membrane is essential for small extracellular vesicle-induced modulation of alpha-synuclein fibrillization, J Extracell Vesicles, 10 (2020) e12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Rohde E, Pachler K, Gimona M, Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing, Cytotherapy, 21 (2019) 581–592. [DOI] [PubMed] [Google Scholar]
- [116].Dong L, Pu Y, Zhang L, Qi Q, Xu L, Li W, Wei C, Wang X, Zhou S, Zhu J, Wang X, Liu F, Chen X, Su C, Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410, Cell Death Dis, 9 (2018) 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Arntz OJ, Pieters BC, Oliveira MC, Broeren MG, Bennink MB, de Vries M, van Lent PL, Koenders MI, van den Berg WB, van der Kraan PM, van de Loo FA, Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models, Mol Nutr Food Res, 59 (2015) 1701–1712. [DOI] [PubMed] [Google Scholar]
- [118].Lamson NG, Berger A, Fein KC, Whitehead KA, Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability, Nat Biomed Eng, 4 (2020) 84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Warren MR, Zhang C, Vedadghavami A, Bokvist K, Dhal PK, Bajpayee AG, Milk exosomes with enhanced mucus penetrability for oral delivery of siRNA, Biomater Sci, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y, Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis, J Transl Med, 13 (2015) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Chrzanowski W, Kim SY, McClements L, Can Stem Cells Beat COVID-19: Advancing Stem Cells and Extracellular Vesicles Toward Mainstream Medicine for Lung Injuries Associated With SARS-CoV-2 Infections, Front Bioeng Biotechnol, 8 (2020) 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Frohlich E, Therapeutic Potential of Mesenchymal Stem Cells and Their Products in Lung Diseases-Intravenous Administration versus Inhalation, Pharmaceutics, 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Geiger A, Walker A, Nissen E, Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice, Biochem Biophys Res Commun, 467 (2015) 303–309. [DOI] [PubMed] [Google Scholar]
- [124].Cabral J, Ryan AE, Griffin MD, Ritter T, Extracellular vesicles as modulators of wound healing, Adv Drug Deliv Rev, 129 (2018) 394–406. [DOI] [PubMed] [Google Scholar]
- [125].Henriques-Antunes H, Cardoso RMS, Zonari A, Correia J, Leal EC, Jimenez-Balsa A, Lino MM, Barradas A, Kostic I, Gomes C, Karp JM, Carvalho E, Ferreira L, The Kinetics of Small Extracellular Vesicle Delivery Impacts Skin Tissue Regeneration, ACS Nano, 13 (2019) 8694–8707. [DOI] [PubMed] [Google Scholar]
- [126].Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV, Exosomes as drug delivery vehicles for Parkinson's disease therapy, J Control Release, 207 (2015) 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang HG, Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain, Mol Ther, 19 (2011) 1769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Cooper JM, Wiklander PB, Nordin JZ, Al-Shawi R, Wood MJ, Vithlani M, Schapira AH, Simons JP, El-Andaloussi S, Alvarez-Erviti L, Systemic exosomal siRNA delivery reduced alpha-synuclein aggregates in brains of transgenic mice, Mov Disord, 29 (2014) 1476–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Teleanu DM, Chircov C, Grumezescu AM, Volceanov A, Teleanu RI, Blood-Brain Delivery Methods Using Nanotechnology, Pharmaceutics, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Finbloom JA, Sousa F, Stevens MM, Desai TA, Engineering the drug carrier biointerface to overcome biological barriers to drug delivery, Adv Drug Deliv Rev, 167 (2020) 89–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, Starmann J, Macas J, Karpova D, Devraj K, Depboylu C, Landfried B, Arnold B, Plate KH, Hoglinger G, Sultmann H, Altevogt P, Momma S, Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation, PLoS Biol, 12 (2014) e1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Gabr H, Elkheir W, Fares A, Farghali H, Mahmoud B, Madbouly M, Free Mesenchymal Stem Cell-Associated Exosomes Induce Better Neuroregeneration than Mesenchymal Stem Cells and Neural Differentiated Mesenchymal Stem Cells in Canine Model of Spinal Cord Injury, J Stem Cell Res Ther, 10 (2020) 463. [Google Scholar]
- [133].Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H, Nanoparticle Uptake: The Phagocyte Problem, Nano Today, 10 (2015) 487–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE, Minimal "Self" peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles, Science, 339 (2013) 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Suk JS, Xu Q, Kim N, Hanes J, Ensign LM, PEGylation as a strategy for improving nanoparticle-based drug and gene delivery, Adv Drug Deliv Rev, 99 (2016) 28–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW, Tannous BA, Breakefield XO, Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter, ACS Nano, 8 (2014) 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T, Matsumoto A, Charoenviriyakul C, Takakura Y, Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice, J Extracell Vesicles, 4 (2015) 26238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kooijmans SAA, Fliervoet LAL, van der Meel R, Fens M, Heijnen HFG, van Bergen En Henegouwen PMP, Vader P, Schiffelers RM, PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time, J Control Release, 224 (2016) 77–85. [DOI] [PubMed] [Google Scholar]
- [139].Yu SS, Lau CM, Thomas SN, Jerome WG, Maron DJ, Dickerson JH, Hubbell JA, Giorgio TD, Size- and charge-dependent non-specific uptake of PEGylated nanoparticles by macrophages, Int J Nanomedicine, 7 (2012) 799–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Mulcahy LA, Pink RC, Carter DR, Routes and mechanisms of extracellular vesicle uptake, J Extracell Vesicles, 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Yang NJ, Hinner MJ, Getting across the cell membrane: an overview for small molecules, peptides, and proteins, Methods Mol Biol, 1266 (2015) 29–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Wasungu L, Hoekstra D, Cationic lipids, lipoplexes and intracellular delivery of genes, J Control Release, 116 (2006) 255–264. [DOI] [PubMed] [Google Scholar]
- [143].Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mager I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE, Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting, J Extracell Vesicles, 4 (2015) 26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Murphy DE, de Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, Vader P, Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking, Exp Mol Med, 51 (2019) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D, Tumour exosome integrins determine organotropic metastasis, Nature, 527 (2015) 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, Mark MT, Molina H, Martin AB, Bojmar L, Fang J, Rampersaud S, Hoshino A, Matei I, Kenific CM, Nakajima M, Mutvei AP, Sansone P, Buehring W, Wang H, Jimenez JP, Cohen-Gould L, Paknejad N, Brendel M, Manova-Todorova K, Magalhaes A, Ferreira JA, Osorio H, Silva AM, Massey A, Cubillos-Ruiz JR, Galletti G, Giannakakou P, Cuervo AM, Blenis J, Schwartz R, Brady MS, Peinado H, Bromberg J, Matsui H, Reis CA, Lyden D, Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation, Nat Cell Biol, 20 (2018) 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Midekessa G, Godakumara K, Ord J, Viil J, Lattekivi F, Dissanayake K, Kopanchuk S, Rinken A, Andronowska A, Bhattacharjee S, Rinken T, Fazeli A, Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability, ACS Omega, 5 (2020) 16701–16710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Charoenviriyakul C, Takahashi Y, Morishita M, Nishikawa M, Takakura Y, Role of Extracellular Vesicle Surface Proteins in the Pharmacokinetics of Extracellular Vesicles, Mol Pharm, 15 (2018) 1073–1080. [DOI] [PubMed] [Google Scholar]
- [149].Matsumoto A, Takahashi Y, Nishikawa M, Sano K, Morishita M, Charoenviriyakul C, Saji H, Takakura Y, Role of Phosphatidylserine-Derived Negative Surface Charges in the Recognition and Uptake of Intravenously Injected B16BL6-Derived Exosomes by Macrophages, J Pharm Sci, 106 (2017) 168–175. [DOI] [PubMed] [Google Scholar]
- [150].Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y, Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection, J Biotechnol, 165 (2013) 77–84. [DOI] [PubMed] [Google Scholar]
- [151].Simonsen JB, Pitfalls associated with lipophilic fluorophore staining of extracellular vesicles for uptake studies, J Extracell Vesicles, 8 (2019) 1582237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Han Z, Liu S, Pei Y, Ding Z, Li Y, Wang X, Zhan D, Xia S, Driedonks T, Witwer KW, Weiss RG, van Zijl PCM, Bulte JWM, Cheng L, Liu G, Highly efficient magnetic labelling allows MRI tracking of the homing of stem cell-derived extracellular vesicles following systemic delivery, Journal of Extracellular Vesicles, 10 (2021) e12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Wu AY, Sung YC, Chen YJ, Chou ST, Guo V, Chien JC, Ko JJ, Yang AL, Huang HC, Chuang JC, Wu S, Ho MR, Ericsson M, Lin WW, Cheung CHY, Juan HF, Ueda K, Chen Y, Lai CP, Multiresolution Imaging Using Bioluminescence Resonance Energy Transfer Identifies Distinct Biodistribution Profiles of Extracellular Vesicles and Exomeres with Redirected Tropism, Adv Sci (Weinh), 7 (2020) 2001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Cheng Y, Wang X, Yang J, Duan X, Yao Y, Shi X, Chen Z, Fan Z, Liu X, Qin S, Tang X, Zhang C, A translational study of urine miRNAs in acute myocardial infarction, J Mol Cell Cardiol, 53 (2012) 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Dinh PC, Paudel D, Brochu H, Popowski KD, Gracieux MC, Cores J, Huang K, Hensley MT, Harrell E, Vandergriff AC, George AK, Barrio RT, Hu S, Allen TA, Blackburn K, Caranasos TG, Peng X, Schnabel LV, Adler KB, Lobo LJ, Goshe MB, Cheng K, Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis, Nat Commun, 11 (2020) 1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, Fu Y, Yang S, Zhang Z, Zhang L, Sun X, Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson's disease, J Control Release, 287 (2018) 156–166. [DOI] [PubMed] [Google Scholar]
- [157].Hu G, Liao K, Niu F, Yang L, Dallon BW, Callen S, Tian C, Shu J, Cui J, Sun Z, Lyubchenko YL, Ka M, Chen XM, Buch S, Astrocyte EV-Induced lincRNA-Cox2 Regulates Microglial Phagocytosis: Implications for Morphine-Mediated Neurodegeneration, Mol Ther Nucleic Acids, 13 (2018) 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Ciullo A, Biemmi V, Milano G, Bolis S, Cervio E, Fertig ET, Gherghiceanu M, Moccetti T, Camici GG, Vassalli G, Barile L, Exosomal Expression of CXCR4 Targets Cardioprotective Vesicles to Myocardial Infarction and Improves Outcome after Systemic Administration, Int J Mol Sci, 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Berger A, Araujo-Filho I, Piffoux M, Nicolas-Boluda A, Grangier A, Boucenna I, Real CC, Marques FLN, de Paula Faria D, do Rego ACM, Broudin C, Gazeau F, Wilhelm C, Clement O, Cellier C, Buchpiguel CA, Rahmi G, Silva AKA, Local administration of stem cell-derived extracellular vesicles in a thermoresponsive hydrogel promotes a pro-healing effect in a rat model of colocutaneous post-surgical fistula, Nanoscale, 13 (2021) 218–232. [DOI] [PubMed] [Google Scholar]
- [160].Brossa A, Fonsato V, Grange C, Tritta S, Tapparo M, Calvetti R, Cedrino M, Fallo S, Gontero P, Camussi G, Bussolati B, Extracellular vesicles from human liver stem cells inhibit renal cancer stem cell-derived tumor growth in vitro and in vivo, Int J Cancer, 147 (2020) 1694–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Das A, Valkov N, Salvador AM, Kur I, Ziegler O, Yeri A, Garcia FC, Lu S, Khamesra A, Xiao C, Rodosthenous R, Li G, Srinivasan S, Toxavidis V, Tigges J, Laurent LC, Momma S, Ghiran I, Das S, Red blood cell-derived extracellular vesicles mediate intercellular communication in ischemic heart failure, bioRxiv, (2019) 624841. [Google Scholar]
- [162].Han C, Xiong N, Guo X, Huang J, Ma K, Liu L, Xia Y, Shen Y, Li J, Jiang H, Wang L, Guo S, Xu X, Zhang G, Liu J, Cao X, Zhang Z, Lin Z, Wang T, Exosomes from patients with Parkinson's disease are pathological in mice, J Mol Med (Berl), 97 (2019) 1329–1344. [DOI] [PubMed] [Google Scholar]
- [163].Joy DC, Limits of SEM Resolution, Microscopy Today, 4 (1996) 10–11. [Google Scholar]
- [164].Hoog JL, Lotvall J, Diversity of extracellular vesicles in human ejaculates revealed by cryoelectron microscopy, J Extracell Vesicles, 4 (2015) 28680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Rikkert LG, Nieuwland R, Terstappen L, Coumans FAW, Quality of extracellular vesicle images by transmission electron microscopy is operator and protocol dependent, J Extracell Vesicles, 8(2019)1555419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Van Deun J, Mestdagh P, Agostinis P, Akay O, Anand S, Anckaert J, Martinez ZA, Baetens T, Beghein E, Bertier L, Berx G, Boere J, Boukouris S, Bremer M, Buschmann D, Byrd JB, Casert C, Cheng L, Cmoch A, Daveloose D, De Smedt E, Demirsoy S, Depoorter V, Dhondt B, Driedonks TA, Dudek A, Elsharawy A, Floris I, Foers AD, Gartner K, Garg AD, Geeurickx E, Gettemans J, Ghazavi F, Giebel B, Kormelink TG, Hancock G, Helsmoortel H, Hill AF, Hyenne V, Kalra H, Kim D, Kowal J, Kraemer S, Leidinger P, Leonelli C, Liang Y, Lippens L, Liu S, Lo Cicero A, Martin S, Mathivanan S, Mathiyalagan P, Matusek T, Milani G, Monguio-Tortajada M, Mus LM, Muth DC, Nemeth A, Nolte-'t Hoen EN, O'Driscoll L, Palmulli R, Pfaffl MW, Primdal-Bengtson B, Romano E, Rousseau Q, Sahoo S, Sampaio N, Samuel M, Scicluna B, Soen B, Steels A, Swinnen JV, Takatalo M, Thaminy S, Thery C, Tulkens J, Van Audenhove I, van der Grein S, Van Goethem A, van Herwijnen MJ, Van Niel G, Van Roy N, Van Vliet AR, Vandamme N, Vanhauwaert S, Vergauwen G, Verweij F, Wallaert A, Wauben M, Witwer KW, Zonneveld MI, De Wever O, Vandesompele J, Hendrix A, EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research, Nat Methods, 14 (2017) 228–232. [DOI] [PubMed] [Google Scholar]
- [167].Egerton R, The scanning electron microscope, Physical Principles of Electron Microscopy, Springer; 2016, pp. 121–147. [Google Scholar]
- [168].Chuo ST, Chien JC, Lai CP, Imaging extracellular vesicles: current and emerging methods, J Biomed Sci, 25 (2018) 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Kondratov KA, Petrova TA, Mikhailovskii VY, Ivanova AN, Kostareva AA, Fedorov AV, A study of extracellular vesicles isolated from blood plasma conducted by low-voltage scanning electron microscopy, Cell and Tissue Biology, 11 (2017) 181–190. [PubMed] [Google Scholar]
- [170].Reimer L, Transmission Electron Microscopy: Physics of Image Formation and Microanalysis, Springer Berlin Heidelberg; 2013. [Google Scholar]
- [171].Chernyshev VS, Rachamadugu R, Tseng YH, Belnap DM, Jia Y, Branch KJ, Butterfield AE, Pease LF 3rd, Bernard PS, Skliar M, Size and shape characterization of hydrated and desiccated exosomes, Anal Bioanal Chem, 407 (2015) 3285–3301. [DOI] [PubMed] [Google Scholar]
- [172].Varga Z, Feher B, Kitka D, Wacha A, Bota A, Berenyi S, Pipich V, Fraikin JL, Size Measurement of Extracellular Vesicles and Synthetic Liposomes: The Impact of the Hydration Shell and the Protein Corona, Colloids Surf B Biointerfaces, 192 (2020) 111053. [DOI] [PubMed] [Google Scholar]
- [173].Yuana Y, Oosterkamp TH, Bahatyrova S, Ashcroft B, Garcia Rodriguez P, Bertina RM, Osanto S, Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles, J Thromb Haemost, 8 (2010) 315–323. [DOI] [PubMed] [Google Scholar]
- [174].Ridolfi A, Brucale M, Montis C, Caselli L, Paolini L, Borup A, Boysen AT, Loria F, van Herwijnen MJC, Kleinjan M, Nejsum P, Zarovni N, Wauben MHM, Berti D, Bergese P, Valle F, AFM-Based High-Throughput Nanomechanical Screening of Single Extracellular Vesicles, Anal Chem, 92 (2020) 10274–10282. [DOI] [PubMed] [Google Scholar]
- [175].Linares R, Tan S, Gounou C, Brisson AR, Imaging and Quantification of Extracellular Vesicles by Transmission Electron Microscopy, Methods Mol Biol, 1545 (2017) 43–54. [DOI] [PubMed] [Google Scholar]
- [176].Bachurski D, Schuldner M, Nguyen PH, Malz A, Reiners KS, Grenzi PC, Babatz F, Schauss AC, Hansen HP, Hallek M, Pogge von Strandmann E, Extracellular vesicle measurements with nanoparticle tracking analysis - An accuracy and repeatability comparison between NanoSight NS300 and ZetaView, J Extracell Vesicles, 8 (2019) 1596016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Mallick ER, Arab T, Huang Y, Dong L, Liao Z, Zhao Z, Smith B, Haughey NJ, Pienta KJ, Slusher BS, Tarwater PM, Tosar JP, Zivkovic AM, Vreeland WN, Paulaitis ME, Witwer KW, Characterization of extracellular vesicles and artificial nanoparticles with four orthogonal single-particle analysis platforms, bioRxiv, (2020) 2020.2008.2004.237156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Filipe V, Hawe A, Jiskoot W, Critical evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates, Pharm Res, 27 (2010) 796–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Vogel R, Willmott G, Kozak D, Roberts GS, Anderson W, Groenewegen L, Glossop B, Barnett A, Turner A, Trau M, Quantitative sizing of nano/microparticles with a tunable elastomeric pore sensor, Anal Chem, 83 (2011) 3499–3506. [DOI] [PubMed] [Google Scholar]
- [180].Anderson W, Lane R, Korbie D, Trau M, Observations of Tunable Resistive Pulse Sensing for Exosome Analysis: Improving System Sensitivity and Stability, Langmuir, 31 (2015) 6577–6587. [DOI] [PubMed] [Google Scholar]
- [181].Coumans FA, van der Pol E, Boing AN, Hajji N, Sturk G, van Leeuwen TG, Nieuwland R, Reproducible extracellular vesicle size and concentration determination with tunable resistive pulse sensing, J Extracell Vesicles, 3 (2014) 25922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Fraikin JL, Teesalu T, McKenney CM, Ruoslahti E, Cleland AN, A high-throughput label-free nanoparticle analyser, Nat Nanotechnol, 6 (2011) 308–313. [DOI] [PubMed] [Google Scholar]
- [183].Anderson W, Kozak D, Coleman VA, Jamting AK, Trau M, A comparative study of submicron particle sizing platforms: accuracy, precision and resolution analysis of polydisperse particle size distributions, J Colloid Interface Sci, 405 (2013) 322–330. [DOI] [PubMed] [Google Scholar]
- [184].Dragovic RA, Gardiner C, Brooks AS, Tannetta DS, Ferguson DJ, Hole P, Carr B, Redman CW, Harris AL, Dobson PJ, Harrison P, Sargent IL, Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis, Nanomedicine, 7 (2011) 780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Sitar S, Kejzar A, Pahovnik D, Kogej K, Tusek-Znidaric M, Lenassi M, Zagar E, Size characterization and quantification of exosomes by asymmetrical-flow field-flow fractionation, Anal Chem, 87 (2015) 9225–9233. [DOI] [PubMed] [Google Scholar]
- [186].Caputo F, Mehn D, Clogston JD, Rosslein M, Prina-Mello A, Borgos SE, Gioria S, Calzolai L, Asymmetric-flow field-flow fractionation for measuring particle size, drug loading and (in)stability of nanopharmaceuticals. The joint view of European Union Nanomedicine Characterization Laboratory and National Cancer Institute - Nanotechnology Characterization Laboratory, J Chromatogr A, 1635 (2021) 461767. [DOI] [PubMed] [Google Scholar]
- [187].Caputo F, Arnould A, Bacia M, Ling WL, Rustique E, Texier I, Mello AP, Couffin AC, Measuring Particle Size Distribution by Asymmetric Flow Field Flow Fractionation: A Powerful Method for the Preclinical Characterization of Lipid-Based Nanoparticles, Mol Pharm, 16 (2019) 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Parot J, Caputo F, Mehn D, Hackley VA, Calzolai L, Physical characterization of liposomal drug formulations using multi-detector asymmetrical-flow field flow fractionation, J Control Release, 320 (2020) 495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Nolan JP, Flow Cytometry of Extracellular Vesicles: Potential, Pitfalls, and Prospects, Curr Protoc Cytom, 73 (2015) 13 14 11–16. [DOI] [PubMed] [Google Scholar]
- [190].van der Pol E, van Gemert MJ, Sturk A, Nieuwland R, van Leeuwen TG, Single vs. swarm detection of microparticles and exosomes by flow cytometry, J Thromb Haemost, 10 (2012) 919–930. [DOI] [PubMed] [Google Scholar]
- [191].Nolte-'t Hoen EN, van der Vlist EJ, Aalberts M, Mertens HC, Bosch BJ, Bartelink W, Mastrobattista E, van Gaal EV, Stoorvogel W, Arkesteijn GJ, Wauben MH, Quantitative and qualitative flow cytometric analysis of nanosized cell-derived membrane vesicles, Nanomedicine, 8 (2012) 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Wiklander OPB, Bostancioglu RB, Welsh JA, Zickler AM, Murke F, Corso G, Felldin U, Hagey DW, Evertsson B, Liang XM, Gustafsson MO, Mohammad DK, Wiek C, Hanenberg H, Bremer M, Gupta D, Bjornstedt M, Giebel B, Nordin JZ, Jones JC, El Andaloussi S, Gorgens A, Systematic Methodological Evaluation of a Multiplex Bead-Based Flow Cytometry Assay for Detection of Extracellular Vesicle Surface Signatures, Front Immunol, 9 (2018) 1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Tian Y, Ma L, Gong M, Su G, Zhu S, Zhang W, Wang S, Li Z, Chen C, Li L, Wu L, Yan X, Protein Profiling and Sizing of Extracellular Vesicles from Colorectal Cancer Patients via Flow Cytometry, ACS Nano, 12 (2018) 671–680. [DOI] [PubMed] [Google Scholar]
- [194].Görgens A, Bremer M, Ferrer-Tur R, Murke F, Tertel T, Horn PA, Thalmann S, Welsh JA, Probst C, Guerin C, Boulanger CM, Jones JC, Hanenberg H, Erdbrügger U, Lannigan J, Ricklefs FL, El-Andaloussi S, Giebel B, Optimisation of imaging flow cytometry for the analysis of single extracellular vesicles by using fluorescence-tagged vesicles as biological reference material, Journal of Extracellular Vesicles, 8 (2019) 1587567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Avci O, Unlu NL, Ozkumur AY, Unlu MS, Interferometric Reflectance Imaging Sensor (IRIS)--A Platform Technology for Multiplexed Diagnostics and Digital Detection, Sensors (Basel), 15 (2015) 17649–17665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Logozzi M, Di Raimo R, Mizzoni D, Fais S, Immunocapture-based ELISA to characterize and quantify exosomes in both cell culture supernatants and body fluids, Methods Enzymol, 645 (2020) 155–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Hartjes TA, Mytnyk S, Jenster GW, van Steijn V, van Royen ME, Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches, Bioengineering (Basel), 6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Garcia-Manrique P, Serrano-Pertierra E, Lozano-Andres E, Lopez-Martin S, Matos M, Gutierrez G, Yanez-Mo M, Blanco-Lopez MC, Selected Tetraspanins Functionalized Niosomes as Potential Standards for Exosome Immunoassays, Nanomaterials (Basel), 10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Pocsfalvi G, Stanly C, Vilasi A, Fiume I, Capasso G, Turiak L, Buzas EI, Vekey K, Mass spectrometry of extracellular vesicles, Mass Spectrom Rev, 35 (2016) 3–21. [DOI] [PubMed] [Google Scholar]
- [200].Chen S, Datta-Chaudhuri A, Deme P, Dickens A, Dastgheyb R, Bhargava P, Bi H, Haughey NJ, Lipidomic characterization of extracellular vesicles in human serum, J Circ Biomark, 8 (2019) 1849454419879848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ, Reassessment of Exosome Composition, Cell, 177 (2019) 428–445 e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Mateescu B, Kowal EJ, van Balkom BW, Bartel S, Bhattacharyya SN, Buzas EI, Buck AH, de Candia P, Chow FW, Das S, Driedonks TA, Fernandez-Messina L, Haderk F, Hill AF, Jones JC, Van Keuren-Jensen KR, Lai CP, Lasser C, Liegro ID, Lunavat TR, Lorenowicz MJ, Maas SL, Mager I, Mittelbrunn M, Momma S, Mukherjee K, Nawaz M, Pegtel DM, Pfaffl MW, Schiffelers RM, Tahara H, Thery C, Tosar JP, Wauben MH, Witwer KW, Nolte-'t Hoen EN, Obstacles and opportunities in the functional analysis of extracellular vesicle RNA - an ISEV position paper, J Extracell Vesicles, 6 (2017) 1286095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Enderle D, Spiel A, Coticchia CM, Berghoff E, Mueller R, Schlumpberger M, Sprenger-Haussels M, Shaffer JM, Lader E, Skog J, Noerholm M, Characterization of RNA from Exosomes and Other Extracellular Vesicles Isolated by a Novel Spin Column-Based Method, PLoS One, 10 (2015) e0136133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Vagner T, Spinelli C, Minciacchi VR, Balaj L, Zandian M, Conley A, Zijlstra A, Freeman MR, Demichelis F, De S, Posadas EM, Tanaka H, Di Vizio D, Large extracellular vesicles carry most of the tumour DNA circulating in prostate cancer patient plasma, J Extracell Vesicles, 7 (2018) 1505403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Bosch S, de Beaurepaire L, Allard M, Mosser M, Heichette C, Chretien D, Jegou D, Bach JM, Trehalose prevents aggregation of exosomes and cryodamage, Sci Rep, 6 (2016) 36162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].De Wever O, Hendrix A, A supporting ecosystem to mature extracellular vesicles into clinical application, EMBO J, 38 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Morishita M, Takahashi Y, Nishikawa M, Sano K, Kato K, Yamashita T, Imai T, Saji H, Takakura Y, Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice, J Pharm Sci, 104 (2015) 705–713. [DOI] [PubMed] [Google Scholar]
- [208].Witwer KW, Wolfram J, Extracellular vesicles versus synthetic nanoparticles for drug delivery, Nature Reviews Materials, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Simonsen JB, A liposome-based size calibration method for measuring microvesicles by flow cytometry, J Thromb Haemost, 14 (2016) 186–190. [DOI] [PubMed] [Google Scholar]
- [210].Geeurickx E, Tulkens J, Dhondt B, Van Deun J, Lippens L, Vergauwen G, Heyrman E, De Sutter D, Gevaert K, Impens F, Miinalainen I, Van Bockstal PJ, De Beer T, Wauben MHM, Nolte-'t-Hoen ENM, Bloch K, Swinnen JV, van der Pol E, Nieuwland R, Braems G, Callewaert N, Mestdagh P, Vandesompele J, Denys H, Eyckerman S, De Wever O, Hendrix A, The generation and use of recombinant extracellular vesicles as biological reference material, Nat Commun, 10 (2019) 3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Geeurickx E, Lippens L, Rappu P, De Geest BG, De Wever O, Hendrix A, Recombinant extracellular vesicles as biological reference material for method development, data normalization and assessment of (pre-)analytical variables, Nat Protoc, (2021). [DOI] [PubMed] [Google Scholar]
- [212].Welsh JA, van der Pol E, Bettin BA, Carter DRF, Hendrix A, Lenassi M, Langlois MA, Llorente A, van de Nes AS, Nieuwland R, Tang V, Wang L, Witwer KW, Jones JC, Towards defining reference materials for measuring extracellular vesicle refractive index, epitope abundance, size and concentration, J Extracell Vesicles, 9 (2020) 1816641. [DOI] [PMC free article] [PubMed] [Google Scholar]